Highlights
-
•
Coronaviruses are RNA viruses that cause systemic diseases in humans and animals.
-
•
There are no approved drugs for the treatment of coronavirus infections.
-
•
Several SARS-CoV inhibitors, with known mechanisms of action, have been identified.
-
•
These inhibitors stand as promising leads for coronavirus therapeutics.
Abstract
Coronaviruses are positive stranded RNA viruses that cause respiratory, enteric and central nervous system diseases in many species, including humans. Until recently, the relatively low burden of disease in humans caused by few of these viruses impeded the development of coronavirus specific therapeutics. However, the emergence of severe acute respiratory syndrome coronavirus (SARS-CoV), and more recently, Middle East respiratory syndrome coronavirus (MERS-CoV), has impelled the development of such drugs. This review focuses on some newly identified SARS-CoV inhibitors, with known mechanisms of action and their potential to inhibit the novel MERS-CoV. The clinical development of optimized versions of such compounds could be beneficial for the treatment and control of SARS-CoV, the current MERS-CoV and other future SARS-like epidemics.
Current Opinion in Virology 2014, 8:45–53
This review comes from a themed issue on Antivirals and resistance
Edited by Luis Menéndez-Arias and Douglas D Richman
For a complete overview see the Issue and the Editorial
Available online 2nd July 2014
http://dx.doi.org/10.1016/j.coviro.2014.06.002
1879-6257/© 2014 Elsevier B.V. All rights reserved.
Introduction
In September 2012, a novel coronavirus (CoV) called Middle East respiratory syndrome CoV (MERS-CoV), was isolated as the causative agent of a severe pneumonia in several patients in the Middle East [1]. Globally, as of May 16, 2014, WHO has been informed of a total of 614 laboratory-confirmed cases of infection with MERS-CoV (including 181 deaths) primarily in the Middle East (Saudi Arabia, Jordan, Qatar, Oman, Kuwait, and the United Arab Emirates), but also in Europe (the UK, France, Italy, Germany, and Greece), North Africa (Tunisia and Egypt), Asia (Malaysia) and the United States of America (http://www.who.int/csr/don/2014_05_16_mers/en/, http://www.cdc.gov/coronavirus/mers/). This CoV is closely related to severe acute respiratory syndrome CoV (SARS-CoV), an epidemic that was short-lived but alarming in 2002–2003 that resulted in approximately 8000 cases and 800 deaths.
SARS-CoV and MERS-CoV both belong to the family Coronaviridae, which are enveloped, positive-stranded RNA viruses with approximately 30,000 nucleotides [2••]. CoVs represent the largest RNA viruses. For the well-characterized SARS-CoV, two overlapping open reading frames (ORF1a and ORF1b), encompass approximately two-thirds of the genome. A translational read-through by a −1 ribosomal frameshift mechanism allows the translation of the overlapping reading frames into a single polyprotein pp1ab, whereas, translation without the −1 ribosomal frameshift mechanism produces pp1a. The polyproteins are later cleaved by two viral proteinases, 3C-like protease (3CLP) and papain-like protease (PLP), to yield non-structural proteins essential for viral replication [3, 4]. The remaining one-third of the genome encodes structural proteins of the virus, which include the spike (S), envelope (E), membrane (M) and nucleocapsid (N) proteins [5, 6].
On the basis of phylogenic analyses, evolutionary studies have shown that SARS-CoV originated most likely from bats. It has been reported to be transmitted to humans by aerosols through intermediate hosts like palm civets infected by the virus [7, 8, 9]. Therefore, the zoonosis of CoV is a threat, due to its ability of interspecies transfer into human population. This has been recapitulated with the novel MERS-CoV, as recent studies have suggested that bats and dromedary camels serve as a reservoir for this virus [10, 11, 12, 13, 14, 15]. MERS-CoV shows SARS-like symptoms following human infections, which include malaise, rigors, fatigues and high fevers, indications similar to influenza, but later progresses to atypical pneumonia in most cases [16].
Although, many antiviral agents have been identified to inhibit SARS in vitro, there are presently, no approved antiviral agents or vaccines available to tackle any potential SARS or SARS-like outbreaks, such as MERS. Different parts of the virus, that are deemed viable targets include, 3CLP, PLP, RNA-dependent RNA polymerase (RdRp) and the 5′–3′ helicase [17, 18•, 19]. Other possible targets include E protein (Orf4), M protein (Orf6), and N protein (Orf9) [17]. This review focuses on the published antiviral inhibitors of coronaviruses, with SARS-CoV being considered as the primary virus. The inhibitors include replication and entry inhibitors, which can be developed for therapeutic purposes, not just against SARS, but other coronaviruses, including the novel MERS-CoV.
Replication inhibitors
Protease inhibitors
The first coronavirus proteins that have been studied in detail include viral proteinases, namely the papain-like protease (PLP) or nsp3 and the 3C-like protease (3CLP, nsp5 or Mpro), which cleave the polyprotein into individual polypeptides that are required for replication and transcription [3, 4]. Following the translation of the messenger RNA to yield the polyproteins, the 3CLP is first auto-cleaved from the polyproteins to become a mature enzyme. The 3CLP further cleaves all the 11 remaining downstream non-structural proteins. Hence, 3CLP is an essential viral protein for the viral replication cycle, and as a result becomes an attractive target for anti-SARS drug development [20, 21••, 22]. 3CLP inhibitors are among the first SARS-CoV inhibitors that were discovered by screening compound libraries using an assay that utilizes a fluorogenic peptide as the substrate and with structure-based design on the basis of the crystal structures of the product-bound form of 3CLP [23, 24, 25]. The compounds identified include zinc or mercury conjugates [26, 27], C2-symmetric diols [28, 29], peptidomimetic α,β-unsaturated esters [30], anilides [31], benzotriazole [32], N-phenyl-2-(2-pyrimidinylthio)acetamide [33], biphenyl sulfone [34], glutamic acid and glutamine peptides possessing a trifluoromethylketone group [35], pyrimidinone [36], and pyrazole analogs that can also inhibit 3Cpro of picornaviruses CV-B3 (coxsackievirus), EV-71 (enterovirus) and RV-14 (rhinovirus) (coronavirus and picornavirus dual inhibitors) [24, 25]. The names and chemical structures of some of the published 3C-protease inhibitors are shown in Figure 1 .
Figure 1.
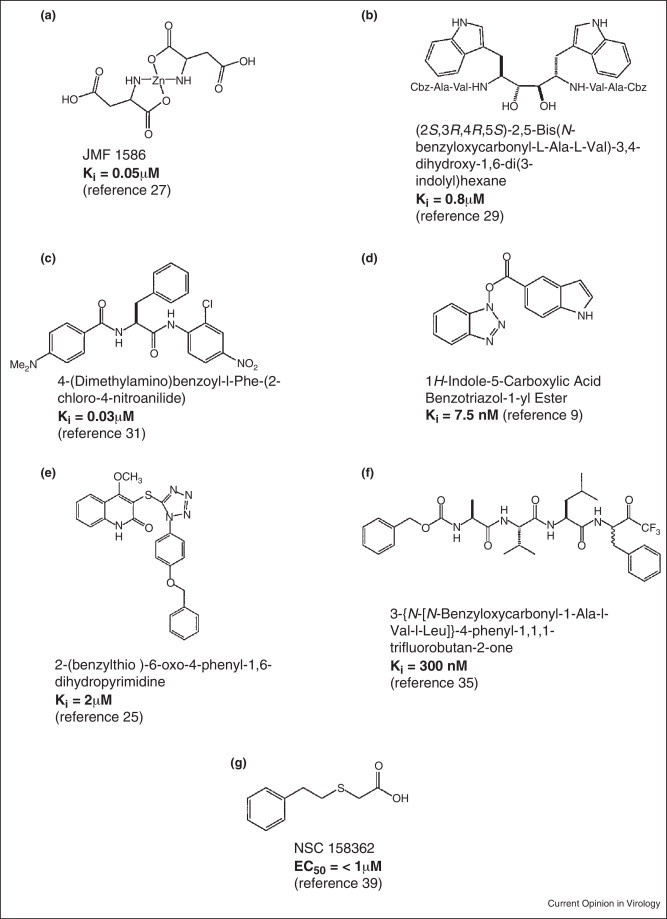
Names and chemical structures of examples of SARS-CoV 3CLP and PLP protease inhibitors.
The papain like protease (PLP) is also an essential component of the SARS-CoV replication machinery. PLP is the nsp3 protein which is part of the synthesized ORF1a polyprotein during replication. nsp3 cleaves protease recognition sites between nsp1/2, nsp2/3 and nsp3/4 [37]. In addition to its protease activity, nsp3 has been shown to have deubiquitination, and interferon antagonist activities in vitro [38]. Since its homologues are found in all coronaviruses, it has also been proposed to be a good target for drug discovery for both SARS-CoV and other human coronaviruses.
Recently, Frieman et al. [39] developed a yeast-based assay to screen for small molecules that block SARS-CoV replication on the basis of their inhibition of nsp3 or PLP. The basis for the screen was that stimulated expression of nsp3 in Saccharomyces cerevisiae causes a pronounced slow growth phenotype. Using this principle, they screened a small molecule library for compounds that specifically prevented the nsp3-induced slow growth phenotype. These compounds were then validated in cell culture models for efficacy against SARS-CoV replication, as well as the known enzymatic functions of nsp3. The authors found five compounds that reversed the slow growth phenotype in yeast. One of the compounds, NSC158362 (Figure 1g), considerably blocked SARS-CoV replication in vitro with an EC50 < 1 μM. This effect was specific for SARS-CoV replication because no effect on influenza virus replication was observed with up to 50 μM of the inhibitory compound. Another compound, NSC158011, was shown to inhibit nsp3-dependent protease activity in a cell culture assay, but could not prevent virus replication. NSC158362, could not inhibit the protease, deubiquitinase or anti-IFN activities of nsp3, therefore suggesting that the compound may be inhibiting a yet unknown novel activity of nsp3 required for viral replication or may be inhibiting some cellular factors that regulate nsp3 function in infected cells.
Helicase inhibitors
Helicases are proteins that catalyze the separation of duplex oligonucleotides into single strands in an ATP-dependent reaction. On the basis of this activity, helicases can be divided into two types: those that unwind duplexes in a 3′→5′ direction, and those that unwind in a 5′→3′ direction. Helicases require a molecular mechanism for transducing the chemical energy generated by the ATPase activity into an oligonucleotide strand separation and displacement activity [40]. The functions of helicase in positive-sense RNA viruses include nucleic acid separations [41••], the melting of highly stable secondary structures within the genomic RNA in order to increase translational efficiency of the polyprotein [42]. Considering all these helicase functions, viral helicases stand as a strong antiviral target.
The helicase of SARS-CoV is called nsp13. A few potential inhibitors of nsp13 have been identified [19, 43, 44, 45, 46]. Some of these inhibitors inhibit nsp13 by interfering with its unwinding and ATPase activities. They include the bananins and the 5-hydroxychromone derivatives [43, 45]. The bananins are a class of antiviral compounds with an exclusive structural signature that incorporates a trioxa-adamantane moiety covalently bound to a pyridoxal derivative [45]. Six members of this class of compounds were synthesized by Tanner et al. [45]. The compounds are bananin, iodobananin, vanillinbananin, ansabananin, eubananin, and adeninobananin. Of the six compounds, bananin, iodobananin, vanillinbananin, and eubananin effectively inhibited the helicase activity of nsp13 by inhibiting the ATPase activity of the helicase with IC50 values in the range 0.5–3 μM. Bananin was also shown to exhibit antiviral activity against SARS-CoV in a cell culture assay, with an EC50 of less than 10 μM [45]. The structure of vanillinbananin is shown in Figure 2a. For the 5-hydroxychromone derivatives, 5-hydroxychromone was used as a scaffold on which two arylmethyloxy substituents were installed. The resulting derivatives include 5-hydroxy-6-(3-chlorobenzyloxy)-chromone with chloro-benzyloxy or iodo-benzyloxy substituents [43] (Figure 2b). These compounds, similar to the bananins, also inhibited the unwinding activities of SARS-CoV nsp13 by inhibiting the ATPase activity of the helicase at comparable EC50s of ∼10 μM [43]. Although these compounds portend to be a promising therapy, the potential ability of these compounds to inhibit the ATPase activity of cellular ATPases or kinases may affect cellular activities, thereby creating a risk of cytotoxicity. A study reported that an aryl diketoacid compound (Figure 2c) selectively inhibited the duplex DNA unwinding activity of SARS-CoV nsp13 without significantly inhibiting its ATPase activity [44]. However, the effects of this compound on nsp13's unwinding activity of double-stranded RNA (dsRNA) and the replication of SARS-CoV were not determined [44].
Figure 2.
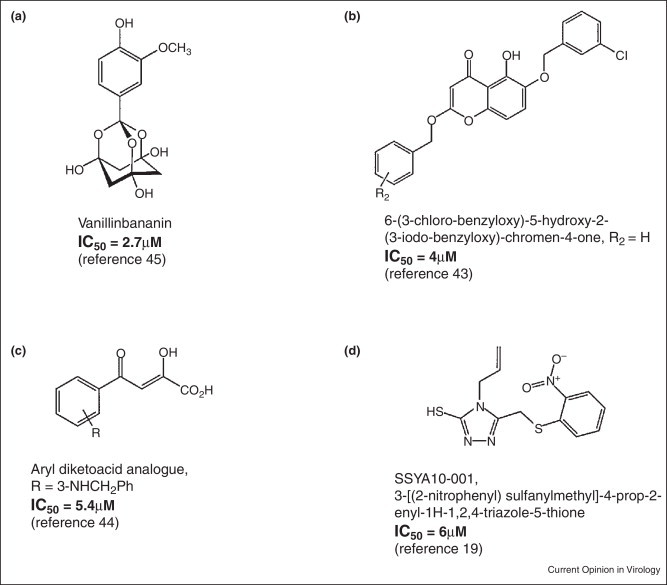
Names and chemical structures of examples of SARS-CoV helicase inhibitors.
A recent report revealed an inhibitor of nsp13 that inhibited the unwinding, but not the ATPase enzymatic and nucleic acid binding activities of nsp13 [19]. Using biochemical analyses, the authors demonstrated that this compound, SSYA10-001 (Figure 2d), is a noncompetitive inhibitor of nsp13 with respect to its major substrates, namely, nucleic acids and ATP. Since this compound did not inhibit nsp13's binding and hydrolysis of nucleic acids and ATP, coupled with the fact that it did not bind the nucleic acid substrate, the authors suggested that, SSYA10-001 may be inhibiting nsp13 unwinding activity by interfering with its conformational changes during the course of the reaction or translocation on the nucleic acid [19]. Moreover, SSYA10-001 was also shown to be an efficient inhibitor of viral replication in a SARS-CoV replicon and live viral assays. More recently, SSYA10-001 was shown to exhibit a broad-spectrum activity against other coronaviruses, including MERS-CoV and mouse hepatitis virus (MHV) [47].
Entry inhibitors
Viral entry is an essential step of the virus replication cycle that can be targeted for therapy [48]. Entry inhibitors of several viruses have been identified. Examples include: RFI 641 and VP-14637, which are small molecules that inhibit the entry of respiratory syncytial virus by binding at a hydrophobic pocket of the fusion (F) glycoprotein [49, 50]; some inhibitors have been reported to block HIV entry by using different strategies. Maraviroc is an entry inhibitor of HIV that targets CCR5, a host protein used as a coreceptor during HIV entry [51, 52, 53]. Enfuvirtide and SC29EK are peptides that can also block viral entry by binding to the viral transmembrane protein gp41 and blocking the final stage of fusion with the target cell [54, 55, 56, 57, 58, 59]. A few monoclonal antibodies are currently in clinical trials, and they include KD-247 and PRO140. They prevent HIV entry by binding either the viral surface glycoprotein gp120 [60, 61] or the CCR5 coreceptor [62, 63].
The surface glycoprotein of SARS-CoV, SARS-S, has two constituents: S1, which comprises the receptor binding domain (RBD), and S2, which comprises the fusion peptide. SARS-CoV gains entry into permissive cells through interactions of the SARS-S RBD with the cell surface receptor, angiotensin converting enzyme 2 (ACE2) (Figure 3 ) [20, 64]. Following these interactions, endocytosis occurs. When the endosomes are within the region of low pH in the cell, SARS-S is cleaved by a cellular protease called cathepsin L (Figure 4 ), leading to the exposure of the S2 domain of the spike protein for membrane fusion [65, 66, 67••, 68, 69••, 70]. Some reports have also suggested the possibility of the fusion of SARS-S-expressing cells with ACE2 receptor-expressing cells in a pH-independent environment at the cell surface [71, 72].
Figure 3.
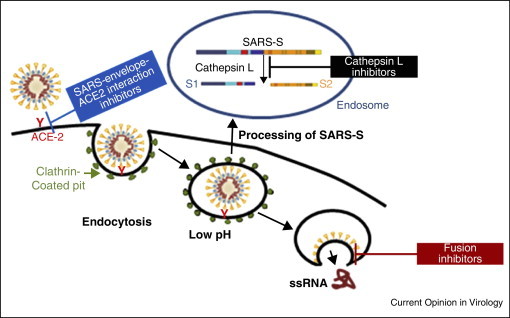
Model for the different stages of SARS-CoV entry that are potential antiviral targets.
Figure 4.
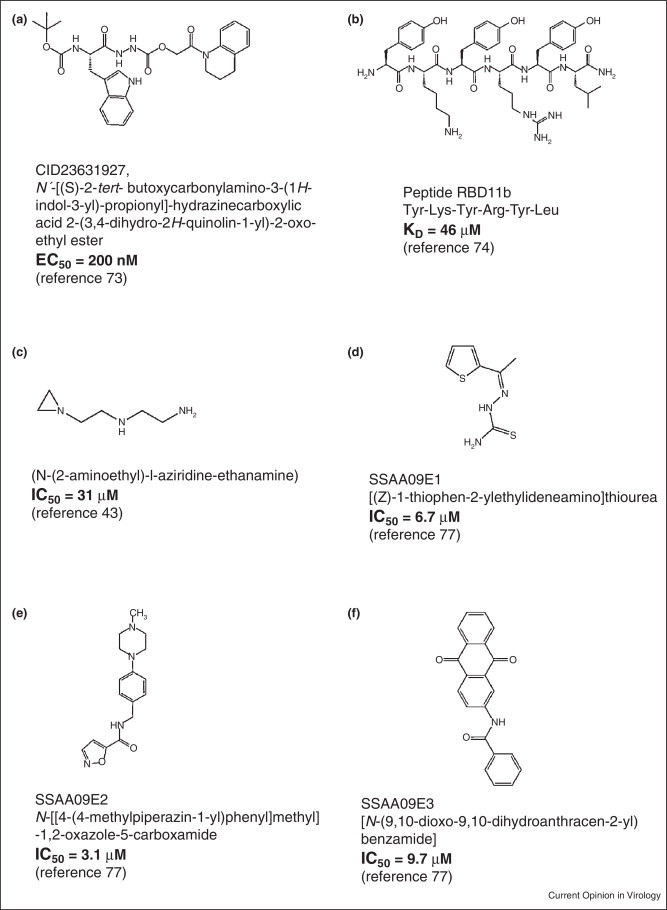
Examples of SARS-CoV entry inhibitors.
The possible targets for SARS viral entry, thus, include cathepsin L, ACE2–SARS-S1 interaction and S2-cell membrane fusion (Figure 3). A number of studies have identified some inhibitors that interfere with these targets. Dipeptide epoxyketones, calpain inhibitor III, oxocarbazate, MDL28170 and SSAA09E1 are all cathepsin L inhibitors (Figure 4) [67••, 73, 74, 75, 76••, 77••]. Although the first four compounds are peptidomimetic in nature (Figure 4a–c), SSAA09E1 (Figure 4d) is a small molecule cathepsin L inhibitor that is likely to have improved bioavailability over others. These compounds have been shown to inhibit SARS viral entry by hindering the ability of cathepsin L to cleave S1 from S2, thereby preventing the fusion of the viral envelope with the host cell membrane, following endocytosis. Other SARS viral entry inhibitors include SSAA09E2 (Figure 4e), SSAA09E3 (Figure 4f), and NAAE (N-(2-aminoethyl)-l-aziridine-ethanamine), all of which are small molecule compounds. SSAA09E3 [77••] prevents fusion of the viral membrane with the host cellular membrane (Figure 3, Figure 4). SSAA09E2 [77••] and NAAE [78] act by blocking early interactions of SARS-S with the receptor for SARS-CoV, angiotensin converting enzyme 2 (ACE2) (Figure 3). NAAE is a unique small molecule entry inhibitor in that, it inhibits both ACE2 catalytic activity and S-protein-induced cell-cell fusion [78]. Although dual inhibitory effects on ACE2 catalytic activity and SARS-CoV binding is not expected, because the catalytic site of ACE2 is distinct from the S-protein-binding domain [71, 79••], NAAE did show antiviral activity. Overexpression of ACE has been shown to cause hypertension, which is counterbalanced by ACE2 (through vasodilation) [80]. Hence, inhibition of ACE2 by NAAE stands as a potential risk for hypertension.
Making a case for clinical development of coronavirus inhibitors
Currently, there are no approved drugs for the treatment of SARS-CoV infection. During the initial outbreak of SARS, a number of medications that included ribavirin with and without corticosteroids [81, 82, 83], interferon (alfacon-1) with corticosteroids [84], and ribavirin with protease inhibitors [85] had some encouraging outcomes, but a definitive treatment regimen was not clearly established. More recently, a combination of IFN-α2b and ribavirin were shown to have some in vitro synergistic activities against MERS-CoV, albeit at higher concentrations [86]. These drugs have known pharmacological properties and are, therefore, widely available, as some of them are used for the treatment of some viral infections, such as hepatitis C virus, albeit with some adverse side effects including hemolytic anemia, elevated transaminase levels and bradycardia [83], depression, suicide, relapse of drug abuse/overdose, bacterial infections and many others (Interferon and ribavirin treatment side effects, http://www.hepatitis.va.gov/provider/reviews/treatment-side-effects.asp#S1X). Hence, with the new outbreak of MERS-CoV infection, optimization and development of some of the aforementioned lead compounds or other inhibitors with known or new mechanisms of action becomes important for therapeutic purposes, as there are no reports of any compound in clinical trials.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
Acknowledgements
We thank Dr. Karen Kirby for help with the figures. This work was supported, in whole or in part, by National Institutes of Health grants AI076119, AI099284, AI100890, AI112417, and GM103368 (SGS). We also acknowledge support from Ministry of Knowledge and Economy, Bilateral International Collaborative R&D Program, Republic of Korea.
References
- 1.Zaki A.M., van Boheemen S., Bestebroer T.M., Osterhaus A.D., Fouchier R.A. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med. 2012;367:1814–1820. doi: 10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]
- 2••.Rota P.A., Oberste M.S., Monroe S.S., Nix W.A., Campagnoli R., Icenogle J.P., Penaranda S., Bankamp B., Maher K., Chen M.H. Characterization of a novel coronavirus associated with severe acute respiratory syndrome. Science. 2003;300:1394–1399. doi: 10.1126/science.1085952. [DOI] [PubMed] [Google Scholar]; This is the first report for the complete sequence of SARS-CoV complete genome with phylogenetic analyses and sequence comparisons indicating its novelty.
- 3.Thiel V., Herold J., Schelle B., Siddell S.G. Viral replicase gene products suffice for coronavirus discontinuous transcription. J Virol. 2001;75:6676–6681. doi: 10.1128/JVI.75.14.6676-6681.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thiel V., Ivanov K.A., Putics A., Hertzig T., Schelle B., Bayer S., Weissbrich B., Snijder E.J., Rabenau H., Doerr H.W. Mechanisms and enzymes involved in SARS coronavirus genome expression. J Gen Virol. 2003;84:2305–2315. doi: 10.1099/vir.0.19424-0. [DOI] [PubMed] [Google Scholar]
- 5.Narayanan K., Maeda A., Maeda J., Makino S. Characterization of the coronavirus M protein and nucleocapsid interaction in infected cells. J Virol. 2000;74:8127–8134. doi: 10.1128/jvi.74.17.8127-8134.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Opstelten D.J., Raamsman M.J., Wolfs K., Horzinek M.C., Rottier P.J. Envelope glycoprotein interactions in coronavirus assembly. J Cell Biol. 1995;131:339–349. doi: 10.1083/jcb.131.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hon C.C., Lam T.Y., Shi Z.L., Drummond A.J., Yip C.W., Zeng F., Lam P.Y., Leung F.C. Evidence of the recombinant origin of a bat severe acute respiratory syndrome (SARS)-like coronavirus and its implications on the direct ancestor of SARS coronavirus. J Virol. 2008;82:1819–1826. doi: 10.1128/JVI.01926-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li W., Shi Z., Yu M., Ren W., Smith C., Epstein J.H., Wang H., Crameri G., Hu Z., Zhang H. Bats are natural reservoirs of SARS-like coronaviruses. Science. 2005;310:676–679. doi: 10.1126/science.1118391. [DOI] [PubMed] [Google Scholar]
- 9.Wang L.F., Eaton B.T. Bats, civets and the emergence of SARS. Curr Top Microbiol Immunol. 2007;315:325–344. doi: 10.1007/978-3-540-70962-6_13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haagmans B.L., Al Dhahiry S.H., Reusken C.B., Raj V.S., Galiano M., Myers R., Godeke G.J., Jonges M., Farag E., Diab A. Middle East respiratory syndrome coronavirus in dromedary camels: an outbreak investigation. Lancet Infect Dis. 2013;14:140–145. doi: 10.1016/S1473-3099(13)70690-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abdel-Moneim A.S. Middle East respiratory syndrome coronavirus (MERS-CoV): evidence and speculations. Arch Virol. 2014 doi: 10.1007/s00705-014-1995-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Annan A., Baldwin H.J., Corman V.M., Klose S.M., Owusu M., Nkrumah E.E., Badu E.K., Anti P., Agbenyega O., Meyer B. Human betacoronavirus 2c EMC/2012-related viruses in bats, Ghana and Europe. Emerg Infect Dis. 2013;19:456–459. doi: 10.3201/eid1903.121503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anthony S.J., Ojeda-Flores R., Rico-Chavez O., Navarrete-Macias I., Zambrana-Torrelio C.M., Rostal M.K., Epstein J.H., Tipps T., Liang E., Sanchez-Leon M. Coronaviruses in bats from Mexico. J Gen Virol. 2013;94:1028–1038. doi: 10.1099/vir.0.049759-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Boheemen S., de Graaf M., Lauber C., Bestebroer T.M., Raj V.S., Zaki A.M., Osterhaus A.D., Haagmans B.L., Gorbalenya A.E., Snijder E.J. Genomic characterization of a newly discovered coronavirus associated with acute respiratory distress syndrome in humans. MBio. 2012:3. doi: 10.1128/mBio.00473-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ithete N.L., Stoffberg S., Corman V.M., Cottontail V.M., Richards L.R., Schoeman M.C., Drosten C., Drexler J.F., Preiser W. Close relative of human Middle East respiratory syndrome coronavirus in bat, South Africa. Emerg Infect Dis. 2013;19:1697–1699. doi: 10.3201/eid1910.130946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peiris J.S., Yuen K.Y., Osterhaus A.D., Stohr K. The severe acute respiratory syndrome. N Engl J Med. 2003;349:2431–2441. doi: 10.1056/NEJMra032498. [DOI] [PubMed] [Google Scholar]
- 17.Barnard D.L., Kumaki Y. Recent developments in anti-severe acute respiratory syndrome coronavirus chemotherapy. Future Virol. 2011;6:615–631. doi: 10.2217/fvl.11.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18•.Liang P.H. Characterization and inhibition of SARS-coronavirus main protease. Curr Top Med Chem. 2006;6:361–376. doi: 10.2174/156802606776287090. [DOI] [PubMed] [Google Scholar]; This review focuses on the detailed characterization of SARS-coronavirus main protease in relation to the inhibitors.
- 19.Adedeji A.O., Singh K., Calcaterra N.E., DeDiego M.L., Enjuanes L., Weiss S., Sarafianos S.G. Severe acute respiratory syndrome coronavirus replication inhibitor that interferes with the nucleic acid unwinding of the viral helicase. Antimicrob Agents Chemother. 2012;56:4718–4728. doi: 10.1128/AAC.00957-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anand K., Ziebuhr J., Wadhwani P., Mesters J.R., Hilgenfeld R. Coronavirus main proteinase (3CLpro) structure: basis for design of anti-SARS drugs. Science. 2003;300:1763–1767. doi: 10.1126/science.1085658. [DOI] [PubMed] [Google Scholar]
- 21••.Yang H., Yang M., Ding Y., Liu Y., Lou Z., Zhou Z., Sun L., Mo L., Ye S., Pang H. The crystal structures of severe acute respiratory syndrome virus main protease and its complex with an inhibitor. Proc Natl Acad Sci U S A. 2003;100:13190–13195. doi: 10.1073/pnas.1835675100. [DOI] [PMC free article] [PubMed] [Google Scholar]; This is the first report on the crystal structures of severe acute respiratory syndrome virus main protease at different pH and its complex with a specific inhibitor.
- 22.Yang H., Xie W., Xue X., Yang K., Ma J., Liang W., Zhao Q., Zhou Z., Pei D., Ziebuhr J. Design of wide-spectrum inhibitors targeting coronavirus main proteases. PLoS Biol. 2005;3:e324. doi: 10.1371/journal.pbio.0030324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ramajayam R., Tan K.P., Liang P.H. Recent development of 3C and 3CL protease inhibitors for anti-coronavirus and anti-picornavirus drug discovery. Biochem Soc Trans. 2011;39:1371–1375. doi: 10.1042/BST0391371. [DOI] [PubMed] [Google Scholar]
- 24.Kuo C.J., Liu H.G., Lo Y.K., Seong C.M., Lee K.I., Jung Y.S., Liang P.H. Individual and common inhibitors of coronavirus and picornavirus main proteases. FEBS Lett. 2009;583:549–555. doi: 10.1016/j.febslet.2008.12.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ramajayam R., Tan K.P., Liu H.G., Liang P.H. Synthesis and evaluation of pyrazolone compounds as SARS-coronavirus 3C-like protease inhibitors. Bioorg Med Chem. 2010;18:7849–7854. doi: 10.1016/j.bmc.2010.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hsu J.T., Kuo C.J., Hsieh H.P., Wang Y.C., Huang K.K., Lin C.P., Huang P.F., Chen X., Liang P.H. Evaluation of metal-conjugated compounds as inhibitors of 3CL protease of SARS-CoV. FEBS Lett. 2004;574:116–120. doi: 10.1016/j.febslet.2004.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee C.C., Kuo C.J., Hsu M.F., Liang P.H., Fang J.M., Shie J.J., Wang A.H. Structural basis of mercury- and zinc-conjugated complexes as SARS-CoV 3C-like protease inhibitors. FEBS Lett. 2007;581:5454–5458. doi: 10.1016/j.febslet.2007.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu C.Y., Jan J.T., Ma S.H., Kuo C.J., Juan H.F., Cheng Y.S., Hsu H.H., Huang H.C., Wu D., Brik A. Small molecules targeting severe acute respiratory syndrome human coronavirus. Proc Natl Acad Sci U S A. 2004;101:10012–10017. doi: 10.1073/pnas.0403596101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shao Y.M., Yang W.B., Peng H.P., Hsu M.F., Tsai K.C., Kuo T.H., Wang A.H., Liang P.H., Lin C.H., Yang A.S. Structure-based design and synthesis of highly potent SARS-CoV 3CL protease inhibitors. Chembiochem. 2007;8:1654–1657. doi: 10.1002/cbic.200700254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shie J.J., Fang J.M., Kuo T.H., Kuo C.J., Liang P.H., Huang H.J., Wu Y.T., Jan J.T., Cheng Y.S., Wong C.H. Inhibition of the severe acute respiratory syndrome 3CL protease by peptidomimetic alpha,beta-unsaturated esters. Bioorg Med Chem. 2005;13:5240–5252. doi: 10.1016/j.bmc.2005.05.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shie J.J., Fang J.M., Kuo C.J., Kuo T.H., Liang P.H., Huang H.J., Yang W.B., Lin C.H., Chen J.L., Wu Y.T. Discovery of potent anilide inhibitors against the severe acute respiratory syndrome 3CL protease. J Med Chem. 2005;48:4469–4473. doi: 10.1021/jm050184y. [DOI] [PubMed] [Google Scholar]
- 32.Wu C.Y., King K.Y., Kuo C.J., Fang J.M., Wu Y.T., Ho M.Y., Liao C.L., Shie J.J., Liang P.H., Wong C.H. Stable benzotriazole esters as mechanism-based inactivators of the severe acute respiratory syndrome 3CL protease. Chem Biol. 2006;13:261–268. doi: 10.1016/j.chembiol.2005.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tsai K.C., Chen S.Y., Liang P.H., Lu I.L., Mahindroo N., Hsieh H.P., Chao Y.S., Liu L., Liu D., Lien W. Discovery of a novel family of SARS-CoV protease inhibitors by virtual screening and 3D-QSAR studies. J Med Chem. 2006;49:3485–3495. doi: 10.1021/jm050852f. [DOI] [PubMed] [Google Scholar]
- 34.Lu I.L., Huang C.F., Peng Y.H., Lin Y.T., Hsieh H.P., Chen C.T., Lien T.W., Lee H.J., Mahindroo N., Prakash E. Structure-based drug design of a novel family of PPARgamma partial agonists: virtual screening, X-ray crystallography, and in vitro/in vivo biological activities. J Med Chem. 2006;49:2703–2712. doi: 10.1021/jm051129s. [DOI] [PubMed] [Google Scholar]
- 35.Shao Y.M., Yang W.B., Kuo T.H., Tsai K.C., Lin C.H., Yang A.S., Liang P.H., Wong C.H. Design, synthesis, and evaluation of trifluoromethyl ketones as inhibitors of SARS-CoV 3CL protease. Bioorg Med Chem. 2008;16:4652–4660. doi: 10.1016/j.bmc.2008.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ramajayam R., Tan K.P., Liu H.G., Liang P.H. Synthesis, docking studies, and evaluation of pyrimidines as inhibitors of SARS-CoV 3CL protease. Bioorg Med Chem Lett. 2010;20:3569–3572. doi: 10.1016/j.bmcl.2010.04.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harcourt B.H., Jukneliene D., Kanjanahaluethai A., Bechill J., Severson K.M., Smith C.M., Rota P.A., Baker S.C. Identification of severe acute respiratory syndrome coronavirus replicase products and characterization of papain-like protease activity. J Virol. 2004;78:13600–13612. doi: 10.1128/JVI.78.24.13600-13612.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barretto N., Jukneliene D., Ratia K., Chen Z., Mesecar A.D., Baker S.C. The papain-like protease of severe acute respiratory syndrome coronavirus has deubiquitinating activity. J Virol. 2005;79:15189–15198. doi: 10.1128/JVI.79.24.15189-15198.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Frieman M., Basu D., Matthews K., Taylor J., Jones G., Pickles R., Baric R., Engel D.A. Yeast based small molecule screen for inhibitors of SARS-CoV. PLoS One. 2011;6:e28479. doi: 10.1371/journal.pone.0028479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gorbalenya A.E.K.E. Helicases: amino acid sequence comparisons and structure function relationships. Curr Opin Struct Biol. 1993;3:419–429. [Google Scholar]
- 41••.Adedeji A.O., Marchand B., Te Velthuis A.J., Snijder E.J., Weiss S., Eoff R.L., Singh K., Sarafianos S.G. Mechanism of nucleic acid unwinding by SARS-CoV helicase. PLoS One. 2012;7:e36521. doi: 10.1371/journal.pone.0036521. [DOI] [PMC free article] [PubMed] [Google Scholar]; This manuscript demonstrates the detailed biochemical mechanism of nuclei acid unwinding by SARS-CoV helicase.
- 42.Paolini C., De Francesco R., Gallinari P. Enzymatic properties of hepatitis C virus NS3-associated helicase. J Gen Virol. 2000;81:1335–1345. doi: 10.1099/0022-1317-81-5-1335. [DOI] [PubMed] [Google Scholar]
- 43.Kim M.K., Yu M.S., Park H.R., Kim K.B., Lee C., Cho S.Y., Kang J., Yoon H., Kim D.E., Choo H. 2,6-Bis-arylmethyloxy-5-hydroxychromones with antiviral activity against both hepatitis C virus (HCV) and SARS-associated coronavirus (SCV) Eur J Med Chem. 2011;46:5698–5704. doi: 10.1016/j.ejmech.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee C., Lee J.M., Lee N.R., Jin B.S., Jang K.J., Kim D.E., Jeong Y.J., Chong Y. Aryl diketoacids (ADK) selectively inhibit duplex DNA-unwinding activity of SARS coronavirus NTPase/helicase. Bioorg Med Chem Lett. 2009;19:1636–1638. doi: 10.1016/j.bmcl.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tanner J.A., Zheng B.J., Zhou J., Watt R.M., Jiang J.Q., Wong K.L., Lin Y.P., Lu L.Y., He M.L., Kung H.F. The adamantane-derived bananins are potent inhibitors of the helicase activities and replication of SARS coronavirus. Chem Biol. 2005;12:303–311. doi: 10.1016/j.chembiol.2005.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Adedeji A.O., Sarafianos S.G. Future treatment strategies for novel Middle East respiratory syndrome coronavirus infection. Future Med Chem. 2013;5:2119–2122. doi: 10.4155/fmc.13.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Adedeji A.O., Singh K., Kassim A., Coleman C.M., Elliott R., Weiss S.R., Frieman M.B., Sarafianos S.G. Evaluation of SSYA10-001 as a replication inhibitor of SARS, MHV and MERS coronaviruses. Antimicrob Agents Chemother. 2014;58(8) doi: 10.1128/AAC.02994-14. PMID: 24841268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bupp K., Roth M.J. Alteration and analyses of viral entry with library-derived peptides. Adv Virus Res. 2005;65:147–172. doi: 10.1016/S0065-3527(05)65005-1. [DOI] [PubMed] [Google Scholar]
- 49.Douglas J.L., Panis M.L., Ho E., Lin K.Y., Krawczyk S.H., Grant D.M., Cai R., Swaminathan S., Cihlar T. Inhibition of respiratory syncytial virus fusion by the small molecule VP-14637 via specific interactions with F protein. J Virol. 2003;77:5054–5064. doi: 10.1128/JVI.77.9.5054-5064.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Razinkov V., Gazumyan A., Nikitenko A., Ellestad G., Krishnamurthy G. RFI-641 inhibits entry of respiratory syncytial virus via interactions with fusion protein. Chem Biol. 2001;8:645–659. doi: 10.1016/s1074-5521(01)00042-4. [DOI] [PubMed] [Google Scholar]
- 51.Lieberman-Blum S.S., Fung H.B., Bandres J.C. Maraviroc: a CCR5-receptor antagonist for the treatment of HIV-1 infection. Clin Ther. 2008;30:1228–1250. doi: 10.1016/s0149-2918(08)80048-3. [DOI] [PubMed] [Google Scholar]
- 52.MacArthur R.D., Novak R.M. Reviews of anti-infective agents: maraviroc: the first of a new class of antiretroviral agents. Clin Infect Dis. 2008;47:236–241. doi: 10.1086/589289. [DOI] [PubMed] [Google Scholar]
- 53.Wood A., Armour D. The discovery of the CCR5 receptor antagonist, UK-427,857, a new agent for the treatment of HIV infection and AIDS. Prog Med Chem. 2005;43:239–271. doi: 10.1016/S0079-6468(05)43007-6. [DOI] [PubMed] [Google Scholar]
- 54.Anonymous Pentafuside. DP 178, T 20. Drugs R D. 1999;2:347–349. doi: 10.2165/00126839-199902050-00016. [DOI] [PubMed] [Google Scholar]
- 55.Berkhout B., Eggink D., Sanders R.W. Is there a future for antiviral fusion inhibitors? Curr Opin Virol. 2012;2:50–59. doi: 10.1016/j.coviro.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 56.Derdeyn C.A., Decker J.M., Sfakianos J.N., Wu X., O’Brien W.A., Ratner L., Kappes J.C., Shaw G.M., Hunter E. Sensitivity of human immunodeficiency virus type 1 to the fusion inhibitor T-20 is modulated by coreceptor specificity defined by the V3 loop of gp120. J Virol. 2000;74:8358–8367. doi: 10.1128/jvi.74.18.8358-8367.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hartt J.K., Liang T., Sahagun-Ruiz A., Wang J.M., Gao J.L., Murphy P.M. The HIV-1 cell entry inhibitor T-20 potently chemoattracts neutrophils by specifically activating the N-formylpeptide receptor. Biochem Biophys Res Commun. 2000;272:699–704. doi: 10.1006/bbrc.2000.2846. [DOI] [PubMed] [Google Scholar]
- 58.Miller M.D., Hazuda D.J. HIV resistance to the fusion inhibitor enfuvirtide: mechanisms and clinical implications. Drug Resist Updat. 2004;7:89–95. doi: 10.1016/j.drup.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 59.Naito T., Izumi K., Kodama E., Sakagami Y., Kajiwara K., Nishikawa H., Watanabe K., Sarafianos S.G., Oishi S., Fujii N. SC29EK, a peptide fusion inhibitor with enhanced alpha-helicity, inhibits replication of human immunodeficiency virus type 1 mutants resistant to enfuvirtide. Antimicrob Agents Chemother. 2009;53:1013–1018. doi: 10.1128/AAC.01211-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Eda Y., Murakami T., Ami Y., Nakasone T., Takizawa M., Someya K., Kaizu M., Izumi Y., Yoshino N., Matsushita S. Anti-V3 humanized antibody KD-247 effectively suppresses ex vivo generation of human immunodeficiency virus type 1 and affords sterile protection of monkeys against a heterologous simian/human immunodeficiency virus infection. J Virol. 2006;80:5563–5570. doi: 10.1128/JVI.02095-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Matsushita S., Takahama S., Shibata J., Kimura T., Shiozaki K., Eda Y., Koito A., Murakami T., Yoshimura K. Ex vivo neutralization of HIV-1 quasi-species by a broadly reactive humanized monoclonal antibody KD-247. Hum Antibodies. 2005;14:81–88. [PubMed] [Google Scholar]
- 62.Cilliers T., Willey S., Sullivan W.M., Patience T., Pugach P., Coetzer M., Papathanasopoulos M., Moore J.P., Trkola A., Clapham P. Use of alternate coreceptors on primary cells by two HIV-1 isolates. Virology. 2005;339:136–144. doi: 10.1016/j.virol.2005.05.027. [DOI] [PubMed] [Google Scholar]
- 63.Dau B., Holodniy M. Novel targets for antiretroviral therapy: clinical progress to date. Drugs. 2009;69:31–50. doi: 10.2165/00003495-200969010-00003. [DOI] [PubMed] [Google Scholar]
- 64.Perlman S., Netland J. Coronaviruses post-SARS: update on replication and pathogenesis. Nat Rev Microbiol. 2009;7:439–450. doi: 10.1038/nrmicro2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bosch B.J., van der Zee R., de Haan C.A., Rottier P.J. The coronavirus spike protein is a class I virus fusion protein: structural and functional characterization of the fusion core complex. J Virol. 2003;77:8801–8811. doi: 10.1128/JVI.77.16.8801-8811.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hofmann H., Geier M., Marzi A., Krumbiegel M., Peipp M., Fey G.H., Gramberg T., Pohlmann S. Susceptibility to SARS coronavirus S protein-driven infection correlates with expression of angiotensin converting enzyme 2 and infection can be blocked by soluble receptor. Biochem Biophys Res Commun. 2004;319:1216–1221. doi: 10.1016/j.bbrc.2004.05.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67••.Simmons G., Gosalia D.N., Rennekamp A.J., Reeves J.D., Diamond S.L., Bates P. Inhibitors of cathepsin L prevent severe acute respiratory syndrome coronavirus entry. Proc Natl Acad Sci U S A. 2005;102:11876–11881. doi: 10.1073/pnas.0505577102. [DOI] [PMC free article] [PubMed] [Google Scholar]; First report that demonstrates cathepsin L is required for SARS-CoV entry, and therefore stands as a potential antiviral target.
- 68.Tripet B., Howard M.W., Jobling M., Holmes R.K., Holmes K.V., Hodges R.S. Structural characterization of the SARS-coronavirus spike S fusion protein core. J Biol Chem. 2004;279:20836–20849. doi: 10.1074/jbc.M400759200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69••.Wong S.K., Li W., Moore M.J., Choe H., Farzan M. A 193-amino acid fragment of the SARS coronavirus S protein efficiently binds angiotensin-converting enzyme 2. J Biol Chem. 2004;279:3197–3201. doi: 10.1074/jbc.C300520200. [DOI] [PMC free article] [PubMed] [Google Scholar]; Identification of receptor binding domain within the SARS-CoV envelope.
- 70.Yang Z.Y., Huang Y., Ganesh L., Leung K., Kong W.P., Schwartz O., Subbarao K. Nabel GJ: pH-dependent entry of severe acute respiratory syndrome coronavirus is mediated by the spike glycoprotein and enhanced by dendritic cell transfer through DC-SIGN. J Virol. 2004;78:5642–5650. doi: 10.1128/JVI.78.11.5642-5650.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dimitrov D.S. The secret life of ACE2 as a receptor for the SARS virus. Cell. 2003;115:652–653. doi: 10.1016/S0092-8674(03)00976-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Xiao X., Chakraborti S., Dimitrov A.S., Gramatikoff K., Dimitrov D.S. The SARS-CoV S glycoprotein: expression and functional characterization. Biochem Biophys Res Commun. 2003;312:1159–1164. doi: 10.1016/j.bbrc.2003.11.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shah P.P., Wang T., Kaletsky R.L., Myers M.C., Purvis J.E., Jing H., Huryn D.M., Greenbaum D.C., Smith A.B., III, Bates P. A small-molecule oxocarbazate inhibitor of human cathepsin L blocks severe acute respiratory syndrome and ebola pseudotype virus infection into human embryonic kidney 293T cells. Mol Pharmacol. 2010;78:319–324. doi: 10.1124/mol.110.064261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Struck A.W., Axmann M., Pfefferle S., Drosten C., Meyer B. A hexapeptide of the receptor-binding domain of SARS corona virus spike protein blocks viral entry into host cells via the human receptor ACE2. Antiviral Res. 2012;94:288–296. doi: 10.1016/j.antiviral.2011.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhou Y., Agudelo J., Lu K., Goetz D.H., Hansell E., Chen Y.T., Roush W.R., McKerrow J., Craik C.S., Amberg S.M. Inhibitors of SARS-CoV entry — identification using an internally-controlled dual envelope pseudovirion assay. Antiviral Res. 2011;92:187–194. doi: 10.1016/j.antiviral.2011.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76••.Simmons G., Reeves J.D., Rennekamp A.J., Amberg S.M., Piefer A.J., Bates P. Characterization of severe acute respiratory syndrome-associated coronavirus (SARS-CoV) spike glycoprotein-mediated viral entry. Proc Natl Acad Sci U S A. 2004;101:4240–4245. doi: 10.1073/pnas.0306446101. [DOI] [PMC free article] [PubMed] [Google Scholar]; Development of SARS-pseudotyped virus to study the mechanism of viral entry and possible antiviral targets.
- 77••.Adedeji A.O., Severson W., Jonsson C., Singh K., Weiss S.R., Sarafianos S.G. Novel inhibitors of severe acute respiratory syndrome coronavirus entry that act by three distinct mechanisms. J Virol. 2013;87:8017–8028. doi: 10.1128/JVI.00998-13. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study reveals the discovery of three small molecule inhibitors of SARS-CoV entry with distinct mechanisms of inhibition.
- 78.Huentelman M.J., Zubcevic J., Hernandez Prada J.A., Xiao X., Dimitrov D.S., Raizada M.K., Ostrov D.A. Structure-based discovery of a novel angiotensin-converting enzyme 2 inhibitor. Hypertension. 2004;44:903–906. doi: 10.1161/01.HYP.0000146120.29648.36. [DOI] [PubMed] [Google Scholar]
- 79••.Li W., Moore M.J., Vasilieva N., Sui J., Wong S.K., Berne M.A., Somasundaran M., Sullivan J.L., Luzuriaga K., Greenough T.C. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]; Identification of ACE2 as the receptor for SARS-CoV entry into permissive cells.
- 80.Danilczyk U., Penninger J.M. Angiotensin-converting enzyme II in the heart and the kidney. Circ Res. 2006;98:463–471. doi: 10.1161/01.RES.0000205761.22353.5f. [DOI] [PubMed] [Google Scholar]
- 81.Peiris J.S., Chu C.M., Cheng V.C., Chan K.S., Hung I.F., Poon L.L., Law K.I., Tang B.S., Hon T.Y., Chan C.S. Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: a prospective study. Lancet. 2003;361:1767–1772. doi: 10.1016/S0140-6736(03)13412-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sung J.J., Wu A., Joynt G.M., Yuen K.Y., Lee N., Chan P.K., Cockram C.S., Ahuja A.T., Yu L.M., Wong V.W. Severe acute respiratory syndrome: report of treatment and outcome after a major outbreak. Thorax. 2004;59:414–420. doi: 10.1136/thx.2003.014076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Booth C.M., Matukas L.M., Tomlinson G.A., Rachlis A.R., Rose D.B., Dwosh H.A., Walmsley S.L., Mazzulli T., Avendano M., Derkach P. Clinical features and short-term outcomes of 144 patients with SARS in the greater Toronto area. JAMA. 2003;289:2801–2809. doi: 10.1001/jama.289.21.JOC30885. [DOI] [PubMed] [Google Scholar]
- 84.Loutfy M.R., Blatt L.M., Siminovitch K.A., Ward S., Wolff B., Lho H., Pham D.H., Deif H., LaMere E.A., Chang M. Interferon alfacon-1 plus corticosteroids in severe acute respiratory syndrome: a preliminary study. JAMA. 2003;290:3222–3228. doi: 10.1001/jama.290.24.3222. [DOI] [PubMed] [Google Scholar]
- 85.Chu C.M., Cheng V.C., Hung I.F., Wong M.M., Chan K.H., Chan K.S., Kao R.Y., Poon L.L., Wong C.L., Guan Y. Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Thorax. 2004;59:252–256. doi: 10.1136/thorax.2003.012658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Falzarano D., de Wit E., Martellaro C., Callison J., Munster V.J., Feldmann H. Inhibition of novel beta coronavirus replication by a combination of interferon-alpha2b and ribavirin. Sci Rep. 2013;3:1686. doi: 10.1038/srep01686. [DOI] [PMC free article] [PubMed] [Google Scholar]


