ABSTRACT
Exposure to environmental toxins is a 21st century global health problem that is often the result of dietary intake. Although efforts are made to reduce dietary toxin levels, they are often unsuccessful, warranting research into novel methods to reduce host exposure. Food-grade microbes that can be delivered to the gastrointestinal tract and that are capable of sequestering toxins present a safe and cost-effective intervention. We sought to investigate the potential for probiotic-supplemented yogurt to lower heavy metal levels in at-risk populations of pregnant women and in children in Mwanza, Tanzania, and to examine the microbiome in relation to toxin levels. Two populations suspected to have high toxic metal exposures were studied. A group of 44 school-aged children was followed over 25 days, and 60 pregnant women were followed over their last two trimesters until birth. A yogurt containing 1010 CFU Lactobacillus rhamnosus GR-1 per 250 g was administered, while control groups received either whole milk or no intervention. Changes in blood metal levels were assessed, and the gut microbiomes of the children were profiled by analyzing 16S rRNA sequencing via the Ion Torrent platform. The children and pregnant women in the study were found to have elevated blood levels of lead and mercury compared to age- and sex-matched Canadians. Consumption of probiotic yogurt had a protective effect against further increases in mercury (3.2 nmol/liter; P = 0.035) and arsenic (2.3 nmol/liter; P = 0.011) blood levels in the pregnant women, but this trend was not statistically significant in the children. Elevated blood lead was associated with increases in Succinivibrionaceae and Gammaproteobacteria relative abundance levels in stool.
IMPORTANCE
Probiotic food produced locally represents a nutritious and affordable means for people in some developing countries to counter exposures to toxic metals. Further research and field trials are warranted to explore this approach in countries where communities are located near mining sites and agricultural areas, two types of areas where toxins are likely to be elevated.
INTRODUCTION
Toxins in the environment are ubiquitous, and exposure is often unavoidable. Their effects on human and animal life are usually seen over time and can be serious. Acute exposure to high toxin levels is particularly detrimental. Anthropomorphic activity has only served to increase levels of toxins, such as heavy metals and pesticides, in the environment (1). Due to lax regulations and exploitation, many environmental toxins disproportionately affect the developing world. Aflatoxin, for example, is ubiquitous in East Africa due to Aspergillus-contaminated cereal and grain crops (2). Metals such as mercury are released due to human activities such as mining, as seen along the shores of Lake Victoria, Africa, where the metal reaches the food web (3). Fish, while not as popular in the Western diet, are one of the most important sources of dietary protein for many cultures (4). The effects of low-level mercury exposure include delayed neurological and cognitive development in children and, more controversially, immune and cardiovascular diseases (5).
Metal-chelating drugs, such as dimercaptosuccinic acid (DMSA) and ethylenediaminetetraacetic acid (EDTA), are indicated for the treatment of acute heavy metal exposure; however, they are not intended for long-term use and there is a lack of regulatory-approved consumer products for chelation. Thus, alternative approaches are needed. Species of lactic acid bacteria, including Lactobacillus rhamnosus strain GR-1, used here in probiotic yogurt, are known to have an affinity for many toxic metals, including lead and cadmium in vitro (6), and we have also found activities of such bacteria against mercury, arsenic, and various organic pesticides (unpublished data). The mechanism is thought to be passive sequestration; however, we have also discovered putative probiotic strains that have active enzymatic pathways for detoxification, such as mercury demethylation and reduction. The concept of probiotic-mediated detoxification has recently been demonstrated in murine models (7), but we explored whether such food-grade microbes could prevent uptake in the gastrointestinal (GI) tract (8).
Logic dictates that if probiotic organisms have these protective capacities, endogenous microbes of the GI tract, termed the microbiota, could also be of importance. Experiments contrasting conventional with germ-free animals have shown the importance of the microbiota in protection against accumulation of mercury (9) and also lead and cadmium (10). Furthermore, levels of indigenous lactobacilli appear to increase in response to metal exposure in murine models (11), perhaps conveying a natural protective effect. We sought to better understand the composition of the human gut microbiota when exposed to toxic metals.
Two of the most vulnerable populations at risk from environmental toxin exposure are pregnant women and children. We suspected that in Mwanza, Tanzania, due to its proximity to Lake Victoria and the population’s fish-rich diet, women and children would have elevated toxic metal exposure and be ideal candidates for intervention. Furthermore, Mwanza is a site with a network of community-run probiotic yogurt kitchens that service economically disadvantaged people (12). The aims of this study were (i) to determine the blood metal levels in the local population and from potential fish sources, (ii) to measure if consumption of a probiotic yogurt had an effect on blood metal levels, and (iii) to characterize the gut microbiome of children to determine if there are bacterial genera associated with these metal levels.
RESULTS
Participant recruitment.
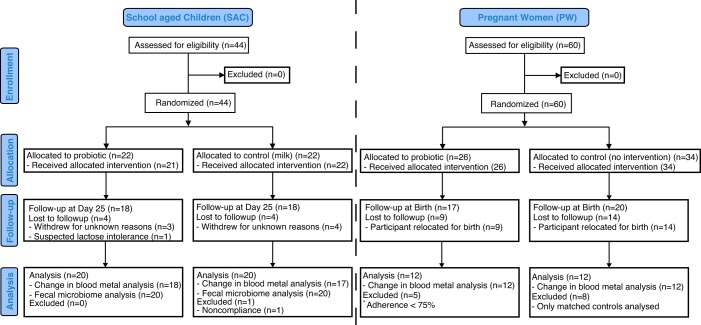
Between November 2012 and December 2012, a total of 44 individuals were recruited into the 25-day study of school-aged children (SAC), with 22 in the control group receiving milk and 22 in the experimental group receiving probiotic yogurt. Eight individuals withdrew during the course of the study, one due to suspected lactose intolerance which was not known to the child’s guardian at the time of enrollment and 7 for unknown reasons, including not being present for final sample collection. A total of 24 individuals were selected for inclusion into blood metal analysis in the pregnant women (PW) group based on adherence of over 75% and matching of nutritional status and fish intake. A summary of recruitment is provided in Fig. 1. Relevant participant demographics are represented in Table 1. Z-scores based on weight for age indicated that the enrolled children were adequately nourished at the point of enrollment. Based on the SAC enrollment questionnaire, 37% of children consumed local fish on a daily basis, 55% consumed multiple courses of fish per week, and 8% consumed fish multiple times per month. No guardians reported that their children did not consume fish regularly as part of their diet.
FIG 1 .
Consort flow diagram, detailing participant enrollment, allocation, follow-up, and analysis.
TABLE 1 .
Participant demographics for PW and SAC controls and yogurt groupsa
| Characteristic | PW |
SAC |
||
|---|---|---|---|---|
| Control | Yogurt | Control | Yogurt | |
| No. of participants | 12 | 12 | 22 | 21 |
| Age (yrs) | 24.5 ± 3.9 | 23.5 ± 3.6 | 8.3 ± 1.1 | 8.4 ± 1.2 |
| Weight (kg) | 58.0 ± 6.8 | 55.0 ± 4.9 | 23.1 ± 3.6 | 23.6 ± 3.2 |
| Height (cm) | 162.0 ± 3.9 | 157.0 ± 5.0 | 119.3 ± 7.5 | 119.6 ± 5.8 |
| BMI (kg/m2) | 22.0 ± 1.9 | 22.3 ± 2.0 | 16.2 ± 1.9 | 16.5 ± 2.0 |
| Z score | −0.4 ± 1.1 | −0.3 ± 0.8 | ||
| Gender (males/females) | 6/16 | 6/15 | ||
| Fish intake (g/day) | 150.4 ± 60.1 | 120.7 ± 52.9 | ||
| Adherence (%) | 90.6 ± 5.4 | 89.8 ± 10.0 | 86.1 ± 11.4 | |
Data are means ± SD. None of the relevant metrics were statistically significantly different between groups. Z scores were calculated from the WHO 5- to 19-year-old children BMI-for-age tables (http://who.int/growthref/who2007_bmi_for_age/en/index.html).
Dietary and blood metal levels.
Levels of metals across fish types for cadmium, lead, total mercury, and total arsenic are shown in Table 2. Total levels reported are the sum of inorganic and organic metal species. Surprisingly, the smaller fish type, silver cyprinid, contained significantly higher levels of mercury and arsenic than the other piscine species tested.
TABLE 2 .
Toxic metal levels in commonly consumed fish in the Mwanza region
| Species | Metal levela (ng/g) in fish |
|||
|---|---|---|---|---|
| Mercury | Lead | Arsenic | Cadmium | |
| Tilapia (Oreochromis niloticus) | 18.3 ± 17.1 | 58.0 ± 13.0 | 22.3 ± 2.5 | 158 ± 254 |
| Nile perch (Lates niloticus) | 56.0 ± 15.1 | 86.7 ± 18.2 | 30.3 ± 14.3 | 33.7 ± 37.5 |
| Silver cyprinid (Rastrineobola argentea) | 77.3 ± 40.5 | 78.0 ± 18.3 | 664.3 ± 159.9 | 113.0 ± 54.7 |
Data are means ± SD. Mercury and arsenic levels are reported as total levels (i.e., sum of inorganic and organic metal species).
Measures of blood lead, total mercury, total arsenic, and cadmium are presented in Table 3 for both the SAC and PW groups. When comparisons were made to levels present in a developed country (Canada) (13), lead and mercury were found to be elevated in both SAC and PW by up to 6.8 times. Levels of arsenic and cadmium appeared on par or lower than the Canadian population values. The PW group tended to display lower levels of metals than the SAC group.
TABLE 3 .
Blood metal levels at the time of recruitment and comparisons to levels found in a developed country
| Study group and heavy metal | Metal level in test group |
Metal level in controls |
Fold difference | ||
|---|---|---|---|---|---|
| Avg ± SD | Range | Canadian avga | Reference rangeb | ||
| SAC | |||||
| Pb (μg/liter) | 47.1 ± 16.2 | 22.5–91.3 | 9.0 | 0.0–17.7 | 5.2 |
| Hg (nmol/liter) | 9.5 ± 5.3 | 3.0–37.4 | 1.4 | 0.0–5.5 | 6.8 |
| As (nmol/liter) | 6.5 ± 2.1 | 2.7–10.8 | 7.8 | 0.0–21.4 | −1.2 |
| Cd (nmol/liter) | 1.2 ± 0.7 | 0.9–4.4 | 0.89 | 0.0–4.6 | 1.3 |
| PW | |||||
| Pb (μg/liter) | 22.6 ± 9.6 | 7.3–40.5 | 8.9 | 0.0–45.0 | 2.5 |
| Hg (nmol/liter) | 8.8 ± 3.1 | 4.0–16.0 | 3.5 | 0.0–18.0 | 2.5 |
| As (nmol/liter) | 3.0 ± 1.6 | 1.3–6.7 | 11.7 | 0.0–21.4 | −3.9 |
| Cd (nmol/liter) | 1.1 ± 0.6 | 0.0–2.7 | 3.2 | 0.0–8.9 | −2.9 |
Canadian averages are geometric means for males and females ages 6 to 11 years (SAC) and of females ages 20 to 39 years (PW) and are based on the Canadian Health Measures Survey (2007-2009 [13]).
Reference ranges were provided by the Trace Elements Laboratory, London Laboratory Services Group.
Effect of probiotic yogurt consumption on blood metal levels.
Before and after intervention, samples were successfully collected from 36 individuals in the SAC group (18 in each group [treatment and controls]). One individual was excluded from the control group after gut microbiome analysis showed a high number of reads presumptively mapping to the probiotic strain, indicating noncompliance. After quantification by high-resolution sector field inductively coupled plasma mass spectrometry (HR-SF-ICP-MS), no statistically significant differences were detected in blood metal levels in SAC receiving the probiotic or milk control, although we noted that there was a weak trend of reduced blood levels of lead and arsenic (Table 4). In the PW cohort, this effect reached a statistically significant level (P < 0.05). It is noteworthy that blood levels of mercury and arsenic increased in the control groups (P < 0.05) but remained stable in the probiotic group, indicating a protective effect of probiotic consumption.
TABLE 4 .
Blood metal levels in control and probiotic groups before and after intervention in SAC and PW study groups
| Study group and metal analyzed | Controls |
Probiotic treated |
Between-group comparisons |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Metal concn |
Data analysis |
Metal concn |
Data analysis |
||||||||||||
| Enrollment | Follow-up | Difference | P valueb | 95% CI | Responder rate (%)c | Enrollment | Follow-up | Difference | P valueb | 95% CI | Responder rate (%)c | Difference (Prob-Con)d | P valuee | 95% CI | |
| SAC | (n = 17) | (n = 18) | |||||||||||||
| Lead (μg/liter) | 48.6 ± 16.1a | 49.7 ± 21.8 | 1.1 | 0.79 | −7.7 to 10.01 | 53 | 46.3 ± 16.7 | 47.3 ± 15.8 | 1.0 | 0.41 | −1.6 to 3.6 | 35 | −0.1 | 0.98 | −9.0 to 8.8 |
| Mercury (nmol/liter) | 8.9 ± 2.8 | 9.4 ± 3.5 | 0.5 | 0.52 | −1.1 to 2.1 | 29 | 10.3 ± 7.5 | 9.7 ± 4.9 | −0.6 | 0.51 | −2.6 to 1.3 | 44 | −1.1 | 0.36 | −3.6 to 1.4 |
| Cadmium (nmol/liter) | 1.4 ± 1.1 | 1.3 ± 1.2 | −0.1 | 0.58 | −0.27 to 0.12 | 13 | 1.2 ± 0.4 | 1.1 ± 0.6 | −0.1 | 0.43 | −0.37 to 0.17 | 22 | 0 | 0.79 | −0.29 to 0.37 |
| Arsenic (nmol/liter) | 6.1 ± 2.3 | 6.3 ± 2.9 | 0.2 | 0.78 | −1.5 to 1.9 | 35 | 6.7 ± 2.2 | 6.3 ± 2.3 | −0.4 | 0.41 | −1.6 to 0.67 | 44 | −0.6 | 0.49 | −2.6 to 1.3 |
| PW | (n = 12) | (n = 12) | |||||||||||||
| Lead (μg/liter) | 25 ± 9.0 | 34 ± 13 | 9 | 0.011 | 2.4 to 15 | 8 | 20 ± 9.7 | 33 ± 19 | 13 | 0.0013 | 6.1 to 19 | 0 | 004 | 0.35 | −4.6 to 12 |
| Mercury (nmol/liter) | 8.2 ± 3.5 | 11 ± 2.5 | 2.8 | 0.042 | 0.12 to 5.6 | 25 | 9.4 ± 2.7 | 9.0 ± 2.5 | −0.4 | 0.60 | −2.1 to 1.2 | 50 | −3.2 | 0.035 | −6.32 to −0.25 |
| Cadmium (nmol/liter) | 1.2 ± 0.59 | 1.3 ± 0.46 | 0.1 | 0.57 | −0.21 to 0.37 | 8 | 1.1 ± 0.65 | 1.4 ± 0.90 | 0.3 | 0.017 | 0.080 to 0.70 | 0 | 0.2 | 0.13 | −0.092 to 0.69 |
| Arsenic (nmol/liter) | 2.4 ± 1.5 | 4.9 ± 2.5 | 2.5 | 0.0032 | 1.0 to 3.9 | 0 | 3.5 ± 1.7 | 3.7 ± 1.1 | 0.2 | 0.68 | −0.85 to 1.3 | 33 | −2.3 | 0.011 | −4.0 to −0.57 |
Values are means ± SD.
A paired t test was used for within-group comparisons.
Responders were defined as persons who showed a decrease in blood metal levels over the study period.
Prob-Con (used for the between-group comparisons) stands for difference between probiotic and control (i.e., probiotic minus control).
A t test was used for between-group comparisons.
Gut microbiome and association with metal levels.
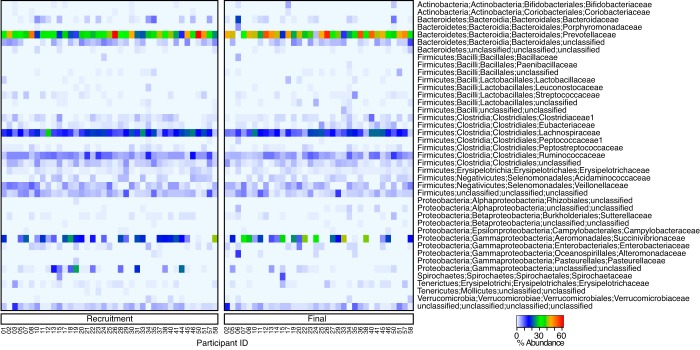
A total of 74 fecal samples from the children were collected and processed for sequencing. For all but one of these samples, 16S rRNA gene sequences were successfully obtained with a total number of reads of 1,150,628, averaging 15,762 reads per sample. With clustering at 97% nucleotide identity, 177 operational taxonomic units (OTUs) were obtained at ≥1% abundance. These data were summarized to the family level and are presented in Fig. 2. Nearly all samples contained Prevotella as the dominant genera and Prevotellaceae as the dominant family. Administration of the probiotic was not observed to have an effect on the gut bacterial community composition based on analysis of weighted and unweighted UniFrac metrics (14) (data not shown), as previously reported (15). The use of 16S rRNA sequencing also gave the unique opportunity to presumptively validate probiotic administration, as one OTU could be mapped back to the genome of L. rhamnosus GR-1 (OTU_140). This OTU was not observed in any sample prior to the start of the study and, with only one exception, was only observed in the group that received probiotic yogurt. The exception (PDTX25) was excluded from analysis of probiotic efficacy in the SAC group.
FIG 2 .
Heat map representation of the gut microbiomes of SAC at the beginning and endpoint of the study. Data were summarized to the family level and plotted in terms of percent abundance. Across nearly all participants, Prevotellaceae were the most dominant family observed, while an unclassified Succinivibrionaceae was also of variably high abundance across many participants.
To analyze the association between blood lead levels and the microbiotas, the initial analysis focused on only the first visit and the most extreme cases (n = 3 per group) based upon visual inspection of the distribution of blood levels. Two OTUs were found to be elevated under conditions of high blood lead and to have a raw P value of <0.05: OTU_1 (P = 0.0205) and OTU_215 (P = 0.0498), representing Succinivibrionaceae and Gammaproteobacteria, respectively. Due to the relatively small sample numbers used in this analysis, significance was not obtained with multiple testing corrections. In order to leverage the full power of the data set, all samples regardless of visit or participant were considered. Quartile values for interquartile 1 (Q1) and Q3 based on blood lead concentrations were used as cutoffs to separate the microbiota samples (n = 16 for “low” blood lead concentrations, and n = 18 for high blood lead concentrations). These two conditions were then compared using a false-discovery rate (FDR) cutoff of 0.05, and again the increased proportional abundance of OTU_1 (2.9-fold; FDR of 0.022) and OTU_215 (3.7-fold; FDR of 0.023) with elevated blood lead levels was found (Fig. 3). Significant associations were not found in the cases of mercury and arsenic.
FIG 3 .
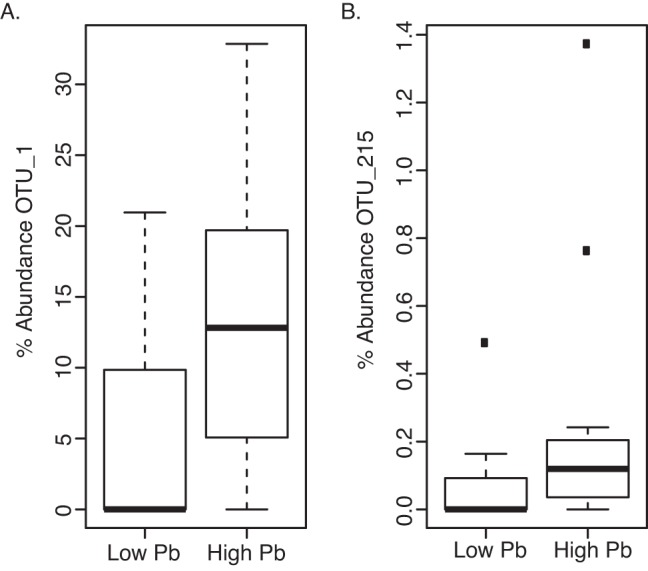
Association of OTU_1 (Succinivibrionaceae) (A) and OTU_215 (Gammaproteobacteria) (B) with elevated blood lead levels in a comparison of upper and lower quartiles of all samples (FDR, <0.05).
DISCUSSION
This is the first study to simultaneously evaluate toxic metal levels in the blood of humans, associated changes in the microbiota, and the potential for probiotics to convey a detoxification effect. It is also the first study to assess the impact of administration of a probiotic food on toxic metal levels in people living in the developing world.
Levels of metals in the fish tested were consistent with previous reports for Lake Victoria (3, 16). Mercury limits in fish have been well described, but they are less well defined in the case of lead, cadmium, and arsenic. It is greatly concerning to observe such high levels of metals in silver cyprinid fish, as daily consumption of this species is common, especially in the economically disadvantaged due to its affordable price. This creates a disproportional burden on these individuals. Furthermore, this goes against the typical dogma that larger fish species are a greater concern for toxic metal exposure due to biomagnification (17).
Metal exposure from dietary fish intake likely explains why we saw elevated blood levels of mercury in both the SAC and PW groups compared to reported levels in Canadians, but the cases of Cd and As are interesting, as these blood levels were not dissimilar between the two countries. It is difficult to speculate why this was the case, and further studies of lake metal levels and concentrations in other foods are needed. Unfortunately, metal levels, particularly lead, were highly elevated in the SAC group, for which their effects may be particularly deleterious. The difference between the adults and children could be due to reduced uptake in adults, as only 5 to 15% of ingested lead is absorbed in the adult gut, while in children absorption can be up to 40% (18).
The studies provided the first positive evidence for the use of probiotics to combat toxic heavy metal exposure in vulnerable human populations. The results comparing the short-term and long-term interventions (SAC versus PW) suggest that probiotic consumption does not have a fast-acting effect, as do DMSA or EDTA, but rather acts over the longer term. This is likely because the mechanism of action involves previention fo uptake into the body from the GI tract, rather than scavenging what is already in the body, as occurs in chelation therapy. Alternatively, it may be reflective of differing metabolic or hormonal differences and/or different indigenous microbes in the PW compared to the SAC group. Further studies involving time course interventions will be necessary to resolve this discrepancy.
Interestingly, in both study groups the mercury and arsenic increased in the control groups. A delay between sample collection and analysis was unavoidable due to the lack of instrumentation locally. But, sample storage of mercury should not interfere with the analysis (19) and is more likely explained by seasonal changes in diet/exposure. Thus, probiotic administration may be especially advocated at peak exposure times.
A high degree of homogeneity in a Prevotella-dominated microbiota is noteworthy. This profile, referred to as enterotype 2 (20), has been previously observed to be predominant in African populations (21) and is presumed to be due to a carbohydrate-rich diet (22). Interestingly, enterotype 2 is often coassociated with Desulfovibrio spp. (22). While we cannot definitively show the presence of these organisms due to their low abundance and lack of sequence diversity in the V6 16S rRNA region, they are associated with mercury methylation through a mechanism that was only recently understood (23) and which could facilitate increased mercury uptake in the gut. In addition, mucin degradation by this microbiome configuration could facilitate increased metal uptake by affecting gut barrier function (24), putting these individuals at greater risk from metal exposure.
There are a number of mechanisms through which the Succinivibrionaceae and Gammaproteobacteria may function to facilitate greater lead uptake, including host interactions and influencing other members of the microbiota. In fact, the mechanism may be relatively simple, since the cell wall structure of Gram-negative bacteria has lower metal-binding activity than Gram-positive organisms (25). Given that probiotic treatment was not found to affect relative abundance of either of these two groups, or any bacterial population, this suggests the mechanism of action is independent of altering the microbiota, at least at the community structure level.
In summary, this work has demonstrated the potential value of long-term probiotic-based interventions to counter mercury and arsenic exposure in vulnerable populations, particularly in pregnant women. This approach can be disseminated at an affordable cost (the equivalent of pennies) in developing countries where individuals are at high risk; however, it could also be applied to developed world citizens and wildlife, for example, those living near mining facilities. We hope that these studies help provide a framework for further human trials. Though it is reasonable to presume health benefits due to reduced toxin levels, long-term multiyear studies would help determine if reductions in toxin levels in the blood via consumption of probiotic foods result in improvements in physical and cognitive development in children.
MATERIALS AND METHODS
Study design and participants.
Two populations were recruited in the Mwanza region, Tanzania for this study: (i) 44 school-aged children aged 6 to 10 years (referred to as SAC) and (ii) a subset of 60 pregnant women in their first trimester who were being recruited for a separate study on nutrition and the microbiome (referred to as PW).
In the SAC group, consent was obtained from the child’s guardian, as identified by school records, and assent was obtained from the child. If a signature could not be provided, a thumbprint was used in its place. Inclusion criteria were that the child was aged 6 to 10 years and in good health, and the only exclusion criteria were known milk allergy and/or lactose intolerance. The guardians were surveyed for basic dietary information about their child, including the frequency with which they consumed fish and the species consumed. Blood was collected for determination of metal levels and feces were collected, stored on ice for <4 h, and stored at −80°C until processing and DNA extraction. Participants were then randomly assigned (using a random number generator) to receive either a locally produced yogurt containing 1 × 1010 CFU Lactobacillus rhamnosus GR-1 per 250 g or an equivalent portion of ultra-heat-treated milk as a control devoid of lactic acid bacteria. For 19 of the next 24 days, the children were supervised during administration of either the yogurt or milk. Five days were missed due to logistical issues in administration/yogurt production. Upon completion of the study, blood and fecal samples were again collected.
As part of a separate study on maternal nutrition and the microbiome (the PW group), 60 pregnant women were recruited, of which 26 received a probiotic yogurt containing 1 × 1010 CFU L. rhamnosus GR-1 per 250 g and supplemented with 4.3 g of Moringa, a micronutrient-rich plant, to enhance maternal nutrition. All women recruited were between 12 and 24 weeks pregnant and aged 18 to 40 years. Until their final visit after birth, individuals in the yogurt group received the product for 6 days a week with an average number of days for consuming yogurt of 102 days ± 19 (standard deviation [SD]). The control group had no form of intervention. For blood trace metal analysis, individuals with ≥75% compliance in the probiotic group were selected, along with controls of appropriate age, nutritional status, and matched fish intake, resulting in 12 PW per group. Given that this was a pilot study, sample size was based upon participant availability.
Both studies were registered with clinicaltrials.gov (NCT01904513 and NCT02021799) and approved in Canada by the Health Sciences Research Ethics Board at Western University (102881 and 18850) and in Tanzania by the Lake Zone Institutional Review Board.
Dietary exposure.
To assess potential dietary exposure to toxic metals via fish consumption, three of the most commonly consumed fish were collected. All samples were obtained from the main fish market in downtown Mwanza in early December 2012. Three specimens of each fish species were collected: Nile perch (Lates niloticus), tilapia (Oreochromis niloticus), and dagaa/silver cyprinid (Rastrineobola argentea). Each was caught from a different area along the Mwanza coastline. Muscle tissue was removed from the Nile perch (Lates niloticus) and tilapia (Oreochromis niloticus) and frozen at −80°C until analysis. Dried R. argentea was frozen whole. Samples were digested in aqua regia and analyzed for lead, mercury, arsenic, and cadmium by ICP-MS (Agilent 7700) at the UWO, Analytical Services Laboratory, London, Canada.
Blood metal quantification.
Blood samples were collected in Vacutainer trace elements blood tubes (Becton, Dickinson) and frozen at −80°C until analysis. Whole-blood samples were digested in ultrapure nitric acid before being analyzed on an Element 2 HR-SF-ICP-MS apparatus (Thermo Scientific) according to the standard operating procedures of the Trace Elements Laboratory of the London Health Sciences Centre for a panel of toxic metals (mercury, arsenic, cadmium, and lead).
Microbiome analysis.
DNA was extracted from frozen fecal samples of the SAC group by using the EZNA stool kit (Omega Bio-tek) according to the manufacturer’s instructions. Amplification of the V6 region of the 16S rRNA gene was carried out by using the primers CCATCTCATCCCTGCGTGTCTCCGACTCAGnnnnnCWACGCGARGAACCTTACC and CCTCTCTATGGGCAGTCGGTGATACRACACGAGCTGACGAC, where “nnnnn” is a sample-specific nucleotide bar code. Amplification was carried out in 42-µl reaction mixtures with 10 µl of 3.2 pmol/µl of each primer, 20 µl GoTaq hot start colorless master mix (Promega), and 2 µl purified DNA. The PCR protocol was 2 min at 95°C and 25 cycles of 1 min each at 95°C, 55°C, and 72°C. PCR yield was assessed with a Qubit flourometer (Life Technologies), and samples were pooled at equimolar concentrations before a final cleanup with the QIAquick PCR purification kit (Qiagen). Library preparation and sequencing were carried out at the London Regional Genomics Centre (London, Canada) on an Ion Torrent personal genome machine (Life Technologies) with 316 chips, following the manufacturer’s instructions.
Resulting reads were extracted, demultiplexed, and grouped into OTUs at 97% identity in the manor previously reported (26). Reads were deposited into the Short Read Archive (BioProject ID PRJNA244107), and barcodes and their corresponding sample IDs are available in Table S1 in the supplemental material. Taxonomic assignments were made by extracting best hits from the Ribosomal Database Project (http://rdp.cme.msu.edu) Seqmatch tool. These were manually curated by comparison to the NCBI Nonredundant Database and the Green Genes database (http://greengenes.lbl.gov). OTU IDs, sequences, and taxonomies are reported in Table S2 in the supplemental material. Further analysis was carried out using the programs QIIME (27) and R (http://R-project.org). To better handle comparisons of compositional data, the centered log ratio transformation described by Aitchison (28) and adapted to microbiome data (29, 30) was used and then tested using an analysis of variance with FDR multiple-testing corrections. Cadmium was excluded from analysis due to the limited range of concentrations observed.
SUPPLEMENTAL MATERIAL
Sample PCR barcodes
OTUs and seed sequences
ACKNOWLEDGMENTS
We thank the Lab Scientists at NIMR Mwanza for providing access to their facility, and we thank the women, children, and their guardians who participated in the study. We also thank the African Probiotic Yogurt Network for logistical support and Mabatini/Tukwamuane, Buswelu, and VSI yogurt kitchens for producing the probiotic yogurts. We appreciate the Trace Elements Laboratory at Victoria Hospital for their valuable guidance and blood sample analysis and the Geoanalysis and SWAP testing lab at the Biotron Climate Change Research Centre for their assistance with food testing. We also express thanks to Jean Macklaim for assistance with analysis of the microbiome data.
J.E.B. and M.E. are recipients of NSERC Canada Graduate Scholarships. Funding was received from the Bill and Melinda Gates Foundation. The funding sources for this work were not involved in the collection, analysis, or decision to publish this work.
J.E.B., J.M., J.C., J.P.B., and G.R. designed the study. J.E.B., M.E., and J.M. collected the samples and oversaw the field studies. J.E.B., M.E., J.M., J.P.B., and G.G. analyzed and interpreted the results. J.E.B., M.E., J.M., J.P.B., and G.R. prepared the manuscript. G.R. had primary responsibility for final content of the manuscript. All authors read and approved the final manuscript. J.E.B., J.P.B., and G.R. are listed as inventors on a patent application entitled “Food grade bacteria for the removal of toxic compounds” (application number PCT/CA2013/000328); however, the contents of the application do not include data generated from these studies.
We report no conflicts of interest.
Footnotes
Citation Bisanz JE, Enos MK, Mwanga JR, Changalucha J, Burton JP, Gloor GB, Reid G. 2014. Randomized open-label pilot study of the influence of probiotics and the gut microbiome on toxic metal levels in Tanzanian pregnant women and school children. mBio 5(5):e01580-14. doi:10.1128/mBio.01580-14.
REFERENCES
- 1. Muir DC, Wang X, Yang F, Nguyen N, Jackson TA, Evans MS, Douglas M, Köck G, Lamoureux S, Pienitz R, Smol JP, Vincent WF, Dastoor A. 2009. Spatial trends and historical deposition of mercury in eastern and northern Canada inferred from lake sediment cores. Environ. Sci. Technol. 43:4802–4809. 10.1021/es8035412 [DOI] [PubMed] [Google Scholar]
- 2. Wagacha JM, Muthomi JW. 2008. Mycotoxin problem in Africa: current status, implications to food safety and health and possible management strategies. Int. J. Food Microbiol. 124:1–12. 10.1016/j.ijfoodmicro.2008.01.008 [DOI] [PubMed] [Google Scholar]
- 3. Campbell L, Dixon DG, Hecky RE. 2003. A review of mercury in Lake Victoria, East Africa: implications for human and ecosystem health. J. Toxicol. Environ. Health B Crit. Rev. 6:325–356. 10.1080/10937400306474 [DOI] [PubMed] [Google Scholar]
- 4. Tidwell JH, Allan GL. 2001. Fish as food: aquaculture’s contribution. Ecological and economic impacts and contributions of fish farming and capture fisheries. EMBO Rep. 2:958–963. 10.1093/embo-reports/kve236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Karagas MR, Choi AL, Oken E, Horvat M, Schoeny R, Kamai E, Cowell W, Grandjean P, Korrick S. 2012. Evidence on the human health effects of low level methylmercury exposure. Environ. Health Perspect. 120:799–806. 10.1289/ehp.1104494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ibrahim F, Halttunen T, Tahvonen R, Salminen S. 2006. Probiotic bacteria as potential detoxification tools: assessing their heavy metal binding isotherms. Can. J. Microbiol. 52:877–885. 10.1139/w06-043 [DOI] [PubMed] [Google Scholar]
- 7. Tian F, Zhai Q, Zhao J, Liu X, Wang G, Zhang H, Zhang H, Chen W. 2012. Lactobacillus plantarum CCFM8661 alleviates lead toxicity in mice. Biol. Trace Elem. Res. 150:264–271. 10.1007/s12011-012-9462-1 [DOI] [PubMed] [Google Scholar]
- 8. Monachese M, Burton JP, Reid G. 2012. Bioremediation and human tolerance to heavy metals through microbial processes: a potential role for probiotics? Appl. Environ. Microbiol. 78:6397–6404. 10.1128/AEM.01665-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nakamura I, Hosokawa K, Tamura H, Miura T. 1977. Reduced mercury excretion with feces in germfree mice after oral administration of methyl mercury chloride. Bull. Environ. Contam. Toxicol. 17:528–533. 10.1007/BF01685974 [DOI] [PubMed] [Google Scholar]
- 10. Breton J, Daniel C, Dewulf J, Pothion S, Froux N, Sauty M, Thomas P, Pot B, Foligné B. 2013. Gut microbiota limits heavy metals burden caused by chronic oral exposure. Toxicol. Lett. 222:132–138. 10.1016/j.toxlet.2013.07.021 [DOI] [PubMed] [Google Scholar]
- 11. Breton J, Massart S, Vandamme P, De Brandt E, Pot B, Foligné B. 2013. Ecotoxicology inside the gut: impact of heavy metals on the mouse microbiome. BMC Pharmacol. Toxicol. 14:62. 10.1186/2050-6511-14-62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Reid MKE, Gough R, Enos M, Reid G. 2013. Social businesses in Tanzania tackling health issues of the Millennium Development Goals, one community kitchen at a time. J. Soc. Bus. 3:24–38 [Google Scholar]
- 13. Health Canada 2010. Report on human biomonitoring of environmental chemicals in Canada: results of the Canadian Health Measures Survey cycle 1 (2007-2009). Health; Canada, Ottawa, Ontario, Canada [Google Scholar]
- 14. Lozupone C, Knight R. 2005. UniFrac: a new phylogenetic method for comparing microbial communities. Appl. Environ. Microbiol. 71:8228–8235. 10.1128/AEM.71.12.8228-8235.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. McNulty NP, Yatsunenko T, Hsiao A, Faith JJ, Muegge BD, Goodman AL, Henrissat B, Oozeer R, Cools-Portier S, Gobert G, Chervaux C, Knights D, Lozupone CA, Knight R, Duncan AE, Bain JR, Muehlbauer MJ, Newgard CB, Heath AC, Gordon JI. 2011. The impact of a consortium of fermented milk strains on the gut microbiome of gnotobiotic mice and monozygotic twins. Sci. Transl. Med. 3:106ra106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Oyoo-Okoth E, Admiraal W, Osano O, Ngure V, Kraak MH, Omutange ES. 2010. Monitoring exposure to heavy metals among children in Lake Victoria, Kenya: environmental and fish matrix. Ecotoxicol. Environ. Saf. 73:1797–1803. 10.1016/j.ecoenv.2010.07.040 [DOI] [PubMed] [Google Scholar]
- 17. Bryan GW, Waldichuk M, Pentreath RJ, Darracott A. 1979. Bioaccumulation of marine pollutants. Philos. Trans. R. Soc. Lond. B 296:483–505. 10.1098/rstb.1979.0042 [DOI] [PubMed] [Google Scholar]
- 18. Cullen G, Dines A, Kolev S. 2014. Lead: information monograph for UK PID. International Programme on Chemical Safety, London, United Kingdom: http://inchem.org/documents/ukpids/ukpids/ukpid25.htm [Google Scholar]
- 19. Varian-Ramos CW, Condon AM, Hallinger KK, Carlson-Drexler KA, Cristol DA. 2011. Stability of mercury concentrations in frozen avian blood samples. Bull. Environ. Contam. Toxicol. 86:159–162. 10.1007/s00128-010-0164-0 [DOI] [PubMed] [Google Scholar]
- 20. Arumugam M, Raes J, Pelletier E, Le Paslier D, Yamada T, Mende DR, Fernandes GR, Tap J, Bruls T, Batto JM, Bertalan M, Borruel N, Casellas F, Fernandez L, Gautier L, Hansen T, Hattori M, Hayashi T, Kleerebezem M, Kurokawa K, Leclerc M, Levenez F, Manichanh C, Nielsen HB, Nielsen T, Pons N, Poulain J, Qin J, Sicheritz-Ponten T, Tims S, Torrents D, Ugarte E, Zoetendal EG, Wang J, Guarner F, Pedersen O, de Vos WM, Brunak S, Doré J, MetaHIT Consortium. Weissenbach J, Ehrlich SD, Bork P, Almeida M, Brechot C, Cara C, Chervaux C, Cultrone A, Delorme C, Denariaz G, Dervyn R, Foerstner KU, Friss C, van de Guchte M, Guedon E, Haimet F, Huber W, van Hylckama-Vlieg J, Jamet A, Juste C, Kaci G, Knol J, Lakhdari O, Layec S, Le Roux K, Maguin E, Mérieux A, Melo Minardi R, M’rini C, Muller J, Oozeer R, Parkhill J, Renault P, Rescigno M, Sanchez N, Sunagawa S, Torrejon A, Turner K, Vandemeulebrouck G, Varela E, Winogradsky Y, Zeller G, Weissenbach J, Ehrlich SD, Bork P. 2011. Enterotypes of the human gut microbiome. Nature 473:174–180. 10.1038/nature09944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. De Filippo C, Cavalieri D, Di Paola M, Ramazzotti M, Poullet JB, Massart S, Collini S, Pieraccini G, Lionetti P. 2010. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc. Natl. Acad. Sci. U. S. A. 107:14691–14696. 10.1073/pnas.1005963107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wu GD, Chen J, Hoffmann C, Bittinger K, Chen Y, Keilbaugh SA, Bewtra M, Knights D, Walters WA, Knight R, Sinha R, Gilroy E, Gupta K, Baldassano R, Nessel L, Li H, Bushman FD, Lewis JD. 2011. Linking long-term dietary patterns with gut microbial enterotypes. Science 334:105–108. 10.1126/science.1208344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Parks JM, Johs A, Podar M, Bridou R, Hurt RA, Smith SD, Tomanicek SJ, Qian Y, Brown SD, Brandt CC, Palumbo AV, Smith JC, Wall JD, Elias DA, Liang L. 2013. The genetic basis for bacterial mercury methylation. Science 339:332–335. 10.1126/science.1226767 [DOI] [PubMed] [Google Scholar]
- 24. Wright DP, Rosendale DI, Robertson M. 2000. Prevotella enzymes involved in mucin oligosaccharide degradation and evidence for a small operon of genes expressed during growth on mucin. FEMS Microbiol. Lett. 190:73–79. 10.1111/j.1574-6968.2000.tb09265.x [DOI] [PubMed] [Google Scholar]
- 25. Beveridge TJ, Fyfe WS. 1985. Metal fixation by bacterial cell walls. Can. J. Earth Sci. 22:1893–1898. 10.1139/e85-204 [DOI] [Google Scholar]
- 26. Gloor GB, Hummelen R, Macklaim JM, Dickson RJ, Fernandes AD, Macphee R, Reid G. 2010. Microbiome profiling by Illumina sequencing of combinatorial sequence-tagged PCR products. PLoS One 5:e15406. 10.1371/journal.pone.0015406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Peña AG, Goodrich JK, Gordon JI, Huttley GA, Kelley ST, Knights D, Koenig JE, Ley RE, Lozupone CA, McDonald D, Muegge BD, Pirrung M, Reeder J, Sevinsky JR, Turnbaugh PJ, Walters WA, Widmann J, Yatsunenko T, Zaneveld J, Knight R. 2010. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 7:335–336. 10.1038/nmeth.f.303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Aitchison J. 1982. The statistical analysis of compositional data. J. R. Stat. Soc. B Stat. Methodol. 44:139–177 [Google Scholar]
- 29. Fernandes AD, Macklaim JM, Linn TG, Reid G, Gloor GB. 2013. ANOVA-like differential expression (ALDEx) analysis for mixed population RNA-seq. PLoS One 8:e67019. 10.1371/journal.pone.0067019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fernandes AD, Reid JNS, Macklaim JM, McMurrough TA, Edgell DR, Gloor GB. 2014. Unifying the analysis of high-throughput sequencing datasets: characterizing RNA-seq, 16S rRNA gene sequencing and selective growth experiments by compositional data analysis. Microbiome 2:15. 10.1186/2049-2618-2-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Sample PCR barcodes
OTUs and seed sequences