Abstract
LDL receptor-related protein 1 (LRP 1) is a transmembrane receptor highly expressed in human placenta. It was recently found to be the receptor for heme and its plasma binding protein hemopexin and is integral to systemic heme clearance. Little is known about systemic concentrations of hemopexin during pregnancy and whether maternal hemopexin and placental LRP1 contributes to fetal iron homeostasis during pregnancy. We hypothesized that placental LRP1 would be up-regulated in maternal/neonatal iron insufficiency and would be related to maternal circulating hemopexin. Placental LRP1 expression was assessed in 57 pregnant adolescents (14–18 y) in relation to maternal and cord blood iron status indicators [hemoglobin, serum ferritin, transferrin receptor], the iron regulatory hormone hepcidin and serum hemopexin. Hemopexin at mid-gestation correlated positively with hemoglobin at mid-gestation (r = 0.35, P = 0.02) and hemopexin at delivery correlated positively with cord hepcidin (r = 0.37, P = 0.005). Placental LRP1 protein expression was significantly higher in women who exhibited greater decreases in serum hemopexin from mid-gestation to term (r = 0.28, P = 0.04). Significant associations were also found between placental LRP1 protein with cord hepcidin (r = − 0.29, P = 0.03) and placental heme exporter FLVCR1 (r = 0.34, P = 0.03). Our data are consistent with a role for placental heme iron utilization in supporting fetal iron demands.
Introduction
The placenta serves as the sole conduit for the transfer of maternal nutrients to the fetus across pregnancy. It is believed that most, if not all, of the iron (Fe) delivered to the placenta is supplied as non-heme Fe. It is unclear whether plasma heme obtained from either intravascular red blood cell (RBC) catabolism (Tolosano et al. 2010), or from macrophage heme export (Yang et al. 2010), might also be utilized to support fetal Fe demands.
Hemopexin (Hx) is a plasma protein with high affinity for heme and contributes to systemic Fe homeostasis by delivery heme to the liver for storage (Tolosano et al. 2010). Decreases in serum Hx reflect a discharge of heme into the circulation and Hx is commonly measured along with the plasma hemoglobin (Hb) scavenge protein, haptoglobin (Hp), to assess the severity of intravascular hemolysis (Delanghe & Langlois 2001). Few data are available on Hx concentrations in pregnant women and it is unclear whether Hx is related to Fe status.
The LDL receptor-related protein 1 (LRP1) is a trans-membrane protein expressed in a variety of cell types including macrophages, hepatocytes, and neurons (Moestrup et al. 1992). Of interest, LRP1 is highly expressed in human placenta (Jensen et al. 1988, Jensen et al. 1989) and has been suggested to play a role in placental lipid transport (Gafvels et al. 1992). Recently, Hvidberg et al. identified LRP1 as the receptor for the Hx-heme complex and revealed a novel role of LRP1 in systemic heme recycling (Hvidberg et al. 2005). These authors suggested that the high expression of LRP1 in the human placenta may enable Fe recovered from maternal circulating heme to be delivered to the fetus (Hvidberg et al. 2005) but no studies have examined this hypothesis.
Fe efflux from the placenta to the fetus is thought to be mediated by the only known mammalian cellular non-heme Fe exporter, ferroportin (FPN) (Donovan et al. 2000, McKie et al. 2000). Consistent with its role in Fe export, FPN is localized to the basolateral side of human placental syncytiotrophoblast (Bastin et al. 2006). FPN is under tight control by the hormone hepcidin, which binds and induces degradation of FPN protein and thus limits cellular Fe export. Whether this FPN-hepcidin model in macrophages applies to the human placenta and whether placental FPN is related to expression of heme Fe transporters remain unknown.
The objectives of this study were: 1) to evaluate placental expression of the heme transporter LRP1 and the Fe export protein FPN in pregnant adolescents delivering at term and 2) to investigate the relationships between placental LRP1 and FPN with placental heme Fe exporter FLVCR1, maternal and neonatal Fe status, and changes in maternal Hx across gestation.
Material and methods
Subjects
Pregnant adolescents in this study were enrolled in a larger prospective study addressing the relationship between maternal and fetal mineral status. All participants were recruited from the Rochester Adolescent Maternity Program in Rochester, NY, USA between 2007 and 2011. Characteristics of this study cohort have been described elsewhere (Young et al. 2011, Young et al. 2012a) and data on placental expression of Fe and vitamin D-related proteins have been published (Young et al. 2010, Jaacks et al. 2011, O’Brien et al. 2014). The Institutional Review Boards at Cornell University and the University of Rochester approved all study procedures and written informed consent was obtained from all participants. A maternal blood sample was taken at mid-gestation (~26 wks) and at delivery (~40 wks) at which time cord blood and placental tissue were also obtained. Placental tissue used in this study was from a subset (n = 57) of the 113 teens who delivered term infants.
Biochemical analysis
Blood Hb was measured using a Cell Dyn 4000 hematology analyzer (Abbott Laboratories, Abbott Park, IL, USA). Serum was separated and stored at −80°C prior to analysis. Serum Hx and Hp were determined by sandwich ELISAs from Genway Biotech (Genway Biotech, San Diego, CA, USA) and ALPCO (ALPCO Diagnostics, Salem, NH, USA), respectively. Serum Hp lower than the normal range (0.3–2.0 g/L) was considered indicative of hemolysis (Delanghe & Langlois 2001). Serum ferritin (SF) and serum soluble transferrin receptor (sTfR) were measured using commercially available ELISA kits (Ramco Laboratories Inc., Stafford, TX, USA). Total body iron (TBI) was calculated from SF and sTfR concentrations using the equation developed by (Cook et al. 2003): TBI (mg/kg) = − [log10 (sTfR/SF) − 2.8229]/0.1207. Maternal ID was defined as either SF < 12 μg/L, sTfR > 8.5 mg/L, or TBI< 0 mg/kg (Akesson et al. 1998, Mei et al. 2011). Because the Hb distribution for African Americans is shifted to the left of that for the Caucasians, we adjusted the Hb cutoffs downward by 0.8 g/dL to define anemia in African American teens as recommended by IOM for black populations (IOM 1993). Specifically, anemia in whites was defined as Hb < 10.5 g/dL in the second trimester and Hb < 11.0 g/dL in the third trimester and anemia in blacks was defined as Hb < 9.7 g/dL in the second and Hb < 0.8 g/dL in the third trimester. Neonatal anemia was defined when cord blood Hb was < 13.0 g/dL (Nathan & Oski 1993). C-reactive protein (CRP) was measured using Immulite 2000 immunoassay system (Seimens Diagnostics, Los Angeles, CA). Intrinsic LifeSciences (La Jolla, CA, USA) measured serum hepcidin using a competitive ELISA specific for the mature peptide. The lower limit for detection of this assay is 5 μg/L. Serum hepcidin concentration below the limit of detection was given a value of 2.5 μg/L for data analysis purposes as previously described for this variable (Young et al. 2012b).
Placental sample collection
Placentas were collected immediately after delivery. Placental weight and dimensions were recorded. Multiple (4–5) placental samples were collected from different quadrants of the placenta, the maternal and fetal membranes removed and the samples then each cut into quarters and then randomly distributed into aliquots. These aliquots were either flash frozen (for western blot analyses) or placed into RNAlater (Ambion, Austin, TX, USA) and kept at −80°C. Placental protein lysates were prepared as previously described (Young et al. 2010, Jaacks et al. 2011) and protein concentrations of lysates were determined using Bio-Rad dye reagent (Bio-Rad, Hercules, CA, USA). Lysates were diluted in SDS-PAGE sample buffer and stored at −80°C until analysis.
Placental tissue Fe content
Total tissue Fe content of the placental tissue was determined using atomic absorption spectrophotometry as detailed before (Jaacks et al. 2011) and expressed as μg Fe/g placental dry weight.
Real-time quantitative reverse transcriptase–polymerase chain reaction (qRT-PCR)
Expression of placental LRP1 mRNA was undertaken in 42 placentas with quality RNA. Total RNA was extracted from the placental tissue samples using the RNeasy Microarray Tissue Mini Kit (Qiagen, Valencia, CA, USA). The extracted RNA was quantified and verified for integrity by the Experion automated electrophoresis system (Bio-Rad, Hercules, CA, USA). RNA purity was checked by the ratio of absorbance at 260 and 280 nm on a Nanodrop spectrophotometer (Thermo Scientific, Wilmington, DE, USA). A total of 1 μg RNA was reverse-transcribed into cDNA with a transcriptor cDNA synthesis kit (Roche Applied Sciences, Indianapolis, IN, USA). All qRT-PCR reactions were set up in a 10 μL reaction mixture containing 2 μL of the cDNA template, 5 μL of SYBR Green I Master reaction mix, and 0.7 μM of primers and run in triplicate in 384-well plates on a LightCycler 480 instrument (Roche Applied Sciences, Indianapolis, IN, USA). The cycling conditions included an initial denaturation step at 95°C for 5 min, followed by 45 cycles at 95°C for 10 sec, annealing at 60°C for 10 sec, and extension at 72°C for 10 sec. Specificity of amplifications was verified by melt curve analysis after PCR cycles. A single peak was consistently observed in all samples. Relative LRP1 mRNA expression was normalized to β-actin and compared to that obtained from a control placenta sample using the 2-ΔΔCt method using the following equations: ΔCt = Ct(LRP1) − Ct(β-actin); ΔΔCt = ΔCt(sample) − ΔCt(control placenta); and Fold Change = 2−ΔΔCt. The primers were designed using the ProbeFinder software from Roche Applied Science using the NCBI sequence ID (NM_002332 for LRP1 and NM_001101 for β-actin) and purchased from Integrated DNA Technologies (Coralville, IA, USA). Sequences of the primer-pairs were as follows: LRP1, Forward: 5′ – GAT GAG ACA CAC GCC AATC TG - 3′, Reverse: 5′-CGG CAC TGG AAC TCA TCA – 3′; β-actin, Forward: 5′– CCA ACC GCG AGA AGA TGA –3′, Reverse: 5′ CCA GAG GCG TAC AGG GAT AG – 3′.
Western blot analysis
Western blot was performed to determine placental protein expression of LRP1 and FPN. Placental lysates were separated by SDS-PAGE using a triple-wide electrophoresis unit (CBS Scientific, Del Mar, CA, USA) and electro-transferred to polyvinylidenedifluoride membranes (Millipore, Billerica, MA, USA). Membranes were blocked in Odyssey Blocking Buffer (Li-Cor, Lincoln, NB, USA) for 1 hr and incubated overnight at 4°C with antibodies against the following targets: mouse anti-LRP1 (1:3000 dilution; ab28320, Abcam, Cambridge, MA, USA), rabbit anti-FPN (1:500 dilution; MTP-11A, Alpha Diagnostics, San Antonio, USA), and β-actin (rabbit anti β-actin: 1:5000 dilution: Abcam, Cambridge, MA, USA or mouse anti β-actin: 1:2000 dilution: Santa Cruz Biotechnology, Santa Cruz, CA, USA). Blots were then incubated with appropriate fluorescence-coupled secondary antibodies (Li-Cor, Lincoln, NE, USA) for 1 hr at room temperature. After washing, fluorescence of the protein bands was quantified by with the Odyssey IR imaging system (Li-Cor, Lincoln, NE, USA). A band at 85 kDa corresponding to the LRP1 β-chain was observed in human placenta and rat liver (Supplemental Fig. 1). A band of a lower molecular weight was evident in the placental lysates and may represent differential phosphorylation of the LRP1 β-chain (May et al. 2003), as similar patterns have been observed in human placenta (Kristensen et al. 1990). The combined fluorescence of the upper and lower bands and that of the upper band was highly correlated (r = 0.96) thus the latter was used for data analysis. The use of combined signal of the upper and lower band did not change study findings. A control placenta sample was loaded in the left, middle, and right part of the gel and the CV for the 3 samples was 2.6% indicating minimal influence of the sample location on band intensity. A representative image of FPN western blot is shown in Supplemental Fig. 2.
Detailed western blotting methods for the determination of placental FLVCR1 expression in this cohort were previously published (Jaacks et al. 2011). Existing data on placental FLVCR1 were used in the present study to explore its relationship with placental LRP1 and FPN.
Statistical analysis
Non-normally distributed variables were transformed to achieve normality prior to data analysis. Paired t-tests were used to compare serum Fe status indicators and Hx between mid-gestation and delivery. Pearson’s correlations were used to examine associations between expression of placental LRP1, FPN, and FLVCR1, and serum Fe status indicators. Multivariate analysis was used to study associations while controlling for possible confounders. Multiple linear regression was used to model predictors of placental LRP1 expression. P values < 0.05 were considered significant. Data were reported as the means ± SDs unless otherwise stated.
Results
Subject characteristics
General characteristics of the study participants are presented in Table 1. Average age at enrollment was 17 y and the majority of adolescents were African American and non-Hispanic. Approximately 19.3% of the adolescents were obese prior to pregnancy and 61.4% gained more than the IOM gestational weight gain recommendations. Of the 57 adolescents, five delivered by Caesarean section. Eleven infants were born small for gestational age and two were large for gestational age.
Table 1.
Characteristics of study participants (n = 57)
| Characteristics | Mean ± SD (Range) |
|---|---|
| Age at enrollment (y) | 17.1 ± 1.1 (14.0–18.7) |
| Gestational age at mid-gestation blood draw (wk) | 25.8 ± 4.0 (20.1–37.2) |
| Gestational age at delivery (wk) | 40.0 ± 1.0 (37.9–41.7) |
| Parity ≥ 1 (%) | 11 |
| Pre-pregnancy BMI (kg/m2) | 25.1 ± 6.0 (17.2–43.5) |
| Gestational weight gain (kg) | 17.6 ± 7.7 (−2.1–43.2) |
| Race (%) | |
| African American | 61 |
| Caucasian | 37 |
| Native American | 2 |
| Ethnicity (%) | |
| Hispanic | 25 |
| Non-Hispanic | 75 |
| Delivery mode (%) | |
| Vaginal delivery | 91 |
| Cesarean section | 9 |
| Placental weight (kg) | 0.61 ± 0.11 (0.35–0.86) |
| Birth weight (kg) | 3.30 ± 0.45 (2.50–4.71) |
| Infant gender (%) | |
| Male | 60 |
| Female | 40 |
Maternal Fe status and Hx concentrations across pregnancy
Suboptimal Fe status in this adolescent cohort was evident (Table 2). Prevalence of anemia was 4.3% at mid-gestation and increased significantly to 10.2 % at term. Serum Hx decreased significantly by 9.9% from mid-gestation to delivery (p = 0.005). Mid-gestation Hx was positively associated with maternal Hb (r = 0.35, p = 0.02) and CRP (r = 0.30, p = 0.03) at mid-gestation and with maternal hepcidin (r = 0.29, p = 0.03) at delivery. Delivery Hx correlated with maternal hepcidin (r = 0.28, p = 0.04) at mid-gestation.
Table 2.
Iron status indicators in 57 pregnant adolescents and their neonates1
| Variable | Mid-Gestation | Delivery | Cord Blood | P value* |
|---|---|---|---|---|
| Hb (g/dL) | 11.4 ± 0.8 (47) a | 11.8 ± 1.3 (49) a | 13.7 ± 2.7 (36) b | < 0.0001 |
| SF (μg/L) | 22.2 ± 19.1 (57) a | 22.6 ± 12.9 (57) a | 133.0 ± 79.4 (57) b | < 0.0001 |
| Serum sTfR (mg/L) | 5.6 ± 5.9 (57) a | 5.4 ± 2.7 (57) a | 8.3 ± 2.5 (57) b | < 0.0001 |
| TBI (mg/kg) | 3.2 ± 3.8 (57) a | 3.6 ± 3.6 (57) a | 8.0 ± 2.6 (57) b | < 0.0001 |
| Serum hepcidin (μg/L) | 28.0 ± 19.9 (57) a | 36.2 ± 28.4 (57) a | 131.8 ± 88.8 (57) b | < 0.0001 |
| Serum Hx (g/L) | 1.0 ± 0.2 (57) a | 0.9 ± 0.3 (54) b | 0.3 ± 0.2 (47) c | < 0.0001 |
| Serum Hp (g/L) | 0.9 ± 0.5 (57) | ND | ND | |
| Serum CRP (mg/L) | 6.6 ± 6.9 (55) a | ND | 2.2 ± 10.8 (44) b | < 0.0001 |
Data are presented as Mean ± SD (sample size). Hb, hemoglobin; SF, serum ferritin; serum sTfR, serum soluble transferrin receptor; TBI, total body iron; Hx, hemopexin; Hp, haptoglobin; CRP, C-reactive protein; ND, not determined. Sample sizes are different due to missing assay (Hb) or sample availability (serum Hx and CRP);
Indicators were compared between groups using a linear regression model with Group (mid-gestation, delivery, and cord) as a fixed effect and Subject as a random effect. Values with different subscripts (a, b, c) are significantly different
Both mid-gestation Hx (r = 0.50, p = 0.002) and delivery Hx (r = 0.52, p = 0.002) correlated strongly with cord Hb and remained positive predictors of cord Hb after controlling for maternal Hb at the respective blood sampling point. Higher maternal Hx at delivery was related to better neonatal Fe status as evidenced by its association with high cord hepcidin (r = 0.37, p = 0.005) and low cord sTfR (r = − 0.27, p = 0.046). There was a non-significant positive correlation between the decrease in Hx from mid-gestation to delivery and the time elapsed between the two blood sampling points (r = 0.26, p = 0.06). Greater decreases in Hx across gestation were seen in women with lower mid-gestation hepcidin (r = − 0.27, p = 0.046) and in neonates with lower hepcidin (r = − 0.30, p = 0.03). These relationships remained significant after controlling for time interval between the mid-gestation and delivery measures. Using all the variables obtained, a multivariate model that included weeks elapsed between the biochemical assessments made at mid-gestation and delivery, maternal hepcidin at mid-gestation, and maternal TBI at delivery was found to capture 23.3% of the variation in changes in Hx from mid-gestation to delivery (p = 0.004).
Serum Hp was assessed as an additional indicator of hemolysis in maternal mid-gestation blood samples. Serum Hp did not correlate with Fe status indexes in the maternal or neonatal circulation. Four of the 57 teens had low Hp levels (< 0.3 g/L) suggestive of hemolysis but there were no differences in Fe status indicators or hepcidin between these 4 teens and the rest of the cohort. Correlation analysis revealed significant positive associations between serum Hp with serum CRP (r = 0.31, p = 0.02) and Hx (r = 0.33, p = 0.01) at mid-gestation.
Neonatal Fe Status and Hx concentrations
Neonatal hematological data are presented in Table 2. Nearly 1/3 of neonates were anemic at birth (cord Hb < 13 g/dL). Neonatal serum Hx was 74% lower than (p < 0.0001), and moderately correlated with maternal Hx at mid-gestation (r = 0.29, p = 0.046) but not at delivery. Cord Hx was not related to cord Fe status indicators or hepcidin.
Associations between placental LRP1 and FPN with Fe status and Hx concentrations
Expression of LRP1 and FPN in the placenta was not related to maternal race, ethnicity, gestational age at delivery, delivery mode (vaginal delivery, C-section), placental weight, or infant weight.
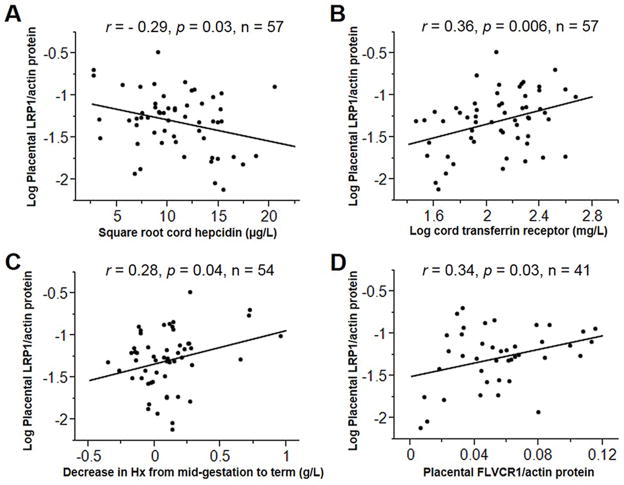
Placental LRP1 protein expression correlated positively with suboptimal neonatal Fe status as indicated by low cord hepcidin (r = − 0.29, p = 0.03; Fig. 1A) and high cord sTfR (r = 0.36, p = 0.006; Fig. 1B). Greater decreases in Hx from mid-gestation to delivery were associated with higher placental LRP1 protein expression (r = 0.28, p = 0.04; Fig. 1C). In the 41 adolescents with data on placental FLVCR1 protein, there was a positive correlation between placental LRP1 and FLVCR1 (r = 0.34, p = 0.03; Fig. 1D). Placental LRP1 mRNA was not significantly correlated with LRP1 protein (p = 0.14). Greater LRP1 mRNA tended to be associated with higher placental weight (r = 0.28, p = 0.08) and birth weight (r = 0.26, p = 0.09). None of the maternal or neonatal Fe status variables were significantly associated with placental LRP1 mRNA.
Fig. 1.
Significant correlates of placental LRP1 protein expression in 57 healthy pregnant adolescents. Placental LRP1 protein expression was assessed as a ratio between LRP1 and β-actin and log-transformed as required for regression analysis. Placental LRP1 protein expression increased in neonates with high Fe demand as indicated by its inverse correlation with cord hepcidin (Panel A) and positive correlation with cord sTfR (Panel B). Placental LRP1 protein expression correlated positively with the decrease in Hx from mid-gestation to term (Panel C) and with placental protein expression of the heme exporter FLVCR1 (Panel D).
Placental FPN protein expression was insignificantly negatively correlated with maternal serum hepcidin (r = − 0.23, p = 0.08) and TBI at mid-gestation (r = − 0.23, p = 0.09). No significant relationship was found between placental FPN with neonatal hepcidin or other Fe status indicators. Similarly, there were no significant relationships between placental FPN and neonatal hepcidin when data were examined in adolescents who were ID or non-ID. Placental Fe content was positively correlated with placental FPN protein expression (r = 0.37, p = 0.01). Neither LRP1 nor FLVCR1 was correlated with FPN protein expression in the placenta.
Discussion
During late pregnancy, Fe flux across the placenta reaches a maximum of 3–8 mg per day (Viteri 1994), a magnitude several times higher than the amount absorbed from the diet. The high Fe transport capacity of the placenta is partly explained by the abundant presence of TfR on the syncytiotrophoblast (Loh et al. 1980) and it is believed that transferrin-bound inorganic Fe is the predominant form of Fe utilized by the placenta to support fetal requirements (van Dijk 1988, McArdle et al. 2011). Recently, attention has been focused on the presence of multiple heme transport proteins in the human placenta (Cao & O’Brien 2013), which suggests that this organ may be an alternate site of heme clearance and/or utilization. In a group of healthy pregnant adolescents, we assessed maternal and neonatal serum Hx in relation to placental expression of the heme-Hx receptor LRP1, the heme exporter FLVCR1, and the non-heme Fe exporter FPN. We documented for the first time a significant positive correlation between two heme transporters LRP1 and FLVCR1 in the human placenta. In addition, placental LRP1 protein expression was higher in neonates with suboptimal Fe status and in women who experienced greater decreases in serum Hx from mid-gestation to delivery. Finally, we report novel associations between maternal Hx with neonatal Hb and hepcidin, adding to the biological plausibility of a role for plasma heme in maintaining Fe homeostasis during pregnancy.
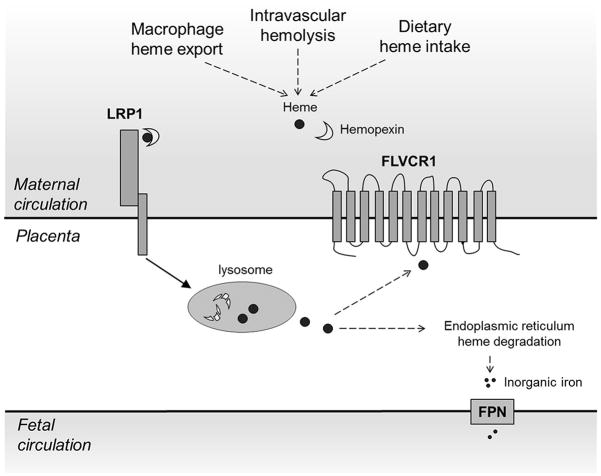
Circulating Hx and liver LRP1 facilitate plasma heme clearance and protect against heme-associated oxidative damage. The importance of Hx in the resolution of hemolysis is evident from knockout studies showing that mice lacking Hx are more sensitive to both induced hemolysis (Tolosano et al. 1999) and heme overload (Vinchi et al. 2008). In addition to systemic heme scavenging, Hx and LRP1 may mediate localized heme clearance in the brain during cerebral hemorrhage (Li et al. 2009). Functional analyses have demonstrated the Hx-heme binding activity of LRP1 purified from human placenta (Hvidberg et al. 2005), but few studies have examined the potential role of placenta as a site of Hx-heme clearance. In our study, placental protein expression of LRP1 was positively associated with that of FLVCR1, a plasma membrane heme exporter essential for erythroid cell survival and macrophage heme Fe recycling (Keel et al. 2008). It is possible that placental heme uptake (mediated by LRP1) and heme export (mediated by FLVCR1) are coordinately regulated to allow for sequestration of circulating heme while preventing accumulation of intracellular heme as depicted in Fig. 2.
Fig. 2.
Proposed pathway of heme Fe utilization in the placenta. Maternal plasma heme may be sourced from heme export from macrophages, oxidation of hemoglobin released during intravascular hemolysis or dietary heme intake. Free heme binds with hemopexin and the complex is taken up by the placental syncytiotrophoblast via LRP1-mediated endocytosis. Upon lysosomal degradation of hemopexin, free heme may be either 1) exported out into the maternal circulation by FLVCR1 or 2) converted to inorganic Fe by heme oxygenase in the endoplasmic reticulum and exported to the fetal circulation by FPN. The figure was modified from the schematic depicting the LRP1-mediated heme uptake pathway by Hvidberg et al. (Hvidberg et al. 2005)
Little is known about the cellular regulation of LRP1 expression by systemic Fe status. Studies in mouse hepatoma cells have found that Fe deprivation doubled the number of LRP1 and increased heme uptake, indicating that LRP1 may be negatively regulated by Fe availability (Smith & Ledford 1988). Consistent with this reciprocal Fe regulation of LRP1 in the liver, we found that high placental LRP1 was associated with suboptimal neonatal Fe status, which may represent a compensatory mechanism to increase placental heme uptake during Fe insufficiency in support of fetal demands.
Knockout experiments and localization studies provide convincing evidence that FPN is the protein that mediates Fe exit from placenta to the fetal circulation (Donovan et al. 2000, Donovan et al. 2005, Bastin et al. 2006). At this time it is unclear whether levels of FPN protein in the placenta are controlled by hepcidin in a similar way to that found in macrophages. Our study, and previous research in rodents (Gambling et al. 2001, Gambling et al. 2009, Cornock et al. 2013) and humans (Li et al. 2008), suggests that placental FPN protein is not regulated by maternal or neonatal Fe status. These data support the notion that FPN-mediated Fe export may not be the rate-limiting step for placental Fe transfer (Gambling et al. 2001). However, it still may be possible that changes in FPN subcellular location may inhibit Fe export without increased proteolytic degradation.
The Hx concentrations observed in maternal and neonatal circulation were within the ranges previously established for healthy adults (Delanghe & Langlois 2001) and term infants (Kanakoudi et al. 1995). Similar to previous reports (Muller-Eberhard et al. 1968, Gitlin & Biasucci 1969), cord Hx concentrations were 74% lower than those observed in maternal circulation, a phenomenon that has been suggested to be a consequence of low fetal Hx production (Lundh et al. 1970).
Kinetic studies demonstrated that extracellular Hx enhances heme export from human macrophages, supporting an emerging role of Hx in systemic heme trafficking (Yang et al. 2010). It has been suggested that the high serum concentration of Hx, which is comparable to that of the non-heme Fe transporter transferrin, is supportive of the importance of Hx in macrophage heme recycling under physiological conditions (Keel et al. 2008). Consistent with a role of Hx in systemic Fe balance in pregnancy, we observed associations between maternal Hx with cord Hb and hepcidin. Few studies have examined possible relationships between Hx and Fe status. In diabetic patients and healthy volunteers (n = 213), serum transferrin was a weak predictor of serum Hx while serum tri-acylglyerol and the diabetic state accounted for the majority of the variation (Van Campenhout et al. 2006). It is possible that the positive association between maternal Hx with maternal and cord Hb is representative of a greater capacity of maternal heme recycling and transport in support of fetal erythropoiesis.
The major strength of this study is the concurrent measurement of placental expression of LRP1 and FLVCR1 protein expression with serum Hx in a group of pregnant adolescents with well-characterized Fe status. Due to the observational nature of this study, we could not determine cellular processes underlying the associations between LRP1, Hx, and Fe status and mechanistic studies are needed to assess the heme transport activity of placental LRP1 and determine whether this process is responsive to changes in maternal/neonatal Fe status.
In summary, this study extends the current discussion of heme utilization to the placenta by documenting a relationship between placental LRP1 with maternal and neonatal Fe status in pregnant adolescents with term delivery. Because of the observational nature of this study, further research is needed to elucidate the mechanisms underlying these associations and examine the significance of placental heme scavenging in supporting fetal Fe demands.
Supplementary Material
Acknowledgments
Funding:
This work was supported by the National Research Initiative or Agriculture and Food Research Initiative of the USDA National Institute of Food and Agriculture, award numbers 2005-35200-15218 and 2008-01857-05171. Additional support was provided by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under award number T32DK007158.
The authors thank Highland Hospital staff and the URMC Midwifery Group for their clinical assistance, Allison W. McIntyre and Hannah Stillings for their assistance in subject recruitment, and the pregnant adolescents for their participation. We are grateful to Dr. Janis L. Abkowitz from the University of Washington for the FLVCR1 antibody.
Abbreviations used
- CRP
C reactive protein
- Fe
iron
- FLVCR
feline leukemia virus C receptor
- FPN
ferroportin
- Hb
hemoglobin
- Hp
haptoglobin
- Hx
hemopexin
- ID
iron deficiency
- LRP1
receptor-related protein 1
- RBC
red blood cells
- SF
serum ferritin
- sTfR
soluble transferrin receptor
- TBI
total body iron
Footnotes
Author contributions:
CC and KOO designed and performed the research, analyzed the data, and wrote the manuscript; EKP and EMC and RG conducted the research and assisted with participant recruitment. MW performed hepcidin analyses.
Disclosure:
None of the authors have any conflicts of interest to disclose.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- Akesson A, Bjellerup P, Berglund M, Bremme K, Vahter M. Serum transferrin receptor: a specific marker of iron deficiency in pregnancy. Am J Clin Nutr. 1998;68:1241–1246. doi: 10.1093/ajcn/68.6.1241. [DOI] [PubMed] [Google Scholar]
- Bastin J, Drakesmith H, Rees M, Sargent I, Townsend A. Localisation of proteins of iron metabolism in the human placenta and liver. Br J Haematol. 2006;134:532–543. doi: 10.1111/j.1365-2141.2006.06216.x. [DOI] [PubMed] [Google Scholar]
- Cao C, O’Brien KO. Pregnancy and iron homeostasis: an update. Nutr Rev. 2013;71:35–51. doi: 10.1111/j.1753-4887.2012.00550.x. [DOI] [PubMed] [Google Scholar]
- Cook JD, Flowers CH, Skikne BS. The quantitative assessment of body iron. Blood. 2003;101:3359–3364. doi: 10.1182/blood-2002-10-3071. [DOI] [PubMed] [Google Scholar]
- Cornock R, Gambling L, Langley-Evans SC, McArdle HJ, McMullen S. The effect of feeding a low iron diet prior to and during gestation on fetal and maternal iron homeostasis in two strains of rat. Reprod Biol Endocrinol. 2013;11:32. doi: 10.1186/1477-7827-11-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delanghe JR, Langlois MR. Hemopexin: a review of biological aspects and the role in laboratory medicine. Clin Chim Acta. 2001;312:13–23. doi: 10.1016/s0009-8981(01)00586-1. [DOI] [PubMed] [Google Scholar]
- Donovan A, Brownlie A, Zhou Y, Shepard J, Pratt SJ, Moynihan J, Paw BH, Drejer A, Barut B, Zapata A, Law TC, Brugnara C, Lux SE, Pinkus GS, Pinkus JL, Kingsley PD, Palis J, Fleming MD, Andrews NC, Zon LI. Positional cloning of zebrafish ferroportin1 identifies a conserved vertebrate iron exporter. Nature. 2000;403:776–781. doi: 10.1038/35001596. [DOI] [PubMed] [Google Scholar]
- Donovan A, Lima CA, Pinkus JL, Pinkus GS, Zon LI, Robine S, Andrews NC. The iron exporter ferroportin/Slc40a1 is essential for iron homeostasis. Cell Metab. 2005;1:191–200. doi: 10.1016/j.cmet.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Gafvels ME, Coukos G, Sayegh R, Coutifaris C, Strickland DK, Strauss JF., 3rd Regulated expression of the trophoblast alpha 2-macroglobulin receptor/low density lipoprotein receptor-related protein. Differentiation and cAMP modulate protein and mRNA levels. J Biol Chem. 1992;267:21230–21234. [PubMed] [Google Scholar]
- Gambling L, Czopek A, Andersen HS, Holtrop G, Srai SK, Krejpcio Z, McArdle HJ. Fetal iron status regulates maternal iron metabolism during pregnancy in the rat. Am J Physiol Regul Integr Comp Physiol. 2009;296:R1063–1070. doi: 10.1152/ajpregu.90793.2008. [DOI] [PubMed] [Google Scholar]
- Gambling L, Danzeisen R, Gair S, Lea RG, Charania Z, Solanky N, Joory KD, Srai SK, McArdle HJ. Effect of iron deficiency on placental transfer of iron and expression of iron transport proteins in vivo and in vitro. Biochem J. 2001;356:883–889. doi: 10.1042/0264-6021:3560883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitlin D, Biasucci A. Development of gamma G, gamma A, gamma M, beta IC-beta IA, C 1 esterase inhibitor, ceruloplasmin, transferrin, hemopexin, haptoglobin, fibrinogen, plasminogen, alpha 1-antitrypsin, orosomucoid, beta-lipoprotein, alpha 2-macroglobulin, and prealbumin in the human conceptus. J Clin Invest. 1969;48:1433–1446. doi: 10.1172/JCI106109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hvidberg V, Maniecki MB, Jacobsen C, Hojrup P, Moller HJ, Moestrup SK. Identification of the receptor scavenging hemopexin-heme complexes. Blood. 2005;106:2572–2579. doi: 10.1182/blood-2005-03-1185. [DOI] [PubMed] [Google Scholar]
- Jaacks LM, Young MF, Essley BV, McNanley TJ, Cooper EM, Pressman EK, McIntyre AW, Orlando MS, Abkowitz JL, Guillet R, O’Brien KO. Placental expression of the heme transporter, feline leukemia virus subgroup C receptor, is related to maternal iron status in pregnant adolescents. J Nutr. 2011;141:1267–1272. doi: 10.3945/jn.110.135798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen PH, Moestrup SK, Gliemann J. Purification of the human placental alpha 2-macroglobulin receptor. FEBS Lett. 1989;255:275–280. doi: 10.1016/0014-5793(89)81105-6. [DOI] [PubMed] [Google Scholar]
- Jensen PH, Moestrup SK, Sottrup-Jensen L, Petersen CM, Gliemann J. Receptors for alpha 2-macroglobulin- and pregnancy zone protein-proteinase complexes in the human placental syncytiotrophoblast. Placenta. 1988;9:463–477. doi: 10.1016/0143-4004(88)90019-7. [DOI] [PubMed] [Google Scholar]
- Kanakoudi F, Drossou V, Tzimouli V, Diamanti E, Konstantinidis T, Germenis A, Kremenopoulos G. Serum concentrations of 10 acute-phase proteins in healthy term and preterm infants from birth to age 6 months. Clin Chem. 1995;41:605–608. [PubMed] [Google Scholar]
- Keel SB, Doty RT, Yang Z, Quigley JG, Chen J, Knoblaugh S, Kingsley PD, De Domenico I, Vaughn MB, Kaplan J, Palis J, Abkowitz JL. A heme export protein is required for red blood cell differentiation and iron homeostasis. Science. 2008;319:825–828. doi: 10.1126/science.1151133. [DOI] [PubMed] [Google Scholar]
- Kristensen T, Moestrup SK, Gliemann J, Bendtsen L, Sand O, Sottrup-Jensen L. Evidence that the newly cloned low-density-lipoprotein receptor related protein (LRP) is the alpha 2-macroglobulin receptor. FEBS Lett. 1990;276:151–155. doi: 10.1016/0014-5793(90)80530-v. [DOI] [PubMed] [Google Scholar]
- Li RC, Saleem S, Zhen G, Cao W, Zhuang H, Lee J, Smith A, Altruda F, Tolosano E, Dore S. Heme-hemopexin complex attenuates neuronal cell death and stroke damage. J Cereb Blood Flow Metab. 2009;29:953–964. doi: 10.1038/jcbfm.2009.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YQ, Yan H, Bai B. Change in iron transporter expression in human term placenta with different maternal iron status. Eur J Obstet Gynecol Reprod Biol. 2008;140:48–54. doi: 10.1016/j.ejogrb.2008.02.012. [DOI] [PubMed] [Google Scholar]
- Loh TT, Higuchi DA, van Bockxmeer FM, Smith CH, Brown EB. Transferrin receptors on the human placental microvillous membrane. J Clin Invest. 1980;65:1182–1191. doi: 10.1172/JCI109773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundh B, Oski FA, Gardner FH. Plasma hemopexin and haptoglobin in hemolytic diseases of the newborn. Acta Paediatr Scand. 1970;59:121–126. doi: 10.1111/j.1651-2227.1970.tb08975.x. [DOI] [PubMed] [Google Scholar]
- May P, Bock HH, Nimpf J, Herz J. Differential glycosylation regulates processing of lipoprotein receptors by gamma-secretase. J Biol Chem. 2003;278:37386–37392. doi: 10.1074/jbc.M305858200. [DOI] [PubMed] [Google Scholar]
- McArdle HJ, Lang C, Hayes H, Gambling L. Role of the placenta in regulation of fetal iron status. Nutr Rev. 2011;69(Suppl 1):S17–22. doi: 10.1111/j.1753-4887.2011.00428.x. [DOI] [PubMed] [Google Scholar]
- McKie AT, Marciani P, Rolfs A, Brennan K, Wehr K, Barrow D, Miret S, Bomford A, Peters TJ, Farzaneh F, Hediger MA, Hentze MW, Simpson RJ. A novel duodenal iron-regulated transporter, IREG1, implicated in the basolateral transfer of iron to the circulation. Mol Cell. 2000;5:299–309. doi: 10.1016/s1097-2765(00)80425-6. [DOI] [PubMed] [Google Scholar]
- Institute of Medicine. Iron Deficiency Anemia:Recommended Guidelines for the Prevention, Detection, and Management Among US Children and Women of Childbearing Age. The National Academies Press; 1993. [PubMed] [Google Scholar]
- Mei Z, Cogswell ME, Looker AC, Pfeiffer CM, Cusick SE, Lacher DA, Grummer-Strawn LM. Assessment of iron status in US pregnant women from the National Health and Nutrition Examination Survey (NHANES), 1999–2006. Am J Clin Nutr. 2011;93:1312–1320. doi: 10.3945/ajcn.110.007195. [DOI] [PubMed] [Google Scholar]
- Moestrup SK, Gliemann J, Pallesen G. Distribution of the alpha 2-macroglobulin receptor/low density lipoprotein receptor-related protein in human tissues. Cell Tissue Res. 1992;269:375–382. doi: 10.1007/BF00353892. [DOI] [PubMed] [Google Scholar]
- Muller-Eberhard U, Javid J, Liem HH, Hanstein A, Hanna M. Plasma concentrations of hemopexin, haptoglobin and heme in patients with various hemolytic diseases. Blood. 1968;32:811–815. [PubMed] [Google Scholar]
- Nathan DG, Oski FA. Hematology of infancy and childhood. Philadelphia: W.B. Saunders; 1993. [Google Scholar]
- O’Brien KO, Li S, Cao C, Kent T, Young BV, Queenan R, Pressman EK, Cooper EM. Placental cyp27b1 and cyp24a1 expression in human placental tissue and their association with maternal and neonatal calcitropic hormones. J Clin Endo Metab. 2014 doi: 10.1210/jc.2013-1366. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A, Ledford BE. Expression of the haemopexin-transport system in cultured mouse hepatoma cells. Links between haemopexin and iron metabolism. Biochem J. 1988;256:941–950. doi: 10.1042/bj2560941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolosano E, Fagoonee S, Morello N, Vinchi F, Fiorito V. Heme scavenging and the other facets of hemopexin. Antioxid Redox Signal. 2010;12:305–320. doi: 10.1089/ars.2009.2787. [DOI] [PubMed] [Google Scholar]
- Tolosano E, Hirsch E, Patrucco E, Camaschella C, Navone R, Silengo L, Altruda F. Defective recovery and severe renal damage after acute hemolysis in hemopexin-deficient mice. Blood. 1999;94:3906–3914. [PubMed] [Google Scholar]
- Van Campenhout A, Van Campenhout C, Lagrou AR, Abrams P, Moorkens G, Van Gaal L, Manuel-y-Keenoy B. Impact of diabetes mellitus on the relationships between iron-, inflammatory- and oxidative stress status. Diabetes Metab Res Rev. 2006;22:444–454. doi: 10.1002/dmrr.635. [DOI] [PubMed] [Google Scholar]
- van Dijk JP. Regulatory aspects of placental iron transfer--a comparative study. Placenta. 1988;9:215–226. doi: 10.1016/0143-4004(88)90018-5. [DOI] [PubMed] [Google Scholar]
- Vinchi F, Gastaldi S, Silengo L, Altruda F, Tolosano E. Hemopexin prevents endothelial damage and liver congestion in a mouse model of heme overload. Am J Pathol. 2008;173:289–299. doi: 10.2353/ajpath.2008.071130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viteri FE. The consequences of iron deficiency and anemia in pregnancy. Nutrient regulation during pregnancy, lactation, and infant growth. 1994:127–139. doi: 10.1007/978-1-4899-2575-6_10. [DOI] [PubMed] [Google Scholar]
- Yang Z, Philips JD, Doty RT, Giraudi P, Ostrow JD, Tiribelli C, Smith A, Abkowitz JL. Kinetics and specificity of feline leukemia virus subgroup C receptor (FLVCR) export function and its dependence on hemopexin. J Biol Chem. 2010;285:28874–28882. doi: 10.1074/jbc.M110.119131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young BE, McNanley TJ, Cooper EM, McIntyre AW, Witter F, Harris ZL, O’Brien KO. Vitamin D insufficiency is prevalent and vitamin D is inversely associated with PTH and calcitriol in pregnant adolescents. J Bone Miner Res. 2011 doi: 10.1002/jbmr.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young BE, McNanley TJ, Cooper EM, McIntyre AW, Witter F, Harris ZL, O’Brien KO. Maternal vitamin D status and calcium intake interact to affect fetal skeletal growth in utero in pregnant adolescents. American Journal of Clinical Nutrition. 2012a;95:1103–1112. doi: 10.3945/ajcn.111.023861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young MF, Griffin I, Pressman E, McIntyre AW, Cooper E, McNanley T, Harris ZL, Westerman M, O’Brien KO. Maternal hepcidin is associated with placental transfer of iron derived from dietary heme and nonheme sources. J Nutr. 2012b;142:33–39. doi: 10.3945/jn.111.145961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young MF, Pressman E, Foehr ML, McNanley T, Cooper E, Guillet R, Orlando M, McIntyre AW, Lafond J, O’Brien KO. Impact of maternal and neonatal iron status on placental transferrin receptor expression in pregnant adolescents. Placenta. 2010;31:1010–1014. doi: 10.1016/j.placenta.2010.08.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.