Abstract
Introduction:
Mupirocin is an effective antibiotic for elimination of methicillin-resistant Staphylococcus aureus (MRSA) from nasal colonization and has been used to control outbreaks. Current reports show an increasing trend of resistance to this antibiotic.
Objective:
This study was conducted to analyze the resistance pattern of MRSA to mupirocin among the patients admitted following trauma to an apex trauma care center of India and to compare the efficacy between two methods of antimicrobial sensitivity testing.
Materials and Methods:
A total of 150 isolates of MRSA from various clinical samples of trauma patients over a period of 2 years were included in this study. These strains were confirmed for MRSA using VITEK® 2 Compact and the Clinical Laboratory Standard Institute disc diffusion methods. The mupirocin susceptibility of the strains was tested by using E-test and 5 μg mupirocin disc in parallel each time, and the results were compared.
Results:
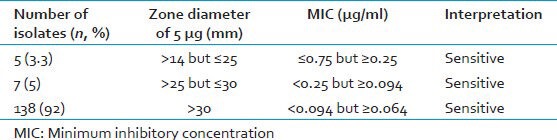
Clear zones of inhibition were observed in both tests. Though, good correlation was observed between the disc diffusion and E-tests in >98%, E-test showed a tendency to show lower minimum inhibitory concentration (MIC) in the remaining. These finding did not affect the final interpretation or outcomes. Of the total 150 strains, 138 (92%) showed sensitivity with the zone size in the range of 30-45 mm by 5 μg disc; rest (8%) showed sensitivity with the zone in the range of 18-30 mm by 5 μg disc, but 143 (95%) showed MIC ≤ 0.094 μg/ml and 8 (5%) gave MIC ≤ 0.75 μg/ml but ≥0.094 μg/ml by E-test. However, when both tests were compared, 5 (3.3%) showed zone size between 14 and 25 mm with ≤0.75 but >0.25 μg/ml MIC; 7 (5%) falling between 25 and 30 mm zone with MIC of ≤0.25 but >0.094 μg/ml and 138 (92%) showed zone >30 mm with MIC ≤0.094 but >0.064 μg/ml.
Conclusions:
All the MRSA isolates in our study were sensitive to mupirocin which is an encouraging finding. Though good screening for sensitivity can be done with 5 μg mupirocin disc, E-test provides a much clear and accurate results in clinical set-up. Hence, disc test can be used in resource poor countries and supplemented with E-test when needed.
Keywords: Antibiotic resistance, disc diffusion, E-test, methicillin-resistant Staphylococcus aureus, mupirocin
INTRODUCTION
Infections due to methicillin-resistant Staphylococcus aureus (MRSA) are on a rise. MRSA is also present as colonizer in the nasal area, which might be responsible for infection in the patient's own wound. Such colonization or superficial infections can be treated effectively with mupirocin. Unfortunately, due to unscrupulous use of this antibiotic, resistance to mupirocin is increasing. In initial clinical trials, a minimum inhibitory concentration (MIC) breakpoint of ≤4 μg/ml, and a corresponding zone diameter breakpoint of ≥18 mm with a 5 mg mupirocin disk was used to define susceptibility.[1] However, investigators have reported false resistance with this zone diameter breakpoint.[2,3] Further studies by other workers suggested an MIC breakpoint of ≤2 μg/ml and a corresponding zone diameter breakpoint of ≥14 mm for the 5-μg mupirocin disk.[4,5] Resistance of MRSA to mupirocin is categorized into two types: Low-level or intermediate resistance (MupI), with MICs of 8-256 μg/ml, and high-level resistance (MupR), with MICs ≥512 μg/ml.[6,7] A plasmid-mediated mupA gene appears to be associated with high-level resistance, while low-level resistance is associated with chromosomal point mutations.[8,9,10] Another novel gene, mupB is also responsible for high-level of mupirocin resistance.[11] Resistance to mupirocin is phenotypically detected by disc diffusion and also by E-tests. Preliminary screening of mupirocin resistance can be done with 5 μg disc, but those isolates, which gives low zones or no zones to 5 μg mupirocin disc can be discriminated for high- and low-level of resistance to mupirocin by using 200 μg disc if available.[6] However, with the introduction of E-tests, this confounding factor is removed and a single E-test can tell accurately the zone of inhibition.
As MRSA is one of the leading causes of infections in trauma wounds, a suitable drug is required to control the infections and its colonization. Resistance to mupirocin is on a rise, but few studies documenting the level of resistance in trauma patients are available, especially in developing countries. Hence, this study tries to explore the resistance pattern of MRSA to mupirocin in patients admitted for various traumas and also compares the results between two tests and their cost-effectiveness.
MATERIALS AND METHODS
Collection of clinical isolates
This study was conducted prospectively in an apex trauma center of North India for a period of 2 years from September, 2010 to August, 2012. A total of 240 S. aureus isolates from various samples were collected during this time.
Antimicrobial sensitivity
All isolates of S. aureus were tested for antimicrobial sensitivity pattern of methicillin susceptibility both by the Clinical Laboratory Standard Institute (CLSI) guidelines[12,13] and using the automated methods by VITEK® 2 compact (bioMιrieux, Durham, US). Those confirmed strains of MRSA were then tested for mupirocin sensitivity. S. aureus ATCC 25923 was used as control strain for mupirocin sensitivity and S. aureus ATCC 43300 was used for MRSA control in VITEK 2 and disc diffusion tests. Both were included with each test. Sensitivity to mupirocin was tested by (1) disc diffusion (CLSI guidelines) and (2) E-test which was performed in parallel. Disc diffusion was done using the 5 μg mupirocin disc on the standard plate. Mueller–Hinton agar (MHA) was used throughout the study. Suspension of freshly cultured MRSA isolates was made up to a turbidity of 0.5 McFarland and later swabbed on the MHA in three directions to give uniform growth. The plates were incubated aerobically at 37°C. All the plates were read independently by two persons to avoid bias.
Disc diffusion method
Disc diffusion was done using the 5 μg mupirocin disc (HiMedia, Mumbai, India) and results read after overnight incubation at 37°C. Here, a clear circular zone of inhibition or lack of it was noted. Depending on the zone of inhibition, which was measured in millimeters, it was categorized as sensitive, intermediate or resistance. For the interpretation of results using 5 μg mupirocin disc, susceptible was take as ≥14 mm, resistant as ≤13 mm and intermediate for those zone size falling in between the two.[4] A duplicate test was put up for those isolates whose zone falls near 14 mm to rule out intermediate resistance.
E-test method
E-test strips (bioMιrieux, Lyon, France) were used with an antibiotic concentration gradient in the range of 0.064 μg/ml to 1024 μg/ml. E-test MIC was determined according to the manufacturer's guidelines. The test was performed and interpreted according to the manufacturer's recommendations. An MIC falling between 8 and 256 μg/ml was taken a low or intermediate resistance, those showing ≥512 μg/ml as highly resistant and those isolates showing MIC <4 μg/ml were interpreted as sensitive.[6,7]
The reading of both the 5 μg disc and E-test were noted and compared regarding its efficacy and its cost-effectiveness for use in poor resource countries in general trauma care centers. Furthermore, the various MRSA isolated from different samples of trauma patients were noted and compared with the mupirocin sensitivity.
RESULTS
After performing the tests for methicillin resistance, 150 isolates of MRSA were identified out of a total of 240 S. aureus isolates. All these isolates were tested for mupirocin sensitivity along with controls in each test batch.
Disc test
A clear circular zone of inhibition was obtained in each plate. All the isolates were found to be sensitive (>14 mm). For better interpretation of results, the zone of inhibition obtained in each was divided into two groups: One with zone size >14 mm but <30 mm, which was near the cut-off level of 14 mm and the other group ≥30 mm showing very large sensitive zone [Figure 1]. Twelve (8%) isolates had zones falling in the first group near the cut-off level of 14 mm and remaining 138 (92%) in the remaining group. This finding obviates the need of using the 200 μg mupirocin disc, which can help to confirm between low- and high-level resistances. A duplicate test has been put up for those whose zone size was <30 mm. Reading taken independently by two different persons was noted and mean taken [Table 1].
Figure 1.
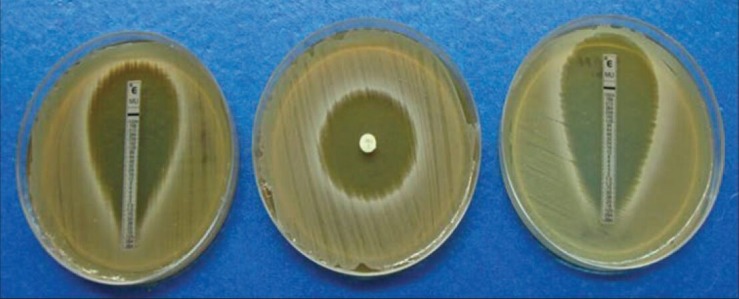
Comparative interpretation of disc diffusion test
Table 1.
Comparative evaluation of zone size with MIC levels by E-test
E-test
Here, an elliptical zone was obtained, and reading was taken by two persons independently. Again, all the MRSA strains were found to be sensitive to mupirocin, though the level of sensitivity varied. All the isolates showed MIC <4 μg/ml. For ease of analysis, the results were divided into two groups: One whose reading falls between ≤0.75 but ≥0.094 μg/ml and the other group where the sensitivity was <0.094, but >0.064 μg/ml [Figure 2]. A total of 8 (5%) showed readings, which falls between ≤0.75 but ≥0.094 μg/ml and remaining 142 (95%) showed zone size falling between <0.094 but >0.064 μg/ml.
Figure 2.
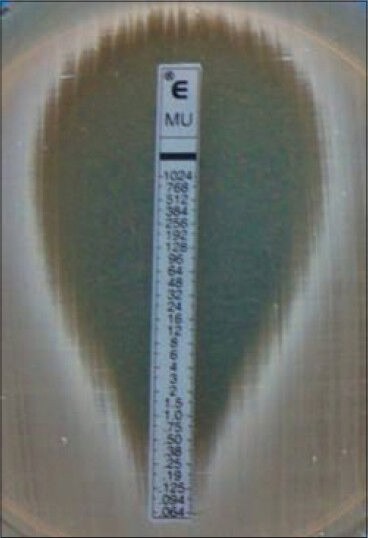
Interpretation of mupirocin E-test
The detail of comparison between zone diameters with MICs is given in Table 1.
Sample wise and patients’ distribution of methicillin-resistant Staphylococcus aureus in relation to the mupirocin sensitivity
Of the total 150 strains, 62% (93) of the MRSA isolates were from pus samples, 25% (39) were from blood samples, 5% (8) from tracheal, 2% (3) were contributed equally by tissue and fluid samples and remaining 1% (2) by urine and tips equally.
Comparison of cost between the 5 μg mupirocin disc and E-test
We have tried to observe the cost-effectiveness between the two methods. A 5 μg mupirocin vial (HiMedia, Mumbai, India) containing 50 discs cost around 150 INR (3 USD). Since a single disc was used per isolate/test, one test costs around 3 INR (0.1 USD). However, a blister packs of mupirocin E-tests strips (bioMιrieux, Lyon, France) containing 100 strips cost around 15,000 INR (254 USD). As a single strip was used per isolate/test, one test costs around 150 INR (3 USD). Hence, it was clearly evident that using a 5 μg mupirocin disc was far cost-effective compared to that of the E-test.
DISCUSSION
Based on our findings, none of the MRSA isolates were mupirocin resistant, and none of the demographic characteristics of the carriers or antibiotic resistance patterns or even the source of the isolates (hospital/community acquired) showed any determinant role in mupirocin sensitivity. The zone diameters of 5 (3.3%) of the strains was 16 mm, which is very close to the resistance zone (<14 mm) in our study.
The emergence of mupirocin resistance among S. aureus isolates has been clearly defined in many parts of the world at different frequencies: Spain 11.3%, USA 13.2%, Trinidad Tobago 26.1%, China 6.6%, India 6%, Turkey 45%, and Korea 5%; however, it does appear to be increasing worldwide.[9,14,15,16,17,18,19] This shows that one of the effective antibiotics to combat the carriage of MRSA is becoming a cause of concern due to rising resistance. Fortunately, all the isolates from our center were sensitive, which is an encouraging finding. This might be due to reason that use of mupirocin indiscriminately is avoided in our set-up. Studies have also shown a strong co-relation between the low rate of high resistance to mupirocin and reduced usage of mupirocin.[20]
A review of literature of Indian studies on mupirocin has shown that approximately 6% of mupirocin resistance is prevalent. High-level and low-level mupirocin resistance was detected in 10 (5%) and 2 (1%) S. aureus strains, respectively.[18] Pulsed-field gel electrophoresis analysis of the high-level mupirocin-resistant MRSA isolates revealed the presence of two clones with the majority of strains belonging to one clone, suggesting clonal dissemination.[18] Another study showed that rates of MuH were found to be 2% in MRSA and 28% in methicillin-resistant coagulase-negative Staphylococcus spp.[21] Another study from Chennai showed that MRSA ST239 isolates showed high-level mupirocin resistance (MIC > 512 mg/L/mupA+), inducible clindamycin resistance and high-level methicillin resistance (MIC = 256 mg/L). Although, there have been individual reports of mupirocin resistance and inducible clindamycin resistance among MRSA, this must be the first report on the emergence of hospital-acquired MRSA with both mupirocin and inducible clindamycin resistance.[22] Another tertiary care center study in India showed only 5 (3.3%) mupirocin resistant Staphylococcus species: Three high-level and two low-level strains were detected. The MICs for the two low-level and three high-level mupirocin resistant strains were 256 mg/L and ≥512 mg/L each, respectively.[23] Though many studies have reported on the different resistance patterns of MRSA to mupirocin; however, there is a paucity of studies which actually deals with the cost-effectiveness of the methods, which is required in routine practice for detection of resistance. Hence, our study can supplement these lacunae.
When sensitivity was tested for mupirocin, it was observed that good sensitivity pattern can be detected with both the 5 μg disc and the E-tests; but more accurate values were observed with E-tests compared to the disc test in clinical set-ups. As already mentioned in the introduction, high-level resistance may be detected by agar based 200 μg mupirocin disc or using the broth microdilution assay (single well-containing the 256 μg/ml of mupirocin). Further high resistance can also be detected using the mupA targeted PCR or the mupirocin E-test. Disc susceptibility, microdilution and E-test each requires 24 h; but, PCR is faster and requires 6-8 h.[24] Though PCR is faster and hence can result in faster initiation of treatment, the problem lies in its cost. The total working cost of PCR is more than 5 times the conventional 5 μg mupirocin disc or E-test, which is used routinely. However, studies have shown that neither 5 μg nor 200 μg shows good results regarding the resistance detection even though both are the forms, which are currently available commercially. Palepou et al. had shown that 25 μg mupirocin disc showed the best results. The best correlation with agar incorporation MIC was obtained with 25 μg mupirocin discs, which classified correctly 98 (95%) isolates, while worse correlations were noted with 5 μg and 200 μg discs, which are the only types currently available commercially, for which there were 47 and 30 minor errors, respectively.[25] The MIC found by E-test were the same or lower than those by using agar incorporation,[25] which is concordant with the findings of our study. The drawback of E-tests is the cost factor, which is a deterrent in routine use in laboratories for testing, especially in low resource country like India. Hence, 5 μg mupirocin disc can be used for routine purposes and supplemented with E-test when results are inconclusive.
CONCLUSIONS
Resistance to mupirocin is on a rise throughout the world, though MRSAs isolated from our set-up are still sensitive. Hence, screening of patients on a routine basis can be helpful to keep a check on it. It was observed that using 5 μg mupirocin disc is more cost-effective compared to E-test in countries where resources are limited. This can be done by using 5 μg disc supplemented with E-test whenever needed.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Finlay JE, Miller LA, Poupard JA. Interpretive criteria for testing susceptibility of staphylococci to mupirocin. Antimicrob Agents Chemother. 1997;41:1137–9. doi: 10.1128/aac.41.5.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Doebbeling BN, Breneman DL, Neu HC, Aly R, Yangco BG, Holley HP, Jr, et al. Elimination of Staphylococcus aureus nasal carriage in health care workers: Analysis of six clinical trials with calcium mupirocin ointment. The Mupirocin Collaborative Study Group. Clin Infect Dis. 1993;17:466–74. doi: 10.1093/clinids/17.3.466. [DOI] [PubMed] [Google Scholar]
- 3.Naguib MH, Naguib MT, Flournoy DJ. Mupirocin resistance in methicillin-resistant Staphylococcus aureus from a veterans hospital. Chemotherapy. 1993;39:400–4. doi: 10.1159/000238984. [DOI] [PubMed] [Google Scholar]
- 4.Fuchs PC, Jones RN, Barry AL. Interpretive criteria for disk diffusion susceptibility testing of mupirocin, a topical antibiotic. J Clin Microbiol. 1990;28:608–9. doi: 10.1128/jcm.28.3.608-609.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barry AL, Pfaller MA, Fuchs PC. Ramoplanin susceptibility testing criteria. J Clin Microbiol. 1993;31:1932–5. doi: 10.1128/jcm.31.7.1932-1935.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eltringham I. Mupirocin resistance and methicillin-resistant Staphylococcus aureus (MRSA) J Hosp Infect. 1997;35:1–8. doi: 10.1016/s0195-6701(97)90162-6. [DOI] [PubMed] [Google Scholar]
- 7.Mondino PJ, Dos Santos KR, de Freire Bastos Mdo C, Giambiagi-deMarval M. Improvement of mupirocin E-test for susceptibility testing of Staphylococcus aureus. J Med Microbiol. 2003;52:385–7. doi: 10.1099/jmm.0.05011-0. [DOI] [PubMed] [Google Scholar]
- 8.Udo EE, Jacob LE, Mathew B. Genetic analysis of methicillin-resistant Staphylococcus aureus expressing high- and low-level mupirocin resistance. J Med Microbiol. 2001;50:909–15. doi: 10.1099/0022-1317-50-10-909. [DOI] [PubMed] [Google Scholar]
- 9.Yun HJ, Lee SW, Yoon GM, Kim SY, Choi S, Lee YS, et al. Prevalence and mechanisms of low- and high-level mupirocin resistance in staphylococci isolated from a Korean hospital. J Antimicrob Chemother. 2003;51:619–23. doi: 10.1093/jac/dkg140. [DOI] [PubMed] [Google Scholar]
- 10.Cookson BD. The emergence of mupirocin resistance: A challenge to infection control and antibiotic prescribing practice. J Antimicrob Chemother. 1998;41:11–8. doi: 10.1093/jac/41.1.11. [DOI] [PubMed] [Google Scholar]
- 11.Seah C, Alexander DC, Louie L, Simor A, Low DE, Longtin J, et al. MupB, a new high-level mupirocin resistance mechanism in Staphylococcus aureus. Antimicrob Agents Chemother. 2012;56:1916–20. doi: 10.1128/AAC.05325-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clinical and Laboratory Standard Institute. 15. Vol. 30. Pennsylvania, USA: CLSI; 2010. Performance standards for Antimicrobial Susceptibility Testing; Twentieth Informational Supplement (June 2010 update) [Google Scholar]
- 13.Clinical and Laboratory Standard Institute. 3. Vol. 32. Pennsylvania, USA: CLSI; 2012. [Last assessed on 2014 May 15]. Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Second Informational Supplement Clinical and. Available from: http://antimicrobianos.com.ar/ATB/wp-content/uploads/2012/11/M100S22E.pdf . [Google Scholar]
- 14.Jones JC, Rogers TJ, Brookmeyer P, Dunne WM, Jr, Storch GA, Coopersmith CM, et al. Mupirocin resistance in patients colonized with methicillin-resistant Staphylococcus aureus in a surgical intensive care unit. Clin Infect Dis. 2007;1(45):541–7. doi: 10.1086/520663. [DOI] [PubMed] [Google Scholar]
- 15.Daskalaki M, Otero JR, Chaves F. Molecular characterization of resistance to mupirocin in methicillin-resistant Staphylococcus aureus isolates in a tertiary hospital in Spain. J Antimicrob Chemother. 2009;63:826–8. doi: 10.1093/jac/dkp025. [DOI] [PubMed] [Google Scholar]
- 16.Liu QZ, Wu Q, Zhang YB, Liu MN, Hu FP, Xu XG, et al. Prevalence of clinical meticillin-resistant Staphylococcus aureus (MRSA) with high-level mupirocin resistance in Shanghai and Wenzhou, China. Int J Antimicrob Agents. 2010;35:114–8. doi: 10.1016/j.ijantimicag.2009.09.018. [DOI] [PubMed] [Google Scholar]
- 17.Sareyyüpoglu B, Ozyurt M, Haznedaroglu T, Ardiç N. Detection of methicillin and mupirocin resistance in staphylococcal hospital isolates with a touchdown multiplex polymerase chain reaction. Folia Microbiol (Praha) 2008;53:363–7. doi: 10.1007/s12223-008-0056-4. [DOI] [PubMed] [Google Scholar]
- 18.Gadepalli R, Dhawan B, Mohanty S, Kapil A, Das BK, Chaudhry R, et al. Mupirocin resistance in Staphylococcus aureus in an Indian hospital. Diagn Microbiol Infect Dis. 2007;58:125–7. doi: 10.1016/j.diagmicrobio.2006.10.012. [DOI] [PubMed] [Google Scholar]
- 19.Orrett FA. The emergence of mupirocin resistance among clinical isolates of methicillin-resistant Staphylococcus aureus in Trinidad: A first report. Jpn J Infect Dis. 2008;61:107–10. [PubMed] [Google Scholar]
- 20.Walker ES, Levy F, Shorman M, David G, Abdalla J, Sarubbi FA. A decline in mupirocin resistance in methicillin-resistant Staphylococcus aureus accompanied administrative control of prescriptions. J Clin Microbiol. 2004;42:2792–5. doi: 10.1128/JCM.42.6.2792-2795.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oommen SK, Appalaraju B, Jinsha K. Mupirocin resistance in clinical isolates of staphylococci in a tertiary care centre in south India. Indian J Med Microbiol. 2010;28:372–5. doi: 10.4103/0255-0857.71825. [DOI] [PubMed] [Google Scholar]
- 22.Abimanyu N, Murugesan S, Krishnan P. Emergence of methicillin-resistant Staphylococcus aureus ST239 with high-level mupirocin and inducible clindamycin resistance in a tertiary care center in Chennai, South India. J Clin Microbiol. 2012;50:3412–3. doi: 10.1128/JCM.01663-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jayakumar S, Meerabai M, Banu SS, Mathew R, Kalyani M, Lal Y B. Prevalence of high- and low level mupirocin resistance among staphylococcal isolates from skin infection in a tertiary care hospital. J Clin Diagn Res. 2013;7:238–42. doi: 10.7860/JCDR/2013/4694.2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Acuna-Villaorduna C, Hardwick MJ, Goucher S, Waga M, Shoham S. Evaluation of mupA Evigene in comparison to disk diffusion for detection of high-level mupirocin resistance in clinical isolates of Staphylococcus aureus. J Clin Microbiol. 2010;48:2953–4. doi: 10.1128/JCM.02237-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Palepou MF, Johnson AP, Cookson BD, Beattie H, Charlett A, Woodford N. Evaluation of disc diffusion and E-test for determining the susceptibility of Staphylococcus aureus to mupirocin. J Antimicrob Chemother. 1998;42:577–83. doi: 10.1093/jac/42.5.577. [DOI] [PubMed] [Google Scholar]


