Abstract
Sup35p of Saccharomyces cerevisiae can form the [PSI+] prion, an infectious amyloid in which the protein is largely inactive. The part of Sup35p that forms the amyloid is the region normally involved in control of mRNA turnover. The formation of [PSI+] by Sup35p’s from other yeasts has been interpreted to imply that the prion-forming ability of Sup35p is conserved in evolution, and thus of survival/fitness/evolutionary value to these organisms. We surveyed a larger number of yeast and fungal species by the same criteria as used previously and find that the Sup35p from many species cannot form prions. [PSI+] could be formed by the Sup35p from Candida albicans, Candida maltosa, Debaromyces hansenii, and Kluyveromyces lactis, but orders of magnitude less often than the S. cerevisiae Sup35p converts to the prion form. The Sup35s from Schizosaccharomyces pombe and Ashbya gossypii clearly do not form [PSI+]. We were also unable to detect [PSI+] formation by the Sup35ps from Aspergillus nidulans, Aspergillus fumigatus, Magnaporthe grisea, Ustilago maydis, or Cryptococcus neoformans. Each of two C. albicans SUP35 alleles can form [PSI+], but transmission from one to the other is partially blocked. These results suggest that the prion-forming ability of Sup35p is not a conserved trait, but is an occasional deleterious side effect of a protein domain conserved for another function.
Keywords: [PSI+] prion, Schizosaccharomyces pombe, Ashbya gossypii, cytoduction
PRIONS are infectious proteins, proteins that are altered in such a way that they can instruct the unaltered form of the same protein to undergo the same alteration. Vertical or horizontal transmission of the altered form to a new individual restarts the process of converting the unaltered form to the altered form. If the presence of the altered form has some toxic or other effect, or if the absence of the unaltered form is detectable, then a phenotype or disease is produced (reviewed in Liebman and Chernoff 2012; Wickner et al. 2013). Most prions are an amyloid form of a normally nonamyloid protein. Amyloid is a linear polymer of a single-protein species, composed largely of β-sheets, with the β-strands perpendicular to the long axis of the filaments.
In yeast, as in other organisms, infection means horizontal transmission to a neighboring cell, not necessarily related to the cell that is the source of the infection. Perhaps because of yeast’s tough cell wall, neither the RNA viruses of yeast nor yeast prions leave one cell to travel through the medium and enter another cell. Rather, they are passed from cell to cell by mating and were first found as nonchromosomal genetic elements. This horizontal spread (infection) is conveniently shown by cytoduction (cytoplasmic mixing), in which two cells mate, but do not fuse their nuclei, which separate in the subsequent cell division. However, the resulting daughter cells with the parental nuclei each have mixed cytoplasms. If one parent strain carried a prion and the other not, both daughter cells will be found to carry it. A self-propagating amyloid that is not passed from cell to cell by this cytoduction process is not infectious. Prions of yeast have several diagnostic properties that distinguish them from viruses or plasmids (Wickner 1994). Overproduction of the prion protein increases the frequency with which the prion arises de novo. Curing of the [PSI+] amyloid-based prion by growth in the presence of guanidine (Tuite et al. 1981), an inhibitor of Hsp104 (Ferreira et al. 2001; Jung and Masison 2001; Jung et al. 2002), is an extremely useful test.
The [PSI+] nonchromosomal gene (Cox 1965) is a prion (infectious protein) of Sup35p (Wickner 1994), a protein whose normal functions include translation termination (Frolova et al. 1994; Stansfield et al. 1995) and regulation of mRNA turnover (Hoshino et al. 1999). Sup35p has three domains, N (residues 1–123), M (124–253), and C (254–685) (N terminal, middle, and C terminal). N and M are dispensable for the essential translation termination function, which is carried out by the C domain (Teravanesyan et al. 1993), but N and M do function in the regulation of mRNA turnover, interacting with the poly(A)-binding protein and the poly(A)-degrading enzyme (Funakoshi et al. 2007). The N domain is necessary and sufficient for propagation of many variants of the [PSI+] prion (Teravanesyan et al. 1994; Bradley and Liebman 2004; Chang et al. 2008), but the M domain is important as well (Liu et al. 2002; Bradley and Liebman 2004; Bateman and Wickner 2012). The prion form of Sup35p is a filamentous β-sheet-rich polymer (amyloid) of this protein (Glover et al. 1997; King et al. 1997; Paushkin et al. 1997; King and Diaz-Avalos 2004; Tanaka et al. 2004). Solid-state NMR studies of infectious amyloid of Sup35NM show a folded parallel in-register β-sheet architecture of most of the N domain (Shewmaker et al. 2006; Shewmaker et al. 2009). Both solid-state NMR data (Shewmaker et al. 2006, 2009) and hydrogen–deuterium exchange data (Toyama et al. 2007) show that some part of the M domain also is highly structured, apparently an in-register parallel β-sheet. Other amyloid-based prions of yeast include [URE3], an amyloid form of the Ure2 protein, a transcription regulator of nitrogen catabolism genes (Wickner 1994; Edskes et al. 1999; Brachmann et al. 2005), and [PIN+], a prion of Rnq1p, whose normal function is not known (Derkatch et al. 1997, 2001; Sondheimer and Lindquist 2000). Like Sup35p, infectious amyloid of both Ure2p and Rnq1p have a folded in-register parallel β-sheet architecture (Baxa et al. 2007; Wickner et al. 2008).
In addition to Saccharomyces cerevisiae, the Sup35p (or parts thereof) of several other yeasts have been shown capable of forming [PSI+] prions in S. cerevisiae. Replacing the S. cerevisiae Sup35 NM or N domain with the corresponding Sup35p domain of Pichia methanolica (Chernoff et al. 2000; Kushnirov et al. 2000; Santoso et al. 2000), S. paradoxus, S. bayanus, S. kudriavzevii, or S. mikatae produces fusion molecules that can form [PSI+] prions in S. cerevisiae (Chen et al. 2007; Afanasieva et al. 2011). Likewise, replacing the S. cerevisiae N domain with that of Kluyveromyces lactis or Candida albicans produced fusion proteins that showed prion-like behavior in S. cerevisiae (Santoso et al. 2000). Using full-length foreign Sup35ps, Nakayashiki et al. (2001) showed that the K. lactis Sup35p could form prions in S. cerevisiae or, importantly, in K. lactis itself. There are no previous reports of Sup35s from any species that cannot form a [PSI+] prion in S. cerevisiae.
The fact that Sup35s of other species can form [PSI+] has been interpreted to imply that this prion must be advantageous to yeast (Santoso et al. 2000; True and Lindquist 2000; Harrison et al. 2007). Of course, without a defined benefit of the prion, such an inference must be considered tentative, as the prion domain of Sup35p has a clear nonprion function (Funakoshi et al. 2007). While beneficial phenotypes of [PSI+] formation have been reported (Eaglestone et al. 1999; True et al. 2004), they have not been reproducible (True et al. 2004; Namy et al. 2008), even using the identical strains. Moreover, the common occurrence of lethal and near-lethal variants of [PSI+] (McGlinchey et al. 2011) and the rare occurrence of [PSI+] in the wild (Chernoff et al. 2000; Resende et al. 2003; Nakayashiki et al. 2005; Halfmann et al. 2012) implies that [PSI+] is generally detrimental to its host (Nakayashiki et al. 2005; Masel and Griswold 2009; Kelly et al. 2012).
Most species of Saccharomyces and C. albicans have Ure2p’s that are capable of forming a [URE3] prion (Baudin-Baillieu et al. 2003; Edskes and Wickner 2002; Edskes et al. 2009, 2011; Engel et al. 2011). However, the Ure2p of S. castellii cannot form [URE3] in S. cerevisiae (Edskes et al. 2009), and those of K. lactis (Safadi et al. 2011) and C. glabrata (Edskes and Wickner 2013) cannot do so in either S. cerevisiae or their native hosts. The Ure2p prion domain is important for stability against degradation of Ure2p in vivo (Shewmaker et al. 2007), a function whose preservation may justify the risk of developing near-lethal [URE3] variants (McGlinchey et al. 2011).
Unlike Ure2p, Sup35p is present from yeast to humans. We sought to explore the [PSI+] prion-forming ability of a wider range of species than had been previously examined in order to assess whether prion-forming ability is indeed conserved.
Materials and Methods
Strains and media
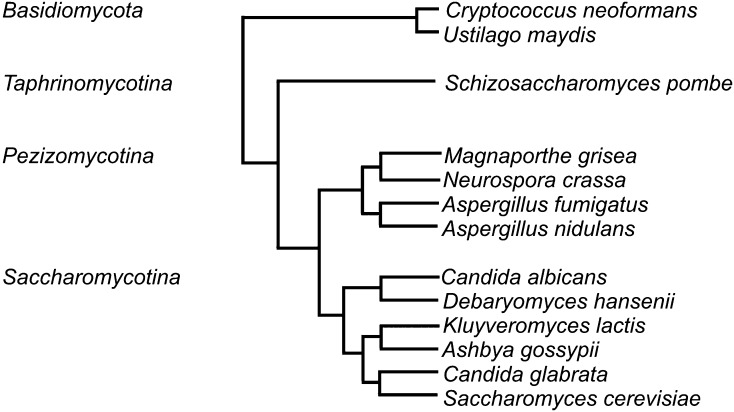
A phylogenetic tree of the yeast and fungal species used can be found in Figure 1. Genotypes of S. cerevisiae strains used are listed in Supporting Information,Table S1 and Table S2. Standard yeast media and methods were used throughout (Sherman 1991). In all experiments yeast were grown at 30°.
Figure 1.
Phylogenetic tree of the species studied. This tree is topologically accurate but does not accurately portray evolutionarily distances (Marcet-Houben and Gabaldon 2009).
Cloning of SUP35
Genomic DNA was isolated after growth in YPAD medium using Plant DNAzol reagent (Invitrogen) or the MasterPure yeast DNA purification kit (Epicentre). PCR was performed using KOD hot start DNA polymerase (EMD Millipore) or PfuUltra II fusion hot start DNA polymerase (Agilent Technologies). Several independent PCR reactions were performed and the products sequenced in order to identify possible PCR-induced mutations. A BamHI site was introduced immediately 5′ of the start codon. The C-terminal domain of S. cerevisiae Sup35p starts at M254. Alignment of Sup35 proteins from other species with the S. cerevisiae protein identified the amino acids corresponding to M254 (Figure S1 and Figure S2). To generate Sup35NM constructs the codon corresponding to M254 in cerevisiae was changed to a TAA stop codon and an XhoI site was placed immediately 3′. The introns present in the Schizosaccharomyces pombe and Neurospora crassa SUP35NMs were removed (Figure S3). To generate fusion proteins, the 5′ domains of SUP35 homologs, up to the codon corresponding to S. cerevisae M254, were linked seamlessly by PCR to the C domain of S. cerevisiae SUP35 starting with codon M254. Directly 3′ of the S. cerevisiae SUP35 stop codon an XhoI site was engineered. The SUP35NM and SUP35NM+C fragments were cloned as BamHI–XhoI fragments into the S. cerevisae expression vectors p1103 (LEU2 CEN PSUP35), pH317 [LEU2 2μ PGAL1; (Edskes and Wickner 2000)], and pH610 [TRP1 2μ PGAL1; (Kryndushkin et al. 2011)]. p1103 was created by replacing the NheI–BamHI-bordered ADH1 promoter from pH124 (Edskes et al. 1999) with a 452-nt SUP35 5′-UTR PCR fragment also flanked by NheI and BamHI (Table S3).
SUP35 integration constructs
A SUP35 promoter fragment of 452 nt was amplified by PCR with flanking NheI and BamHI restriction sites. A SUP35 3′-UTR fragment starting 6 nt upstream of the stop codon and ending 399 nt downstream of the stop codon was amplified by PCR. TRP1 containing 278 nt 5′-UTR and 46 nt 3′-UTR was amplified by PCR with the addition of flanking loxP sites (loxP sequence: ATAACTTCGTATAGCATACATTATACGAAGTTAT). The three fragments were combined in pTIF2 (Edskes et al. 2009) creating NheI–PSUP35–BamHI–XhoI–LoxP–TRPI–LoxP–SUP35 3′-UTR–PmeI. In the integrative vector pCW042 a BsmBI site is present between NheI and PSUP35 and between SUP35 3′-UTR and PmeI. In the integrative vector pHK025 a PmeI site is present between NheI and PSUP35. SUP35NM+C fragments were cloned as BamHI/XhoI fragments into the same window of pCW042 or pHK025 (Table S3).
Sup35NM–GFP constructs
SUP35NM fragments were amplified by PCR creating a BamHI site immediately upstream of the ATG start codon. Following the last codon of SUP35NM the sequence AGCGGCCGC was added. This creates a NotI site and would generate the peptide SGR when translated. SUP35NM fragments bordered by BamHI and NotI sites were ligated into the same window of pH327 (Edskes et al. 1999) creating a SUP35NM–GFP fusion separated by the codons for S,G, and R. The fusion ORF was moved as a BamHI–XhoI fragment into the same window of plasmid pH317 [LEU2 2μ PGAL1; (Edskes and Wickner 2000)].
Bacterial expression constructs
SUP35NM fragments were amplified by PCR creating a NheI site at the ATG start codon. Six histidine codons were added at the 3′ end followed by a TAA stop codon and an XhoI site. The Aspergillus nidulans SUP35NM codon Y89 was changed from TAT to TAC to remove an internal NdeI site. PCR fragments were cloned into the NdeI–XhoI window of pET21a(+) (EMD Millipore). Plasmid constructs are listed in Table S3 and described further in File S1.
[PSI+] induction
Yeast cells were transformed (Geitz and Schiestl 2007) with the inducing plasmids or the vector control and plated on SD medium. Transformants were inoculated into 4 ml SD medium and grown for 2 days to saturation. A total of 3 × 107 cells were used to inoculate 4 ml minimal medium with 2% galactose and 2% raffinose as the only carbon sources and grown overnight. Cells were washed with water, diluted to 3 × 107 cells/ml, and plated in 10-fold dilutions on minimal medium lacking adenine. Colonies were counted after 5 and 6 days of incubation at 30°. To check for dilution accuracy and viability a calculated 100 cells were plated on YPAD medium. In all the experiments we found ∼100 colonies on these viability test plates, indicating that overexpression of Sup35 was not extremely toxic under these conditions (Bradley et al. 2002).
Cytoductions
A cycloheximide-resistant CYH2 allele (Q38K) was amplified by PCR from strain L2598 (Taneja et al. 2007). The PCR product was transformed into S. cerevisiae and after overnight recovery in YPAD, transformants were selected on YPAD plates containing 3 μg/ml cycloheximide. Cycloheximide-resistant strains were made rhoo by growth on YPAD containing 30 μg/ml ethidium bromide. Donor cells placed in grids on plates lacking adenine were grown for 2 days at 30°. A lawn of rhoo, cycloheximide-resistant, recipient cells was grown for 7 hr on a YPAD plate at 30°. Donor cells were transferred by replica plating onto the lawn of recipient cells and the mating mixture was incubated for 24 hr at 30°. Cytoductants were recovered by replica plating to YPG plates containing 3 μg/ml cycloheximide. After growth at 30° the adenine phenotype was checked by replica plating to SD medium lacking adenine but containing 10 μg/ml cycloheximide.
Prion curing
Cells were cured of the [PSI+] prion by growth on media containing 3–5 mM guanidine hydrochloride. Prion- and non-prion-containing cells can conveniently be identified on the same plate by the accumulation of red pigment in the ade2-1 cells lacking [PSI+] grown on adenine limiting medium. On this YES medium (Edskes et al. 2009) [PSI+] cells form white colonies.
GFP fluorescence
Cells expressing GFP fusion proteins were examined by fluorescent microscopy using a Zeis Axiovert 200. Quantitation of the fluorescent signal of the cell population was performed using a Molecular Devices SpectroMax M5 multi-mode microplate reader. Cells were grown as described for the [PSI+] inductions and washed three times with water, and 300 μl of 5 × 107 cells/ml was pipetted in Costar 3915 plates. Fluorescence was measured using an excitation wavelength of 485 nm, emission wavelength of 510 nm, and a cut off filter set at 495 nm.
Protein expression and amyloid formation
Expression plasmids were transformed into BL21-CodonPlus(DE3)-RIPL cells (Agilent Technologies); transformants were inoculated in LB medium at an OD600 of 0.1 and grown until an OD600 of 0.4, at which point IPTG was added to 1 mM. Four hours later cells were harvested, resuspended in lysis buffer (15 ml/500 ml culture; 8 M guanidine hydrochloride, 150 mM NaCl, 100 mM Tris–HCl pH 8.0, 10 mM imidazole, one tablet protease inhibitor without EDTA, Roche), and incubated for 1 hr at 4°. Lysates were cleared by centrifugation at 30,000 rpm for 45 min in a SW45Ti rotor and the supernatant was mixed with 12 ml Ni-NTA (Qiagen), resuspended in equilibration buffer (8 M urea, 0.1 M Tris–HCl pH 8.0, 150 mM NaCl). Ater 4 hr gentle shaking at 4° the slurry was added to a column (Bio-Rad) and washed with 250 ml wash buffer (8 M urea, 0.1 M Tris–HCl pH 8.0, 150 mM NaCl, and 20 mM imidazole). Proteins were eluted with 12 ml buffer (8 M urea, 0.1 M Tris–HCl pH 8.0, 150 mM NaCl, 200 mM imidazole). Proteins were desalted using PD-10 columns (GE Healthcare) and concentrated to 0.5 mg/ml in 100 mM sodium phosphate pH 7.4 and 0.1 M NaCl using Amicon Ultra-15 filters. Preparations were incubated at room temperature with rotation for 1 week followed by 3 days without rotation.
Electron microscopy
Amyloid fibril suspensions were adsorbed to a carbon-coated copper grid for 2 min, washed for 1 min with H2O, stained for 1 min with 3% uranyl acetate, blotted, and air dried. The stained samples were examined using an FEI Morgagni transmission electron microscope (FEI, Hillsboro, OR) operating at accelerating potential of 80 kV.
Western blotting
To check Sup35p expression levels, yeast cells were grown to log phase in minimal glucose medium and harvested by centrifugation. If galactose induction was used to express proteins, cells were transferred to minimal galactose medium and grown for one doubling before harvesting. Protein lysates were prepared in buffer (8 M urea, PBS pH 7.4, 1 mM PMSF, protease inhibitor cocktail tablet, Roche) using a mini-beadbeater-8 (BioSpec). Western blots were probed with Sup35 C-terminal monoclonal antibody BE4 produced by Virivan Prapapanich (Bagriantsev and Liebman 2006).
Results
[PSI+] is assayed by the partial impairment of Sup35p’s translation termination function (Cox 1965). The ade2-1 allele is a TAA (ochre) termination codon early in the ADE2 open reading frame and SUQ5 is a weak serine-inserting tRNA ochre suppressor mutation (Liebman et al. 1975). The suppressor tRNA is in competition with the translation termination complex (Sup35p–Sup45p), and SUQ5 is so weak a suppressor that only if the termination complex is impaired, by Sup35p being largely tied up in amyloid filaments in this case, is there sufficient suppression of the ade2-1 nonsense mutation to allow cells to grow in the absence of adenine. The substrate of Ade2, phosphoribosylaminoimidazole, forms a red pigment whose accumulation reflects the degree of termination. The presence of [PSI+] can be distinguished from other mutations that produce an Ade+ phenotype in such strains because [PSI+] is efficiently cured by growth in the presence of 3 mM guanidine, an inhibitor of Hsp104, and because [PSI+] is efficiently transferred by cytoplasmic mixing (cytoduction).
Choice of species
The Sup35p N-terminal domains of a wide range of species are rich in glutamine and asparagine residues, but there are yeast and fungal prions with prion domains that are not Q/N rich (Balguerie et al. 2003; Suzuki et al. 2012), so we did not restrict our search to Q/N-rich Sup35ps (Figure S1). We examined a wide range of ascomycete yeasts, filamentous ascomycetes, and basiomycete yeasts, important model organisms and significant pathogens whose genomic sequences were available (Figure 1).
Both N and M of the S. cerevisiae Sup35p determine prion formation/propagation and thus should be viewed as a unit. Assaying N without M might not be biologically relevant. The border between M and C is easily delineated because of the strong sequence identity in the C-terminal domains (Figure S2). The border between N and M is much harder to pin down because of the lack of sequence identity.
Except for S. pombe, all NM domains of the Sup35 proteins have amino acid composition similarities with the protein of S. cerevisiae: (a) nearly all the aromatic residues are present in the N-terminal part of NM and (b) most of the aliphatic and charged residues are in the C-terminal parts of the NM domain (Table S5). The A. nidulans, N. crassa, Magnaporthe grisea, Aspergillus fumigatus, Ashbya gossypii, Cryptococcus neoformans, and, to a lesser degree, Debaromyces hansenii proteins have a clustering of charged residues in their N-terminal domains. In the Sup35 protein of S. pombe, the aromatic residues are still limited to the N-terminal part of NM, but the aliphatic and charged residues are spread throughout the protein.
We decided to create fusion proteins in which the NM domains of fungal Sup35p homologs were fused to the C-terminal domain of S. cerevisiae. As the C-terminal domain of Sup35p is sufficient to perform the essential translation termination function, fusion proteins would be predicted to affect the mechanics of this function less than full-length fungal homologs. The Sup35NM domains of several species were fused to S. cerevisiae Sup35C in a series of plasmid constructs. Introns were removed from the NM domains of the SUP35NM of S. pombe and N. crassa (Figure S3). These fusion proteins are designated Sup35NMspecies+C (e.g., Sup35NMcer, Sup35NMhan, Sup35NMalb1, or Sup35NMalb2 for the S. cerevisiae, D. hansenii, or the two C. albicans alleles examined here, respectively, and Sup35NMhan+C, etc., where linked to the S. cerevisiae Sup35C domain). Each such construct was used to replace pJ533 (CEN URA3 S.cerevisiae SUP35) in a strain with a deletion of the chromosomal locus (sup35::kanMX). In each case, the fusion protein was sufficiently active to allow growth, but the fusions of N. crassa and C. glabrata Sup35NMs with the S. cerevisiae Sup35C-made cells partially Ade+, indicating a partially inactive fusion protein. These cases were necessarily excluded from further study.
Induction of [PSI+] using plasmid-based strains
Overproduction of a prion protein induces the de novo formation of the prion (Chernoff et al. 1993; Wickner 1994), an effect amplified by deletion of the nonprion part of the molecule (Masison and Wickner 1995; Kochneva-Pervukhova et al. 1998). Strains carrying the fusion plasmids were transformed with a second plasmid expressing Sup35NMcer, Sup35NM of the other species, or Sup35NM of the other species fused to Sup35Ccer (= Sup35NMspecies+C), each under control of the inducible GAL1 promoter (Table 1). For the S. cerevisiae SUP35, the frequency of Ade+ colonies increased ∼100-fold by overexpression of either Sup35NMcer or Sup35NMcer+C. As expected, cytoduction from each of 48 Ade+ isolates to a recipient with the same Sup35p sequence resulted in transmission of the Ade+ phenotype in nearly all cases, indicating that these clones were [PSI+]. Note that even the Ade+ clones formed without overexpression of Sup35cer were nearly all [PSI+] as judged by the high-frequency transmission of the Ade+ phenotype by cytoduction. Similar robust induction of Ade+ clones by the appropriate Sup35NMspecies or Sup35NMspecies+C, with transmission by cytoduction from many Ade+ isolates, was observed for the C. albicans Sup35NMalb1 and Sup35NMalb2 and D. hansenii Sup35NMhan (Table 1). Sup35NMmal+C showed substantial induction of Ade+ colonies by overproduction of Sup35NMmal or Sup35NMmal+C, but only 2 of the 95 isolates tested after specific induction showed transmission by cytoduction. Thus, while nearly all of the Ade+ isolates without induction with Sup35cer were [PSI+], none, or almost none, of those with Sup35NMalb1,2+C, Sup35NMhan+C, or Sup35NMmal+C were [PSI+species], although all three can rarely form prions. This suggests that conversion to the prion form is much less frequent for Sup35p from these species than for the S. cerevisiae Sup35.
Table 1. [PSI+] generation by SUP35NM homologs in a plasmid based system.
| Source of SUP35NM | Strain | Inducing plasmid | Ade+/ 106 cells | n | Cytoduct. recipient | Total Ade+ isolates tested | Isolates giving Ade+ cytoductants | |
|---|---|---|---|---|---|---|---|---|
| S. cerevisiae | YHE1227 | pH610 | vector | 348 | 4 | YHE1409 | 48 | 46 |
| pH952 | NM cer | 31,000 | 4 | 48 | 43 | |||
| pH1337 | NM cer + C | 26,700 | 4 | 48 | 41 | |||
| C. albicans-1 | YAG1 | pH610 | vector | 17 | 4 | YHE1430 | 48 | 0 |
| pH952 | NM cer | 23 | 4 | 47 | 2 | |||
| pH1361 | NM alb1 | 33,000 | 4 | 48 | 19 | |||
| pH1344 | NM alb1 + C | 9,700 | 4 | 48 | 15 | |||
| C. albicans-2 | YAG2 | pH610 | vector | 80 | 4 | YHE1431 | 48 | 6 |
| pH952 | NM cer | 80 | 4 | 48 | 2 | |||
| p987A | NM alb2 | 55,000 | 4 | 48 | 46 | |||
| pH1343 | NM alb2 + C | 38,000 | 4 | 48 | 42 | |||
| K. lactis | YAG2 | pH610 | vector | 69 | 4 | YHE1435 | 48 | 0 |
| pH952 | NM cer | 73 | 4 | 48 | 0 | |||
| pAG105 | NM lac | 63 | 4 | 48 | 0 | |||
| pH1345 | NM lac + C | 45 | 4 | 96 | 0 | |||
| D. hansenii | YAG9 | pH610 | vector | 2 | 4 | YHE1432 | 48 | 0 |
| pH952 | NM cer | 6 | 4 | 48 | 0 | |||
| pAG184 | NM han | 4,620 | 4 | 188 | 75 | |||
| pH1346 | NM han + C | 22,000 | 4 | 48 | 27 | |||
| C. maltosa | YAG11 | pH610 | vector | 6 | 4 | YHE1434 | 48 | 0 |
| pH952 | NM cer | 6 | 4 | 48 | 0 | |||
| pCW025 | NM mal | 7,500 | 4 | 47 | 2 | |||
| pH1347 | NM mal + C | 2,800 | 4 | 48 | 0 | |||
| A. gossypii | YAG12 | pH610 | vector | 4 | 4 | YHE1433 | 48 | 0 |
| pH952 | NM cer | 3 | 4 | 47 | 0 | |||
| pAG56 | NM gos | 3 | 4 | 80 | 0 | |||
| pH1349 | NM gos + C | 1 | 4 | 25 | 0 | |||
| A. nidulans | YAG6 | pH610 | vector | 2 | 3 | YHE1459 | 48 | 0 |
| pH952 | NM cer | 7 | 4 | 47 | 0 | |||
| pAG178 | NM nid | 2 | 4 | 47 | 0 | |||
| pH1348 | NM nid + C | 9 | 4 | 47 | 0 | |||
| A. fumigatus | YAG4 | pH610 | vector | 40 | 4 | YHE1411 | 48 | 0 |
| pH952 | NM cer | 47 | 4 | 48 | 0 | |||
| pH976 | NM fum | 52 | 4 | 48 | 0 | |||
| pH1339 | NM fum + C | 40 | 4 | 48 | 0 | |||
| M. grisea | YAG3 | pH610 | vector | 3 | 4 | YHE1412 | 35 | 0 |
| pH952 | NM cer | 5 | 4 | 48 | 0 | |||
| p992A | NM gri | 2 | 4 | 26 | 0 | |||
| pH1341 | NM gri + C | 2 | 4 | 28 | 0 | |||
| S. pombe | YAG7 | pH610 | vector | 1 | 4 | YHE1436 | 23 | 0 |
| pH952 | NM cer | 3 | 4 | 34 | 0 | |||
| pAG173 | NM pom | 1 | 4 | 8 | 0 | |||
| pH1359 | NM pom + C | 1 | 4 | 7 | 0 | |||
| U. maydis | YHE1228 | pH610 | vector | 5 | 4 | YHE1413 | 29 | 0 |
| pH952 | NM cer | 7 | 4 | 42 | 0 | |||
| pH951 | NM may | 5 | 4 | 48 | 0 | |||
| pH1338 | NM may + C | 8 | 4 | 48 | 0 | |||
| C. neoformans | YAG14 | pH610 | vector | 3 | 4 | YHE1410 | 18 | 0 |
| pH952 | NM cer | 2 | 3 | 14 | 0 | |||
| p990A | NM neo | 3 | 4 | 13 | 0 | |||
| pH1340 | NM neo + C | 1 | 4 | 11 | 0 | |||
The indicated S. cerevisiae strain carried the indicated SUP35NMspecies+C gene with the S. cerevisiae SUP35 promoter on a plasmid as well as an inducing plasmid with SUP35NMcer (pH952) or SUP35NMspecies or SUP35NMspecies+C (or just the vector), each with the GAL1 promoter. Cells were grown in galactose to induce prion formation by overproduction of the fusion protein or NM and plated on –Ade. Clones were counted at 6 days and the results shown are the average of n experiments. The “total” number of Ade+ isolates was used as cytoduction donors, the cytoduction recipients also having a plasmid-based SUP35, strain YHE1407 with the corresponding plasmid with PSUP35SUP35NMspecies+C CEN LEU2 (Table S2). In plating cells expressing Sup35NM from U. maydis, A. fumigatus, M. grisea, C. neoformans, or A. nidulans, there was a significant background of tiny red colonies. However, some such colonies were also tested by cytoduction and did not have the properties of a [PSI+] isolate.
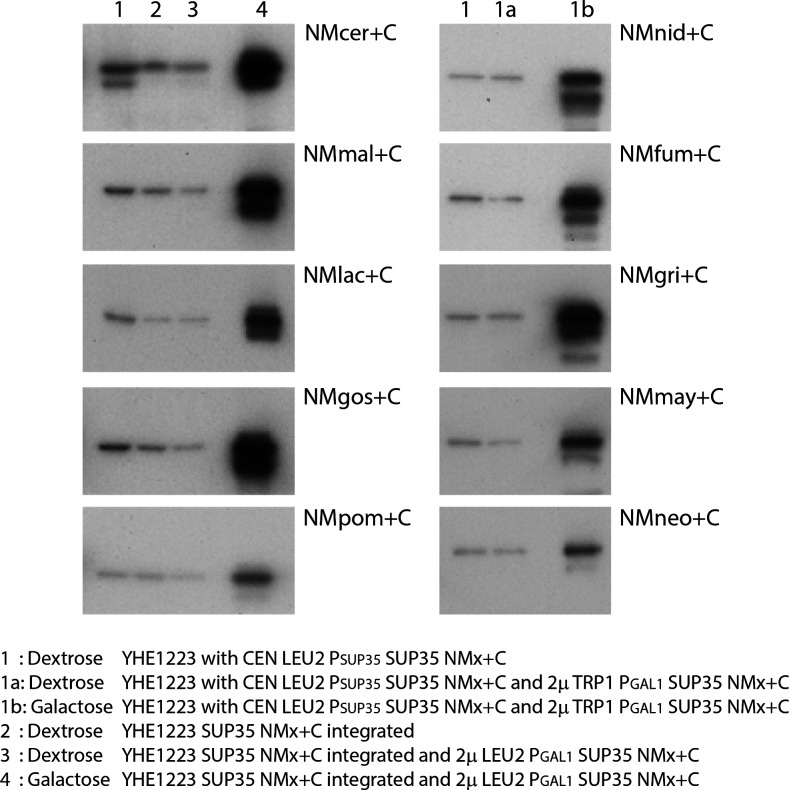
The fusion Sup35s from K. lactis, A. gossypii, A. nidulans, A. fumigatus, M. grisea, S. pombe, Ustilago maydis, and C. neoformans showed neither induction of Ade+ colonies by overexpression of the corresponding Sup35NM nor transfer of the Ade+ phenotype from any of the few Ade+ colonies arising (Table 1). As a check that plasmids constructed to overproduce Sup35NMspecies were in fact doing so, we measured GFP fluorescence from the same constructs but with GFP added to their C termini. Sup35NM–GFP constructs for species whose Sup35NM did not form [PSI+] all showed strong overproduction on galactose medium (Table 2). A second approach to testing for protein overproduction, useful on the Sup35NMspecies+C proteins without including the GFP appendage, is Western blot of extracts (Figure 2). Using a monoclonal antibody to Sup35Ccer prepared by Virivan Prapapanich (Bagriantsev and Liebman 2006), we found that the Sup35NMspecies+C proteins were dramatically overproduced in all cases (Figure 2).
Table 2. Overproduction of Sup35NMspeciesGFP measured by fluorescence of whole cells.
| Plasmid | NM–GFP | Fold expression |
|---|---|---|
| pH317 | Vector | 1 |
| pAZ018 | GFP | 41 |
| pAZ017 | S. cerevisiae | 16 |
| pAZ15 | K. lactis | 33 |
| pH1301 | C. albicans1 | 1 |
| pH1302 | C. albicans2 | 7 |
| pH1303 | U. maydis | 33 |
| pH1304 | A. fumigatus | 68 |
| pH1305 | C. neoformans | 22 |
| pH1306 | D. hansenii | 18 |
| pH1307 | N. crassa | 39 |
| pH1308 | C. glabrata | 16 |
| pH1309 | C. maltosa | 2 |
| pH1310 | A. gossypii | 40 |
| pH1311 | S. pombe | 45 |
| pH1312 | A. nidulans | 50 |
| pH1313 | M. grisiae | 64 |
Plasmids used to overexpress Sup35NMspecies for prion induction were modified to encode GFP at the C terminus of the Sup35NM. Strain HJK088 carrying these plasmids was grown under the same conditions used to induce [PSI+] in the same strain. Fold expression was measured by GFP fluorescence (see Materials and Methods) compared to cells carrying the vector alone. Expression of Sup35NMs that did not produce [PSI+] was at least as good as those that did.
Figure 2.
Western blots of Sup35NMspecies+C. Cells of indicated genotype were grown on dextrose or galactose, and extracts made and Western blots prepared as described in Methods. “x” is the species that is the source of NM. cer = S. cerevisiae, mal = C. maltosa, lac = K. lactis, gos = A. gossypii, pom = S. pombe, nid = A. nidulans, fum = A. fumigatus, gri = M. grisea, may = U. maydis, neo = C. neoformans. (1) Dextrose YHE1223 with pCEN LEU2 PSUP35 SUP35NMx+C. (1a) Dextrose YHE1223 with pCEN LEU2 PSUP35 SUP35NMx+C and 2μ pTRP1 PGAL1 SUP35NMx+C. (1b) Galactose YHE1223 with pCEN LEU2 PSUP35 SUP35NMx+C and 2μ TRP1 PGAL1 SUP35NMx+C. (2) Dextrose YHE1223 SUP35NMx+C integrated. (3) Dextrose YHE1223 SUP35NMx+C integrated and 2μ LEU2 PGAL1 SUP35NMx+C. (4) Galactose YHE1223 SUP35NMx+C integrated and 2μ LEU2 PGAL1 SUP35NMx+C.
Integrated SUP35NMspecies+C strains
To assure a more stable source of Sup35p, and thus to more closely simulate the natural environment in which a [PSI+] prion exists, we constructed integrated versions of the hybrid genes producing Sup35NMspecies+C proteins to test again for prion formation. We integrated versions of the hybrid genes with NM segments from C. albicans (SUP35alb1), K. lactis, C. maltosa, A. gossypii, and S. pombe. A robust induction of Ade+ colony formation was observed on overproduction of NM for S. cerevisiae (>1000-fold), a very real (10-fold) increase for SUP35NMalb1+C, and only a marginal increase for Sup35NMlac+C (Table 3). Nearly all Ade+ clones from the all-S. cerevisiae Sup35 were infectious, but only a small minority of those from Sup35NMalb1+C or Sup35NMlac+C were transmitted (Table 3). All seven transmissible Sup35NMlac+C isolates were also guanidine curable, confirming that they were [PSI+lac]. Sup35NMmal+C showed no significant increase in Ade+ clones and no evidence of infectivity of those that did arise. As in the plasmid-based experiments above, Sup35NMgos+C and Sup35NMpom+C showed no evidence of either induction or transmission by the few Ade+ clones arising.
Table 3. [PSI+] generation by SUP35NM homologs integrated at the genomic SUP35 locus.
| Source of SUP35NM | Strain | Inducing plasmid | Ade+/106 cells | n | Cytoduct. Recipient | Total Ade+ isolates tested | Isolates giving Ade+ cytoductants | |
|---|---|---|---|---|---|---|---|---|
| S. cerevisiae | HJK088 | pH317 | vector | 31 | 8 | YAZ013 | 44 | 0 |
| pHK006 | NM cer | 23,900 | 8 | 47 | 45 | |||
| pH1294 | NM cer + C | 82,900 | 8 | 47 | 45 | |||
| C. albicans-1 | HJK089 | pH317 | vector | 143 | 8 | YHE1387 | 144 | 0 |
| pHk006 | NM cer | 162 | 8 | 144 | 0 | |||
| pHK007 | NM alb1 | 1,850 | 8 | 144 | 12 | |||
| pH1295 | NM alb1 + C | 1,470 | 8 | 144 | 3 | |||
| K. lactis | HJK092 | pH317 | vector | 79 | 8 | YHE1425 | 144 | 0 |
| pHK006 | NM cer | 136 | 8 | 144 | 0 | |||
| pHK016 | NM lac | 169 | 8 | 144 | 0 | |||
| pH1296 | NM lac + C | 637 | 8 | 144 | 7 | |||
| C. maltosa | HJK122 | pH317 | vector | 13 | 4 | YHE1389 | 48 | 0 |
| PHK006 | NM cer | 23 | 4 | 48 | 0 | |||
| pHK012 | NM mal | 32 | 4 | 48 | 0 | |||
| pH1299 | NM mal + C | 24 | 4 | 95 | 0 | |||
| A. gossypii | HJK111 | pH317 | vector | 77 | 4 | YHE1391 | 48 | 0 |
| pHK006 | NM cer | 115 | 4 | 48 | 0 | |||
| pHK018 | NM gos | 129 | 4 | 48 | 0 | |||
| pH1300 | NM gos + C | 84 | 4 | 48 | 0 | |||
| S. pombe | HJK110 | pH317 | vector | 24 | 3 | YHE1383 | 48 | 0 |
| pHK006 | NM cer | 41 | 4 | 47 | 0 | |||
| pHK010 | NM pom | 24 | 4 | 48 | 0 | |||
| pH1297 | NM pom + C | 12 | 4 | 48 | 0 | |||
The indicated SUP35NMspecies+C fusion genes were integrated into strain YHE1223 replacing the sup35::kanMX marker, and pJ533 (CEN URA3 SUP35cer) was eliminated. SUP35 homologs not listed could not be integrated into the genome of this strain. In general these are the same SUP35 homologs that showed poor suppression of ade2-1. An inducing plasmid with SUP35NMcer (pH952), SUP35NMspecies, or SUP35NMspecies+C (or just the vector), each with the GAL1 promoter, was introduced and cells were grown in galactose to induce prion formation by overproduction of the fusion protein or NM. Cells were plated on –Ade, clones were counted at 6 days, and the results shown are the average of n experiments. Cytoduction recipients have the same SUP35 integrated at the genomic SUP35 locus. The cytoduction recipients are not isogenic as they were created by meiotic crosses. The “total” number of Ade+ isolates were used as cytoduction donors.
To retest and confirm the prion properties of Ade+ colonies isolated using the plasmid system, we cytoduced Ade+ isolates into strains carrying the same hybrid SUP35 gene on a plasmid or integrated at the normal SUP35 chromosomal site (Table S4). We found that for those species for which specific induction of Ade+ colonies was observed in the plasmid-based system, only a fraction showed transmission by cytoduction using either the plasmid-based recipient or the integrated recipient. No transmission was observed from the few Ade+ colonies appearing with Sup35NMlac+C, Sup35NMgos+C, or Sup35NMpom+C.
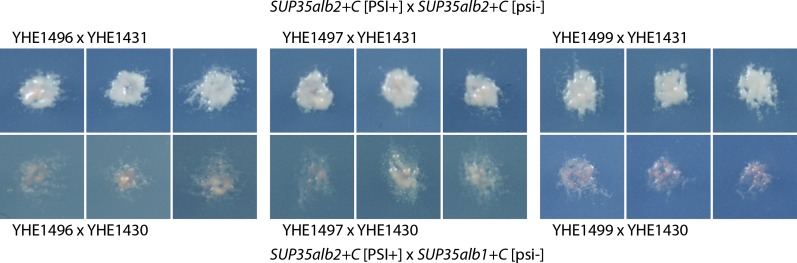
The SN100 strain of C. albicans, a yeast found naturally only as a diploid, had two distinct SUP35 alleles, differing only in their M domains (Figure S4). Each could form [PSI+alb], but cytoduction from a SUP35alb2+C [PSI+alb2] strain to a SUP35alb1+C [psi-] recipient showed only inefficient transmission of the prion (Table 4). This intraspecies barrier was observed, to different extents, for each of the four [PSI+alb2] variants tested. This intraspecies transmission barrier may partially prevent [PSI+alb] appearance or transmission in a heterozygous diploid, like the decreased appearance and transmission of human prions to individuals heterozygous for the M/V polymorphisms at PrP residue 129 (Mead et al. 2003). Indeed, diploids formed by mating SUP35alb2+C [PSI+alb] × SUP35alb2+C [psi-] are all [PSI+alb2] (48 Ade+ of 48), but heterozygous diploids formed by mating SUP35alb2+C [PSI+alb2] × SUP35alb1+C [psi−] are mostly [psi−] (e.g., 17 of 48) or weakly [PSI+alb] (30 of 48), with only a few heterozygous diploid clones being fully [PSI+alb] (1 of 48) (Figure 3). The asymmetry of the transmission barrier is reminiscent of asymmetry in the species barrier for [URE3] between Ure2p of S. cerevisiae and that of S. bayanus (Edskes et al. 2009) or the intraspecies barriers between polymorphs of the Sup35p of S. cerevisiae (Bateman and Wickner 2012). Because Sup35alb1 and Sup35alb2 differ only in the M domain, this result is also of interest as a new example of a central role of the Sup35M domain in [PSI+] transmission.
Table 4. Transmission barrier of [PSI+alb2] to Sup35NMalb1.
| Recipient NMalb1 | Recipient NMalb2 | |||
|---|---|---|---|---|
| Donor | Ade+ | Total | Ade+ | Total |
| NMalb1 | ||||
| YHE1464 | 24 | 24 | 24 | 24 |
| YHE1465 | 23 | 24 | 24 | 24 |
| YHE1466 | 24 | 24 | 24 | 24 |
| NMalb2 | ||||
| YHE1496 | 1 | 24 | 22 | 24 |
| YHE1497 | 2 | 24 | 24 | 24 |
| YHE1498 | 20 | 24 | 22 | 24 |
| YHE1499 | 0 | 24 | 24 | 24 |
NMalb1 donor strains are derived from HJK089. Strains YHE1464 and YHE1465 are [PSI+alb1] inductants obtained after NMalb1 overexpression using pHK007 while strain YHE1466 was obtained after NMab1+C overexpression using pH1295. NMalb2 donor strains are derived from YAG2. Strains YHE1498 and YHE1499 are [PSI+alb2] inductants obtained after NMalb2 overexpression using p987A while strains YHE1496 and YHE1497 were obtained after NMalb2+C overexpression using pH1343. The NMalb1 recipient strain is YHE1430 and the NMalb2 recipient strain is YHE1431. Cells were allowed to mate on YPAD for 7 hr and cytoductants were identified after growth to single clonies on YPG+3mM cycloheximide.
Figure 3.
SUP35alb2+C/SUP35alb1+C heterozygous diploids lose [PSI+alb2]. Three [PSI+alb2] SUP35alb2+C isolates YHE1496, YHE1497, and YHE1499 (see Table 4) were mated with the [psi−] SUP35alb2+C strain 1431 or the SUP35alb1+C strain 1430. Diploids were isolated and replicaplated to –Ade plates. Most heterozygous diploids were Ade− or weakly Ade+ (below) but all homozygous diploids were Ade+ (above).
Filament formation by Sup35NMspecies
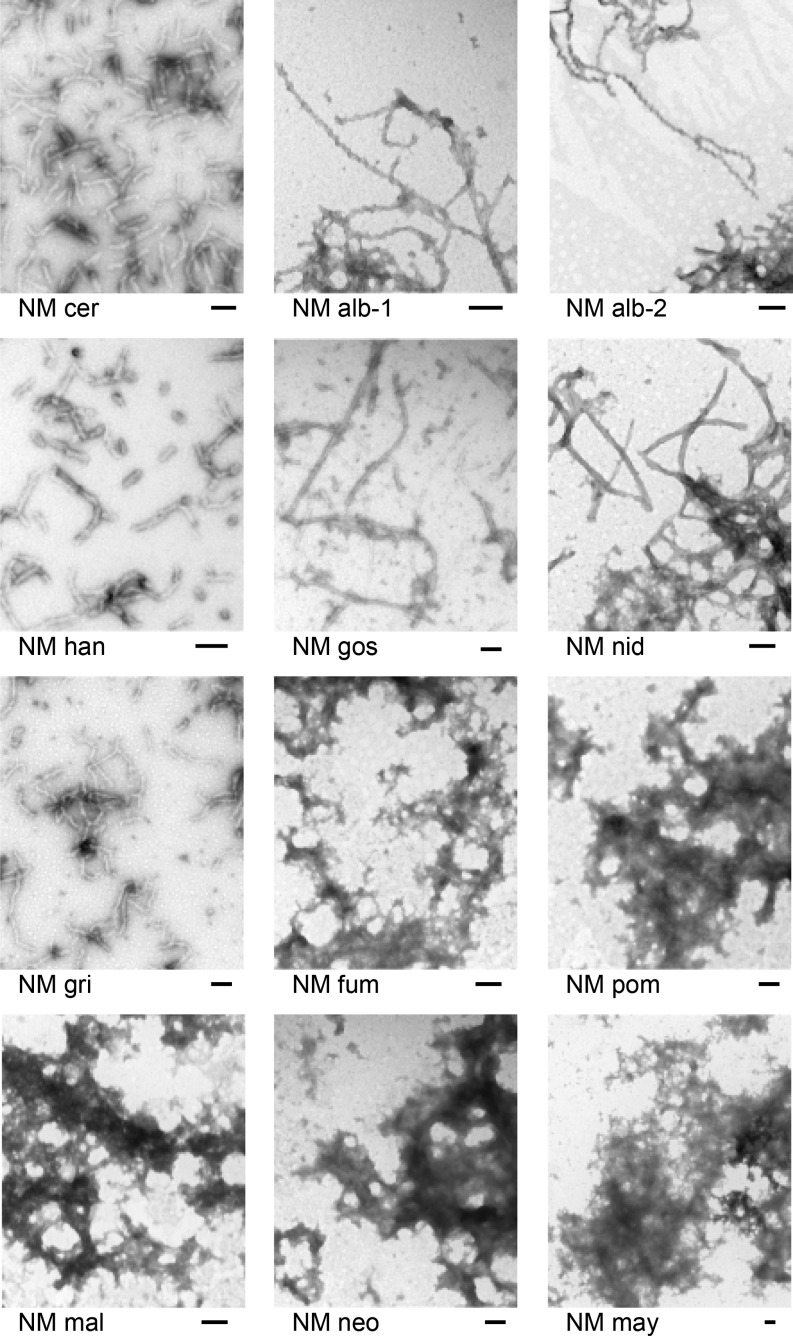
It is likely that a wide array of proteins, perhaps any protein, can form amyloid under some conditions (reviewed in Vendruscolo et al. 2011). Furthermore, many amyloidoses are not infectious. Nevertheless, we determined whether the Sup35NMs studied here could form fibrils in neutral buffer with rotation, conditions under which the S. cerevisiae Sup35NM readily forms infectious amyloid. We purified His-tagged Sup35NMs from each species (except K. lactis, which was unsuccessful). We found that Sup35NMs of S. cerevisiae, D. hansenii, and C. albicans (Sup35alb1 and Sup35alb2) could form filaments in vitro and prions in vivo (Figure 3 and Table 1). In contrast, Sup35NMs of A. fumigatus, U. maydis, S. pombe, and C. neoformans could form neither amyloids nor prions (Figure 4 and Table 1). Although C. maltosa did rarely form prions, it did not form amyloid under our conditions. The A. gossipyii, A. nidulans, and M. grisea Sup35NMs did form fibrils in vitro, but did not form prions in vivo.
Figure 4.
Amyloid formation by Sup35NM domains. Preparations of the indicated Sup35NM’s were allowed to form filaments and examined by electron microscopy as described in Materials and Methods.
Discussion
It is possible that by using S. cerevisiae to examine the prion-forming ability of a foreign protein, one has detected prions made by a protein that cannot do so in its native environment. Similarly, a protein unable to form a prion in S. cerevisiae may do so in cells of its own species. However, the Hsp104–Hsp70–NEF chaperone disaggregation apparatus from S. pombe, or even Escherichea coli, can propagate yeast prions in S. cerevisiae, suggesting that prions are using for their propagation a facility provided by a wide range of organisms (Reidy and Masison 2012; Reidy et al. 2013). The prion domains of Ure2p and Sup35p form prions in vivo more readily than do the corresponding full-length proteins (Masison and Wickner 1995; Kochneva-Pervukhova et al. 1998). Thus, using fragments of proteins fused to S. cerevisiae Sup35MC or Sup35C raises the possibility that prions are observed that would not occur were the full-length (nonhybrid) protein examined. Up to the present, the use of S. cerevisiae as a test bed for prion formation has not led to inconsistencies, but chaperones, the Btn2/Cur1 anti-[URE3] systems and other factors may well be crucial to prion-forming ability and must vary in their properties between species. Only a few cases have been tested in their native environment, largely because of the effort needed to develop the genetic tools in each species.
The induction of prion formation by overproduction of the prion protein (Wickner 1994), particularly by the prion domain (Masison and Wickner 1995; Kochneva-Pervukhova et al. 1998), is a reliable diagnostic criteria for a prion. All yeast amyloid-based prions are cured by guanidine (Tuite et al. 1981), but guanidine also induces mitochondrial mutations (Villa and Juliani 1980), so this agent must be used with caution. A prion must be infectious, by definition, so demonstrating horizontal transmission, most easily by cytoduction, is essential.
Previous studies of prion formation by the Sup35N of C. albicans did not include studies of infectivity (Santoso et al. 2000; Chien and Weissman 2001; Resende et al. 2002). We find that on overexpression of the C. albicans fusion protein, there was a dramatic increase in Ade+ colonies, and a portion of these were indeed infectious. The Sup35 of K. lactis formed aggregates in K. lactis that made cells carrying ade1–14 become Ade+ (Nakayashiki et al. 2001). We found that S. cerevisiae expressing Sup35NMlac+C showed only weak specific induction of Ade+ colonies, and only a small fraction of these showed infectivity. D. hansenii Sup35NM+C gave robust, specific induction of Ade+ colonies, with transmission of the Ade+ phenotype by cytoplasmic mixing, indicating [PSI+han] formation. Sup35NMmal+C gave substantial specific induction of Ade+ clones in the plasmid system, but not for the integrated gene. Only a small fraction of the Ade+ clones transmitted their phenotype to plasmid-based or integrated recipients. Except for very inefficient prion formation of Sup35NMalb1,2+C when Sup35NMcer was overexpressed, no cross seeding was observed.
Prion formation did not occur with the Sup35NMspecies+C fusion proteins from A. gossypii or S. pombe, using either the plasmid-based system or the integrated hybrid gene. There was no specific induction of Ade+ clones, and the few clones that did arise were not transmissible, indicating that they were not due to prions. We saw no evidence of prion formation with the fusion proteins from A. nidulans, A. fumigatus, M. grisea, C. neoformans, or U. maydis, but the incomplete complementation by the Sup35NMx+C’s in this group may have obscured a very rare occurrence of [PSI+]. As has been found for [URE3] formation by Ure2p from various organisms, ability to form [PSI+] is sporadically distributed among species, not necessarily conserved. Even some Q/N-rich N domains do not form [PSI+].
Although the sequence of the NM domains are not conserved, there is a remarkable conservation of amino acid composition (Table S5). In all proteins, the N domain can be identified due to overrepresentation of N + Q and the aromatic amino acids (F, Y, and W). The M domain can be identified from the overrepresentation of the charged residues (D, E, K, R), the aliphatic residues (I, L, V), and S + T. The notable exception is the S. pombe sequence, as charged and aliphatic residues are distributed throughout. However, aromatic residues are still clustered in the N-terminal domain. When the S. pombe sequence is analyzed with those of other Schizosaccharomyces species (Kuramae et al. 2006), a border can be discerned separating typical N and M domains (Table S6). Apparently, this amino acid compositional distribution is important for the normal functioning of these domains.
The sequence of the prion domains of Sup35p and Ure2p are not conserved and in fact show far more rapid variability in evolution than does the remainder of the molecules (Jensen et al. 2001; Edskes and Wickner 2002; Baudin-Baillieu et al. 2003; Bateman and Wickner 2012). The sequence changes produce barriers to transmission of [PSI+] within S. cerevisiae (Bateman and Wickner 2012), as well as between species for both prions (Chen et al. 2007; Edskes et al. 2009). Here we show that deletions in the C. albicans Sup35M domain produce an intraspecies transmission barrier. These domains have nonprion functions (Funakoshi et al. 2007; Shewmaker et al. 2007), but one can explain the rapid sequence changes as selected to produce these transmission barriers, much as sequence changes in PrP have been selected to produce resistance to transmission of human prion diseases (Mead et al. 2009). A majority of [PSI+] isolates in one study are severely toxic or lethal (McGlinchey et al. 2011), providing sufficient reason why prion resistance might be selected.
Supplementary Material
Acknowledgments
This work was supported by the Intramural Program of the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health.
Footnotes
Supporting information is available online at http://www.genetics.org/lookup/suppl/doi:10.1534/genetics.114.166538/-/DC1.
Communicating editor: E. U. Selker
Literature Cited
- Afanasieva E. G., Kushnirov V. V., Tuite M. F., Ter-Avanesyan M. D., 2011. Molecular basis for transmission barrier and interference between closely related prion proteins in yeast. J. Biol. Chem. 286: 15773–15780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagriantsev S., Liebman S. W., 2006. Modulation of Abeta42 low-n oligomerization using a novel yeast reporter system. BMC Biol. 4: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balguerie A., Dos Reis S., Ritter C., Chaignepain S., Coulary-Salin B., et al. , 2003. Domain organization and structure-function relationship of the HET-s prion protein of Podospora anserina. EMBO J. 22: 2071–2081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateman D. A., Wickner R. B., 2012. [PSI+] prion transmission barriers protect Saccharomyces cerevisiae from infection: intraspecies ’species barriers.’ Genetics 190: 569–579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudin-Baillieu A., Fernandez-Bellot E., Reine F., Coissac E., Cullin C., 2003. Conservation of the prion properties of Ure2p through evolution. Mol. Biol. Cell 14: 3449–3458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxa U., Wickner R. B., Steven A. C., Anderson D., Marekov L., et al. , 2007. Characterization of β-sheet structure in Ure2p1–89 yeast prion fibrils by solid state nuclear magnetic resonance. Biochemistry 46: 13149–13162 [DOI] [PubMed] [Google Scholar]
- Brachmann A., Baxa U., Wickner R. B., 2005. Prion generation in vitro: amyloid of Ure2p is infectious. EMBO J. 24: 3082–3092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley M. E., Liebman S. W., 2004. The Sup35 domains required for maintenance of weak, strong or undifferentiated yeast [PSI+] prions. Mol. Microbiol. 51: 1649–1659 [DOI] [PubMed] [Google Scholar]
- Bradley M. E., Edskes H. K., Hong J. Y., Wickner R. B., Liebman S. W., 2002. Interactions among prions and prion “strains” in yeast. Proc. Natl. Acad. Sci. USA 99(Suppl. 4): 16392–16399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang H.-Y., Lin J.-Y., Lee H.-C., Wang H.-L., King C.-Y., 2008. Strain-specific sequences required for yeast prion [PSI+] propagation. Proc. Natl. Acad. Sci. USA 105: 13345–13350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B., Newnam G. P., Chernoff Y. O., 2007. Prion species barrier between the closely related yeast proteins is detected despite coaggregation. Proc. Natl. Acad. Sci. USA 104: 2791–2796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernoff Y. O., Derkach I. L., Inge-Vechtomov S. G., 1993. Multicopy SUP35 gene induces de-novo appearance of psi-like factors in the yeast Saccharomyces cerevisiae. Curr. Genet. 24: 268–270 [DOI] [PubMed] [Google Scholar]
- Chernoff Y. O., Galkin A. P., Lewitin E., Chernova T. A., Newnam G. P., et al. , 2000. Evolutionary conservation of prion-forming abilities of the yeast Sup35 protein. Mol. Microbiol. 35: 865–876 [DOI] [PubMed] [Google Scholar]
- Chien P., Weissman J. S., 2001. Conformational diversity in a yeast prion dictates its seeding specificity. Nature 410: 223–227 [DOI] [PubMed] [Google Scholar]
- Cox B. S., 1965. PSI, a cytoplasmic suppressor of super-suppressor in yeast. Heredity 20: 505–521 [Google Scholar]
- Derkatch I. L., Bradley M. E., Zhou P., Chernoff Y. O., Liebman S. W., 1997. Genetic and environmental factors affecting the de novo appearance of the [PSI+] prion in Saccharomyces cerevisiae. Genetics 147: 507–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derkatch I. L., Bradley M. E., Hong J. Y., Liebman S. W., 2001. Prions affect the appearance of other prions: the story of [PIN] Cell 106: 171–182 [DOI] [PubMed] [Google Scholar]
- Eaglestone S. S., Cox B. S., Tuite M. F., 1999. Translation termination efficiency can be regulated in Saccharomyces cerevisiae by environmental stress through a prion-mediated mechanism. EMBO J. 18: 1974–1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edskes H. K., Wickner R. B., 2000. A protein required for prion generation: [URE3] induction requires the Ras-regulated Mks1 protein. Proc. Natl. Acad. Sci. USA 97: 6625–6629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edskes H. K., Wickner R. B., 2002. Conservation of a portion of the S. cerevisiae Ure2p prion domain that interacts with the full-length protein. Proc. Natl. Acad. Sci. USA 99(Suppl. 4): 16384–16391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edskes H. K., Wickner R. B., 2013. The [URE3] prion in Candida. Eukaryot. Cell 12: 551–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edskes H. K., Gray V. T., Wickner R. B., 1999. The [URE3] prion is an aggregated form of Ure2p that can be cured by overexpression of Ure2p fragments. Proc. Natl. Acad. Sci. USA 96: 1498–1503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edskes H. K., Mccann L. M., Hebert A. M., Wickner R. B., 2009. Prion variants and species barriers among Saccharomyces Ure2 proteins. Genetics 181: 1159–1167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edskes H. K., Engel A., McCann L. M., Brachmann A., Tsai H.-F., et al. , 2011. Prion-forming ability of Ure2 of yeasts is not evolutionarily conserved. Genetics 188: 81–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel A., Shewmaker F., Edskes H. K., Dyda F., Wickner R. B., 2011. Amyloid of the Candida albicans Ure2p prion domain is infectious and has a parallel in-register β-sheet structure. Biochemistry 50: 5971–5978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira P. C., Ness F., Edwards S. R., Cox B. S., Tuite M. F., 2001. The elimination of the yeast [PSI+] prion by guanidine hydrochloride is the result of Hsp104 inactivation. Mol. Microbiol. 40: 1357–1369 [DOI] [PubMed] [Google Scholar]
- Frolova L., Legoff X., Rasmussen H. H., Cheperegin S., Drugeon G., et al. , 1994. A highly conserved eukaryotic protein family possessing properties of polypeptide chain release factor. Nature 372: 701–703 [DOI] [PubMed] [Google Scholar]
- Funakoshi Y., Doi Y., Hosoda N., Uchida N., Osawa M., et al. , 2007. Mechanism of mRNA deadenylation: evidence for a molecular interplay between translation termination factor eRF3 and mRNA deadenylases. Genes Dev. 21: 3135–3148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geitz R. D., Schiestl R. H., 2007. High-efficiency yeast transformation using the LiAc/SS carrier DNA/PEG method. Nat. Protoc. 2: 31–34 [DOI] [PubMed] [Google Scholar]
- Glover J. R., Kowal A. S., Shirmer E. C., Patino M. M., Liu J.-J., et al. , 1997. Self-seeded fibers formed by Sup35, the protein determinant of [PSI+], a heritable prion-like factor of S. cerevisiae. Cell 89: 811–819 [DOI] [PubMed] [Google Scholar]
- Halfmann R., Jarosz D. F., Jones S. K., Chang A., Lancster A. K., et al. , 2012. Prions are a common mechanism for phenotypic inheritance in wild yeasts. Nature 482: 363–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison L. B., Yu Z., Stajich J. E., Dietrich F. S., Harrison P. M., 2007. Evolution of budding yeast prion-determinant sequences across diverse fungi. J. Mol. Biol. 368: 273–282 [DOI] [PubMed] [Google Scholar]
- Hoshino S., Imai M., Kobayashi T., Uchida N., Katada T., 1999. The eukaryotic polypeptide chain releasing factor (eRF3/GSPT) carrying the translation termination signal to the 3′-poly(A) tail of mRNA. J. Biol. Chem. 274: 16677–16680 [DOI] [PubMed] [Google Scholar]
- Jensen M. A., True H. L., Chernoff Y. O., Lindquist S., 2001. Molecular population genetics and evolution of a prion-like protein in Saccharomyces cerevisiae. Genetics 159: 527–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung G., Masison D. C., 2001. Guanidine hydrochloride inhibits Hsp104 activity in vivo: a possible explanation for its effect in curing yeast prions. Curr. Microbiol. 43: 7–10 [DOI] [PubMed] [Google Scholar]
- Jung G., Jones G., Masison D. C., 2002. Amino acid residue 184 of yeast Hsp104 chaperone is critical for prion-curing by guanidine, prion propagation, and thermotolerance. Proc. Natl. Acad. Sci. USA 99: 9936–9941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly A. C., Shewmaker F. P., Kryndushkin D., Wickner R. B., 2012. Sex, prions and plasmids in yeast. Proc. Natl. Acad. Sci. USA 109: E2683–E2690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King C. Y., Diaz-Avalos R., 2004. Protein-only transmission of three yeast prion strains. Nature 428: 319–323 [DOI] [PubMed] [Google Scholar]
- King C.-Y., Tittmann P., Gross H., Gebert R., Aebi M., et al. , 1997. Prion-inducing domain 2–114 of yeast Sup35 protein transforms in vitro into amyloid-like filaments. Proc. Natl. Acad. Sci. USA 94: 6618–6622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochneva-Pervukhova N. V., Poznyakovski A. I., Smirnov V. N., Ter-Avanesyan M. D., 1998. C-terminal truncation of the Sup35 protein increases the frequency of de novo generation of a prion-based [PSI+] determinant in Saccharmyces cerevisiae. Curr. Genet. 34: 146–151 [DOI] [PubMed] [Google Scholar]
- Kryndushkin D. S., Engel A., Edskes H. K., Wickner R. B., 2011. Molecular chaperone Hsp104 can promote yeast prion generation. Genetics 188: 339–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuramae E. E., Robert V., Snel B., Boekout T., 2006. Conflicting phylogenetic position of Schizosaccharomyces pombe. Genomics 88: 387–393 [DOI] [PubMed] [Google Scholar]
- Kushnirov V. V., Kochneva-Pervukhova N. V., Cechenova M. B., Frolova N. S., Ter-Avanesyan M. D., 2000. Prion properties of the Sup35 protein of yeast Pichia methanolica. EMBO J. 19: 324–331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebman S. W., Chernoff Y. O., 2012. Prions in yeast. Genetics 191: 1041–1072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebman S. W., Stewart J. W., Sherman F., 1975. Serine substitutions caused by an ochre suppressor in yeast. J. Mol. Biol. 94: 595–610 [DOI] [PubMed] [Google Scholar]
- Liu J.-J., Sondheimer N., Lindquist S., 2002. Changes in the middle region of Sup35p profoundly alter the nature of epigenetic inheritance for the yeast prion. [PSI+] Proc. Natl. Acad. Sci. USA 99: 16446–16453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcet-Houben M., Gabaldon T., 2009. The tree vs. the forest: the fungal tree of life and the topological diversity within the yeast phylome. PLoS ONE 4: e4357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masel J., Griswold C. K., 2009. The strength of selection against the yeast prion. [PSI+] Genetics 181: 1057–1063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masison D. C., Wickner R. B., 1995. Prion-inducing domain of yeast Ure2p and protease resistance of Ure2p in prion-containing cells. Science 270: 93–95 [DOI] [PubMed] [Google Scholar]
- McGlinchey R., Kryndushkin D., Wickner R. B., 2011. Suicidal [PSI+] is a lethal yeast prion. Proc. Natl. Acad. Sci. USA 108: 5337–5341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mead S., Stumpf M. P., Whitfield J., Beck J. A., Poulter M., et al. , 2003. Balancing selection at the prion protein gene consistent with prehistoric kurulike epidemics. Science 300: 640–643 [DOI] [PubMed] [Google Scholar]
- Mead S., Whitfield J., Poulter M., Shah P., Uphill J., et al. , 2009. A novel protective prion protein variant that colocalizes with kuru exposure. N. Engl. J. Med. 361: 2056–2065 [DOI] [PubMed] [Google Scholar]
- Nakayashiki T., Ebihara K., Bannai H., Nakamura Y., 2001. Yeast [PSI+] “prions” that are crosstransmissible and susceptible beyond a species barrier through a quasi-prion state. Mol. Cell 7: 1121–1130 [DOI] [PubMed] [Google Scholar]
- Nakayashiki T., Kurtzman C. P., Edskes H. K., Wickner R. B., 2005. Yeast prions [URE3] and [PSI+] are diseases. Proc. Natl. Acad. Sci. USA 102: 10575–10580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namy O., Galopier A., Martini C., Matsufuji S., Fabret C., et al. , 2008. Epigenetic control of polyamines by the prion. [PSI+] Nat. Cell Biol. 10: 1069–1075 [DOI] [PubMed] [Google Scholar]
- Paushkin S. V., Kushnirov V. V., Smirnov V. N., Ter-Avanesyan M. D., 1997. In vitro propagation of the prion-like state of yeast Sup35 protein. Science 277: 381–383 [DOI] [PubMed] [Google Scholar]
- Reidy M., Masison D. C., 2012. Prokaryotic chaperones support yeast prions and thermotolerance and define disaggregation machinery interactions. Genetics 192: 185–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reidy M., Sharma R., Masison D. C., 2013. Schizosaccharomyces pombe disaggregation machinery chaperones support Saccharomyces cerevisiae growth and prion propagation. Eukaryot. Cell 12: 739–745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resende C., Parham S. N., Tinsley C., Ferreira P. C., Duarte J. A. B., et al. , 2002. The Candida albicans Sup35p protein (CaSup35p): function, prion-like behavior and an associated polyglutamine length polymorphism. Microbiology 148: 1049–1060 [DOI] [PubMed] [Google Scholar]
- Resende C. G., Outeiro T. F., Sands L., Lindquist S., Tuite M. F., 2003. Prion protein gene polymorphisms in Saccharomyces cerevisiae. Mol. Microbiol. 49: 1005–1017 [DOI] [PubMed] [Google Scholar]
- Safadi R. A., Talarek N., Jacques N., Aigle M., 2011. Yeast prions: could they be exaptations?: the URE2/[URE3] system in Kluyveromyces lactis. FEMS Yeast Res. 11: 151–153 [DOI] [PubMed] [Google Scholar]
- Santoso A., Chien P., Osherovich L. Z., Weissman J. S., 2000. Molecular basis of a yeast prion species barrier. Cell 100: 277–288 [DOI] [PubMed] [Google Scholar]
- Sherman F., 1991. Getting started with yeast, pp. 3–21 in Guide to Yeast Genetics and Molecular Biology, edited by Guthrie C., Fink G. R. Academic Press, San Diego [Google Scholar]
- Shewmaker F., Wickner R. B., Tycko R., 2006. Amyloid of the prion domain of Sup35p has an in-register parallel β-sheet structure. Proc. Natl. Acad. Sci. USA 103: 19754–19759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shewmaker F., Mull L., Nakayashiki T., Masison D. C., Wickner R. B., 2007. Ure2p function is enhanced by its prion domain in Saccharomyces cerevisiae. Genetics 176: 1557–1565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shewmaker F., Kryndushkin D., Chen B., Tycko R., Wickner R. B., 2009. Two prion variants of Sup35p have in-register β-sheet structures, independent of hydration. Biochemistry 48: 5074–5082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sondheimer N., Lindquist S., 2000. Rnq1: an epigenetic modifier of protein function in yeast. Mol. Cell 5: 163–172 [DOI] [PubMed] [Google Scholar]
- Stansfield I., Jones K. M., Kushnirov V. V., Dagkesamanskaya A. R., Poznyakovski A. I., et al. , 1995. The products of the SUP45 (eRF1) and SUP35 genes interact to mediate translation termination in Saccharomyces cerevisiae. EMBO J. 14: 4365–4373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki G., Shimazu N., Tanaka M., 2012. A yeast prion, Mod5, promotes acquired drug resistance and cell survival under environmental stress. Science 336: 355–359 [DOI] [PubMed] [Google Scholar]
- Tanaka M., Chien P., Naber N., Cooke R., Weissman J. S., 2004. Conformational variations in an infectious protein determine prion strain differences. Nature 428: 323–328 [DOI] [PubMed] [Google Scholar]
- Taneja V., Maddelein M. L., Talarek N., Saupe S. J., Liebman S. W., 2007. A non-Q/N-rich prion domain of a foreign prion, [Het-s], can propagate as a prion in yeast. Mol. Cell 27: 67–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teravanesyan M. D., Kushnirov V. V., Dagkesamanskaya A. R., Didichenko S. A., Chernoff Y. O., et al. , 1993. Deletion analysis of the SUP35 gene of the yeast Saccharomyces cerevisiae reveals two non-overlapping functional regions in the encoded protein. Mol. Microbiol. 7: 683–692 [DOI] [PubMed] [Google Scholar]
- Teravanesyan A., Dagkesamanskaya A. R., Kushnirov V. V., Smirnov V. N., 1994. The SUP35 omnipotent suppressor gene is involved in the maintenance of the non-Mendelian determinant [psi+] in the yeast Saccharomyces cerevisiae. Genetics 137: 671–676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyama B. H., Kelly M. J., Gross J. D., Weissman J. S., 2007. The structural basis of yeast prion strain variants. Nature 449: 233–237 [DOI] [PubMed] [Google Scholar]
- True H. L., Lindquist S. L., 2000. A yeast prion provides a mechanism for genetic variation and phenotypic diversity. Nature 407: 477–483 [DOI] [PubMed] [Google Scholar]
- True H. L., Berlin I., Lindquist S. L., 2004. Epigenetic regulation of translation reveals hidden genetic variation to produce complex traits. Nature 431: 184–187 [DOI] [PubMed] [Google Scholar]
- Tuite M. F., Mundy C. R., Cox B. S., 1981. Agents that cause a high frequency of genetic change from [psi+] to [psi-] in Saccharomyces cerevisiae. Genetics 98: 691–711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vendruscolo M., Knowles T. P., Dobson C. M., 2011. Protein solubility and protein homeostasis: a generic view of protein misfolding disorders. Cold Spring Harb. Perspect. Biol. 3: a010454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villa L. L., Juliani M. H., 1980. Mechanism of rho- induction in Saccharomyces cerevisiae by guanidine hydrochloride. Mutat. Res. 7: 147–153 [DOI] [PubMed] [Google Scholar]
- Wickner R. B., 1994. [URE3] as an altered URE2 protein: evidence for a prion analog in S. cerevisiae. Science 264: 566–569 [DOI] [PubMed] [Google Scholar]
- Wickner R. B., Dyda F., Tycko R., 2008. Amyloid of Rnq1p, the basis of the [PIN+] prion, has a parallel in-register β-sheet structure. Proc. Natl. Acad. Sci. USA 105: 2403–2408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner R. B., Edskes H. K., Bateman D. A., Kelly A. C., Gorkovskiy A., et al. , 2013. Amyloids and yeast prion biology. Biochemistry 52: 1514–1527 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.