Abstract
Dendritic cells (DCs) undergo maturation during virus infection and thereby become potent stimulators of cell-mediated immunity. HIV-1 replicates in immature DCs, but we now find that infection is not accompanied by many components of maturation in either infected cells or uninfected bystanders. The infected cultures do not develop potent stimulating activity for the mixed leukocyte reaction (MLR), and the DCs producing HIV-1 gag p24 do not express CD83 and DC–lysosome-associated membrane protein maturation markers. If different maturation stimuli are applied to DCs infected with HIV-1, the infected cells selectively fail to mature. When DCs from HIV-1-infected patients are infected and cultured with autologous T cells, IL-10 was produced in 6 of 10 patients. These DC–T cell cocultures could suppress another immune response, the MLR. The regulation was partially IL-10-dependent and correlated in extent with the level of IL-10 produced. Suppressor cells only developed from infected patients, rather than healthy controls, and the DCs had to be exposed to live virus rather than HIV-1 gag peptides or protein. These results indicate that HIV-1-infected DCs have two previously unrecognized means to evade immune responses: maturation can be blocked reducing the efficacy of antigen presentation from infected cells, and T cell-dependent suppression can be induced.
Dendritic cells (DCs) mediate several protective responses to viral infection. DCs provide innate resistance, e.g., by producing high levels of interferons, and DCs set the stage for adaptive immunity by efficiently processing and presenting antigens acquired through direct infection or capture of inactive virions and dying virus infected cells (reviewed in ref. 1). Importantly, as a result of infection, DCs can undergo an extensive differentiation process called maturation, which is initiated within the cell (2, 3) or after ligation of toll-like receptors, e.g., TLR3 (4–6) and TLR9 (7–9), and IFN receptors (5) at the cell surface or from within the endocytic system. This maturation is critical, not only for DCs to become stimulators of cell mediated immunity but also to avoid the induction of tolerance, a function of different types of immature DCs (iDCs) in the steady state (reviewed in refs. 10 and 11). Several changes occur during DC maturation, many of which can be monitored at the single cell level by fluorescent-activated cell sorter (FACS) analyses, e.g., the up-regulation of antigen presenting MHC class II and CD86 costimulatory molecules, and the de novo expression of cell surface CD83 and the lysosomal antigen DC–LAMP (lysosome-associated membrane protein). Importantly, mature DCs become potent stimulators for T cell-mediated immunity, which is often assessed by stimulation of the mixed leukocyte reaction (MLR) as a model. In trying to interface HIV-1 infection of DCs with these general principles of DC function during virus infection, we began with prior evidence that iDCs derived from blood and from skin preferentially replicate HIV-1 (12–16). Mature DCs, in contrast, when challenged with HIV-1, do not replicate virus, in part because of a low level of surface CCR5 expression (17–19) and also the result of reverse transcription and postintegration blocks (20). We therefore have studied the effects of HIV-1 infection on the maturation of monocyte-derived iDCs. We will show that HIV-1 infection does not induce many features of DC maturation and even retards it, and that infected DCs interact with T cells from HIV-1-infected patients to stimulate IL-10 production and immune suppression.
Materials and Methods
Patient Samples. Study subjects were normal healthy control volunteers or HIV-1-infected patients. Eight HIV-1 patients (Table 2, which is published as supporting information on the PNAS web site) were receiving antiretroviral drugs and had been clinically, immunologically, and virologically stable for at least the year before study. Blood was obtained in Institutional Review Board approved protocols.
DCs. CD14+ monocytes were positively selected from blood mononuclear cells by using CD14 magnetic beads (Miltenyi Biotec, Auburn, CA). A total of 2 × 106 cells were plated in six-well dishes (Falcon) in 3 ml of RPMI medium 1640 with 5% human serum AB (Gemini Biological Products, Calabasas, CA), recombinant granulocyte–macrophage colony-stimulating factor (100 units/ml, Leukine, Immunex), and rIL-4 (20 ng/ml, R & D Systems). Cells were fed on days 2 and 4 with the same concentration of cytokines. At day 6, most nonadherent cells were immature HLA-DR+, CD3-, CD14-, and CD83- DCs. Mature DCs were induced by adding cytokines (IL-1β, tumor necrosis factor α, and IL-6 at 10 ng/ml;R&D Systems) together with PGE2 (1 μg/ml; Sigma) or CD40L-expressing cells provided by J. Banchereau (Baylor Institute, Dallas).
Cell Lines. MAGI, a HeLa cell clone expressing CD4, CCR5, and HIV-LTR-β gal was obtained from National Institutes of Health A IDS Research Reagent Program and maintained in DMEM/5% FCS with selection medium (0.2 mg/ml G418/0.1 mg/ml hygromycin B/1 μg/ml puromycin).
Viruses and Infection of DCs. The Ba-L HIV-1 isolate was grown in mitogen-stimulated PBMC and titered in MAGI cells to determine infectious units (IU). In general, 105 iDCs were pulsed with 300 pg of HIV Ba-L or 3,000 IU for 2 h at 37°C. 3′-Azido-3′-deoxythymidine AZT, 1 μM, National Institutes of Health AIDS Reagent Program) was added 1 h before infection and maintained throughout infection. Alternatively, DCs were infected with 250 hemagglutinin units per ml of live influenza A virus strain Aichi/68 (SPAFAS) in RPMI medium 1640. After 1 h, 5% human serum was added to block infection, and the cells cultured an additional 2–3 days.
Detection of HIV-1 Reverse Transcripts and p24-Expressing Cells. Infected cultures were lysed and PCR amplified to detect early (R/U5) and late (LTR/gag) reverse transcripts (21). Globin sequences were amplified in parallel to control for DNA input. For FACS assays, infected DCs were fixed with 4% paraformaldehyde in PBS for 30 min on ice. Cells were permeabilized for 15 min in 0.1% saponin and double stained with FITC anti-HIV gag p24 (clone KC 57, Coulter) or anti-influenza HA (C102, Abcam, Cambridge, MA), and phycoerythrin (PE)-labeled antibodies to CD3 (clone SK7, BD), CD14 (clone MφP9, BD), HLA-DR (clone 243, BD), DC-SIGN/CD209 (clone 120507, R & D Systems), CD86 (clone 2331, Pharmingen), or DC–LAMP/CD207 (clone 104.64, Immunotech, Luminy, France). Samples were analyzed on a FACSort (Becton Dickinson) with cellquest software.
MLR. Immature or mature DCs were pretreated or not with AZT (1 μM) and infected with HIV-1. After 5 days, DCs were cultured with 5,6-carboxyfluorescein diacetate-succinimyl ester (CFSE)-labeled allogeneic T cells (ratio 1:10) from another blood donor, and the MLR was assessed by CFSE dilution.
DC–T Cell Cocultures. Infected and uninfected, immature and mature DCs were cultured with autologous T cells (2 × 105) from healthy adults or HIV-1-infected donors in round-bottom microtest wells at ratio of 1:30 for 7 days. Alternatively, iDCs were treated with a panel of 122 peptides spanning the entire length of p55 gag (National Institutes of Health AIDS Reagent Program) at 2 μM final concentration, or recombinant gag protein at 5 μg/ml (Protein Science, Meriden, CT).
Cytokine Assays. IFN-γ and IL-10 secreting cells were quantified by a 36-h enzyme-linked immunospot (ELISPOT) assay initiated at day 0, upon mixing DCs and T cells, or after 7 days of coculture. ELISA assays (R & D Systems) also were used to quantify cytokine released into culture supernatants.
Transwell Cultures to Detect Immune Regulation. Mixtures of DCs and T cells, obtained from HIV-1-infected donors and cultured for 7 days, were tested as suppressors of MLR proliferation. The MLR was induced by adding mature DCs to CFSE-labeled, negatively selected, allogeneic CD4+ T cells (using the Untouched CD4 T cell selection, Miltenyi Biotec) at ratio 1:10. A total of 105 MLR cells were placed in the bottom of a 96-well plate, whereas 5 × 104 cells obtained from the 7-day DC/HIV plus T cell coculture were placed in the top of the transwell chamber (0.2 μm, Nunc Roskilde). In some experiments, inhibitory anti-IL-10 and control nonreactive antibodies were added.
Results
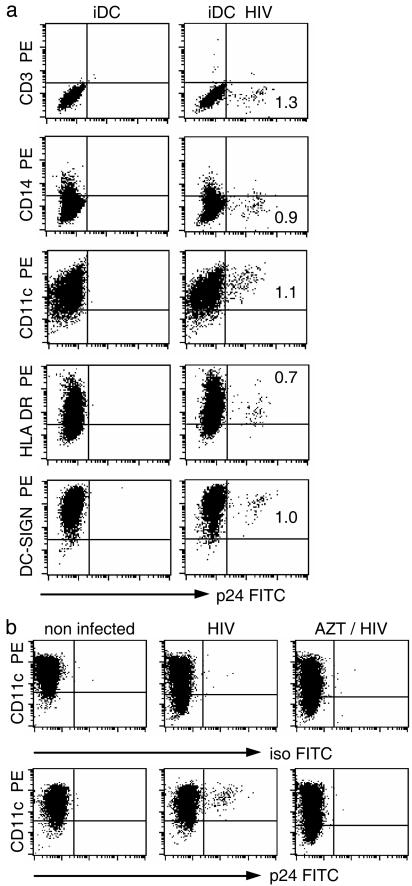
HIV-1 Infects a Fraction of iDCs. iDCs were generated from CD14+ blood monocytes by culture with rIL-4 and recombinant granulocyte-macrophage colony-stimulating factor for 6 days. HIV-1 was then added for 4–6 days. Productively infected cells were identified by gag p24 staining in the FACS. To confirm that the small p24+ subset was DCs, we double stained for p24 and lineage markers. The p24+ cells were negative for CD3 (T cells), CD14 (monocytes), and CD19 (B cells), but positive for DC markers like HLA DR, CD11c, and DC-SIGN (Fig. 1a). Interestingly, p24+ DCs were MHC class II-low. Because cell surface MHC II increases during maturation, this indicates preferential infection of the least mature DCs. To prove that p24 staining was not caused by added virions, we showed that AZT, a reverse transcriptase inhibitor, blocked formation of p24+ DCs (Fig. 1b). Thus HIV-1 productively infects a small fraction of immature monocyte-derived DCs.
Fig. 1.
p24 expression by HIV-1-infected iDCs. (a) DCs were exposed to the BaL isolate of HIV-1 for 2 h at 37°C. The FACS was used to monitor HIV infection (FITC anti-gag p24) and surface antigens (y axis) 5 days later. (b) DCs were infected without or with 1 μM AZT and stained for CD11c and p24. One of six similar experiments is shown.
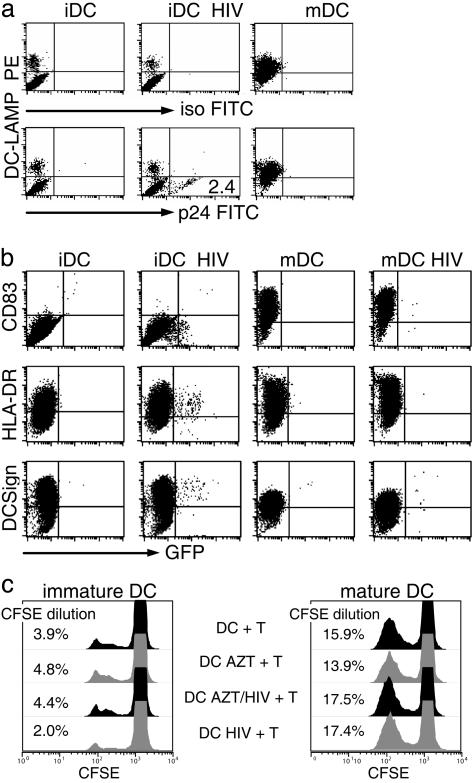
HIV-1 Infection Does Not Mature DCs. To determine whether DCs mature as a consequence of viral infection, we first emphasized the DC–LAMP intracellular (lysosomal) marker for maturation (22), identified in the same cells permeabilized to detect HIV-1 gag p24. If DCs were infected at the immature stage, the p24+ cells remained DC–LAMP negative (Fig. 2a). To monitor surface markers of maturation in infected DCs, we used GFP expressing HIV-1, engineered from the YU2 virus. Again, maturation did not develop in the infected DCs or bystander cells, whereas DCs matured with either cytokines or CD40 ligation (data not shown) up-regulated surface CD83 and HLA–DR, and down regulated DC–SIGN/CD209 (Fig. 2b). HIV-1 did not replicate if added to DCs matured with cytokines (Fig. 2b), CD40 ligation, or lipopolysaccharide (LPS) (data not shown), consistent with prior reports (13, 23). In contrast to HIV-1, influenza-virus-infected DCs did mature (Fig. 6, which is published as supporting information on the PNAS web site) as reported (24). As a functional assay for maturation, we added the DCs to allogeneic T cells. iDCs, infected or not with HIV-1, were equally weak MLR stimulators when compared to mature DCs (Fig. 2c). These data indicate that HIV-1, in contrast to many viruses, does not induce maturation of cultured monocytederived DCs.
Fig. 2.
HIV-1 infection does not mature DCs. (a) iDC and DCs matured with a cytokine mixture (mDC) were infected with BaL and stained for the DC–LAMP maturation marker and p24 (intracellular staining). Similar findings were made in eight experiments, including other maturation stimuli like LPS and CD40 ligation (Table 2). (b) The iDCs and mDCs were infected with Yu2-HIV-1/GFP and analyzed for maturation markers (y axis) by FACS after 5 days (surface staining). (c) Allo MLR stimulation was monitored by CFSE dilution by using CFSE-labeled T cells and the indicated DCs. The experiment shown is representative of three or more experiments.
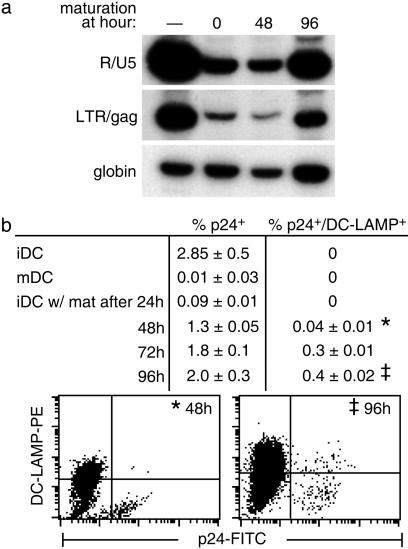
Maturation Stimuli Do Not Mature HIV-1-Infected DCs. We next studied the effect of maturation stimuli on viral replication as a function of time. iDCs were infected, and maturation cytokines were added immediately or at 24-h intervals for up to 4 days. If maturation stimuli were added at 0–48 h after infection, viral replication was absent or very low, whereas at later time points (72–96 h), significant numbers of full-length DNA transcripts (Fig. 3a, right lane) and p24+ cells (Fig. 3b) were noted relative to the iDCs. Importantly, in eight experiments of this type, most p24+ DCs selectively remained DC–LAMP low (see Fig. 3b) in the presence of different maturation stimuli, i.e., cytokines, LPS, and CD40 ligation (Table 2). Therefore, infected DCs shut down virus production if maturation stimuli are added shortly after infection, or maturation of infected cells is selectively blocked when the maturation stimulus is encountered later on.
Fig. 3.
Effect of maturation stimuli added to HIV-1-infected DCs. iDCs were infected for 2 h. After washing, maturation cytokines (or LPS or CD40L, Table 2) were added immediately (time 0) or at the indicated times. Cells were analyzed at 5 days by PCR for HIV-1 reverse transcripts (a) and FACS for expression of p24 and the maturation marker DC–LAMP (b).
HIV-1-Infected iDCs Induce IL-10 Production Upon Coculture with T Cells. There is evidence that iDCs can induce IL-10-producing regulatory cells (25). To evaluate this possibility, we infected iDCs differentiated from HIV-1-infected patients and healthy controls for 4 days and added these (or aliquots of uninfected DCs) to autologous T cells (see Fig. 7, which is published as supporting information on the PNAS web site, for experimental design). When the DCs were HIV-1 infected, IL-10 and IFN-γ were secreted by the DC–T cell cocultures, but DCs pulsed with gag peptides rather than live virus stimulated mostly IFN-γ. Infected cultures matured with cytokines elicited IFN-γ but not IL-10 (Fig. 7 Right). To determine whether the observed responses were caused by direct infection of DCs, cells were pretreated with AZT before HIV-1 infection. Treatment with AZT inhibited cytokine secretion, indicating that direct infection of DCs was primarily responsible for the cytokine secretion (Fig. 7). T cells or DCs alone did not secrete cytokines (Fig. 7 Left), and the removal of natural killer cells did not alter the outcome of IFN-γ-secreting cells (not shown).
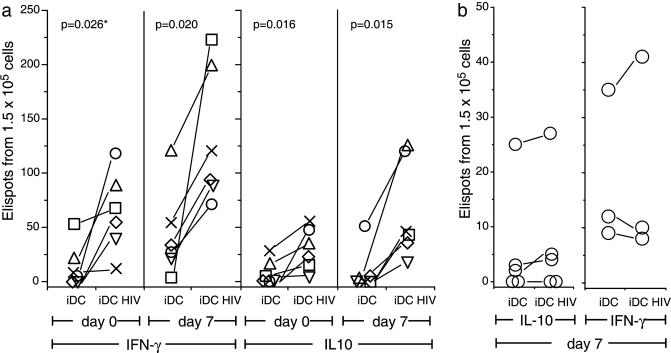
With this system, we tested cells from 10 consecutive HIV-1-infected patients to look for IL-10 production immediately or after 7 days of DC–T cell coculture. The antigen-presenting cells in the ELISPOT assays for IFN-γ and IL-10 were the autologous infected or uninfected DCs. Six of 10 patients showed a boost in IL-10 after 7 days of coculture by ELISA (Table 3, which is published as supporting information on the PNAS web site) or ELISPOT (Fig. 4a) assays, whereas four of these six showed a significant increase in IFN-γ- and IL-10-producing ELISPOTs when measured during the first 36 h (day 0, Fig. 4a). Similar results were obtained when five of these six patients were studied for IL-10 production on a second occasion, but secreted transforming growth factor β by ELISA was not detected (data not shown). In contrast, cells from five healthy donors did not show an HIV-1-dependent increase in IFN-γ- and IL-10-producing cells by ELISA (data not shown) or ELISPOT assays (Fig. 4b). Therefore, iDCs infected with HIV-1 induce IL-10 production upon culture with T cells from HIV-1-infected patients.
Fig. 4.
Detection and expansion of T cells secreting IFN-γ and IL-10. iDCs were generated from HIV-1-infected patients (a) or uninfected donors (b), infected with HIV-1 for 4 days, and added to autologous T cells. IFN-γ and IL-10 production were assessed by ELISPOT immediately (day 0) or after 7 days of coculture. Each symbol is a different patient. Statistical comparisons (P values at top) between uninfected and infected DCs were made by using Student's t tests.
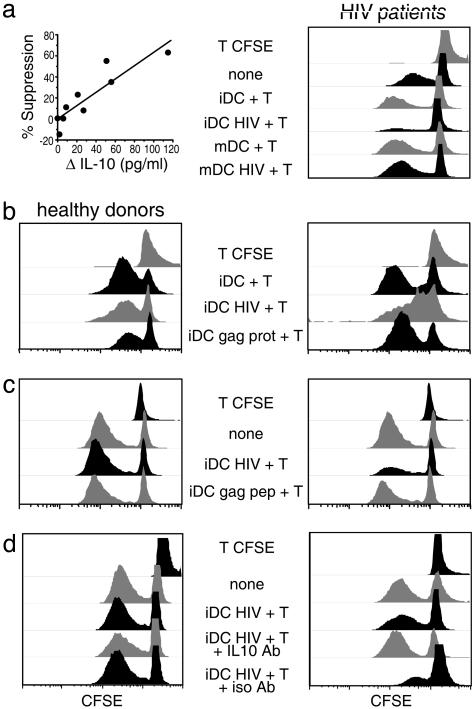
Cocultures of Immature HIV-1-Infected DCs and T Cells Suppress Allogeneic Responses. Next, we evaluated whether the T cells generated during 7 days of coculture had regulatory properties. Cells from 7-day cocultures of DC with or without HIV plus T cells were added in transwells to a “third party” MLR to test suppression by the release of soluble mediators. The MLR used CFSE-labeled CD4+ T cells and allogeneic mature DCs, both from healthy noninfected donors, so that proliferation in the MLR could be monitored by CFSE dilution. As shown in Fig. 5a Right, T cells generated in the cocultures with immature HIV-1-infected DCs suppressed proliferation in the MLR (fourth row), whereas T cells derived from cultures of uninfected immature or mature DCs did not suppress. In 10 patients studied in this way (Table 3), the degree of suppression correlated directly with the amount of IL-10 produced in parallel cultures (Fig. 5a Left). Next, we observed that HIV-1 specifically induced this regulation, because DCs pulsed with gag protein or gag peptides did not elicit comparable responses (Fig. 5 b and c and Table 1). Fig. 5 b Left and c Left also show that regulation did not occur when immature HIV-1-infected DCs and T cells from healthy donors were added to the MLR across a transwell filter. In view of the correlation between MLR suppression and amount of IL-10 produced, we tested blocking anti-IL-10 antibodies. Blocking antibody, but not a control nonreactive antibody, reduced or totally reversed the suppressive activity in four of six patients tested (Fig. 5d). Thus, immature virus-infected DCs can generate IL-10-dependent suppression upon coculture with autologous T cells.
Fig. 5.
Cocultures of infected immature DCs and T cells inhibit a MLR. DCs from HIV-1-infected patients were infected or not with HIV-1 for 4 days and then added to autologous T cells for 7 days. The indicated cells (Center) were then placed in a transwell chamber. In the lower wells, an MLR was generated, and proliferation was assessed by CFSE dilution. T CFSE indicates T cells cultured without DCs. (A)(Left) Correlation between suppression of the MLR and production of IL-10 (Table 2). (Right) Comparison between immature and mature DCs to show the suppression by iDC HIV + T (fourth row). (b and c) Comparison between iDCs infected with HIV-1 or pulsed with gag protein (b) or peptides (c). (d) Neutralizing anti-IL-10 or isotype control antibody was added at 10 μg/ml, and healthy controls studied in parallel (Left).
Table 1. IL-10 (pg/ml) production by T cells cocultured with autologous DC.
| iDC + T | iDC HIV + T | iDC gag pep + T | mDC + T | mDC + HIV + T | mDC gag pep + T |
|---|---|---|---|---|---|
| 0 | 0 | 0 | ND | ND | ND |
| 0 | 130 | 0 | ND | ND | ND |
| 0 | 205 | 5 | 0 | 15 | 1 |
| 26 | 85 | 31 | ND | ND | ND |
| 157 | 163 | ND | 0 | 8 | 0 |
| 0 | 54 | 110 | 0 | 7 | ND |
| 0 | 280 | 0 | ND | ND | ND |
| 0 | 77 | 8 | 0 | 3 | 1 |
DCs were prepared from eight HIV-1 patients. Immature (iDC) and mature (mDC) DCs were infected with HIV-1 or pulsed with gag peptides (2 μM) overnight. The different populations of DCs were cultured with autologous T cells for 7 days. Supernatants were assessed for IL-10 released by ELISA. ND, not done because of insufficient cells.
Discussion
The consequences of virus infection on DC maturation depend on the virus type. Herpes simplex virus, vaccinia virus, and hepatitis C virus inhibit DC maturation (26–28), whereas infection with influenza (24) and dengue virus (29) leads to DC maturation. Cytomegalovirus (30) and Epstein–Barr virus (31) inhibit monocyte differentiation to iDCs. Ebola (32) and measles virus (33, 34) also impair DC function. HIV-1 is already known to have several mechanisms for immune evasion (reviewed in ref. 35), such as nef-dependent down modulation of MHC class I expression, mutation of T cell epitopes, and a decrease in the total number of DCs in blood. Nef HIV-1 and simian immunodeficiency virus can stimulate cultures of iDCs to produce cytokines and chemokines (36). Here we have examined the effects of HIV-1 on DCs derived from blood monocytes, emphasizing the fraction of cells that are productively infected in the cultures. We have found two mechanisms that would further allow HIV-1 to evade immunity. One is a block in many key features of DC maturation, whereas the other is a T cell inhibitory function that depends in part on the suppressive cytokine IL-10 (reviewed in ref. 37).
Prior in vitro studies of the interaction of HIV-1 with DCs have emphasized that these cells are a driving force for virus replication. A major pathway entails the capacity of DCs to sequester and transmit HIV-1 to T cells with which the DCs are interacting. This leads to enhanced viral replication in the T cells. In the case of DCs derived from blood monocytes, the virus transmission function involves a calcium-dependent lectin DC-SIGN/CD209 that binds and internalizes HIV-1, thereby retaining the virus in an infectious state for days before transmission to the T cell (38–40). There is another pathway whereby DCs drive virus replication: DCs themselves serve as a direct site for productive infection. This has been observed with iDCs from three sources: Langerhans cells (15, 16), monocyte-derived DCs (13), and CD34+-derived DCs (23). In these instances, only a small fraction of the immature population is productively infected, as indicated by the accumulation of HIV gag protein. Here, we have studied the direct infection of immature monocyte-derived DCs, and have uncovered additional consequences that may contribute to disease pathogenesis through immune evasion.
We began by evaluating HIV-1-infected, p24+ iDCs. Maturation by viruses is now realized to proceed by many pathways, including stimulation of toll-like receptors, e.g., TLR9 by viral DNA (7, 9, 41), TLR3 by double-stranded RNA (4–6), cytoplasmic protein kinase R (3), and IFN-γ receptors (5). However, HIV-1 infection did not lead directly to maturation. All of the infected cells expressing viral p24 did not acquire DC maturation markers such as DC–LAMP and CD83 (Figs. 1 and 2). Likewise, bystander uninfected DCs in the culture did not mature, as assessed by cell surface remodeling and increased stimulation of the MLR. Because mature DCs are the form that initiates T cell immunity, both T helper 1 type CD4+ helpers and CD8+ cytotoxic T cells, infected DCs are likely to be compromised in their capacity to directly initiate T cell immunity to newly synthesized viral antigens.
We then identified an additional dimension to the lack of maturation, which became apparent with the DC maturation marker DC–LAMP. It is known that mature DC–LAMP+ DCs are difficult to infect with HIV-1. This entails reductions at the level of viral entry, for example, reduced expression of CCR5 chemokine coreceptor, the subsequent viral life cycle, and both reverse transcription and DNA integration. We now find that immature productively infected DCs (expressing HIV-1 gag protein), if given different maturation stimuli 2 days after infection (cytokines, LPS, CD40 ligand; Table 2), selectively fail to express the maturation marker DC–LAMP, in contrast to the noninfected DCs in the same cultures (Fig. 3B). Therefore, infected monocyte-derived DCs seem to evade, at several levels, the chance to mature and to become immunogenic for the viral proteins that are being synthesized.
There are two potential consequences of these findings. First, immunogenicity might be delayed if the infected DCs could not mature and present viral antigens, although it would not be totally blocked; this is because DCs should still be able to present viral antigens from dying infected cells and inactive virions (42, 43). However, such presentation would be delayed with respect to the time required for the DCs to take up and process viral antigens, and would allow other cells to replicate virus beforehand. A second consequence is the possibility of more active immunosuppression via infected iDCs. When we obtained DCs and T cells from chronically infected donors (as opposed to healthy noninfected donors, Fig. 5), and infected the DCs before addition to autologous T cells, we found that the DC–T cell cocultures started to produce IL-10, and furthermore, the cocultures could suppress another or “third party” immune response, the MLR, by secreting soluble factors including IL-10. We have been unable at this point to determine whether the DCs and/or T cells were the source of IL-10 in the cocultures, but we presume that the requirement for T cells from infected donors signifies that these donors have generated a Tr1 type of IL-10-producing suppressors (reviewed in ref. 44). Interestingly, both IL-10 production and suppression were greatly enhanced when the source of antigen was live virus vs. viral peptides (Table 1). Possibly virus infection up-regulates ICOS-L on DCs, because ICOS-L is implicated in the induction of IL-10+ regulatory T cells (45).
Our findings add to a considerable existing literature on IL-10 in HIV-1 infection. Elevated IL-10 production and serum levels have been reported (46–50). Monocytes are a major cell type producing IL-10 in blood cells from HIV-1-infected patients (51), and IL-10 production can be induced by HIV-1 nef (52). DCs have the potential to produce IL-10, but, interestingly, HIV-1-infected DCs produce less of this cytokine (53). IL-10 up-regulates the expression of CCR5 on monocytes, leading to more efficient HIV-1 infection (54). In view of these data, as well as recent studies indicating that different types of iDCs can induce T cell tolerance, e.g., by stimulating regulatory T cells (45, 55–57), we would speculate that HIV-1-infected iDCs are more tolerogenic then immunogenic.
Supplementary Material
Acknowledgments
We thank Mark Muesing (Aaron Diamond AIDS Research Center, New York) for the gift of Yu2 HIV–GFP plasmid. This work was supported by Direct Effect, Center for AIDS Research Grant 5 P30 AI 42848-04, and National Institutes of Health Grants R01 AI40045 and MO-1 RR00102 (to The Rockefeller University General Clinical Research Center).
Abbreviations: DC, dendritic cell; iDC, immature DC; MLR, mixed leukocyte reaction; AZT, 3′-azido-3′-deoxythymidine; FACS, fluorescence-activated cell sorting; CFSE, 5,6-carboxyfluorescein diacetate-succinimyl ester; ELISPOT, enzyme-linked immunospot; LPS, lipopolysaccharide; LAMP, lysosome-associated membrane protein.
References
- 1.Carbone, F. R. & Heath, W. R. (2003) Curr. Opin. Immunol. 15, 416-420. [DOI] [PubMed] [Google Scholar]
- 2.Lopez, C. B., Garcia-Sastre, A., Williams, B. R. G. & Moran, T. M. (2003) J. Infect. Dis. 187, 1126-1136. [DOI] [PubMed] [Google Scholar]
- 3.Diebold, S. S., Montoya, M., Unger, H., Alexopoulou, L., Roy, P., Haswell, L. E., Al-Shamkhani, A., Flavell, R., Borrow, P. & Reis e Sousa, C. (2003) Nature 424, 324-328. [DOI] [PubMed] [Google Scholar]
- 4.Hoebe, K., Janssen, E. M., Kim, S. O., Alexopoulou, L., Flavell, R. A., Han, J. & Beutler, B. (2003) Nat. Immunol. 4, 1223-1229. [DOI] [PubMed] [Google Scholar]
- 5.Honda, K., Sakaguchi, S., Nakajima, C., Watanabe, A., Yanai, H., Matsumoto, M., Ohteki, T., Kaisho, T., Takaoka, A., Akira, S., et al. (2003) Proc. Natl. Acad. Sci. USA 100, 10872-10877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang, L., Smith, D., Bot, S., Dellamary, L., Bloom, A. & Bot, A. (2002) J. Clin. Invest. 110, 1175-1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ichikawa, H. T., Williams, L. P. & Segal, B. M. (2002) J. Immunol. 169, 2781-2787. [DOI] [PubMed] [Google Scholar]
- 8.Krug, A., Luker, G. D., Barchet, W., Leib, D. A., Akira, S. & Colonna, M. (2004) Blood 103, 1433-1437. [DOI] [PubMed] [Google Scholar]
- 9.Spies, B., Hochrein, H., Vabulas, M., Huster, K., Busch, D. H., Schmitz, F., Heit, A. & Wagner, H. (2003) J. Immunol. 171, 5908-5912. [DOI] [PubMed] [Google Scholar]
- 10.Heath, W. R. & Carbone, F. R. (2001) Annu. Rev. Immunol. 19, 47-64. [DOI] [PubMed] [Google Scholar]
- 11.Steinman, R. M., Hawiger, D. & Nussenzweig, M. C. (2003) Annu. Rev. Immunol. 21, 685-711. [DOI] [PubMed] [Google Scholar]
- 12.Weissman, D., Li, Y., Ananworanich, J., Zhou, L.-J., Adelsberger, J., Tedder, T. F., Baseler, M. & Fauci, A. S. (1995) Proc. Natl. Acad. Sci. USA 92, 826-830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Granelli-Piperno, A., Delgado, E., Finkel, V., Paxton, W. & Steinman, R. M. (1998) J. Virol. 72, 2733-2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Canque, B., Rosenzwajg, M., Camus, S., Yagello, M., Bonnet, M.-L., Guigon, M. & Gluckman, J. C. (1996) Blood 88, 4215-4228. [PubMed] [Google Scholar]
- 15.Zaitseva, M., Blauvelt, A., Lee, S., Lapham, C. K., Klaus-Kovtun, V., Mostowski, H., Manischewitz, J. & Golding, H. (1997) Nat. Med. 3, 1369-1375. [DOI] [PubMed] [Google Scholar]
- 16.Kawamura, T., Gulden, F. O., Sugaya, M., McNamara, D. T., Borris, D. L., Lederman, M. M., Orenstein, J. M., Zimmerman, P. A. & Blauvelt, A. (2003) Proc. Natl. Acad. Sci. USA 100, 8401-8406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Delgado, E., Finkel, V., Baggiolini, M., Clark-Lewis, I., Mackay, C. R., Steinman, R. M. & Granelli-Piperno, A. (1998) Immunobiology 198, 490-500. [DOI] [PubMed] [Google Scholar]
- 18.Lin, Y. L., Mettling, C., Portales, P., Reynes, J., Clot, J. & Corbeau, P. (2002) Proc. Natl. Acad. Sci. USA 99, 15590-15595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee, B., Sharron, M., Montaner, L. J., Weissman, D. & Doms, R. W. (1999) Proc. Natl. Acad. Sci. USA 96, 5215-5220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bakri, Y., Schiffer, C., Zennou, V., Charneau, P., Kahn, E., Benjouad, A., Gluckman, J. C. & Canque, B. (2001) J. Immunol. 166, 3780-3788. [DOI] [PubMed] [Google Scholar]
- 21.Granelli-Piperno, A., Moser, B., Pope, M., Chen, D., Wei, Y., Isdell, F., O'Doherty, U., Paxton, W., Koup, R., Mojsov, S., et al. (1996) J. Exp. Med. 184, 2433-2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Saint-Vis, B., Vincent, J., Vandenabeele, S., Vanbervliet, B., Pin, J.-J., Ait-Yahia, S., Patel, S., Mattei, M.-G., Banchereau, J., Zurawski, S., et al. (1998) Immunity 9, 325-336. [DOI] [PubMed] [Google Scholar]
- 23.Canque, B., Bakri, Y., Camus, S., Yagello, M., Benjouad, A. & Gluckman, J. C. (1999) Blood 93, 3866-3875. [PubMed] [Google Scholar]
- 24.Cella, M., Salio, M., Sakakibara, Y., Langen, H., Julkunen, I. & Lanzavecchia, A. (1999) J. Exp. Med. 189, 821-829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jonuleit, H., Schmitt, E., Schuler, G., Knop, J. & Enk, A. H. (2000) J. Exp. Med. 192, 1213-1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Salio, M., Cella, M., Suter, M. & Lanzavecchia, A. (1999) Eur. J. Immunol. 29, 3245-3253. [DOI] [PubMed] [Google Scholar]
- 27.Engelmayer, J., Larsson, M., Subklewe, M., Chahroudi, A., Schmaljohn, A., William, C., Steinman, R. M. & Bhardwaj, N. (1999) J. Immunol. 163, 6762-6768. [PubMed] [Google Scholar]
- 28.Sarobe, P., Lasarte, J. J., Zabaleta, A., Arribillaga, L., Arina, A., Melero, I., Borras-Cuesta, F. & Prieto, J. (2003) J. Virol. 77, 10862-10871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ho, L.-J., Wang, J.-J., Shaio, M.-F., Kao, C.-L., Chang, D.-M., Han, S.-W. & Lai, J.-H. (2001) J. Immunol. 166, 1499-1506. [DOI] [PubMed] [Google Scholar]
- 30.Moutaftsi, M., Mehl, A. M., Borysiewicz, L. K. & Tabi, Z. (2002) Blood 99, 2913-2921. [DOI] [PubMed] [Google Scholar]
- 31.Li, L., Liu, D., Hutt-Fletcher, L., Morgan, A., Masucci, M. G. & Levitsky, V. (2002) Blood 99, 3725-3734. [DOI] [PubMed] [Google Scholar]
- 32.Mahanty, S., Hutchinson, K., Agarwal, S., McRae, M., Rollin, P. E. & Pulendran, B. (2003) J. Immunol. 170, 2797-2801. [DOI] [PubMed] [Google Scholar]
- 33.Schnorr, J.-J., Xanthakos, S., Keikavoussi, P., Kämpgen, E., Meulen, V. T. & Schneider-Schaulies, S. (1997) Proc. Natl. Acad. Sci. USA 94, 5326-5331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fugier-Vivier, I., Rivailler, P., Rissoan, M.-C., Liu, Y.-J., Rabourdin-Combe, C., Warnarr, S. O., van de Velde, C. J. H. & Melief, C. J. M. (1997) J. Exp. Med. 186, 813-823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Letvin, N. L. & Walker, B. D. (2003) Nat. Med. 9, 861-866. [DOI] [PubMed] [Google Scholar]
- 36.Messmer, D., Jacque, J. M., Santisteban, C., Bristow, C., Han, S.-Y., Villamide-Herrera, L., Mehlhop, E., Marx, P. A., Steinman, R. M., Gettie, A. & Pope, M. (2002) J. Immunol. 169, 4172-4182. [DOI] [PubMed] [Google Scholar]
- 37.Moore, K. W., de Waal Malefyt, R., Coffman, R. L. & O'Garra, A. (2001) Annu. Rev. Immunol. 19, 683-765. [DOI] [PubMed] [Google Scholar]
- 38.Geijtenbeek, T. B. H., Kwon, D. S., Torensma, R., van Vliet, S. J., van Duijnhoven, G. C. F., Middel, J., Cornelissen, I. L., Nottet, H. S., KewalRamani, V. N., Littman, D. R., et al. (2000) Cell 100, 587-597. [DOI] [PubMed] [Google Scholar]
- 39.Kwon, D. S., Gregario, G., Bitton, N., Hendrickson, W. A. & Littman, D. R. (2002) Immunity 16, 135-144. [DOI] [PubMed] [Google Scholar]
- 40.Trumpfheller, C., Park, C. G., Finke, J., Steinman, R. M. & Granelli-Piperno, A. (2003) Int. Immunol. 15, 289-298. [DOI] [PubMed] [Google Scholar]
- 41.Hacker, H., Mischak, H., Miethke, T., Liptay, S., Schmid, R., Sparwasser, T., Heeg, K., Lipford, G. B. & Wagner, H. (1998) EMBO J. 17, 6230-6240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Larsson, M., Fonteneau, J. F., Lirvall, M., Haslett, P., Lifson, J. D. & Bhardwaj, N. (2002) AIDS 16, 1319-1329. [DOI] [PubMed] [Google Scholar]
- 43.Buseyne, F., Gall, S. L., Boccaccio, C., Abastado, J. P., Lifson, J. D., Arthur, L. O., Riviere, Y., Heard, J. M. & Schwartz, O. (2001) Nat. Med. 7, 344-349. [DOI] [PubMed] [Google Scholar]
- 44.Roncarolo, M.-G., Levings, M. K. & Traversari, C. (2001) J. Exp. Med. 193, F5-F9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Akbari, O., Freeman, G. J., Meyer, E. H., Greenfield, E. A., Chang, T. T., Sharpe, A. H., Berry, G., DeKruyff, R. H. & Umetsu, D. T. (2002) Nat. Med. 8, 1024-1032. [DOI] [PubMed] [Google Scholar]
- 46.Ameglio, F., Cordiali Fei, P., Solmone, M., Bonifati, C., Prignano, G., Giglio, A., Caprilli, F., Gentili, G. & Capobianchi, M. R. (1994) J. Biol. Regul. Homeost. Agents 8, 48-52. [PubMed] [Google Scholar]
- 47.Ostrowski, M. A., Gu, J. X., Kovacs, C., Freedman, J., Luscher, M. A. & MacDonald, K. S. (2001) J. Infect. Dis. 184, 1268-1278. [DOI] [PubMed] [Google Scholar]
- 48.Altfeld, M., Addo, M. M., Kreuzer, K. A., Rockstroh, J. K., Dumoulin, F. L., Schliefer, K., Leifeld, L., Sauerbruch, T. & Spengler, U. (2000) J. Acquir. Immune Defic. Syndr. 23, 287-294. [DOI] [PubMed] [Google Scholar]
- 49.Havlir, D. V., Torriani, F. J., Schrier, R. D., Huang, J. Y., Lederman, M. M., Chervenak, K. A. & Boom, W. H. (2001) J. Clin. Microbiol. 39, 298-303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Clerici, M., Wynn, T. A., Berzofsky, J. A., Blatt, S. P., Hendrix, C. W., Sher, A., Coffman, R. L. & Shearer, G. M. (1994) J. Clin. Invest. 93, 768-775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Barcova, M., Kacani, L., Speth, C. & Dierich, M. P. (1998) J. Infect. Dis. 177, 905-913. [DOI] [PubMed] [Google Scholar]
- 52.Tangsinmankong, N., Day, N. K., Good, R. A. & Haraguchi, S. (2000) Cytokine 12, 1506-1511. [DOI] [PubMed] [Google Scholar]
- 53.Kawamura, T., Gatanaga, H., Borris, D. L., Connors, M., Mitsuya, H. & Blauvelt, A. (2003) J. Immunol. 170, 4260-4266. [DOI] [PubMed] [Google Scholar]
- 54.Sozzani, S., Ghezzi, S., Iannolo, G., Luini, W., Borsatti, A., Polentarutti, N., Sica, A., Locati, M., Mackay, C., Wells, T. N. C., et al. (1998) J. Exp. Med. 187, 439-444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jonuleit, H., Schmitt, E., Steinbrink, K. & Enk, A. H. (2001) Trends Immunol. 22, 394-400. [DOI] [PubMed] [Google Scholar]
- 56.Groux, H., Bigler, M., DeVries, J. E. & Roncarolo, M.-G. (1996) J. Exp. Med. 184, 19-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wakkach, A., Fournier, N., Brun, V., Breittmayer, J. P., Cottrez, F. & Groux, H. (2003) Immunity 18, 605-617. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.