Abstract
The hyperactivity of the brain renin-angiotensin system (RAS) has been implicated in the development and maintenance of hypertension in several types of experimental and genetic hypertension animal models. We previously reported that in the murine brain, aminopeptidase A (APA) is involved in the conversion of angiotensin II (AngII) to AngIII and that AngIII is one of the main effector peptides of the brain RAS in the control of vasopressin release. Here we report that brain AngIII exerts a tonic stimulatory effect on blood pressure in a model of salt-dependent hypertension, the DOCA-salt rat, characterized by a depressed systemic but a hyperactive brain RAS. Similar high blood pressure accompanied by a low systemic renin state was described in some patients, especially in hypertensive African Americans who are resistant to treatment by blockers of the systemic RAS. We developed RB150, a prodrug of the specific and selective APA inhibitor, EC33. RB150 given i.v. is able to cross the blood–brain barrier, to inhibit brain APA, and to block the formation of central AngIII. A single dose of systemic RB150 (15 mg/kg, i.v.) in conscious DOCA-salt rats inhibited brain APA activity and markedly reduced blood pressure for up to 24 h. These results demonstrate the crucial role of brain APA as a candidate target for the treatment of hypertension and suggest that RB150, a potent systemically active APA inhibitor, could be the prototype of a new class of antihypertensive agents for the treatment of certain forms of hypertension.
Hypertension is a major cardiovascular risk factor affecting ≈10% of the population. Treatment of hypertension can effectively reduce cardiovascular morbidity and mortality, even in the case of isolated systolic hypertension or mild to moderate forms of hypertension (1, 2). Historically, the first antihypertensive drugs used were sympathicolytic agents and diuretics. Centrally active drugs that act by stimulating bulbar α2-adrenoreceptors (α-methyldopa, clonidine) or by inhibiting central α1-adrenoreceptors (carvedilol) and stimulating 5-HT1A serotoninergic receptors (indorenate) are still being used. However, they cause a number of secondary side effects and are thus not the first choice of drugs. Blockers of the renin-angiotensin system (RAS), either angiotensin I-converting enzyme (ACE) inhibitors or angiotensin II (AngII) receptor type 1 (AT1) antagonists, have proved to be efficient and safe (3). ACE inhibitors cause cough and more rarely angioedema (4–6), and renal function may deteriorate with both ACE inhibitors and AT1 receptor antagonists in cases of underlying renal artery stenosis (7–9). In addition, blockers of the RAS are poorly effective in some patients, especially in African Americans in whom high blood pressure (BP) is accompanied by a low-renin state and is usually responsive to salt-depletion (10, 11). Thus, the development of new classes of antihypertensive agents with different mechanisms of action remains an important goal.
The hyperactivity of the brain RAS has been implicated in the development and maintenance of hypertension in several types of experimental and genetic hypertension animal models, such as spontaneously hypertensive rats (SHR), DOCA-salt hypertensive rats (12, 13), and transgenic animals harboring the mouse renin Ren 2d gene (14, 15) or overexpressing both human angiotensinogen and human renin (16, 17). The activity of the systemic RAS is normal in the SHR model, depressed in DOCA-salt rats, and high in transgenic animals.
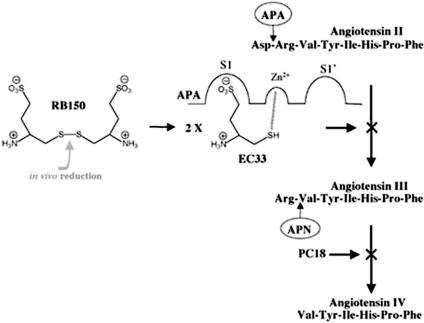
We previously reported that in the murine brain, aminopeptidase A (APA) (EC 3.4.11.7), a membrane-bound zinc-metal-loprotease (18–21), hydrolyzes in vivo the N-terminal aspartate of AngII (Ang 1–8) to generate AngIII (Ang 2–8), whereas aminopeptidase N (APN, EC 3.4.11.2), another zinc-metal-loprotease, hydrolyzes the N-terminal arginine of AngIII to generate AngIV (Ang 3–8) (22) (Fig. 1). We developed specific and selective APN and APA inhibitors, PC18 and EC33, respectively (23, 24), and used these tools to demonstrate that AngIII, but not AngII as shown in the periphery, is one of the main effector peptides of the brain RAS in the control of vasopressin release (25–27). Moreover, brain AngIII exerts a tonic stimulatory action on the control of BP in the conscious SHR (26), suggesting that APA, generating brain AngIII, could constitute a new candidate target for the treatment of hypertension. In this study, we demonstrated that the intracerebroventricular (i.c.v.) administration of the APA inhibitor EC33 [(S)-3-amino-4-mercapto-butyl sulfonic acid] decreases BP in DOCA-salt rats, a salt- and volume-dependent but renin-independent (low plasma renin levels) model of hypertension, resistant to RAS blockers (28). We then designed RB150 {4,4′-dithio[bis(3-aminobutyl sulfonic acid)]}, a systemically active prodrug of EC33 obtained by dimerization through a disulfide bond. We showed that RB150 is able to inhibit brain APA activity, to block the formation of brain AngIII, and to decrease arterial BP in conscious DOCA-salt hypertensive rats after i.v. administration. Thus, this potent systemically active APA inhibitor prodrug could be the prototype of a new class of antihypertensive agents for the treatment of certain forms of hypertension.
Fig. 1.
Metabolic pathways of AngII and AngIII in the brain involving APA and APN. Structures of the APA inhibitor EC33, the corresponding prodrug RB150, and the APN inhibitor PC18.
Materials and Methods
Drugs. Human AngII, α-l-glutamyl-β-naphthylamide (GluNA), bestatin, Fast Green dye, and penicillin were purchased from Sigma, and [tyrosyl-3,5-3H]AngII (5-l-isoleucine) was purchased from Amersham Pharmacia. All compounds administered i.c.v. or i.v. were dissolved in sterile 0.9% saline, and the pH was adjusted to 7.0 with 0.5 M NaOH.
Animals. Brain APA activity measurements and in vivo angiotensin metabolism experiments were performed on male Swiss mice weighing 18–20 g (Iffa Credo). For BP measurements, we used male WKY and DOCA-salt rats weighing 250–300 g (Iffa Credo). Hypertension was induced in unilaterally nephrectomized WKY rats by the s.c. implantation of a DOCA pellet (200 mg per kg of body weight, Innovative Research of America, DOCA-salt rats). Sham rats corresponded to unilaterally nephrectomized WKY rats. After surgery, rats received a standard rat chow diet and tap water supplemented with 0.9% NaCl and 0.2% KCl. Hypertension occurred 3 weeks later. All animal experiments were carried out in accordance with institutional guidelines.
Enzymatic Activity Measurements. APA activity was determined by measuring the rate of hydrolysis of a synthetic substrate, GluNA, as described in ref. 24. Recombinant APA (29) was incubated for 30 min at 37°C in the presence of 200 μM GluNA with or without increasing concentrations of RB150 or EC33 [prepared in 50 mM Tris·HCl buffer (pH 7.4) containing 100 eq DTT per eq of inhibitor], in a final volume of 100 μl of 50 mM Tris·HCl buffer (pH 7.4) containing 4 mM CaCl2 (24).
In vivo, in mice, EC33 or RB150 were administered i.c.v. (1–100 μg, in a volume of 10 μl) or i.v. (50 mg/kg, in a volume of 200 μl). For each condition, three to six mice were used. Mice were decapitated 5, 15, 30, and 60 min after the APA inhibitor injection. Their brains were immediately removed and homogenized by sonication in 10 vol of ice-cold 50 mM Tris·HCl buffer (pH 7.4). APA enzymatic activity was measured on brain homogenates. For this purpose, aliquots of the tissue homogenate (16 μl) were incubated for 30 min at 37°C with 200 μM of GluNA, 4 mM CaCl2, and 1 μM bestatin inhibitor, with or without 5 μM EC33, in a total volume of 100 μl of 50 mM Tris·HCl buffer (pH 7.4), as described in ref. 30. Similar in vivo experiments were performed in DOCA-salt rats that received either i.v. saline or RB150 (15 mg/kg) injection in a volume of 200 μl. Rats were decapitated 60 min after the injection, and their brains were homogenized and assayed as described above.
In Vivo Metabolism Studies. A mixture of 1.5 × 106 cpm [3H]AngII and 30 μg of unlabeled AngII was administrated i.c.v. in a volume of 5 μl, either alone (control) or with EC33 or RB150 (30 μg). At different times after the injection, mice were decapitated. Their brains were immediately removed, and the hypothalamus was dissected out and homogenized in six vol of cold 0.1 HCl M, as described in refs. 22 and 27. To determine the amount of hypothalamic [3H]AngII and [3H]AngIII, we separated AngII and AngIII by cation-exchange chromatography.
Surgical Method. At least 24 h before the experimental day, anesthetized (pentobarbital sodium, 60 mg/kg, i.p., Sanofi) rats were surgically implanted with femoral arterial and/or venous catheters and assigned to two different groups. In the first group, a guide cannula was positioned in the lateral cerebral ventricle, as described by Reaux et al. (26). In the second group, a catheter was introduced into the femoral vein for i.v. injection of APA inhibitors. Immediately afterward, an additional catheter was inserted into the femoral artery of all animals, as described in ref. 31, for continuously recording arterial BP. The catheters were tunneled s.c. to exit from the neck. Animals were treated with penicillin (100,000 units, i.p.), and their temperature was maintained at 37°C throughout their recovery from the anesthesia and surgery.
BP Recording. Arterial BP was continuously recorded as described in ref. 26: for 7–8 h on the first day, then between 9:00 and 10:00 a.m. for the next 2 days. Animals received either an i.c.v. injection of EC33 or RB150 or an i.v. injection of RB150 (0.1, 0.5, 1, 7.5, 15, and 30 mg/kg) 1 h after the beginning of the BP recording. In the first group of animals, the correct placement of the i.c.v. cannula was checked as reported in ref. 26.
Statistical Analysis. Data are presented as means ± SEM. APA activities in the different groups were compared by using a Student's unpaired t test. Intragroup comparisons of mean arterial BP (MABP) and heart rate (HR) were made by using an ANOVA followed by a least-significant difference Fisher's t test. Differences were considered to be statistically significant at P < 0.05.
Results
Synthesis of RB150. A solution of 7 g (23 mmol, 1.2 eq) of iodine in 100 ml of methanol was added drop-wise at room temperature to a solution of 7 g (38 mmol) of EC33 in 100 ml of MeOH until the appearance of a persistent yellow color. The precipitate obtained was filtered, washed with cold MeOH, and dried under reduced pressure. A white solid (5.4 g, 77%) was isolated. TLC Rf (iPrOH/H2O/AcOH = 8/2/1) = 0.26; [α]20D =+194.5° (C = 1.33 in H2O). CHNS analysis showed the following composition: C = 25.81%, H = 5.60%, N = 7.39%, S = 34.50% (analysis calculated, C = 26.07%, H = 5.47%, N = 7.60%, S = 34.81%). NMR (D2O) δ ppm 2.10 (2H, CH2β); 2.85 (1H) and 32.10 (1H), CH2γ; 2.95 (2H, CH2(SH)); 3.10 (1H, CHα). Mass spectrum electrospray ionization (ESI)-, (M - H) = 367.
Ability of RB150 to Inhibit Recombinant Purified APA. In vitro studies on recombinant purified mouse APA showed that the inhibitory potency of the reduced form of RB150 obtained in the presence of DTT (Ki = 2.0 ± 0.2 × 10-7 M) was similar to that of EC33 (Ki = 3.0 ± 0.1 × 10-7 M). EC33 was previously shown to be 100-fold less active in vitro on APN (24). In the absence of DTT, RB150 with intact disulfide bridge was inactive on APA (Ki > 10-5 M). Furthermore, the concentration of DTT used was inactive on APA.
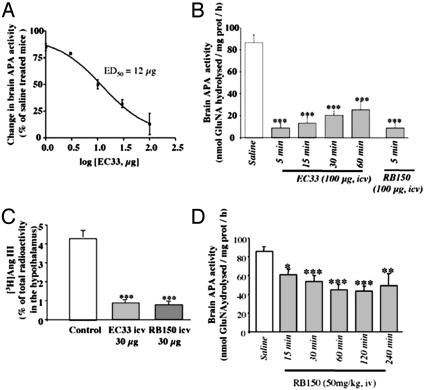
Inhibitory Effects of i.c.v. EC33 and RB150 on Mouse Brain APA Activity. We first established a standard curve of inhibition of brain APA by EC33 and showed that EC33 (1–100 μg, i.c.v.) inhibited brain APA activity in a dose-dependant manner with an IC50 of 12 μg (50 nmol) (Fig. 2A). Inhibition was maximal (-90%) 5 min after the injection for doses between 50 and 100 μg (205–412 nmol) of EC33, compared to control brain APA activity (86.5 ± 6.5 nmol GluNA hydrolyzed per mg of protein per h). At 100 μg, the inhibition was still pronounced (-75%) 1 h after the injection (Fig. 2B). In the same experimental conditions, central injection of RB150 (100 μg, 272 nmol) similarly inhibited the activity of brain APA by 90 ± 9%; the mouse brain being homogenized in 2.5 ml, this corresponded to a brain concentration of RB150 of 109 μM.
Fig. 2.
Effects of the APA inhibitors, EC33 and RB150, on mouse brain APA activity and hypothalamic [3H]AngIII formation. (A) Dose–response inhibition curve of ex vivo brain APA activity after i.c.v. injection of EC33 (1–100 μg). (B) Time course of brain APA ex vivo activity inhibition after i.c.v. injection of EC33 or RB150 (100 μg). (C) Percentage of [3H]AngIII formation in the hypothalamus 1.5 min after i.c.v. injection of [3H]AngII in the absence or presence of EC33 or RB150 (30 μg). Values after APA inhibitor treatments were compared to control values obtained after saline injection. (D) Time course of brain APA ex vivo activity inhibition after i.v. injection of RB150 (50 mg/kg). Mean ± SEM of three to eight animals for each condition. *, P < 0.05; **, P < 0.01; and ***, P < 0.001 vs. control values.
Effect of RB150 on the Production of AngIII in the Mouse Hypothalamus. In control animals, hypothalamic [3H]AngIII could be detected as early as 0.5 min after the i.c.v. injection of [3H]AngII. The amount of hypothalamic [3H]AngIII peaked at 1.5 min and then progressively decreased during the following 10 min (data not shown). In contrast, EC33 (30 μg, 123 nmol, i.c.v.) immediately blocked the formation of hypothalamic [3H]AngIII by 86 ± 2%. RB150 (30 μg, 81 nmol, i.c.v.) induced a similar blockade of hypothalamic [3H]AngIII formation (Fig. 2C). We verified that the i.c.v. injection of EC33 or RB150 together with [3H]AngII did not modify the total amount of radioactivity found in the hypothalamus.
Inhibition of Brain APA by i.v. RB150 in Mice and Rats. We next assessed the ability of i.v. RB150 to block brain APA in conscious mice by measuring inhibition of brain APA activity. RB150 (50 mg/kg, i.e., 2720 nmol per mouse, i.v.) progressively inhibited brain APA activity (Fig. 2D) that was maximally decreased by 50% after 60 min (43.3 ± 5.7 nmol of GluNA hydrolyzed per mg of protein per h vs. 86.5 ± 6.5, P < 0.001). Thus, RB150 is able to cross the blood–brain barrier. Comparison with the standard curve constructed with EC33 showed that this inhibition corresponded to the generation of 12 μg (50 nmol) of EC33 in the mouse brain, representing ≈1.8% of the total amount of RB150 injected i.v. and resulting in a brain concentration of RB150 of ≈20 μM. Similarly, i.v. injection of RB150 (15 mg/kg, 12,000 nmol per rat) in DOCA-salt rats highly significantly decreased brain APA activity that was inhibited by 62% after 60 min compared to DOCA-salt rats receiving i.v. saline injection [52.3 ± 8.8 nmol of GluNA hydrolyzed per mg of protein per h vs. 135.8 ± 32.8 (P < 0.05)].
Effects of i.c.v. Injections of EC33 and RB150 on Arterial Blood Pressure in Conscious DOCA-Salt Rats. We first assessed whether the central injection of EC33 decreased BP in DOCA-salt rats. MABP and HR were significantly higher in these animals than in normotensive WKY or sham-operated rats [MABP in DOCA-salt rats, 160.9 ± 4.2 mmHg (1 mmHg = 133 Pa), n = 10; vs. WKY rats, 105.1 ± 2.8, n = 5, and sham rats, 107.2 ± 1.9, n = 9(P < 0.001); HR in DOCA-salt rats, 350.2 ± 9.6 bpm; HR in WKY rats, 315.9 ± 12.9; HR in sham rats, 304.3 ± 5.4 (P < 0.01)] (32). The systemic RAS activity was lower in DOCA-salt rats than in sham-operated rats as indicated by the significantly lower plasma renin activity [0.6 ± 0.1 ng of AngI produced per ml per h, n = 6, vs. 1.7 ± 0.3, n = 6 (P < 0.05)]. In contrast, the brain RAS was hyperactive in DOCA-salt rats as previously shown by the increased density of AT1 receptor binding sites observed in the brain of these animals as compared to normotensive WKY rats (33). This hyperactivity could account for the higher pressor response to i.c.v. AngII (10 ng) in DOCA-salt rats (+47.0 ± 1.1 mmHg) than in WKY rats (+20.0 ± 1.3 mmHg, P < 0.001) or sham-operated rats (+25.2 ± 0.6 mmHg, P < 0.001).
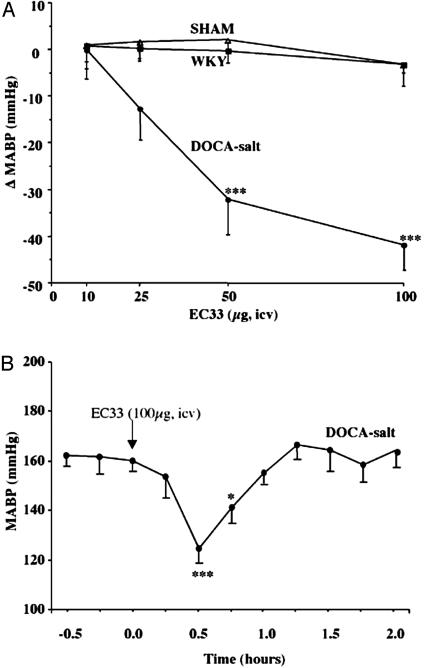
EC33 (1–100 μg, i.c.v.), by blocking endogenous AngIII formation, decreased BP in a dose-dependent manner in conscious DOCA-salt rats with an ED50 of 40 μg (164 nmol), although it did not modify BP in conscious WKY or shamoperated rats (Fig. 3A). Blood pressure was decreased by 41.9 ± 4.8 mmHg 30 min after the injection of 100 μg of EC33, leading to a normalization of BP. BP returned to baseline 90 min after EC33 injection (Fig. 3B). We never observed any significant changes in HR in WKY or sham-operated rats, whatever the dose of EC33 tested. Conversely, HR decreased slightly when DOCA-salt rats were injected with 25 μg of EC33 (103 nmol) (data not shown). The i.c.v. injection of RB150 (100 μg, 272 nmol) induced a similar hypotensive effect as EC33 (-33.0 ± 1.9 mmHg) in conscious DOCA-salt rats. This effect started 5 min after the injection and the maximal decrease occurred between 30 and 60 min. Interestingly, the i.c.v. injection of RB150 had no effect on BP in conscious normotensive WKY or sham-operated rats and did not change HR in any of the three groups of animals (Table 1).
Fig. 3.
Central effect of the APA inhibitor EC33 on MABP in conscious rats. (A) Maximal MABP variations, recorded between 15 and 30 min after i.c.v. injection of EC33 (10, 25, 50, and 100 μg), in conscious WKY, sham, and DOCA-salt rats are shown. Filled squares, WKY rats (n = 5); open triangles, sham rats (n = 6); filled circles, DOCA-salt rats (n = 6). (B) Time course of MABP after i.c.v. injection of EC33 (100 μg) in conscious DOCA-salt rats (n = 6). *, P < 0.05; and ***, P < 0.001 vs. basal values.
Table 1. Maximal changes and durations of MABP and HR after i.c.v. injection of RB150 (100 μg).
| MABP, mmHg
|
HR, bpm
|
||||||
|---|---|---|---|---|---|---|---|
| Rats | Basal | After infusion | Variation | Basal | After infusion | Variation | Duration, min |
| WKY | 98.7 ± 2.1 | 97.0 ± 3.0 | -1.7 ± 4.7 | 273.2 ± 0.8 | 286.4 ± 5.6 | 13.2 ± 5.0 | 36.7 ± 20.8 |
| Sham | 110.0 ± 8.3 | 109.5 ± 8.3 | -0.5 ± 1.9 | 301.9 ± 6.3 | 305.8 ± 11.5 | 3.8 ± 8.5 | 34.0 ± 27.1 |
| DOCA | 154.4 ± 7.6* | 121.3 ± 9.5** | -33.1 ± 1.9*** | 399.1 ± 14.9** | 392.0 ± 2.3* | -7.1 ± 16.9 | 56.0 ± 12.8 |
, P < 0.001 vs. WKY and sham-operated rats
, P < 0.01 vs. WKY and P < 0.05 vs. sham-operated rats
, P < 0.01 vs. WKY and P < 0.001 vs. sham-operated rats
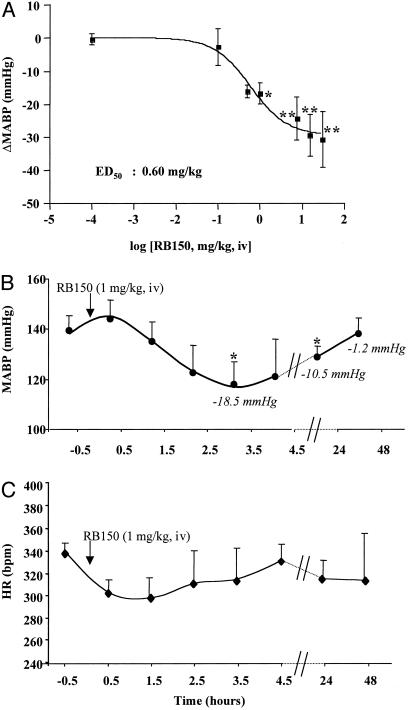
Effects of i.v. Injection of EC33 and RB150 on Arterial Blood Pressure in Conscious DOCA-Salt Rats. MABP and HR were 145.3 ± 4.4 mmHg and 348.6 ± 9.5 bpm, respectively, in DOCA-salt rats and 115.8 ± 4.6 mmHg and 313.2 ± 3.7 bpm, respectively, in sham-operated rats. Intravenous injection of EC33 (10 mg/kg, corresponding to 41 μmol/kg) did not modify BP or HR in conscious DOCA-salt rats (ΔMABP = -6.0 ± 5.6 mmHg and ΔHR = -4.0 ± 11.7 bpm, n = 5). We next verified that RB150 given i.v. can cross the blood–brain barrier and decrease BP. RB150 (0.1–30 mg/kg, i.v.) decreased arterial BP in a dose-dependent manner in conscious DOCA-salt rats with an ED50 of 0.6 mg/kg (Fig. 4A) but did not modify HR (data not shown). The minimal dose of RB150 required to normalize BP (ΔMABP = -29.4 ± 6.3 mmHg) was 15 mg/kg (41 μmol/kg). Given the degree of penetration of RB150 in the brain (1.8%), this dose corresponds to the presence of 221 nmol of this compound, a concentration that blocks brain APA activity and the formation of brain AngIII by 85–90%. The hypotensive effect induced by RB150 (1 mg/kg, i.v.) appeared 1 h and 30 min after the injection, was maximal at 3 h and 30 min, remained significantly reduced after 24 h, and disappeared after 48 h (ΔMABP = -1.2 ± 6.7 mmHg) (Fig. 4B). This dose of RB150 did not significantly affect HR in DOCA-salt rats (Fig. 4C). No effects on BP or HR were observed in conscious sham-operated rats, whatever the dose of RB150 used (data not shown).
Fig. 4.
Effect of RB150 on MABP in conscious DOCA-salt rats. (A) Mean ± SEM variation in MABP after i.v. injection of RB150 (0.1, 0.5, 1.0, 7.5, 15.0, and 30.0 mg/kg) in conscious DOCA-salt rats (n = 5 for each dose). *, P < 0.05; and **, P < 0.01 vs. variation in MABP values obtained with the same i.v. dose of RB150 in sham rats. (B and C) Time course of mean ± SEM MABP and HR changes after a single i.v. injection of RB150 (1 mg/kg) in conscious DOCA-salt rats. *, P < 0.05 vs. basal values.
Discussion
This study describes a way of acutely normalizing BP in a salt-dependent model of hypertension by blocking the brain RAS activity. This was achieved by designing a systemically active APA inhibitor prodrug, RB150, that is able to cross the blood–brain barrier and block brain APA activity. This prevents brain AngIII formation and decreases BP.
We first demonstrated the role of brain APA in the control of BP by showing that the central injection of EC33 (30–100 μg) into DOCA-salt rats leads to an immediate and total inhibition of brain APA activity, thus blocking in the brain the conversion of AngII to AngIII and producing a hypotensive effect. The decrease in BP was marked as a single central injection of 100 μg of EC33 normalized BP (-40 mmHg) in DOCA-salt rats for 45 min. This acute hypotensive effect was similar to the 7-day central infusion (15–30 μg per day) of an angiotensin I-converting enzyme (ACE) inhibitor, captopril, or an AT1 receptor antagonist, candesartan, required to decrease BP by 20 mmHg in DOCA-salt rats (34, 35). In contrast, a high i.v. dose of EC33 did not change BP, demonstrating that the i.c.v. EC33-induced decrease in BP is not due to a peripheral effect. This finding suggests that brain APA could be a new target for the control of hypertension.
If APA inhibitors are to be used as central antihypertensive agents, they must be able to cross the blood–brain barrier and inhibit brain APA activity when given by systemic route. The central bioavailability of thiol inhibitors of zinc metal-lopeptidases such as neutral endopeptidase 24.11 (NEP) or APN (36) is strongly enhanced by designing prodrugs obtained by dimerizing the compound through disulfide bridge formation. This procedure was achieved with EC33 yielding a systemically active APA inhibitor prodrug, RB150. As the thiol group of RB150 is engaged in a disulfide bridge, it is unable to interact with the zinc atom present in the active site of APA (18, 36, 37). However, in vitro, in the presence of DTT, the thiol inhibitor EC33 is generated from RB150, whereas in vivo the disulfide bridge of the prodrug can be cleaved by brain reductases (36), generating EC33. This explains why equal i.c.v. doses of RB150 and EC33 are similarly efficient at blocking brain APA activity and hypothalamic AngIII formation (Fig. 3 B and C). Furthermore, the i.c.v. injection of RB150 induces an intense and sustained decrease in BP with a similar time course as EC33 in conscious DOCA-salt rats, demonstrating that RB150 is rapidly cleaved to generate EC33 in the brain. In mice and DOCA-salt rats, the rapid decrease in brain APA activity (-50% and -62%, respectively) after the i.v. injection of RB150 demonstrates the ability of RB150 to cross the blood–brain barrier. However, in mice the decrease in brain APA activity was weaker after i.v. injection of RB150 than after i.c.v. injection (-90%). Comparison of the inhibition of brain APA activity obtained after i.v. injection of RB150 (Fig. 3D) with the dose–response inhibition curve obtained after i.c.v. administration of EC33 (Fig. 3A) suggests that ≈1.8% of the prodrug penetrates in the brain. Under these conditions, the final brain concentration of RB150 obtained after its i.v. injection is 5.5-fold weaker than that obtained after its i.c.v. injection. This result can account for the differences in brain APA activity inhibition observed after systemic or central RB150 administration. The inhibition of brain APA activity in DOCA-salt rats after the i.v. administration of 15 mg/kg of RB150 (corresponding to the presence of 220 nmol of the prodrug in the brain) results in a decrease of arterial BP of 30 mmHg equivalent to that observed with the i.c.v. administration of EC33 (150–200 nmol). The hypotensive effect is dose-dependent and long-lasting because at a dose close to the ED50 value, the reduction in BP is still significant 24 h after the administration of the prodrug. Although the amplitude of the hypotensive effect is high, HR is not modified, suggesting that the baroreflex is inhibited by brain AngIII, in agreement with the study of Lin et al. (38).
Our data show that the systemic administration of RB150 blocks brain RAS activity and consequently decreases BP. The brain RAS controls BP via three different mechanisms, as proposed by Phillips (39): an increase in vasopressin release, an increase in sympathetic neuron activity, and a decrease of the baroreflex. Because RAS hyperactivity has been observed in the brains of DOCA-salt rats, resulting in increased vasopressin release (35) and increased sympathetic neuron activity (40), this hyperactivity could account for the high efficiency with which APA inhibitors decreased BP in this model. Finally, the participation of APA to the conversion of AngII into AngIII at the periphery remains unclear. Previous studies (41) with nonspecific and nonselective APA inhibitors such as bestatin and amastatin did not allow for the conclusion that APA is a component of the systemic RAS. However, current experiments of our laboratory, in rats treated with EC33 by i.v. route, showed a very limited blockade of the conversion of AngII into AngIII in the blood circulation (unpublished work) compared to an almost total inhibition after i.c.v. EC33 treatment (22).
This discrepancy can be explained by the following: [nlist]
APA is not the main enzyme responsible for the conversion of AngII into AngIII at the periphery.
Systemic AngIII is more rapidly degraded than brain AngIII. Thus, inhibition of systemic AngIII formation is undetectable even after systemic APA inhibition, explaining the minor role of circulating AngIII in the control of BP despite its high affinity for AT1 receptors.
Alternative degradation pathways of systemic AngII exist other than APA. Such pathways would explain the absence of systemic AngII-induced BP increase after i.v. injection of EC33. In this context, the recent report of Mitsui et al. (42) showing a slight elevation of systolic BP in APA genetically deficient mice cannot be easily explained. However, on one hand, the mechanism by which APA deficiency results in hypertension was not addressed in that paper, and on the other, one must consider that the chronic inactivation of APA achieved in APA-deficient mice cannot be easily compared to an acute blockade of this enzyme as in the present study. Indeed, the total absence of APA during fetal and adult life could elicit compensatory mechanisms leading to this slight hypertensive effect that is not contradictory with our hypothesis that APA plays a major role in the conversion of Ang II to AngIII in the brain, whereas this role appears minor at the periphery.
In conclusion, RB150 is a systemically active prodrug that inhibits the brain but not the systemic RAS activity, normalizing BP several hours after a single injection without changing HR, in an experimental salt-dependent model of hypertension.
Thus, APA inhibitors could constitute a new class of central antihypertensive agents and could be clinically tested as an additional therapy for the treatment of hypertensive patients. This treatment may be particularly beneficial in hypertensive patients with low renin and high vasopressin plasma levels that are resistant to the usual antihypertensive medication (43).
Acknowledgments
We thank Professor J. Menard for critical reading of the manuscript. This research was supported by a grant from Glaxo Smith Kline Laboratories and by the Institut National de la Santé et de la Recherche Médicale and Centre National de la Recherche Scientifique Institutes.
Abbreviations: Ang, angiotensin; APA, aminopeptidase A; APN, aminopeptidase N; BP, blood pressure; HR, heart rate; i.c.v., intracerebroventricular(ly); MABP, mean arterial BP; RAS, renin-angiotensin system.
References
- 1.Levine, C. B., Fahrbach, K. R., Frame, D., Connelly, J. E., Estok, R. P., Stone, L. R. & Ludensky, V. (2003) Clin. Ther. 25, 35-57. [DOI] [PubMed] [Google Scholar]
- 2.Smith, D. H. (2002) Clin. Ther. 24, 1484-1501. [DOI] [PubMed] [Google Scholar]
- 3.Corvol, P. & Plouin, P. F. (2002) Drugs 62, 53-64. [PubMed] [Google Scholar]
- 4.Fried, M. R., Eastlund, T., Christie, B., Mullin, G. T. & Key, N. S. (1996) Transfusion 36, 900-903. [DOI] [PubMed] [Google Scholar]
- 5.Israili, Z. H. & Hall, W. D. (1992) Ann. Intern. Med. 117, 234-242. [DOI] [PubMed] [Google Scholar]
- 6.Owen, H. G. & Brecher, M. E. (1994) Transfusion 34, 891-894. [DOI] [PubMed] [Google Scholar]
- 7.Holm, E. A., Randlov, A. & Strandgaard, S. (1996) Blood Press. 5, 360-362. [DOI] [PubMed] [Google Scholar]
- 8.Saine, D. R. & Ahrens, E. R. (1996) Ann. Intern. Med. 124, 775 (lett.). [DOI] [PubMed] [Google Scholar]
- 9.Johansen, T. L. & Kjaer, A. (2001) BMC Nephrol. 2, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saunders, E. J. & Saunders, J. A. (1990) Health Care Women Int. 11, 423-432. [DOI] [PubMed] [Google Scholar]
- 11.Radevski, I., Skudicky, D., Candy, G., Sathekge, S., Strugo, V. & Sareli, P. (1999) Am. J. Hypertens. 12, 194-203. [DOI] [PubMed] [Google Scholar]
- 12.Ganten, D., Herman, K., Bayer, C., Unger, T. & Lang, R. E. (1983) Science 221, 869-871. [DOI] [PubMed] [Google Scholar]
- 13.Basso, N., Ruiz, P., Mangiarua, E. & Taquini, A. C. (1981) Hypertension 3, II-14-7. [DOI] [PubMed] [Google Scholar]
- 14.Averill, D. B., Matsumura, K., Ganten, D. & Ferrario, C. M. (1996) Hypertension 27, 591-597. [DOI] [PubMed] [Google Scholar]
- 15.Senanayake, P. D., Moriguchi, A., Kumagai, H., Ganten, D., Ferrario, C. M. & Brosnihan, K. B. (1994) Peptides 15, 919-926. [DOI] [PubMed] [Google Scholar]
- 16.Morimoto, S., Cassell, M. D., Beltz, T. G., Johnson, A. K., Davisson, R. L. & Sigmund, C. D. (2001) Circ. Res. 89, 365-372. [DOI] [PubMed] [Google Scholar]
- 17.Davisson, R. L., Yang, G., Beltz, T. G., Cassell, M. D., Johnson, A. K. & Sigmund, C. D. (1998) Circ. Res. 83, 1047-1058. [DOI] [PubMed] [Google Scholar]
- 18.Vazeux, G., Wang, J., Corvol, P. & Llorens-Cortes, C. (1996) J. Biol. Chem. 271, 9069-9074. [DOI] [PubMed] [Google Scholar]
- 19.Wu, Q., Lahti, J. M., Air, G. M., Burrows, P. D. & Cooper, M. D. (1990) Proc. Natl. Acad. Sci. USA 87, 993-997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nanus, D. M., Engelstein, D., Gastl, G. A., Gluck, L., Vidal, M. J., Morrison, M., Finstad, C. L., Bander, N. H. & Albino, A. P. (1993) Proc. Natl. Acad. Sci. USA 90, 7069-7073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li, L., Wang, J. & Cooper, M. D. (1993) Genomics 17, 657-664. [DOI] [PubMed] [Google Scholar]
- 22.Zini, S., Fournie-Zaluski, M.-C., Chauvel, E., Roques, B. P., Corvol, P. & Llorens-Cortes, C. (1996) Proc. Natl. Acad. Sci. USA 93, 11968-11973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fournie-Zaluski, M.-C., Coric, P., Turcaud, S., Bruetschy, L., Lucas, E., Noble, F. & Roques, B. P. (1992) J. Med. Chem. 35, 1259-1266. [DOI] [PubMed] [Google Scholar]
- 24.Chauvel, E. N., Coric, P., Llorens-Cortes, C., Wilk, S., Roques, B. P. & Fournie-Zaluski, M.-C. (1994) J. Med. Chem. 37, 1339-1346. [DOI] [PubMed] [Google Scholar]
- 25.Zini, S., Demassey, Y., Fournie-Zaluski, M.-C., Bischoff, L., Corvol, P., Llorens-Cortes, C. & Sanderson, P. (1998) NeuroReport 9, 825-828. [DOI] [PubMed] [Google Scholar]
- 26.Reaux, A., Fournie-Zaluski, M.-C., David, C., Zini, S., Roques, B. P., Corvol, P. & Llorens-Cortes, C. (1999) Proc. Natl. Acad. Sci. USA 96, 13415-13420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reaux, A., de Mota, N., Zini, S., Cadel, S., Fournie-Zaluski, M.-C., Roques, B. P., Corvol, P. & Llorens-Cortes, C. (1999) Neuroendocrinology 69, 370-376. [DOI] [PubMed] [Google Scholar]
- 28.Morton, J. J., Casals-Stenzel, J., Lever, A. F., Millar, J. A., Riegger, A. J. & Tree, M. (1979) Br. J. Clin. Pharmacol. 7, 233S-241S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iturrioz, X., Vazeux, G., Celerier, J., Corvol, P. & Llorens-Cortes, C. (2000) Biochemistry 39, 3061-3068. [DOI] [PubMed] [Google Scholar]
- 30.Zini, S., Masdehors, P., Lenkei, Z., Fournie-Zaluski, M.-C., Roques, B. P., Corvol, P. & Llorens-Cortes, C. (1997) Neuroscience 78, 1187-1193. [DOI] [PubMed] [Google Scholar]
- 31.Gaudet, E., Blanc, J. & Elghozi, J. L. (1996) Am. J. Physiol. 270, R1265-R1272. [DOI] [PubMed] [Google Scholar]
- 32.Park, C. G. & Leenen, F. H. (2001) J. Korean Med. Sci. 16, 553-557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gutkind, J. S., Kurihara, M. & Saavedra, J. M. (1988) Am. J. Physiol. 255, H646-H650. [DOI] [PubMed] [Google Scholar]
- 34.Itaya, Y., Suzuki, H., Matsukawa, S., Kondo, K. & Saruta, T. (1986) Am. J. Physiol. 251, H261-H268. [DOI] [PubMed] [Google Scholar]
- 35.Nishimura, M., Ohtsuka, K., Sakamoto, M., Nanbu, A., Takahashi, H. & Yoshimura, M. (1998) J. Hypertens. 16, 1175-1185. [PubMed] [Google Scholar]
- 36.Fournie-Zaluski, M.-C., Coric, P., Turcaud, S., Lucas, E., Noble, F., Maldonado, R. & Roques, B. P. (1992) J. Med. Chem. 35, 2473-2481. [DOI] [PubMed] [Google Scholar]
- 37.Rozenfeld, R., Iturrioz, X., Maigret, B. & Llorens-Cortes, C. (2002) J. Biol. Chem. 277, 29242-29252. [DOI] [PubMed] [Google Scholar]
- 38.Lin, K. S., Chan, J. Y. & Chan, S. H. (1997) Am. J. Physiol. 272, H2204-2210. [DOI] [PubMed] [Google Scholar]
- 39.Phillips, M. I. (1987) Annu. Rev. Physiol. 49, 413-435. [DOI] [PubMed] [Google Scholar]
- 40.Masuyama, Y., Tsuda, K., Kuchii, M. & Nishio, I. (1986) J. Hypertens. Suppl. 4, S189-S192. [PubMed] [Google Scholar]
- 41.Ahmad, S. & Ward, P. E. (1990) J. Pharmacol. Exp. Ther. 252, 643-650. [PubMed] [Google Scholar]
- 42.Mitsui, T., Nomura, S., Okada, M., Ohno, Y., Kobayashi, H., Nakashima, Y., Murata, Y., Takeuchi, M., Kuno, N., Nagasaka, T., et al. (2003) Mol. Med. 9, 57-62. [PMC free article] [PubMed] [Google Scholar]
- 43.Bakris, G., Bursztyn, M., Gavras, I., Bresnahan, M. & Gavras, H. (1997) J. Hypertens. 15, 545-550. [DOI] [PubMed] [Google Scholar]