Vps21 plays a role in autophagy in addition to its role in endocytosis. Individual deletions of members of the endocytic Vps21 module, including a GEF and four effectors, result in autophagy defects and accumulation of autophagosomal clusters. Therefore the endocytic Vps21 module regulates autophagy.
Abstract
In autophagy, the double-membrane autophagosome delivers cellular components for their degradation in the lysosome. The conserved Ypt/Rab GTPases regulate all cellular trafficking pathways, including autophagy. These GTPases function in modules that include guanine-nucleotide exchange factor (GEF) activators and downstream effectors. Rab7 and its yeast homologue, Ypt7, in the context of such a module, regulate the fusion of both late endosomes and autophagosomes with the lysosome. In yeast, the Rab5-related Vps21 is known for its role in early- to late-endosome transport. Here we show an additional role for Vps21 in autophagy. First, vps21∆ mutant cells are defective in selective and nonselective autophagy. Second, fluorescence and electron microscopy analyses show that vps21∆ mutant cells accumulate clusters of autophagosomal structures outside the vacuole. Third, cells with mutations in other members of the endocytic Vps21 module, including the GEF Vps9 and factors that function downstream of Vps21, Vac1, CORVET, Pep12, and Vps45, are also defective in autophagy and accumulate clusters of autophagosomes. Finally, Vps21 localizes to PAS. We propose that the endocytic Vps21 module also regulates autophagy. These findings support the idea that the two pathways leading to the lysosome—endocytosis and autophagy—converge through the Vps21 and Ypt7 GTPase modules.
INTRODUCTION
Autophagy is a process in which cellular components are delivered to the lysosome for degradation and reuse of their building blocks. Whereas nonselective autophagy is induced by stress, selective autophagy targets specific cellular components and is important for cell homeostasis. Both selective and nonselective autophagy start with the formation of the double-membrane organelle termed the autophagosome (AP). Autophagosomal formation begins with assembly of the preautophagosomal structure (PAS), proceeds through extension to engulf parts of the cytoplasm, goes through the vague process of maturation, and finally fuses with the lysosome (Nakatogawa et al., 2009; Reggiori and Klionsky, 2013). The origin of the autophagosomal membrane has been a source of ongoing debate. Whereas the endoplasmic reticulum (ER) is considered a major contributor for AP membrane, nearly all other membrane-bound compartments have been implicated too, including mitochondria, Golgi, the plasma membrane (PM), and endosomes (Lamb et al., 2012; Rubinsztein et al., 2012).
A set of conserved proteins, known as Atgs, is specifically required for autophagy (Xie and Klionsky, 2007; Mizushima et al., 2011). Additional factors involved in other cellular processes are also required for autophagy. The latter factors include Ypt/Rab GTPases, which regulate all membrane-trafficking events in eukaryotic cells (Segev, 2001b; Stenmark, 2009). These GTPases function in the context of modules that include activators and effectors. The activators are guanine-nucleotide exchange factors (GEFs) that stimulate the switch of the GTPase from the GDP- to the GTP‑bound form. Once in the active GTP-bound form, the GTPase can interact with multiple downstream effectors, which in turn mediate the membrane-trafficking events (Segev, 2001a; Zerial and McBride, 2001). Recently multiple Ypt/Rabs were implicated in autophagy (Ao et al., 2014). In yeast, a Ypt1 module regulates the first step in autophagy, PAS assembly (Lipatova et al., 2012; Lipatova and Segev, 2012). A Ypt7 module regulates the last step of autophagy—fusion of autophagosomes with the lysosome (Kim et al., 1999; Kirisako et al., 1999; Meiling-Wesse et al., 2002). The established role of Ypt1 and its human functional homologue, Rab1, is in ER-to-Golgi transport (Segev et al., 1988; Pind et al., 1994). The established role of Ypt7 and its human homologue, Rab7, is in the last step of endocytosis—fusion of late endosomes with the lysosome (Epp et al., 2011). Thus autophagy uses components of both the exocytic and endocytic pathways. However, whereas Ypt7 functions in endocytosis and autophagy in the context of the same module, Ypt1 regulates ER-to-Golgi and autophagy through two separate modules (Lipatova et al., 2012; Hyttinen et al., 2013).
Rab5 regulates early steps of the endocytic pathway, and Rab5-to-Rab7 switch was proposed to integrate the different steps of the endocytic pathway (Epp et al., 2011; Solinger and Spang, 2013). In yeast, of the three Rab5-related Ypts—Vps21/Ypt51, Ypt52, and Ypt53—only Vps21 is required for early endocytosis (Singer-Kruger et al., 1994). Endocytic machinery components are not essential for yeast cell viability. Deletion of members of the Vps21 module results in a class D Vps phenotype, which is caused by a defect in endosome maturation and characterized by an enlarged vacuole (the yeast lysosome). In contrast, deletion of members of the Ypt7 module results in a class B Vps phenotype, with a defect in endosome fusion with the lysosome and a fragmented vacuole (Epp et al., 2011). Here we address the question of whether, like Ypt7, the Rab5-related Vps21 plays a role in autophagy in addition to its role in endocytosis, and, if it does, whether it does that in the context of the same module. We show that individual deletions of each member of the endocytic Vps21 module, including a GEF and four different factors that function downstream of Vps21, result in autophagy defects and accumulation of autophagosomal clusters. Our results suggest that the endocytic Vps21 module plays a role in autophagy in a step between AP formation and fusion. We propose that autophagy and endocytosis, which start as two separate pathways originating from different cellular compartments, converge and use common machinery for fusion with the lysosome.
RESULTS
vps21∆ mutant cells are defective in selective and nonselective autophagy
To determine whether the Rab5-related Ypts play a role in autophagy, cells deleted for VPS21, YPT52, and YPT53, alone or in combinations, were tested for their ability to deliver proteins for degradation in the vacuole through the autophagic pathway. Deletion of VPS21 results in a slight growth defect at 37°C (Singer-Kruger et al., 1994); for all experiments done here, cells were grown at 26°C. Nonselective autophagy was tested in cells starved for nitrogen using three established assays: Pho8∆60 alkaline phosphatase (ALP) activity, green fluorescent protein (GFP)–Atg8 processing, and Ape1 processing. Cells deleted for VPS21 exhibit defects in all three assays (Figure 1). vps21∆ mutant cells also exhibit a defect in processing of Ape1 in rich medium (yeast extract/peptone/dextrose [YPD]; Figure 1C, top), showing that these mutant cells are defective in cytoplasm-to-vacuole targeting, a selective autophagy pathway, in addition to nonselective autophagy. Whereas vps21∆ mutant cells exhibit ∼90% block in selective autophagy, they show 50–70% block in the nonselective autophagy. Both GFP-Atg8 and Ape1 processing defects of vps21∆ mutant cells can be complemented by expression of Vps21 from a plasmid (Figure 1, B and C).
FIGURE 1:
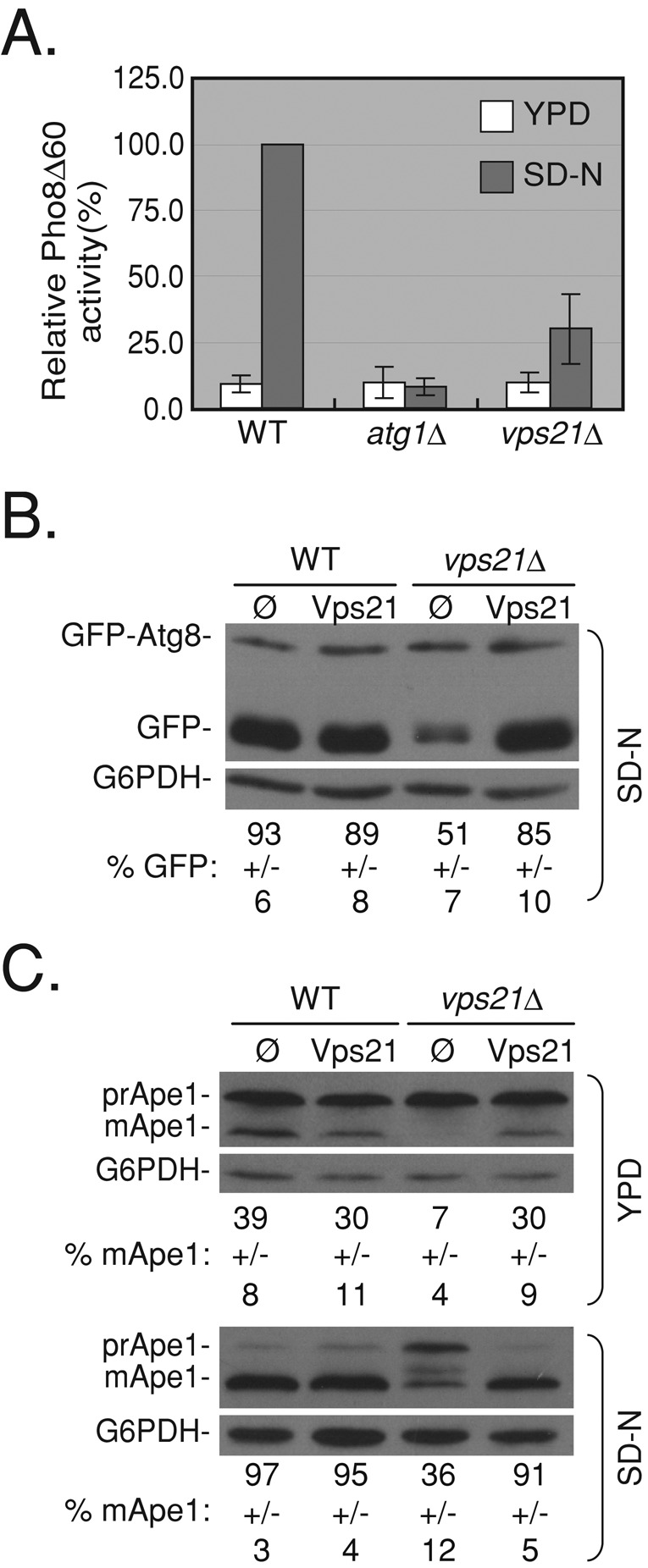
vps21∆ mutant cells are defective in selective and nonselective autophagy. (A) vps21∆ mutant cells are defective in nonselective autophagy measured by Pho8∆60 alkaline phosphatase (ALP) activity. ALP activity was determined in lysates of wild-type, atg1∆ (as a negative control), and vps21∆ mutant cells grown in rich medium (YPD, white bars) or starved for nitrogen (SD-N, gray bars). Vps21∆ mutant cells exhibit an autophagy defect under starvation when compared with wild-type cells (p < 0.001). (B) Vps21 suppresses the GFP-Atg8–processing defect of vps21∆ mutant cells under nitrogen starvation. GFP-Atg8 was integrated into the genome of wild-type and vps21∆ mutant cells. Cells transformed with a 2 μ plasmid for overexpression of Vps21 (empty plasmid [ø] as negative control) were grown in rich medium and shifted to SD‑N medium. GFP-Atg8 processing was determined in cell lysates using immunoblot analysis with anti-GFP antibodies (G6PDH serves as a loading control). In wild-type cells or in vps21∆ mutant cells expressing Vps21 from a plasmid, most of the GFP-Atg8 is processed to GFP. In vps21∆ mutant cells this processing is defective (p < 0.0001). (C) Vps21 suppresses the Ape1-processing defect of vps21∆ mutant cells grown in either rich or starvation medium. The experiment was done as described in B, except that Ape1 processing was determined using anti-Ape1 antibodies. In wild-type or vps21∆ mutant cells expressing Vps21 from a plasmid, premature Ape1 (prApe1) is processed to mature Ape1 (mApe1) in rich and starvation medium. In vps21∆ mutant cells, this processing is defective (p < 0.0008). Error bars and ± represent standard deviation (STD). Results represent at least three independent experiments.
GFP-Atg8 processing was also tested in vps21ts mutant cells (Gerrard et al., 2000). Whereas at the permissive temperature (26°C), vps21ts mutant cells process GFP-Atg8 like wild-type cells, when shifted to their nonpermissive temperature (37°C), they exhibit a defect in GFP-Atg8 processing (Supplemental Figure S1A). This result supports the idea that Vps21 plays a role in autophagy.
Cells deleted for the two other Rab5-related Ypts, YPT52 and YPT53, do not exhibit defects in selective and nonselective autophagy (Supplemental Figure S2, A–C). Deletion of YPT53 in combination with VPS21 does not cause enhancement of the VPS21 deletion phenotype. Of interest, cells deleted for both VPS21 and YPT52 exhibit a more severe GFP-Atg8 processing defect under starvation than that of the single VPS21 deletion (Supplemental Figure S2B). This result is in agreement with the more severe Ape1 processing defect observed in the vps21∆ ypt52∆ double mutant compared with the vps21∆ single-mutant phenotype (Nickerson et al., 2012; Supplemental Figure S2B). It suggests that Ypt52 can partially complement the function of Vps21 in autophagy. On the basis of the defects exhibited by vps21∆ mutant cells shown in the foregoing, we conclude that proper function of Vps21 is required for selective and nonselective autophagy.
vps21∆ mutant cells accumulate clusters of autophagosomes outside their vacuoles
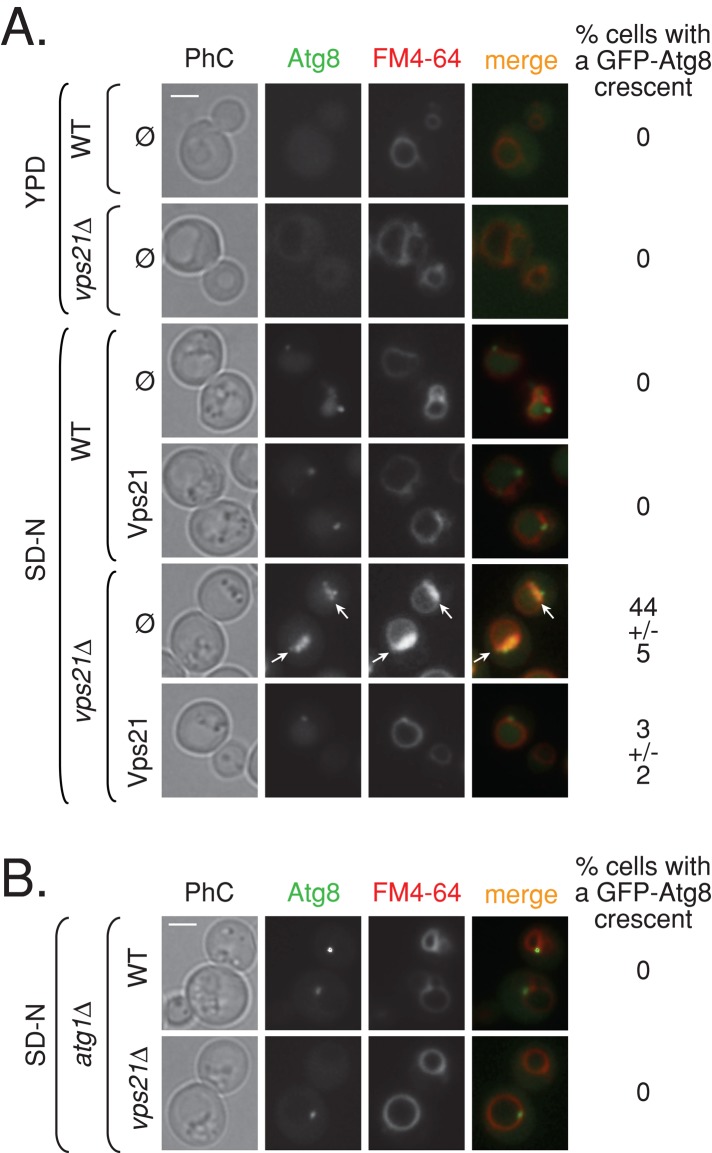
To gain insight into the autophagic step blocked in vps21∆ mutant cells under starvation, we determined the vacuolar morphology and localization of GFP-Atg8 by live-cell fluorescence microscopy. GFP-Atg8 that resides on the inner membrane of APs is delivered to the vacuole after the AP and vacuole fuse, where it is degraded (Kirisako et al., 1999; Huang et al., 2000). When grown in rich medium (YPD), GFP-Atg8 is diffuse, with an occasional dot near the vacuole of both wild-type and vps21∆ mutant cells. Under starvation (synthetic defined medium that lacks nitrogen and amino acid [SD-N]), wild-type cells generally contain one or two dots per cell of GFP-Atg8 near the vacuole representing the AP, and a substantial amount is delivered to the vacuole for degradation (as indicated by GFP fluorescence observed inside the vacuoles). In vps21∆ mutant cells starved for nitrogen, some cells contain green fluorescence in their vacuoles (Figure 2A), which is in agreement with the ∼50% processed GFP found in these cells (Figure 1B). Of importance, after 4 h of nitrogen starvation, ∼45% of the vps21∆ mutant cells accumulate GFP-Atg8 in crescent-like structures near the vacuole (stained by FM4-64). This GFP-Atg8 accumulation phenotype of vps21∆ mutant cells occurs only under starvation and can be complemented by expression of Vps21 from a plasmid (Figure 2A). A similar phenotype was observed in vps21ts mutant cells at 37°C (Supplemental Figure S1B). Analysis of the two other Rab5-related genes, YPT52 and YPT53, revealed that in cells deleted for these genes, the GFP-Atg8 pattern is similar to that seen in wild- type cells (Supplemental Figure S2D).
FIGURE 2:
Atg1-dependent accumulation of GFP-Atg8 crescent-like structures in vps21∆ mutant cells. (A) Vps21 suppresses accumulation of GFP-Atg8 crescents in vps21∆ mutant cells under nitrogen starvation. Wild-type and vps21∆ mutant cells expressing GFP-Atg8 were transformed with a plasmid for expression of Vps21 (as in Figure 1B). The cells were shifted from rich (YPD, top) to starvation medium (SD-N, bottom), their vacuoles were stained with FM4-64, and they were analyzed by live-cell fluorescence microscopy. In wild-type and vps21∆ mutant cells grown in rich medium (YPD), GFP-Atg8 is diffuse and occasionally localizes to a single dot next to the vacuole. In wild-type cells starved for nitrogen (SD-N), GFP-Atg8 localizes inside the vacuole membrane, which is marked by FM4-64, and to a single dot of the AP per cell. However, whereas GFP-Atg8 can be seen in the vacuole of some vps21∆ mutant cells under nitrogen starvation, in ∼45% of these cells, GFP-Atg8 accumulates in crescent-like structures, which overlaps with the FM4-64 signal. Expression of Vps21 in mutant cells suppresses this accumulation (number of cells visualized for each strain: >300 in YPD; >800 in SD-N). Left to right, PhC, GFP-Atg8, FM4-64, merge, and percentage of cells with a GFP-Atg8 crescent. Arrows point to GFP-Atg8 crescents in vps21∆ mutant cells. (B) Accumulation of GFP-Atg8 crescents in vps21∆ mutant cells depends on Atg1. ATG1 was deleted in wild-type and vps21∆ mutant cells. GFP-Atg8 accumulation and vacuolar morphology were determined as described in A. In atg1∆ mutant cells, GFP-Atg8 accumulates in a single dot outside the vacuole, and crescent-like structures of GFP-Atg8 were not observed in the atg1∆ vps21∆ double-mutant cells (number of cells visualized for each strain, >500). Bar, 2 μm. Results represent two independent experiments.
To ensure that the crescent-like GFP-Atg8 structures accumulating in vps21∆ mutant cells represent an autophagy defect, we used a double-mutant epistasis analysis. Atg1 is required for AP function but not for the assembly of other PAS components (Xie and Klionsky, 2007; Mizushima et al., 2011). In cells deleted for ATG1, GFP-Atg8 is not delivered to the vacuole and accumulates in a dot next to the vacuole, distinguished from the crescents seen in vps21∆ mutant cells. In atg1∆ vps21∆ double-mutant cells, GFP-Atg8 crescents do not accumulate (Figure 2B). This result shows that accumulation of GFP-Atg8 crescent-like structures in vps21∆ mutant cells is dependent on the function of Atg1 and supports the idea that these structures represent APs.
To test the idea that the crescent GFP-Atg8 structures that accumulate in vps21∆ mutant cells represent multiple autophagosomes, we visualized the dynamics of GFP-Atg8 accumulation using time-lapse live microscopy for 2 h after cells were shifted to medium without nitrogen. In wild-type cells, one or two bright dots can be seen, but these dots are very dynamic and appear and disappear within minutes. In contrast, bright GFP-Atg8 dots that appear ∼20 min after the shift do not disappear, and new dots appear and stay for the duration of the experiment (Figure 3 and Supplemental Movies S1 and S2). This result suggests that crescent-like structures observed in vps21∆ mutant cells after starvation represent clusters of APs marked with GFP-Atg8.
FIGURE 3:
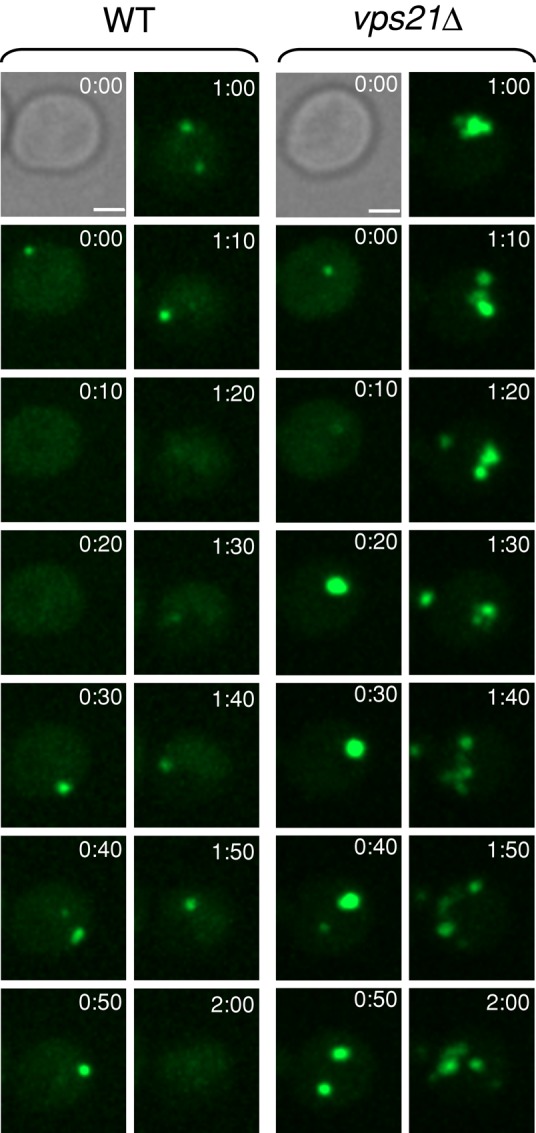
Accumulation of multiple autophagosomes in vps21∆ mutant cells. The dynamics of autophagosomes marked with GFP-Atg8 was determined in wild-type and vps21∆ mutant cells using time-lapse live microscopy. Sequential frames of GFP-Atg8 dots (in 10-min intervals) are shown, with differential interference contrast at time 0. WT and vps21∆ mutant cells were grown in YPD medium until log phase and shifted to SD-N medium for 5 min before the cells were sampled on 2% agar in SD-N for observation by time-lapse video microscopy. Bar, 2 μm. See videos of GFP-Atg8 in WT (Supplemental Movie S1) and GFP-Atg8 in vps21∆ mutant cells (Supplemental Movie S2) for 20-s intervals, playing at 12 frames/s (fps). Results represent three independent experiments.
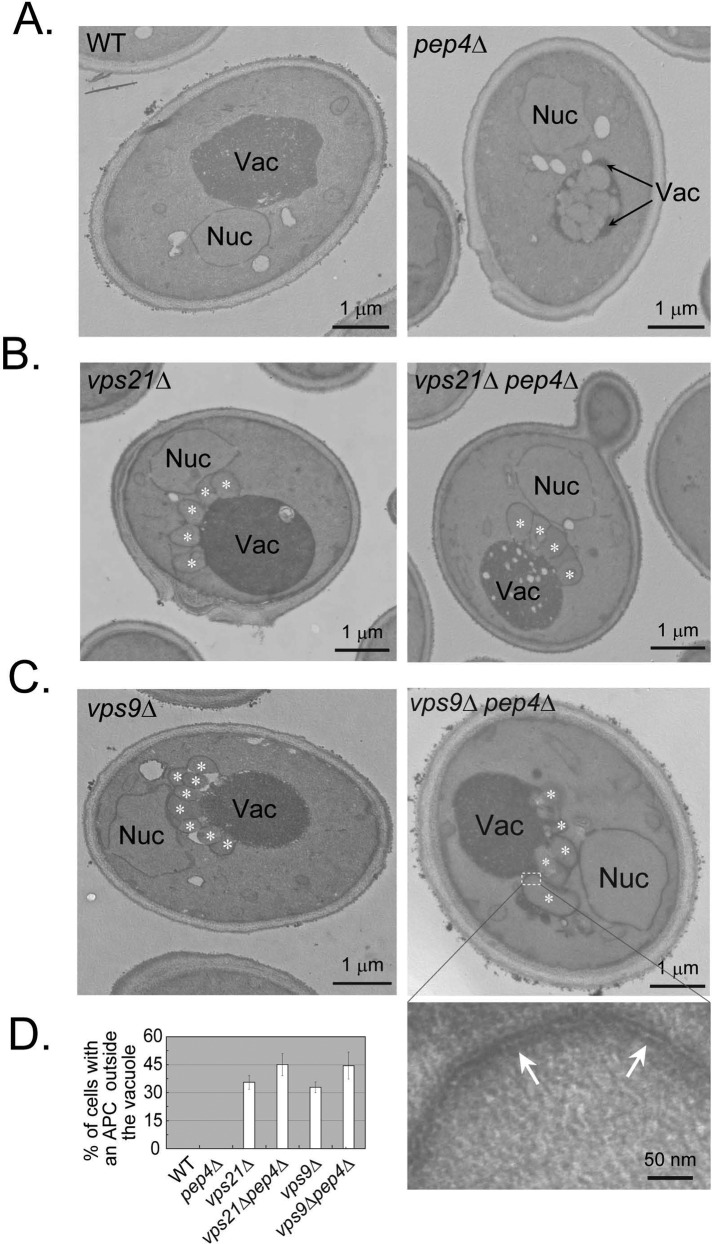
To confirm that multiple APs accumulate in vps21∆ mutant cells under starvation, we determined the ultrastructure of cells using transmission electron microscopy. In wild-type cells, even under starvation, APs do not accumulate because they are cleared in the vacuole. Accumulation of undegraded APs, or AP bodies, can be observed inside the vacuoles of nitrogen-starved pep4∆ mutant cells, which are defective in vacuolar degradation (Figure 4A; Takeshige et al., 1992). Of importance, under nitrogen starvation, clusters of autophagosomes were observed outside the vacuole in ∼40% of the vps21∆ mutant cell sections, with an average of ≥3 APs/cluster. These structures represent autophagosomes because, as previously shown for ypt7∆ mutant cells (Kirisako et al., 1999), they are surrounded by a double membrane that can be seen in a higher magnification (see examples in Figure 4C, bottom, and Supplemental Figure S4). Autophagosomal clusters (APCs) are present in vps21∆ mutant cells that are either PEP4 or pep4∆, supporting the idea that this accumulation is caused by a defect in a step preceding AP fusion with the vacuole, in which the Pep4 protease functions (Figure 4, B and D).
FIGURE 4:
Pep4-independent accumulation of autophagosomal clusters in vps21∆ and vps9∆ mutant cells. The ultrastructure of cells after a 2-h shift to starvation medium was determined by electron microscopy. (A–C) Representative cells. (A) Deletion of PEP4 results in accumulation of autophagosomal bodies inside the vacuole of VPS21 wild-type cells. (B) APCs appear outside the vacuole of both vps21∆ and vps21∆ pep4∆ mutant cells. (C) APCs appear outside the vacuole of both vps9∆ and vps9∆ pep4∆ mutant cells. Bar, 1 μm. Nuc, nucleus; Vac, vacuole; white asterisks mark individual APs in a cluster. Bottom, right: a portion of the vps9∆ pep4∆ double-mutant cell in higher magnification (bar, 50 nm); white arrows point to double membrane of an autophagosome. Double membrane was observed in higher magnification around autophagosomes in the four strains shown in B and C (at least 20 cells observed for each strain). (D) Quantification of results shown in A–C. Between 30 and 45% of the vps21∆ and vps9∆ mutant cell slices, PEP4 or pep4∆, contain APCs. Cell slices with ≥2 neighboring APs were scored as containing an APC, with average number of 3.15 ± 0.54; ≥500 cells were visualized for each strain; error bars represent STD. Results represent three independent experiments.
Accumulation of dispersed APs in the cytoplasm of mutant cells defective in the function of Ypt7 (Kirisako et al., 1999), its GEF, and effectors was previously shown (Noda et al., 2009). The dispersed GFP-Atg8 pattern observed in ypt7∆ and vam3∆ mutant cells is different from the crescents observed in vps21∆ mutant cells (Supplemental Figure S3). It is possible that the difference in AP accumulation pattern between vps21∆ and ypt7∆ mutant cells is due to the fact that in ypt7∆ (and vam3∆) mutant cells the vacuole is fragmented (Haas et al., 1995; Wada et al., 1997; Supplemental Figure S3, FM4-64). Therefore the pattern of AP accumulation cannot be used in a meaningful epistasis analysis of vps21∆ and ypt7∆.
Together fluorescence and electron microscopy analyses show that APCs are present in the cytoplasm of vps21∆ mutant cells next to the vacuole.
Deletion of Vps9, the endocytic Vps21 GEF, results in autophagy defects and APC appearance
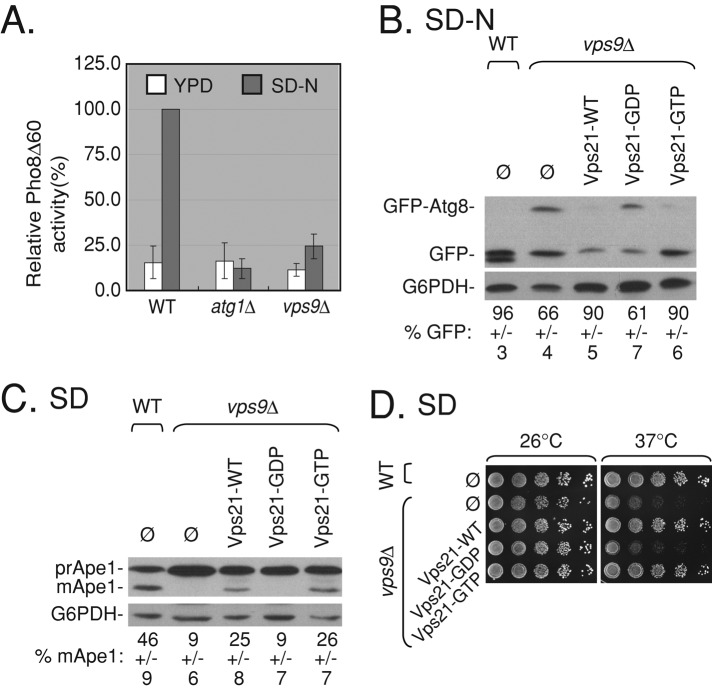
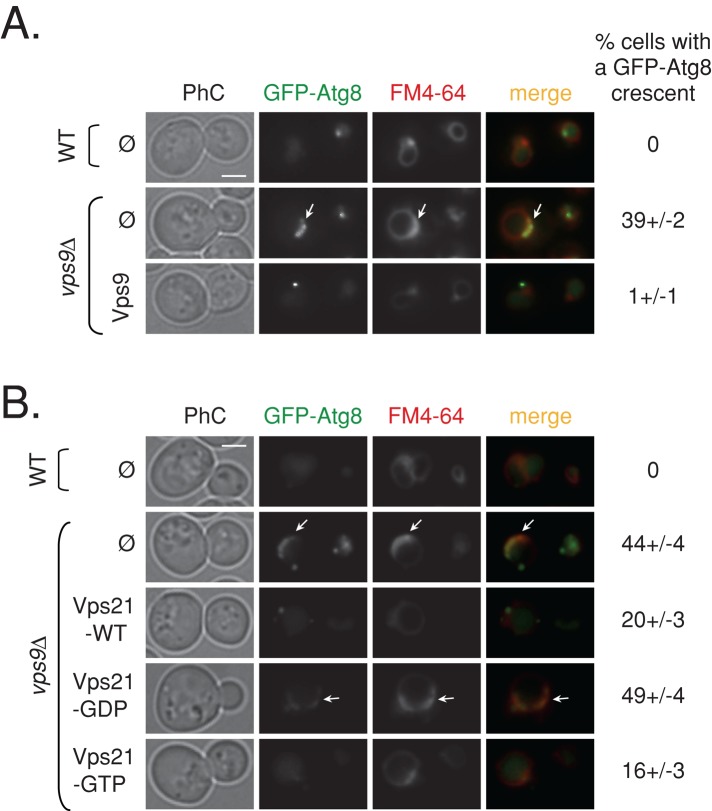
Vps9 is the GEF that activates Vps21 in endocytosis (Hama et al., 1999). To determine whether Vps9 also activates Vps21 in autophagy, we first determined whether vps9∆ mutant cells exhibit defects in autophagy. Assays described earlier showed that vps9∆ mutant cells exhibit defects in selective and nonselective autophagy similar to those observed for vps21∆ mutant cells (Figure 5, A–C, and Supplemental Figure S5, B and C). Moreover, fluorescence and electron microscopy analyses show that, like vps21∆ mutant cells, ∼40% of the vps9∆ mutant cells contain APCs outside their vacuoles under starvation (Figures 6A and 4, C and D, respectively). Remarkably, the autophagic defects displayed by vps9∆ mutant cells are very similar to those of vps21∆ mutant cells.
FIGURE 5:
Autophagic phenotypes of vps9∆ mutant cells can be suppressed by active Vps21. (A) vps9∆ mutant cells are defective in nonselective autophagy measured by ALP activity. ALP activity was determined as described in Figure 1A in wild-type, atg1∆ (as a negative control), and vps9∆ mutant cells. Vps9∆ mutant cells exhibit a 75% defect (p < 0.0005). (B) The GFP-Atg8 processing defect of vps9∆ mutant cells is suppressed by expression of the Vps21 wild-type or the putative GTP-locked mutant proteins but not the putative GDP-locked mutant protein. GFP-Atg8 processing was determined as described in Figure 1B in wild-type and vps9∆ mutant cells transformed with empty plasmid (ø) or plasmids for overexpression of wild-type Vps21 (Vps21-WT), Vps21-S21N (putative GDP-locked Vps21 mutant protein), or Vps21-Q66L (putative GTP-locked Vps21 mutant). Vps9∆ mutant cells (ø or putative GDP-locked Vps21) exhibit a defect in GFP-Atg8 processing compared with wild-type cells (p < 0.002). (C) The Ape1 processing defect of vps9∆ mutant cells is partially suppressed by expression of wild type or the putative GTP-locked Vps21 mutant, but not the putative GDP-locked mutant. Ape1 processing was determined (as in Figure 1C, top) in wild-type and vps9∆ mutant cells transformed with plasmids for Vps21 expression (as in B). Vps9∆ mutant cells (ø or putative Vps21-GDP locked) exhibit a defect in Ape1 processing compared with wild-type cells (p < 0.005). (D) The growth defect of vps9∆ mutant cells is suppressed by expression of wild-type or GTP-locked, but not the putative GDP-locked, Vps21. The growth of wild-type and vps9∆ mutant cells transformed with plasmids for Vps21 expression (as in B) was determined on SD plates at 26 and 37°C (1/10 serial dilution from left to right). Vps9∆ mutant cells (ø or the putative GDP-locked Vps21) exhibit a growth defect at 37°C when compared with wild-type cells or vps9∆ mutant cells expressing Vps21-WT or Vps21-GTP. Error bars and ± represent STD. Results represent three independent experiments.
FIGURE 6:
Accumulation of GFP-Atg8 crescents in vps9∆ mutant cells can be suppressed by active Vps21. (A) Vps9 suppresses accumulation of GFP-Atg8 crescents in vps9∆ mutant cells. GFP-Atg8 was integrated into the genome of wild-type and vps9∆ mutant cells. Cells transformed with a 2 μ plasmid for overexpression of Vps9 (or empty plasmid [ø] as a negative control) were analyzed for GFP-Atg8 pattern and vacuolar morphology as described for Figure 2. About 40% of the vps9∆ mutant cells accumulate GFP-Atg8 in crescents that overlap with the vacuole rim, and this phenotype is suppressed by overexpression of Vps9 from a plasmid (number of cells visualized for each strain, >450). (B) Active Vps21 partially suppresses the accumulation of GFP-Atg8 crescents in vps9∆ mutant cells. The experiment was done as in A, except that vps9∆ mutant cells were transformed with plasmids for overexpression of the different nucleotide-locked forms of Vps21 (as in Figure 5, B–D). Fewer vps9∆ mutant cells accumulate GFP-Atg8 crescents when Vps21-WT and the GTP-locked Vps21 mutant protein, but not the putative GDP-locked Vps21, are overexpressed (<20% and >40%, respectively; number of cells visualized for each strain, >650). Arrows point to GFP-Atg8 crescents; bar, 2 μm; ± represents STD. Results represent two independent experiments.
If the autophagic defects exhibited by vps9∆ mutant cells are caused by the inability to activate Vps21, it is expected that expression of active Vps21 in these cells would suppress these phenotypes. Wild-type Vps21 protein or mutants locked in the GTP- or GDP-bound configuration were expressed in vps9∆ mutant cells. Expression of the wild-type or the putative GTP-locked (“active”) mutant form can suppress the temperature-sensitive growth and the selective autophagy defects of vps9∆ mutant cells (Figure 5, C and D). Expression of active Vps21 proteins also partially suppressed the nonselective autophagy phenotype and APC appearance in vps9∆ mutant cells (Figures 5B and 6B). In contrast, expression of the putative GDP-locked (“inactive”) mutant form of Vps21 does not suppress the growth, autophagy, and APC appearance phenotypes of vps9∆ mutant cells (Figures 5 and 6 and Supplemental Figure S5). Together these results support the idea that Vps9, as in endocytosis, acts as the GEF for Vps21 in autophagy.
Recently Muk1, which contains a Vps9 domain, was shown to act as a second GEF for the three yeast Rab5-related Ypts—Vps21, Ypt52 and Ypt53. In vivo, Vps9 and Muk1 are partially redundant (Cabrera et al., 2013; Paulsel et al., 2013). The possibility that Muk1 plays a role in autophagy was tested using three assays: GFP-Atg8 and Ape1 processing and GFP-Atg8 intracellular localization. In all three assays, muk1∆ mutant cells do not exhibit a defect in autophagy (Supplemental Figure S5 and S6). Therefore Muk1 is not required for autophagy. However, deletion of MUK1 in vps9∆ mutant cells results in more-severe growth and autophagy phenotypes compared with those of vps9∆ mutant cells. Specifically, in vps9∆ muk1∆ double-mutant cells, GFP-Atg8 processing is ∼10% (∼60% in vps9∆), Ape1 maturation is ∼6% (20% in vps9∆), and GFP-Atg8 crescents accumulate in 75% of the cells (∼45% in vps9∆; Supplemental Figures S5 and S6). Thus, as in endocytosis (Paulsel et al., 2013), the functions of Muk1 and Vps9 in autophagy are partially redundant.
Of interest, the vps9∆ muk1∆ double-mutant autophagic phenotypes are more severe than those of the vps21∆ mutant cells—for example, 75 and 45% of cells with APC accumulation, respectively. This might mean that Vps9 and Muk1 play an additional role in autophagy independent of Vps21 activation.
In addition, whereas the growth and autophagic phenotypes of vps9∆ mutant cells can be suppressed by overexpression of active Vps21 (wild type or the putative GTP-locked form), those of vps9∆ muk1∆ double-mutant cells cannot (Supplemental Figures S5 and S6). We interpret the ability of overexpressed wild-type Vps21 to suppress deletion of the single GEF but not the double GEF to mean that, as in endocytosis (Cabrera et al., 2013; Paulsel et al., 2013), at least one of these GEFs is required for the function of Vps21 in autophagy. The inability of the Vps21-QL mutant to suppress the double GEF deletion might mean that this mutant is not locked in the GTP-bound configuration (Langemeyer et al., 2014). Alternatively, this inability might uncover another GEF function in addition to nucleotide exchange—for example, stable localization of Vps21 to the right location.
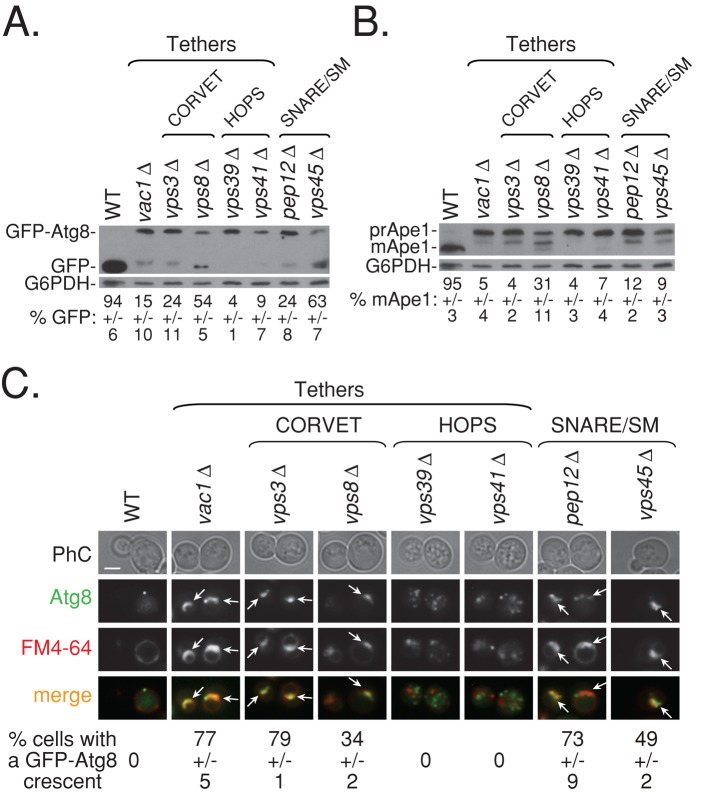
Deletion of endocytic proteins that function downstream of Vps21 also results in autophagy defects and APC appearance
To accomplish its role in endocytosis, activated Vps21 recruits a group of factors that include the class C core vacuole/endosome tethering complex (CORVET), the tether Vac1, the soluble N-ethylmaleimide–sensitive factor attachment protein receptor (SNARE) Pep12, and the Sec1/Munc18 (SM) protein Vps45. Mutations in genes encoding all of these proteins, grouped together with Vps21 as class D Vps, cause endocytic phenotypes similar to those of vps21∆ mutant cells (Stack et al., 1995; Tall et al., 1999; Epp et al., 2011). To determine whether Vps21 recruits the same group of factors in autophagy, we determined the effect of mutations in their genes on GFP-Atg8 and Ape1 processing and GFP-Atg8 localization. The Vps21-specific tethering complex CORVET shares four subunits with homotypic fusion and vacuole protein sorting complex (HOPS), a Ypt7 effector. However, each complex has two specific subunits: Vps3 and Vps8 are specific for CORVET, and Vps39 and Vps41 are specific for HOPS (Epp et al., 2011). Mutations in all of the endocytic Vps21 downstream factors and the HOPS subunits cause defects in GFP-Atg8 and Ape1 processing under starvation (Figure 7, A and B). However, only mutations in the Vps21 downstream factors, but not the HOPS-specific subunits Vps39 and Vps41, result in appearance of APCs under starvation (Figure 7C). Thus Vps21, together with its downstream endocytic factors, plays a role in autophagy. Of interest, deletion of three Vps21 downstream factors—Vps3, Vac1, and Pep12—results in autophagy defects and APC appearance that are more severe than those exhibited by vps21∆ mutant cells (Figure 7).
FIGURE 7:
Cells deleted for factors that function downstream of Vps21 exhibit autophagic phenotypes similar to or greater than those of vps21∆ mutant cells. Cells were deleted for CORVET-specific components, Vps3 and Vps8; HOPS-specific components, Vps39 and Vps41; and other class D Vps members, Pep12, Vac1, and Vps45. Wild-type and mutant cells were shifted to SD-N medium and their autophagic phenotypes, GFP-Atg8 processing (A), Ape1 processing (B), and GFP-Atg8 accumulation (C), were determined as described in the legends to Figures 1 and 2. All mutant cells are defective in GFP-Atg8 (A) and Ape1 (B) processing (same blot was probed with the antibodies sequentially), with vps8∆ and vps45∆ mutant cells showing the weakest phenotypes (p < 0.04 when compared with wild type). (C) Mutant cells deleted for Vps21-related factors, Vps3, Vps8, Vac1, and Vps45, and the SNARE Pep12, but not Ypt7-related factors, Vps39 and Vps41, accumulate GFP-Atg8 crescents (>70% for vps3∆, pep12∆, and vac1∆, and ∼35% for vps8∆ and vps45∆; number of cells visualized for each strain, >700 cells). ± represents STD; bar, 2 μm; arrows point to GFP-Atg8 crescents. Results represent three independent experiments.
Vps21 localizes to PAS
Vps21 is a marker for endosomes (Epp et al., 2011). If Vps21 plays a role in autophagy, it is expected to localize also to autophagosomes. To test this idea, we determined the colocalization of fluorescently tagged Vps21 with two AP markers, Ape1 and Atg8. Vps21 tagged at its N-terminus with either GFP or red fluorescent protein (RFP) was expressed from the chromosome (as the only copy) or from a plasmid (under the PHO5 promoter), respectively. GFP-Vps21 is functional because the tagging did not result in temperature-sensitive growth or autophagic (Ape1 delivery to the vacuole) phenotypes characteristic of vps21∆ mutant cells. Similarly, RFP-Vps21 is functional based on its ability to suppress the temperature-sensitive growth and autophagic (Atg8 delivery to the vacuole) phenotypes of vps21∆ mutant cells (Supplemental Figure S7).
Appearance of Ape1 and Atg8 in a single AP dot occurs in a fraction of wild-type cells. In ∼15% of wild-type cells with an AP dot, Vps21 colocalizes with these autophagosomal markers (Figure 8, A and B). The relatively low colocalization of Vps21 with AP markers can be attributed to the fact that Vps21 is present on APs transiently. If true, an increased level of colocalization should be seen in cells that accumulate APs, especially if the Vps21-mediated step is blocked. Therefore we tested the colocalization of Vps21 with Ape1 and Atg8 in pep12∆ mutant cells. Because the Pep12 functions as a SNARE in the Vps21-mediated step, the Vps21-mediated step is blocked in pep12∆ mutant cells. An increase of colocalization of Vps21 with Ape1 is seen in pep12∆ mutant cells (Figure 8A). Moreover, accumulation of GFP-Atg8 crescents in >70% of pep12∆ mutant cells (Figure 7C) facilitates observation of the colocalization of Vps21 with GFP-Atg8. Indeed, under starvation, Vps21 colocalizes with dots or crescents of GFP-Atg8 in >40% of the pep12∆ mutant cells (Figure 8B). The colocalization of Vps21 with two autophagosomal markers supports the idea that Vps21 plays a role in autophagy.
FIGURE 8:
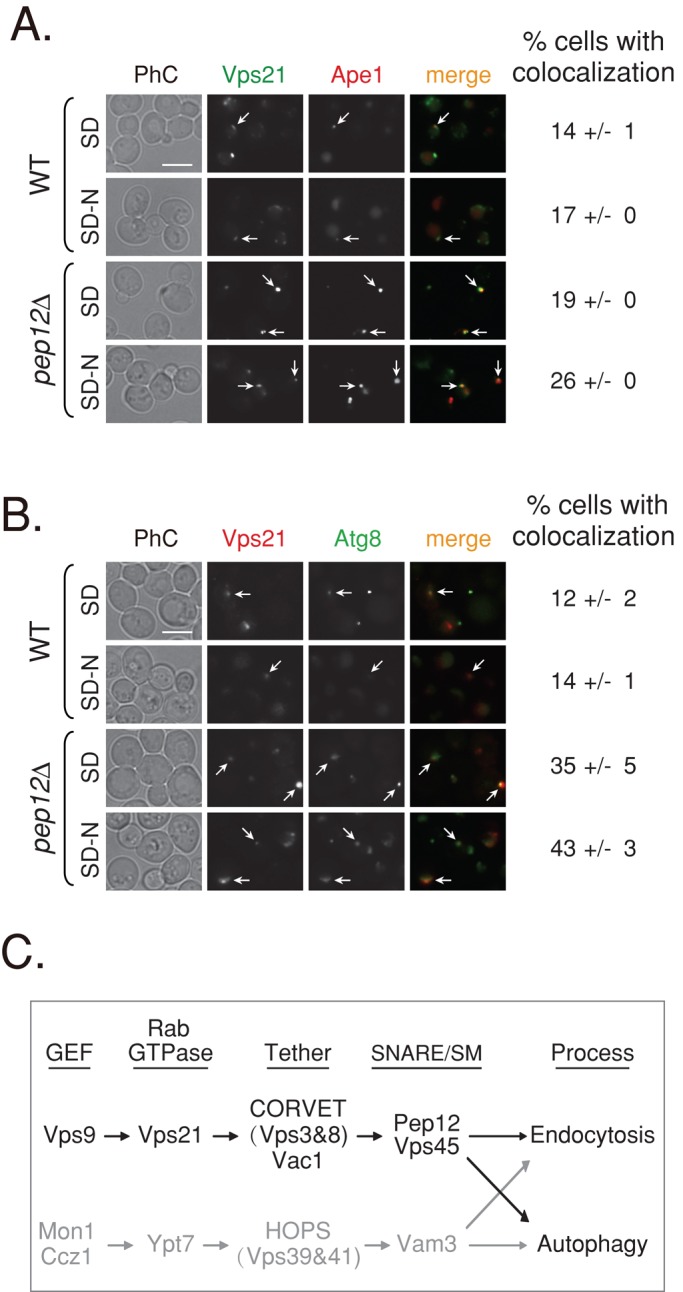
Vps21 colocalizes with autophagosomal markers. (A) Colocalization of GFP-Vps21 with RFP-Ape1 in wild-type and pep12∆ mutant cells. GFP-Vps21 and RFP-Ape1 were integrated into the genome of wild-type (top) and pep12∆ mutant (bottom) cells. Cells grown in SD or shifted to SD-N were visualized by live-cell fluorescence microscopy. Left to right, GFP-Vps21, RFP-Ape1, merge, and percentage of cells with colocalization. In wild-type cells, Ape1 is found inside the vacuole (Shintani et al., 2002) and localizes to a single dot of the AP. In pep12∆ mutant cells, Ape1 accumulates outside the vacuole in APs. Vps21 accumulates in multiple dots or a cluster per cell, and in 14–26% of the cells, one of the Vps21 dots colocalizes with Ape1. (B) Colocalization of RFP-Vps21 with GFP-Atg8 in wild-type and pep12∆ mutant cells. GFP-Atg8 was integrated into the genome of wild-type (top) and pep12∆ mutant (bottom) cells, and RFP-Vps21 was expressed from a plasmid (Markgraf et al., 2009). The experiment was performed as described in A. Left to right, RFP-Vps21, GFP-Atg8, merge, and percentage of cells with colocalization. In wild-type cells, Atg8 is found inside the vacuole and localizes to a single dot of the AP. In pep12∆ mutant cells, Atg8 accumulates outside the vacuole, in SD medium to a single dot, and in SD-N to a cluster (as in vps21∆ mutant cells). RFP-Vps21 localizes to multiple dots or a cluster, and one of them colocalizes with GFP-Atg8 in ∼14 and ∼43% of wild-type and pep12∆ mutant cells, respectively. Percentage of cells with colocalization of the Vps21 with the AP marker was determined in cells that contain both green and red puncta; >130 wild-type cells; >350 pep12∆ mutant cells. Arrows point to areas of colocalization of Vps21 with the AP marker; bar, 5 μm; ± represents SD. Results in A represent three independent experiments and in B four independent experiments. (C) Convergence of the endocytic and autophagic pathways. We propose that the two pathways leading to the lysosome converge through two Ypt/Rab modules: Vps21 (black) and Ypt7 (gray). Ypt7, its GEF, and effectors were previously shown to regulate both endocytosis and autophagy (Noda et al., 2009). A role for Vps21, its GEF, and effectors was established in endocytosis (Stack et al., 1995; Epp et al., 2011). Here we show that the endocytic Vps21 module also regulates autophagy. Factors that function downstream of Vps21 include a number of effectors, the tethering complex CORVET (Vps3 and Vps8), the adaptor/tether Vac1, and the SM protein Vps45, as well as the SNARE Pep12.
DISCUSSION
On the basis of deletion and temperature-sensitive mutant analyses presented here, we propose that the Rab5-related GTPase Vps21 plays a role in autophagy. The localization of Vps21 to AP supports this idea. Vps21 does that in the context of the same module that regulates endocytosis: with Vps9 as a GEF and CORVET, Vac1, Pep12, and Vps45 as its downstream factors (model, Figure 8C). Mutant cells deleted for genes that encode each member of this module are defective in selective and nonselective autophagy and accumulate clusters of APCs outside their vacuole. Of importance, all of the autophagic phenotypes of vps9∆ mutant cells can be suppressed by overexpression of active Vps21, supporting the GEF-GTPase connection between the two in autophagy. Appearance of APCs next to the vacuole was shown by live-cell fluorescence and electron microscopy and is dependent on induction of autophagy and the function of Atg1. This suggests that the Vps21 module plays a role in autophagy after Atg1-dependent AP formation and before AP fusion with the vacuole.
Deletion of genes encoding the other two Rab5-related Ypts, Ypt52 and Ypt53, does not result in autophagic phenotypes. However, deletion of YPT52 together with VPS21 exacerbates the vps21∆ phenotype (Nickerson et al., 2012; Supplemental Figure S2). This is similar to the endocytic phenotypes previously described for deletions of the three Rab5-related Ypts (Singer-Kruger et al., 1994). Therefore it seems that Ypt52 can partially complement the function of Vps21 in both endocytosis and autophagy. A parallel relationship exists between the two suggested Vps21 GEFs, Vps9 and Muk1. Although only Vps9 is required for autophagy, vps9∆ muk1∆ double-mutant cells exhibit more-severe autophagy defects than those of the vps9∆ mutant cells. Thus it is possible that the functions of Muk1 and Vps9 are partially redundant in both endocytosis and autophagy: Whereas Vps9 can fully supplement the function of Muk1 in muk1∆ mutant cells, Muk1 can only partially complement the function of Vps9 in vps9∆ mutant cells.
Factors that function downstream of Vps21 and Ypt7 fall into similar functional groups. They include two Vps21 and Ypt7 effector tethering complexes, which share four of their six subunits, CORVET and HOPS, respectively, and two SNAREs, Pep12 and Vam3, respectively. Two other endocytic factors that function in the Vps21-mediated step described here are the adaptor/tether Vac1 and the SM protein Vps45. Of interest, deletion of genes encoding three of the Vps21 downstream factors, including the CORVET subunit Vps3, Vac1, and Pep12, results in more-severe autophagic phenotypes than those of vps21∆ or vps21∆ ypt52∆ mutant cells. Comparison of CPY transport defects in different studies suggests that depletion of Vps3, Vac1, and Pep12 also results in more-severe endocytic phenotypes than depletion of Vps21 (Raymond et al., 1990; Tall et al., 1999; Gerrard et al., 2000). This suggests that there is another Vps21-independent way to recruit these factors to both endosomes and APs. The observation that overexpression of active Vps21 cannot suppress the autophagic defects of the vps9∆ muk1∆ double-mutant cells is in agreement with this idea.
The Ypt7 GTPase module, which includes the Mon1/Ccz1 GEF and the HOPS effector and the Vam3 SNARE, also plays a role in both endocytosis and autophagy (Figure 8C). This idea is also based on deletion mutant analysis (Kirisako et al., 1999; Meiling-Wesse et al., 2002; Noda et al., 2009). Mutations in genes encoding members of the Vps21 and Ypt7 modules result in different distribution patterns of the APs: clusters and dispersed, respectively. Because APCs appear next to the vacuole, we propose that the different pattern is a result of the single large vacuole observed in mutant cells defective in the Vps21 module versus fragmented vacuoles observed in Ypt7 module mutants. Alternatively, it is possible that a difference in an AP characteristic contributes to the different distribution patterns in the two groups of mutant cells.
It is not clear why members of the Vps21 and Ypt7 GTPase modules were not identified in the multiple screens for autophagy mutants that were done in multiple laboratories. Some of these new mutants, such as vps21∆, which have only partial autophagy defects, might have not passed the strict criteria of those screens.
One obvious open question is which autophagy step is regulated by Vps21. Ypt7 regulates fusion of either late endosomes or autophagosomes with the vacuole (Noda et al., 2009). In endocytosis, Vps21 and Ypt7 function in a GTPase switch that coordinates two successive steps: early- to late-endosome maturation and fusion of late endosomes with the vacuole, respectively (Poteryaev et al., 2010). Future studies should determine whether Vps21 functions before Ypt7 in autophagy as well and the specific Vps21-dependent step between AP formation and fusion.
Endocytosis and autophagy convergence
Regardless of whether Vps21 functions before Ypt7 and of the specific step it regulates in autophagy, the involvement of the same Vps21 module in endocytosis and autophagy highlights the convergence between these two pathways. There are two possible reasons for such convergence. The first is that fusion of any membrane-bound compartment—endosomes or autophagosomes—with the vacuole requires the same machinery, including the Vps21 and Ypt7 GTPase modules. The second is that autophagosomes acquire endocytic membranes in an early stage of their formation (Lamb et al., 2012) and therefore require endosomal machinery for fusion with the lysosome. The observation that APCs seen in vps21∆ or vps9∆ mutant cells are stained with the fluorescent dye FM4-64, which stains endocytic membranes (Figure 2), supports this idea. Elucidation of mechanisms by which Rab5 and Rab7 mediate autophagy should help distinguish between these possibilities.
Because most of the evidence for a role for Vps21, Rab5, Ypt7, and their accessory factors in autophagy is based on autophagic phenotypes caused by the loss of a protein, it is possible that their effects are indirect. For example, proper function of the endocytic pathway might be important for the ability of the lysosomal membrane to fuse with APs. We favor the idea that their role in autophagy is direct, for the following three reasons. First, the colocalization of Vps21 with AP markers, shown here, supports a direct role. Second, two early endocytic mutants, end3∆ and rvs167∆, are not defective in autophagy (Nair et al., 2011), suggesting that proper function of the endocytic pathway is not required for autophagy. Third, cumulative evidence suggests a role for endosomes and endosomal membranes in autophagy (Lamb et al., 2012, 2013).
A new Ypt/Rab paradigm
Rab GTPases are considered compartment specific (Zerial and McBride, 2001; Barr, 2013). The idea that an individual Rab GTPase can regulate more than one trafficking pathway has emerged only recently. One example is of the Rab1 homologue, Ypt1, which can regulate both ER-to-Golgi transport and autophagy. We showed that Ypt1 does so in the context of two different modules, with different GEFs and effectors (Lipatova et al., 2012). In that case, we proposed that the two Ypt1 modules regulate two transport steps diverging from the Ypt1-specific compartment, ER-derived membranes (Lipatova et al., 2013). Here we present a different paradigm in which a Ypt/Rab apparently can regulate two different cellular processes in the context of the same module. Specifically, Vps21 and Ypt7 play a role in autophagy and endocytosis, each in the context of the same module, which includes a GEF and effectors. We propose that in this case too, the Ypt/Rab GTPases function in a specific compartment—Vps21 in endosomes/autophagosomes and Ypt7 in lysosomes—and the two pathways converge.
Conservation
In addition to its role in endocytosis, Rab5, either directly or through its interactors, has been implicated in autophagy relevant for Huntington disease and hepatitis C infection (Ravikumar et al., 2008). However, this role is not clear because whereas in fly and human cells it was suggested to regulate autophagosome formation (Ravikumar et al., 2008; Li et al., 2013), in Caenorhabditis elegans its disruption was suggested to induce autophagy (Dwivedi et al., 2011). It is possible that Rab5 is involved in autophagy at two different levels: first, through Rab5-mediated signaling that controls induction of autophagy, and second, in a Rab5-mediated membrane-trafficking step of the autophagy pathway. Here we provide evidence for the latter case. Because of the amazing conservation of the membrane-trafficking and autophagy machineries from yeast to humans, we speculate that convergence of the endocytotic and autophagic processes proposed here is conserved as well.
MATERIALS AND METHODS
Strains, plasmids, and reagents
Yeast strains and plasmids used in this study are listed in Supplemental Table S1. Plasmid constructions were done as follows. Open reading frames and 500 base pairs of the 5′ promoter region and 200 base pairs of the 3′ untranslated region (3’ UTR) of VPS21 and VPS9 were amplified by PCR from yeast genomic DNA, cloned into the BamHI/XhoI sites of pRS425 (2 μ, LEU2), and transformed into yeast for complementation analyses. Guanine nucleotide–locked Vps21 mutants in pFBT9 (2 μ, TRP1; Markgraf et al., 2009) were a gift from C. Ungermann (University of Osnabrück, Osnabrück, Germany). All yeast and Escherichia coli transformations were as previously described (Liang et al., 2007).
For live-cell microscopy, p1K-GFP-Atg8-406 was linearized and integrated in strains as previously described (Zou et al., 2013). For obtaining single, double, or triple mutants of vps21∆, ypt52∆, and ypt53∆ with tagged GFP-Atg8 (YLY2696-YLY2703), p1K-GFP-Atg8-406 was linearized and integrated in wild type (YLY2560), and the obtained strain (YLY2688) was mated with a vps21∆ypt52∆ypt53∆ strain (YLY2567) for dissection. The dissected spores were examined with PCR to select desired deletions.
Antibodies included mouse anti-GFP (sc-9996, Santa Cruz Biotechnology, Dallas, TX); rabbit anti-Ape1 (a gift from Y. Ohsumi, Tokyo Institute of Technology, Tokyo, Japan); rabbit anti–glucose-6-phosphate dehydrogenase (G6PDH; A9521; Sigma-Aldrich, St. Louis, MO); horseradish peroxidase (HRP)–linked goat anti-rabbit (GAR0072; Multisciences, Hangzhou, China); and HRP-linked goat anti-mouse immunoglobulin G (GAM0072; Multisciences).
All chemical reagents were purchased from Ameresco (Fair Lawn, NJ), except for SynaptoRed, also known as FM4-64 (70021; Biotium, Hayward, CA), geneticin (A1720; Sigma-Aldrich), Hygromycin B (10843555001; Roche Diagnostics, Basel, Switzerland), and restriction enzymes and buffers (Takara Biotechnology, Dalian, China).
Yeast culture conditions and live-cell microscopy
For complementation analyses, cells transformed with empty plasmid (pFBT9, 2 μ, TRP1) or Vps21-expressing plasmids (WT; putative GDP bound, S21N; or GTP bound, Q66L) were grown in SD-Trp overnight and then spotted onto SD-Trp plates (in 10-fold serial dilutions) and incubated at indicated temperatures. For live-cell fluorescence microscopy, yeast cultures were grown at permissive temperature (26°C) in rich medium (YPD) or selection medium (when plasmid was used, or as indicated in growth conditions) to mid log phase. For cells subjected to nitrogen starvation (SD-N; 0.17% yeast nitrogen base without amino acid and ammonium sulfate with 2% glucose), cells grown at 26°C to mid log phase were washed and shifted to SD-N at 26°C for 2 or 4 h as described previously (Shintani and Reggiori, 2008). The vps21 temperature-sensitive (vps21ts) mutant cells were grown to mid log phase at 26°C, shifted to 37°C for 0.5 h, washed with prewarmed distilled H2O, and incubated in SD-N at 37°C for 2 h. When indicated, FM4-64 was added to a final concentration of 1.6 μM to stain the vacuole in the last hour of incubation before collection of the cells. Cells on slides were examined with a Nikon (Melville, NY) inverted research microscope Eclipse Ti as previously described (Zou et al., 2013). More than five fields were visualized for each sample, and data were quantified as indicated in the figure legends.
Time-lapse imaging
Cells were grown to mid log phase in YPD medium at permissive temperature (26°C), then shifted to SD-N medium for 5 min before seeding on 2% agar prepared in SD-N liquid medium. Images were acquired at a fixed rate of 20 s/time point for two channels (differential interference contrast and 488 nm) and four Z-slices (0.967-μm step size) using a 100× oil lens objective on an inverted fluorescence microscope (Nikon Eclipse Ti-E) with an UltraVIEW spinning-disk confocal scanner unit (PerkinElmer, Waltham, MA) for 2 h. Exposure time was set to 232 ms, with autofocus at each position. The images were processed using Volocity 6.3.0 software. GFP frames at every 10 min of the movie were picked to show as still pictures. Three independent experiments were carried out.
Pho8Δ60 ALP activity assay
Pho8Δ60 ALP assay was performed using a spectrophotometry as previously described (Noda and Klionsky, 2008). Briefly, cells were grown to mid log phase in YPD at 26°C, and half the cells were grown in rich medium at 26°C for 1.5 h. The other half was starved in SD-N medium at 26°C for 4 h. Protein extracts were prepared and the Pho8Δ60 ALP assay was conducted as previously described (Noda and Klionsky, 2008); three independent experiments were carried out with duplicates for each sample.
Immunoblot analysis of yeast cell lysates
Yeast cells were lysed as previously described (Chen et al., 2005; Cheong and Klionsky, 2008). Immunoblot assays were conducted as previously described (Zou et al., 2013). Blots were probed with anti-Ape1 antibody to determine the conversion of Ape1 from prApe1 (precursor form of Ape1) to mApe1 (mature Ape1) or with anti-GFP antibody to determine GFP-Atg8 processing. Bands from anti-G6PDH antibody or Ponceau S staining were used as a loading control. Immunoblot bands were quantified using ImageJ software (National Institutes of Health, Bethesda, MD). The percentage of processed GFP was calculated as [GFP/(GFP-Atg8 + GFP)] × 100%. The percentage of mature Ape1 was calculated as [mApe1/(preApe1 + mApe1)] × 100%. The data are presented as the mean ± STD of each variable.
Transmission electron microscopy analysis
Cells were grown to mid log phase in YPD medium at permissive temperature (26°C) and then starved in SD-N medium for 2 h (as for live-cell fluorescence microscopy). Cells were fixed and stained with potassium permanganate as previously described (Wright, 2000; Liang et al., 2007). Images were acquired with a transmission electron microscope H7700 (Hitachi High-Tech Corp., Tokyo, Japan). Three independent transmission electron microscopy experiments were carried out.
Supplementary Material
Acknowledgments
We thank D. Taussig and A. U. Hain for critical reading of the manuscript. We thank M. Zerial (Max Planck Institute of Molecular Cell Biology and Genetics, Dresden, Germany), C. Ungermann, and Y. Ohsumi for strains, plasmids, and antibodies. We thank the electron microscopy facility of the School of Biomedical Sciences Core Laboratory, Chinese University of Hong Kong, for transmission electron microscope images. We also thank PerkinElmer Instruments (Shanghai, China) for financial and technical support in time-lapse microscopy. This work was supported by grants from the Natural Science Foundation of China (31271520 to Y.L., 31301173 to S.Z., 31222034 and 31171285 to Z.X., and 31070242 to SL); Fundamental Research Funds for the Central Universities (KYZ201215 to Y.L.; Y0201300236 to S.Z.); the Project sponsored by the Scientific Research Foundation for the Returned Overseas Chinese Scholars, State Education Ministry (2011/508 to Y.L.); Nanjing Agricultural University (680-804094-521 to Y.L.); the Ministry of Education in China (NCET-12-0283 to Z.X.); the National Key Basic Research Program of China (2011CB910100 to Z.X.); and the National Institutes of Health (GM-45444 to N.S.). Y.C. was supported by the Program for Scientific Innovation Research of College Graduate in Jiangsu Province (CXLX12_0267).
Abbreviations used:
- ALP
alkaline phosphatase
- AP
autophagosome
- APC
autophagosomal cluster
- CORVET
class C core vacuole/endosome tethering complex
- ER
endoplasmic reticulum
- GEF
guanine-nucleotide exchange factor
- HOPS
homotypic fusion and vacuole protein sorting complex
- PAS
preautophagosomal structure
- PM
plasma membrane
- SD
synthetic defined medium
- SM
Sec1/Munc18
- SNARE
the soluble N-ethylmaleimide–sensitive factor attachment protein receptor
- STD
standard deviation
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E14-04-0917) on August 20, 2014.
REFERENCES
- Ao X, Zou L, Wu Y. Regulation of autophagy by the Rab GTPase network. Cell Death Differ. 2014;21:348–358. doi: 10.1038/cdd.2013.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr FA. Review series: Rab GTPases and membrane identity: causal or inconsequential. J Cell Biol. 2013;202:191–199. doi: 10.1083/jcb.201306010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabrera M, Arlt H, Epp N, Lachmann J, Griffith J, Perz A, Reggiori F, Ungermann C. Functional separation of endosomal fusion factors and the class C core vacuole/endosome tethering (CORVET) complex in endosome biogenesis. J Biol Chem. 2013;288:5166–5175. doi: 10.1074/jbc.M112.431536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SH, Chen S, Tokarev AA, Liu F, Jedd G, Segev N. Ypt31/32 GTPases and their novel F-box effector protein Rcy1 regulate protein recycling. Mol Biol Cell. 2005;16:178–192. doi: 10.1091/mbc.E04-03-0258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheong H, Klionsky DJ. Biochemical methods to monitor autophagy-related processes in yeast. Methods Enzymol. 2008;451:1–26. doi: 10.1016/S0076-6879(08)03201-1. [DOI] [PubMed] [Google Scholar]
- Dwivedi M, Sung H, Shen H, Park BJ, Lee S. Disruption of endocytic pathway regulatory genes activates autophagy in C. elegans. Mol Cells. 2011;31:477–481. doi: 10.1007/s10059-011-1035-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epp N, Rethmeier R, Kramer L, Ungermann C. Membrane dynamics and fusion at late endosomes and vacuoles—Rab regulation, multisubunit tethering complexes and SNAREs. Eur J Cell Biol. 2011;90:779–785. doi: 10.1016/j.ejcb.2011.04.007. [DOI] [PubMed] [Google Scholar]
- Gerrard SR, Bryant NJ, Stevens TH. VPS21 controls entry of endocytosed and biosynthetic proteins into the yeast prevacuolar compartment. Mol Biol Cell. 2000;11:613–626. doi: 10.1091/mbc.11.2.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas A, Scheglmann D, Lazar T, Gallwitz D, Wickner W. The GTPase Ypt7p of Saccharomyces cerevisiae is required on both partner vacuoles for the homotypic fusion step of vacuole inheritance. EMBO J. 1995;14:5258–5270. doi: 10.1002/j.1460-2075.1995.tb00210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hama H, Tall GG, Horazdovsky BF. Vps9p is a guanine nucleotide exchange factor involved in vesicle-mediated vacuolar protein transport. J Biol Chem. 1999;274:15284–15291. doi: 10.1074/jbc.274.21.15284. [DOI] [PubMed] [Google Scholar]
- Huang WP, Scott SV, Kim J, Klionsky DJ. The itinerary of a vesicle component, Aut7p/Cvt5p, terminates in the yeast vacuole via the autophagy/Cvt pathways. J Biol Chem. 2000;275:5845–5851. doi: 10.1074/jbc.275.8.5845. [DOI] [PubMed] [Google Scholar]
- Hyttinen JM, Niittykoski M, Salminen A, Kaarniranta K. Maturation of autophagosomes and endosomes: a key role for Rab7. Biochim Biophys Acta. 2013;1833:503–510. doi: 10.1016/j.bbamcr.2012.11.018. [DOI] [PubMed] [Google Scholar]
- Kim J, Dalton VM, Eggerton KP, Scott SV, Klionsky DJ. Apg7p/Cvt2p is required for the cytoplasm-to-vacuole targeting, macroautophagy, and peroxisome degradation pathways. Mol Biol Cell. 1999;10:1337–1351. doi: 10.1091/mbc.10.5.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirisako T, Baba M, Ishihara N, Miyazawa K, Ohsumi M, Yoshimori T, Noda T, Ohsumi Y. Formation process of autophagosome is traced with Apg8/Aut7p in yeast. J Cell Biol. 1999;147:435–446. doi: 10.1083/jcb.147.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb CA, Dooley HC, Tooze SA. Endocytosis and autophagy: shared machinery for degradation. Bioessays. 2012;35:34–45. doi: 10.1002/bies.201200130. [DOI] [PubMed] [Google Scholar]
- Lamb CA, Yoshimori T, Tooze SA. The autophagosome: origins unknown, biogenesis complex. Nat Rev Mol Cell Biol. 2013;14:759–774. doi: 10.1038/nrm3696. [DOI] [PubMed] [Google Scholar]
- Langemeyer L, Nunes Bastos R, Cai Y, Itzen A, Reinisch KM, Barr FA. Diversity and plasticity in Rab GTPase nucleotide release mechanism has consequences for Rab activation and inactivation. eLife. 2014;3:e01623. doi: 10.7554/eLife.01623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Zhao Y, Hu J, Xiao J, Qu L, Wang Z, Ma D, Chen Y. A novel ER-localized transmembrane protein, EMC6, interacts with RAB5A and regulates cell autophagy. Autophagy. 2013;9:150–163. doi: 10.4161/auto.22742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Y, Morozova N, Tokarev AA, Mulholland JW, Segev N. The role of Trs65 in the Ypt/Rab guanine nucleotide exchange factor function of the TRAPP II complex. Mol Biol Cell. 2007;18:2533–2541. doi: 10.1091/mbc.E07-03-0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipatova Z, Belogortseva N, Zhang XQ, Kim J, Taussig D, Segev N. Regulation of selective autophagy onset by a Ypt/Rab GTPase module. Proc Natl Acad Sci USA. 2012;109:6981–6986. doi: 10.1073/pnas.1121299109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipatova Z, Segev N. A Ypt/Rab GTPase module makes a PAS. Autophagy. 2012;8:1271–1272. doi: 10.4161/auto.20872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipatova Z, Shah AH, Kim JJ, Mulholland JW, Segev N. Regulation of ER-phagy by a Ypt/Rab GTPase module. Mol Biol Cell. 2013;24:3133–3144. doi: 10.1091/mbc.E13-05-0269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markgraf DF, Ahnert F, Arlt H, Mari M, Peplowska K, Epp N, Griffith J, Reggiori F, Ungermann C. The CORVET subunit Vps8 cooperates with the Rab5 homolog Vps21 to induce clustering of late endosomal compartments. Mol Biol Cell. 2009;20:5276–5289. doi: 10.1091/mbc.E09-06-0521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meiling-Wesse K, Barth H, Voss C, Barmark G, Muren E, Ronne H, Thumm M. Yeast Mon1p/Aut12p functions in vacuolar fusion of autophagosomes and cvt-vesicles. FEBS Lett. 2002;530:174–180. doi: 10.1016/s0014-5793(02)03456-7. [DOI] [PubMed] [Google Scholar]
- Mizushima N, Yoshimori T, Ohsumi Y. The role of Atg proteins in autophagosome formation. Annu Rev Cell Dev Biol. 2011;27:107–132. doi: 10.1146/annurev-cellbio-092910-154005. [DOI] [PubMed] [Google Scholar]
- Nair U, Jotwani A, Geng J, Gammoh N, Richerson D, Yen WL, Griffith J, Nag S, Wang K, Moss T, et al. SNARE proteins are required for macroautophagy. Cell. 2011;146:290–302. doi: 10.1016/j.cell.2011.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatogawa H, Suzuki K, Kamada Y, Ohsumi Y. Dynamics and diversity in autophagy mechanisms: lessons from yeast. Nat Rev Mol Cell Biol. 2009;10:458–467. doi: 10.1038/nrm2708. [DOI] [PubMed] [Google Scholar]
- Nickerson DP, Russell MR, Lo SY, Chapin HC, Milnes JM, Merz AJ. Termination of isoform-selective Vps21/Rab5 signaling at endolysosomal organelles by Msb3/Gyp3. Traffic. 2012;13:1411–1428. doi: 10.1111/j.1600-0854.2012.01390.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda T, Fujita N, Yoshimori T. The late stages of autophagy: how does the end begin. Cell Death Differ. 2009;16:984–990. doi: 10.1038/cdd.2009.54. [DOI] [PubMed] [Google Scholar]
- Noda T, Klionsky DJ. The quantitative Pho8Δ60 assay of nonspecific autophagy. Methods Enzymol. 2008;451:33–42. doi: 10.1016/S0076-6879(08)03203-5. [DOI] [PubMed] [Google Scholar]
- Paulsel AL, Merz AJ, Nickerson DP. Vps9 family protein Muk1 is the second Rab5 guanosine nucleotide exchange factor in budding yeast. J Biol Chem. 2013;288:18162–18171. doi: 10.1074/jbc.M113.457069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pind SN, Nuoffer C, McCaffery JM, Plutner H, Davidson HW, Farquhar MG, Balch WE. Rab1 and Ca2 +are required for the fusion of carrier vesicles mediating endoplasmic reticulum to Golgi transport. J Cell Biol. 1994;125:239–252. doi: 10.1083/jcb.125.2.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poteryaev D, Datta S, Ackema K, Zerial M, Spang A. Identification of the switch in early-to-late endosome transition. Cell. 2010;141:497–508. doi: 10.1016/j.cell.2010.03.011. [DOI] [PubMed] [Google Scholar]
- Ravikumar B, Imarisio S, Sarkar S, O'Kane CJ, Rubinsztein DC. Rab5 modulates aggregation and toxicity of mutant huntingtin through macroautophagy in cell and fly models of Huntington disease. J Cell Sci. 2008;121:1649–1660. doi: 10.1242/jcs.025726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond CK, O'Hara PJ, Eichinger G, Rothman JH, Stevens TH. Molecular analysis of the yeast VPS3 gene and the role of its product in vacuolar protein sorting and vacuolar segregation during the cell cycle. J Cell Biol. 1990;111:877–892. doi: 10.1083/jcb.111.3.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reggiori F, Klionsky DJ. Autophagic processes in yeast: mechanism, machinery and regulation. Genetics. 2013;194:341–361. doi: 10.1534/genetics.112.149013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinsztein DC, Shpilka T, Elazar Z. Mechanisms of autophagosome biogenesis. Curr Biol. 2012;22:R29–R34. doi: 10.1016/j.cub.2011.11.034. [DOI] [PubMed] [Google Scholar]
- Segev N. Ypt and Rab GTPases: insight into functions through novel interactions. Curr Opin Cell Biol. 2001a;13:500–511. doi: 10.1016/s0955-0674(00)00242-8. [DOI] [PubMed] [Google Scholar]
- Segev N. Ypt/rab gtpases: regulators of protein trafficking. Sci STKE. 2001b;2001:re11. doi: 10.1126/stke.2001.100.re11. [DOI] [PubMed] [Google Scholar]
- Segev N, Mulholland J, Botstein D. The yeast GTP-binding YPT1 protein and a mammalian counterpart are associated with the secretion machinery. Cell. 1988;52:915–924. doi: 10.1016/0092-8674(88)90433-3. [DOI] [PubMed] [Google Scholar]
- Shintani T, Huang WP, Stromhaug PE, Klionsky DJ. Mechanism of cargo selection in the cytoplasm to vacuole targeting pathway. Dev Cell. 2002;3:825–837. doi: 10.1016/s1534-5807(02)00373-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shintani T, Reggiori F. Fluorescence microscopy-based assays for monitoring yeast Atg protein trafficking. Methods Enzymol. 2008;451:43–56. doi: 10.1016/S0076-6879(08)03204-7. [DOI] [PubMed] [Google Scholar]
- Singer-Kruger B, Stenmark H, Dusterhoft A, Philippsen P, Yoo JS, Gallwitz D, Zerial M. Role of three Rab5-like GTPases, Ypt51p, Ypt52p, and Ypt53p, in the endocytic and vacuolar protein sorting pathways of yeast. J Cell Biol. 1994;125:283–298. doi: 10.1083/jcb.125.2.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solinger JA, Spang A. Tethering complexes in the endocytic pathway: CORVET and HOPS. FEBS J. 2013;280:2743–2757. doi: 10.1111/febs.12151. [DOI] [PubMed] [Google Scholar]
- Stack JH, Horazdovsky B, Emr SD. Receptor-mediated protein sorting to the vacuole in yeast: roles for a protein kinase, a lipid kinase and GTP-binding proteins. Annu Rev Cell Dev Biol. 1995;11:1–33. doi: 10.1146/annurev.cb.11.110195.000245. [DOI] [PubMed] [Google Scholar]
- Stenmark H. Rab GTPases as coordinators of vesicle traffic. Nat Rev Mol Cell Biol. 2009;10:513–525. doi: 10.1038/nrm2728. [DOI] [PubMed] [Google Scholar]
- Takeshige K, Baba M, Tsuboi S, Noda T, Ohsumi Y. Autophagy in yeast demonstrated with proteinase-deficient mutants and conditions for its induction. J Cell Biol. 1992;119:301–311. doi: 10.1083/jcb.119.2.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tall GG, Hama H, DeWald DB, Horazdovsky BF. The phosphatidylinositol 3-phosphate binding protein Vac1p interacts with a Rab GTPase and a Sec1p homologue to facilitate vesicle-mediated vacuolar protein sorting. Mol Biol Cell. 1999;10:1873–1889. doi: 10.1091/mbc.10.6.1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada Y, Nakamura N, Ohsumi Y, Hirata A. Vam3p, a new member of syntaxin related protein, is required for vacuolar assembly in the yeast Saccharomyces cerevisiae. J Cell Sci. 1997;110:1299–1306. doi: 10.1242/jcs.110.11.1299. [DOI] [PubMed] [Google Scholar]
- Wright R. Transmission electron microscopy of yeast. Microsc Res Tech. 2000;51:496–510. doi: 10.1002/1097-0029(20001215)51:6<496::AID-JEMT2>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Xie Z, Klionsky DJ. Autophagosome formation: core machinery and adaptations. Nat Cell Biol. 2007;9:1102–1109. doi: 10.1038/ncb1007-1102. [DOI] [PubMed] [Google Scholar]
- Zerial M, McBride H. Rab proteins as membrane organizers. Nat Rev Mol Cell Biol. 2001;2:107–117. doi: 10.1038/35052055. [DOI] [PubMed] [Google Scholar]
- Zou S, Chen Y, Liu Y, Segev N, Yu S, Min G, Ye M, Zeng Y, Zhu X, Hong B, et al. Trs130 participates in autophagy through GTPases Ypt31/32 in Saccharomyces cerevisiae. Traffic. 2013;14:233–246. doi: 10.1111/tra.12024. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.