Abstract
Hypothalamic leptin signaling plays a central role in maintaining body weight homeostasis. Here, we show that clusterin/ApoJ, recently identified as an anorexigenic neuropeptide, is an important regulator in the hypothalamic leptin signaling pathway. Coadministration of clusterin potentiates the anorexigenic effect of leptin and boosts leptin-induced hypothalamic Stat3 activation. In cultured neurons, clusterin enhances receptor binding and subsequent endocytosis of leptin. These effects are mainly mediated through the LDL receptor-related protein-2 (Lrp2). Notably, inhibition of hypothalamic clusterin, Lrp2 or endocytosis abrogates anorexia and hypothalamic Stat3 activation caused by leptin. These findings propose a novel regulatory mechanism in central leptin signaling pathways.
Keywords: clusterin, endocytosis, leptin, Lrp2, Stat3
Introduction
Leptin is a representative hormone that signals adiposity to the hypothalamus, a key organ controlling appetite and energy metabolism. By acting on the hypothalamus, leptin reduces food intake but increases energy expenditure, thereby returning the increased body fat level to normal 1. Impaired leptin signaling in the hypothalamus has been suggested as an important underlying mechanism for appetite dysregulation in obese individuals 1.
In the hypothalamus neurons, leptin activates the signal transduction-activated transcript-3 (Stat3) signaling pathway 2. Replacement of amino acid (Tyr1138 to Ser1138) in the long form leptin receptor (Leprb) that is critical for Stat3 activation caused severe obese phenotype as seen in Leprb-deficient mice 3. Thus, Stat3 activation through Leprb is thought to be an important signaling event for the central metabolic actions of leptin. Leptin-caused Stat3 phosphorylation was significantly reduced in the hypothalamus of obese mice 4, but underlying mechanisms have not been fully unveiled.
Clusterin/apolipoprotein J (ApoJ) is a 70- to 80-kD glycoprotein that is highly expressed in the central nervous system and plasma 5. Clusterin was isolated as a leptin-binding protein from the plasma 6 although only a small portion of plasma leptin is associated with clusterin 7. We recently reported that intracerebroventricular (ICV) administration of clusterin to rodents causes a significant reduction in food intake and body weight. Moreover, ICV injection of clusterin activated Stat3 signaling in the hypothalamus, a well-known leptin signaling pathway 8. Interestingly, clusterin treatment triggered a molecular interaction between Leprb and a potential clusterin receptor, the LDL receptor-related protein-2 (Lrp2) in cultured neuron cells. Anorexigenic and weigh-reducing effects of clusterin were dependent on both hypothalamic Lrp2 and Leprb, suggesting that clusterin signaling pathway is intimately linked to leptin signaling in hypothalamic neurons. These findings led us to examine a possible engagement of clusterin in hypothalamic leptin signaling.
Results & Discussion
Functional relationship between clusterin and leptin
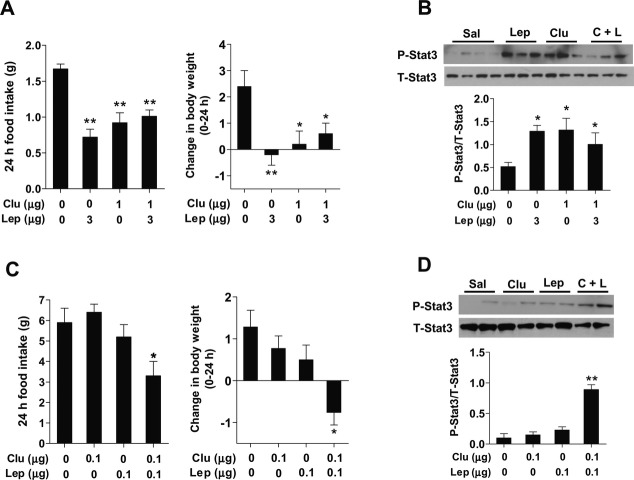
We firstly investigated a functional link between leptin and clusterin in central feeding regulation. For this, we injected leptin (3 μg) with or without clusterin (1 μg) via ICV-implanted cannulae in overnight-fasted mice. As reported 1,8, leptin or clusterin-alone treatment potently repressed fast-induced hyperphagia and inhibited body weight gain for 24 h post-injection (Fig 1A). However, coadministration of leptin (3 μg) and clusterin (1 μg) had no additive or synergistic effect on food intake and body weight (Fig 1A). Similarly, hypothalamic Stat3 phosphorylation in mice coinjected with leptin and clusterin was not greater than in mice injected with either clusterin or leptin (Fig 1B). These data raised the possibility that leptin and clusterin may share the signaling pathway, as maximum activation of the signaling pathway by one may prevent further activation by the other.
Figure 1. Functional relationship between leptin and clusterin.
A, B Effects of coadministration of leptin and clusterin at effective doses on food intake, body weight, and hypothalamic Stat3 signaling (n = 6–7). Peptides were administered into the cerebroventricle via implanted cannulae in overnight-fasted mice. Food intake and body weight were monitored for 24 h post-injection. Mediobasal hypothalamus was collected 30 min after injection to measure hypothalamic Stat3 phosphorylation using immunoblotting.
C, D Effects of coadministration of leptin and clusterin at subeffective doses on food intake, body weight, and hypothalamic Stat3 signaling (n = 6–7).
Data information: Data represent the mean ± SEM. Comparisons between the groups were analyzed using one-way ANOVA followed by a post-hoc LSD test, *P < 0.05, **P < 0.01 versus saline-injected control.
Source data are available online for this figure.
Consistent with this notion, coadministration of leptin and clusterin at subeffective doses (0.1 μg each) provoked a significant reduction in both food intake and body weight (Fig 1C). Similarly, we observed marked activation of the hypothalamic Stat3 signaling pathway 30 min after coadministration of leptin and clusterin (Fig 1D). These findings indicate that clusterin may potentiate hypothalamic leptin signaling and actions by acting on a common signaling pathway.
Reduced hypothalamic leptin activity in clusterin-deficient mice
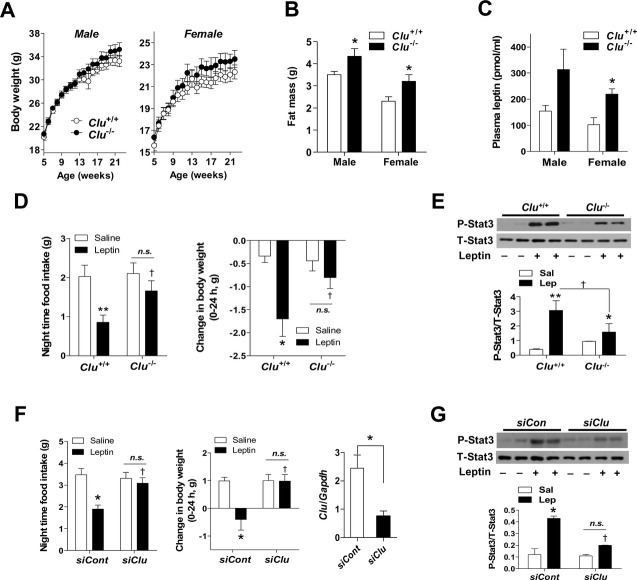
To further define the interaction between leptin and clusterin, we examined the effects of leptin in generalized clusterin-deficient (Clu−/−) mice. These mice displayed delayed-onset mild obesity, which was more pronounced in female mice (Fig 2A). Despite normal body weights, young Clu−/− mice had increased fat mass and higher plasma leptin levels compared to wild littermates (Fig 2B and C), suggesting that they might develop leptin resistance. To test leptin sensitivity in Clu−/− mice, food intake, body weight, and hypothalamic Stat3 activation were determined following ICV administration of leptin (1 μg). As previously reported 9, ICV injection of leptin caused anorexia and weight loss in Clu+/+ mice and stimulated phospho-Stat3 levels in the hypothalamus of Clu+/+ mice (Fig 2D and E). However, these effects were significantly attenuated in Clu−/− mice (Fig 2D and E), implying decreased hypothalamic leptin activity in clusterin-deficient mice. Chronic obesity may render animals less sensitive to leptin 4. To ensure an important role of hypothalamic clusterin in central leptin signaling, we acutely depleted hypothalamic clusterin expression by microinjecting the small inhibitory RNA (siRNA) targeting murine clusterin in the bilateral mediobasal hypothalamus. ICV leptin-induced anorexia, weight loss, and Stat3 phosphorylation were significantly reduced in mice treated with clusterin siRNA (Fig 2F and G). These findings support the notion that hypothalamic clusterin is needed for adequate responses to leptin.
Figure 2. Reduced leptin actions in mice lacking hypothalamic clusterin.
A Body weights were measured once a week in male and female Clu+/+ and Clu−/− mice (n = 5–6).
B, C Fat mass and fasting plasma leptin concentrations were determined in 12-week-old Clu+/+ and Clu−/− mice (n = 5–6). *P < 0.05 versus Clu+/+ mice.
D ICV leptin (1 μg)-induced reduction in food intake and body weight were attenuated in Clu−/− mice (n = 4–5). *P < 0.05, **P < 0.005 versus saline-injected Clu+/+ mice; †P < 0.05 versus leptin-injected Clu+/+ mice; n.s., not significant.
E Hypothalamic Stat3 phosphorylation 30 min after ICV administration of leptin (1 μg) in young Clu+/+ and Clu−/− mice (n = 5). *P < 0.05, **P < 0.005 versus saline-injected Clu+/+ mice; †P < 0.05 versus leptin-injected Clu+/+ mice.
F, G Effects of ICV leptin (1 μg) on food intake, body weight, and hypothalamic Stat3 phosphorylation in mice injected with control siRNA or clusterin siRNA in mediobasal hypothalamus (n = 5). *P < 0.01 versus control siRNA- and saline-injected mice; †P < 0.05 versus control siRNA- and leptin-injected mice. n.s., not significant. Successful knockdown was confirmed by measuring hypothalamic clusterin mRNA expression at the end of the study.
Data information: Data represent the mean ± SEM. Comparisons between the groups were analyzed using two-way ANOVA or the Student’s t-test.
Source data is available online for this figure.
Clusterin enhances leptin receptor binding
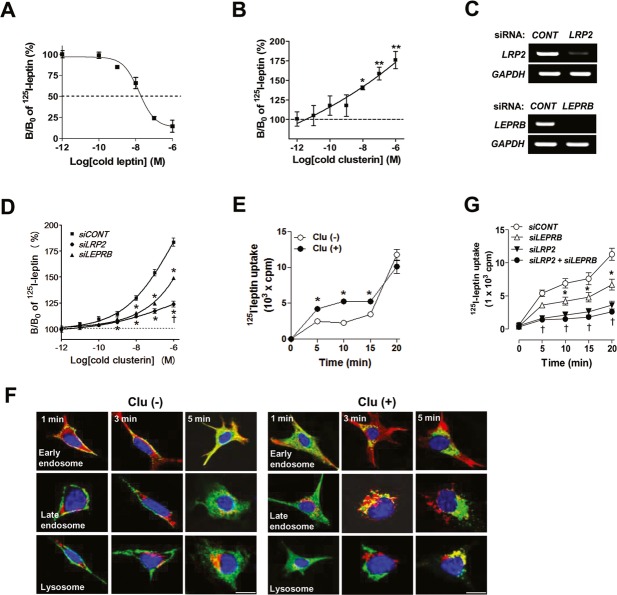
One potential mechanism through which clusterin and leptin may functionally interact may be effects on receptor binding. To test this, we conducted the binding assay of leptin in the presence or absence of clusterin in SH-SY5Y human neuronal cells that expressed both LEPRB and LRP2 8. The specific binding affinity (Kd) of leptin was 8.5 × 10−7 M (Fig 3A). Notably, the specific binding of 125I-leptin was significantly increased by cotreatment of clusterin in a dose-dependent manner (Fig 3B), indicating that clusterin stimulates leptin receptor binding.
Figure 3. Clusterin stimulates leptin receptor binding and endocytosis through Lrp2-mediated mechanisms.
A, B Receptor binding assays of 125I-leptin were performed in SH-SY5Y cells. SH-SY5Y neuron cells were incubated with iodinated leptin (0.1 nM) in the presence or absence of unlabeled leptin or clusterin (1 fM–1 μM) at 37°C for 30 min (n = 3). B/Bo = leptin specific binding.
C The mRNA expression levels of LRP2 and LEPRB in cells transfected with LEPRB siRNA (siLEPRB) and/or LRP2 siRNA (siLRP2) (n = 2). *P < 0.01 versus control siRNA (siCONT)-treated cells.
D Receptor binding assays of 125I-leptin were performed in SH-SY5Y cells transfected with siLEPRB and/or siLRP2 and treated with various concentrations of clusterin peptide (n = 3). *P < 0.05, **P < 0.005 versus cold peptide-untreated controls (B/Bo = 100%).
E Intracellular uptake of 125I-leptin was assessed in SH-SY5Y cells with or without clusterin (1 nM) treatment for indicated time (n = 3). *P < 0.05 versus clusterin-untreated control at each time point.
F Internalization of FITC-leptin (0.5 μg/ml) was allowed for indicated time in SH-SY5Y cells (n = 2). Cells were fixed, immunostained with antibodies against EEA1 (early endosome marker), mannose-6-phosphate receptor (late endosome marker), and LAP2 (lysosome marker) and observed using confocal microscopy. Scale bar = 10 μm.
G Intracellular uptake of 125I-leptin was assessed in SH-SY5Y cells transfected with siLEPRB and siLRP2 (n = 3). *P < 0.05 versus siCONT at each time point; †P < 0.05 versus siLEPRB at each time point.
Data information: Data represent the mean ± SEM. Comparisons between the groups were analyzed using repeated ANOVA.
Clusterin binds LRP2 with a high affinity in SH-SY5Y neuronal cells 8. Moreover, clusterin treatment rapidly induced molecular interactions between LEPRB and LRP2. To clarify which receptor among LRP2 and LEPRB mediate these effects, the binding assays were conducted in SH-SY5Y cells in which LEPRB or LRP2 expression was depleted using siRNAs targeting LRP2 and LEPRB. Treatment with siRNAs decreased the mRNA levels of LEPRB and LRP2 more than 90% (Fig 3C). The ability of clusterin to enhance leptin binding was inhibited to a greater extent by LRP2 siRNA treatment compared to LEPRB siRNA treatment (68% versus 40%, Fig 3D). These data suggest a predominant role of LRP2 in clusterin-mediated enhancement of leptin receptor binding.
Clusterin stimulates leptin endocytosis in neuronal cells
LRP family proteins were originally identified as endocytic receptors 10, and both leptin and Leprs have been shown to undergo endocytosis in cultured cells 6,11. Moreover, leptin is internalized and accumulated in the neuronal cytoplasm when administered into the rat cerebroventricle 12, providing in vivo evidence for leptin endocytosis. We thus examined whether clusterin may regulate leptin endocytosis in neuronal cells. Intracellular uptake of 125I-labeled leptin in SH-SY5Y cells was enhanced by clusterin treatment (Fig 3E). Endocytosis of fluorescein isothiocyanate (FITC)-labeled leptin was also accelerated in the presence of clusterin (1 nM; Fig 3F). In the absence of clusterin, FITC-leptin localized to the submembranous early endosome 1–3 min after the initiation of endocytosis and by 5 min was located in early endosomes in the cytosol. Upon clusterin treatment, FITC-leptin localized primarily to cytosolic early endosomes at 1 min, late endosomes at 3 min, and finally the lysosome by 5 min.
To identify the receptor mediating leptin endocytosis, 125I-leptin uptake was examined in cells depleted of LEPRB and/or LRP2. Leptin endocytosis was reduced by the depletion of LEPRB (by around 30%), LRP2 (by around 68%), and both LEPRB and LRP2 (by around 77%), which indicated that leptin endocytosis was mediated primarily by LRP2 in this system (Fig 3G).
Lrp2-mediated endocytosis is essential for leptin signaling and actions
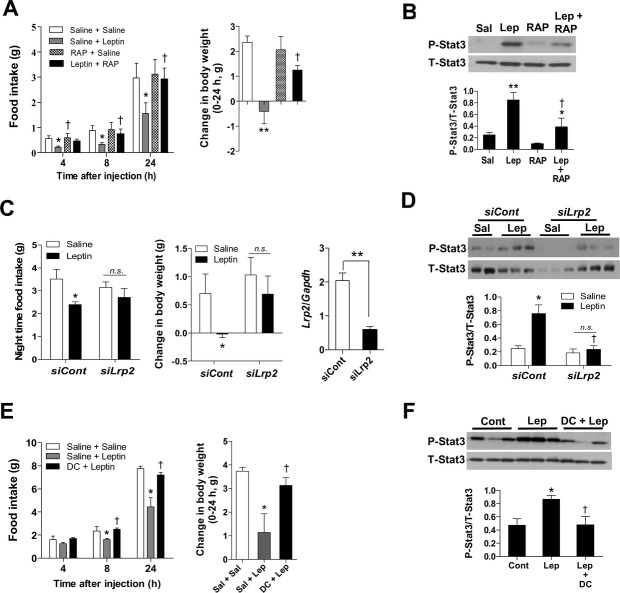
Positron emission topography (PET) has demonstrated that leptin binds to Lrp2 in renal tubules 13, which is essential in leptin renal reuptake 14. Lrp2 also mediates leptin transport across the blood-CSF barrier into the CSF 15. To investigate a role for Lrp2 in hypothalamic leptin signaling in vivo, we administered receptor-associated protein (RAP, 2 μg), known to strongly inhibit the ligand binding to Lrp2 16, into the bilateral mediobasal hypothalamus 30 min prior to the injection of leptin (0.5 μg) in overnight-fasted mice. In fasted mice having low levels of endogenous leptin signals, administration of RAP alone did not significantly alter food intake, but did significantly inhibit a decrease in food intake and body weight caused by exogenous leptin (Fig 4A). Moreover, prior RAP treatment significantly attenuated hypothalamic Stat3 phosphorylation induced by leptin injection (Fig 4B).
Figure 4. Role for hypothalamic Lrp2 and endocytosis in central leptin actions.
A, B Either saline or RAP was administered 30 min prior to the injection of saline or leptin in overnight-fasted mice in the early light phase. Food intake and body weight were monitored for 24 h post-injection. As a separate experiment, mediobasal hypothalamus was collected 30 min after the second injection (n = 6–7). *P < 0.05, **P < 0.01 versus saline; †P < 0.05 versus leptin-alone group.
C, D The siRNA specific to Lrp2 was injected into bilateral mediobasal hypothalamus of mice. ICV leptin was injected just before light-off on the second day of siRNA injection. Food intake and body weight during the dark period were monitored (n = 5–6). In a separate experiment, hypothalamic Stat3 phosphorylation was determined 30 min after leptin injection in mice injected with Lrp2 siRNA (n = 4–5). *P < 0.05 versus control siRNA (siCont)- and saline-injected controls; †P < 0.05 versus siCont- and leptin-injected groups. **P < 0.01 between indicated group. n.s., not significant.
E, F Either saline or dansylcadaverine (DC), a clathrin-dependent endocytosis inhibitor, was administered 30 min prior to the injection of leptin (n = 6–7). *P < 0.05 versus saline; †P < 0.05 versus leptin alone.
Data information: Data represent the mean ± SEM. Comparisons between the groups were analyzed using one-way (A, B, E, F), two-way (C, D) and repeated ANOVA (A, E) or the Student’s t-test (C).
Source data are available online for this figure
To further elucidate the role for Lrp2, we injected small inhibitory RNA (siRNA) specific to murine Lrp2 (1 μmol each side, Dharmacon) into bilateral mediobasal hypothalamus. Mice in control group were injected with the same amount of non-targeting scrambled siRNA. In mice with reduced hypothalamic Lrp2 expression, the effects of IP leptin (3 mg/kg) on food intake, body weight, and Stat3 activation were significantly attenuated (Fig 4C and D). These findings highlight a critical role for Lrp2 in mediating hypothalamic leptin actions.
Given that Lrp2 mediates leptin endocytosis in vitro, we tested the importance of endocytosis in hypothalamic leptin signaling pathway. For this, we administered clathrin-dependent endocytosis inhibitor dansylcadaverine (25 μmol) into the mediobasal hypothalamus 30 min before the injection of leptin (0.5 μg) in overnight-fasted mice. Intrahypothalamic injection of dansylcadaverine completely prevented leptin-induced reduction in food intake and body weight and hypothalamic Stat3 activation (Fig 4E and F). These findings suggested that endocytosis of ligand-receptor complexes may be a pivotal step in hypothalamic leptin signal transduction pathways and thus leptin-mediated regulation of feeding and body weight.
From the results of the present study, we propose a new model of hypothalamic leptin signaling. In the extracellular space of the hypothalamus, clusterin may form a complex with leptin. Leptin-clusterin complex binds to Leprb and Lrp2 expressed on the plasma membrane of hypothalamic leptin-target neurons. These ligand-receptor complexes may then undergo endocytosis and interact with signaling molecules (e.g., Jak2, Stat3) on the membrane of endosomal vesicles during endosomal shuttling from the plasma membrane to the perinuclear area. Endosome-associated bulk shuttling of ligand-receptor-signaling molecules might facilitate more efficient and rapid signal transduction than the shuttling of individual signaling molecules. According to our new model, leptin is present inside endosomal vesicles where it either remains bound to Leprb, as in the case of epidermal growth factor (EGF)-EGF receptor, or dissociates from Leprb and loses its biological activity when the endosomal pH becomes acidic. Although further studies are required to confirm leptin activity in different endosomal compartments, leptin in early endosomes may be functionally active and activate Stat3 signaling. In this model, clusterin and Lrp2 may function as a cofactor-coreceptor system of leptin-Leprb signaling. A direct interaction of Lrp1 and Leprb was also demonstrated. Moreover, deletion of neuronal Lrp1 caused obesity phenotype and leptin resistance 17. Thus, hypothalamic Lrp1 and Lrp2 may have similar functions in central leptin signaling.
Reduced leptin-induced Stat3 phosphorylation in the hypothalamic arcuate nucleus (ARC) of diet-induced obese (DIO) mice is a key feature of obesity-associated central leptin resistance 4. Identifying the mechanism behind impaired leptin activity in the hypothalamus has been a major focus in the field of obesity research. In our previous study 8, clusterin-induced Stat3 activation was restricted to the hypothalamic ARC, suggesting that clusterin-mediated augmentation of leptin signaling occurs in this region. Moreover, DIO mice had inability to increase hypothalamic clusterin levels following leptin administration 8, which would result in reduced Stat3 activation in this area. These data suggest that dysregulation of hypothalamic clusterin may contribute to the development of central leptin resistance in obesity.
Despite a marked impairment in central leptin signaling, generalized deletion of the clusterin gene resulted in a mild obese phenotype in mice, which contradicts the importance of leptin signaling in body weight regulation. Since clusterin is widely expressed in peripheral tissues 18, the peripheral metabolic effects of clusterin deficiency may compensate for the central effects of clusterin deficiency, thereby minimizing changes in adiposity. Thus, there is a need to generate tissue-specific clusterin-knockout mice to clarify the central and peripheral effects of clusterin in body weight homeostasis.
This study shows that clusterin plays an important role in the hypothalamic leptin signaling pathway. However, central clusterin administration could suppress food intake in leptin-deficient ob/ob mice (Supplementary Fig S1), suggesting that clusterin can regulate feeding without leptin. Further studies are required to elucidate the leptin-independent signaling pathways involved in clusterin-mediated feeding regulation. In ob/ob mice, suppression of hypothalamic clusterin expression by injecting clusterin siRNA increased food intake (Supplementary Fig S2). Unexpectedly, we found that ob/ob mice had higher clusterin expression levels in the hypothalamus compared to normal mice (Supplementary Fig S3). Thus increased clusterin expression in ob/ob mice may be a compensatory mechanism to reduce hyperphagia. In conclusion, we have identified hypothalamic clusterin as a novel anorexigenic molecule that can potentiate central leptin activity. Treatment targeting hypothalamic clusterin could lead to new approaches to the management of leptin resistance in obese subjects.
Materials and Methods
Cell culture
SH-SY5Y neuroblastoma cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal calf serum, penicillin and streptomycin (100 units/ml each).
Animals
Adult male C57BL/6 mice 8–10 weeks of age were obtained from Orient Bio (Seoul, Korea). Clu−/− mice were obtained from the Jackson Laboratory (Bar Harbor, ME, USA). Mice were fed a standard chow diet (Samyang, Seoul, Korea) ad libitum unless indicated. Mice were housed in a controlled temperature (22 ± 1°C) and were subjected to a 12-h light/dark cycle, with light from 07:00 to 19:00 h. Fat and lean body masses of Clu+/+ and Clu−/− mice were assessed at 12 weeks of age using dual-energy X-ray absorptiometry (QDR-4500A, Hologic, Waltham, MA, USA). Fasting plasma leptin concentrations were measured by enzyme-linked immunosorbent assay (ALPCO Diagnostics). All of the procedures were approved by the Institutional Animal Care and Use Committee of the Asan Institute for Life Sciences (Seoul, Korea).
Cannulation and injection
Cannulation and injection into the 3rd cerebroventricle and mediobasal hypothalamus was performed as previously described 19. Only mice in which cannulae had been correctly positioned were included in data analysis. Clusterin (AdipoGen #AG-40A-0050, Incheon, Korea), leptin (R & D systems, Minneapolis, MN, USA), and RAP (Molecular Innovations, Southfield, MI, USA) were dissolved in 0.9% (w/v) saline, and dansylcadaverine (Sigma) was dissolved in 10% DMSO. Peptides and chemicals were administered in a total volume of 3 μl for intracerebroventricular injection and 1 μl for intrahypothalamic injection, respectively. Most experiments were performed in the early light phase (9–11 am) on overnight-fasted mice unless indicated. Food intake and body weight were monitored for 24 h post-injection.
Immunoblotting
For phosphorylated Stat3 immunoblotting, mediobasal hypothalami were collected 30 min after ICV or IP injection. The mediobasal hypothalami were collected as previously described 20. Hypothalamic lysates (30 μg protein) were subjected to immunoblotting using antibodies against the phospho-Y705-Stat3 and total Stat3 (Cell Signaling). Band density was measured with a densitometer (VersaDoc Multi Imaging Analyzer System, Bio-Rad).
Small interfering RNA (siRNA)
The siRNA particles targeting murine clusterin (Dharmacon, E-043966-00-0020) and Lrp2 (Dharmacon, M-045961-01-0020) were resuspended in RNase-free water and mixed with Lipofectamine (9:1 v/v, Invitrogen) to a final concentration of 1 mM. The siRNA solution (0.5 μl each side) was injected bilaterally into the mediobasal hypothalamus over a 15-min period during stereotaxic surgery as previously described 19. The same amount of non-targeting scrambled control siRNA (Dharmacon, D-001910-10-20) was administered to the control group. When hypothalamic Lrp2 expression levels were less than 30% of the average of the control group, gene knockdown was considered successful. Animals showing a successful gene knockdown were included for data analysis. For LRP2 and LEPRB knockdown in the SH-SY5Y human neuron cells, cells were transfected with siRNA against human LEPRB (Dharmacon, M-008015-02) and LRP2 (Dharmacon, M-012673-01-0020; 100 nM using Lipofectamine 48 h before assays). For control group, cells were transfected with non-targeting scrambled siRNA (Dharmacon).
Receptor binding assay
125I-human leptin was purchased from Amersham (Arlington Heights, IL, USA). After serum starvation for 2 h, SH-SY5Y neuron cells were incubated with iodinated leptin (0.1 nM) in the presence or absence of unlabeled leptin or clusterin (1 fM–1 μM) dissolved in binding medium (DMEM containing 1% bovine serum albumin) at 37°C for 30 min. Cells were washed with phosphate-buffered saline three times and treated with 0.1 N NaOH. Radioactivity (cpm) was quantified using a gamma counter (Perkin-Elmer, Waltham, MA, USA). Specific binding is represented as B (binding [cpm] in the presence of a certain amount of unlabeled peptide)/Bo (binding [cpm] in the absence of unlabeled peptide) (%). Curve fitting was performed using the Prism software program (GraphPad).
Endocytosis of 125I-leptin
The internalization of 125I-leptin was measured in SH-SY5Y cells. Cells were incubated with 125I-leptin (130 nCi/well) with or without 0.1 nM clusterin in binding buffer (DMEM containing 25 mM HEPES, pH 7.4, and 1% bovine serum albumin) for the indicated times at 37°C. Cells were washed twice with cold PBS and then subjected to an acid wash in DMEM containing 25 mM HEPES, pH 3.0 twice to remove cell surface-associated leptin. Cells were solubilized with 0.4 N NaOH, and intracellular radioactivity was measured using a gamma counter.
Endocytosis of FITC-leptin
SH-SY5Y cells were grown on glass coverslips. After serum starvation for 2 h, cells were treated with 0.5 μg/ml FITC-leptin for 45 min at 4°C to induce ligand-receptor binding at the cell surface. Cells were then warmed to allow endocytosis for 1, 3 and 5 min. Where indicated, cells were pretreated with 10 nM clusterin for 30 min before being exposed to FITC-leptin. Cells were fixed in methanol and then incubated at 4°C overnight with rabbit anti-EEA1 (early endosome marker; 1:250), mouse anti-mannose 6 phosphate receptor (late endosome marker; 1:500), or rat anti-LAP2 (lysosome marker; 1:100) antibodies (Abcam). Cells were rinsed with PBS and then incubated for 1 h at room temperature with AlexaFluor 555-conjugated secondary antibodies (1:500, Invitrogen). After washing with PBS, tissue slices were mounted using Vectashield mounting media (Vector Laboratories) and viewed using an LSM 700 confocal microscope (Carl Zeiss).
Measurement of mRNA expression
Total RNA was extracted from tissues and cells using Trizol reagents (Invitrogen). The mRNA expression levels of Lrp2 and Leprb were determined by qPCR using the primers 8. The expression of each mRNA was normalized to that of glyceraldehyde 3-phosphate dehydrogenase (Gapdh) mRNA.
Statistical analysis
Data are presented as the mean ± standard error mean (SEM). Statistical analysis was performed using SPSS-PC14 (Chicago, IL, USA). Statistical significance among the groups was tested using one-way, two-way, or repeated analysis of variance (ANOVA) followed by a post-hoc least significant difference (LSD) test, or an unpaired Student’s t-test when appropriate. Significance was defined as P < 0.05.
Acknowledgments
This study was supported by grants from the National Research Foundation of Korea (NRF-2013R1A1A3010137) and the Asan Institute for Life Science (2011-326). It was also supported by a grant from the American Diabetes Association (7-12-BS-094) to Y.B.K.
Author contributions
KHB, SYG, BL, Y-BK, and M-SK designed research; SYG, CN, B-SY, HH, M-SS, GMK, and H-KK performed experiments; KHB, SYG, BL, Y-BK, and M-SK analyzed the data and wrote the manuscript.
Conflict of interest
Financial disclosure: Patent application (PCT:KR2007-002232). There is no conflict of interest as the patents were declined.
Supporting Information
Supplementary information for this article is available online: http://embor.embopress.org
References
- Halaas JL, Gajiwala KS, Maffei M, Cohen SL, Chait BT, Rabinowitz D, Lallone RL, Burley SK, Friedman JM. Weight-reducing effects of the plasma protein encoded by the obese gene. Science. 1995;269:543–546. doi: 10.1126/science.7624777. [DOI] [PubMed] [Google Scholar]
- Vaisse C, Halaas JL, Horvath CM, Darnell JE, Stoffel M, Friedman JM. Leptin activation of Stat3 in the hypothalamus of wild-type and ob/ob mice but not db/db mice. Nat Genet. 1996;14:95–97. doi: 10.1038/ng0996-95. [DOI] [PubMed] [Google Scholar]
- Bates SH, Stearns WH, Dundon TA, Schubert M, Tso AW, Wang Y, Banks AS, Lavery HJ, Haq AK, Maratos-Flier E, et al. STAT3 signalling is required for leptin regulation of energy balance but not reproduction. Nature. 2003;421:856–859. doi: 10.1038/nature01388. [DOI] [PubMed] [Google Scholar]
- Munzberg H, Flier JS, Bjørbaek C. Region-specific leptin resistance within the hypothalamus of diet-induced obese mice. Endocrinology. 2004;145:4880–4889. doi: 10.1210/en.2004-0726. [DOI] [PubMed] [Google Scholar]
- Danik M, Chabot JG, Hassan-Gonzalez D, Suh M, Quirion R. Localization of sulfated glycoprotein-2/clusterin mRNA in the rat brain by in situ hybridization. J Comp Neurol. 1993;334:209–227. doi: 10.1002/cne.903340205. [DOI] [PubMed] [Google Scholar]
- Bajari TM, Strasser V, Nimpf J, Schneider WJ. A model for modulation of leptin activity by association with clusterin. FASEB J. 2003;17:1505–1507. doi: 10.1096/fj.02-1106fje. [DOI] [PubMed] [Google Scholar]
- Arnold T, Brandlhofer S, Vrtikapa K, Stangl H, Hermann M, Zwiauer K, Mangge H, Karwautz A, Huemer J, Koller D, et al. Effect of obesity on plasma clusterin, [corrected] a proposed modulator of leptin action. Pediatr Res. 2011;69:237–242. doi: 10.1203/PDR.0b013e31820930cb. [DOI] [PubMed] [Google Scholar]
- Gil SY, Youn BS, Byun K, Huang H, Namkoong C, Jang PG, Lee JY, Jo YH, Kang GM, Kim HK. Clusterin and LRP2 are critical components of the hypothalamic feeding regulatory pathway. Nat Commun. 2013;4:1862. doi: 10.1038/ncomms2896. [DOI] [PubMed] [Google Scholar]
- Schwartz MW, Seeley RJ, Campfield LA, Burn P, Baskin DG. Identification of targets of leptin action in rat hypothalamus. J Clin Invest. 1996;98:1101–1106. doi: 10.1172/JCI118891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kounnas MZ, Loukinova EB, Stefansson S, Harmony JA, Brewer BH, Strickland DK, Argraves WS. Identification of glycoprotein 330 as an endocytic receptor for apolipoprotein J/clusterin. J Biol Chem. 1995;270:13070–13075. doi: 10.1074/jbc.270.22.13070. [DOI] [PubMed] [Google Scholar]
- Barr VA, Lane K, Taylor SI. Subcellular localization and internalization of the four human leptin receptor isoforms. J Biol Chem. 1999;274:21416–21424. doi: 10.1074/jbc.274.30.21416. [DOI] [PubMed] [Google Scholar]
- Fernández-Galaz MC, Diano S, Horvath TL, Garcia-Segura LM. Leptin uptake by serotonergic neurones of the dorsal raphe. J Neuroendocrinol. 2002;14:429–434. doi: 10.1046/j.1365-2826.2002.00783.x. [DOI] [PubMed] [Google Scholar]
- Ceccarini G, Flavell RR, Butelman ER, Synan M, Willnow TE, Bar-Dagan M, Goldsmith SJ, Kreek MJ, Kothari P, Vallabhajosula S, et al. PET imaging of leptin biodistribution and metabolism in rodents and primates. Cell Metab. 2009;10:148–159. doi: 10.1016/j.cmet.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hama H, Saito A, Takeda T, Tanuma A, Xie Y, Sato K, Kazama JJ, Gejyo F. Evidence indicating that renal tubular metabolism of leptin is mediated by megalin but not by the leptin receptors. Endocrinology. 2004;145:3935–3940. doi: 10.1210/en.2004-0074. [DOI] [PubMed] [Google Scholar]
- Dietrich MO, Spuch C, Antequera D, Rodal I, de Yébenes G, Molina JA, Bermejo F, Carro E. Megalin mediates the transport of leptin across the blood-CSF barrier. Neurobiol Aging. 2008;29:902–912. doi: 10.1016/j.neurobiolaging.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Morales CR, Igdoura SA, Wosu UA, Boman J, Argraves WS. Low density lipoprotein receptor-related protein-2 expression in efferent duct and epididymal epithelia: evidence in rats for its in vivo role in endocytosis of apolipoprotein J/clusterin. Biol Reprod. 1996;55:676–683. doi: 10.1095/biolreprod55.3.676. [DOI] [PubMed] [Google Scholar]
- Liu Q, Zhang J, Zerbinatti C, Zhan Y, Kolber BJ, Herz J, Muglia LJ, Bu G. Lipoprotein receptor LRP1 regulates leptin signaling and energy homeostasis in the adult central nervous system. PLoS Biol. 2011;9:e1000575. doi: 10.1371/journal.pbio.1000575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Silva HV, Harmony JA, Stuart WD, Gil CM, Robbins J. Apolipoprotein J: structure and tissue distribution. Biochemistry. 1990;29:5380–5389. doi: 10.1021/bi00474a025. [DOI] [PubMed] [Google Scholar]
- Kim MS, Pak YK, Jang PG, Namkoong C, Choi YS, Won JC, Kim KS, Kim SW, Kim HS, Park JY, et al. Role of hypothalamic Foxo1 in the regulation of food intake and energy homeostasis. Nat Neurosci. 2006;9:901–906. doi: 10.1038/nn1731. [DOI] [PubMed] [Google Scholar]
- Kim MS, Park JY, Namkoong C, Jang PG, Ryu JW, Song HS, Yun JY, Namgoong IS, Ha J, Park IS, et al. Anti-obesity effects of alpha-lipoic acid mediated by suppression of hypothalamic AMP-activated protein kinase. Nat Med. 2004;10:727–733. doi: 10.1038/nm1061. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.