Abstract
Collective migration of cells in the zebrafish posterior lateral line primordium (PLLp) along a path defined by Cxcl12a expression depends on Cxcr4b receptors in leading cells and on Cxcr7b in trailing cells. Cxcr7b-mediated degradation of Cxcl12a by trailing cells generates a local gradient of Cxcl12a that guides PLLp migration. Agent-based computer models were built to explore how a polarized response to Cxcl12a, mediated by Cxcr4b in leading cells and prevented by Cxcr7b in trailing cells, determines unidirectional migration of the PLLp. These chemokine signaling-based models effectively recapitulate many behaviors of the PLLp and provide potential explanations for the characteristic behaviors that emerge when the PLLp is severed by laser to generate leading and trailing fragments. As predicted by our models, the bilateral stretching of the leading fragment is lost when chemokine signaling is blocked in the PLLp. However, movement of the trailing fragment toward the leading cells, which was also thought to be chemokine dependent, persists. This suggested that a chemokine-independent mechanism, not accounted for in our models, is responsible for this behavior. Further investigation of trailing cell behavior shows that their movement toward leading cells depends on FGF signaling and it can be re-oriented by exogenous FGF sources. Together, our observations reveal the simple yet elegant manner in which leading and trailing cells coordinate migration; while leading cells steer PLLp migration by following chemokine cues, cells further back play follow-the-leader as they migrate toward FGFs produced by leading cells.
Keywords: FGF, Agent-based modeling, Chemokines, Collective migration, Lateral line, Zebrafish
INTRODUCTION
The lateral line is a sensory system in fish and amphibians, which detects water flow over the surface of the animal. It consists of sensory organs called neuromasts arrayed in a stereotyped pattern over the body. Each neuromast contains sensory hair cells at its center, tuned to detect water flow in a particular direction. Establishment of the posterior lateral line system in zebrafish is pioneered during the second day of development by the posterior lateral line primordium (PLLp), a cluster of ∼125 cells that forms near the otic vesicle. From its position caudal to the otic vesicle, the cell cohort of the PLLp migrates collectively along the horizontal myoseptum to the tip of the tail, periodically depositing neuromasts, sequentially referred to as the L1, L2, L3, etc. neuromasts (Fig. 1A) (Gompel et al., 2001).
Fig. 1.
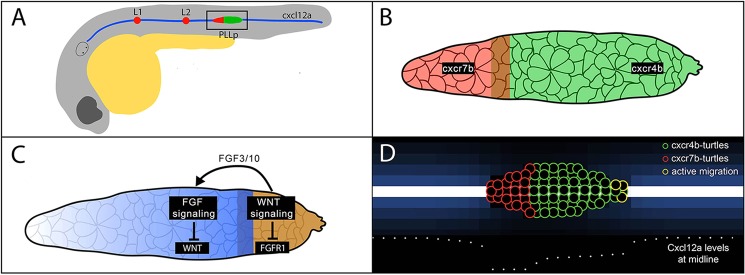
Schematic of PLLp migration, gene expression domains and example model output. (A) Schematic of PLLp migration along the myoseptum. (B) Detail of the region outlined in A, showing approximate expression domains of cxcr4b and cxcr7b. (C) Schematic of juxtaposed Wnt and FGF signaling domains in the PLLp. Arrow indicates diffusion of FGF3 and FGF10A. (D) Example of PLLp computational model. In all model figures green circles represent cxcr4b-turtles, red circles represent cxcr7b-turtles and yellow circles represent actively migrating turtles. Blue shades show levels of Cxcl12a in patches.
The PLLp in the zebrafish, Danio rerio, has emerged as an extraordinary model system in which to study collective cell migration, a type of migration broadly observed in a variety of developmental and disease contexts, including gastrulation, branching morphogenesis, epithelial wound healing and tissue repair, and invasive growth of tumor strands (for a review, see Friedl and Gilmour, 2009). During collective migration, direction-sensing and migratory behavior must be coordinated between many cells, while maintaining cohesive interactions. How this is achieved in various contexts is now only beginning to be understood. Chemokine signaling in the PLLp guides leading cells (David et al., 2002; Haas and Gilmour, 2006; Li et al., 2004); however, questions remain as to how exactly this system determines directional migration and how trailing cells follow leading cells of the PLLp.
Establishment of polarized Wnt and FGF signaling systems coordinates many aspects of morphogenesis, migration and cell fate specification in the PLLp (Fig. 1C; supplementary material Fig. S1A-E). Formation of nascent neuromasts or ‘protoneuromasts’ in the migrating PLLp is initiated by Wnt signaling-dependent secretion of FGFs by leading cells. However, Wnt signaling also induces expression of intracellular inhibitors of FGF receptor signaling such as Sef and Mkp3 (Aman and Piotrowski, 2008; Matsuda et al., 2013). So the FGFs, secreted in response to Wnt signaling in leading cells, are unable to activate FGF receptor signaling and sustain FGF receptor expression in these cells. Instead, the FGFs are delivered to an adjacent trailing domain, where activation of FGF receptors initiates protoneuromast formation and maintains FGF receptor expression. As additional protoneuromasts periodically form toward the leading end, previously formed protoneuromasts mature and are eventually deposited from the trailing end by the migrating primordium (Aman and Piotrowski, 2008; Nechiporuk and Raible, 2008).
The PLLp is guided along the horizontal myoseptum by the transient expression of the chemokine Cxcl12a (also known as Sdf1a) (David et al., 2002; Li et al., 2004). Directionality of PLLp migration is not encoded by graded expression of this chemoattractant signal, instead, directional migration requires the differential expression of two chemokine receptors, Cxcr4b and Cxcr7b (Dambly-Chaudiere et al., 2007; Valentin et al., 2007; Fig. 1B; supplementary material Fig. S1F,G). cxcr4b transcripts are most prominent in a leading domain, whereas cxcr7b transcripts are restricted to a trailing domain. However, there is a significant overlap between these two expression domains, and in reality Cxcr4b protein is distributed throughout the PLLp, including the trailing domain (Dona et al., 2013).
Several models for how these receptors impart directional movement to the PLLp have been proposed based on either differences in their ability to allow cells to respond to chemokines with migratory behavior or in their role in facilitating internalization and subsequent degradation of the chemokines. One model suggests that whereas Cxcr4b is capable of binding Cxcl12a and triggering migratory behavior, Cxcr7b is not. Instead, it promotes the rapid internalization and degradation of Cxcl12a (Burns et al., 2006; Boldajipour et al., 2008; Naumann et al., 2010). In the context of the PLLp, this hypothesis implies that Cxcr7b in the trailing domain locally depletes the chemokine, thus creating a gradient of chemokine availability along the length of the PLLp (Dambly-Chaudiere et al., 2007; Ghysen and Dambly-Chaudiere, 2007). This, it has been suggested, ensures that Cxcr4b-expressing cells steer migration by responding to the relatively high levels of chemokine they see at the leading end of the PLLp. Another model suggests that if all the PLLp cells were to internalize and degrade Cxcl12a, then, as the PLLp migrates, it would degrade Cxcl12a in its path, leaving less in its wake. The resulting asymmetry in the distribution of Cxcl12a, this model suggests, could also account for directed PLLp migration (Streichan et al., 2011).
Recent studies have directly examined the Cxcl12a gradient during primordium migration (Dona et al., 2013; Venkiteswaran et al., 2013). For example, using tools that measure the lifetime of the Cxcr4b receptor, Dona et al. infer the presence of a gradient of Cxcr4b internalization along the length of the PLLp by demonstrating a shorter receptor lifetime in leading cells compared with trailing cells. Internalization of Cxcr4b is determined by its interaction with Cxcl12a. The average lifetime of the Cxcr4b receptor in this study has therefore been interpreted to reflect a gradient in the availability of Cxcl12a in the surrounding environment. These data support a model by which trailing cells act as a sink to polarize Cxcl12a availability to Cxcr4b along the length of the PLLp. However, these studies leave important questions unanswered: is the primary purpose of Cxcl12a internalization by Cxcr7b in trailing cells to provide directional information via a chemokine gradient to leading cells in order to polarize their migration; or is the primary function of ligand degradation by Cxcr7b to prevent Cxcr4b activation in trailing cells?
Evidence from transplant experiments shows that even a small number of transplanted wild-type cells can rescue Cxcl12a-mediated migration in a cxcr4b mutant PLLp. However, they appear to do so only when positioned at the leading edge of the PLLp (Valentin et al., 2007). This suggests that Cxcr4b is essential only in cells that are at the leading end of the PLLp and that Cxcr4b-mediated chemokine signaling may operate to determine a migratory response only when Cxcr4b-expressing cells are at one or more edges of the PLLp. On the other hand, when transplanted cxcr4b mutant cells are not at the leading end of a wild-type PLLp and are in the interior of the PLLp, they are able to migrate and behave indistinguishably from their wild-type counterparts (Haas and Gilmour, 2006). This suggests that movement of trailing cells in the PLLp does not require Cxcr4b-mediated chemokine signaling and that alternate mechanisms govern collective migration of trailing cells within the PLLp.
In the present study, agent-based computational models were built to explore how a migratory response to Cxcl12a, which is mediated by Cxcr4b in leading cells, could determine unidirectional migration of the PLLp when coupled with local depletion of Cxcl12a by chemokine receptors. The models were able to recapitulate PLLp behavior in a variety of situations. We describe experimental manipulations of the PLLp designed to test some of the predictions of the chemokine signaling-based model of PLLp migration. Analysis of these experiments now suggests that the primary role of Cxcr7b-mediated degradation by trailing cells is to ensure a polarized response to Cxcl12a by ensuring Cxcr4b receptors in trailing cells do not have access to the chemokine.
Although our chemokine signaling-based models were able to account for various behaviors of leading cells, they could not account for specific chemokine-independent behaviors of trailing cells in the PLLp. Further experimental investigation of trailing cell behavior in this study now reveals the simple yet elegant manner in which leading and trailing cells coordinate their migration. We show that while leading cells steer PLLp migration by following chemokine cues, trailing cells play follow-the-leader as they migrate toward FGFs produced by leading cells. Our study illustrates how the development of an agent-based model has allowed us to visualize how chemokine signaling determines collective migration of the PLLp and to account for some complex emergent behaviors of the PLLp. More importantly, however, the study illustrates how the failure of the model to recapitulate specific behaviors has played a key role in inspiring a series of experiments that now provide a more-integrated view of how leading and trailing cells cooperate to guide collective migration of the PLLp.
RESULTS
Construction of an agent-based model of PLLp migration
We constructed agent-based computational models of the PLLp in the modeling environment Netlogo (Wilensky, 1999) to explore the potential roles of leading Cxcr4b- and trailing Cxcr7b-expressing cells in degrading Cxcl12a and guiding migration of the PLLp. This programming environment consists of individual mobile agents called turtles, where multiple breeds of turtles can be defined that behave according to distinct sets of rules. In addition there are links that form visco-elastic connections between defined sets of turtles and individual non-motile agents called patches, which define the environment in which the turtles operate.
In our models, patches are used to represent the substrate upon which the PLLp migrates and to define the source of Cxcl12a. Patches in a horizontal stripe one-cell wide along the middle of our model world, representing the horizontal myoseptum, produce Cxcl12a (Fig. 1D). It diffuses and is degraded at a defined rate by the patches. The PLLp is made of two breeds of turtles (Fig. 1D): a leading compartment of Cxcr4-turtles (green), representing cells that only express Cxcr4b; and a trailing compartment of Cxcr7b-turtles (red) that represent cells expressing both Cxcr4b and Cxcr7b. As transplant experiments suggest that only cells at the edges of the PLLp move in response to Cxcl12a in their environment, we replicate this in the model so that only turtles at a free edge respond to Cxcl12a.
Links connect adjacent turtles and model adhesive interactions between cells of the PLLp. Although movement of the PLLp is initiated by the movement of turtles at the edge of the PLLp, the visco-elastic links indirectly determine the movement of the remaining cells of the model PLLp. This implementation assumes that coordinated movement of cells within the PLLp is determined by the adhesive or mechanical coupling of cells. Though this simplification ignores additional mechanisms that determine active migration of trailing cells, it allowed us to focus on chemokine-dependent migratory behavior in these models. The model file (pLLP model.zip) and details on its construction can be found in the supplementary material. Readers are encouraged to experiment with the model using the Netlogo software, available at http://ccl.northwestern.edu/netlogo/.
The absolute level, rather than a gradient of Cxcl12a, determines the migratory response
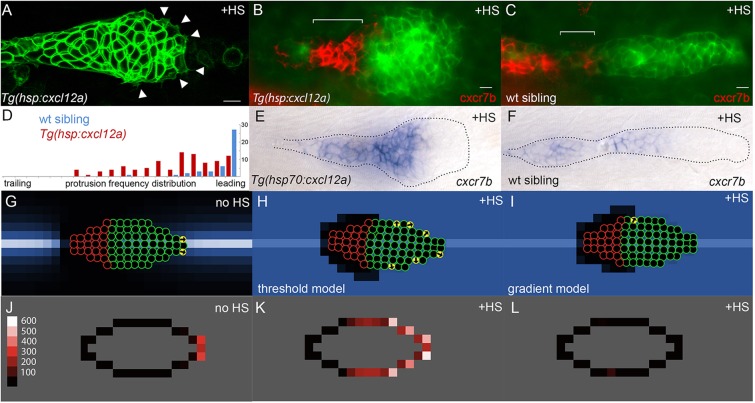
During the construction of agent-based models, we had to decide whether some minimal gradient or some threshold level of Cxcl12a determines migratory behavior of Cxcr4b-turtles in the PLLp. To test which of these determines protrusive cell behavior in the actual PLLp, we used heatshock to induce broad ectopic expression of Cxcl12a in Tg(hsp:cxcl12a) embryos (Knaut et al., 2005). Broad ectopic expression was expected to increase the absolute level of Cxcl12a, while at the same time flatten the endogenous gradient (Dona et al., 2013). Protrusive activity was no longer primarily observed at the leading end of the PLLp following heatshock-induced Cxcl12a expression; instead, protrusive activity was seen around the entire edge of a leading domain of the PLLp, where cxcr7b was not expressed (Fig. 2A-D; supplementary material Movie 1 and Fig. S2). This suggested that protrusive activity is determined by the level of Cxcl12a present, rather than by the steepness of the Cxcl12a gradient. However, protrusive activity remained low in the trailing area, where cxcr7b is expressed (Fig. 2B,D). This suggested that, despite relatively high levels of induced expression, Cxcr7b-expressing cells prevent Cxcr4b receptors in trailing cells from having access to levels of Cxcl12a that are high enough to stimulate chemokine-dependent protrusive activity.
Fig. 2.
Experimental and simulated overexpression of Cxcl12a. (A) Morphology of PLLp in Tg(hsp:cxcl12a) after 1 h of heatshock (corresponding to t=78 min in supplementary material Movie 1). Arrowheads indicate protrusions. (B,C) False-color overlay of cxcr7b in situ hybridization (red) in Tg(hsp70:cxcl12a) (B) and wild-type sibling embryo (C) after heatshock for 1 h and recovery for 2 h. (D) Frequency of protrusions observed from the leading to the trailing end of the PLLp in embryos with heatshock-induced cxcl12a expression (red bars, six embryos) compared with that in control siblings (blue, six embryos). Each bar represents the frequency of protrusions observed in 5% increments of the relative distance from the leading end of the PLLp observed. (E,F) In situ hybridization for cxcr7b transcript in Tg(hsp:cxcl12a) (E) and wild-type sibling (F) PLLp after a 30 min heatshock at 37.5°C and 4 h of recovery. (G) Model PLLp without heatshock. (H) Model PLLp after heatshock when turtles are migrating in response to above-threshold levels of Cxcl12a. (I) Model PLLp after heatshock when turtles are migrating in response to the gradient of Cxcl12a. (J-L) Time-averaged position of actively migrating turtles within model PLLp for the conditions in G-I, respectively. Scale (‘protrusions’/1000 model iterations) is in J. Scale bars: 10 µm.
Based on these observations, we implemented migratory behavior in our models that was determined by the absolute level of Cxcl12a encountered by the cxcr4b-turtles, rather than the steepness of the Cxcl12a gradient. Specifically, at each time step, each cxcr4b-turtle orients in a random direction, checks that there is no turtle ahead (confirming it is an edge of the PLLp), and then, if the absolute level of Cxcl12a in the empty patch ahead exceeds some defined threshold, it moves ahead (see model description in the supplementary material for details). In our models, the green cxcr4b-turtles undergoing this migratory response to Cxcl12a transiently assume a yellow color so their ‘protrusive’ activity can be monitored in simulations.
We also assumed relatively high levels of Cxcl12a degradation by trailing cxcr7b-turtles. This prevented trailing cells from being exposed to levels of Cxcl12a that exceed the threshold required to determine a migratory response, even in the context of broad exaggerated Cxcl12a expression. Simulations incorporating these parameters illustrate how protrusive activity in the model PLLp, represented by the migratory response of individual turtles, recapitulates what is seen in the actual PLLp, both in control embryos and in embryos with heatshock-induced Cxcl12a expression (Fig. 2G-K, compare with D). By contrast, when the migratory response was determined by the steepness of the Cxcl12a gradient, heatshock-induced Cxcl12a expression flattened the gradient and this was accompanied by reduced protrusive activity around the edges of the model PLLp, not increased protrusive activity as in the actual PLLp (Fig. 2I,L).
It was initially surprising to observe that, when high levels of Cxcl12a expression are induced with heatshock, trailing cxcr7b-expressing cells remain relatively unresponsive. However, 4 h after heatshock induction of cxcl12a expression there was a significant increase in cxcr7b transcript expression, which nevertheless remained confined to a trailing domain of the PLLp (Fig. 2E,F; quantified in supplementary material Fig. S3). This suggests that autoregulation of cxcr7b expression by Cxcl12a is part of a robust mechanism that ensures Cxcr4b receptors in trailing cells are not exposed to high levels of Cxcl12a.
Chemokine-dependent models recapitulate the results of laser-dissection of the PLLp
Simulations of PLLp migration with models based on the assumptions described above were able to recapitulate directed migration (supplementary material Movie 2). To challenge our models, we asked how effectively they were able to recapitulate the behavior of the PLLp following experimental manipulations in which the PLLp was cut into individual fragments. The first such manipulation was based on an experiment initially described by Gilmour et al. (2009). In it, laser ablation of PLLp cells, just in front of the leading protoneuromast, was used to create two fragments: a small leading fragment with 10-20 cells, shown to contain only cxcr4b-expressing cells; and a larger trailing fragment, containing both leading cxcr4b- and trailing cxcr7b-expressing cells (supplementary material Fig. S4). The purpose of this experiment was to determine whether degradation of Cxcl12a by trailing PLLp cells creates a broad enough local depression in Cxcl12a availability to polarize migration of the leading cxcr4b-expressing fragment.
The outcome of this manipulation in vivo is complex and the two PLLp fragments display variable but reproducible behaviors following the laser cut. In most cases, the leading fragment moves forward a short distance and then stalls (Fig. 3A; supplementary material Movie 3). The stalling of the leading fragment suggests that clearance of Cxcl12a by trailing Cxcr7b-expressing cells is not adequate to maintain polarized caudal migration of the leading fragment, at least over long distances. Stalling of the leading fragment is accompanied by protrusive activity in both directions and stretching of the leading fragment (arrowheads in Fig. 3A). This stretching behavior is independent of the influence of the trailing fragment and was also observed when we performed an additional ablation wherein all trailing cells were removed (Fig. 3C). In this context, we observed the same stalling and bilateral stretching of the remaining leading fragment over the course of several (>7) hours of time-lapse imaging. In contrast to the stalling of the leading fragment, the polarized large trailing fragment initially stalls and then, following some delay, always resumes caudal migration (Fig. 3B). When the leading and trailing fragments come close enough to each other they eventually rejoin and the PLLp in its entirety resumes its migration (supplementary material Movie 3; Gilmour et al., 2009). When the gap between fragments is very short, no stalling is seen. Instead, the leading fragment continues to move forward, the trailing fragment follows, eventually catches up and joins, and the reconstituted PLLp resumes its caudal migration (data not shown).
Fig. 3.
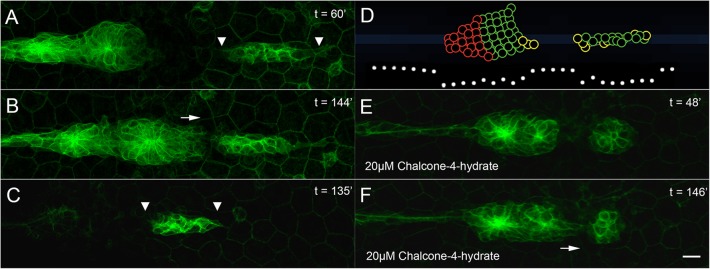
PLLp morphology following laser ablation. (A) PLLp fragments 60 min after ablation to separate leading cells from trailing cells. Arrowheads indicate stretching of the leading fragment. (B) As in A, 144 min after ablation. Arrow indicates migration of trailing cells toward leading cells. (C) PLLp 135 min after complete ablation of trailing cells. Arrowheads indicate stretching of leading fragment. (D) Model ablation showing cxcr4b (green) and cxcr7b (red) turtles. White dots indicate the levels of Cxcl12a along the midline. (E) PLLp fragments 90 min after ablation in the presence of 20 µM chalcone-4-hydrate. Leading cells adopt a rounded morphology. (F) Same embryo as in E, 148 min after ablation. Arrow indicates migration of trailing cells toward leading cells. Scale bar: 10 µm.
Our agent-based model was able to easily reproduce many aspects of the behavior of both leading and trailing fragments (Fig. 3D; supplementary material Movie 4) and provided a potential explanation for it. The bi-directional stretching is consistent with the unpolarized cxcr4b-expressing leading fragment responding to chemokine cues at either end with protrusive activity. On the other hand, build up of newly secreted Cxcl12a between severed fragments could account for the movement of the trailing and leading fragments toward each other following their separation. These potential explanations suggested that both bilateral stretching of the leading fragment and the movement of the trailing fragment toward the leading fragment would be lost if responses to Cxcl12a were blocked.
To test these predictions, we repeated these experiments in the presence of chalcone-4-hydrate, an inhibitor of Cxcl12a activity (Hachet-Haas et al., 2008). We confirmed the efficacy of this inhibitor by first demonstrating that treatment with 10 μM of chalcone-4-hydrate resulted in slowing and eventual stalling of PLLp migration (data not shown). In PLLps severed to create leading and trailing fragments after treatment with chalcone-4-hydrate to inhibit Cxcl12a signaling, we failed to observe bilateral stretching of the leading fragment. Instead, it appeared as a compact cluster of cells [average circularity 0.72±0.09 for treated (n=13) and 0.43±0.13 for DMSO treated (n=5) leading fragments after ablation, P<0.001, Fig. 3E]. These data confirm that stretching of the leading fragment is due to a bilateral response to Cxcl12a by both ends of the cxcr4b-expressing leading fragment, as suggested by our model.
On the other hand, exposure to chalcone-4-hydrate did not prevent the caudal migration of the trailing fragment toward the leading fragment (Fig. 3F; supplementary material Movie 5, 10/13 embryos). The persistent movement of the trailing fragment toward the leading fragment in the presence of an inhibitor of chemokine signaling suggested that this behavior did not depend on the polarized response of the trailing fragment to Cxcl12a, but may instead be related to the influence of leading cells on the trailing fragment. This possibility was supported by the observation that in some cases the trailing fragment appeared to leap toward the leading fragment (supplementary material Movie 3, lower panel), a phenomenon that could not be accounted for by our chemokine-based migration models.
Laser ablation reveals a role for leading cells in guiding trailing cell migration
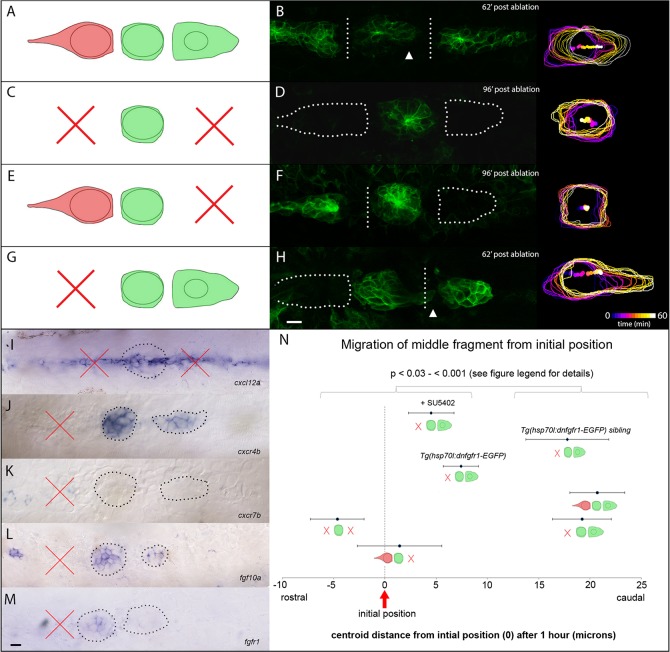
To further explore how leading cells influence trailing cells, we selected embryos at a stage when the PLLp has three protoneuromasts and laser ablated a stripe of cells both rostral and caudal to the central protoneuromast, mechanically isolating this protoneuromast from the remaining leading and trailing cells of the PLLp (Fig. 4A,B). This separated the PLLp into a trailing, middle and leading fragment. When examined, only the trailing fragment had cxcr7b expression (data not shown). Despite having no obvious physical link to either trailing or leading cells, the isolated middle fragment consistently moved toward the leading fragment of the PLLp (Fig. 4A,B,N), allowing it to reconnect eventually with the leading fragment (four out of four embryos; supplementary material Movie 6, top panel). This behavior is consistent with the leading fragment being the source of chemoattractive cues for the isolated middle fragment. On the other hand, it is also consistent with Cxcr7b in the trailing fragment locally degrading Cxcl12a and decreasing the likelihood of migratory behavior of the middle fragment toward trailing cells. A third possibility is that it is related to some inherent polarity in cells of the middle fragment, which allows them to continue migration for a short distance in their original direction of migration. It is important to note that the severing of physical connections between leading and trailing cell populations in this experiment suggests that adhesive mechanical connections are not essential for leading cells to initiate directional migration of trailing cells in this context.
Fig. 4.
PLLp morphology following double laser ablation. (A,B) Schematic of ablation and PLLp fragments 62 min after ablation to separate leading, middle and trailing fragments. Arrowhead indicates migration of middle fragment toward leading fragment. (C,D) Schematic of ablation and PLLp fragments 96 min after ablation to remove leading and trailing cells. (E,F) Schematic of ablation and PLLp fragments 96 min after ablation to separate middle from trailing cells, and remove leading cells. (G,H) Schematic of ablation and PLLp fragments 62 min after ablation to separate middle from leading cells, and remove trailing cells. Arrowhead indicates migration of trailing cells toward leading cells. Right panels in B,D,F,H show outlines and centroid position of middle fragments over time (see scale in H). (I) Expression of cxcl12a in the horizontal myoseptum after ablation. (J,K) Expression of cxcr4b (J) and cxcr7b (K) in PLLp immediately following ablation to generate isolated leading and middle fragments. (L,M) Expression of fgf10a (L) and fgfr1 (M) in PLLp immediately following ablation to generate isolated leading and middle fragments. In I-M, dashed lines indicate PLLp fragments. Crosses indicate ablated fragments. (N) Quantification of middle fragment centroid movement in all experimental conditions, as indicated by diagrams (mean±s.e.m.). Statistical significances of all pairwise comparisons between the indicated groups fall between P<0.03 and P<0.001, Student's t-test. See supplementary material Table S2 for all pairwise statistical comparisons. Scale bars: 10 µm.
To test these three hypotheses, we re-examined the behavior of isolated middle fragments following complete ablation of the leading and/or trailing fragments. When both leading and trailing fragments were ablated, the isolated middle fragment lost its polarized caudal migratory behavior (12 out of 13 embryos, Fig. 4C,D,N; supplementary material Movie 6, second panel). This argues against any inherent polarity determining this behavior, and suggests that leading and/or trailing fragments provide directional cues to cells in the middle fragment.
When the leading fragment was ablated but the trailing fragment was left intact, we did not observe polarized caudal movement of the middle fragment (Fig. 4E,F,N; supplementary material Movie 6, third panel, four out of five embryos). As the trailing fragment has the bulk of the Cxcr7b-expressing cells, its failure to significantly polarize migratory behavior of the middle fragment when they are separated suggests that degradation of Cxcl12a by Cxcr7b-expressing cells is insufficient to polarize chemokine-dependent migration of the middle fragment, at a distance, on its own.
When the trailing fragment was ablated, leaving the leading fragment intact, the middle fragment consistently moved caudally toward the leading fragment, and the two fragments eventually rejoined (Fig. 4G,H,N; supplementary material Movie 6, bottom panel, five out of five embryos). We confirmed by RNA in situ hybridization that the ablation did not affect expression of cxcl12a in the underlying myoseptum (Fig. 4I). Furthermore, the directional migration was not due to polarized expression of cxcr4b and cxcr7b in the middle fragment (Fig. 4J,K). Together, these observations demonstrate that movement of the middle fragment toward the leading fragment is likely to be a response to attractive cues from the leading fragment.
FGFs mediate attraction of trailing cells toward leading cells
What guidance cue might leading cells provide for trailing cells? As noted earlier, cells in the leading zone are a source of FGF ligands (Aman and Piotrowski, 2008). FGFs serve as migratory cues in a variety of other contexts (Kadam et al., 2012; Vitorino and Meyer, 2008), making them strong candidates for providing a chemoattractive cue for trailing cells. Previous work has already shown that cryptic lamellipodia in trailing cells of the intact PLLp are normally polarized toward the leading edge, and that this polarization is lost upon inhibition of FGF signaling by treatment with the Fgfr1 inhibitor SU5402 (Lecaudey et al., 2008; Sun et al., 1999). It has also been shown that at an early stage of PLLp formation, around 18 hpf, isolated leading primordial cells are attracted toward the trailing PLLp cells by FGF signals (Breau et al., 2012). We determined by in situ hybridization that fgf10a expression is maintained in leading cells after ablation (Fig. 4L) and, furthermore, that fgfr1 expression was restricted to trailing cells, as in the intact PLLp (Fig. 4M).
To test whether FGFs secreted by the leading cells are providing a chemoattractive cue for trailing cells during migration we first asked whether inhibition of FGF signaling prevents cells in a middle fragment from migrating toward the leading fragment. We pre-treated embryos with SU5402 for 2-4 h and then performed laser ablation to isolate a middle fragment of the PLLp. The trailing fragment was then ablated, leaving the middle and leading fragments intact. In the presence of SU5402 there was significantly less caudal movement of the middle fragment toward the leading fragment (Fig. 4N and Fig. 5A,B; supplementary material Movie 7, upper panel, n=9). However, in embryos in which SU5402 was washed out after ablation, we observed a gradual recovery of movement of the middle fragment toward the leading fragment and eventual joining of these two fragments in six out of seven embryos examined (Fig. 5C,D; supplementary material Movie 7, lower panel). Similarly, in transgenic Tg(hsp70l:dn-fgfr1) embryos subjected to a 1 h heatshock, movement toward leading cells was also significantly inhibited compared with non-transgenic control sibling embryos (data not shown, quantification in Fig. 4N).
Fig. 5.
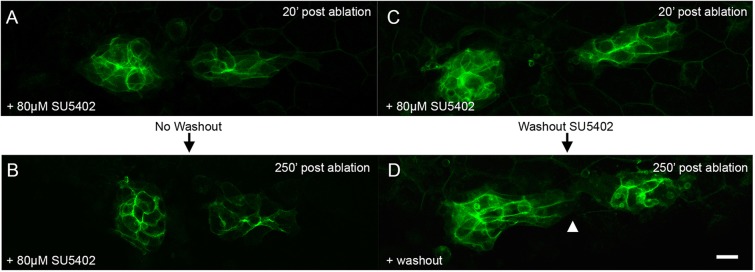
FGF signaling is required for polarized migration of the middle fragment. (A,B) PLLp fragments 20 and 250 min after ablation, but in the presence of 80 µM SU5402. (C,D) PLLp fragment 20 and 250 min after ablation, with subsequent wash-out of SU5402. Arrowhead indicates migration of trailing cells toward leading cells. Scale bar: 10 µm.
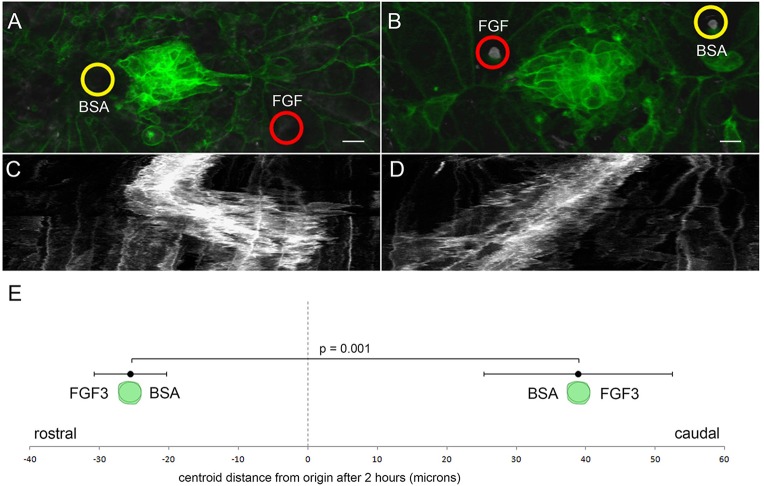
To demonstrate more directly that FGF can act as a chemotactic signal, we repeated the experiments with an isolated middle fragment to see whether the FGF-soaked beads could elicit the same polarized chemoattractive response from cells in the middle fragment that we had previously demonstrated could be elicited by the leading fragment. We again used laser ablation to isolate a middle fragment and then eliminated the remaining leading and trailing cells of the PLLp. After this ablation, we transplanted beads soaked in either FGF3 or BSA on opposing sides of the fragment and recorded the subsequent behavior of the cells by time-lapse microscopy (Fig. 6A,B). While the BSA-soaked bead provided a control for the effects of FGF, placement of two beads, one rostral and one caudal to the middle fragment, ensured that biased movement of the middle fragment was not related to unilateral tissue damage incurred during placement of beads. When FGF beads were placed caudally to the isolated middle fragment it migrated in the caudal direction just as they do toward an isolated leading fragment (six out of six embryos; Fig. 6A,C,E; another example is shown in supplementary material Movie 8, upper panel). Furthermore, when the FGF-soaked bead was placed rostrally, where the trailing fragment would normally be located, the cells reversed direction to migrate back toward the rostrally placed FGF3-soaked bead (five out of six embryos, Fig. 6B,D,E; supplementary material Movie 8, lower panel). These data suggest that exogenous FGF signals are sufficient to polarize migration of cells in an isolated middle fragment.
Fig. 6.
PLLp migration polarized by FGF-soaked beads. (A) Morphology of the middle PLLp fragment after insertion of a FGF3-soaked (red circle) bead caudal to and a BSA soaked (yellow circle) bead rostral to the PLLp fragment. (B) Similar to A with position of the FGF3-soaked (red circle) and BSA-soaked (yellow circle) beads reversed. (C,D) Kymograph of cell behavior during a ∼400 min time-lapse in A and B, respectively. (E) Quantification of movement of centroid position of cells (mean±s.e.m.) with FGF3-soaked beads either rostral or caudal to cells, as indicated. Scale bars: 10 µm.
DISCUSSION
This study began with the development of an agent-based model of the PLLp to explore how polarized expression of the chemokine receptors, Cxcr4b and Cxcr7b, coupled with local depletion of the chemokine Cxcl12a by these receptors, determines directed collective migration of cells in the PLLp along a relatively uniform stripe of Cxcl12a expression. To test the agent-based model, we asked how well it recapitulates the behavior of a small leading and larger trailing fragment of the PLLp generated by laser ablation. As the agent-based model was able to recapitulate many of the observed in vivo behaviors of leading and trailing fragments, it was initially thought that their behaviors could be accounted for by the differential response of Cxcr4b and Cxcr7b receptors to dynamic changes in Cxcl12a expression. When this hypothesis was tested with a chemical inhibitor of Cxcl12a function, characteristic bilateral stretching of the leading fragment was lost. On the other hand, the movement of the trailing fragment toward the leading fragment persisted, suggesting that this behavior is independent of Cxcl12a-mediated chemokine signaling and that the leading fragment is a source of additional cues that determine polarized behavior of trailing cells. An investigation of this possibility revealed that FGFs produced by PLLp cells in a leading domain can influence the direction in which cells in more trailing domains migrate.
Previous studies have suggested Cxcr7b acts as a ‘decoy’ receptor by binding, internalizing and degrading Cxcl12a, rather than facilitating a protrusive migratory response to the chemokine. Other studies have shown that just a few Cxcr4b cells at the leading end or Cxcr7b cells at the trailing end are adequate to determine this polarized response of the PLLp (Valentin et al., 2007). However, our observations suggest it is unlikely that local degradation of Cxcl12a by trailing cells can establish a gradient that is broad enough to determine polarized migratory behavior of individual leading Cxcr4b-expressing cells at a distance. For example, a leading fragment of the PLLp generated by laser ablation, typically stretches bi-directionally in response to local Cxcl12a and does not maintain polarized caudal protrusions. Similarly, when laser ablation was used to generate a middle and trailing fragment, the trailing fragment, containing primarily Cxcr7b-expressing cells, was unable to deplete Cxcl12a in a manner that polarizes migration of the middle fragment.
Although local depletion by the Cxcr7b receptor may not be able to establish a Cxcl12a gradient that can influence migratory behavior of Cxcr4b-expressing cells at a distance, its local depletion by Cxcr7b receptors is likely to ensure that Cxcr4b receptors, also expressed in trailing cells, are unable respond to trailing Cxcl12a and determine migratory behavior in the opposite direction. Indeed our Cxcl12a heatshock experiments indicate that when cells are exposed to artificially high levels of Cxcl12a, this eventually induces higher levels of cxcr7b expression in trailing cells, ensuring the trailing domain with cxcr7b expression remains relatively unresponsive to the chemotactic influence of this chemokine.
If the primary role of Cxcr7b-dependent depletion of Cxcl12a is to prevent trailing Cxcr4b-expressing cells from responding to Cxcl12a, this could be achieved either by out competing Cxcr4b for binding to Cxcl12a, thereby reducing the available ligand, or by heterodimerizing with Cxcr4b to dampen downstream signaling (Levoye et al., 2009). A recent study has shown that Cxcr7b expressed exogenously in the lateral line nerve, which travels underneath the migrating PLLp, is able to non-autonomously rescue PLLp migration in a cxcr7b mutant (Dona et al., 2013). This supports the notion that lack of ligand availability to Cxcr4b may be adequate to prevent Cxcr4b activation in trailing cells. A predominantly local role for Cxcr7b in determining the response of trailing cells helps explain why restoring even a few Cxcr7b-expressing cells at the trailing end can restore directed migration of a cxcr7b mutant PLLp (Valentin et al., 2007).
We have described a chemoattractive role for FGF in polarizing the migration of trailing cells during collective migration of the lateral line primordium in zebrafish embryos. Previous studies showed that inhibition of FGF signaling by SU5402 results in loss of polarization of cryptic lamellipodia extended by trailing cells (Lecaudey et al., 2008). However, as these observations were made in an intact PLLp, they could be interpreted to suggest that FGF signaling orients processes extended by trailing cells based on a mechanism that requires transmission of mechanical tension from leading cells to trailing cells. As a severed leading fragment secreting FGFs or an ectopic source of FGFs can influence the migration of trailing cells, our study suggests FGF signaling organizes directed collective migration of trailing cells through a mechanism that can operate at a distance. However, this observation does not rule out an additional role for mechanical tension generated between in PLLp cells, determined by FGF signaling or other factors, in coordinating trailing cell migration, either within the intact PLLp or within the smaller fragments examined here. It is important to note that when the PLLp was severed to create a leading and middle fragment, fgf10a expression was seen in both middle and leading fragments, whereas fgfr1 expression was absent in the leading fragment. Hence, it is not only the expression of FGFs by cells in the leading zone that determines polarized migration but also the polarized expression of the FGF receptor, the expression of which is significantly less in leading cells (supplementary material Fig. S1E; Fig. 4M). The mechanism by which FGF signals polarize migratory behavior remains unclear, as FGF does not simply promote protrusive migratory behavior. Rather, the presence of a sustained source of FGFs in the leading zone of the migrating PLLp appears to polarize migratory behavior in trailing cells that would otherwise be present but unpolarized in the absence of FGF signaling (Lecaudey et al., 2008).
Agent-based models provide a platform for understanding how similar behaviors emerge when cells interact by following specific rules, independent of the molecular pathways mediating these interactions. Similar strategies appear to be in operation during the collective migration of PLLp and neural crest cells. Long-range migration of cranial neural crest cells is determined by leading cells following VEGF cues secreted by the overlying ectoderm (McLennan et al., 2010). As with Cxcl12a in the lateral line system, leading crest cells do not follow an inherent VEGF concentration gradient, instead VEGF degradation by migrating neural crest cells ensures leading cells always encounter the highest levels of VEGF ahead in their path. However, depletion of VEGF prevents it from serving as an effective guidance cue for trailing cells, so trailing cells follow leading cells by a different strategy. Neural crest cells secrete a factor, C3a, which serves as a short-range chemoattractant (Carmona-Fontaine et al., 2011) that ensures crest cells migrate together. In this manner, though very different signaling systems are involved, collective migration in both the PLLp and neural crest systems involves a similar follow-the-leader strategy.
In the agent-based models described in this study, visco-elastic links between the turtles play a key role in determining collective migration. However, this aspect of the model is an over simplification. Though intercellular adhesions are likely to contribute in some way to collective migration in the PLLp, the trailing cells are not simply dragged along by leading cells. Instead, they actively migrate toward leading cells. Our experiments suggest that, in part, this is determined by the chemoattractive role of FGFs secreted by leading cells. However, the mechanisms that define cohesive migration in the PLLp remain poorly understood.
Future studies will determine how mechanical tension mediated by adhesion molecules, or by chemoattractants operating at short range, contribute to cohesive migration. The ideas discussed above will play a key role in the construction of the next generation of agent-based models of the PLLp. The iterative construction of such in silico models coupled with rigorous experimental validation promises a progressively deeper understanding of collective migration of the zebrafish PLLp system.
MATERIALS AND METHODS
Fish lines, chemical treatment and in situ hybridization
Zebrafish were maintained under standard conditions and staged according to Kimmel et al. (1995). All experiments used Tg(clbnb:lynGFP) transgenic embryos (Haas and Gilmour, 2006) generated by natural spawning. For heatshock induction of Cxcl12a expression, we used Tg(hsp:cxcl12a) embryos in a Tg(clbnb:lynGFP) background, heatshocked for 1 h at 37.5°C. For FGF receptor inhibition, embryos were treated with 20-80 μM SU5402 (Tocris) with shaking for 2-4 h prior to ablation. In practice, we observed significant manufacturer-to-manufacturer and batch-to-batch variability in the toxicity of SU5402, and as such each stock solution was independently titrated and concentrations between 20 and 80 µM were used. Tg(hsp70l:dn-fgfr1) (Lee et al., 2005) embryos were heatshocked for 1 h prior to ablation. For inhibition with chalcone-4-hydrate, embryos were placed in 10-20 μM inhibitor 30 min before ablation. In situ hybrization was performed as described elsewhere (Matsuda and Chitnis, 2010). To generate the false-color pictures in Fig. 2, Tg(clbnb:lynGFP) embryos were stained after in situ with anti-GFP antibody (Abcam). DIC images of NBT/BCIP in situ staining were inverted and false-colored in ImageJ (Schneider et al., 2012), then overlaid onto fluorescent images of anti-GFP staining.
Laser ablation and time-lapse microscopy
To quantify protrusions following induction of cxcl12a expression, individual frames from time-lapse movies over ∼3 h were examined and protrusions manually annotated. Protrusions were identified as dynamic membrane displacements, at least half a cell diameter in width, from the edge of the PLLp. The frequency of protrusions was measured based on the relative distance of each protrusion from the leading end of PLLp. Laser ablation was performed either using a Mai-Tai DeepSee 2-photon laser at 800 nm on a Leica SP5 confocal microscope, or a 488 nm laser attached to a Zeiss Axioplan2 compound microscope. Embryos were anesthetized in 600 μM MS-222 (Sigma) and mounted in 0.8% low melting temperature agarose (NuSieve GTG) and placed in a solution of egg water with 600 μM tricaine for imaging. In most cases, the lateral line ganglion and part of the lateral line nerve adjacent to the otic vesicle was ablated before PLLp ablations were conducted. To standardize these manipulations as much as possible, we selected embryos between deposition of L1 and L3 neuromasts, at which time there are three protoneuromasts in the PLLp. We separated the PLLp into three fragments by ablating a ∼20 μm patch of cells on either side of the central protoneuromast within the migrating PLLp to create the isolated middle fragment. Time-lapse images were acquired on a Leica SP5 confocal microscope and images were processed using ImageJ and Fluorender (SCI Institute, http://www.sci.utah.edu/software/46-documentation/137-fluorender.html). Kymographs were made using ImageJ. Circularity was determined in ImageJ using the ‘Circularity’ measurement [circularity=4π(area/perimeter2)] on PLLp fragments. Centroid positions were determined in ImageJ by manual outlining of cell fragments.
Bead transplantation and implantation
Approximately 50 μl of a suspension of 10 μm polystyrene beads (Polysciences) were rinsed twice in filter-sterilized PBS and pre-soaked in 0.5 mg/ml heparin solution for 20 min at room temperature. After removal of heparin, beads were incubated in 10 μl of either 0.1% BSA (for control beads) or 0.25 μg/μl recombinant FGF3 or FGF10 protein (R&D Systems) for 2 h at 16°C with shaking. Beads were implanted under the skin of ∼30-32 hpf embryos using a cell transplantation apparatus. For bilateral transplants, ablations were performed first, and then BSA- and FGF-soaked beads transplanted to either side of the remaining PLLp fragment.
Supplementary Material
Acknowledgements
We thank lab members for helpful comments, Chongmin Wang for fish care and Darren Gilmour for the ClaudinB-lyn GFP transgenic fish.
Footnotes
Competing interests
The authors declare no competing financial interests.
Author contributions
D.D.N. and A.B.C. conceived most of the experiments and wrote the manuscript. D.D.N. performed most of the experiments and developed models. K.S. helped with ablations, S.R. helped in coding models, M.M. performed some heatshock cxcl12a experiments with help from M.R.-F. and E.R.
Funding
The Intramural Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) and National Institutes of Health (NIH) [HD001012] supported this work. Deposited in PMC for release after 12 months.
Supplementary material
Supplementary material available online at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.106690/-/DC1
References
- Aman A., Piotrowski T. (2008). Wnt/beta-catenin and Fgf signaling control collective cell migration by restricting chemokine receptor expression. Dev. Cell 15, 749-761 10.1016/j.devcel.2008.10.002 [DOI] [PubMed] [Google Scholar]
- Boldajipour B., Mahabaleshwar H., Kardash E., Reichman-Fried M., Blaser H., Minina S., Wilson D., Xu Q., Raz E. (2008). Control of chemokine-guided cell migration by ligand sequestration. Cell 132, 463-473 10.1016/j.cell.2007.12.034 [DOI] [PubMed] [Google Scholar]
- Breau M. A., Wilson D., Wilkinson D. G., Xu Q. (2012). Chemokine and Fgf signalling act as opposing guidance cues in formation of the lateral line primordium. Development 139, 2246-2253 10.1242/dev.080275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns J. M., Summers B. C., Wang Y., Melikian A., Berahovich R., Miao Z., Penfold M. E. T., Sunshine M. J., Littman D. R., Kuo C. J., et al. (2006). A novel chemokine receptor for SDF-1 and I-TAC involved in cell survival, cell adhesion, and tumor development. J. Exp. Med. 203, 2201-2213 10.1084/jem.20052144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmona-Fontaine C., Theveneau E., Tzekou A., Tada M., Woods M., Page K. M., Parsons M., Lambris J. D., Mayor R. (2011). Complement fragment C3a controls mutual cell attraction during collective cell migration. Dev. Cell 21, 1026-1037 10.1016/j.devcel.2011.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dambly-Chaudière C., Cubedo N., Ghysen A. (2007). Control of cell migration in the development of the posterior lateral line: antagonistic interactions between the chemokine receptors CXCR4 and CXCR7/RDC1. BMC Dev. Biol. 7, 23 10.1186/1471-213X-7-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- David N. B., Sapede D., Saint-Etienne L., Thisse C., Thisse B., Dambly-Chaudiere C., Rosa F. M., Ghysen A. (2002). Molecular basis of cell migration in the fish lateral line: role of the chemokine receptor CXCR4 and of its ligand, SDF1. Proc. Natl. Acad. Sci. USA 99, 16297-16302 10.1073/pnas.252339399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donà E., Barry J. D., Valentin G., Quirin C., Khmelinskii A., Kunze A., Durdu S., Newton L. R., Fernandez-Minan A., Huber W., et al. (2013). Directional tissue migration through a self-generated chemokine gradient. Nature 503, 285-289 10.1038/nature12635 [DOI] [PubMed] [Google Scholar]
- Friedl P., Gilmour D. (2009). Collective cell migration in morphogenesis, regeneration and cancer. Nat. Rev. 10, 445-457 10.1038/nrm2720 [DOI] [PubMed] [Google Scholar]
- Ghysen A., Dambly-Chaudiere C. (2007). The lateral line microcosmos. Genes Dev. 21, 2118-2130 10.1101/gad.1568407 [DOI] [PubMed] [Google Scholar]
- Gilmour D., Haas P., Lecaudey V., Streichan S., Valentin G. (2009). S20-05 Dissecting the role of extrinsic and intrinsic cues in coordinating collective cell migration. Mechanisms of Development. 126, Supplement, S21 10.1016/j.mod.2009.06.1006 [DOI] [Google Scholar]
- Gompel N., Cubedo N., Thisse C., Thisse B., Dambly-Chaudière C., Ghysen A. (2001). Pattern formation in the lateral line of zebrafish. Mech. Dev. 105, 69-77 10.1016/S0925-4773(01)00382-3 [DOI] [PubMed] [Google Scholar]
- Haas P., Gilmour D. (2006). Chemokine signaling mediates self-organizing tissue migration in the zebrafish lateral line. Dev. Cell 10, 673-680 10.1016/j.devcel.2006.02.019 [DOI] [PubMed] [Google Scholar]
- Hachet-Haas M., Balabanian K., Rohmer F., Pons F., Franchet C., Lecat S., Chow K. Y. C., Dagher R., Gizzi P., Didier B., et al. (2008). Small neutralizing molecules to inhibit actions of the chemokine CXCL12. J. Biol. Chem. 283, 23189-23199 10.1074/jbc.M803947200 [DOI] [PubMed] [Google Scholar]
- Kadam S., Ghosh S., Stathopoulos A. (2012). Synchronous and symmetric migration of Drosophila caudal visceral mesoderm cells requires dual input by two FGF ligands. Development 139, 699-708 10.1242/dev.068791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmel C. B., Ballard W. W., Kimmel S. R., Ullmann B., Schilling T. F. (1995). Stages of embryonic development of the zebrafish. Dev. Dyn. 203, 253-310 10.1002/aja.1002030302 [DOI] [PubMed] [Google Scholar]
- Knaut H., Blader P., Strähle U., Schier A. F. (2005). Assembly of trigeminal sensory ganglia by chemokine signaling. Neuron 47, 653-666 10.1016/j.neuron.2005.07.014 [DOI] [PubMed] [Google Scholar]
- Lecaudey V., Cakan-Akdogan G., Norton W. H. J., Gilmour D. (2008). Dynamic Fgf signaling couples morphogenesis and migration in the zebrafish lateral line primordium. Development 135, 2695-2705 10.1242/dev.025981 [DOI] [PubMed] [Google Scholar]
- Lee Y., Grill S., Sanchez A., Murphy-Ryan M., Poss K. D. (2005). Fgf signaling instructs position-dependent growth rate during zebrafish fin regeneration. Development 132, 5173-5183 10.1242/dev.02101 [DOI] [PubMed] [Google Scholar]
- Levoye A., Balabanian K., Baleux F., Bachelerie F., Lagane B. (2009). CXCR7 heterodimerizes with CXCR4 and regulates CXCL12-mediated G protein signaling. Blood 113, 6085-6093 10.1182/blood-2008-12-196618 [DOI] [PubMed] [Google Scholar]
- Li Q., Shirabe K., Kuwada J. Y. (2004). Chemokine signaling regulates sensory cell migration in zebrafish. Dev. Biol. 269, 123-136 10.1016/j.ydbio.2004.01.020 [DOI] [PubMed] [Google Scholar]
- Matsuda M., Chitnis A. B. (2010). Atoh1a expression must be restricted by Notch signaling for effective morphogenesis of the posterior lateral line primordium in zebrafish. Development 137, 3477-3487 10.1242/dev.052761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda M., Nogare D. D., Somers K., Martin K., Wang C., Chitnis A. B. (2013). Lef1 regulates Dusp6 to influence neuromast formation and spacing in the zebrafish posterior lateral line primordium. Development 140, 2387-2397 10.1242/dev.091348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLennan R., Teddy J. M., Kasemeier-Kulesa J. C., Romine M. H., Kulesa P. M. (2010). Vascular endothelial growth factor (VEGF) regulates cranial neural crest migration in vivo. Dev. Biol. 339, 114-125 10.1016/j.ydbio.2009.12.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naumann U., Cameroni E., Pruenster M., Mahabaleshwar H., Raz E., Zerwes H.-G., Rot A., Thelen M. (2010). CXCR7 functions as a scavenger for CXCL12 and CXCL11. PLoS ONE 5, e9175 10.1371/journal.pone.0009175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nechiporuk A., Raible D. W. (2008). FGF-dependent mechanosensory organ patterning in zebrafish. Science 320, 1774-1777 10.1126/science.1156547 [DOI] [PubMed] [Google Scholar]
- Schneider C. A., Rasband W. S., Eliceiri K. W. (2012). NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9, 671-675 10.1038/nmeth.2089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streichan S. J., Valentin G., Gilmour D., Hufnagel L. (2011). Collective cell migration guided by dynamically maintained gradients. Phys. Biol. 8, 045004 10.1088/1478-3975/8/4/045004 [DOI] [PubMed] [Google Scholar]
- Sun L., Tran N., Liang C., Tang F., Rice A., Schreck R., Waltz K., Shawver L. K., McMahon G., Tang C. (1999). Design, synthesis, and evaluations of substituted 3-[(3- or 4-carboxyethylpyrrol-2-yl)methylidenyl]indolin-2-ones as inhibitors of VEGF, FGF, and PDGF receptor tyrosine kinases. J. Med. Chem. 42, 5120-5130 10.1021/jm9904295 [DOI] [PubMed] [Google Scholar]
- Valentin G., Haas P., Gilmour D. (2007). The chemokine SDF1a coordinates tissue migration through the spatially restricted activation of Cxcr7 and Cxcr4b. Curr. Biol. 17, 1026-1031 10.1016/j.cub.2007.05.020 [DOI] [PubMed] [Google Scholar]
- Venkiteswaran G., Lewellis S. W., Wang J., Reynolds E., Nicholson C., Knaut H. (2013). Generation and dynamics of an endogenous, self-generated signaling gradient across a migrating tissue. Cell 155, 674-687 10.1016/j.cell.2013.09.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitorino P., Meyer T. (2008). Modular control of endothelial sheet migration. Genes Dev. 22, 3268-3281 10.1101/gad.1725808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilensky U. (1999). NetLogo. http://ccl.northwestern.edu/netlogo/ Center for Connected Learning and Computer-Based Modeling, Northwestern University, Evanston, IL USA. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.