Abstract
Branching morphogenesis is the developmental program that builds the ramified epithelial trees of various organs, including the airways of the lung, the collecting ducts of the kidney, and the ducts of the mammary and salivary glands. Even though the final geometries of epithelial trees are distinct, the molecular signaling pathways that control branching morphogenesis appear to be conserved across organs and species. However, despite this molecular homology, recent advances in cell lineage analysis and real-time imaging have uncovered surprising differences in the mechanisms that build these diverse tissues. Here, we review these studies and discuss the cellular and physical mechanisms that can contribute to branching morphogenesis.
Keywords: Pattern, Tension, Mechanical stress, Proliferation, Bifurcation
Introduction
Arborized epithelial networks are found ubiquitously in the organ systems of animals as diverse as insects and mammals. Such branched epithelial tubes create conduits that permit the flow and exchange of gases and fluids within the body. Branching morphogenesis is the developmental program that generates these tree- or bush-like organ geometries through recursive rounds of self-similar bifurcations of the epithelium into its surrounding mesenchyme (Ochoa-Espinosa and Affolter, 2012). The molecular signals that are important for branching morphogenesis have been investigated for over 40 years, beginning with studies of vertebrate organs, including the embryonic lung and salivary gland. In recent years, our understanding of branching morphogenesis has been accelerated by genetic studies of the Drosophila tracheal system, which permit elegant piecemeal dissection of the various steps taken by groups of cells as they form a branched network (Affolter and Caussinus, 2008). Together, these molecular and genetic studies have revealed that branching morphogenesis appears to be controlled by a conserved set of molecules, including fibroblast growth factors (FGFs), which signal through the mitogen-activated protein kinase (MAPK) cascade. Recent studies have also indicated a role for miRNAs in branching morphogenesis of the lung, kidney, salivary gland and vasculature (Biyashev et al., 2012; Chu et al., 2014; Hayashi et al., 2011; Jiang et al., 2013; Mujahid et al., 2013; Rebustini et al., 2012; Yu, 2014).
Despite this rich molecular description, the physical processes that drive branching are still being elucidated (Nelson and Gleghorn, 2012). Up until ∼10 years ago, morphogenesis itself was largely inferred from images of specimens that were fixed at various points in time. The advent of fluorescent reporter strategies, including tissue-specific promoter-driven transgenic expression, and of mosaic reporters has begun to reveal the dynamics and kinematics of branching morphogenesis in a variety of model organs (Chi et al., 2009; Schnatwinkel and Niswander, 2013). These studies suggest that branching morphogenesis is much more dynamic than would be expected from still images and fixed samples: epithelial cells either move dynamically within the tissue during branching (Chi et al., 2009) or extend and retract as the branch progresses (Larsen et al., 2006). Furthermore, a surge of interest from biophysicists and engineers has led to an increase in computational models of branching, which are helping to unravel the systems biology of branching morphogenesis at both the biochemical reaction level (Menshykau and Iber, 2013; Menshykau et al., 2012) and the mechanical level (Kim et al., 2013; Lubkin, 2008; Wyczalkowski et al., 2012). Together, these approaches have shown that a variety of cellular processes, including differential growth, cell invasion, epithelial folding and matrix-driven branching, can contribute to branching morphogenesis in different contexts. Here, we review the different cellular and physical mechanisms that can drive branching, and discuss recent revelations from both experiment and theory for a few specific examples.
Differential growth
One of the earliest hypotheses put forth to explain branching morphogenesis was that new branches result from a local increase in the proliferation of cells within the parent branch. The development of many branched organs depends crucially upon cell proliferation (Goldin, 1980), with new cells providing the raw material needed to construct additional generations of branches. Notably, early studies revealed that this mode of epithelial growth relies on interactions with the surrounding mesenchyme. For example, using developing mouse salivary glands, Grobstein demonstrated that isolated epithelial explants fail to branch in culture (Grobstein, 1953a). When recombined with their mesenchyme, however, these explants resume essentially normal morphogenesis (Grobstein, 1953a) and the quantity of mesenchyme present in culture was found to modulate the rate of proliferation within the epithelium (Alescio and Colombo Piperno, 1967; Alescio and Di Michele, 1968).
Following these early studies, investigators began using a variety of different grafting techniques to assess how mesenchymal interactions might instruct epithelial growth (Grobstein, 1967). Remarkably, it was shown that epithelial growth and branching can be stimulated by heterologous mesenchyme from a variety of sources. Salivary mesenchyme, for example, was found to support thymic (Auerbach, 1960), pancreatic (Golosow and Grobstein, 1962) and mammary (Kratochwil, 1969) branching in culture. Of these grafting experiments, those using developing lungs presented a unique advantage, as ectopic buds could be stimulated to form along the usually non-branching, tracheal epithelium (Alescio and Cassini, 1962). Sections of tracheal mesenchyme were dissected away and replaced with grafts of mesenchyme from various organs, including lung, salivary gland, stomach and mammary gland (Wessells, 1970). Whereas each of these promoted the formation of an ectopic bud, only lung mesenchyme was sufficient to induce subsequent branching morphogenesis (Spooner and Wessells, 1970b). These supernumerary buds were then used as proxies to investigate the stimulatory effects of lung mesenchyme on the branching epithelium. In particular, Wessells and colleagues sought to determine whether local increases in cell proliferation along the epithelium accompanied the formation of supernumerary buds (Goldin et al., 1984; Goldin and Opperman, 1980; Goldin and Wessells, 1979; Wessells, 1970). Although initial experiments failed to detect significant spatial differences in the incorporation of tritiated thymidine along the epithelium (Wessells, 1970), subsequent studies reported levels of proliferation in supernumerary buds that increased with time, as well as decreasing rates of proliferation in the adjacent (non-branching) tracheal epithelium (Goldin and Wessells, 1979). Furthermore, agarose beads loaded with epidermal growth factor (EGF) stimulated the formation of supernumerary buds when they were placed adjacent to the tracheal epithelium in cultured embryonic chicken lungs (Goldin and Opperman, 1980). Consistently, treatment of these cultured explants with the DNA synthesis inhibitor aphidicolin disrupted the formation of supernumerary buds (Goldin et al., 1984). Elevated levels of proliferation were also reported in the morphogenetically active tips of extending branches in the mouse salivary gland (Bernfield et al., 1972, 1973), mammary gland (Bresciani, 1968) and, much more recently, in the developing kidney (Michael and Davies, 2004).
Taken together, these data led early investigators to conclude that local stimulation of epithelial cell proliferation was involved in the formation of new branches (Ettensohn, 1985) (Fig. 1A). Even so, the observation that elevated proliferation is confined to branch tips does not, in general, indicate that differential proliferation is the driving force behind branch initiation. It remains possible that patterns of differential growth are simply a consequence of branch formation, and not the actual underlying physical mechanism. Among the early investigators, Goldin and Wessells were particularly cognizant of this possibility, acknowledging that: ‘Although it is evident that the continuing high level of mitotic activity in the induced buds is due, in some sense, to the influence of the bronchial mesenchyme, there is no clear evidence to suggest that the initial outpocketing of the bud from the tracheal epithelium was achieved through an active localized stimulation of mitotic activity’ (Goldin and Wessells, 1979).
Fig. 1.
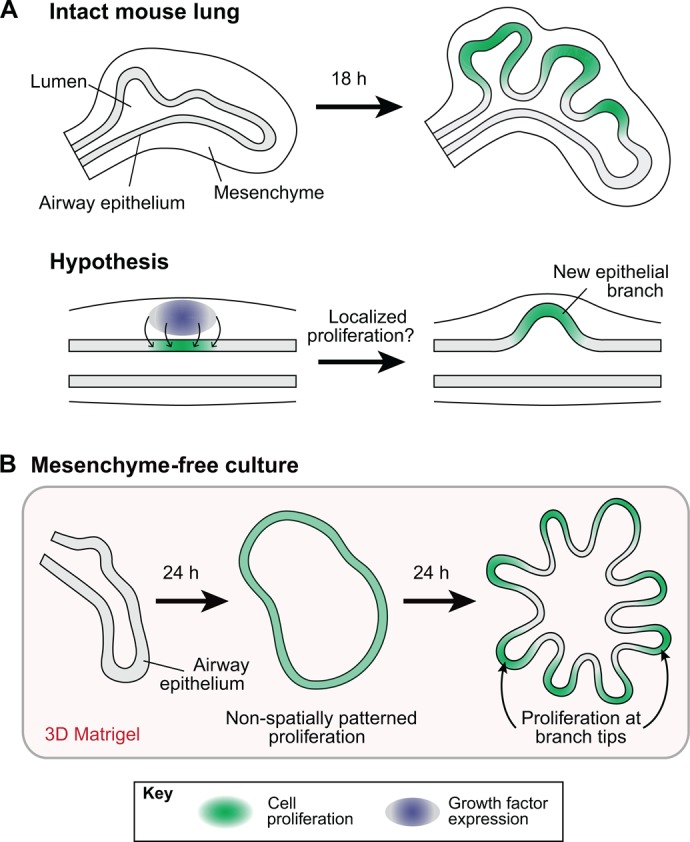
Branching via patterned or differential cell proliferation. Differential rates of cell proliferation have been hypothesized to induce branching in a number of developing organs, including the lung, kidney and salivary gland. In each of these cases, elevated levels of proliferation have been observed in nascent epithelial buds. (A) As a representative example, the developing mouse lung is shown. Given the observed patterns of proliferation, it is generally thought that growth factor expression (blue) in the neighboring mesenchyme stimulates localized growth/cell proliferation (green) in the epithelium, which initiates the formation of new epithelial branches. (B) Lung epithelial explants, denuded of mesenchyme and embedded in 3D gels of reconstituted basement membrane protein, have also been shown to branch in culture. Here again, elevated proliferation was observed in incipient branches, but only after these branches had already formed. No proliferative pre-pattern was observed in the epithelium prior to branching.
Clear support for a mechanism based on differential growth would require evidence of patterned cell division prior to (or during) branch initiation, i.e. the presence of a proliferative precursor that presages the emergence of actual epithelial buds.
To further probe the role of differential growth and proliferation, mesenchyme-free culture assays were developed to examine the effects of different mesenchymal growth factors on epithelial proliferation and branching. When embedded in three-dimensional (3D) gels of reconstituted basement membrane protein, isolated epithelial explants branch in the presence of exogenously applied growth factors (Nogawa and Ito, 1995; Qiao et al., 1999). Explanted embryonic epithelium, for example, branches when treated with fibroblast growth factor 1 (Fgf1) (Cardoso et al., 1997) or Fgf10 (Bellusci et al., 1997). The patterns of proliferation observed within these cultures were similar to those reported in intact organs: spatial variations in cell proliferation were not observed until branches had already formed (Nogawa et al., 1998) and zones of elevated proliferation were restricted to the tips of extending branches (Fig. 1B). These results were surprising, because it is typically assumed that focal patterns of growth factor expression in the mesenchyme stimulates localized proliferation in the airway epithelium, but in these explanted cultures the applied growth factors are ubiquitous (Makarenkova et al., 2009), and no mesenchymal pre-pattern is present to stimulate epithelial proliferation locally. Mesenchyme-free branching is not unique to the developing airway epithelium; similar branching patterns have been observed during 3D culture of epithelia isolated from the developing salivary gland (Morita and Nogawa, 1999; Nogawa and Takahashi, 1991), kidney (Qiao et al., 2001, 1999) and lacrimal gland (Makarenkova et al., 2009). In each of these culture systems too, spatial patterns of proliferation were not observed until branches had already formed. Moreover, these spatial patterns, when present, were all similar topologically: elevated proliferation was localized to the branch tips. If, however, growth factor-loaded beads were embedded into the gel adjacent to mesenchyme-free explants, new epithelial branches extended toward these local sources of growth factor (Park et al., 1998; Weaver et al., 2000), and cell proliferation was elevated in the branches extending towards the bead (Weaver et al., 2000). However, in these studies, patterns of proliferation were not reported prior to branch formation, so it is unclear whether differential growth preceded branch initiation.
In each of the aforementioned studies, investigators examined patterns of proliferation in explants that were fixed at different stages of branching. Recent advances in live imaging, however, have made it possible to investigate this process dynamically. For example, Schnatwinkel and Niswander used a line of transgenic reporter mice expressing GFP-tagged histones in airway epithelial cells to quantify patterns of proliferation in cultured lung explants (Schnatwinkel and Niswander, 2013). By dynamically tracking mitotic events from time-lapse images, the authors reported elevated levels of proliferation during the formation of lateral branches, but not during the formation of terminal bifurcations. In addition, the alignment of the observed cell divisions was found to vary spatially along the epithelium. This oriented growth, which is mediated by ERK1/2 signaling, has been shown to dramatically influence the overall morphology of the developing murine airways (Tang et al., 2011). Conversely, in the embryonic chicken lung, although spatial patterns of proliferation were observed during branch formation, proliferation was not required for the initiation of new epithelial buds (Kim et al., 2013).
Given these seemingly contradictory data and the inherent complexity of morphogenesis, physical models are necessary to resolve the mechanical contribution of patterned cell proliferation to branching morphogenesis (Gleghorn et al., 2013; Wyczalkowski et al., 2012). In this spirit, Kim and colleagues developed a computational model to determine the physical role of differential growth during monopodial branching in the developing chicken lung (Kim et al., 2013). Patterned proliferation was modeled using a continuum mechanical theory for finite volumetric growth (Rodriguez et al., 1994), which decomposed the overall deformation of the tissue into a component due to growth and a component due to elastic deformation. This approach was strictly focused on behaviors at the tissue level, and any cell- or molecular-level details were ‘lumped’ into model parameters. This framework has been used to simulate several morphogenetic processes, including head-fold formation (Varner et al., 2010) and cardiac looping (Shi et al., 2014; Voronov et al., 2004). Importantly, the observed patterns of epithelial proliferation in the chicken lung were not sufficient to simulate the observed changes in epithelial morphology during branching morphogenesis (Kim et al., 2013). These computational data were further supported by the experimental observation that inhibiting cell division did not disrupt bud formation in the developing chicken lung, suggesting that proliferation does not drive the formation of branches in this species. Similar computational studies in the developing mouse lung (and other branching systems) are thus warranted.
Whether or not differential proliferation initiates branching, once branches have formed it is unclear how distal populations of proliferating cells are maintained within the extending epithelium. In the mouse lung, expression of the transcription factor N-myc (Mycn – Mouse Genome Informatics) is restricted to the distal epithelium, where it is thought to help maintain this population of proliferating undifferentiated cells (Okubo et al., 2005). Transgenic mice overexpressing an N-myc-EGFP fusion protein exhibit expanded domains of proliferating cells with attenuated levels of cell differentiation. This suggests an interplay between the regulation of cell proliferation and differentiation during branching morphogenesis. Recent evidence has also implicated the microRNA cluster miR-17-92 during this process, which functions in part by regulating the expression of retinoblastoma-like 2 (Rbl2/p130) (Lu et al., 2007), which is important for cell cycle control. By contrast, distal pools of proliferating cells in the pancreas are regulated by reciprocal feedback between Rho and MAPK signaling (Petzold et al., 2013). Finally, during renal branching, time-lapse microscopy has revealed that proliferating cells in branch tips delaminate from the epithelium and divide within the lumen before reincorporating back into the epithelium (Packard et al., 2013). The reincorporation of these dividing cells contributes to cell intercalation in the branch tips, which is involved in epithelial expansion and branch growth. It remains to be seen whether similar signaling networks and cell behaviors are used to maintain the distal populations of dividing cells in other species and organ systems. Thus, although the epithelium is clearly proliferating during the branching morphogenesis of most epithelial trees, a role for differential proliferation in branch initiation still remains hypothetical.
Invasive branching
One of the best-understood modes of branching morphogenesis is that of invasive branching, wherein the tip of a new epithelial branch translocates forward into the surrounding tissue via an invasive migratory process. The cell that occupies the leading edge of this extending branch, the so-called ‘tip cell’ or ‘leader cell’, often exhibits an extended morphology that is characterized by membrane protrusions at its invasive front. The cells that lag behind the tip cell, sometimes referred to as ‘follower cells’, ‘trailing cells’ or ‘stalk cells’, maintain cadherin-mediated adhesion to each other as well as to the tip cell. Invasive branching has been examined in detail at both the physical and genetic level in studies of Drosophila tracheal morphogenesis (Casanova, 2007; Ghabrial et al., 2003; Schottenfeld et al., 2010; Uv et al., 2003), but has also been investigated during sprouting angiogenesis in the vertebrate vasculature.
The Drosophila tracheal system is a network of tubes that conduct oxygen and other gases from the larval surface to the cells deep within the tissues of the body (Ghabrial et al., 2003). The trachea has three generations of branches: primary, secondary and terminal. The tracheal system is unusual among branching organs in that it develops into a branched network from a starting population of 20 clusters (known as tracheal sacs, each containing 80 epithelial cells), without cell division or cell death (Ghabrial et al., 2003; Samakovlis et al., 1996). The Drosophila FGF homolog Branchless (Bnl) is expressed focally by epidermal and mesodermal cells adjacent to the tracheal sac (Fig. 2A) in locations where each primary branch will bud (Sutherland et al., 1996). Bnl binds to its receptor, the FGF receptor homolog Breathless (Btl), which is expressed on the epithelial cells and acts as a chemoattractant, inducing a subset of the 80 cells to form a bud, organize into a tube, migrate out of the tracheal sac and form the primary (first generation) branches (Klambt et al., 1992). Genetic analysis has revealed that Bnl specifies the leader: the cell with the highest amount of Btl signaling becomes the tip cell, produces the highest levels of the Notch ligand Delta and actively prevents its neighbors from acquiring tip cell fate via lateral inhibition through Notch signaling (Ghabrial and Krasnow, 2006; Llimargas, 1999). Consistently, mathematical modeling of Delta/Notch signaling in the primary branch cells of the Drosophila trachea suggests that this mode of lateral inhibition increases the robustness of the Bnl-mediated selection of tip cell phenotype (Koizumi et al., 2012). Furthermore, Bnl activates the expression of genes such as pointed, blistered and escargot, which encode transcription factors that are required for morphogenesis of secondary and terminal branches and branch fusion, respectively (Guillemin et al., 1996; Samakovlis et al., 1996). A genetic screen recently identified ∼70 genes that are required for the development of the tracheal system (Ghabrial et al., 2011), in addition to the 100 or so genes that had been identified previously by other studies. The current estimated minimum number of genes required to form an arborized epithelium is thus ∼200, in addition to genes involved in cell survival and housekeeping.
Fig. 2.
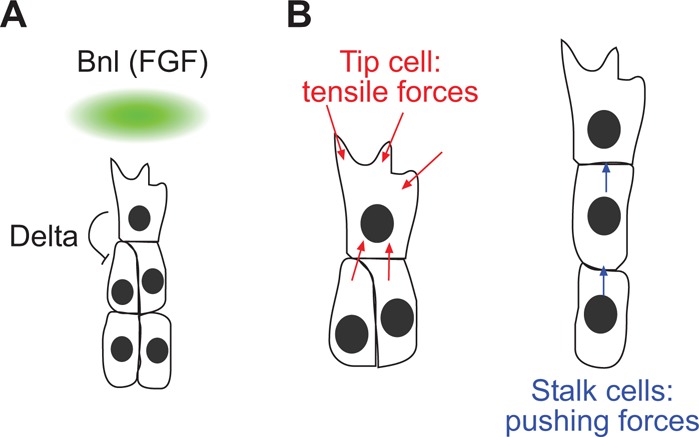
Branching via invasion. Collective cell migration drives branching morphogenesis in the Drosophila trachea and vertebrate vasculature. (A) In the Drosophila trachea, the expression of the FGF homolog Branchless (Bnl, green) specifies the tip cell, which inhibits tip cell phenotype in the neighboring stalk cells through Delta-Notch signaling. The collective migrates toward the source of Bnl. (B) This collective migration is mediated by at least two types of mechanical force. The tip cell induces tensile forces (red arrows) on the surrounding tissue, and pulls the stalk cells forward. At the same time, the stalk cells intercalate with each other, and these cellular rearrangements generate sufficient pushing forces (blue arrows) to move the collective forward.
Morphogenesis of the Drosophila trachea is a form of collective cell migration (Rørth, 2009). Live imaging has revealed that Bnl induces tip cells to undergo cytoskeletal reorganization, enriching actin at their basal surface (Lebreton and Casanova, 2014). Bnl also induces the activation of Rac and the expression of fascin, an actin-bundling protein required for the formation of filopodia at the leading edge (Okenve-Ramos and Llimargas, 2014). By contrast, the trailing stalk cells exhibit high levels of actin at their apical surfaces (Lebreton and Casanova, 2014). In addition to these cytoskeletal changes, the primary branch appears to extend into the surrounding tissue through a combination of physical forces (Fig. 2B). Live imaging of fluorescently labeled cells has revealed that filopodia actively protrude from the leading edge of the tip cell into the surrounding tissue (Caussinus et al., 2008; Ribeiro et al., 2002). The forces generated by this tip cell are thought to induce a mechanical strain and pull the stalk cells forward, while also inducing additional rearrangements of the stalk cells with respect to each other, thus giving rise to geometric lengthening of the branch (Caussinus et al., 2008). Initially, the lumen of the primary branch is surrounded by multiple stalk cells around its circumference. The stalk cells within this branch then rearrange to generate an epithelium in which the lumen is surrounded by a single cell, an intercalation event that both lengthens the branch and reduces its caliber. E-cadherin is actively reduced in the stalk cells via endocytosis (Shaye et al., 2008), which facilitates intercalation, as disrupting endocytic trafficking of E-cadherin blocks stalk cell intercalation and tracheal extension. This is consistent with the finding that genes involved in vesicle trafficking are required for tracheal morphogenesis (Ghabrial et al., 2011). Laser ablation experiments have also revealed that pulling forces from the tip cell are required to induce stalk cell intercalation (Caussinus et al., 2008): the stalk cells are subjected to tensile forces by the tip cell and ablating their connection with the tip cell not only prevents subsequent intercalation, but also causes the stalk cells to retract.
How tensile forces exerted by the tip cell lead to intercalation by the stalk cells remains unclear. An obvious hypothesis is that pulling by the tip cell activates the endocytic machinery in the stalk cells and thereby reduces the cell-surface levels of E-cadherin, thus increasing adhesion dynamics and facilitating intercalation. This process also depends on signaling downstream of the planar cell polarity (PCP) pathway, specifically via Frizzled, which appears to induce the turnover of junctional E-cadherin by activating the small GTPase RhoA via the Drosophila Rho guanine nucleotide exchange factor RhoGEF2 (Warrington et al., 2013). RhoGEF2 is part of a family that includes the mammalian p115-RhoGEF and LARG (Mulinari and Hacker, 2010), the latter of which is activated by tensional forces exerted on focal adhesion complexes in mammalian cells in culture (Guilluy et al., 2011). Furthermore, the levels of E-cadherin within the stalk cells are also controlled by signaling through Src: activated Src reduces the accumulation of E-cadherin at the membrane of stalk cells, permitting them to intercalate with their neighbors (Shindo et al., 2008). Src activation responds to tension (Arthur et al., 2000), suggesting a second possible pathway by which tip cells can induce intercalation of their neighboring stalk cells. Together, these findings highlight that physical forces are crucial for the repositioning of stalk cells during branch elongation.
The physical and molecular mechanisms of branching morphogenesis in the Drosophila trachea are similar to those that drive sprouting angiogenesis in the vertebrate vasculature (Ochoa-Espinosa and Affolter, 2012). Here, tip cells are selected by a different ligand, vascular endothelial growth factor (VEGF) A, which induces signaling downstream of VEGF receptor 2 (Vegfr2) to upregulate the expression of Delta-like 4 (Dll4) (Hellstrom et al., 2007; Noguera-Troise et al., 2006; Suchting et al., 2007). Cells with the highest levels of Dll4 become tip cells and lead the growing branch (Jakobsson et al., 2010). Activation of Notch signaling in the adjacent cells reduces their expression of Vegfr2, thus inducing them to become stalk cells (Kume, 2009). Moreover, this Notch-mediated inhibition of the tip cell phenotype can extend beyond adjacent cells, as Dll4 can be incorporated into small extracellular vesicles known as exosomes that are taken up by cells at a distance (Sheldon et al., 2010) to prevent the tip cell phenotype and even induce retraction of a capillary sprout (Sharghi-Namini et al., 2014). As with the Drosophila tracheal system, computational models have also lent support to the model of Notch-mediated inhibitory signaling in stalk cells (Bentley et al., 2009), and have suggested that drag (pulling) forces from the tip cell may contribute to this signaling pathway (Wang et al., 2013). It would be interesting to use laser ablation to disrupt the connections between tip cells and stalk cells during angiogenesis to test the physical limits of the homology between tracheal branching in flies and angiogenic sprouting in vertebrates.
Epithelial folding
In other developing organs, branching epithelia do not exhibit an invasive or migratory phenotype. Instead, the epithelium folds as a coherent sheet, and the leading edge exhibits neither filopodial nor lamellopodial protrusions. This epithelial folding can be driven by active forces such as apical constriction or differential growth, or by external forces such as mechanical buckling (Taber, 1995) (Fig. 3), and has been postulated to occur in the developing mammary gland (Ewald et al., 2008) and, more recently, in the embryonic mouse lung (Schnatwinkel and Niswander, 2013).
Fig. 3.
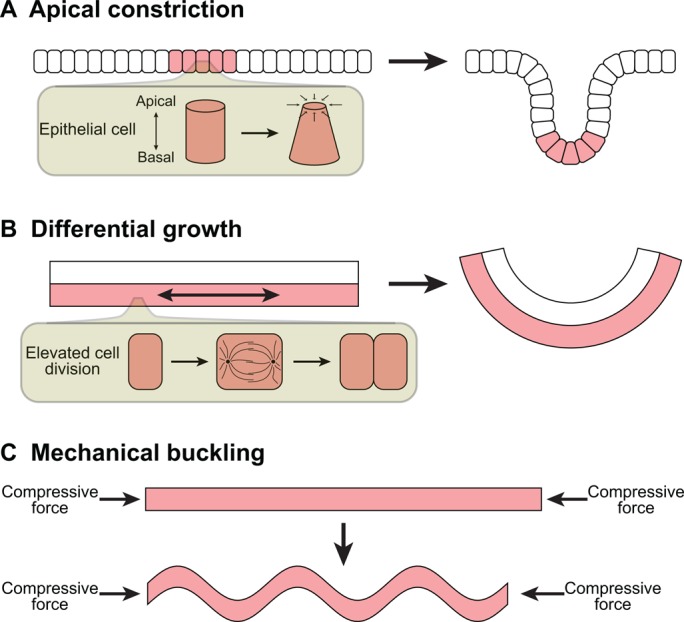
Branching via epithelial folding. The collective folding of cells in an epithelium can produce new branches. Changes in epithelial shape can occur by: (A) apical constriction of cells within a localized region (pink) of the epithelium; (B) differential growth, caused by increased cell division in the epithelium (pink) relative to an adjacent tissue (white); or (C) mechanical buckling, which casts the epithelium into a wave-like morphology when loaded with sufficient compressive force, possibly produced by adjacent tissues.
Because many of the signaling pathways involved in branching are conserved across organs and species (Davies, 2002), it is often assumed that the physical mechanisms of morphogenesis are similarly conserved (Lubkin, 2008). In the lung, for example, incipient branches extend toward localized regions of Fgf10 expressed by the adjacent mesenchyme (Bellusci et al., 1997; Park et al., 1998). This is phenomenologically similar to the molecular mechanisms that regulate tracheal branching in Drosophila, whereby new tracheal branches form as cells extend filopodia and migrate toward focal regions of expression of the FGF homolog Bnl (Metzger and Krasnow, 1999; Sutherland et al., 1996). This molecular homology has led researchers to speculate that a similar migratory phenotype underpins the mechanics of airway branching in the mammalian lung (Metzger and Krasnow, 1999; Sutherland et al., 1996). This hypothesis gained ground in the wake of experiments demonstrating that isolated epithelial explants extend branches toward Fgf10-soaked beads in 3D culture (Park et al., 1998; Weaver et al., 2000). It was argued that FGFs exert a ‘chemoattractant effect’ on the branching epithelium, but it remains unclear how this chemoattraction manifests itself in the context of a coherent epithelium. During invasive branching, chemoattraction drives the directed migration of cells in the epithelium (Sutherland et al., 1996). But during epithelial folding, if the constituent cells are not crawling towards a local source of growth factors, how does the epithelium deform in a direction-dependent manner?
Recent data suggest that apical constriction of cells in the airway epithelium is sufficient to initiate the formation of monopodial (lateral) branches in the developing chicken lung (Kim et al., 2013). New epithelial buds form along the dorsal aspect of the primary bronchial tube, as airway epithelial cells constrict their apices and adopt wedge-shaped profiles. These coordinated changes in cell shape drive directional folding of the airway epithelium via a process that is both dependent on actomyosin contraction and downstream of FGF signaling (Kim et al., 2013). Although epithelial proliferation is not required for the formation of new buds, it is needed for the subsequent stages of branch elongation. This is supported by evidence from the developing mouse lung, which suggests that oriented cell division in the airway epithelium is responsible for the directional elongation of these tubes (Tang et al., 2011). However, whether apical constriction-mediated branching occurs in the mouse or in any other species remains to be determined. In addition, several other developing organs, including the kidney and mammary gland, branch via non-invasive mechanisms; but the physical mechanisms that drive epithelial folding in these systems remain to be investigated quantitatively.
It has also been hypothesized that mechanical buckling is involved in the branching morphogenesis of developing epithelia (Ettensohn, 1985). In his seminal review paper, Ettensohn suggested that epithelial growth, constrained by the surrounding mesenchyme, may cause the epithelium to ‘buckle inward, forming clefts at several sites’ (Ettensohn, 1985). This possibility is supported by recent reports suggesting that different aspects of gut morphogenesis, including gut looping (Savin et al., 2011) and villus morphogenesis (Shyer et al., 2013), are generated via mechanical buckling. It will be interesting to determine whether physical instabilities drive epithelial branching morphogenesis in other organs.
Matrix-driven branching
In most developing organs, the epithelium is surrounded by mesenchymal cells as well as by extracellular matrix (ECM). Not surprisingly, therefore, the ECM has been shown to play an instructive role in branching in various contexts. A regulatory role for the ECM in branching morphogenesis was recognized more than 30 years ago (Williams and Daniel, 1983), and has been most deeply investigated for the morphogenesis of the salivary and mammary glands.
In mice, one of the three pairs of major salivary glands, the submandibular glands, begins to develop at E11, when the bulb-shaped anlage grows out from the oral epithelium and swells into the surrounding mandibular mesenchyme (Tucker, 2007). One day later, indentations form on the surface of the epithelial bud and progress inwardly, clefting the epithelium into multiple daughter buds (Fig. 4A). At the same time, proliferation of the epithelial cells causes the daughter buds to extend (Patel et al., 2006). This process repeats itself to form the mature salivary epithelial tree, which consists of clusters of terminal buds connected to a system of ducts that deliver saliva to the oral cavity (Harunaga et al., 2011). Branching morphogenesis of the salivary epithelium has been studied most extensively in culture ex vivo, by placing the developing gland on top of filter paper floating on medium, during which time the explant continues to branch (Borghese, 1950; Grobstein, 1953b, 1956). This approach reveals that cleft formation requires actomyosin contraction, as treatment with cytochalasin B disrupts morphogenesis (Spooner and Wessells, 1970a, 1972). More recently, time-lapse imaging analysis of salivary branching has shown that each cleft first initiates as a gap between adjacent epithelial cells (Kadoya and Yamashina, 2010; Larsen et al., 2006), in a process that appears to depend on ERK1/2 signaling (Koyama et al., 2008) downstream of EGF from the surrounding mesenchyme (Kashimata et al., 2000; Nogawa and Takahashi, 1991). Once clefts have initiated on the surface of the epithelium, they appear to be stabilized when the epithelial cells lining the clefts form cell-ECM adhesions that contain activated focal adhesion kinase (FAK) (Daley et al., 2011). Here, FAK appears to be acting as a mechanosensor, and is required for the subsequent assembly of ECM fibrils within the growing cleft. The stabilized clefts then progress deeper into the epithelial bud (Kadoya and Yamashina, 2010) via Rho-associated kinase (ROCK)-induced actomyosin contraction, which leads to the assembly of fibronectin fibrils at the basal surface of the epithelial cells lining the growing cleft (Daley et al., 2009).
Fig. 4.
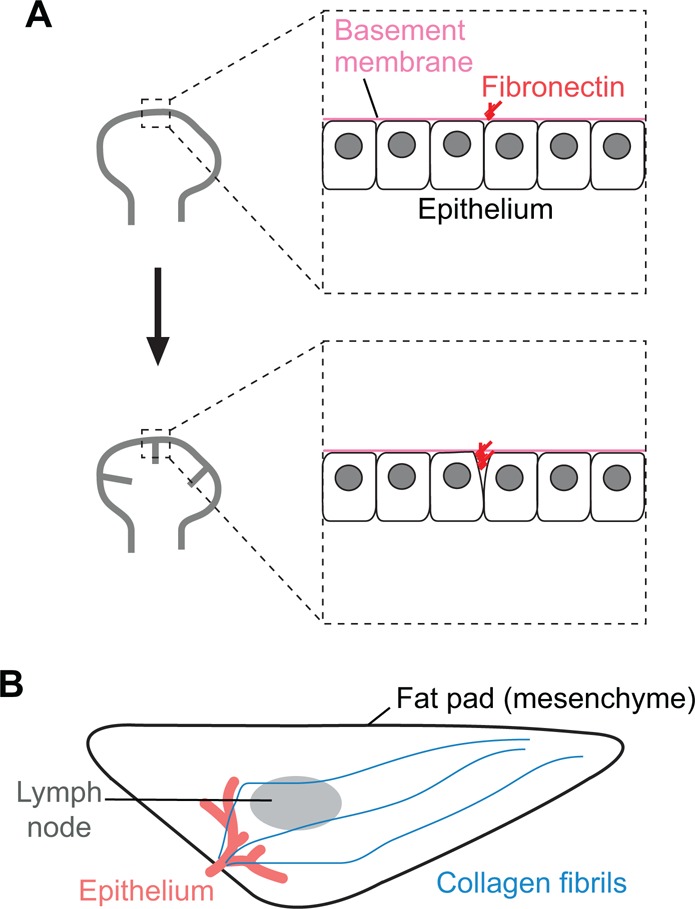
Matrix-mediated branching. The ECM plays an instructive role in branching morphogenesis in some organs. (A) In the salivary gland, fibrils of fibronectin (red) are deposited at the sites of new clefts (gray) in the epithelium. (B) In the mammary gland, fibrils of collagen (blue) present within the stroma may act as guidance cues for the elongation of nascent epithelial branches (red) during morphogenesis.
Cleft elongation is a dynamic process (Harunaga et al., 2011), and timelapse imaging suggests that not only do the epithelial cells themselves move dynamically at the basal surface of the growing bud (Hsu et al., 2013), but also that the growing end of the cleft ‘wiggles’ as the adjacent epithelial cells move and change shape (Kadoya and Yamashina, 2010). Small clefts can also regress after initiation, suggesting that the sites at which clefts first form are not determined precisely (Larsen et al., 2006). Furthermore, the spatial progression of a cleft deeper into the epithelium mirrors the increase in cell-ECM adhesions formed by the epithelial cells, which preferentially lose membrane localization of E-cadherin in these regions, denoting a reduction in cell-cell adhesion (Sakai et al., 2003). The presence of fibronectin in the stabilized cleft stimulates integrin-mediated signaling and proliferation of the adjacent epithelial cells, which helps to deepen the cleft (Daley et al., 2009, 2011). Fibronectin also induces the expression of Btbd7 in the adjacent epithelial cells, which in turn represses E-cadherin and activates Slug (Onodera et al., 2010). Epithelial proliferation requires signaling through FGF receptor 1 (Fgfr1) (Hoffman et al., 2002) and, accordingly, inhibiting Fgfr1 kinase activity disrupts salivary branching by halting proliferation, although it has no effect on the initiation of clefts. Inhibiting ROCK, myosin II ATPase, or FAK also stalls branching after cleft initiation (Daley et al., 2009, 2011). E-cadherin probably also plays a signaling role, as its association with the Hippo pathway effector TAZ (transcriptional co-activator with PDZ-binding motif) regulates branching of salivary explants (Enger et al., 2013).
Dynamic changes in the localization of ECM proteins thus appear to be crucial for cleft initiation and progression during branching morphogenesis of the murine submandibular gland. The epithelium contacts its basement membrane during salivary development (Bernfield and Banerjee, 1982), with ECM turnover being higher at the distal ends of the buds than within the stalks. Fibrils of interstitial collagen (Fukuda et al., 1988; Nakanishi et al., 1988) and fibronectin (Sakai et al., 2003) accumulate preferentially at sites of cleft initiation, and exogenous fibronectin in the culture medium is sufficient to induce cleft formation in salivary explants. Consistently, the epithelium increases its expression of integrins α6, β1 and β4 during the branching process, and in response to EGF (Kashimata and Gresik, 1997). Simultaneously, the epithelial cells themselves also move dynamically, and apparently randomly, within the clefting bud (Larsen et al., 2006; Wei et al., 2007), but the motility of those contacting the basement membrane is higher and depends on signaling downstream of integrins α6 and β1, as well as on myosin (Hsu et al., 2013). Epithelial clefting thus results from the combined movement of epithelial cells and the translocation of ECM fibrils.
Although cleft formation appears to be rather unique to the developing salivary gland, fibronectin is also required for branching morphogenesis of the lung and kidney (De Langhe et al., 2005; Roman, 1997; Sakai et al., 2003). Fibronectin is expressed during the pseudoglandular (branching) stage of embryonic lung development in mice (E11-E16), with highest intensity of fibronectin staining occurring at branch points (Roman and McDonald, 1992). Disrupting fibronectin association using function-blocking antibodies reduces branching of embryonic lung explants, whereas exogenous addition of fibronectin enhances branching (De Langhe et al., 2005; Sakai et al., 2003). Consistently, treatments that cause mislocalization of fibronectin fibrils block airway branching (Prince et al., 2005). Fibronectin is also implicated in branching morphogenesis in the developing kidney, where fibronectin expression is enhanced in the metanephric mesenchyme adjacent to the branching ureteric bud, and induces branching tubulogenesis of kidney epithelial cells (Santos and Nigam, 1993) and embryonic ureteric bud cells (Ye et al., 2004) in culture. Our understanding of the physical role of fibronectin in the branching morphogenesis of the lung and kidney is still rudimentary, but some of the factors involved in fibronectin-dependent branching have been uncovered. In particular, fibronectin deposition was shown to be regulated by Wnt in the embryonic lung (De Langhe et al., 2005).
Whereas fibrils of fibronectin appear to bisect the salivary epithelium like a cheese wire, the ECM provides a different type of guidance cue for branching morphogenesis of the mammary gland. Unlike the other organs discussed thus far, the mammary gland develops into a fully elaborated epithelial tree postnatally, during puberty. In mouse and human, the epithelial anlage present at birth is a small simply branched rudiment, embedded in a complex mesenchyme that contains adipose and fibrous regions (Gjorevski and Nelson, 2011). The gland remains quiescent until the onset of puberty, at which time hormones such as ovarian estrogens induce rapid proliferation, expansion and bifurcation of the epithelium until it reaches the limits of the fat pad (Sternlicht et al., 2006). The epithelial tree thus expands from the nipple to the margins of the mesenchyme, and does so along the long axis of the fat pad (Brownfield et al., 2013). Recent quantitative imaging analysis revealed that fibers of type I collagen are present within the mouse mammary gland prior to the onset of pubertal branching, with the majority of these fibers oriented parallel to the long axis of the gland (Brownfield et al., 2013). Importantly, epithelial branches orient themselves along the collagen fibers as they extend (Fig. 4B). Consistently, branching increases with collagen density (Nguyen-Ngoc and Ewald, 2013) and collagen fibril orientation is sufficient to direct the angle of branch elongation in culture (Brownfield et al., 2013; Guo et al., 2012). Similarly, genetic manipulations in mice that result in a decrease in collagen synthesis or assembly, such as knockout of matrix metalloproteinase (MMP) 11 (Tan et al., 2013), lead to defects in mammary branching. These data suggest that collagen fibers act as patterning cues for branching morphogenesis of the mammary epithelium, although how these fibers themselves become patterned remains unclear.
The mammary epithelium interacts with its surrounding ECM primarily through β1 integrin. In both cell culture and in vivo, β1 integrin associates with the transmembrane Mmp14, and silencing either of these molecules in mammary epithelial cells prevents branching morphogenesis (Mori et al., 2013). Mmp14 is expressed at high levels by epithelial cells located within the terminal buds of the pubertal mouse mammary gland (Mori et al., 2013), and is also expressed at high levels by cells at branch sites in culture (Mori et al., 2009). Importantly, the transmembrane/cytoplasmic domains of Mmp14 appear to be crucial for its interaction with β1 integrin and for downstream signaling to MAPK, which induces branching in the collagen-rich microenvironment surrounding the mammary epithelium. In addition to inducing signaling, Mmp14 also degrades the ECM at the invasive front of mammary epithelial branches in culture, which has been postulated to reduce the steric and/or mechanical resistance of the surrounding matrix to expansion by the epithelium (Alcaraz et al., 2011). Given the above-mentioned reports of mammary epithelium extending along collagen fibers in vivo and in culture, a more likely possibility is that Mmp14 helps the epithelium to remodel the surrounding ECM as the cells find their mechanical guidance cues.
Recent data suggest that mechanical stresses exerted by the mammary epithelium are also crucial for directing branching morphogenesis, as well as for initiating nascent branches (Gjorevski and Nelson, 2010, 2012). Individual cells contract isometrically within the mammary epithelium, and the stresses generated by this contraction become concentrated at specific sites along the epithelial tree, depending on its geometry (Gjorevski and Nelson, 2010). New branches initiate at sites of highest mechanical stress, and this branch initiation is stimulated in part by local activation of FAK within these cells. The mechanical properties (elastic modulus and crosslinking) of the surrounding ECM regulate the profile of mechanical stress and branch initiation (Gjorevski and Nelson, 2010), and are also affected by matrix remodeling induced by contraction of the epithelium (Gjorevski and Nelson, 2012). Such ECM-mediated mechanical stresses regulate the expression of mesenchymal genes, including those encoding transcription factors such as Snail and Slug, within branch sites (Lee et al., 2011). As these genes are also expressed at branch sites within the mammary epithelium in vivo, it is possible that they are induced by the collagen fibrils already present in the mesenchyme.
Conclusions
In summary, recent timelapse imaging, quantitative morphometric analysis and computational models have together revealed that branching morphogenesis is not a single physical process. Instead, a group of several different physical mechanisms can all generate branched epithelial trees. It is important to note that these mechanisms are not mutually exclusive, and may indeed be found to act synergistically during branching. Furthermore, the exact mechanisms that drive morphogenesis of many branching epithelia remain to be elucidated. Based on our discussions above, we identify four major conclusions that we detail below.
The pattern of branching depends on a pre-pattern within the surrounding mesenchyme … or does it?
Most model branching systems show some evidence of a complementary pattern of gene expression or physical factors in the mesenchyme prior to branch initiation. Focal expression of growth factors is the most common mesenchymal pre-pattern, but the aligned fibrils of collagen in the stroma of the mammary gland also lend support to the idea of physical pre-patterning of branching morphogenesis. However, the one problem with this concept comes from mesenchyme-free culture models, which undergo branching morphogenesis in the absence of any pre-patterned signal. Either the latter model does not reflect the situation in vivo, or there is some element of self-assembly to the pre-patterning process. Either way, we are still left with glaring questions. What induces the pre-pattern of signal in the mesenchyme? How does this arise, both spatially and temporally, during development of the branching epithelial tree? What feedback mechanisms are needed to couple the mesenchymal pre-pattern to the elaboration of the epithelium? Computational models may provide some guidance in addressing this last question (Iber and Menshykau, 2013; Ray et al., 2013), which is challenging to investigate experimentally using intact organs.
Branches are unlikely to form due to spatial patterns in proliferation
More than 40 years of investigation have failed to yield incontrovertible evidence that local increases in the growth of an epithelium can generate a new branch. Clearly, proliferation is necessary to increase the number of cells within the branch once it has formed and to promote branch extension (at least, in organs other than the Drosophila trachea). Intriguingly, across a large number of vertebrate organs, this increase in proliferation is concentrated at the tip of the growing branch. But, neither static images nor timelapse analysis have revealed an increase in cell proliferation in the epithelium at branch sites before branches have formed. This suggests that conceptual and computational models that rely solely on growth factor-induced proliferation to generate an epithelial branch need to be reconsidered. Furthermore, several questions still remain unanswered. For example, all examples of branching morphogenesis examined thus far require the presence of one or more growth factors (e.g. FGF, VEGF, EGF), but how does the developing epithelium distinguish between the capacity of these molecules to act as mitogens, and the capacity to induce a branch? Furthermore, once the branch has formed, why is proliferation universally increased in the cells at the tip of the branch? What additional signals are needed for this patterning? Timelapse image analysis of tissue-specific reporter mice may yield some clues to answer these questions.
Molecular homology does not equal mechanistic homology
Despite the fact that many of the same signaling molecules are used during branching morphogenesis of the Drosophila trachea and vertebrate lung, the physical mechanisms that generate branches are distinct. Bnl (FGF) induces a sprouting morphogenesis process in the trachea of the fly, and can clearly be considered as a chemoattractant, or at least a motogen, in this system. By contrast, FGF induces apical constriction and folding of the airway epithelium in the embryonic chicken lung, whereas it represses epithelial differentiation in the embryonic mouse lung (Volckaert et al., 2013). There is no evidence to suggest that FGF acts as either a classical chemoattractant or as a motogen in the lungs of birds or mammals. Given this major distinction, future investigations may wish to avoid drawing too many conclusions based on molecular homology alone. Of course, this begs the question: how do homologous signaling pathways yield such a diversity of physical mechanisms? Have the downstream molecular details changed over the course of evolution? Or, is the molecular homology simply misleading our search for blueprints that govern the development of branching epithelia? These questions are not limited to branching systems. For example, similar instances of molecular similarity despite physical dissimilarity have been observed in the vertebrate heart. Many of the transcription factors involved in early cardiogenesis, including Nkx2.5 and Gata5, are conserved across species, but the tissue deformations that drive heart development can be very different, as demonstrated by the major differences in cardiogenesis between fish and birds.
There is no ‘paradigmatic’ branching epithelium
Although comparisons can be made between organ systems, the differences between them are large enough to suggest that no single branching epithelium can be considered as representative of the development of all branching systems. As such, we suggest that increased attention should be given to the precision of the language used to describe branching morphogenesis. For example, despite the fact that many branching epithelia lack obvious membrane protrusions, morphogenesis of these organs is often described as an ‘invasive’ process, without clarification. Perhaps this is a relic of the comparisons between lung branching in mice and tracheal development in Drosophila. Nonetheless, imprecise descriptions may hinder progress in this field if inaccurate or inappropriate physical mechanisms are errantly associated with branching. Again, this realization prompts additional questions. Why are distinct physical mechanisms used to create very similar epithelial architectures? Why do the same organs in different species develop using very different physical mechanisms? How many different branching ‘morphogeneses’ have been realized over the course of evolution? Increased attention on quantitative analysis and new model systems will likely shed light on these, and other, unanswered questions.
Footnotes
Competing interests
The authors declare no competing financial interests.
Funding
Work in the authors' group was supported in part by grants from the National Institutes of Health, by the David & Lucile Packard Foundation, by the Alfred P. Sloan Foundation and by the Henry & Camille Dreyfus Foundation. C.M.N. holds a Career Award at the Scientific Interface from the Burroughs Wellcome Fund. Deposited in PMC for release after 12 months.
References
- Affolter M., Caussinus E. (2008). Tracheal branching morphogenesis in Drosophila: new insights into cell behaviour and organ architecture. Development 135, 2055-2064. 10.1242/dev.014498 [DOI] [PubMed] [Google Scholar]
- Alcaraz J., Mori H., Ghajar C. M., Brownfield D., Galgoczy R., Bissell M. J. (2011). Collective epithelial cell invasion overcomes mechanical barriers of collagenous extracellular matrix by a narrow tube-like geometry and MMP14-dependent local softening. Integr. Biol. (Camb.) 3, 1153-1166. 10.1039/c1ib00073j [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alescio T., Cassini A. (1962). Induction in vitro of tracheal buds by pulmonary mesenchyme grafted on tracheal epithelium. J. Exp. Zool. 150, 83-94. 10.1002/jez.1401500202 [DOI] [PubMed] [Google Scholar]
- Alescio T., Colombo Piperno E. (1967). A quantitative assessment of mesenchymal contribution to epithelial growth rate in mouse embryonic lung developing in vitro. J. Embryol. Exp. Morphol. 17, 213-227 [PubMed] [Google Scholar]
- Alescio T., Di Michele M. (1968). Relationship of epithelial growth to mitotic rate in mouse embryonic lung developing in vitro. J. Embryol. Exp. Morphol. 19, 227-237 [PubMed] [Google Scholar]
- Arthur W. T., Petch L. A., Burridge K. (2000). Integrin engagement suppresses RhoA activity via a c-Src-dependent mechanism. Curr. Biol. 10, 719-722. 10.1016/S0960-9822(00)00537-6 [DOI] [PubMed] [Google Scholar]
- Auerbach R. (1960). Morphogenetic interactions in the development of the mouse thymus gland. Dev. Biol. 2, 271-284. 10.1016/0012-1606(60)90009-9 [DOI] [PubMed] [Google Scholar]
- Bellusci S., Grindley J., Emoto H., Itoh N., Hogan B. L. (1997). Fibroblast growth factor 10 (FGF10) and branching morphogenesis in the embryonic mouse lung. Development 124, 4867-4878 [DOI] [PubMed] [Google Scholar]
- Bentley K., Mariggi G., Gerhardt H., Bates P. A. (2009). Tipping the balance: robustness of tip cell selection, migration and fusion in angiogenesis. PLoS Comput. Biol. 5, e1000549. 10.1371/journal.pcbi.1000549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernfield M., Banerjee S. D. (1982). The turnover of basal lamina glycosaminoglycan correlates with epithelial morphogenesis. Dev. Biol. 90, 291-305. 10.1016/0012-1606(82)90378-5 [DOI] [PubMed] [Google Scholar]
- Bernfield M. R., Banerjee S. D., Cohn R. H. (1972). Dependence of salivary epithelial morphology and branching morphogenesis upon acid mucopolysaccharide-protein (proteoglycan) at the epithelial surface. J. Cell Biol. 52, 674-689. 10.1083/jcb.52.3.674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernfield M. R., Cohn R. H., Banerjee S. D. (1973). Glycosaminoglycans and Epithelial Organ Formation. Am. Zool. 13, 1067-1083 [Google Scholar]
- Biyashev D., Veliceasa D., Topczewski J., Topczewska J. M., Mizgirev I., Vinokour E., Reddi A. L., Licht J. D., Revskoy S. Y., Volpert O. V. (2012). miR-27b controls venous specification and tip cell fate. Blood 119, 2679-2687. 10.1182/blood-2011-07-370635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borghese E. (1950). The development in vitro of the submandibular and sublingual glands of Mus musculus. J. Anat. 84, 287-302 [PMC free article] [PubMed] [Google Scholar]
- Bresciani F. (1968). Topography of DNA synthesis in the mammary gland of the C3H mouse and its control by ovarian hormones: an autoradiographic study. Cell Tissue Kinet. 1, 51-63 [Google Scholar]
- Brownfield D. G., Venugopalan G., Lo A., Mori H., Tanner K., Fletcher D. A., Bissell M. J. (2013). Patterned collagen fibers orient branching mammary epithelium through distinct signaling modules. Curr. Biol. 23, 703-709. 10.1016/j.cub.2013.03.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoso W. V., Itoh A., Nogawa H., Mason I., Brody J. S. (1997). FGF-1 and FGF-7 induce distinct patterns of growth and differentiation in embryonic lung epithelium. Dev. Dyn. 208, 398-405. [DOI] [PubMed] [Google Scholar]
- Casanova J. (2007). The emergence of shape: notions from the study of the Drosophila tracheal system. EMBO Rep. 8, 335-339. 10.1038/sj.embor.7400942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caussinus E., Colombelli J., Affolter M. (2008). Tip-cell migration controls stalk-cell intercalation during Drosophila tracheal tube elongation. Curr. Biol. 18, 1727-1734. 10.1016/j.cub.2008.10.062 [DOI] [PubMed] [Google Scholar]
- Chi X., Michos O., Shakya R., Riccio P., Enomoto H., Licht J. D., Asai N., Takahashi M., Ohgami N., Kato M., et al. (2009). Ret-dependent cell rearrangements in the Wolffian duct epithelium initiate ureteric bud morphogenesis. Dev. Cell 17, 199-209. 10.1016/j.devcel.2009.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu J. Y. S., Sims-Lucas S., Bushnell D. S., Bodnar A. J., Kreidberg J. A., Ho J. (2014). Dicer function is required in the metanephric mesenchyme for early kidney development. Am. J. Physiol. Renal Physiol. 306, F764-F772. 10.1152/ajprenal.00426.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daley W. P., Gulfo K. M., Sequeira S. J., Larsen M. (2009). Identification of a mechanochemical checkpoint and negative feedback loop regulating branching morphogenesis. Dev. Biol. 336, 169-182. 10.1016/j.ydbio.2009.09.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daley W. P., Kohn J. M., Larsen M. (2011). A focal adhesion protein-based mechanochemical checkpoint regulates cleft progression during branching morphogenesis. Dev. Dyn. 240, 2069-2083. 10.1002/dvdy.22714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies J. A. (2002). Do different branching epithelia use a conserved developmental mechanism? Bioessays 24, 937-948. 10.1002/bies.10161 [DOI] [PubMed] [Google Scholar]
- De Langhe S. P., Sala F. G., Del Moral P.-M., Fairbanks T. J., Yamada K. M., Warburton D., Burns R. C., Bellusci S. (2005). Dickkopf-1 (DKK1) reveals that fibronectin is a major target of Wnt signaling in branching morphogenesis of the mouse embryonic lung. Dev. Biol. 277, 316-331. 10.1016/j.ydbio.2004.09.023 [DOI] [PubMed] [Google Scholar]
- Enger T. B., Samad-Zadeh A., Bouchie M. P., Skarstein K., Galtung H. K., Mera T., Walker J., Menko A. S., Varelas X., Faustman D. L., et al. (2013). The Hippo signaling pathway is required for salivary gland development and its dysregulation is associated with Sjogren's syndrome. Lab. Invest. 93, 1203-1218. 10.1038/labinvest.2013.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettensohn C. A. (1985). Mechanisms of epithelial invagination. Q. Rev. Biol. 60, 289-307. 10.1086/414426 [DOI] [PubMed] [Google Scholar]
- Ewald A. J., Brenot A., Duong M., Chan B. S., Werb Z. (2008). Collective epithelial migration and cell rearrangements drive mammary branching morphogenesis. Dev. Cell 14, 570-581. 10.1016/j.devcel.2008.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda Y., Masuda Y., Kishi J., Hashimoto Y., Hayakawa T., Nogawa H., Nakanishi Y. (1988). The role of interstitial collagens in cleft formation of mouse embryonic submandibular gland during initial branching. Development 103, 259-267 [DOI] [PubMed] [Google Scholar]
- Ghabrial A. S., Krasnow M. A. (2006). Social interactions among epithelial cells during tracheal branching morphogenesis. Nature 441, 746-749. 10.1038/nature04829 [DOI] [PubMed] [Google Scholar]
- Ghabrial A., Luschnig S., Metzstein M. M., Krasnow M. A. (2003). Branching morphogenesis of the Drosophila tracheal system. Annu. Rev. Cell Dev. Biol. 19, 623-647. 10.1146/annurev.cellbio.19.031403.160043 [DOI] [PubMed] [Google Scholar]
- Ghabrial A. S., Levi B. P., Krasnow M. A. (2011). A systematic screen for tube morphogenesis and branching genes in the Drosophila tracheal system. PLoS Genet. 7, e1002087. 10.1371/journal.pgen.1002087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gjorevski N., Nelson C. M. (2010). Endogenous patterns of mechanical stress are required for branching morphogenesis. Integr. Biol. (Camb.) 2, 424-434. 10.1039/c0ib00040j [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gjorevski N., Nelson C. M. (2011). Integrated morphodynamic signalling of the mammary gland. Nat. Rev. Mol. Cell Biol. 12, 581-593. 10.1038/nrm3168 [DOI] [PubMed] [Google Scholar]
- Gjorevski N., Nelson C. M. (2012). Mapping of mechanical strains and stresses around quiescent engineered three-dimensional epithelial tissues. Biophys. J. 103, 152-162. 10.1016/j.bpj.2012.05.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleghorn J. P., Manivannan S., Nelson C. M. (2013). Quantitative approaches to uncover physical mechanisms of tissue morphogenesis. Curr. Opin. Biotechnol. 24, 954-961. 10.1016/j.copbio.2013.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin G. V. (1980). Towards a mechanism for morphogenesis in epithelio-mesenchymal organs. Q. Rev. Biol. 55, 251-265. 10.1086/411856 [DOI] [PubMed] [Google Scholar]
- Goldin G. V., Opperman L. A. (1980). Induction of supernumerary tracheal buds and the stimulation of DNA synthesis in the embryonic chick lung and trachea by epidermal growth factor. J. Embryol. Exp. Morphol. 60, 235-243 [PubMed] [Google Scholar]
- Goldin G. V., Wessells N. K. (1979). Mammalian lung development: the possible role of cell proliferation in the formation of supernumerary tracheal buds and in branching morphogenesis. J. Exp. Zool. 208, 337-346. 10.1002/jez.1402080310 [DOI] [PubMed] [Google Scholar]
- Goldin G. V., Hindman H. M., Wessells N. K. (1984). The role of cell proliferation and cellular shape change in branching morphogenesis of the embryonic mouse lung: analysis using aphidicolin and cytochalasins. J. Exp. Zool. 232, 287-296. 10.1002/jez.1402320216 [DOI] [PubMed] [Google Scholar]
- Golosow N., Grobstein C. (1962). Epitheliomesenchymal interaction in pancreatic morphogenesis. Dev. Biol. 4, 242-255. 10.1016/0012-1606(62)90042-8 [DOI] [PubMed] [Google Scholar]
- Grobstein C. (1953a). Analysis in vitro of the early organization of the rudiment of the mouse submandibular gland. J. Morphol. 93, 19-43. 10.1002/jmor.1050930103 [DOI] [Google Scholar]
- Grobstein C. (1953b). Inductive epithelio-mesenchymal interaction in cultured organ rudiments of the mouse. Science 118, 52-55. 10.1126/science.118.3054.52 [DOI] [PubMed] [Google Scholar]
- Grobstein C. (1956). Trans-filter induction of tubules in mouse metanephrogenic mesenchyme. Exp. Cell Res. 10, 424-440. 10.1016/0014-4827(56)90016-7 [DOI] [PubMed] [Google Scholar]
- Grobstein C. (1967). Mechanisms of organogenetic tissue interaction. Natl. Cancer Inst. Monogr. 26, 279-299 [PubMed] [Google Scholar]
- Guillemin K., Groppe J., Ducker K., Treisman R., Hafen E., Affolter M., Krasnow M. A. (1996). The pruned gene encodes the Drosophila serum response factor and regulates cytoplasmic outgrowth during terminal branching of the tracheal system. Development 122, 1353-1362 [DOI] [PubMed] [Google Scholar]
- Guilluy C., Swaminathan V., Garcia-Mata R., O'Brien E. T., Superfine R., Burridge K. (2011). The Rho GEFs LARG and GEF-H1 regulate the mechanical response to force on integrins. Nat. Cell Biol. 13, 724-729. 10.1038/ncb2254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo C.-L., Ouyang M., Yu J.-Y., Maslov J., Price A., Shen C.-Y. (2012). Long-range mechanical force enables self-assembly of epithelial tubular patterns. Proc. Natl. Acad. Sci. U.S.A. 109, 5576-5582. 10.1073/pnas.1114781109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harunaga J., Hsu J. C., Yamada K. M. (2011). Dynamics of salivary gland morphogenesis. J. Dent. Res. 90, 1070-1077. 10.1177/0022034511405330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T., Koyama N., Azuma Y., Kashimata M. (2011). Mesenchymal miR-21 regulates branching morphogenesis in murine submandibular gland in vitro. Dev. Biol. 352, 299-307. 10.1016/j.ydbio.2011.01.030 [DOI] [PubMed] [Google Scholar]
- Hellström M., Phng L.-K., Hofmann J. J., Wallgard E., Coultas L., Lindblom P., Alva J., Nilsson A.-K., Karlsson L., Gaiano N., et al. (2007). Dll4 signalling through Notch1 regulates formation of tip cells during angiogenesis. Nature 445, 776-780. 10.1038/nature05571 [DOI] [PubMed] [Google Scholar]
- Hoffman M. P., Kidder B. L., Steinberg Z. L., Lakhani S., Ho S., Kleinman H. K., Larsen M. (2002). Gene expression profiles of mouse submandibular gland development: FGFR1 regulates branching morphogenesis in vitro through BMP- and FGF-dependent mechanisms. Development 129, 5767-5778. 10.1242/dev.00172 [DOI] [PubMed] [Google Scholar]
- Hsu J. C., Koo H., Harunaga J. S., Matsumoto K., Doyle A. D., Yamada K. M. (2013). Region-specific epithelial cell dynamics during branching morphogenesis. Dev. Dyn. 242, 1066-1077. 10.1002/dvdy.24000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iber D., Menshykau D. (2013). The control of branching morphogenesis. Open Biol. 3, 130088. 10.1098/rsob.130088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobsson L., Franco C. A., Bentley K., Collins R. T., Ponsioen B., Aspalter I. M., Rosewell I., Busse M., Thurston G., Medvinsky A., et al. (2010). Endothelial cells dynamically compete for the tip cell position during angiogenic sprouting. Nat. Cell Biol. 12, 943-953. 10.1038/ncb2103 [DOI] [PubMed] [Google Scholar]
- Jiang Q., Lagos-Quintana M., Liu D., Shi Y., Helker C., Herzog W., le Noble F. (2013). miR-30a regulates endothelial tip cell formation and arteriolar branching. Hypertension 62, 592-598. 10.1161/HYPERTENSIONAHA.113.01767 [DOI] [PubMed] [Google Scholar]
- Kadoya Y., Yamashina S. (2010). Cellular dynamics of epithelial clefting during branching morphogenesis of the mouse submandibular gland. Dev. Dyn. 239, 1739-1747. 10.1002/dvdy.22312 [DOI] [PubMed] [Google Scholar]
- Kashimata M., Gresik E. W. (1997). Epidermal growth factor system is a physiological regulator of development of the mouse fetal submandibular gland and regulates expression of the alpha6-integrin subunit. Dev. Dyn. 208, 149-161. [DOI] [PubMed] [Google Scholar]
- Kashimata M., Sayeed S., Ka A., Onetti-Muda A., Sakagami H., Faraggiana T., Gresik E. W. (2000). The ERK-1/2 signaling pathway is involved in the stimulation of branching morphogenesis of fetal mouse submandibular glands by EGF. Dev. Biol. 220, 183-196. 10.1006/dbio.2000.9639 [DOI] [PubMed] [Google Scholar]
- Kim H. Y., Varner V. D., Nelson C. M. (2013). Apical constriction initiates new bud formation during monopodial branching of the embryonic chicken lung. Development 140, 3146-3155. 10.1242/dev.093682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klambt C., Glazer L., Shilo B. Z. (1992). breathless, a Drosophila FGF receptor homolog, is essential for migration of tracheal and specific midline glial cells. Genes Dev. 6, 1668-1678. 10.1101/gad.6.9.1668 [DOI] [PubMed] [Google Scholar]
- Koizumi Y., Iwasa Y., Hirashima T. (2012). Mathematical study of the role of Delta/Notch lateral inhibition during primary branching of Drosophila trachea development. Biophys. J. 103, 2549-2559. 10.1016/j.bpj.2012.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama N., Hayashi T., Ohno K., Siu L., Gresik E. W., Kashimata M. (2008). Signaling pathways activated by epidermal growth factor receptor or fibroblast growth factor receptor differentially regulate branching morphogenesis in fetal mouse submandibular glands. Develop. Growth Differ. 50, 565-576 [DOI] [PubMed] [Google Scholar]
- Kratochwil K. (1969). Organ specificity in mesenchymal induction demonstrated in the embryonic development of the mammary gland of the mouse. Dev. Biol. 20, 46-71. 10.1016/0012-1606(69)90004-9 [DOI] [PubMed] [Google Scholar]
- Kume T. (2009). Novel insights into the differential functions of Notch ligands in vascular formation. J. Angiogenes. Res. 1, 8. 10.1186/2040-2384-1-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen M., Wei C., Yamada K. M. (2006). Cell and fibronectin dynamics during branching morphogenesis. J. Cell Sci. 119, 3376-3384. 10.1242/jcs.03079 [DOI] [PubMed] [Google Scholar]
- Lebreton G., Casanova J. (2014). Specification of leading and trailing cell features during collective migration in the Drosophila trachea. J. Cell Sci. 127, 465-474. 10.1242/jcs.142737 [DOI] [PubMed] [Google Scholar]
- Lee K., Gjorevski N., Boghaert E., Radisky D. C., Nelson C. M. (2011). Snail1, Snail2, and E47 promote mammary epithelial branching morphogenesis. EMBO J. 30, 2662-2674. 10.1038/emboj.2011.159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llimargas M. (1999). The Notch pathway helps to pattern the tips of the Drosophila tracheal branches by selecting cell fates. Development 126, 2355-2364 [DOI] [PubMed] [Google Scholar]
- Lu Y., Thomson J. M., Wong H. Y. F., Hammond S. M., Hogan B. L. M. (2007). Transgenic over-expression of the microRNA miR-17-92 cluster promotes proliferation and inhibits differentiation of lung epithelial progenitor cells. Dev. Biol. 310, 442-453. 10.1016/j.ydbio.2007.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubkin S. R. (2008). Branched organs: mechanics of morphogenesis by multiple mechanisms. Curr. Top. Dev. Biol. 81, 249-268. 10.1016/S0070-2153(07)81008-8 [DOI] [PubMed] [Google Scholar]
- Makarenkova H. P., Hoffman M. P., Beenken A., Eliseenkova A. V., Meech R., Tsau C., Patel V. N., Lang R. A., Mohammadi M. (2009). Differential interactions of FGFs with heparan sulfate control gradient formation and branching morphogenesis. Sci. Signal. 2, ra55. 10.1126/scisignal.2000304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menshykau D., Iber D. (2013). Kidney branching morphogenesis under the control of a ligand-receptor-based Turing mechanism. Phys. Biol. 10, 046003. 10.1088/1478-3975/10/4/046003 [DOI] [PubMed] [Google Scholar]
- Menshykau D., Kraemer C., Iber D. (2012). Branch mode selection during early lung development. PLoS Comput. Biol. 8, e1002377. 10.1371/journal.pcbi.1002377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger R. J., Krasnow M. A. (1999). Genetic control of branching morphogenesis. Science 284, 1635-1639. 10.1126/science.284.5420.1635 [DOI] [PubMed] [Google Scholar]
- Michael L., Davies J. A. (2004). Pattern and regulation of cell proliferation during murine ureteric bud development. J. Anat. 204, 241-255. 10.1111/j.0021-8782.2004.00285.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori H., Gjorevski N., Inman J. L., Bissell M. J., Nelson C. M. (2009). Self-organization of engineered epithelial tubules by differential cellular motility. Proc. Natl. Acad. Sci. U.S.A. 106, 14890-14895. 10.1073/pnas.0901269106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori H., Lo A. T., Inman J. L., Alcaraz J., Ghajar C. M., Mott J. D., Nelson C. M., Chen C. S., Zhang H., Bascom J. L., et al. (2013). Transmembrane/cytoplasmic, rather than catalytic, domains of Mmp14 signal to MAPK activation and mammary branching morphogenesis via binding to integrin beta1. Development 140, 343-352. 10.1242/dev.084236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita K., Nogawa H. (1999). EGF-dependent lobule formation and FGF7-dependent stalk elongation in branching morphogenesis of mouse salivary epithelium in vitro. Dev. Dyn. 215, 148-154. [DOI] [PubMed] [Google Scholar]
- Mujahid S., Nielsen H. C., Volpe M. V. (2013). MiR-221 and miR-130a regulate lung airway and vascular development. PLoS ONE 8, e55911. 10.1371/journal.pone.0055911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulinari S., Häcker U. (2010). Rho-guanine nucleotide exchange factors during development: force is nothing without control. Small GTPases 1, 28-43. 10.4161/sgtp.1.1.12672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi Y., Nogawa H., Hashimoto Y., Kishi J., Hayakawa T. (1988). Accumulation of collagen III at the cleft points of developing mouse submandibular epithelium. Development 104, 51-59 [DOI] [PubMed] [Google Scholar]
- Nelson C. M., Gleghorn J. P. (2012). Sculpting organs: mechanical regulation of tissue development. Annu. Rev. Biomed. Eng. 14, 129-154. 10.1146/annurev-bioeng-071811-150043 [DOI] [PubMed] [Google Scholar]
- Nguyen-Ngoc K.-V., Ewald A. J. (2013). Mammary ductal elongation and myoepithelial migration are regulated by the composition of the extracellular matrix. J. Microsc. 251, 212-223. 10.1111/jmi.12017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogawa H., Ito T. (1995). Branching morphogenesis of embryonic mouse lung epithelium in mesenchyme-free culture. Development 121, 1015-1022 [DOI] [PubMed] [Google Scholar]
- Nogawa H., Takahashi Y. (1991). Substitution for mesenchyme by basement-membrane-like substratum and epidermal growth factor in inducing branching morphogenesis of mouse salivary epithelium. Development 112, 855-861 [DOI] [PubMed] [Google Scholar]
- Nogawa H., Morita K., Cardoso W. V. (1998). Bud formation precedes the appearance of differential cell proliferation during branching morphogenesis of mouse lung epithelium in vitro. Dev. Dyn. 213, 228-235. [DOI] [PubMed] [Google Scholar]
- Noguera-Troise I., Daly C., Papadopoulos N. J., Coetzee S., Boland P., Gale N. W., Lin H. C., Yancopoulos G. D., Thurston G. (2006). Blockade of Dll4 inhibits tumour growth by promoting non-productive angiogenesis. Nature 444, 1032-1037. 10.1038/nature05355 [DOI] [PubMed] [Google Scholar]
- Ochoa-Espinosa A., Affolter M. (2012). Branching morphogenesis: from cells to organs and back. Cold Spring Harb. Perspect. Biol. 4, a008243. 10.1101/cshperspect.a008243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okenve-Ramos P., Llimargas M. (2014). Fascin links Btl/FGFR signalling to the actin cytoskeleton during Drosophila tracheal morphogenesis. Development 141, 929-939. 10.1242/dev.103218 [DOI] [PubMed] [Google Scholar]
- Okubo T., Knoepfler P. S., Eisenman R. N., Hogan B. L. M. (2005). Nmyc plays an essential role during lung development as a dosage-sensitive regulator of progenitor cell proliferation and differentiation. Development 132, 1363-1374. 10.1242/dev.01678 [DOI] [PubMed] [Google Scholar]
- Onodera T., Sakai T., Hsu J. C.-f., Matsumoto K., Chiorini J. A., Yamada K. M. (2010). Btbd7 regulates epithelial cell dynamics and branching morphogenesis. Science 329, 562-565. 10.1126/science.1191880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packard A., Georgas K., Michos O., Riccio P., Cebrian C., Combes A. N., Ju A., Ferrer-Vaquer A., Hadjantonakis A.-K., Zong H., et al. (2013). Luminal mitosis drives epithelial cell dispersal within the branching ureteric bud. Dev. Cell 27, 319-330. 10.1016/j.devcel.2013.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park W. Y., Miranda B., Lebeche D., Hashimoto G., Cardoso W. V. (1998). FGF-10 is a chemotactic factor for distal epithelial buds during lung development. Dev. Biol. 201, 125-134. 10.1006/dbio.1998.8994 [DOI] [PubMed] [Google Scholar]
- Patel V. N., Rebustini I. T., Hoffman M. P. (2006). Salivary gland branching morphogenesis. Differentiation 74, 349-364. 10.1111/j.1432-0436.2006.00088.x [DOI] [PubMed] [Google Scholar]
- Petzold K. M., Naumann H., Spagnoli F. M. (2013). Rho signalling restriction by the RhoGAP Stard13 integrates growth and morphogenesis in the pancreas. Development 140, 126-135. 10.1242/dev.082701 [DOI] [PubMed] [Google Scholar]
- Prince L. S., Dieperink H. I., Okoh V. O., Fierro-Perez G. A., Lallone R. L. (2005). Toll-like receptor signaling inhibits structural development of the distal fetal mouse lung. Dev. Dyn. 233, 553-561. 10.1002/dvdy.20362 [DOI] [PubMed] [Google Scholar]
- Qiao J., Sakurai H., Nigam S. K. (1999). Branching morphogenesis independent of mesenchymal-epithelial contact in the developing kidney. Proc. Natl. Acad. Sci. U.S.A. 96, 7330-7335. 10.1073/pnas.96.13.7330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao J., Bush K. T., Steer D. L., Stuart R. O., Sakurai H., Wachsman W., Nigam S. K. (2001). Multiple fibroblast growth factors support growth of the ureteric bud but have different effects on branching morphogenesis. Mech. Dev. 109, 123-135. 10.1016/S0925-4773(01)00592-5 [DOI] [PubMed] [Google Scholar]
- Ray S., Yuan D., Dhulekar N., Oztan B., Yener B., Larsen M. (2013). Cell-based multi-parametric model of cleft progression during submandibular salivary gland branching morphogenesis. PLoS Comput. Biol. 9, e1003319. 10.1371/journal.pcbi.1003319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebustini I. T., Hayashi T., Reynolds A. D., Dillard M. L., Carpenter E. M., Hoffman M. P. (2012). miR-200c regulates FGFR-dependent epithelial proliferation via Vldlr during submandibular gland branching morphogenesis. Development 139, 191-202. 10.1242/dev.070151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro C., Ebner A., Affolter M. (2002). In vivo imaging reveals different cellular functions for FGF and Dpp signaling in tracheal branching morphogenesis. Dev. Cell 2, 677-683. 10.1016/S1534-5807(02)00171-5 [DOI] [PubMed] [Google Scholar]
- Ribeiro C., Neumann M., Affolter M. (2004). Genetic control of cell intercalation during tracheal morphogenesis in Drosophila. Curr. Biol. 14, 2197-2207. 10.1016/j.cub.2004.11.056 [DOI] [PubMed] [Google Scholar]
- Rodriguez E. K., Hoger A., McCulloch A. D. (1994). Stress-dependent finite growth in soft elastic tissues. J. Biomech. 27, 455-467. 10.1016/0021-9290(94)90021-3 [DOI] [PubMed] [Google Scholar]
- Roman J. (1997). Fibronectin and fibronectin receptors in lung development. Exp. Lung Res. 23, 147-159. 10.3109/01902149709074027 [DOI] [PubMed] [Google Scholar]
- Roman J., McDonald J. A. (1992). Expression of fibronectin, the integrin alpha 5, and alpha-smooth muscle actin in heart and lung development. Am. J. Respir. Cell Mol. Biol. 6, 472-480. 10.1165/ajrcmb/6.5.472 [DOI] [PubMed] [Google Scholar]
- Rørth P. (2009). Collective cell migration. Annu. Rev. Cell Dev. Biol. 25, 407-429. 10.1146/annurev.cellbio.042308.113231 [DOI] [PubMed] [Google Scholar]
- Sakai T., Larsen M., Yamada K. M. (2003). Fibronectin requirement in branching morphogenesis. Nature 423, 876-881. 10.1038/nature01712 [DOI] [PubMed] [Google Scholar]
- Samakovlis C., Hacohen N., Manning G., Sutherland D. C., Guillemin K., Krasnow M. A. (1996). Development of the Drosophila tracheal system occurs by a series of morphologically distinct but genetically coupled branching events. Development 122, 1395-1407 [DOI] [PubMed] [Google Scholar]
- Santos O. F. P., Nigam S. K. (1993). HGF-induced tubulogenesis and branching of epithelial cells is modulated by extracellular matrix and TGF-beta. Dev. Biol. 160, 293-302. 10.1006/dbio.1993.1308 [DOI] [PubMed] [Google Scholar]
- Savin T., Kurpios N. A., Shyer A. E., Florescu P., Liang H., Mahadevan L., Tabin C. J. (2011). On the growth and form of the gut. Nature 476, 57-62. 10.1038/nature10277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnatwinkel C., Niswander L. (2013). Multiparametric image analysis of lung-branching morphogenesis. Dev. Dyn. 242, 622-637. 10.1002/dvdy.23961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schottenfeld J., Song Y., Ghabrial A. S. (2010). Tube continued: morphogenesis of the Drosophila tracheal system. Curr. Opin. Cell Biol. 22, 633-639. 10.1016/j.ceb.2010.07.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharghi-Namini S., Tan E., Ong L.-L. S., Ge R., Asada H. H. (2014). Dll4-containing exosomes induce capillary sprout retraction in a 3D microenvironment. Sci. Rep. 4, 4031. 10.1038/srep04031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaye D. D., Casanova J., Llimargas M. (2008). Modulation of intracellular trafficking regulates cell intercalation in the Drosophila trachea. Nat. Cell Biol. 10, 964-970. 10.1038/ncb1756 [DOI] [PubMed] [Google Scholar]
- Sheldon H., Heikamp E., Turley H., Dragovic R., Thomas P., Oon C. E., Leek R., Edelmann M., Kessler B., Sainson R. C. A., et al. (2010). New mechanism for Notch signaling to endothelium at a distance by Delta-like 4 incorporation into exosomes. Blood 116, 2385-2394. 10.1182/blood-2009-08-239228 [DOI] [PubMed] [Google Scholar]
- Shi Y., Yao J., Xu G., Taber L. A. (2014). Bending of the looping heart: differential growth revisited. J. Biomech. Eng. (in press). 10.1115/1.4026645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shindo M., Wada H., Kaido M., Tateno M., Aigaki T., Tsuda L., Hayashi S. (2008). Dual function of Src in the maintenance of adherens junctions during tracheal epithelial morphogenesis. Development 135, 1355-1364. 10.1242/dev.015982 [DOI] [PubMed] [Google Scholar]
- Shyer A. E., Tallinen T., Nerurkar N. L., Wei Z., Gil E. S., Kaplan D. L., Tabin C. J., Mahadevan L. (2013). Villification: how the gut gets its villi. Science 342, 212-218. 10.1126/science.1238842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spooner B. S., Wessells N. K. (1970a). Effects of cytochalasin B upon microfilaments involved in morphogenesis of salivary epithelium. Proc. Natl. Acad. Sci. U.S.A. 66, 360-364. 10.1073/pnas.66.2.360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spooner B. S., Wessells N. K. (1970b). Mammalian lung development: interactions in primordium formation and bronchial morphogenesis. J. Exp. Zool. 175, 445-454. 10.1002/jez.1401750404 [DOI] [PubMed] [Google Scholar]
- Spooner B. S., Wessells N. K. (1972). An analysis of salivary gland morphogenesis: role of cytoplasmic microfilaments and microtubules. Dev. Biol. 27, 38-54. 10.1016/0012-1606(72)90111-X [DOI] [PubMed] [Google Scholar]
- Sternlicht M. D., Kouros-Mehr H., Lu P., Werb Z. (2006). Hormonal and local control of mammary branching morphogenesis. Differentiation 74, 365-381. 10.1111/j.1432-0436.2006.00105.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suchting S., Freitas C., le Noble F., Benedito R., Breant C., Duarte A., Eichmann A. (2007). The Notch ligand Delta-like 4 negatively regulates endothelial tip cell formation and vessel branching. Proc. Natl. Acad. Sci. U.S.A. 104, 3225-3230. 10.1073/pnas.0611177104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland D., Samakovlis C., Krasnow M. A. (1996). branchless encodes a Drosophila FGF homolog that controls tracheal cell migration and the pattern of branching. Cell 87, 1091-1101. 10.1016/S0092-8674(00)81803-6 [DOI] [PubMed] [Google Scholar]
- Taber L. A. (1995). Biomechanics of growth, remodeling, and morphogenesis. Appl. Mech. Rev. 48, 487-545. 10.1115/1.3005109 [DOI] [Google Scholar]
- Tan J., Buache E., Alpy F., Daguenet E., Tomasetto C. L., Ren G. S., Rio M. C. (2013). Stromal matrix metalloproteinase-11 is involved in the mammary gland postnatal development. Oncogene (in press). 10.1038/onc.2013.434 [DOI] [PubMed] [Google Scholar]
- Tang N., Marshall W. F., McMahon M., Metzger R. J., Martin G. R. (2011). Control of mitotic spindle angle by the RAS-regulated ERK1/2 pathway determines lung tube shape. Science 333, 342-345. 10.1126/science.1204831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker A. S. (2007). Salivary gland development. Semin. Cell Dev. Biol. 18, 237-244. 10.1016/j.semcdb.2007.01.006 [DOI] [PubMed] [Google Scholar]
- Uv A., Cantera R., Samakovlis C. (2003). Drosophila tracheal morphogenesis: intricate cellular solutions to basic plumbing problems. Trends Cell Biol. 13, 301-309. 10.1016/S0962-8924(03)00083-7 [DOI] [PubMed] [Google Scholar]
- Varner V. D., Voronov D. A., Taber L. A. (2010). Mechanics of head fold formation: investigating tissue-level forces during early development. Development 137, 3801-3811. 10.1242/dev.054387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volckaert T., Campbell A., Dill E., Li C., Minoo P., De Langhe S. (2013). Localized Fgf10 expression is not required for lung branching morphogenesis but prevents differentiation of epithelial progenitors. Development 140, 3731-3742. 10.1242/dev.096560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voronov D. A., Alford P. W., Xu G., Taber L. A. (2004). The role of mechanical forces in dextral rotation during cardiac looping in the chick embryo. Dev. Biol. 272, 339-350. 10.1016/j.ydbio.2004.04.033 [DOI] [PubMed] [Google Scholar]
- Wang M., Ong L. L., Dauwels J., Asada H. H. (2013). 2D data-driven stalk cell prediction model based on tip-stalk cell interaction in angiogenesis. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2013, 4537-4540 [DOI] [PubMed] [Google Scholar]
- Warrington S. J., Strutt H., Strutt D. (2013). The Frizzled-dependent planar polarity pathway locally promotes E-cadherin turnover via recruitment of RhoGEF2. Development 140, 1045-1054. 10.1242/dev.088724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver M., Dunn N. R., Hogan B. L. (2000). Bmp4 and Fgf10 play opposing roles during lung bud morphogenesis. Development 127, 2695-2704 [DOI] [PubMed] [Google Scholar]
- Wei C., Larsen M., Hoffman M. P., Yamada K. M. (2007). Self-organization and branching morphogenesis of primary salivary epithelial cells. Tissue Eng. 13, 721-735. 10.1089/ten.2006.0123 [DOI] [PubMed] [Google Scholar]
- Wessells N. K. (1970). Mammalian lung development: interactions in formation and morphogenesis of tracheal buds. J. Exp. Zool. 175, 455-466. 10.1002/jez.1401750405 [DOI] [PubMed] [Google Scholar]
- Williams J. M., Daniel C. W. (1983). Mammary ductal elongation: differentiation of myoepithelium and basal lamina during branching morphogenesis. Dev. Biol. 97, 274-290. 10.1016/0012-1606(83)90086-6 [DOI] [PubMed] [Google Scholar]
- Wyczalkowski M. A., Chen Z., Filas B. A., Varner V. D., Taber L. A. (2012). Computational models for mechanics of morphogenesis. Birth Defects Res. C Embryo Today 96, 132-152. 10.1002/bdrc.21013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye P., Habib S. L., Ricono J. M., Kim N.-H., Choudhury G. G., Barnes J. L., Abboud H. E., Arar M. Y. (2004). Fibronectin induces ureteric bud cells branching and cellular cord and tubule formation. Kidney Int. 66, 1356-1364. 10.1111/j.1523-1755.2004.00897.x [DOI] [PubMed] [Google Scholar]
- Yu J. (2014). miRNAs in mammalian ureteric bud development. Pediatr. Nephrol. 29, 745-749. 10.1007/s00467-013-2734-y [DOI] [PubMed] [Google Scholar]


