Abstract
BACKGROUND & AIMS
Bone morphogenetic protein (BMP)4 is a mesenchymal peptide that regulates cells of the gastric epithelium. We investigated whether BMP signaling pathways affect gastric inflammation after bacterial infection of mice.
METHODS
We studied transgenic mice that express either the BMP inhibitor noggin or the β- galactosidase gene under the control of a BMP-responsive element and BMP4βgal/+ mice. Gastric inflammation was induced by infection of mice with either Helicobacter pylori or Helicobacter felis. Eight to 12 weeks after inoculation, gastric tissue samples were collected and immunohistochemical, quantitative, reverse-transcription polymerase chain reaction and immunoblot analyses were performed. We used enzyme-linked immunosorbent assays to measure cytokine levels in supernatants from cultures of mouse splenocytes and dendritic cells, as well as from human gastric epithelial cells (AGS cell line). We also measured the effects of BMP-2, BMP-4, BMP-7, and the BMP inhibitor LDN-193189 on the expression of interleukin (IL)8 messenger RNA by AGS cells and primary cultures of canine parietal and mucus cells. The effect of BMP-4 on NFkB activation in parietal and AGS cells was examined by immunoblot and luciferase assays.
RESULTS
Transgenic expression of noggin in mice increased H pylori– or H felis–induced inflammation and epithelial cell proliferation, accelerated the development of dysplasia, and increased expression of the signal transducer and activator of transcription 3 and activation-induced cytidine deaminase. BMP-4 was expressed in mesenchymal cells that expressed α-smooth muscle actin and activated BMP signaling pathways in the gastric epithelium. Neither BMP-4 expression nor BMP signaling were detected in immune cells of C57BL/6, BRE–β-galactosidase, or BMP-4βgal/+ mice. Incubation of dendritic cells or splenocytes with BMP-4 did not affect lipopolysaccharide-stimulated production of cytokines. BMP-4, BMP-2, and BMP-7 inhibited basal and tumor necrosis factor α–stimulated expression of IL8 in canine gastric epithelial cells. LDN-193189 prevented BMP4-mediated inhibition of basal and tumor necrosis factor α–stimulated expression of IL8 in AGS cells. BMP-4 had no effect on TNFα-stimulated phosphorylation and degradation of IκBα, or on TNFα induction of a NFκβ reporter gene.
CONCLUSIONS
BMP signaling reduces inflammation and inhibits dysplastic changes in the gastric mucosa after infection of mice with H pylori or H felis.
Keywords: BRE, STAT3, Differentiation, Immune Regulation
The bone morphogenetic proteins (BMPs) are important regulators of a broad array of biological actions during both embryonic and postnatal vertebrate development.1–13 BMP-2, BMP-4, and BMP-7 appear to be significantly expressed in gastrointestinal tissues, where they have been shown to play a significant role in the regulation of cellular proliferation and differentiation.1–13 The clinical relevance of these observations has been underscored by studies conducted in human tissues that have demonstrated an important role for BMP signaling in the inhibition of gastrointestinal tumor growth.4,9–12
The actions of the BMPs can be specifically blocked, by inhibitory proteins that are expressed in tissues to modulate the level of activation of BMP signaling.1–14 Of these, noggin, a secreted polypeptide present in several mammalian tissues, has been shown to bind to, and inhibit, the actions of BMP-2, BMP-4, and, to a lesser degree, BMP-7.1,2,5,14 In addition, small molecule inhibitors of BMP type I receptors have been recently described.15 These compounds have been employed both in vivo and in vitro to manipulate a broad array of physiological functions, underscoring the importance of BMP signaling in both physiology and disease.15
BMP-4, in particular, appears to be expressed in the mesenchymal layers of the gastric mucosa and to exert significant regulatory effects on gastric physiology.3,16–18 Studies from our laboratory have shown that incubation of cultured parietal cells with this peptide leads to stimulation of H+/K+-adenosine triphosphatase α-subunit gene expression and to enhancement of secretagogue-stimulated gastric acid production.16 In a series of recent in vivo investigations, we and others also have shown that inhibition of BMP signaling in the gastric epithelium causes profound aberrations in the normal mechanisms that regulate the proliferation, maturation, and differentiation of several lineages of gastric epithelial cells, underscoring the importance of BMP signaling in the regulation of gastric epithelial homeostasis.17,18
In addition to these effects, studies have shown that the BMPs might exert widespread anti-inflammatory actions and therefore they could represent novel and hitherto poorly characterized regulators of gastrointestinal inflammation.19–21 In support of this hypothesis, the gastric mucosa of patients infected with Helicobacter pylori shows increased expression of both BMP-2 and BMP-4,22 indicating that these peptides might be involved in Helicobacter-induced gastric inflammation. Similarly, BMP-7 has been shown to ameliorate the severity of colonic inflammation and to accelerate the healing of colitis in rats exposed to trinitrobenzene sulfonic acid, a well-established inducer of experimental colitis in rodents.19,20 Finally, transgenic expression of BMP-4 in the skin of mice treated with both the carcinogen N-methyl-N’-nitrosoguanidine and the tumor promoter 12-O-teradecanoylphorbol-13-acetate leads to a marked decrease in the degree of cellular hyperproliferation and inflammation induced by these agents.24
Exposure of the gastric epithelium to inflammatory stimuli leads to the release of cytokines and chemokines, peptides known to play a prominent role in the induction and maintenance of gastric mucosal damage.23–27 The chemokine Interleukin (IL)8, in particular, is one of the best-characterized mediators of Helicobacter-induced gastric inflammation.23–26 IL8 is released by cells of the gastric epithelium in response to both infection with Helicobacter and to stimulation with IL1β, tumor necrosis factor-α (TNF-α), and interferon-γ (IFN-γ),23–27 cytokines released by activated macrophages and T helper 1 (Th1) lymphocytes, respectively.
Although the significance of gastric inflammation in the pathogenesis of peptic ulcer and gastric cancer has been appreciated, the factors and the signaling pathways involved in the development of these diseases only partially have been characterized. In particular, the function and localization of BMP-4 and the cellular targets of the BMP signal transduction pathway in the inflamed stomach currently are unknown.
Accordingly, we took advantage of several lines of genetically engineered mice and of well-established primary cultures of gastric epithelial cells to test the hypothesis that BMP-4 expression and signaling are modulated by inflammation and that the BMP signal transduction pathway negatively regulates the response of the gastric mucosa to inflammatory stimuli.
Material and Methods
Mice
H pylori and Helicobacter felis Culture and Infection
H pylori Lipopolysaccharide Isolation
Primary Cell Culture
Generation of Bone Marrow–Derived Dendritic Cells
Quantitative Reverse-Transcription Polymerase Chain Reaction Analysis
Enzyme-Linked Immunosorbent Assay
Histochemical Analysis and Image Acquisition
Northern Blots
Western Blots
Data Analysis
Data are expressed as means ± standard error. Statistical analysis was performed using the Student t test. P values less than .05 were considered significant.
Results
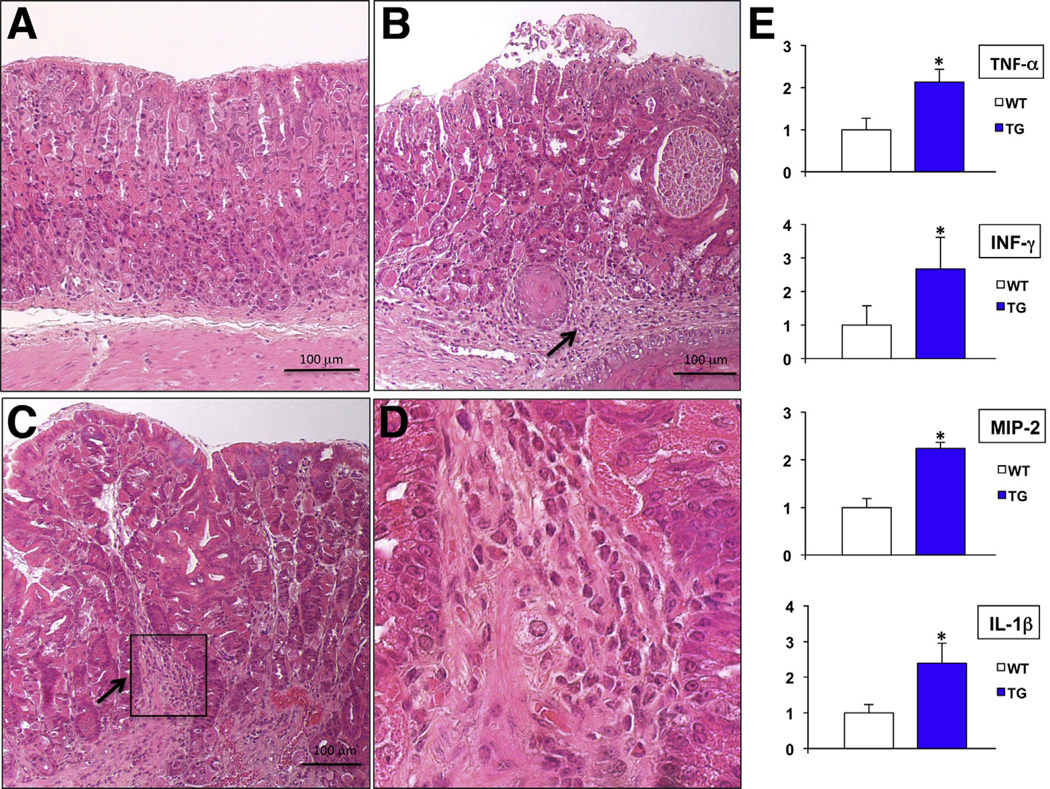
In order to test the hypothesis that the BMPs inhibit gastric inflammation, we took advantage of the promoter of the mouse H+/K+-ATPase β-subunit gene to express the secreted BMP inhibitor noggin in the gastric epithelium of mice.17 Microscopic analysis of H&E-stained sections of the fundic mucosa of the transgenic, but not of wild-type, control mice (Figure 1A) revealed the presence of foci of mild to moderate inflammatory infiltrates (Figure 1B–D). Measurement by QRT-PCR of TNF-α, IFN-γ, macrophage inflammatory protein-2 (MIP-2), and IL1β messenger RNAs (mRNAs) demonstrated that inhibition of BMP signaling causes a significant increase in the expression of these inflammatory molecules (Figure 1E). In contrast to these findings, a previously published study indicated that transgenic expression of noggin in the gastric epithelium by means of the Keratin 19 promoter (K19-Nog mice) does not lead to the expression of a significant gastric phenotype.35 As previously reported,17 it is possible that this discrepant phenotypic outcome might have been due to differences between our transgenic vector and that used in the K19-Nog mice.
Figure 1.
Inflammation in noggin TG mice. Representative H&E-stained paraffin sections of the corpus of (A) 12-week old WT and (B and C) TG mice. Arrows point to inflammatory cells. (D) Magnified window depicting inflammatory cells. (E) TNF-α, IFN-γ, MIP-2, and IL-1β mRNA abundance in WT mice was compared with that detected in TG mice using QRT-PCR and displayed as a fold-increase over the non-TG (WT) negative controls. Values are shown as means ± standard error (n = 4). *P < .05.
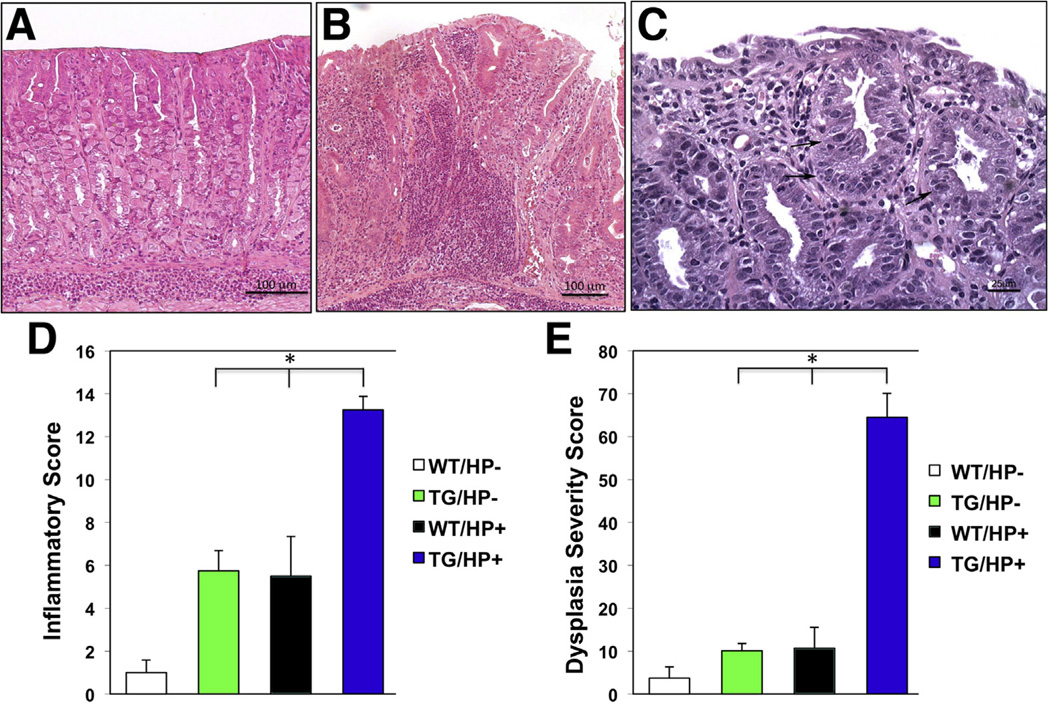
To better characterize the significance of these observations we challenged 3-month-old noggin transgenic mice with the Sydney strain 1 (SS1) strain of H pylori, which is known to induce an inflammatory response in the gastric mucosa of mice.30–34 Microscopic and morphometric analysis of H&E-stained sections of the fundic mucosa of the transgenic mice 3 months after H pylori infection showed a significant increase in the severity of the inflammatory infiltrates and the presence of areas of dysplastic mucosa when compared with nontransgenic/noninfected, nontransgenic/H pylori–infected, and transgenic/noninfected, age-matched littermates (Figure 2A–E).
Figure 2.
Enhanced inflammation and accelerated dysplasia in the gastric epithelium of Helicobacfer-infected noggin TG mice. Representative H&E-stained gastric paraffin sections of the corpus of (A) H pylori (HP)-infected WT and (B) TG mice. (C) Dysplastic changes in Helicobacter-infected TG mice. Arrows point to areas of dysplastic epithelium. Bars represent the (D) inflammatory and (E) dysplasia severity scores calculated in both WT and TG mice in the (D) presence and absence of H pylori (HP). Values are shown as means ± standard error (n = 4). *P < .05.
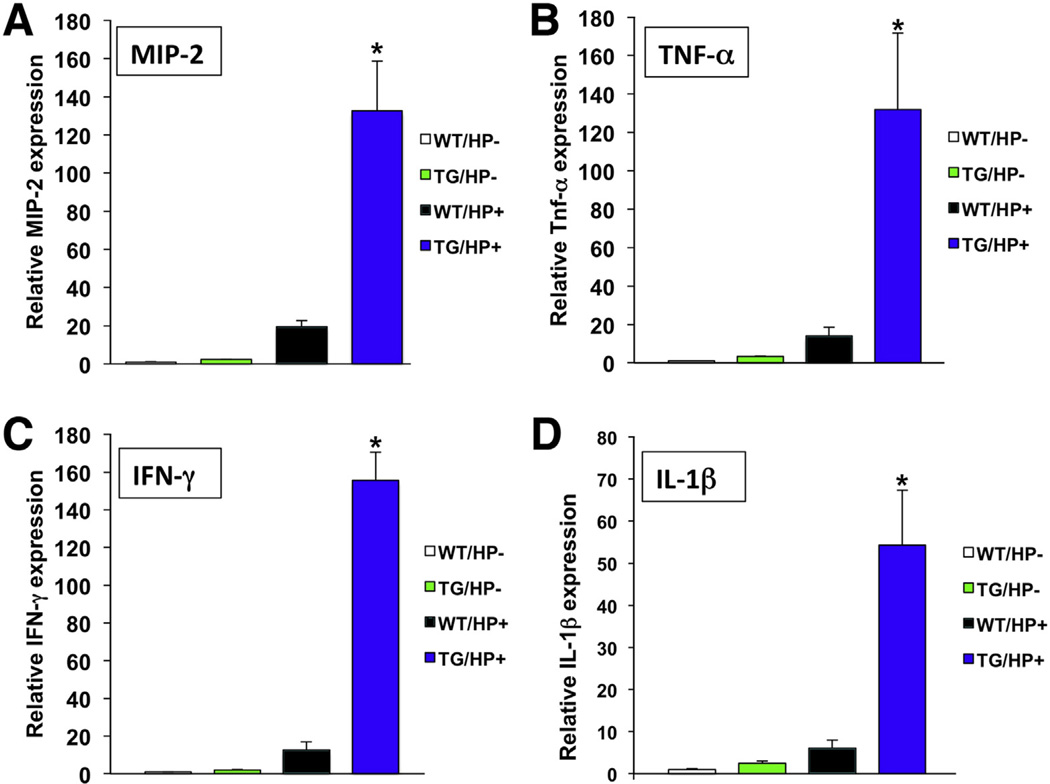
In agreement with these observations, exposure of the gastric mucosa of the transgenic mice to H pylori led to enhanced expression of MIP-2, TNF-α, IFN-γ, and IL1β mRNAs (Figure 3A–D). Thus, inhibition of BMP signaling in the gastric epithelium leads to a proinflammatory state, resulting in extreme responses and in accelerated development of dysplasia with H pylori infection.
Figure 3.
Helicobacter infection increases the expression of proinflammatory cytokines in noggin TG mice. (A) MIP-2, (B) TNF-α, (C) IFN-γ, and (D) IL-1β mRNA signals in WT mice were compared with those detected in TG mice in the presence and absence of H pylori (HP) using QRT-PCR and displayed as a fold-increase over the WT-negative controls. Values are shown as means ± standard error (n = 4). *P < .05.
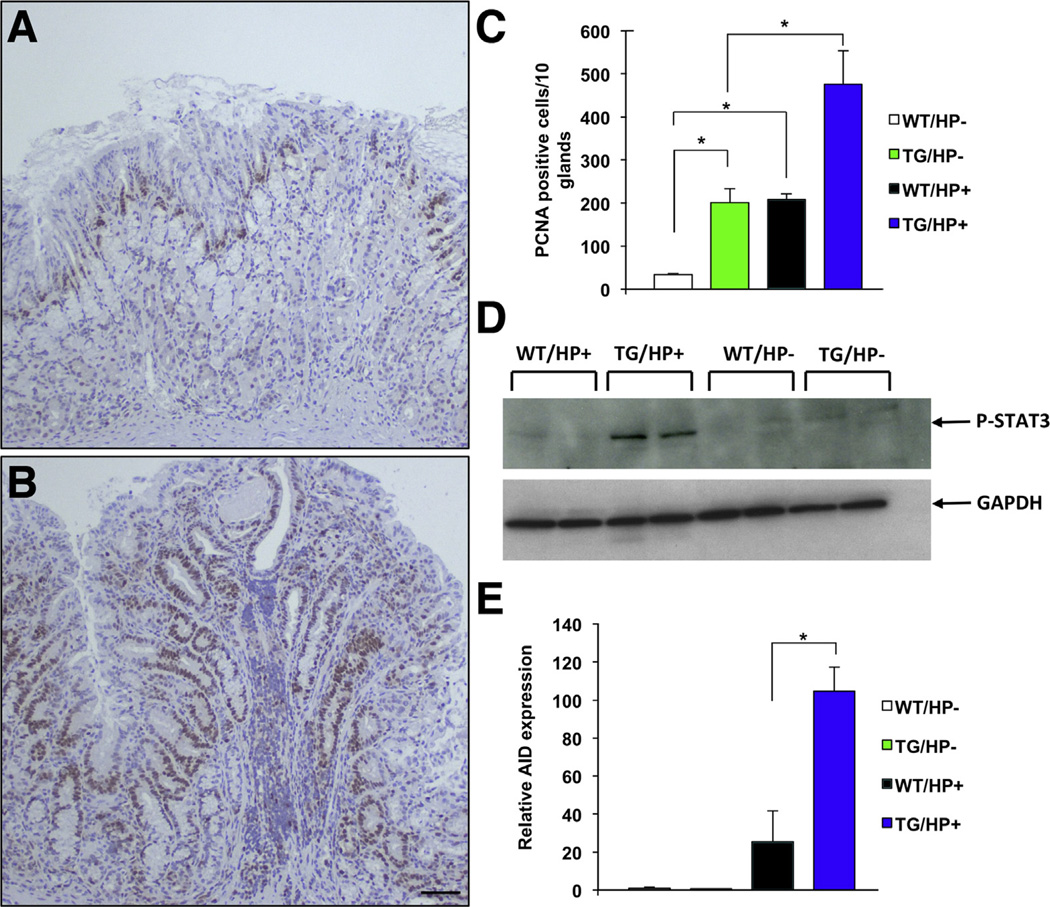
In order to investigate if enhanced inflammation leads to increased cell proliferation, we stained sections of the fundic mucosa with antibodies directed against both proliferating cell nuclear antigen and Ki 6717 (and data not shown), well-established markers of cell proliferation. As shown in Figure 4A and data not shown, in agreement with previously published reports, both infection with H pylori33,36 and inhibition of BMP signaling17 led to a significant increase in the number of proliferating cell nuclear antigen–positive nuclei, an effect that was enhanced markedly by infection of the transgenic mice with H pylori (Figure 4A–C). We then examined the role of BMP signaling on the expression of molecules, such as STAT3, which are known to mediate inflammatory and proliferative signals in the gastric mucosa.37 Accordingly, using Western blots with anti–phospho-STAT3 antibodies, we measured the activation of STAT3 in the gastric mucosa of both transgenic and nontransgenic mice in the presence and absence of H pylori. As depicted in Figure 4D, infection of the transgenic mice with H pylori led to a dramatic increase in the level of phosphorylation of STAT3. In agreement with these observations, immuno-histochemical analysis with anti-P-STAT3 antibodies, confirmed the presence of positively stained nuclei in clusters of inflammatory and epithelial cells in the H pylori-infected transgenic-mice but not in the other groups of animals (Supplementary Figure 1A and B and data not shown). Similar results were observed when we measured by QRT-PCR the expression of AID, a molecule that has been shown to mediate some of the pro-oncogenic actions of H pylori in the stomach38 (Figure 4E). Thus, inhibition of BMP signaling and heightened gastric inflammation induce the development of a pro-oncogenic environment characterized by increased cell proliferation and by enhanced expression of STAT3 and AID.
Figure 4.
Increased cell proliferation and expression of pro-oncogenic molecules in Helicobacfer-infected noggin TG mice. Gastric paraffin sections from (A) noninfected and (B) H pylori (HP)-infected TG mice were stained with anti–proliferating cell nuclear antigen (PCNA) antibodies. Scale bar: 50 µm. (C) Graph bars represent the number of PCNA-positive nuclei detected in WT and TG mice in the presence and absence of HP. Values are shown as means ± standard error (n = 4). *P < .05. (D) Phosphorylation and activation of STAT3 in WT and TG mice in the presence and absence of H pylori was studied by Western blots using an anti-phospho-STAT3 antibody. (E) AID mRNA signals in WT mice were compared with those detected in TG mice in the presence and absence of HP using QRT-PCR and displayed as a fold-increase over the WT negative controls. Values are shown as means ± standard error (n = 4). *P < .05. GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Trefoil factor 2 (TFF2) is a peptide growth factor that, in the normal stomach, is expressed in deep gland cells of the antrum and in the mucus neck cells of the fundic glands.17,39 Moreover, aberrant expression of TFF2 at the base of the fundic glands has been associated with the occurrence of spasmolytic polypeptide-expressing metaplasia, an event linked to the development of both dysplasia and neoplasia in Helicobacter-infected mice.39,40 In previously published studies we reported that inhibition of BMP signaling in the stomach leads to increased TTF2 mRNA expression and spasmolytic polypeptide-expressing metaplasia.17 In this report we observed that H pylori infection significantly enhanced the expression of TFF2 mRNA in transgenic mice when compared with both infected nontransgenic, and noninfected transgenic controls (Supplementary Figure 2A). In contrast, Helicobacter infection did not alter the level of MUC6 mRNA, another mucus neck cell marker whose expression is increased significantly in transgenic mice (Supplementary Figure 2B). Thus, inhibition of BMP signaling and H pylori–induced inflammation appear to exert a synergistic and specific effect on TFF2 mRNA expression.
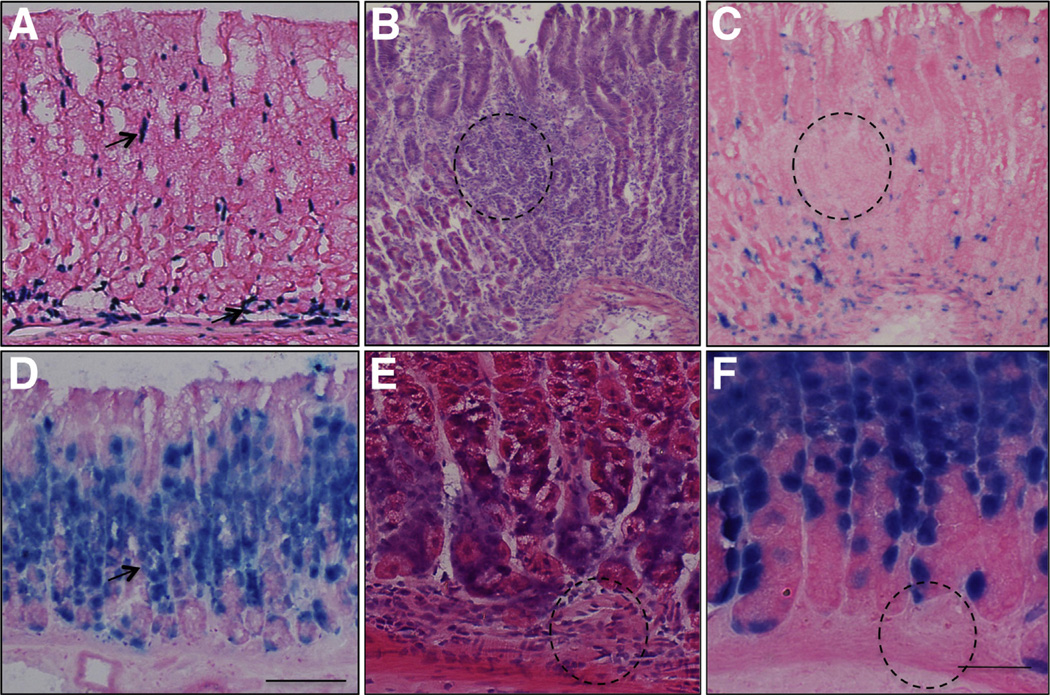
We examined the localization of BMP-4–expressing cells in the gastric mucosa both in the presence and absence of inflammation. As shown in Figure 5A, X-gal staining of the gastric mucosa of BMP-4βgal/+ mice indicated that BMP-4 is expressed in mesenchymal cells located both under and between the glands. To analyze the pattern of expression of BMP-4 during inflammation, we infected the BMP-4β-gal/+ mice with H felis for 2 months. For these studies we elected to use H felis because it induces more rapid and robust inflammation than H pylori in mice.36 H felis induced the expression of TNF-α, MIP-2, and IFN-γ mRNAs in these mice (data not shown), and, as shown on the representative H&E-stained frozen sections depicted in Figure 5B, it led to the development of significant foci of inflammatory infiltrates in the mucosa of the corpus. Immunohistochemical analysis of these sections using anti-F4/80 antibodies confirmed the presence of inflammatory cells (Supplementary Figure 3). Moreover, staining of corresponding sections with X-gal showed, as depicted in Figure 5C, that BMP-4 is expressed in clusters of cells that appear to be localized in the mesenchymal layers of the mucosa, adjacent to, but not in the inflammatory infiltrates. To define the localization of cells receiving BMP-generated signals, we used BRE–β-galactosidase mice. As shown in Figure 5D, X-gal positively stained cells could be detected, mostly at the level of the isthmus and neck of the glands, but not in the mesenchyme, both in the absence (Figure 5D) and presence of inflammation, as shown in the H&E- and matching X-gal–stained sections shown in Figure 5E and F. Staining with anti-H+,K+-ATPase a-subunit antibodies confirmed that these sections were obtained from the oxyntic mucosa (Supplementary Figure 4). Similar results were observed when we stained paraffin sections of the gastric mucosa of H pylori–infected mice with antibodies recognizing phosphorylated and active forms of the BMP-4 signal transducing proteins, Smad1, 5, and 81,2 (Supplementary Figure 5A and B). Thus, studies conducted with 2 different experimental approaches, in the presence of 2 types of Helicobacter organisms, confirmed the notion that BMP-generated signals specifically target cells located in the epithelium but not in the mesenchyme or in the inflammatory infiltrates.
Figure 5.
Expression of BMP-4 and localization of BMP-activated signaling in gastric inflammation. (A) Gastric frozen sections from BMP-4β-gal/+ mice stained with X- gal. (B) H&E and (C) corresponding X-gal–stained frozen sections from H felis–infected BMP-4β-gal/+ mice. (D) Gastric frozen sections from BRE–β-gal mice stained with X-gal. (E) H&E- and (F) corresponding X-gal–stained frozen sections from H felis–infected BRE–β-gal mice. Arrows point to X-gal–stained (A) mesenchymal and (D) epithelial cells. (B, C, E, and F) Dotted circles mark matching areas of the sections with inflammatory infiltrates. Scale bars: (A–D) 100 µm, (E and F) 50 µm.
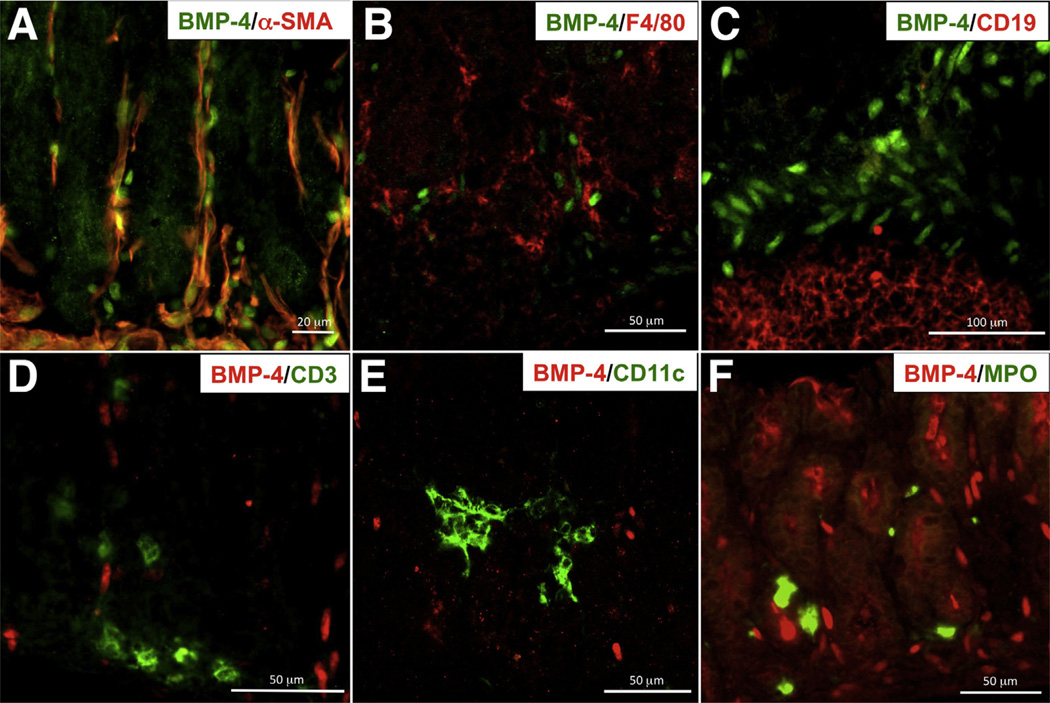
To better define the type of cells that express BMP-4 in the inflamed stomach, we performed an immunohistochemical analysis of sections of the fundic mucosa of H felis–infected BMP-4β-gal/+ mice. Although, as indicated in Figure 6A, BMP-4 expression could be detected predominantly in α-smooth muscle antibody (SMA)–positive cells, a few rare β-galactosidase–positive, α-SMA–negative cells also were noted, suggesting that BMP-4 expression is not restricted to α-SMA–expressing mesenchymal cells. Similar results were observed in the gastric mucosa of noninfected animals (data not shown). No significant BMP-4 expression could be identified in cells expressing macrophage, B, T, dendritic, and neutrophil markers (Figure 6B–F). Thus, myofibroblasts, but not immune cells, appear to represent the main source of BMP-4 expression in the gastric mucosa.
Figure 6.
Localization of BMP-4 in mesenchymal and inflammatory cells. (A–C) Gastric frozen sections from H felis-infected BMP-4β-gal/+ mice were stained with anti–β-galactosidase primary antibodies and fluorescein isothiocyanate (FITC)-conjugated secondary antibodies (green), together with Cy3-conjugated anti-actin, (A) α-smooth muscle antibodies (red), (B) anti-F4/80, and (C) anti-CD19 primary antibodies, and Alexa 594–conjugated secondary antibodies (red). (D–F) Gastric frozen sections from H felis-infected BMP-4β-gal/+ mice were stained anti–β-galactosidase primary antibodies and Alexa 555–conjugated secondary antibodies (red), together with (D) fluorescein isothiocyanate (FITC)-conjugated anti-CD3, (E) FITC-conjugated anti-CD11c, and (F) FITC-conjugated anti-myeloperoxidase (MPO) antibodies (green). Results similar to those depicted in the figure were observed in at least 3 other separate experiments.
Helicobacter-induced inflammation is a complex process that involves the activation of several types of inflammatory cells.36 Among these, dendritic cells are known to play a crucial role in the pathophysiology of the gastric inflammatory response.30–32,36 To confirm that BMP-4–generated signals do not target inflammatory cells, we treated cultures of dendritic cells with H pylori LPS, either alone or in association with BMP-4. As shown in Supplementary Figure 6A and B, BMP-4 did not have any effect on both basal and LPS-stimulated IL12 and IL23. We investigated whether BMP-4 could modulate the process of immune cell stimulation by activated dendritic cells. Accordingly, we cultured populations of freshly isolated splenocytes, which are enriched in CD4+ T lymphocytes, together with dendritic cells that were primed with H pylori LPS. As shown in Supplementary Figure 6C and D, BMP-4 had no effect on IFN-γ and IL17-α release from dendritic cell–activated splenocytes, confirming the notion that BMP-4 does not modulate the function of these immune cells. Similar results were observed in experiments performed using flow cytometry, in which BMP-4 did not have any significant effect on the percentage of CD4+ T lymphocytes that were primed to produce IFN-γ in response to LPS stimulation (data not shown).
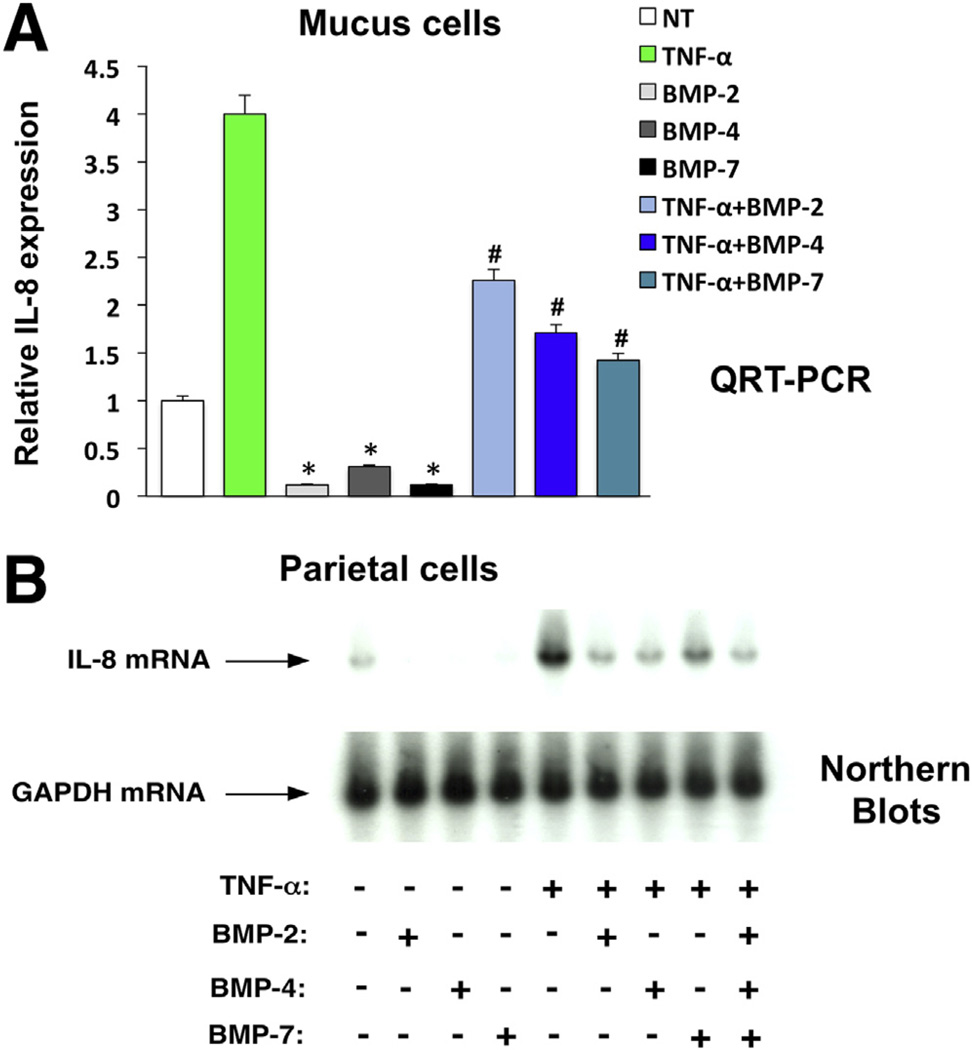
We investigated if the BMPs exert direct inhibitory effects on the expression of proinflammatory molecules in gastric epithelial cells. Accordingly, we performed experiments using primary cultures of canine parietal and mucus cells. We elected to use these cell types because we showed that these cells appear to be the major target of BMP-generated signals in the gastric mucosa of mice.17 We showed that BMP-4, BMP-2, and BMP-7 are all capable of stimulating Smad1, 5, and 8 phosphorylation after 24, 48, and 72 hours, respectively, in the parietal cells16 (and data not shown). Similar results were observed in mucus cells stimulated with these BMPs for 24, 48, and 72 hours (data not shown). Moreover, we observed that the stimulatory action of BMP-4 on P-Smad1, phosphorylation was completely blocked by treatment of the cells with noggin (Supplementary Figure 7A). The specificity of this effect was underscored by the observation that noggin failed to block transforming growth factor-β–induced phosphorylation of Smad2 (Supplementary Figure 7B). We examined if the BMPs inhibit the expression of the IL8 gene, a well-established mediator of Helicobacter-induced gastric inflammation.23–27,36 As shown on Northern blots and in the QRT-PCR assays depicted in Figure 7A and B, BMP-2, BMP-4, and BMP-7 significantly inhibited both basal and TNF-α–stimulated IL8 gene expression after 72 hours of incubation in both cell types. Exposure of the cells to a combination of all 3 BMPs did not induce any potentiating or additive effect, suggesting that these agents are likely to share common signaling mechanisms.
Figure 7.
Inhibition of basal and TNF-α–stimulated IL8 gene expression by bone morphogenetic proteins in isolated canine gastric epithelial cells. IL-8 mRNA abundance in (A) canine mucus and (B) parietal cells stimulated with TNF-α (10 ng/mL), in the presence and absence of BMP-2 (20 ng/mL), BMP-4 (20 ng/mL), and BMP-7 (20 ng/mL), alone or in combination, was measured by QRT-PCR and Northern blots, respectively. Values are shown as means ± standard error (n = 3). *P < .05 and #P < .05 vs basal and TNF-α-stimulated IL8 gene expression, respectively.
To confirm the significance of this effect in human pathophysiology, we performed experiments with AGS cells, a well-established in vitro system that has been used extensively to study the effects of inflammatory stimuli on the production of chemokines in human gastric epithelial cells.36 As indicated in Supplementary Figure 8, BMP-4 significantly inhibited both basal and TNF-α–stimulated IL8 gene expression in AGS cells. In addition, LDN-193189, a specific BMP-receptor type 1 inhibitor, blocked BMP-4 inhibition of IL8 gene expression, confirming the specificity of this effect. BMP-4 also interfered with the release of the IL8 protein from the AGS cells (Supplementary Figure 9), underscoring the notion that activation of BMP signaling restrains both the expression and the release of inflammatory chemokines in human epithelial cells.
Discussion
In this article we report a series of novel observations that underscore the importance of BMP signaling in the regulation of gastric inflammation and epithelial homeostasis. In particular, we present evidence, using both in vivo and in vitro approaches, that inhibition of BMP signaling leads to an enhanced response to inflammatory stimuli and to the accelerated development of dysplastic changes of the gastric mucosa.
Several investigations have underscored the importance of inflammation in the pathophysiology of diseases of the stomach such as gastritis, peptic ulcer, and gastric cancer.41 One of the best-characterized models of gastric inflammation is that caused by infection of the gastric mucosa with Helicobacter organisms such as H pylori and H felis. Inflammation induced by these organisms can induce profound alterations in the normal homeostatic mechanisms of the gastric mucosa, leading to the development of dysplasia and neoplasia.40,41 In our study we reported that transgenic expression of the BMP-inhibitor noggin during H pylori infection leads to a dramatic increase in the expression of the cytokines IL1β, TNF-α, and IFN-γ, and of the chemokine MIP-2, molecules known to cause significant alterations in epithelial cell differentiation, maturation, proliferation, and apoptosis.23–27,36 We also showed that inhibition of BMP signaling in the presence of Helicobacter organisms leads to the accelerated development of dysplasia, to enhancement of gastric epithelial cell proliferation, and to the expression STAT3 and AID, molecules that are activated during inflammation and that contribute to the development of dysplastic and neoplastic changes.37,38 STAT3, in particular, is a transcription factor that regulates several growth-regulatory and anti-apoptotic genes such as cyclin D1, c-Myc, survivin, and Bcl-XL. Activation of STAT3 has been linked to aberrant cellular growth and proliferation and to the development of gastrointestinal neoplasias.37 Interestingly, STAT3 also has been shown to regulate cellular fate and lineage determination because, in the pancreas, it appears to promote conversion of quiescent adult pancreatic epithelial cells to metaplastic cells with a progenitor-like phenotype that is more susceptible to K-Ras–mediated transformation.37 Similarly, the enzyme AID has been shown to alter P53 function causing significant aberrations in the normal mechanisms that regulate gastric epithelial cell apoptosis during Helicobacter-mediated inflammation.38 Taken together, these observations suggest that BMP signaling inhibits the expression of proinflammatory and pro-oncogenic molecules in the gastric mucosa and that loss of this important homeostatic mechanism might be responsible for the development of metaplastic and dysplastic changes. Another interesting finding of our study was the observation that infection with Helicobacter specifically and potently enhances noggin-induced stimulation of TFF2 gene expression. A recent study has suggested that expression of TFF2 mRNA might mark a novel population of gastric progenitor cells that can give rise to several lineages of specialized epithelial cells.42 An intriguing possibility is that inflammation and lack of BMP signals might be responsible for the expansion of TFF2-labeled progenitors that could, over time, contribute to the development of metaplasia and dysplasia. It is clear that additional experiments with lineage tracing studies will be necessary to test this possibility.
In our article we showed that BMP-4 is a mesenchymal peptide expressed in α-SMA–positive cells and that during inflammation, BMP-4–positive myofibroblasts can be seen in clusters located adjacent to, but not in, the inflammatory infiltrates. Interestingly, we also noted that BMP-4–positive staining could be detected in a few α-SMA–negative cells. Although the meaning of this observation is currently unclear, it is conceivable that BMP-4 could be expressed in different types of mesenchymal cells. Because fibroblasts appear to play an important role in the generation of cellular niches that can lead to the development of neoplastic transformation,43 future studies will be directed toward a more in-depth elucidation of the phenotypic and functional characteristics of these cells.
A novel finding of our study was that BMP-4 cannot be detected in cells labeled with macrophage, neutrophil, or T- and B-cell–specific markers, suggesting that BMP-4 is not expressed in these types of inflammatory cells. Interestingly, a previous immunohistochemical study performed in biopsy specimens from patients with H pylori gastritis indicated that BMP-2– and, to a lesser degree, BMP-4–positive staining could be seen in cells of the inflammatory infiltrates.22 Several interpretations could be considered to explain these observations. One possibility is that there could be species-specific differences regarding the expression of BMPs in inflammatory cells. It also is conceivable that BMP-2 and BMP-4 might be expressed in inflammatory cells that do not express F4/80, CD11c, myeloperoxidase, CD19, and CD3. Finally, because the biopsy specimens were not stained with specific immune cell markers, the BMP-positive cells could have represented fibroblasts located in the proximity of the inflammatory infiltrates.
One important finding of our study was that BMP signaling specifically targets epithelial but not mesenchymal and immune cells and that induction of BMP signaling in the epithelium leads to inhibition of IL8 production, a chemokine that plays a crucial role in the response of the epithelium to inflammatory stimuli.23–27,36 The observation that this effect could be seen in both canine primary epithelial cells and in human AGS cells underscores the relevance of these results in gastric pathophysiology. Accordingly, BMP-4 is likely to represent an anti-inflammatory peptide released by the mesenchyme to modulate the inflammatory response of the gastric epithelium.
The NF-κB signal transduction pathway plays a crucial role in the mediation of TNF-α stimulation of IL-8 gene expression.26,36 Interestingly, in our study, we observed that BMP-4 did not interfere with the process of IκB-regulated NF-κB activation (see supplemental material). Since induction NF-κB is a complex biological process, 26,36 it is possible that BMP-4 could regulate other mechanisms that lead to the activation of NF-κB. It also conceivable that BMP-4 might cause complex epigenetic modifications of the IL-8 gene that could lead to its inhibition through NF-κB-independent pathways. Elucidation of the mechanisms responsible for BMP-mediated inhibition of IL-8 gene expression will be the focus of future investigations.
An attractive hypothesis is that inflammatory stimuli could induce the expression of BMP-4 and, possibly, of other BMPs, to activate self-safe mechanisms aimed at tampering and limiting the severity of the inflammatory response. The finding that clusters of BMP-4–positive cells can be detected adjacent to the inflammatory infiltrates and the recently published observation that mice infected with Helicobacter show increased expression of BMP-4 in the inflamed gastric mucosa, would support this possibility.44 Another consideration that stems from the finding that there is no BMP-4 expression and signaling in the inflammatory infiltrates, is that, over time, there could be a progressive loss of BMP signaling in the inflamed stomach, leading to the development of a pro-oncogenic environment, similar to that seen in the H pylori–infected noggin transgenic mice, that ultimately could be responsible for the development of dysplastic and neoplastic changes.
In conclusion, our study provides evidence for the existence of a mechanism based on the release of a mesenchymal peptide, BMP-4, that specifically activates a signaling pathway in the gastric epithelium that exerts inhibitory effects on the expression of proinflammatory mediators. These findings underscore the importance of BMP signaling in the regulation of gastric inflammation and epithelial homeostasis, providing new clues for a better understanding of the pathophysiological mechanisms that lead to the development of both dysplastic and neoplastic lesions in the stomach. Additional investigations and possible manipulations of the BMP signal transduction pathway therefore might offer novel opportunities for the treatment of gastric inflammation and carcinogenesis.
Supplementary Material
Acknowledgments
The authors thank Jung Park, Alexander Basing, Saravanan Ramamoorthy, and Kathy McClinchey for technical assistance.
Hidehiko Takabayashi was responsible for the study concept and design, acquisition of data, analysis and interpretation of data, critical revision of the manuscript for important intellectual content, and statistical analysis; Masahiko Shinohara was responsible for the study concept and design, acquisition of data, analysis and interpretation of data, critical revision of the manuscript for important intellectual content, and statistical analysis; Maria Mao was responsible for the acquisition of data, analysis and interpretation of data, technical support, and statistical analysis; Piangwarin Phaosawasdi was responsible for the acquisition of data, analysis and interpretation of data, and technical support; Mohamad El-Zaatari was responsible for the acquisition of data, analysis and interpretation of data, and technical support; Min Zhang was responsible for the acquisition of data, analysis and interpretation of data, technical support, and statistical analysis; Tuo Ji was responsible for the acquisition of data, analysis and interpretation of data, technical support, and statistical analysis; Kathryn Eaton was responsible for the acquisition, analysis and interpretation of data, and critical revision of the manuscript for important intellectual content; Duyen Dang was responsible for the analysis and interpretation of data, critical revision of the manuscript for important intellectual content, and obtained funding; John Kao was responsible for the acquisition, analysis and interpretation of data, critical revision of the manuscript for important intellectual content, and obtained funding; and Andrea Todisco was responsible for the study concept and design, acquisition of data, analysis and interpretation of data, critical revision of the manuscript for important intellectual content; statistical analysis, obtained funding, study supervision, and writing of the manuscript.
Funding
Supported by the National Institute for Diabetes and Digestive and Kidney Diseases (R56058312-06A2 and RO1DK083373 to AT., and RO1DK087708 to J.K.), by the University of Michigan Gastrointestinal Peptide Research Center (P30-DK-34933), by the Funderburg Award in Gastric Biology Related to Cancer (AT.), from the Foundation for Digestive Health and Nutrition, and by funds from the US Department of Veterans Affairs, Australian Government (D.D.).
Abbreviations used in this paper
- AID
activation-induced cytidine deaminase
- BMP
bone morphogenetic protein
- IL
interleukin
- LPS
lipopolysaccharide
- MIP-2
macrophage inflammatory protein-2
- mRNA
messenger RNA
- QRT-PCR
quantitative reverse-transcription polymerase chain reaction
- SMA
smooth muscle antibody
- STAT
signal transducer and activator of transcription
- TFF2
trefoil factor 2
- TG
transgenic
- TNF
tumor necrosis factor
- WT
wild-type
Footnotes
Supplementary Material
Note: To access the supplementary material accompanying this article, visit the online version of Gastroenterology at www.gastrojournal.org, and at http://dx.doi.Org/10.1053/j.gastro.2014.04.015.
Conflicts of interest
The authors disclose no conflicts.
References
- 1.Bragdon B, Moseychuk O, Saldanha S, et al. Bone morphogenetic proteins: a critical review. Cell Signal. 2011;23:609–620. doi: 10.1016/j.cellsig.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 2.Kawabata M, Imamura T, Miyazono K. Signal transduction by bone morphogenetic proteins. Cytokine Growth Factor Rev. 1998;9:49–61. doi: 10.1016/s1359-6101(97)00036-1. [DOI] [PubMed] [Google Scholar]
- 3.van den Brink GR, Hardwick JC, Tytgat GN, et al. Sonic hedgehog regulates gastric gland morphogenesis in man and mouse. Gastroenterology. 2001;121:317–328. doi: 10.1053/gast.2001.26261. [DOI] [PubMed] [Google Scholar]
- 4.Zhou XP, Woodford -Richens K, Lehtonen R, et al. Germline mutations in BMPR1A/ALK3 cause a subset of cases of juvenile polyposis syndrome and of Cowden and Bannayan-Riley-Ruvalcaba syndromes. Am J Hum Genet. 2001;69:704–711. doi: 10.1086/323703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haramis AP, Begthel H, van den Born M, et al. De novo crypt formation and juvenile polyposis on BMP inhibition in mouse intestine. Science. 2004;303:1684–1686. doi: 10.1126/science.1093587. [DOI] [PubMed] [Google Scholar]
- 6.Auclair BA, Benoit YD, Rivard N, et al. Bone morphogenetic protein signaling is essential for terminal differentiation of the intestinal secretory cell lineage. Gastroenterology. 2007;133:887–896. doi: 10.1053/j.gastro.2007.06.066. [DOI] [PubMed] [Google Scholar]
- 7.Itoh K, Kataoka H, Sasaki M, et al. Bone morphogenetic protein 2 induced differentiation toward superficial epithelial cells in the gastric mucosa. J Gastroenterol. 2006;41:1064–1075. doi: 10.1007/s00535-006-1899-6. [DOI] [PubMed] [Google Scholar]
- 8.Takaku K, Miyoshi H, Matsunaga A, et al. Gastric and duodenal polyps in Smad4 (Dpc4) knockout mice. Cancer Res. 1999;59:6113–6117. [PubMed] [Google Scholar]
- 9.Wen XZ, Miyake S, Akiyama Y, et al. BMP-2 modulates the proliferation and differentiation of normal and cancerous gastric cells. Biochem Biophys Res Commun. 2004;316:100–106. doi: 10.1016/j.bbrc.2004.02.016. [DOI] [PubMed] [Google Scholar]
- 10.Wen XZ, Akiyama Y, Baylin SB, et al. Frequent epigenetic silencing of the bone morphogenetic protein 2 gene through methylation in gastric carcinomas. Oncogene. 2006;25:2666–2673. doi: 10.1038/sj.onc.1209297. [DOI] [PubMed] [Google Scholar]
- 11.Shirai Y, Ehata S, Yashiro M, et al. Bone morphogenetic protein-2 and −4 play tumor suppressive roles in human diffuse-type gastric carcinoma. Am J Pathol. 2011;179:2920–2930. doi: 10.1016/j.ajpath.2011.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beck SE, Jung BH, Fiorino A, et al. Bone morphogenetic protein signaling and growth suppression in colon cancer. Am J Physiol Gastrointest Liver Physiol. 2006;291:G135–G145. doi: 10.1152/ajpgi.00482.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bleuming SA, He XC, Kodach LL, et al. Bone morphogenetic protein signaling suppresses tumorigenesis at gastric epithelial transition zones in mice. Cancer Res. 2007;67:8149–8155. doi: 10.1158/0008-5472.CAN-06-4659. [DOI] [PubMed] [Google Scholar]
- 14.Zimmerman LB, De Jesus-Escobar JM, Harland RM. The Spemann organizer signal noggin binds and inactivates bone morphogenetic protein 4. Cell. 1996;86:599–606. doi: 10.1016/s0092-8674(00)80133-6. [DOI] [PubMed] [Google Scholar]
- 15.Hong CC, Yu PB. Applications of small molecule BMP inhibitors in physiology and disease. Cytokine Growth Factor Rev. 2009;20:409–418. doi: 10.1016/j.cytogfr.2009.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nitsche H, Ramamoorthy S, Sareban M, et al. Functional role of bone morphogenetic protein-4 in isolated canine parietal cells. Am J Physiol Gastrointest Liver Physiol. 2007;293:G607–G614. doi: 10.1152/ajpgi.00194.2006. [DOI] [PubMed] [Google Scholar]
- 17.Shinohara M, Mao M, Keeley TM, et al. Bone morphogenetic protein signaling regulates gastric epithelial cell development and proliferation in mice. Gastroenterology. 2010;139:2050–2060. doi: 10.1053/j.gastro.2010.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maloum F, Allaire JM, Gagné-Sansfaçon J, et al. Epithelial BMP signaling is required for proper specification of epithelial cell lineages and gastric endocrine cells. Am J Physiol Gastrointest Liver Physiol. 2011;300:G1065–G1079. doi: 10.1152/ajpgi.00176.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maric I, Poljak L, Zoricic S, et al. Bone morphogenetic protein-7 reduces the severity of colon tissue damage and accelerates the healing of inflammatory bowel disease in rats. J Cell Physiol. 2003;196:258–264. doi: 10.1002/jcp.10275. [DOI] [PubMed] [Google Scholar]
- 20.Maric I, Kucic N, Turk Wensveen T, et al. BMP signaling in the rats with TNBS induced colitis following BMP7 therapy. Am J Physiol Gastrointest Liver Physiol. 2012;302:G1151–G1162. doi: 10.1152/ajpgi.00244.2011. [DOI] [PubMed] [Google Scholar]
- 21.Blessing M, Nanney LB, King LE, et al. Chemical skin carcinogenesis is prevented in mice by the induced expression of a TGF-beta related transgene. Teratog Carcinog Mutagen. 1995;15:11–21. doi: 10.1002/tcm.1770150103. [DOI] [PubMed] [Google Scholar]
- 22.Bleuming SA, Kodach LL, Garcia Leon MJ, et al. Altered bone morphogenetic protein signaling in the Helicobacter pylori-infected stomach. J Pathol. 2006;209:190–197. doi: 10.1002/path.1976. [DOI] [PubMed] [Google Scholar]
- 23.Ernst PB, Crowe SE, Reyes VE. How does Helicobacter pylori cause mucosal damage? The inflammatory response. Gastroenterology. 1997;113:S35–S42. doi: 10.1016/s0016-5085(97)80009-1. [DOI] [PubMed] [Google Scholar]
- 24.Crabtree JE. Role of cytokines in pathogenesis of Helicobacter pylori-induced mucosal damage. Dig Dis Sci. 1998;43:46S–55S. [PubMed] [Google Scholar]
- 25.Mukaida N, Harada A, Matsushima K. lnterleukin-8 (IL-8) and monocyte chemotactic and activating factor (MCAF/ MCP-1), chemokines essentially involved in inflammatory and immune reactions. Cytokine Growth Factor Rev. 1998;9:9–23. doi: 10.1016/s1359-6101(97)00022-1. [DOI] [PubMed] [Google Scholar]
- 26.Peek RM., Jr Helicobacter pylori strain-specific activation of signal transduction cascades related to gastric inflammation. Am J Physiol Gastrointest Liver Physiol. 2001;280:G525–G530. doi: 10.1152/ajpgi.2001.280.4.G525. [DOI] [PubMed] [Google Scholar]
- 27.Obonyo M, Guiney DG, Harwood J, et al. Role of gamma interferon in Helicobacter pylori induction of inflammatory mediators during murine infection. Infect Immun. 2002;70:3295–3299. doi: 10.1128/IAI.70.6.3295-3299.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blank U, Seto ML, Adams DC, et al. An in vivo reporter of BMP signaling in organogenesis reveals targets in the developing kidney. BMC Dev Biol. 2008;8:86. doi: 10.1186/1471-213X-8-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lawson KA, Dunn NR, Roelen BA, et al. Bmp4 is required for the generation of primordial germ cells in the mouse embryo. Genes Dev. 1999;13:424–436. doi: 10.1101/gad.13.4.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kao JY, Rathinavelu S, Eaton KA, et al. Helicobacter pylori-secreted factors inhibit dendritic cell IL-12 secretion: a mechanism of ineffective host defense. Am J Physiol Gastrointest Liver Physiol. 2006;291:G73–G81. doi: 10.1152/ajpgi.00139.2005. [DOI] [PubMed] [Google Scholar]
- 31.Eaton KA, Benson LH, Haeger J, et al. Role of transcription factor T-bet expression by CD4+ cells in gastritis due to Helicobacter pylori in mice. Infect Immun. 2006;74:4673–4684. doi: 10.1128/IAI.01887-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kao JY, Zhang M, Miller MJ, et al. Helicobacter pylori immune escape is mediated by dendritic cell-induced Treg skewing and Th17 suppression in mice. Gastroenterology. 2010;138:1046–1054. doi: 10.1053/j.gastro.2009.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eaton KA, Ringler SR, Danon SJ. Murine splenocytes induce severe gastritis and delayed-type hypersensitivity and suppress bacterial colonization in Helicobacter pylori-infected SCID mice. Infect Immun. 1999;67:4594–4602. doi: 10.1128/iai.67.9.4594-4602.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eaton KA, Danon SJ, Krakowka S, et al. A reproducible scoring system for quantification of histologic lesions of inflammatory disease in mouse gastric epithelium. Comp Med. 2007;57:57–65. [PubMed] [Google Scholar]
- 35.Oshima H, Itadani H, Kotani H, et al. Induction of prostaglandin E2 pathway promotes gastric hamartoma development with suppression of bone morphogenetic protein signaling. Cancer Res. 2009;69:2729–2733. doi: 10.1158/0008-5472.CAN-08-4394. [DOI] [PubMed] [Google Scholar]
- 36.Peek RM, Fiske C, Wilson KT. Role of innate immunity in Helicobacter pylori- induced gastric malignancy. Physiol Rev. 2010;90:831–858. doi: 10.1152/physrev.00039.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li N, Grivennikov SI, Karin M. The unholy trinity: inflammation, cytokines, and STAT3 shape the cancer micro-environment. Cancer Cell. 2011;19:429–431. doi: 10.1016/j.ccr.2011.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Matsumoto Y, Marusawa H, Kinoshita K, et al. Helicobacter pylori infection triggers aberrant expression of activation-induced cytidine deaminase in gastric epithelium. Nat Med. 2007;13:470–476. doi: 10.1038/nm1566. [DOI] [PubMed] [Google Scholar]
- 39.Nomura S, Baxter T, Yamaguchi H, et al. Spasmolytic polypeptide expressing metaplasia to preneoplasia in H. felis-infected mice. Gastroenterology. 2004;127:582–594. doi: 10.1053/j.gastro.2004.05.029. [DOI] [PubMed] [Google Scholar]
- 40.Goldenring JR, Nomura S. Differentiation of the gastric mucosa III. Animal models of oxyntic atrophy and metaplasia. Am J Physiol Gastrointest Liver Physiol. 2006;291:G999–G1004. doi: 10.1152/ajpgi.00187.2006. [DOI] [PubMed] [Google Scholar]
- 41.Fox JG, Wang TC. Inflammation, atrophy, and gastric cancer. J Clin Invest. 2007;117:60–69. doi: 10.1172/JCI30111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Quante M, Marrache F, Goldenring JR, et al. TFF2 mRNA transcript expression marks a gland progenitor cell of the gastric oxyntic mucosa Gastroenterology. 2010;139:2018–2027. doi: 10.1053/j.gastro.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Quante M, Tu SP, Tomita H, et al. Bone marrow-derived myofibroblasts contribute to the mesenchymal stem cell niche and promote tumor growth. Cancer Cell. 2011;19:257–272. doi: 10.1016/j.ccr.2011.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Camilo V, Barros R, Sousa S, et al. Helicobacter pylori and the BMP pathway regulate CDX2 and SOX2 expression in gastric cells. Carcinogenesis. 2012;33:1985–1992. doi: 10.1093/carcin/bgs233. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.