Abstract
Background
Transcatheter Aortic Valve Replacement (TAVR) has emerged as a less invasive option for valve replacement for high-risk patients with severe aortic stenosis. However, not all patients derive a mortality or quality of life (QoL) benefit from TAVR. As such, we sought to build and validate a prediction model to identify patients at high risk for a poor outcome after TAVR, using a novel definition of outcome that integrates QoL with mortality.
Methods and Results
We examined QoL and mortality outcomes among 2137 patients who underwent TAVR in the PARTNER randomized trial or the associated continued access registry. QoL was assessed using the Overall Summary Scale of the Kansas City Cardiomyopathy Questionnaire (KCCQ-OS, range 0-100; higher=better) at baseline and at 1, 6, and 12 months. A poor 6-month outcome—defined as death, KCCQ-OS <45, or a decrease in KCCQ-OS by ≥10 points compared with baseline—occurred in 704 patients (33%). A multivariable model was constructed using a split-sample design to identify a parsimonious set of covariates to identify patients at high-risk for poor outcome. The resulting model demonstrated adequate discrimination (c-index=0.66) and good calibration with the observed data and performed similarly in the separate validation cohort. Based on pre-procedure characteristics alone, the model identified 211 patients (10% of the population) with a ≥50% likelihood of a poor outcome after TAVR. These individuals were more likely to have low body weights, low mean aortic valve gradients, oxygen-dependent lung disease, and poor baseline functional and cognitive status. A second model that explored predictors of poor outcome at 1 year identified similar predictors as the original model and was able to identify 1102 patients (52%) with ≥50% likelihood and 178 (8%) with ≥70% likelihood of a poor 1-year outcome after TAVR
Conclusions
Using a large, multicenter cohort of patients undergoing TAVR, we have developed and validated predictive models that can identify patients at high-risk for a poor outcome after TAVR. These models may help guide treatment choices and offer patients realistic expectations of outcomes based on their presenting characteristics.
Keywords: aortic valve stenosis, quality of life, transcatheter aortic valve, valve
Among patients with severe aortic stenosis, transcatheter aortic valve replacement (TAVR) has emerged as a less invasive option for aortic valve replacement and offers substantial reductions in mortality and improvement in quality of life (QoL) compared with medical therapy1-2 and similar long-term outcomes to surgical valve replacement.3-4 With increasing experience, however, it has become evident that some patients do not improve functionally or live longer following TAVR. For example, in the Placement of AoRTic TraNscathetER Valve (PARTNER) Trial, approximately 1 in 4 patients who were treated with TAVR were dead at 1 year.1, 3 Furthermore, there were a number of patients who received TAVR who, while alive at 1 year, continued to have very poor QoL after TAVR, with significant heart failure symptoms and functional limitation.2, 4
In light of these observations, there has been a recognition by practitioners,5-6 regulators,7 and third party payers8 that TAVR should not be offered to patients in whom valve replacement would not be expected to positively impact either their survival or quality of life. To date, however, there has been little guidance as to how best to identify these patients. To address this need, several efforts have focused on predicting mortality after TAVR, with the goal of trying to identify patients at high risk for poor outcomes.9-10 However, in this elderly population of patients, often with extensive comorbidity and impaired health status, it is likely that prolonged survival alone (without improved QoL) would not be viewed as a desirable outcome. Therefore, any definition of a successful outcome of TAVR (and conversely, of a poor outcome) must consider both survival and QoL. The goal of this study was to build and validate a prediction model to prospectively identify patients at high risk of a poor outcome (including death or very poor QoL) after TAVR.
METHODS
Study Population and Protocol
The study population was derived from patients with severe symptomatic AS who were enrolled in Cohort A or Cohort B of the PARTNER trial or in the associated non-randomized continued access PARTNER registry. Enrolled patients in both the trial and the registry had severe aortic stenosis (aortic valve area of <0.8 cm2 with either a mean aortic valve gradient ≥40 mmHg or a peak aortic jet velocity ≥4.0 m/s); New York Heart Association (NYHA) class II or greater heart failure symptoms; and high surgical risk based on the Society for Thoracic Surgeons (STS) mortality risk score and other factors.1, 3 Patients with severe (4+) mitral or aortic regurgitation were excluded. Eligible patients were classified into 2 cohorts: Cohort A patients were high-risk but suitable for surgical aortic valve replacement3 whereas Cohort B patients were deemed ineligible for cardiac surgery due to coexisting medical or anatomic conditions associated with a predicted probability of perioperative death or permanent disability ≥50%.1 In the randomized trial, Cohort A patients were randomized to surgical AVR or TAVR, and Cohort B patients were randomized to medical therapy or TAVR. For this study, we included only patients who received TAVR. After enrollment in the PARTNER trial was complete but before commercial approval of the Edwards-Sapien valve, a limited number of patients who would have been eligible for the PARTNER randomized trial were allowed to receive TAVR, via either the transfemoral or transapical route as clinically indicated, as part of a continued access registry. Inclusion and exclusion criteria, study protocols, and follow-up procedures were identical in the registry as in the randomized trial.
Patients were assessed for clinical factors and QoL/health status at baseline and at 1, 6, and 12 months after randomization. Baseline QoL/health status questionnaires were administered before randomization (or study registration for the continued access registries), and follow-up questionnaires were administered during in-person visits to the enrolling centers or by mail. The study was approved by the institutional review board at each participating site, and all patients provided written informed consent for baseline and follow-up assessments.
Health Status Data
Disease-specific health status (i.e., symptoms, functional status, and QoL) was assessed with the Kansas City Cardiomyopathy Questionnaire (KCCQ),11 a 23-item self-administered questionnaire that assesses specific health domains pertaining to heart failure that yields an overall summary scale, which was the primary health status outcome for our study. Values for the KCCQ overall summary score (KCCQ-OS) range from 0 to 100 with higher scores indicating less symptom burden and better QoL. Linguistically and culturally validated translations of the KCCQ were provided to non-English speakers. Previous studies have suggested that KCCQ-OS scores correlate roughly with New York Heart Association Functional Class as follows: Class I ≈ KCCQ-OS 75-100; Class II ≈ 60-74; Class III ≈ 45-59; and Class IV ≈ 0-44.12 Among outpatients with heart failure, small, moderate, and large clinical improvements/deteriorations, as rated by treating physicians, correspond to changes in the KCCQ-OS of approximately 5, 10, and 20 points, respectively.12 The KCCQ has undergone extensive reliability and validity testing in various heart failure populations11, 13-14 as well as in patients with severe aortic stenosis.15
Functional status was assessed objectively by means of a 6-minute walk test (6MWT). If a patient could not perform the test, the value for the 6MWT distance was set to zero. Generic health status was assessed with the physical and mental summary scores of the Medical Outcomes Study Short-Form 12 (SF-12) Health Survey.16 Since the SF-12 physical summary score represents functional status, which was also assessed by means of the KCCQ and the 6-minute walk test, we did not include this as a potential predictor for poor outcome.
Definition of a Poor Outcome
While any single definition of a “poor outcome” after TAVR is a somewhat arbitrary construct, we have previously examined several potential definitions that combine mortality and QoL.17 For the purposes of this study, we used outcomes at 6 months after TAVR as the basis for our primary endpoint, as we believe that survival for at least 6 months with a reasonable QoL would be the minimum acceptable outcome for the procedure. As previously described, for our primary analysis, a poor outcome was defined as any of the following at 6 months after TAVR (Definition #1): 1) death; 2) KCCQ overall summary score <45; or 3) decrease of ≥10 points in the KCCQ-OS score from baseline to 6 months.17-18
From a conceptual standpoint, this definition means that if a patient had a very poor QoL prior to TAVR, his or her QoL would have to improve to a minimum threshold (~NYHA Class III symptoms or better) in order to be considered an acceptable outcome. On the other hand, if a patient had a satisfactory QoL prior to TAVR, then survival for at least 6 months with a QoL that had not deteriorated substantially from baseline would be considered an acceptable result. This combined definition integrates the two potential benefits of TAVR, reduced mortality and improved QoL, and recognizes that patients that have good QoL at baseline may not improve symptomatically after TAVR but could still derive a mortality benefit (which would represents a clinically meaningful benefit of the procedure). In addition, we constructed an alternative, expanded definition of poor outcome (Definition #2) that included any of the following at 1 year after TAVR: 1) death; 2) KCCQ overall summary score <60; or 3) decrease of ≥10 points in the KCCQ-OS score from baseline to 1 year. This definition allows for a longer time frame of analysis and may be beneficial to patients and providers in conjunction with, or as an alternative to, Definition #1 depending on the patient’s goals of care.
Statistical Analysis
Demographics, cardiac and non-cardiac comorbidities, and echocardiographic variables were compared between patients who had a poor outcome and patients who had an acceptable outcome after TAVR using t-tests for continuous variables and chi square tests for categorical variables. We then randomly split the cohort of TAVR patients into 2 groups: 2/3 for model derivation and 1/3 for validation. In the derivation cohort, we developed a multivariable logistic regression model to predict a poor 6-month outcome (using Definition #1 as described above). Variables for the prediction model were selected from 25 candidate variables (Table 1). Baseline data had a high rate of completion, with an average of 0.55 missing data items per patient, which were imputed with a single imputation dataset using IVEware (Institute for Social Research, University of Michigan, Ann Arbor, MI).
Table 1. Baseline characteristics of patients with acceptable vs. poor outcomes according to Definition #1.
| All Patients n=2137 |
Acceptable Outcome n=1433 |
Poor Outcome n=704 |
P- Value |
|
|---|---|---|---|---|
| Age (y) | 84.4 (7.2) | 84.3 (7.2) | 84.6 (7.0) | 0.357 |
| Male (%) | 52.8 | 51.3 | 56.0 | 0.042 |
| Coronary artery disease (%) | 78.2 | 78.1 | 78.6 | 0.807 |
| Cerebrovascular disease (%) | 26.6 | 26.5 | 26.8 | 0.872 |
| Carotid disease (%) | 27.0 | 27.6 | 25.7 | 0.364 |
| Peripheral vascular disease (%) | 42.2 | 42.0 | 42.6 | 0.791 |
| Diabetes mellitus (%) | 36.6 | 38.4 | 33.1 | 0.017 |
| Major arrhythmia* (%) | 50.9 | 47.5 | 57.8 | <0.001 |
| Creatinine (mg/dL) | 1.31 (0.48) | 1.29 (0.46) | 1.36 (0.51) | 0.002 |
| Hemoglobin (g/dL) | 11.7 (1.5) | 11.8 (1.5) | 11.6 (1.5) | 0.033 |
| Mean arterial pressure (mmHg) | 87.1 (13.1) | 87.2 (12.8) | 87.0 (13.4) | 0.656 |
| Body mass index (kg/m2) | 26.9 (6.3) | 27.1 (6.1) | 26.6 (6.8) | 0.093 |
| Oxygen-dependent lung disease (%) | 10.9 | 9.3 | 14.3 | <0.001 |
| Pulmonary hypertension (%) | 40.0 | 39.2 | 41.5 | 0.316 |
| Mitral regurgitation (>1+) (%) | 25.3 | 24.8 | 26.3 | 0.473 |
| Aortic regurgitation (>1+) (%) | 10.5 | 10.8 | 9.9 | 0.536 |
| Mean aortic gradient (mmHg) | 43.8 (14.3) | 45.1 (14.3) | 41.2 (14.0) | <0.001 |
| Ejection fraction (%) | 52.0 (13.1) | 52.4 (13.0) | 51.2 (13.4) | 0.053 |
| Stroke volume (mL/beat) | 64.5 (21.1) | 65.1 (20.0) | 63.2 (23.1) | 0.053 |
| Mini-Mental Status Exam score | 27.4 (3.0) | 27.5 (2.9) | 27.1 (3.0) | 0.008 |
| 6-Min Walk Test (% able to perform) | 65.0 | 70.1 | 54.8 | <0.001 |
| 6-Min Walk Test distance (m) | 110.8 (118.1) | 123.9 (122.2) | 84.0 (104.3) | <0.001 |
| KCCQ Overall Summary score | 42.0 (21.7) | 43.8 (20.8) | 38.4 (23.0) | <0.001 |
| SF-12 Mental Summary score | 47.6 (11.2) | 48.3 (11.0) | 46.2 (11.5) | <0.001 |
| STS mortality risk score | 11.5 (4.4) | 11.4 (4.5) | 11.9(4.1) | 0.005 |
STS, Society of Thoracic Surgeon
Defined as a history of atrial fibrillation or flutter, supraventricular tachycardia, ventricular arrhythmias, or high-degree AV block Values in parentheses represent standard deviations
Harrell’s backward selection strategy was used to select a parsimonious set of variables for the final model.19 The contribution of each covariate in the multivariable model was ranked by F-value, and variables with the smallest contribution to the model were sequentially eliminated. This iterative process continued until further variable elimination led to a greater than 5% loss in model prediction, as compared with the initial model. The remaining covariates comprised the final parsimonious model and explained over 95% of the variance of the full model. This selection strategy supports inclusion of only variables that provide incremental prognostic value, avoids over-fitting, and maximizes the potential clinical usefulness of the model.19 Non-linear spline terms were considered for all continuous variables. Model discrimination was assessed with the c-index, and model calibration was assessed by plotting deciles of predicted risk against the observed event rate and comparing the regression line to theline of unity. These analyses were performed in both the derivation and validation cohorts and were repeated using Definition #2 of a poor outcome.
As sensitivity analyses, to further explore the associations of the covariates with the outcome, we applied the model to each of the component endpoints to explore its ability to predict: 1) death or 2) poor QoL or QoL decline (among survivors). Second, to ensure that our results were not heavily influenced by the consequences of early complications (which might be considered outliers), we conducted a sensitivity analysis excluding patients who had a major peri-procedural complication (i.e., major stroke, bleeding complication, vascular complication, or surgical aortic valve replacement within 7 days of the TAVR procedure). Third, we applied the model separately to patients according to access site (transfemoral or transapical) to ensure that model performance did not vary markedly by site of valve delivery. Finally, we constructed an alternative model including the KCCQ instead of the 6MWT, for ease of implementation. All statistical analyses were performed with the use of SAS software, version 9.2 (SAS Institute, Inc., Cary, NC).
RESULTS
Patient Population
Of the 1057 patients with severe aortic stenosis who were enrolled in the PARTNER randomized trial, 527 patients underwent TAVR (Figure 1; 348 in Cohort A, 179 in Cohort B), and an additional 2068 patients underwent TAVR in the non-randomized continued access registry via the transfemoral (n=1503) or transapical route (n=1092). Of these, 400 died within 6 months of TAVR. Among the 2195 who survived 6 months, KCCQ data were available for 1737 (79%). Thus, our analytic population included 2137 patients who underwent TAVR and were either dead or were assessed with the KCCQ at 6 months after their procedure. Most baseline characteristics were similar for patients with vs. without complete KCCQ data. However, patients with missing KCCQ data had lower baseline 6 minute walk distances (85 vs. 119 m, p<0.001), lower body mass indices (26.3 vs. 27.2, p=0.008), and lower hemoglobin levels (11.6 vs. 11.8, p=0.026).
Figure 1. Patient flow.
The mean age of the analytic population was 84 years, and 53% were male (Table 1). The mean aortic valve gradient was 44 mmHg, and 93% were classified as NYHA Class III-IV. At 6-month follow-up, among the 2137 study patients, 400 (19%) had died, 260 (12%) had a very poor QoL (i.e., KCCQ-OS <45), and an additional 44 (2%) had worsened QoL (i.e., decrease in KCCQ-OS of ≥10 points) (Figure 1). Thus, a total of 704 patients (33%) had a poor 6-month outcome. The baseline characteristics of patients with an acceptable vs. poor outcome after TAVR are summarized in Table 1.
Model Development
After backward stepwise elimination, the final predictive model consisted of 10 covariates, which are summarized in Table 2 (model parameters in Supplemental Table 1). According to F-values, the distance walked on the 6MWT had the strongest association with poor outcome after TAVR, with each additional 10 meters walked associated with a 3% lower risk of poor outcome (adjusted OR 0.97, 95% CI 0.96-0.98). Higher mean aortic valve gradients were also strongly associated with a lower risk of poor outcome; each 10 mmHg increase in mean gradient was associated with a 18% lower odds of a poor outcome (adjusted OR 0.82, 95% CI 0.75-0.89). Other baseline factors associated with a poor outcome after TAVR included oxygen-dependent chronic lung disease, renal dysfunction, decreased cognition, and cardiac arrhythmias (defined as a history of atrial fibrillation or flutter, supraventricular tachycardia, ventricular arrhythmia, or high-degree AV block). The c-index of the model was 0.66, indicating adequate discriminatory capacity.
Table 2. Association of pre-procedure factors with poor outcomes after TAVR.
| Poor Outcome at 6 Months | Poor Outcome at 1 Year | |||
|---|---|---|---|---|
| (Definition #1) | (Definition #2) | |||
| Predictor | OR (95% CI) | P-Value | OR (95% CI) | P-Value |
| Male sex | 1.23 (0.96-1.57) | 0.097 | 1.22 (0.97-1.53) | 0.097 |
| Diabetes mellitus | 0.82 (0.63-1.06) | 0.130 | ||
| Major arrhythmia | 1.29 (1.02-1.63) | 0.036 | 1.13 (0.91-1.40) | 0.280 |
| Serum creatinine (per 1 mg/dL) | 1.32 (1.03-1.70) | 0.028 | 1.41 (1.11-1.79) | 0.005 |
| Mean arterial pressure (per 1 mmHg) | 1.01 (1.00-1.02) | 0.209 | ||
| Body mass index (per 1 kg/m2) | 0.98 (0.96-1.00) | 0.104 | 1.00 (0.98-1.02) | 0.791 |
| Oxygen-dependent lung disease | 1.77 (1.23-2.54) | 0.002 | 1.80 (1.25-2.61) | 0.002 |
| Mean aortic valve gradient (per 10 mmHg) | 0.82 (0.75-0.89) | <0.001 | 0.84 (0.77-0.90) | <0.001 |
| Mini-Mental Status Exam (per 1 point) | 0.96 (0.92-1.00) | 0.036 | 0.94 (0.90-0.97) | 0.001 |
| 6-Minute Walk Test distance (per 10 m) | 0.97 (0.96-0.98) | <0.001 | 0.97 (0.96-0.98) | <0.001 |
The observed versus predicted risk of poor outcome after TAVR within risk deciles is shown in Figure 2a. In general, the model demonstrated good calibration with the observed outcomes with an intercept of −0.01 (p-value for difference from 0=0.806), a slope of 1.03 (p-value for difference from 1=0.793), and an R2 of 93%.
Figure 2. Calibration plots for prediction of poor outcome at 6 months after TAVR (Definition #1) in the derivation cohort (a) and validation cohort (b).
(a) Intercept of −0.01 (SE 0.04; p-value [for difference from 0]=0.806), a slope of 1.03 (SE 0.10; p-value [for difference from 1]=0.793), and R2 of 93%. (b) Intercept of 0.07 (SE 0.06; p-value [for difference from 0]=0.277), a slope of 0.75 (SE 0.18; p-value [for difference from 1]=0.203), and R2 of 69%.
Model Validation
The baseline characteristics of the derivation vs. validation cohort are presented in Supplemental Table 2. Patients were similar in terms of baseline demographic and clinical characteristics, except that patients in the validation cohort had somewhat worse QoL at baseline (KCCQ-OS: 40 vs. 43, p=0.007). The model performed well in the validation cohort, with adequate discrimination (c-index=0.64) and reasonable calibration (Figure 2b), with an intercept of 0.07 (p-value for difference from 0=0.277) and a slope of 0.75 (p-value for difference from 1=0.203).
Sensitivity Analyses
When the same covariates were applied to the individual components of the composite endpoint (i.e., mortality and poor QoL), there was general concordance of the 2 models such that the direction of association of each covariate with the 2 endpoint components was similar (although the magnitude of the association varied; Supplemental Table 3). When patients with major peri-procedural complications (n=194) were excluded from the analytic population, the model c-index was unchanged at 0.66 and the calibration remained good (Supplemental Figure 1). When the transfemoral and transapical cohorts were analyzed separately (baseline characteristics of 2 groups, the model demonstrated similar discrimination within each access site group (c-index 0.65 vs. 0.66), although the calibration was worse within the transapical group, with somewhat higher rates of poor outcomes than predicted (Supplemental Figure 2). Finally, when we substituted the KCCQ-OS for the 6MWT distance (for ease of implementation), the discrimination decreased slightly (c-index 0.64; Supplemental Table 4).
Outcomes According to Predicted Risk
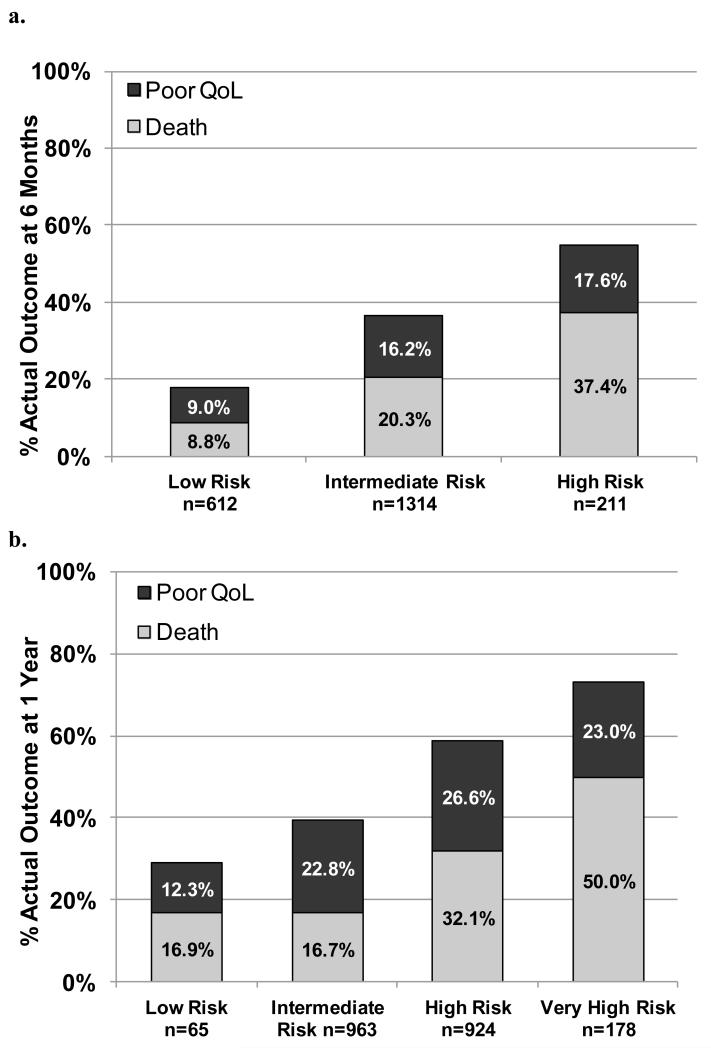
In order to better understand the ability of the model to inform clinical decisions, we stratified patients into 3 groups according to their predicted risk of a poor outcome: low risk (<25%; n=612), intermediate risk (25% to <50%; n=1314), and high risk (≥50%; n=211). The baseline characteristics of the 3 groups are shown in Table 3. Compared with patients at low risk of a poor outcome, high-risk patients had less diabetes, lower body mass indices, worse kidney function, more frequent oxygen-dependent lung disease, lower mean aortic valve gradients, worse cognitive function, worse functional status, and worse QoL at baseline. In the 6 months after TAVR, 31% of high-risk patients died, and an additional 24% had very poor QoL or a decline in QoL. In contrast, among intermediate risk patients, 17% had died, and an additional 20% had a very poor QoL or a decline in QoL, while among low-risk patients, only 8% had died and 10% had a very poor QoL or a decline in QoL (p<0.001; Figure 3a).
Table 3. Patient characteristics according to predicted risk of poor outcome (Definition #1).
| Predicted Risk* |
||||
|---|---|---|---|---|
| Low n=612 |
Intermediate n=1314 |
High n=211 |
P-Value | |
| Age (y) | 83.4 (8.2) | 85.0 (6.6) | 84.0 (7.0) | <0.001 |
| Male (%) | 43.0 | 53.3 | 78.2 | <0.001 |
| Coronary artery disease (%) | 77.6 | 78.4 | 79.1 | 0.878 |
| Cerebrovascular disease (%) | 25.3 | 27.1 | 27.5 | 0.685 |
| Carotid disease (%) | 30.1 | 25.8 | 25.1 | 0.119 |
| Peripheral vascular disease (%) | 42.5 | 42.8 | 37.9 | 0.410 |
| Diabetes mellitus (%) | 45.1 | 34.2 | 27.5 | <0.001 |
| Major arrhythmia (%) | 29.1 | 57.3 | 74.4 | <0.001 |
| Creatinine (mg/dL) | 1.17 (0.41) | 1.32 (0.46) | 1.60 (0.60) | <0.001 |
| Hemoglobin (g/dL) | 11.9 (1.5) | 11.7 (1.5) | 11.5 (1.6) | 0.018 |
| Mean arterial pressure (mmHg) | 86.6 (12.2) | 87.4 (13.2) | 86.9 (13.7) | 0.420 |
| Body mass index (kg/m2) | 28.0 (7.4) | 26.6 (5.9) | 25.6 (5.2) | <0.001 |
| Oxygen-dependent lung disease (%) | 2.5 | 9.5 | 44.5 | <0.001 |
| Pulmonary hypertension (%) | 35.8 | 41.6 | 42.2 | 0.043 |
| Mitral regurgitation (>1+) (%) | 20.9 | 26.6 | 30.3 | 0.006 |
| Aortic regurgitation (>1+) (%) | 9.2 | 11.5 | 8.5 | 0.181 |
| Mean aortic gradient (mmHg) (%) | 53.0 (15.2) | 41.3 (12.0) | 32.7 (9.7) | <0.001 |
| Ejection fraction (%) | 55.5 (11.2) | 51.2 (13.3) | 46.6 (15.0) | <0.001 |
| Stroke volume (mL/beat) | 68.9 (24.0) | 62.9 (19.4) | 61.1 (20.1) | <0.001 |
| Mini-Mental Status Exam score | 28.1 (2.1) | 27.3 (2.9) | 25.3 (4.3) | <0.001 |
| 6-Min Walk Test (% able to perform) | 89.2 | 61.2 | 19.0 | <0.001 |
| 6-Min Walk Test distance (m) | 208.9 (128.9) | 80.7 (88.6) | 13.2 (33.1) | <0.001 |
| KCCQ Overall Summary score | 50.2 (21.6) | 40.3 (20.8) | 29.4 (18.5) | <0.001 |
| SF-12 Mental Summary score | 49.6 (10.9) | 47.3 (11.2) | 43.9 (11.1) | <0.001 |
| STS mortality risk score | 10.7 (4.3) | 11.8 (4.3) | 12.6 (4.6) | <0.001 |
| Outcomes at 6 Months | ||||
| Dead (%) | 8.8 | 20.3 | 37.4 | <0.001 |
| Poor Outcome (%) | 17.8 | 36.5 | 55.0 | <0.001 |
KCCQ-OS, Kansas City Cardiomyopathy Questionnaire Overall Summary; STS, Society of Thoracic Surgeons
Low Risk: <25%, Intermediate Risk: 25 to <50%, High Risk: ≥50%
Figure 3. Outcomes of patients by their predicted risk of poor outcome at baseline at 6 months (Definition #1; Panel a) and 1 year (Definition #2; Panel b).
Definition #2 for Poor Outcome
For Definition #2, the analytic population consisted of 2130 patients who had available QOL data or had died by 1 year follow-up. Of these, 1073 (50%) had a poor 1-year outcome based on either death (n=558), poor QoL (n=503) or QoL decline (n=12). After backward stepwise elimination, the final predictive model consisted of 8 covariates, which are summarized in Table 2 (model parameters in Supplemental Table 1). In general, the predictors were similar to those for Definition #1—both in the variables that were included and their magnitude of association, except that the alternative model did not include mean arterial pressure or diabetes mellitus.
The c-index of the model was again 0.66, but the calibration of the model was better than the 6-month model (Supplemental Figure 3a) with an intercept of −0.01 (p-value for difference from 0=0.875), a slope of 1.01 (p-value for difference from 1=0.871), and an R2 of 97%. The model also performed well in the validation cohort, with a c-index of 0.62 and good calibration (Supplemental Figure 3b). We then divided the patients into 4 groups based on their predicted risk of poor outcome at 1 year: low risk (<25%; n=65), intermediate risk (25% to <50%; n=963), high risk (50% to <70%; n=924), and very high risk (≥70%, n=178). At 1 year after TAVR, 50% of very high-risk patients had died, and an additional 23% had poor QoL or a decline in QoL. In contrast, among low-risk patients, only 17% had died and 12% had a poor QoL or a decline in QoL (p<0.001; Figure 3b).
DISCUSSION
Among patients with severe aortic stenosis, TAVR is highly effective at relieving the hemodynamic obstruction and can lead to excellent outcomes in many patients. However, there are some patients who do not achieve either a survival or functional benefit from the intervention. In this study, we have identified a set of covariates and an associated prediction model that can prospectively identify patients at high-risk for poor outcomes after TAVR. Within the PARTNER trial, we found that one-third of patients had a poor outcome at 6 months using Definition #1 (conservative definition) and one-half of patients had a poor outcome at 1 year using Definition #2 (expanded definition). The most important predictors of poor outcomes, using either definition, were poor functional capacity (as assessed with the distance walked on the 6-minute walk test) and lower mean aortic valve gradients. Other important predictors were oxygen-dependent lung disease, renal dysfunction, and poorer baseline cognitive function. Of note, the STS mortality risk score was not a predictor in either model. Taken together in a validated statistical model, these factors allowed us to predict each patient’s probability of a poor outcome after TAVR. Such information could help inform clinical decision making for patients and their physicians when considering TAVR and could also help patients and their families set realistic expectations when they choose to undergo TAVR.
While our definitions for poor outcome after TAVR are unique among prior studies, we believe that the optimal definition for a poor outcome after TAVR should reflect a failure to achieve the goals of the intervention and therefore must include both a mortality and a QoL component. Combining these two endpoints into a single definition can be challenging, however.17 Nonetheless, in this elderly population of patients with multiple comorbidities, inclusion of QoL as a component of the determination of a poor (or acceptable) outcome is critical, since improved QoL can be the primary treatment goal of many patients considering TAVR. We believe that the definitions of poor outcome that we considered reflect the treatment goals of patients considering TAVR, since patients who have minimal symptoms choose TAVR primarily to achieve an expected improvement in survival while patients with substantial symptom burden most likely undergo TAVR for an expected improvement in symptoms.
There have been a number of previous efforts to determine the predictors of poor outcome after TAVR. To date, all of these analyses have been restricted to identifying predictors of mortality or specific complications, such as stroke or kidney dysfunction.9, 20-22 Despite differences in the study populations and settings, some factors have been consistently found to be prognostically important among patients undergoing TAVR. In a single-center German study, older age, lower BMI, NYHA class IV symptoms, depressed left ventricular function, and higher estimated surgical risk score were independently predictive of 1-year mortality.20 In a large, multicenter French registry, higher estimated surgical risk score, NYHA class III-IV symptoms, transapical approach, and post-implant aortic regurgitation were associated with reduced survival.9 In a multicenter Canadian study, chronic lung disease, chronic kidney disease, atrial fibrillation, and a subjective assessment of frailty were all associated with long-term mortality after TAVR.22 Finally, in a single-center Canadian study, male sex, chronic lung disease, and the 6-minute walk test distance were associated with 1-year mortality after TAVR.23
While our model also included many of these same factors (body mass index, lung disease, kidney disease, arrhythmias, functional and cognitive decline [components of frailty]), our study offers several important advantages compared with these previous studies. First, our model was based on pre-procedural factors alone and therefore can be used prospectively to inform clinical decision making. Second, ours is one of the few studies that performed model validation, which provides greater assurance of reliability. Finally, ours is the first such study to incorporate patient-reported outcomes as an explicit component of the endpoint. By integrating QoL into our definition of a poor outcome, we believe that our model may be better aligned with the goals of many patients who are considering TAVR. Although further research is needed to understand how best to present the predicted risk of poor outcome to patients and physicians, this information could be invaluable to patients who are considering whether or not to undergo TAVR. With these additional insights, these models (or future iterations) could eventually be applied in routine clinical care in order to improve decisional quality, reduce anxiety associated with the treatment decision, and provide patients and their families realistic expectations of recovery.
Our study has several important limitations. First, our study population was derived from the PARTNER trial, which included patients with a mean STS mortality risk score of ~12%—much higher than most previous studies. Consequently, it is uncertain whether our model applies to moderate risk patients. Nonetheless, since many of the factors that were included in our final model had been previously identified as associated with poor outcomes even in moderate risk TAVR patients, there is reason to believe such extrapolation may be reasonable until future studies evaluating the performance of our model in lower risk patients are performed. Second, the discriminative ability of our model was only modest. While this level of discrimination is similar to other prediction models in cardiology,24-25 there may be additional patient factors that are associated with poor outcomes after TAVR but were not available for inclusion in our models. For example, when the appropriate dataset is available, it will be particularly interesting to determine the incremental prognostic information gained from the addition of other markers of frailty, such as grip strength and gait speed, as such measures have been predictive of poor outcomes with other therapies.26-27 Since TAVR is evolving rapidly, novel predictors of poor outcomes will certainly continue to be discovered. When such factors are identified in the future, their incremental predictive value can be tested with our model, thereby leading to further refinement and improvement in the model over time.
In conclusion, using a large, multicenter cohort of patients with severe, symptomatic aortic stenosis who underwent TAVR, we have created and validated 2 related prediction models for poor outcome after TAVR based on readily available pre-procedure patient characteristics. Using these models, it is possible to identify subsets of patients who are relatively unlikely to derive meaningful survival or quality of life benefit from TAVR. In the future, providing this information prospectively to patients could offer valuable guidance as patients and their families decide whether or not to undergo TAVR. Further studies are necessary to understand the incremental value of additional risk factors to the models’ discrimination and to understand how the model performs in lower risk TAVR populations.
Supplementary Material
Acknowledgments
Funding Source
The PARTNER trial was sponsored by Edwards Lifesciences. This current study was self-funded, and the funding organization for the trial did not play a role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, or approval of the manuscript.
Reynolds: research support from Edwards Lifesciences and Medtronic; consulting income from Medtronic
Kodali: consulting income from Edwards Lifesciences, Medtronic; advisory board member Thubrikar Aortic Valve, Inc., Paieon Medical, and St. Jude Medical
Thourani: research support from Edwards Lifesciences, Sorin Medical; consulting income from DirectFlow, St. Jude Medical, Sorin Medical; royalties/intellectual property rights from Apica
Rodés-Cabau: consulting income from Edwards Lifesciences, St. Jude Medical
Leon: travel reimbursements from Edwards Lifesciences for activities related to his position on the Executive Committee of the PARTNER Trial
Mack: travel reimbursements from Edwards Lifesciences for activities related to his position on the Executive Committee of the PARTNER Trial
Cohen: research support from Edwards Lifesciences, Medtronic, Boston Scientific, Abbott Vascular, MedRad, Merck/Schering-Plough, and Eli Lilly-Daiichi Sankyo; consulting income from Schering-Plough, Eli Lilly, Medtronic, and Cordis; and speaking honoraria from Eli Lilly, The Medicines Company, and St. Jude Medical.
Footnotes
Conflict of Interest Disclosures:
The other authors report no potential conflicts.
REFERENCES
- 1.Leon MB, Smith CR, Mack M, Miller DC, Moses JW, Svensson LG, Tuzcu EM, Webb JG, Fontana GP, Makkar RR, Brown DL, Block PC, Guyton RA, Pichard AD, Bavaria JE, Herrmann HC, Douglas PS, Petersen JL, Akin JJ, Anderson WN, Wang D, Pocock S. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010;363:1597–1607. doi: 10.1056/NEJMoa1008232. [DOI] [PubMed] [Google Scholar]
- 2.Reynolds MR, Magnuson EA, Lei Y, Leon MB, Smith CR, Svensson LG, Webb JG, Babaliaros VC, Bowers BS, Fearon WF, Herrmann HC, Kapadia S, Kodali SK, Makkar RR, Pichard AD, Cohen DJ. Health-related quality of life after transcatheter aortic valve replacement in inoperable patients with severe aortic stenosis. Circulation. 2011;124:1964–1972. doi: 10.1161/CIRCULATIONAHA.111.040022. [DOI] [PubMed] [Google Scholar]
- 3.Smith CR, Leon MB, Mack MJ, Miller DC, Moses JW, Svensson LG, Tuzcu EM, Webb JG, Fontana GP, Makkar RR, Williams M, Dewey T, Kapadia S, Babaliaros V, Thourani VH, Corso P, Pichard AD, Bavaria JE, Herrmann HC, Akin JJ, Anderson WN, Wang D, Pocock SJ. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med. 2011;364:2187–2198. doi: 10.1056/NEJMoa1103510. [DOI] [PubMed] [Google Scholar]
- 4.Reynolds MR, Magnuson EA, Wang K, Thourani VH, Williams M, Zajarias A, Rihal CS, Brown DL, Smith CR, Leon MB, Cohen DJ. Health-related quality of life after transcatheter or surgical aortic valve replacement in high-risk patients with severe aortic stenosis: Results from the partner (placement of aortic transcatheter valve) trial (cohort a) J Am Coll Cardiol. 2012;60:548–558. doi: 10.1016/j.jacc.2012.03.075. [DOI] [PubMed] [Google Scholar]
- 5.Vahanian A, Alfieri O, Al-Attar N, Antunes M, Bax J, Cormier B, Cribier A, De Jaegere P, Fournial G, Kappetein AP, Kovac J, Ludgate S, Maisano F, Moat N, Mohr F, Nataf P, Pierard L, Pomar JL, Schofer J, Tornos P, Tuzcu M, van Hout B, Von Segesser LK, Walther T. Transcatheter valve implantation for patients with aortic stenosis: A position statement from the european association of cardio-thoracic surgery (eacts) and the european society of cardiology (esc), in collaboration with the european association of percutaneous cardiovascular interventions (eapci) Eur Heart J. 2008;29:1463–1470. doi: 10.1093/eurheartj/ehn183. [DOI] [PubMed] [Google Scholar]
- 6.Holmes DR, Jr., Mack MJ, Kaul S, Agnihotri A, Alexander KP, Bailey SR, Calhoon JH, Carabello BA, Desai MY, Edwards FH, Francis GS, Gardner TJ, Kappetein AP, Linderbaum JA, Mukherjee C, Mukherjee D, Otto CM, Ruiz CE, Sacco RL, Smith D, Thomas JD. 2012 accf/aats/scai/sts expert consensus document on transcatheter aortic valve replacement. J Am Coll Cardiol. 2012;59:1200–1254. doi: 10.1016/j.jacc.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 7.U.S. Food and Drug Administration [July 20, 2011];Fda executive summary: Edwards sapien™ transcatheter heart valve. Www.Fda.Gov/downloads/.../ucm262930.Pdf
- 8.Centers for Medicare & Medicaid Services . Decision memo for transcatheter aortic valve replacement (tavr) (cag-00430n) May 5, 2012. [Google Scholar]
- 9.Gilard M, Eltchaninoff H, Iung B, Donzeau-Gouge P, Chevreul K, Fajadet J, Leprince P, Leguerrier A, Lievre M, Prat A, Teiger E, Lefevre T, Himbert D, Tchetche D, Carrie D, Albat B, Cribier A, Rioufol G, Sudre A, Blanchard D, Collet F, Dos Santos P, Meneveau N, Tirouvanziam A, Caussin C, Guyon P, Boschat J, Le Breton H, Collart F, Houel R, Delpine S, Souteyrand G, Favereau X, Ohlmann P, Doisy V, Grollier G, Gommeaux A, Claudel JP, Bourlon F, Bertrand B, Van Belle E, Laskar M. Registry of transcatheter aortic-valve implantation in high-risk patients. N Engl J Med. 2012;366:1705–1715. doi: 10.1056/NEJMoa1114705. [DOI] [PubMed] [Google Scholar]
- 10.Durand E, Borz B, Godin M, Tron C, Litzler PY, Bessou JP, Dacher JN, Bauer F, Cribier A, Eltchaninoff H. Performance analysis of euroscore ii compared to the original logistic euroscore and sts scores for predicting 30-day mortality after transcatheter aortic valve replacement. Am J Cardiol. 2013;111:891–897. doi: 10.1016/j.amjcard.2012.11.056. [DOI] [PubMed] [Google Scholar]
- 11.Green CP, Porter CB, Bresnahan DR, Spertus JA. Development and evaluation of the kansas city cardiomyopathy questionnaire: A new health status measure for heart failure. J Am Coll Cardiol. 2000;35:1245–1255. doi: 10.1016/s0735-1097(00)00531-3. [DOI] [PubMed] [Google Scholar]
- 12.Spertus J, Peterson E, Conard MW, Heidenreich PA, Krumholz HM, Jones P, McCullough PA, Pina I, Tooley J, Weintraub WS, Rumsfeld JS. Monitoring clinical changes in patients with heart failure: A comparison of methods. Am Heart J. 2005;150:707–715. doi: 10.1016/j.ahj.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 13.Pettersen KI, Reikvam A, Rollag A, Stavem K. Reliability and validity of the kansas city cardiomyopathy questionnaire in patients with previous myocardial infarction. Eur J Heart Fail. 2005;7:235–242. doi: 10.1016/j.ejheart.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 14.Spertus JA, Jones PG, Kim J, Globe D. Validity, reliability, and responsiveness of the kansas city cardiomyopathy questionnaire in anemic heart failure patients. Qual Life Res. 2008;17:291–298. doi: 10.1007/s11136-007-9302-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arnold SV, Spertus JA, Lei Y, Allen KB, Chhatriwalla AK, Leon MB, Smith CR, Reynolds MR, Webb JG, Svensson LG, Cohen DJ. Utility of the kansas city cardiomyopathy questionnaire for monitoring health status in patients with aortic stenosis. Circ Heart Fail. 2012 doi: 10.1161/CIRCHEARTFAILURE.112.970053. [DOI] [PubMed] [Google Scholar]
- 16.Ware J, Jr., Kosinski M, Keller SD. A 12-item short-form health survey: Construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34:220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 17.Arnold SV, Spertus JA, Lei Y, Green P, Kirtane AJ, Kapadia S, Thourani VH, Herrmann HC, Beohar N, Zajarias A, Mack MJ, Leon MB, Cohen DJ. How to define a poor outcome after transcatheter aortic valve replacement: Conceptual framework and empirical observations from the placement of aortic transcatheter valve (partner) trial. Circ Cardiovasc Qual Outcomes. 2013;6:591–597. doi: 10.1161/CIRCOUTCOMES.113.000354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Allen LA, Gheorghiade M, Reid KJ, Dunlay SM, Chan PS, Hauptman PJ, Zannad F, Konstam MA, Spertus JA. Identifying patients hospitalized with heart failure at risk for unfavorable future quality of life. Circ Cardiovasc Qual Outcomes. 2011;4:389–398. doi: 10.1161/CIRCOUTCOMES.110.958009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harrell FE. Regression modeling strategies: With applications to linear models, logistic regression, and survival analysis. Springer-Verlag; New York: 2001. [Google Scholar]
- 20.Seiffert M, Schnabel R, Conradi L, Diemert P, Schirmer J, Koschyk D, Linder M, Kersten JF, Grosser A, Wilde S, Blankenberg S, Reichenspurner H, Baldus S, Treede H. Predictors and outcomes after transcatheter aortic valve implantation using different approaches according to the valve academic research consortium definitions. Catheter Cardiovasc Interv. 2012 doi: 10.1002/ccd.24751. [DOI] [PubMed] [Google Scholar]
- 21.Moat NE, Ludman P, de Belder MA, Bridgewater B, Cunningham AD, Young CP, Thomas M, Kovac J, Spyt T, MacCarthy PA, Wendler O, Hildick-Smith D, Davies SW, Trivedi U, Blackman DJ, Levy RD, Brecker SJ, Baumbach A, Daniel T, Gray H, Mullen MJ. Long-term outcomes after transcatheter aortic valve implantation in high-risk patients with severe aortic stenosis: The u.K. Tavi (united kingdom transcatheter aortic valve implantation) registry. J Am Coll Cardiol. 2011;58:2130–2138. doi: 10.1016/j.jacc.2011.08.050. [DOI] [PubMed] [Google Scholar]
- 22.Rodes-Cabau J, Webb JG, Cheung A, Ye J, Dumont E, Osten M, Feindel CM, Natarajan MK, Velianou JL, Martucci G, Devarennes B, Chisholm R, Peterson M, Thompson CR, Wood D, Toggweiler S, Gurvitch R, Lichtenstein SV, Doyle D, Delarochelliere R, Teoh K, Chu V, Bainey K, Lachapelle K, Cheema A, Latter D, Dumesnil JG, Pibarot P, Horlick E. Long-term outcomes after transcatheter aortic valve implantation: Insights on prognostic factors and valve durability from the canadian multicenter experience. J Am Coll Cardiol. 2012;60:1864–1875. doi: 10.1016/j.jacc.2012.08.960. [DOI] [PubMed] [Google Scholar]
- 23.Mok M, Nombela-Franco L, Urena M, Delarochelliere R, Doyle D, Ribeiro HB, Cote M, Pibarot P, Delarochelliere H, Laflamme L, Poirier P, Dumont E, Rodes-Cabau J. Prognostic value of exercise capacity as evaluated by the 6-minute walk test in patients undergoing transcatheter aortic valve implantation. J Am Coll Cardiol. 2013;61:897–898. doi: 10.1016/j.jacc.2012.10.050. [DOI] [PubMed] [Google Scholar]
- 24.Krumholz HM, Merrill AR, Schone EM, Schreiner GC, Chen J, Bradley EH, Wang Y, Lin Z, Straube BM, Rapp MT, Normand SL, Drye EE. Patterns of hospital performance in acute myocardial infarction and heart failure 30-day mortality and readmission. Circ Cardiovasc Qual Outcomes. 2009;2:407–413. doi: 10.1161/CIRCOUTCOMES.109.883256. [DOI] [PubMed] [Google Scholar]
- 25.Yeh RW, Normand SL, Wolf RE, Jones PG, Ho KK, Cohen DJ, Cutlip DE, Mauri L, Kugelmass AD, Amin AP, Spertus JA. Predicting the restenosis benefit of drug-eluting versus bare metal stents in percutaneous coronary intervention. Circulation. 2011;124:1557–1564. doi: 10.1161/CIRCULATIONAHA.111.045229. [DOI] [PubMed] [Google Scholar]
- 26.Afilalo J, Eisenberg MJ, Morin JF, Bergman H, Monette J, Noiseux N, Perrault LP, Alexander KP, Langlois Y, Dendukuri N, Chamoun P, Kasparian G, Robichaud S, Gharacholou SM, Boivin JF. Gait speed as an incremental predictor of mortality and major morbidity in elderly patients undergoing cardiac surgery. J Am Coll Cardiol. 2010;56:1668–1676. doi: 10.1016/j.jacc.2010.06.039. [DOI] [PubMed] [Google Scholar]
- 27.Sundermann S, Dademasch A, Praetorius J, Kempfert J, Dewey T, Falk V, Mohr FW, Walther T. Comprehensive assessment of frailty for elderly high-risk patients undergoing cardiac surgery. Eur J Cardiothorac Surg. 2011;39:33–37. doi: 10.1016/j.ejcts.2010.04.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.