Abstract
Objective
The study aims were to assess the influence of provider recommendations on parental vaccine perceptions and identify the most potent parent vaccine perceptions for HPV vaccine series initiation considering provider recommendation strength.
Methods
We administered a questionnaire and assessed HPV vaccine claims among a stratified-random sample of parents of 9-17 year old girls enrolled in Florida's Medicaid and the Children's Health Insurance Program. Using multivariate analyses, we evaluated the associations between: (1) parent vaccine perceptions and provider recommendation strength, and (2) parent vaccine perceptions and HPV vaccine series initiation (≥ 1 vaccine claim or positive parental report) controlling for provider recommendation strength.
Results
The majority of the 2,422 participating parents agreed that the HPV vaccine was safe (61%), would not make girls more likely to have sex (69%), and prevented cervical cancer (71%). About half (44%) reported receiving a strong provider recommendation. Compared to parents without recommendations, parents with strong recommendations had 2 to 7 times higher odds of agreeing that: vaccines are safe, the HPV vaccine is safe, not concerned about side effects, and the vaccine prevents cervical cancer. Even when considering provider recommendation strength, HPV vaccine series initiation was more likely among girls of parents who agreed rather than disagreed that the HPV vaccine was safe [Odds Ratio (OR) =5.8, 95% Confidence Interval (CI) = 3.1, 11.1), does not cause sex (OR=2.0, 95% CI = 1.2, 3.4), prevents cervical cancer (OR=2.0, 95% CI = 1.0, 3.4), and prevents HPV infections (OR=1.8, 95% CI = 1.0, 3.0).
Conclusions
Parent concerns about HPV vaccine are similar to their concerns about other vaccines. Providers should focus HPV vaccine discussions with parents on vaccine safety and illness prevention.
Keywords: HPV, Vaccine, Adolescents, Provider Recommendations, Parent Attitudes
Human papillomavirus (HPV) is the most common sexually transmitted infection in the United States with 7.1 million women acquiring infections annually [1]. Among women, HPV leads to 17,400 cancer cases each year including nearly all (91%) cervical and anal cancer cases and approximately three quarters (range 70-75%) of vulvar, vaginal, and oropharyngeal cancers [2]. HPV vaccines are recommended to adolescent girls for cervical cancer (Gardasil® and Cervarix™) and genital warts prevention (Gardasil®) [3, 4].
Within the United States, HPV vaccination rates remain well below Healthy People 2020 goals (80%) and coverage rates for other adolescent vaccines (74% meningococcal conjugate and 85% tetanus, diphtheria, and acellular pertussis vaccine) [5, 6]; depending on dataset assessed, national estimates for receipt of at least one dose (i.e., initiation) range from 38% to 53% and receipt of all three recommended doses (i.e., completion) range from 31% to 33% [7, 8]. Nationally, HPV vaccine series initiation is lowest in Florida (39%) and likely even lower among Florida publicly insured populations – enrollees in Florida Medicaid and Children's Health Insurance Programs (CHIP) [5, 9].
Parents primarily decide whether an adolescent receives the HPV vaccine, especially among 11-12 year olds, the subpopulation targeted for universal coverage [3, 4, 10]. Social cognitive theory and the literature suggest parents' decisions to vaccinate are influenced by their perceptions of vaccine risks (e.g., safety, side effects, influence adolescents to have sex), vaccine benefits (e.g., prevention of cervical cancer, HPV, genital warts), and HPV-specific knowledge (e.g., symptoms and transmission routes) [11-15] . Parents' vaccination decisions are heavily influenced by recommendations from their child's medical provider[15-17]. For example, in a national study, compared to no recommendation, receiving a provider recommendation increased the odds of receiving the vaccine five times [17]. In light of the overwhelming influence of provider recommendations, the relative importance of parent vaccine perceptions is unclear. Because time needed to discuss the vaccine is a barrier for providers recommending the vaccine [18], it is essential to focus provider discussions on the most potent perceptions.
To identify the most potent topics for providers to discuss with parents to improve HPV vaccine series initiation among girls enrolled in Florida's Medicaid and CHIP, we assessed the influence of: (1) provider recommendation strength on parental vaccine perceptions of risks, benefits, and knowledge and (2) parent vaccine perceptions on HPV vaccine series initiation considering provider recommendation strength. Increased understanding of crucial topics for parents will help maximize providers’ and health promotion intervention efforts.
Methods
Design and Sample
We selected a stratified-random sample of 8,422 adolescent girls among 9-17 year olds enrolled in Florida's Medicaid or CHIP during December 2009 and at least 10 of the prior 12 months. We focused on 9-17 year olds to include all vaccine-eligible ages who require parental consent [3]. For the sample pool, we randomly selected one girl from each family and stratified by two-year age group and vaccine initiation; determined by the presence of at least one claim for Cevarix (ICD-9 code 90650) or Gardasil (ICD-9 code 90649) by December 2009.
We administered a survey via telephone and re-contacted non-responders by mail. Survey questions were primarily adopted from the Health Information National Trends Survey, the Youth Risk Behavior Survey, and HPV vaccine surveys [16, 19-21]. Between March and August 2010, trained female interviewers called primary caregivers up to ten times. Participants received $10. Overall, 936 participated, 714 refused, 6573 did not respond, and 199 were ineligible (e.g., business numbers or unable to participate).
Between March and May 2011, we sent paper surveys to the 6,414 non-responders with verifiable address information. Following best survey practices [22], we sent full-color notification postcards, used an attractive survey booklet with personalized questions, provided hand-stamped return envelopes, sent surveys via FedEx, and included $5. Overall, 1,486 completed the paper survey, 152 refused, 132 were ineligible, and 774 were undeliverable.
A total of 2,422 caregivers participated. Based on the American Association for Public Opinion Research's Response Rate 4 [23] method and assuming 48% of unreachable parents were eligible (percentage eligible in telephone survey), our overall response rate was 43%. We limited analysis to the 2,127 caregivers (88%) who had heard of the vaccine, and thus, were asked further questions. Caregivers were primarily parents (89%); thus, we refer to caregivers as parents and girls as daughters. The University of Florida Institutional Review Board approved this project and all participants provided informed consent.
HPV Vaccine Initiation
To assess vaccine initiation, we including all claims from June 2006 to the survey date (between March 2010 and May 2011). Because vaccines can be obtained without receiving a Medicaid or CHIP claim (e.g., at the Department of Health), we also considered the 317 girls without a claim, but with parental report of vaccination as having initiated the series.
Parent vaccine perceptions
We measured parents’ agreement with seven vaccine risk/benefit statements. To limit participant refusal and confusion, all questions referred to the cervical cancer vaccine. For clarity, HPV vaccine is used hereafter. Vaccine risks included: 1) “Vaccines are safe”; 2) The HPV vaccine is safe; 3) concerned about vaccine side effects; and 4) The HPV vaccine would make girls more likely to have sex. Vaccine benefits included the vaccine prevents: 1) cervical cancer, 2) HPV infections, and 3) genital warts. For analysis, we collapsed responses into: agree (strongly agree or agree), neutral, disagree (disagree or strongly disagree), and don't know.
We assessed HPV-specific knowledge with a nine-item scale. Participants answered yes, no, or don't know to whether: they had heard of HPV, specific symptoms could be caused by HPV (genital warts, cough, cervical cancer, heart attack, and infected without symptoms), and transmission occurs during (sex, someone coughing or sneezing on you, or eating uncooked meat). Among participants who had heard of HPV, we summed the number of correct responses. Cronbach's alpha for correct answers was 0.89. We analyzed approximate quartiles: not heard of HPV, little knowledge (≤ 4 correct), some knowledge (5 or 6 correct), and high knowledge (7 or 8 correct).
Provider Recommendation Strength
To assess provider recommendation strength, we asked three questions adapted from the Health Information National Trends Survey and Rosenthal et al. (2011) [16, 19]: Health care provider, such as doctor or nurse: 1) talked about or 2) recommended, and 3) expressed importance for the HPV vaccine. Similar to Rosenthal et al. (2011) [16], we created a four category summary variable. We categorized parents as having no recommendation if their provider did not discuss (n=552) or recommended against the vaccine (n=49). Neutral recommendations were assigned to parents whose provider discussed the vaccine, but did not recommend the vaccine (n=182) or recommended the vaccine as unimportant (n=37). Moderate recommendations were assigned to parents whose provider recommended the vaccine as somewhat important (n=353) or of unknown importance (n=2) . Parents with strong recommendations reported providers recommended the vaccine as very important (n=915).
Covariates
Based on the literature [8, 9], we included daughter's age, parent's race/ethnicity, area of residence, and type of insurance as covariates. Daughter's age was calculated by subtracting her birth date from the date of survey completion. When parents’ self-reported race/ethnicity was unavailable, we used daughters’ race/ethnicity from the Medicaid or CHIP enrollment files if available (n=40). We restricted analyses considering race/ethnicity to non-Hispanic white, non-Hispanic black, or Hispanic race/ethnicity because other racial/ethnic groups were too diverse to combine and too small (n < 20) to analyze separately. Type of government insurance was daughters’ enrolled program within two months prior to the survey date. We considered Medicaid programs separately because of key differences in service delivery structure and provider reimbursement [9]. We categorized residential zip codes into four categories using Rural-Urban Communing Area Codes version 2.0 [24]. We combined large rural, small rural, and isolated because less than 3% lived in small rural or isolated areas.
Statistical Analysis
Models were determined a priori. We used SAS software version 9.3 (SAS Institute, Inc., Cary, NC) for all analyses and assigned statistical significance at p<0.05. We used Chi-square tests to access differences in the frequency of vaccination by demographic characteristics. To estimate the association between parental vaccine perceptions and provider recommendation strength, we used ordinal logistic regression adjusting for: 1) daughter's age only and 2) daughter's age plus other important demographics (parent's race/ethnicity, urban or rural residence, and health program type). We used age-squared in all models to adjust for hypothesized quadratic trends with daughter's age. We used logistic regression to estimate the association between HPV vaccine series initiation and each parent vaccine perception adjusting for demographics, the other parent perceptions, and provider recommendation strength. Since mode of data collection may influence data comparability [25], we also considered mode of data collection (i.e., mail vs. telephone) as a potential confounder and interaction term in adjusted models. Similar to expectations by chance alone, one of the eighteen mode interaction terms was marginally significant (p=0.04), and thus, interaction terms were omitted from final models.
Results
While we targeted a population with 50% of parents within each age stratum having vaccinated daughters, 64% of participating parents initiated the HPV vaccine series for their daughter. Over 150 parents participated in each one-year age group, except 9-year-olds (n=45) and 19-years-old (n=39) (Table 1). Parents of each racial/ethnic group participated: 40% non-Hispanic White, 36% Hispanic, and 24% non-Hispanic Black. Most (92%) participants were from urban areas. Demographic distributions were similar by data collection mode except telephone survey participants were more likely than mail responders to be non-Hispanic white (46% vs. 37%) and live in rural or isolated areas (11% vs. 6%).”
Table 1.
Characteristics of Participating Parents of girls enrolled in Florida Medicaid or CHIP (n=2127)
| Number of parents | Percent vaccinated | Chi-square test p-value | |
|---|---|---|---|
| Age of daughter (years) | <.0001 | ||
| 9 | 45 | 22% | |
| 10 | 177 | 39% | |
| 11 | 234 | 51% | |
| 12 | 283 | 60% | |
| 13 | 259 | 66% | |
| 14 | 268 | 77% | |
| 15 | 238 | 68% | |
| 16 | 227 | 74% | |
| 17 | 207 | 72% | |
| 18 | 150 | 74% | |
| 19 | 39 | 64% | |
| Race/ethnicity | |||
| Non-Hispanic White | 804 | 62% | .0006 |
| Non-Hispanic Black | 477 | 61% | |
| Hispanic | 714 | 70% | |
| Area of Residence | .71 | ||
| Urban | 1929 | 64% | |
| Rural | 168 | 65% | |
| Health plan | <.0001 | ||
| CHIP | 846 | 66% | |
| Medicaid | |||
| Managed Care | 437 | 52% | |
| MediPass | 487 | 71% | |
| FFS | 94 | 63% | |
| CMSN | 65 | 82% | |
| Reform | 61 | 61% | |
| PSN | 23 | 48% | |
| No longer enrolled | 114 | 59% |
FFS = fee for service; CMSN = children's medical services network; PSN = provider service network.
Parent perceptions
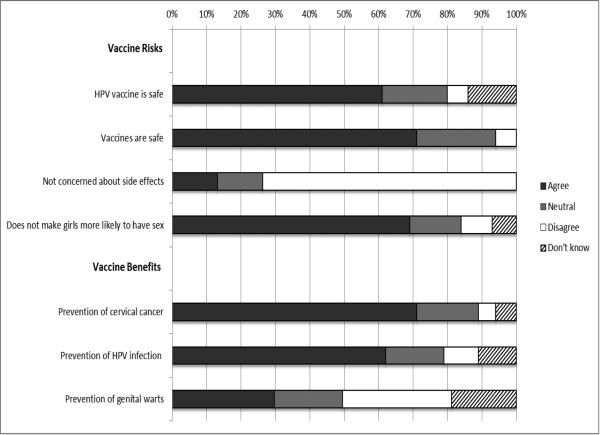
The majority of parents (62-71%) demonstrated positive perceptions about vaccination and the HPV vaccine, but most parents (73%) were concerned about vaccine side effects (Figure 1). Among the 80% of parents who heard of HPV, most (71-77%) correctly identified HPV transmission routes. Regarding symptoms of HPV, most parents (74%) identified cervical cancer and infection without symptoms (71%), but approximately half identified genital warts and incorrectly identified cough or heart attack. Most parents (74%) reported that a health care provider had talked to them or their daughter about the HPV vaccine, but only half (44%) reported receiving a strong vaccine recommendation.
Figure 1.
Percent of parents who endorse vaccine risks and benefits
Influence of provider recommendation strength on parent vaccine perceptions
Comparisons between parental neutral and don't know perceptions to disagreement did not substantively change conclusions; thus, for clarity, only results for comparisons between parent agreement and disagreement are presented. Furthermore, results were similar regardless of survey mode adjustment. The odds of being comfortable with vaccine risks was two to seven times more likely among parents with strong provider recommendations than parents without a recommendation (Table 2). Compared to parents without a recommendation, parents with a strong recommendation had three times higher odds of agreeing vaccines are safe (OR = 3.1, 95% Confidence Interval (CI) = 1.7 to 5.4) and seven times higher odds of agreeing the HPV vaccine is safe (OR=7.1, 95% CI = 3.8 to 13.0). Parents with strong compared to no recommendations had twice the odds of agreeing they were not concerned about side effects (OR=2.0, 95% CI = 1.4 to 2.8) and the vaccine did not make girls more likely to have sex (OR=1.7, 95% CI = 1.1 to 2.5).
Table 2.
Influence provider's recommendation strength on parents’ vaccine perception agreement compared to disagreement
| Neutral Recommendation Adjusteda Odds Ratio | Moderate Recommendation Adjusteda Odds Ratio | Strong Recommendation Adjusteda Odds Ratio | |
|---|---|---|---|
| Vaccine risks | |||
| Vaccines are safe | 0.6 (0.4, 1.2) | 1.2 (0.7, 2.3) | 3.1 (1.7, 5.4)* |
| HPV vaccine is safe | 0.9 (0.5, 1.6) | 1.2 (0.7, 2.1) | 7.1 (3.8, 13.0)* |
| Not Concerned about side effectsa | 1.4 (0.8, 2.3) | 1.0 (0.6, 1.6) | 2.0 (1.4, 2.8)* |
| Does Not make girls more likely to have sexa | 1.3 (0.7, 2.3) | 1.5 (0.9, 2.5) | 1.7 (1.1, 2.5)* |
| Vaccine Benefits | |||
| Prevention of cervical cancer | 0.4 (0.2, 0.7)* | 0.7 (0.4, 1.4) | 3.3 (1.7, 6.3)* |
| Prevention of HPV infections | 0.6 (0.4, 1.1) | 1.2 (0.7, 2.1) | 1.4 (0.9, 2.1) |
| Prevention of genital warts | 0.5 (0.3, 0.8)* | 0.8 (0.6, 1.2) | 1.2 (0.9, 1.6) |
| HPV-specific knowledge | |||
| High vs. Never heard of | 1.7 (1.0, 2.8)* | 1.9 (1.2, 2.9)* | 2.0 (1.5, 2.9)* |
| Some vs. Never heard of | 2.0 (1.2, 3.3)* | 2.0 (1.3, 3.2)* | 2.1 (1.5, 2.9)* |
| Little vs. Never heard of | 1.5 (0.9, 2.4) | 1.6 (1.0, 2.4)* | 1.7 (1.2, 2.4)* |
statistically significant at p< 0.05
Compared to no recommendation and adjusted for all parent's race/ethnicity, quadratic function of daughter's age, area of residence, and government health program type
Provider recommendation strength was also associated with parents’ perceived benefits of the HPV vaccine and HPV-specific knowledge (Table 2). The odds of agreeing that the vaccine prevents cervical cancer were three times higher among parents with a strong recommendation than parents without a recommendation (OR=3.3, 95% CI = 1.7 to 6.3). Compared to parents without a recommendation, parents with any strength of recommendation had twice the odds of increased HPV-specific knowledge.
Influence of parent vaccine perceptions on HPV vaccine initiation
The parent vaccine perception most strongly associated with HPV vaccine initiation was HPV vaccine safety (OR=5.8, 95% CI = 3.1, 11.1) (Table 3). We found approximately two-fold increased odds of HPV vaccine series initiation with decreased concerns about side effects, concerns that the vaccine will make girls more likely to have sex, and believing the vaccine prevents cervical cancer and HPV infections. After adjusting for demographics, HPV vaccine initiation was more likely among daughters of parents with a recommendation than without: Strong OR=26.3 (95% CI =19.2 to 35.9), Moderate OR=5.4 (95% CI =3.9 to 7.4), and Neutral OR=3.2 (95% CI =2.2 to 4.6).
Table 3.
Odds of daughter's initiating the HPV vaccine series by parents’ vaccine perceptions agreement compared to disagreement
| Age-adjusted Odds Ratio | + Parent perceptions and other Demographics Adjusteda Odds Ratio | + Provider recommendation Adjustedb Odds Ratio | |
|---|---|---|---|
| Vaccine risks | |||
| Vaccines are safe | 5.1 (3.4, 7.8)* | 1.6 (0.9, 2.7) | 1.7 (0.9, 3.2) |
| HPV vaccine is safe | 12.3 (7.8, 19.5)* | 8.0 (4.5, 14.3)* | 5.8 (3.1, 11.1)* |
| Not Concerned about side effects | 2.8 (2.0, 3.9)* | 1.8 (1.2, 2.6)* | 1.9 (1.2, 3.0)* |
| Does Not make girls more likely to have sex | 2.0 (1.4, 2.7)* | 2.1 (1.3, 3.2)* | 2.0 (1.2, 3.4)* |
| Vaccine Benefits | |||
| Prevention of cervical cancer | 5.0 (3.2, 7.9)* | 2.3 (1.2, 4.3)* | 2.0 (1.0, 4.0)* |
| Prevention of HPV infections | 2.3 (1.7, 3.2)* | 1.4 (0.9, 2.3) | 1.8 (1.0, 3.0)* |
| Prevention of genital warts | 1.3 (1.0, 1.6)* | 0.7 (0.5, 1.0) | 0.7 (0.5, 1.1) |
| HPV-specific knowledge | |||
| High vs. Never heard of | 1.2 (0.9, 1.5) | 0.9 (0.6, 1.3) | 0.6 (0.4, 0.9)* |
| Some vs. Never heard of | 1.2 (0.9, 1.6) | 1.1 (0.8, 1.6) | 0.8 (0.5, 1.2) |
| Little vs. Never heard of | 1.1 (0.8, 1.4) | 0.9 (0.6, 1.2) | 0.6 (0.4, 0.9)* |
statistically significant at p< 0.05
adjusted for all parent perceptions, parent's race/ethnicity, quadratic function of daughter's age, area of residence, and government health program type
adjusted for provider recommendation plus all parent perceptions, parent's race/ethnicity, quadratic function of daughter's age, area of residence, and government health program type
While similar overall, a few differences are notable when provider recommendation was considered (Table 3). First, increased HPV-specific knowledge decreased the likelihood of HPV vaccine initiation. Second, the association of parental agreement with HPV vaccine safety with vaccine initiation attenuated from 8.0 to 5.8. The full model with all three constructs was more predictive of HPV vaccine initiation than models with either parent perceptions or provider recommendations alone (Likelihood ratio test: full model vs. provider and demographics model P < 0.0001 and full model vs. parent perceptions and demographics model P < 0.0001). In the full model, age, health plan, and race/ethnicity remained significantly associated with vaccine initiation.
Discussion
Parent beliefs of HPV-specific vaccine safety and illness prevention remain important for HPV vaccine series initiation even when considering provider recommendation strength. Provider discussions are associated with parents’ perceptions of safety and, to a lesser degree, prevention benefits. The small percentage of parents who did not initiate the vaccine series because of concerns about the vaccine's influence on sexual activity do not appear swayed by providers’ recommendations. To improve HPV vaccine initiation rates among low-income adolescent girls, intervention strategies should emphasize providers' focus discussion on the safety and illness prevention benefits of the HPV vaccine.
Based on parent safety concerns influencing childhood vaccinations [26] and parents citing safety concerns as a primary reason for vaccine refusal [12, 27-29], it is not surprising that parents' concerns about HPV vaccine safety are highly predictive of HPV vaccine initiation. Providers may be convincing parents that the vaccine is safe, and in some cases, a provider recommendation alone may convince some parents that the vaccine is safe.
Similar to parents citing prevention benefits as the main reason for accepting the vaccine [12, 27-29] we found parents who believed the vaccine prevented cervical cancer were more likely to vaccinate. Our findings of the stronger influence of provider recommendation on parent vaccine perceptions for cervical cancer prevention compared to HPV and genital warts prevention complement findings that providers discuss prevention of cervical cancer more frequently than other vaccine benefits [30].
Consistent with other studies [31], a small percent of parents believed the vaccine could promote sexual activity. Our findings demonstrate that a concern about sexual activity was a barrier for vaccine initiation. While 25% of parents report discussing sexual transmission during their vaccine conversation with providers [30], we found limited influence of provider recommendations on parents’ beliefs about the vaccine's influence on sexual activity. Given providers’ discomfort discussing sex with parents as a primary reason for delaying vaccination [10], providers should consider targeting only concerned parents or omitting discussions about sex in vaccine recommendations.
The consistent and nearly two-fold increase in knowledge for each strata of provider recommendation suggests most providers are discussing HPV transmission and symptoms with parents when recommending the vaccine. Yet, consistent with prior findings of limited vaccine-specific knowledge among vaccinating parents and considerable knowledge among resisting parents [32-34], we found knowledge was not essential and could be detrimental to parent's decisions to vaccinate. To insure providers do not inadvertently discourage vaccination, further research is needed to identify how to best communicate HPV-vaccine specific knowledge to parents.
Despite the clear importance of provider recommendations on HPV vaccine initiation, the adolescents’ age, race/ethnicity, and health plan type remain important. Consistent with national surveillance and prior studies within Florida Medicaid [7, 9, 35] , Hispanic adolescent girls were more likely to be vaccinated than non-Hispanic whites and non-Hispanic blacks supporting suggestions of cultural differences in HPV vaccine acceptability [36, 37] . Health plan organizational structures and priorities also likely influence vaccination rates [9, 38].
This study has three important limitations. First, our results may not generalize to all adolescent girls enrolled in Florida Medicaid or CHIP because only 43% of selected parents participated in the survey. Yet, the achieved response rate is typical of surveys in similar populations [39-41]. Second, while it is likely reasonable to assume that providers influenced parents’ beliefs, we are unable to establish the temporal association because of the case-control design. Third, identified beliefs associated with vaccine series initiation may have changed since 2010-2011 and may differ from beliefs associated with series completion [31]. Since we assessed parent beliefs, the vaccine has been universally recommended for boys and protection for non-cervical cancers has received wider recognition [2, 42]. Yet, national vaccination rates among girls do not reflect significant changes following these advances [7].
This study has three important strengths. First, our measurement of vaccination with insurance claims supplemented with parent-report is more accurate than parent-report alone [43]. Second, our large (n=2127) low-income population of parents of vaccinated and unvaccinated 9-18 year old girls allowed for: simultaneous assessment of multiple predictors, identification of common parental attitudes across ages [44, 45], and consideration of girls with increased risk of late stage cervical cancer diagnosis [46]. Third, because cost of the vaccine is a frequently identified barrier to HPV vaccination [31] and the Medicaid and CHIP families have been eligible for free HPV vaccine since approval [47, 48], studies within Medicaid and CHIP may be more applicable than privately insured populations to the post-Affordable Care Act era when the HPV vaccine is available without cost to all girls [49].
Providers need to have fast and efficient strategies to recommend the HPV vaccine because adolescents primarily seek acute care or athletic physical exams [50]. Focusing on the most salient topics for parents – HPV vaccine safety and prevention benefits – may maximize vaccinations within the time constraints. Providers need additional tools to discuss the vaccine with the minority of families concerned about the vaccine and sex. While providers are treating the HPV vaccine differently than other vaccines [10], the majority of parents’ concerns about the HPV vaccine are similar to their concerns about other vaccines.
Highlights.
Most parents expressed positive attitudes about HPV vaccine safety and prevention
Provider recommendation strength influences parents’ vaccine perceptions
Safety and prevention are the most salient parent concerns for HPV vaccination
Acknowledgements
The work was funded in part by the University of Florida Cancer Center and the Florida Agency for Health Care Administration. Partial funding for Dr. Staras and Ms. Patel was provided by NIH K01 AA018255. The authors would like to thank Alana Christou, MPH; Katie Eddleton, MPH; Chris Delcher MS, Ashley Sanders MS, and Serena Joseph for contributions to the data collection, literature review, and data management.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions: SASS, RP, and SV conceived the study. SASS, SV, and ES collected the data. SASS and RP and completed the analysis. All authors contributed to the interpretation of results, the writing of the paper, and have approved the final draft.
Conflict of interest: All authors declare no conflict of interest that may have influenced this work.
References
- 1.Satterwhite CL, et al. Sexually transmitted infection among US women and men: Prevalence and incidence estimates, 2008. Sex Transm Dis. 2013;40:187–93. doi: 10.1097/OLQ.0b013e318286bb53. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention [July 1 2014];Human Papillomavirus (HPV) - Associated Cancers: How many cancers are linked with HPV each year? 2014 Available at: http://www.cdc.gov/cancer/hpv/statistics/cases.htm.
- 3.Centers for Disease Control and Prevention Quadrivalent human papillomavirus vaccine: Recommendations of the Advisory Committee on Immunization Practices (ACIP). Morbidity and Mortality Weekly Report. 2007;56:1–24. [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention FDA Licensure of Bivalent Human Papillomavirus Vaccine (HPV2, Cervarix) for Use in Females and Updated HPV Vaccination Recommendations from ACIP. MMWR Morb Mortal Wkly Rep. 2010 [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention National and State Vaccination Converage Among Adolescents Aged 13-17 Years - United States, 2012. Morbidity and Mortality Weekly Report. 2013;62:685–93. [PMC free article] [PubMed] [Google Scholar]
- 6.U.S. Department of Health and Human Services [July 1 2014];Healthy People 2020. 2014 Available at: http://healthypeople.gov/2020/topicsobjectives2020/pdfs/HP2020objectives.pdf.
- 7.Centers for Disease Control and Prevention Human papillomavirus vaccination coverage among adolescent girls, 2007–2012, and postlicensure vaccine safety monitoring, 2006–2013 — United States. Morbidity and Mortality Weekly Report. 2013;62:591–5. [PMC free article] [PubMed] [Google Scholar]
- 8.Schmidt MA, Gold R, Kurosky SK, Daley MF, Irving SA, Gee J, et al. Uptake, coverage, and completion of quadrivalent human papillomavirus vaccine in the vaccine safety datalink, July 2006-June 2011. The Journal of adolescent health : official publication of the Society for Adolescent Medicine. 2013;53:637–41. doi: 10.1016/j.jadohealth.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Staras SA, Vadaparampil ST, Haderxhanaj LT, Shenkman EA. Disparities in human papillomavirus vaccine series initiation among adolescent girls enrolled in Florida medicaid programs, 2006-2008. Journal of Adolescent Health. 2010;47:381–8. doi: 10.1016/j.jadohealth.2010.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hughes C, Jones A, Feemster K, Fiks A. HPV vaccine decision making in pediatric primary care: A semi-structured interview study. BMC Pediatr. 2011;11:74. doi: 10.1186/1471-2431-11-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bandura A. Social foundations of thought and action: A social cognitive theory. Prentice-Hall, Inc; Englewood Cliffs, NJ: 1986. [Google Scholar]
- 12.Rand CM, Humiston SG, Schaffer SJ, Albertin CS, Shone LP, Blumkin AK, et al. Parent and adolescent perspectives about adolescent vaccine delivery: Practical considerations for vaccine communication. Vaccine. 2011;29:7651–8. doi: 10.1016/j.vaccine.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 13.Hendry M, Lewis R, Clements A, Damery S, Wilkinson C. “HPV? Never heard of it!”: a systematic review of girls’ and parents’ information needs, views and preferences about human papillomavirus vaccination. Vaccine. 2013;31:5152–67. doi: 10.1016/j.vaccine.2013.08.091. [DOI] [PubMed] [Google Scholar]
- 14.Sperber NR, Brewer NT, Smith JS. Influence of parent characteristics and disease outcome framing on HPV vaccine acceptability among rural, Southern women. Cancer Causes Control. 2008;19:115–8. doi: 10.1007/s10552-007-9074-9. [DOI] [PubMed] [Google Scholar]
- 15.Holman DM, Benard V, Roland KB, Watson M, Liddon N, Stokley S. Barriers to human papillomavirus vaccination among US adolescents: A systematic review of the literature. JAMA Pediatr. 2014;168:76–82. doi: 10.1001/jamapediatrics.2013.2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosenthal SL, Weiss TW, Zimet GD, Ma L, Good MB, Vichnin MD. Predictors of HPV vaccine uptake among women aged 19–26: Importance of a physician's recommendation. Vaccine. 2011;29:890–5. doi: 10.1016/j.vaccine.2009.12.063. [DOI] [PubMed] [Google Scholar]
- 17.Ylitalo KR, Lee H, Mehta NK. Health care provider recommendation, human papillomavirus vaccination, and race/ethnicity in the US National Immunization Survey. Am J Public Health. 2013;103:164–9. doi: 10.2105/AJPH.2011.300600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bynum SA, Staras SA, Malo TL, Giuliano AR, Shenkman E, Vadaparampil ST. Factors associated With Medicaid providers' recommendation of the HPV vaccine to low-income adolescent girls. The Journal of adolescent health : official publication of the Society for Adolescent Medicine. 2014;54:190–6. doi: 10.1016/j.jadohealth.2013.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.National Cancer Institute [2009];Health Information National Trends Survey. 2014 Available at: http://hints.cancer.gov/instrument.aspx.
- 20.Rosenthal SL, Rupp R, Zimet GD, Meza HM, Loza ML, Short MB, et al. Uptake of HPV vaccine: Demographics, sexual history and values, parenting style, and vaccine attitudes. J Adolescent Health. 2008;43:239–45. doi: 10.1016/j.jadohealth.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 21.Centers for Disease Control and Prevention Youth Risk Behavior Survey. 2009 2009 Available at: http://www.cdc.gov/HealthyYouth/yrbs/index.htm.
- 22.Edwards PJ, Roberts I, Clarke MJ, DiGuiseppi C, Wentz R, Kwan I, et al. Methods to increase response to postal and electronic questionnaires. Cochrane Database Syst Rev. 2009 doi: 10.1002/14651858.MR000008.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.American Association for Public Opinion Research [06/06/2012];Standard definitions: Final dispositions of case codes and cutcome rates for surveys. 2009 Available at: http://www.aapor.org/Standard_Definitions2.htm.
- 24.Health Resources and Service Administration, United States Department of Agriculture, University of Washington [07/20/2012];RUCA (Rural-Urban Commuting Area Codes) Version 2.0. 2005 Available at: http://depts.washington.edu/uwruca/index.php.
- 25.Jäckle A, Roberts C, Lynn P. Assessing the effect of data collection mode on measurement. International Statistical Review. 2010;78:3–20. [Google Scholar]
- 26.Gust DA, Darling N, Kennedy A, Schwartz B. Parents with doubts about vaccines: which vaccines and reasons why. Pediatrics. 2008;122:718–25. doi: 10.1542/peds.2007-0538. [DOI] [PubMed] [Google Scholar]
- 27.Dorell C, Yankey D, Jeyarajah J, Stokley S, Fisher A, Markowitz L, et al. Delay and Refusal of Human Papillomavirus Vaccine for Girls, National Immunization Survey-Teen, 2010. Clin Pediatr (Phila) 2014 doi: 10.1177/0009922813520070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dempsey AF, Abraham LM, Dalton V, Ruffin M. Understanding the reasons why mothers do or do not have their adolescent daughters vaccinated against human papillomavirus. Annals of epidemiology. 2009;19:531–8. doi: 10.1016/j.annepidem.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kester L, Zimet G, Fortenberry JD, Kahn J, Shew M. A national study of HPV vaccination of adolescent girls: Rates, predictors, and reasons for non-vaccination. Matern Child Health J. 2012:1–7. doi: 10.1007/s10995-012-1066-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rand CM, Schaffer SJ, Humiston SG, Albertin CS, Shone LP, Heintz EV, et al. Patient-provider communication and human papillomavirus vaccine acceptance. Clin Pediatr. 2011;50:106–13. doi: 10.1177/0009922810379907. [DOI] [PubMed] [Google Scholar]
- 31.Holman DM, Benard V, Roland KB, Watson M, Liddon N, Stokley S. Barriers to Human Papillomavirus Vaccination Among US Adolescents: A Systematic Review of the Literature. JAMA pediatrics. 2013 doi: 10.1001/jamapediatrics.2013.2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dube E, Laberge C, Guay M, Bramadat P, Roy R, Bettinger J. Vaccine hesitancy: an overview. Hum Vaccin Immunother. 2013;9:1763–73. doi: 10.4161/hv.24657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Evans M, Stoddart H, Condon L, Freeman E, Grizzell M, Mullen R. Parents' perspectives on the MMR immunisation: a focus group study. The British journal of general practice : the journal of the Royal College of General Practitioners. 2001;51:904–10. [PMC free article] [PubMed] [Google Scholar]
- 34.Cassell JA, Leach M, Poltorak MS, Mercer CH, Iversen A, Fairhead JR. Is the cultural context of MMR rejection a key to an effective public health discourse? Public health. 2006;120:783–94. doi: 10.1016/j.puhe.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 35.Cook RL, Zhang J, Mullins J, Kauf T, Brumback B, Steingraber H, et al. Factors associated with initiation and completion of human papillomavirus vaccine series among young women enrolled in medicaid. J Adolescent Health. 2010;47:596–9. doi: 10.1016/j.jadohealth.2010.09.015. [DOI] [PubMed] [Google Scholar]
- 36.Watts LA, Joseph N, Wallace M, Rauh-Hain JA, Muzikansky A, Growdon WB, et al. HPV vaccine: A comparison of attitudes and behavioral perspectives between Latino and non-Latino women. Gynecologic oncology. 2009;112:577–82. doi: 10.1016/j.ygyno.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 37.Sanderson M, Coker AL, Eggleston KS, Fernandez ME, Arrastia CD, Fadden MK. HPV Vaccine Acceptance among Latina Mothers by HPV Status. J Womens Health (Larchmt) 2009;18:1793–9. doi: 10.1089/jwh.2008.1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smith PJ, Santoli JM, Chu SY, Ochoa DQ, Rodewald LE. The association between having a medical home and vaccination coverage among children eligible for the vaccines for children program. Pediatrics. 2005;116:130–9. doi: 10.1542/peds.2004-1058. [DOI] [PubMed] [Google Scholar]
- 39.Fredrickson DD, Jones TL, Molgaard CA, Carman CG, Schukman J, Dismuke SE, et al. Optimal design features for surveying low-income populations. Journal of health care for the poor and underserved. 2005;16:677–90. doi: 10.1353/hpu.2005.0096. [DOI] [PubMed] [Google Scholar]
- 40.McMenamin SB, Halpin HA, Bellows NM. Knowledge of Medicaid coverage and effectiveness of smoking treatments. Am J Prev Med. 2006;31:369–74. doi: 10.1016/j.amepre.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 41.Hall AG, Landry AY, Lemak CH, Boyle EL, Duncan RP. Reported experiences with Medicaid managed care models among parents of children. Matern Child Health J. 2014;18:544–53. doi: 10.1007/s10995-013-1270-5. [DOI] [PubMed] [Google Scholar]
- 42.Centers for Disease Control and Prevention Use of HPV4 in Males. MMWR Morb Mortal Wkly Rep. 2011 [Google Scholar]
- 43.Attanasio L, McAlpine D. Accuracy of Parental Reports of Children's HPV Vaccine Status: Implications for Estimates of Disparities, 2009-2010. Public health reports (Washington, DC : 1974) 2014;129:237–44. doi: 10.1177/003335491412900305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vadaparampil ST, Kahn JA, Salmon D, Lee J-H, Quinn GP, Roetzheim R, et al. Missed clinical opportunities: Provider recommendations for HPV vaccination for 11–12 year old girls are limited. Vaccine. 2011;29:8632–41. doi: 10.1016/j.vaccine.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kahn JA, Cooper HP, Vadaparampil ST, Pence BC, Weinberg AD, LoCoco SJ, et al. Human papillomavirus vaccine recommendations and agreement with mandated human papillomavirus vaccination for 11-to-12-year-old girls: A statewide survey of Texas physicians. Cancer Epidem Biomar. 2009;18:2325–32. doi: 10.1158/1055-9965.EPI-09-0184. [DOI] [PubMed] [Google Scholar]
- 46.Singh GK, Miller BA, Hankey BF, Edwards BK. Persistent area socioeconomic disparities in U.S. incidence of cervical cancer, mortality, stage, and survival, 1975-2000. Cancer. 2004;101:1051–7. doi: 10.1002/cncr.20467. [DOI] [PubMed] [Google Scholar]
- 47.Centers for Disease Control and Prevention Vaccines Included in the VFC Program - Vaccines for Children Program. 2006 2014 Available at: http://www.cdc.gov/vaccines/programs/vfc/downloads/resolutions/0606-vaccines.pdf.
- 48.Association of Maternal and Child Health Programs AMCHP Fact Sheet - The HPV Vaccine: Background, Coverage & Benefits. 2007 2014 Available at: http://www.amchp.org/programsandtopics/AdolescentHealth/resources/Documents/adolescent-health-HPV.pdf.
- 49.U.S. Department of Health and Human Services The Affordable Care Act and Immunization. 2012 2014 Available at: http://www.hhs.gov/healthcare/facts/factsheets/2010/09/The-Affordable-Care-Act-and-Immunization.html.
- 50.Humiston SG, Rosenthal SL. Challenges to Vaccinating Adolescents: Vaccine Implementation Issues. The Pediatric Infectious Disease Journal. 2005;24:S134–S40. doi: 10.1097/01.inf.0000166161.12087.94. [DOI] [PubMed] [Google Scholar]