Abstract
Glutathione is generally accepted as the principal electron donor for dehydroascorbate (DHA) reduction. Moreover, both glutathione and DHA affect cell cycle progression in plant cells. But other mechanisms for DHA reduction have been proposed. To investigate the connection between DHA and glutathione, we have evaluated cellular ascorbate and glutathione concentrations and their redox status after addition of dehydroascorbate to medium of tobacco (Nicotiana tabacum) L. cv Bright Yellow-2 (BY-2) cells. Addition of 1 mm DHA did not change the endogenous glutathione concentration. Total glutathione depletion of BY-2 cells was achieved after 24-h incubation with 1 mm of the glutathione biosynthesis inhibitor l-buthionine sulfoximine. Even in these cells devoid of glutathione, complete uptake and internal reduction of 1 mm DHA was observed within 6 h, although the initial reduction rate was slower. Addition of DHA to a synchronized BY-2 culture, or depleting its glutathione content, had a synergistic effect on cell cycle progression. Moreover, increased intracellular glutathione concentrations did not prevent exogenous DHA from inducing a cell cycle shift. It is therefore concluded that, together with a glutathione-driven DHA reduction, a glutathione-independent pathway for DHA reduction exists in vivo, and that both compounds act independently in growth control.
Ascorbate (ASC) and glutathione (GSH) are well known antioxidants, participating in the cellular defense of plants against oxidative stress. Biotic and abiotic stress conditions therefore generally affect cellular ASC levels and/or the ASC redox status (defined here as the percentage of reduced ASC upon the total pool of ASC and the oxidized dehydroascorbate [DHA]). For example, the ASC content and redox status of shoots and roots of Phaseolus vulgaris is sensitive to zinc stress (Cuypers et al., 2001), and ASC is oxidized in tomato (Lycopersicon esculentum) plants infected with Botrytis cinerea (Kuźniak and Skłodowska, 1999, 2001). Especially in the apoplast, the ASC redox status is quite sensitive to stress conditions. Different types of environmental stress result in an increased oxidation of the apoplastic ASC pool (Castillo and Greppin, 1988; Luwe et al., 1993; Takahama, 1993; Gossett et al., 1994; Plöchl et al., 2000). In turn, GSH has been implicated in herbicide and heavy metal defense (Kömives et al., 1998). For example, GSH is oxidized after copper treatment in Lemna minor (Teisseire and Vernet, 2000) or under ozone fumigation in Spinacia oleracea (Luwe et al., 1993), and GSH metabolism is enhanced under different forms of stress (May and Leaver, 1993; Xiang and Olivier, 1998).
In any case, ASC and GSH are thought to be intimately connected. In the chloroplast, GSH functions as an electron donor for DHA reductase, regenerating ASC through DHA reduction (the so-called ascorbate-glutathione cycle, Foyer and Halliwell, 1976; Noctor and Foyer, 1998). The oxidized form of GSH (designated GSSG) that results from this reaction is subsequently reduced by an NADPH-dependent GSSG reductase. The components of this ASC regenerating pathway have also been demonstrated in the cytoplasm (Borracino et al., 1986; Noctor and Foyer, 1998). Moreover, transformed plants overexpressing GSSG reductase show higher foliar ASC levels and improved tolerance to oxidative stress. Conversely, reduced GSSG reductase activity resulted in increased stress sensitivity (Noctor and Foyer, 1998). Furthermore, the cellular GSH pool is drained upon addition of exogenous DHA, at least in roots of white lupin (Lupinus albus) and onion (Allium cepa; Paciolla et al., 2001). Last, recent experiments from Chen et al. (2003) show that enhanced expression of a GSH-dependent DHA reductase increases tissue concentrations of ASC.
In addition to their functioning as antioxidants, ASC and GSH are essential in different physiological phenomena in plant cells (May et al., 1998; Noctor et al., 1998; Arrigoni and De Tullio, 2000). For example, an ever-increasing body of evidence demonstrates the role of ASC and DHA in the regulation of cell growth and division (Potters et al., 2002). GSH has also been implicated in the regulation of plant and animal cell growth and division. GSH metabolism is intimately linked to the control of root hair development and root cell growth (Sánchez-Fernández et al., 1997). Normal cell cycle progression in a tobacco (Nicotiana tabacum) cell suspension was blocked after addition of l-buthionine sulfoximine (BSO). This compound is a nontoxic, highly specific inhibitor of γ-glutamylcysteine synthase, which is the first step in GSH biosynthesis (Griffith and Meister, 1979; May and Leaver, 1993; May et al., 1998; Vernoux et al., 2000). Similar findings have been reported in animal systems (Shaw and Chou, 1986; Atzori et al., 1994; Poot et al., 1995). Moreover, in exemplary cases ASC and GSH even are involved in cell cycle and growth control in similar ways; the size of the ASC pool in the Zea mays quiescent center is markedly decreased, compared to the pool in the meristematic cells close by (Kerk and Feldman, 1995), but also the level of GSH in the Arabidopsis quiescent center is markedly lower, again compared to the surrounding tissues (May et al., 1998). This suggests that ASC and GSH may be closely linked when it comes to growth control.
We have previously demonstrated that exogenous DHA, but not ASC, when added during the G1 phase of the tobacco L. cv Bright Yellow-2 (BY-2) cell cycle, is able to delay normal cell cycle progression (Potters et al., 2000). However, internal DHA concentrations were not affected by this treatment, whereas ASC levels increased, suggesting a rapid reduction of DHA to ASC. The DHA-mediated increase of cellular ASC and effect on cell cycle progression shows an interesting specificity. A mere increase of cellular ASC may result in faster cell proliferation rates (Liso et al., 1988; Arrigoni et al., 1989; Innocenti et al., 1990; Kerk and Feldman, 1995; Davey et al., 1999; De Pinto et al., 1999) but has never been reported to delay the cell cycle. We therefore suggested that the fact that the cell's need to invest in DHA reduction, rather than the increased levels of ASC, may influence cell cycle progression.
This system opens a way to assess the role of the GSH/GSSG redox pair in the DHA-mediated halt in cell cycle progression in BY-2 suspension cells. Hopefully, it provides information on the extent of the intimate entanglement of ASC and GSH, in terms of DHA reduction and in terms of growth control. The aim of this work, therefore, is to challenge the hypothesis that GSH is the first and foremost reductant for DHA, and that the action of either ASC or GSH on a given physiological phenomenon should always involve changes in the other component's concentration or redox status.
RESULTS
Effect of DHA Addition on GSH Redox Status in BY-2 Cells
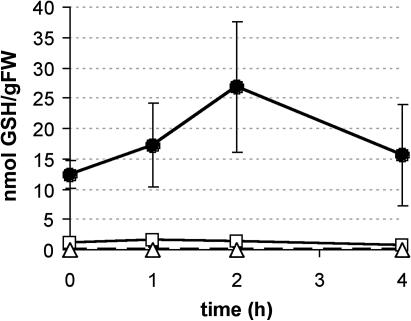
Addition of 1 mm DHA to the medium of an exponentially growing BY-2 culture led to a strong increase in intracellular ASC levels. Although only the oxidized form was taken up, no increase in intracellular DHA concentration could be observed, suggesting a rapid uptake and internal reduction (Potters et al., 2000). To study the contribution of GSH in this DHA reduction, changes in intracellular ASC, DHA, GSH, and GSSG levels were followed after addition of 1 mm DHA to an exponentially growing BY-2 culture. Samples were collected with 1-h intervals, with the “0-h” sample taken immediately before DHA addition. A control culture, where extra medium was added instead of DHA, was sampled as well. Although the internal ASC concentration increased 20-fold (data not shown for this set of experiments; see Fig. 4), no significant change could be observed in the intracellular GSH pool of control cells and DHA-treated cells up to 6 h after treatment (P < 0.05; Fig. 1). In addition, no changes were observed in the total amount of glutathione (GSH plus GSSG), or in its redox status (data not shown). The high variability of our data is surprising. However, this variation is for the most part due to variation between different cultures, since sampling the same culture flask produced fairly comparable data. Since the GSH content in BY-2 cells is drastically decreased during this period, as is GSSG reductase (de Pinto et al., 2000), this may cause the huge differences between cell cultures.
Figure 4.
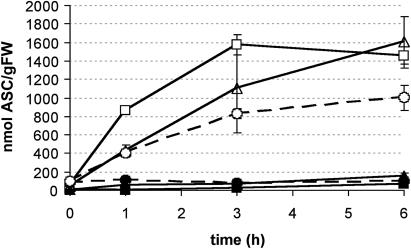
ASC generation in GSH-depleted cells. Intracellular ASC content (black symbols, no DHA added; white symbols, after addition of 1 mm DHA) in control cultures (squares), in cultures treated with BSO (triangles) or DEM (circles). The T = 0 point is the point at which DHA was added and represents also the start of sampling, as in Figure 3, to permit comparison. BSO has been added 24 h before, DEM 2 h before t = 0. Both compounds remained in the medium during the remainder of the experiment. Results presented here are the average of two independent results; bars indicate se.
Figure 1.
DHA addition has no effect on the intracellular GSH concentration. Intracellular GSH content in control conditions (black diamonds) or after addition of 1 mm DHA (white squares). Average of seven independent culture flasks (except for time [t] = 3 h, where n = 3). Bars indicate se; t0 = immediately after DHA addition.
Nitrosourea Fails to Inhibit the Plant GSSG Reductase But Causes Cell Death
The above-presented results possibly indicate that GSH may not be involved in the rapid reduction of DHA taken up by the cells. An alternative explanation is that GSSG may itself have been rapidly re-reduced to GSH masking changes in its redox status, even after the first hour of sampling. To evaluate this possibility, we applied 1,3-bis(2-chloroethyl)-1-nitrosourea (BCNU), a known inhibitor of the animal GSSG reductase (for example, Kehrer, 1983; Harlan et al., 1984; Kaneko et al., 2002) and its plant counterpart (Gullner and Dodge, 2000; Piquery et al., 2002).
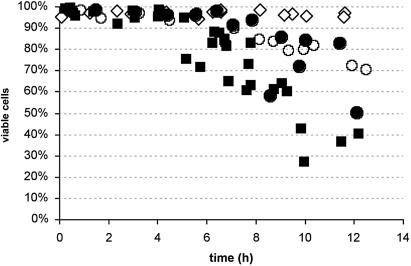
Concentrations ranging from 50 to 500 μm of BCNU were added to the BY-2 culture medium in the absence or presence of 1 mm DHA, and samples were collected every hour after addition. In animal cells, concentrations of 500 μm BCNU were effective in GSSG reductase inhibition (Riley, 1984; Ueda-Kawamitsu et al., 2002). In our BY-2 culture, even after addition of 500 μm NU, no changes were observed in the cellular GSH or GSSG content, as compared to the control cells, even after addition of 500 μm BCNU (data not shown). Unfortunately, the cells started to die after 6 h of exposure to the compound (Fig. 2), which is just after the time interval during which the culture was sampled. The rate at which the cells died was concentration dependent (percentages of viable cells after addition of both 500 and 400 μm BCNU are shown in Fig. 2). Cell viability was indistinguishable from the control culture even after 24 h of incubation with 250 μm BCNU (or all concentrations below). As an aside, addition of 1 mm DHA seemed to alleviate the cell death rates after application of 500 mm BCNU. However, this trend could not be confirmed statistically. Furthermore, DHA has no effect on cell death upon application together with lower BCNU concentrations (400 μm). As a control experiment, we have run a native gel confirming that BCNU does inhibit GSSG reductase, but only slightly, even at the 500-μm concentration (data not shown).
Figure 2.
BCNU affects plant cell viability. Cell viability, as measured in Evans blue-stained cell aliquots, of cells under control conditions (white diamonds), or after addition of 400 μm (circles) or 500 μm (squares) BCNU (black symbols) or BCNU plus 1 mm DHA (white symbols) to the cell population. Values (measured in four independent cultures) are all represented on the chart to properly indicate trends in the data. Abscissa represents time after the start of the experiment.
Uptake and Internal Reduction of DHA Proceeds Even in GSH-Depleted Cells
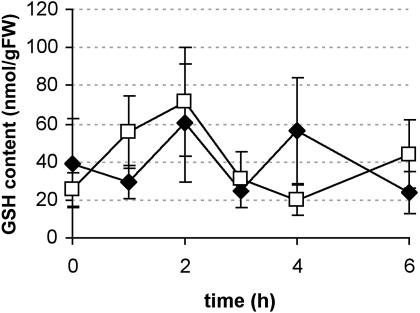
As an alternative approach to investigate the role of GSH in the rapid reduction of intracellular DHA, we attempted to decrease the cellular GSH content. Diethylmaleate (DEM) covalently binds GSH, thereby lowering the physiologically active intracellular levels (Mulder and Ouwerkerk-Mahadevan, 1997; Nagai et al., 2002). Pretreatment of BY-2 cells for 2 h with 1 mm DEM decreases the intracellular GSH content in BY-2 cells to one tenth of the control value (Fig. 3; 0 h meaning 2 h after addition of DEM, to permit comparison with Fig. 4).
Figure 3.
Depletion of the GSH pool in BY-2 cells with DEM and BSO. Intracellular GSH content in control cultures (black circles), or in cultures treated with 1 mm DEM (white squares) or with 1 mm BSO (white triangles; dashed line). The t = 0 point is the start of sample analysis, as in Figure 4, to permit comparison. BSO has been added 24 h before, DEM 2 h before t = 0. Both compounds remained in the medium for the remainder of the experiment. Results are the average of two independent culture flasks; bars show se.
An alternative method to decrease the intracellular GSH content involves the addition of the GSH biosynthesis inhibitor BSO. Addition of 1 mm BSO, 24 h before the start of the actual measurements, resulted in the complete depletion of cellular GSH (Fig 3; 0 h meaning 24 h after addition of BSO, to permit comparison with Fig. 4). Cell viability was not affected, neither by BSO nor by DEM treatments, up to 36 h and 14 h after the addition, respectively (all values in treated and control cultures between 90% and 95%).
The effect of both compounds on ASC metabolism was checked as a control experiment. Apparently, endogenous ASC concentrations in BY-2 cells were not affected by DEM or BSO addition only (Fig. 4). The effect of DHA addition was tested on DEM- and BSO-treated cells. DEM and BSO were kept in the culture medium after DHA addition to ensure continuously low or zero levels of GSH in the cells (compared with Fig. 3). Addition of DHA to control cells led to a 20-fold increase of the intracellular ASC concentration within 3 h (Fig. 4). No changes were observed in the internal DHA concentration, confirming the intracellular capacity to efficiently reduce DHA (as described before, Potters et al., 2000). The accumulation of intracellular ASC was significantly lower 1 h after DHA treatment of cells pretreated with DEM and BSO (P < 0.05, Fig. 4). However, over the course of the experiment, all DHA was effectively taken up into the cells and reduced to ASC. Six hours after DHA treatment the ASC content of the DEM- or BSO-treated cells was not significantly different from the ASC content in the control cells (again, P < 0.05). These results demonstrated that cells effectively depleted from GSH levels by DEM or BSO treatment were still capable of reducing high intracellular DHA levels, although at different initial rates when compared to control cells. Measurements of the DHA uptake (transport) rates were performed, showing that neither BSO nor DEM did affect the DHA uptake capacity of the cells (data not shown).
Effect of GSH Depletion or Addition on Cell Cycle Progression
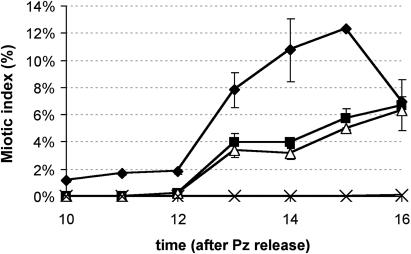
To investigate the effect of altered intracellular GSH levels on the DHA-mediated arrest in cell cycle progression, synchronized BY-2 cells were treated with DHA and BSO. DHA treatment (1 mm) of cells synchronously passing through the G1 phase resulted in a strong delay in cell division, confirming our previous results (Fig. 5; Potters et al., 2000). Interestingly, treatment of synchronized cells with 1 mm BSO, effectively lowering the intracellular GSH concentrations, delayed cell cycle progression to a similar extent (Fig. 5). Simultaneous application of DHA and BSO resulted in the complete block of mitotic activity.
Figure 5.
Effect of DHA and BSO on the cell cycle. Changes in cell cycle progression after addition of 1 mm DHA (black squares), 1 mm BSO (white triangles), or both (crosses) versus control culture (black diamonds). Abscissa represents the time after release from the propyzamide-induced cell cycle block (at which time point cells are restrained in M phase). The average of two synchronized cultures is presented here; bars indicate se.
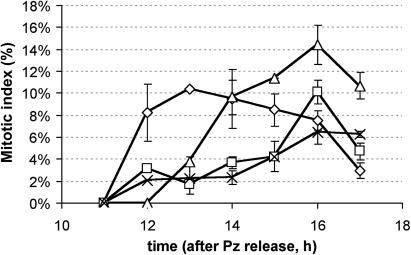
To further determine the role of intracellular GSH in cell cycle progression, 1 mm of GSH was added to M-phase synchronized cells, in the absence and presence of DHA. GSH is readily taken up by the cell (Vernoux et al., 2000). As observed before, addition of 1 mm DHA caused a delay in the cell cycle progression (Fig. 6). The combined addition of 1 mm GSH and 1 mm DHA resulted in a delay similar to that with DHA alone. Surprisingly, however, addition of extra GSH alone also caused a delay in the cell cycle progression, though not as large as that caused by DHA addition.
Figure 6.
Effect of DHA and GSH on the cell cycle. Cell cycle progression in synchronized cells, under control circumstances (diamonds) or after addition of 1 mm DHA (squares), 1 mm GSH (triangles) or 1 mm DHA plus 1 mm GSH (crosses). Abscissa represents the time after release from the propyzamide-induced cell cycle block (at which time point cells are restrained in M phase). Values are the average of three independent cultures; bars indicate se.
DISCUSSION
Without GSH, BY-2 Cells Still Possess DHA Reduction Capacity
GSH is a well-known antioxidant, implicated in the enzymatic regeneration of ASC through the reduction of DHA (Foyer and Halliwell, 1976, 1977; Jiménez et al., 1997; Noctor and Foyer, 1998). This role is, for example, illustrated by the decrease of GSH levels in onion and white lupin roots upon DHA treatment (Paciolla et al., 2001), and by the identification of different GSH-consuming DHA reductases (for review, see Potters et al., 2002). On the other hand, different proteins have been proposed to carry out DHA reduction without using GSH in animals (for review, see Potters et al., 2002), and plant thioredoxins apparently show DHA reducing activity in a native gel assay (Morell et al., 1997; Foyer and Mullineaux, 1998). In general, it is supposed that thiol-containing proteins, and most likely those containing a dicysteinyl motif, are capable of fulfilling this function. (Paciolla et al., 2001; De Tullio et al., 2002; Wilson, 2002; Banhegyi et al., 2003). This raised the questions about the identity of the possible other reductant(s) of DHA and about the extent of the contribution of GSH in this set of DHA reducing agents.
The first part of this work involved the verification of GSH as an electron donor for DHA. In previous work, we have demonstrated that upon addition of a large amount of DHA (100 μmol in 100 mL BY-2 suspension), all DHA is taken up and internally reduced. Remarkably (and contrary to the results of Paciolla et al., 2001), this treatment did not affect the GSH pool in our BY-2 system (Fig. 1). Several possibilities could explain this discrepancy: a rapid turnover of cellular GSH, either by reduction of GSSG or by an increased biosynthesis, or because GSH has a limited role in DHA reduction in these cells.
To assess the role of GSH in DHA reduction, we have tried to influence cellular GSH levels by “scavenging” GSH with DEM, by inhibiting GSH biosynthesis with BSO, and by blocking GSSG reductase activity by BCNU. BCNU is a good inhibitor for the animal GSSG reductase and has even some effect on the plant enzyme, but, unfortunately, prolonged exposure of BY-2 cells caused the cells to die (Fig. 2), and a concentration of 500 μm, which is quite effective in animal cells, does not affect GSH levels in BY-2 cells. It should therefore be questioned whether this compound is as useful in plants as in animal systems. Perhaps BCNU should only be used for long-term events (as performed by Piquery et al., 2002), which was impossible in our system. The cell death we encountered seems a rather unspecific event.
Scavenging of cellular GSH levels by DEM was effective in the BY-2 cells and reduced free GSH concentrations to 10% of that in control cells. Since only redox active molecules are detected in our HPLC analysis, our measurements indicate the effective reduction of the reducing capacity of GSH in the cells. Although the reduction rate of DHA to ASC was lowered by the DEM treatment, similar levels of reduction were reached as in the untreated cells at the end of the experiment (Fig. 4). The possibility that even small GSH concentrations might have been enough to reduce DHA due to a fast re-reduction of GSSG cannot be ruled out completely.
Experiments using the GSH biosynthesis inhibitor, BSO, however, provided further evidence that GSH is not the primary reductant for the observed DHA reduction. After a 24-h BSO treatment, cells were completely devoid of GSH (Fig. 3). Similar to the DEM-treated cells, BSO treatment did not affect the uptake rates but resulted in slower reduction of intracellular DHA to ASC (Fig. 4). The observations that addition of DHA did not change intracellular GSH levels and that depletion of intracellular GSH did not affect the reduction of DHA to ASC strongly support the idea that GSH is not the sole, and maybe even not the primary, reductant for DHA in BY-2 cells.
For example, the observation that the appearance of ASC was slower in GSH-depleted BY-2 cells (Fig. 4) can be explained in two ways (which are not mutually exclusive). It is possible that the observed DHA reduction involves different pathways, including one which is GSH dependent. Both in the case of DEM- and BSO-treated cells, DHA reduction proceeds significantly slower than under control conditions (Fig. 4). It might be argued that this difference is due to the lack of GSH in these cells. In that case we estimate that, based on the comparison of reduction rates, around 50% of the incoming DHA is being reduced with the aid of GSH. One may even speculate that the GSH-independent reduction of DHA is proportional to the area under the BSO-plus-DHA curve in Fig. 4, and the area between the control curve (1 mm DHA) and the BSO-plus-DHA curve proportional to the GSH-dependent DHA reduction. The fact that there seems to be a difference (not statistically proven) between the DEM-plus-DHA and the BSO-plus-DHA curve might then be attributed to the fact that DEM also impacts on other thiol groups.
On the other hand, it is also possible that depleting the GSH pool forces the reductants involved in the reduction of DHA to be used in other physiological processes slowing down the DHA reduction. Although our data do not allow us to distinguish between these two possibilities, this does not affect the major conclusion of the involvement of a GSH-independent pathway in cellular DHA reduction. Any distinction between both explanations requires a definitive identification of the components of the DHA reduction pathways.
Similar experiments performed on different animal cell types before have demonstrated the existence of different mechanisms for DHA reduction. For example, DHA uptake and internal reduction were severely hampered in GSH-depleted human (HepG2) and rat (H4IIE) liver cells (Li et al., 2001). DHA reduction was also shown to be impaired in GSH-depleted erythrocytes (May et al., 2001b) or endothelial cells (May et al., 2001a), suggesting the involvement of GSH in DHA reduction. However, GSH did apparently not play any role in ASC recycling in human HaCaT keratinocytes (Savini et al., 2000), or in HL-60 cells (Guaiquil et al., 1997). It should therefore be interesting to check the involvement of GSH in DHA reduction in different plant cell types. Indicative for the presence of different mechanisms in different tissues are the results on onion and white lupin root tips, demonstrating that GSH is involved in DHA reduction (Paciolla et al., 2001). It must be noted, however, that in BY-2 cells, this effect on thiol groups could not be duplicated (G. Potters and N. Horemans, unpublished results).
ASC and GSH Are Not Linked for Cell Cycle Control
Using the tools to affect cellular GSH levels, we also explored the connection between the effect of DHA on cell cycle progression and the possible connection to GSH. GSH depletion as a result of a BSO treatment blocks the cell cycle in BY-2 cells (Potters et al., 2000). Therefore, we wanted to check whether the observed DHA-mediated delay of the cell cycle was in fact mediated by a change in GSH content (or redox status). However, an increase in internal GSH did not prevent the observed DHA-mediated cell cycle delay (Fig. 6). Moreover, GSH depletion and DHA addition were shown to have an additive effect, strongly suggesting that both compounds act in a different pathway, each ultimately influencing the cell cycle in its own fashion. Therefore, previous observations demonstrating the involvement of ASC and GSH in cell cycle progression (Liso et al., 1988; De Gara and Tommasi, 1999; De Pinto et al., 1999, 2000; Kato and Esaka, 1999; Potters et al., 2000; Vernoux et al., 2000, Paciolla et al., 2001) might probably also be the result of independent pathways. Also, the cells are completely devoid of GSH—there is no GSH left to be influenced by the exogenous DHA. That DHA still affects the cell cycle progression under these conditions is an additional indication for the general independence of both compounds.
Surprisingly, we also noted that the mere addition of GSH did influence the cell cycle slightly, although in an inhibitory way. This is in apparent contradiction to the results of Vernoux et al. (2000) or Xiang et al. (2001)—where in both instances a lower GSH content in the cell is linked to an inhibition of cell division—as well as to our own results (Fig. 5). This indicates that, more than the mere presence of a compound, its proper concentration is crucial for optimal functioning of the cell's physiology. If this equilibrium is shifted in either direction, the cell loses its balance and normal functioning is disturbed or even disrupted. In the actual case of GSH, Creissen et al. (1999) already demonstrated that plants overproducing GSH are surprisingly more sensitive to oxidative stress.
Possible Alternative Mechanisms for DHA Reduction and Growth Control?
Our hypothesis is—albeit indirectly—also supported by other results; plants with 20% of the control GSH levels did not show altered tolerance to oxidative stress (May et al., 1998). Apparently, defense against stress situations sometimes occurs irrespective of the GSH concentration. In addition, plants with only 5% of the wild-type GSH level grew normally (Xiang et al., 2001). On the other hand, in the Arabidopsis vtc-1 mutant (which contains only 30% of a wild-type plant's ASC level), both stress resistance and growth are affected (Veljovic-Jovanovic et al., 2001). This also suggests that GSH and ASC are not necessarily linked to one another and that they rather have distinct functions to fulfill.
Of course, the question remains as to which compound(s) will assist in GSSG-independent DHA reduction. Both in animal and plant cells, the thioredoxin/thioredoxin reductase system, glutaredoxin, protein disulfide isomerase, and even less-known proteins all have been suggested to possess DHA-reduction activity (Wells et al., 1990; Del Bello et al., 1994; Trümper et al., 1994; Hou and Lin, 1997; May et al., 1997; Morell et al., 1997; Hou et al., 1999; Potters et al., 2002). Therefore different sources of electrons for DHA reduction should be considered in future work concerning the effects of ASC and DHA on cell physiology.
In any case, the involvement of the thioredoxin/thioredoxin reductase system may be a suitable working hypothesis, explaining the inhibition of cell cycle progression by DHA. Apparently, DHA is only capable of slowing down cell cycle progression when added in G1 phase. Addition of 1 mm DHA in G2 phase does not affect the cell cycle at all (G. Potters and N. Horemans, unpublished results). This suggests that DHA influences the cell cycle through processes which are specific for either G1 or S phase. Interestingly, the thioredoxin/thioredoxin reductase system is involved in the deliverance of deoxyribonucleotides (obviously necessary for the passage through S phase). A competition for reducing equivalents between DHA and ribonucleotides, which both need to be reduced, might slow down the supply for deoxyribonucleotides, and therefore effectively halts S phase progression. Unfortunately, up to now no known inhibitor of the animal thioredoxin/thioredoxin reductase system has been proven to inhibit DHA reduction in our BY-2 cells, and this hypothesis remains highly uncertain.
On the other hand, the thioredoxin/thioredoxin reductase system has even in plants been shown to interact directly or indirectly with DHA, both on a protein level and on an mRNA level. For example, both DHA and GSSG deactivated thioredoxin f-activated enzymes (Nishizawa and Buchanan, 1981), and ASC influences the expression of genes, coding for thioredoxin-activated or -repressed proteins (Kiddle et al., 2003). Further research is thus needed to look for the proper integration of ASC/DHA metabolism in thiol-mediated redox signaling and regulation.
CONCLUSION
Our results demonstrate that, similar to animal cells, different pathways for DHA reduction exist in plant cells, the nature of which remains unknown. It is true that one cannot ignore the many examples where a functional link between GSH and ASC metabolism has been crucial in understanding what happens (see the many examples listed in the introduction). Indeed, GSH is probably a team player in the whole network of DHA reducing reactions. Nevertheless, we feel that the question about DHA reduction should be broadened; whereas GSH and DHA are most likely connected, for example, in stress resistance, their influence on growth regulation may be mediated by other, distinct pathways. As an aside, we also suggest that GSH and ASC have apparently a distinct function in the regulation of cell cycle progression and may each be part of a different oxidative stress-sensitive pathway (Reichheld et al., 1999). Further research will have to identify and specify the different components on which each molecule acts.
MATERIALS AND METHODS
Plant Material
A tobacco (Nicotiana tabacum) L. cv Bright Yellow-2 (BY-2) cell suspension was propagated as described by Nagata et al. (1992), in Murashige and Skoog (1962) medium, supplemented with 0.2 g L−1 KH2PO4, 30 g L−1 sucrose, 0.2 mg L−1 2,4-dichlorophenoxyacetic acid, 0.01 g L−1 thiamine-HCl, and 0.1 g L−1 myoinositol, set at pH 5.8 with KOH. Cells were cultured in a rotary shaker at 130 rpm and at 27°C, in the dark. Weekly subculturing was initiated by transferring 4 mL of a 7-d-old stationary culture to 100 mL of fresh medium. DHA uptake experiments were performed with cells in full exponential growth (4 d after inoculation) to ensure a valid comparison with a synchronized (dividing) culture, as used in the cell cycle experiments. Treatments were either added exactly at the beginning of the measurements (1 mm DHA, 1 mm ASC, BCNU) or at the time specified in the “Results” section (1 mm BSO, 1 mm DEM).
HPLC Measurement of ASC and GSH
Intracellular and extracellular ASC and GSH content was determined by reversed phase HPLC separation, followed by amperometric detection. Cells were collected on a Büchner filter (Haldenwanger, Berlin) at intervals of 1 h. Aliquots of around 0.3 g fresh weight were resuspended in 1 mL of extraction medium, consisting of 6% m-phosphoric acid and 1 mm EDTA. Insoluble polyvinylpyrridoline (1%) was added, and the cells were subsequently snap-frozen in liquid nitrogen. ASC, DHA, GSH, and GSSG were subsequently extracted through three cycles of freezing and thawing; the homogenate was centrifuged at 50,000g for 15 min at 4°C. ASC determination in the supernatant was carried out by reverse phase HPLC (RP type C-18 column, LiChroSpher, Alltech, Deerfield, IL; isocratic pump, 0.8 mL min−1, LC-10ADVP, Shimadzu, Columbia, MD) coupled to an electrochemical detection system (reference potential 1,000 mV; supplied by Prof. Dr. L. Nagels, University of Antwerp, Belgium). Chromatogram analysis was performed with the Class VP software package (ClassVP 5.0, Shimadzu). Samples were diluted 1:4 in mobile phase (2 mm KCl, pH set at 2.5 with H3PO4) prior to injection. Total ASC (ASC plus DHA) was determined by reducing 100 μL of each sample with 100 μL of a 200-mm dithiothreitol plus 400-mm Tris solution (at a final pH of 6, checked in random samples). After 1-h incubation at room temperature, samples were acidified again by addition of 300-μL mobile phase and kept at 4°C until injection. The DHA concentration was estimated as the difference between the reduced and total ASC concentration.
Synchronization of BY-2 Cells
BY-2 cell cultures were blocked in their cell cycle with aphidicolin and propyzamide (Nagata et al., 1992; Samuels et al., 1998) and released when synchronized at the beginning of M phase. Two hours after propyzamide release, during G1 phase, 1 mm DHA, 1 mm GSH and/or 1 mm BSO were added to the cell culture. The concentrations of these compounds were chosen in accordance to existing literature (Potters et al., 2000; Vernoux et al., 2000).
Determination of Mitotic Index and Viability
Every hour after synchronization, a 500-μL aliquot of the cell suspension was taken from the culture and put on ice to allow the cells to sediment. The remaining medium was removed 10 min after harvesting. The cells were then fixed in a 3:1 ethanol:acetic acid mixture and stored at 4°C until further analysis. Mitotic indices were determined by staining the chromatin with orcein (2% w/v in a 1:1 lactic acid:propionic acid mixture) and determining the percentage of cells displaying one of the mitotic phases under a bright field microscope. At least 500 cells were counted in every sample.
To assess the viability of the cells after different treatments, Evans blue was added (in a final concentration of 0.5%) to 500-μL aliquots of cell suspension. Stained cells were considered dead; 500 cells were counted in each sample.
Uptake of DHA
Uptake of radiolabeled [14C]ASC and [14C]DHA into protoplasts of BY-2 cells was performed as described by Horemans et al. (1998).
Native PAGE GSSG Reductase Assay
Soluble proteins were extracted from 2 g of a 3-d-old BY-2 culture with 20 mL of the buffer described in De Gara et al. (1997). Native gel electrophoresis and subsequent staining for GSSG reductase activity was performed according to Foyer et al. (1991). Every gel was cut in three pieces; before the actual activity stain, each gel strip was soaked for 15 min in buffer, buffer plus 250 μm BCNU, or buffer plus 500 μm BCNU. These concentrations of BCNU were also added to the staining mixture afterwards.
Acknowledgments
G.P. and N.H. are postdoctoral researchers at the Fund for Scientific Research, Flanders (F.W.O.-Vlaanderen). Mr. Eddy Biebaut is gratefully acknowledged for his technical assistance. Mrs. Inge Van Dyck was a great help in preparing the figures. Dr. J. Kapila is acknowledged for helpful discussion. This paper is a contribution of the University of Nebraska Agricultural Research Division, Lincoln, Nebraska.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.103.033548.
References
- Arrigoni O, Bitonti MB, Cozza R, Innocenti AM, Liso R, Veltri R (1989) Ascorbic acid effect on pericycle cell line in Allium cepa root. Caryologia 42: 213–216 [Google Scholar]
- Arrigoni O, De Tullio MC (2000) The role of ascorbic acid in cell metabolism: between gene-directed functions and unpredictable chemical reactions. J Plant Physiol 157: 481–488 [Google Scholar]
- Atzori L, Dypbukt JM, Hybbinette SS, Moldeus P, Grafstrom RC (1994) Modifications of cellular thiols during growth and squamous differentiation of cultured human bronchial epithelial cells. Exp Cell Res 211: 115–120 [DOI] [PubMed] [Google Scholar]
- Bánhegyi G, Csala M, Szarka A, Varsányi M, Benedetti A, Mandl J (2003) Role of ascorbate in oxidative protein folding. Biofactors 17: 37–46 [DOI] [PubMed] [Google Scholar]
- Borracino G, Dipierro S, Arrigoni O (1986) Purification and properties of ascorbate free radical reductase from potato tubers. Planta 167: 521–526 [DOI] [PubMed] [Google Scholar]
- Castillo FJ, Greppin H (1988) Extracellular ascorbic acid and enzyme activities related to ascorbic acid metabolism in Sedum album L. leaves after ozone exposure. Environ Exp Bot 28: 231–238 [Google Scholar]
- Chen Z, Young TE, Ling J, Chang SC, Gallie DR (2003) Increasing vitamin C content of plants through enhanced ascorbate recycling. Proc Natl Acad Sci USA 100: 3525–3530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creissen G, Firmin J, Fryer M, Kular B, Leyland N, Reynolds H, Pastori G, Wellburn F, Baker N, Wellburn A, Mullineaux P (1999) Elevated glutathione biosynthetic capacity in the chloroplasts of transgenic tobacco plants paradoxically causes increased oxidative stress. Plant Cell 11: 1277–1292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuypers A, Vangronsveld J, Clijsters H (2001) The redox status of plant cells (AsA and GSH) is sensitive to zinc imposed oxidative stress in roots and primary leaves of Phaseolus vulgaris. Plant Physiol Biochem 39: 657–664 [Google Scholar]
- Davey MW, Gilot C, Persiau G, Østergaard J, Han Y, Bauw GC, Van Montagu MC (1999) Ascorbate biosynthesis in Arabidopsis cell suspension culture. Plant Physiol 121: 535–544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Gara L, de Pinto MC, Arrigoni O (1997) Ascorbate synthesis and ascorbate peroxidase activity during the early stage of wheat germination. Physiol Plant 100: 894–900 [Google Scholar]
- De Gara L, Tommasi F (1999) Ascorbate redox enzymes: a network of reactions involved in plant development. Recent Res Devel Phytochem 3: 1–15 [Google Scholar]
- De Pinto MC, Francis D, De Gara L (1999) The redox state of the ascorbate-dehydroascorbate pair as a specific sensor of cell division in tobacco BY-2 cells. Protoplasma 209: 90–97 [DOI] [PubMed] [Google Scholar]
- De Pinto MC, Tommasi F, De Gara L (2000) Enzymes of the ascorbate biosynthesis and ascorbate-glutathione cycle in cultured cells of tobacco Bright Yellow-2. Plant Physiol Biochem 38: 541–550 [Google Scholar]
- De Tullio MC, Paciolla C, Arrigoni O (2002) Identification and analysis of proteins sharing dehydroascorbate reductase activity. Biol Plant 45: 145–147 [Google Scholar]
- Del Bello B, Maellaro E, Sugherini L, Santucci A, Comporti M, Casini AF (1994) Purification of NADPH-dependent dehydroascorbate reductase from rat liver and its identification with 3α-hydroxysteroid dehydrogenase. Biochem J 304: 385–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foyer CH, Halliwell B (1976) Presence of glutathione and glutathione reductase in chloroplasts: a proposed role in ascorbic acid metabolism. Planta 133: 21–25 [DOI] [PubMed] [Google Scholar]
- Foyer CH, Halliwell B (1977) Purification and properties of dehydroascorbate reductase from spinach leaves. Phytochemistry 16: 1347–1350 [Google Scholar]
- Foyer CH, Lelandais M, Galap C, Kunert KJ (1991) Effects of elevated cytosolic glutathione reductase activity on the cellular glutathione pool and photosynthesis in leaves under normal and stress conditions. Plant Physiol 97: 863–872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foyer CH, Mullineaux PM (1998) The presence of dehydroascorbate and dehydroascorbate reductase in plant tissues. FEBS Lett 425: 528–529 [DOI] [PubMed] [Google Scholar]
- Gossett DR, Millhollon EP, Lucas MC (1994) Antioxidant response to NaCl stress in salt-tolerant and salt-sensitive genotypes of cotton. Crop Sci 34: 706–714 [Google Scholar]
- Griffith OW, Meister A (1979) Potent and specific inhibition of glutathione synthesis by buthionine sulfoximine (S-n-butyl homocysteine sulfoximine). J Biol Chem 254: 7558–7560 [PubMed] [Google Scholar]
- Guaiquil VH, Farber CM, Golde DW, Vera JC (1997) Efficient transport and accumulation of vitamin C in HL-60 cells depleted of glutathione. J Biol Chem 272: 9915–9921 [DOI] [PubMed] [Google Scholar]
- Gullner G, Dodge AD (2000) Accumulation of glutathione in pea leaf discs exposed to the photo-oxidative herbicides acifluorfen and 5-aminolevulinic acid. J Plant Physiol 156: 111–117 [Google Scholar]
- Harlan JM, Levine JD, Callahan KS, Schwartz BR, Harker LA (1984) Glutathione redox cycle protects cultured endothelial cells against lysis by extracellularly generated hydrogen peroxide. J Clin Invest 73: 706–713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horemans N, Potters G, Caubergs RJ, Asard H (1998) Transport of ascorbate into protoplasts of Nicotiana tabacum Bright Yellow-2 cell line. Protoplasma 205: 114–121 [Google Scholar]
- Hou WC, Chen HJ, Lin YH (1999) Dioscorins, the major tuber storage proteins of yam (Dioscorea batatas Decne), with dehydroascorbate reductase and monodehydroascorbate reductase activities. Plant Sci 149: 151–156 [Google Scholar]
- Hou WC, Lin YH (1997) Dehydroascorbate reductase and monodehydroascorbate reductase activities of trypsin inhibitors, the major sweet potato (Ipomoea batatas (L.) Lam) root storage protein. Plant Sci 128: 151–158 [Google Scholar]
- Innocenti AM, Bitonti MB, Arrigoni O, Liso R (1990) The size of quiescent centre in roots of Allium cepa L. grown with ascorbic acid. New Phytol 110: 507–509 [DOI] [PubMed] [Google Scholar]
- Jiménez A, Hernandez JA, del Rio LA, Sevilla F (1997) Evidence for the presence of the ascorbate-glutathione cycle in mitochondria and peroxisomes of pea leaves. Plant Physiol 114: 275–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko T, Iuchi Y, Kobayashi T, Fujii T, Saito H, Kurachi H, Fujii J (2002) The expression of glutathione reductase in the male reproductive system of rats supports the enzymatic basis of glutathione function in spermatogenesis. Eur J Biochem 269: 1570–1578 [DOI] [PubMed] [Google Scholar]
- Kato N, Esaka M (1999) Changes in ascorbate oxidase gene expression and ascorbate levels in cell division and cell elongation in tobacco cells. Physiol Plant 105: 321–329 [Google Scholar]
- Kehrer JP (1983) The effect of BCNU (carmustine) on tissue glutathione reductase activity. Toxicol Lett 17: 63–68 [DOI] [PubMed] [Google Scholar]
- Kerk NM, Feldman LJ (1995) A biochemical model for the initiation and maintenance of the quiescent center: implications for organization of root meristems. Development 121: 2825–2833 [Google Scholar]
- Kiddle G, Pastori GM, Bernard S, Pignocchi C, Antoniw J, Verrier PJ, Foyer CH (2003) Effects of leaf ascorbate content on defense and photosynthesis gene expression in Arabidopsis thaliana. Antioxid Redox Signal 5: 23–32 [DOI] [PubMed] [Google Scholar]
- Kömives T, Gullner G, Király Z (1998) Role of glutathione and glutathione-related enzymes in response of plants to environmental stress. In P Csermely, ed, Stress of Life: From Molecules to Man, Vol. 851. The New York Academy of Sciences, New York, pp. 251–258
- Kuźniak E, Skłodowska M (1999) The effect of Botrytis cinerea infection on ascorbate-glutathione cycle in tomato leaves. Plant Sci 148: 69–76 [Google Scholar]
- Kuźniak E, Skłodowska M (2001) Ascorbate, glutathione and related enzymes in chloroplasts of tomato infected by Botrytis cinerea. Plant Sci 160: 723–731 [DOI] [PubMed] [Google Scholar]
- Li X, Qu ZC, May JM (2001) GSH is required to recycle ascorbic acid in cultured liver cell lines. Antiox Redox Signal 3: 1089–1097 [DOI] [PubMed] [Google Scholar]
- Liso R, Innocenti AM, Bitonti MB, Arrigoni O (1988) Ascorbic acid-induced progression of quiescent centre cells from G1 to S phase. New Phytol 110: 469–471 [Google Scholar]
- Luwe MWF, Takahama U, Heber U (1993) Role of ascorbate in detoxifying ozone in the apoplast of spinach (Spinacia oleracea L.) leaves. Plant Physiol 101: 969–976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- May MJ, Leaver CJ (1993) Oxidative stimulation of glutathione synthesis in Arabidopsis thaliana suspension cultures. Plant Physiol 103: 621–627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- May JM, Mendiretta S, Hill KE, Burk RF (1997) Reduction of dehydroascorbate to ascorbate by the selenoenzyme thioredoxin reductase. J Biol Chem 272: 22607–22610 [DOI] [PubMed] [Google Scholar]
- May JM, Qu Z, Li X (2001. a) Requirement for GSH in recycling of ascorbic acid in endothelial cells. Biochem Pharmacol 62: 873–881 [DOI] [PubMed] [Google Scholar]
- May JM, Qu Z, Morrow JD (2001. b) Mechanisms of ascorbic acid recycling in human erythrocytes. Biochim Biophys Acta 1528: 159–166 [DOI] [PubMed] [Google Scholar]
- May MJ, Vernoux T, Leaver C, Van Montagu M, Inzé D (1998) Glutathione homeostasis in plants: implications for environmental sensing and plant development. J Exp Bot 49: 649–667 [Google Scholar]
- Morell S, Follmann H, De Tullio M, Häberlein I (1997) Dehydroascorbate and dehydroascorbate reductase are phantom indicators of oxidative stress in plants. FEBS Lett 414: 567–570 [DOI] [PubMed] [Google Scholar]
- Mulder GJ, Ouwerkerk-Mahadevan S (1997) Modulation of glutathione conjugation in vivo: how to decrease glutathione conjugation in vivo or in intact cellular systems in vitro. Chem Biol Interact 105: 17–34 [DOI] [PubMed] [Google Scholar]
- Murashige TR, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol Plant 15: 473–497 [Google Scholar]
- Nagai H, Matsumura K, Feng G, Kaplowitz N (2002) Reduced glutathione depletion causes necrosis and sensitisation to tumor necrosis factor-alpha-induced apoptosis in cultured mouse hepatocytes. Hepatology 366: 55–64 [DOI] [PubMed] [Google Scholar]
- Nagata T, Nemoto Y, Hasezawa S (1992) Tobacco BY-2 cell line as the “HeLa” cell in the cell biology of higher plants. Int Rev Cytol 132: 1–30 [Google Scholar]
- Nishizawa AN, Buchanan BB (1981) Enzyme regulation in C4 photosynthesis. Purification and properties of thioredoxin-linked fructose bisphosphatase and sedoheptulose bisphosphatase from corn leaves. J Biol Chem 256: 6119–6126 [PubMed] [Google Scholar]
- Noctor G, Arisi ACM, Jouanin L, Kunert KJ, Rennenberg H, Foyer CH (1998) Glutathione: biosynthesis, metabolism and relationship to stress tolerance explored in transformed plants. J Exp Bot 49: 623–647 [Google Scholar]
- Noctor G, Foyer CH (1998) Ascorbate and glutathione: keeping active oxygen under control. Annu Rev Plant Physiol Plant Mol Biol 49: 249–279 [DOI] [PubMed] [Google Scholar]
- Paciolla C, De Tullio MC, Chiappetta A, Innocenti AM, Bitonti MB, Liso R, Arrigoni O (2001) Short- and long-term effects of dehydroascorbate in Lupinus albus and Allium cepa roots. Plant Cell Physiol 42: 857–863 [DOI] [PubMed] [Google Scholar]
- Piquery L, Huault C, Billard J-P (2002) Ascorbate-glutathione cycle and H2O2 detoxification in elongating leaf bases of ryegrass: effect of inhibition of glutathione reductase activity on foliar regrowth. Physiol Plant 116: 406–415 [Google Scholar]
- Plöchl M, Lyons T, Ollerenshaw J, Barnes J (2000) Simulating ozone detoxification in the leaf apoplast through the direct reaction with ascorbate. Planta 210: 454–467 [DOI] [PubMed] [Google Scholar]
- Poot M, Teubert H, Rabinovitch PS, Kavanagh TJ (1995) De novo synthesis of glutathione is required for both entry into and progression through the cell cycle. J Cell Physiol 163: 555–560 [DOI] [PubMed] [Google Scholar]
- Potters G, De Gara L, Asard H, Horemans N (2002) Ascorbate and glutathione: guardians of the cell cycle, partners in crime? Plant Physiol Biochem 40: 537–548 [Google Scholar]
- Potters G, Horemans N, Caubergs RJ, Asard H (2000) Ascorbate and dehydroascorbate influence cell cycle progression in a tobacco cell suspension. Plant Physiol 124: 17–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichheld JP, Vernoux T, Lardon F, Van Montagu M, Inzé D (1999) Specific checkpoints regulate plant cell cycle progression in response to oxidative stress. Plant J 17: 647–656 [Google Scholar]
- Riley MV (1984) A role for glutathione and glutathione reductase in control of corneal hydration. Exp Eye Res 39: 751–758 [DOI] [PubMed] [Google Scholar]
- Samuels AL, Meehl J, Lipe M, Staehelin LA (1998) Optimizing conditions for tobacco BY-2 cell cycle synchronization. Protoplasma 202: 232–236 [Google Scholar]
- Sánchez-Fernández R, Fricker M, Corben LB, White NS, Sheard N, Leaver CJ, Van Montagu M, Inzé D, May MJ (1997) Cell proliferation and hair tip growth in the Arabidopsis root under mechanistically different forms of redox control. Proc Natl Acad Sci USA 94: 2745–2750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savini I, Duflot S, Avigliano L (2000) Dehydroascorbic acid uptake in a human keratinocyte cell line (HaCaT) is glutathione-independent. Biochem J 345: 665–672 [PMC free article] [PubMed] [Google Scholar]
- Shaw JP, Chou IN (1986) Elevation of intracellular glutathione content associated with mitogenic stimulation of quiescent fibroblasts. J Cell Physiol 129: 193–198 [DOI] [PubMed] [Google Scholar]
- Takahama U (1993) Redox state of ascorbic acid in the apoplast of stems of Kalanchoe daigremontiana. Physiol Plant 89: 791–798 [Google Scholar]
- Teisseire H, Vernet G (2000) Ascorbate and glutathione contents in duckweed, Lemna minor, as biomarkers of the stress generated by copper, folpet and diuron. Biomarkers 5: 263–273 [DOI] [PubMed] [Google Scholar]
- Trümper S, Follmann H, Häberlein I (1994) A novel dehydroascorbate reductase from spinach chloroplasts homologous to plant trypsin inhibitor. FEBS Lett 352: 159–162 [DOI] [PubMed] [Google Scholar]
- Ueda-Kawamitsu H, Lawson TA, Gwilt PR (2002) In vitro pharmacokinetics and pharmacodynamics of 1,3-bis(2-chloroethyl)-1-nitrosourea. Biochem Pharmacol 63: 1209–1218 [DOI] [PubMed] [Google Scholar]
- Veljovic-Jovanovic SD, Pignocchi C, Noctor G, Foyer CH (2001) Low ascorbic acid in the vtc-1 mutant of Arabidopsis is associated with decreased growth and intracellular redistribution of the antioxidant system. Plant Physiol 127: 426–435 [PMC free article] [PubMed] [Google Scholar]
- Vernoux T, Wilson RC, Seeley KA, Reichheld JP, Muroy S, Brown S, Maughan SC, Cobbett CS, Van Montagu M, Inze D, May MJ, Sung ZR (2000) The ROOT MERISTEMLESS1/CADMIUM SENSITIVE2 gene defines a glutathione-dependent pathway involved in initiation and maintenance of cell division during postembryonic root development. Plant Cell 12: 97–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells WW, Xu DP, Yanf Y, Rocque PA (1990) Mammalian thioltransferase (glutaredoxin) and protein disulfide isomerase have dehydroascorbate reductase activity. J Biol Chem 265: 15361–15364 [PubMed] [Google Scholar]
- Wilson JX (2002) The physiological role of dehydroascorbic acid. FEBS Lett 527: 5–9 [DOI] [PubMed] [Google Scholar]
- Xiang C, Olivier DJ (1998) Glutathione metabolic genes coordinately respond to heavy metal and jasmonic acid in Arabidopsis. Plant Cell 10: 1539–1550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang C, Werner BL, Christensen EM, Olivier DJ (2001) The biological functions of glutathione revisited in Arabidopsis transgenic plants with altered glutathione levels. Plant Physiol 126: 564–574 [DOI] [PMC free article] [PubMed] [Google Scholar]