Abstract
Salivary secretion is crucial for successful tick feeding, and it is the mediator of pathogen transmission. Salivation functions to inhibit various components of the host immune system and remove excess water and ions during the ingestion of large blood meals. Control of salivary glands involves autocrine/paracrine dopamine, which is the most potent inducer of tick salivation. Previously, we reported the presence of two dopamine receptors in the salivary glands of the blacklegged tick (Ixodes scapularis): dopamine receptor (D1) and invertebrate specific D1-like dopamine receptor (InvD1L). Here, we investigated the different physiological roles of the dopamine receptors in tick salivary glands by using pharmacological tools that discriminate between the two distinct receptors. Heterologous expressions followed by reporter assays of the dopamine receptors identified receptor-specific antagonists and agonists. These pharmacological tools were further used to discriminate the physiological role of each receptor by using in vitro assays: measuring salivary secretions of isolated salivary glands and monitoring dynamic changes in the size of individual salivary gland acini. We propose that the D1 receptor acts on salivary gland acini epithelial cells for inward fluid transport. InvD1L controls (or modulates) each acinus for expelling saliva from the acini to the salivary ducts, presumably through the actions of myoepithelial cells and valves for pumping/gating. We conclude that dopamine acts on the D1 and the InvD1L receptors and leads different physiological actions to orchestrate tick salivary secretion.
KEY WORDS: Tick salivary secretion, Osmoregulation, G-protein-coupled receptor
INTRODUCTION
Ticks are obligatory ectoparasites of many vertebrates and transmit pathogens that cause numerous diseases, including Lyme disease, Rocky Mountain spotted fever (RMSF), and southern tick-associated rash illness (STARI). Lyme disease transmitted by Ixodes scapularis Say 1821 is the most commonly reported vector-borne disease in the United States. Ticks generally remain attached to the host for up to ~14 days depending on species for blood meals, unlike hematophagous insects, such as mosquitoes, which feed for ~3 min. Female ticks can normally increase their body weight up to 100 times compared with unfed ticks (Kaufman, 2007). A successful long tick feeding duration, which may be the major reason that they are such efficient disease vectors, requires that the tick be capable of overcoming host defensive responses, mainly through salivation.
Tick salivary secretion contains various bioactive components, such as anti-haemostatic molecules, anti-inflammatory compounds and immunomodulators that compromise the host immune responses (Ribeiro, 1987; Ribeiro, 1989; Nuttall et al., 2006; Francischetti et al., 2009). Salivation also functions to maintain homeostasis by removing excessive water and ions that are processed from large blood meals (Tatchell, 1967; Kaufman and Phillips, 1973a). A pair of salivary glands is located at the anterolateral regions of the tick, and they resemble a cluster of grapes, which consist of three types of acini, types I, II and III. Based on the cellular structure, each type is thought to have different functions: type I for secretion of hyperosmotic fluid for uptake of atmospheric water vapour in free-living unfed ticks; type II for secretion of bioactive proteins on the host; and type III for excretion of hyposmotic saliva on the host (Rudolph and Knülle, 1974; Gaede and Knülle, 1997).
In on-host osmoregulation that removes excess water and ions during blood feeding, the majority of water and sodium ions were secreted back into the host via salivary secretion (Tatchell, 1967; Kaufman and Phillips, 1973a). Homogenates of synganglia (tick brain) and dopamine induced fluid secretion in isolated salivary glands (Kaufman, 1976; Kaufman, 1977). Octopamine or cyclic AMP (cAMP) demonstrated low activities on salivary secretion (Kaufman, 1976; Needham and Pannabecker, 1983; Pannabecker and Needham, 1985), whereas acetylcholine, serotonin and glutamate did not show activity from isolated salivary glands (Kaufman and Phillips, 1973b; Kaufman, 1976).
Earlier studies found that the activity of dopamine on salivary secretion is dependent on extracellular Ca2+ (Kaufman, 1976; Needham and Sauer, 1979). In addition, cAMP was also involved in dopamine-mediated salivary secretion (Schmidt et al., 1981; Krolak et al., 1983; Hume et al., 1984). Recently, we characterised two dopamine receptors: dopamine receptor (D1) and invertebrate specific D1-like dopamine receptor (InvD1L) in both acini types II and III (Šimo et al., 2011; Šimo et al., 2014). Receptor localisation and downstream coupling in the heterologous expression system suggested that the D1 receptor is probably coupled to cAMP elevation in epithelial cells, and the InvD1L receptor is coupled to Ca2+ mobilisation in myoepithelial cells (Šimo et al., 2011; Šimo et al., 2014). Therefore, two different dopamine actions mediated through different acini cell types were proposed, although experimental verifications of the suggested downstream cellular actions and physiological roles of each receptor were not possible because RNA interference targeting those receptors were not practicable in those studies.
In this study, we identified pharmacological agents that distinguish D1 and InvD1L receptors in heterologous receptor expression. The agents discriminating the two dopamine receptors successfully revealed different physiological actions in salivary gland acini in vitro.
List of symbols and abbreviations
- Ace
acepromazine
- Apo
apomorphine
- (−)Bu
(−)-butaclamol
- (+)Bu
(+)-butaclamol
- cAMP
cyclic AMP
- CHO
Chinese hamster ovary
- Clo
clozapine
- D1
dopamine receptor
- DA
dopamine
- DA-3
dopamine-3-O-sulfate
- DA-4
dopamine-4-O-sulfate
- EDTA
ethylenediaminetetraacetic acid
- Flu
fluphenazine
- Hal
haloperidol
- InvD1L
invertebrate specific D1-like dopamine receptor
- MIP
myoinhibitory peptide
- SCH
SCH23390
- SKF
- Sul
sulpiride
RESULTS
Receptor-specific agonists and antagonists identified in heterologous expressions of D1 and InvD1L
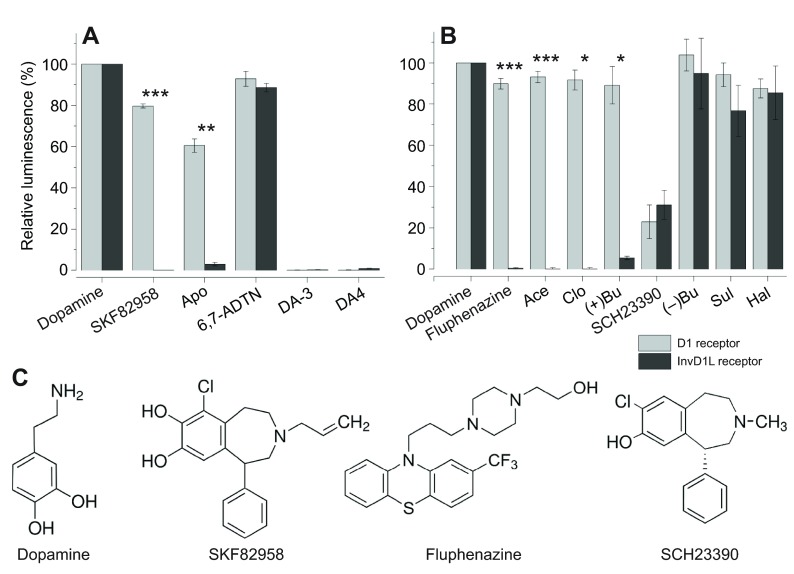
To identify receptor-specific agonists and antagonists of two dopamine receptors, we expressed the receptors in Chinese hamster ovary cell (CHO) for receptor-mediated calcium mobilisation reporter assays as described previously (Šimo et al., 2011; Šimo et al., 2014). Among candidate chemicals that were well known to be active on mammalian D1 receptors, we determined that SKF82958 and apomorphine specifically activated the D1 receptor but not the InvD1L receptor at 10 μmol l−1 (Fig. 1A). SKF82958 and apomorphine activated only the D1 receptor by 80 and 60% of dopamine (10 μmol l−1) activity, respectively, while they had no effect on the InvD1L receptor, showing 0.02 and 3% of dopamine (10 μmol l−1) activity, respectively (Fig. 1A). Another agonist, (±)-2-amino-6,7-dihydroxy-1,2,3,4-tetrahydronaphthalene hydrobromide (6,7-ADTN, 10 μmol l−1), activated both D1 and InvD1L receptors by 93 and 89% of dopamine activity. We also tested sulfate-conjugated dopamine, dopamine-3-O-sulfate (DA-3S) and dopamine-4-O-sulfate (DA-4S), which were highly abundant in the tick salivary gland in our previous study (Koči et al., 2014). However, both DA-3S and DA-4S did not show any activity on either the D1 or the InvD1L receptor in our assay system.
Fig. 1.
Calcium mobilisation assay using aequorin reporter for D1 and InvD1L receptors expressed in Chinese hamster ovary cells (CHO-K1). The light grey bars represent the D1 receptor results, and the dark grey bars represent the InvD1L receptor results. (A) Agonistic activities of various chemicals (10 μmol l−1) on the D1 receptor and InvD1L receptor. The first group of bars represents the full luminescence responses of D1 and InvD1L receptors to 10 μmol l−1 dopamine. (B) Antagonistic activities of various chemicals (10 μmol l−1) on the D1 and InvD1L receptors. The luminescence responses of D1 and InvD1L receptors to 10 μmol l−1 dopamine are shown in the first column, and the responses to 10 μmol l−1 dopamine after pre-incubation with different chemicals (10 μmol l−1) for 15 min are shown in the remaining columns. The bars in the graphs indicate the averages with s.e.m. for three replicates. (C) Structures of the pharmacological discriminators used in this study: dopamine, SKF82958 (D1-specific agonist), fluphenazine (InvD1L-specific antagonist) and SCH23390 (common antagonist). Apo, apomorphine; 6,7-ADTN, (±)-2-amino-6,7-dihydroxy-1,2,3,4-tetrahydronaphthalene hydrobromide; DA-3, dopamine-3-O-sulfate; DA-4, dopamine-4-O-sulfate; Ace, acepromazine; Clo, clozapine; (+)Bu, (+)-butaclamol; (−)Bu, (−)-butaclamol; Sul, sulpiride; Hal, haloperidol. Asterisks indicate statistical significance in the paired t-test: ***P<0.0001, **P<0.001 and *P<0.01 for differences between D1 and InvD1L.
In search of receptor-specific antagonists, which measured the reduction in dopamine activity on the receptor after 15 min pre-incubation with the test compound (10 μmol l−1), we identified four antagonists that were specific only to the InvD1L receptor: fluphenazine, acepromazine, clozapine and (+)-butaclamol, while (−)-butaclamol, sulpiride and haloperidol had no or low antagonistic activities on either receptor. We also found a common antagonist (SCH23390) that showed 77 and 69% antagonism on D1 and InvD1L, respectively (Fig. 1B), which was also previously described for dopamine receptors of mosquito and tick (Meyer et al., 2011; Meyer et al., 2012).
Taken together, we identified three groups of pharmacological discriminators: D1-specific agonists, InvD1L-specific antagonists, and a common antagonist. However, there was no InvD1L-specific agonist and D1-specific antagonist among the ligands assessed in this study. In subsequent in vitro experiments on isolated salivary glands, we utilised only SKF82958 as D1-specific agonist, fluphenazine as InvD1L-specific antagonist, and SCH23390 as a common antagonist (Fig. 1C).
Activities of agonists and antagonists on the secretory activity of isolated salivary glands
To investigate the responses of salivary glands to pharmacological discriminators, we quantified the volumes of secreted saliva from isolated salivary glands by developing the assay method modified from Ramsay's original assay (Fig. 2) (Ramsay, 1954). The salivary glands that were pre-incubated with buffer only did not show any secretory activity. Dopamine (1, 10 and 100 μmol l−1) induced significant salivary secretion in a dose-dependent manner (Fig. 3A,E). The rate of salivary secretion was increased immediately after agonist treatment, peaked at 10 or 15 min, and then gradually decreased until 30 min, which was the end of our observation (Fig. 3A–D). The maximal total salivary secretion volume for 30 min was ~1.2 μl at 100 μmol l−1 dopamine (Fig. 3E).
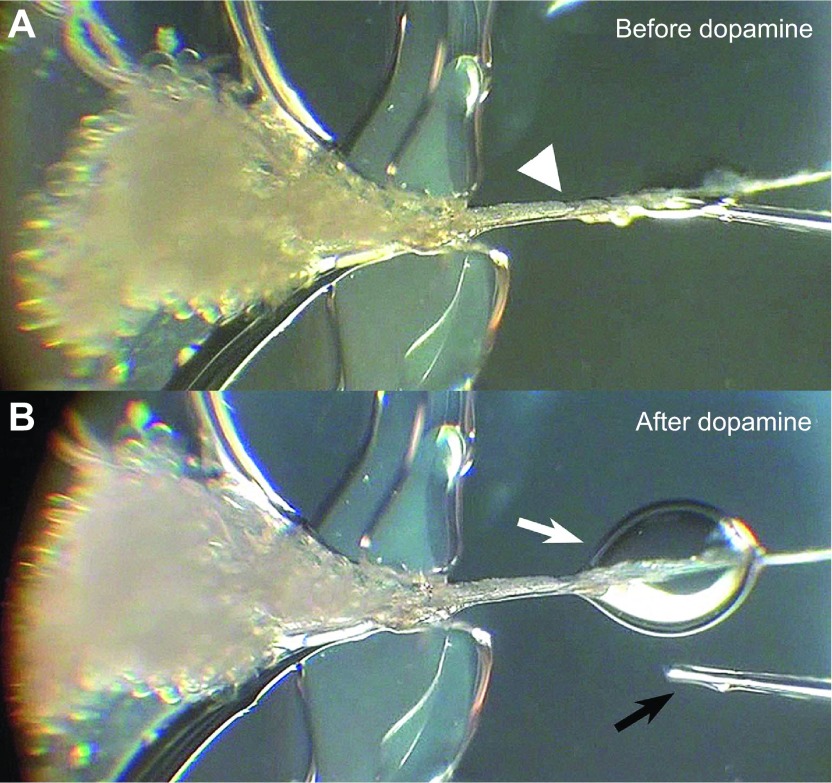
Fig. 2.
Example fluid secretion assay using a whole salivary gland. A salivary gland preparation is shown before (A) and after dopamine treatment (B). The arrowhead indicates the main salivary gland duct that was pulled out from the Hanks' saline reservoir across a narrow vacuum grease dam and attached onto the Sylgard plate. The white arrow indicates the drop of saliva that was secreted through the main duct after treatment. The black arrow is the tip of microcapillary tube that was used for quantifying the secretion volume every 5 min for 30 min.
Fig. 3.
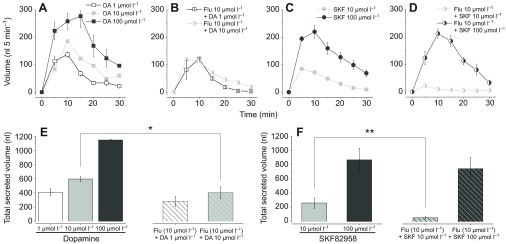
Fluid secretion assay using a whole salivary gland with dopamine, D1-specific agonist SKF82958 and InvD1L-specific antagonist fluphenazine. The salivary secretion pattern over 30 min duration after treatments (A) by distinct dopamine doses (DA), (B) by 25 min pre-treatment and DA co-incubation with fluphenazine (Flu, 10 μmol l−1), (C) by SKF82958 (SKF), and (D) by 25 min pre-treatment and SKF co-incubation with fluphenazine (10 μmol l−1). (E) Total volume of secreted saliva in 30 min by different DA doses (filled bars) and combined treatment of DA and Flu (hatched bars), which corresponded to A and B, respectively. (F) Total volume of secreted saliva by different SKF doses (filled bars) and combination of SKF and Flu (hatched bars), which corresponded to C and D, respectively. The bars indicate averages with s.e.m. for three and six replicates (only for DA 1 μmol l−1 and Flu 10 μmol l−1 + DA 1 μmol l−1). Asterisks indicate statistical significance in a paired t-test: **P<0.01 and *P<0.05.
SKF82958, a D1-specific agonist activating only the D1 but not the InvD1L receptor, also showed significant, but slightly lower activities than dopamine. At SKF82958 doses of 10 and 100 μmol l−1, the secretory activity was 44 and 76% of dopamine at the same respective concentrations (Fig. 3E,F). The general patterns of secretory activities over time in the post-treatments were similar to the patterns observed in the dopamine treatments.
Pre-incubation of the isolated salivary gland with the InvD1L-specific antagonist fluphenazine (10 μmol l−1) followed by co-incubation of fluphenazine with dopamine or SKF82958 significantly suppressed secretory activity, while the salivary secretion pattern peak at ~10 min was similar to agonist treatment alone (Fig. 3A–D). We observed ~30% reductions at both 1 and 10 μmol l−1 dopamine treatment (Fig. 3E). The reduction in SKF82958 treatment was also significant at 10 μmol l−1 but not at 100 μmol l−1 (Fig. 3F).
Co-incubation of the isolated salivary gland with SCH23390 (10 μmol l−1), a common antagonist for both D1 and InvD1L, and dopamine (10 μmol l−1) almost completely abolished secretory activity (Fig. 4). The antagonistic activity of SCH23390 was reverted after replacing it with dopamine only (100 μmol l−1) without the antagonist (Fig. 4).
Fig. 4.
Effect of the common antagonist SCH23390 (SCH) on dopamine (DA)-mediated salivary gland fluid secretion. DA-mediated salivary secretion was abolished by SCH in the first 15 min. Subsequent washing and treatment with DA alone induced significant salivary secretion.
Agonist activity on individual salivary gland acini
Microscopic observation of each acinus after pharmacological agent treatment revealed dynamic changes occurring in each acinus. In the initial observations, we found that the acini sizes dynamically changed, especially when they were treated with dopamine. The changes in acini sizes were driven by the changes in luminal acini volumes but not by the changes in sizes of the cells composing the acini (Fig. 5A). Therefore changes in acini sizes were measured and calculated for volume and percentage volume change. Based on the volume calculated, each acinus luminal space increased up to ~4 nl by dopamine treatment.
Fig. 5.
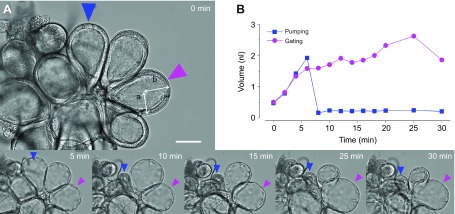
Dynamic responses of individual salivary gland type III acini to dopamine treatment. (A) An example indicating the changes of salivary gland acini recorded by a video camera with 200× magnification for 30 min after 10 μmol l−1 dopamine treatment. Arrowheads indicate two randomly selected acini for measuring the volume change. The lengths a and b demonstrate the axes that were used for calculation of the volume described in Materials and methods. Colours of arrowheads correspond to the line colours in B. (B) Each line indicates changes in individual acini volume to dopamine treatment for 30 min. Blue and purple lines indicate pumping and gating, respectively. Scale bar, 50 μm.
Patterns of the changes over time were used to infer physiological changes of the acini: increased sizes were interpreted as water-solute influx into the acinar lumen and decreased sizes as pumping or gating for saliva discharge through the acinar duct, respectively (Fig. 5). Following influx of water-solute, rapid or slow reductions in acini sizes occurred and were referred to as pumping or gating, respectively, and together as pumping/gating. We set the threshold for counting the pumping/gating category based on the statistics of samples with mock treatments and a rate of reduction higher than 3.5% per minute (see further details in Materials and Methods). However, based on our close observations, this method was unavoidable from the false-negative cases, i.e. the cases where true pumping/gating were not counted because the fluid expulsion rate was occasionally masked by equal or higher fluid influx rates, although the positive pumping/gating counts were accurate.
As an example of dynamic changes in acini, dopamine triggered changes in acini sizes (Fig. 5A; supplementary material Movie 1) and volume (Fig. 5B). Typical pumping is shown by rapid reduction of the acini size that appeared, such as a squeezing muscular contraction of the acini (supplementary material Movie 2) (Coons et al., 1994). In some cases, slow reduction in size occurred over a relatively long duration. This case was considered to be gating because subtle movements of the acinar duct valve in concurrence with slow volume reduction were observed in some cases (supplementary material Movie 3).
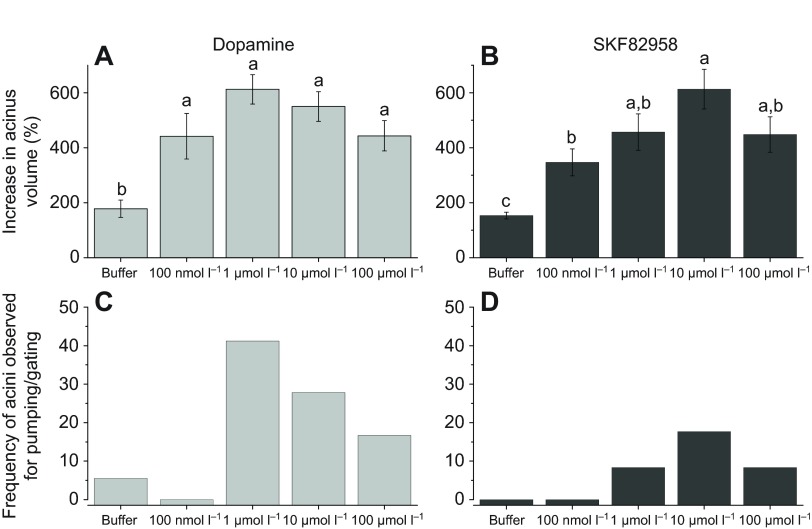
The maximum sizes of the acini that were activated by dopamine at the four different doses (100 nmol l−1 to 100 μmol l−1) demonstrated a typical dose–response of size increase up to 1 μmol l−1 but slight reductions at the higher concentrations of 10 and 100 μmol l−1 (Fig. 6A). Similarly, the D1-specific agonist SKF82958 also showed significant activities for the increased acini size at all doses but with slightly lower activities than dopamine. Similar to the dopamine treatments, the higher doses induced stronger responses up to 10 μmol l−1, but the highest dose (100 μmol l−1) showed a slightly reduced degree of response. The pumping/gating frequency was highest in the 1 μmol l−1 dopamine treatment (42%) but was progressively reduced in the higher dopamine doses (Fig. 6C). In the SKF82958 treatments, pumping/gating was much less frequent than that of dopamine with a maximum count of 18% of acini at the 10 μmol l−1 dose (Fig. 6D).
Fig. 6.
Dynamic responses of individual acini to dopamine receptor agonists. (A) Percentage increase in acini volume calculated for the maximum size reached within 30 min after dopamine treatment. (B) Percentage increase in acini volume after SKF82958 treatment. (C,D) Frequencies of acini observed for pumping/gating in each treatment. The data in A,B are averaged with the s.e.m. for at least three biological replicates. The data in C,D are for frequencies in observations of more than 18 acini for each data point. The data were analysed by an ANOVA–Tukey–Kramer honestly significant difference (HSD) test (P=0.05). Hanks' saline (buffer) indicated mock treatment.
Effects of antagonists on agonist-mediated individual salivary gland acini
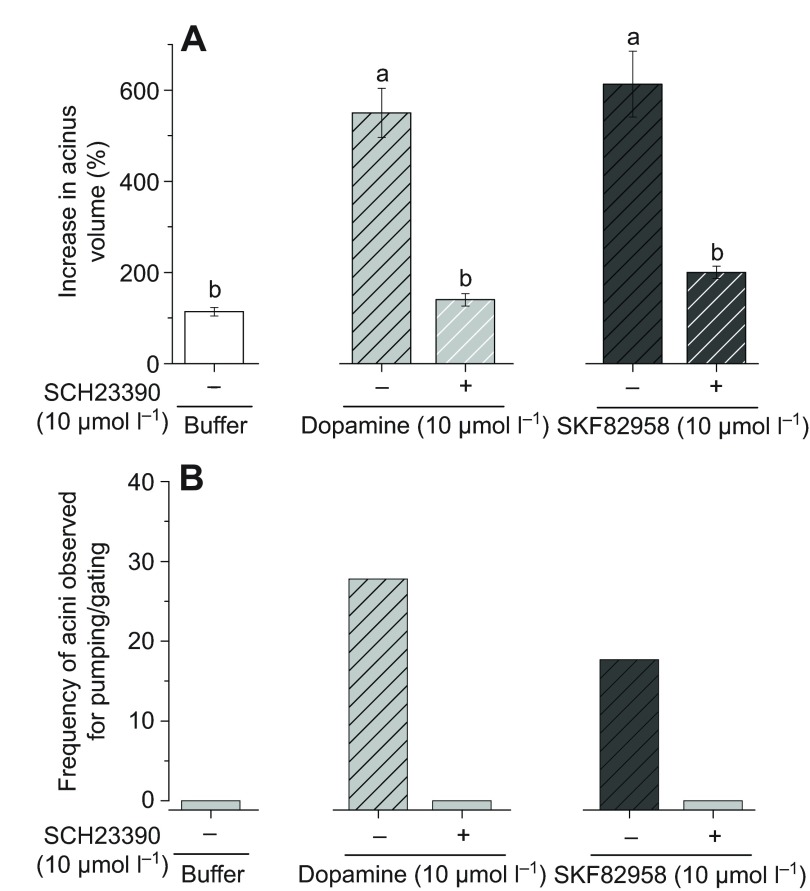
The activities of dopamine or SKF82958 for increased acini volume were completely abolished by SCH23390, the common antagonist of both D1 and InvD1L (Fig. 7A). Consequently, the acini that were pre-treated with SCH23390 did not show any pumping/gating activity (Fig. 7B).
Fig. 7.
Effects of common dopamine receptor antagonist SCH23390 (SCH) on dopamine- or SKF82958-mediated salivary gland acini dynamics. (A) Percentage increase in acini volume after various combined treatments. (B) Frequencies of acini observed for pumping/gating in each treatment. The data in A are the average with the s.e.m. for at least three biological replicates. The data in B are frequencies in observations of more than six acini for each data point. The data were analysed by an ANOVA–Tukey–Kramer HSD test (P=0.05).
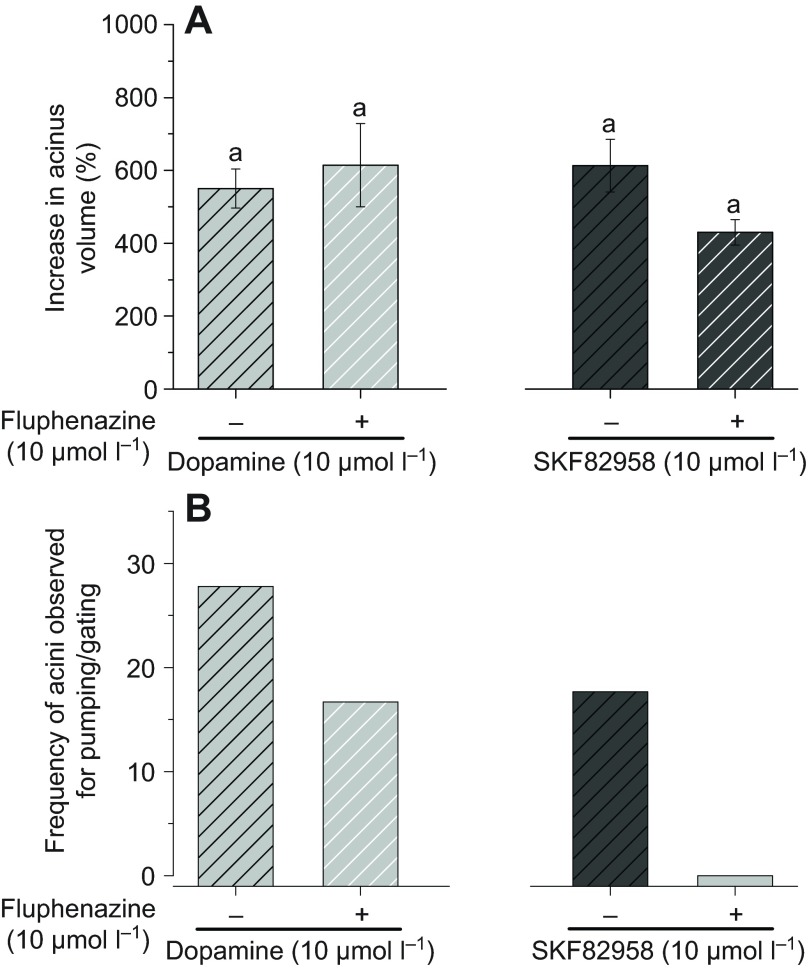
Pre-treatments of acini with the InvD1L-specific antagonist fluphenazine followed by dopamine or SKF82958 treatments did not significantly reduce the maximum acini sizes (Fig. 8A). However, reduced frequencies of pumping/gating by the pre-treatments were apparent in both dopamine and SKF82958 treatments (Fig. 8B). The frequency of dopamine-induced pumping/gating was reduced from 28 to 17% by fluphenazine pre-treatment. Pre-treatment eliminated SKF82958 treatment-mediated pumping/gating (from 18% to 0%) (Fig. 8B).
Fig. 8.
Effects of InvD1L-specific blocker fluphenazine (Flu) on dopamine- or SKF82958-mediated salivary gland acini dynamics. (A) Percentage increase in acini volume after various combined treatments. (B) Frequencies of acini observed for pumping/gating in each treatment. The data in A are the averages with s.e.m. for at least three biological replicates. The data in B are frequencies in observations of more than 18 acini for each data point. The data were analysed by an ANOVA–Tukey–Kramer HSD test (P=0.05).
DISCUSSION
In dopamine-mediated osmoregulatory salivary secretion by the tick, we determined that two combinatory physiological actions occur in the type III salivary gland acini: inward fluid transport and release of the luminal content. Dopamine treatment immediately activated fluid influx as indicated by increased luminal volume (Fig. 5). In contrast to the description made in Ixodes ricinus, where increased luminal size accommodated the fluid influx without increased acini size (Bowman and Sauer, 2004), we observed rapid acini expansion up to approximately five times because of increased luminal volume (Fig. 5, supplementary material Movie 1). Visually detectable levels of fluid efflux through the acinar duct in this study found mainly two main categories of efflux patterns in different acini: acini with slowly reduced sizes over time (gating) and acini with rapid collapse for expelling fluid (pumping). On rare occasions, we observed subtle movements of acinar valve flaps in the gating acini (supplementary material Movie 3). In contrast, the pumping acini, when it occurred in the intermediate size before the acini was fully expanded, appeared to be driven by a squeezing contraction of the acini (supplementary material Movie 2). These were difficult to distinguish when the acini were fully expanded because rapid size reduction could be partly caused by release of high internal pressure in the absence of the contraction. Summing up the observations, two components of salivary secretion are epithelial cell fluid transport for water-solute influx and myoepithelial cell contraction (Coons et al., 1994) for generating internal pressure with valve opening allowing saliva efflux through the acinar duct. The full salivary secretion rate required both processes based on the experiment using pharmacological tools (Fig. 3 and see below). Observation of the salivary glands in real time provided physiological events that were involved in salivary secretion, although we do not know whether the observed levels of acini expansion occur in an in vivo system that is enclosed in the rigid body wall.
Use of receptor-specific pharmacological tools in this study suggests that the downstream physiological events are mediated through different dopamine receptors D1 and InvD1L, which were previously characterised in tick salivary glands (Šimo et al., 2011; Šimo et al., 2014). The results of this study support the function of D1 for water-solute influx and InvD1L for pumping/gating in type III acini. The most pronounced result was demonstrated in the acini that were treated with the combination of D1-specific agonist SKF82958 and InvD1L-specific antagonist fluphenazine, abolishing the pumping/gating with normal acini size increase (Fig. 8) and significantly diminishing fluid secretion by whole salivary glands (Fig. 3F). Treatment with dopamine and fluphenazine in combination and SKF82958 alone, however, reduced but did not completely abolish the pumping/gating frequencies and fluid secretion, although fluphenazine and SKF82958 clearly distinguished recombinant receptors in the heterologous system at the 10 μmol l−1 dose. This leaky activity in suppression of pumping/gating suggests the presence of other signalling pathways that interact with dopamine signalling for pumping/gating. Such candidates could be the neuropeptides myoinhibitory peptide (MIP) and SIFamide, which were found in the neural projections reaching the basal region of acini (Šimo et al., 2009; Šimo et al., 2013). Alternatively, an unidentified signalling system acting through the octopamine receptor, which is probably cross-activated by dopamine, could have also been involved in this process.
Previous studies in dopamine signalling pathways (Schmidt et al., 1981; Hume et al., 1984; Sauer et al., 2000) and in characterising the D1 and InvD1L receptors in tick salivary glands (Šimo et al., 2011; Šimo et al., 2014) and the present study are all congruent and provide further insight into the mechanisms of tick salivary gland control. Dopamine activates crucial Ca2+ (Kaufman, 1976; Needham and Sauer, 1979) and cAMP (Hume et al., 1984) downstream signalling pathways in tick salivary secretion. Heterologous receptor expression determined that D1 has higher dopamine sensitivity and was mainly coupled to cAMP elevation, while InvD1L was coupled to Ca2+ elevation with lower dopamine sensitivity (Šimo et al., 2011; Šimo et al., 2014). Immunohistochemistry revealed that D1 is located in the acini epithelial cells and InvD1L is located in axonal projections reaching to the basal region of acini and in another projection further extending to the apical region of acini (Šimo et al., 2014; Šimo et al., 2011). These characteristics of D1 and InvD1L suggest that an initial low dose of dopamine activates D1 in epithelial cells leading the cAMP-mediated fluid transport. High dopamine doses acting on InvD1L trigger (or modulate) Ca2+-mediated myoepithelial cell contraction and/or acinar valve flap for saliva efflux through the salivary duct.
While this study focused on dopamine actions in type III acini, the roles of the two distinct dopamine receptors in type II acini are thought to be similar. Although type II acini are thought to function for secretion of the precursor of cement and bioactive molecules to manipulate host immune responses (Binnington, 1978; Walker et al., 1985), they also contain epithelial cell types with similar immunoreactive patterns for D1 and InvD1L receptors. Visual investigation of fluid flow through type II acini was not possible due to lack of dopamine-induced acinar expansion as in acini type III. Employing additional experimental tools, such as electrophysiology, will be required to understand the physiological mechanism underlying dopamine and its receptors in type II acini.
The autocrine/paracrine dopamine (Koči et al., 2014) and downstream systems built in the salivary glands also probably interact with neural commands. The neural projections reaching to the basal region of acini containing MIP/SIFamide are also positive with InvD1L antibodies (Šimo et al., 2014). The co-localisation suggests that dopamine action includes modulation of MIP/SIFamide neuronal secretory activities at axon terminals. Candidate functions of MIP and SIFamide were previously suggested as controls of acinar valves, myoepithelial cells and dopamine secretion, based on the anatomy of the axon terminal in acini (Šimo et al., 2009; Šimo et al., 2013). Therefore we conclude that osmoregulatory salivary secretion in ticks involves the orchestration of interactions of multiple components for dynamic controls.
MATERIALS AND METHODS
Functional expression and pharmacological assay of two dopamine receptors: D1 and InvD1L
The full open reading frame (ORF) for each D1 and InvD1L were inserted into the pcDNA 3.1 (+) expression vector (Invitrogen, Carlsbad, CA, USA). Expression plasmids were prepared for high purity and concentrations (>1 μg μl−1) and were then sequenced. Functional G-protein-coupled receptor expression was performed based on a previous description with modifications (Park et al., 2003; Šimo et al., 2011; Jiang et al., 2013; Šimo et al., 2014). A one-to-one mixture of the receptor-containing plasmid and the codon-optimised human apoaequorin-containing plasmid (Vernon and Printen, 2002) were used for transfections of Chinese Hamster Ovary (CHO) cells using TransIT®-LT1 (Mirus, Madison, WI, USA). Approximately 24–48 h after the transfection, cells were harvested with trypsin-EDTA (Gibco, Grand Island, NY, USA) and resuspended in 3 ml Dulbecco's modified Eagle medium: nutrient mixture F-12 (DMEM/F12) with 0.1% bovine serum albumin (BSA; Amresco, Solon, OH, USA). Coelenterazine h (final concentration 5 μmol l−1; AAT Bioquest, Sunnyvale, CA, USA) was added to the resuspended cells. After a 3 h incubation, cells were additionally incubated for 30 min followed by addition of 27 ml DMEM/F12 media, including 0.1% BSA (Amresco). For the agonist assay, various dopamine agonists (final concentration 10 μmol l−1) were prepared in a 96-well plate, upon which transfected cells (~15,000 cells in 50 μl) were applied. For the antagonist assay, the cells were pre-incubated with various dopamine antagonists (final concentration 10 μmol l−1) for 15 min, followed by 10 μmol l−1 dopamine treatment. Luminescence, the indicator of calcium mobilisation, was monitored for 20 s (100 ms exposure in every 100 ms) immediately following the application of either transfected cells (for the agonist assay) or dopamine (for the antagonist assay). Luminescence values were integrated over time for 20 s and normalised to the largest positive control response and to the negative control background values.
Chemicals
The following chemicals were used for the agonist and antagonist assays. Dopamine agonists included SKF82958, 6,7-ADTN [(±)-2-amino-6,7-dihydroxy-1,2,3,4-tetrahydronaphthalene hydrobromide], apomorphine (Sigma-Aldrich, St Louis, MO, USA), dopamine-3-O-sulfate and dopamine-4-O-sulfate (RTI International, Research Triangle Park, NC, USA). Dopamine antagonists included acepromazine, fluphenazine, clozapine, sulpiride, haloperidol, SCH23390, (+)-butaclamol and (−)-butaclamol (Sigma-Aldrich).
Tick and salivary gland preparation
The blacklegged tick I. scapularis was purchased from the Tick Rearing Center at Oklahoma State University (Stillwater, OK, USA). Partially engorged female ticks (weighing 12–19 mg, 4–5 days blood-fed) were prepared from an artificial feeding system that was modified from the method developed by Kröber and Guerin (Kröber and Guerin, 2007). Tick feeding chambers were maintained at 37°C with a 16 h:8 h light:dark photoperiod (Kröber and Guerin, 2007). The defibrinated bovine blood (Cedarlane, Burlington, NC, USA) contained 10 μmol l−1 gentamycin solution (Sigma-Aldrich) and 100 μmol l−1 ATP (Sigma-Aldrich), and we replaced blood meals every 12 h. Salivary glands of partially engorged female ticks were dissected and pre-incubated in Hanks' saline (Sigma-Aldrich). For microscopic video recording of individual acini, whole salivary glands were cut into small pieces, each containing ~10 acini per group. For the in vitro fluid secretion assay, whole salivary glands were examined following pre-incubation in Hanks' saline for 1 h.
In vitro fluid secretion assays
A modified Ramsay assay (Ramsay, 1954) was established for tick salivary glands that have much shorter ducts than insect Malpighian tubules. All experiments in this study were performed with female salivary glands. Salivary glands were placed in a droplet (30 μl) of Hanks' saline on a 60 mm × 15 mm Petri dish coated with Sylgard® (World Precision Instruments, Inc., Sarasota, FL, USA) after pre-incubation of isolated intact salivary glands in Hanks' saline for 1 h. The main duct was pulled out from the Hanks' saline droplet crossing over a narrow dam (0.5–1 mm) made of high vacuum grease (Dow Corning Corporation, Midland, MI, USA) and immobilised on the Sylgard Petri dish surface. Heavy mineral oil (Fisher Scientific, Fair Lawn, NJ, USA) covered both the Hanks' saline and the main salivary gland duct to collect secreted saliva (Fig. 2A). To establish basal levels of excretion induced by Hanks' saline, measurements were taken in the first 5 min after preparation of the fluid secretion assay. A micro-injector (Nanoliter 2000, World Precision Instruments) controlled by a micro-syringe pump controller (Micro4, World Precision Instruments) was used for withdrawing the secretion formed at the tip of the main duct in the heavy mineral oil, and the volume withdrawn was recorded every 5 min for 30 min. For the agonist assay, we measured secreted saliva for 30 min after drug treatment. For the antagonist assay, salivary glands were pre-incubated in antagonist for 25 min, and then agonists were administered to salivary glands in the presence of the antagonists (Fig. 2B).
Measurement of the individual type III acini responses to pharmacological treatments
It was possible to perform multiple experiments for different treatments from a salivary gland by dividing it into several groups containing ~10 acini per group. Data points were obtained from 6–18 salivary glands (from 3–9 individuals). The size changes occurring in type III acini after treatment with pharmacological agents were video recorded. The video record was replayed for plotting volume changes over time (for ~30 min) every 2 min. The acinus volume (V) was calculated by the equation for an ellipsoid volume:
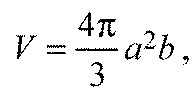 |
(1) |
where a and b are outer radii demonstrated in Fig. 5A. For an unbiased observation, we randomly pre-selected two individual type III acini on the focal plane of the microscope, measured the changes and calculated acini volumes. Increased acini size was generally interpreted as inward fluid transport, while reduced size demonstrated two different patterns: pumping and gating.
For statistical analysis of the frequencies of pumping/gating of acini, we established thresholds for different degrees of reduction over time. The thresholds were determined by the percentage volume reductions that were observed in P<0.01 of the mock treatments. Acini categorized as gating showed more than average 3.15% of reduction of the volume per minute from the maximum to the lowest in the 30 min observation. Thereby, the formula for the threshold of gating was:
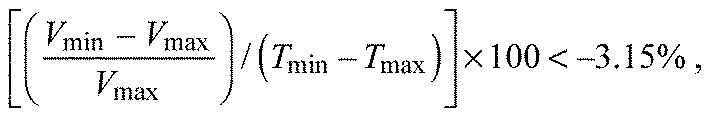 |
(2) |
where Vmax and Tmax are the volume and time at the peak size, and Vmin and Tmin are the volume and time after the acini had fully reduced in size.
Acini categorized as pumping showed a reduction of volume of more than 12.04% within 2 min with the formula:
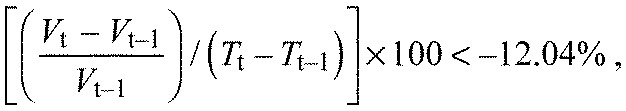 |
(3) |
where V is volume and T is time after treatment.
For the case of acini showing both pumping and gating, the acini were counted as pumping action because the criteria for pumping action are generally inclusive of the gating action. In results, pumping and gating were combined for the expression of frequency of discharging the fluid. For the antagonist assay, salivary glands were pre-incubated in antagonist for 25 min followed by administration of agonist to salivary glands in the presence of antagonists.
Supplementary Material
ACKNOWLEDGEMENTS
This paper is contribution no. 15-114-J from the Kansas Agricultural Experiment Station.
FOOTNOTES
Competing interests
The authors declare no competing financial interests.
Funding
This work was supported by the National Institutes of Health grant RO1AI090062 to Y.P. Deposited in PMC for release after 12 months.
Supplementary material
Supplementary material available online at: http://jeb.biologists.org/lookup/suppl/doi:10.1242/jeb.109462/-/DC1.
References
- Binnington K. C. (1978). Sequential changes in salivary gland structure during attachment and feeding of the cattle tick, Boophilus microplus. Int. J. Parasitol. 8, 97-115 [DOI] [PubMed] [Google Scholar]
- Bowman A. S., Sauer J. R. (2004). Tick salivary glands: function, physiology and future. Parasitology 129 Suppl., S67-S81 [DOI] [PubMed] [Google Scholar]
- Coons L. B., Lessman C. A., Ward M. W., Berg R. H., Lamoreaux W. J. (1994). Evidence of a myoepithelial cell in tick salivary glands. Int. J. Parasitol. 24, 551-562 [DOI] [PubMed] [Google Scholar]
- Francischetti I. M., Sa-Nunes A., Mans B. J., Santos I. M., Ribeiro J. M. (2009). The role of saliva in tick feeding. Front. Biosci. 14, 2051-2088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaede K., Knülle W. (1997). On the mechanism of water vapour sorption from unsaturated atmospheres by ticks. J. Exp. Biol. 200, 1491-1498 [DOI] [PubMed] [Google Scholar]
- Hume M. E., Essenberg R. C., McNew R. W., Bantle J. A., Sauer J. R. (1984). Adenosine-3′,5′-monophosphate in salivary glands of unfed and feeding female lone star ticks, Amblyomma americanum (L.). Comp. Biochem. Physiol. 79C, 47-50 [DOI] [PubMed] [Google Scholar]
- Jiang H., Lkhagva A., Daubnerová I., Chae H.-S., Šimo L., Jung S.-H., Yoon Y.-K., Lee N.-R., Seong J. Y., Žitňan D., et al. (2013). Natalisin, a tachykinin-like signaling system, regulates sexual activity and fecundity in insects. Proc. Natl. Acad. Sci. USA 110, E3526-E3534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman W. (1976). The influence of various factors on fluid secretion by in vitro salivary glands of ixodid ticks. J. Exp. Biol. 64, 727-742 [DOI] [PubMed] [Google Scholar]
- Kaufman W. R. (1977). The influence of adrenergic agonists and their antagonists on isolated salivary glands of ixodid ticks. Eur. J. Pharmacol. 45, 61-68 [DOI] [PubMed] [Google Scholar]
- Kaufman W. R., Phillips J. E. (1973a). Ion and water-balance in ixodid tick Dermacentor andersoni. 1. Routes of ion and water excretion. J. Exp. Biol. 58, 523-536 [Google Scholar]
- Kaufman W. R., Phillips J. E. (1973b). Ion and water-balance in ixodid tick Dermacentor andersoni. 2. Mechanism and control of salivary secretion. J. Exp. Biol. 58, 537-547 [Google Scholar]
- Koči J., Šimo L., Park Y. (2014). Autocrine/paracrine dopamine in the salivary glands of the blacklegged tick Ixodes scapularis. J. Insect Physiol. 62, 39-45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kröber T., Guerin P. (2007). The tick blood meal: from a living animal or from a silicone membrane? ALTEX 24, 39-41 [PubMed] [Google Scholar]
- Krolak J. M., Ownby C. L., Barker D. M., Sauer J. R. (1983). Immunohistochemical localization of adenosine 3′,5′-cyclic monophosphate in female ixodid tick Amblyomma americanum (L.) salivary glands. J. Parasitol. 69, 152-157 [PubMed] [Google Scholar]
- Meyer J. M., Ejendal K. F., Watts V. J., Hill C. A. (2011). Molecular and pharmacological characterization of two D1-like dopamine receptors in the Lyme disease vector, Ixodes scapularis. Insect Biochem. Mol. Biol. 41, 563-571 [DOI] [PubMed] [Google Scholar]
- Meyer J. M., Ejendal K. F., Avramova L. V., Garland-Kuntz E. E., Giraldo-Calderón G. I., Brust T. F., Watts V. J., Hill C. A. (2012). A ‘genome-to-lead’ approach for insecticide discovery: pharmacological characterization and screening of Aedes aegypti D1-like dopamine receptors. PLoS Negl. Trop. Dis. 6, e1478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Needham G. R., Pannabecker T. L. (1983). Effects of octopamine, chlordimeform, and demethylchlordimeform on amine-controlled tick salivary-glands isolated from feeding Amblyomma americanum (L). Pestic. Biochem. Physiol. 19, 133-140 [Google Scholar]
- Needham G. R., Sauer J. R. (1979). Involvement of calcium and cyclic AMP in controlling ixodid tick salivary fluid secretion. J. Parasitol. 65, 531-542 [PubMed] [Google Scholar]
- Nuttall P. A., Trimnell A. R., Kazimirova M., Labuda M. (2006). Exposed and concealed antigens as vaccine targets for controlling ticks and tick-borne diseases. Parasite Immunol. 28, 155-163 [DOI] [PubMed] [Google Scholar]
- Pannabecker T., Needham G. R. (1985). Effects of octopamine on fluid secretion by isolated salivary glands of a feeding ixodid tick. Arch. Insect Biochem. Physiol. 2, 217-226 [Google Scholar]
- Park Y., Kim Y. J., Dupriez V., Adams M. E. (2003). Two subtypes of ecdysis-triggering hormone receptor in Drosophila melanogaster. J. Biol. Chem. 278, 17710-17715 [DOI] [PubMed] [Google Scholar]
- Ramsay J. A. (1954). Active transport of water by the malpighian tubules of the stick insect, Dixippus morosus (Orthoptera, Phasmidae). J. Exp. Biol. 31, 104-113 [Google Scholar]
- Kaufman W. R. (2007). Gluttony and sex in female ixodid ticks: how do they compare to other blood-sucking arthropods? J. Insect Physiol. 53, 264-273 [DOI] [PubMed] [Google Scholar]
- Ribeiro J. M. (1987). Role of saliva in blood-feeding by arthropods. Annu. Rev. Entomol. 32, 463-478 [DOI] [PubMed] [Google Scholar]
- Ribeiro J. M. (1989). Role of saliva in tick/host interactions. Exp. Appl. Acarol. 7, 15-20 [DOI] [PubMed] [Google Scholar]
- Rudolph D., Knülle W. (1974). Site and mechanism of water vapour uptake from the atmosphere in ixodid ticks. Nature 249, 84-85 [DOI] [PubMed] [Google Scholar]
- Sauer J. R., Essenberg R. C., Bowman A. S. (2000). Salivary glands in ixodid ticks: control and mechanism of secretion. J. Insect Physiol. 46, 1069-1078 [DOI] [PubMed] [Google Scholar]
- Schmidt S. P., Essenberg R. C., Sauer J. R. (1981). Evidence for a D1 dopamine receptor in the salivary glands of Amblyomma americanum (L.). J. Cyclic Nucleotide Res. 7, 375-384 [PubMed] [Google Scholar]
- Šimo L., Žitňan D., Park Y. (2009). Two novel neuropeptides in innervation of the salivary glands of the black-legged tick, Ixodes scapularis: myoinhibitory peptide and SIFamide. J. Comp. Neurol. 517, 551-563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Šimo L., Koči J., Žitňan D., Park Y. (2011). Evidence for D1 dopamine receptor activation by a paracrine signal of dopamine in tick salivary glands. PLoS ONE 6, e16158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Šimo L., Koči J., Park Y. (2013). Receptors for the neuropeptides, myoinhibitory peptide and SIFamide, in control of the salivary glands of the blacklegged tick Ixodes scapularis. Insect Biochem. Mol. Biol. 43, 376-387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Šimo L., Koči J., Kim D., Park Y. (2014). Invertebrate specific D1-like dopamine receptor in control of salivary glands in the black-legged tick Ixodes scapularis. J. Comp. Neurol. 522, 2038-2052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatchell R. J. (1967). Salivary secretion in the cattle tick as a means of water elimination. Nature 213, 940-941 [Google Scholar]
- Vernon W. I., Printen J. A. (2002). Assay for intracellular calcium using a codon-optimized aequorin. Biotechniques 33, 730, 732, 734 [DOI] [PubMed] [Google Scholar]
- Walker A. R., Fletcher J. D., Gill H. S. (1985). Structural and histochemical changes in the salivary glands of Rhipicephalus appendiculatus during feeding. Int. J. Parasitol. 15, 81-100 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.