Abstract
Subunit vaccination benefits from improved safety over attenuated or inactivated vaccines, but their limited capability to elicit long-lasting, concerted cellular and humoral immune responses is a major challenge. Recent studies have demonstrated that antigen delivery via nanoparticle formulations significantly improve immunogenicity of vaccines due to either intrinsic immunostimulatory properties of the materials or by co-entrapment of molecular adjuvants such as Toll-like receptor agonists. These studies have collectively shown that nanoparticles designed to mimic biophysical and biochemical cues of pathogens offer new exciting opportunities to enhance activation of innate immunity and elicit potent cellular and humoral immunity with minimal cytotoxicity. In this review, we present key research advances that were made within the last 5 years in the field of nanoparticle vaccine delivery systems. In particular, we focus on the impact of biomaterials composition, size, and surface charge of nanoparticles on modulation of particle biodistribution, delivery of antigens and immunostimulatory molecules, trafficking and targeting of antigen presenting cells, and overall immune responses in systemic and mucosal tissues. This review describes recent progresses in the design of nanoparticle vaccine delivery carriers, including liposomes, lipid-based particles, micelles and nanostructures composed of natural or synthetic polymers, and lipid-polymer hybrid nanoparticles.
Keywords: Nanoparticle, vaccination, subunit vaccine, liposomes, polymeric particles
1. Introduction
Vaccination is considered to be the most cost-effective strategy for controlling infectious diseases (1). Ever since the first documented case of vaccination performed by Edward Jenner in 1796 against small pox, vaccination has been instrumental in reducing fatalities associated with life-threatening diseases including polio, measles, and diphtheria. Despite such huge success, there are still a large number of infectious pathogens, including human immunodeficiency virus (HIV), Hepatitis C, and Mycobacterium tuberculosis, for which effective vaccines are not yet available (2,3). Although traditional vaccine formulations, including live-attenuated or inactivated/killed pathogens, are very effective at generating high avidity and long-lasting immune responses, their clinical translation for fatal pathogens such as HIV have been challenging because of safety concerns and risks stemming from incomplete inactivation process, potential reversion to virulent form, and pre-existing anti-vector immunity (4,5).
In contrast, the new generation subunit vaccines, including peptides, recombinant proteins, and DNA, can potentially address these long-standing challenges. Subunit vaccines either synthesized or purified from pathogens are easy to manufacture and safe to administer with minimal toxicity. However, their major drawbacks are weak immunogenicity and short-term immune responses. This necessitates formulation of subunit vaccines with immunopotentiating adjuvants, which can be classified into two major categories; 1) delivery vehicles (e.g. emulsions and micro/nanoparticles) that enhance antigen delivery and presentation by antigen-presenting cells (APCs), and 2) immunostimulators (e.g. ligands for Toll-like receptors (TLRs)) that activate innate immunity and stimulate potent adaptive immune responses. Although numerous experimental adjuvants have been explored over the last few decades, their clinical translation has been extremely slow. Alum-based mineral salts that have been used since the 1930’s still continue to be the major adjuvants used in the United States (6). Alum-based adjuvants may be sufficient for eliciting humoral immune responses with acceptable safety profiles, but they are poor immunostimulators of CD4+ and CD8+ T cell-mediated immune responses, hence limiting their potential use in vaccines designed for intracellular pathogens and cancer (2). Thus, there is an urgent need for novel adjuvants and formulations that can induce long-term humoral and cellular immune responses without toxicity and virulence typically associated with traditional microbe-based vaccines.
Recent advances in our understanding of antigen presentation by innate immune cells and their interaction with adaptive immune system have facilitated a rational approach for design and development of vaccine delivery systems. The key elements of an effective vaccine are 1) an antigen against which adaptive immune responses are elicited, 2) an immunopotentiator to stimulate the innate immune system, and 3) a delivery system to ensure targeting of antigen and immunopotentiator to APCs. In this aspect, nanoparticle-based vaccine delivery systems engineered to meet these criteria have multi-fold advantages over traditional vaccines; 1) encapsulation of antigens in particles prevents antigen degradation and increases their stability; 2) co-encapsulation of antigen and immunostimulatory agent in particles enhances immunogenicity and potency of vaccines; 3) APCs can readily phagocytose and process particles; 4) particles designed for cytosolic delivery of antigens enhance cross-presentation and MHC-I presentation of antigens, thereby promoting cytotoxic T-cell lymphocyte (CTL) responses; 5) multivalent presentation of antigens on the surfaces of particles allows crosslinking of B cell receptor for enhanced humoral immune responses; and 6) surface modifications of particles with functional moieties and targeting ligands permit organ- and cell-specific targeting to lymphoid organs and APCs.
As reflected by the substantial increase in the number of publications on this topic, immunization strategies based on synthetic particulate carriers have gained considerable interest in recent years. Indeed, intense efforts in this research area have advanced our understanding of the design criteria for synthesis of biocompatible particle systems that can mimic biophysical and biochemical characteristics of pathogens and elicit robust immune responses without toxicity and anti-vector immunity. In this review, we first outline innate and adaptive immune responses that nanoparticle-based vaccines aim to modulate. We then focus our discussion on new emerging particle-based vaccine delivery systems categorized by their biomaterial platforms. Since there are a number of excellent recent reviews that provide a broad overview of this rapidly evolving field (7-11), we aim to highlight the latest key research progresses that were published within the last 5 years and discuss their potential clinical utility and impact on future vaccine design and development. Summary of our review with references to research articles is provided in Table 1.
Table 1. Recent advances in particle-based vaccines.
| Challenges | Solutions | References | |
|---|---|---|---|
| Site of Administration |
Induction of mucosal immunity via oral and intranasal administration |
Nanoparticles coated with or composed of mucoadhesive biopolymers | 68, 81, 102 |
| Use of pH-responsive particles for protection of antigens from stomach acids and subsequent release in lower digestive tract |
61, 90 | ||
| Antigen delivery to lymph nodes |
Instability of particles in vivo Ineffective lymphatic transport of particles |
Increasing stability of lipid vesicles via interbilayer crosslinking | 49-52 |
| Design of small (< 100 nm) and PEGylated nanoparticles for enhanced lymphatic drainage |
35, 36, 117-120 | ||
| Direct intranodal injection of particle vaccines | 95 | ||
| Activation of APCs |
Promotion of APC activation and maturation to avoid immune tolerance |
Co-delivery of antigens and danger signals within particles | 57, 90, 91, etc. |
| Use of intrinsically immunostimulatory materials as building blocks |
72, 103, 117, 118 |
||
| Intracellular antigen release |
Antigens trapped in lysosomes are degraded |
Cell-penetrating peptides for cytosolic antigen delivery | 40, 42, 44 |
| pH-responsive polymers for endosome escape |
45, 46, 113- 115, 125 |
||
| Reduction-sensitive conjugation of antigens to particles | 113-120 | ||
| Vaccine preparation |
Loss of antigenicity and immunogenicity during particle synthesis |
Antigen loading into polymeric particles in aqueous conditions via self-healing process Use of cell membrane-decorated particles for adsorption and inactivation of bacterial toxin |
99, 100 130 |
2. Principles of innate and adaptive immunity
The major goal of vaccination is to provide long-term protection against pathogen and infection by triggering the immune system to eliminate infectious agents and toxic products from the body (1). The human immune system is broadly classified into innate and adaptive components. The innate immune system includes nonspecific, constitutive set of defenses that are activated quickly after infection (within minutes). Such responses are mediated by soluble factors, such as complement proteins, and cellular effectors, such as granulocytes, mast cells, macrophages, dendritic cells (DCs) and natural killer (NK) cells. On the other hand, the adaptive immune responses take several days or weeks to become effective but provide antigenic specificity and immunological memory, both of which are required for complete elimination of pathogens. The induction of immunological memory allows rapid immunological response to subsequent exposures with the same antigen and forms the basis for successful immunization (12).
Until recently, innate immunity was merely considered as the first line of defense against pathogens. However, it is now clear that the strength and type of adaptive immune responses highly depend on the initial ‘danger’ signals recognized by the innate immune system (13). The innate immune system recognizes signatures from pathogens called pathogen associated molecular patterns (PAMPs) (14). This detection is mediated by diverse and evolutionarily conserved families of receptors called pattern-recognition receptors (PRRs) such as Toll-like receptors (TLRs) and Nod-like receptors (NLRs), expressed on a wide variety of immune cells including DCs, neutrophils, macrophages, NK cells, and B cells (15,16). Danger signals bound to PRRs activate macrophages and DCs and promote release of pro-inflammatory cytokines and chemokines and upregulation of CD80 and CD86 maturation markers, leading to initiation of antigen-specific adaptive immune responses and functional differentiation of T cells. Synthetic danger signals frequently employed in particulate vaccine design include the following molecular adjuvants. Polyinosinic:cytidylic acid (polyI:C) is a synthetic analogue of double-stranded viral RNA, known to promote strong T cell priming by activating TLR3 in macrophages and DCs (17,18). Monophosphoryl lipid A (MPLA) is a synthetic TLR4 agonist derived from lipopolysaccharide, and MPLA adsorbed to aluminum salts, also known as AS04, is currently licensed for the hepatitis B vaccine Fendrix® and human papilloma virus vaccine Cervarix® (both GlaxoSmithKline Biologicals) (13,19). Imidazoquinoline molecules such as imiquimod or gardiquimod are synthetic TLR7/8 agonists (20). Imiquimod (Aldara® Imiquimod 5% cream) is approved for external genital warts, superficial basal cell carcinoma and actinic keratosis, and a number of studies have demonstrated its potential as a vaccine adjuvant and immunotherapeutic drug (21-23). Synthetic oligonucleotide containing unmethylated CpG motifs (CpG) is a ligand for TLR9 and is known to stimulate T helper type 1 (Th1)-based cellular immune responses by inducing IL-12 production in APCs (24,25). Alpha-galactosyl ceramide (α-galcer) is a glycolipid ligand for invariant natural killer T-cells (NKT) when presented in the context of CD1d (26), and it has been shown to promote both CD4+ and CD8+ T-cell responses by inducing IFN-γ secretion from NKT cells (27).
The adaptive immune system is divided into two main types: humoral and cell-mediated immunity. Humoral immunity is mediated by antibodies (IgG, IgA, IgE, IgM, and IgD) produced by B lymphocytes and provides the principal defense mechanism against extracellular antigens. In contrast, the main component of cell-mediated immune system is T cells, whose activation is dependent on antigen-presenting cells (APCs). DCs are the most efficient APCs that capture antigens, process them, and migrate to local lymph nodes where they present antigenic peptides to T cells (28). T cells are subdivided into CD4+ T helper cells (Th) and CD8+ cytotoxic T lymphocytes (CTLs). CD8+ T cells directly kill infected cells and play a crucial role in combatting intracellular infections and cancer. CD8+ T cells recognize and respond to foreign antigens presented by major histocompatibility complex (MHC) class I molecules. On the other hand, CD4+ T-Helper cells (Th1 and Th2) recognize foreign antigens presented by MHC class II molecules. IFN-γ produced by Th1 cells has an important role in the initiation of cell-mediated immune responses and stimulates production of IgG2 antibodies. In contrast, the cytokines secreted by Th2 cells (IL-4, IL-5, and IL-10) play an important role in controlling the activation and differentiation of B cells and production of IgE and IgG1 antibodies.
3. Lipid-based delivery vehicles
3.1 Liposomes
Liposomes have been investigated extensively for drug delivery applications due to their proven clinical safety, biocompatibility, and ease of manufacturing and scale-up (29). Recent studies have focused on the impact of physicochemical characteristics of liposomal carriers on particle biodistribution, targeting of lymphoid organs and immune cells, and ultimately immune activation (30). Positively charged liposomes have been associated with a depot effect via ionic interactions with negatively charged cellular membranes, leading to prolonged antigen release at the site of injection (31). Although antigen persistence is important, co-delivery of high concentration of antigen and adjuvant was determined to be necessary to prevent tolerance (32,33). Incorporation of 10-25% poly(ethylene glycol) (PEG) on the surface of positively charged liposomes has been shown to reduce the antigen depot by decreasing particle size, screening the surface charge, and increasing clearance from the site of injection and greater accumulation in the local lymph nodes by at least 3-fold (34). Similarly, 1% PEGylation of liposomes consisting of 1,2-dioleoyl-3-trimethylammoninum propane (DOTAP) led to faster clearance from the site of injection and retention in draining lymph nodes (dLNs), resulting in 10- to 300-fold increase in IgG2a and IgG2b response compared with soluble antigen vaccination (35). Increasing PEG amount to 5% led to even faster clearance from the site of injection and dLNs, enhancing systemic circulation and accumulation in the spleen. Compared with 1% PEG formulation, immunization with 5% PEG liposomes resulted in similar IgG2a and IgG2b primary response (7 days post vaccination) but increased antibody titers by 1.5-fold three weeks after vaccination. Both formulations led to a 2-fold increase in IFN-γ production following restimulation with antigen, compared with non-PEGylated liposomes.
Positively charged liposomes formed with cationic lipid DOTAP have also been extensively investigated for delivery of plasmid DNA. DOTAP mixed with DNA spontaneously formed a virus-like structure with the condensed DNA located inside the lipid membranes, and the resulting structures promoted DC maturation and augmented anti-tumoral cellular immune responses (36). The same liposomal platform was used to deliver E7 protein and peptide antigens derived from human papillomavirus (37,38). These studies collectively have demonstrated immunogenicity of cationic liposomes. To identify cationic liposomal formulations optimized for vaccine applications, vaccinations were performed with liposomes composed of 1,2-Dipalmitoyl-sn-glycero-3-phosphocholine (DPPC) and a cationic lipid component (Dimethyl dioctadecyl-ammonium (DDA), 1,2-Dipalmitoyl-3-trimethylammonium-propane (DPTAP), 1,2-Diacyl-sn-glycero-3-ethylphosphocholine (eDPPC), or 3β-[N-(N’,N’-Dimethylaminoethane)-carbamoyl] cholesterol (DC-Chol)). DC-Chol had the most pronounced effect on antibody titers and DC maturation (39). Although the specific cause for this improved action is still under investigation, liposomes containing DC-Chol are distinctive from other liposome formulations since DC-Chol contains a sterol group and a tertiary amine head group, which affected the organization of the liposomal bilayer and prevented phase transition.
Aside from zeta potential, the size of liposomal carriers has been shown to greatly affect biodistribution. For liposomes modified with cell-penetrating peptide octaarganine (R8), PEGylation and size increase from 98 nm to 273 nm led to 4-fold increase in their localization to the spleen and IFN-γ production (40). Additionally, immunization with liposomes greater than 400 nm in diameter led to a 2-fold increase in the IgG2a/IgG1 ratio indicative of a Th1-skewed response, compared with 100 nm liposomes (41). On the other hand, studies from another group have reported contradictory findings, indicating that size does not play a role, at least for cationic liposome CAF01, composed of dimethyldioctadecylammonium (DDA) and immunostimulatory trehalose dibehenate (TDB). Regardless of liposomal size (ranging from 300 nm to 4 m), CAF01 liposomes did not show any liposome-size dependent uptake or IgG1/2 responses in vivo, whereas size-dependent increase in cell proliferation was noted with larger particles possibly due to their increased retention in lymph nodes (31).
In addition to increased antigen uptake and protection, liposomal systems offer the opportunity to co-deliver antigen and immunopotentiator for enhanced immune responses (32,33). R8-liposomes incorporated with polyinosinic:polycytidylic acid (polyI:C), a synthetic dsRNA which can signal through TLR3, promoted significantly higher CTL responses and reduced tumor growth, compared with antigen formulated with soluble polyI:C or complete Freund’s adjuvant (CFA) (42). Adsorption of polyI:C onto DOTAP liposomes containing tumor lysate led to TLR3-dependent DC activation, resulting in significant anti-tumor effects and higher CD8+ tumor infiltration (43).
Targeted intracellular antigen trafficking has been achieved by varying lipid composition of liposomes. Liposomes modified with fusogenic lipid dioleoyl phosphatidyl ethanolamine (DOPE) and cell-penetrating peptide R8 released a significantly increased amount of antigen to the cytosol and induced a 10-fold increase in antigen presentation via MHC-I pathway with at least 10-fold less dose of antigen, compared with pH-responsive or cationic liposomes (44). Further modifications of these liposomes with alpha-galactosyl-ceramide (αGC), a synthetic glycolipid and a potent activator of NKT cells, allowed efficient presentation of αGC by CD1d. Systemic administration of these liposomes increased the frequency of NKT cells by at least 3-fold compared with soluble αGC treatment group. When administered into mice bearing B16F10 lung tumors, R8/αGC-liposomes drastically reduced the number of lung tumor metastases compared with soluble αGC treatment group, demonstrating the therapeutic efficacy of liposome-based approach to boost NKT responses for cancer immunotherapy (40).
Overall, liposomes are among the most extensively investigated vaccine delivery systems owing to their biocompatible, biodegradable, and non-toxic nature. One of the main advantages of liposomes is their ability to co-deliver both antigen and adjuvant to the same antigen presenting cell, which is considered crucial for inducing potent immune response. The physiochemical properties such as size, surface charge, lipid composition, and bilayer rigidity highly influence liposome biodistribution and persistence, and these parameters can be tuned to elicit a specific type and strength of the immune response. In addition, liposomes can protect the antigens from degradation, enhance their uptake by APCs, promote release into the cytosol, and even serve an immunostimulatory function. The effectiveness of liposomes as vaccine delivery vehicles can be greatly enhanced by increasing their stability in physiological condition, which is the focus of many recent development described below.
3.2 Polymer-modified lipid vesicles
Post modification of preformed liposomes with polymers of specific function is a facile and convenient method to synthesize liposomes with desired characteristics for vaccine delivery applications. For example, liposomes have been surface-modified with pH-sensitive fusogenic polymers, such as succinylated poly(glycidol) and 3-methylglutarylated poly(glycidol), to confer endosome disruptive capability to liposomes (45). Indeed, liposomes coated with 3-methylglutarylated poly(glycidol) enhanced cytosolic delivery of antigens in DCs and induced MHC Class I antigen presentation, resulting in strong CTL activation comparable to that induced by CFA, but without adverse side effects and inflammatory reactions associated with it (45). In a follow-up study, the authors further modified their system by varying the structure of fusogenic poly(glycidol) derivatives to either linear or hyperbranched forms (Figure 1) (46). Liposomes incorporated with linear polymer structures delivered a model antigen ovalbumin (OVA) more efficiently into cytosols of DCs compared with liposomes modified with hyperbranched structures. Administration of these polymer-modified liposomes containing OVA induced strong OVA-specific CTL responses that rejected the engraftment of E.G7-OVA in a prophylactic condition and reduced tumor growth in tumor-bearing mice. In a separate study, liposomes have also been complexed with polyethyleneimine (PEI), which allowed surface adsorption of proteins with high affinity. These nanocarriers saturated with protein antigens promoted antigen uptake and presentation by APCs and induced upregulation of co-stimulatory markers and secretion of proinflammatory cytokines (47). Moreover, these liposome-polymer hybrid structures preferentially activated Th1 immune responses, and inclusion of immunomodulators, such as CpG and MPLA, within liposomes dramatically enhanced the IgA and IgG2a responses.
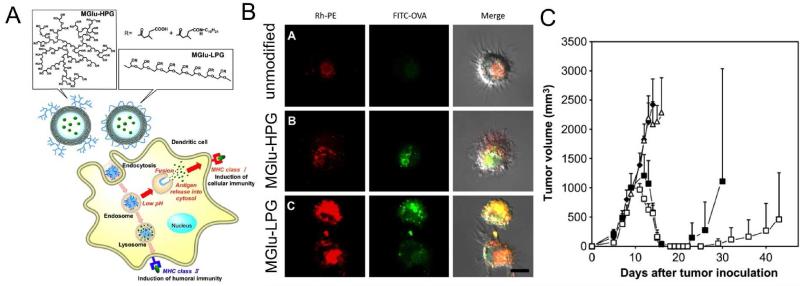
Figure 1. Fusogenic polymer-modified liposomes for cytosolic delivery of antigens.
(A) Liposomes were incorporated with pH-responsive, fusogenic poly(glycidol) derivatives in either a linear (MGlu-LPG) or hyperbranched structure (MGlu-HPG). (B) Confocal images of BMDCs treated with liposomes containing rhodamine-labeled phospholipid (Rh-PE) and FITC-OVA. MGlu-LPG induced more pronounced cytosolic delivery of antigens, compared with MGlu-HPG. (C) Mice were inoculated with E.G7-OVA cells on day 0, and vaccinated with 50 g of OVA either in unmodified liposomes (open triangles), MGlu-LPG-liposomes (open squares), or MGlu-HPG-liposomes (closed squares) on days 7 and 14. MGlu-LPG-liposomes reduced tumor volume more effectively compared with other formulations. Reproduced with permission (46). Copyright 2013, Elsevier.
3.3 Lipid-based nanoparticles
One of the major limitations of lipid vesicles for vaccine applications has been their instability in the presence of serum components (48); administration of liposomes in vivo results in rapid leakage of encapsulated macromolecules, leading to premature vesicle rupture and loss of antigens prior to reaching DCs in lymphoid organs. To address this limitation, Moon et al. developed a new approach to stabilize lipid vesicles by forming crosslinks between lipid headgroups within multilayered liposomes (49). The resulting lipid nanoparticles, called interbilayer-crosslinked multilamellar vesicles (ICMVs), encapsulated a large of amount of protein antigen, exhibited good serum stability with zero-order antigen release for more than 30 days in serum-containing media, and dramatically improved antigen delivery and uptake by DCs in lymphoid tissues compared with traditional liposomal vehicles. Importantly, ICMVs composed of crosslinked phospholipids underwent rapid degradation in an endolysosomal condition containing phospholipase, and this endosomal instability is postulated to enhance intracellular delivery of antigens and cross-presentation of antigens by DCs. Following a subcutaneous vaccination regimen consisting of a prime and two booster immunizations, ICMVs loaded with OVA and MPLA expanded OVA-specific CD8+ T cells to ~30% of the total CD8+ T cells in the systemic compartment (49). In addition, ICMVs incorporated with a candidate malaria antigen, VMP001, derived from Plasmodium vivax sporozoites, elicited significantly higher antibody titers lasting more than a year in mice with greater avidity and durability than soluble antigens mixed with conventional adjuvants, such as MPLA, alum, or Montanide (50). Stability of ICMVs also allowed deposition of these nanostructures on the surfaces of microneedles via layer-by-layer approach for transcutaneous vaccine delivery (51). Notably, non-invasive mucosal route of vaccination with ICMVs was the subject of a recent study by Li et al., who have demonstrated that pulmonary ICMV vaccination primed 13-fold more CTLs than equivalent dose of soluble vaccine and generated CD8+ T cells with mucosal homing phenotype (integrin α4β +7) (Figure 2) (52). CD8+ T cells expanded with ICMVs disseminated to both local and distant mucosal tissues, including lungs, cervico-vaginal and gastrointestinal tracts and established long-lived effector memory populations (Figure 2B). To demonstrate the protective efficacy of these memory CD8+ T cells, mice were immunized with ICMVs carrying minimal CD8+ T cell epitope antigens derived from simian immunodeficiency virus (SIV) and challenged with vaccinia virus expressing the target antigen. Mice immunized with ICMVs via pulmonary route were protected against the viral challenge and exhibited significantly reduced viral titers, whereas mice immunized with soluble vaccines succumbed to the challenge (Figure 2C) (52). These studies have highlighted the potency of ICMVs as a subunit vaccine platform for induction of systemic and mucosal immunity, and efforts to test the clinical efficacy of this new vaccine technology are underway at Vedantra Pharmaceuticals.
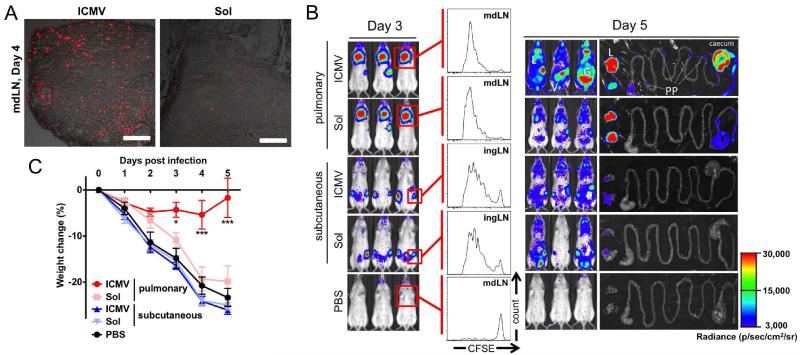
Figure 2. Elicitation of potent mucosal CD8+ T cell responses with pulmonary nanoparticle vaccination.
(A) Intratracheal administration of ICMVs loaded with fluorescent OVA (red) resulted in efficient antigen delivery to mediastinal lymph nodes draining lungs by day 4. (B) Expansion and migration of OVA-specific OT-I CD8+ T cells expressing luciferase was monitored after vaccination. Pulmonary administration of ICMVs led to robust expansion of OT-I CD8+ T cells in the lungs by day 3, and their dissemination to the lungs (L), gastrointestinal tracts (G), Peyer’s patches (PP), and vaginal tract (V) by day 5. Mice immunized via pulmonary route with soluble antigen or subcutaneous route with particles had significantly reduced signals from OT-I T cells in mucosal tissues. (C) Pulmonary vaccination with ICMVs loaded with SIV gag antigen, AL11, and PADRE peptide on days 0 and 28 protected mice against challenge by intratracheal administration of AL11-expressing vaccinia virus, whereas pulmonary vaccination with soluble antigens or subcutaneous vaccination with particles failed to protect the animals. Reproduced with permission (52). Copyright 2013, The American Association for Advancement of Science.
There are other examples of lipid-based nanoparticles that are offering an alternative drug delivery platform to colloidal drug delivery carriers. For instance, solid lipid nanoparticles have gained increasing interest for topical cosmetic and pharmaceutical applications (53). Formed by either high-pressure homogenization of lipid molecules or microemulsion technique, these solid lipid nanoparticles offer opportunities to sustain release of lipophilic and hydrophilic drugs. Cationic solid lipid nanoparticles (cSLN) capable of adsorbing negatively charged DNA have been recently developed for delivery of coding sequences for three different types of cysteine proteinases in Leishmania major (54). These lipid-based nanoparticles effectively protected the cargo from nucleases in vitro, and particles with ~250 nm diameter and positive zeta potential enhanced DC targeting, phagocytosis, and transfection efficiency. Additionally, augmented DC activation and maturation were achieved by incorporation of a positively charged lipid with known adjuvant characteristics (DOTAP) and CpG, which were integrated in pDNA. Administration of cSLN-pDNA led to 1.76-fold greater IFNγ/IL-5 ratio compared with pDNA alone, indicating promotion of a Th1 response necessary for protection against L. major. Enhanced IgG2a/IgG1 ratio with cSLN-pDNA vaccination also corroborated Th1-skewed response. In the end, cSLN-pDNA vaccine successfully decreased parasite burden by 86.3%, which is a significant reduction compared with the previously reported mean average of parasite load reduction of 59.2% for DNA alone vaccination.
Another class of emerging lipid-based nanoparticles for vaccine delivery includes cubosomes, which consist of multiple lipid bilayers interrupted by numerous aqueous channels (55). Cubosomes have high loading capacity for both hydrophilic and hydrophobic bioactives due to their aqueous core and lipid matrix with large surface area, and they have been shown to retard drug release in physiological condition. Cubosomes can be prepared by high-energy mechanical dispersion of lipids self-assembled into viscous cubic phase. Alternatively, they can be formed by solvent dilution method of dispersing liquid crystal-forming lipid, polymers, and hydrotrope in excess water. Cubosomes formed with minimal energy input with the solvent dilution method have been recently investigated for vaccine applications (56,57). Cubosomes were shown to efficiently encapsulate OVA along with MPLA and imiquimod (TLR4 and TLR7 agonists, respectively) and sustain their release over 1 week in vitro. Cubosomes with adjuvants were more effective at generating OVA-specific humoral immune responses and CD8+ and CD4+ T cell responses, compared with conventional liposomes or antigen adsorbed with alum (57).
The recent studies highlighted in this section focused on improving physical stability of lipid-based vaccine delivery carriers. These emerging nano-formulations have high serum stability, resistance to enzyme-mediated degradation, good drug loading efficiency, and strong immunogenic properties, offering new versatile platform technology for vaccine development.
4. Natural polymer-based particles
4.1 Chitosan
Chitosan is a natural polyaminosaccharide derived primarily from exoskeletons of crustaceans. It consists of copolymers of β-(1-4)-linked D-glucosamine (deacetylated unit) and N-acetyl-D-glucosamine (acetylated unit) monomers obtained by deacetylation of chitin (58). Chitosan has attractive properties for vaccine delivery applications, including high availability, biodegradability, and biocompatibility. In addition, high density of amino groups in chitosan allows facile chemical modification, complexation with negatively charged antigens, and muco-adhesiveness (59,60). Chitosan has been formulated into nano/microparticles without the use of organic solvents or high shear stress via ionotropic gelation and self-assembly of polyelectrolytes, preserving immunogenicity of antigens (61,62). Antigen-loaded chitosan nanoparticles significantly enhanced upregulation of co-stimulatory markers on APCs and release of pro-inflammatory cytokines (e.g. IL-1β, IL-6, TNF-α) compared with soluble antigen. Chitosan particles carrying OVA promoted antigen presentation via MHC-I and MHC-II pathways in DCs and induced higher proliferation of antigen-specific CD4+ and CD8+ T cells than equivalent dose of soluble antigen (63). Interestingly, both macrophages and DCs exhibited maximum antigen uptake with 1 μm diameter chitosan particles, while 300 nm and 3 μm diameter particles had decreased antigen uptake in vitro (63).
High density of amino groups in chitosan permits its interaction with negatively charged antigens, plasmid DNA, and other anionic biopolymers. Exploiting these charge-mediated ionic interactions, a wide variety of proteins and DNA vaccines have been incorporated into chitosan nano/micro particles. Among these, chitosan nanoparticles coated with alginic acid have unique pH-responsive properties that make them an ideal platform for oral vaccination (61). To synthesize these particles, amino groups within chitosan-plasmid DNA complexes were reacted with carboxyl groups in alginic acid, resulting in a crosslinked nanoparticle system stable at pH 7.0. In acidic conditions of simulated gastric fluid (pH 1.5), the particles aggregated into micrometer-sized complexes while excess alginic acid, which is insoluble at pH 1.5, self-assembled into amorphous shell on the surfaces of particles, thereby protecting the encapsulated DNA against enzymatic and acidic degradation. As pH increased to 7.0, the aggregated particles dispersed into nanoparticles. Oral administration of particles carrying DNA for legumain, an asparaginyl endopeptidase overexpressed in tumor cells, enhanced DNA uptake by macrophages and DCs in intestinal Peyer’s patches, and increased the frequency of legumain-specific cytotoxic T cells (CD8+CD25+) while inhibiting regulatory T cells (CD4+CD25+). When mice bearing orthotopic 4T1 breast tumor were vaccinated with these particles via oral gavage, significant reduction in tumor volumes was observed, suggesting suitability of this system for oral DNA vaccination (61). Chitosan core nanogels coated with mannosylated alginate, which is an effective targeting ligand for C type lectin receptor expressed by DCs have also been prepared (64). Surface adsorption of antigens on these particles significantly enhanced antigen uptake by DCs, while incorporation of adjuvants allowed modulation of immune responses. Alginate-chitosan nanogels loaded with Pam3Cys-SK4 (a TLR2 agonist) enhanced IL-1β production, whereas particles admixed with CpG impaired production of IFN-α, IL-6, and TNF-α, demonstrating the importance of selecting optimal combination of delivery vehicle and immunostimulatory agents for ensuring appropriate immune activation. High density of cationic charges on chitosan can also facilitate interaction with negatively charged mucin on mucosal surfaces, providing molecular attractive forces necessary for mucoadhesion. The extent of mucoadhesion of chitosan microspheres was shown to be highly dependent upon their zeta potential; chitosan microspheres formed by emulsification and ionotropic gelation had higher cationic surface charge and superior mucoadhesiveness, compared with chitosan particles formed by thermal crosslinking, glutaraldehyde crosslinking, or tripolyphosphate crosslinking (65).
Despite its salient features as a vaccine delivery platform, one of the major limitations of chitosan for biomedical applications is its low solubility in physiological conditions. Chitosan with high density of acetylated monomers is soluble only in acidic medium with pH < 5 (66). To overcome this limitation, several chitosan derivatives such as trimethyl chitosan and hydroxyethyl chitosan have been developed (59). Among these, trimethyl chitosan (TMC), formed by quaternization (methylation) of amino groups in chitosan, has been extensively investigated for vaccine applications. TMC nanoparticles co-encapsulating OVA antigen and immunostimulatory agents, such as lipopolysaccharide, NOD-like receptor 2 ligand muramyl dipeptide, and CpG, resulted in higher IgG, IgG1 and secretory IgA levels than non-adjuvanted TMC/OVA particles after intranasal or intradermal vaccination (67). TMC has also been further modified with thiol groups, which allowed self-assembly process with thiolated hyaluronic acid via ionic interactions (68). Spontaneous disulfide formation between thiolated TCM and hyaluronic acid led to formation of chitosan/hyaluronic acid hybrid nanoparticles. These TMC-S-S-HA particles were coupled with maleimide PEG on their surfaces to shield the surface charge. The resulting particles higher stability in saline solutions, and vaccination with these particles via intranasal or intradermal routes enhanced elicitation of IgG responses, compared with non-stabilized particles (68).
Overall, these studies suggest great potential of chitosan based nano/micro particles for vaccine delivery. In particular, their high bioavailabiliy, mucoadhesiveness, and biodegradable nature, combined with compatibility for oral and intransal route of administration, position chitosan-based particles as an ideal vaccine carrier for mucosal vaccination. We expect that future studies focused on addressing their low aqueous solubility at physiological conditions, irregular particle size distribution, and low target specificity can lead to highly effective vaccine formulations.
4.2 Gamma polyglutamic acid
Gamma polyglutamic acid (γ-PGA), a high molecular weight polypeptide composed of linked glutamic acid units and carboxylate side chains, is produced by certain strains of Bacillus subtilis, which are natural components of a traditional Japanese food item “natto” (69). The major advantages of γ-PGA polymer for biomedical applications include its water solubility and biodegradability. Recent reports have described modification of γ-PGA with hydrophobic L-phenylalanine ethyl ester, which converts γ-PGA into amphiphilic biopolymers with an ability to self-assemble into nanoparticles. A variety of antigens, including HIV-1 p24, HIV-1 gp120, and Japanese encephalitis envelope protein have been encapsulated and delivered via γ-PGA nanoparticles (69-73). These studies have demonstrated that γ-PGA nanoparticles can potently activate both humoral and cellular immune responses. In addition, γ-PGA nanoparticles were efficiently internalized by immature human monocyte-derived DCs and promoted their maturation and secretion of chemokines and inflammatory cytokines (74). Mice immunized with OVA-loaded γ-PGA nanoparticles had significantly higher frequency of antigen-specific CD8+ T cells, compared with mice immunized with soluble OVA alone, OVA + alum, or OVA + MPLA (75). In a follow-up study, γ-PGA nanoparticles without any additional adjuvant molecules were found to induce innate and adaptive immune responses via TLR4 and MyD88 signaling pathways, suggesting their intrinsic immunostimulatory properties (72). Strategies to further enhance immunogenicity of γ-PGA nanoparticles include incorporation of danger signals. Stable encapsulation of CpG into poly(γ-glutamic acid)-graft-L-phenylalanine ethyl ester (γ-PGA-Phe) nanoparticles was achieved with polycationic protamine that stabilized CpG (76). This system allowed synergistic stimulation of TLR4 and TLR9 in macrophages by γ-PGA-Phe nanoparticles and induced potent antigen-specific cellular immunity.
Size of γ-PGA nanoparticles has been shown to influence antigen uptake by APCs and their maturation and migration to lymph nodes (76). γ-PGA nanoparticles with 40 nm diameter promoted highest activation of DCs and their migration to lymph nodes as compared with 100 nm or 200 nm nanoparticles. Using a pH-insensitive, self-quenched OVA conjugate (DQ-OVA) that exhibits bright fluorescence upon proteolytic degradation, the authors have shown that free DQ-OVA degraded much faster than DQ-OVA delivered via γ-PGA nanoparticles. Interestingly, DQ-OVA formulated into larger (200 nm diameter) γ-PGA nanoparticles degraded at a much faster rate, compared with protein delivered via 40 nm nanoparticles. This is in contrast to a previous study that showed faster rate of degradation when antigens were delivered via small polystyrene nanoparticles (50 nm) compared with larger 500 nm and 3 m particles (77). Although the detailed mechanism is not clear yet, the authors suggested that the unique amide bond in γ-PGA formed between α-amino and γ-carboxylic acid groups may have contributed to the polymer’s inherent resistance to intracellular proteases and distinctive degradation kinetics.
γ-PGA nanoparticles also have been utilized as a vaccine platform for cancer immunotherapy. Intranasal vaccination with OVA-loaded γ-PGA led to rapid particle uptake by nasopharyngeal-associated lymphoid tissue, antigen delivery to the cervical lymph nodes, and induction of OVA-specific IFN-γ producing cells in the spleen and lymph nodes (78). Furthermore, mice vaccinated intranasally with OVA/γ-PGA nanoparticles were able to resist challenge by E.G7-OVA tumor cells and completely suppressed the formation of lung metastasis by B16-OVA cells. In a separate study, γ-PGA nanoparticles complexed with benzalkonium chloride permitted efficient loading of OVA (79). Mice immunized with these particles generated balanced Th1 and Th2 antibody titers and were protected against challenge with E.G7 cells expressing OVA. Although their efficacy in therapeutic tumor models is yet to be demonstrated, these studies have suggested the potential clinical utility of γ-PGA nanoparticles for cancer vaccine.
In short, γ-PGA nanoparticles have great potential not only as enhanced antigen delivery system but also as an effective vaccine adjuvant that generates both humoral and cellular immune response. For successful development of γ-PGA-based vaccines, more studies are warranted to better understand the mechanism for intracellular degradation of γ-PGA and their interaction with APCs.
4.3 Hyaluronic acid
Hyaluronan (or hyaluronic acid, HA) is a linear polysaccharide composed of repeating disaccharide units of D-glucuronic acid and N-acetyl-D-glucosamine. HA is a normal component of cartilage, and its high density of carboxylate groups helps to retain water and provide cartilage’s resistance to compression. In addition, HA plays critical roles in immune responses by modulating leukocyte trafficking into inflamed skin, maturation and migration of epidermal dendritic cells, and T-cell activation during antigen presentation (80). Due to its biocompatibility, biodegradability, muco-adhesiveness, and good safety profile, HA-based delivery systems have been widely explored for vaccine applications. Intranasal immunization with HA-based microspheres loaded with influenza hemagglutinin antigen and a mucosal adjuvant Escherichia Coli Heat-Labile Toxin (LTK63) was shown to enhance serum IgG levels and hemagglutination inhibition titers (81).
HA, which is found in normal skin tissues, has been utilized as a base material for preparation of transcutaneous microneedles. MicroHyala®, a microneedle patch comprising of sodium hyaluronate has been used to deliver a wide range of antigens derived from tetanus, diphtheria, malaria sporozoites, and influenza virus through skin (82). Antigen-loaded microneedle patches fabricated by micromolding technologies were designed to undergo degradation after insertion into skin, effectively delivering antigens into stratum corneum. Vaccination with microneedles generated comparable IgG antibody titers as vaccination via subcutaneous injection. Biodegradable characteristic of HA-based microneedles offers a superior alternative to the conventional microneedles with regard to safety, offering a simple, effective, and non-invasive vaccine delivery system (82).
An additional advantage of HA is its ability to reduce growth of tumors, including colorectal carcinoma, which is postulated to be mediated by activation of DCs (83). Administration of DCs preconditioned with low molecular weight HA led to dramatically enhanced DC trafficking to tumor-regional lymph nodes in vivo (84). Vaccination of tumor-bearing mice with DCs preconditioned with HA and pulsed with colorectal carcinoma tumor lysate resulted in significantly increased anti-tumor CTL responses and superior long-term protection against tumor recurrence, compared with administration of DCs pulsed with tumor lysate alone.
In summary, HA and other natural polymers are attractive materials for vaccine delivery applications due to their high abundance in nature, hydrophilicity, biodegradability, and tissue-specific interactions. Recent studies focused on improving the physical stability of natural polymer-based nanoparticles have led to new vaccine formulations with enhanced efficiency of antigen delivery, especially for mucosal routes of vaccination. Future studies should focus on utilization of tissue-targeting properties of these biopolymers and elaboration of mechanism of action, including inherent immunogenic properties of these biopolymers.
5. Synthetic polymer-based particles
5.1 Poly(lactic-co-glycolic acid)
Biodegradable polymeric nanoparticles have gained significant attention for their potential in drug and vaccine delivery applications. Synthetic polymers that are commonly used in biomedical applications include aliphatic polyesters such as poly(lactic acid), poly(glycolic acid), poly(ε-caprolactone), poly(hydroxybutyrate), and their copolymers. Among these, poly(lactic-co-glycolic acid) (PLGA) copolymer has been explored extensively for vaccine delivery due to its safety, biocompatibility, and biodegradability (85). PLGA polymer undergoes hydrolysis upon in vivo administration, forming biologically compatible and metabolizable moieties i.e. lactic acid and glycolic acid, which are eventually removed from the body by citric acid cycle (86). PLGA particles have been utilized to deliver a wide range of protein and peptide antigens, including Hepatitis B surface antigen, VMP001 malaria protein, HIV antigens, tumor-associated antigens, and whole tumor lysate (87-93). The unique capability of PLGA particles to sustain release of antigens and adjuvant molecules for several weeks to months is thought to prolong and enhance immune stimulation (32). Long-term, potent immune responses were achieved by single administration of antigen-loaded PLGA particles via subcutaneous or intramuscular route (94) or by direct injection of adjuvant-loaded PLGA particles into regional lymph nodes (95). Furthermore, the kinetics of antigen release from PLGA particles can be easily controlled by modifying the composition and molecular weight of PLGA copolymers (96). Fine-tuning of their molecular composition led to the development of PLGA particles that can release antigens and adjuvants in several phases after a single vaccination, thus avoiding the need for multiple boost injections (97,98). Yet, one of the major drawbacks for the PLGA particle system has been the potential exposure of antigens to harsh organic solvents since conventional technique for loading antigens into PLGA particles involves formation of single or double emulsion (O/W or W/O/W) and solvent evaporation. However, with the recent advances in micro-encapsulation of biomolecules into pre-formed, self-healing PLGA particles, antigens can be loaded into PLGA vaccine particles in aqueous condition with minimal loss of their integrity and antigenicity (99,100).
An important attribute of PLGA nanoparticles for vaccine applications includes their ability to co-encapsulate antigens and adjuvants. Oral administration of PLGA nanoparticles loaded with OVA antigen and MPLA adjuvant was shown to induce significantly higher IgG and IgA antibody titers when compared with OVA solution or PLGA nanoparticles loaded with OVA alone (101). Primard et al. have demonstrated effective induction of mucosal immunity after intranasal administration of PLGA nanoparticles coated with muco-adhesive chitosan and loaded with antigen (bovine serum albumin - BSA) and TLR7 agonist (imiquimod) (Figure 3A) (102). Co-encapsulating BSA and imiquimod into particles prevented their enzymatic degradation and promoted their intracellular release, enhancing activation of TLRs. Systemic and intranasal immunization with PLGA nanoparticles elicited similar titers of BSA-specific IgG, but interestingly, nasal immunization elicited significantly higher levels of IgA antibodies in nasal washes, vaginal washes, and fecal samples, compared with systemic immunization, suggesting that the route of vaccination has a critical role in induction of tissue-specific immune responses. Another study has examined intranasal administration of PLGA nanoparticles coated with glycol chitosan (89). It was found that muco-adhesiveness of glycol chitosan coating prolonged the nasal residence time of PLGA nanoparticles, improved antigen uptake, and induced potent mucosal and systemic immunity against Hepatitis B surface antigen.
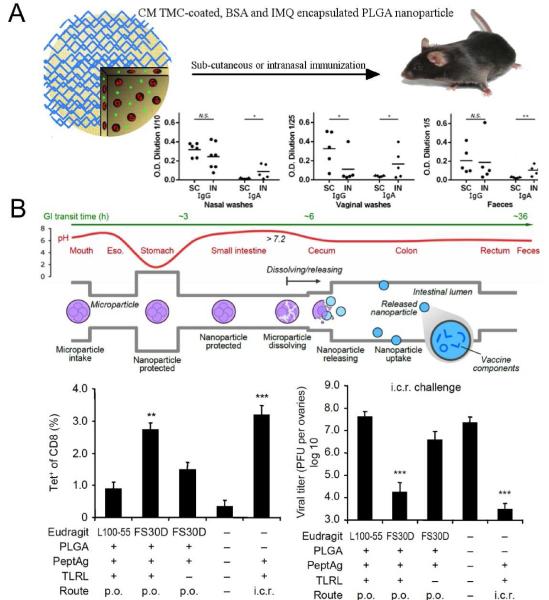
Figure 3. PLGA vaccine particles for co-delivery of antigens and adjuvant molecules.
(A) PLGA nanoparticles were coated with a chitosan derivative and co-loaded with bovine serum albumin and TLR7 agonist, imiquimod. Humoral immune responses were evaluated after three immunizations with the particles via either subcutaneous or intranasal route of administration. Local administration of PLGA particles via intranasal route induced higher IgA titers in mucosal surfaces. (B) PLGA microparticles were designed to undergo degradation in the terminal ileum, allowing uptake of particles co-loaded with antigens and TLR agonists in the large intestine. Oral administration of the particles coated with FS30D, a biodegradable polymer at pH > 7.0, led to elicitation of antigen-specific CD8+ T cells in the colorectum. However, particles coated with L100-55, which undergoes degradation at pH > 5.5 and allows particle uptake in small intestine, promoted significantly reduced frequency of tetramer+ CD8+ T cells. Orally delivered FS30D-coated PLGA particles conferred T cell-mediated resistance and reduced viral load following an intra-colorectal viral challenge. Panels (A) reproduced with permission (102). Copyright 2013, American Chemical Society. Panels (B) reproduced with permission (90). Copyright 2012, Nature Publishing Group.
PLGA nanoparticles can be rationally designed with specific physiochemical and biological properties that allows tissue specific delivery of vaccines and modulation of local immune responses. Zhu et al. have recently demonstrated an elegant strategy for oral vaccination that can protect HIV antigen and immunostimulatory molecules from the low pH and enzymatic destruction in the upper gastrointestinal tract and achieve selective vaccine delivery to the lower gastrointestinal tract (Figure 3B) (90). PLGA microparticles (≥10 m in diameter) were coated with pH responsive methacrylate-based Eudragit FS30D21 polymer. This anionic tripolymer composed of poly-(methyl acrylate, methyl methacrylate, methacrylic acid) is soluble only at pH > 7.0, thus preventing encapsulated cargo materials from being released too early in the stomach, while promoting content release in the large intestine. PLGA particles were designed to release HIV peptide antigens together with poly(I:C) and CpG in the large intestine, but not small intestine. Oral vaccination with these particles generated strong colorectal immunity and protected mice against rectal and vaginal viral challenge. Importantly, by using particles with distinctive degradation properties, this study for the first time has identified large intestine as the potential target site after oral vaccination for elicitation of protective mucosal immune responses against mucosal pathogens. To summarize, PLGA based micro/nanoparticles have emerged as promising vaccine delivery candidates due to their current clinical usage, biodegradable nature, ability to prolong antigen release and co-encapsulate antigen and immunopotentiator. We anticipate that the recent advances in self-healing process of drug encapsulation into PLGA particles, coupled with targeting capability of surface-modified PLGA particles, will allow effective delivery of vaccine components to lymphoid tissues with minimal loss of antigenicity and immunogenicity.
5.2. Polyethyleneimine
PEI is a cationic polymer, synthesized in various forms including linear or branched, and high or low molecular weight species. It is widely used as an agent for non-viral gene delivery due to its ability to form nanoscale complexes (polyplexes) with nucleic acids by electrostatic interactions (103). These polyplexes can bind heparan sulfate proteoglycans present on the surface of cells including APCs and become internalized via endocytosis. During endosomal acidification, buffering action of PEI (i.e. proton sponge effect) results in osmotic rupture of endosomes, allowing cytosolic entry of polyplexes and release of cargo. Thus, PEI-DNA polyplexes are thought to increase uptake of plasmid DNA and improve its expression, generating enhanced immune responses. Additional advantages of PEI-based DNA vaccine delivery system include its ease of preparation, stability, and high DNA loading efficiency. Indeed, it was shown that intravenous administration of PEI-DNA plasmid complexes increased the levels of gene expression by 20-400 fold and enhanced elicitation of epitope-specific CD8+ T-cell responses by 10-25 fold with higher frequency of antigen-specific Th1-helper cells (104). Memory cellular immune responses elicited by PEI-DNA complexes were able to respond rapidly and protect immunized animals against a lethal dose of recombinant Listeria monocytogenes.
Several studies have exploited the ability of PEI to promote cross-presentation through the MHC Class I pathway (105,106). Treatment of mice with PEI complexed DNA encoding for OVA resulted in antigen-specific target cell lysis and activation of B3Z cells (OVA/Kb-specific CD8+ cytotoxic T cell clone that recognizes the target cells through the class I MHC molecules), indicative of greater rates of OVA cross-presentation (106). Mice immunized with DNA/PEI complexes either before or after tumor cell inoculation suppressed tumor growth with prolonged survival rate, suggesting the efficacy of PEI-mediated DNA vaccination for anti-cancer therapy.
PEI-based vaccines have been investigated for their efficacy for elicitation of mucosal immune responses. Intranasally administered PEI/DNA vaccine induced potent mucosal and systemic immune responses against hemagglutinin from influenza A H5N1 and H1N1 2009 viruses (107). Robust CD4+ and CD8+ memory T cell responses were observed in spleen and lungs, along with secretion of IFN-γ, TNF-α, and IL-2 from splenocytes as well as lung T cells. Moreover, the H5N1 vaccine elicited full protection against the parental strain and partial cross-protection against a distinct highly pathogenic H5N1 strain. Similarly, PEI-mediated intranasal immunization with plasmid DNA for influenza hemagglutinin or herpes simplex virus type-2 (HSV-2) glycoprotein D elicited robust IgG and IgA antibody titers and provided superior protection against lethal challenge with influenza virus, compared with a strong experimental mucosal adjuvant cholera toxin B (103). The authors have attributed the enhanced immunogenicity of PEI-based vaccines to 1) increased antigen uptake by APCs, 2) improved trafficking of DCs to dLNs, 3) induction of non-proinflammatory cytokines including IL-4, IL-5 and IL-13, and 4) stimulation of Irf3-dependent signaling.
To improve vaccine delivery, a multifunctional system consisting of a protein antigen and its encoding plasmid linked to maltosylated PEI nanocomplexes has been developed (108). High charge density on PEI enabled complex formation with plasmid DNA, while the maltosylated moieties induced high-affinity bonding with protein antigens. Immunization with PEI nanocomplexes triggered potent antibody responses and IFN-γ-producing CD8+ T cells against human papillomavirus. Since sugar receptors are abundantly expressed on APCs, modification of PEI with sugar moieties may have contributed to receptor-mediated endocytosis of PEI nanocomplexes (109). In a separate study, PEI complexed with alginate has been synthesized into biodegradable and non-biodegradable nanogel formulations. The nanogels were shown to increase in vitro antigen uptake by DCs as compared to soluble antigen alone, with the biodegradable formulation being more effective at cytosolic antigen release (110). Additionally, this formulation was effective at inducing DC maturation in vitro and significantly increased IgG and IFN-γ production and frequency of antigen-specific CD8+ T-cells in vivo.
PEI-based systems, which have been traditionally used as a transfection agent, may provide an effective platform for DNA immunogens, and recent studies indicating their potent mucosal adjuvant activity further highlight the potential of PEI in vaccine applications. Future studies should be directed to address cytotoxicity associated with PEI for its clinical use in biomedical applications.
5.3 Acrylic acid based polymers
Polyalkyl acrylate based polymers including polymethylmethacrylate (PMMA), poly(ethylacrylic acid), poly(propylacrylic acid) and poly(butylacrylic acid) have been investigated for vaccine delivery, and several studies have demonstrated good adjuvant effect of polyacrylate-based nanoparticles with a considerable number of antigens (111,112). The ease of preparation, good safety record, and biodegradable nature make them an excellent vaccine delivery vehicle. Notably, Flanary et al. reported development of a vaccine delivery system based on poly(propylacrylic acid) (PPAA) with pH-sensitive and membrane disruptive properties (113). PPAA becomes protonated at low pH of endosomes and gains the ability to destabilize membranes - a unique feature that mimics membrane fusion induced by pathogenic proteins such as hemagglutinin and diphtheria toxin. OVA was conjugated to PPAA polymer by a disulfide bond, which allowed antigen release by glutathione reduction in cytoplasm. Antigen-polymer nano-conjugate increased intracellular accumulation of OVA and enhanced MHC-I presentation and subsequent CTL activation by 22-fold as compared with free OVA. In a subsequent study, antigen-PPAA conjugate was condensed into a particulate formulation by ionic complexation of anionic PPAA-OVA conjugate with cationic poly(dimethylaminoethyl methacrylate) (114). In-vivo administration of PPAA-containing formulations induced 8-fold increase in generation of OVA-specific CD8+ T cells and 11-fold increase in production of anti-OVA IgG as compared with saline or OVA solution. Using an EG.7-OVA mouse tumor model, the authors showed that PPAA-containing carriers suppressed tumor growth and increased tumor-free survival rate by 3.5-fold compared with saline or OVA solution.
Building upon these results, Wilson et al. recently reported development of pH-responsive, endosome-lytic polymer micelle structures designed for dual-delivery of antigen and immunostimulatory DNA oligonucleotides (Figure 4) (115). To construct the micellar structure, amphiphilic diblock copolymer with two multifunctional modules was synthesized. The first block consisted of cationic dimethylaminoethyl methacrylate and pyridyl disulfide ethyl methacrylate for electrostatic interaction with CpG and reversible disulfide bond formation with thiol-bearing antigens, respectively. The second block composed of PAA, DMAEMA, and butyl methacrylate had hydrophobic and endosome-lytic properties that promoted micelle assembly and cytosolic antigen delivery. The resulting micelles were ~30 nm in diameter and exhibited potent pH-dependent membrane destabilizing activity. Conjugation of OVA to the micellar structures significantly enhanced antigen cross-presentation and elicited significantly higher CD8+ T cell response as compared with free OVA or physical mixture of components. Incorporation of CpG into the micelles further increased the CD8+ and CD4+ T cell responses by ~7-fold as compared with OVA conjugates and elicited balanced IgG1/IgG2c antibody responses.
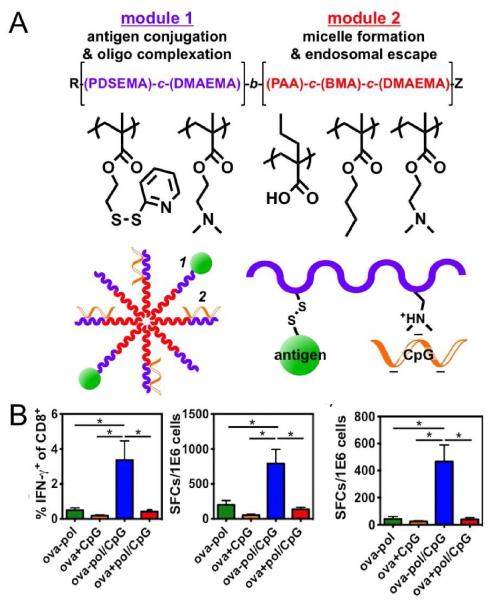
Figure 4. Nanoparticle vaccine based on pH-responsive copolymers for cytosolic delivery of antigen and immunostimulatory molecules.
(A) Schematic for the amphiphilic diblock copolymers with (i) hydrophilic and cationic block for conjugation of antigen and electrostatic complexation with CpG and (ii) hydrophobic and endosomolytic block for micelle assembly and cytosolic delivery of antigens. (B) Mice were immunized with conjugates (ova-pol), ova mixed with free CpG (ova+CpG), dual-delivery vehicles (ova-pol/CpG), and free ova mixed with CpG/micelle complexes (ova+pol/CpG). Splenocytes were restimulated ex vivo with free OVA257-264 for IFN-γ production among CD8+ T cells detected via intracellular cytokine staining (left panel) and ELISPOT (middle panel). ELISPOT quantification of IFN-γ production among CD4+ T cells after splenocytes restimulation with OVA323-339 (right panel). Reproduced with permission (115). Copyright 2013, American Chemical Society.
In summary, acrylic acid based polymers with ‘smart’ capability for pH-responsive membrane-destabilization allows delivery of therapeutic peptides, proteins, and nucleic acids into the cytoplasm of targeted cells, and this unique feature may be exploited for enhancing cross-presentation of antigens and elicitation of cellular immune responses.
5.4 Polypropylene sulfide
Fully synthetic and biodegradable Pluronic-stabilized polypropylene sulfide (PPS) nanoparticles have been developed as a new antigen nanocarrier system. (116). Surface of PPS nanoparticles can be precisely engineered to display specific chemical moieties or antigens in a disulfide-sensitive manner. It was shown that surface chemistry on PPS nanoparticles can directly influence activation of complement cascade, thereby modulating generation of danger signals in situ and initiation of adaptive immune responses (117,118). The size of PPS nanoparticles has been shown to be a major factor determining their lymphatic transport and uptake by APCs. After intradermal injection, PPS nanoparticles in the size range of 30 nm drained rapidly through lymphatics to the dLNs, whereas lymphatic transport of 100 nm nanoparticles was only 10% as efficient (117). Similarly, intranasal vaccination with PPS nanoparticles resulted in penetration of particles through the nasal epithelium, transition via M cells, and particle uptake by APCs in the nasal-associated lymphoid tissue (119). Interestingly, for intranasal route of vaccination, as the size of PPS nanoparticles was increased from 30 nm to 200 nm, antigen was more effectively delivered via both MHC-I and MHC-II presentation pathways (119). Moreover, intranasal immunization with 200 nm nanoparticles significantly increased the frequency of antigen-specific lung CD4+ T cells producing IFN-γ, TNF-α, or IL-2 and enhanced systemic and mucosal humoral responses in the lungs compared with 30 nm particles. As noted by the authors of this study, this seemingly contrasting impact of particle size on the resulting immune responses suggests that physiology of the site of vaccination is the major determinant for draining of particles to lymphoid tissues and antigen delivery to APCs.
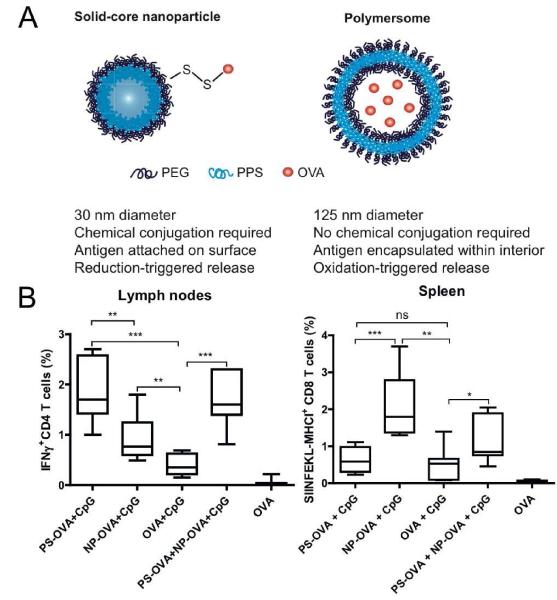
Two different platforms composed of PPS polymer were compared for their potency as vaccine vehicles (Figure 5A). Solid-core Pluronic-stabilized PPS nanoparticles were conjugated with protein antigens on their surface via a disulfide linker, while PEG17-bl-PPS30 polymersomes were loaded with antigens within their aqueous core (120). Polymersomes are stable self-assembled polymeric shells capable of releasing hydrophobic as well as hydrophilic moieties (121,122). Mice immunized with polymersomes exhibited enhanced induction of IFN-γ-producing CD4+T cells in spleen, lymph nodes, and lungs compared with mice immunized with PPS nanoparticles (Figure 5B). On the other hand, PPS nanoparticles surface-displaying OVA significantly enhanced the clonal expansion of antigen-specific CD8+ T cells than polymersomes. Taking advantage of the distinct behaviors of two nanocarriers, the authors combined the two systems (nanoparticles and polymersomes) in a single vaccine formulation, which generated concerted CD4+ and CD8+ T cell responses, thus positioning PPS-based delivery systems as ideal vaccine nanocarriers for induction of robust cellular immune responses (120).
Figure 5. Nanoparticle vaccine based on pH-responsive copolymers for cytosolic delivery of antigen and immunostimulatory molecules.
(A) Solid-core nanoparticles and aqueous core polymersomes were compared for induction of immune responses. Solid-core nanoparticles had antigens conjugated on their surface via a reduction-sensitive disulfide bond. Polymersomes loaded with antigens in the aqueous core were synthesized by self-assembly of a block copolymer, consisted of hydrophilic poly(ethylene glycol) in the outer and inner core and hydrophobic poly(propylene sulfide) in the center of the lamella. (B) Mice immunized with polymersomes (PS-OVA+CpG) or nanoparticles (NP-OVA+CpG) were analyzed for OVA-specific CD4+ and CD8+ T cell responses with intracellular cytokine staining and tetramer staining. Polymersomes were more effective than nanoparticles at induction of IFN-γ producing CD4+ T cells. In contrast, nanoparticles elicited higher frequency of OVA-specific CD8+ T cells than polymersomes. Reproduced with permission (120). Copyright 2013, Elsevier.
6. Lipid coated polymeric particles
Lipid-polymer hybrid nanoparticles (LPNs) have emerged as a promising vaccine delivery platform in the past few years. LPNs combine the advantages of polymeric nanoparticles with physical stability and liposomes with biomimetic characteristics (123,124). In general, LPNs have the following three components; 1) a polymer core, 2) phospholipid layer enveloping the polymer core, and 3) an outer lipid-PEG layer. Therapeutic drugs and proteins can be encapsulated inside the polymeric core while lipid layer provides biocompatibility and biomimetic properties to the polymeric core. The outermost lipid-PEG coating provides steric stabilization and prolongs the in-vivo circulation time of particles (123).
LPNs consisting of poly-(β-amino ester) (PBAE) core enveloped by a phospholipid bilayer shell were prepared for delivery of mRNA-based vaccines (125). PBAE is a pH-responsive polymer capable of promoting endosomal disruption in acidic condition. Lipid-enveloped PBAE particles, surface-adsorbed with mRNA via electrostatic interaction were shown to disrupt endosomes in DCs, allowing cytosolic delivery of mRNA. Intranasal administration of these particles significantly enhanced the expression of reporter protein luciferase within 6 hrs as compared to naked mRNA, suggesting their potential utility for mRNA-based in vivo transfection for vaccine applications.
Similarly, pathogen-mimicking polymeric vaccine delivery system consisting of PLGA nanoparticle core coated by a lipid membrane has been reported (126,127). PLGA particles with antigen conjugated on the lipid envelope elicited high antigen-specific IgG titers (>106) that were sustained for over 100 days after two immunizations with just 2.5 ng of antigen. Insertion of lipophilic danger signals, such as MPLA and αGC further enhanced antibody titers by ~12-fold, thus providing a potent dose-sparing vaccine delivery system with reduced risk of reactogenicity. These PLGA-lipid hybrid nanoparticles were subsequently applied for vaccination against malaria Plasmodium vivax sporozoites (88). Nanoparticles co-delivering MPLA and malaria vaccine candidate, VMP001, on the outer lipid envelope promoted germinal center formation and elicited long-lasting Th1/Th2 balanced humoral immune responses. Antibodies raised by particle vaccination were able to agglutinate live Plasmodium vivax sporozoites. In a separate study, PLGA-lipid hybrid nanostructures were developed for delivery of multiple melanoma tumor-associated antigen peptides for cancer immunotherapy. Prophylactic administration of lipid-enveloped PLGA particles carrying TRP2, p15E, and hgp100 peptides suppressed growth of B16 melanoma tumor in mice (128).
In efforts to engineer biomimetic nanoparticle system, nanocarriers coated with red blood cell membrane recently gained much attention. PLGA nanoparticles “camouflaged” with erythrocyte membrane exhibited superior circulation half-life of 9.6 hr compared with 6.5 hr for PEGylated nanoparticles (129). This nanocarrier system has been utilized to adsorb staphylococcal α-haemolysin (Hla), a bacterial pore-forming toxin, on their lipid surfaces for vaccine applications (130). Hla admixed with erythrocyte membrane-coated PLGA nanoparticles lost their virulence and became incorporated into the lipid layer. Notably, compared with conventional toxoid vaccines prepared by heat-induced inactivation, this procedure was much more effective at preserving immunogenicity and antigenicity of Hla. Compared with heat-treated Hla, immunization with these novel nanotoxoids elicited higher sera IgG titers with superior capability to block haemolysis by Hla, and conferred improved protection against intravenous and subcutaneous toxin challenge. Thus, this biomimetic polymer/lipid hybrid nanoparticles offer a unique and promising approach to enhance immunogenicity and efficacy of bacterial antitoxin vaccines while minimizing the potential safety issues associated with virulence of toxoids.
The overall advantages of LPNs include controlled particle size, surface functionality, their ability to entrap a wide range of molecules, high encapsulation efficiency, tunable drug release profile, and good serum stability. In particular, LPNs can encapsulate hydrophilic antigens inside the polymeric core and entrap hydrophobic antigens and/or adjuvants in the lipid envelope, mimicking features of pathogens and providing a versatile materials platform for vaccine delivery.
7. Conclusions and future outlook
Subunit vaccines benefit greatly from nanoparticle formulations due to improved antigen uptake and targeting to APCs as well as enhanced immunogenicity stemming from either immunostimulatory features of nanocarriers themselves or adjuvant molecules co-delivered by the particles. Most particulate vaccine platforms in consideration are biodegradable and biocompatible with minimal toxicity, offering safe and effective alternatives to traditional microorganism-based vaccines. We covered a wide range of distinct nanoparticle formulations differing in composition, size, charge, and route of administration, all of which have been shown to play crucial roles in modulation of biodistribution, cellular trafficking, and overall immune response. In the end, the majority of particulate vaccines delivering subunit antigens and immunostimulatory agents improved immune responses over equivalent dose of soluble vaccines. In particular, nanoparticle vaccines with the ability to enhance cross-presentation of subunit antigens and generate strong cellular immunity provide significant improvement over traditional vaccines. Thus, these new vaccine nano-formulations may play a crucial role in future clinical development of vaccines against cancer and intracellular infections, such as HIV, TB, and Hepatitis C. In addition, a greater understanding of adjuvants and their molecular mechanisms of immune activation is needed to further refine the biophysical and biochemical features of nanoparticles for improved immunostimulation. It is also important to consider the manufacturability of these systems, as economic and facile scale-up with reproducibility in industrial settings are critical considerations for their translation into the clinic.
Although it is a beyond the scope of this review article, there have been key advances in the design of artificial antigen-presenting cells (aAPCs) based on micro/nanoparticles. For instance, the shape of PLGA microparticles has been found to directly impact CD8+ T cell responses, with stretched, ellipsoidal PLGA aAPCs decorated with anti-CD28 and MHC-IgG dimer improving CD8+ T-cell expansion by 2- to 20-fold, compared with spherical aAPCs (131). In addition, Perica et al. have recently reported the development of nanoscale aAPCs synthesized from 50-100 nm iron-dextran paramagnetic particles and 30 nm quantum dot nanocrystals (132). Both nano-aAPC systems induced antigen specific T cell proliferation and functional responses in vitro (132), with iron-dextran paramagnetic particles even allowing magnetic field-induced T cell activation (133). Notably, when administered in vivo, nano-aAPCs effectively primed adoptively transferred CTLs to attenuate tumor growth in vivo. Unlike conventional microparticle-based aAPCs, nano-aAPCs can drain efficiently via lymphatics to lymph nodes and preferentially accumulate in tumor through enhanced permeability and retention, suggesting that in situ cancer immunotherapy may benefit greatly from in vivo administration of nano-aAPCs.
In conclusion, the recent studies we have highlighted in this review represent great strides in application of nanoparticle drug delivery platforms toward prophylactic and therapeutic vaccinations. The field of nanotechnology will continue to address challenges remaining in immunology and provide innovative strategies in the future of vaccine design and development.
Acknowledgement
This study was supported by the Michigan Institute for Clinical & Health Research (MICHR) Pilot Grant Program and by the National Institute of Health grant 1K22AI097291-01.
Abbreviations
- α-galcer/αGC
alpha-galactosyl ceramide
- aAPC
artificial antigen presenting cell
- APC
antigen-presenting cell
- BSA
bovine serum albumin
- CFA
complete Freund’s adjuvant
- CpG
oligonucleotide with unmethylated CpG motifs
- cSLN
cationic solid lipid nanoparticles
- CTL
cytotoxic T-cell lymphocyte
- DC
dendritic cell
- DC-Chol
3β-[N-(N’,N’-Dimethylaminoethane)-carbamoyl] cholesterol
- DDA
dimethyl dioctadecyl-ammonium
- dLNs
draining lymph nodes
- DOPE
dioleoyl phosphatidyl ethanolamine
- DOTAP
1,2-dioleoyl-3-trimethylammoninum propane
- DPPC
1,2-Dipalmitoyl-sn-glycero-3-phosphocholine
- DPTAP
1,2-Dipalmitoyl-3-trimethylammonium-propane
- dsRNA
double stranded RNA
- eDPPC
1,2-Diacyl-sn-glycero-3-ethylphosphocholine
- γ-PGA
gamma polyglutamic acid
- HA
hyaluronic acid
- HIV
human immunodeficiency virus
- Hla
staphylococcal α-haemolysin
- HPV
human papillomavirus
- ICMVs
interbilayer-crosslinked multilamellar vesicles
- LPNs
lipid-polymer hybrid nanoparticles
- MHC-I
major histocompatibility complex class I
- MHC-II
major histocompatibility complex class I
- MPLA
monophosphoryl lipid A
- NK
natural killer
- NKT
natural killer T-cell
- NLR
nod-like receptor
- OVA
ovalbumin
- PAMP
pathogen associated molecular pattern
- PBAE
poly-(β-amino ester)
- PCL
poly(ε-caprolactone)
- PEG
poly(ethylene glycol)
- PEI
polyethyleneimine
- PGA
poly(glycolic acid)
- PHB
poly(hydroxybutyrate)
- PLA
poly(lactic acid)
- PLGA
poly(lactic-co-glycolic acid)
- PMMA
polymethylmethacrylate
- polyI:C
polyinosinic:cytidylic acid
- PPAA
poly(propylacrylic acid)
- PPS
polypropylene sulfide
- PRR
pattern-recognition receptor
- R8
octaarganine
- SIV
simian immunodeficiency virus
- TB
Mycobacterium tuberculosis
- TDB
trehalose dibehenate
- Th1
T helper type 1
- Th2
T helper type 2
- TLR
Toll-like receptor
- TMC
trimethyl chitosan
References
- 1.Pulendran B, Ahmed R. Immunological mechanisms of vaccination. Nat Immunol. 2011;12(6):509–517. doi: 10.1038/ni.2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reed SG, Bertholet S, Coler RN, Friede M. New horizons in adjuvants for vaccine development. Trends Immunol. 2009;30(1):23–32. doi: 10.1016/j.it.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 3.Mbow ML, De Gregorio E, Valiante NM, Rappuoli R. New adjuvants for human vaccines. Curr Opin Immunol. 2010;22(3):411–416. doi: 10.1016/j.coi.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 4.Brave A, Ljungberg K, Wahren B, Liu MA. Vaccine delivery methods using viral vectors. Mol Pharm. 2007;4(1):18–32. doi: 10.1021/mp060098+. [DOI] [PubMed] [Google Scholar]
- 5.Barouch DH. Challenges in the development of an HIV-1 vaccine. Nature. 2008;455(7213):613–619. doi: 10.1038/nature07352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gupta RK. Aluminum compounds as vaccine adjuvants. Adv Drug Deliv Rev. 1998;32(3):155–172. doi: 10.1016/s0169-409x(98)00008-8. [DOI] [PubMed] [Google Scholar]
- 7.Peek LJ, Middaugh CR, Berkland C. Nanotechnology in vaccine delivery. Adv Drug Deliv Rev. 2008;60(8):915–928. doi: 10.1016/j.addr.2007.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moon JJ, Huang B, Irvine DJ. Engineering nano- and microparticles to tune immunity. Advanced Materials. 2012;24(28):3724–3746. doi: 10.1002/adma.201200446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Swartz MA, Hirosue S, Hubbell JA. Engineering approaches to immunotherapy. Sci Transl Med. 2012;4(148):148rv149. doi: 10.1126/scitranslmed.3003763. [DOI] [PubMed] [Google Scholar]
- 10.Irvine DJ, Swartz MA, Szeto GL. Engineering synthetic vaccines using cues from natural immunity. Nat Mater. 2013;12(11):978–990. doi: 10.1038/nmat3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith DM, Simon JK, Baker JR., Jr. Applications of nanotechnology for immunology. Nat Rev Immunol. 2013;13(8):592–605. doi: 10.1038/nri3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sallusto F, Lanzavecchia A, Araki K, Ahmed R. From vaccines to memory and back. Immunity. 2010;33(4):451–463. doi: 10.1016/j.immuni.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guy B. The perfect mix: recent progress in adjuvant research. Nat Rev Microbiol. 2007;5(7):505–517. doi: 10.1038/nrmicro1681. [DOI] [PubMed] [Google Scholar]
- 14.Kawai T, Akira S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity. 2011;34(5):637–650. doi: 10.1016/j.immuni.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 15.Iwasaki A, Medzhitov R. Regulation of adaptive immunity by the innate immune system. Science. 2010;327(5963):291–295. doi: 10.1126/science.1183021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11(5):373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 17.Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature. 2001;413(6857):732–738. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- 18.Trumpfheller C, Caskey M, Nchinda G, Longhi MP, Mizenina O, Huang Y, et al. The microbial mimic poly IC induces durable and protective CD4+ T cell immunity together with a dendritic cell targeted vaccine. Proc Natl Acad Sci U S A. 2008;105(7):2574–2579. doi: 10.1073/pnas.0711976105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mata-Haro V, Cekic C, Martin M, Chilton PM, Casella CR, Mitchell TC. The vaccine adjuvant monophosphoryl lipid A as a TRIF-biased agonist of TLR4. Science. 2007;316(5831):1628–1632. doi: 10.1126/science.1138963. [DOI] [PubMed] [Google Scholar]
- 20.Vasilakos JP, Tomai MA. The use of Toll-like receptor 7/8 agonists as vaccine adjuvants. Expert Rev Vaccines. 2013;12(7):809–819. doi: 10.1586/14760584.2013.811208. [DOI] [PubMed] [Google Scholar]
- 21.Hemmi H, Kaisho T, Takeuchi O, Sato S, Sanjo H, Hoshino K, et al. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat Immunol. 2002;3(2):196–200. doi: 10.1038/ni758. [DOI] [PubMed] [Google Scholar]
- 22.van Stipdonk MJ, Badia-Martinez D, Sluijter M, Offringa R, van Hall T, Achour A. Design of agonistic altered peptides for the robust induction of CTL directed towards H-2 Db in complex with the melanoma-associated epitope gp100. Cancer Res. 2009;69(19):7784–7792. doi: 10.1158/0008-5472.CAN-09-1724. [DOI] [PubMed] [Google Scholar]
- 23.Ly LV, Sluijter M, Versluis M, Luyten GP, van Stipdonk MJ, van der Burg SH, et al. Peptide vaccination after T-cell transfer causes massive clonal expansion, tumor eradication, and manageable cytokine storm. Cancer Res. 2010;70(21):8339–8346. doi: 10.1158/0008-5472.CAN-10-2288. [DOI] [PubMed] [Google Scholar]
- 24.Krieg AM, Yi AK, Matson S, Waldschmidt TJ, Bishop GA, Teasdale R, et al. CpG motifs in bacterial DNA trigger direct B-cell activation. Nature. 1995;374(6522):546–549. doi: 10.1038/374546a0. [DOI] [PubMed] [Google Scholar]
- 25.Takeshita F, Gursel I, Ishii KJ, Suzuki K, Gursel M, Klinman DM. Signal transduction pathways mediated by the interaction of CpG DNA with Toll-like receptor 9. Semin Immunol. 2004;16(1):17–22. doi: 10.1016/j.smim.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 26.Kawano T, Cui J, Koezuka Y, Toura I, Kaneko Y, Motoki K, et al. CD1d-restricted and TCR-mediated activation of valpha14 NKT cells by glycosylceramides. Science. 1997;278(5343):1626–1629. doi: 10.1126/science.278.5343.1626. [DOI] [PubMed] [Google Scholar]
- 27.Hermans IF, Silk JD, Gileadi U, Salio M, Mathew B, Ritter G, et al. NKT cells enhance CD4+ and CD8+ T cell responses to soluble antigen in vivo through direct interaction with dendritic cells. J Immunol. 2003;171(10):5140–5147. doi: 10.4049/jimmunol.171.10.5140. [DOI] [PubMed] [Google Scholar]
- 28.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392(6673):245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 29.Torchilin VP. Recent advances with liposomes as pharmaceutical carriers. Nat Rev Drug Discov. 2005;4(2):145–160. doi: 10.1038/nrd1632. [DOI] [PubMed] [Google Scholar]
- 30.Watson DS, Endsley AN, Huang L. Design considerations for liposomal vaccines: influence of formulation parameters on antibody and cell-mediated immune responses to liposome associated antigens. Vaccine. 2012;30(13):2256–2272. doi: 10.1016/j.vaccine.2012.01.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Henriksen-Lacey M, Devitt A, Perrie Y. The vesicle size of DDA:TDB liposomal adjuvants plays a role in the cell-mediated immune response but has no significant effect on antibody production. J Control Release. 2011;154(2):131–137. doi: 10.1016/j.jconrel.2011.05.019. [DOI] [PubMed] [Google Scholar]
- 32.Demento SL, Cui W, Criscione JM, Stern E, Tulipan J, Kaech SM, et al. Role of sustained antigen release from nanoparticle vaccines in shaping the T cell memory phenotype. Biomaterials. 2012;33(19):4957–4964. doi: 10.1016/j.biomaterials.2012.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kamath AT, Mastelic B, Christensen D, Rochat AF, Agger EM, Pinschewer DD, et al. Synchronization of dendritic cell activation and antigen exposure is required for the induction of Th1/Th17 responses. J Immunol. 2012;188(10):4828–4837. doi: 10.4049/jimmunol.1103183. [DOI] [PubMed] [Google Scholar]
- 34.Kaur R, Bramwell VW, Kirby DJ, Perrie Y. Manipulation of the surface pegylation in combination with reduced vesicle size of cationic liposomal adjuvants modifies their clearance kinetics from the injection site, and the rate and type of T cell response. J Control Release. 2012;164(3):331–337. doi: 10.1016/j.jconrel.2012.07.012. [DOI] [PubMed] [Google Scholar]
- 35.Zhuang Y, Ma Y, Wang C, Hai L, Yan C, Zhang Y, et al. PEGylated cationic liposomes robustly augment vaccine-induced immune responses: Role of lymphatic trafficking and biodistribution. J Control Release. 2012;159(1):135–142. doi: 10.1016/j.jconrel.2011.12.017. [DOI] [PubMed] [Google Scholar]
- 36.Cui Z, Han SJ, Vangasseri DP, Huang L. Immunostimulation mechanism of LPD nanoparticle as a vaccine carrier. Mol Pharm. 2005;2(1):22–28. doi: 10.1021/mp049907k. [DOI] [PubMed] [Google Scholar]
- 37.Cui Z, Huang L. Liposome-polycation-DNA (LPD) particle as a carrier and adjuvant for protein-based vaccines: therapeutic effect against cervical cancer. Cancer Immunol Immunother. 2005;54(12):1180–1190. doi: 10.1007/s00262-005-0685-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen W, Huang L. Induction of cytotoxic T-lymphocytes and antitumor activity by a liposomal lipopeptide vaccine. Mol Pharm. 2008;5(3):464–471. doi: 10.1021/mp700126c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barnier Quer C, Elsharkawy A, Romeijn S, Kros A, Jiskoot W. Cationic liposomes as adjuvants for influenza hemagglutinin: more than charge alone. Eur J Pharm Biopharm. 2012;81(2):294–302. doi: 10.1016/j.ejpb.2012.03.013. [DOI] [PubMed] [Google Scholar]
- 40.Nakamura T, Yamazaki D, Yamauchi J, Harashima H. The nanoparticulation by octaarginine-modified liposome improves alpha-galactosylceramide-mediated antitumor therapy via systemic administration. J Control Release. 2013;171(2):216–224. doi: 10.1016/j.jconrel.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 41.Badiee A, Khamesipour A, Samiei A, Soroush D, Shargh VH, Kheiri MT, et al. The role of liposome size on the type of immune response induced in BALB/c mice against leishmaniasis: rgp63 as a model antigen. Exp Parasitol. 2012;132(4):403–409. doi: 10.1016/j.exppara.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 42.Nakamura T, Moriguchi R, Kogure K, Harashima H. Incorporation of polyinosine-polycytidylic acid enhances cytotoxic T cell activity and antitumor effects by octaarginine-modified liposomes encapsulating antigen, but not by octaarginine-modified antigen complex. Int J Pharm. 2013;441(1-2):476–481. doi: 10.1016/j.ijpharm.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 43.Wang C, Zhuang Y, Zhang Y, Luo Z, Gao N, Li P, et al. Toll-like receptor 3 agonist complexed with cationic liposome augments vaccine-elicited antitumor immunity by enhancing TLR3-IRF3 signaling and type I interferons in dendritic cells. Vaccine. 2012;30(32):4790–4799. doi: 10.1016/j.vaccine.2012.05.027. [DOI] [PubMed] [Google Scholar]
- 44.Nakamura T, Moriguchi R, Kogure K, Shastri N, Harashima H. Efficient MHC class I presentation by controlled intracellular trafficking of antigens in octaarginine-modified liposomes. Mol Ther. 2008;16(8):1507–1514. doi: 10.1038/mt.2008.122. [DOI] [PubMed] [Google Scholar]
- 45.Yuba E, Kojima C, Harada A, Tana, Watarai S, Kono K. pH-Sensitive fusogenic polymer-modified liposomes as a carrier of antigenic proteins for activation of cellular immunity. Biomaterials. 2010;31(5):943–951. doi: 10.1016/j.biomaterials.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 46.Yuba E, Harada A, Sakanishi Y, Watarai S, Kono K. A liposome-based antigen delivery system using pH-sensitive fusogenic polymers for cancer immunotherapy. Biomaterials. 2013;34(12):3042–3052. doi: 10.1016/j.biomaterials.2012.12.031. [DOI] [PubMed] [Google Scholar]
- 47.Chen CH, Lin YL, Liu YK, He PJ, Lin CM, Chiu YH, et al. Liposome-based polymer complex as a novel adjuvant: enhancement of specific antibody production and isotype switch. Int J Nanomedicine. 2012;7:607–621. doi: 10.2147/IJN.S28097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Allen TM, Mumbengegwi DR, Charrois GJ. Anti-CD19-targeted liposomal doxorubicin improves the therapeutic efficacy in murine B-cell lymphoma and ameliorates the toxicity of liposomes with varying drug release rates. Clin Cancer Res. 2005;11(9):3567–3573. doi: 10.1158/1078-0432.CCR-04-2517. [DOI] [PubMed] [Google Scholar]
- 49.Moon JJ, Suh H, Bershteyn A, Stephan MT, Liu H, Huang B, et al. Interbilayer-crosslinked multilamellar vesicles as synthetic vaccines for potent humoral and cellular immune responses. Nat Mater. 2011;10(3):243–251. doi: 10.1038/nmat2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moon JJ, Suh H, Li AV, Ockenhouse CF, Yadava A, Irvine DJ. Enhancing humoral responses to a malaria antigen with nanoparticle vaccines that expand Tfh cells and promote germinal center induction. Proc Natl Acad Sci U S A. 2012;109(4):1080–1085. doi: 10.1073/pnas.1112648109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.DeMuth PC, Moon JJ, Suh H, Hammond PT, Irvine DJ. Releasable layer-by-layer assembly of stabilized lipid nanocapsules on microneedles for enhanced transcutaneous vaccine delivery. ACS Nano. 2012;6(9):8041–8051. doi: 10.1021/nn302639r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li AV, Moon JJ, Abraham W, Suh H, Elkhader J, Seidman MA, et al. Generation of effector memory T cell-based mucosal and systemic immunity with pulmonary nanoparticle vaccination. Sci Transl Med. 2013;5(204):204ra130. doi: 10.1126/scitranslmed.3006516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Almeida AJ, Souto E. Solid lipid nanoparticles as a drug delivery system for peptides and proteins. Adv Drug Deliv Rev. 2007;59(6):478–490. doi: 10.1016/j.addr.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 54.Doroud D, Zahedifard F, Vatanara A, Najafabadi AR, Taslimi Y, Vahabpour R, et al. Delivery of a cocktail DNA vaccine encoding cysteine proteinases type I, II and III with solid lipid nanoparticles potentiate protective immunity against Leishmania major infection. Journal of Controlled Release. 2011;153(2):154–162. doi: 10.1016/j.jconrel.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 55.Shah JC, Sadhale Y, Chilukuri DM. Cubic phase gels as drug delivery systems. Adv Drug Deliv Rev. 2001;47(2-3):229–250. doi: 10.1016/s0169-409x(01)00108-9. [DOI] [PubMed] [Google Scholar]
- 56.Rizwan SB, Assmus D, Boehnke A, Hanley T, Boyd BJ, Rades T, et al. Preparation of phytantriol cubosomes by solvent precursor dilution for the delivery of protein vaccines. Eur J Pharm Biopharm. 2011;79(1):15–22. doi: 10.1016/j.ejpb.2010.12.034. [DOI] [PubMed] [Google Scholar]
- 57.Rizwan SB, McBurney WT, Young K, Hanley T, Boyd BJ, Rades T, et al. Cubosomes containing the adjuvants imiquimod and monophosphoryl lipid A stimulate robust cellular and humoral immune responses. J Control Release. 2013;165(1):16–21. doi: 10.1016/j.jconrel.2012.10.020. [DOI] [PubMed] [Google Scholar]
- 58.Illum L. Chitosan and its use as a pharmaceutical excipient. Pharm Res. 1998;15(9):1326–1331. doi: 10.1023/a:1011929016601. [DOI] [PubMed] [Google Scholar]
- 59.van der Merwe SM, Verhoef JC, Verheijden JH, Kotze AF, Junginger HE. Trimethylated chitosan as polymeric absorption enhancer for improved peroral delivery of peptide drugs. Eur J Pharm Biopharm. 2004;58(2):225–235. doi: 10.1016/j.ejpb.2004.03.023. [DOI] [PubMed] [Google Scholar]
- 60.Islam MA, Firdous J, Choi YJ, Yun CH, Cho CS. Design and application of chitosan microspheres as oral and nasal vaccine carriers: an updated review. Int J Nanomedicine. 2012;7:6077–6093. doi: 10.2147/IJN.S38330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liu Z, Lv D, Liu S, Gong J, Wang D, Xiong M, et al. Alginic acid-coated chitosan nanoparticles loaded with legumain DNA vaccine: effect against breast cancer in mice. PLoS One. 2013;8(4):e60190. doi: 10.1371/journal.pone.0060190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Prego C, Paolicelli P, Diaz B, Vicente S, Sanchez A, Gonzalez-Fernandez A, et al. Chitosan-based nanoparticles for improving immunization against hepatitis B infection. Vaccine. 2010;28(14):2607–2614. doi: 10.1016/j.vaccine.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 63.Koppolu B, Zaharoff DA. The effect of antigen encapsulation in chitosan particles on uptake, activation and presentation by antigen presenting cells. Biomaterials. 2013;34(9):2359–2369. doi: 10.1016/j.biomaterials.2012.11.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Demoulins T, Bassi I, Thomann-Harwood L, Jandus C, Kaeuper P, Simon HU, et al. Alginate-coated chitosan nanogel capacity to modulate the effect of TLR ligands on blood dendritic cells. Nanomedicine. 2013;9(6):806–817. doi: 10.1016/j.nano.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 65.Dhawan S, Singla AK, Sinha VR. Evaluation of mucoadhesive properties of chitosan microspheres prepared by different methods. AAPS PharmSciTech. 2004;5(4):e67. doi: 10.1208/pt050467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Aranaz I, Mengibar M, Harris R, Panos I, Miralles B, Acosta N, et al. Functional Characterization of Chitin and Chitosan. Current Chemical Biology. 2009;3(2):203–230. [Google Scholar]
- 67.Bal SM, Slutter B, Verheul R, Bouwstra JA, Jiskoot W. Adjuvanted, antigen loaded N-trimethyl chitosan nanoparticles for nasal and intradermal vaccination: adjuvant- and site-dependent immunogenicity in mice. Eur J Pharm Sci. 2012;45(4):475–481. doi: 10.1016/j.ejps.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 68.Verheul RJ, Slutter B, Bal SM, Bouwstra JA, Jiskoot W, Hennink WE. Covalently stabilized trimethyl chitosan-hyaluronic acid nanoparticles for nasal and intradermal vaccination. J Control Release. 2011;156(1):46–52. doi: 10.1016/j.jconrel.2011.07.014. [DOI] [PubMed] [Google Scholar]
- 69.Akagi T, Kaneko T, Kida T, Akashi M. Preparation and characterization of biodegradable nanoparticles based on poly(gamma-glutamic acid) with l-phenylalanine as a protein carrier. J Control Release. 2005;108(2-3):226–236. doi: 10.1016/j.jconrel.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 70.Akagi T, Kaneko T, Kida T, Akashi M. Multifunctional conjugation of proteins on/into bio-nanoparticles prepared by amphiphilic poly(gamma-glutamic acid) J Biomater Sci Polym Ed. 2006;17(8):875–892. doi: 10.1163/156856206777996871. [DOI] [PubMed] [Google Scholar]
- 71.Wang X, Uto T, Akagi T, Akashi M, Baba M. Poly(gamma-glutamic acid) nanoparticles as an efficient antigen delivery and adjuvant system: potential for an AIDS vaccine. J Med Virol. 2008;80(1):11–19. doi: 10.1002/jmv.21029. [DOI] [PubMed] [Google Scholar]
- 72.Uto T, Akagi T, Yoshinaga K, Toyama M, Akashi M, Baba M. The induction of innate and adaptive immunity by biodegradable poly(gamma-glutamic acid) nanoparticles via a TLR4 and MyD88 signaling pathway. Biomaterials. 2011;32(22):5206–5212. doi: 10.1016/j.biomaterials.2011.03.052. [DOI] [PubMed] [Google Scholar]
- 73.Okamoto S, Yoshii H, Matsuura M, Kojima A, Ishikawa T, Akagi T, et al. Poly-gamma-glutamic acid nanoparticles and aluminum adjuvant used as an adjuvant with a single dose of Japanese encephalitis virus-like particles provide effective protection from Japanese encephalitis virus. Clin Vaccine Immunol. 2012;19(1):17–22. doi: 10.1128/CVI.05412-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Broos S, Lundberg K, Akagi T, Kadowaki K, Akashi M, Greiff L, et al. Immunomodulatory nanoparticles as adjuvants and allergen-delivery system to human dendritic cells: Implications for specific immunotherapy. Vaccine. 2010;28(31):5075–5085. doi: 10.1016/j.vaccine.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 75.Uto T, Akagi T, Toyama M, Nishi Y, Shima F, Akashi M, et al. Comparative activity of biodegradable nanoparticles with aluminum adjuvants: antigen uptake by dendritic cells and induction of immune response in mice. Immunol Lett. 2011;140(1-2):36–43. doi: 10.1016/j.imlet.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 76.Shima F, Uto T, Akagi T, Akashi M. Synergistic stimulation of antigen presenting cells via TLR by combining CpG ODN and poly(gamma-glutamic acid)-based nanoparticles as vaccine adjuvants. Bioconjug Chem. 2013;24(6):926–933. doi: 10.1021/bc300611b. [DOI] [PubMed] [Google Scholar]
- 77.Tran KK, Shen H. The role of phagosomal pH on the size-dependent efficiency of cross-presentation by dendritic cells. Biomaterials. 2009;30(7):1356–1362. doi: 10.1016/j.biomaterials.2008.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Matsuo K, Koizumi H, Akashi M, Nakagawa S, Fujita T, Yamamoto A, et al. Intranasal immunization with poly(gamma-glutamic acid) nanoparticles entrapping antigenic proteins can induce potent tumor immunity. J Control Release. 2011;152(2):310–316. doi: 10.1016/j.jconrel.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 79.Kurosaki T, Kitahara T, Nakamura T, Nishida K, Fumoto S, Kodama Y, et al. Development of effective cancer vaccine using targeting system of antigen protein to APCs. Pharm Res. 2012;29(2):483–489. doi: 10.1007/s11095-011-0571-x. [DOI] [PubMed] [Google Scholar]
- 80.Mummert ME. Immunologic roles of hyaluronan. Immunol Res. 2005;31(3):189–206. doi: 10.1385/IR:31:3:189. [DOI] [PubMed] [Google Scholar]
- 81.Singh M, Briones M, O’Hagan DT. A novel bioadhesive intranasal delivery system for inactivated influenza vaccines. J Control Release. 2001;70(3):267–276. doi: 10.1016/s0168-3659(00)00330-8. [DOI] [PubMed] [Google Scholar]
- 82.Matsuo K, Yokota Y, Zhai Y, Quan YS, Kamiyama F, Mukai Y, et al. A low-invasive and effective transcutaneous immunization system using a novel dissolving microneedle array for soluble and particulate antigens. J Control Release. 2012;161(1):10–17. doi: 10.1016/j.jconrel.2012.01.033. [DOI] [PubMed] [Google Scholar]
- 83.Alaniz L, Rizzo M, Malvicini M, Jaunarena J, Avella D, Atorrasagasti C, et al. Low molecular weight hyaluronan inhibits colorectal carcinoma growth by decreasing tumor cell proliferation and stimulating immune response. Cancer Lett. 2009;278(1):9–16. doi: 10.1016/j.canlet.2008.12.029. [DOI] [PubMed] [Google Scholar]
- 84.Alaniz L, Rizzo M, Garcia MG, Piccioni F, Aquino JB, Malvicini M, et al. Low molecular weight hyaluronan preconditioning of tumor-pulsed dendritic cells increases their migratory ability and induces immunity against murine colorectal carcinoma. Cancer Immunol Immunother. 2011;60(10):1383–1395. doi: 10.1007/s00262-011-1036-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jain S, O’Hagan DT, Singh M. The long-term potential of biodegradable poly(lactide-co-glycolide) microparticles as the next-generation vaccine adjuvant. Expert Rev Vaccines. 2011;10(12):1731–1742. doi: 10.1586/erv.11.126. [DOI] [PubMed] [Google Scholar]
- 86.Panyam J, Labhasetwar V. Biodegradable nanoparticles for drug and gene delivery to cells and tissue. Adv Drug Deliv Rev. 2003;55(3):329–347. doi: 10.1016/s0169-409x(02)00228-4. [DOI] [PubMed] [Google Scholar]
- 87.Zhang Z, Tongchusak S, Mizukami Y, Kang YJ, Ioji T, Touma M, et al. Induction of anti-tumor cytotoxic T cell responses through PLGA-nanoparticle mediated antigen delivery. Biomaterials. 2011;32(14):3666–3678. doi: 10.1016/j.biomaterials.2011.01.067. [DOI] [PubMed] [Google Scholar]
- 88.Moon JJ, Suh H, Polhemus ME, Ockenhouse CF, Yadava A, Irvine DJ. Antigen-displaying lipid-enveloped PLGA nanoparticles as delivery agents for a Plasmodium vivax malaria vaccine. PLoS One. 2012;7(2):e31472. doi: 10.1371/journal.pone.0031472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pawar D, Mangal S, Goswami R, Jaganathan KS. Development and characterization of surface modified PLGA nanoparticles for nasal vaccine delivery: effect of mucoadhesive coating on antigen uptake and immune adjuvant activity. Eur J Pharm Biopharm. 2013;85(3 Pt A):550–559. doi: 10.1016/j.ejpb.2013.06.017. [DOI] [PubMed] [Google Scholar]
- 90.Zhu Q, Talton J, Zhang G, Cunningham T, Wang Z, Waters RC, et al. Large intestine-targeted, nanoparticle-releasing oral vaccine to control genitorectal viral infection. Nat Med. 2012;18(8):1291–1296. doi: 10.1038/nm.2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ali OA, Huebsch N, Cao L, Dranoff G, Mooney DJ. Infection-mimicking materials to program dendritic cells in situ. Nat Mater. 2009;8(2):151–158. doi: 10.1038/nmat2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ali OA, Doherty E, Bell WJ, Fradet T, Hudak J, Laliberte MT, et al. Biomaterial-based vaccine induces regression of established intracranial glioma in rats. Pharm Res. 2011;28(5):1074–1080. doi: 10.1007/s11095-010-0361-x. [DOI] [PubMed] [Google Scholar]
- 93.De Temmerman ML, Rejman J, Vandenbroucke RE, De Koker S, Libert C, Grooten J, et al. Polyelectrolyte LbL microcapsules versus PLGA microparticles for immunization with a protein antigen. J Control Release. 2012;158(2):233–239. doi: 10.1016/j.jconrel.2011.10.029. [DOI] [PubMed] [Google Scholar]
- 94.Eyles JE, Williamson ED, Alpar HO. Immunological responses to nasal delivery of free and encapsulated tetanus toxoid: studies on the effect of vehicle volume. Int J Pharm. 1999;189(1):75–79. doi: 10.1016/s0378-5173(99)00239-2. [DOI] [PubMed] [Google Scholar]
- 95.Jewell CM, Lopez SC, Irvine DJ. In situ engineering of the lymph node microenvironment via intranodal injection of adjuvant-releasing polymer particles. Proc Natl Acad Sci U S A. 2011;108(38):15745–15750. doi: 10.1073/pnas.1105200108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Makadia HK, Siegel SJ. Poly Lactic-co-Glycolic Acid (PLGA) as Biodegradable Controlled Drug Delivery Carrier. Polymers (Basel) 2011;3(3):1377–1397. doi: 10.3390/polym3031377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Johansen P, Estevez F, Zurbriggen R, Merkle HP, Gluck R, Corradin G, et al. Towards clinical testing of a single-administration tetanus vaccine based on PLA/PLGA microspheres. Vaccine. 2000;19(9-10):1047–1054. doi: 10.1016/s0264-410x(00)00343-1. [DOI] [PubMed] [Google Scholar]
- 98.Johansen P, Moon L, Tamber H, Merkle HP, Gander B, Sesardic D. Immunogenicity of single-dose diphtheria vaccines based on PLA/PLGA microspheres in guinea pigs. Vaccine. 1999;18(3-4):209–215. doi: 10.1016/s0264-410x(99)00191-7. [DOI] [PubMed] [Google Scholar]
- 99.Reinhold SE, Desai KG, Zhang L, Olsen KF, Schwendeman SP. Self-healing microencapsulation of biomacromolecules without organic solvents. Angew Chem Int Ed Engl. 2012;51(43):10800–10803. doi: 10.1002/anie.201206387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Desai KG, Schwendeman SP. Active self-healing encapsulation of vaccine antigens in PLGA microspheres. J Control Release. 2013;165(1):62–74. doi: 10.1016/j.jconrel.2012.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sarti F, Perera G, Hintzen F, Kotti K, Karageorgiou V, Kammona O, et al. In vivo evidence of oral vaccination with PLGA nanoparticles containing the immunostimulant monophosphoryl lipid A. Biomaterials. 2011;32(16):4052–4057. doi: 10.1016/j.biomaterials.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 102.Primard C, Poecheim J, Heuking S, Sublet E, Esmaeili F, Borchard G. Multifunctional PLGA-based nanoparticles encapsulating simultaneously hydrophilic antigen and hydrophobic immunomodulator for mucosal immunization. Mol Pharm. 2013;10(8):2996–3004. doi: 10.1021/mp400092y. [DOI] [PubMed] [Google Scholar]
- 103.Wegmann F, Gartlan KH, Harandi AM, Brinckmann SA, Coccia M, Hillson WR, et al. Polyethyleneimine is a potent mucosal adjuvant for viral glycoprotein antigens. Nat Biotechnol. 2012;30(9):883–888. doi: 10.1038/nbt.2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Grant EV, Thomas M, Fortune J, Klibanov AM, Letvin NL. Enhancement of plasmid DNA immunogenicity with linear polyethylenimine. Eur J Immunol. 2012;42(11):2937–2948. doi: 10.1002/eji.201242410. [DOI] [PubMed] [Google Scholar]
- 105.Chen J, Li Z, Huang H, Yang Y, Ding Q, Mai J, et al. Improved antigen cross-presentation by polyethyleneimine-based nanoparticles. Int J Nanomedicine. 2011;6:77–84. doi: 10.2147/IJN.S15457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ma YF, Yang YW. Delivery of DNA-based cancer vaccine with polyethylenimine. Eur J Pharm Sci. 2010;40(2):75–83. doi: 10.1016/j.ejps.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 107.Torrieri-Dramard L, Lambrecht B, Ferreira HL, Van den Berg T, Klatzmann D, Bellier B. Intranasal DNA vaccination induces potent mucosal and systemic immune responses and cross-protective immunity against influenza viruses. Mol Ther. 2011;19(3):602–611. doi: 10.1038/mt.2010.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cho HJ, Han SE, Im S, Lee Y, Kim YB, Chun T, et al. Maltosylated polyethylenimine-based triple nanocomplexes of human papillomavirus 16L1 protein and DNA as a vaccine co-delivery system. Biomaterials. 2011;32(20):4621–4629. doi: 10.1016/j.biomaterials.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 109.Park IY, Kim IY, Yoo MK, Choi YJ, Cho MH, Cho CS. Mannosylated polyethylenimine coupled mesoporous silica nanoparticles for receptor-mediated gene delivery. Int J Pharm. 2008;359(1-2):280–287. doi: 10.1016/j.ijpharm.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 110.Li P, Luo Z, Liu P, Gao N, Zhang Y, Pan H, et al. Bioreducible alginate-poly(ethylenimine) nanogels as an antigen-delivery system robustly enhance vaccine-elicited humoral and cellular immune responses. Journal of Controlled Release. 2013;168(3):271–279. doi: 10.1016/j.jconrel.2013.03.025. [DOI] [PubMed] [Google Scholar]
- 111.Kreuter J, Speiser PP. New adjuvants on a polymethylmethacrylate base. Infect Immun. 1976;13(1):204–210. doi: 10.1128/iai.13.1.204-210.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kreuter J, Mauler R, Gruschkau H, Speiser PP. The use of new polymethylmethacrylate adjuvants for split influenza vaccines. Exp Cell Biol. 1976;44(1):12–19. doi: 10.1159/000162849. [DOI] [PubMed] [Google Scholar]
- 113.Flanary S, Hoffman AS, Stayton PS. Antigen delivery with poly(propylacrylic acid) conjugation enhances MHC-1 presentation and T-cell activation. Bioconjug Chem. 2009;20(2):241–248. doi: 10.1021/bc800317a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Foster S, Duvall CL, Crownover EF, Hoffman AS, Stayton PS. Intracellular delivery of a protein antigen with an endosomal-releasing polymer enhances CD8 T-cell production and prophylactic vaccine efficacy. Bioconjug Chem. 2010;21(12):2205–2212. doi: 10.1021/bc100204m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wilson JT, Keller S, Manganiello MJ, Cheng C, Lee CC, Opara C, et al. pH-Responsive nanoparticle vaccines for dual-delivery of antigens and immunostimulatory oligonucleotides. ACS Nano. 2013;7(5):3912–3925. doi: 10.1021/nn305466z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.van der Vlies AJ, O’Neil CP, Hasegawa U, Hammond N, Hubbell JA. Synthesis of pyridyl disulfide-functionalized nanoparticles for conjugating thiol-containing small molecules, peptides, and proteins. Bioconjug Chem. 2010;21(4):653–662. doi: 10.1021/bc9004443. [DOI] [PubMed] [Google Scholar]
- 117.Reddy ST, van der Vlies AJ, Simeoni E, Angeli V, Randolph GJ, O’Neil CP, et al. Exploiting lymphatic transport and complement activation in nanoparticle vaccines. Nat Biotechnol. 2007;25(10):1159–1164. doi: 10.1038/nbt1332. [DOI] [PubMed] [Google Scholar]
- 118.Thomas SN, van der Vlies AJ, O’Neil CP, Reddy ST, Yu SS, Giorgio TD, et al. Engineering complement activation on polypropylene sulfide vaccine nanoparticles. Biomaterials. 2011;32(8):2194–2203. doi: 10.1016/j.biomaterials.2010.11.037. [DOI] [PubMed] [Google Scholar]
- 119.Stano A, van der Vlies AJ, Martino MM, Swartz MA, Hubbell JA, Simeoni E. PPS nanoparticles as versatile delivery system to induce systemic and broad mucosal immunity after intranasal administration. Vaccine. 2011;29(4):804–812. doi: 10.1016/j.vaccine.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 120.Stano A, Scott EA, Dane KY, Swartz MA, Hubbell JA. Tunable T cell immunity towards a protein antigen using polymersomes vs. solid-core nanoparticles. Biomaterials. 2013;34(17):4339–4346. doi: 10.1016/j.biomaterials.2013.02.024. [DOI] [PubMed] [Google Scholar]
- 121.Discher BM, Won YY, Ege DS, Lee JC, Bates FS, Discher DE, et al. Polymersomes: tough vesicles made from diblock copolymers. Science. 1999;284(5417):1143–1146. doi: 10.1126/science.284.5417.1143. [DOI] [PubMed] [Google Scholar]
- 122.Holowka EP, Sun VZ, Kamei DT, Deming TJ. Polyarginine segments in block copolypeptides drive both vesicular assembly and intracellular delivery. Nat Mater. 2007;6(1):52–57. doi: 10.1038/nmat1794. [DOI] [PubMed] [Google Scholar]
- 123.Hadinoto K, Sundaresan A, Cheow WS. Lipid-polymer hybrid nanoparticles as a new generation therapeutic delivery platform: a review. Eur J Pharm Biopharm. 2013;85(3 Pt A):427–443. doi: 10.1016/j.ejpb.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 124.Mandal B, Bhattacharjee H, Mittal N, Sah H, Balabathula P, Thoma LA, et al. Core-shell-type lipid-polymer hybrid nanoparticles as a drug delivery platform. Nanomedicine. 2013;9(4):474–491. doi: 10.1016/j.nano.2012.11.010. [DOI] [PubMed] [Google Scholar]
- 125.Su X, Fricke J, Kavanagh DG, Irvine DJ. In Vitro and in Vivo mRNA Delivery Using Lipid-Enveloped pH-Responsive Polymer Nanoparticles. Mol Pharm. 2011;8(3):774–787. doi: 10.1021/mp100390w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Bershteyn A, Chaparro J, Yau R, Kim M, Reinherz E, Ferreira-Moita L, et al. Polymer-supported lipid shells, onions, and flowers. Soft Matter. 2008;4(9):1787–1791. doi: 10.1039/b804933e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bershteyn A, Hanson MC, Crespo MP, Moon JJ, Li AV, Suh H, et al. Robust IgG responses to nanograms of antigen using a biomimetic lipid-coated particle vaccine. J Control Release. 2012;157(3):354–365. doi: 10.1016/j.jconrel.2011.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Tan S, Sasada T, Bershteyn A, Yang K, Ioji T, Zhang Z. Combinational delivery of lipid-enveloped polymeric nanoparticles carrying different peptides for anti-tumor immunotherapy. Nanomedicine (Lond) 2013 doi: 10.2217/nnm.13.67. [DOI] [PubMed] [Google Scholar]
- 129.Hu CM, Zhang L, Aryal S, Cheung C, Fang RH, Zhang L. Erythrocyte membrane-camouflaged polymeric nanoparticles as a biomimetic delivery platform. Proc Natl Acad Sci U S A. 2011;108(27):10980–10985. doi: 10.1073/pnas.1106634108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hu CM, Fang RH, Luk BT, Zhang L. Nanoparticle-detained toxins for safe and effective vaccination. Nat Nanotechnol. 2013;8(12):933–938. doi: 10.1038/nnano.2013.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sunshine JC, Perica K, Schneck JP, Green JJ. Particle shape dependence of CD8+ T cell activation by artificial antigen presenting cells. Biomaterials. 2014;35(1):269–277. doi: 10.1016/j.biomaterials.2013.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Perica K, De Leon Medero A, Durai M, Chiu YL, Bieler JG, Sibener L, et al. Nanoscale artificial antigen presenting cells for T cell immunotherapy. Nanomedicine. 2014;10(1):119–129. doi: 10.1016/j.nano.2013.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Perica K, Tu A, Richter A, Bieler JG, Edidin M, Schneck JP. Magnetic field-induced T cell receptor clustering by nanoparticles enhances T cell activation and stimulates antitumor activity. ACS Nano. 2014;8(3):2252–2260. doi: 10.1021/nn405520d. [DOI] [PMC free article] [PubMed] [Google Scholar]