Abstract
SLUG represses E‐cadherin to promote epithelial–mesenchymal transition (EMT) in various cancers. Mechanisms that regulate SLUG/E‐cadherin pathway remain poorly understood, especially during tumorigenesis in vivo. Here we report that p19Arf (p14ARF in human) stabilizes Slug to inhibit E‐cadherin in prostate cancer mouse models. Inactivation of p19Arf reduces Slug levels, resulting in increased E‐cadherin expression and delaying the onset and progression of prostate cancer in Pten/Trp53 double null mice. Mechanistically, p14ARF stabilizes SLUG through increased sumoylation at lysine residue 192. Importantly, levels of SLUG and p14ARF are positively correlated in human prostate cancer specimens. These data demonstrated that ARF modulates the SLUG/E‐cadherin signaling axis for augmenting prostate tumorigenesis in vivo, revealing a novel paradigm where the oncogenic functions of SLUG require ARF to target E‐cadherin in prostate cancer. Collectively, our findings further support that ARF has dual tumor suppressive/oncogenic roles in cancers in a context‐dependent manner.
Keywords: SLUG, Prostate cancer, SUMO1, Mouse model
Highlights
p19Arf deficiency inhibits prostate tumorigenesis of Pten/Trp53 mice.
p19Arf inactivation results in Slug decrease and E‐cadherin increase in vivo.
ARF stabilizes SLUG through SUMO1 interaction.
SLUG sumoylation at K192 residue is essential for the stability.
Levels of SLUG and ARF are positively correlated in human prostate cancer specimens.
Abbreviations
- EMT
epithelial–mesenchymal transition
- PCa
prostate cancer
- PTEN
phosphatase and tensin homolog deleted on chromosome 10
- SUMO1
small ubiquitin-like modifier 1
- ARF
alternative reading frame
1. Introduction
Prostate cancer (PCa) is driven by aberrant regulations of multiple pathways involving many tumor suppressors and oncogenes (Thomas et al., 2008). PTEN (phosphatase and tensin homolog deleted on chromosome 10) is frequently mutated and deleted in human cancers including PCa (Whang et al., 1998). Inactivation of PTEN contributes to hyper‐activation of the AKT‐mTOR (mammalian target of rapamycin) signaling pathway and downstream targets (Cantley and Neel, 1999). Complete loss of Pten triggers a cellular senescence response through the aberrant activation of the ARF‐p53 pathway (Chen et al., 2005; Yilmaz et al., 2006), but eventually results in invasive PCa in mice (Pourmand et al., 2007). Inactivation of this senescence pathway through loss of Trp53 augments cell proliferation, leading to a lethal cancer phenotype (Chen et al., 2005; Zheng et al., 2008). By contrast, inactivation of p19Arf reduces, instead of accelerates, prostate tumorigenesis driven by Pten‐deficiency (Chen et al., 2009), indicating the oncogenic potential of ARF in the prostate. Yet, the mechanism contributed by ARF remains elusive.
ARF (p14ARF in human or p19Arf in mouse) is an alternative transcript of the ARF‐INK4a locus (CDKN2a) on human chromosome 9p21 that encodes two cyclin‐dependent kinase inhibitors, p14ARF and p16INK4a (den Besten et al., 2006; Kamijo et al., 1999). CDKN2a deficiency causes spontaneous or increased susceptibility to carcinogen‐induced tumors (Bardeesy et al., 2006; Sharpless et al., 2004). ARF is functionally coupled to p53 through antagonization of the Mdm2‐mediated p53 degradation, and overexpression of p19Arf results in a p53‐dependent growth arrest in fibroblasts (Chen et al., 2010; Ha et al., 2007). Increasing evidence reveals that ARF may function in both tumor suppressive and oncogenic roles in cancers depending on the context (Abida and Gu, 2008; Humbey et al., 2008; Khoo et al., 2007). For example, p19 Arf‐deficient mice develop sarcomas, lymphomas, pulmonary and mammary adenocarcinomas; but the morphology of prostate remains normal (Kamijo et al., 1999). ARF protein is dramatically accumulated in cells upon oncogenic insults such as loss of Pten, or activation of Ras or Myc (Lowe and Sherr, 2003). We have previously shown that increased ARF expression is correlated with aggressive disease in human PCa specimens (Chen et al., 2009), and recent studies reveal that ARF absence causes apoptosis in spermatogonia of mice (Churchman et al., 2011). These results suggest that ARF may execute a unique oncogenic function or may be required for sustained cell proliferation under specific contexts.
SLUG promotes cell migration and cancer metastasis through repression of E‐cadherin (Hajra et al., 2002) and the regulation of epithelial–mesenchymal transition (EMT) (Shih and Yang, 2011). Even though, the underlying mechanisms regulating SLUG during PCa progression are still poorly understood (Rodriguez et al., 2012), particularly in the context of Pten loss. Sumoylation is mediated via SUMO1 or SUMO2/3 to regulate kinase activity as well as protein trafficking and stability (Geiss‐Friedlander and Melchior, 2007; Kho et al., 2011), and ARF is involved in protein post‐translational regulation including sumoylation (den Besten et al., 2006; Tago et al., 2005). We hypothesized that the oncogenic function of ARF in PCa may be associated with its role in the post‐translational regulation of SLUG. Here we define a novel mechanism in that ARF enhances SLUG sumoylation resulting in the repression of E‐cadherin during PCa progression.
2. Materials and methods
The detailed information is provided in the Supplementary methods.
2.1. Mutant mice, genotyping and tumor analysis
Mice were bred and maintained in an AAALAC accredited facility and in accordance with IACUC guidelines of Meharry Medical College. Pten lox/lox, Trp53 lox/lox, PB‐Cre4, and p19 Arf−/− mice were maintained and genotyped as previously described (Chen et al., 2009, 2005, 1999). All genotypes were verified by polymerase chain reaction (PCR) with primers and conditions as previously described (Chen et al., 2005; Kamijo et al., 1999).
2.2. Cell proliferation and migration
For cell proliferation, 3000 cells were seeded into each well of 96‐well plates. For migration assay, cells were seeded at a density of 5 × 104/well for PC3 cells into the upper chamber with 8 μm polyethylene terephthalate membrane filters (Becton–Dickinson).
2.3. ARF knockdown by small hairpin RNA (shRNA)
ShRNA constructs and knockdown of p14ARF in PC3 prostate cancer cells were performed as previously described (Lu et al., 2013).
2.4. Western blotting, co‐immunoprecipitation assay, protein stability assay, immunofluorescence (IF), immunohistochemistry (IHC) and prostate tissue microarrays (TMA)
For Western blotting, cell lysates were prepared in RIPA buffer [1× PBS, 1% Nonidet P‐40, 0.5% sodium deoxycholate, 0.1% SDS, and protease inhibitor cocktail (Roche)], and were subjected to standard procedures of SDS‐PAGE and antibody detection. For co‐immunoprecipitation assay, cell lysates were prepared in RIPA buffer for overexpressed proteins or denatured buffer (8 M Urea, 0.1 M Na2HPO4/NaH2PO4) for endogenous proteins. Insoluble materials were removed by centrifugation. Lysates in denatured buffer were diluted by 50 times in PBS, and incubated with 0.2 μg/ml primary antibodies overnight at 4 °C. The immunocomplexes were collected using protein A/G agarose beads (Santa Cruz), and then the beads were washed three times with IP buffer (20 mM Tris–HCl, pH 7.4, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% Triton X‐100) with protease inhibitor cocktail (Roche) at 4 °C. To study protein stability, cells were treated with cycloheximide (CHX, Sigma–Aldrich) at 50 μg/ml, and lysates were harvested at indicated time points. Human TMA containing 35 of paraffin‐embedded prostate tumor tissues and 5 of normal prostate tissue samples were purchased from US Biomax with stage and grade information (40 cases/80 cores, PR808). The intensity and extent of the staining was evaluated blindly and graded as described in the reference (Ji et al., 2009).
2.5. Plasmid
SLUG‐Myc, SLUG‐pGL2 and SNAIL‐pGL2 promoter‐Luc reporter plasmids were purchased from Addgene (31694, 31695, 31698). SUMO1, SUMO2/3 and SUMO mutant plasmids were kindly provided from Dr. Angela Chen (National Sun Yat‐Sen University, Kaohsiung, Taiwan) through Dr. Ifeanyi J. Arinze (Meharry Medical College).
2.6. Statistical analysis
Statistical analysis was performed using two‐tailed Student's t‐test unless otherwise stated, and values of p ≤ 0.05 were considered statistically significant. The significance of differences in human prostate specimens was evaluated using Pearson correlation test.
3. Results
3.1. p19 Arf deficiency suppresses prostate tumor progression in Pten/Trp53 double‐null mice
We have previously shown that Pten/Trp53 mutant mice develop aggressive PCa, and increased ARF expression is observed in this mouse model as well as PCa specimens with malignant phenotypes. To understand the role of p19Arf in prostate tumorigenesis, we generated prostate specific Pten/Trp53/p19 Arf triple knockout mice. As Pten/Trp53 mutant mice developed PCa after puberty, prostate tissues of mutant mice from two groups were collected at 3 months of age, and were subjected to histopathological analysis. Interestingly, the mass of the anterior prostate lobes (AP) in Pten/Trp53/p19 Arf mutant mice (both p19 Arf+/−and p19 Arf−/−) were reduced by approximately 50% compared to that of age‐matched Pten/Trp53 mice, though Pten/Trp53/p19 Arf mice exhibited enlarged prostates (Supplementary Figure S1A and B, n = 12, p = 0.059). Pathological analysis revealed that Pten/Trp53/p19 Arf mice developed high‐grade prostatic intraepithelial neoplasia (HG‐PIN) but not invasive cancer in all three prostate lobes, compared to Pten/Trp53 mice that displayed a significantly increased volume of HG‐PIN and invasive cancer (Supplementary Figure S1C). These results indicated that deletion of p19Arf resulted in a marginal suppression on the initiation of prostate tumorigenesis of Pten/Trp53 mice at 3 months of age. In contrast, the growth of prostate tumors of Pten/Trp53/p19 Arf mice was remarkably suppressed as compared to that of Pten/Trp53 mice at 6 months of age (Figure 1A). The average tumor weight of Pten/Trp53/p19 Arf mice was reduced 3.7‐fold as compared to that of Pten/Trp53 mice (Figure 1B; n = 12, p < 0.001, t‐test). To evaluate the sustained impact of p19Arf in tumors of Pten/Trp53 mice, we followed a cohort of 52 mice over 9 months for cumulative survival analysis. Remarkably, the reduction of p19Arf prolonged the overall survival rate of Pten/Trp53 mice (Figure 1C), and loss of one allele of p19 Arf showed a suppressive effect on tumor growth (Figure 1A–C). Therefore, p19Arf inactivation significantly suppresses the PCa progression in Pten/Trp53 mice.
Figure 1.
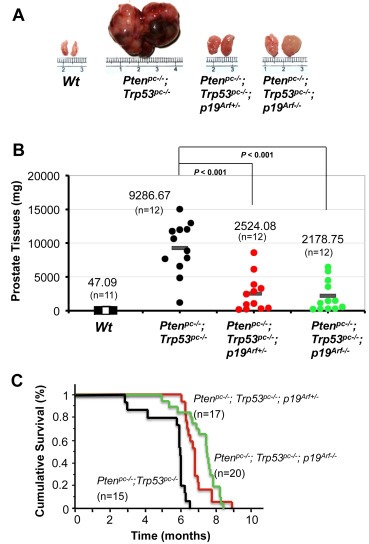
p19Arf deficiency significantly suppresses prostate tumorigenesis in Pten/Trp53 mice. (A) Actual sizes of representative tumors from anterior prostates (APs) of Wt, Ptenpc–/–; Trp53pc–/– double‐mutant, and Ptenpc–/–; Trp53pc–/–; p19Arf–/– triple‐mutant mice at 6 months of age. (B) Comparison of AP tumor masses from Ptenpc–/–; Trp53pc–/–, Ptenpc–/–; Trp53pc–/–; p19Arf+/– and Ptenpc–/–; Trp53pc–/–; p19Arf–/– mice at 6 months of age. p19Arf deficiency inhibits prostate tumorigenesis in Pten/Trp53 mutant mice (N = 12 for each group, p < 0.001). (C) Cumulative survival analysis (Kaplan–Meier plot) for Ptenpc–/–; Trp53pc–/– (black line), Ptenpc–/–; Trp53pc–/–; p19Arf+/– (red line), and Ptenpc–/–; Trp53pc–/–; p19Arf–/– (green line) cohort mice.
3.2. p19Arf inactivation represses the EMT phenotype of Pten/Trp53 mice through regulation of SLUG
Next we performed molecular pathological analyses on the prostate tumors of Pten/Trp53 and Pten/Trp53/p19 Arf mice at 6 months of age. Our results showed that prostate tumors of Pten/Trp53/p19 Arf mice maintain differentiated proliferations of prostate epithelial cells, and Pten/Trp53 mice develop de‐differentiated histologies displaying an EMT‐like phenotype with the transition from epithelial to mesenchymal (sarcomatoid) (Figure 2A, Supplementary Figure S2A). Importantly, IHC staining showed that Slug, a marker of EMT, was strikingly elevated in tumors of Pten/Trp53 mice, and p19Arf inactivation significantly decreased Slug levels (Figure 2B). As SLUG targets the down‐regulation of E‐cadherin in cancers (Hajra et al., 2002), we examined E‐cadherin levels in the tumors. As expected, E‐cadherin levels were markedly reduced in Pten/Trp53 mice where levels of p19Arf and Slug were elevated, but were not increased in Pten/Trp53/p19 Arf mice where p19 Arf is deleted and Slug levels were low (Figure 2C, Supplementary Figure S2B). Prostatic cells of Pten/Trp53/p19 Arf mice showed decreased proliferations when compared to Pten/Trp53 mice, as evidenced by Ki67 staining (Figure 2D). Thus our results suggest that p19Arf deficiency restricts tumorigenesis through regulation of Slug and E‐cadherin.
Figure 2.
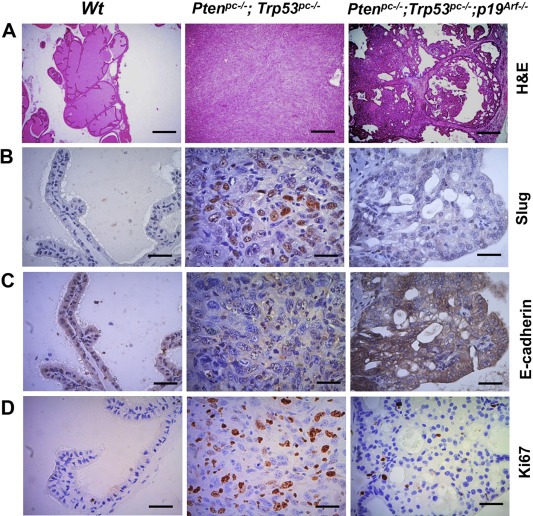
Loss of p19Arf suppresses prostate cancer progression by down‐regulating Slug in mice. (A) Histopathology analysis (H&E staining) of anterior prostates in Wt, Ptenpc–/–; Trp53pc–/–, Ptenpc–/–; Trp53pc–/–; p19Arf–/– mutant mice at 6 months of age. Immunohistochemical staining (IHC) of Slug (B), E‐cadherin (C) and Ki67 (D) in prostate tissue (A). Bars equal 500 μm (A), 50 μm (B–D).
3.3. ARF determines SLUG protein levels in human prostate cancer cells
To investigate the regulation of SLUG by ARF, we knocked down p14ARF in PC3 cells that have high levels of ARF and SLUG (data not shown). Our results showed that p14ARF knockdown resulted in a striking reduction of SLUG protein levels as compared to the control, and the down‐stream expression of E‐cadherin was increased while Vimentin levels were decreased (Figure 3A). Furthermore, p14ARF knockdown resulted in a significant reduction of cell proliferation (Figure 3B), wound closure, and cell migration (Figure 3C and D), and a tightly adhesive morphology of epithelial cells when compared to control cells (Supplementary Figure S3). In agreement with previous reports (Chen et al., 2009; Churchman et al., 2011), our results reveal that p14ARF regulates SLUG protein levels thereby promoting cell proliferation and migration in PCa.
Figure 3.

Effects of p14ARF knockdown on SLUG, cell growth and migration in prostate cancer cells. (A) SLUG reduction upon p14ARF knockdown in PC3 cells. E‐cadherin is increased and Vimentin is decreased. Quantifications on Western blot results were shown at the right panel. (B) Decreased proliferation of PC3 cells upon p14ARF knockdown. (C) Reduced wound‐healing ability of PC3 cells by p14ARF knockdown. Bars equal 100 μm. (D) Decreased migration of PC3 cells upon p14ARF knockdown. Migrated cells were counted from three fields and presented as mean values ± s.d. **p < 0.01 indicates the statistical significance, Student's t‐test, n = 3.
3.4. ARF regulates SLUG levels through induction of sumoylation in prostate cancer cells
To understand the mechanism of ARF‐mediated SLUG regulation, we examined whether ARF regulates SLUG gene transcription. Our results showed that p14ARF overexpression did not result in the transactivation of luciferase reporter gene driven by the SLUG‐promoter (Figure 4A), indicating SLUG regulation by p14ARF may not occur at the transcriptional level. We reasoned that SLUG may be regulated by ARF in a post‐transcriptional fashion. As demonstrated, p14ARF knockdown resulted in a remarkable reduction of the half‐life of SLUG protein levels as compared to the control (Figure 4B), suggesting that ARF is essential in stabilizing the SLUG protein. Given the role of ubiquitination on protein degradation, we tested if MG‐132, a proteasome inhibitor, would prevent the observed decrease in SLUG protein levels caused by ARF knockdown. Surprisingly, MG‐132 treatment failed to reverse the impact of ARF knockdown (Supplementary Figure S4), suggesting that ARF may stabilize SLUG through a mechanism independent of ubiquitin‐mediated proteasomal degradation.
Figure 4.
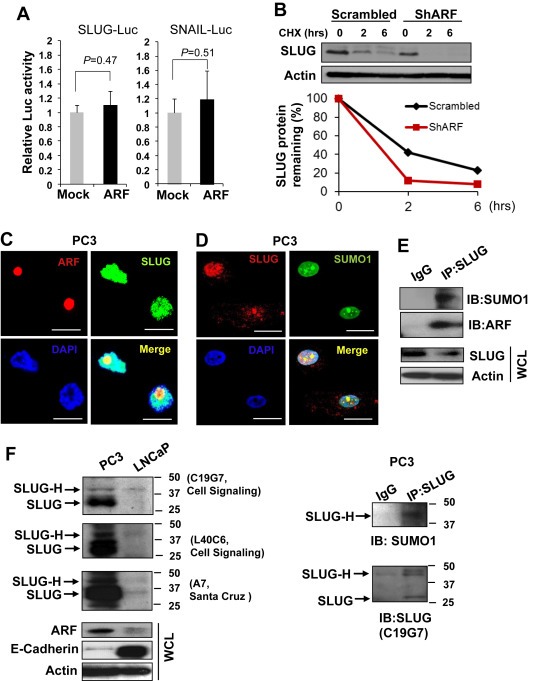
The association of ARF with SLUG determines SLUG stability. (A) ARF overexpression does not affect the promoter activities of SLUG and SNAIL genes in Luciferase reporter assay. (B) ARF knockdown results in a shortened half‐life of SLUG protein in PC3 cells. (C) Co‐localization of ARF and SLUG proteins in nucleus of PC3 cells. Bars equal 25 μm. (D) Co‐localization of SLUG and SUMO1 in PC3 cells. (E) Co‐immunoprecipitation (IP) was performed using SLUG (L40C6) antibody, and immunoblot (IB) was probed using ARF (4C6/4) or SLUG (C19G7) antibody. (F) Left panel: detection of endogenous SLUG using three different antibodies to show a higher molecular weight band (SLUG‐H) at 42 kDa and lower molecular weight band (SLUG) at 30 kDa in prostate cancer cells. Right panel: co‐IP using SLUG (A7) antibody and IB using SLUG (C19G7) or SUMO1 to show the SLUG‐H and SUMO1 band in PC3 cells.
Since ARF plays a role on protein sumoylation (den Besten et al., 2006), we investigated whether ARF stabilizes SLUG through sumoylation. We first determined whether ARF associates with SLUG protein in its sumoylated form. Our results demonstrated that SLUG was co‐localized with both ARF and SUMO1 in nucleus of PC3 cells (Figure 4C and D), and SLUG interacted with ARF and SUMO1 (Figure 4E). To further confirm the SLUG sumoylation, cell lysates were subjected to the detection of SLUG modifications in Western blot by three different antibodies. As shown, in addition to the SLUG bands, higher molecular weight bands of SLUG (SLUG‐H) were detected by all three antibodies in PC3 cells but not in LNCaP cells in which SLUG levels are very low (Sun et al., 2012) (Figure 4F). Moreover, SLUG‐H expression was correlated with ARF elevation and a decrease in E‐cadherin (Figure 4F, left panel), and endogenous SLUG‐H was conjugated with SUMO1 (Figure 4F, right panel). Our results indicate that endogenous SLUG is sumoylated in PC3 cells, and ARF interacts with SLUG to promote its sumoylation.
3.5. ARF‐promoted SLUG sumoylation is essential for protein stability
To evaluate the effects of SLUG sumoylation on protein stability, we co‐transfected Myc‐SLUG with SUMO1, SUMO1 glycine‐deleted mutant (ΔGG), or SUMO2/3 into 293FT cells followed by co‐immunoprecipitation (co‐IP). We found that SUMO1 interacted with SLUG, and SUMO1 mutation at ΔGG abolished the interaction, while SUMO2/3 had no interaction with SLUG (Figure 5A). We determined the lysine residues of SLUG that are for sumoylated modifications. The SUMOplot™ Analysis Program (Abgent) predicted that K192 and K258 were two candidate sites of SLUG sumoylation (Supplementary Figure S5A). We then generated K192R and K258R mutants of SLUG, and found that protein levels of the SLUGK192R mutant were remarkably decreased as compared to that of SLUGwt and SLUGK258R (Figure 5B). In addition, the SLUGK192R mutant maintained a similar pattern of cellular localization to that of SLUGwt (Figure 5C). These results suggested that K192 could be the major site of SLUG sumoylation in regulating SLUG protein levels. To examine the effect of K192R mutation on SLUG stability, we performed CHX chase experiments and found that the K192R mutation decreased the half‐life of SLUG protein (Figure 5D), suggesting that SLUG sumoylation at K192 is essential for its stability. Consistent with the sumoylated SLUG normally seen as a higher band in Western blotting, the K192R mutation showed a decreased level of the high molecular weight form of SLUG (SLUG‐H) (Supplementary Figure S5B). SLUGK192R mutation reduced its interaction with SUMO1 compared to SLUGwt (Figure 5E). Our results suggest that the SLUG sumoylation predominately occurs at K192 by SUMO1 to increase protein stability.
Figure 5.
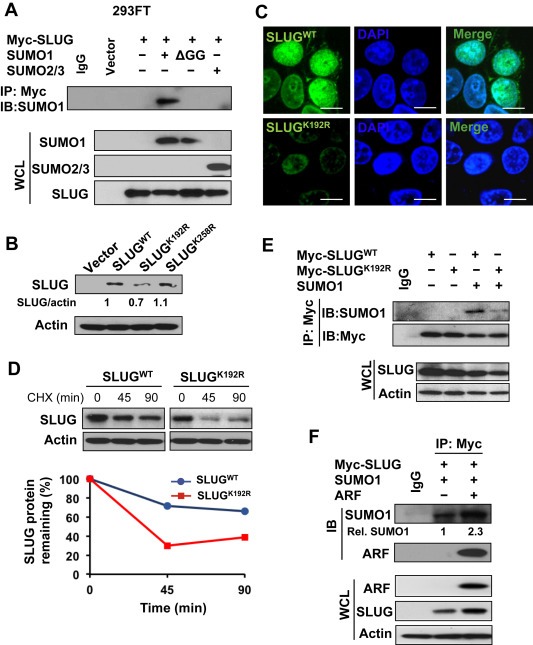
SLUG sumoylation is essential for its protein stability. (A) Interaction between SLUG and SUMO1 for sumoylation. (B) SLUG mutation at K192R decreases SLUG protein levels. (C) Localization of SLUGK192R in nucleus of 293FT cells. Myc‐tag antibody was used to detect Myc‐SLUG. Bars equal 10 μm. (D) SLUGK192R decreases the protein stability compared to SLUGWT. (E) SLUGK192R decreases the interaction with SUMO1 compared to SLUGWT. (F) ARF promotes the interaction between SUMO1 and SLUG for sumoylation.
As SLUG sumoylation is required for its stability, we asked if ARF stabilized the SLUG protein through increasing the interaction between SLUG and SUMO1 for sumoylation. The co‐IP assay revealed that ARF overexpression resulted in a 2‐fold increase of SUMO1 binding with SLUG as compared to the control (Figure 5F). Consistent with the results in PC3 cells, overexpression of SLUG also showed an association with ARF and SUMO1 (Figure 5F). Similarly, ARF knockdown by two individual shRNA constructs dramatically decreased SLUG and SLUG‐H (Data not shown). Together, our results demonstrated that ARF promotes SLUG sumoylation to increase the protein stability of SLUG.
3.6. SLUG protein levels correlate with ARF expression in human prostate cancer specimens
The novel function of ARF on SLUG in vitro and in vivo encouraged us to assess the correlation of their protein levels in human PCa specimens. IHC staining of prostate tissue microarrays revealed that ARF and SLUG protein levels were significantly higher in advanced stages of PCa (IV) compared to normal tissues (Figure 6A). Our results are in agreement with previous reports that either ARF or SLUG levels alone are significantly elevated in advanced cancers (Chen et al., 2009; Liu et al., 2012). Importantly, we found that the correlation between ARF and SLUG protein levels were statistically significant in PCa specimens (Figure 6B, r = 0.6891, p < 0.01). PCa samples with elevated SLUG displayed higher levels of ARF (Figure 6C). Thus, ARF may be an important factor in maintaining increased levels of SLUG during PCa progression.
Figure 6.

The correlation between ARF and SLUG in human prostate cancer. (A) Levels of ARF and SLUG expression among normal, stages II and IV of prostate cancer. **, p < 0.01 and *, p < 0.05 indicate the statistical significance by Student's t‐test. (B) Statistical analysis of the correlations between ARF and SLUG, in human prostate cancer specimens. (C) IHC staining of ARF and SLUG in human prostate cancer tissues. Bars equal 50 μm. (D) The signaling network of ARF, SLUG, SUMO1 and E‐cadherin for prostate cancer progression.
4. Discussion
ARF was recently reported to show pro‐proliferative or oncogenic‐promoting roles in cells under compromised physiologic or genetic contexts (Chen et al., 2009; Humbey et al., 2008; Mulholland et al., 2012). In this study, we demonstrated in vitro and in vivo that ARF contributes to PCa progression by regulation of the SLUG and E‐cadherin axis. Furthermore, we identified mechanistically that ARF regulates the post‐translational modification of SLUG.
Loss of E‐cadherin, a hallmark of cancer, is primarily controlled by elevation of SNAIL and SLUG proteins (Cano et al., 2000). Deletions and mutations of PTEN and p53 are frequently found in various cancers including advanced PCa (Shen and Abate‐Shen, 2010), and inactivation of both PTEN and p53 in the prostate leads to the lethal cancer in mice. Loss of PTEN can induce an EMT to accelerate PCa progression in the RAS‐activated mouse model (Dubrovska et al., 2009; Mulholland et al., 2012). Additionally, p53 deficiency promotes an EMT in breast cancer cells through miR‐200c (Chang et al., 2011). It has been observed that loss of PTEN and p53 promotes an EMT in PCa, yet the mechanism was not well understood. Our results reveal that the EMT‐like phenotype induced by PTEN and p53 loss is an ARF‐dependent oncogenic cascade that induces SLUG sumoylation to increase the protein stability resulting in down‐regulation of E‐cadherin. This mechanism underscores the oncogenic role of ARF in cell motility and malignancy in the prostate, which is different from that of other tissue contexts where p19Arf is reported to inhibit cell motility by the suppression of Pten/PI3K deregulation or Rac1 GTPase (Guo et al., 2003; Yu et al., 2009). Our study reveals a novel network among PTEN/PI3K/AKT, ARF, and EMT signaling in PCa.
One noteworthy finding in this study is that ARF stabilizes SLUG protein to potentiate its oncogenic functions in PCa cells. Given the essential role of the SLUG/E‐cadherin axis in cancer progression, our discovery that ARF modulates the protein interactions between SUMO1 and SLUG for SLUG sumoylation resulting in decreased E‐cadherin provides valuable insight into understanding the role of sumoylation in malignancy. Our studies have defined that the aberrant elevation of ARF upon PTEN and p53 inactivation determines the oncogenic switch from p53‐dependent senescence to SLUG‐driven EMT (Kuilman et al., 2010) (Figure 6D). Collectively, our findings highlight that ARF‐mediated stabilization of SLUG is important for the repression of cell–cell junctions and E‐cadherin during cancer progression (Figure 6D). Further investigation will reveal how ARF overexpression cooperates with SLUG signaling to regulate cancer cell invasion and metastasis, and the impact of SLUG sumoylation deficiency in PCa in vivo. We previously reported that ARF inhibits androgen receptor (AR) activity by direct disruption of NH and COOH terminal interactions of AR (Lu et al., 2013). AR is reported to regulate an EMT by targeting E‐cadherin (Liu et al., 2008). Here we demonstrated that ARF regulates SLUG and E‐cadherin both in AR positive (data not shown) and AR negative PCa cells, indicating that ARF can stabilize SLUG to repress E‐cadherin in both an AR dependent and independent manner (Sun et al., 2012). This suggests that ARF plays distinct roles in mediating AR action and EMTs.
We previously reported that ARF correlates with Gleason scores in human prostate cancer specimens (Chen et al., 2009). Here we showed ARF correlates with SLUG, an EMT marker, in advanced stages of PCa, highlighting that SLUG and ARF have the potential of being biomarkers for aggressive PCa. Our findings on the association of SLUG sumoylation with EMT constitute important clues with implications in clinical application. Chemotherapeutic compounds targeting ubiquitination machinery to control cancers have been in clinical trials. With a similar strategy, inhibitors preventing SLUG sumoylation may constitute a novel strategy for reversal of EMT to thereby block cancer metastasis. Furthermore, due to the oncogenic functions of ARF, treatments targeting both ARF and SLUG sumoylation would be of potential therapeutic strategy to improve the efficacy for PCa control.
Conflict of interest
The authors declare no conflict of interest.
Supporting information
The following are the supplementary data related to this article:
Supplementary data
Supplementary data
Acknowledgments
We would like to thank Drs. Simon W. Hayward and LaMonica Stewart for providing the prostate cell lines, Dr. Paul Wade for plasmids through Addgene, and Dr. Angela Chen for plasmids through Dr. Ifeanyi J. Arinze. This work was supported in part by NIH grants MD004038 (to Z.C.), G12 MD007586, U54 CA163069 (to Meharry), UL1 RR024975‐01 and UL1 TR000445‐06 (to Vanderbilt CTSA). Microscopy experiments and data analysis were performed through the use of Meharry Medical College Morphology Core supported in part by NIH grants U54MD007593 and S10RR0254970.
Supplementary data 1.
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.molonc.2014.05.006.
Xie Yingqiu, Liu Shenji, Lu Wenfu, Yang Qing, Williams Kieosha D., Binhazim Awadh A., Carver Brett S., Matusik Robert J., Chen Zhenbang, (2014), Slug regulates E‐cadherin repression via p19Arf in prostate tumorigenesis, Molecular Oncology, 8, doi: 10.1016/j.molonc.2014.05.006.
References
- Abida, W.M. , Gu, W. , 2008. p53-Dependent and p53-independent activation of autophagy by ARF. Cancer Res. 68, 352–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardeesy, N. , Aguirre, A.J. , Chu, G.C. , Cheng, K.H. , Lopez, L.V. , Hezel, A.F. , 2006. Both p16(Ink4a) and the p19(Arf)-p53 pathway constrain progression of pancreatic adenocarcinoma in the mouse. Proc. Natl. Acad. Sci. U.S.A. 103, 5947–5952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cano, A. , Perez-Moreno, M.A. , Rodrigo, I. , Locascio, A. , Blanco, M.J. , del Barrio, M.G. , 2000. The transcription factor snail controls epithelial–mesenchymal transitions by repressing E-cadherin expression. Nat. Cell Biol. 2, 76–83. [DOI] [PubMed] [Google Scholar]
- Cantley, L.C. , Neel, B.G. , 1999. New insights into tumor suppression: PTEN suppresses tumor formation by restraining the phosphoinositide 3-kinase/AKT pathway. Proc. Natl. Acad. Sci. U.S.A. 96, 4240–4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang, C.J. , Chao, C.H. , Xia, W. , Yang, J.Y. , Xiong, Y. , Li, C.W. , 2011. p53 regulates epithelial–mesenchymal transition and stem cell properties through modulating miRNAs. Nat. Cell Biol. 13, 317–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, D. , Shan, J. , Zhu, W.G. , Qin, J. , Gu, W. , 2010. Transcription-independent ARF regulation in oncogenic stress-mediated p53 responses. Nature. 464, 624–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Z. , Carracedo, A. , Lin, H.K. , Koutcher, J.A. , Behrendt, N. , Egia, A. , 2009. Differential p53-independent outcomes of p19(Arf) loss in oncogenesis. Sci. Signal. 2, ra44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Z. , Trotman, L.C. , Shaffer, D. , Lin, H.K. , Dotan, Z.A. , Niki, M. , 2005. Crucial role of p53-dependent cellular senescence in suppression of Pten-deficient tumorigenesis. Nature. 436, 725–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchman, M.L. , Roig, I. , Jasin, M. , Keeney, S. , Sherr, C.J. , 2011. Expression of Arf tumor suppressor in spermatogonia facilitates meiotic progression in male germ cells. PLoS Genet. 7, e1002157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Besten, W. , Kuo, M.L. , Tago, K. , Williams, R.T. , Sherr, C.J. , 2006. Ubiquitination of, and sumoylation by, the Arf tumor suppressor. Isr. Med. Assoc. J. 8, 249–251. [PubMed] [Google Scholar]
- Dubrovska, A. , Kim, S. , Salamone, R.J. , Walker, J.R. , Maira, S.M. , Garcia-Echeverria, C. , 2009. The role of PTEN/Akt/PI3K signaling in the maintenance and viability of prostate cancer stem-like cell populations. Proc. Natl. Acad. Sci. U.S.A. 106, 268–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiss-Friedlander, R. , Melchior, F. , 2007. Concepts in sumoylation: a decade on. Nat. Rev. Mol. Cell Biol. 8, 947–956. [DOI] [PubMed] [Google Scholar]
- Guo, F. , Gao, Y. , Wang, L. , Zheng, Y. , 2003. p19Arf-p53 tumor suppressor pathway regulates cell motility by suppression of phosphoinositide 3-kinase and Rac1 GTPase activities. J. Biol. Chem. 278, 14414–14419. [DOI] [PubMed] [Google Scholar]
- Ha, L. , Ichikawa, T. , Anver, M. , Dickins, R. , Lowe, S. , Sharpless, N.E. , 2007. ARF functions as a melanoma tumor suppressor by inducing p53-independent senescence. Proc. Natl. Acad. Sci. U.S.A. 104, 10968–10973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajra, K.M. , Chen, D.Y. , Fearon, E.R. , 2002. The SLUG zinc-finger protein represses E-cadherin in breast cancer. Cancer Res. 62, 1613–1618. [PubMed] [Google Scholar]
- Humbey, O. , Pimkina, J. , Zilfou, J.T. , Jarnik, M. , Dominguez-Brauer, C. , Burgess, D.J. , 2008. The ARF tumor suppressor can promote the progression of some tumors. Cancer Res. 68, 9608–9613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji, H. , Wang, J. , Nika, H. , Hawke, D. , Keezer, S. , Ge, Q. , 2009. EGF-induced ERK activation promotes CK2-mediated disassociation of alpha-catenin from beta-catenin and transactivation of beta-catenin. Mol. Cell. 36, 547–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamijo, T. , Bodner, S. , van de Kamp, E. , Randle, D.H. , Sherr, C.J. , 1999. Tumor spectrum in ARF-deficient mice. Cancer Res. 59, 2217–2222. [PubMed] [Google Scholar]
- Kho, C. , Lee, A. , Jeong, D. , Oh, J.G. , Chaanine, A.H. , Kizana, E. , 2011. SUMO1-dependent modulation of SERCA2a in heart failure. Nature. 477, 601–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoo, C.M. , Carrasco, D.R. , Bosenberg, M.W. , Paik, J.H. , Depinho, R.A. , 2007. Ink4a/Arf tumor suppressor does not modulate the degenerative conditions or tumor spectrum of the telomerase-deficient mouse. Proc. Natl. Acad. Sci. U.S.A. 104, 3931–3936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuilman, T. , Michaloglou, C. , Mooi, W.J. , Peeper, D.S. , 2010. The essence of senescence. Genes Dev. 24, 2463–2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Y.N. , Abou-Kheir, W. , Yin, J.J. , Fang, L. , Hynes, P. , Casey, O. , 2012. Critical and reciprocal regulation of KLF4 and SLUG in transforming growth factor beta-initiated prostate cancer epithelial–mesenchymal transition. Mol. Cell. Biol. 32, 941–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Y.N. , Liu, Y. , Lee, H.J. , Hsu, Y.H. , Chen, J.H. , 2008. Activated androgen receptor downregulates E-cadherin gene expression and promotes tumor metastasis. Mol. Cell. Biol. 28, 7096–7108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe, S.W. , Sherr, C.J. , 2003. Tumor suppression by Ink4a-Arf: progress and puzzles. Curr. Opin. Genet. Dev. 13, 77–83. [DOI] [PubMed] [Google Scholar]
- Lu, W. , Xie, Y. , Ma, Y. , Matusik, R.J. , Chen, Z. , 2013. ARF represses androgen receptor transactivation in prostate cancer. Mol. Endocrinol. 27, 635–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulholland, D.J. , Kobayashi, N. , Ruscetti, M. , Zhi, A. , Tran, L.M. , Huang, J. , 2012. Pten loss and RAS/MAPK activation cooperate to promote EMT and metastasis initiated from prostate cancer stem/progenitor cells. Cancer Res. 72, 1878–1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pourmand, G. , Ziaee, A.A. , Abedi, A.R. , Mehrsai, A. , Alavi, H.A. , Ahmadi, A. , 2007. Role of PTEN gene in progression of prostate cancer. Urol. J. 4, 95–100. [PubMed] [Google Scholar]
- Rodriguez, F.J. , Lewis-Tuffin, L.J. , Anastasiadis, P.Z. , 2012. E-cadherin's dark side: possible role in tumor progression. Biochim. Biophys. Acta. 1826, 23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpless, N.E. , Ramsey, M.R. , Balasubramanian, P. , Castrillon, D.H. , DePinho, R.A. , 2004. The differential impact of p16(INK4a) or p19(ARF) deficiency on cell growth and tumorigenesis. Oncogene. 23, 379–385. [DOI] [PubMed] [Google Scholar]
- Shen, M.M. , Abate-Shen, C. , 2010. Molecular genetics of prostate cancer: new prospects for old challenges. Genes Dev. 24, 1967–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih, J.Y. , Yang, P.C. , 2011. The EMT regulator slug and lung carcinogenesis. Carcinogenesis. 32, 1299–1304. [DOI] [PubMed] [Google Scholar]
- Sun, Y. , Wang, B.E. , Leong, K.G. , Yue, P. , Li, L. , Jhunjhunwala, S. , 2012. Androgen deprivation causes epithelial–mesenchymal transition in the prostate: implications for androgen-deprivation therapy. Cancer Res. 72, 527–536. [DOI] [PubMed] [Google Scholar]
- Tago, K. , Chiocca, S. , Sherr, C.J. , 2005. Sumoylation induced by the Arf tumor suppressor: a p53-independent function. Proc. Natl. Acad. Sci. U.S.A. 102, 7689–7694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas, G. , Jacobs, K.B. , Yeager, M. , Kraft, P. , Wacholder, S. , Orr, N. , 2008. Multiple loci identified in a genome-wide association study of prostate cancer. Nat. Genet. 40, 310–315. [DOI] [PubMed] [Google Scholar]
- Whang, Y.E. , Wu, X. , Suzuki, H. , Reiter, R.E. , Tran, C. , Vessella, R.L. , 1998. Inactivation of the tumor suppressor PTEN/MMAC1 in advanced human prostate cancer through loss of expression. Proc. Natl. Acad. Sci. U.S.A. 95, 5246–5250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yilmaz, O.H. , Valdez, R. , Theisen, B.K. , Guo, W. , Ferguson, D.O. , Wu, H. , 2006. Pten dependence distinguishes haematopoietic stem cells from leukaemia-initiating cells. Nature. 441, 475–482. [DOI] [PubMed] [Google Scholar]
- Yu, J. , Zhang, S.S. , Saito, K. , Williams, S. , Arimura, Y. , Ma, Y. , 2009. PTEN regulation by Akt-EGR1-ARF-PTEN axis. EMBO J. 28, 21–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng, H. , Ying, H. , Yan, H. , Kimmelman, A.C. , Hiller, D.J. , Chen, A.J. , 2008. p53 and Pten control neural and glioma stem/progenitor cell renewal and differentiation. Nature. 455, 1129–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The following are the supplementary data related to this article:
Supplementary data
Supplementary data


