Abstract
Chondrocytes in articular cartilage are surrounded by a narrow pericellular matrix (PCM) that is both biochemically and biomechanically distinct from the extracellular matrix (ECM) of the tissue. While the PCM was first observed nearly a century ago, its role is still under investigation. In support of early hypotheses regarding its function, increasing evidence indicates that the PCM serves as a transducer of biochemical and biomechanical signals to the chondrocyte. Work over the past two decades has established that the PCM in adult tissue is defined biochemically by several molecular components, including type VI collagen and perlecan. On the other hand, the biomechanical properties of this structure have only recently been measured. Techniques such as micropipette aspiration, in situ imaging, computational modeling, and atomic force microscopy have determined that the PCM exhibits distinct mechanical properties as compared to the ECM, and that these properties are influenced by specific PCM components as well as disease state. Importantly, the unique relationships among the mechanical properties of the chondrocyte, PCM, and ECM in different zones of cartilage suggest that this region significantly influences the stress-strain environment of the chondrocyte. In this review, we discuss recent advances in the measurement of PCM mechanical properties and structure that further increase our understanding of PCM function. Taken together, these studies suggest that the PCM plays a critical role in controlling the mechanical environment and mechanobiology of cells in cartilage and other cartilaginous tissues, such as the meniscus or intervertebral disc.
Keywords: Chondron, type II collagen, type VI collagen, perlecan, aggrecan, osteoarthritis, territorial matrix, decorin, mechanobiology, mechanotransduction
Introduction
Articular cartilage provides a nearly frictionless, lubricating, load-bearing surface that supports and distributes the forces generated during loading and motion of the diarthrodial joints. The mechanical properties of cartilage are conferred by the tissue's extensive extracellular matrix (ECM) that is maintained by a single population of cells known as chondrocytes. Chondrocyte physiology and control of matrix turnover are influenced by a number of environmental factors, including matrix composition, soluble mediators (e.g., growth factors and cytokines), and biophysical factors engendered by mechanical loading of the joint (Grodzinsky et al., 2000; Guilak and Hung, 2005). Due to the avascular nature of adult articular cartilage, the immediate pericellular environment of the chondrocytes appears to play a critical role in regulating cell activity.
Each chondrocyte is surrounded by a narrow pericellular matrix (PCM) that together with the enclosed cell(s) is referred to as a chondron (Figure 1) (Benninghoff, 1925; Poole et al., 1987; Szirmai, 1968; Vanden Berg-Foels et al., 2012). Although the complete function of the PCM in cartilage has yet to be elucidated, growing evidence suggests that the PCM serves as a transducer of both biomechanical and biochemical signals for the chondrocyte (Chen et al., 2013; Guilak et al., 2006; Macri et al., 2007; Vincent et al., 2007). The results of previous theoretical models (Alexopoulos et al., 2005a; Guilak and Mow, 2000; Haider et al., 2006; Julkunen et al., 2009; Korhonen and Herzog, 2008; Michalek and Iatridis, 2007; Mow et al., 1994; Sibole and Erdemir, 2012; Wu and Herzog, 2000) and experimental studies (Choi et al., 2007; Hing et al., 2002; Knight et al., 1998) support an important functional role for the ECM and PCM in the regulation of the mechanical and physiochemical environments at multiple scales, which in turn influence chondrocyte metabolism, cartilage homeostasis, and overall joint health (Halloran et al., 2012).
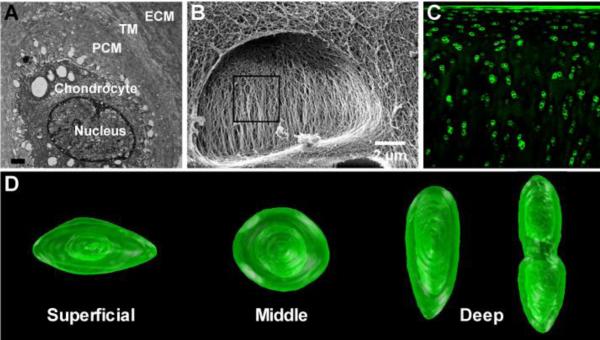
Figure 1. Structure and morphology of the PCM.
(A) Transmission electron microscopy (TEM) showing the chondrocyte within the pericellular, territorial, and extracellular matrices (scale bar = 1 μm). (B) Helium ion microscopy of a cell void within a cross-section of articular cartilage showing the basket-like morphology of the collagen in the pericellular matrix [adapted from (Vanden Berg-Foels et al., 2012), with permission]. (C) Immunohistochemistry for type VI collagen in the full thickness of articular cartilage. (D) Three-dimensional reconstruction of articular cartilage chondrons defined by type VI collagen [adapted from (Choi et al., 2007), with permission].
The Mechanical Properties and Function of the Pericellular Matrix
The quantification of PCM biomechanical properties is technically challenging due to the low cell density of articular cartilage (Stockwell, 1971) and the micrometer length scale of the PCM (Hunziker et al., 1997; Youn et al., 2006). As chondrons are embedded within the cartilage ECM, measurement of PCM properties requires extraction of the chondron, sectioning of the tissue, or indirect methods that couple measures of microscale deformation with theoretical modeling. In this regard, over the last two decades, a variety of experimental techniques have been developed to provide a means for evaluating PCM properties either using isolated chondrons or in situ testing methods (Figure 2). Together, these studies have contributed to a growing understanding of the micromechanical environment of the chondrocyte.
Figure 2. Methods of mechanical testing for determining the micromechanical properties of the PCM.
(A) Isolated chondrons tested using micropipette aspiration [from (Alexopoulos et al., 2003), with permission]. (B) Immunofluorescence-guided atomic force microscopy of cartilage sections labeled for PCM molecules [adapted from (Wilusz and Guilak, 2014)]. (C) 3D reconstruction of compressed chondrons and computational modeling of chondron deformation [adapted from (Choi et al., 2007; Kim et al., 2010), with permission].
Several techniques that have been used to characterize the mechanical properties of the PCM require physical extraction of chondrons from the tissue, notably deformation of isolated chondrons embedded within hydrogels (Hing et al., 2002; Knight et al., 1998; Knight et al., 2001), single chondron compression (Ng et al., 2007; Nguyen et al., 2009; Nguyen et al., 2010), and micropipette aspiration (Alexopoulos et al., 2003; Alexopoulos et al., 2005b; Alexopoulos et al., 2009; Guilak et al., 2005; Guilak et al., 1999). These techniques demonstrated that the mechanical integrity of chondrons isolated using enzymatic digestion is significantly reduced as compared to chondrons isolated using mechanical methods (Guilak et al., 1999; Knight et al., 2001). Nonetheless, such measurements provide important information for potential applications in tissue engineering, wherein large numbers of enzymatically-isolated chondrons may be used for seeding of constructs or cartilage repair (Bekkers et al., 2013; Vonk et al., 2010).
Micropipette aspiration studies (Figure 2a) have provided some of the first direct quantitative measurements of PCM elastic (Alexopoulos et al., 2003; Guilak et al., 2005; Guilak et al., 1999) and biphasic (Alexopoulos et al., 2005a; Alexopoulos et al., 2005b) properties, demonstrating that PCM moduli (40 – 70 kPa) are two orders of magnitude greater than those of chondrocytes [~0.5 kPa; (Darling et al., 2006; Guilak et al., 1999)] but as much as an order of magnitude lower than those of the surrounding ECM, depending on the zone of cartilage [0.1 – 2 MPa (Schinagl et al., 1997)]. In addition, micropipette aspiration of mechanically isolated chondrons from canine (Guilak et al., 2005) and human (Alexopoulos et al., 2003; Alexopoulos et al., 2005b) cartilage demonstrated that PCM properties exhibit zonal uniformity, despite significant variations in ECM properties through the depth of the tissue (Schinagl et al., 1997).
Although techniques designed for isolated chondrons provide quantitative measurements of PCM mechanical properties, it is not known how the extraction of chondrons from the ECM alters their mechanical properties. In situ imaging studies of chondron deformation within cartilage explants have been useful tools to investigate the relative deformations of the cell, PCM, and ECM (Figure 2b) (Choi et al., 2007). These studies have demonstrated that the PCM may have multiple biomechanical functions by regulating either the amplification or the shielding of forces, depending on a cell's position within the tissue. For example, in the superficial zone of cartilage, which exhibits the lowest ECM modulus, the presence of a PCM can actually decrease the cellular level strains, whereas in the deep zones, the difference in ECM and PCM moduli results in an amplification of cellular strains (Choi et al., 2007). These findings suggest that an important role for the PCM may be to provide a relatively uniform strain environment for the chondrocytes despite large zonal variations in ECM strain during loading.
Furthermore, “triphasic” models that incorporate the ionic phases of cartilage in addition to the solid and fluid phases (Lai et al., 1991) suggest that another important role for the PCM and ECM may be to enhance and regulate the conversion of mechanical loading to physicochemical changes that can be sensed by the chondrocytes (Mow et al., 1999). The proteoglycan content of the PCM is generally higher than that of the surrounding ECM. Therefore, tissue deformation and the associated changes in interstitial water content that occur during loading (Coleman et al., 2013) will result in dynamic changes in the physicochemical and osmotic environment of the chondrocyte (Haider et al., 2006). Importantly, a growing body of evidence suggests that osmotic changes may provide critical signals for regulating the chondrocyte response to loading (Chao et al., 2006; O'Conor et al., 2014).
Data from imaging studies of PCM morphology has also been used to facilitate indirect quantification of PCM properties through the development and application of computational models (Kim et al., 2010; Michalek and Iatridis, 2007). In this manner, the mechanical properties of the PCM can be estimated in situ during loading, without sectioning or extraction of the tissue. In a recent study, the elastic modulus of porcine PCM was determined using an inverse boundary element analysis coupled with three-dimensional confocal microscopy (Figure 2c) (Kim et al., 2010). This study showed that the in situ properties of the PCM in the middle zone measured using this method (24 – 59 kPa) were similar to those measured for mechanically isolated chondrons (Alexopoulos et al., 2005b; Guilak et al., 2005).
Direct quantification of PCM properties in situ with minimal disruption of native matrix integration between the PCM and ECM has recently been reported through applications of atomic force microscopy (AFM)-based microindentation (Figure 2b) (Allen and Mao, 2004; Darling et al., 2010; McLeod et al., 2013; Wilusz et al., 2012a, b; Wilusz and Guilak, 2014; Wilusz et al., 2013). Using a force spectroscopy technique known as stiffness or force-volume mapping (Radmacher et al., 1992), AFM can be used to collect arrays of indentation curves and map spatial variations in elastic modulus in the micromechanical environment of the chondrocyte. PCM elastic moduli from porcine [13 – 75 kPa; (Darling et al., 2010; McLeod et al., 2013)] and human [27 – 205 kPa; (Darling et al., 2010; Wilusz et al., 2013)] cartilage obtained using AFM-based stiffness mapping were comparable to micropipette aspiration of mechanically isolated chondrons (Alexopoulos et al., 2003) and computational methods based on in situ deformation (Kim et al., 2010). AFM-based methods also provide a means for evaluating PCM anisotropy with a recent study reporting that when pooled through the tissue depth, PCM moduli were greatest in the direction parallel to the split-line orientation (McLeod et al., 2013).
Furthermore, in situ AFM-based methods allow for detailed evaluation of regions beyond the PCM such as the territorial matrix (TM), the structural transition region between the PCM and ECM. Quantitative evaluation of stiffness maps has revealed a modulus gradient outward from the PCM to the ECM (Figure 3) (Wilusz et al., 2012a, b; Wilusz and Guilak, 2014; Wilusz et al., 2013). The combination of AFM with fluorescence microscopy (Sen and Kumar, 2009) has provided a means for direct correlation among structural features, biochemical composition, and biomechanical properties of the PCM (Wilusz et al., 2012a, b; Wilusz et al., 2013).
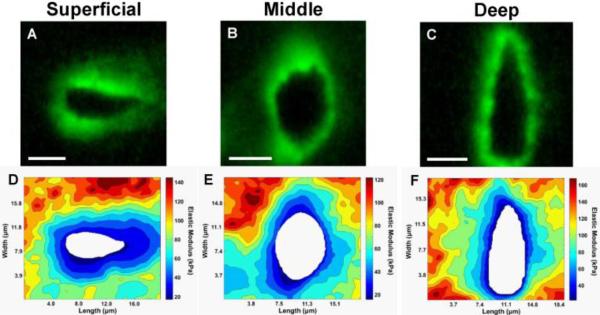
Figure 3. Stiffness mapping of the PCM in the superficial, middle, and deep zones of articular cartilage.
(A-C) Representative immunofluorescence images showing the distribution of type VI collagen around cell-sized voids in superficial, middle, and deep zones. (D-F) Representative contour maps of calculated elastic moduli of the PCM in each zone. The spatial distribution of type VI collagen correlates well with lower modulus regions within each scan. Scale bar = 5 μm. [From (Wilusz et al., 2012b), with permission].
The Relationship between the Composition and Biomechanical Properties of the PCM
A number of matrix molecules, most notably type VI collagen (Hagiwara et al., 1993; Poole et al., 1992; Poole et al., 1988; Youn et al., 2006), perlecan (Melrose et al., 2006; SundarRaj et al., 1995), aggrecan monomers and small aggregates (Poole et al., 1982; Poole et al., 1991a), hyaluronan (Knudson, 1993; Poole et al., 1991a), biglycan (Kavanagh and Ashhurst, 1999), and type IX collagen (Hu et al., 2006; Poole et al., 1997), are found either exclusively or at higher concentrations in the PCM as compared to the surrounding ECM (Heinegard and Oldberg, 1989). Interactions among these molecular constituents contribute to the distinct mesh-like capsule ultrastructure of the PCM (Hunziker et al., 1997; Poole et al., 1984, 1987) and its biomechanical properties.
In normal adult articular cartilage, the PCM is typically defined by the exclusive presence and localization of type VI collagen around the chondrocyte (Poole et al., 1988; Youn et al., 2006). Multiple studies have focused on the important biomechanical role of type VI collagen. In a study using AFM stiffness mapping guided by immunofluorescence labeling, regions of porcine articular cartilage rich in type VI collagen and regions exhibiting low elastic moduli co-localized to the pericellular space and exhibited lower elastic moduli than regions lacking type VI collagen (Wilusz et al., 2012b). Additional studies have utilized Col6a1 knockout mice to further elucidate the role of type VI collagen in the PCM. Somewhat surprisingly, intact chondrons were successfully isolated from Col6a1+/− and Col6a1−/− mice using a pipette-based mechanical extraction technique, showing that a distinct PCM can form despite the disruption or absence of type VI collagen (Alexopoulos et al., 2009). Micropipette aspiration of these isolated chondrons revealed that PCM moduli were reduced by one-third in heterozygous Col6a1+/− mice and by two-thirds in Col6a1−/− mice. These studies confirm that type VI collagen has an important functional role in the biochemical and biomechanical definitions of the PCM and also suggest that additional matrix components contribute to PCM structural integrity and mechanical properties.
One candidate molecule that likely contributes significantly to PCM properties is perlecan, a large heparan sulfate proteoglycan. Perlecan is found exclusively in the PCM of normal articular cartilage (Melrose et al., 2006; SundarRaj et al., 1995) and co-localizes with type VI collagen (Vincent et al., 2007). Though its exact role in cartilage is unknown, perlecan is essential for normal cartilage development. Dysfunction of the perlecan gene results in potentially lethal skeletal dysplasias (Arikawa-Hirasawa et al., 1999) and a cartilage phenotype similar to that of type II collagen knockout mice (Kvist et al., 2006). In a study using dual immunofluorescence-guided AFM stiffness mapping (Wilusz et al., 2012a), perlecan and type VI collagen were found to co-localize to lower modulus regions adjacent to the chondrocyte in the PCM interior. The lower elastic moduli observed in these regions were directly related to the presence of heparan sulfate, providing additional support for heparan sulfate and perlecan as a pericellular depot of fibroblast growth factor-2 (FGF-2) (Vincent et al., 2002; Vincent et al., 2004) which may play a role in mechanotransduction in cartilage through release of FGF-2 with loading (Vincent et al., 2007). Peripheral PCM regions positive for type VI collagen alone exhibited higher elastic moduli than dual-labeled regions, suggesting a defining role for perlecan as the boundary of the PCM with regions of type VI collagen alone marking the transition to the adjacent TM (Wilusz et al., 2012a).
Furthermore, as other growth factors and matrix proteins pass to or from the cell, they may be retained in the PCM where their structure or action may be further modified (Ruoslahti and Yamaguchi, 1991). For example, the influence of matrilin-3 on chondrocytes switches from an anti-anabolic to a pro-anabolic effect once it is integrated in the PCM (Vincourt et al., 2012). Thus, changes in the microscale transport properties of the PCM (Bougault et al., 2013; Leddy et al., 2008) may also influence the activity of the chondrocytes, potentially by altering growth factor transport in response to loading (Vincent et al., 2007). Furthermore, alterations in PCM structure or properties may influence the response of the enclosed cells to various chemical or physical mediators (Farnsworth et al., 2012; Steward et al., 2013). Overall, these studies suggest the presence of direct relationships between spatial variations in biochemical composition and the biomechanical, biophysical, and biological properties of the PCM in the chondrocyte microenvironment.
Insights into PCM Development, Turnover, and Degeneration
As the limb develops and the growth plate and articular cartilage emerge, the PCM becomes distinct from its surrounding matrix. The formation of the PCM can be observed by tracking one of the most abundant PCM components, type VI collagen, over time. During early development, type VI collagen is abundant throughout the cartilage matrix and then gradually localizes around the cell until it is found exclusively in the PCM of adult cartilage (Alexopoulos et al., 2009; Morrison et al., 1996; Sherwin et al., 1999). This process also occurs throughout the growth plate as the limb develops and is thought to play an important role in organizing the growth plate's proliferative zone (Plumb et al., 2011). While the localization of PCM components during development is a well-known phenomenon, the mechanism by which these molecules are organized and assembled remains an important unanswered question.
In healthy adult articular cartilage, matrix turnover is a slow, continuous process, and maintenance of matrix architecture is achieved through a balance of anabolic and catabolic activities. As the PCM surrounds every chondrocyte, all matrix components and enzymes secreted by chondrocytes must pass through this region. Thus, an understanding of the influence of various matrix enzymes on the mechanical properties of the PCM and ECM could provide important insights into the functional properties of these regions under normal or pathologic conditions. For example, collagenase digestion has been utilized to isolate intact chondrons from the tissue as type VI collagen is resistant to this enzyme (Lee et al., 1997); however, this process, which leads to a loss of type II collagen and proteoglycan, significantly reduces chondron mechanical properties (Guilak et al., 1999; Knight et al., 2001). Aggrecan plays an important role in cartilage matrix turnover due to its high abundance, relatively short half-life in situ (Maroudas et al., 1998), and interactions with a number of PCM and ECM matrix components (Hedlund et al., 1999; Junqueira and Montes, 1983; Scott, 1988; Wiberg et al., 2003).
Interestingly, the structure and composition of the PCM appears to provide it with resistance to enzymatic degradation. In fact, protocols for enzymatic isolation of chondrons have taken advantage of the resistance of the PCM, and more specifically type VI collagen, to digestion with dispase and collagenase (Lee et al., 1997). A recent study using targeted digestion with aggrecanase-1 (ADAMTS-4), bacterial hyaluronidase, and chondroitinase ABC demonstrated that PCM mechanical properties exhibit high resistance to aggrecan-targeted digestion, despite significant alterations in ECM properties (Wilusz and Guilak, 2014). This resistance may be an important property of the chondrocyte microenvironment by providing a mechanism for enzyme transport from the chondrocyte to the ECM during normal matrix turnover without mechanical disruption of the PCM.
Abnormal matrix turnover is a hallmark of osteoarthritis (OA), a joint disease characterized by progressive degradation and loss of articular cartilage, ultimately resulting in severe pain and disability. While most investigations of alterations in cartilage properties in OA focus on the macroscale properties of the ECM, characterization of the morphological, biochemical, and biomechanical changes in the PCM can improve our understanding of the specific contributions of mechanical loading to cartilage degeneration in OA. In this regard, alterations in PCM appear to modify the response of chondrocytes to soluble mediators and matrix proteins (Peters et al., 2011; Vonk et al., 2011; Xu et al., 2011)
Despite localized matrix degradation by chondrocyte-derived matrix metalloproteinases and aggrecanases (Aigner et al., 2003; Song et al., 2007), enlarged, loosely-organized chondrons are prevalent in OA (Lee et al., 2000; Poole et al., 1991b). These changes are associated with a significant loss of biomechanical properties. Micropipette aspiration studies revealed that human chondrons isolated from OA cartilage exhibit Young's moduli that are 30 – 40% lower than those exhibited by chondrons isolated from healthy tissue (Alexopoulos et al., 2003; Alexopoulos et al., 2005b). When measured in situ, human PCM in early OA cartilage exhibited Young's moduli 30% lower than macroscopically normal cartilage (Wilusz et al., 2013). This study also demonstrated that the chondrocyte biomechanical microenvironment was significantly altered in early OA cartilage showing a low gradient in the change of modulus from the PCM to the ECM, as compared to the sharper transition observed in normal cartilage (Figure 4). Interestingly, similar alterations in the PCM micromechanical environment were observed following digestion with human leukocyte elastase (Wilusz and Guilak, 2014), providing support for elastase as a potential contributor to PCM degeneration in OA (Elsaid et al., 2003). Additional studies with Col6a1 knockout mice have provided indirect evidence that alterations in PCM properties or Col6a1−/− derived joint laxity can accelerate the progression of OA in the hip (Alexopoulos et al., 2009), but not the knee (Christensen et al., 2012). Furthermore, recent work using fluorescence-guided AFM stiffness mapping techniques has shown that in situ mechanical properties of Col6a1−/− PCM is significantly lower than wild type PCM as early as 2 months of age, and that these changes are accompanied by altered calcium signaling in chondrocytes and increased cell swelling in response to osmotic stress (Zelenski et al., 2014). These studies demonstrate that disease-related PCM structure and biomechanical properties can be measured at the microscale, and that these changes may affect the way chondrocytes sense and respond to the diseased environment, perhaps contributing to further matrix degradation and disease progression.
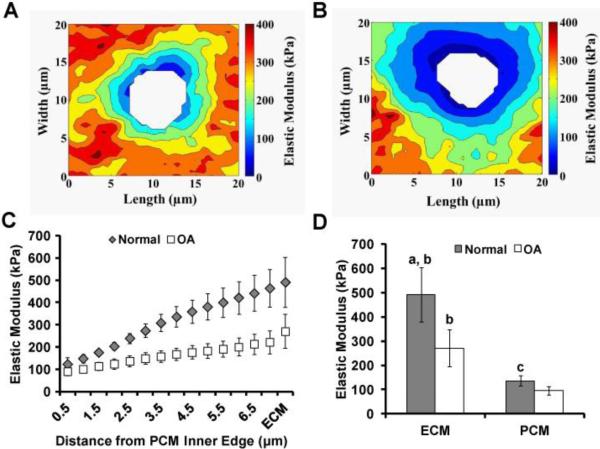
Figure 4. Alterations in the mechanical properties of the PCM with OA.
(A, B) Representative contour maps of calculated elastic moduli of the PCM of normal (A) or osteoarthritic (B) cartilage. (C) Mean elastic modulus values with distance from the PCM inner edge of with and without OA. (D) Mean elastic moduli of the ECM and PCM, with and without OA, showing a loss of mechanical properties with OA. [Adapted from (Wilusz et al., 2013), with permission].
Conclusion
Although the “chondron” was initially observed nearly a century ago (Benninghoff, 1925), little direct research was performed on this entity until it was discovered that intact chondrons could be isolated as a by-product of cartilage homogenization (Szirmai, 1968). Following this discovery, Poole and co-workers performed an extensive body of work on the biochemical composition and structure of the chondron, proposing important hypotheses on the potential function of this matrix compartment [e.g., (Poole, 1997; Poole et al., 1988; Poole et al., 1987; Poole et al., 1997; Poole et al., 1991a; Poole et al., 1991b)]. Over the past two decades, increasing evidence on the structure, biomechanical properties, and function of the PCM has confirmed the role of the chondron as a distinct functional compartment within articular cartilage. As outlined in this review, the PCM plays a role in a number of important processes throughout the development, maintenance, and degeneration of articular cartilage, including organizing the growth plate, modulating enzyme and growth factor activity, transducing biophysical signals, and undergoing changes that contribute to the progression of OA. The disparity in mechanical properties between the PCM and ECM, and within the PCM itself, confers the ability of the PCM to modulate stress and strain in the unique micromechanical environment of each chondrocyte within the tissue.
As more studies evaluate the PCM and generate new insights into its ultrastructure, biochemical composition, and biomechanical properties, a more complete characterization of the chondrocyte mechanical environment in cartilage will continue to emerge. Further investigations focused on the roles of individual PCM components and how they contribute to chondrocyte mechanotransduction in health and disease can help to elucidate factors contributing to the progression of OA. These findings may be relevant to the mechanobiology of other cartilaginous tissues such as the meniscus and intervertebral disc (Setton and Chen, 2004; Upton et al., 2003), which possess similar PCM-like structures that are rich in type VI collagen and surround individual cells within the tissue (Figure 5) (Cao et al., 2007; Sanchez-Adams et al., 2013). Furthermore, the detailed characterization of PCM structure and properties gained from these studies can be applied to computational modeling of cell-PCM-ECM interactions to further our understanding of the specific mechanical stresses experienced by the chondrocyte during joint loading and advance our knowledge of mechanotransduction mechanisms in articular cartilage and other tissues.
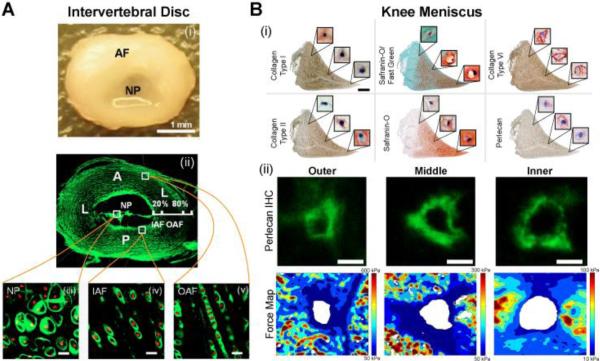
Figure 5. The pericellular matrix in other cartilaginous tissues.
(A) (i) The intervertebral disc is composed of the annulus fibrosis (AF) and nucleus pulposus (NP); (ii) immunofluorescence for type VI collagen in the disc; (iii-v) collagen type VI surrounding cells in the NP, inner AF, and outer AF (scale bar = 20 μm). [Adapted from (Cao et al., 2007), with permission]. (B) (i) Knee meniscus histology and immunohistochemistry showing the variation in ECM and PCM labeling with meniscus region (scale = 0.2 mm, cell view: 20×20 μm); (ii) representative images of perlecan immunolabeling and elastic modulus maps in the outer, middle and inner regions of the knee meniscus [adapted from (Sanchez-Adams et al., 2013), with permission].
Acknowledgments
We would like to dedicate this work to Dr. Dick Heinegård. We are deeply saddened by his loss, and we will miss him tremendously as a friend, scientist, and collaborator. His enormous impact and influence on the field of matrix biology will continue to be felt, despite his absence. This work was supported in part by a National Science Foundation Graduate Research Fellowship (REW), the Arthritis Foundation, and National Institutes of Health grants AG15768, AR48182, AR50245, AR48852, and AG46927.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aigner T, Zien A, Hanisch D, Zimmer R. Gene expression in chondrocytes assessed with use of microarrays. J Bone Joint Surg Am. 2003;85-A(Suppl 2):117–123. doi: 10.2106/00004623-200300002-00016. [DOI] [PubMed] [Google Scholar]
- Alexopoulos LG, Haider MA, Vail TP, Guilak F. Alterations in the mechanical properties of the human chondrocyte pericellular matrix with osteoarthritis. J Biomech Eng. 2003;125:323–333. doi: 10.1115/1.1579047. [DOI] [PubMed] [Google Scholar]
- Alexopoulos LG, Setton LA, Guilak F. The biomechanical role of the chondrocyte pericellular matrix in articular cartilage. Acta Biomater. 2005a;1:317–325. doi: 10.1016/j.actbio.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Alexopoulos LG, Williams GM, Upton ML, Setton LA, Guilak F. Osteoarthritic changes in the biphasic mechanical properties of the chondrocyte pericellular matrix in articular cartilage. J Biomech. 2005b;38:509–517. doi: 10.1016/j.jbiomech.2004.04.012. [DOI] [PubMed] [Google Scholar]
- Alexopoulos LG, Youn I, Bonaldo P, Guilak F. Developmental and osteoarthritic changes in Col6a1-knockout mice: biomechanics of type VI collagen in the cartilage pericellular matrix. Arthritis Rheum. 2009;60:771–779. doi: 10.1002/art.24293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen DM, Mao JJ. Heterogeneous nanostructural and nanoelastic properties of pericellular and interterritorial matrices of chondrocytes by atomic force microscopy. J Struct Biol. 2004;145:196–204. doi: 10.1016/j.jsb.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Arikawa-Hirasawa E, Watanabe H, Takami H, Hassell JR, Yamada Y. Perlecan is essential for cartilage and cephalic development. Nat Genet. 1999;23:354–358. doi: 10.1038/15537. [DOI] [PubMed] [Google Scholar]
- Bekkers JE, Tsuchida AI, van Rijen MH, Vonk LA, Dhert WJ, Creemers LB, Saris DB. Single-stage cell-based cartilage regeneration using a combination of chondrons and mesenchymal stromal cells: comparison with microfracture. Am J Sports Med. 2013;41:2158–2166. doi: 10.1177/0363546513494181. [DOI] [PubMed] [Google Scholar]
- Benninghoff A. Form und bau der Gelenkknorpel in ihren Beziehungen Zur Funktion. Zweiter Teil: der Aufbau des Gelenkknorpels in sienen Bezienhungen zur Funktion. 1925;2:783–862. [Google Scholar]
- Bougault C, Cueru L, Bariller J, Malbouyres M, Paumier A, Aszodi A, Berthier Y, Mallein-Gerin F, Trunfio-Sfarghiu AM. Alteration of cartilage mechanical properties in absence of beta1 integrins revealed by rheometry and FRAP analyses. J Biomech. 2013;46:1633–1640. doi: 10.1016/j.jbiomech.2013.04.013. [DOI] [PubMed] [Google Scholar]
- Cao L, Guilak F, Setton LA. Three-dimensional morphology of the pericellular matrix of intervertebral disc cells in the rat. J Anat. 2007;211:444–452. doi: 10.1111/j.1469-7580.2007.00784.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao PH, West AC, Hung CT. Chondrocyte intracellular calcium, cytoskeletal organization, and gene expression responses to dynamic osmotic loading. Am J Physiol Cell Physiol. 2006;291:C718–725. doi: 10.1152/ajpcell.00127.2005. [DOI] [PubMed] [Google Scholar]
- Chen C, Tambe DT, Deng L, Yang L. Biomechanical properties and mechanobiology of the articular chondrocyte. Am J Physiol Cell Physiol. 2013;305:C1202–1208. doi: 10.1152/ajpcell.00242.2013. [DOI] [PubMed] [Google Scholar]
- Choi JB, Youn I, Cao L, Leddy HA, Gilchrist CL, Setton LA, Guilak F. Zonal changes in the three-dimensional morphology of the chondron under compression: the relationship among cellular, pericellular, and extracellular deformation in articular cartilage. J Biomech. 2007;40:2596–2603. doi: 10.1016/j.jbiomech.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen SE, Coles JM, Zelenski NA, Furman BD, Leddy HA, Zauscher S, Bonaldo P, Guilak F. Altered trabecular bone structure and delayed cartilage degeneration in the knees of collagen VI null mice. PLoS One. 2012;7:e33397. doi: 10.1371/journal.pone.0033397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman JL, Widmyer MR, Leddy HA, Utturkar GM, Spritzer CE, Moorman CT, 3rd, Guilak F, Defrate LE. Diurnal variations in articular cartilage thickness and strain in the human knee. J Biomech. 2013;46:541–547. doi: 10.1016/j.jbiomech.2012.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darling EM, Wilusz RE, Bolognesi MP, Zauscher S, Guilak F. Spatial mapping of the biomechanical properties of the pericellular matrix of articular cartilage measured in situ via atomic force microscopy. Biophys J. 2010;98:2848–2856. doi: 10.1016/j.bpj.2010.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darling EM, Zauscher S, Guilak F. Viscoelastic properties of zonal articular chondrocytes measured by atomic force microscopy. Osteoarthritis Cartilage. 2006;14:571–579. doi: 10.1016/j.joca.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Elsaid KA, Jay GD, Chichester CO. Detection of collagen type II and proteoglycans in the synovial fluids of patients diagnosed with non-infectious knee joint synovitis indicates early damage to the articular cartilage matrix. Osteoarthritis Cartilage. 2003;11:673–680. doi: 10.1016/s1063-4584(03)00151-1. [DOI] [PubMed] [Google Scholar]
- Farnsworth N, Bensard C, Bryant SJ. The role of the PCM in reducing oxidative stress induced by radical initiated photoencapsulation of chondrocytes in poly(ethylene glycol) hydrogels. Osteoarthritis Cartilage. 2012;20:1326–1335. doi: 10.1016/j.joca.2012.06.015. [DOI] [PubMed] [Google Scholar]
- Grodzinsky AJ, Levenston ME, Jin M, Frank EH. Cartilage tissue remodeling in response to mechanical forces. Annu Rev Biomed Eng. 2000;2:691–713. doi: 10.1146/annurev.bioeng.2.1.691. [DOI] [PubMed] [Google Scholar]
- Guilak F, Alexopoulos LG, Haider MA, Ting-Beall HP, Setton LA. Zonal uniformity in mechanical properties of the chondrocyte pericellular matrix: micropipette aspiration of canine chondrons isolated by cartilage homogenization. Ann Biomed Eng. 2005;33:1312–1318. doi: 10.1007/s10439-005-4479-7. [DOI] [PubMed] [Google Scholar]
- Guilak F, Alexopoulos LG, Upton ML, Youn I, Choi JB, Cao L, Setton LA, Haider MA. The pericellular matrix as a transducer of biomechanical and biochemical signals in articular cartilage. Ann N Y Acad Sci. 2006;1068:498–512. doi: 10.1196/annals.1346.011. [DOI] [PubMed] [Google Scholar]
- Guilak F, Hung CT. Physical regulation of cartilage metabolism. In: Mow VC, Huiskes R, editors. Basic Orthopaedic Biomechanics and Mechano-Biology. 3rd ed. Lippincott Williams & Wilkins; Philadelphia: 2005. pp. 179–207. [Google Scholar]
- Guilak F, Jones WR, Ting-Beall HP, Lee GM. The deformation behavior and mechanical properties of chondrocytes in articular cartilage. Osteoarthritis Cartilage. 1999;7:59–70. doi: 10.1053/joca.1998.0162. [DOI] [PubMed] [Google Scholar]
- Guilak F, Mow VC. The mechanical environment of the chondrocyte: a biphasic finite element model of cell-matrix interactions in articular cartilage. J Biomech. 2000;33:1663–1673. [PubMed] [Google Scholar]
- Hagiwara H, Schroter-Kermani C, Merker HJ. Localization of collagen type VI in articular cartilage of young and adult mice. Cell Tissue Res. 1993;272:155–160. doi: 10.1007/BF00323581. [DOI] [PubMed] [Google Scholar]
- Haider MA, Schugart RC, Setton LA, Guilak F. A mechano-chemical model for the passive swelling response of an isolated chondron under osmotic loading. Biomech Model Mechanobiol. 2006;5:160–171. doi: 10.1007/s10237-006-0026-1. [DOI] [PubMed] [Google Scholar]
- Halloran JP, Sibole S, van Donkelaar CC, van Turnhout MC, Oomens CW, Weiss JA, Guilak F, Erdemir A. Multiscale mechanics of articular cartilage: potentials and challenges of coupling musculoskeletal, joint, and microscale computational models. Ann Biomed Eng. 2012;40:2456–2474. doi: 10.1007/s10439-012-0598-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedlund H, Hedbom E, Heinegrd D, Mengarelli-Widholm S, Reinholt FP, Svensson O. Association of the aggrecan keratan sulfate-rich region with collagen in bovine articular cartilage. J Biol Chem. 1999;274:5777–5781. doi: 10.1074/jbc.274.9.5777. [DOI] [PubMed] [Google Scholar]
- Heinegard D, Oldberg A. Structure and biology of cartilage and bone matrix noncollagenous macromolecules. FASEB J. 1989;3:2042–2051. doi: 10.1096/fasebj.3.9.2663581. [DOI] [PubMed] [Google Scholar]
- Hing WA, Sherwin AF, Poole CA. The influence of the pericellular microenvironment on the chondrocyte response to osmotic challenge. Osteoarthritis Cartilage. 2002;10:297–307. doi: 10.1053/joca.2002.0517. [DOI] [PubMed] [Google Scholar]
- Hu K, Xu L, Cao L, Flahiff CM, Brussiau J, Ho K, Setton LA, Youn I, Guilak F, Olsen BR, Li Y. Pathogenesis of osteoarthritis-like changes in the joints of mice deficient in type IX collagen. Arthritis Rheum. 2006;54:2891–2900. doi: 10.1002/art.22040. [DOI] [PubMed] [Google Scholar]
- Hunziker EB, Michel M, Studer D. Ultrastructure of adult human articular cartilage matrix after cryotechnical processing. Microsc Res Tech. 1997;37:271–284. doi: 10.1002/(SICI)1097-0029(19970515)37:4<271::AID-JEMT3>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Julkunen P, Wilson W, Jurvelin JS, Korhonen RK. Composition of the pericellular matrix modulates the deformation behaviour of chondrocytes in articular cartilage under static loading. Med Biol Eng Comput. 2009;47:1281–1290. doi: 10.1007/s11517-009-0547-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junqueira LC, Montes GS. Biology of collagen-proteoglycan interaction. Arch Histol Jpn. 1983;46:589–629. doi: 10.1679/aohc.46.589. [DOI] [PubMed] [Google Scholar]
- Kavanagh E, Ashhurst DE. Development and aging of the articular cartilage of the rabbit knee joint: Distribution of biglycan, decorin, and matrilin-1. J Histochem Cytochem. 1999;47:1603–1616. doi: 10.1177/002215549904701212. [DOI] [PubMed] [Google Scholar]
- Kim E, Guilak F, Haider MA. An axisymmetric boundary element model for determination of articular cartilage pericellular matrix properties in situ via inverse analysis of chondron deformation. J Biomech Eng. 2010;132:031011. doi: 10.1115/1.4000938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight MM, Lee DA, Bader DL. The influence of elaborated pericellular matrix on the deformation of isolated articular chondrocytes cultured in agarose. Biochim Biophys Acta. 1998;1405:67–77. doi: 10.1016/s0167-4889(98)00102-5. [DOI] [PubMed] [Google Scholar]
- Knight MM, Ross JM, Sherwin AF, Lee DA, Bader DL, Poole CA. Chondrocyte deformation within mechanically and enzymatically extracted chondrons compressed in agarose. Biochim Biophys Acta. 2001;1526:141–146. doi: 10.1016/s0304-4165(01)00118-0. [DOI] [PubMed] [Google Scholar]
- Knudson CB. Hyaluronan receptor-directed assembly of chondrocyte pericellular matrix. J Cell Biol. 1993;120:825–834. doi: 10.1083/jcb.120.3.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korhonen RK, Herzog W. Depth-dependent analysis of the role of collagen fibrils, fixed charges and fluid in the pericellular matrix of articular cartilage on chondrocyte mechanics. J Biomech. 2008;41:480–485. doi: 10.1016/j.jbiomech.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Kvist AJ, Johnson AE, Morgelin M, Gustafsson E, Bengtsson E, Lindblom K, Aszodi A, Fassler R, Sasaki T, Timpl R, Aspberg A. Chondroitin sulfate perlecan enhances collagen fibril formation. Implications for perlecan chondrodysplasias. J Biol Chem. 2006;281:33127–33139. doi: 10.1074/jbc.M607892200. [DOI] [PubMed] [Google Scholar]
- Lai WM, Hou JS, Mow VC. A triphasic theory for the swelling and deformation behaviors of articular cartilage. J Biomech Eng. 1991;113:245–258. doi: 10.1115/1.2894880. [DOI] [PubMed] [Google Scholar]
- Leddy HA, Christensen SE, Guilak F. Microscale diffusion properties of the cartilage pericellular matrix measured using 3D scanning microphotolysis. Journal of Biomechanical Engineering. 2008;130:061002. doi: 10.1115/1.2979876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee GM, Paul TA, Slabaugh M, Kelley SS. The incidence of enlarged chondrons in normal and osteoarthritic human cartilage and their relative matrix density. Osteoarthritis Cartilage. 2000;8:44–52. doi: 10.1053/joca.1999.0269. [DOI] [PubMed] [Google Scholar]
- Lee GM, Poole CA, Kelley SS, Chang J, Caterson B. Isolated chondrons: a viable alternative for studies of chondrocyte metabolism in vitro. Osteoarthritis Cartilage. 1997;5:261–274. doi: 10.1016/s1063-4584(97)80022-2. [DOI] [PubMed] [Google Scholar]
- Macri L, Silverstein D, Clark RA. Growth factor binding to the pericellular matrix and its importance in tissue engineering. Adv Drug Deliv Rev. 2007;59:1366–1381. doi: 10.1016/j.addr.2007.08.015. [DOI] [PubMed] [Google Scholar]
- Maroudas A, Bayliss MT, Uchitel-Kaushansky N, Schneiderman R, Gilav E. Aggrecan turnover in human articular cartilage: use of aspartic acid racemization as a marker of molecular age. Arch Biochem Biophys. 1998;350:61–71. doi: 10.1006/abbi.1997.0492. [DOI] [PubMed] [Google Scholar]
- McLeod MA, Wilusz RE, Guilak F. Depth-dependent anisotropy of the micromechanical properties of the extracellular and pericellular matrices of articular cartilage evaluated via atomic force microscopy. J Biomech. 2013;46:586–592. doi: 10.1016/j.jbiomech.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melrose J, Roughley P, Knox S, Smith S, Lord M, Whitelock J. The structure, location, and function of perlecan, a prominent pericellular proteoglycan of fetal, postnatal, and mature hyaline cartilages. J Biol Chem. 2006;281:36905–36914. doi: 10.1074/jbc.M608462200. [DOI] [PubMed] [Google Scholar]
- Michalek AJ, Iatridis JC. A numerical study to determine pericellular matrix modulus and evaluate its effects on the micromechanical environment of chondrocytes. J Biomech. 2007;40:1405–1409. doi: 10.1016/j.jbiomech.2006.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison EH, Ferguson MW, Bayliss MT, Archer CW. The development of articular cartilage: I. The spatial and temporal patterns of collagen types. J Anat. 1996;189(Pt 1):9–22. [PMC free article] [PubMed] [Google Scholar]
- Mow VC, Bachrach N, Setton LA, Guilak F. Stress, strain, pressure, and flow fields in articular cartilage. In: Mow VC, Guilak F, Tran-Son-Tay R, Hochmuth R, editors. Cell Mechanics and Cellular Engineering. Springer Verlag; New York: 1994. pp. 345–379. [Google Scholar]
- Mow VC, Wang CC, Hung CT. The extracellular matrix, interstitial fluid and ions as a mechanical signal transducer in articular cartilage. Osteoarthritis Cartilage. 1999;7:41–58. doi: 10.1053/joca.1998.0161. [DOI] [PubMed] [Google Scholar]
- Ng L, Hung HH, Sprunt A, Chubinskaya S, Ortiz C, Grodzinsky A. Nanomechanical properties of individual chondrocytes and their developing growth factor-stimulated pericellular matrix. J Biomech. 2007;40:1011–1023. doi: 10.1016/j.jbiomech.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Nguyen BV, Wang Q, Kuiper NJ, El Haj AJ, Thomas CR, Zhang Z. Strain-dependent viscoelastic behaviour and rupture force of single chondrocytes and chondrons under compression. Biotechnol Lett. 2009;31:803–809. doi: 10.1007/s10529-009-9939-y. [DOI] [PubMed] [Google Scholar]
- Nguyen BV, Wang QG, Kuiper NJ, El Haj AJ, Thomas CR, Zhang Z. Biomechanical properties of single chondrocytes and chondrons determined by micromanipulation and finite-element modelling. J R Soc Interface. 2010;7:1723–1733. doi: 10.1098/rsif.2010.0207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Conor C, Leddy HA, Benefield H, Liedtke W, Guilak F. TRPV4-mediated mechanotransduction regulates the metabolic response of chondrocytes to dynamic loading. Proceedings of the National Academy of Sciences USA. 2014;111:1316–1321. doi: 10.1073/pnas.1319569111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters HC, Otto TJ, Enders JT, Jin W, Moed BR, Zhang Z. The protective role of the pericellular matrix in chondrocyte apoptosis. Tissue Eng Part A. 2011;17:2017–2024. doi: 10.1089/ten.TEA.2010.0601. [DOI] [PubMed] [Google Scholar]
- Plumb DA, Ferrara L, Torbica T, Knowles L, Mironov A, Jr., Kadler KE, Briggs MD, Boot-Handford RP. Collagen XXVII organises the pericellular matrix in the growth plate. PLoS One. 2011;6:e29422. doi: 10.1371/journal.pone.0029422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole AR, Pidoux I, Reiner A, Rosenberg L. An immunoelectron microscope study of the organization of proteoglycan monomer, link protein, and collagen in the matrix of articular cartilage. J Cell Biol. 1982;93:921–937. doi: 10.1083/jcb.93.3.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole CA. Articular cartilage chondrons: form, function and failure. J Anat. 1997;191:1–13. doi: 10.1046/j.1469-7580.1997.19110001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole CA, Ayad S, Gilbert RT. Chondrons from articular cartilage. V. Immunohistochemical evaluation of type VI collagen organisation in isolated chondrons by light, confocal and electron microscopy. J Cell Sci. 1992;103(Pt 4):1101–1110. doi: 10.1242/jcs.103.4.1101. [DOI] [PubMed] [Google Scholar]
- Poole CA, Ayad S, Schofield JR. Chondrons from articular cartilage: I. Immunolocalization of type VI collagen in the pericellular capsule of isolated canine tibial chondrons. J Cell Sci. 1988;90(Pt 4):635–643. doi: 10.1242/jcs.90.4.635. [DOI] [PubMed] [Google Scholar]
- Poole CA, Flint MH, Beaumont BW. Morphological and functional interrelationships of articular cartilage matrices. J Anat. 1984;138(Pt 1):113–138. [PMC free article] [PubMed] [Google Scholar]
- Poole CA, Flint MH, Beaumont BW. Chondrons in cartilage: ultrastructural analysis of the pericellular microenvironment in adult human articular cartilages. J Orthop Res. 1987;5:509–522. doi: 10.1002/jor.1100050406. [DOI] [PubMed] [Google Scholar]
- Poole CA, Gilbert RT, Herbage D, Hartmann DJ. Immunolocalization of type IX collagen in normal and spontaneously osteoarthritic canine tibial cartilage and isolated chondrons. Osteoarthritis Cartilage. 1997;5:191–204. doi: 10.1016/s1063-4584(97)80014-3. [DOI] [PubMed] [Google Scholar]
- Poole CA, Glant TT, Schofield JR. Chondrons from articular cartilage. (IV). Immunolocalization of proteoglycan epitopes in isolated canine tibial chondrons. J Histochem Cytochem. 1991a;39:1175–1187. doi: 10.1177/39.9.1717545. [DOI] [PubMed] [Google Scholar]
- Poole CA, Matsuoka A, Schofield JR. Chondrons from articular cartilage. III. Morphologic changes in the cellular microenvironment of chondrons isolated from osteoarthritic cartilage. Arthritis Rheum. 1991b;34:22–35. doi: 10.1002/art.1780340105. [DOI] [PubMed] [Google Scholar]
- Radmacher M, Tillamnn RW, Fritz M, Gaub HE. From molecules to cells: imaging soft samples with the atomic force microscope. Science. 1992;257:1900–1905. doi: 10.1126/science.1411505. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E, Yamaguchi Y. Proteoglycans as modulators of growth factor activities. Cell. 1991;64:867–869. doi: 10.1016/0092-8674(91)90308-l. [DOI] [PubMed] [Google Scholar]
- Sanchez-Adams J, Wilusz RE, Guilak F. Atomic force microscopy reveals regional variations in the micromechanical properties of the pericellular and extracellular matrices of the meniscus. J Orthop Res. 2013;31:1218–1225. doi: 10.1002/jor.22362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schinagl RM, Gurskis D, Chen AC, Sah RL. Depth-dependent confined compression modulus of full-thickness bovine articular cartilage. J Orthop Res. 1997;15:499–506. doi: 10.1002/jor.1100150404. [DOI] [PubMed] [Google Scholar]
- Scott JE. Proteoglycan-fibrillar collagen interactions. Biochem J. 1988;252:313–323. doi: 10.1042/bj2520313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen S, Kumar S. Combining mechanical and optical approaches to dissect cellular mechanobiology. J Biomech. 2009 doi: 10.1016/j.jbiomech.2009.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setton LA, Chen J. Cell mechanics and mechanobiology in the intervertebral disc. Spine (Phila Pa 1976) 2004;29:2710–2723. doi: 10.1097/01.brs.0000146050.57722.2a. [DOI] [PubMed] [Google Scholar]
- Sherwin AF, Carter DH, Poole CA, Hoyland JA, Ayad S. The distribution of type VI collagen in the developing tissues of the bovine femoral head. Histochem J. 1999;31:623–632. doi: 10.1023/a:1003811310619. [DOI] [PubMed] [Google Scholar]
- Sibole SC, Erdemir A. Chondrocyte deformations as a function of tibiofemoral joint loading predicted by a generalized high-throughput pipeline of multi-scale simulations. PLoS One. 2012;7:e37538. doi: 10.1371/journal.pone.0037538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song RH, Tortorella MD, Malfait AM, Alston JT, Yang Z, Arner EC, Griggs DW. Aggrecan degradation in human articular cartilage explants is mediated by both ADAMTS-4 and ADAMTS-5. Arthritis Rheum. 2007;56:575–585. doi: 10.1002/art.22334. [DOI] [PubMed] [Google Scholar]
- Steward AJ, Wagner DR, Kelly DJ. The pericellular environment regulates cytoskeletal development and the differentiation of mesenchymal stem cells and determines their response to hydrostatic pressure. Eur Cell Mater. 2013;25:167–178. doi: 10.22203/ecm.v025a12. [DOI] [PubMed] [Google Scholar]
- Stockwell RA. The interrelationship of cell density and cartilage thickness in mammalian articular cartilage. J Anat. 1971;109:411–421. [PMC free article] [PubMed] [Google Scholar]
- SundarRaj N, Fite D, Ledbetter S, Chakravarti S, Hassell JR. Perlecan is a component of cartilage matrix and promotes chondrocyte attachment. J Cell Sci. 1995;108(Pt 7):2663–2672. doi: 10.1242/jcs.108.7.2663. [DOI] [PubMed] [Google Scholar]
- Szirmai JA. Structure of Cartilage. In: Engel A, Larsson T, editors. Aging of Connective and Skeletal Tissue. Nordiska Bokhandelns Forlag; Stockholm: 1968. pp. 163–184. [Google Scholar]
- Upton ML, Chen J, Guilak F, Setton LA. Differential effects of static and dynamic compression on meniscal cell gene expression. J Orthop Res. 2003;21:963–969. doi: 10.1016/S0736-0266(03)00063-9. [DOI] [PubMed] [Google Scholar]
- Vanden Berg-Foels WS, Scipioni L, Huynh C, Wen X. Helium ion microscopy for high-resolution visualization of the articular cartilage collagen network. J Microsc. 2012;246:168–176. doi: 10.1111/j.1365-2818.2012.03606.x. [DOI] [PubMed] [Google Scholar]
- Vincent T, Hermansson M, Bolton M, Wait R, Saklatvala J. Basic FGF mediates an immediate response of articular cartilage to mechanical injury. Proc Natl Acad Sci U S A. 2002;99:8259–8264. doi: 10.1073/pnas.122033199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent TL, Hermansson MA, Hansen UN, Amis AA, Saklatvala J. Basic fibroblast growth factor mediates transduction of mechanical signals when articular cartilage is loaded. Arthritis Rheum. 2004;50:526–533. doi: 10.1002/art.20047. [DOI] [PubMed] [Google Scholar]
- Vincent TL, McLean CJ, Full LE, Peston D, Saklatvala J. FGF-2 is bound to perlecan in the pericellular matrix of articular cartilage, where it acts as a chondrocyte mechanotransducer. Osteoarthritis Cartilage. 2007;15:752–763. doi: 10.1016/j.joca.2007.01.021. [DOI] [PubMed] [Google Scholar]
- Vincourt JB, Etienne S, Grossin L, Cottet J, Bantsimba-Malanda C, Netter P, Mainard D, Libante V, Gillet P, Magdalou J. Matrilin-3 switches from anti- to pro-anabolic upon integration to the extracellular matrix. Matrix Biol. 2012;31:290–298. doi: 10.1016/j.matbio.2012.03.004. [DOI] [PubMed] [Google Scholar]
- Vonk LA, Doulabi BZ, Huang C, Helder MN, Everts V, Bank RA. Preservation of the chondrocyte's pericellular matrix improves cell-induced cartilage formation. J Cell Biochem. 2010;110:260–271. doi: 10.1002/jcb.22533. [DOI] [PubMed] [Google Scholar]
- Vonk LA, Doulabi BZ, Huang C, Helder MN, Everts V, Bank RA. Collagen-induced expression of collagenase-3 by primary chondrocytes is mediated by integrin α1 and discoidin domain receptor 2: a protein kinase C-dependent pathway. Rheumatology (Oxford) 2011;50:463–472. doi: 10.1093/rheumatology/keq305. [DOI] [PubMed] [Google Scholar]
- Wiberg C, Klatt AR, Wagener R, Paulsson M, Bateman JF, Heinegard D, Morgelin M. Complexes of matrilin-1 and biglycan or decorin connect collagen VI microfibrils to both collagen II and aggrecan. J Biol Chem. 2003;278:37698–37704. doi: 10.1074/jbc.M304638200. [DOI] [PubMed] [Google Scholar]
- Wilusz RE, Defrate LE, Guilak F. A biomechanical role for perlecan in the pericellular matrix of articular cartilage. Matrix Biol. 2012a;31:320–327. doi: 10.1016/j.matbio.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilusz RE, DeFrate LE, Guilak F. Immunofluorescence-guided atomic force microscopy to measure the micromechanical properties of the pericellular matrix of porcine articular cartilage. J R Soc Interface. 2012b;9:2997–3007. doi: 10.1098/rsif.2012.0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilusz RE, Guilak F. High resistance of the mechanical properties of the chondrocyte pericellular matrix to proteoglycan digestion by chondroitinase, aggrecanase, or hyaluronidase. J Mech Behav Biomed Mater. 2014 doi: 10.1016/j.jmbbm.2013.09.021. http://dx.doi.org/10.1016/j.jmbbm.2013.09.021. [DOI] [PMC free article] [PubMed]
- Wilusz RE, Zauscher S, Guilak F. Micromechanical mapping of early osteoarthritic changes in the pericellular matrix of human articular cartilage. Osteoarthritis Cartilage. 2013;21:1895–1903. doi: 10.1016/j.joca.2013.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu JZ, Herzog W. Finite element simulation of location- and time-dependent mechanical behavior of chondrocytes in unconfined compression tests. Ann Biomed Eng. 2000;28:318–330. doi: 10.1114/1.271. [DOI] [PubMed] [Google Scholar]
- Xu L, Polur I, Servais JM, Hsieh S, Lee PL, Goldring MB, Li Y. Intact pericellular matrix of articular cartilage is required for unactivated discoidin domain receptor 2 in the mouse model. Am J Pathol. 2011;179:1338–1346. doi: 10.1016/j.ajpath.2011.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youn I, Choi JB, Cao L, Setton LA, Guilak F. Zonal variations in the three-dimensional morphology of the chondron measured in situ using confocal microscopy. Osteoarthritis Cartilage. 2006;14:889–897. doi: 10.1016/j.joca.2006.02.017. [DOI] [PubMed] [Google Scholar]
- Zelenski N, Leddy HA, Sanchez-Adams J, Bonaldo P, Liedtke W, Guilak F. Collagen VI: the link between the extracellular matrix and chondrocyte mechanotranduction. Trans Orthop Res Soc. 2014;39:192. [Google Scholar]