Abstract
Insulin resistance is a pathophysiological component of type 2 diabetes and obesity and also occurs in states of stress, infection, and inflammation associated with an upregulation of cytokines. Here we show that in both obesity and lipopolysaccharide (LPS)-induced endotoxemia there is an increase in suppressor of cytokine signaling (SOCS) proteins, SOCS-1 and SOCS-3, in liver, muscle, and, to a lesser extent, fat. In concordance with these increases by LPS, tyrosine phosphorylation of the insulin receptor (IR) is partially impaired and phosphorylation of the insulin receptor substrate (IRS) proteins is almost completely suppressed. Direct overexpression of SOCS-3 in liver by adenoviral-mediated gene transfer markedly decreases tyrosine phosphorylation of both IRS-1 and IRS-2, while SOCS-1 overexpression preferentially inhibits IRS-2 phosphorylation. Neither affects IR phosphorylation, although both SOCS-1 and SOCS-3 bind to the insulin receptor in vivo in an insulin-dependent fashion. Experiments with cultured cells expressing mutant insulin receptors reveal that SOCS-3 binds to Tyr960 of IR, a key residue for the recognition of IRS-1 and IRS-2, whereas SOCS-1 binds to the domain in the catalytic loop essential for IRS-2 recognition in vitro. Moreover, overexpression of either SOCS-1 or SOCS-3 attenuates insulin-induced glycogen synthesis in L6 myotubes and activation of glucose uptake in 3T3L1 adipocytes. By contrast, a reduction of SOCS-1 or SOCS-3 by antisense treatment partially restores tumor necrosis factor alpha-induced downregulation of tyrosine phosphorylation of IRS proteins in 3T3L1 adipocytes. These data indicate that SOCS-1 and SOCS-3 act as negative regulators in insulin signaling and serve as one of the missing links between insulin resistance and cytokine signaling.
Insulin resistance is observed in a wide variety of pathophysiological states. In obesity, infection, and inflammation, this is associated with elevated levels of cytokines (37, 52). Tumor necrosis factor alpha (TNF-α) has been shown to play an important role for insulin resistance in obese animal models (20), and loss of TNF-α signaling can reduce insulin resistance in ob/ob mice (51). Moreover, recent studies have revealed that elevated concentrations of cytokines, such as interleukin-6, are associated with development of type 2 diabetes (15, 38). Several mechanisms may play a role in cytokine-induced insulin resistance. TNF-α stimulation increases serine phosphorylation of insulin receptor substrate 1 (IRS-1), decreasing its tyrosine phosphorylation by the insulin receptor (IR) kinase (2, 19). Although these phosphorylation events are very rapid, full TNF-α-mediated inhibition develops over several hours (13), suggesting the involvement of other mechanisms, such as transcription-mediated regulation.
The suppressor of cytokine signaling (SOCS; also known as JAB and SSI) family is composed of SOCS-1 to -7 and the cytokine-inducible src homology 2 domain-containing protein (CIS) (56). These are thought to participate in negative feedback loops in cytokine signaling by multiple mechanisms (12, 33, 45). SOCS-1 and SOCS-3 have been shown to bind JAK tyrosine kinase and attenuate its ability to phosphorylate signal transducer and activator of transcription (STAT) proteins (34, 55), while CIS and SOCS-3 bind phosphorylated cytokine receptors and competitively interfere with binding of other src homology 2 domain-containing proteins (41). Expression of the SOCS proteins is increased by cytokine signaling through activation of STAT- and NF-κB-mediated pathways (12, 33, 40, 45). Thus, the negative feedback loop via SOCS proteins is doubly regulated in both a phosphorylation-dependent manner and a transcription-dependent manner.
Recent studies using the yeast two-hybrid system and molecular reconstitution in cultured cells have shown that SOCS-1, SOCS-3, and SOCS-6 can bind IR (11, 31) and that SOCS-2 and SOCS-3 can bind the insulin-like growth factor 1 receptor (9, 58). If SOCS proteins could attenuate insulin signaling in vivo, they would be attractive candidate molecules linking elevated cytokine levels and decreased insulin sensitivity in insulin-resistant states. Indeed, recently it has been shown that SOCS-1 knockout mice have decreased glucose levels and that cells derived from these mice seem to exhibit enhanced insulin signaling (25), although it is difficult to determine insulin sensitivity in vivo by using these mice because they die within 3 weeks of birth (32, 44).
In this study, we show that SOCS-1 and SOCS-3 are increased in insulin-resistant states, such as endotoxemia and obesity. The increased SOCS-1 and SOCS-3 bind to the distinct domains of IR, thereby differently inhibiting phosphorylation of IRS-1 and IRS-2 without affecting tyrosine phosphorylation of IR in vivo and in vitro. This attenuation of insulin signaling by SOCS-1 or SOCS-3 results in reductions of activation of glycogen synthesis and glucose transport in cultured cells, and reducing levels of these proteins restores the decreased tyrosine phosphorylation of IRS proteins caused by TNF-α.
MATERIALS AND METHODS
Animals.
Eight-week-old female C57BLKS/Jdb/db mice and C57BLKS/J mice were purchased from Jackson Laboratories, and 8-week-old male C57BL/6 mice were purchased from Taconic. All animals were housed on a 12-h light-dark cycle and were fed a standard rodent chow. All protocols for animal use and euthanasia were approved by the Animal Care Use Committee of the Joslin Diabetes Center and Harvard Medical School in accordance with National Institutes of Health guidelines.
RNA isolation from mice and cultured cells.
Total RNA was isolated from mouse tissues and cultured cells by using an RNeasy kit (QIAGEN). To induce endotoxemia, mice were starved overnight and treated with 20 mg of lipopolysaccharide (LPS; Sigma)/kg of body weight by intraperitoneal injection for the indicated period before sacrifice (47). For in vitro experiments, the cultured cells were starved for 18 h followed by treatment with 25 ng of TNF-α/ml or 100 nM insulin for 4 h, after which RNA was isolated as described above.
Semiquantitative RT-PCR.
Five hundred nanograms of the total RNA was applied to reverse transcription-PCR (RT-PCR) by using a One-Step RT-PCR system (Invitrogen). The primer pairs were the following: 5′-TCCGATTACCGGCGCATCACG-3′ and 5′-CTCCAGCAGCTCGAAAAGGCA-3′ for SOCS-1; 5′-CACAGCAAGTTTCCCGCCGCC-3′ and 5′-GTGCACCAGCTTGAGTACACA-3′ for SOCS-3; and 5′-ACCACCATGGAGAAGGCCGG-3′ and 5′-CTCAGTGTAGCCCAAGATGC-3′ for glyceraldehyde-3-phosphate dehydrogenase (GAPDH). The PCR profile was as follows: 1 cycle at 94°C for 5 min followed by 38 cycles (for db/db mice and cells) or 35 cycles (for LPS- and adenovirus-treated mice) at 94°C for 1 min, 60°C for 30 s, and 72°C for 1 min, and finally 1 cycle at 72°C for 10 min.
Generation of recombinant adenoviruses.
The cDNAs of SOCS-1 and SOCS-3 were subcloned between BamHI and EcoRI sites of pCMV-Tag2 vector, respectively, and amplified the full-length SOCS-1 and SOCS-3 cDNAs with an N-terminal FLAG tag by using the primer pairs 5′-GCCGCCACCATGGATTACAAGGAT-3′ and 5′-TCAGATCTGGAAGGGGAAGGAACTCAG-3′ for SOCS-1 and 5′-GCCGCCACCATGGATTACAAGGAT-3′ and 5′-CTAAAGTGGAGCATCATACTGATC-3′ for SOCS-3. After confirming the sequences, we treated the amplified fragments with Klenow enzyme and subcloned them into the SwaI site of the pAdex1CAwt cosmid cassette. The recombinant adenoviruses, Adex1CASOCS-1-FLAG and Adex1CASOCS-3-FLAG, were constructed by homologous recombination between the expression cosmid cassette and parental virus genome, as described previously (49).
Adenovirus-mediated gene transfer.
Eight-week-old male C57BL/6 mice were injected with the adenoviruses at a concentration of 5 × 108 PFU/g of body weight in a suspension of 200 μl of phosphate-buffered saline through the tail vein as described previously (50). We performed intravenous insulin injection at day 8. For in vitro experiments, Fao cells were grown to confluence and then were treated with the adenovirus at a multiplicity of infection (MOI) of 50 for 48 h followed by 18 h of starvation and insulin stimulation. For L6 myotubes and 3T3L1 adipocytes, we induced the cells into full differentiation as described previously (49) and then treated them with the adenovirus at an MOI of 50 and 250, respectively, for 48 h.
In vivo insulin stimulation and analysis of insulin signaling.
Mice were starved overnight, anesthetized with pentobarbital, and injected with 5 U of regular human insulin (Lilly) into the inferior vena cava. Five minutes after injection the liver was removed and frozen in liquid nitrogen. Immunoprecipitation and immunoblot analysis of insulin-signaling molecules were performed by using tissue homogenates extracted with buffer A, which contained 25 mM Tris-HCl (pH 7.4), 10 mM Na3VO4, 100 mM NaF, 50 mM Na4P2O7, 10 mM EGTA, 10 mM EDTA, 5 μg of leupeptin/ml, 5 μg of aprotinin/ml, 2 mM phenylmethylsulfonyl fluoride, and 1% Nonidet-P 40, as previously described (50).
Antibodies.
Polyclonal anti-SOCS-1 antibody (αSOCS-1), αSOCS-3, and polyclonal anti-Akt1 and -2 antibody (αAkt) were purchased from Santa Cruz Biotechnology. Rabbit polyclonal anti-IRS-1 antibody (αIRS-1), anti-IRS-2 antibody (αIRS-2), and anti-IR antibody (αIR) were generated as described previously (4). Monoclonal anti-phosphotyrosine antibody (αPY) and monoclonal anti-FLAG antibody (αFLAG) were purchased from Upstate Biotechnology, Inc., and Sigma, respectively.
In vitro kinase assays.
Tissue homogenates or cells were extracted with buffer A and were subjected to immunoprecipitation with αIRS-1, αIRS-2, or αPY antibodies followed by phosphatidylinositol (PI) 3-kinase assay as described previously (50). Akt kinase activity in the immunoprecipitates with αAkt was determined by using Crosstide as a substrate, as described previously (50).
GST pull-down assay.
The bacterial expression vector, pGEX-TY, encoding the major tyrosine phosphorylation sites in the catalytic loop of the human IR (1141-Met-Thr-Arg-Asp-Ile-Tyr-Glu-Thr-Asp-Tyr-Tyr-Arg-Lys-Gly-Gly-Lys-Gly-Leu-1158) fused to glutathione S-transferase (GST-TY), was generated by subcloning the annealed two oligonucleotides, 5′-GATCCATGACCAGAGACATCTATGAAACGGATTACTACCGGAAAGGGGGCAAGGGTCTGG-3′ and 5′-AATTCCAGACCCTTGCCCCCTTTCCGGTAGTAATCCGTTTCATAGATGTCTCTGGTCATG-3′, between BamHI and EcoRI sites of pGEX4T-1 vector. The activated IRs were purified and immobilized on wheat germ agglutinin beads from CHO-IR cells treated with 100 nM insulin for 5 min, as described previously (48). To phosphorylate the tyrosine residues of GST-TY (pGST-TY), GST-TY was incubated with the purified IR in buffer containing 50 mM Tris-HCl (pH 7.4), 5 mM MnCl2, and 20 μM ATP, as described previously (48). Lysates (1 mg of protein) form Fao cells overexpressing SOCS-1 or SOCS-3 by adenovirus-mediated gene transfer following 18 h of serum starvation were incubated with 1 μg of pGST-TY or GST-TY fusion protein that was previously immobilized on glutathione Sepharose beads. The protein complexes on the beads were extensively washed with buffer A followed by immunoblotting with anti-FLAG antibody.
Metabolic studies.
Glycogen synthase activity was assayed by using L6 myotubes treated with the indicated adenovirus, as described previously (49). The results were expressed as the fractional activity determined by dividing the activity measured with 0.25 mM glucose-6-phosphate (G6P) by the activity measured with 10 mM G6P. 2-Deoxy glucose uptake assays were performed by using 3T3L1 adipocytes treated with the indicated adenovirus, as described previously (49).
Antisense treatment.
Two oligonucleotides, designated AS1 and AS3, were synthesized for antisense treatment against SOCS-1 and SOCS-3, respectively. AS1 was designed as a 26-bp single-strand oligonucleotide, covering the −5 to approximately +21 region of murine SOCS-1 mRNA (5′-CACCTGGTTGCGTGCTACCATCCTAC-3′), while AS3 was designed to cover the −5 to approximately +21 region of the murine SOCS-3 mRNA (5′-AAACTTGCTGTGGGTGACCATGGCGC-3′) (3). Two oligonucleotides, designated C1 (5′-CAGCTCGTAGCGAGCAACCATCGTAC-3′, a six-base mismatch to AS1) and C3 (5′-AATCTAGCTCTGCGTGAGCATCGCGC-3′, a six-base mismatch to AS3), were also synthesized for controls. All oligonucleotides were synthesized as uniform phosphorothioate chimeric oligonucleotides, with 2′-O-methoxyethyl groups on bases 1 to 5 and 22 to 26. Fully differentiated 3T3L1 adipocytes were transfected with the oligonucleotides at the final concentration of 500 nM in serum-free Dulbecco's modified Eagle medium (DMEM) and FuGENE6 (Roche) as described previously (5). After being cultured in DMEM plus 10% fetal bovine serum for 48 h, cells were starved for 18 h followed by treatment with 25 ng of TNF-α/ml for 5 h and then were stimulated with 100 nM insulin for 2 min.
RESULTS
Upregulation of SOCS-1 and SOCS-3 in insulin-sensitive tissues in insulin-resistant states.
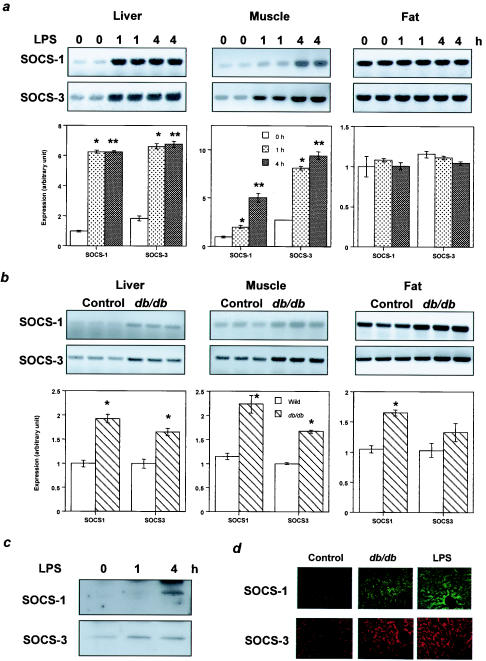
Endotoxemia in animals caused by bacterial LPS injection provides an experimental model for sepsis and a tool to investigate the relationship between the effects by elevated cytokine levels and insulin resistance, because LPS is known to cause systemic insulin resistance associated with elevated cytokine levels, such as interleukin-6 and TNF-α (6, 26, 29, 46, 53). Thus, to assess potential roles of SOCS proteins in insulin resistance states, we investigated changes in expression levels of SOCS-1 and SOCS-3 in insulin-sensitive tissues during endotoxemia. We treated mice with LPS, which has been shown to rapidly induce severe insulin resistance in liver and skeletal muscle (47, 53). We found that expression of both SOCS-1 and SOCS-3 in liver, as estimated by RT-PCR, was markedly increased, reaching a maximal level at 1 h after injection of LPS, and this was sustained at 4 h (Fig. 1a). At the protein level, SOCS-1 was maximally induced at 4 h, while SOCS-3 increased more rapidly, reaching its maximal level by 1 h (Fig. 1c). SOCS-1 and SOCS-3 were also significantly increased in muscle tissue at 1 h after injection of LPS, and further increase continued at 4 h (Fig. 1a). By contrast, LPS treatment produced no changes in SOCS-1 or SOCS-3 expression in fat (Fig. 1a).
FIG. 1.
Increased expression levels of SOCS-1 and SOCS-3 in insulin-sensitive tissues in insulin-resistant states. (a) Induction of SOCS-1 and SOCS-3 in liver and muscle by endotoxemia. The top panels show representative results of RT-PCR. In the bottom panels the data are quantified and each bar represents the means ± standard errors (n = 6) of the SOCS expression standardized to GAPDH expression. *, P < 0.05 for 0 h versus 1 h of treatment; **, P < 0.05 for 0 h versus 4 h of treatment. (b) Upregulation of SOCS-1 and SOCS-3 expression in db/db mice. The top panels show representative results of RT-PCR, and in the bottom panels each bar represents the means ± standard errors (n = 4) of the SOCS expression standardized to GAPDH expression. *, P < 0.05 for control versus db/db mice.
It has been previously shown that the insulin resistance of obesity may have a component mediated by cytokines, such as TNF-α (20). In muscle and liver of the obese insulin-resistant db/db mouse, SOCS-1 and SOCS-3 mRNAs were upregulated by 60 to 120%. In this case, there was also a significant increase in SOCS-1 and also a trend to increased levels of SOCS-3 in fat compared to levels in the controls (Fig. 1b). At the protein level, both SOCS-1 and SOCS-3 proteins were increased in livers of db/db mice compared to levels in their controls, but the level of increase appeared to be less than that induced by LPS as evidenced by immunostaining (Fig. 1d).
Impaired insulin signaling in liver with endotoxemia.
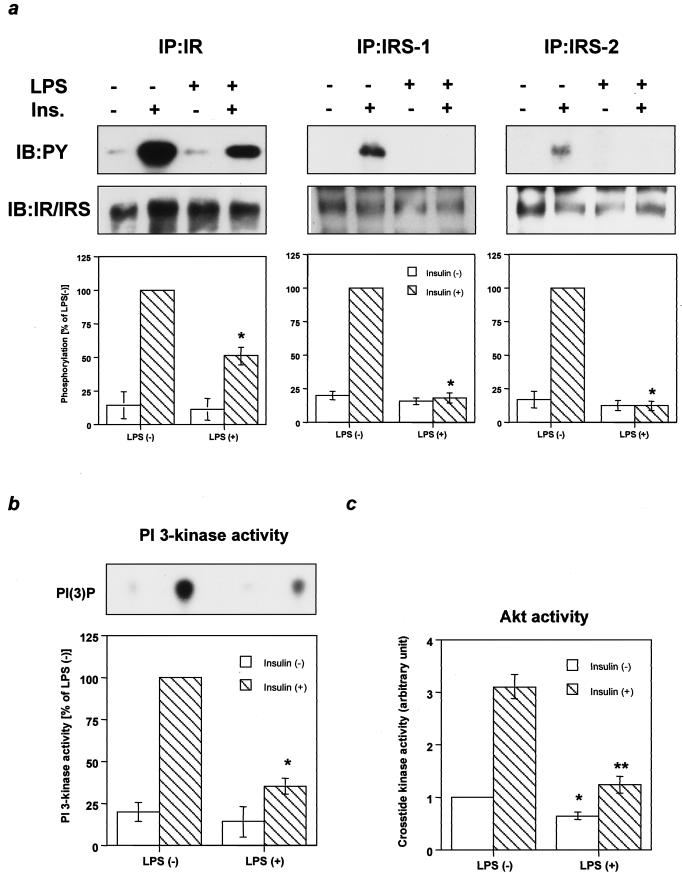
Although biological responses to insulin are known to be severely impaired during sepsis or endotoxemia (6, 26, 53), the specific alterations in insulin signaling have been poorly explored. To assess the mechanism of insulin resistance caused by endotoxemia and the possible role of the increase in SOCS proteins, we investigated insulin-signaling events in the livers of mice treated with LPS at 4 h, a time when insulin resistance has been demonstrated (6, 26, 53) and SOCS proteins are upregulated (Fig. 1c). At this time, phosphorylation of IR in liver was decreased by 50% in mice treated with LPS compared to controls (Fig. 2a). The phosphorylation of IRS-1 and IRS-2 was more profoundly decreased to almost undetectable levels by LPS treatment (Fig. 2a), suggesting that another inhibitory mechanism might be exerted in addition to the decrease in phosphorylation of IR. Reductions in phosphorylation of IR and IRS proteins were not associated with alterations in the levels of these proteins. Parallel with the decrease in phosphorylation of IRS proteins, PI 3-kinase activity associated with phosphotyrosine complexes was decreased by ∼65% in livers of mice treated with LPS (Fig. 2b). Consistent with the decrease in PI 3-kinase activity, basal- and insulin-induced Akt/ protein kinase B activity were markedly decreased in liver treated with LPS. Akt lies downstream of PI 3-kinase and has been shown to play an important role in inhibition of hepatic glucose production (7). This may contribute to the increased hepatic glucose production observed in animals during endotoxemia (6, 47).
FIG. 2.
Endotoxemia impairs insulin signaling at early steps. (a) Tyrosine phosphorylation of IR and its substrates. The immunoprecipitates (IP) with αIR, αIRS-1, or αIRS-2 from liver lysates with or without insulin stimulation were immunoblotted (IB) with αPY (top panels) or the same antibody (bottom panels). (b) PI 3-kinase activity associated with phosphotyrosine complexes. The top panel shows a representative result. In the bottom panel the data are quantified and each bar represents the means ± standard errors (n = 4). *, P < 0.05 for LPS negative versus LPS positive with insulin treatment. (c) Insulin-induced Akt activity. Each bar represents the means ± standard errors (n = 4). *, P < 0.05 for LPS negative versus LPS positive without insulin treatment; **, P < 0.05 LPS negative versus LPS positive with insulin treatment.
Impaired insulin signaling in liver overexpressing SOCS-1 or SOCS-3.
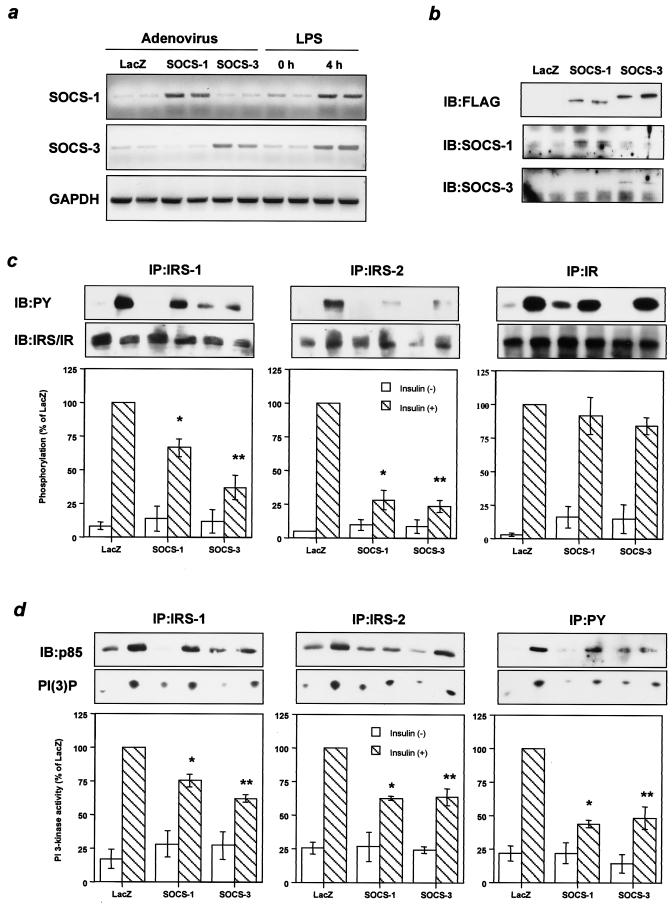
To assess whether the increase in SOCS-1 or SOCS-3 could mimic the attenuation of insulin signaling observed in endotoxemia, we treated three groups of mice with the adenoviruses encoding SOCS-1, SOCS-3, or LacZ, respectively. In each group the expression of protein following intravenous injection of the virus was primarily detected in liver, and the infection efficiency estimated by β-galactosidase assay with the liver treated with LacZ adenovirus was more than 90% (data not shown). The levels of overexpression of SOCS-1 and SOCS-3 under these conditions of infection were similar to those induced by LPS (Fig. 3a). Because both SOCS proteins were FLAG tagged, immunoblotting with an anti-FLAG antibody revealed that the protein level of introduced SOCS-3 was slightly higher than that of the introduced SOCS-1 (Fig. 3b).
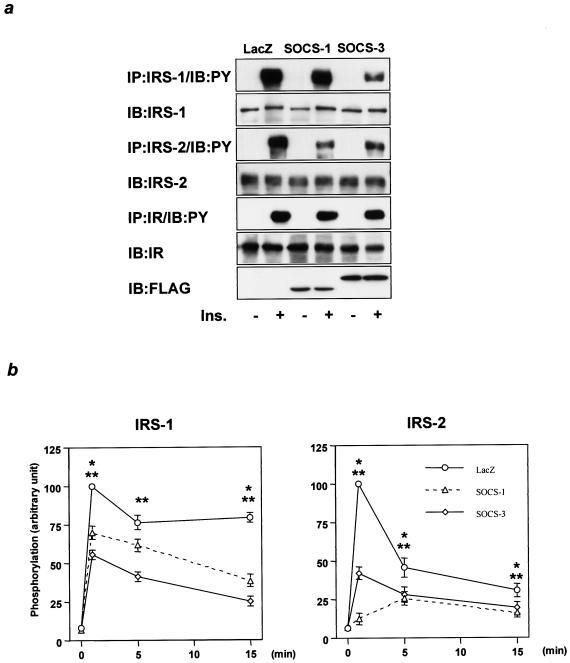
FIG. 3.
Increased SOCS-1 or SOCS-3 inhibits tyrosine phosphorylation of IRS proteins and downstream signaling in liver. (a) Comparable expression of SOCS-1 and SOCS-3 introduced by adenovirus injection to those induced by LPS treatment. The panels show representative results of RT-PCR using RNA from liver with the indicated treatment. (b) Liver contents of SOCS-1 and SOCS-3. The immunoprecipitates (IP) with αSOCS-1, αSOCS-3, or αFLAG from liver lysates were immunoblotted (IB) with the same antibody. (c) Tyrosine phosphorylation of IRS-1, IRS-2, and IR. The immunoprecipitates with αIRS-1, αIRS-2, or αIR antibodies from liver lysates with or without insulin stimulation were immunoblotted with αPY (top) or the same antibody (middle). (d) Insulin-induced PI 3-kinase activity. p85 subunit of PI 3-kinase bound to tyrosine-phosphorylated proteins (top) and PI 3-kinase activity (middle) were estimated by using the immunoprecipitates with αIRS-1, αIRS-2, or αPY from liver lysates with or without insulin stimulation. The top and middle panels show representative results; in the bottom panels each bar represents the means ± standard errors (n = 3). *, P < 0.05 for LacZ versus SOCS-1; **, P < 0.05 for LacZ versus SOCS-3 adenovirus treatment.
To assess the effects of overexpression of SOCS-1 and SOCS-3, 8 days after adenoviral treatment mice were given an intravenous insulin injection and 5 min later livers were removed to study insulin signaling. Overexpression of either SOCS-1 or SOCS-3 modestly inhibited phosphorylation of IRS-1, with SOCS-3 being more potent than SOCS-1. On the other hand, both profoundly decreased phosphorylation of IRS-2, while phosphorylation of IR was not altered in either case (Fig. 3c). Overexpression of SOCS-3 also produced a small reduction in the levels of IRS-1 and IRS-2 proteins, whereas SOCS-1 had almost no effect on IRS protein levels (Fig. 3c), indicating that reductions in IRS proteins could not account for the decrease in IRS phosphorylation related to increased SOCS expression. Indeed, these reductions in IRS proteins appeared to be secondary to the chronic hyperinsulinemia that occurs in the insulin-resistant state and is known to downregulate IRS-1 protein and IRS-2 mRNA (18, 43), because these did not occur in cultured cells overexpressing SOCS-1 or SOCS-3 (Fig. 4c). In parallel with the decrease in IRS protein phosphorylation, PI 3-kinase activities associated with IRS-1 and IRS-2 were significantly decreased in livers overexpressing SOCS-1 and SOCS-3 (Fig. 3d). As a result, total PI 3-kinase activity associated with phosphotyrosine complexes was decreased by ∼50% in livers overexpressing SOCS-1 and SOCS-3 (Fig. 3d), and insulin-induced Akt activity was reduced by ∼30% (data not shown). Thus, overexpression of SOCS-1 or SOCS-3 inhibits tyrosine phosphorylation of IRS proteins and impairs subsequent downstream signaling without directly inhibiting IR phosphorylation.
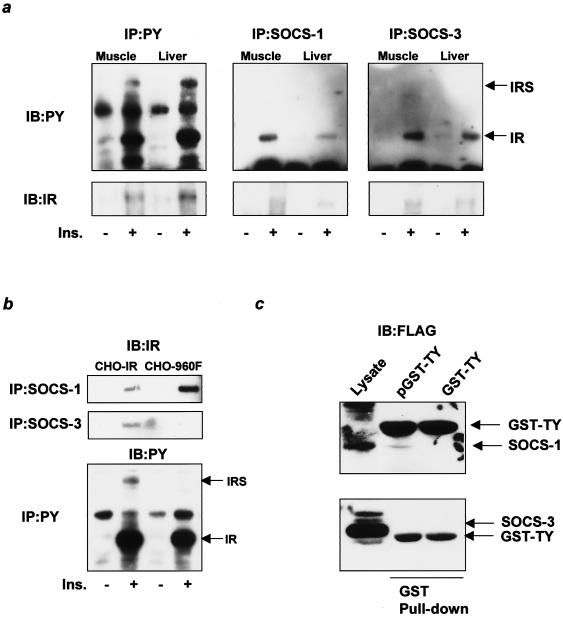
FIG. 4.
SOCS-1 and SOCS-3 bind to distinct sites of the IR and inhibit phosphorylation of IRS-1 and IRS-2 in different fashions. (a) Insulin-dependent binding of SOCS-1 and SOCS-3 to the IR in vivo. The immunoprecipitates (IP) with αPY (left), αSOCS-1 (middle), or αSOCS-3 (right) from liver or muscle lysates with or without insulin stimulation were immunoblotted (IB) with αPY (top) or αIR (bottom). (b) SOCS-3, but not SOCS-1, binds to Tyr960 of the IR. The immunoprecipitates with αSOCS-1 (top) or αSOCS-3 (middle) from CHO-IR cells or CHO-960F cells were immunoblotted with αIR, and the immunoprecipitates with αPY (bottom) were immunoblotted with αPY. (c) SOCS-1, but not SOCS-3, binds to the kinase domain of the IR. Total cell lysates (left lane), the pulled-down proteins with GST-TY (middle lane), or the phosphorylated GST-TY (pGST-TY, right lane) were blotted with αFLAG. Ins., insulin.
Inhibitory mechanisms of SOCS proteins in insulin signaling.
SOCS proteins are known to interact with JAK tyrosine kinase and inhibit phosphorylation of JAK substrates, such as the STAT proteins involved in cytokine signaling (34, 55). To clarify the inhibitory mechanisms of SOCS-1 and SOCS-3 on insulin signaling, we investigated the interaction of SOCS-1 or SOCS-3 with tyrosine-phosphorylated proteins induced by insulin in vivo. Following insulin stimulation, coprecipitation experiments revealed that the major insulin-dependent phosphorylated protein interacting with SOCS-1 or SOCS-3 in liver and muscle was migrated at the 95-kDa range where the β subunit of the IR (IRβ) was located (Fig. 4a, top). Indeed, this band was identified as IRβ by immunoblotting with the specific antibody for the IR (Fig. 4a, bottom). In these experiments, insulin-induced phosphorylation of JAK kinases was not detected (data not shown). Because the increased SOCS proteins attenuate phosphorylation of IRS proteins without affecting the IR phosphorylation, it is likely that SOCS proteins bind to the residue(s) of IR that is important for substrate recognition. We and others have previously shown that Tyr960 of IR is the key residue for recognition of IRS-1 by IR (10, 54). To address the possibility that SOCS proteins interact with IR via this residue (thereby inhibiting phosphorylation of IRS proteins), we assessed the interaction between SOCS-1 or SOCS-3 and a mutant IR in which the Tyr at 960 was replaced with Phe (IR960F). In cells expressing IR960F, the interaction between the mutant IR and SOCS-3 was almost abolished (Fig. 4b), suggesting that Tyr960 is the major binding site of IR for SOCS-3. This is consistent with the results of studies that used the yeast two-hybrid system (11). By contrast, the interaction between SOCS-1 and the mutant IR was actually increased, suggesting that SOCS-1 binds to another site that cooperates with Tyr960 in recognition of IRS proteins. Indeed, it is known that the kinase domain of the IR including the three major tyrosine phosphorylation sites (Tyr1146, -1150, and -1151) is required for the interaction between IR and IRS-2 in addition to Tyr960 (17, 42). To assess the possibility that SOCS-1 binds to this region of IR, we conducted pull-down assays with the GST fusion protein containing the sequence of the IR kinase domain including the three tyrosine residues (GST-TY) by using the lysates from the cells overexpressing SOCS-1 or SOCS-3. These experiments revealed that phosphorylated GST-TY (pGST-TY), but not unphosphorylated GST-TY, bound to SOCS-1 but not SOCS-3 (Fig. 4c) in vitro, indicating that SOCS-1 can bind to the kinase domain and residues which are important for IRS-2 recognition.
These distinct patterns of the interaction with IR may account for the difference in the inhibitory affinity for IRS-1 and IRS-2 of SOCS-1 and SOCS-3. Indeed, in Fao hepatoma cells, overexpression of SOCS-1 more potently inhibited phosphorylation of IRS-2 than that of IRS-1, while overexpression of SOCS-3 almost equally affected phosphorylation of both IRS-1 and IRS-2 (Fig. 5a). The inhibition of phosphorylation did not accompany any detectable reductions of IR or IRS proteins (Fig. 5a), supporting the molecular mechanism of inhibition proposed above. Furthermore, time courses of IRS-1 and IRS-2 phosphorylation in cells expressing SOCS-1 and SOCS-3 more clearly demonstrated the difference of the inhibitory effects of SOCS-1 and SOCS-3 on each IRS protein (Fig. 5b).
FIG. 5.
Differential inhibitory effects of SOCS-1 and SOCS-3 on IRS-1 and IRS-2 phosphorylation. (a) Effects of overexpression of SOCS-1 or SOCS-3 on the phosphorylation of IR, IRS-1, and IRS-2. The immunoprecipitates (IP) with αIRS-1, αIRS-2, or αIR from Fao cells infected with the indicated adenovirus were immunoblotted (IB) with αPY or the same antibody, and the lysates were immunoprecipitated with αFLAG. (b) Time course of inhibitory effects of SOCS-1 and SOCS-3 on IRS-1 and IRS-2 phosphorylation. The immunoprecipitates with αIRS-1, αIRS-2, or αIR from Fao cells infected with the indicated adenovirus stimulated with insulin (Ins.) for the indicated period were immunoblotted with αPY. Results were expressed as means ± standard errors (n = 3) of the percent maximal phosphorylation in control cells treated with LacZ. *, P < 0.05 for LacZ versus SOCS-1; **, P < 0.05 for LacZ versus SOCS-3 adenovirus treatment.
Inhibition of biological responses to insulin by SOCS-1 and SOCS-3 in cultured muscle cells and adipocytes.
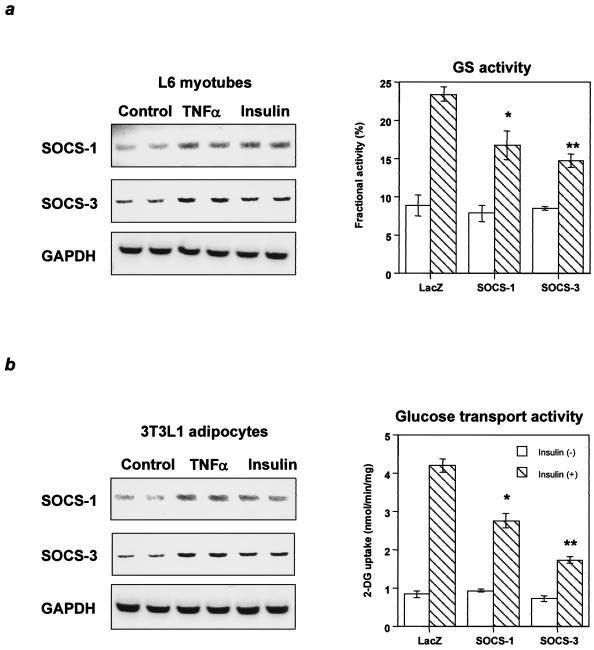
The effects of the SOCS proteins were further explored in cultured L6 myotubes and 3T3L1 adipocytes. In these cells, SOCS-1 and SOCS-3 were induced by TNF-α and insulin (Fig. 6a and b), two substances which are increased in insulin-resistant states. Furthermore, adenoviral-mediated overexpression of SOCS-1 or SOCS-3 in L6 myotubes and 3T3L1 adipocytes inhibited phosphorylation of IRS proteins as observed in liver and cultured hepatoma cells (data not shown). In parallel with the decreased IRS protein phosphorylation, glycogen synthase activity was inhibited by SOCS-1 and SOCS-3 in L6 myotubes (Fig. 6a) and glucose transport activity was significantly decreased by SOCS-1 and SOCS-3 in 3T3L1 adipocytes (Fig. 6b). Thus, both SOCS-1 and SOCS-3 have an inhibitory role on insulin action in muscle and fat in insulin-resistant states in which insulin and TNF-α are increased.
FIG. 6.
Inhibitory effects of SOCS-1 and SOCS-3 on biological responses to insulin in muscle cells and adipocytes. (a) Increased SOCS-1 or SOCS-3 inhibits glycogen synthase activity in L6 myotubes. (b) Increased SOCS-1 or SOCS-3 inhibits glucose transport activity in 3T3L1 adipocytes. The left panels show representative results of RT-PCR using RNA with the indicated treatment, and in the right graphs each bar represents the means ± standard errors (n = 4). *, P < 0.05 for LacZ versus SOCS-1; **, P < 0.05 for LacZ versus SOCS-3 adenovirus treatment.
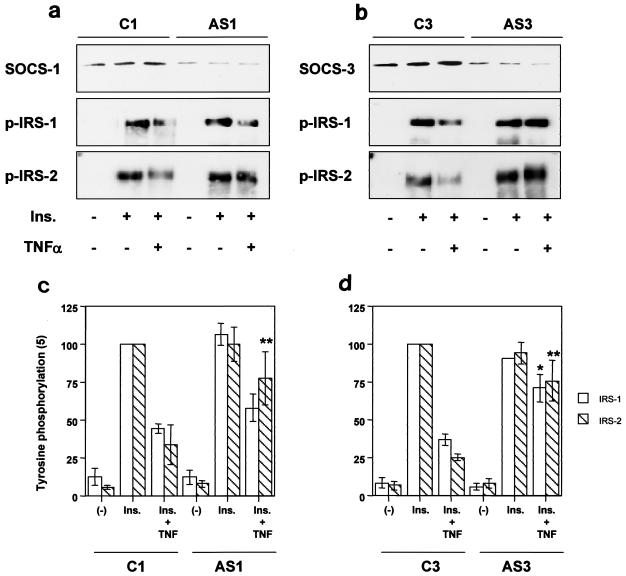
Amelioration of TNF-α-induced impaired phosphorylation of IRS proteins by suppressing SOCS-1 and SOCS-3.
To address whether inhibition of SOCS-1 or SOCS-3 could ameliorate impaired insulin signaling caused by cytokines, we treated 3T3L1 adipocytes with antisense oligonucleotides against SOCS-1 and SOCS-3. Treatment with antisense oligonucleotide for SOCS-1 (AS1) or SOCS-3 (AS3) reduced the level of the targeted protein and completely inhibited the elevation by TNF-α compared to that of the control treatment with C1 or C3, respectively. Suppression of SOCS-1 partially but significantly restored phosphorylation of IRS-2 inhibited by TNF-α while it had only modest effect on IRS-1 phosphorylation (Fig. 7, left). Suppression of SOCS-3, on the other hand, also significantly rescued TNF-α-induced inhibition of IRS protein phosphorylation with equal effects on both proteins, consistent with the discrete inhibitory mechanisms of each SOCS protein (Fig. 7, right).
FIG. 7.
Effects of antisense treatment of SOCS-1 or SOCS-3 on TNF-α-induced inhibition of IRS phosphorylation. The immunoprecipitates with αSOCS-1 (a, top row), αSOCS-3 (b, top row), αIRS-1 (a and b, middle rows), and αIRS-2 (a and b, bottom rows) from 3T3L1 adipocytes transfected with the indicated oligonucleotide treated with the indicated reagent were immunoblotted with the same antibody (for αSOCS-1 and αSOCS-3) or αPY (for αIRS-1 and αIRS-2). In the graphs, each bar represents the means ± standard errors (n = 4). *, P < 0.05 for C3 (Ins. + TNF) versus AS3 (Ins. + TNF); **, P < 0.05 for C1 (Ins. + TNF) versus AS1 (Ins. + TNF) or C3 (Ins. + TNF) versus AS3 (Ins. + TNF). Ins., insulin.
DISCUSSION
Insulin resistance is a central feature of many physiological and pathological states and can be the result of a wide variety of biochemical defects in the insulin action cascade (52). In the present study, we demonstrate that endotoxin-induced insulin resistance elevates SOCS-1 and SOCS-3 expression in liver and muscle. In liver, insulin signaling is impaired at very early steps, including phosphorylation of IR and IRS proteins, leading to decreased downstream insulin signaling. Overexpression of SOCS-1 or SOCS-3 by adenovirus-mediated gene transfer mimics most of these features by decreasing phosphorylation of IRS-1 and IRS-2. In contrast to the effect of LPS treatment, however, for overexpression by gene transfer the phosphorylation of IR is not altered, suggesting that LPS treatment attenuates insulin signaling by additional mechanisms at additional steps.
There are several possible mechanisms that may play a role following LPS treatment, including downregulation of the IR by transient hyperinsulinemia and increasing serine phosphorylation of the IR by protein kinase C (21, 24). In addition, IκB kinase β (IKKβ) has recently been proposed to be involved in cytokine- and obesity-induced insulin resistance through decreasing phosphorylation of IR (57), and LPS is known to activate the IKK/NF-κB pathway (16). Because SOCS expression is controlled by the NF-κB pathway (40), it is possible that activation of the IKK pathway by cytokines upregulates SOCS proteins as well as other inhibitory effects on insulin signaling. Nevertheless, the decrease in phosphorylation of IRS proteins produced by SOCS proteins is associated with downregulation of PI 3-kinase and Akt activities, leading to upregulation of gluconeogenic enzymes and hepatic glucose output (7). In L6 myotubes and 3T3L1 adipocytes, increased SOCS-1 and SOCS-3 also impair glycogen synthesis and glucose transport, consistent with the decreased insulin sensitivity observed in muscle and fat in insulin-resistant states, such as endotoxemia (26, 53).
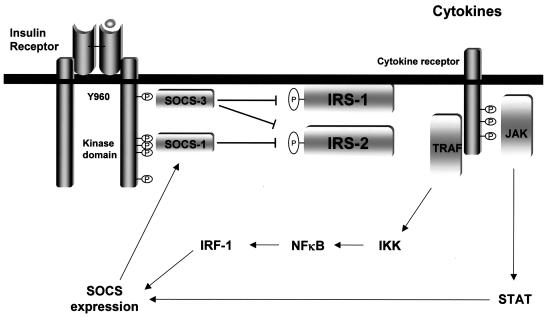
Although IR phosphorylation is not blocked, the mechanism of inhibition of insulin signaling by SOCS-1 and SOCS-3 appears to depend on the interaction between SOCS proteins and IR. This interaction has been shown by the yeast two-hybrid system and reconstitution system in cultured cells (11, 31, 35) and is also shown in vivo for the first time in the present study. In the case of SOCS-3, the binding site on IR appears to be Tyr960. Tyr960 is a key residue for recognition of the phosphotyrosine-binding (PTB) domains of IRS-1 and IRS-2, based on experiments with CHO cells expressing IR960F (54), studies of the crystal structures of the PTB domain of IRS-1 and the juxtamembrane domain of IR (10), and the yeast two-hybrid system (17, 42). This suggests that the interaction between SOCS-3 and IR equally inhibits recognition for IRS-1 and IRS-2 and is consistent with the inhibitory patterns of phosphorylation of IRS proteins by SOCS-3. SOCS-1, on the other hand, binds even better to IR960F than to wild-type IR. This is probably because the binding site for SOCS-1 is not Tyr960, and in cells expressing IR960F lesser amounts of IRS proteins (because of a lack of interaction through their PTB domains) compete with SOCS-1 for its binding site for IR, leading to the increase in binding of SOCS-1 to the mutant IR. Indeed, the binding site of SOCS-1 appears to be in the kinase domain of IR, because SOCS-1 binds to the phosphorylated fusion protein containing the three major tyrosine residues in the kinase domain in vitro. This region is known to be important for the recognition of IRS-2 by IR in addition to the juxtamembrane domain containing Tyr960 (17, 42), consistent with the finding that SOCS-1 inhibits phosphorylation of IRS-2 much more than IRS-1. It is difficult to definitively identify the binding site for SOCS-1 in vivo by using an IR with mutations of the tyrosine residues in this region, because these mutants have reduced kinase activity and reduced phosphorylation of other tyrosine residues on IR which might interact with SOCS-1. However, the sequence similarity between the kinase domain of IR, especially that surrounding Tyr1150 (ETDYYRK [the designated site of phosphorylation described is italicized]), and the binding site for SOCS-1 in the catalytic loop (Tyr1007) of JAK2 (DKEYYKV) (55) also supports the idea that the kinase domain important for IRS-2 recognition is the binding site of SOCS-1 in IR. Taken together, it is likely that SOCS-1 binds to the IRS-2 recognition site in the kinase domain of IR and mainly inhibits IRS-2-mediated insulin signaling, while SOCS-3 binds to Tyr960 of IR and inhibits both IRS-1- and IRS-2-mediated signaling (Fig. 8).
FIG. 8.
Model of discrete mechanisms of inhibition of insulin signaling by SOCS-1 and SOCS-3 induced by proinflammatory cytokines.
SOCS-1, but not SOCS-3, has been reported to interact with and promote ubiquitin-mediated degradation of some proteins through its carboxyl-terminal region (SOCS-box) as part of a ubiquitin-ligase complex (8, 22, 23). Recently, White and coworkers have reported that SOCS-1 and SOCS-3 bind to IRS proteins and lead them to ubiquitin-mediated protein degradation in cultured cells and liver (39). However, in the present study we could not detect interaction between SOCS and IRS proteins and any significant decrease in IR or IRS proteins in cultured cells, although there were modest reductions in IRS proteins in liver overexpressing SOCS protein. This suggests that the changes in vivo are not directly caused by SOCS proteins but rather are secondary to other factors, such as hyperinsulinemia. Indeed, we and others have found that chronic insulin treatment decreases mRNA of IRS-2 in cultured hepatocytes in a PI 3-kinase/Akt-dependent manner, whereas IRS-1 is decreased mainly due to protein degradation (18, 30, 43).
Proinflammatory cytokines, such as TNF-α, have been shown to inhibit insulin signaling by suppressing phosphorylation of IR and IRS proteins (14, 36). Although serine phosphorylation of IRS-1 by Jun N-terminal kinase (JNK) (1) appears to be one of the inhibitory mechanisms, our data suggest that SOCS protein-mediated inhibition of IRS phosphorylation is also involved in TNF-α-mediated inhibition of insulin signaling. Indeed, suppression of SOCS-1 or SOCS-3 in 3T3L1 adipocytes partially attenuates TNF-α-induced inhibition of tyrosine phosphorylation of IRS proteins, and the residual inhibition, including JNK-mediated inhibition, can be due to other pathways. In a separate study it was found that reducing SOCS-1 and SOCS-3 in vivo by treatment with antisense oligonucleotides partially, but not completely, normalizes IRS phosphorylation and hepatic steatosis in livers of db/db mice whose insulin resistance seems to be largely mediated by TNF-α signaling (51), consistent with the hypothesis that SOCS-mediated inhibition of insulin signaling is one of several inhibitory mechanisms produced by cytokines. This is supported by the fact that SOCS-1 knockout mice and cells seem to exhibit enhanced insulin signaling (25), although it is difficult to determine the role of SOCS-1 and SOCS-3 in mice by disruption of these genes because of their lethality (27, 28, 32, 44).
In summary, expression of SOCS-1 and SOCS-3 is elevated in insulin-sensitive tissues in obesity and endotoxemia. Increased SOCS-1 and SOCS-3 levels differently inhibit the phosphorylation of IRS-1 and IRS-2 and subsequent downstream signaling, leading to insulin resistance. The present study demonstrates that these two SOCS proteins have unique mechanisms of attenuation of insulin signaling, suggesting that both SOCS-1 and SOCS-3 may serve as therapeutic targets for type 2 diabetes and other insulin-resistant states.
Acknowledgments
We thank M. Kaneki (Massachusetts General Hospital) for helpful suggestions and T. Naka (Osaka University) for SSI (SOCS) expression vectors.
This work was supported by NIH grants DK33201 and DK55545, by the Joslin DERC grant DK34834 to C.R.K., and by a Grant-in-Aid for the 21st Century COE program from the Ministry of Education, Culture, Sports, Science, and Technology to K.U.
REFERENCES
- 1.Aguirre, V., T. Uchida, L. Yenush, R. Davis, and M. F. White. 2000. The c-Jun NH(2)-terminal kinase promotes insulin resistance during association with insulin receptor substrate-1 and phosphorylation of Ser(307). J. Biol. Chem. 275:9047-9054. [DOI] [PubMed] [Google Scholar]
- 2.Aguirre, V., E. D. Werner, J. Giraud, Y. H. Lee, S. E. Shoelson, and M. F. White. 2002. Phosphorylation of Ser307 in insulin receptor substrate-1 blocks interactions with the insulin receptor and inhibits insulin action. J. Biol. Chem. 277:1531-1537. [DOI] [PubMed] [Google Scholar]
- 3.Barkai, U., A. Prigent-Tessier, C. Tessier, G. B. Gibori, and G. Gibori. 2000. Involvement of SOCS-1, the suppressor of cytokine signaling, in the prevention of prolactin-responsive gene expression in decidual cells. Mol. Endocrinol. 14:554-563. [DOI] [PubMed] [Google Scholar]
- 4.Bruning, J. C., J. Winnay, S. Bonner-Weir, S. I. Taylor, D. Accili, and C. R. Kahn. 1997. Development of a novel polygenic model of NIDDM in mice heterozygous for IR and IRS-1 null alleles. Cell 88:561-572. [DOI] [PubMed] [Google Scholar]
- 5.Butler, M., R. A. McKay, I. J. Popoff, W. A. Gaarde, D. Witchell, S. F. Murray, N. M. Dean, S. Bhanot, and B. P. Monia. 2002. Specific inhibition of PTEN expression reverses hyperglycemia in diabetic mice. Diabetes 51:1028-1034. [DOI] [PubMed] [Google Scholar]
- 6.Chang, C. K., S. F. Moskal, Jr., K. S. Srivenugopal, and W. Schumer. 1993. Altered levels of mRNA encoding enzymes of hepatic glucose metabolism in septic rats. Circ. Shock 41:35-39. [PubMed] [Google Scholar]
- 7.Cho, H., J. Mu, J. K. Kim, J. L. Thorvaldsen, Q. Chu, E. B. Crenshaw III, K. H. Kaestner, M. S. Bartolomei, G. I. Shulman, and M. J. Birnbaum. 2001. Insulin resistance and a diabetes mellitus-like syndrome in mice lacking the protein kinase Akt2 (PKB beta). Science 292:1728-1731. [DOI] [PubMed] [Google Scholar]
- 8.Desepulveda, P., S. Ilangumaran, and R. Rottapel. 2000. Suppressor of cytokine signaling-1 inhibits VAV function through protein degradation. J. Biol. Chem. 275:14005-14008. [DOI] [PubMed] [Google Scholar]
- 9.Dey, B. R., S. L. Spence, P. Nissley, and R. W. Furlanetto. 1998. Interaction of human suppressor of cytokine signaling (SOCS)-2 with the insulin-like growth factor-I receptor. J. Biol. Chem. 273:24095-24101. [DOI] [PubMed] [Google Scholar]
- 10.Eck, M. J., S. Dhe-Paganon, T. Trub, R. T. Nolte, and S. E. Shoelson. 1996. Structure of the IRS-1 PTB domain bound to the juxtamembrane region of the insulin receptor. Cell 85:695-705. [DOI] [PubMed] [Google Scholar]
- 11.Emanuelli, B., P. Peraldi, C. Filloux, D. Sawka-Verhelle, D. Hilton, and E. Van Obberghen. 2000. SOCS-3 is an insulin-induced negative regulator of insulin signaling. J. Biol. Chem. 275:15985-15991. [DOI] [PubMed] [Google Scholar]
- 12.Endo, T. A., M. Masuhara, M. Yokouchi, R. Suzuki, H. Sakamoto, K. Mitsui, A. Matsumoto, S. Tanimura, M. Ohtsubo, H. Misawa, T. Miyazaki, N. Leonor, T. Taniguchi, T. Fujita, Y. Kanakura, S. Komiya, and A. Yoshimura. 1997. A new protein containing an SH2 domain that inhibits JAK kinases. Nature 387:921-924. [DOI] [PubMed] [Google Scholar]
- 13.Engelman, J. A., A. H. Berg, R. Y. Lewis, M. P. Lisanti, and P. E. Scherer. 2000. Tumor necrosis factor alpha-mediated insulin resistance, but not dedifferentiation, is abrogated by MEK1/2 inhibitors in 3T3-L1 adipocytes. Mol. Endocrinol. 14:1557-1569. [DOI] [PubMed] [Google Scholar]
- 14.Feinstein, R., H. Kanety, M. Z. Papa, B. Lunenfeld, and A. Karasik. 1993. Tumor necrosis factor-alpha suppresses insulin-induced tyrosine phosphorylation of insulin receptor and its substrates. J. Biol. Chem. 268:26055-26058. [PubMed] [Google Scholar]
- 15.Fernandez-Real, J. M., M. Broch, J. Vendrell, C. Gutierrez, R. Casamitjana, M. Pugeat, C. Richart, and W. Ricart. 2000. Interleukin-6 gene polymorphism and insulin sensitivity. Diabetes 49:517-520. [DOI] [PubMed] [Google Scholar]
- 16.Guha, M., and N. Mackman. 2001. LPS induction of gene expression in human monocytes. Cell Signal. 13:85-94. [DOI] [PubMed] [Google Scholar]
- 17.He, W., A. Craparo, Y. Zhu, T. J. O'Neill, L. M. Wang, J. H. Pierce, and T. A. Gustafson. 1996. Interaction of insulin receptor substrate-2 (IRS-2) with the insulin and insulin-like growth factor I receptors. Evidence for two distinct phosphotyrosine-dependent interaction domains within IRS-2. J. Biol. Chem. 271:11641-11645. [DOI] [PubMed] [Google Scholar]
- 18.Hirashima, Y., K. Tsuruzoe, S. Kodama, M. Igata, T. Toyonaga, K. Ueki, C. R. Kahn, and E. Araki. 2003. Insulin down-regulates insulin receptor substrate-2 expression through the phosphatidylinositol 3-kinase/Akt pathway. J. Endocrinol. 179:253-266. [DOI] [PubMed] [Google Scholar]
- 19.Hotamisligil, G. S., P. Peraldi, A. Budavari, R. Ellis, M. F. White, and B. M. Spiegelman. 1996. IRS-1-mediated inhibition of insulin receptor tyrosine kinase activity in TNF-alpha- and obesity-induced insulin resistance. Science 271:665-668. [DOI] [PubMed] [Google Scholar]
- 20.Hotamisligil, G. S., N. S. Shargill, and B. M. Spiegelman. 1993. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science 259:87-91. [DOI] [PubMed] [Google Scholar]
- 21.Kahn, C. R., and J. Roth. 1975. Cell membrane receptors for polypeptide hormones. Application to the study of disease states in mice, and men. Am. J. Clin. Pathol. 63:656-667. [DOI] [PubMed] [Google Scholar]
- 22.Kamizono, S., T. Hanada, H. Yasukawa, S. Minoguchi, R. Kato, M. Minoguchi, K. Hattori, S. Hatakeyama, M. Yada, S. Morita, T. Kitamura, H. Kato, K. Nakayama, and A. Yoshimura. 2001. The SOCS box of SOCS-1 accelerates ubiquitin-dependent proteolysis of TEL-JAK2. J. Biol. Chem. 276:12530-12538. [DOI] [PubMed] [Google Scholar]
- 23.Kamura, T., S. Sato, D. Haque, L. Liu, W. G. Kaelin, Jr., R. C. Conaway, and J. W. Conaway. 1998. The elongin BC complex interacts with the conserved SOCS-box motif present in members of the SOCS, ras, WD-40 repeat, and ankyrin repeat families. Genes Dev. 12:3872-3881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karasik, A., P. L. Rothenberg, K. Yamada, M. F. White, and C. R. Kahn. 1990. Increased protein kinase C activity is linked to reduced insulin receptor autophosphorylation in liver of starved rats. J. Biol. Chem. 265:10226-10231. [PubMed] [Google Scholar]
- 25.Kawazoe, Y., T. Naka, M. Fujimoto, H. Kohzaki, Y. Morita, M. Narazaki, K. Okumura, H. Saitoh, R. Nakagawa, Y. Uchiyama, S. Akira, and T. Kishimoto. 2001. Signal transducer and activator of transcription (STAT)-induced STAT inhibitor 1 (SSI-1)/suppressor of cytokine signaling 1 (SOCS1) inhibits insulin signal transduction pathway through modulating insulin receptor substrate 1 (IRS-1) phosphorylation. J. Exp. Med. 193:263-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ling, P. R., B. R. Bistrian, B. Mendez, and N. W. Istfan. 1994. Effects of systemic infusions of endotoxin, tumor necrosis factor, and interleukin-1 on glucose metabolism in the rat: relationship to endogenous glucose production and peripheral tissue glucose uptake. Metabolism 43:279-284. [DOI] [PubMed] [Google Scholar]
- 27.Marine, J. C., C. McKay, D. Wang, D. J. Topham, E. Parganas, H. Nakajima, H. Pendeville, H. Yasukawa, A. Sasaki, A. Yoshimura, and J. N. Ihle. 1999. SOCS3 is essential in the regulation of fetal liver erythropoiesis. Cell 98:617-627. [DOI] [PubMed] [Google Scholar]
- 28.Marine, J. C., D. J. Topham, C. McKay, D. Wang, E. Parganas, D. Stravopodis, A. Yoshimura, and J. N. Ihle. 1999. SOCS1 deficiency causes a lymphocyte-dependent perinatal lethality. Cell 98:609-616. [DOI] [PubMed] [Google Scholar]
- 29.Martich, G. D., A. J. Boujoukos, and A. F. Suffredini. 1993. Response of man to endotoxin. Immunobiology 187:403-416. [DOI] [PubMed] [Google Scholar]
- 30.Michael, M. D., R. N. Kulkarni, C. Postic, S. F. Previs, G. I. Shulman, M. A. Magnuson, and C. R. Kahn. 2000. Loss of insulin signaling in hepatocytes leads to severe insulin resistance and progressive hepatic dysfunction. Mol. Cell 6:87-97. [PubMed] [Google Scholar]
- 31.Mooney, R. A., J. Senn, S. Cameron, N. Inamdar, L. M. Boivin, Y. Shang, and R. W. Furlanetto. 2001. Suppressors of cytokine signaling-1 and -6 associate with and inhibit the insulin receptor. A potential mechanism for cytokine-mediated insulin resistance. J. Biol. Chem. 276:25889-25893. [DOI] [PubMed] [Google Scholar]
- 32.Naka, T., T. Matsumoto, M. Narazaki, M. Fujimoto, Y. Morita, Y. Ohsawa, H. Saito, T. Nagasawa, Y. Uchiyama, and T. Kishimoto. 1998. Accelerated apoptosis of lymphocytes by augmented induction of Bax in SSI-1 (STAT-induced STAT inhibitor-1) deficient mice. Proc. Natl. Acad. Sci. USA 95:15577-15582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Naka, T., M. Narazaki, M. Hirata, T. Matsumoto, S. Minamoto, A. Aono, N. Nishimoto, T. Kajita, T. Taga, K. Yoshizaki, S. Akira, and T. Kishimoto. 1997. Structure and function of a new STAT-induced STAT inhibitor. Nature 387:924-929. [DOI] [PubMed] [Google Scholar]
- 34.Narazaki, M., M. Fujimoto, T. Matsumoto, Y. Morita, H. Saito, T. Kajita, K. Yoshizaki, T. Naka, and T. Kishimoto. 1998. Three distinct domains of SSI-1/SOCS-1/JAB protein are required for its suppression of interleukin 6 signaling. Proc. Natl. Acad. Sci. USA 95:13130-13134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peraldi, P., C. Filloux, B. Emanuelli, D. J. Hilton, and E. Van Obberghen. 2001. Insulin induces suppressor of cytokine signaling-3 tyrosine phosphorylation through janus-activated kinase. J. Biol. Chem. 276:24614-24620. [DOI] [PubMed] [Google Scholar]
- 36.Peraldi, P., G. S. Hotamisligil, W. A. Buurman, M. F. White, and B. M. Spiegelman. 1996. Tumor necrosis factor (TNF)-alpha inhibits insulin signaling through stimulation of the p55 TNF receptor and activation of sphingomyelinase. J. Biol. Chem. 271:13018-13022. [DOI] [PubMed] [Google Scholar]
- 37.Peraldi, P., and B. Spiegelman. 1998. TNF-alpha and insulin resistance: summary and future prospects. Mol. Cell. Biochem. 182:169-175. [PubMed] [Google Scholar]
- 38.Pradhan, A. D., J. E. Manson, N. Rifai, J. E. Buring, and P. M. Ridker. 2001. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA 286:327-334. [DOI] [PubMed] [Google Scholar]
- 39.Rui, L., M. Yuan, D. Frantz, S. Shoelson, and M. F. White. 2002. SOCS-1 and SOCS-3 block insulin signaling by ubiquitin-mediated degradation of IRS1 and IRS2. J. Biol. Chem. 277:42394-42398. [DOI] [PubMed] [Google Scholar]
- 40.Saito, H., Y. Morita, M. Fujimoto, M. Narazaki, T. Naka, and T. Kishimoto. 2000. IFN regulatory factor-1-mediated transcriptional activation of mouse STAT-induced STAT inhibitor-1 gene promoter by IFN-gamma. J. Immunol. 164:5833-5843. [DOI] [PubMed] [Google Scholar]
- 41.Sasaki, A., H. Yasukawa, T. Shouda, T. Kitamura, I. Dikic, and A. Yoshimura. 2000. CIS3/SOCS-3 suppresses erythropoietin (EPO) signaling by binding the EPO receptor and JAK2. J. Biol. Chem. 275:29338-29347. [DOI] [PubMed] [Google Scholar]
- 42.Sawka-Verhelle, D., S. Tartare-Deckert, M. F. White, and E. Van Obberghen. 1996. Insulin receptor substrate-2 binds to the insulin receptor through its phosphotyrosine-binding domain and through a newly identified domain comprising amino acids 591-786. J. Biol. Chem. 271:5980-5983. [DOI] [PubMed] [Google Scholar]
- 43.Shimomura, I., M. Matsuda, R. E. Hammer, Y. Bashmakov, M. S. Brown, and J. L. Goldstein. 2000. Decreased IRS-2 and increased SREBP-1c lead to mixed insulin resistance and sensitivity in livers of lipodystrophic and ob/ob mice. Mol. Cell. 6:77-86. [PubMed] [Google Scholar]
- 44.Starr, R., D. Metcalf, A. G. Elefanty, M. Brysha, T. A. Willson, N. A. Nicola, D. J. Hilton, and W. S. Alexander. 1998. Liver degeneration and lymphoid deficiencies in mice lacking suppressor of cytokine signaling-1. Proc. Natl. Acad. Sci. USA 95:14395-14399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Starr, R., T. A. Willson, E. M. Viney, L. J. Murray, J. R. Rayner, B. J. Jenkins, T. J. Gonda, W. S. Alexander, D. Metcalf, N. A. Nicola, and D. J. Hilton. 1997. A family of cytokine-inducible inhibitors of signalling. Nature 387:917-921. [DOI] [PubMed] [Google Scholar]
- 46.Suffredini, A. F., G. Fantuzzi, R. Badolato, J. J. Oppenheim, and N. P. O'Grady. 1999. New insights into the biology of the acute phase response. J. Clin. Immunol. 19:203-214. [DOI] [PubMed] [Google Scholar]
- 47.Sugita, H., M. Kaneki, E. Tokunaga, M. Sugita, C. Koike, S. Yasuhara, R. G. Tompkins, and J. A. Martyn. 2002. Inducible nitric oxide synthase plays a role in LPS-induced hyperglycemia and insulin resistance. Am. J. Physiol. Endocrinol. Metab. 282:E386-E394. [DOI] [PubMed] [Google Scholar]
- 48.Takayama, S., M. F. White, and C. R. Kahn. 1988. Phorbol ester-induced serine phosphorylation of the insulin receptor decreases its tyrosine kinase activity. J. Biol. Chem. 263:3440-3447. [PubMed] [Google Scholar]
- 49.Ueki, K., R. Yamamoto-Honda, Y. Kaburagi, T. Yamauchi, K. Tobe, B. M. Burgering, P. J. Coffer, I. Komuro, Y. Akanuma, Y. Yazaki, and T. Kadowaki. 1998. Potential role of protein kinase B in insulin-induced glucose transport, glycogen synthesis, and protein synthesis. J. Biol. Chem. 273:5315-5322. [DOI] [PubMed] [Google Scholar]
- 50.Ueki, K., T. Yamauchi, H. Tamemoto, K. Tobe, R. Yamamoto-Honda, Y. Kaburagi, Y. Akanuma, Y. Yazaki, S. Aizawa, R. Nagai, and T. Kadowaki. 2000. Restored insulin-sensitivity in IRS-1-deficient mice treated by adenovirus-mediated gene therapy. J. Clin. Investig. 105:1437-1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Uysal, K. T., S. M. Wiesbrock, M. W. Marino, and G. S. Hotamisligil. 1997. Protection from obesity-induced insulin resistance in mice lacking TNF-alpha function. Nature 389:610-614. [DOI] [PubMed] [Google Scholar]
- 52.Virkamaki, A., K. Ueki, and C. R. Kahn. 1999. Protein-protein interaction in insulin signaling and the molecular mechanisms of insulin resistance. J. Clin. Investig. 103:931-943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Virkamaki, A., and H. Yki-Jarvinen. 1994. Mechanisms of insulin resistance during acute endotoxemia. Endocrinology 134:2072-2078. [DOI] [PubMed] [Google Scholar]
- 54.White, M. F., J. N. Livingston, J. M. Backer, V. Lauris, T. J. Dull, A. Ullrich, and C. R. Kahn. 1988. Mutation of the insulin receptor at tyrosine 960 inhibits signal transmission but does not affect its tyrosine kinase activity. Cell 54:641-649. [DOI] [PubMed] [Google Scholar]
- 55.Yasukawa, H., H. Misawa, H. Sakamoto, M. Masuhara, A. Sasaki, T. Wakioka, S. Ohtsuka, T. Imaizumi, T. Matsuda, J. N. Ihle, and A. Yoshimura. 1999. The JAK-binding protein JAB inhibits Janus tyrosine kinase activity through binding in the activation loop. EMBO J. 18:1309-1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yasukawa, H., A. Sasaki, and A. Yoshimura. 2000. Negative regulation of cytokine signaling pathways. Annu. Rev. Immunol. 18:143-164. [DOI] [PubMed] [Google Scholar]
- 57.Yuan, M., N. Konstantopoulos, J. Lee, L. Hansen, Z. W. Li, M. Karin, and S. E. Shoelson. 2001. Reversal of obesity- and diet-induced insulin resistance with salicylates or targeted disruption of IKKβ. Science 293:1673-1677. [DOI] [PubMed] [Google Scholar]
- 58.Zong, C. S., J. Chan, D. E. Levy, C. Horvath, H. B. Sadowski, and L. H. Wang. 2000. Mechanism of STAT3 activation by insulin-like growth factor I receptor. J. Biol. Chem. 275:15099-15105. [DOI] [PubMed] [Google Scholar]