Abstract
Although recent evidence supports a tumor-suppressive role for the GTPase RhoB, little is known about its regulation by signal transduction pathways. Here we demonstrate that Ras downregulates RhoB expression by a phosphatidylinositol 3-kinase (PI3K)- and Akt- but not Mek-dependent mechanism. Furthermore, genetic and pharmacological blockade of PI3K/Akt results in upregulation of RhoB expression. We also provide evidence for the importance of the downregulation of RhoB in oncogenesis by demonstrating that RhoB antagonizes Ras/PI3K/Akt malignancy. Ectopic expression of RhoB, but not the close relative RhoA, inhibits Ras, PI3K, and Akt induction of transformation, migration, and invasion and induces apoptosis and anoikis. Finally, RhoB inhibits melanoma metastasis to the lung in a mouse model. These studies identify suppression of RhoB as a mechanism by which the Ras/PI3K/Akt pathway induces tumor survival, transformation, invasion, and metastasis.
RhoB shares 86% amino acid sequence identity with RhoA, yet the roles of these low-molecular-weight GDP/GTP binding GTPases in oncogenesis are quite different. While RhoA, like other GTPase family members such as Ras, Rac1, and Cdc42, promotes oncogenesis, invasion, and metastasis (23, 33, 39, 40), emerging evidence points to a tumor-suppressive role for RhoB (7, 10-12, 27, 28). For example, RhoB, but not RhoA, inhibits proliferation, induces apoptosis, and inhibits tumor growth in a nude mouse xenograft model (7, 11, 12). Consistent with the tumor-suppressive activity of RhoB is the finding that in lung as well as head and neck and brain cancer patient biopsies, RhoB expression is dramatically decreased as tumors become more aggressive (1, 13, 30). Furthermore, preclinically, RhoB, unlike RhoA, which is constitutively expressed, has been shown to be induced by physical (UV and γ irradiation) and chemical (H2O2, methyl methanesulfonate, and cisplatin) agents (15, 16). Interestingly, RhoB is also induced by growth factors such as epidermal growth factor (EGF) and platelet-derived growth factor (PDGF) (18). Finally, RhoB appears to be required for stress-induced apoptosis, as cultured fibroblasts derived from RhoB−/− knockout mice are resistant to physical and chemical agent-induced apoptosis (27, 28). Taken together, the evidence points to RhoB as a gene that may play a critical role in protecting cells against stress as well as a novel role as a gene with tumor-suppressive activity. This prompted us to suggest that certain oncogenic and tumor survival pathways that become aberrantly activated during cancer progression may have to overcome RhoB tumor-suppressive activity as one of the steps leading to oncogenesis.
Two major pathways believed to play a pivotal role in human cancer progression are the phosphatidylinositol 3-kinase (PI3K)/Akt and the mitogen-activated Mek/extracellular signal-related kinase (Erk) pathways (8). Both of these pathways are activated by the low-molecular-weight GTP/GDP binding GTPase Ras, which is found oncogenically mutated in 30% of all human cancers (3). The ability of the Ras/Raf/Mek/Erk and Ras/PI3K/Akt pathways to induce uncontrolled deregulated proliferation and tumor survival in human cancer cells may depend not only on activating genes that stimulate cellular proliferation and survival but also on antagonizing those genes that suppress proliferation and/or induce apoptosis. Recently we have shown that EGF receptor (EGFR), ErbB2, and Ras but not Src inhibit RhoB expression (21). In this article, we demonstrate that oncogenic Ras downregulates RhoB expression by a PI3K- and Akt- but not a Mek-dependent mechanism. Furthermore, ectopic expression of RhoB, but not its close relative, RhoA, antagonizes Ras/PI3K/Akt-dependent transformation, apoptosis resistance, migration, and invasion as well as metastasis in an animal model.
MATERIALS AND METHODS
Cell culture, antibodies, and reagents.
NIH 3T3 cells were maintained in Dulbecco's minimum essential medium (DMEM) supplemented with 5% calf serum and 100 μg of penicillin-streptomycin ml−1. NIH 3T3 cells stably transfected with constitutively active H-Ras61L (H-Ras/NIH 3T3) were cultured in DMEM complete medium containing 400 μg of Geneticin ml−1. PANC-1 and PC3 human cancer cell lines were obtained from the American Type Culture Collection and cultured in DMEM supplemented with 10% fetal bovine serum and penicillin-streptomycin. B16-F10 mouse melanoma cells were cultured in RPMI 1640 supplemented with 10% fetal bovine serum and penicillin-streptomycin.
Antibodies to RhoB and RhoA, P110, Mek1, and Mek2 were purchased from Santa Cruz, Inc., Santa Cruz, Calif. Rabbit anti-phospho-Erk1/2, anti-phospho-Akt (Ser473) and anti-Akt were purchased from Cell Signaling Technology, Inc., Beverly, Mass. Anti-hemagglutinin (anti-HA) antibody (12AC5) was purchased from Roche. Monoclonal antibody to β-actin was obtained from Sigma. LY294002 and PD98059 were purchased from Calbiochem, La Jolla, Calif.
Gene constructs and RhoB promoter transcriptional activity assay.
Human RhoA and RhoB cDNA sequences as well as H-Ras61L were subcloned into HA-tagged pcDNA3 (10, 23, 34). The orientations and sequences of the genes were confirmed by DNA sequencing facilities at the H. Lee Moffitt Cancer Center, Tampa, Fla. Mouse RhoB promoter construct pGEI was kindly provided by Y. Monden (Banyu Tsukuba Research, Tsukuba, Japan) (31). The plasmids containing constitutively active or function-deficient PI3K, Mek1, Mek2, or Akt were kindly provided by Julie Y. Djeu (H. Lee Moffitt Cancer and Research Institute). Mek1 and -2 plasmids were originally provided by Michael J. Weber (6), PI3K constructs were originally provided by Anke Klippel (25), and Akt constructs were originally provided by Jin Cheng (20). β-Galactosidase activity and luciferase assay kits were purchased from Promega Corporation, Madison, Wis. The serum response element (SRE) reporter has been described before (21). DNA transfection was performed according to the Trans-IT-3T3 protocols for NIH 3T3 and H-Ras/NIH 3T3 cells or Trans-IT-LT1 protocols for PANC-1 cells (Mirus Corporation, Madison, Wis.). For B16-F10 cells, DNA transfection was performed with standard Lipofectamine protocols (Invitrogen, Grand Island, N.Y.). RhoB and SRE promoter transcriptional activity assays were performed according to the protocols accompanying the kits (Promega Corporation, Madison, Wis.). All the samplings were performed in triplicate, and the averages of three independent experiments are reported here.
Focus formation assay.
NIH 3T3 cells were seeded into 60-mm-diameter plates, and each plate was transfected with 0.1 μg of each oncogene construct plus 0.9 μg of pcDNA-RhoA, pcDNA-RhoB, or pcDNA3 vector control. Two days later, the cells were seeded into 60-mm-diameter plates at a density of 2.5 × 103 cells per plate and maintained in DMEM containing 1.5% calf bovine serum. The medium was changed every 3 days. Four weeks later, the cells were fixed, stained with crystal violet solution, and photographed. All the samplings were performed in triplicate, and a representative of three independent experiments is reported here.
Cell migration and invasion assay.
Cellular migration and invasion assays were performed with conventional Boyden transwell methods. Briefly, the cells were transfected and serum starved overnight and then treated with either dimethyl sulfoxide (DMSO) vehicle control or LY294002 (20 μM) or PD98059 (20 μM) for 30 min. Equal numbers of cells were added to the upper side of the transfilter (poly-hydrocarbonate membrane, 6-μm pore size) precoated with collagen type I for migration assays or reduced Matrigel plus collagen type I for invasion assays and placed in a 37°C tissue culture incubator for 12 h (H-Ras/NIH 3T3) or 8 h (PANC-1). The cells that migrated to the lower surface of the transfilter were stained and counted to determine the effect of a transfected gene or the inhibitor treatment.
Western blot analysis.
Whole-cell lysates were prepared in a lysis buffer containing 20 mM Tris-HCl (pH 7.5), 150 mM NaCl, 1% NP-40, 1 mM phenylmethylsulfonyl fluoride, 1.5 μg each of aprotinin and leupeptin per ml, 10 mM NaF, and 10 mM NaPPi. Fifty micrograms of the lysates was loaded into sodium dodecyl sulfate-polyacrylamide gel electrophoresis gel and analyzed for each sample. Antigen-bound antibody was detected with an enhanced chemiluminescence Western blotting kit (Amersham Pharmacia Biotech, Piscataway, N.J).
Cell proliferation and apoptosis assay.
Cells were collected and stained with annexin V and 7-AAD according to the manufacturer's recommendation (PharMingen, San Diego, Calif.). Data acquisition and analysis were performed by the Flow Cytometry Core Facility at the H. Lee Moffitt Cancer Center. In parallel, cells were also examined for cell number and viability by trypan blue exclusion and hemocytometer counting at the time intervals as indicated. All samplings were performed in triplicate, and the averages of three independent experiments are reported.
Anoikis assay.
The tissue culture plates were precoated twice with 50 mg of poly-(2-hydroxyethyl methacrylate) (poly-HEMA) ml−1 (Sigma, St. Louis, Mo.). The cells were washed in serum-free medium and seeded onto the precoated plates, and viability was examined at different time points by apoptotic assays with annexin V and 7-AAD labeling as described above. All samplings were performed in duplicate, and a representative of three independent experiments is reported here.
Melanoma metastasis assay.
pcDNA3, pcDNA-RhoB, and pcDNA-RhoA were transfected into B16-F10 cells as described above. The cells were harvested 20 h posttransfection, 5 × 105 cells from each group were analyzed for transfection efficiency by Western blotting, and another 5 × 105 cells were injected into the tail veins of C57/BL6 mice (6-week-old females). The mice were sacrificed after 21 days, and the nodules growing in the lungs were counted.
RESULTS
Ras downregulation of RhoB promoter transcriptional activity is mediated by PI3K and Akt but not Mek1/2.
The fact that RhoB has tumor-suppressive activity (7, 11, 12) and that RhoB levels decrease dramatically with the aggressiveness of tumors (1, 13, 30) prompted us to test the hypothesis that oncogenic and tumor survival pathways downregulate RhoB as a step leading to malignant transformation. Recently we have shown that EGFR, ErbB2, and Ras but not Src inhibit RhoB expression (21). Here we investigated the role of the PI3K/Akt and Mek limbs of the Ras pathways in this RhoB downregulation. To this end, NIH 3T3 cells were transfected with a RhoB promoter firefly luciferase reporter and SRE-Renilla reporter, along with various DNA constructs as described in Materials and Methods. Figure 1a shows that transfection with oncogenic H-Ras resulted in a 78% inhibition of RhoB promoter activity. This suppression of RhoB promoter activity was rescued in a concentration-dependent manner by dominant-negative forms of PI3K (DN-PI3K) and Akt1 (DN-Akt), suggesting that PI3K and Akt1 are required for H-Ras to inhibit RhoB promoter activity. Similar results were obtained with DN-Akt2 (data not shown). Figure 1a also shows that while DN-PI3K and DN-Akt inhibited the ability of Ras to suppress RhoB promoter activity by 79 and 76%, DN-Mek1/2 was only able to inhibit activity by 30%. In contrast, the ability of Ras to induce SRE promoter activity was equally inhibited by DN-PI3K, DN-Akt, and DN-Mek1/2. Therefore, the Ras suppression of RhoB promoter activity is primarily mediated by the PI3K/Akt pathway. Consistent with this is the demonstration that constitutively activated forms of PI3K (CA-PI3K) and Akt1 (CA-Akt), but not CA-Mek1/2, inhibited RhoB promoter activity (Fig. 1b). Finally we have also shown that the downregulation of RhoB by two receptor tyrosine kinases, EGFR and ErbB2, also requires PI3K and Akt, but not Mek (data not shown). To document that the CA and DN forms of PI3K, Akt, and Mek1/2 as well as H-Ras are expressed and that their expression results in their intended effects, we have blotted the lysates from the various conditions with antibodies that recognize P110, Akt, Mek, P-Akt, and P-ERK as well as antibodies that recognize HA (H-Ras) and actin (Fig. 1a and b).
FIG. 1.
Oncogenic PI3K/Akt, but not Mek1/2 mediates Ras suppression of RhoB promoter transcriptional activity in NIH 3T3 cells. NIH 3T3 cells were transiently transfected with various oncogene constructs (H-Ras, DN-PI3K, DN-Akt1, DN-Mek1/2 in panel a and CA-PI3K, CA-Akt1, and CA-Mek1/2 in panel b) or pcDNA3 vector control along with full-length RhoB promoter-firefly luciferase reporter and SRE-Renilla luciferase reporter for 36 h, and the cell lysates were processed for determination of RhoB and SRE promoter activity (upper panels) or for determination of expression of transfected genes as well as their effects on Akt and ERK phosphorylation (lower panels) as described in Materials and Methods. The data are reported as ratios of luciferase to β-galactosidase from the cells transfected by the oncogene(s) over those in pcDNA3-transfected cells.
Activation of the Ras/PI3K/Akt pathway inhibits the induction of RhoB promoter activity by the anticancer drug 5-FU.
Figure 1 demonstrated that the Ras/PI3K/Akt pathway inhibits the basal level (unstimulated) of RhoB promoter transcriptional activity. However, RhoB is usually expressed at very low levels but is induced by physical (UV and γ irradiation) and chemical (paclitaxel cisplatin, and H2O2) agents (15, 16). Therefore we next determined whether the Ras/PI3K/Akt pathway also suppresses the induction of RhoB. To this end, NIH 3T3 cells were transfected with RhoB promoter reporter, SRE promoter reporter, and treated with either DMSO or the anticancer drug 5-fluorouracil (5-FU) as described in Materials and Methods. Figure 2a shows that treatment of NIH 3T3 cells with 5-FU induced RhoB promoter activity by twofold, whereas transfection with oncogenic H-Ras, CA-PI3K, and CA-Akt but not CA-Mek1/2 inhibited RhoB promoter activity by 76, 72, and 70%, respectively. Furthermore, in the presence of oncogenic H-Ras, CA-PI3K, or CA-Akt, 5-FU was unable to stimulate RhoB promoter activity (Fig. 2a). In contrast, CA-Mek1/2 did not inhibit 5-FU induction of RhoB (Fig. 2a). Figure 2b shows that 5-FU had little effect on RhoB promoter activity in NIH 3T3 cells that stably express oncogenic H-Ras. Figure 2b also shows that treatment of H-Ras/NIH 3T3 cells with LY294002, a pharmacological inhibitor of PI3K (38, 41), alone induced RhoB promoter activity by 1.7-fold, whereas treatment with both 5-FU and LY294002 induced this activity by 3.5-fold, suggesting that inhibition of PI3K sensitizes H-Ras/NIH 3T3 cells to 5-FU. Taken together, the data from Fig. 2a and b demonstrate that the H-Ras/PI3K/Akt pathway downregulates RhoB in NIH 3T3 cells.
FIG. 2.
The ability of 5-FU to induce RhoB promoter activity is antagonized by oncogenic Ras, PI3K, and Akt in NIH 3T3 cells. (a) NIH 3T3 cells were transiently transfected with RhoB-promoter luciferase and SRE-luciferase constructs in the presence or absence of H-Ras61L, CA-PI3K, CA-Akt, or CA-MEK1/2 for 24 h, and then the cells were treated with either DMSO or 2.0 μM 5-FU for another 12 h. The cell lysates were processed as in Fig. 1. H-Ras/NIH 3T3 (b) and A549 cells (d) were transiently transfected with RhoB-promoter luciferase and SRE-luciferase constructs and cultured for 24 h; the cells were then treated with either DMSO, LY294002, 5-FU, or LY294002 plus 5-FU for 12 h. The cell lysates were processed as in Fig. 1. (c) PANC-1 cells were transiently transfected with CA-PI3K, CA-Akt, or pcDNA3 vector control for 24 h and then treated with either DMSO, LY294002, 5-FU, or LY294002 plus 5-FU for 12 h. The cell lysates were processed as in Fig. 1.
To determine whether this holds true for human cancer cells, we performed similar experiments with pancreatic (PANC-1) and lung (A549) cancer cells, both of which express mutated K-Ras. Figure 2c and d show that both cell lines are resistant to 5-FU and that treatment with LY294002 sensitize these cells to 5-FU induction of RhoB promoter activity. Furthermore, transfection of PANC-1 cells with CA-PI3K and CA-Akt inhibits both basal and 5-FU-induced RhoB promoter activity, and LY294002 reverses the CA-PI3K but not the CA-Akt suppression (Fig. 2c).
Blocking the H-Ras/PI3K/Akt pathway induces RhoB protein levels.
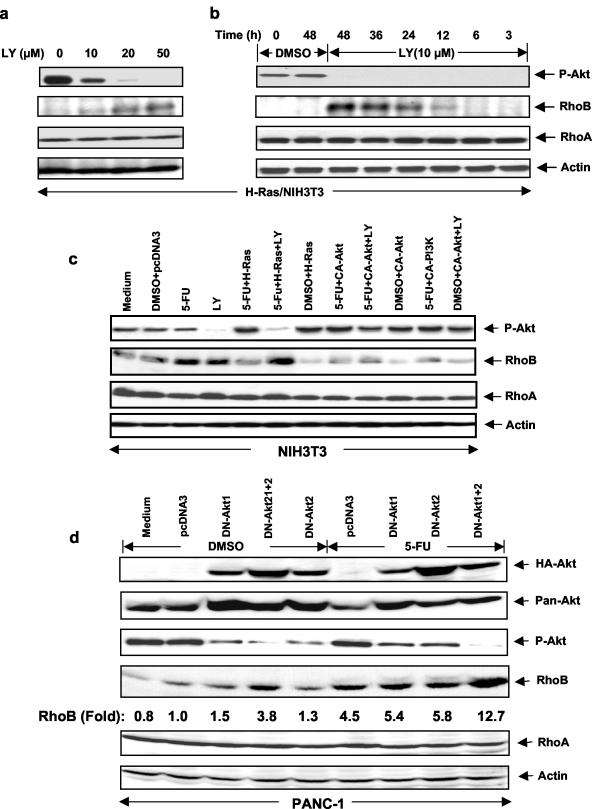
Figures 1 and 2 demonstrate that the Ras/PI3K/Akt pathway downregulates RhoB promoter transcriptional activity. The relevance of this important finding to endogenous RhoB protein was documented by showing that in the absence of LY294002, H-Ras/NIH 3T3 cells contained phosphorylated Akt (P-Akt) and expressed little RhoB, while treatment with LY294002 inhibited P-Akt levels and increased RhoB protein levels (Fig. 3a). In contrast, the levels of RhoA, a closely related family member, did not change following LY294002 treatment. Figure 3b shows that the induction of RhoB protein levels was detectable as early as 12 h after LY294002 treatment. We next analyzed parental NIH 3T3 cells and found that 5-FU induces RhoB protein levels and that oncogenic H-Ras, CA-PI3K, and CA-Akt all decreased both basal and 5-FU-induced RhoB protein levels (Fig. 3c). Treatment with LY294002 sensitizes oncogenic H-Ras- but not CA-Akt-transfected cells to 5-FU induction of RhoB expression, consistent with H-Ras being upstream, whereas Akt is downstream of PI3K, the target for LY294002. Treatment with PD98059 or U0126 (Mek inhibitors) did not sensitize the cells to 5-FU induction of RhoB (data not shown). The relevance of these findings to human cancer cells is documented in Fig. 3d, e, and f. Figure 3d shows that the DN forms of Akt1 and Akt2 induced RhoB protein levels slightly when used alone, but the induction was greater when both DN-Akt1 and DN-Akt2 were transfected into PANC-1 cells. Figure 3d also shows that transfection of PANC-1 cells with both DN-Akt1 and DN-Akt2 sensitized these cells to 5-FU induction of RhoB. Figure 3e shows that treatment of PANC-1 cells with LY294002 induced RhoB, but not RhoA, protein levels by eightfold. Treatment of PANC-1 cells with PD98059 resulted in no induction. Figure 3f shows that in another human cancer cell line, A549, LY294002 treatment similarly sensitized these cells to 5-FU induction of RhoB protein levels. Treatment with 5-FU or LY294002 alone induced RhoB only slightly (1.3- and 1.2-fold, respectively). However, cotreatment with 5-FU and LY294002 induced RhoB protein levels 3.9-fold (Fig. 3f).
FIG. 3.
Blocking H-Ras/PI3K/Akt pathway induces RhoB expression in NIH 3T3 cells and human cancer cell lines. H-Ras/NIH 3T3 cells were treated with different concentrations of LY294002 for 48 h (a) or LY294002 for different time intervals (b). The cells were then lysed and analyzed for P-Akt, RhoB, and RhoA protein levels by Western blotting with anti-RhoB and anti-RhoA; the same filter was reprobed with anti-β-actin for a loading control. (c) NIH 3T3 cells were transiently transfected with various genes as indicated for 24 h and then treated with either DMSO vehicle, LY294002, or 5-FU alone or in combination for another 48 h. The cells were analyzed for P-Akt, RhoB, RhoA, and β-actin protein levels as described above. (d) PANC-1 cells were transiently transfected with DN-Akt1 and DN-Akt2 genes as indicated for 24 h and then treated with either DMSO vehicle or 5-FU alone or in combination for another 48 h. The cells were analyzed for P-Akt, RhoB, and RhoA protein levels by Western blotting with anti-P-Akt, anti-RhoB, and anti-RhoA; the same filter was reprobed with anti-β-actin, anti-HA, and anti-pan-Akt antibodies. PANC-1 (e) and A549 (f) cells were treated with PD, LY294002, or 5-FU as indicated for 48 h and then analyzed for RhoB and RhoA protein levels by Western blotting as described above.
The ability of oncogenic H-Ras, PI3K, and Akt to transform NIH 3T3 cells is antagonized by ectopic expression of RhoB not RhoA.
Figures 1 through 3 clearly demonstrate that the H-Ras/PI3K/Akt pathway downregulates RhoB at the promoter as well as the protein levels. If downregulation of RhoB is a critical step for the H-Ras/PI3K/Akt pathway to mediate malignant transformation, then ectopic expression of RhoB should antagonize this transformation. To evaluate this possibility, we transfected NIH 3T3 cells with DNA constructs containing RhoA, RhoB, H-Ras61L, CA-PI3K, and CA-Akt either alone or in combination and monitored the ability of these cells to form foci as described in Materials and Methods. Figure 4a shows that parental NIH 3T3 cells as expected grew no colonies, but those transfected with either H-Ras61L, CA-PI3K, or CA-Akt grew numerous colonies. Cotransfection with RhoB but not RhoA along with the above genes resulted in significant inhibition of colony formation (see actual colony numbers in Fig. 4a). RhoB and RhoA were expressed at similar levels (Fig. 4a).
FIG. 4.
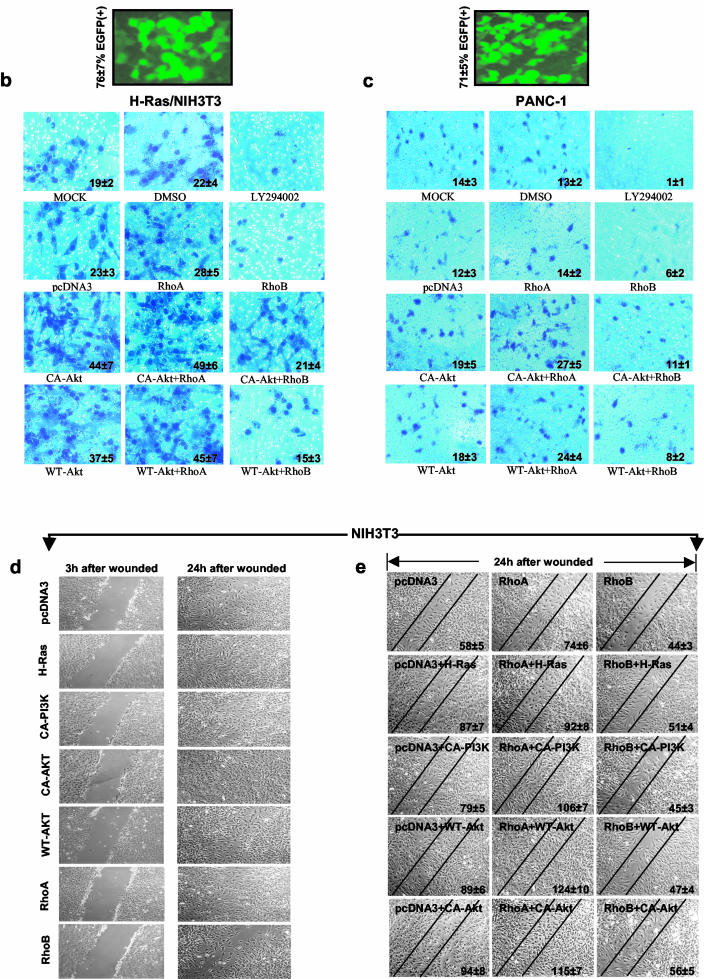
Ectopic expression of RhoB, not RhoA, inhibits Ras/PI3K/Akt-mediated transformation and migration. (a) Parental NIH 3T3 cells were transiently transfected with pcDNA3 vector control, H-Ras61L, CA-PI3K, or CA-Akt in the presence or absence of RhoA, RhoB, or pcDNA3 for 36 h. The cells were split, and an aliquot of the cells was evaluated for expression of transfected RhoA and RhoB by Western blotting. The rest of the cells were then cultured for another 4 weeks. The plates were examined for focus formation by staining. H-Ras/NIH 3T3 (b) and PANC-1 (c) cells were transiently transfected with pcDNA3 vector control, CA-Akt, or WT-Akt in the presence or absence of RhoA or RhoB or pcDNA3 for 36 h, internal ribosome entry site-EGFP was cotransfected as a transfection indicator. The cells were then split and analyzed for their migration capabilities through collagen type I-coated transfilters as described in Materials and Methods. (d and e) NIH 3T3 cells were transiently transfected with pcDNA3 vector control, H-Ras61L, CA-Akt, or WT-Akt in the presence or absence of RhoA, RhoB, or pcDNA3 for 36 h. The monolayers were then scratched with a yellow tip and microphotographed at the time points indicated to analyze their capability to migrate into and fill the wounded area.
The ability of oncogenic H-Ras, PI3K, and Akt to induced migration is inhibited by RhoB not RhoA.
Among the hallmarks of malignant transformation is the ability of cancer cells to migrate, invade, and metastasize, and the Ras/PI3K/Akt pathway is well known to be intimately involved in these processes (2, 9, 24, 26, 32, 37). Based on the results shown in Fig. 1, 2, and 3, we reasoned that Ras/PI3K/Akt may have to suppress RhoB to induce migration and invasion and, therefore, ectopic RhoB expression may block the ability of this pathway to induce migration and invasion. To further explore this possibility, we first examined whether RhoB inhibits Ras/PI3K/Akt-induced cellular migration. To this end, oncogenic H-Ras/NIH 3T3 cells and PANC-1 cells were analyzed for their capabilities to migrate through collagen type I in the presence or absence of ectopically expressed RhoA or RhoB as described in Materials and Methods. Figure 4b and c show that mock-, pcDNA3-, and RhoA-transfected as well as DMSO-treated cells migrated (through collagen type I) to the lower side of the transfilter. In contrast, the ability of LY294002-treated H-Ras/NIH 3T3 and PANC-1 cells as well as cells transfected with RhoB was dramatically hindered (see the actual number of cells within each panel of Fig. 4b and c). Furthermore, CA-Akt or wild-type Akt (WT-Akt) alone or with RhoA enhanced the ability to migrate, while RhoB inhibited the ability of CA-Akt and WT-Akt to enhance cell migration (Fig. 4b and c). Using enhanced green fluorescent protein (EGFP) transfections, we have determined transfection efficiencies to be 76% ± 7% and 71% ± 5% for H-Ras/NIH 3T3 cells and PANC-1 cells, respectively (Fig. 4b and 4c).
The ability of RhoB to inhibit cancer cell migration was further confirmed in a different assay in which cells are induced to migrate by physical wounding of cells plated on fibronectin-precoated plates. Figure 4d shows that 24 h after wounding, NIH 3T3 cells transfected with pcDNA3 were able to grow and fill the wounded area. Figure 4d also shows that oncogenic H-Ras, CA-PI3K, CA-Akt, WT-Akt, and RhoA transfection accelerated whereas RhoB inhibited the wound healing. Furthermore, RhoB also inhibited the ability of oncogenic H-Ras, CA-PI3K, CA-Akt, and WT-Akt to enhance wound healing (see the actual number of cells within each panel of Fig. 4e).
RhoB, not RhoA, inhibited H-Ras/PI3K/Akt-mediated cell invasion.
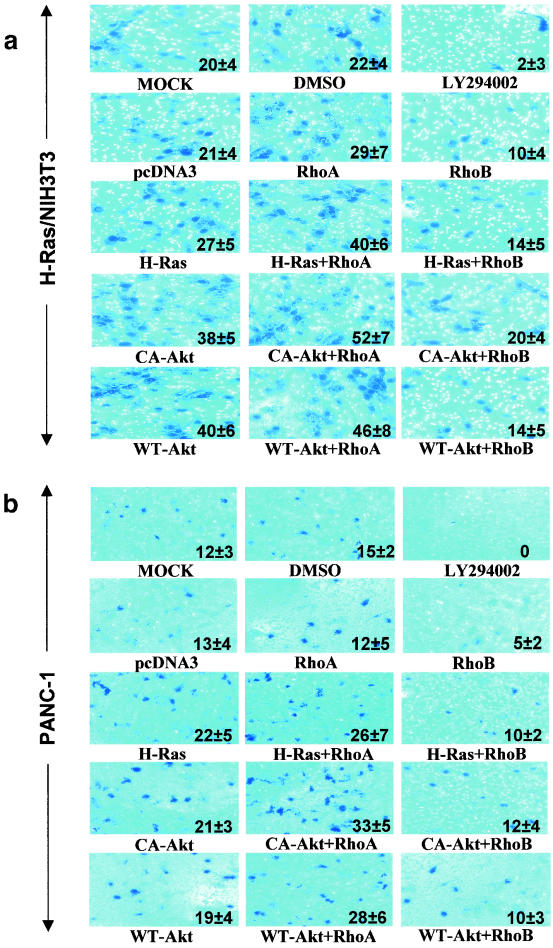
Figure 4 demonstrated that ectopic expression of RhoB antagonizes cell migration. We next evaluated whether RhoB can also antagonize cell invasion. To this end, H-Ras/NIH 3T3 and PANC-1 cells were transfected as described in the legend to Fig. 4 with various oncogenes along with RhoA or RhoB and then seeded onto Matrigel-coated poly-hydrocarbonate filters mounted in the middle of a Boyden transwell apparatus as described in Materials and Methods. Figure 5a and b show that mock-transfected or DMSO-treated cells efficiently invaded through Matrigel-collagen. In contrast, LY294002-treated H-Ras/NIH 3T3 and PANC-1 cells did not invade. In addition, cells transfected with pcDNA3 or pcDNA3-RhoA, but not pcDNA3-RhoB, invaded. Furthermore, transfection with CA-Akt or WT-Akt enhanced invasion, and RhoB, not RhoA, inhibited this enhancement of invasion (see the actual number of cells within each panel of Fig. 5a and b).
FIG. 5.
RhoB, but not RhoA, inhibits H-Ras/PI3K/Akt-mediated cell invasion. H-Ras/NIH 3T3 (a) and PANC-1 (b) cells were obtained from the samples shown in Fig. 4b and c that were transiently transfected with pcDNA3 vector control, H-Ras61L, CA-Akt, or WT-Akt in the presence or absence of RhoA or RhoB or pcDNA3 for 36 h. The cells were then split and analyzed for their invading capabilities through Matrigel-coated transfilters as described in Materials and Methods.
RhoB, not RhoA, reverses Ras/PI3K/Akt-mediated resistance to 5-FU-induced apoptosis and protects against anoikis.
Another hallmark of cancer cells is to resist apoptosis and promote tumor survival. The ability of 5-FU to induce RhoB is antagonized by the Ras/PI3K/Akt pathway (Fig. 2 and 3), and this coupled with the previously reported role of RhoB in apoptosis (7, 27) prompted us to determine the role of RhoB and the Ras/PI3/Akt pathway in 5-FU-induced apoptosis. To this end, NIH 3T3 cells were transiently transfected with pcDNA3, H-Ras61L, Akt, RhoA, or RhoB for 24 h and treated with DMSO vehicle or 5-FU for an additional 48 h and apoptosis was analyzed by annexin V labeling and flow cytometry as described in Materials and Methods. In experiments similar to those of Fig. 4a and b, using EGFP, we have determined the transfection efficiency in NIH 3T3 cells to be 71% ± 6% (Fig. 6a). Figure 6a also shows that 5-FU treatment induced 28 to 30% apoptosis in NIH 3T3 cells. Transfection with H-Ras61L or Akt decreased the 5-FU apoptosis rate to 15 or 17%, respectively. Furthermore, while RhoA slightly protected it, RhoB enhanced the ability of 5-FU to induce apoptosis (Fig. 6a). Importantly, RhoB, but not RhoA, reversed Akt-mediated resistance to 5-FU-induced apoptosis.
FIG. 6.
RhoB, but not RhoA, inhibits H-Ras/PI3K/Akt-mediated cell survival and metastasis. (a) Parental NIH 3T3 cells were transiently transfected with pcDNA3 vector, H-Ras61L, or Akt in the presence or absence of RhoA or RhoB or pcDNA3 for 36 h. Internal ribosome entry site-EGFP was cotransfected as a transfection indicator. The cells were then split and treated with 5-FU for another 48 h. The cells were thenexamined for their susceptibility to 5-FU-induced apoptosis by annexin V labeling and flow cytometry apoptosis assays as described in Materials and Methods. (b) Thirty-six hours after the transfection, a fraction of the cells from panel a were washed and resuspended in serum-free medium and then seeded onto poly-HEMA-precoated plates and examined for viability at different time points by annexin V and 7-AAD labeling. (c and d) B16-F10 melanoma cells were treated with LY294002 for 48 h, and 50 μg of the whole-cell lysates was analyzed for RhoB induction by Western blotting. In parallel, B16-F10 cells were transiently transfected with pcDNA3 vector, pcDNA-RhoA, or pcDNA-RhoB. Twenty hours later, the cells were examined for gene delivery by Western blotting with anti-HA, anti-RhoA, and anti-RhoB. A total of 5 × 105 of the cells were injected into the tail veins of C57/BL6 mice. The metastatic nodules growing in the lungs were counted and photographed at day 21. The numbers of metastatic nodules per mouse lung represent the average ± standard error from five mice per group.
In addition to 5-FU-induced apoptosis, we examined the effects of RhoB on apoptosis induced by depriving cells from substratum attachment (anoikis). Figure 6b shows that 12 or 24 h after seeding onto poly-HEMA-coated culture plates, RhoB-transfected cells displayed significantly higher cell death induced by lack of attachment than pcDNA3-transfected cells. However, H-Ras61L-, Akt-, and RhoA-transfected cells showed a much lower rate of anoikis. Notably, RhoB reversed Akt-mediated resistance to this type of apoptosis (Fig. 6b).
RhoB, not RhoA, inhibits melanoma metastasis to the lung in a mouse model.
The work described above clearly shows that in cultured cells RhoB is a potent suppressor of transformation, migration, and invasion of cancer cells. To give further support to this in vivo, we transfected the highly metastatic melanoma cells B16-F10 with either pcDNA3, pcDNA3-RhoA, or pcDNA3-RhoB, injected the cells into the tail vein of C57 black mice, and determined lung metastasis after 3 weeks as described in Materials and Methods. First we documented that RhoB expression is regulated by the PI3K/Akt pathway in B16-F10 cells by demonstrating that treatment with LY294002 strongly induced RhoB protein levels (Fig. 6c). Furthermore, the transfected HA-RhoA and HA-RhoB were readily expressed in B16-F10 cells as determined by Western blotting (Fig. 6c). Importantly, Fig. 6d shows that pcDNA3-transfected cells were highly metastatic and grew 14.8 ± 1.9 metastatic colonies per lung. Similarly pcDNA3-RhoA-transfected B16-F10 cells grew 13 ± 4.5 colonies per lung. In contrast, pcDNA3-RhoB-transfected B16-F10 cells grew only 2 ± 0.7 colonies.
DISCUSSION
The ability of oncogenic Ras to transform cells depends not only on activating proliferative pathways but also on inhibiting tumor-suppressive pathways. Recently we have shown that EGFR, ErbB2, and Ras, but not Src, inhibit RhoB expression (21). The work presented in this article provides support for the downregulation of RhoB as one of the steps taken by oncogenic Ras to transform cells and that the PI3K/Akt limb and not the Mek limb of the Ras signaling pathway is the mediator for this downregulation. Furthermore, we demonstrate that ectopic expression of RhoB, but not its close GTPase relative, RhoA, blocks activated H-Ras, PI3K- and Akt-mediated malignant transformation, resistance to anticancer drug-induced apoptosis and anoikis, cell migration, and invasion. Importantly, in an animal model, ectopic expression of RhoB in the highly metastatic melanoma cell line B16-F10 dramatically inhibited its metastasis to the lung. These results clearly demonstrate an antagonistic interaction between the oncogenic Ras/PI3K/Akt tumor survival pathway and RhoB (Fig. 6e). Similar results also showed an antagonistic interaction between RhoB and two receptor tyrosine kinases, EGFR and ErbB2 (data not shown). These findings are critical, since they identify RhoB as a negative regulator of Ras, EGFR, and ErbB2, three components of signal transduction that are intimately involved in human cancers, and further suggest that RhoB is functioning as a tumor suppressor against carcinogenesis mediated by receptor tyrosine kinases and their downstream effectors. This is consistent with a recent report showing that RhoB−/− mice are more prone to skin chemical carcinogenesis (28).
This is the first report documenting the anti-invasive and antimetastatic activities of RhoB. This is an important finding, since most GTPases studied to date, such as Ras, RhoA, Rac1, and Cdc42, promote rather than inhibit invasiveness and metastasis (4, 17, 19, 22, 35). The ability of RhoB to inhibit motility, invasion, and metastasis is consistent with the fact that RhoB expression is decreased dramatically as tumors progress from the noninvasive carcinoma stages to the highly invasive, deeply infiltrating and metastatic stage in head and neck, brain, and lung cancer patient biopsies (1, 13, 30). Furthermore, RhoB decreased the levels of matrix metalloprotease 2 (MMP-2) (data not shown), one of the matrix metalloproteinases that tumors secrete to degrade extracellular matrix components, a step required for tumor cells to migrate and invade surrounding tissue as well as distant sites (24, 36, 42). PI3K and Akt have recently been shown to induce the expression of MMP-2 and MMP-9 by a mechanism involving Akt activation of NF-κB binding to the MMP promoter (24, 32). Thus, one possible mechanism by which RhoB inhibits tumor migration and invasion is by blocking the ability of the Ras/PI3K/Akt pathway to activate NF-κB. Consistent with this is the demonstration by Fritz et al. that ectopic expression of RhoB inhibits NF-κB-dependent transcriptional activation (14). Finally, the PI3K/Akt-induced resistance of nonadherent cells to apoptosis (anoikis) is antagonized by RhoB, giving further support to the notion that the prosurvival Ras/PI3K/Akt pathway must suppress RhoB expression for nonadherent cancer cells to migrate and invade.
Anticancer drug resistance, a major obstacle to cancer treatment, is often due to constitutive activation of the oncogenic and tumor survival pathway, and the Ras/PI3K/Akt pathway is a major contributor to resistance of human cancers to common anticancer drugs such as paclitaxel (5, 29). In this study, we have shown that this pathway induces resistance to another commonly used anticancer drug, 5-FU. Importantly, ectopic expression of RhoB counteracted Akt-mediated resistance and sensitized cells to 5-FU. Consistent with this is a recent study demonstrating that RhoB−/− cells are resistant to radiation and anticancer drug therapy (27). Taken together, these findings suggest that RhoB can be used in combination therapy studies to overcome anticancer drug resistance.
In summary, we demonstrated that the GTPase RhoB negatively regulates the ability of the Ras/PI3K/Akt pathway to induce transformation, migration, and invasion as well as resistance to anticancer drug apoptosis and nonadherent cell death (anoikis). We further demonstrated that Ras downregulates the expression of RhoB by a mechanism involving PI3K and Akt and not Mek. Based on these results and our recently reported studies of human biopsies, we propose that tumor cells may have to downregulate RhoB expression and thus suppress its ability to inhibit transformation, invasion, and metastasis as one of the steps necessary for reaching a highly malignant phenotype (Fig. 6e). The discovery of this novel antagonistic interaction between a major oncogenic/tumor survival pathway and RhoB further enhances our understanding of oncogenesis and has far-reaching implications for the treatment of advanced cancer by targeting tumor cell invasion/metastasis as well as drug resistance.
Acknowledgments
This work is supported by NIH grant CA67771.
We thank Cassandra Martin for technical assistance. We also thank the Molecular Biology Core, the Image Core, and the FACS facility at the H. Lee Moffitt Cancer Center and Research Institute for technical assistance.
REFERENCES
- 1.Adnane, J., C. Muro-Cacho, L. Mathews, S. M. Sebti, and T. Munoz-Antonia. 2002. Suppression of rho B expression in invasive carcinoma from head and neck cancer patients. Clin. Cancer Res. 8:2225-2232. [PubMed] [Google Scholar]
- 2.Arboleda, M. J., J. F. Lyons, F. F. Kabbinavar, M. R. Bray, B. E. Snow, R. Ayala, M. Danino, B. Y. Karlan, and D. J. Slamon. 2003. Overexpression of AKT2/protein kinase Bbeta leads to up-regulation of beta1 integrins, increased invasion, and metastasis of human breast and ovarian cancer cells. Cancer Res. 63:196-206. [PubMed] [Google Scholar]
- 3.Barbacid, M. 1987. ras genes. Annu. Rev. Biochem. 56:779-827. [DOI] [PubMed] [Google Scholar]
- 4.Bouzahzah, B., C. Albanese, F. Ahmed, F. Pixley, M. P. Lisanti, J. D. Segall, J. Condeelis, D. Joyce, A. Minden, C. J. Der, A. Chan, M. Symons, and R. G. Pestell. 2001. Rho family GTPases regulate mammary epithelium cell growth and metastasis through distinguishable pathways. Mol. Med. 7:816-830. [PMC free article] [PubMed] [Google Scholar]
- 5.Bowers, D. C., S. Fan, K. A. Walter, R. Abounader, J. A. Williams, E. M. Rosen, and J. Laterra. 2000. Scatter factor/hepatocyte growth factor protects against cytotoxic death in human glioblastoma via phosphatidylinositol 3-kinase- and AKT-dependent pathways. Cancer Res. 60:4277-4283. [PubMed] [Google Scholar]
- 6.Catling, A. D., H.-J. Schaeffer, C. W. M. Reuter, G. R. Reddy, and M. J. Weber. 1995. A proline-rich sequence unique to MEK1 and MEK2 is required for Raf binding and regulates MEK function. Mol. Cell. Biol. 15:5214-5225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen, Z., J. Sun, A. Pradines, G. Favre, J. Adnane, and S. M. Sebti. 2000. Both farnesylated and geranylgeranylated RhoB inhibit malignant transformation and suppress human tumor growth in nude mice. J. Biol. Chem. 275:17974-17978. [DOI] [PubMed] [Google Scholar]
- 8.Cox, A. D., and C. J. Der. 2002. Ras family signaling: therapeutic targeting. Cancer Biol. Ther. 1:599-606. [DOI] [PubMed] [Google Scholar]
- 9.Davies, M. A., S. J. Kim, N. U. Parikh, Z. Dong, C. D. Bucana, and G. E. Gallick. 2002. Adenoviral-mediated expression of MMAC/PTEN inhibits proliferation and metastasis of human prostate cancer cells. Clin. Cancer Res. 8:1904-1914. [PubMed] [Google Scholar]
- 10.Delarue, F. L., B. S. Taylor, and S. M. Sebti. 2001. Ras and RhoA suppress whereas RhoB enhances cytokine-induced transcription of nitric oxide synthase-2 in human normal liver AKN-1 cells and lung cancer A-549 cells. Oncogene 20:6531-6537. [DOI] [PubMed] [Google Scholar]
- 11.Du, W., P. F. Lebowitz, and G. C. Prendergast. 1999. Cell growth inhibition by farnesyltransferase inhibitors is mediated by gain of geranylgeranylated RhoB. Mol. Cell. Biol. 19:1831-1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Du, W., and G. C. Prendergast. 1999. Geranylgeranylated RhoB mediates suppression of human tumor cell growth by farnesyltransferase inhibitors. Cancer Res. 59:5492-5496. [PubMed] [Google Scholar]
- 13.Forget, M. A., R. R. Desrosiers, M. Del, R. Moumdjian, D. Shedid, F. Berthelet, and R. Beliveau. 2002. The expression of rho proteins decreases with human brain tumor progression: potential tumor markers. Clin. Exp. Metastasis 19:9-15. [DOI] [PubMed] [Google Scholar]
- 14.Fritz, G., and B. Kaina. 2001. Ras-related GTPase Rhob represses NF-kappaB signaling. J. Biol. Chem. 276:3115-3122. [DOI] [PubMed] [Google Scholar]
- 15.Fritz, G., and B. Kaina. 1997. rhoB encoding a UV-inducible Ras-related small GTP-binding protein is regulated by GTPases of the Rho family and independent of JNK, ERK, and p38 MAP kinase. J. Biol. Chem. 272:30637-30644. [DOI] [PubMed] [Google Scholar]
- 16.Fritz, G., B. Kaina, and K. Aktories. 1995. The ras-related small GTP-binding protein RhoB is immediate-early inducible by DNA damaging treatments. J. Biol. Chem. 270:25172-25177. [DOI] [PubMed] [Google Scholar]
- 17.Jaffe, A. B., and A. Hall. 2002. Rho GTPases in transformation and metastasis. Adv. Cancer Res. 84:57-80. [DOI] [PubMed] [Google Scholar]
- 18.Jähner, D., and T. Hunter. 1991. The ras-related gene rhoB is an immediate-early gene inducible by v-Fps, epidermal growth factor, and platelet-derived growth factor in rat fibroblasts. Mol. Cell. Biol. 11:3682-3690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Janda, E., K. Lehmann, I. Killisch, M. Jechlinger, M. Herzig, J. Downward, H. Beug, and S. Grunert. 2002. Ras and TGFβ cooperatively regulate epithelial cell plasticity and metastasis: dissection of Ras signaling pathways. J. Cell Biol. 156:299-313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiang, K., D. Coppola, N. C. Crespo, S. V. Nicosia, A. D. Hamilton, S. M. Sebti, and J. Q. Cheng. 2000. The phosphoinositide 3-OH kinase/AKT2 pathway as a critical target for farnesyltransferase inhibitor-induced apoptosis. Mol. Cell. Biol. 20:139-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiang, K., F. L. Delarue, and S. M. Sebti. 2004. EGFR, ErbB2 and Ras but not Src suppress RhoB expression while ectopic expression of RhoB antagonizes oncogene-mediated transformation. Oncogene 23:1136-1145. [DOI] [PubMed] [Google Scholar]
- 22.Keely, P. J., J. K. Westwick, I. P. Whitehead, C. J. Der, and L. V. Parise. 1997. Cdc42 and Rac1 induce integrin-mediated cell motility and invasiveness through PI(3)K. Nature 390:632-636. [DOI] [PubMed] [Google Scholar]
- 23.Khosravi-Far, R., P. A. Solski, G. J. Clark, M. S. Kinch, and C. J. Der. 1995. Activation of Rac1, RhoA, and mitogen-activated protein kinases is required for Ras transformation. Mol. Cell. Biol. 15:6443-6453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim, D., S. Kim, H. Koh, S. O. Yoon, A. S. Chung, K. S. Cho, and J. Chung. 2001. Akt/PKB promotes cancer cell invasion via increased motility and metalloproteinase production. FASEB J. 15:1953-1962. [DOI] [PubMed] [Google Scholar]
- 25.Klippel, A., W. M. Kavanaugh, D. Pot, and L. T. Williams. 1997. A specific product of phosphatidylinositol 3-kinase directly activates the protein kinase Akt through its pleckstrin homology domain. Mol. Cell. Biol. 17:338-344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kubiatowski, T., T. Jang, M. B. Lachyankar, R. Salmonsen, R. R. Nabi, P. J. Quesenberry, N. S. Litofsky, A. H. Ross, and L. D. Recht. 2001. Association of increased phosphatidylinositol 3-kinase signaling with increased invasiveness and gelatinase activity in malignant gliomas. J. Neurosurg. 95:480-488. [DOI] [PubMed] [Google Scholar]
- 27.Liu, A., G. J. Cerniglia, E. J. Bernhard, and G. C. Prendergast. 2001. RhoB is required to mediate apoptosis in neoplastically transformed cells after DNA damage. Proc. Natl. Acad. Sci. USA 98:6192-6197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu, A. X., N. Rane, J.-P. Liu, and G. C. Prendergast. 2001. RhoB is dispensable for mouse development, but it modifies susceptibility to tumor formation as well as cell adhesion and growth factor signaling in transformed cells. Mol. Cell. Biol. 21:6906-6912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.MacKeigan, J. P., D. J. Taxman, D. Hunter, H. S. Earp III, L. M. Graves, and J. P. Ting. 2002. Inactivation of the antiapoptotic phosphatidylinositol 3-kinase-Akt pathway by the combined treatment of taxol and mitogen-activated protein kinase kinase inhibition. Clin. Cancer Res. 8:2091-2099. [PubMed] [Google Scholar]
- 30.Mazieres, A. T., G. Daste, C. Muro-Cacho, D. Berchery, V. Tillement, A. Pradines, S. M. Sebti, and G. Favre. 2004. Loss of RhoB expression in human lung cancer progression. Clin. Cancer Res. 10:2742-2750. [DOI] [PubMed]
- 31.Nakamura, T., M. Asano, N. Shindo-Okada, S. Nishimura, and Y. Monden. 1996. Cloning of the RhoB gene from the mouse genome and characterization of its promoter region. Biochem. Biophys. Res. Commun. 226:688-694. [DOI] [PubMed] [Google Scholar]
- 32.Park, B. K., X. Zeng, and R. I. Glazer. 2001. Akt1 induces extracellular matrix invasion and matrix metalloproteinase-2 activity in mouse mammary epithelial cells. Cancer Res. 61:7647-7653. [PubMed] [Google Scholar]
- 33.Pruitt, K., and C. J. Der. 2001. Ras and Rho regulation of the cell cycle and oncogenesis. Cancer Lett. 171:1-10. [DOI] [PubMed] [Google Scholar]
- 34.Quilliam, L. A., K. Kato, K. M. Rabun, M. M. Hisaka, S. Y. Huff, S. Campbell-Burk, and C. J. Der. 1994. Identification of residues critical for Ras(17N) growth-inhibitory phenotype and for Ras interaction with guanine nucleotide exchange factors. Mol. Cell. Biol. 14:1113-1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schmitz, A. A., E. E. Govek, B. Bottner, and L. Van Aelst. 2000. Rho GTPases: signaling, migration, and invasion. Exp. Cell Res. 261:1-12. [DOI] [PubMed] [Google Scholar]
- 36.Stamenkovic, I. 2000. Matrix metalloproteinases in tumor invasion and metastasis. Semin. Cancer Biol. 10:415-433. [DOI] [PubMed] [Google Scholar]
- 37.Stewart, A. L., A. M. Mhashilkar, X. H. Yang, S. Ekmekcioglu, Y. Saito, K. Sieger, R. Schrock, E. Onishi, X. Swanson, J. B. Mumm, L. Zumstein, G. J. Watson, D. Snary, J. A. Roth, E. A. Grimm, R. Ramesh, and S. Chada. 2002. PI3 kinase blockade by Ad-PTEN inhibits invasion and induces apoptosis in RGP and metastatic melanoma cells. Mol. Med. 8:451-461. [PMC free article] [PubMed] [Google Scholar]
- 38.Vlahos, C. J., W. F. Matter, K. Y. Hui, and R. F. Brown. 1994. A specific inhibitor of phosphatidylinositol 3-kinase, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002). J. Biol. Chem. 269:5241-5248. [PubMed] [Google Scholar]
- 39.Westwick, J. K., Q. T. Lambert, G. J. Clark, M. Symons, L. Van Aelst, R. G. Pestell, and C. J. Der. 1997. Rac regulation of transformation, gene expression, and actin organization by multiple, PAK-independent pathways. Mol. Cell. Biol. 17:1324-1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Whitehead, I. P., K. Abe, J. L. Gorski, and C. J. Der. 1998. CDC42 and FGD1 cause distinct signaling and transforming activities. Mol. Cell. Biol. 18:4689-4697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yano, H., T. Agatsuma, S. Nakanishi, Y. Saitoh, Y. Fukui, Y. Nonomura, and Y. Matsuda. 1995. Biochemical and pharmacological studies with KT7692 and LY294002 on the role of phosphatidylinositol 3-kinase in Fc epsilon RI-mediated signal transduction. Biochem. J. 312:145-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yoon, S. O., S. J. Park, C. H. Yun, and A. S. Chung. 2003. Roles of matrix metalloproteinases in tumor metastasis and angiogenesis. J. Biochem. Mol. Biol. 36:128-137. [DOI] [PubMed] [Google Scholar]