Abstract
Myogenesis is an intricate process that coordinately engages multiple intracellular signaling cascades. The Rho family GTPase RhoA is known to promote myogenesis, however, the mechanisms controlling its regulation in myoblasts have yet to be fully elucidated. We show here that the SH2-containing protein tyrosine phosphatase, SHP-2, functions as an early modulator of myogenesis by regulating RhoA. When MyoD was expressed in fibroblasts lacking functional SHP-2, muscle-specific gene activity was impaired and abolition of SHP-2 expression by RNA interference inhibited muscle differentiation. By using SHP-2 substrate-trapping mutants, we identified p190-B RhoGAP as a SHP-2 substrate. When dephosphorylated, p190-B RhoGAP has been shown to stimulate the activation of RhoA. During myogenesis, p190-B RhoGAP was tyrosyl dephosphorylated concomitant with the stimulation of SHP-2's phosphatase activity. Moreover, overexpression of a catalytically inactive mutant of SHP-2 inhibited p190-B RhoGAP tyrosyl dephosphorylation, RhoA activity, and myogenesis. These observations strongly suggest that SHP-2 dephosphorylates p190-B RhoGAP, leading to the activation of RhoA. Collectively, these data provide a mechanistic basis for RhoA activation in myoblasts and demonstrate that myogenesis is critically regulated by the actions of SHP-2 on the p190-B Rho GAP/RhoA pathway.
Skeletal muscle differentiation involves a highly orchestrated set of biological processes that are regulated through numerous extracellular cues. A wealth of evidence has now established a paradigm for the process of skeletal muscle differentiation that begins when a skeletal muscle precursor cell activates muscle-specific genes such as muscle regulatory factors (MRFs) of the basic helix-loop-helix family and the muscle enhancer factor 2 family (11, 25, 32, 33). The transcription factors, MyoD, myogenin, Myf5, and MRF4, promote skeletal muscle differentiation by activating muscle-specific genes that include the myosin light and heavy chains, desmin, and troponin T (11, 32). Concomitant with the upregulation of muscle-specific genes, muscle precursor cells withdraw from the cell cycle, engage in a dramatic change in the cytoskeleton architecture, in order to align, elongate, and fuse to form multinucleated myotubes.
We previously raised the possibility that the Src homology 2 (SH2) domain-containing protein tyrosine phosphatase (PTP), SHP-2, is involved in myogenesis because of the findings that SHP-2 expression levels increase and that it interacts with the SHP-2 substrate 1 (SHPS-1) during myogenesis (24). SHP-2 contains two SH2 domains, a catalytic domain, and a C-terminal tail containing two tyrosyl phosphorylation sites (29, 30). SHP-2 has been implicated in positive signaling in numerous biological processes such as cell proliferation (3, 28), cell adhesion (31, 54), apoptosis (8, 50, 56), and development (36). In most, but not all, cases the catalytic activity of SHP-2 is essential for the initiation of positive signaling in response to a variety of extracellular stimuli leading to the activation of the Ras/mitogen-activated protein kinases (29), PI3K/Akt (50, 55, 56), JAK/STAT (21, 38, 52), and c-Src (31) pathways. The requirement for the catalytic activity of SHP-2 in these signaling pathways has led to the notion that SHP-2 could signal via the dephosphorylation of a positive signaling molecule that is retained in an inhibitory state by tyrosyl phosphorylation. Conversely, SHP-2 may signal via the dephosphorylation of a negative signaling molecule that is retained in an active state by tyrosyl phosphorylation. Despite the identification of several putative substrates for SHP-2 (15, 53, 55), none yet fully explain how SHP-2 exerts its positive signaling effects on the many biological processes with which it has been implicated (30).
The Rho family of GTPases which includes, Rho, Rac, and Cdc42 functions as a critical signal integrator in a wide range of cellular processes (12, 44). In skeletal muscle, the Rho family of GTPases has been implicated in the regulation of distinct signaling pathways that lead either to the promotion and/or attenuation of myogenesis. There is strong evidence to support the notion that RhoA positively regulates myogenesis by stimulating the serum response factor, which in turn promotes the activation of MRFs and ultimately skeletal muscle differentiation (5, 7, 41, 42, 45, 48, 49). In contrast, Rac and Cdc42, appear to play more antagonistic roles in myogenesis through stimulation of signaling pathways that are either dependent or independent of the activation of the c-Jun amino-terminal kinase (16, 27). Despite the fact that these observations implicate the Rho family of GTPases in the regulation of myogenesis, how Rho, Rac or Cdc42 are regulated during myogenesis still remains to be fully defined.
We demonstrate here a role for SHP-2 in myogenesis, and we provide a mechanism for its positive signaling effect in this process. We show that the phosphatase activity of SHP-2 is required for the activation of muscle-specific genes and myogenesis. Using substrate-trapping approaches, we have identified p190-B RhoGAP as a SHP-2 substrate. Significantly, tyrosyl dephosphorylation of p190-B RhoGAP activates the Rho family GTPase, RhoA (40). Although it has been suggested that p190-B RhoGAP regulates RhoA to promote myogenesis, how p190-B RhoGAP is regulated during myogenesis is unclear. Our data show that the catalytic activity of SHP-2 is required to promote p190-B RhoGAP dephosphorylation and RhoA activity in myoblasts. These data define a mechanism in which SHP-2 regulates myogenesis by directly modulating the p190-B RhoGAP/RhoA signaling pathway.
MATERIALS AND METHODS
Cell lines and reagents.
C2C12 myoblasts were purchased from the American Tissue Culture Collection (Rockville, Md.) and were cultured and induced to differentiate as described previously (23). Fibroblasts derived from mice containing either a deletion in exon 3 of SHP-2 (SHP-2Ex3−/−) or fibroblasts from their littermate controls containing wild-type SHP-2 (SHP-2+/+) were provided by Gen-Shen Feng (Burnham Institute, La Jolla, Calif.). C2C12 myoblasts were visualized by using a Zeiss Axiovert 100 inverted microscope and photographed by using a SPOT charge-coupled device camera (Diagnostic Instruments, Sterling Heights, Mich.). Insulin-like growth factor 1 (IGF-1) was obtained from Calbiochem (San Diego, Calif.). Antiphosphotyrosine antibodies (4G10) were purchased from Upstate Biotechnology Incorporated (Lake Placid, N.Y.). Polyclonal antibodies used for immunoprecipitating SHP-2 were purchased from Santa Cruz Biotechnology (Santa Cruz, Calif.) and, for immunoblotting, both mouse monoclonal SHP-2 and p190-B RhoGAP antibodies were obtained from BD Transduction Laboratories (Lexington, Ky.). Phospho-specific antibodies to Erk1/2 were obtained from New England Biolabs (Beverly, Mass.). Anti-Erk1/2 antibodies (C1) were kindly provided by John Blenis (Harvard Medical School, Boston, Mass.). Antibodies to MyoD and myosin heavy chain (MHC) were purchased from the Developmental Studies Hybridoma Bank (Iowa City, Iowa). p70S6K antibodies were purchased from Santa Cruz Biotechnology. Anti-PTP-1B antibodies were kindly provided by Benjamin Neel (Beth Israel Deaconess Hospital, Mass.). A rabbit polyclonal antibody to p190-B RhoGAP was provided by Jeffrey Settleman (Massachusetts General Hospital Cancer Center) and was used for immunoprecipitation (40). Horseradish peroxidase-conjugated secondary antibodies to mouse and rabbit were purchased from Amersham-Pharmacia Biotechnology (Arlington Heights, Ill.) and detected by using enhanced chemiluminescence.
SHP-2 plasmids and adenoviral generation.
A summary of the various SHP-2 mutants used in these studies is provided in Table 1. SHP-2 expression was established by subcloning wild type, the catalytically inactive mutant of SHP-2 (CS), the phosphatase-deleted mutant of SHP-2 (ΔP), and the double substrate trapping mutant of SHP-2 (Asp425 to Ala425, Gln506 to Ala506; DAQA) into the pIRES-green-fluorescent protein (GFP) vector (Invitrogen, Carlsbad, Calif.). The CS and ΔP mutant constructs of SHP-2 have been described previously (3). The SHP-2-DAQA mutant was constructed by performing site-directed mutagenesis by using a QuikChange mutagenesis kit (Stratagene, La Jolla, Calif.) according to the manufacturer's instructions. The sequence of the resultant construct was confirmed by automated sequencing. Recombinant wild-type (Ad-WT); phosphatase-deleted (Ad-ΔP); and catalytically inactive, non-substrate-trapping (Ad-RM) SHP-2 adenoviruses were generated by using the pAdEasy method (17). The catalytically inactive, non-substrate-trapping mutant of SHP-2 (Arg465 to Met465), SHP-2-RM, was generated by site-directed mutagenesis. Glutathione S-transferase (GST) fusion proteins encoding either the tandem (GST-N+C) or the N-SH2 (N-SH2) domain alone have been described previously (3). For GST substrate-trapping experiments, GST fusion proteins that represented the PTP domain alone of SHP-2 (amino acids 218 to 528) were constructed by PCR amplification with wild-type SHP-2 as a template. The resultant PCR product was subcloned into the pGEX-2TK vector to generate pGEX-2TK-PTP-WT (GST-PTP-WT). The substrate-trapping variant of SHP-2, Asp425 to Ala425, was generated by performing site-directed mutagenesis on GST-PTP-WT to generate pGEX-2TK-PTP-DA (GST-PTP-DA).
TABLE 1.
SHP-2 mutants used in this study
| SHP-2 mutant | Description |
|---|---|
| CS (Cys459→Ser459) | Catalytically inactive |
| ΔP (PTP domain internal deletion) | Catalytically inactive, non-substrate trapping |
| RM (Arg465→Met465) | Catalytically inactive, non-substrate trapping |
| DA (Asp425→Ala425) | Substrate trapping |
| DAQA (Asp425→Ala425/Gln506→Ala506) | Substrate trapping |
Immunoprecipitation and immunoblotting.
Immunoprecipitation and immunoblotting experiments were performed by lysing cells on ice in 1 ml of NP-40 lysis buffer containing 1.0% NP-40, 150 mM NaCl, 50 mM Tris-HCl (pH 7.4), 5 μg of leupeptin/ml, 5 μg of aprotinin/ml, 1 μg of pepstatin A/ml, 1 mM phenylmethylsulfonyl fluoride (PMSF), 1 mM benzamidine, and 0.2 mM Na3VO4 or 1 ml of radioimmunoprecipitation assay lysis buffer (1.0% NP-40, 0.1% sodium dodecyl sulfate [SDS], 0.5% sodium deoxycholate, 25 mM Tris-HCl [pH 7.4], 150 mM NaCl, 5 μg of leupeptin/ml, 5 μg of aprotinin/ml, 1 μg of pepstatin A/ml, 1 mM PMSF, 1 mM benzamidine, and 0.2 mM Na3VO4). Lysates were collected and centrifuged at 20,800 × g for 15 min at 4°C. Supernatants were precleared for 15 min with 2 μl of pansorbin (Calbiochem) and spun down at 12,100 × g for 1 min, and these supernatants were collected and measured for protein content by using the Bradford Coomassie assay (Pierce, Rockford, Ill.). Immunoblotting was conducted as described previously (23). After electrophoretic transfer of proteins onto Immobilon-P membranes (Millipore, Mass.), protein loading was verified by Ponceau S staining of membranes. Membranes were incubated with primary antibodies (anti-SHP-2 at 1:1,000, anti-MHC at 1:12, anti-MyoD at 1:100, antimyogenin at 1:12, anti-Erk1/2 at 1:10,000, anti-p190-B RhoGAP at 1:250, and anti-p70S6K at 1:1,000) diluted in 2.5% nonfat dry milk plus Tris-buffered saline-Tween 20 (TBST). Antiphosphotyrosine 4G10 antibodies were diluted 1:2,000 in 2.5% bovine serum albumin-TBST. Anti-phospho-Erk1/2 antibodies were diluted 1:1,000 in 2.5% nonfat dry milk-TBST. Anti-PTP-1B antibodies were diluted 1:1,000 in 2.5% nonfat dry milk-TBST. Primary antibodies were incubated with the membranes for either 3 h at room temperature or overnight at 4°C. Primary antibodies were visualized by using enhanced chemiluminescence reagents (Amersham Pharmacia Biotech, Piscataway, N.J.). Densitometric analyses on immunoblots were performed by using the LabWorks 4.0 Image Analysis Software (UVP, Inc., Upland, Calif.). Lysates prepared from proliferating and differentiating myoblasts were subjected to immunoprecipitation with anti-SHP-2 antibodies. These SHP-2 immune complexes were assayed for phosphatase activity as described previously (23).
Transient-transfection and luciferase reporter assays.
For the assessment of muscle-specific gene expression, mammalian expression plasmids were transfected into wild-type (SHP-2+/+) and SHP-2 exon 3-deleted (SHP-2Ex3−/−) fibroblast cell lines by using Lipofectamine 2000 (Invitrogen) at 70 to 80% confluence with 0.5 μg of simian virus 40 β-galactosidase, 0.5 μg of the M isozyme of the creatine kinase (MCK) promoter from rabbit skeletal muscle fused to luciferase (MCK-luc) (46), 2.5 μg of pBABE-MyoD, and 4.0 μg of wild-type or mutant SHP-2 expression plasmids as indicated. At 24 h after transfection, these fibroblasts were either left in the presence of 10% fetal bovine serum or were stimulated with 100 ng of IGF-1 (Calbiochem)/ml for another 24 h. Lysates were then prepared, and the β-galactosidase and luciferase activities were measured by using the Luciferase Assay System Kit from Promega (Madison, Wis.). Muscle-specific gene expression was also measured by using the avian skeletal α-actin promoter fused to luciferase (SK-luc) (48, 49), which was provided by George Porter (Yale University School of Medicine). C2C12 myoblasts maintained in Dulbecco modified Eagle medium with 10% fetal bovine serum were plated at a density of 2 × 105 cells per well of a six-well dish and were cotransfected with 0.1 μg of SK-luc and 0.01 μg of pRL-Renilla, along with 2.5 μg of the indicated expression plasmid for pIRES-GFP-SHP-2 and 1.5 μg of pCA-Myc-RhoA(V14) or both. Transfected C2C12 myoblasts were initiated to undergo differentiation in differentiation medium (DM; Dulbecco modified Eagle medium [Invitrogen] with 0.1% fetal bovine serum, 5 μg of insulin/ml, and 5 μg of transferrin/ml); cells were harvested after 48 h, and luciferase activities were measured.
Generation of stable cell lines and transfection of SHP-2 RNA interference (RNAi) oligonucleotides.
To generate stable cell lines overexpressing the SHP-2 substrate-trapping mutant, either wild type or the double substrate-trapping mutant of SHP-2 (SHP-2-DAQA) was subcloned into the pIRES-Neo vector (Invitrogen) to generate pIRES-Neo-WT and pIRES-Neo-DAQA, respectively. Stable cell lines were generated by transiently transfecting pIRES-Neo, pIRES-Neo-WT, or pIRES-Neo-DAQA into C2C12 myoblasts, which were subjected to selection with G418 (700 U/ml). Pools of clones for each of these stable transfectants were collected, and the expression of SHP-2 was determined by immunoblotting with anti-SHP-2 antibodies. SHP-2 expression was suppressed as described previously (56) by using the following target sequences: 1, 5′-AACACTGGGGACTACTATGAC-3′; 4, 5′-AAATGTGTCAAGTACTGGCCT-3′; and 2, 5′-AAAAGAAGCAGAGAAGCTGCT-3′.
SHP-2 substrate-trapping and GST affinity precipitations.
C2C12 myoblasts transfected with the SHP-2 substrate-trapping mutant (SHP-2-DAQA) were lysed in 1% NP-40 lysis buffer and immunoprecipitated for SHP-2 by using anti-SHP-2 antibodies. Anti-SHP-2 immune complexes were resolved by SDS-PAGE and tyrosyl-phosphorylated proteins were detected by immunoblotting with antiphosphotyrosine antibodies. For in vitro substrate trapping, proliferating C2C12 myoblasts were lysed in the presence of 1% NP-40 without Na3VO4 but with 5 mM iodoacetic acid. Lysates were neutralized of the iodoacetic acid by incubation with 10 mM dithiothreitol and then incubated with either GST alone, GST-PTP-WT, or GST-PTP-DA for 3 h at 4°C. GST affinity complexes were dissociated by boiling and resolved by SDS-polyacrylamide gel electrophoresis (PAGE), and proteins were transferred to Immobilon-P membranes. GST-SH2 domain affinity precipitations were performed in a similar manner, except that Na3VO4 was included in the lysis buffer instead of iodoacetic acid. These affinity complexes either were immunoblotted for p190-B RhoGAP and for phosphotyrosine by using 4G10 antibodies. For vanadate blocking experiments, GST-PTP-DA Sepharose beads were incubated with 10 mM Na3VO4 for 10 min at 4°C prior to incubation with C2C12 lysates.
Assay for detection of activated RhoA.
C2C12 myoblasts were infected with Ad-GFP, Ad-WT, or Ad-RM for 24 h at 37°C in growth medium (GM) containing Dulbecco modified Eagle medium (Invitrogen) with 10% fetal bovine serum, 1 mM sodium pyruvate, and antibiotics (5 U of penicillin and 50 μg of streptomycin/ml). Cells were washed twice with ice-cold Tris-buffered saline and lysed (50 mM Tris [pH 8.0], 350 mM NaCl, 1% Triton X-100, 0.5% deoxycholate, 0.1% SDS, 10 mM MgCl2, 1 mM sodium vanadate, 0.2 mM PMSF, 10 μg of aprotinin/ml, 10 μg of leupeptin/ml) for 30 min at 4°C. Lysates were cleared by centrifugation at 20,800 × g for 10 min at 4°C. Whole-cell lysates were incubated for 45 min at 4°C with 30 μg of a 50% slurry of Rhotekin coupled to glutathione-Sepharose beads (Upstate Biotechnology) to precipitate GTP-bound Rho. Affinity complexes were washed three times in cold wash buffer (50 mM Tris [pH 8.0], 150 mM NaCl, 1% Triton X-100, 10 mM MgCl2, 0.2 mM PMSF, 10 μg of aprotinin/ml, 10 μg of leupeptin/ml), eluted with loading buffer, and separated by 10% SDS-PAGE. Proteins were visualized by immunoblotting by using 0.5 μg of mouse monoclonal antibody to RhoA (Santa Cruz Biochemicals)/ml.
RESULTS
SHP-2 is required for the activation of MyoD-dependent muscle-specific gene expression.
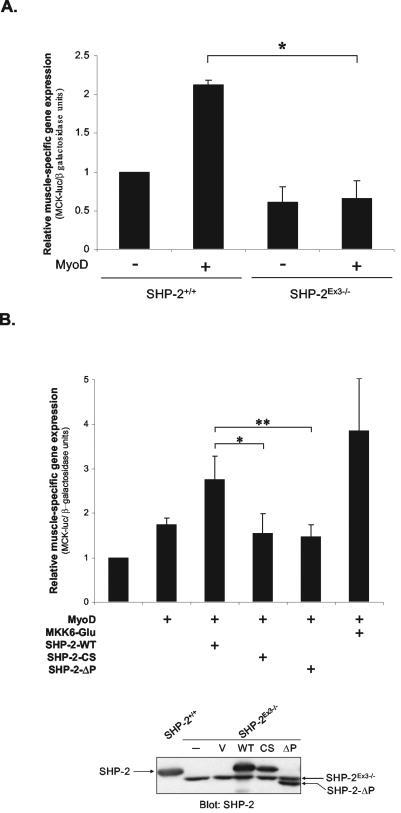
To define a role for SHP-2 during myogenesis, we investigated whether SHP-2 is required for muscle-specific gene expression. MyoD can confer heterologous muscle-specific gene expression from E-box-containing promoters when expressed in nonmuscle cells (10). Therefore, we transiently expressed MyoD in fibroblasts derived from mice containing a deletion within exon 3 of SHP-2 (SHP-2Ex3−/−) that removes the amino-terminal SH2 domain (36). These SHP-2Ex3−/− fibroblasts have been shown previously to exhibit properties consistent with a loss-of-function SHP-2 mutant (39, 55, 56). MyoD expressing wild-type fibroblasts (SHP-2+/+) and SHP-2Ex3−/− fibroblasts were compared for their ability to activate muscle-specific gene expression through the muscle creatine kinase (MCK) promoter (Fig. 1A). SHP-2+/+ and SHP-2Ex3−/− fibroblasts cultured in 10% fetal bovine serum were transiently transfected with either vector control or MyoD, and muscle-specific gene expression was assessed by using the MCK promoter fused to the luciferase gene (MCK-luc). These experiments revealed that, relative to vector control transfectants, expression of MyoD in SHP-2+/+ fibroblasts induced MCK-mediated gene expression by ∼2-fold (Fig. 1A). In contrast, the expression of MyoD in SHP-2Ex3−/− fibroblasts failed to significantly induce muscle-specific gene expression (Fig. 1A). MyoD was expressed to comparable levels in both SHP-2+/+ and SHP-2Ex3−/− fibroblasts (data not shown). These data imply that SHP-2 is required for the activation of muscle-specific genes.
FIG. 1.
The catalytic activity of SHP-2 is required for muscle-specific gene expression. (A) SHP-2+/+ and SHP-2Ex3−/− fibroblasts were transiently transfected with MCK-luciferase (MCK-luc), β-galactosidase, and pBabe-MyoD. Transfections were performed in triplicate and MCK-luc activity was normalized to β-galactosidase. The results represent the mean ± the standard error of the mean (SEM) from three separate experiments; statistical significances (*, P < 0.05) between SHP-2+/+ and SHP-2Ex3−/− MyoD transfectants were determined by using a two-tailed Student t test. (B) SHP-2Ex3−/− fibroblasts were transiently transfected with MCK-luc, β-galactosidase, pBabe-MyoD, and either pIRES-GFP, wild-type SHP-2 (SHP-2-WT), catalytically inactive SHP-2 (SHP-2-CS), catalytically inactive PTP deletion of SHP-2 (SHP-2-ΔP), or a constitutively active mutant of MKK6 (MKK6-Glu). Cells were stimulated with IGF-1 (100 ng/ml) for 24 h, and MCK-luc and β-galactosidase activities were determined. Transfections were performed in triplicate and normalized to vector control. The results represent the mean ± the SEM from four separate experiments. Statistical significances between SHP-2-WT versus SHP-2-CS (*, P < 0.01) and SHP-2-WT versus SHP-2-ΔP (**, P < 0.05) [MyoD versus MKK6(Glu); P < 0.1] were determined by a two-way analysis of variance. The lower panel shows the expression of transiently trans-fected SHP-2-WT, SHP-2-CS, and SHP-2-ΔP in SHP-2Ex3−/− fibroblasts. Arrows show the relative migration of the endogenous N-terminal SH2 domain deleted SHP-2 (SHP-2Ex3−/−) and exogenous wild-type and mutant SHP-2.
The IGFs are known to be critical for myogenic progression (14). In addition, SHP-2 has been shown to mediate IGF-1-dependent signaling (39, 55, 56). Therefore, we sought to determine whether SHP-2 participates in IGF-1-dependent muscle-specific gene expression. To address this, we assessed the ability of SHP-2 to rescue muscle-specific gene expression in SHP-2Ex3−/− fibroblasts in the presence of IGF-1. Expression of MyoD alone, in the presence of IGF-1, led to a small but consistent activation of MCK-luc activity that was enhanced when the constitutively active mutant of MKK6 (MKK6-Glu) was expressed (Fig. 1B). Reintroduction of wild-type SHP-2 into SHP-2Ex3−/− fibroblasts in the presence of IGF-1 stimulated MCK-luc activity relative to MyoD alone. However, neither SHP-2-CS nor SHP-2-ΔP was able to do so, despite their appropriate expression (Fig. 1B). These data demonstrate that reintroduction of wild-type SHP-2, but not a catalytically inactive mutant of SHP-2, into SHP-2Ex3−/− fibroblasts is sufficient to restore MyoD-dependent muscle-specific gene activity in an IGF-1-dependent manner.
The catalytic activity of SHP-2 is required for myogenesis.
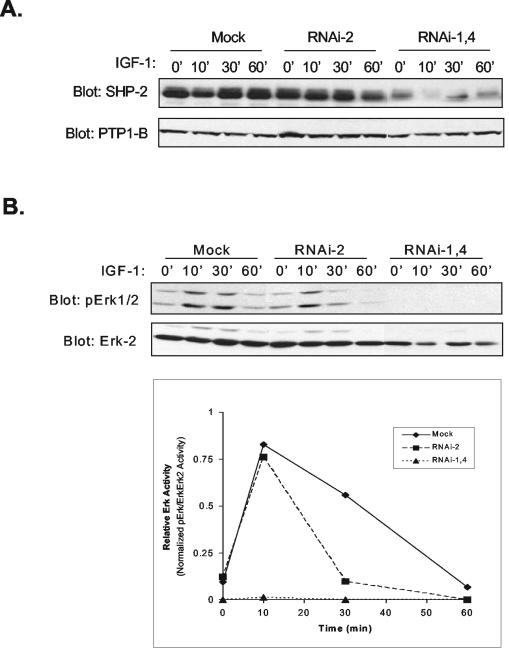
In order to determine whether SHP-2 is required for myogenesis, we used RNAi to downregulate SHP-2 expression in C2C12 myoblasts. We utilized two RNAi oligonucleotides against SHP-2, designated RNAi-1 and RNAi-4, that were directed to the SH2 and to the PTP domain of SHP-2, respectively. Upon transfection into C2C12 myoblasts, RNAi-1 and RNAi-4 were each only moderately effective in suppressing SHP-2 expression (data not shown). However, when both RNAi-1 and RNAi-4 (RNAi-1,4) were cotransfected into C2C12 myoblasts, SHP-2 expression was dramatically inhibited (Fig. 2A). Control cultures that were either mock transfected or were transfected with an RNAi sequence derived against SHP-2 that was found to be essentially ineffective at inhibiting SHP-2 expression (RNAi-2) were unaffected in their levels of SHP-2 expression (Fig. 2A). As a control for PTP specificity, these lysates were immunoblotted for the expression of PTP-1B. Figure 2A (lower panel) shows that RNAi-1,4 transfection into C2C12 myoblasts does not affect the level of PTP-1B expression.
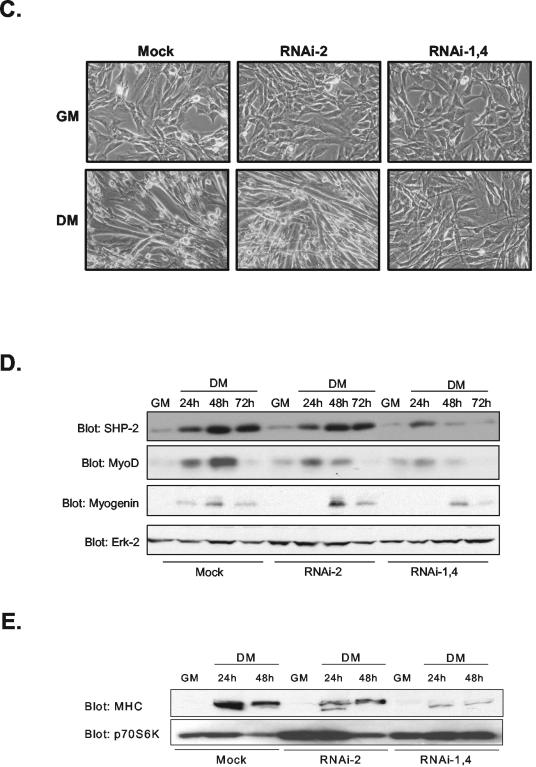
FIG. 2.
RNAi-mediated knockdown of SHP-2 expression inhibits IGF-1 signaling and myogenesis. (A) C2C12 myoblasts were either left untransfected (Mock) or were transiently transfected with either RNAi-2 or an equimolar concentration of both RNAi-1 and RNAi-4 (RNAi-1,4). C2C12 myoblasts were either left unstimulated or were stimulated with IGF-1 (100 ng/ml). Cell lysates were resolved and immunoblotted with antibodies to detect for the expression of SHP-2 and PTP-1B. (B) Erk activity was assessed by using anti-phospho-Erk1/2 and anti-Erk2 antibodies. The graph in the lower panel is a representative densitometric analysis of normalized phospho-Erk2 levels after SHP-2 RNAi transfection into C2C12 myoblasts in response to IGF-1. (C) C2C12 myoblasts were either mock transfected or were transfected with either RNAi-2 or RNAi-1,4. RNAi-transfected myoblasts were either cultured in GM or initiated to undergo differentiation by transfer to DM. Shown are representative photomicrographs of mock-, RNAi-2-, and RNAi-1,4-transfected C2C12 myoblasts 48 h after culture in DM. (D) Cell lysates prepared from panel C were immunoblotted with antibodies for the detection of either SHP-2, MyoD, myogenin, or Erk-2. (E) C2C12 myoblasts were either mock transfected or were transfected with either RNAi-2 or RNAi-1,4, cultures were either left undifferentiated (GM) or were differentiated (DM) for the indicated times. Cell lysates were prepared and analyzed by immunoblotting for the expression of MHC. These membranes were reprobed for p70S6K as a loading control.
SHP-2 has been shown to be required for IGF-1-dependent Erk activation (39, 55, 56), and so we tested the efficacy of SHP-2 RNAi knockdown in C2C12 myoblasts to block IGF-1-dependent activation of Erk. We transfected myoblasts with either RNAi-1,4 or as a control RNAi-2. Cultures that were mock transfected did not receive RNAi oligonucleotides. Myoblasts were serum starved and then restimulated with IGF-1 (100 ng/ml). Transfection of RNAi-1,4 abolished IGF-1-induced Erk activation compared to either mock transfected or RNAi-2-transfected cultures (Fig. 2B). These data confirmed the effectiveness of RNAi-1,4 to perturb endogenous SHP-2 signaling and demonstrate that SHP-2 is required for IGF-1-mediated Erk activation in myoblasts.
To determine whether SHP-2 is required for myogenesis, we sought to determine whether RNAi knockdown of SHP-2 inhibits C2C12 myogenesis. We found that RNAi-1,4 inhibited not only endogenous SHP-2 in proliferating myoblasts (Fig. 2A) but also the induction and sustained expression of SHP-2 when C2C12 myoblasts were initiated to undergo myogenesis (Fig. 2D). Although the mock- and RNAi-2-transfected myoblasts differentiated normally (Fig. 2C), RNAi-1,4-transfected myoblasts were inhibited from undergoing differentiation, as depicted by the lack of multinucleated myotube formation (Fig. 2C). Analysis of muscle-specific gene expression in these cultures indicated that RNAi-1,4 transfectants exhibited reduced expression of MyoD, myogenin, and MHC compared to mock- and RNAi-2-transfected myoblasts (Fig. 2D and E). Thus, both at the level of multinucleated myotube formation and at the level of muscle-specific gene expression, our data implicate SHP-2 as a critical component of the myogenic program.
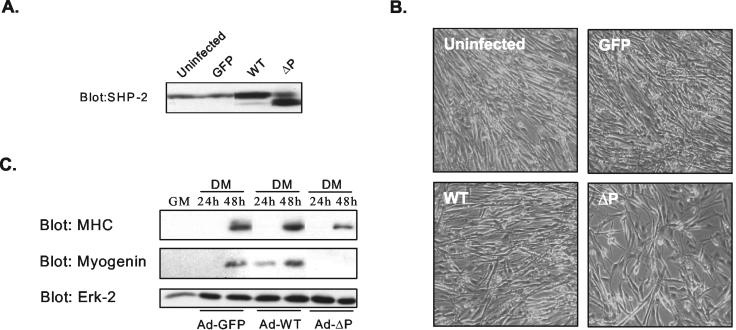
Next, we sought to determine whether the catalytic activity of SHP-2 is required for myogenesis by infecting C2C12 myoblasts with either Ad-GFP control adenovirus, adenoviruses expressing wild type (Ad-WT), or a catalytically inactive mutant of SHP-2 (Ad-ΔP) (Table 1). SHP-2 was overexpressed throughout differentiation, as confirmed by immunoblotting for the expression of SHP-2 in terminally differentiated myotubes (Fig. 3A). We observed that uninfected, Ad-GFP-infected, and Ad-WT-infected myoblasts all differentiated appropriately, as indicated by the formation of multinucleated myotubes and the expression of MHC and myogenin (Fig. 3B and C). In contrast, C2C12 myoblasts infected with Ad-ΔP failed to form multinucleated myotubes (Fig. 3B). Myogenin and MHC also exhibited reduced expression in the Ad-ΔP-infected myoblasts compared to uninfected, Ad-GFP-infected, and Ad-WT-infected myoblasts (Fig. 3C). These data revealed that the PTP domain, and hence the catalytic activity of SHP-2, is required for myogenesis.
FIG. 3.
The catalytic activity of SHP-2 is required for myogenesis. (A) C2C12 myoblasts were either left uninfected or were infected with adenoviral vectors expressing GFP (Ad-GFP), wild-type SHP-2 (Ad-WT), or a catalytically inactive mutant of SHP-2 (Ad-ΔP). Lysates were prepared after adenoviral transduction of C2C12 myoblasts that were cultured in DM for 72 h and immunoblotted with anti-SHP-2 antibodies. (B) Adenovirus-infected C2C12 myoblasts from panel A were visualized for the formation of multinucleated myotubes by phase-contrast microscopy. (C) Differentiated C2C12 myoblasts infected with adenoviral vectors as described for panel A were lysed at the indicated times. Cell lysates were resolved and immunoblotted with anti-MHC, antimyogenin, and anti-Erk-2 antibodies.
SHP-2 substrate-trapping mutant blocks myogenesis and complexes with potential substrates.
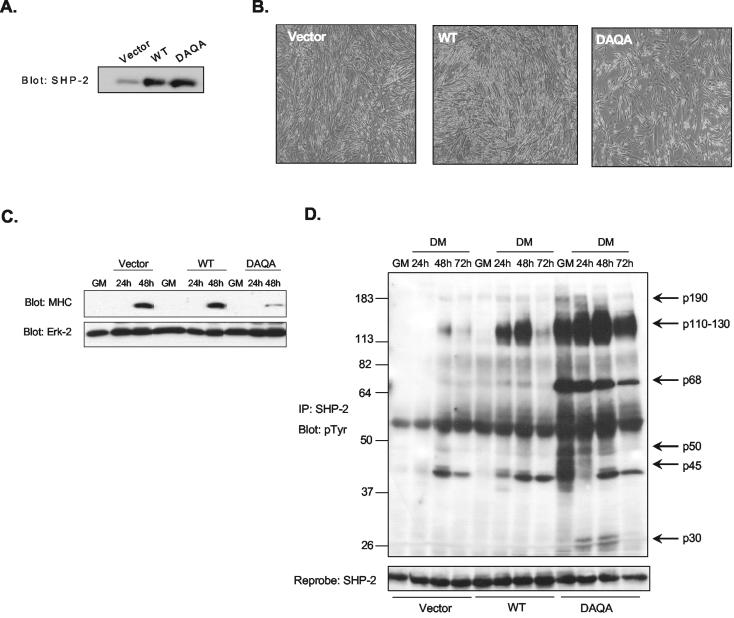
To identify SHP-2 substrates involved in myogenesis, we constructed a SHP-2 substrate-trapping mutant in which the invariant aspartic acid and glutamine residues within the PTP domain are mutated to alanine (13, 51). A double mutant of SHP-2 was generated in which Asp425 and Gln506 were mutated to Ala425 and Ala506, respectively (SHP-2-DAQA) (Table 1). First, we sought to determine whether overexpression of SHP-2-DAQA inhibits muscle differentiation in C2C12 myoblasts through the physical trapping of potential SHP-2 substrates that are required for differentiation. We generated stable C2C12 cell lines overexpressing either wild type (SHP-2-WT) or the substrate-trapping mutant of SHP-2 (SHP-2-DAQA) and a vector alone control. These pools of stable transfectants overexpressed SHP-2-WT and SHP-2-DAQA to levels ∼3-fold greater than endogenous SHP-2 (Fig. 4A). Although the vector and SHP-2-WT transfected myoblasts underwent normal differentiation, stable SHP-2-DAQA C2C12 myoblast transfectants were inhibited in their ability to form multinucleated myotubes and express the terminal differentiation marker, MHC (Fig. 4B and C). These data show that the substrate-trapping mutant of SHP-2 perturbs myogenesis by sequestering SHP-2 substrates that are required for differentiation.
FIG. 4.
The SHP-2 substrate-trapping mutant inhibits myogenesis and complexes with phosphotyrosyl-containing proteins. (A) Expression of SHP-2 in C2C12 myoblasts stably expressing either wild-type SHP-2 (WT) or the substrate-trapping SHP-2 mutant (DAQA) was determined by immunoblotting lysates prepared from these stable transfectants with anti-SHP-2 antibodies. (B) Representative photomicrographs of vector, wild-type, and DAQA-overexpressing C2C12 myoblasts that were induced to differentiate for 72 h. (C) Vector, wild-type, and DAQA C2C12 myoblast lines were differentiated for the indicated times, and cell lysates were prepared for analysis by immunoblotting for the expression of MHC. (D) C2C12 myoblasts were transiently transfected with either vector, wil-type, or DAQA expression vectors in GM. Transfected myoblasts were either left in GM or were switched to DM for 24, 48, and 72 h. SHP-2 was immunoprecipitated with polyclonal anti-SHP-2 antibodies and immune complexes were resolved and immunoblotted with antiphosphotyrosine antibodies (pTyr). These immunoblots were reprobed with anti-SHP-2 antibodies as a control for SHP-2 levels. The p68 tyrosyl-phosphorylated protein was identified to be SHP-2 (data not shown).
In order to identify potential SHP-2 substrates, we sought to establish whether the SHP-2-DAQA mutant complexed with tyrosyl phosphorylated proteins during C2C12 myogenesis. To accomplish this, vector control, wild-type SHP-2, and SHP-2-DAQA were transiently transfected into C2C12 myoblasts that were subsequently initiated to undergo differentiation in response to growth factor withdrawal. We found that SHP-2-DAQA was able to complex with multiple tyrosyl-phosphorylated proteins that were either hyper-tyrosyl phosphorylated or were not detectable in either vector control or wild-type SHP-2 transfectants (Fig. 4D). These tyrosyl-phosphorylated SHP-2 substrate-trapped proteins exhibited apparent molecular masses of 30, 45, 50, 68, 120 to 130, and 190 kDa (Fig. 4D). We identified by immunoblotting that the 68-kDa tyrosyl-phosphorylated protein is SHP-2 (data not shown), suggesting that SHP-2 autodephosphorylates during myogenesis.
Tyrosyl-phosphorylated p190-B RhoGAP is a SHP-2 substrate.
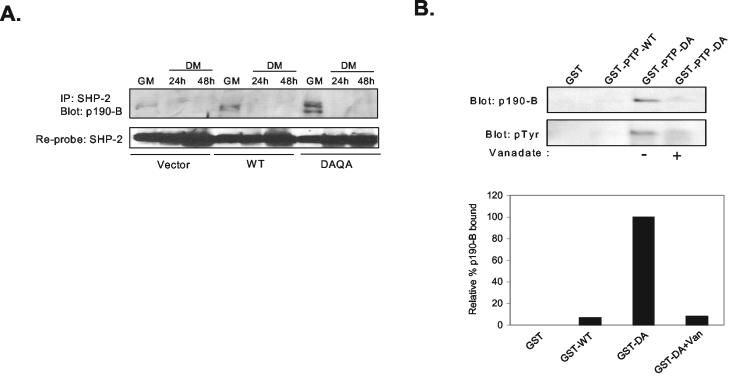
In order to identify SHP-2 substrates that are involved in myogenesis, we attempted to make predictions on the identity of these SHP-2 substrate-trapped proteins based on their apparent molecular mass (Fig. 4D). Recently, it has been suggested that SHP-2 may be a candidate PTP to mediate p190-B RhoGAP tyrosyl dephosphorylation during myogenesis (40). Tyrosyl dephosphorylation of p190-B RhoGAP is thought to prevent its localization to lipid rafts, thus facilitating the activation of RhoA which resides there (40). In addition, several reports indicate that RhoA positively regulates myogenesis (5, 7, 41, 42, 45, 48, 49). We noted that during C2C12 myogenesis a tyrosyl-phosphorylated protein with an approximate molecular mass of 190 kDa (p190) formed an enzyme-substrate complex with SHP-2-DAQA that was not detectable in either vector control or wild-type SHP-2 immune complexes (Fig. 4D). To test the possibility that this p190 could be p190-B RhoGAP, C2C12 myoblasts were transiently transfected with either wild-type SHP-2, SHP-2-DAQA, or vector control. Transfected C2C12 myoblasts were either left undifferentiated in GM or were differentiated by transferring these cultures to DM for various lengths of time, and SHP-2 immune complexes derived from these transfectants were immunoblotted with anti-p190-B RhoGAP antibodies. We found that p190-B RhoGAP associated with SHP-2 in wild-type transfectants, indicating that p190-B RhoGAP interacted with SHP-2 in an active-site-independent manner (Fig. 5A). However, in GM, SHP-2-DAQA also coprecipitated with p190-B RhoGAP, to a much greater extent, than either wild-type or vector control transfectants (Fig. 5A). Similar results also were obtained by using the SHP-2-DA substrate-trapping mutant (data not shown). The interpretation from these experiments is that p190-B RhoGAP is able to form an enzyme-substrate complex with SHP-2 in vivo. To further substantiate that p190-B RhoGAP forms an active site-mediated complex with SHP-2, we sought to determine whether p190-B RhoGAP complexes with the SHP-2 substrate-trapping PTP domain alone and whether this interaction is disrupted by vanadate, an active-site PTP inhibitor (18). When lysates prepared from proliferating C2C12 myoblasts were subjected to affinity precipitation with either GST-SHP-2 PTP domain (GST-PTP-WT) or the SHP-2 PTP domain substrate-trapping mutant (GST-PTP-DA), we found that tyrosyl-phosphorylated p190-B RhoGAP bound GST-PTP-DA but that p190-B RhoGAP was not detectable in GST-PTP-WT affinity precipitations (Fig. 5B). Significantly, preincubation of GST-PTP-DA with vanadate reduced the interaction between GST-PTP-DA and p190-B RhoGAP by up to 90% (Fig. 5B). These data demonstrate that tyrosyl phosphorylated p190-B RhoGAP forms an active site-mediated complex with SHP-2 both in vitro and within a cellular context in C2C12 myoblasts.
FIG. 5.
SHP-2 substrate-trapping mutant forms an active-site mediated complex with tyrosyl-phosphorylated p190-B RhoGAP in myoblasts. (A) C2C12 myoblasts were transfected in GM with either vector, wild-type SHP-2 (WT), or the substrate-trapping SHP-2 mutant (DAQA). Lysates prepared from the various stages of differentiation were subjected to immunoprecipitation with anti-SHP-2 antibodies; immune complexes were immunoblotted for the detection of p190-B RhoGAP. Immunoblots were reprobed with anti-SHP-2 antibodies (lower panel). (B) Lysates prepared from C2C12 myoblasts in GM were incubated with either 10 μg of GST, GST-PTP-WT, or GST-PTP-DA. GST affinity-purified complexes were resolved by SDS-PAGE and immunoblotted with either anti-p190-B RhoGAP or antiphosphotyrosine (pTyr) antibodies. The GST-PTP-DA affinity complexes also were pretreated with vanadate (10 mM). The graph below shows the relative p190-B RhoGAP bound to PTP-DA set to 100% relative to that of GST, PTP-WT, and PTP-DA+vanadate. Similar results were obtained in at least three independent experiments.
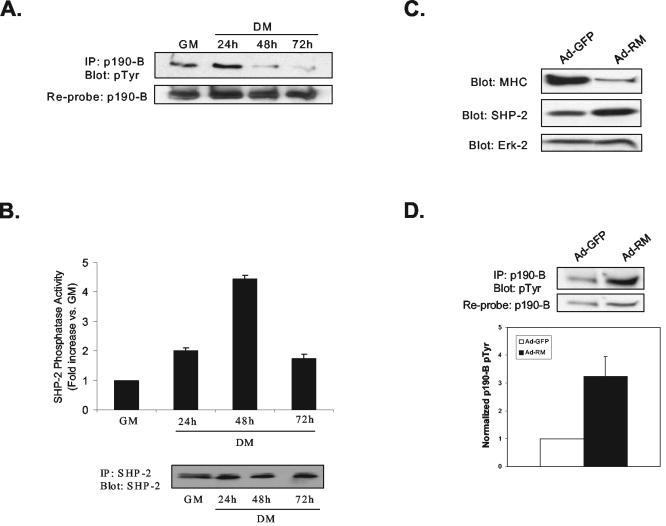
If SHP-2 dephosphorylates p190-B RhoGAP, then the catalytic activity of SHP-2 should be induced concomitantly with p190-B RhoGAP tyrosyl dephosphorylation during myogenesis. To test this, p190-B RhoGAP tyrosyl phosphorylation and SHP-2 phosphatase activity were assessed during myogenesis. As shown in Fig. 6A, p190-B RhoGAP undergoes tyrosyl dephosphorylation between 24 and 48 h during C2C12 differentiation. We next performed immune complex phosphatase assays against SHP-2 during C2C12 differentiation. We found that as myoblasts differentiated into multinucleated myotubes, the activity of SHP-2 increased within the first 24 h postdifferentiation by ∼2-fold and then by ∼5-fold between 24 h and 48 h, relative to undifferentiated myoblasts, and then declined thereafter (Fig. 6B). Thus, SHP-2 undergoes catalytic activation early on during myogenesis, within 24 h postdifferentiation, and exhibited maximal activation after 48 h postdifferentiation, coincident with that of the tyrosyl dephosphorylation of p190-B RhoGAP. These data, therefore, support the interpretation that SHP-2 dephosphorylates p190-B RhoGAP during myogenesis.
FIG. 6.
SHP-2 regulates p190-B RhoGAP tyrosyl phosphorylation during myogenesis. (A) p190-B RhoGAP was immunoprecipitated from proliferating (GM) and differentiating (DM) C2C12 myoblasts, and at the indicated times p190-B RhoGAP immune complexes were immunoblotted with antiphosphotyrosine antibodies (pTyr). The immunoblot was reprobed with anti-p190-B RhoGAP antibodies. (B) Immune complex SHP-2 phosphatase assays were performed from proliferating (GM) and differentiating (DM) C2C12 myoblasts. The graph shows the increase in SHP-2 phosphatase activity relative to GM, these data are representative of the mean ± the SEM from three separate experiments. As a control, these SHP-2 immune complexes were immunoblotted with anti-SHP-2 antibodies (lower panel). (C) C2C12 myoblasts were infected with either Ad-GFP control or Ad-RM (catalytically inactive, non-substrate-trapping mutant; SHP-2-RM) in GM. Adenoviral transduced cultures were then differentiated by switching to DM for 48 h. SHP-2-RM expression and C2C12 terminal differentiation were ascertained 48 h after differentiation by immunoblotting with anti-SHP-2 and anti-MHC antibodies, respectively. Anti-Erk2 immunoblots are shown as a control for protein loading. (D) Lysates prepared from C2C12 myoblasts treated as in panel C were subjected to p190-B RhoGAP immunoprecipitation, followed by antiphosphotyrosine immunoblotting (pTyr). Membranes were reprobed with monoclonal anti-p190-B RhoGAP antibodies. The graph below shows densitometric analyses of p190-B RhoGAP tyrosyl phosphorylation levels normalized to total immunoprecipitated p190-B RhoGAP. The data are representative of the mean ± the SEM of three separate experiments.
We next sought to determine whether overexpression of a catalytically inactive mutant of SHP-2 prevents p190-B RhoGAP tyrosyl dephosphorylation during myogenesis. C2C12 myoblasts were either infected with control adenovirus (Ad-GFP) or an adenovirus expressing a catalytically inactive and non-substrate-trapping mutant of SHP-2 (Ad-RΜ) (Table 1). We found that Ad-RM-infected myoblasts overexpressing SHP-2-RM were inhibited in their ability to undergo terminal differentiation, as demonstrated by the reduced level of MHC expression compared to Ad-GFP-infected controls (Fig. 6C). Significantly, SHP-2-RM overexpressing myoblasts were also inhibited in their ability to dephosphorylate p190-B RhoGAP since p190-B RhoGAP was hyper-tyrosyl phosphorylated in SHP-2-RM-expressing myoblasts compared to GFP-expressing myotubes (Fig. 6D). Taken together, these data demonstrate that p190-B RhoGAP tyrosyl dephosphorylation is inhibited upon overexpression of a catalytically inactive mutant of SHP-2, thus supporting the conclusion that p190-B RhoGAP is specifically dephosphorylated by SHP-2 during myogenesis.
SHP-2 complexes with p190-B RhoGAP and positively regulates Rho activity in myoblasts.
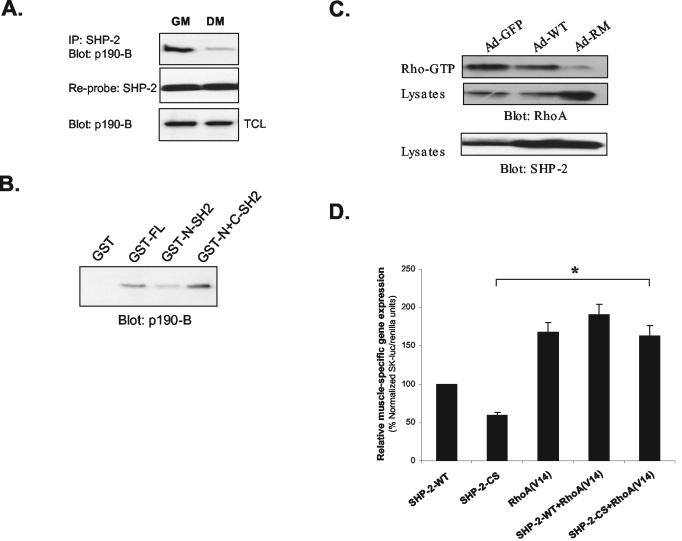
In order to provide additional mechanistic insight into how SHP-2 regulates p190-B RhoGAP tyrosyl phosphorylation, we tested whether SHP-2 associates with p190-B RhoGAP endogenously in myoblasts. We found that p190-B RhoGAP formed a complex with SHP-2 in proliferating myoblasts (GM) and to a much lower extent in terminally differentiated myotubes (DM) (Fig. 7A). The association of SHP-2 with p190-B RhoGAP in proliferating and terminally differentiated myotubes correlated with the levels of p190-B RhoGAP tyrosyl phosphorylation under these conditions (Fig. 6A). This observation suggested that SHP-2 may interact with p190-B RhoGAP in a phosphotyrosyl-dependent manner, possibly via its SH2 domains. To test this, we generated GST fusion proteins encoding either full-length SHP-2 (GST-FL), the tandem NH2- and COOH-terminal SH2 domains (N+C-SH2), or the NH2-terminal SH2 domain alone (N-SH2). Lysates prepared from proliferating myoblasts were subjected to affinity precipitation with these GST fusion proteins, and the ability of p190-B RhoGAP to bind either full-length SHP-2 or the SH2 domains of SHP-2 was assessed by immunoblotting with anti-p190-B RhoGAP antibodies. As expected, the full-length SHP-2 bound p190-B RhoGAP, and the N+C-SH2 domains also bound p190-B RhoGAP to comparable levels (Fig. 7B). In addition, the N-SH2 domain alone bound p190-B RhoGAP, although to lower levels, than either full-length SHP-2 or N+C-SH2 domains (Fig. 7B). These data demonstrate that the SH2 domains of SHP-2 interact with p190-B RhoGAP in myoblasts.
FIG. 7.
SHP-2 interacts with p190-B RhoGAP and positively regulates RhoA activity in myoblasts. (A) Lysates prepared from C2C12 myoblasts cultured either in GM or DM for 72 h were subjected to immunoprecipitation with anti-SHP-2 antibodies. Immune complexes were immunoblotted for p190-B RhoGAP and SHP-2 with anti-p190-B RhoGAP and SHP-2 antibodies, respectively. (B) Lysates prepared from proliferating C2C12 myoblasts were subjected to affinity precipitation with GST fusion proteins of either full-length SHP-2 (GST-FL), the tandem SH2 domains (GST-N+C-SH2) or the amino-terminal SH2 domain alone (GST-N-SH2) of SHP-2. Detection of p190-B RhoGAP in these complexes was achieved by immunoblotting with anti-p190-B RhoGAP antibodies. (C) C2C12 myoblasts cultured in GM were infected either with Ad-GFP, Ad-WT, or Ad-RM adenoviruses. GST-Rhotekin bound RhoA and total RhoA in cell lysates was detected by immunoblotting with anti-RhoA antibodies. (D) C2C12 myoblasts were transfected with SK-luc and pRL-Renilla along with either vector, SHP-2-WT, or SHP-2-CS and/or RhoA(V14) as indicated. Transfected C2C12 myoblasts were then harvested after 48 h in DM. Transfections were performed in triplicate, and the results are the means ± the SEM of five to six independent experiments. SK-luc/Renilla luciferase units were normalized as a percentage of SHP-2-WT, and the statistical analysis was performed by using a two-tailed Student t test SHP-2-CS versus SHP-2-CS+RhoA(V14) (*, P < 0.001).
If SHP-2 serves to dephosphorylate p190-B RhoGAP leading to RhoA activation, then SHP-2's catalytic activity should be required for RhoA activity in myoblasts. We tested this directly by measuring Rho-GTP levels after infection of myoblasts with either Ad-GFP control, Ad-WT, or Ad-RM. When overexpressed in myoblasts, SHP-2-RM inhibited RhoA activity relative to that of wild-type SHP-2 (Fig. 7C). These data demonstrate that in myoblasts the catalytic activity of SHP-2 is required to promote RhoA activity and suggests that SHP-2 functions upstream of RhoA. To test whether SHP-2 is positioned upstream of RhoA, we sought to determine whether the inhibitory effects of the catalytically inactive SHP-2 mutant on myogenesis could be rescued by a constitutively active mutant of RhoA. The constitutively activated mutant of RhoA, RhoA(V14), has been shown to stimulate the activity of the skeletal α-actin promoter (42, 48, 49). We therefore, transiently transfected C2C12 myoblasts in DM for 48 h with either SHP-2-CS alone or SHP-2-CS plus RhoA(V14). As expected, expression of SHP-2-CS suppressed the activity of the skeletal α-actin promoter (SK-luc) relative to that of wild-type SHP-2 (Fig. 7D). However, this inhibitory effect of SHP-2-CS on SK-luc relative to wild-type SHP-2 was rescued upon coexpression with RhoA(V14) to levels equivalent to that of the expression of RhoA(V14) alone (Fig. 7D). Together, these data support the interpretation that SHP-2 lies upstream of RhoA in the activation of muscle-specific genes via a pathway that involves the dephosphorylation of p190-B RhoGAP.
DISCUSSION
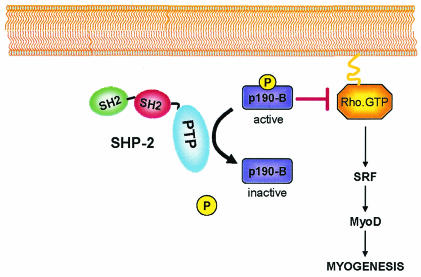
We show here that SHP-2 is a critical early regulator of myogenesis by acting as an upstream mediator of the RhoA GTPase. The use of substrate-trapping approaches has revealed that p190-B RhoGAP is a substrate for SHP-2 and that regulation of p190-B RhoGAP tyrosyl phosphorylation by SHP-2 promotes RhoA-mediated stimulation of myogenesis (Fig. 8).
FIG. 8.
Model for SHP-2 regulation of myogenesis. SHP-2 associates with p190-B RhoGAP and stimulation of SHP-2's phosphatase activity leads to tyrosyl dephosphorylation of p190-B RhoGAP. Tyrosyl dephosphorylation of p190-B RhoGAP by SHP-2 facilitates the activation of RhoA which promotes muscle-specific gene activation and myogenesis.
In order to investigate whether SHP-2 is involved in muscle-specific gene activation, we determined whether MyoD could induce heterologous muscle-specific gene activity when expressed in fibroblasts in which the N-SH2 domain of SHP-2 had been deleted. These experiments revealed that, whereas MyoD was capable of activating muscle-specific gene expression in wild-type fibroblasts, it was incapable of doing so in fibroblasts lacking the N-SH2 domain of SHP-2. Presumably, the truncated N-SH2 domain form of SHP-2 is crippled in its ability to appropriately interact with and/or be localized to substrates that participate in mediating MyoD-dependent muscle-specific gene expression. Nevertheless, these data demonstrate that the N-SH2 domain of SHP-2 is required to promote muscle-specific gene activity.
Given that we have identified several potential SHP-2 substrates in myoblasts, it is likely that, in addition to p190-B RhoGAP, other SHP-2 substrates contribute to myogenesis. In this regard, we show that SHP-2 can participate in regulating muscle-specific gene activity downstream of IGF-1, since wild-type SHP-2 is able to rescue the ability of SHP-2 loss-of-function fibroblasts to stimulate MyoD-dependent muscle-specific gene expression. These gain-of-function experiments and overexpression of catalytically inactive mutants of SHP-2 demonstrate that it is SHP-2's catalytic activity that is required for muscle-specific gene activation. Other studies have shown that the IGF-1/PI3K/Akt pathway promotes myogenesis (9, 20, 34, 35). SHP-2 has also been implicated in the regulation of the IGF-1/PI3K/Akt pathway in fibroblasts (50, 55), and we have recently shown that the catalytic activity of SHP-2 is required for IGF-1-dependent activation of PI3K (56). Thus, it is likely that SHP-2 may also regulate muscle-specific gene activation via the IGF-1/PI3K/Akt pathway. It is intriguing that, although SHP-2 is required for IGF-1-dependent activation of Erk in myoblasts, it somehow disengages from the Erk pathway during myogenesis. The activity of SHP-2 increases early during myogenesis, whereas Erk activity decreases at this stage (4, 9). It is not clear at this juncture how SHP-2 selectively disables itself from stimulating the Erk pathway early during myogenesis. These data suggest an unanticipated complexity in the signaling mechanisms of SHP-2. The answer to this apparent paradox may well lie in the identification of these additional SHP-2 substrates.
When overexpressed in differentiating C2C12 myoblasts, a “double” DAQA SHP-2 substrate-trapping mutant resulted in the identification of several complexed tyrosyl phosphorylated proteins of apparent molecular masses of 30, 45, 50, 68, 120 to 130, and 190 kDa. We found that the 68-kDa substrate-trapped protein is SHP-2, suggesting that, during myogenesis, SHP-2 undergoes trans- and/or autodephosphorylation. It has been suggested that SHP-2 can engage in at least intermolecular dephosphorylation and that tyrosyl phosphorylation within its C terminus stimulates its activity (26, 47). Thus, it is conceivable that later on during myogenesis SHP-2 participates in feedback inhibition of myogenic signaling by undergoing autodephosphorylation. Consistent with this idea, SHP-2 is tyrosyl dephosphorylated in terminally differentiated myotubes (24).
The other SHP-2 substrate-trapped protein identified was p190-B RhoGAP. We speculated that, based on previous observations that p190-B RhoGAP undergoes tyrosyl dephosphorylation during myogenesis (40), the observed p190 tyrosyl-phosphorylated protein complexed with SHP-2-DAQA could be p190-B RhoGAP. The substrate-trapped p190 was in fact p190-B RhoGAP. Both in vitro and in vivo experiments confirmed that p190-B RhoGAP was capable of forming a direct active-site-mediated complex with SHP-2. Moreover, p190-B RhoGAP fails to undergo tyrosyl dephosphorylation when myogenesis is inhibited upon overexpression of a catalytically inactive mutant of SHP-2. Finally, the catalytic activity of SHP-2 is required to promote RhoA activity in myoblasts. Together, these data support the interpretation that SHP-2 dephosphorylates p190-B RhoGAP and subsequently facilitates the activation of RhoA. SHP-2 has been implicated in the regulation of RhoA, in some cases SHP-2 negatively regulates RhoA (22, 37) and in others it is proposed that SHP-2 positively regulates RhoA (19). However, in these cases a mechanism for the effects of SHP-2 on RhoA was not defined. Our findings that SHP-2 dephosphorylates p190-B RhoGAP now provide a mechanistic basis for how SHP-2 regulates RhoA. A mechanism for how tyrosyl phosphorylation regulates p190-B RhoGAP was provided by Sordella et al. (40), who showed that tyrosyl phosphorylation of p190-B RhoGAP promotes its relocalization to lipid rafts where RhoA resides, thereby facilitating the inactivation of RhoA. In myoblasts, tyrosyl dephosphorylation of p190-B RhoGAP by SHP-2 presumably excludes it from lipid rafts and thus promotes RhoA activation. Of course, other potential mechanisms for regulating p190-B RhoGAP activity in response to its tyrosyl dephosphorylation may also be operative. Interestingly, recent work has suggested that SHP-2 controls Ras activation by preventing the relocalization of p120 RasGAP to the plasma membrane in response to epidermal growth factor (EGF) (1). It is proposed that SHP-2 dephosphorylates the EGF receptor at a site which is required for the recruitment of p120 RasGAP to the receptor. Thus, the control of p120 RasGAP localization to the plasma membrane and subsequently Ras activity appears to be modulated indirectly by SHP-2-mediated tyrosyl dephosphorylation of the EGF receptor (1). It is thus reasonable to suggest that a similar mechanism for how SHP-2-mediates RhoA activity might exist in myoblasts. In this case, however, the regulation of p190-B RhoGAP localization occurs by SHP-2 directly dephosphorylating p190-B RhoGAP rather than by dephosphorylating a receptor and/or scaffold protein. Further studies will be required in order to fully determine the precise mechanism for how tyrosyl dephosphorylation of p190-B RhoGAP by SHP-2 regulates RhoA activity.
An important question that remains to be addressed is how SHP-2 is regulated during myogenesis. Previously, we demonstrated that SHP-2 complexes with SHPS-1 during myogenesis (24). Interestingly, the kinetics of SHP-2's interaction with SHPS-1, as well as its interaction with other tyrosyl-phosphorylated proteins (also within the 120- to 130-kDa range), coincides with its catalytic activation during C2C12 differentiation. Thus, the recruitment of SHP-2 to SHPS-1 or some other tyrosyl-phosphorylated protein may serve to activate SHP-2 by providing a docking site for its N-SH2 domain. This raises a provocative issue regarding how SHP-2 complexes with p190-B RhoGAP in proliferating myoblasts without apparently dephosphorylating it. One possibility is that SHP-2 interacts with p190-B RhoGAP through its C-SH2 domain predominantly, leaving the N-SH2 domain free to activate SHP-2 upon binding to its phosphotyrosyl target. The C-SH2 domain may thus serve as a docking site for p190-B RhoGAP to facilitate its dephosphorylation by SHP-2. This model is consistent with the C-SH2 domain playing a minimal role in the catalytic activation of SHP-2 (2). Thus, it will be important to identify the phosphotyrosyl targets for the N-SH2 domain of SHP-2 during myogenesis in order to further define its mechanism of p190-B RhoGAP dephosphorylation.
In summary, these data indicate a novel role for SHP-2 in myogenesis and demonstrate a mechanism for how SHP-2 regulates skeletal muscle differentiation by directly coupling to the Rho family GTPase RhoA. Given the pleiotropic nature of RhoA in cellular signaling, our data may yield valuable insight into the role of SHP-2 not only in skeletal muscle disorders but in other diseases such as Noonan syndrome, an autosomal-dominant disease that displays a high frequency of activating SHP-2 mutations (43), and breast cancer in which p190-B RhoGAP has been suggested to play a role (6). Further investigation into the identity of these additional SHP-2 substrates, as well as an understanding of the spatiotemporal kinetics of SHP-2 activity during myogenesis, is warranted in order to fully decipher SHP-2's signaling role in skeletal muscle.
Acknowledgments
We thank all of the members of the Bennett lab for comments on this study and Lei Zhang for technical assistance in adenovirus generation.
M.I.K. and C.I.Z. were supported by NIH training grants T32-NS07136 and T32-CA09085, respectively. A.M.B. was supported by NIH grants R01-AR46504 and P01-DK57751 and a New Investigators Award from Burroughs Wellcome Fund.
REFERENCES
- 1.Agazie, Y. M., and M. J. Hayman. 2003. Molecular mechanism for a role of SHP2 in epidermal growth factor receptor signaling. Mol. Cell. Biol. 23:7875-7886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barford, D., and B. G. Neel. 1998. Revealing mechanisms for SH2 domain mediated regulation of the protein tyrosine phosphatase SHP-2. Structure 6:249-254. [DOI] [PubMed] [Google Scholar]
- 3.Bennett, A. M., S. F. Hausdorff, A. M. O'Reilly, R. M. Freeman, and B. G. Neel. 1996. Multiple requirements for SHPTP2 in epidermal growth factor mediated cell cycle progression. Mol. Cell. Biol. 16:1189-1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bennett, A. M., and N. K. Tonks. 1997. Regulation of distinct stages of skeletal muscle differentiation by mitogen-activated protein kinases. Science 278:1288-1291. [DOI] [PubMed] [Google Scholar]
- 5.Carnac, G., M. Primig, M. Kitzmann, P. Chafey, D. Tuil, N. Lamb, and A. Fernandez. 1998. RhoA GTPase and serum response factor control selectively the expression of MyoD without affecting Myf5 in mouse myoblasts. Mol. Biol. Cell 9:1891-1902. [DOI] [PMC free article] [PubMed]
- 6.Chakravarty, G., D. Roy, M. Gonzales, J. Gay, A. Contreras, and J. M. Rosen. 2000. p190-B, a Rho-GTPase-activating protein, is differentially expressed in terminal end buds and breast cancer. Cell Growth Differ. 11:343-354. [PubMed] [Google Scholar]
- 7.Charrasse, S., M. Meriane, F. Comunale, A. Blangy, and C. Gauthier-Rouviere. 2002. N-cadherin-dependent cell-cell contact regulates Rho GTPases and beta-catenin localization in mouse C2C12 myoblasts. J. Cell Biol. 158:953-965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chauhan, D., P. Pandey, T. Hideshima, S. Treon, N. Raje, F. E. Davies, Y. Shima, Y. T. Tai, S. Rosen, S. Avraham, S. Kharbanda, and K. C. Anderson. 2000. SHP2 mediates the protective effect of interleukin-6 against dexamethasone-induced apoptosis in multiple myeloma cells. J. Biol. Chem. 275:27845-27850. [DOI] [PubMed] [Google Scholar]
- 9.Coolican, S. A., D. S. Samuel, D. Z. Ewton, F. J. McWade, and J. R. Florini. 1997. The mitogenic and myogenic actions of insulin-like growth factors utilize distinct signaling pathways. J. Biol. Chem. 272:6653-6662. [DOI] [PubMed] [Google Scholar]
- 10.Davis, R. L., H. Weintraub, and A. B. Lassar. 1987. Expression of a single transfected cDNA converts fibroblasts to myoblasts. Cell 51:987-1000. [DOI] [PubMed] [Google Scholar]
- 11.Dias, P., M. Dilling, and P. Houghton. 1994. The molecular basis of skeletal muscle differentiation. Semin. Diagn. Pathol. 11:3-14. [PubMed] [Google Scholar]
- 12.Etienne-Manneville, S., and A. Hall. 2002. Rho GTPases in cell biology. Nature 420:629-635. [DOI] [PubMed] [Google Scholar]
- 13.Flint, A. J., T. Tiganis, D. Barford, and N. K. Tonks. 1997. Development of “substrate-trapping” mutants to identify physiological substrates of protein tyrosine phosphatases. Proc. Natl. Acad. Sci. USA 94:1680-1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Florini, J. R., D. Z. Ewton, K. A. Magri, and F. J. Mangiacapra. 1993. IGFs and muscle differentiation. Adv. Exp. Med. Biol. 343:319-326. [DOI] [PubMed] [Google Scholar]
- 15.Fujioka, Y., T. Matozaki, T. Noguchi, A. Iwamatsu, T. Yamao, N. Takahashi, M. Tsuda, T. Takada, and M. Kasuga. 1996. A novel membrane glycoprotein, SHPS-1, that binds the SH2-domain-containing protein tyrosine phosphatase SHP-2 in response to mitogens and cell adhesion. Mol. Cell. Biol. 16:6887-6899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gallo, R., M. Serafini, L. Castellani, G. Falcone, and S. Alema. 1999. Distinct effects of rac1 on differentiation of primary avian myoblasts. Mol. Biol. Cell 10:3137-3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.He, T. C., S. Zhou, L. T. da Costa, J. Yu, K. W. Kinzler, and B. Vogelstein. 1998. A simplified system for generating recombinant adenoviruses. Proc. Natl. Acad. Sci. USA 95:2509-2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huyer, G., S. Liu, J. Kelly, J. Moffat, P. Payette, B. Kennedy, G. Tsaprailis, M. J. Gresser, and C. Ramachandran. 1997. Mechanism of inhibition of protein-tyrosine phosphatases by vanadate and pervanadate. J. Biol. Chem. 272:843-851. [DOI] [PubMed] [Google Scholar]
- 19.Inagaki, K., T. Yamao, T. Noguchi, T. Matozaki, K. Fukunaga, T. Takada, T. Hosooka, S. Akira, and M. Kasuga. 2000. SHPS-1 regulates integrin-mediated cytoskeletal reorganization and cell motility. EMBO J. 19:6721-6731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaneko, S., R. I. Feldman, L. Yu, Z. Wu, T. Gritsko, S. A. Shelley, S. V. Nicosia, T. Nobori, and J. Q. Cheng. 2002. Positive feedback regulation between Akt2 and MyoD during muscle differentiation. Cloning of Akt2 promoter. J. Biol. Chem. 277:23230-23235. [DOI] [PubMed] [Google Scholar]
- 21.Kim, H., and H. Baumann. 1999. Dual signaling role of the protein tyrosine phosphatase SHP-2 in regulating expression of acute-phase plasma proteins by interleukin-6 cytokine receptors in hepatic cells. Mol. Cell. Biol. 19:5326-5338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kodama, A., T. Matozaki, A. Fukuhara, M. Kikyo, M. Ichihashi, and Y. Takai. 2000. Involvement of an SHP-2-Rho small G protein pathway in hepatocyte growth factor/scatter factor-induced cell scattering. Mol. Biol. Cell 11:2565-2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kontaridis, M. I., X. Liu, L. Zhang, and A. M. Bennett. 2002. Role of SHP-2 in fibroblast growth factor receptor-mediated suppression of myogenesis in C2C12 myoblasts. Mol. Cell. Biol. 22:3875-3891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kontaridis, M. I., X. Liu, L. Zhang, and A. M. Bennett. 2001. SHP-2 complex formation with the SHP-2 substrate-1 during C2C12 myogenesis. J. Cell Sci. 114:2187-2198. [DOI] [PubMed] [Google Scholar]
- 25.Lassar, A. B., S. X. Skapek, and N. Bennett. 1994. Regulatory mechanisms that coordinate skeletal muscle differentiation and cell cycle withdrawal. Curr. Opin. Cell Biol. 6:788-794. [DOI] [PubMed] [Google Scholar]
- 26.Lu, W., K. Shen, and P. A. Cole. 2003. Chemical dissection of the effects of tyrosine phosphorylation of SHP-2. Biochemistry 42:5461-5468. [DOI] [PubMed] [Google Scholar]
- 27.Meriane, M., P. Roux, M. Primig, P. Fort, and C. Gauthier-Rouviere. 2000. Critical activities of Rac1 and Cdc42Hs in skeletal myogenesis: antagonistic effects of JNK and p38 pathways. Mol. Biol. Cell 11:2513-2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Milarski, K. L., and A. R. Saltiel. 1994. Expression of catalytically inactive Syp phosphatase in 3T3 cells blocks stimulation of mitogen-activated protein kinase by insulin. J. Biol. Chem. 269:21239-21243. [PubMed] [Google Scholar]
- 29.Neel, B., and N. Tonks. 1997. Protein tyrosine phosphatases in signal transduction. Curr. Opin. Cell Biol. 9:193-204. [DOI] [PubMed] [Google Scholar]
- 30.Neel, B. G., H. Gu, and L. Pao. 2003. The 'Shp'ing news: SH2 domain-containing tyrosine phosphatases in cell signaling. Trends Biochem. Sci. 28:284-293. [DOI] [PubMed] [Google Scholar]
- 31.Oh, E. S., H. Gu, T. M. Saxton, J. F. Timms, S. Hausdorff, E. U. Frevert, B. B. Kahn, T. Pawson, B. G. Neel, and S. M. Thomas. 1999. Regulation of early events in integrin signaling by protein tyrosine phosphatase SHP-2. Mol. Cell. Biol. 19:3205-3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Olson, E., and W. Klein. 1994. bHLH factors in muscle development: dead lines and commitments, what to leave in and what to leave out. Genes Dev. 8:1-8. [DOI] [PubMed] [Google Scholar]
- 33.Olson, E. N. 1990. MyoD family: a paradigm for development? Genes Dev. 4:1454-1461. [DOI] [PubMed] [Google Scholar]
- 34.Rommel, C., S. C. Bodine, B. A. Clarke, R. Rossman, L. Nunez, T. N. Stitt, G. D. Yancopoulos, and D. J. Glass. 2001. Mediation of IGF-1-induced skeletal myotube hypertrophy by PI(3)K/Akt/mTOR and PI(3)K/Akt/GSK3 pathways. Nat. Cell Biol. 3:1009-1013. [DOI] [PubMed] [Google Scholar]
- 35.Rommel, C., B. A. Clarke, S. Zimmermann, L. Nunez, R. Rossman, K. Reid, K. Moelling, G. D. Yancopoulos, and D. J. Glass. 1999. Differentiation stage-specific inhibition of the raf-MEK-ERK pathway by Akt. Science 286:1738-1741. [DOI] [PubMed] [Google Scholar]
- 36.Saxton, T. M., M. Henkemeyer, S. Gasca, R. Shen, D. J. Rossi, F. Shalaby, G. S. Feng, and T. Pawson. 1997. Abnormal mesoderm patterning in mouse embryos mutant for the SH2 tyrosine phosphatase Shp-2. EMBO J. 16:2352-2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schoenwaelder, S. M., L. A. Petch, D. Williamson, R. Shen, G. Feng, and K. Burridge. 2000. The protein tyrosine phosphatase shp-2 regulates RhoA activity. Curr. Biol. 10:1523-1526. [DOI] [PubMed] [Google Scholar]
- 38.Servidei, T., Y. Aoki, S. E. Lewis, A. Symes, J. S. Fink, and S. A. Reeves. 1998. Coordinate regulation of STAT signaling and c-fos expression by the tyrosine phosphatase SHP-2. J. Biol. Chem. 273:6233-6241. [DOI] [PubMed] [Google Scholar]
- 39.Shi, Z. Q., W. Lu, and G. S. Feng. 1998. The Shp-2 tyrosine phosphatase has opposite effects in mediating the activation of extracellular signal-regulated and c-Jun NH2-terminal mitogen-activated protein kinases. J. Biol. Chem. 273:4904-4908. [DOI] [PubMed] [Google Scholar]
- 40.Sordella, R., W. Jiang, G. C. Chen, M. Curto, and J. Settleman. 2003. Modulation of Rho GTPase signaling regulates a switch between adipogenesis and myogenesis. Cell 113:147-158. [DOI] [PubMed] [Google Scholar]
- 41.Soulez, M. S., C. G. Rouviere, P. Chafey, D. Hentzen, M. Vandromme, N. Lautredou, N. Lamb, A. Kahn, and D. Tuil. 1996. Growth and differentiation of C2 myogenic cells are dependent on serum response factor. Mol. Cell. Biol. 16:6065-6074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Takano, H., I. Komuro, T. Oka, I. Shiojima, Y. Hiroi, T. Mizuno, and Y. Yazaki. 1998. The Rho family G proteins play a critical role in muscle differentiation. Mol. Cell. Biol. 18:1580-1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tartaglia, M., E. L. Mehler, R. Goldberg, G. Zampino, H. G. Brunner, H. Kremer, I. van der Burgt, A. H. Crosby, A. Ion, S. Jeffery, K. Kalidas, M. A. Patton, R. S. Kucherlapati, and B. D. Gelb. 2001. Mutations in PTPN11, encoding the protein tyrosine phosphatase SHP-2, cause Noonan syndrome. Nat. Genet. 29:465-468. [DOI] [PubMed] [Google Scholar]
- 44.Van Aelst, L., and C. D. Souza-Schorey. 1997. Rho GTPases and signaling networks. Genes Dev. 11:2295-2322. [DOI] [PubMed] [Google Scholar]
- 45.Vandromme, M., C. Gauthier-Rouviere, G. Carnac, N. Lamb, and A. Fernandez. 1992. Serum response factor p67SRF is expressed and required during myogenic differentiation of both C2 and rat L6 muscle cell lines. J. Cell Biol. 118:1489-1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vincent, C. K., A. Gualberto, C. V. Patel, and K. Walsh. 1993. Different regulatory sequences control creatine kinase-M gene expression in directly injected skeletal and cardiac muscle. Mol. Cell. Biol. 13:1264-1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vogel, W., R. Lammers, J. Huang, and A. Ullrich. 1993. Activation of a phosphotyrosine phosphatases by tyrosine phosphorylation. Science 259:1611-1614. [DOI] [PubMed] [Google Scholar]
- 48.Wei, L., W. Zhou, J. D. Croissant, F.-E. Johansen, R. Prywes, A. Balasubramanyam, and R. J. Schwartz. 1998. RhoA signaling via serum response factor plays an obligatory role in myogenic differentiation. J. Biol. Chem. 273:30287-30294. [DOI] [PubMed] [Google Scholar]
- 49.Wei, L., W. Zhou, L. Wang, and R. J. Schwartz. 2000. β1-integrin and PI 3-kinase regulate RhoA-dependent activation of skeletal alpha-actin promoter in myoblasts. Am. J. Physiol. Heart Circ. Physiol. 278:H1736-H1743. [DOI] [PubMed] [Google Scholar]
- 50.Wu, C. J., D. M. O'Rourke, G. S. Feng, G. R. Johnson, Q. Wang, and M. I. Greene. 2001. The tyrosine phosphatase SHP-2 is required for mediating phosphatidylinositol 3-kinase/Akt activation by growth factors. Oncogene 20:6018-6025. [DOI] [PubMed] [Google Scholar]
- 51.Xie, L., Y. L. Zhang, and Z. Y. Zhang. 2002. Design and characterization of an improved protein tyrosine phosphatase substrate-trapping mutant. Biochemistry 41:4032-4039. [DOI] [PubMed] [Google Scholar]
- 52.You, M., D. H. Yu, and G. S. Feng. 1999. Shp-2 tyrosine phosphatase functions as a negative regulator of the interferon-stimulated Jak/STAT pathway. Mol. Cell. Biol. 19:2416-2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yu, C. L., Y. J. Jin, and S. J. Burakoff. 2000. Cytosolic tyrosine dephosphorylation of STAT5. Potential role of SHP-2 in STAT5 regulation. J. Biol. Chem. 275:599-604. [DOI] [PubMed] [Google Scholar]
- 54.Yu, D. H., C. K. Qu, O. Henegariu, X. Lu, and G. S. Feng. 1998. Protein-tyrosine phosphatase Shp-2 regulates cell spreading, migration, and focal adhesion. J. Biol. Chem. 273:21125-21131. [DOI] [PubMed] [Google Scholar]
- 55.Zhang, S. Q., W. G. Tsiaras, T. Araki, G. Wen, L. Minichiello, R. Klein, and B. G. Neel. 2002. Receptor-specific regulation of phosphatidylinositol 3′-kinase activation by the protein tyrosine phosphatase Shp2. Mol. Cell. Biol. 22:4062-4072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zito, C. I., M. I. Kontaridis, M. Fornaro, G. S. Feng, and A. M. Bennett. 2004. SHP-2 regulates the phosphatidylinositide 3′-kinase/Akt pathway and suppresses caspase 3-mediated apoptosis. J. Cell Physiol. 199:227-236. [DOI] [PubMed]