Abstract
Although the link between transcription and DNA repair is well established, defects in the core transcriptional complex itself have not been shown to elicit a DNA damage response. Here we show that a cell line with a temperature-sensitive defect in TBP-associated factor 1 (TAF1), a component of the TFIID general transcription complex, exhibits hallmarks of an ATR-mediated DNA damage response. Upon inactivation of TAF1, ATR rapidly localized to subnuclear foci and contributed to the phosphorylation of several downstream targets, including p53 and Chk1, resulting in cell cycle arrest. The increase in p53 expression and the G1 phase arrest could be blocked by caffeine, an inhibitor of ATR. In addition, dominant negative forms of ATR but not ATM were able to override the arrest in G1. These results suggest that a defect in TAF1 can elicit a DNA damage response.
The ts13 and tsBN462 cell lines have been used as model systems to study the relationship between transcription and cell cycle control. These lines were originally isolated in screens to identify cells that underwent a cell cycle arrest upon a shift in temperature from 33 to 39°C (28, 45). It was subsequently discovered that both lines contain the same point substitution mutation (G690D) in TBP-associated factor 1 (TAF1) (TAFII250/CCG1), a key member of the TFIID complex (32, 35, 36). TAF1 is the largest of several TAFs, forming a scaffold between TBP and other TAFs and contributing to activated transcription (9). The ability of TAF1 to bind to TBP and other TAFs appears to be unaffected in ts13 cells shifted to the restrictive temperature (14, 37). However, TAF1 may lose the ability to bind to TBP at the cyclin D1 promoter in cells shifted to the restrictive temperature (18).
TAF1 is associated with at least three enzymatic activities. The N- and C-terminal ends of TAF1 contain a kinase activity that phosphorylates RAP74, a subunit of TFIIF (12). This kinase activity appears to be unaffected by the ts13 mutation (30). The central domain of TAF1 contains a histone acetyltransferase (HAT) activity that can acetylate TFIIEβ and histones H3 and H4 (21, 26). The ts13 and tsBN462 point mutation in TAF1 is located within the HAT domain, and a mutation of the corresponding residue in human TAF1 resulted in temperature-sensitive elimination of the HAT activity in vitro (14). TAF1 also contains an E1 and E2 ubiquitin activating and conjugating activity that participates in the monoubiquitination of histone H1 (23). It is not known whether this activity of TAF1 is affected by the ts13 and tsBN462 mutation.
Since TAF1 contributes to activated transcription, loss-of-function mutations could be expected to have significant effects on the transcription profile. Surprisingly, the ts13 and tsBN462 point mutation affected the transcription of a limited set of genes, including those that encode the cyclin-dependent kinase Cdk1/Cdc2 and cyclins D1, D3, and A (44, 49, 50). The transcription of several other genes, including c-fos and c-myc, was not reduced at the restrictive temperature (49). Since several cell cycle-related genes were found to be downregulated upon a shift to the restrictive temperature, it had been proposed that the decreased expression of cell cycle-related genes accounted for the cell cycle arrest, linking a defect in a general transcription factor to cell cycle regulation.
In contrast to the decreased transcription of cell cycle-related genes, several reports have suggested that the transcription of p53-dependent genes increased in ts13 cells after a shift to the restrictive temperature. For example, expression of the cyclin kinase inhibitor p21Cip1 increased in ts13 cells shifted to the restrictive temperature (33, 37). A microarray analysis of gene expression revealed that although 18% of the more than 4,000 genes examined had decreased expression, the mRNA levels of several p53-dependent genes, including those that encode Gadd45 and Pai-1, increased dramatically at the restrictive temperature (29). Finally, it has been reported that temperature restriction of tsBN462 cells led to increased expression of p53 itself (53). These reports suggested the possibility that the cell cycle arrest of ts13 cells might be mediated by a p53-dependent pathway.
p53 can be activated in response to a variety of cellular stresses, including hypoxia, heat shock, and DNA damage (17, 43). DNA damage, in particular, leads to the stabilization and activation of p53 by phosphorylation and acetylation of specific residues (8, 40). For example, two mediators of the DNA damage response, the ATM (ataxia-telangiectasia mutated) and ATR (ATM and Rad3 related) kinases, phosphorylate p53 on the serine 15 residue, inhibiting the ability of p53 to bind to and become degraded by Mdm2 (40, 46).
ATR and ATM are members of a family of large phosphatidylinositol 3-kinase-related protein kinases. Both ATR and ATM are activated in the presence of abnormal DNA structures, including stalled replication forks and double-strand breaks. In response to activation, these kinases phosphorylate a number of checkpoint and DNA repair proteins, including p53, the kinases Chk1 and Chk2, Rad17, Rad9, and BRCA1 (1, 6). Phosphorylation of these substrates contributes to cell cycle arrest and repair of DNA damage.
Here we provide evidence that the cell cycle arrest of ts13 cells is dependent on the rapid activation of p53 in response to a DNA damage checkpoint. Furthermore, ATR is specifically activated in response to the TAF1 defect and contributes to the phosphorylation of p53 and the G1 phase arrest. These results indicate that a defect in TAF1 can trigger the activation of a DNA damage pathway.
MATERIALS AND METHODS
Plasmids.
Wild-type (WT) and kinase-defective (KD) ATM (27) and ATR (54) plasmids have been previously described.
Cells.
ts13 and ts13R cells have been described previously (28, 49). Cells were cultured in Dulbecco modified Eagle medium containing 10% fetal bovine serum (HyClone) and penicillin and streptomycin at the permissive temperature of 33.5°C. To shift cells to the restrictive temperature, cells were fed with Dulbecco modified Eagle medium plus fetal bovine serum prewarmed to 39.5°C and transferred to a 39.5°C incubator.
Western blot assay and antibodies.
Cells were lysed in radioimmunoprecipitation assay buffer (200 mM Tris-HCl [pH 7.4], 130 mM NaCl, 10% glycerol, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate [SDS], 1% Triton X-100, 10 μl of aprotinin per ml, 10 μg of leupeptin per ml, 0.1 mM phenylmethylsulfonyl fluoride, 4 mM sodium fluoride, 0.1 mM sodium orthovanadate). Lysates were cleared by centrifugation at 14,000 × g, and protein concentrations were determined by the Bradford assay (Bio-Rad). A 50- to 200-μg portion of each sample was separated by SDS-polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes (Bio-Rad). The membranes were blocked for 1 h in 5% nonfat dry milk and 1% goat serum in TBS-T (10 mM Tris-HCl [pH 8.0], 150 mM NaCl, 0.1% Tween 20) before overnight incubation with the primary antibody in TBS-T containing 1% goat serum at 4°C.
The antibodies used in this study were specific for p53 (D01/Neomarkers), cyclin D1 (Neomarkers), cyclin A (H-432/Santa Cruz), p21Cip1 (C-19/Santa Cruz), α-tubulin (DM 1A/Sigma), p53 P-S15 and P-S20 (Cell Signaling Technology), Chk1 P-S345 (Cell Signaling Technology), Chk1 (FL-476/Santa Cruz), ATR (Santa Cruz), γ-H2AX (UBI), P-ATM (Roche), RPA34 (Ab-2/Oncogene), Mdm2 (Oncogene), and vinculin (Sigma).
Detection of proteins was performed with the appropriate horseradish peroxidase-conjugated secondary rabbit or mouse antibody (Pierce) at a 1:2,000 dilution in TBS-T containing 2.5% milk and 1% goat serum. Immunoblots were developed by enhanced chemiluminescence (Pierce).
Indirect immunofluorescence.
After growth on coverslips for 24 to 48 h at 33.5°C, ts13 cells were shifted to 39.5°C for 2 h or treated with hydroxyurea (HU) or 10 Gy of ionizing radiation. Where indicated, cells were pretreated with 2 mM caffeine or 2.5 mM 2-aminopurine (2-AP) for 2 h prior to the shift to the restrictive temperature. The coverslips were rinsed twice with phosphate-buffered saline (PBS) and fixed for 20 min in PBS containing 3% paraformaldehyde and 2% sucrose. For RPA34 staining, cells were rinsed with a PBS solution containing 1% Triton X-100 prior to fixation. After fixation, slides were rinsed in PBS, permeabilized for 20 min in PBS containing 1% Triton X-100, and exposed to the primary antibody for 1 h. The coverslips were washed five times in PBS, exposed to the appropriate secondary antibodies (Jackson Immuno) for 30 min, and washed thoroughly in PBS before mounting.
Fluorescence-activated cell sorting (FACS).
Cells were cotransfected with an ATR or ATM plasmid and a green fluorescent protein (GFP) reporter plasmid that served as a marker of transfected cells (42). One day after transfection, cells were shifted to the restrictive temperature or maintained at the permissive temperature for an additional 24 h. Cells were harvested in PBS containing 0.5 μM EDTA. A fraction of the cells was lysed and used for Western blotting to determine the level of transfected protein expression. The remaining cells were washed with PBS and fixed in PBS containing 2% glucose and 3% paraformaldehyde for 10 min at 4°C, washed with PBS twice, and resuspended in 70% ethanol for 1 to 2 h at 4°C. Cells were washed twice with PBS and resuspended in a solution containing 40 mM sodium citrate, 50 μg of propidium iodide per ml, and 10 μg of RNase A per ml.
RESULTS
p53 expression is transiently increased at the restrictive temperature.
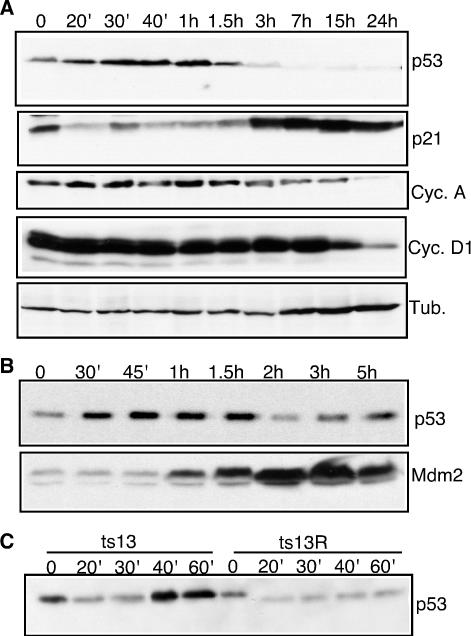
At the permissive temperature of 33.5°C, ts13 cells proliferate indefinitely. In contrast, these cells arrest in the G1 phase of the cell cycle within 24 h after a shift to the restrictive temperature of 39.5°C. To investigate the role of p53, ts13 cells were harvested at several time points after a shift to the restrictive temperature. As shown in Fig. 1A, p53 levels rapidly increased in ts13 cells within 20 to 40 min after the shift to the restrictive temperature. p53 levels continued to rise during the first hour after temperature restriction. Remarkably, the levels of p53 then dropped abruptly over the next few hours and it was nearly undetectable by 7 h after the temperature shift.
FIG. 1.
Rapid activation of p53 in ts13 cells. ts13 (A, B, and C) and ts13R (C) cells were cultured at 33.5°C (time zero) or 39.5°C for the indicated periods of time. Equal amounts of lysates were separated by SDS-polyacrylamide gel electrophoresis, transferred to nitrocellulose, and probed with the indicated antibodies. Cyc., cyclin; Tub., tubulin.
Notably, levels of Mdm2 began to rise soon after p53 levels peaked. The rise in Mdm2 levels and corresponding ubiquitin ligase activity directed toward p53 could account for the rapid decrease in p53 levels (Fig. 1B) (53). p21Cip1 levels increased significantly within 3 h after temperature restriction and remained elevated for 24 h (Fig. 1A). In contrast, cyclin D1 protein levels decreased at a much slower pace, with significantly reduced levels of cyclin D1 observed 15 h after temperature restriction. Cyclin A levels also decreased, but not until more than 15 h after the temperature shift. The decrease in the cyclin A and D1 protein levels most likely reflected the decreased activity of these promoters at the restrictive temperature (18, 44, 49, 50). However, by 15 h after a shift to the restrictive temperature, most of the ts13 cells have started to arrest in G1, so the differences in the cyclin A and D1 levels may reflect the shift in cell cycle phase. Thus, ts13 cells responded to the temperature restriction with a rapid increase in p53, followed by slower increases in Mdm2 and p21Cip1 and a very slow decrease in cyclins D1 and A.
To determine if the increase in p53 levels was due to the specific defect in TAF1, we examined the p53 levels in ts13R cells, a ts13-derived cell line that stably expresses WT human TAF1 (hTAF1) (49). Expression of hTAF1 completely rescued the transcriptional and cell cycle defects of the ts13 cells (49). As shown in Fig. 1C, while ts13 cells showed an increased level of p53 within 40 min after a shift to 39.5°C, ts13R cells showed no increase in p53 levels at any time after a shift to 39.5°C, suggesting that the up-regulation of p53 was a specific response to the defect in TAF1.
p53 is specifically phosphorylated on serine 15 and serine 20 in ts13 cells.
Since p53 levels increased rapidly in ts13 cells at the restrictive temperature, we reasoned that the change was likely not dependent on transcriptional regulation but rather due to a change in protein stability. The stability of p53 can be regulated by phosphorylation of certain N-terminal residues in response to stress. Among the best-characterized p53 phosphorylation sites are serine 15 (P-S15) and serine 20 (P-S20). The hamster and human p53 proteins are highly homologous, including the region encompassing residues S15 and S20. We sequenced a p53 cDNA isolated from ts13 cells and found that it was identical to WT hamster p53 (data not shown).
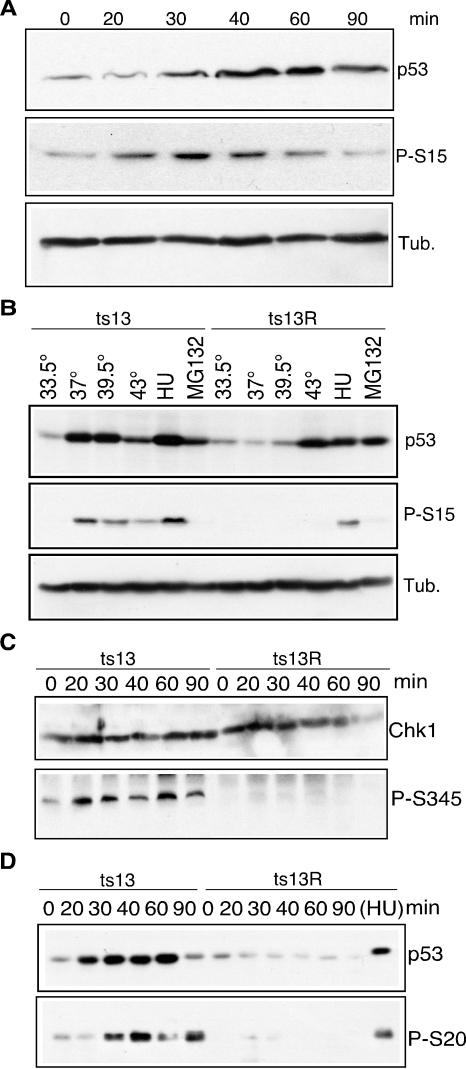
We examined whether the shift of ts13 cells from the permissive to the restrictive temperature resulted in a change in the phosphorylation of p53 by using phosphospecific antibodies to P-S15. As shown in Fig. 2A, P-S15 was detected in ts13 cells within 20 min after a shift to the restrictive temperature. P-S15 was detected concurrently with the increase in p53 levels and became less prominent by 90 min after the shift in temperature, when total p53 levels also declined.
FIG. 2.
Phosphorylation of p53 and activation of Chk1. (A) ts13 cells were incubated at 33.5°C (0 min) or 39.5°C for the indicated times. Lysates were immunoblotted with the indicated antibodies. (B) ts13 and ts13R cells were incubated at the indicated temperatures for 45 min or treated with 100 μM HU or 3 μg of MG132 per ml for 20 h. Equal amounts of lysates were immunoblotted and probed with the indicated antibodies. (C) ts13 or ts13R cells were incubated at 33.5°C (0 min) or 39.5°C for the indicated times and then lysed and immunoblotted with the indicated antibodies. (D) Lysates from ts13 or ts13R cells were immunoprecipitated with anti-p53 D01 antibody cross-linked to protein A-Sepharose beads, followed by immunoblotting with anti-p53 or anti-P-S20 antibodies. Tub., tubulin.
It has been reported that p53 levels can increase in response to heat shock (43). Furthermore, a previous report raised the possibility that the cell cycle arrest of tsBN462 cells was caused by an up-regulation of p53 in response to heat shock (53). To determine whether heat shock could account for the P-S15 induction in ts13 cells, we compared the levels of total p53 and P-S15 in ts13 and ts13R cells cultured at 33.5, 37, 39.5, and 43°C for 45 min. As shown in Fig. 2B, ts13 cells contained increased levels of p53 and P-S15 at temperatures of 37°C and above. In contrast, ts13R cells showed an increase in total p53 levels only at the heat shock temperature of 43°C but without significant levels of P-S15 present. Notably, HU induced an increase in total p53 and P-S15 levels, indicating that activation of p53 by the DNA replication checkpoint remained intact in both cell lines. Conversely, increases in p53 levels induced by inhibition of the proteasome with MG132 did not result in increased P-S15 levels. This experiment strongly supports the idea that the phosphorylation of S15 in p53 in ts13 cells was dependent on the cellular response to the TAF1 mutation and not to heat shock.
Given the rapid and specific phosphorylation of p53 on S15, we explored the possibility that other substrates of ATM and ATR became phosphorylated in ts13 cells after temperature restriction. ATR and ATM can phosphorylate the checkpoint kinase Chk1 on residue serine 345 (P-S345), resulting in the activation of Chk1 kinase activity (24). As shown in Fig. 2C, although total Chk1 levels did not change at the restrictive temperature, P-S345 was detected within 20 to 30 min after a shift to the restrictive temperature. In contrast, when ts13R cells were shifted to the restrictive temperature, no evidence of Chk1 phosphorylation was observed, suggesting that activation reflected a specific defect in TAF1.
Activated Chk1 has been shown to phosphorylate p53 on serine residue 20 (P-S20) (8, 39). After a shift to the restrictive temperature, P-S20 was detected in ts13 cells but not in ts13R cells (Fig. 2D). This result is consistent with the specific activation of Chk1 in ts13, but not ts13R, cells after a shift to the restrictive temperature. Although the Chk1-related kinase Chk2 could also phosphorylate S20 of p53, we were unable to test its role owing to a lack of antibodies cross-reactive with hamster Chk2.
Activation of ATR in ts13 cells.
The rapid phosphorylation of S15 and S20 of p53 and S345 of Chk1 suggested that ATM or ATR might be activated in ts13 cells shifted to the restrictive temperature. The ATM and ATR DNA damage pathways are characterized by the recruitment of signaling and repair factors to sites of DNA damage. For example, phosphorylated ATM (P-ATM) localizes to sites of DNA damage after treatment with IR (2). ATR can be recruited to sites of DNA damage induced by UV irradiation or to stalled replication forks in cells treated with HU (3, 47). Upon activation, ATR and ATM phosphorylate H2AX (γ-H2AX), a variant form of histone H2A, at sites of DNA damage (7, 31, 52).
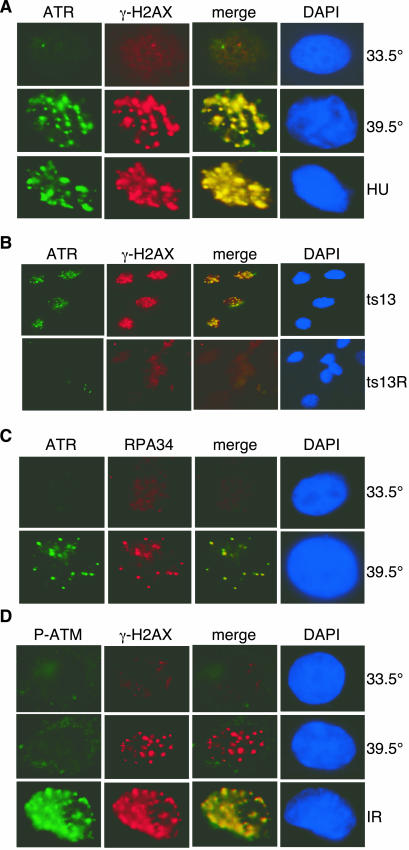
To examine whether there was evidence of specific activation of ATR and ATM in ts13 cells, we performed immunostaining at the permissive and restrictive temperatures. We observed that ATR formed subnuclear foci that colocalized with γ-H2AX in ts13 cells shifted to the restrictive temperature but not in cells maintained at the permissive temperature (Fig. 3A). ATR foci were present in 82.5% ± 4.2% of the ts13 cells within 2 h after the temperature shift, while only 11.8% ± 6.8% of the cells at the permissive temperature showed subnuclear foci. ATR and γ-H2AX did not form subnuclear foci in ts13R cells at 39.5°C, indicating that the effect was caused by the inactivation of TAF1 (Fig. 3B).
FIG. 3.
DNA damage foci in ts13 cells at the restrictive temperature. (A) ts13 cells were cultured at the permissive temperature (33.5°C), shifted to the restrictive temperature (39.5°C) for 2 h, or incubated in 300 μM HU at 33.5°C for 2 h. Cells were immunostained with antibodies to ATR (1:200) or γ-H2AX (1:10,000) and counterstained with 4′,6′-diamidino-2-phenylindole (DAPI). Images for ATR and γ-H2AX were merged. (B) ts13 and ts13R cells were cultured at 39.5°C for 2 h and immunostained with ATR and γ-H2AX antibodies and counterstained with DAPI. (C) ts13 cells were cultured at 39.5°C for 2 h and then briefly extracted with detergent prior to fixation and immunostaining with ATR and RPA34 (1:10,000) antibodies and counterstaining with DAPI. (D) ts13 cells were cultured at 39.5°C for 2 h or treated with IR (10 Gy) and incubated at the permissive temperature (33.5°C) for 90 min. Cells were fixed and stained with antibodies to P-ATM (1:100) and γ-H2AX and counterstained with DAPI.
Since ATR can be activated by stalled replication forks, it was possible that the ATR foci seen in ts13 cells were induced by a DNA synthesis arrest and not by the transcriptional defect. However, more than 80% of the ts13 cells showed ATR foci within 2 h after the shift to the restrictive temperature, suggesting that activation of ATR occurred in all phases of the cell cycle. To determine if the relocalization of ATR could occur in the G0/G1 phase of the cell cycle, ts13 cells were serum starved for 48 h at the permissive temperature prior to a shift to the restrictive temperature. After serum starvation, more than 85% of the ts13 cells were in the G0/G1 phase of the cell cycle as determined by FACS analysis (data not shown). When serum-starved cells were shifted to the restrictive temperature, ATR and γ-H2AX foci appeared within 2 h and P-S15 appeared within 45 min (data not shown). These results indicate that TAF1 can activate ATR in the G0/G1 phase of the cell cycle.
It has been reported that ATR can be recruited into subnuclear foci by replication protein A (RPA), a trimeric protein complex that binds specifically to single-stranded DNA (ssDNA) and that is required for both DNA synthesis and repair (3, 56). Using antibodies specific for the 34-kDa subunit of RPA (RPA34), we observed colocalization of RPA with ATR in subnuclear foci in ts13 cells (Fig. 3C). This result suggests that the defect in TAF1 resulted in the appearance of ssDNA that was recognized by RPA.
Certain forms of DNA damage result in the activation of ATM by autophosphorylation (2). P-ATM is then recruited to subnuclear foci, where it colocalizes with other DNA repair proteins. We did not observe the appearance of P-ATM within subnuclear foci of ts13 cells shifted to the restrictive temperature (Fig. 3D). In contrast, P-ATM appeared rapidly as subnuclear foci within 2 h after ts13 cells were treated with IR at the permissive temperature (Fig. 3D). Similarly, P-ATM was not detected in Western blots of lysates prepared from ts13 cells shifted to the restrictive temperature although it was present after ts13 cells were exposed to IR (data not shown). Although ATM can be appropriately activated and recruited to sites of DNA damage after IR exposure in ts13 cells, there was no suggestion that ATM was activated in response to inactivation of TAF1.
ATR activation is required for cell cycle arrest.
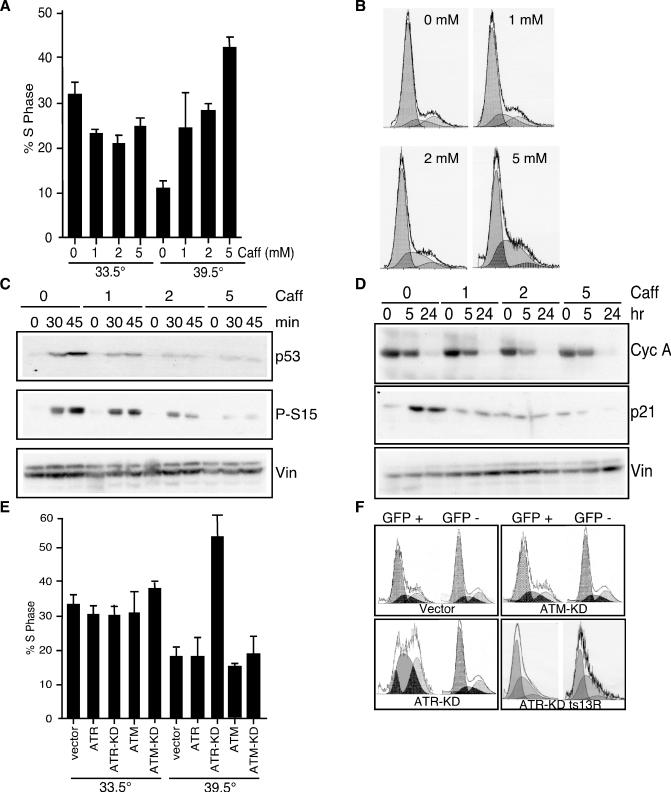
When ts13 cells are shifted to the restrictive temperature, they undergo growth arrest in the G1 phase of the cell cycle. The change in the cell cycle profile of ts13 cells can be observed within 16 to 24 h after the temperature shift. To determine whether ATR activity was required for the growth arrest at the restrictive temperature, ts13 cells were exposed to caffeine, an inhibitor of ATR (5, 16, 34). Caffeine treatment reduced the G1 phase arrest at the restrictive temperature, resulting in a dose-dependent increase in the percentage of cells in S phase from 15% to 25 to 45% (Fig. 4A and B, 39.5°C). In contrast, caffeine had little effect on the cell cycle profile of ts13 cells at the permissive temperature (Fig. 4A, 33.5°C).
FIG. 4.
Role of ATR in growth arrest of ts13 cells. ts13 cells were incubated with the indicated concentrations of caffeine (Caff) for 2 h and either maintained at the permissive temperature (33.5°C) or shifted to the restrictive temperature (39.5°C) for an additional 24 h. Cells were fixed and stained for DNA content with propidium iodide. (A) Graph showing the percentage of cells in S phase in the presence of the indicated concentrations of caffeine. (B) FACS profiles of DNA content of caffeine-treated ts13 cells at the restrictive temperature for 24 h. (C and D) Cells were pretreated with caffeine for 2 h at 33.5°C, shifted to 39.5°C, and then cultured for the indicated for the indicated periods of time. Lysates were prepared and blotted with the indicated antibodies. (E) ts13 cells were cotransfected with WT or KD derivatives of ATR or ATM and GFP, which served as a marker of transfected cells. Cells were incubated at 33.5°C for 24 h and then either maintained at 33.5°C or shifted to 39.5°C for an additional 24 h. The graph shows the percentage of GFP-positive (transfected) cells in S phase. (F) FACS profiles of GFP-positive (transfected) and GFP-negative (nontransfected) cells cultured at the restrictive temperature for 24 h. Cyc, cyclin; Vin, vinculin.
We examined the contribution of ATR to p53 activation by treating ts13 cells with caffeine. Treatment with caffeine eliminated the increase in p53 levels and the appearance of P-S15 at the restrictive temperature (Fig. 4C). In addition, exposure of cells to increasing amounts of caffeine prevented the increase in p21Cip1 levels (Fig. 4D). Notably, caffeine did not block the decrease in cyclin A or D1 levels at the restrictive temperature, suggesting that the decreased expression of these genes was independent of the DNA damage response (Fig. 4D and data not shown). This suggests that caffeine did not restore the activity of TAF1 on the cyclin D1 and A promoters.
To further test the requirement for ATR activity in the cell cycle arrest of ts13 cells, we transfected WT and KD mutant ATR and ATM constructs into ts13 cells (10, 27, 46, 54). The KD mutant constructs are dominant negative and have been previously shown to inhibit the ability of endogenous ATM and ATR to respond to DNA damage (10, 27, 46, 54). At the permissive temperature, none of the constructs had a significant effect on the percentage of cells in S phase (Fig. 4E). Furthermore, ATR-WT or ATM-WT had no effect on the percentage of ts13 cells in S phase at the restrictive temperature (Fig. 4E and F). In contrast, expression of ATR-KD in ts13 cells led to a significant increase in the percentage of cells in S phase at the restrictive temperature while ATM-KD had no effect on the cell cycle profile (Fig. 4E and F). Notably, ATR-KD did not affect the cell cycle profile of ts13R cells at any temperature (Fig. 4F).
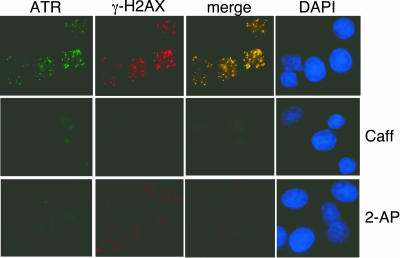
Caffeine and 2-AP treatment can block the activation of ATR and prevent the formation of subnuclear foci (19). Treatment of ts13 cells with 2 mM caffeine or 2.5 mM 2-AP blocked the recruitment ATR and γ-H2AX to subnuclear foci after the shift to the restrictive temperature (Fig. 5). Thus, inhibition of ATR activity blocked the formation of ATR and γ-H2AX foci, the up-regulation of p53, and the appearance of P-S15 and prevented the G1 phase arrest of ts13 cells. We therefore conclude that ATR signals to initiate the G1 arrest of ts13 cells shifted to the restrictive temperature.
FIG. 5.
Role of ATR in ts13 cells. Cells were cultured in the absence (top panels) or presence of caffeine (Caff; 2 mM) or 2-AP (2.5 mM) for 2 h and then shifted to the restrictive temperature for an additional 2 h. Cells were then fixed and processed as described in the legend to Fig. 1A. DAPI, 4′,6′-diamidino-2-phenylindole.
DISCUSSION
In this report, we have demonstrated that a temperature-sensitive defect in the transcription initiation factor TAF1 induces a DNA damage response that contributes to an arrest in the G1 phase of the cell cycle. Evidence for the checkpoint response in ts13 cells includes the rapid phosphorylation of p53 and Chk1. In addition, ATR was rapidly relocalized to subnuclear foci that also contained RPA and γ-H2AX, two factors that are activated at sites of DNA damage. The appearance of these subnuclear foci is a hallmark of ATR activation (3, 11, 47). This response was specific to ATR, as we did not observe foci of P-ATM upon temperature restriction. Furthermore, dominant negative ATR-KD, but not ATM-KD, could prevent ts13 cells from arresting in G1 at the restrictive temperature. Treatment of cells with caffeine, an inhibitor of ATR, could prevent a G1 phase arrest, p53 activation, and recruitment of ATR to subnuclear foci. This indicates that ATR was essential for the activation of p53 and the G1 phase arrest in response to the TAF1 defect.
We have not determined whether inhibition of ATR activity could fully overcome the cell cycle defect in ts13 cells or whether inhibition of ATR merely allowed cells to progress from G1 to S phase. Treatment with caffeine or overexpression of ATR-KD was not sufficient to prevent ts13 cells from undergoing apoptosis when held at the restrictive temperature for more than 24 h. Notably, it has been reported that cells cannot survive long without ATR activity (10, 54). In addition, cyclin A and D1 levels are not restored in caffeine-treated ts13 cells, suggesting that the cell cycle progression of these cells is not normal. It is still possible that the decrease in transcription of a number of cell cycle genes contributes to the growth arrest of ts13 cells and that the inhibition of ATR activity is merely sufficient to override the cell cycle arrest in G1.
The ability of caffeine to block the rapid activation of p53 and p21Cip1 and override the cell cycle arrest without restoring the expression of cyclins D1 and A suggests that the G1 arrest was dependent on the DNA damage response and not the decrease in cyclin expression. In addition, the late decrease in the levels of cyclins D1 and A also suggests that the cell cycle arrest was not caused by the transcriptional defect in the cyclin D1 and A promoters. However, both of these experiments were conducted with asynchronous cells. Since transcription from the cyclin D1 and A promoters is tightly regulated by the phase of the cell cycle, it is possible that the changes in transcription from these promoters occurred at earlier time points in some cells, affecting their ability to progress through the cell cycle.
It remains a possibility that decreased TAF1-dependent transcription of an especially labile mRNA triggers the activation of the ATR pathway and the DNA damage response in ts13 cells. However, the rapid activation of the ATR checkpoint within minutes of the temperature shift suggests that ATR may be responding to changes that occur directly at promoters in the presence of a mutant form of TAF1. It is possible that loss of HAT activity or some other function of TAF1 at an active promoter could cause the formation of DNA structures that can be recognized by ATR. Alternatively, loss of a TAF1 function could signal directly to ATR.
Several models could be proposed for how the TAF1 defect leads to ATR activation. First, the defect in TAF1 may cause a transcriptional stop or pause that is sufficient to induce a checkpoint response. Notably, p53 becomes up-regulated in response to certain compounds that interfere with transcription, including 5,6-dichloro-1-β-d-ribofuranosylbenzimidazole and α-amanitin (25). Although the mechanism for p53 activation under these conditions is not known, it is possible that ATR becomes specifically activated in response to a pause or a defect in transcription caused by inhibitors of RNA polymerase II or by the ts13 mutation of TAF1. A second possibility is that the defect in TAF1 induces specific structural changes at the sites of transcriptional initiation that resemble DNA damage. Defective TAF1 may induce the formation of an ssDNA transcription bubble recognized by RPA. For example, in vivo genomic footprinting of the cyclin D1 promoter in ts13 cells held at the restrictive temperature revealed a change in the structure of a 33-bp region within the cyclin D1 promoter suggesting the appearance of ssDNA (18). The third and most intriguing possibility is that TAF1 itself plays a role in monitoring changes in DNA structure at sites of active transcription. The temperature-sensitive defect in TAF1 could disrupt this activity, resulting in unresolved DNA damage that can be recognized by ATR.
The DNA damage checkpoint observed in ts13 cells may provide a glimpse into a much broader connection between transcription and DNA damage signaling. Other general transcription factors recognize and repair DNA damage. For example, nucleotide excision repair of DNA depends on the general transcription factor TFIIH (13, 51). At least three components of TFIIH, including XPB, XPD, and the cyclin-activating kinase CAK, have roles in transcription and nucleotide excision repair (15, 20, 38, 41).
Broader connections between transcription and checkpoint responses have been suggested by reports that in yeast meiotic recombination and double-strand breaks in DNA occur at sites of transcriptionally active promoters. Furthermore, higher rates of transcription increase the rate of recombination events that are mediated by double-strand breaks (48, 55). In mammalian B lymphocytes, the rate of immunoglobulin gene locus rearrangement is influenced by the presence of active transcription (4, 22). Therefore, the activation of a DNA damage checkpoint upon inactivation of a temperature-sensitive allele of TAF1 may reflect a broad but not yet fully understood connection between transcription and DNA damage signaling.
Acknowledgments
We thank Julie Sullivan and Ping Hua for excellent technical assistance and members of the DeCaprio laboratory for advice and support. We gratefully acknowledge the gifts of cells, plasmids, and antibodies from Peter Howley, Michael Kastan, Patrick Concannon, and Stephen Elledge.
A.M.B. was supported by NIH training grant 2T32CA09361 and NRSA fellowship F32CA81745. J.R.S. is supported by an NSF Graduate Research Fellowship. J.A.D. is a Scholar of the Leukemia and Lymphoma Society. This work was supported in part by Public Health Service grants RO1-CA63113 and PO1-CA50661.
REFERENCES
- 1.Abraham, R. T. 2001. Cell cycle checkpoint signaling through the ATM and ATR kinases. Genes Dev. 15:2177-2196. [DOI] [PubMed]
- 2.Bakkenist, C. J., and M. B. Kastan. 2003. DNA damage activates ATM through intermolecular autophosphorylation and dimer dissociation. Nature 421:499-506. [DOI] [PubMed] [Google Scholar]
- 3.Barr, S. M., C. G. Leung, E. E. Chang, and K. A. Cimprich. 2003. ATR kinase activity regulates the intranuclear translocation of ATR and RPA following ionizing radiation. Curr. Biol. 13:1047-1051. [DOI] [PubMed] [Google Scholar]
- 4.Blackwell, T. K., M. W. Moore, G. D. Yancopoulos, H. Suh, S. Lutzker, E. Selsing, and F. W. Alt. 1986. Recombination between immunoglobulin variable region gene segments is enhanced by transcription. Nature 324:585-589. [DOI] [PubMed] [Google Scholar]
- 5.Blasina, A., B. D. Price, G. A. Turenne, and C. H. McGowan. 1999. Caffeine inhibits the checkpoint kinase ATM. Curr. Biol. 9:1135-1138. [DOI] [PubMed] [Google Scholar]
- 6.Bradbury, J. M., and S. P. Jackson. 2003. ATM and ATR. Curr. Biol. 13:R468. [DOI] [PubMed] [Google Scholar]
- 7.Burma, S., B. P. Chen, M. Murphy, A. Kurimasa, and D. J. Chen. 2001. ATM phosphorylates histone H2AX in response to DNA double-strand breaks. J. Biol. Chem. 276:42462-42467. [DOI] [PubMed] [Google Scholar]
- 8.Chehab, N. H., A. Malikzay, E. S. Stavridi, and T. D. Halazonetis. 1999. Phosphorylation of Ser-20 mediates stabilization of human p53 in response to DNA damage. Proc. Natl. Acad. Sci. USA 96:13777-13782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen, J. L., L. D. Attardi, C. P. Verrijzer, K. Yokomori, and R. Tjian. 1994. Assembly of recombinant TFIID reveals differential coactivator requirements for distinct transcriptional activators. Cell 79:93-105. [DOI] [PubMed] [Google Scholar]
- 10.Cliby, W. A., C. J. Roberts, K. A. Cimprich, C. M. Stringer, J. R. Lamb, S. L. Schreiber, and S. H. Friend. 1998. Overexpression of a kinase-inactive ATR protein causes sensitivity to DNA-damaging agents and defects in cell cycle checkpoints. EMBO J. 17:159-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cortez, D., S. Guntuku, J. Qin, and S. J. Elledge. 2001. ATR and ATRIP: partners in checkpoint signaling. Science 294:1713-1716. [DOI] [PubMed] [Google Scholar]
- 12.Dikstein, R., S. Ruppert, and R. Tjian. 1996. TAFII250 is a bipartite protein kinase that phosphorylates the basal transcription factor RAP74. Cell 84:781-790. [DOI] [PubMed] [Google Scholar]
- 13.Drapkin, R., J. T. Reardon, A. Ansari, J. C. Huang, L. Zawel, K. Ahn, A. Sancar, and D. Reinberg. 1994. Dual role of TFIIH in DNA excision repair and in transcription by RNA polymerase II. Nature 368:769-772. [DOI] [PubMed] [Google Scholar]
- 14.Dunphy, E. L., T. Johnson, S. S. Auerbach, and E. H. Wang. 2000. Requirement for TAFII250 acetyltransferase activity in cell cycle progression. Mol. Cell. Biol. 20:1134-1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feaver, W. J., J. Q. Svejstrup, N. L. Henry, and R. D. Kornberg. 1994. Relationship of CDK-activating kinase and RNA polymerase II CTD kinase TFIIH/TFIIK. Cell 79:1103-1109. [DOI] [PubMed] [Google Scholar]
- 16.Hall-Jackson, C. A., D. A. Cross, N. Morrice, and C. Smythe. 1999. ATR is a caffeine-sensitive, DNA-activated protein kinase with a substrate specificity distinct from DNA-PK. Oncogene 18:6707-6713. [DOI] [PubMed] [Google Scholar]
- 17.Hammond, E. M., N. C. Denko, M. J. Dorie, R. T. Abraham, and A. J. Giaccia. 2002. Hypoxia links ATR and p53 through replication arrest. Mol. Cell. Biol. 22:1834-1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hilton, T. L., and E. H. Wang. 2003. Transcription factor IID recruitment and Sp1 activation: dual function of TAF1 in cyclin D1 transcription. J. Biol. Chem. 278:12992-13002. [DOI] [PubMed] [Google Scholar]
- 19.Huang, S., L. K. Qu, A. R. Cuddihy, R. Ragheb, Y. Taya, and A. E. Koromilas. 2003. Protein kinase inhibitor 2-aminopurine overrides multiple genotoxic stress-induced cellular pathways to promote cell survival. Oncogene 22:3721-3733. [DOI] [PubMed] [Google Scholar]
- 20.Hwang, J. R., V. Moncollin, W. Vermeulen, T. Seroz, H. van Vuuren, J. H. Hoeijmakers, and J. M. Egly. 1996. A 3′ → 5′ XPB helicase defect in repair/transcription factor TFIIH of xeroderma pigmentosum group B affects both DNA repair and transcription. J. Biol. Chem. 271:15898-15904. [DOI] [PubMed] [Google Scholar]
- 21.Imhof, A., X. J. Yang, V. V. Ogryzko, Y. Nakatani, A. P. Wolffe, and H. Ge. 1997. Acetylation of general transcription factors by histone acetyltransferases. Curr. Biol. 7:689-692. [DOI] [PubMed] [Google Scholar]
- 22.Lauster, R., C. A. Reynaud, I. L. Martensson, A. Peter, D. Bucchini, J. Jami, and J. C. Weill. 1993. Promoter, enhancer and silencer elements regulate rearrangement of an immunoglobulin transgene. EMBO J. 12:4615-4623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leung, W., A. R. Chen, R. C. Klann, T. J. Moss, J. M. Davis, S. J. Noga, K. J. Cohen, A. D. Friedman, D. Small, C. L. Schwartz, M. J. Borowitz, M. D. Wharam, C. N. Paidas, C. A. Long, S. Karandish, J. D. McMannis, M. B. Kastan, and C. I. Civin. 1998. Frequent detection of tumor cells in hematopoietic grafts in neuroblastoma and Ewing's sarcoma. Bone Marrow Transplant. 22:971-979. [DOI] [PubMed] [Google Scholar]
- 24.Liu, Q., S. Guntuku, X. S. Cui, S. Matsuoka, D. Cortez, K. Tamai, G. Luo, S. Carattini-Rivera, F. DeMayo, A. Bradley, L. A. Donehower, and S. J. Elledge. 2000. Chk1 is an essential kinase that is regulated by Atr and required for the G2/M DNA damage checkpoint. Genes Dev. 14:1448-1459. [PMC free article] [PubMed] [Google Scholar]
- 25.Ljungman, M., F. Zhang, F. Chen, A. J. Rainbow, and B. C. McKay. 1999. Inhibition of RNA polymerase II as a trigger for the p53 response. Oncogene 18:583-592. [DOI] [PubMed] [Google Scholar]
- 26.Mizzen, C. A., X. J. Yang, T. Kokubo, J. E. Brownell, A. J. Bannister, T. Owen-Hughes, J. Workman, L. Wang, S. L. Berger, T. Kouzarides, Y. Nakatani, and C. D. Allis. 1996. The TAFII250 subunit of TFIID has histone acetyltransferase activity. Cell 87:1261-1270. [DOI] [PubMed] [Google Scholar]
- 27.Morgan, S. E., C. Lovly, T. K. Pandita, Y. Shiloh, and M. B. Kastan. 1997. Fragments of ATM which have dominant-negative or complementing activity. Mol. Cell. Biol. 17:2020-2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nishimoto, T., T. Sekiguchi, R. Kai, K. Yamashita, T. Takahashi, and M. Sekiguchi. 1982. Large-scale selection and analysis of temperature-sensitive mutants for cell reproduction from BHK cells. Somatic Cell Genet. 8:811-824. [DOI] [PubMed] [Google Scholar]
- 29.O'Brien, T., and R. Tjian. 2000. Different functional domains of TAFII250 modulate expression of distinct subsets of mammalian genes. Proc. Natl. Acad. Sci. USA 97:2456-2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O'Brien, T., and R. Tjian. 1998. Functional analysis of the human TAFII250 N-terminal kinase domain. Mol. Cell 1:905-911. [DOI] [PubMed] [Google Scholar]
- 31.Paull, T. T., E. P. Rogakou, V. Yamazaki, C. U. Kirchgessner, M. Gellert, and W. M. Bonner. 2000. A critical role for histone H2AX in recruitment of repair factors to nuclear foci after DNA damage. Curr. Biol. 10:886-895. [DOI] [PubMed] [Google Scholar]
- 32.Ruppert, S., E. H. Wang, and R. Tjian. 1993. Cloning and expression of human TAFII250: a TBP-associated factor implicated in cell-cycle regulation. Nature 362:175-179. [DOI] [PubMed] [Google Scholar]
- 33.Rushton, J. J., R. A. Steinman, and P. D. Robbins. 1997. Differential regulation of transcription of p21 and cyclin D1 conferred by TAFII250. Cell Growth Differ. 8:1099-1104. [PubMed] [Google Scholar]
- 34.Sarkaria, J. N., E. C. Busby, R. S. Tibbetts, P. Roos, Y. Taya, L. M. Karnitz, and R. T. Abraham. 1999. Inhibition of ATM and ATR kinase activities by the radiosensitizing agent, caffeine. Cancer Res. 59:4375-4382. [PubMed] [Google Scholar]
- 35.Sauve, G. J., Y. M. Shen, M. Zannis-Hadjopoulos, C. D. Chang, R. Baserga, and R. Hand. 1987. Isolation of a human sequence which complements a mammalian G1-specific temperature-sensitive mutant of the cell cycle. Oncogene Res. 1:137-147. [PubMed] [Google Scholar]
- 36.Sekiguchi, T., T. Miyata, and T. Nishimoto. 1988. Molecular cloning of the cDNA of human X chromosomal gene (CCG1) which complements the temperature-sensitive G1 mutants, tsBN462 and ts13, of the BHK cell line. EMBO J. 7:1683-1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sekiguchi, T., E. Noguchi, T. Hayashida, T. Nakashima, H. Toyoshima, T. Nishimoto, and T. Hunter. 1996. D-type cyclin expression is decreased and p21 and p27 CDK inhibitor expression is increased when tsBN462 CCG1/TAFII250 mutant cells arrest in G1 at the restrictive temperature. Genes Cells 1:687-705. [DOI] [PubMed] [Google Scholar]
- 38.Serizawa, H., T. P. Makela, J. W. Conaway, R. C. Conaway, R. A. Weinberg, and R. A. Young. 1995. Association of Cdk-activating kinase subunits with transcription factor TFIIH. Nature 374:280-282. [DOI] [PubMed] [Google Scholar]
- 39.Shieh, S. Y., J. Ahn, K. Tamai, Y. Taya, and C. Prives. 2000. The human homologs of checkpoint kinases Chk1 and Cds1 (Chk2) phosphorylate p53 at multiple DNA damage-inducible sites. Genes Dev. 14:289-300. [PMC free article] [PubMed] [Google Scholar]
- 40.Shieh, S. Y., M. Ikeda, Y. Taya, and C. Prives. 1997. DNA damage-induced phosphorylation of p53 alleviates inhibition by MDM2. Cell 91:325-334. [DOI] [PubMed] [Google Scholar]
- 41.Shiekhattar, R., F. Mermelstein, R. P. Fisher, R. Drapkin, B. Dynlacht, H. C. Wessling, D. O. Morgan, and D. Reinberg. 1995. Cdk-activating kinase complex is a component of human transcription factor TFIIH. Nature 374:283-287. [DOI] [PubMed] [Google Scholar]
- 42.Subramanian, S., and F. Srienc. 1996. Quantitative analysis of transient gene expression in mammalian cells using the green fluorescent protein. J. Biotechnol. 49:137-151. [DOI] [PubMed] [Google Scholar]
- 43.Sugano, T., M. Nitta, H. Ohmori, and M. Yamaizumi. 1995. Nuclear accumulation of p53 in normal human fibroblasts is induced by various cellular stresses which evoke the heat shock response, independently of the cell cycle. Jpn. J. Cancer Res. 86:415-418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Suzuki-Yagawa, Y., M. Guermah, and R. G. Roeder. 1997. The ts13 mutation in the TAFII250 subunit (CCG1) of TFIID directly affects transcription of D-type cyclin genes in cells arrested in G1 at the nonpermissive temperature. Mol. Cell. Biol. 17:3284-3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Talavera, A., and C. Basilico. 1977. Temperature sensitive mutants of BHK cells affected in cell cycle progression. J. Cell. Physiol. 92:425-436. [DOI] [PubMed] [Google Scholar]
- 46.Tibbetts, R. S., K. M. Brumbaugh, J. M. Williams, J. N. Sarkaria, W. A. Cliby, S. Y. Shieh, Y. Taya, C. Prives, and R. T. Abraham. 1999. A role for ATR in the DNA damage-induced phosphorylation of p53. Genes Dev. 13:152-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tibbetts, R. S., D. Cortez, K. M. Brumbaugh, R. Scully, D. Livingston, S. J. Elledge, and R. T. Abraham. 2000. Functional interactions between BRCA1 and the checkpoint kinase ATR during genotoxic stress. Genes Dev. 14:2989-3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Voelkel-Meiman, K., R. L. Keil, and G. S. Roeder. 1987. Recombination-stimulating sequences in yeast ribosomal DNA correspond to sequences regulating transcription by RNA polymerase I. Cell 48:1071-1079. [DOI] [PubMed] [Google Scholar]
- 49.Wang, E. H., and R. Tjian. 1994. Promoter-selective transcriptional defect in cell cycle mutant ts13 rescued by hTAFII250. Science 263:811-814. [DOI] [PubMed] [Google Scholar]
- 50.Wang, E. H., S. Zou, and R. Tjian. 1997. TAFII250-dependent transcription of cyclin A is directed by ATF activator proteins. Genes Dev. 11:2658-2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang, Z., J. Q. Svejstrup, W. J. Feaver, X. Wu, R. D. Kornberg, and E. C. Friedberg. 1994. Transcription factor b (TFIIH) is required during nucleotide-excision repair in yeast. Nature 368:74-76. [DOI] [PubMed] [Google Scholar]
- 52.Ward, I. M., and J. Chen. 2001. Histone H2AX is phosphorylated in an ATR-dependent manner in response to replicational stress. J. Biol. Chem. 276:47759-47762. [DOI] [PubMed] [Google Scholar]
- 53.Wasylyk, C., and B. Wasylyk. 2000. Defect in the p53-Mdm2 autoregulatory loop resulting from inactivation of TAFII250 in cell cycle mutant tsBN462 cells. Mol. Cell. Biol. 20:5554-5570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wright, J. A., K. S. Keegan, D. R. Herendeen, N. J. Bentley, A. M. Carr, M. F. Hoekstra, and P. Concannon. 1998. Protein kinase mutants of human ATR increase sensitivity to UV and ionizing radiation and eliminate cell cycle checkpoint control. Proc. Natl. Acad. Sci. USA 95:7445-7450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wu, T. C., and M. Lichten. 1994. Meiosis-induced double-strand break sites determined by yeast chromatin structure. Science 263:515-518. [DOI] [PubMed] [Google Scholar]
- 56.Zou, L., and S. J. Elledge. 2003. Sensing DNA damage through ATRIP recognition of RPA-ssDNA complexes. Science 300:1542-1548. [DOI] [PubMed] [Google Scholar]