Abstract
The Reg regulon from Rhodobacter capsulatus and Rhodobacter sphaeroides encodes proteins involved in numerous energy-generating and energy-utilizing processes such as photosynthesis, carbon fixation, nitrogen fixation, hydrogen utilization, aerobic and anaerobic respiration, denitrification, electron transport, and aerotaxis. The redox signal that is detected by the membrane-bound sensor kinase, RegB, appears to originate from the aerobic respiratory chain, given that mutations in cytochrome c oxidase result in constitutive RegB autophosphorylation. Regulation of RegB autophosphorylation also involves a redox-active cysteine that is present in the cytosolic region of RegB. Both phosphorylated and unphosphorylated forms of the cognate response regulator RegA are capable of activating or repressing a variety of genes in the regulon. Highly conserved homologues of RegB and RegA have been found in a wide number of photosynthetic and nonphotosynthetic bacteria, with evidence suggesting that RegB/RegA plays a fundamental role in the transcription of redox-regulated genes in many bacterial species.
INTRODUCTION
RegB and RegA were originally described as cognate members of a two-component signal transduction system that are involved in anaerobic synthesis of the photosystem in Rhodobacter capsulatus (55, 78). However, it is now known that RegB and RegA constitute a highly conserved global regulatory system that provides an overlying layer of redox control on a variety of energy-generating and energy-utilizing biological processes. Specifically, photosynthesis, carbon fixation, nitrogen fixation, hydrogen oxidation, denitrification, aerobic and anaerobic respiration, electron transport, and aerotaxis are now known to be part of the RegB/RegA regulon.
In addition to studies revealing the extent of the RegB/RegA regulon, there has been a substantial amount of biochemical research on mechanistic functions of these regulators. RegB, a histidine sensor kinase, has been characterized in terms of kinetics, necessary components, and conditions needed to observe redox-regulated in vitro autophosphorylation. RegA has also been studied in terms of the effect of phosphorylation on DNA-binding activity, transcriptional modulation and interactions with other regulators. In addition, a consensus RegA DNA-binding motif has been identified.
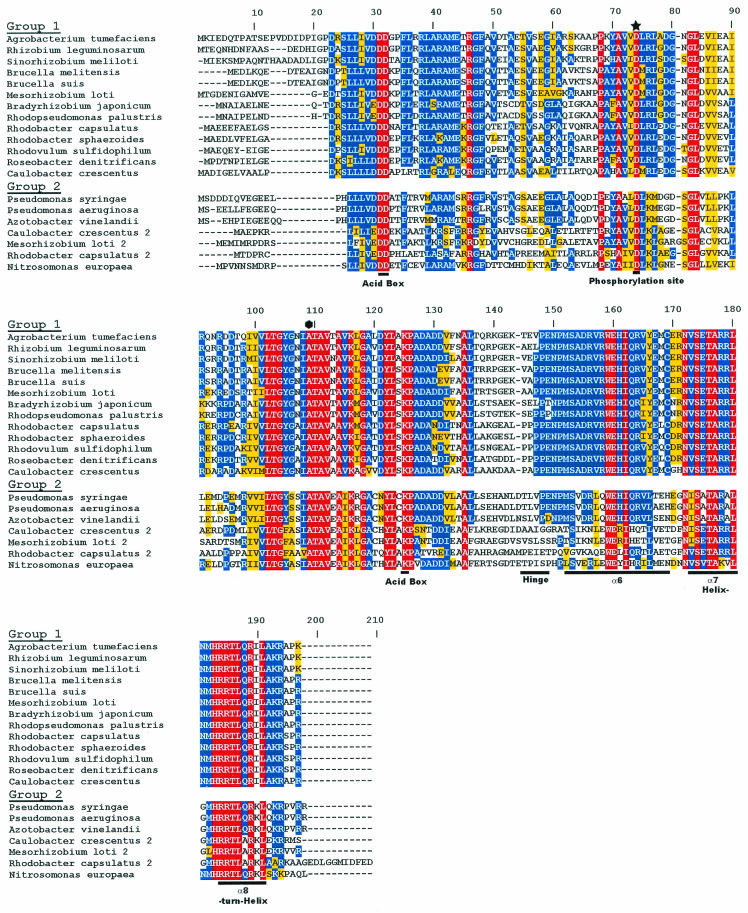
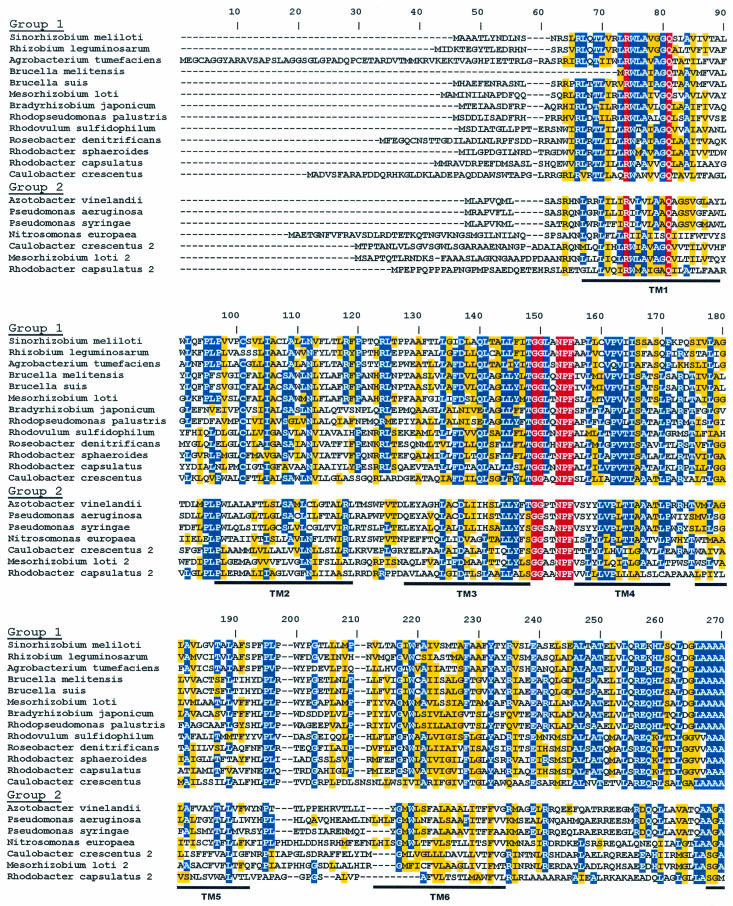
The R. capsulatus RegB/RegA system has provided the groundwork for the discovery and characterization of homologues found in a wide variety of photosynthetic and nonphotosynthetic bacteria. Among α-proteobacteria, there is an unprecedented 100% conservation of the helix-turn-helix (HTH) DNA-binding motif of RegA homologues, suggesting that there are significant constraints on the ability of RegA to undergo genetic drift (see Fig. 1). Additionally, a variety of RegB homologues show conservation of a “redox box” that is at least partially responsible for controlling RegB kinase activity in response to changes in environmental redox conditions (see Fig. 2). Below we describe our current understanding of this multivalent system.
FIG. 1.
Alignment of RegA homologues present in genome databases. The input domain contains a conserved phosphate-accepting aspartate residue, denoted by a star. The output domain contains the three-helical bundle DNA-binding domain, denoted by α6, α7, and α8. The flexible linker region, which connects the input and output domains, is labeled Hinge. The alanine responsible for the RegA* phenotype is indicated by an octagon. Red residues represent 100% conserved amino acids, blue residues represent 50% or higher conserved amino acids, and yellow residues represent conservative amino acid changes.
FIG. 2.
Alignment of RegB homologues present in genome databases. Membrane-spanning domains are indicated as regions TM1 through TM6. The site of histidine phosphorylation is denoted by a star within the H-box. The threonine residue important for phosphatase activity is indicated by a square. The location of the redox-active cysteine is denoted by a circle within the redox box. The ATP-binding domains are indicated as the N, G1, F, and G2 boxes. The color scheme is as described in the legend to Fig. 1.
RegB/RegA TWO-COMPONENT REGULATORY SYSTEM
Discovery
In many species of purple photosynthetic bacteria, synthesis of the photosynthetic apparatus occurs predominantly under conditions of low oxygen tension (12). To define the mechanism behind redox regulation of photosystem synthesis, genetic screens were used to isolate R. capsulatus mutants that have reduced pigmentation after prolonged growth under dark anaerobic conditions. This screen led to the isolation of regA and regB mutants exhibiting a defect in anaerobic induction of the photosystem (55, 78). Null mutations in regB and regA were subsequently shown to be defective in high-level anaerobic expression of the puh, puf, and puc operons that encode apoproteins for the light-harvesting I, light-harvesting II, and reaction center complexes (55, 78). The similar phenotypes displayed by these mutants led to the hypothesis that they may be cognate trans-acting partners constituting a two-component regulatory system. This was confirmed by sequence analysis which demonstrated that RegB exhibits homology to histidine protein kinases (55, 70, 81, 82) and that RegA exhibits homology to DNA-binding response regulators (70, 78, 81, 82). Disruption of regB affected photosystem gene expression less dramatically than did disruption of regA, suggesting that another sensor kinase(s) may also phosphorylate RegA (55) at a low level or that RegA may obtain phosphates from small-molecule donors, such as acetyl phosphate or carbamyl phosphate (82).
Homologous Systems
Soon after the discovery of RegB/RegA from R. capsulatus, homologues were found in Rhodobacter sphaeroides that were called either RegB/RegA (71) or PrrB/PrrA (29, 30). Mutations in the R. sphaeroides RegB/RegA homologues exhibited defects in photosystem synthesis that were similar to those caused by RegB/RegA mutations in R. capsulatus (29, 30, 71). Homologous two-component regulatory systems have also been genetically characterized in many other species such as the RegS/RegR system from Bradyrhizobium japonicum (6), ActS/ActR from Sinorhizobium meliloti (89), RoxS/RoxR from Pseudomonas aeruginosa (15), and RegB/RegA from Rhodovulum sulfidophilum and Roseobacter denitrificans (53). Genome sequence studies have identified RegA and RegB homologues in many other photosynthetic as well as nonphotosynthetic α- and γ-proteobacterial species (Fig. 1 and 2, respectively).
Sequence analysis indicated that regB and regA genes from most species are localized to a similar region of the chromosome. In R. capsulatus, R. sphaeroides, R. sulfidophilum, and R. denitrificans, the regB gene is divergently transcribed from a putative senC-regA-hvrA operon (10, 29, 30, 31, 53, 55). A variation occurs in Caulobacter crescentus, where a putative regB-regA operon is transcribed divergently from senC. The other organisms listed in Fig. 1 and 2 have regB and regA genes linked in a single putative regB-regA operon without senC or hvrA genes being found within close proximity on the chromosome (details of SenC and HvrA are discussed below).
As shown in the sequence alignments of RegB (Fig. 2) and RegA (Fig. 1) homologues, RegB and RegA proteins are highly conserved among photosynthetic and nonphotosynthetic α- and γ-proteobacteria. The RegA homologues can be placed into two groups based on the sequence similarity of their output domain. Group 1 members have 100% conservation of the HTH DNA-binding motif, while group 2 homologues have a similar but nonidentical HTH region (Fig. 1). Overall, group 1 homologues exhibit a remarkable 78.7 to 84.2% sequence identity to RegA from R. capsulatus, with 93% overall identity in the C-terminal output domain. This level of conservation suggests that there are significant constraints on the variability of RegA to undergo mutational change in these species. In contrast to extensive analysis of group 1 homologues, there has been limited characterization of group 2 homologues. Mutational analysis of roxR, which is a group 2 RegA homologue in P. aeruginosa, indicates that RoxR controls respiratory gene expression in P. aeruginosa as does the RegB/RegA system in R. capsulatus (15, 83). Interestingly, several photosynthetic and nonphotosynthetic species, such as C. cresentus, R. capsulatus, and Mesorhizobium loti, contain both group 1 and group 2 homologues (Fig. 1).
Among RegB homologues, there are also two identifiable groups composed of similar members, as discussed for RegA homologues (Fig. 2). Differences between group 1 and group 2 RegB homologues occur in the regions between transmembrane 6 and the H box (amino acids 245 to 269), between the redox box and the N box (amino acids 335 to 355), and at the carboxyl terminus (amino acid 455 to end) (Fig. 2).
In addition to RegB and RegA proteins having a high level of sequence identity, they have functional similarity. For example, Emmerich et al. (25) demonstrated that RegB and RegA homologues from R. capsulatus, B. japonicum, and S. meliloti are interchangeable. More precisely, phosphotransfer was observed between the B. japonicum RegB homologue (RegS) and R. capsulatus RegA. The RegA homologue from B. japonicum (RegR) was shown to bind to the R. capsulatus puf and puc operon promoters. R. capsulatus RegA, and its homologue from S. meliloti (ActR), can activate transcription of the fixR nifA operon from B. japonicum in vivo (25). Furthermore, Comolli and Donohue (15) demonstrated that RegA homologues from R. sphaeroides (PrrA) and P. aeruginosa (RoxR) also succeeded in heterologous complementation. These studies demonstrate that homologous Reg proteins are interchangeable in vitro as well as in vivo.
Mutational analysis of several α-proteobacterial RegA homologues indicates that they control a similar set of target genes such as respiratory and nitrogenase genes, details of which are discussed below. Thus, it appears that the RegB/RegA system is involved in global regulation in many diverse species of bacteria.
SENSOR KINASE RegB
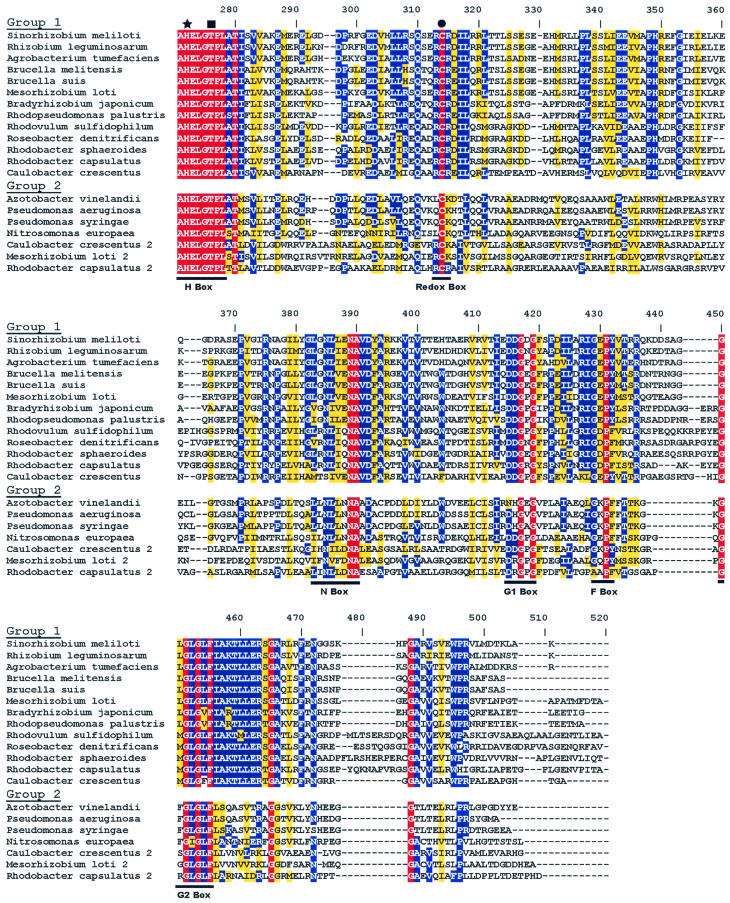
The R. capsulatus regB gene encodes a 460-amino-acid (50.1-kDa) protein that is composed of two domains; a N-terminal transmembrane domain and a C-terminal cytoplasmic “transmitter” domain that typifies histidine protein kinases (Fig. 2). The presence of six hydrophobic membrane-spanning regions in the N terminus was predicted by hydrophobicity profile topological modeling and confirmed by alkaline phosphatase fusions to both R. capsulatus and R. sphaeroides RegB homologues (11, 68). Regions of conservation in the transmitter domain include the H-box of the dimerization domain, containing the conserved site of autophosphorylation (His225), and the N, G1, F, and G2 boxes, which define the nucleotide-binding cleft (11, 30, 55, 68, 82).
Whereas the transmitter domain constitutes the catalytic phosphorylation domain, the role of the amino-terminal transmembrane domain is still under investigation. Insertion of RegB into the membrane has been suggested to enhance the dimerization of the protein and/or be important for regulation of the catalytic activities of RegB (41). Comparisons of in vitro properties of N-terminally truncated cytosolic and full-length proteins indicate that the transmembrane domain may play a regulatory role (74). Furthermore, a mutant with a mutation in the R. sphaeroides RegB homologue in the cytoplasmic loop between the second and third membrane-spanning domains (leucine 78 to proline) exhibits constitutive (oxygen-insensitive) kinase activity in vivo (30). There is also a significant region of conservation located in the second periplasmic loop between the third and fourth transmembrane domain of RegB that could constitute a potential redox-sensing component of this protein.
Kinase Activity
Initial kinase assays demonstrated that a His-tagged cytosolic domain of R. capsulatus RegB was capable of autophosphorylation in vitro as well as phosphotransfer to its cognate response regulator, RegA. The rate of autophosphorylation was initially reported to be low, with half-maximal phosphorylation observed after 45 min of incubation with [γ-32P]ATP (7, 41). Since the kinetics were not affected by the ATP concentration, it was suggested that the rate-limiting step of RegB autophosphorylation was phosphotransfer from bound ATP to the histidine residue or the dimerization of the protein, rather than binding of ATP (7). Recent results from Swem et al. (84) demonstrate that a non-His-tagged (truncated) version of RegB exhibits a significantly higher autophosphorylation rate, reaching half-maximal phosphorylation within 5 min. Similar results were seen with truncated cytosolic forms of RegB homologues from R. sphaeroides (14) and B. japonicum (26), as well as with a recently purified full-length version from R. sphaeroides (74). Phosphorylated full-length RegB exhibits decreased the stability of the phosphate compared to the truncated version of RegB (half-lives of about 34 min and 5.5 to 6 h, respectively), pointing to a role of the transmembrane domain in regulating the phosphorylation state of RegB (14, 74). Since mutations in the N-terminal transmembrane domain led to RegB proteins with constitutive kinase activity in vivo, the “unsignaled” state of the protein has been proposed to be “autophosphorylation dominant” (30, 67).
The first demonstration of phosphotransfer from R. capsulatus RegB∼P to RegA was reported by Inoue et al. (41). Phosphotransfer studies showed that the transfer of phosphate from the cytosolic domain of RegB to RegA in vitro is rapid (<1 min) (7). Similar phosphotransfer kinetics have been reported by investigators using truncated cytosolic and full-length versions of R. sphaeroides RegB homologues. No back-transfer of phosphate from RegA∼P to RegB has been observed (14, 74). A soluble truncated form of the RegB homologue (RegSC) in B. japonicum was shown to autophosphorylate at residue His219 in the presence of [γ-32P]ATP and to transfer the phosphate to the Asp63 residue of the RegA homologue (RegR) (26). Since phosphotransfer is very rapid, autophosphorylation of RegB appears to be the rate-limiting step in the phosphorylation of RegA.
Phosphatase Activity
Like many histidine kinases, RegB can modulate the level of phosphorylated RegA, not only through phosphorylation but also by exerting phosphatase activity on RegA∼P. Dephosphorylation of RegA∼P in vitro is dependent on the amount of unphosphorylated RegB, which is a good indication that RegB possesses phosphatase activity toward RegA (7). Similar results were observed for the B. japonicum RegB homologue (RegS) (26) and for the full-length version of RegB from R. sphaeroides (74). Phosphatase activity has also been characterized for a truncated soluble form of R. sphaeroides RegB by measuring the stability of the phosphate on RegA∼P in the presence and absence of RegB. Results showed that the presence of RegB resulted in a >16-fold reduction in the stability of the phosphate on RegA∼P (14).
Because both truncated and full-length RegB exhibit the same phosphatase activity, it is assumed that modulation of phosphatase activity does not require the N-terminal domain of RegB (74). However, further studies are required to determine if phosphatase activity is redox regulated.
In the E. coli sensor kinase EnvZ, it is apparent that a threonine residue positioned 4 residues downstream of the conserved phosphorylated histidine is important for phosphatase activity (42). This threonine residue is positioned on the same α-helical face directly below the phosphorylated histidine. It is thought that the histidine residue may deprotonate the threonine hydroxyl group to form a good nucleophile that attacks the phosphoryl group bound to the aspartate residue of the cognate response regulator. Interestingly, RegB homologues also contain a 100% conserved threonine residue located 4 residues downstream of the phosphorylated histidine (Fig. 2), suggesting that RegB may exhibit a similar phosphatase mechanism with RegA. Nonetheless, Potter et al. (74) point out that a significant difference between RegB and EnvZ phosphatase activity exists, given that EnvZ has a requirement for ATP or a nonhydrolyzable analogue as a cofactor for phosphatase activity while RegB lacks this nucleotide requirement.
Redox-Sensing Capabilities
The RegB/RegA system was originally described as a two-component regulatory system that was required for anaerobic activation of photosynthetic gene expression (55, 78). For some time, it has been presumed that RegB kinase activity is directly inhibited by oxygen. Although this may be true to some degree, it is also clear that RegB is capable of responding to additional redox signals beyond the simple presence or absence of oxygen. For example, R. capsulatus is fully capable of derepressing pigment biosynthesis under chemiautotrophic growth conditions involving growth in the presence of oxygen, hydrogen, and carbon dioxide (51). This clearly indicates that RegB is not directly affected by oxygen and, instead, may simply respond to the redox state of the cell.
In vivo redox control.
One redox signal that has been proposed to regulate RegB is the redox state of the aerobic respiratory chain (29, 31, 43, 60, 64, 76). This conclusion is based on the observation that mutations that disrupt R. sphaeroides and R. capsulatus cytochrome cbb3 oxidase lead to elevated aerobic expression of RegB/RegA-regulated genes (9, 29). It has therefore been suggested that cytochrome cbb3 oxidase generates an “inhibitory” signal that represses the RegB/RegA two-component system. This signal may either inhibit the kinase activity or stimulate the phosphatase activity of RegB, which, in turn, controls the amount of phosphorylated RegA (60, 63). It has also been proposed that electron flow through the cytochrome cbb3 oxidase could be monitored by CcoQ, the smallest subunit of cbb3 oxidase that is thought to stabilize the CcoP subunit from proteolytic degradation under aerobic conditions (66). In this model, CcoQ would function as a “transponder,” relaying an inhibitory signal to RegB (63). This model, however, must be viewed with caution since it has also been reported that there is no effect of disrupting the ccoNOQP genes (cytochrome cbb3 oxidase) on RegA-dependent expression of the two cbb promoters in R. capsulatus (33). The same investigators also observed no effect on cytochrome cbb expression after disruption of the ccoQ gene in R. sphaeroides (33). Clearly, additional experimentation is needed to sort out the role of cytochrome cbb3 oxidase, if any, on controlling the activity of RegB.
Genetic studies also implicate SenC (also called PrrC) in the transduction of a “redox signal” in R. sphaeroides and in R. capsulatus. Specifically, inactivation of senC (prrC), which is cotranscribed with regA, results in an oxygen-insensitive phenotype in R. sphaeroides (29, 31, 43, 60, 64, 76). The senC gene encodes a 23.2-kDa membrane-spanning protein containing a highly conserved motif, CPDVCP, with the cysteine residues thought to be involved in copper binding (54). Interestingly, SenC also has sequence similarity to a family of oxidoreductases that are involved in disulfide bond oxidation and reduction (54). One possibility is that SenC could be directly involved in modulation of the oxidation and reduction of a redox-active cysteine residue within RegB (84). Furthermore, eukaryotic homologues of SenC (called ScoI) are thought to be associated with cytochrome c oxidase assembly (35), so it has also been proposed that a redox signal could be passed from cytochrome cbb3 oxidase to RegB via SenC (64).
It is also interesting that RegA controls expression of the regB gene, the senC-regA-hvrA operon, and the ccoNOQP operon that codes for cytochrome cbb3 oxidase (19, 83). Hence, not only does the RegB/RegA system down-regulate its own synthesis, but it also controls transcription of components that may modulate its own activity.
In vitro rcdox control.
Initially, the in vitro kinase activity of cytosolic truncated RegB was shown to be unaffected by redox changes (7, 14, 41). It was therefore assumed that the redox-sensing ability of RegB lay within its transmembrane-spanning region. Although there may indeed be a role for the membrane-spanning region in redox sensing in vivo, recent analysis indicates that the cytosolic domain of RegB also plays a critical role in controlling the autophosphorylation activity of the kinase. An advance in our molecular understanding of the control of RegB activity occurred when Swem et al. (83, 84) demonstrated that RegB requires the coordination of a metal, such as copper, to respond to redox. This crucial component was not observed previously because of the addition of the metal chelator, EDTA, to the protein purification mixtures. Additionally, it was demonstrated that RegB contains a redox-responsive Cys (Cys265) located in the dimerization interface, more precisely in the “redox box” (Fig. 2), which seems to be involved in the redox-sensing mechanism of RegB (84). It is proposed that this Cys residue undergoes modification such as oxygen-dependent formation of a sulfenic acid (Cys-S-OH) and/or disulfide bond formation in vivo that inhibits kinase activity. In vitro disulfide bond formation was shown to require the presence of a divalent metal ion, with ligand coordination of the metal playing a structural role necessary to align RegB monomers into a conformation allowing the redox-active Cys residue to function properly (84).
A copper requirement for RegB in vivo is also supported by the observation that mutations in the hypothetical copper ATPase-type transporter protein, RdxI, and a copper oxidase, RdxB (CcoG), which is involved in intracellular oxidation of Cu+ to Cu2+ (47), lead to constitutive RegB autophosphorylation as assayed by measuring the elevated aerobic expression of genes under control of the RegB/RegA system (61, 76). The involvement of an intermolecular disulfide bridge in the control of RegB activity is also supported by an increase in in vitro RegB phosphorylation in the presence of dithiothreitol (74).
In vitro analysis indicates that an intermolecular disulfide bond forms between RegB dimers, resulting in a tetramer when oxidized and shutting off kinase activity (84). However, the redox state of this cysteine in vivo has not been elucidated. Nonetheless, substitution of alanine for this cysteine leads to significantly elevated aerobic expression of RegB/RegA-targeted genes in vivo, indicating its importance for redox sensing.
Other input signals.
Although the redox-responding properties of the RegB/RegA system are well established, a recent study by Abada et al. (1) has revealed that the R. capsulatus system may also respond to other stimuli, specifically a flow of intermediates through the bacteriochlorophyll (Bchl) biosynthetic pathway. Their study demonstrated that synthesis of the pigment-binding proteins encoded by the RegB/RegA-regulated puf and puc operons is reduced when Bchl biosynthesis is defective. They further demonstrated that Bchl-dependent expression of puf and puc depends on the RegB/RegA system, since disruption of either gene affects Bchl-dependent transcription. Interestingly, a regA mutant does not sense the absence of Bchl, whereas a regB mutant does. One possibility is that RegA directly or indirectly senses the amounts of photopigments being synthesized through its receiver domain.
RESPONSE REGULATOR RegA
R. capsulatus RegA is a 184-amino-acid 20.4-kDa protein containing several highly conserved residues that are typically found in two-component response regulators. Conserved residues include a phosphate-accepting aspartate and an “acid pocket” containing two highly conserved aspartate residues in the N-terminal receiver domain (Fig. 1). The receiver domain is linked by a four-proline hinge to a 50-amino-acid C-terminal output domain that contains a three-helix bundle HTH DNA-binding motif (18, 49, 78). As discussed above, RegA homologues are highly conserved among numerous α-proteobacterial species, with an unprecedented complete conservation of the DNA-binding domain.
Effect of Phosphorylation
DNA-binding activity of RegA was initially demonstrated by using a constitutively active variant of RegA called RegA* (18). RegA* was isolated in a RegB-null mutant strain by selecting for a suppressor mutation that exhibited elevated photosynthesis gene expression in the absence of RegB. Analysis of RegA* revealed that this form of RegA was capable of binding DNA and activating transcription independently of phosphorylation (7, 8, 18). Sequence analysis of RegA* revealed a mutation at amino acid 95 that substitutes serine for a conserved alanine. It is hypothesized that this mutation alters the conformation of RegA such that it mimics the phosphorylated state of the wild-type protein (7). Interestingly, Asp63 of RegA* is still capable of accepting a phosphate from RegB, with the phosphoryl group on RegA* being much more stable than the phosphoryl group on wild-type RegA (7). As a consequence of a shift in equilibrium between the phosphorylated and unphosphorylated states of the proteins, RegA* is capable of being phosphorylated to a greater extent in vitro than is wild-type RegA.
The inherent stability of the aspartyl-phosphate among the RegA homologues, as a measure of half-life, is high relative to that observed with other response regulators. The half-life was about 90 min for R. capsulatus RegA∼P (7), about 162 min for the RegA homologue (RegR∼P) from B. japonicum (26), and 330 min for R. sphaeroides RegA∼P (14). RegA from R. sphaeroides has also been shown to use the small phosphodonor acetyl∼P in vitro (14). Autophosphorylation of R. capsulatus RegA in the presence of acetyl∼P was also reported by Hemschemeir et al. (39).
DNase I footprint analysis of RegA binding to the puc promoter region revealed that phosphorylated and unphosphorylated wild-type RegA, and RegA* protect identical regions with different affinities. This observation indicates that phosphorylation does not affect the points of protein-DNA interaction but, rather, affects the affinity for the binding site (7). Indeed, phosphorylation is reported to increase the DNA-binding affinity of RegA by at least 16-fold. Interestingly, unphosphorylated RegA* exhibits DNA-binding activity comparable to that of phosphorylated wild type, supporting in vivo evidence that RegA* is able to promote transcription activation even in the absence of phosphotransfer from RegB. A further six-fold increase in the DNA-binding activity of RegA* is achieved upon phosphorylation (7). These results are consistent with what was reported for the B. japonicum RegA homologue (RegR), whose DNA-binding activity is increased by at least eightfold on phosphorylation (26). Phosphorylation-induced stimulation of DNA-binding activity was also reported for P. aeruginosa RoxR (15). However, Hemschemeier et al. (40) have reported the same affinity (KD, 50 to 70 nM) for phosphorylated and unphosphorylated RegA when using the puc promoter as a probe in vitro. The reason for this discrepancy is not clear. In addition, they showed that deletion of the last 13 amino acids, which contain the DNA-binding motif of RegA, inhibited DNA-binding ability in vitro as well as the activation of puc and puf expression in vivo. This confirms the importance of DNA binding for the transcriptional activation activity of RegA.
Analysis of mutations at the site of phosphorylation (Asp63) in R. capsulatus RegA, as well as with RegA homologues from B. japonicum and R. sphaeroides, has been performed. A D63N mutation in RegR of B. japonicum rendered the protein unable to be phosphorylated and also unable to bind DNA, as demonstrated by gel retardation experiments with a fixR-nifA promoter probe (26). In contrast, experiments involving a D63K RegA mutant from R. capsulatus showed that the mutant protein was capable of binding DNA, despite an inability to be phosphorylated. This indicated that phosphorylation might not affect the DNA-binding ability but, rather, might facilitate a conformational change allowing appropriate interactions of RegA with RNA polymerase (38). This theory was further supported by in vitro transcription assays by Comolli et al. (14), involving wild-type PrrA and a D63A mutant of PrrA from R. sphaeroides. Their studies revealed that both unphosphorylated and phosphorylated wild-type PrrA are able to activate the transcription of cycA P2, with phosphorylated PrrA exhibiting greater activity than unphosphorylated PrrA. Interestingly, the D63A form of PrrA was unable to activate any detectable amounts of transcription. These data suggest that phosphorylation of Asp63 and the mere presence of Asp63 may affect several steps of activation such as the DNA binding of RegA and the interaction of RegA∼P with RNA polymerase.
Superimposed on the effect of phosphorylation are numerous in vivo observations that unphosphorylated RegA is also capable of affecting transcription. Specifically, mutational analysis indicates that phosphorylated RegA functions as an anaerobic repressor of cytochrome cbb3 oxidase expression while unphosphorylated RegA functions as an aerobic activator of cytochrome cbb3 oxidase (83, 85). In addition, both phosphorylated and unphosphorylated RegA are involved in activation of ubiquinol oxidase expression (85), activation of cbb operon expression (75), and repression of hupSLC expression (23). Additional DNA-binding studies with both phosphorylated and unphosphorylated RegA are clearly needed to obtain an understanding of the mechanism of activation or repression by unphosphorylated RegA.
DNA-Binding Sites
DNase I footprint analysis initially demonstrated that purified wild-type RegA and RegA* bind to identical specific sites in the puf and puc promoters (18, 40). Subsequent DNase I footprint analysis demonstrated that RegA also binds to a plethora of other operons under the direct regulation of the RegB/RegA system, including nifA2, hupSLC (23), regB, senC-regA- hvrA (19), petABC, cycA, cycY, cydAB, ccoNOPQ (83), cbbI and cbbII (93), and cheOp2 (77). DNA-binding activity has also been reported for the phosphorylated forms of RegR in B. japonicum (26) and RoxR in P. aeruginosa (14) by using gel mobility retardation experiments.
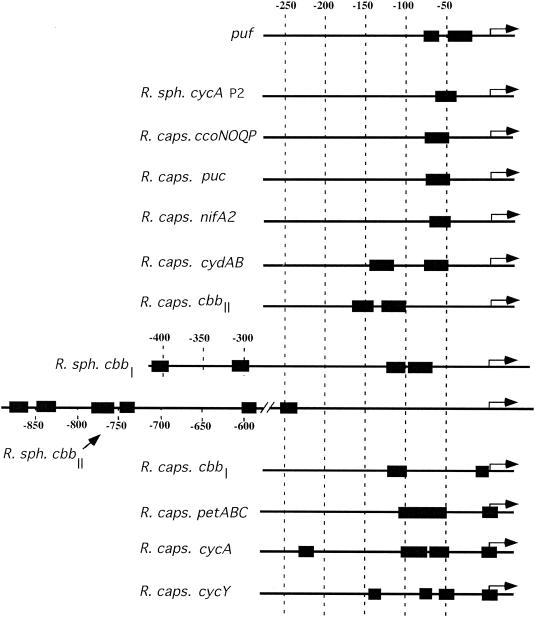
The number of RegA DNA-binding sites on target promoters ranges between 1 and 6, based on DNase I protection assays (Fig. 3). Individual sites in the promoter regions have different affinities, as determined by the amount of RegA needed to obtain half-maximal protection of individual sites (83). This finding leads to a classification of RegA-regulated promoters into three types based on the location of the RegA-binding sites. The first type, exemplified by the R. capsulatus puf promoter, has RegA DNA-binding sites that extend from bp −22 to −80 relative to the start site of transcription (Fig. 3). The second type, comprising the R. capsulatus puc, nifA2, cydAB, ccoNOQP, and cbbII promoters and the R. sphaeroides cbbI, cbbII, and cycA promoters, possesses several DNA-binding sites that extend from approximately bp −50 to −890 upstream from the transcriptional start site. The third type, represented by the R. capsulatus cbbI, petABC, cycA, and cycY promoters, contains one site overlapping the start site of transcription (from bp −19 to +22) as well as DNA-binding sites from bp −50 to −225. Although it is possible that additional DNA-binding sites have been left unrevealed, these different binding locations may allow RegA to interact with RNA polymerase in more than one manner at these promoters. This is especially true in comparisons of promoters that have RegA DNA-binding sites at the region from bp +20 to −50 with promoters that have binding sites just in the region from bp −300 to −890. For example, RegA binding to the puc promoter is centered at around −60, which presumably favors the interaction of RegA with the α subunit of RNA polymerase (18). In contrast, RegA binds to the region of the puf promoter from bp −22 to −52 (18), which presumably favors interaction with the σ subunit of the RNA polymerase. There is also RegA binding to the region of the puf promoter from bp −68 to −80, which would favor an additional interaction with the α subunit of the RNA polymerase. These interactions should overcome the presence of poor σ70 recognition sequences harbored by the puf promoter, which uses the σ70 housekeeping RNA polymerase (8). Clearly, additional detailed analyses of the mechanism of RegA activation and repression at these different classes of promoters is warranted, and indeed needed, to better understand how RegA interacts with RNA polymerase and controls gene expression.
FIG. 3.
Location of RegA-binding sites (black boxes) that have been located by DNase I footprint protection analysis. The location of transcription initiation is indicated by an arrow. R. sph., R. sphaeroides; R. caps., R. capsulatus.
Initially it was hypothesized that RegA recognizes structural features of the DNA rather than a specific nucleotide sequence for DNA binding. Lack of sequence specificity was proposed as a result of the failure of early DNA-binding studies to recognize a consensus sequence that had a limited number of protection sites for analysis (18). However, the alignment of 21 RegA-binding sites from R. capsulatus and R. sphaeroides revealed that RegA indeed binds to a consensus sequence of 5′-G(C/T)G(G/C)(G/C)(G/A)NN(T/A)(T/A)NNC(G/A)C-3′ (83). An in vitro method was also used to determine a DNA-binding consensus sequence for RegR from B. japonicum (28). The selection of RegR-interacting oligonucleotides from a randomized oligonucleotide pool (SELEX), coupled with site-directed mutagenesis of known RegR DNA-binding sites on the fixR nifA promoter, revealed a “RegR box,” 5′-GNG(A/G)C(A/G)TTNNGNCGC-3′ that contains an imperfect inverted repeat with 4 nucleotides per half-site. The 5-bp spacing sequence was shown to also contain critical nucleotides for RegR DNA-binding activity. The RegR box is similar to the consensus sequence obtained from R. capsulatus RegA-protected promoter sequences, which is not surprising given that there is total identity of the putative HTH DNA-binding motifs between RegA and RegR (53).
A nuclear magnetic resonance (NMR) spectral structure was recently solved for the DNA-binding output domain of RegA bound to DNA (49). This structure demonstrated that the RegA DNA-binding domain is composed of a three-helix bundle encompassing an HTH motif (49). The three helices within the three-helix bundle are labeled in Fig. 1 as α6, α7, and α8, with α7 and α8 comprising the predicted DNA-binding HTH motif. This structure is similar to the three-helix bundle found in the E. coli Fis family of DNA-binding proteins (49). This DNA-binding effector domain of RegA is more structurally similar to Fis than to the effector domain of the response regulator, NtrC (49). The NMR structure has also provided new insights into the DNA recognition and DNA-binding ability of RegA. As demonstrated previously by DNase I protection assays and SELEX (systemic evolution of ligands by exponential enrichment) RegA has a distinct affinity for a GCG…CGC palindromic sequence (28, 83). The NMR structure of a RegA-DNA cocomplex has confirmed that the RegA consensus recognition sequence consists of YGCGRCRxTATAxGNCGC (with x denoting a variable number of bases) (49). Surprisingly, the GCG inverted repeat can be separated by a 3- to 9-nucleotide AT-rich spacer region (49). This AT-rich spacer region could presumably allow for DNA bending, which aids RegA recognition and binding (49). The NMR structure clearly identifies the α8 helix and the DNA recognition helix with this helix binding specifically to the GCG inverted repeat sequence within the major DNA groove. The α6 helix appears to undergo nonspecific interactions with the DNA phosphate backbone (49). These data provide a very revealing picture of how RegA recognizes and binds to the numerous promoters under RegB/RegA transcriptional control.
Transcription Regulation
In vitro transcription assays demonstrated that housekeeping σ70-RNA polymerase in the presence of purified RegA* can activate transcription from puc and puf promoters (8). Unphosphorylated RegA* was shown to stimulate puc expression fourfold over basal-level expression observed in the absence of RegA. Phosphorylation of RegA* by RegB∼P leads to a further 12- to 60-fold increase in transcription, depending on the RNA polymerase concentration (8). This result is surprising, given that RegA* is fully active in vivo in the absence of RegB. It was also observed that only the major RegA-binding site was required for RegA-dependent in vitro transcription, even though the puc promoter has both major and minor DNA-binding sites for RegA (7, 18).
As detailed in the following section, it is a common occurrence that other transcription factors regulate operons found within the RegB/RegA regulon. For instance, RegA regulates the transcription of photosystem promoters in concert with the histone-like proteins, integration host factor (IHF), and HvrA (10, 46, 58), as well as with CrtJ (8, 24, 72, 73) and AerR (17) (details of these additional transcription factors are covered in references 3 through 5). The occurrence of additional regulators of genes that are also controlled by RegA is common. Thus, RegA constitutes a global regulator that provides an overarching layer of redox control on operons that have additional transcription factors that respond to photosynthesis, respiratory electron transport, organic carbon, fixed nitrogen, hydrogen, etc.
Reg REGULON
Photosynthesis
The RegB/RegA system was discovered by selecting for mutations that exhibited reduced synthesis of the photosystem ini R. capsulatus (55, 78). An intact copy of regA was shown to be absolutely required for R. capsulatus to grow photosynthetically under dim light, which is a growth condition that requires maximum transcription of the puh, puf, and puc operons, which code for apoproteins of the light-harvesting and reaction center complexes. As is the case for RegA, RegB is also necessary for anaerobic induction of the puc, puf, and puh operons (55). Mutations in the RegB and RegA homologues from R. sphaeroides show similar effects with respect to the control of puh, puf, and puc expression, with the difference that RegA is indispensable for photosynthetic growth under all light intensities (29).
As is the case for many RegB/RegA-regulated promoters, numerous other transcription factors have been found to control puf, puh, and puc operon expression in R. capsulatus (3, 4). DNase I footprint assays showed that CrtJ and RegA compete for binding to overlapping sites in the puc promoter (8). An additional aerobic repressor called AerR also represses the puf operon; the site of AerR repression has not yet been determined (17). In addition to CrtJ and AerR, the H-NS-like protein HvrA regulates the puf and puh promoters, where it functions as an activator in R. capsulatus and as a repressor in R. sphaeroides (10, 59, 80). The location of HvrA binding in R. sphaeroides is unknown, but in R. capsulatus, the HvrA DNA-binding site is adjacent to RegA DNA-binding sites (48). In both R. capsulatus and R. sphaeroides, the histone-like protein IHF is required for maximal puc operon transcription (58, 96). FnrL in R. sphaeroides also anaerobically activates puc expression, whereas inactivation of fnrL in R. capsulatus has no noticeable affect on photosynthetic growth (reviewed in reference 96). Thus, there are numerous potential protein-protein interactions that might exist between RegA and a number of other transcription factors that control these photosystem promoters. Currently, little is known about the nature of these interactions or their importance in controlling photosystem gene expression.
In addition to controlling the synthesis of light-harvesting and reaction center apoproteins, RegA affects the expression of the bchE promoter in R. sphaeroides, which encodes an enzyme in the bacteriochlorophyll biosynthesis pathway (62). There are also reports that RegA from R. sphaeroides controls the expression of hemA, hemZ, and hemN (62, 65), which encode one δ-aminolevulinic acid (ALA) synthase isoenzyme and two coproporphyrinogen III oxidases, respectively: enzymes involved in the Mg- and Fe-tetrapyrrole biosynthetic pathways. Abada et al. (1) have also reported an involvement of the R. capsulatus RegB/RegA system in bacteriochlorophyll-dependent expression of the puc, puf, and bchC operons. Therefore, not only does RegB/RegA control the synthesis of the photosystem and cytochrome apoproteins (the role of RegB/RegA in cytochrome biogenesis is discussed below), but also they control synthesis of bacteriochlorophyll and heme that are bound by these respective apoproteins.
Electron Transfer System
An important discovery was made in 1994 that hinted at the global nature of the RegB/RegA signal transduction cascade. The RegA homologue from R. sphaeroides (PrrA) was found to positively regulate the expression of cycA, which encodes cytochrome c2 (29). Cytochrome c2 is an important element of the electron transfer system shared by several energy-generating pathways including those of photosynthesis and respiration. Primer extension and in vitro transcription studies have indicated that PrrA positively controls the P2 promoter, which is one of three promoters driving the expression of cycA under both aerobic and anaerobic conditions. Expression from P2 is responsible for basal expression under aerobiosis as well as for induction under anaerobic conditions (45). Evidence that RegA directly controls cycA expression was also provided by DNase I protection assays, which showed that RegA* from R. capsulatus binds to a region of P2 centered at bp −50 from the start site of transcription (45). Furthermore, in vitro transcription assays confirmed that R. sphaeroides PrrA directly activates cycA transcription (14).
Swem et al. (83) demonstrated that RegB/RegA controls synthesis of cytochrome c2 as well as cytochrome cy and the cytochrome bc1 complex in R. capsulatus. The expression patterns of these different cytochrome genes were compared in wild-type and regA-disrupted strains, which revealed that RegA activates the biosynthesis of cytochromes bc1 and c2 under anaerobic, semiaerobic, and aerobic growth conditions, whereas it activates cytochrome cy only under semiaerobic and anaerobic conditions. DNase I protection assays also demonstrated that RegA binds to two sites on the promoter of the pet (bc1) operon and to four sites on the promoters of the cycA and cycY genes encoding cytochrome c2 and cytochrome cy, respectively (83) (Fig. 3). These three promoters belong to the type III class of RegA-regulated promoters (discussed above), where there is one DNA-binding site that overlaps the start site of transcription and one site located just upstream from the −35 promoter region. The in vivo involvement of these different RegA-binding sites still needs to be determined.
Differences in expression patterns of these various cytochromes suggest that additional transcription factors may also regulate the expression of these genes. For example, evidence suggests the involvement of another protein besides RegA, that regulates cycA expression in the absence of O2 in R. sphaeroides (45). There is also a response regulator and a putative repressor located just upstream of the pet operon, which is suspected to be involved in controlling the expression of cytochrome bc1 apoproteins (91). Thus, as with other cellular processes, RegB/RegA appears to be just one component of a more complex regulatory network that controls the biosynthesis of cytochrome apoproteins.
Aerobic Respiration
Like many bacterial species, R. capsulatus possesses a branched respiratory chain involving two different terminal oxidases. In one branch, the ubiquinol (ubihydroquinone) oxidase takes electrons directly from the quinone pool to reduce O2 to H2O. The second branch, which is similar to the mitochondrial electron transfer chain, is composed of the cytochrome bc1 complex, cytochromes c2 or cy, and a cbb3-type cytochrome c oxidase (37). Cytochromes cy, c2, and bc1 are also involved in photosynthetic electron transfer events, shuttling electrons from cytochrome bc1 back to the photosynthetic reaction center either by the membrane-associated cytochrome cy or by the soluble cytochrome c2 (37).
Both of the terminal oxidases are maximally synthesized under semiaerobic growth conditions. However, the two oxidases are differentially regulated in regards to high and no (or very low) oxygen levels, with the ubiquinol oxidase exhibiting a low level of expression under aerobic conditions and a higher level under anaerobic conditions. The converse is true for cytochrome cbb3 oxidase, which exhibits higher expression under aerobic than anaerobic growth conditions (83, 85). This expression pattern indicates that cytochrome cbb3 oxidase from R. capsulatus may have a lower affinity for oxygen than does the ubiquinol oxidase (87, 95).
RegA has been observed to activate cytochrome cbb3 oxidase expression semiaerobically and aerobically while repressing expression anaerobically. The mechanism and the significance of this observation are not yet well understood (83). DNase I footprint analysis revealed that RegA directly controls the synthesis of cytochrome cbb3 oxidase by binding to a site on the ccoNOQP promoter located just upstream from the −35 sequence (Fig. 3) (83). Although there was an early report that FnrL does not affect cytochrome cbb3 oxidase synthesis in R. capsulatus (96), more recent analysis indicates that expression of ccoNOPQ is indeed lower in an fnrL-disrupted mutant under semiaerobic and anaerobic conditions (85).
The expression pattern of the cydAB operon, encoding ubiquinol oxidase, suggests that the enzyme has a higher affinity for oxygen than does cytochrome cbb3 oxidase (83, 85). As observed for the ccoNOQP operon, RegB/RegA is involved in the regulation of cydAB expression, with RegA being required for activation of cydAB transcription under all growth conditions tested. DNase footprint assays indicate that RegA binds to two sites upstream from the −35 region of the promoter (Fig. 3) (83).
Recently, RegB/RegA homologues from P. aeruginosa (RoxS/RoxR) were reported to be involved in the control of aerobic respiration (15). More precisely, RoxS/RoxR controls the induction of the cyanide-insensitive oxidase in the presence of cyanide. It is proposed that RoxR coregulates the cioAB promoter with another anaerobic regulator, ANR, thereby permitting the integration of different stimuli in the control of cyanide-insensitive oxidase expression.
Anaerobic Respiration
R. capsulatus and R. sphaeroides are both capable of anaerobic respiration using dimethyl sulfoxide (DMSO) as a terminal electron acceptor (95). The reduction of DMSO is catalyzed by a membrane-bound DMSO reductase enzyme that is encoded by the dorCDA operon. The dor operon is under the transcriptional control of a two-component signal transduction system, DorS/DorR, that responds to the availability of DMSO (56, 57, 79). The sensor kinase, DorS, is known to autophosphorylate in the presence of DMSO, with the phosphate transferred to the response regulator, DorR, which then activates dorCDA expression. The dorCDA operon is known to also be under the control of the RegB/RegA system, with RegA acting as a repressor of the dorCDA operon during photoheterotrophic growth in the presence of malate as a carbon source (44). However, RegA seems to lose control of the dorCDA operon if the cells are grown on pyruvate rather than malate. This indicates that another, unidentified, regulator can suppress the regA mutant phenotype in cells grown on pyruvate but not in cells grown on malate. It is not yet known if the effect of RegA on the dorCDA operon is direct or indirect. However, since no obvious RegA DNA-binding sequence has been found in the dorCDA promoter, the influence of RegA on dorCDA expression may be indirect. Nonetheless, this appears to be another instance in which the RegB/RegA system exerts transcriptional control over a system that is responsible for energy generation.
Carbon Fixation
The Calvin-Benson-Bassham reductive pentose phosphate pathway allows the production of organic carbon via the assimilation of CO2. Carbon fixation also plays an important role under photoheterotrophic growth conditions, where it acts as an electron sink that is needed to balance the redox potential of the cell. Consequently, mutants of R. capsulatus and R. sphaeroides that are devoid of a functional Calvin cycle do not grow photoheterotrophically unless exogenous electron acceptors such as DMSO are provided (reviewed in references 86 and 89 and references therein).
Enzymes of the Calvin cycle are encoded by the cbbI and cbbII operons. Transcription of these operons is regulated in response to carbon by the transcriptional activator CbbR, which is a member of the LysR family of transcription factors. CbbR is absolutely required for expression of the cbbI operon, since inactivation of the R. sphaeroides cbbR gene leads to the absence of transcription through the cbb1 operon and to a strong reduction of cbbII expression (34). CbbR directly regulates cbbI expression by binding to two sites located in the promoter-proximal region, as demonstrated by in vitro DNase I footprint experiments (20, 21).
An involvement of the RegB/RegA system in the biosynthesis of Calvin cycle enzymes was first discovered in R. sphaeroides by screening for mutants that derepress an alternative CO2 fixation pathway (75). From studies of these mutants, it was demonstrated that RegB (PrrB) of R. sphaeroides was required for positive regulation of the cbb operons, both anaerobically in the light and aerobically in the dark (75). Using purified R. capsulatus RegA*, Dubbs et al. (20, 22) demonstrated that RegA directly controls R. sphaeroides cbb expression by binding to four sites in the cbbI promoter and to six sites on the cbbII promoter (Fig. 3). The authors hypothesized that the locations of RegA binding could allow direct interactions with CbbR and/or with RNA polymerase. Furthermore, binding of RegA to the two sites located in the upstream activating sequence in the cbbI promoter appears responsible for a RegA-mediated 41-fold enhancement in cbbI expression (20).
Gibson et al. (33) demonstrated that chemoautotrophically grown regA (prrA) mutants of R. sphaeroides differentially express the two cbb operons with expression of the cbbII promoter being severely reduced and expression of the cbbI promoter being enhanced in the prrA mutant strain. This result indicates that PrrA functions as an activator of cbbII and a repressor of cbbI. Analysis of promoter mutants suggests that RegA may bind to distinct regions in cbbII and in cbbI during photoautotrophic and chemoautotrophic growth.
In R. capsulatus, the RegB/RegA system also controls the expression of the two cbb operons that are present in this species (93). The cbbI and cbbII operons are regulated by cognate CbbR proteins encoded by the cbbRI and cbbRII genes. The cbbRI and cbbRII genes are located upstream of, and are divergently transcribed from, the cbbI and cbbII operons, respectively. The CbbRI protein is able to control its own expression (down regulation) as well as cbbII expression under certain conditions (69, 93). On both the cbbI and cbbII operon promoters, CbbR binds to a site that overlaps the −35 region, which suggests that protein-protein interactions between CbbR and the RNA polymerase are required for transcriptional activation. Inactivation of regA and regB affects cbbI and cbbII expression, with only 14 and 10% of wild-type levels, respectively, found in a regA-disrupted strain under photoautotrophic growth conditions. RegA* was also shown to bind to two DNA-binding sites in both the cbbI and cbbII promoter regions. There is a major high-affinity RegA binding site located at bp −102 to −121, upstream of the cbbI transcription start site, that is assumed to be involved in transcriptional activation in concert with CbbRI. A low-affinity RegA binding site is located at positions −4 to −19, overlapping a CbbRI DNA-binding site located at positions −18 to −79. Presumably the low-affinity RegA binding site that overlaps the CbbR site plays a negative role as a result of RegA-mediated occlusion of CbbRI binding to this region. On the cbbII promoter, there are two high-affinity RegA-binding sites, one located at positions −101 to −116 and the second located at positions −124 to −169. The upstream location of these binding sites suggests that they are involved in activation. The cbbII promoter also contains a CbbRII DNA-binding site located at positions −19 to −78.
It has been reported that RegA homologues from B. japonicum (RegR) and S. meliloti (ActR) also function as activators of cbb operons in concert with CbbR (27, 32). Thus, as was demonstrated for R. capsulatus, RegA homologues appear to control CO2 fixation in a number of photosynthetic and nonphotosynthetic bacteria.
Nitrogen Fixation
Conditions of nitrogen and oxygen limitation are known to activate the expression of nif genes, which are required for the biosynthesis of molybdenum nitrogenase (reviewed in reference 52). Joshi and Tabita (43) made the surprising discovery that RegA (PrrA) from R. sphaeroides was also involved in the control of nitrogen fixation, underscoring the global role of the RegB/RegA system. Specifically, they observed that nitrogenase synthesis is derepressed in the presence of excess ammonium in strains that lacked a functional CO2 fixation pathway. They reasoned that CO2 fixation normally functions as an electron sink to dissipate excess reducing equivalents generated by photoheterotrophic growth. In the absence of CO2 fixation, they concluded that nitrogenase becomes derepressed to serve as an alternative secondary electron sink. Interestingly, a functional regB gene is required for derepression of nitrogenase in the absence of carbon fixation.
Elsen et al. (23) shed light on the mechanism of derepression of nitrogenase in R. capsulatus by showing that the RegB/RegA system indirectly controls expression of the nifHDK operon, which encodes the molybdenum-containing nitrogenase complex. In R. capsulatus and in many other species, nitrogenase expression is regulated by nitrogen limitation through the NtrB/NtrC two-component system. Under nitrogen-limiting conditions NtrB phosphorylates NtrC, which then activates nifA transcription by binding to two tandem sites centered >100 bp upstream of the transcriptional start site. NifA then activates the expression of numerous nif genes, including nifHKD (reviewed in reference 52). In R. capsulatus, there are two functional copies of nifA, nifA1 and nifA2, either of which can activate nifHDK expression. Elsen et al. (23) demonstrated that RegA binds to the nifA2 promoter between the tandem NtrC DNA-binding sites and the −35 and −10 promoter sequences. Interestingly, RegA-mediated activation of nifA2 transcription requires NtrC, indicating that RegA∼P alone is not sufficient to stimulate nifA2 expression (23). Thus, RegA appears to provide an overarching layer of redox control on top of the control of nitrogen availability provided by NtrC.
In B. japonicum, the RegB/RegA homologues (RegS/RegR) are required for the aerobic and anaerobic expression of the fixRnifA operon (6). Interestingly, a mutation that disrupts the response regulator RegR reduces fixR nifA expression and consequently nitrogen fixation activity. However, no related phenotype was observed on disruption of the sensor kinase, RegS. RegR mutants of B. japonicum form nodules, but the nodules are functionally incapable of fixing nitrogen (a Fix− phenotype). Electron micrographic analysis indicates that nodules formed on infection by the RegR-disrupted strain failed to produce bacteroids, which are differentiated B. japonicum cells that undertake nitrogen fixation (6).
Denitrification
Recently, the R. sphaeroides RegB/RegA system (PrrB/PrrA) was shown to control the expression of nitrite reductase, which is a terminal electron acceptor involved in denitrification (50). Specifically, RegB- and RegA-disrupted strains reduced the expression of the nitrite reductase structural gene, nirK, which resulted in an inability to grow anaerobically on nitrite-containing medium. nir expression is also regulated by nitrite availability through the transcription factor NnrR, which is a member of the FNR/Crp family. Thus, RegA presumably acts in concert with NnrR to coordinate nirK expression.
Hydrogen Oxidation
R. capsulatus possesses the hupSLC operon, which codes for a membrane-bound uptake [NiFe]hydrogenase that catalyzes H2 oxidation. This enzyme allows the bacterium to grow autotrophically with H2 as the sole electron source (reviewed in reference 94). Hydrogenase can also recycle electrons from H2 to nitrogenase under photoheterotrophic growth conditions. Biosynthesis of hydrogenase is regulated by growth conditions, with expression and activity being highest in the presence of its substrate, H2 (13).
H2 regulation is mediated by the two-component regulatory system HupT/HupR, with the response regulator HupR directly activating hupSLC transcription in the presence of H2 (16). The nonphosphorylated form, HupR, binds to the hupSLC promoter at a palindromic sequence centered at bp −157 with respect to the transcription start site. Maximal expression of hupSLC also requires the binding of IHF between the HupR and RNA polymerase DNA-binding sites (reference 92 and references therein).
Elsen et al. (23) demonstrated that RegA is involved in repressing hupSLC expression under both aerobic and anaerobic heterotrophic growth conditions. A major DNA-binding site of RegA was shown to be located close to the −35 promoter recognition sequence, with a second, lower-affinity RegA-binding site overlapping the IHF DNA-binding region. At that location, it is possible that RegA could prevent either the RNA polymerase or the IHF protein, or both, from binding to the hupSLC promoter. Interestingly, a deletion in RegB can be suppressed by addition of multiple copies of the sensor kinase HupT (36). Presumably, increased amounts of HupT are capable of phosphorylating RegA in the absence of RegB.
Dehydrogenases
Glutathione-dependent formaldehyde dehydrogenase plays an important role in the detoxification of formaldehyde by converting it to formate. Analysis of the expression of the glutathione-dependent formaldehyde dehydrogenase gene, adhI, demonstrated that adhI expression is under the control of several effectors that respond to formaldehyde, methanol, or other formaldehyde adducts (2). This enzyme is absolutely required for growth with carbon sources such as methanol, that generate formaldehyde. Formaldehyde oxidation creates reducing power in the form of NADH, thereby providing cellular energy as a product. Interestingly, RegA (PrrA) was shown to be essential for normal aerobic expression of the adhI gene in R. sphaeroides (2). Analysis of RegA binding to the adhI promoter has not been undertaken, so it is not yet certain whether RegA directly or indirectly affects the expression of formaldehyde dehydrogenase.
In S. meliloti, the RegB and RegA homologues, ActS and ActR, control the biosynthesis of three dehydrogenases, formaldehyde dehydrogenase, formate dehydrogenase, and methanol dehydrogenase, as well as CO2 fixation (32). The ActS/ActR system is also involved in acid tolerance (90).
Aerotaxis
Romagnoli et al. (77) reported that the aerotactic motility response of R. sphaeroides is partially under the control of the RegB/RegA system. Their study indicated that aerotaxis in R. sphaeroides involves the second chemosensory operon cheOp2, which is one of three che clusters present in R. sphaeroides. Deletion of cheOp2 genes results in complete loss of aerotaxis, while deletion of regB (prrB) results in partial loss of aerotaxis. Deletions of the three linked regulatory genes regB, regA, and senC (prrBCA) restores the aerotactic ability of a cheOp2 deletion (77). It is not well understood why a deletion of the sensor kinase gene, regB, results in a nonaerotactic phenotype whereas deletion of the entire reg operon allows aerotaxis.
It will take further analysis to determine the exact role of RegB and RegA in controlling aerotaxis, such as the ability of RegB to directly affect phosphorylation of the chemotaxis cascade or simply modulate the abundance of the chemosensory apparatus. Nevertheless, aerotactic control going through the RegB/RegA cascade is certainly novel.
CONCLUDING REMARKS
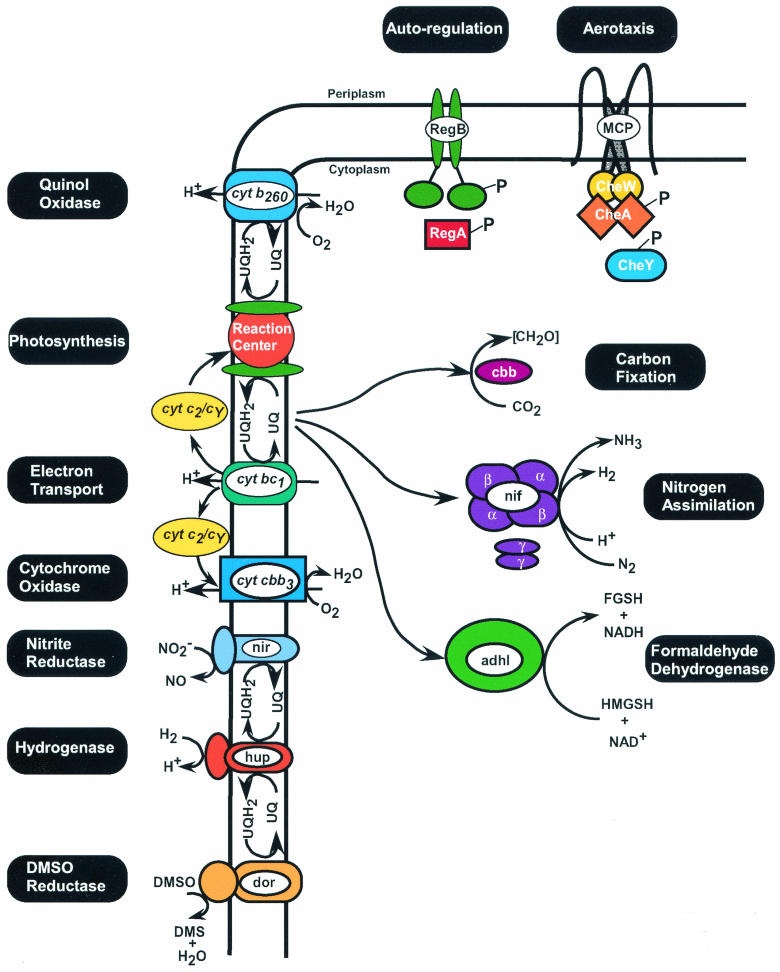
Genetic and biochemical analyses have revealed that the RegB/RegA system is a major global regulator of numerous energy-generating and energy-utilizing cellular processes. The systems controlled by RegB/RegA in R. capsulatus and R. sphaeroides include such fundamental and diverse processes as photosynthesis, CO2 fixation, N2 assimilation, hydrogen utilization, denitrification, dehydrogenases, electron transport, and aerotaxis (Fig. 4). The Reg regulon is continuously growing, and it is likely that there are many more, yet to be discovered, target genes under the control of RegB/RegA in these metabolically diverse bacteria.
FIG. 4.
Diagram of the various RegB/RegA-controlled systems that have been identified in R. capsulatus and R. sphaeroides. UQH2, reduced ubiquinol; UQ, oxidized ubiquinone; FGSH, S-formylglutathione; HMGSH, hydroxymethylglutathione; DMS, dimethyl sulfate.
Inspection of known members of the Reg regulon, as shown in Fig. 4, reveals an interesting interrelationship among the various regulated components. Specifically, photosynthesis, respiration (oxygen and DMSO mediated), and hydrogen oxidation all directly affect the oxidation-reduction state of the ubiquinone pool. Processes such as carbon fixation, nitrogen assimilation, and formaldehyde dehydration all can function as electron sinks. In addition, formation of hydrogen by nitrogenase can be used as a substrate by the uptake hydrogenase system. Likewise, carbon generated by dehydration of formaldehyde can be used by the Calvin cycle during carbon fixation. Indeed, evidence suggests that RegA can function as a “master controller” that is responsible for coordinating these various redox-responding systems (88). For example, the carbon-fixing Calvin cycle becomes derepressed under photoheterotrophic conditions involving light plus organic compounds. Under these growth conditions, carbon fixation is thought to function as an electron sink that bleeds off excess reducing power. If carbon fixation is incapacitated, such as when RubisCO is mutated, then nitrogenase becomes derepressed even in the presence of excess ammonium, so that nitrogenase can take over the role of functioning as an electron sink in the absence of carbon fixation. Importantly, derepression of carbon fixation and nitrogen fixation under conditions of excess carbon and ammonium are RegB/RegA-dependent events (43). This clearly underscores the importance of RegB/RegA in controlling the overall cellular redox poise.
The observation that highly conserved RegB and RegA homologues exist in many other bacterial species also indicates that the RegB/RegA system constitutes an important redox control element that is not easily replaced by other regulators. Although many questions regarding the function of RegB and RegA in other systems still need to be addressed, evidence is mounting that they control a similar set of target genes in a number of bacterial species.
Finally, there are many questions regarding the molecular mechanism of redox sensing by RegB and the specific involvement of upstream elements such as SenC and cytochrome cbb3 oxidase in the redox control of RegB activity. There also remain many outstanding questions about the mechanism of transcription activation and repression by phosphorylated and dephosphorylated RegA, as well as the nature of the possible interactions of RegA with other transcription factors at target promoters. Hopefully, the decade to come will be as fruitful in unraveling the mysteries of the Reg regulon as was the decade that followed its discovery.
Acknowledgments
We thank the numerous investigators who work on the RegB-RegA system for sending us recently published papers and communicating unpublished data.
RegB-RegA work from the Bauer laboratory is supported by grant GM 40941 from the National Institutes of Health.
REFERENCES
- 1.Abada, E. M., A. Balzer, A. Jager, and G. Klug. 2002. Bacteriochlorophyll-dependent expression of genes for pigment-binding proteins in Rhodobacter capsulatus involves the RegB/RegA two-component system. Mol. Genet. Genomics 267:202-209. [DOI] [PubMed] [Google Scholar]
- 2.Barber, D. R., and T. J. Donohue. 1998. Pathways for transcriptional activation of a glutathione-dependent formaldehyde dehydrogenase gene. J. Mol. Biol. 280:775-784. [DOI] [PubMed] [Google Scholar]
- 3.Bauer, C. E. 2001. Regulating synthesis of the purple bacterial photosystem, p. 67-83. In E.-M. Aro and B. Andersson (ed.), Regulation of photosynthesis. Kluwer Academic Publishers, Dordrecht, The Netherlands.
- 4.Bauer, C. E., and T. H. Bird. 1996. Regulatory circuits controlling photosynthesis gene expression. Cell 85:5-8. [DOI] [PubMed] [Google Scholar]
- 5.Bauer, C. E., S. Elsen, L. R. Swem, D. L. Swem, and S. Masuda. 2002. Redox and light regulation of gene expression in photosynthetic prokaryotes. Philos. Trans. R. Soc. London. Ser. B 358:147-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bauer, E., T. Kaspar, H. M. Fischer, and H. Hennecke. 1998. Expression of the fixR-nifA operon in Bradyrhizobium japonicum depends on a new response regulator, RegR. J. Bacteriol. 180:3853-3863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bird, T. H., S. Du, and C. E. Bauer. 1999. Autophosphorylation, phosphotransfer and DNA- binding properties of the RegB/RegA two-component regulatory system in Rhodobacter capsulatus. J. Biol. Chem. 274:16343-16348. [DOI] [PubMed] [Google Scholar]
- 8.Bowman, W. C., S. Du, C. E. Bauer, and R. G. Kranz. 1999. In vitro activation and repression of photosynthesis gene transcription in Rhodobacter capsulatus. Mol. Microbiol. 33:429-437. [DOI] [PubMed] [Google Scholar]
- 9.Buggy, J. J., and C. E. Bauer. 1995. Cloning and characterization of senC, a gene involved in both aerobic respiration and photosynthesis gene expression in Rhodobacter capsulatus. J. Bacteriol. 177:6958-6965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buggy, J. J., M. W. Sganga, and C. E. Bauer. 1994. Characterization of a light-responding trans-activator responsible for differentially controlling reaction center and light-harvesting I gene expression in Rhodobacter capsulatus. J. Bacteriol. 176:6936-6943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen, W., A. Jager, and G. Klug. 2000. Correction of the DNA sequence of the regB gene of Rhodobacter capsulatus with implications for the membrane topology of the sensor kinase RegB. J. Bacteriol. 182:818-820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cohen-Bazire, G. W., W. R. Sistrom, and R. Y. Stanier. 1957. Kinetic studies of pigment synthesis by non-sulfur purple bacteria. J. Cell. Comp. Physiol. 49:25-68. [DOI] [PubMed] [Google Scholar]
- 13.Colbeau, A., and P. M. Vignais. 1992. Use of hupS::lacZ gene fusion to study regulation of hydrogenase expression in Rhodobacter capsulatus: stimulation by H2. J. Bacteriol. 174:4258-4264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Comolli, J. C., A. J. Carl, C. Hall, and T. J. Donohue. 2002. Transcriptional activation of the Rhodobacter sphaeroides cytochrome c2 gene P2 promoter by the response regulator PrrA. J. Bacteriol. 184:390-399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Comolli, J. C., and T. J. Donohue. 2002. Pseudomonas aeriginosa RoxR, a response regulator related to Rhodobacter sphaeroides PrrA, activates expression of the cyanide-insensitive terminal oxidase. Mol. Microbiol. 45:755-768. [DOI] [PubMed] [Google Scholar]
- 16.Dischert, W., P. M. Vignais, and A. Colbeau. 1999. The synthesis of Rhodobacter capsulatus HupSL hydrogenase is regulated by the two-component HupT/HupR system. Mol. Microbiol. 34:995-1006. [DOI] [PubMed] [Google Scholar]
- 17.Dong, C., S. Elsen, L. R. Swem, and C. E. Bauer. 2002. AerR, a second aerobic repressor of photosynthesis geen expression in Rhodobacter capsulatus. J. Bacteriol. 184:2805-2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Du, S., T. H. Bird, and C. E. Bauer. 1998. DNA binding characteristics of RegA*. A constitutively active anaerobic activator of photosynthesis gene expression in Rhodobacter capsulatus. J. Biol. Chem. 273:18509-18513. [DOI] [PubMed] [Google Scholar]
- 19.Du, S., J.-L. Kouadio, and C. E. Bauer. 1999. Regulated expression of a highly conserved regulatory gene cluster is necessary for controlling photosynthesis gene expression in response to anaerobiosis in Rhodobacter capsulatus. J. Bacteriol. 181:4334-4341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dubbs, J. M., T. H. Bird, C. E. Bauer, and F. R. Tabita. 2000. Interaction of CbbR and RegA* transcription regulators with the Rhodobacter sphaeroides cbb1 promoter-operator region. J. Biol. Chem. 275:19224-19230. [DOI] [PubMed] [Google Scholar]
- 21.Dubbs, J. M., and F. R. Tabita. 1998. Two functionally distinct regions upstream of the cbb1 operon of Rhodobacter sphaeroides regulate gene expression. J. Bacteriol. 180:4903-4911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dubbs, J. M., and F. R. Tabita. 2003. Interactions of the cbbII promoter-operator region with CbbR and RegA (PrrA) regulators indicate distinct mechanisms to control expression of the two cbb operons of Rhodobacter sphaeroides. J. Biol. Chem. 278:16443-16450. [DOI] [PubMed] [Google Scholar]
- 23.Elsen, S., W. Dischert, A. Colbeau, and C. E. Bauer. 2000. Expression of uptake hydrogenase and molybdenum nitrogenase in Rhodobacter capsulatus is coregulated by the RegB-RegA two-component regulatory system. J. Bacteriol. 182:2831-2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Elsen, S., S. N. Ponnampalam, and C. E. Bauer. 1998. CrtJ bound to distant binding sites interacts cooperatively to aerobically repress photopigment biosynthesis and light harvesting II gene expression in Rhodobacter capsulatus. J. Biol. Chem. 273:30762-30769. [DOI] [PubMed] [Google Scholar]
- 25.Emmerich, R., H. Hennecke, and H. M. Fischer. 2000. Evidence for a functional similarity between the two-component regulatory systems RegSR, ActSR, and RegBA (PrrBA) in α-Proteobacteria. Arch. Microbiol. 174:307-313. [DOI] [PubMed] [Google Scholar]
- 26.Emmerich, R., K. Panglungtshang, P. Strehler, H. Hennecke, and H. M. Fischer. 1999. Phosphorylation, dephosphorylation and DNA-binding of the Bradyrhizobium japonicum RegSR two-component regulatory proteins. Eur. J. Biochem. 263:455-463. [DOI] [PubMed] [Google Scholar]
- 27.Emmerich, R., P. Strehler, E. Bauer, H. M. Fischer, and H. Hennecke. 2000. Functional analysis of the Bradyrhizobium japonicum RegSR two-component regulatory system, p. 89-90. In F. O. Pedrosa, M. Hungria, G. Yates, and W. E. Newton (ed.), Nitrogen fixation: from molecules to crop productivity. Kluwer, London, United Kingdom.
- 28.Emmerich, R., P. Strehler, H. Hennecke, and H. M. Fischer. 2000. An imperfect inverted repeat is critical for DNA binding of the response regulator RegR of Bradyrhizobium japonicum. Nucleic Acids Res. 28:4166-4171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eraso, J. M., and S. Kaplan. 1994. PrrA, a putative response regulator involved in oxygen regulation of photosynthesis gene expression in Rhodobacter sphaeroides. J. Bacteriol. 176:32-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eraso, J. M., and S. Kaplan. 1995. Oxygen-insensitive synthesis of the photosynthetic membranes of Rhodobacter sphaeroides: a mutant histidine kinase. J. Bacteriol. 177:2695-2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eraso, J. M., and S. Kaplan. 2000. From redox flow to gene regulation: role of the PrrC protein of Rhodobacter sphaeroides 2.4.1. Biochemistry 39:2052-2062. [DOI] [PubMed] [Google Scholar]
- 32.Fenner, B. J., R. P. Tiwari, W. G. Reeve, M. J. Dilworth, and A. R. Glenn. 2000. ActR is a global genetic regulator in Sinorhizobium meliloti. p. 488. In F. O. Pedrosa, M. Hungria, G. Yates, and W. E. Newton (ed.), Nitrogen fixation: from molecules to crop productivity. Kluwer Academic Publisher London, United Kingdom.
- 33.Gibson, J. L., J. M. Dubbs, and F. R. Tabita. 2002. Differential expression of the CO2 fixation operons of Rhodobacter sphaeroides by the Prr/Reg two-component system during chemoautotrophic growth. J. Bacteriol. 184:6654-6664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gibson, J. L., and F. R. Tabita. 1993. Nucleotide sequence and functional analysis of CbbR, a positive regulator of the calvin cycle operons of Rhodobacter sphaeroides. J. Bacteriol. 175:5778-5784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Glerum, D. M., and A. Shtanko, and A. Tzagoloff. 1996. SCO1 and SCO2 act as high copy suppressors of a mitochondrial copper recruitment defect in Saccharomyces cerevisiae. J. Biol. Chem. 271:20531-20535. [DOI] [PubMed] [Google Scholar]
- 36.Gomelsky, M., and S. Kaplan. 1995. Isolation of regulatory mutants in photosynthesis gene expression in Rhodobacter sphaeroides 2.4.1 and partial complementation of a PrrB mutant by the HupT histidine-kinase. Microbiology 141:1805-1819. [DOI] [PubMed] [Google Scholar]
- 37.Gray, K. A., and F. Daldal. 1995. Mutational studies of the cytochrome bc1 complexes, p. 747-774. In R. E. Blankenship, M. T. Madigan and C. E. Bauer (ed.), Anoxygenic photosynthetic bacteria. Kluwer Academic Publishers, Dordrecht, The Netherlands.
- 38.Hemschemeier, S. K., U. Ebel, A. Jager, A. Balzer, M. Kirndorfer, and G. Klug. 2000. In vivo and in vitro analysis of RegA response regulator mutants of Rhodobacter capsulatus. J. Mol. Microbiol. Biotechnol. 2:291-300. [PubMed] [Google Scholar]
- 39.Hemschemeier, S. K., M. Kirndörfer, U. Ebel, and G. Klug. 1999. Transcriptional regulation of puf and puc operon expression in Rhodobacter capsulatus by the DNA binding protein RegA, p. 127-130. In G. A. Peschek, W. Löffelhardt, and G. Schmetterer (ed.), The phototrophic prokaryotes. Kluwer Academic, Plenum Publishers, New York.
- 40.Hemschemeier, S. K., M. Kirndörfer, M. Hebermchl, and G. Klug. 2000. DNA binding of wild-type RegA protein and its differential effect on the expression of pigment binding proteins in Rhodobacter capsulatus. J. Mol. Microbiol. Biotechnol. 2:235-243. [PubMed] [Google Scholar]
- 41.Inoue, K., J.-L. Kouadio, C. S. Mosley, and C. E. Bauer. 1995. Isolation and in vitro phosphorylation of sensory transduction components controlling anaerobic induction of light harvesting and reaction center gene expression in Rhodobacter capsulatus. Biochemistry 34:391-396. [DOI] [PubMed] [Google Scholar]
- 42.Inouye, M., R. Dutta, and Y. Zhu. 2003. Regulation of porins in Escherichia coli by the osmosensing histidine kinase/phosphatase EnvZ, p. 25-46. In M. Inouye and R. Dutta (ed.), Histidine kinases in signal transduction. Academic Press, Inc., San Diego, Calif.
- 43.Joshi, H. M., and F. R. Tabita. 1996. A global two-component signal transduction system that integrates the control of photosynthesis, carbon dioxide assimilation, and nitrogen fixation. Proc. Natl. Acad. Sci. USA 93:14515-14520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kappler, U., W. M. Huston, and A. G. McEwan. 2002. Control of dimethylsulfoxide reductase expression in Rhodobacter capsulatus: the role of carbon metabolites and the response regulators DorR and RegA. Microbiology 148:605-614. [DOI] [PubMed] [Google Scholar]
- 45.Karls, R. K., J. R. Wolf, and T. J. Donohue. 1999. Activation of the cycA P2 promoter for the Rhodobacter sphaeroides cytochrome c2 gene by the photosynthesis response regulator. Mol. Microbiol. 34:822-835. [DOI] [PubMed] [Google Scholar]
- 46.Kirndörfer, M., A. Jäger, and G. Klug. 1998. Integration host factor affects the oxygen- regulated expression of photosynthesis gene in Rhodobacter capsulatus. Mol. Gen. Genet. 258:297-305. [DOI] [PubMed] [Google Scholar]
- 47.Koch H. G., C. Winterstein, A. S. Saribas, J. O. Alben, and F. Daldal. 2000. Roles of the ccoGHIS gene products in the biogenesis of the cbb(3)-type cytochrome c oxidase. J. Mol. Biol. 297:49-65. [DOI] [PubMed] [Google Scholar]
- 48.Kouadio, J. L., 1997. Functional characterization of transcription factors involved in photosynthesis from the purple non-sulfur bacterium Rhodobacter capsulatus. Ph.D. thesis. Indiana University, Bloomington.
- 49.Laguri, C., M. K. Phillips-Jones, and M. P. Williamson. 2003. Solution structure and DNA binding of the effector domain from the global regulator PrrA (RegA) from Rhodobacter sphaeroides: insights into DNA binding specificity. Nucleic Acids Res. 31:6778-6787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Laratta, W. P., P. S. Choi, I. E. Tosques, and J. P. Shapleigh. 2002. Involvement of the PrrB/PrrA two-component system in nitrite respiration in Rhodobacter sphaeroides 2.4.3: evidence for transcriptional regulation. J. Bacteriol. 184:3521-3529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Madigan, M. T., and H. Gest. 1979. Growth of the photosynthetic bacterium Rhodopseudomonas capsulatus chemoautotrophically in darkness with H2 as the energy source. J. Bacteriol. 137:524-530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Masepohl, B., and W. Klipp. 1996. Organization and regulation of genes encoding the molybdenum nitrogenase and the alternative nitrogenase in Rhodobacter capsulatus. Arch. Microbiol. 165:80-90. [Google Scholar]
- 53.Masuda, S., Y. Matsumoto, K. V. P. Nagashima, K. Shimada, K. Inoue, C. E. Bauer, and K. Matsuura. 1999. Structural and functional analyses of photosynthetic regulatory genes regA and regB from Rhodovulum sulfidophilum, Roseobacter denitrificans and Rhodobacter capsulatus. J. Bacteriol. 181:4205-4215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McEwan, A. G., A. Lewin, S. L. Davy, R. Boetzel, A. Leech, D. Walker, T. Wood, and G. R. Moore. 2002. PrrC from Rhodobacter sphaeroides, a homologue of eukaryotic Sco proteins, is a copper-binding protein and may have a thiol-disulfide oxidoreductase activity. FEBS Lett. 518:10-16. [DOI] [PubMed] [Google Scholar]
- 55.Mosley, C. S., J. Y. Suzuki, and C. E. Bauer. 1994. Identification and molecular genetic characterization of a sensor kinase responsible for coordinately regulating light harvesting and reaction center gene expression in response to anaerobiosis. J. Bacteriol. 176:7566-7573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mouncey, N. J., and S. Kaplan. 1998. Redox-dependent gene regulation in Rhodobacter sphaeroides 2.4.1: effects on dimethylsulfoxide reductase (dor) gene expression. J. Bacteriol. 180:5612-5618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mouncey, N. J., M. Choudhary, and S. Kaplan. 1997. Characterization of the genes encoding dimethylsulfoxide reductase of Rhodobacter sphaeroides 2.4.1: an essential metabolic gene function encoded by chromosome II. J. Bacteriol. 179:7617-7624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nickens, D. G., and C. E. Bauer. 1998. Analysis of the puc operon promoter from Rhodobacter capsulatus. J. Bacteriol. 180:4270-4277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nishimura K., H. Shimada, S. Hatanaka, H. Mizoguchi, H. Ohta, T. Masuda, and K. Takamiya. 1998. Growth, pigmentation, and expression of the puf and puc operons in a light- responding-repressor (SPB)-disrupted Rhodobacter sphaeroides. Plant Cell Physiol. 39:411-417. [DOI] [PubMed] [Google Scholar]
- 60.O'Gara, J. P., J. M. Eraso, and S. Kaplan. 1998. Evidence for the role of redox carriers in photosynthesis gene expression and carotenoid biosynthesis in Rhodobacter sphaeroides 2.4.1. J. Bacteriol. 179:1951-1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.O'Gara, J. P., and S. Kaplan. 1997. A redox-responsive pathway for aerobic regulation of photosynthesis gene expression in Rhodobacter sphaeroides 2.4.1. J. Bacteriol. 180:4044-4050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Oh, J.-I., J. M. Eraso, and S. Kaplan. 2000. Interacting regulatory circuits involved in orderly control of photosynthesis gene expression in Rhodobacter sphaeroides 2.4.1. J. Bacteriol. 182:3081-3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Oh, J.-I., and S. Kaplan. 1999. The cbb3 terminal oxidase of Rhodobacter sphaeroides 2.4.1: structural and functional implications for the regulation of spectral complex formation. Biochemistry 38:2688-2696. [DOI] [PubMed] [Google Scholar]
- 64.Oh, J.-I., and S. Kaplan. 2000. Redox signaling: globalization of gene expression. EMBO J. 19:4237-4247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Oh, J.-I., and S. Kaplan. 2001. Generalized approach to the regulation and integration of gene expression. Mol. Microbiol. 39:1116-1123. [DOI] [PubMed] [Google Scholar]
- 66.Oh, J.I., and S. Kaplan. 2002. Oxygen adaptation. The role of the CcoQ subunit of the cbb3 cytochrome c oxidase of Rhodobacter sphaeroides 2.4.1. J. Biol. Chem. 277:16220-16228. [DOI] [PubMed] [Google Scholar]
- 67.Oh, J. I., I. J. Ko, and S. Kaplan. 2001. The default state of the membrane-localized histidine kinase PrrB of Rhodobacter sphaeroides 2.4.1 is in the kinase-positive mode. J. Bacteriol. 183:6807-6814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ouchane, S., and S. Kaplan. 1999. Topological analysis of the membrane-localized redox- responsive sensor kinase PrrB from Rhodobacter sphaeroides 2.4.1. J. Biol. Chem. 274:17290-17296. [DOI] [PubMed] [Google Scholar]
- 69.Paoli, G. C., P. Vichivanives, and F. R. Tabita. 1998. Physiological control and regulation of the Rhodobacter capsulatus cbb operons. J. Bacteriol. 180:4258-4269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Parkinson, J. S., and E. C. Kofoid. 1992. Communication modules in bacterial signaling proteins. Annu. Rev. Genet. 26:71-112. [DOI] [PubMed] [Google Scholar]
- 71.Phillips-Jones, M. K., and C. N. Hunter. 1994. Cloning and nucleotide sequence of regA, a putative response regulator gene of Rhodobacter sphaeroides. FEMS Microbiol. Lett. 116:269-275. [DOI] [PubMed] [Google Scholar]
- 72.Ponnampalam, S. N., J. J. Buggy, and C. E. Bauer. 1995. Characterization of an aerobic repressor that coordinately regulates bacteriochlorophyll, carotenoid, and light harvesting-II expression in Rhodobacter capsulatus. J. Bacteriol. 177:2990-2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ponnampalam, S. N., and C. E. Bauer. 1997. DNA Binding characteristics of CrtJ: a redox-responding repressor of bacteriochlorophyll, carotenoid and light harvesting-II gene expression in Rhodobacter capsulatus. J. Biol. Chem. 272:9671-9676. [DOI] [PubMed] [Google Scholar]
- 74.Potter, C. A., A. Ward, C. Laguri, M. P. Williamson, P. J. Henderson, and M. K. Phillips-Jones. 2002. Expression, purification and characterisation of full-length histidine protein kinase RegB from Rhodobacter sphaeroides. J. Mol. Biol. 320:201-213. [DOI] [PubMed] [Google Scholar]
- 75.Qian, Y., and F. R. Tabita. 1996. A global signal transduction system regulates aerobic and anaerobic CO2 fixation in Rhodobacter sphaeroides. J. Bacteriol. 178:12-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Roh, J. H., and S. Kaplan. 2000. Genetic and phenotypic analyses of the rdx locus of Rhodobacter sphaeroides 2.4.1. J. Bacteriol. 182:3475-3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Romagnoli, S., H. L. Packer, and J. P. Armitage. 2002. Tactic responses to oxygen in the phototrophic bacterium Rhodobacter sphaeroides WS8N. J. Bacteriol. 184:5590-5598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sganga, M. W., and C. E. Bauer. 1992. Regulatory factors controlling photosynthetic reaction center and light-harvesting gene expression in Rhodobacter capsulatus. Cell 68:945-954. [DOI] [PubMed] [Google Scholar]
- 79.Shaw, A. L., S. Leimkuhler, W. Klipp, G. R. Hanson, and A. G. McEwan. 1999. Mutational analysis of the dimethylsulfoxide respiratory (dor) operon of Rhodobacter capsulatus. Microbiology 145:1409-1420. [DOI] [PubMed] [Google Scholar]
- 80.Shimada, H., T. Wada, H. Handa, H. Ohta, H. Mizoguchi, K. Nishimura, T. Masuda, Y. Shioi, and K. Takamiya. 1996. A transcription factor with a leucine-zipper motif involved in light-dependent inhibition of expression of the puf operon in the photosynthetic bacterium Rhodobacter sphaeroides. Plant Cell Physiol. 37:515-521. [DOI] [PubMed] [Google Scholar]
- 81.Stock, J. B., A. J. Ninfa, and A. M. Stock. 1989. Protein phosphorylation and regulation of adaptive responses in bacteria. Microbiol. Rev. 53:450-490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Stock, A. M., V. L. Robinson, and P. N. Goudreau. 2000. Two-component signal transduction. Annu. Rev. Biochem. 69:183-215. [DOI] [PubMed] [Google Scholar]
- 83.Swem, L., S. Elsen, T. H. Bird, H.-G. Koch, H. Myllykallio, F. Daldal, and C. E. Bauer. 2001. The RegB/RegA two-component regulatory system controls synthesis of photosynthetic and respiratory electron transfer components in Rhodobacter capsulatus. J. Mol. Biol. 309:121-138. [DOI] [PubMed] [Google Scholar]
- 84.Swem, L. R., B. J. Kraft, D. L. Swem, A. T. Setterdahl, S. Masuda, D. B. Knaff, J. M. Zalesk, and C. E. Bauer. 2003. Signal transduction by the global regulator RegB is mediated by a redox-active cysteine. EMBO J. 22:4699-4708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Swem, D. L., and C. E. Bauer. 2002. Coordination of ubiquinol oxidase and cytochrome cbb3 oxidase expression by multiple regulators in Rhodobacter capsulatus. J. Bacteriol. 184:2815-2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tabita, F. R. 1995. The biochemistry and metabolic regulation of carbon metabolism and CO2 fixation in purple bacteria, p. 885-914. In R. E. Blankenship, M. T. Madigan, and C. E. Bauer (ed.), Anoxygenic photosynthetic bacteria. Kluwer Academic Publishers, Dordrecht, The Netherlands.
- 87.Thöny-Meyer, L., C. Beck, O. Preisig, and H. Hennecke. 1994. The ccoNOQP gene cluster codes for a cb-type cytochrome oxidase that functions in aerobic respiration of Rhodobacter capsulatus. Mol. Microbiol. 174:705-716. [DOI] [PubMed] [Google Scholar]
- 88.Tichi, M. A., and F. R. Tabita. 2001. Interactive control of Rhodobacter capsulatus redox-balancing systems during phototrophic metabolism. J. Bacteriol. 183:6344-6354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tichi, M. A., and F. R. Tabita. 2000. Maintenance and control of redox poise in Rhodobacter capsulatus strains deficient in the Calvin-Benson-Bassham pathway. Arch. Microbiol. 174:322-333. [DOI] [PubMed] [Google Scholar]
- 90.Tiwari, R. P., W. G. Reeve, M. J. Dilworth, and A. R. Glenn. 1996. Acid tolerance in Rhizobium meliloti strain WSM419 involves a two-component sensor-regulator system. Microbiology 142:1693-1704. [DOI] [PubMed] [Google Scholar]
- 91.Tokito, M. K., and F. Daldal. 1992. petR, located upstream of the fbcFBC operon encoding the cytochrome bc1 complex, is homologous to bacterial response regulators and necessary for photosynthetic and respiratory growth of Rhodobacter capsulatus. Mol. Microbiol. 6:1645-1654. [DOI] [PubMed] [Google Scholar]
- 92.Toussaint, B., R. de Sury d'Aspremont, I. Delic-Attree, V. Berchet, S. Elsen, A. Colbeau, W. Dischert, Y. Lazzaroni, and P. M. Vignais. 1997. The Rhodobacter capsulatus hupSLC promoter: identification of cis-acting regulatory elements and of trans-activating factors involved in H2 activation of hupSLC transcription. Mol. Microbiol. 26:927-937. [DOI] [PubMed] [Google Scholar]
- 93.Vichivanives, P., T. H. Bird, C. E. Bauer, and F. R. Tabita. 2000. Multiple regulators and their interactions in vivo and in vitro with the cbb regulons of Rhodobacter capsulatus. J. Mol. Biol. 300:1079-1099. [DOI] [PubMed] [Google Scholar]
- 94.Vignais, P. M., B. Toussaint, and A. Colbeau. 1995. Regulation of hydrogenase gene expression, p. 1175-1190. In R. E. Blankenship, M. T. Madigan, and C. E. Bauer (ed.), Anoxygenic photosynthetic bacteria. Kluwer Academic Publishers, Dordrecht, The Netherlands.
- 95.Zannoni, D. 1995. Aerobic and anaerobic transport chains in anoxygenic phototrophic bacteria, p. 949-971. In R. E. Blankenship, M. T. Madigan, and C. E. Bauer (ed.), Anoxygenic photosynthetic bacteria. Kluwer Academic Publishers, Dordrecht, The Netherlands.
- 96.Zeilstra-Ryalls, J., K. Gabbert, N. J. Mouncey, S. Kaplan, and R. G. Kranz. 1997. Analysis of the fnrL gene and its function in Rhodobacter capsulatus. J. Bacteriol. 179:7264-7273. [DOI] [PMC free article] [PubMed] [Google Scholar]