Abstract
The bacterium Myxococcus xanthus undergoes multicellular development during times of nutritional stress and uses extracellular signals to coordinate cell behavior. C-signal affects gene expression late in development, including that of Ω4499, an operon identified by insertion of Tn5 lac into the M. xanthus chromosome. The Ω4499 promoter region has several sequences in common with those found previously to be important for expression of other C-signal-dependent promoters. To determine if these sequences are important for Ω4499 promoter activity, the effects of mutations on expression of a downstream reporter gene were tested in M. xanthus. Although the promoter resembles those recognized by Escherichia coli σ54, mutational analysis implied that a σ70-type σ factor likely recognizes the promoter. A 7-bp sequence known as a C box and a 5-bp element located 6 bp upstream of the C box have been shown to be important for expression of other C-signal-dependent promoters. The Ω4499 promoter region has C boxes centered at −33 and −55 bp, with 5-bp elements located 7 and 8 bp upstream, respectively. A multiple-base-pair mutation in any of these sequences reduced Ω4499 promoter activity more than twofold. Single base-pair mutations in the C box centered at −33 bp yielded a different pattern of effects on expression than similar mutations in other C boxes, indicating that each functions somewhat differently. An element from about −81 to −77 bp exerted a twofold positive effect on expression but did not appear to be responsible for the C-signal dependence of the Ω4499 promoter. Mutations in sigD and sigE, which are genes that encode σ factors, reduced expression from the Ω4499 promoter. The results provide further insight into the regulation of C-signal-dependent genes, demonstrating both shared and unique properties among the promoter regions so far examined.
Myxococcus xanthus is a gram-negative, rod-shaped bacterium that is found in most soils. It has the ability to undergo multicellular development (21, 23, 45, 48), distinguishing it from most other bacteria. Under starvation conditions on a solid surface, M. xanthus cells move in a coordinated fashion called rippling and accumulate at foci. When approximately 105 cells have aggregated, mound-shaped structures called fruiting bodies are built, inside which some of the cells differentiate into heat- and desiccation-resistant, spherical myxospores.
The developmental process is believed to be regulated by several extracellular signals (21, 23, 45, 48), including the A- and C-signals, which are the best characterized. A-signaling early in development leads to the production of extracellular proteases, peptides, and amino acids, which are thought to provide a mechanism for cell density sensing (24, 34, 35, 41). C-signaling is the latest acting of the known signals and is required for rippling, aggregation, and sporulation (28, 37, 47). Signaling also leads to changes in gene expression during development (12, 31, 33).
Genes expressed during M. xanthus development have been identified by transposition of Tn5 lac into the chromosome (30, 32). Tn5 lac contains a promoterless lacZ gene whose transcription can come under the control of a promoter outside the transposon. Among 2,374 Tn5 lac insertions, 29 were shown to be developmentally regulated (32), and 15 of these were shown to depend on C-signaling for full expression (31). The 15 fusions are expressed at various times after 6 h into development. Several were shown to depend absolutely on C-signaling for expression (e.g., Ω4403). Others, such as Ω4400 and Ω4499, were shown to depend partially on C-signaling (i.e., expression was reduced, but not abolished, in the absence of C-signaling).
To gain insight into the differential regulation of C-signal-dependent genes, the promoter regions upstream of Tn5 lac insertions Ω4403 (9), Ω4400 (4), and Ω4499 (8) have been identified and searched for conserved sequence elements. Mutational analysis of the Ω4403 (53) and Ω4400 (56) promoter regions has revealed important cis-acting DNA elements. In both promoter regions, the identical 7-bp sequence (CATCCCT), which has been called a C box (consensus sequence CAYYCCY, in which Y means pyrimidine), is centered at −49 bp, and a 5-bp element (consensus sequence GAACA) is centered at −61 bp. Both the C boxes and the 5-bp elements were found to be essential for promoter activity. However, single base pair changes in these elements had different effects on promoter activity, suggesting that different transcription factors bind to these regions. Activity of the Ω4403 promoter also required a 10-bp element centered at −74.5 bp. Activity of the Ω4400 promoter required a large region from approximately −63 to −31 bp, which encompasses the 5-bp element, the C box, and adjoining DNA. In addition, a small region from approximately −86 to −81 bp exerted a twofold to fourfold positive effect on expression and was shown to be at least partially responsible for the C-signal dependence of the Ω4400 promoter.
Tn5 lac Ω4499 is an insertion in the second gene of an operon that is predicted to code for reductase and oxidase components of a cytochrome P-450 system (8). The insertion does not cause a developmental defect, but expression of lacZ is strongly induced during development. The timing of expression is similar to that from Tn5 lac Ω4400 (32). Expression from both the Ω4499 and Ω4400 promoters was reduced in a csgA mutant (31), which fails to produce the CsgA protein involved in C-signaling (29, 36, 39), and expression was restored by codeveloping the csgA mutant with wild-type cells, which supplied the C-signal (4, 8). Moreover, expression from both promoters has been shown to correlate closely with the altered levels of CsgA produced in act mutants (13, 56). Examination of the Ω4499 promoter region revealed three sequences that match the C box consensus sequence, centered at −55, −33, and −1 bp (8). In addition, centered at −65 bp is a sequence that matches a sequence in the Ω4400 promoter region in eight of nine positions. The sequence is centered at −80 bp in the Ω4400 promoter region and is in the opposite orientation relative to the start site of transcription but, interestingly, it includes the region shown to mediate, at least in part, the response to C-signaling (56).
Here, we report the results of mutational analysis of the Ω4499 promoter region. We found some similarities between the Ω4499 and Ω4400 promoter regions in terms of overall organization, but the effects of single base pair changes were different in many cases from either the Ω4400 (56) or Ω4403 (53) promoter regions, indicating that DNA elements similar in sequence function uniquely to regulate transcription from the three promoters.
MATERIALS AND METHODS
Bacterial strains and plasmids.
Strains and plasmids that were used in this study are listed in Table 1.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant characteristic(s) | Source or reference |
|---|---|---|
| E. coli DH5α | φ80 lacZΔM15 ΔlacU169 recA1 endA1 hsdR17 supE44 thi-1 gyrA relA1 | 16 |
| M. xanthus | ||
| DK1622 | Wild type | 22 |
| MDB01 | attB::pDB01 | 8 |
| MDY1727 | attB::pREG1727 | 56 |
| MDY101 | attB::pDY101 (pREG1727 with 268-bp XhoI-BamHI fragment from pDY100)a | This study |
| MDY103 | attB::pDY103 (pREG1727 with 121-bp XhoI-BamHI fragment from pDO2) | This study |
| MDY104 | attB::pDY104 (pREG1727 with 111-bp XhoI-BamHI fragment from pDO3) | This study |
| MDY40 | attB::pDY40 (pREG1727 with 268-bp XhoI-BamHI fragment from pDY39) | This study |
| MDY42 | attB::pDY42 (pREG1727 with 268-bp XhoI-BamHI fragment from pDY41) | This study |
| MDY44 | attB::pDY44 (pREG1727 with 268-bp XhoI-BamHI fragment from pDY43) | This study |
| MDY46 | attB::pDY46 (pREG1727 with 268-bp XhoI-BamHI fragment from pDY45) | This study |
| MDY48 | attB::pDY48 (pREG1727 with 268-bp XhoI-BamHI fragment from pDY47) | This study |
| MDY50 | attB::pDY50 (pREG1727 with 268-bp XhoI-BamHI fragment from pDY49) | This study |
| MDY52 | attB::pDY52 (pREG1727 with 150-bp XhoI-BamHI fragment from pDY51) | This study |
| MDY106 | attB::pDY106 (pREG1727 with 268-bp XhoI-BamHI fragment from pDY105) | This study |
| MDY108 | attB::pDY108 (pREG1727 with 268-bp XhoI-BamHI fragment from pDY107) | This study |
| MDY110 | attB::pDY110 (pREG1727 with 268-bp XhoI-BamHI fragment from pDY109) | This study |
| MDY112 | attB::pDY112 (pREG1727 with 268-bp XhoI-BamHI fragment from pDY111) | This study |
| MDY114 | attB::pDY114 (pREG1727 with 268-bp XhoI-BamHI fragment from pDY113) | This study |
| MDY116 | attB::pDY116 (pREG1727 with 268-bp XhoI-BamHI fragment from pDY115) | This study |
| MDY118 | attB::pDY118 (pREG1727 with 268-bp XhoI-BamHI fragment from pDY117) | This study |
| MDY120 | attB::pDY120 (pREG1727 with 268-bp XhoI-BamHI fragment from pDY119) | This study |
| MDY122 | attB::pDY122 (pREG1727 with 268-bp XhoI-BamHI fragment from pDY121) | This study |
| MDY124 | attB::pDY124 (pREG1727 with 268-bp XhoI-BamHI fragment from pDY123) | This study |
| MDY126 | attB::pDY126 (pREG1727 with 268-bp XhoI-BamHI fragment from pDY125) | This study |
| MDY128 | attB::pDY128 (pREG1727 with 268-bp XhoI-BamHI fragment from pDY127) | This study |
| MDY130 | attB::pDY130 (pREG1727 with 268-bp XhoI-BamHI fragment from pDY129) | This study |
| MDY132 | attB::pDY132 (pREG1727 with 268-bp XhoI-BamHI fragment from pDY131) | This study |
| MDY134 | attB::pDY134 (pREG1727 with 268-bp XhoI-BamHI fragment from pDY133) | This study |
| MDY136 | attB::pDY136 (pREG1727 with 268-bp XhoI-BamHI fragment from pDY135) | This study |
| MDY138 | attB::pDY138 (pREG1727 with 268-bp XhoI-BamHI fragment from pDY137) | This study |
| MDY140 | attB::pDY140 (pREG1727 with 268-bp XhoI-BamHI fragment from pDY139) | This study |
| MDY142 | attB::pDY142 (pREG1727 with 268-bp XhoI-BamHI fragment from pDY141) | This study |
| MDY144 | attB::pDY144 (pREG1727 with 268-bp XhoI-BamHI fragment from pDY143) | This study |
| DK5208 | csgA::Tn5-132 (Tcr) Ω205 | 46 |
| MDY5208-103 | csgA::Tn5-132 (Tcr) Ω205 attB::pDY103 | This study |
| MDY5208-134 | csgA::Tn5-132 (Tcr) Ω205 attB::pDY134 | This study |
| ΔsigD | ΔsigD | 51 |
| MDY4499.SD | attB::pDY101 | This study |
| ΔsigE | ΔsigE | 52 |
| MDY4499.SE | attB::pDY101 | This study |
| Plasmids | ||
| pGEM7Zf | Aprlacα | Promega |
| pREG1727 | Apr Kmr P1-inc attP ′lacZ | 9 |
| pMF0051 | pGEM7Zf with 3.2-kb PstI-BamHI fragment from pMF002 | 8 |
| pDY100 | pGEM7Zf with 268-bp fragment from −218 to +50 bp of Ω4499 DNA generated by PCR using pMF0051 as template, inserted as a XhoI-BamHI fragment | This study |
| pDO2 | pGEM7Zf with 121-bp fragment from −71 to +50 bp of Ω4499 DNA generated by PCR using pDY100 as a template, inserted as a XhoI-BamHI fragment | This study |
| pDO3 | pGEM7Zf with 111-bp fragment from −61 to +50 bp of Ω4499 DNA generated by PCR using pDY100 as a template, inserted as a XhoI-BamHI fragment | This study |
| pDY39 | pDY100 with C-to-A mutation at −36 bp | This study |
| pDY41 | pDY100 with C-to-A mutation at −32 bp | This study |
| pDY43 | pDY100 with C-to-A mutation at −31 bp | This study |
| pDY45 | pDY100 with CATTCCT-to-ACGGAAG mutation from −36 to −30 bp | This study |
| pDY47 | pDY100 with GAAC-to-TCCA mutation from −48 to −45 bp | This study |
| pDY49 | pDY100 with TCATTC-to-GACGGA mutation from −59 to −54 bp | This study |
| pDY51 | pGEM7Zf with 150-bp fragment from −100 to +50 bp of Ω4499 DNA generated by PCR using pDY100 as template, inserted as a XhoI-BamHI fragment | This study |
| pDY105 | pDY100 with CGA-to-TAT mutation from −12 to −10 bp | This study |
| pDY107 | pDY100 with T-to-G mutation at −25 bp | This study |
| pDY109 | pDY100 with T-to-G mutation at −30 bp | This study |
| pDY111 | pDY100 with T-to-G mutation at −33 bp | This study |
| pDY113 | pDY100 with T-to-G mutation at −34 bp | This study |
| pDY115 | pDY100 with A-to-C mutation at −35 bp | This study |
| pDY117 | pDY100 with C-to-A mutation at −37 bp | This study |
| pDY119 | pDY100 with T-to-G mutation at −44 bp | This study |
| pDY121 | pDY100 with CCTTC-to-AAGGA mutation from −53 to −49 bp | This study |
| pDY123 | pDY100 with TCA-to-GAC mutation from −59 to −57 bp | This study |
| pDY125 | pDY100 with CCGG-to-AATT mutation from −63 to −60 bp | This study |
| pDY127 | pDY100 with ACCA-to-CAAC mutation from −67 to −64 bp | This study |
| pDY129 | pDY100 with GGAC-to-TTCA mutation from −71 to −68 bp | This study |
| pDY131 | pDY100 with TCGCT-to-GATAG mutation from −76 to −72 bp | This study |
| pDY133 | pDY100 with GCCGC-to-TAATA mutation from −81 to −77 bp | This study |
| pDY135 | pDY100 with CATAC-to-ACGCA mutation from −86 to −82 bp | This study |
| pDY137 | pDY100 with GCGTT-to-TATGG mutation from −91 to −87 bp | This study |
| pDY139 | pDY100 with AGATT-to-CTCGG mutation from −96 to −92 bp | This study |
| pDY141 | pDY100 with CGAGG-to-ATCTT mutation from −101 to −97 bp | This study |
| pDY143 | pDY100 with CCC-to-AAA mutation from −29 to −27 bp | This study |
Where possible, the plasmid description is given in parentheses after the strain description.
Growth and development.
Escherichia coli DH5α strains were grown at 37°C in Luria-Bertani medium (44) containing 50 μg of ampicillin per ml. M. xanthus strains were grown at 32°C in CTT broth or agar (1.5%) plates (1% Casitone, 10 mM Tris-HCl [pH 8.0], 1 mM KH2PO4, 8 mM MgSO4 [final pH = 7.6]) (18). When necessary, 40 μg of kanamycin (Km) per ml was used for selection. Fruiting body development was performed on TPM agar (1.5%) plates (10 mM Tris-HCl [pH 8.0], 1 mM KH2PO4-K2HPO4, 8 mM MgSO4 [final pH = 7.6]) as described previously (32).
Construction of plasmids.
A PCR fragment containing the Ω4499 promoter region from −218 bp to +50 bp relative to the start site of transcription was generated using pMF0051 as the template. The PCR fragment was ligated into XhoI-BamHI-digested pGEM7Zf to form pDY100. Additional deletion constructs were created by PCR using pDY100 and primers designed to produce a product with a XhoI restriction site at the upstream end and a BamHI restriction site at the downstream end. PCR products were then digested with XhoI and BamHI, gel purified, and ligated into pGEM7Zf, and the ligation products were electroporated into E. coli DH5α cells. Ampicillin-resistant transformants were selected, and plasmid DNA was sequenced at the Michigan State University Genomics Technology Support Facility to confirm the sequence and end points of the M. xanthus DNA insert.
The QuikChange site-directed mutagenesis kit (Stratagene) was used to create mutations in the Ω4499 promoter region that, in most cases, were A↔ C or T↔ G single-base-pair or multiple-base-pair transversion mutations. The plasmid pDY100 described above was used as a template in PCRs with various combinations of mutagenic primers. The M. xanthus DNA insert was sequenced to ensure only the proper mutations had been created.
Each mutant derivative of pDY100 was digested with XhoI and BamHI, gel purified, and ligated into pREG1727 previously cut with the same enzymes. The ligated constructs were introduced into E. coli DH5α by electroporation, and ampicillin-resistant transformants were selected. A transformant containing the mutant Ω4499 plasmid was identified using colony PCR with primers to ensure proper orientation. The transformants containing the mutated Ω4499 promoter regions were then used to prepare plasmid DNA for introduction into M. xanthus.
Construction of M. xanthus strains and determination of lacZ expression during development.
Strains containing pREG1727 derivatives integrated at the Mx8 phage attachment site (designated attB in Table 1) were constructed by electroporation (25) of M. xanthus, and transformants were selected on CTT-Km plates. Based on previous experience in our laboratory (4, 8, 9), the majority of transformants have a single copy of the plasmid integrated at attB. To eliminate colonies with unusual developmental lacZ expression, we screened at least 10 transformants on TPM agar plates containing 40 μg of 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside per ml. Any colonies with unusual expression of lacZ were discarded and, of the remaining candidates, three independent isolates of each mutant construct were chosen for development. In all cases, the three transformants gave similar results (see Table 2, below) when developmental β-galactosidase activity was measured as described previously (32).
TABLE 2.
Summary of activities of mutant Ω4499 promoters
| Promoter assayed | Avg maximum β-galactosidase sp act during developmenta | % Wild-type activity measured in the same exptb |
|---|---|---|
| Vector (no insert) | 11 ± 4 | |
| Wild-type 4499 promoter | 39 ± 18 | |
| Deletions | ||
| −100 to +50 bp | 51 ± 10 | 141 ± 10 |
| −71 to +50 bp | 25 ± 5 | 41 ± 15 |
| −61 to +50 bp | 13 ± 2 | 4 ± 6 |
| Mutationsc | ||
| CGA −12 to −10 TAT | 161 ± 10 | 877 ± 58 |
| T −25 G | 28 ± 5 | 49 ± 15 |
| CCC −29 to −27 AAA | 45 ± 6 | 178 ± 30 |
| CATTCCT −36 to −30 ACGGAAG | 12 ± 1 | 12 ± 4 |
| T −30 G | 27 ± 10 | 46 ± 28 |
| C −31 A | 31 ± 6 | 53 ± 19 |
| C −32 A | 29 ± 6 | 59 ± 21 |
| T −33 G | 7 ± 0.6 | 0 |
| T −34 G | 7 ± 2 | 0 |
| A −35 C | 17 ± 2 | 61 ± 10 |
| C −36 A | 55 ± 9 | 134 ± 26 |
| C −37 A | 11 ± 2 | 0 |
| T −44 G | 17 ± 3 | 63 ± 15 |
| GAAC −48 to −45 TCCA | 19 ± 4 | 18 ± 13 |
| CCTTC −53 to −49 AAGGA | 10 ± 1 | 0 |
| TCATTC −59 to −54 GACGGA | 12 ± 2 | 10 ± 6 |
| TCA −59 to −57 GAC | 9 ± 1 | 0 |
| CCGG −63 to −60 AATT | 7 ± 1 | 5 ± 6 |
| ACCA −67 to −64 CAAC | 10 ± 0.8 | 0 |
| GGAC −71 to −68 TTCA | 7 ± 2 | 0 |
| TCGCT −76 to −72 GATAG | 36 ± 4 | 170 ± 23 |
| GCCGC −81 to −77 TAATA | 19 ± 1 | 45 ± 6 |
| CATAC −86 to −82 ACGCA | 44 ± 8 | 214 ± 47 |
| GCGTT −91 to −87 TATGG | 41 ± 14 | 155 ± 72 |
| AGATT −96 to −92 CTCGG | 48 ± 7 | 235 ± 40 |
| CGAGG −101 to −97 ATCTT | 30 ± 3 | 99 ± 15 |
The maximum β-galactosidase specific activity in nanomoles of o-nitrophenyl phosphate per minute per milligram of protein (average ± 1 standard deviation) is listed for three independently isolated M. xanthus transformants (one determination each) in the case of mutant promoter regions and for one isolate in the case of the wild-type promoter (16 determinations) and vector controls (11 determinations). Samples were assayed at 0, 6, 12, 18, 24, 30, 36, and 48 h during development.
The wild-type promoter and vector-only strains were included in each experiment. The maximum for each mutant promoter region is expressed as a percentage of the maximum observed for the wild-type promoter in the same experiment, after subtracting from both values the maximum observed for vector only in that experiment. The average percentage ± 1 standard deviation is listed. A zero indicates that the expression from the mutant promoter region was equal to or slightly less than that observed for the vector-only control.
For example, mutant CGA −12 to −10 TAT has a mutation changing CGA at positions −12 to −10 bp to TAT, and mutant T −25 G has a mutation changing T at position −25 bp to G.
RESULTS
Deletion analysis of the Ω4499 promoter region.
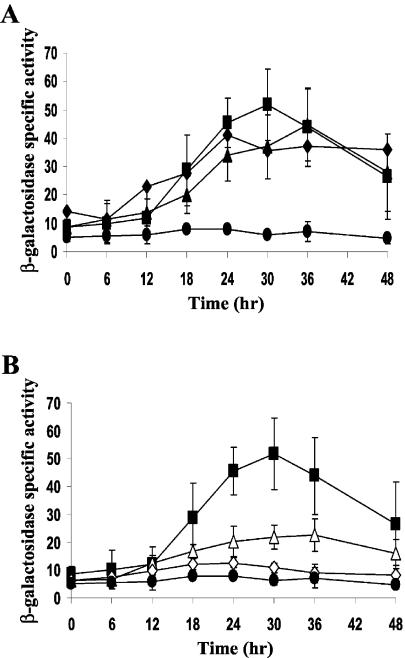
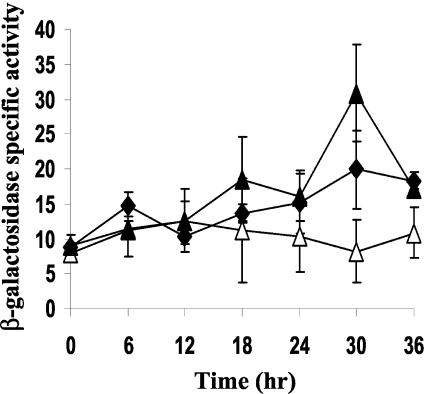
Previous analysis of the Ω4499 regulatory region showed that a segment containing from −218 bp to +2.68 kbp relative to the start site of transcription, fused to the E. coli lacZ gene and integrated at the Mx8 phage attachment site in the M. xanthus chromosome, showed a similar pattern of developmental lacZ expression as the M. xanthus strain, DK4299, which contains Tn5 lac Ω4499 (8). A 5′ deletion to −49 bp with the same 3′ end resulted in a dramatic decrease in expression. To further define the minimal region required for Ω4499 promoter activity, a DNA fragment spanning from −218 to +50 bp of the Ω4499 promoter region was generated by PCR, fused to lacZ, and tested for developmental expression (see Materials and Methods). Figure 1A shows that the segment from −218 to +50 bp directed a similar level of β-galactosidase production during development as the segment from −218 bp to +2.68 kbp. This demonstrates that the region between +50 bp and +2.68 kbp is not essential for Ω4499 promoter activity.
FIG. 1.
Deletion analysis of the Ω4499 promoter region. (A) Developmental lacZ expression was determined for M. xanthus strains bearing integrated plasmids with Ω4499 DNA from −218 bp to +2.68 kbp (⧫), −218 to +50 bp (▴), or −100 to +50 bp (▪), along with a vector, no-insert control (•). (B) The 5′-deletion constructs contained Ω4499 DNA from −71 to +50 bp (▵) or −61 to +50 bp (□). Constructs containing −100 to +50 bp (▪) or no insert (•) were included as controls. The data for the −100 to +50 bp construct are the same in both panels and represent six independent determinations made in three separate experiments. The average β-galactosidase activity is expressed as nanomoles of o-nitrophenyl phosphate per minute per milligram of protein. Error bars show 1 standard deviation of the data.
To further characterize the upstream boundary of the Ω4499 regulatory region, 5′ deletions were made to −100, −71, and −61 bp in the context of a 3′ end at +50 bp. The segment from −100 to +50 bp showed comparable developmental expression as the segment from −218 to +50 bp (Fig. 1A), indicating that DNA between −218 and −100 bp is not necessary for Ω4499 promoter activity. The deletion to −71 bp led to a 60% decrease in activity compared to the −218 to +50 bp promoter region (Fig. 1B and Table 2), indicating that DNA between −100 and −71 bp is important for Ω4499 activity. Furthermore, the 5′ deletion to −61 bp retained only 4% of wild-type promoter activity (Fig. 1B and Table 2), so DNA between −71 and −61 bp is essential for expression of the Ω4499 promoter.
Effects of mutations in the −25 to −10 bp region of the Ω4499 promoter.
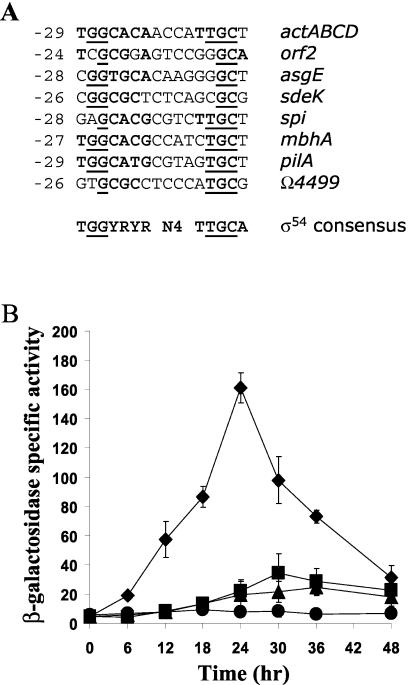
The product of the rpoN gene, σ54 (27), is believed to recognize several promoters in M. xanthus, including those for mbhA (43), sdeK (11), pilA (55), spi (15, 26), actABCD (14), and asgE and orf2 (10). An alignment of these promoter regions with the consensus sequence found in E. coli σ54-dependent promoters (49) is shown in Fig. 2A. The Ω4499 promoter matches the consensus sequence at four of seven positions in the −24 region and at three of five positions in the −12 region (Fig. 2A), suggesting that the Ω4499 promoter may be recognized by σ54 RNA polymerase. To test this hypothesis, two mutations were created in the context of the Ω4499 promoter region from −218 to +50 bp. One mutation was a T-to-G transversion at position −25 bp, which creates a better match to the E. coli σ54 consensus sequence (49) in the −24 region. This mutation decreased Ω4499 promoter activity by 50% (Fig. 2B and Table 2). In contrast, a mutation of CGA to TAT at −12 to −10 bp, which changes the highly conserved C in the −12 region to T and creates a perfect match in the −10 region to the consensus sequence recognized by E. coli σ70 (TATAAT) (38), resulted in a dramatic increase in promoter activity (Fig. 2B and Table 2). These results suggest that a σ factor in the σ70 family, rather than σ54, recognizes the Ω4499 promoter.
FIG. 2.
(A) Comparison of the Ω4499 promoter region to promoters of M. xanthus genes believed to be transcribed by σ54 RNA polymerase (see text for references). Also shown is the consensus sequence to which E. coli σ54 binds (49). The numbers to the left indicate the location within the promoter relative to the start site of transcription. The bold nucleotides indicate those that match the consensus sequence, and the underlined nucleotides match those most highly conserved in the consensus sequence. (B) Mutational analysis of the −25 to −10 bp region of the Ω4499 promoter. Developmental lacZ expression was determined for M. xanthus strains bearing integrated plasmids with a mutation at −12 to −10 bp from CGA to TAT (⧫) or a mutation at −25 bp from T to G (▴). The Ω4499 wild-type promoter region from −218 to +50 bp (▪) (four determinations in two experiments) and the vector with no promoter insert (•) were included as controls. The meaning of points and error bars is the same as described in the Fig. 1 legend.
Effects of mutations in the C box centered at −33 bp and adjacent regions.
The Ω4499 promoter region contains three sequences that match the C box consensus sequence (8). Among these, the one centered at −33 bp is 7 bp downstream of a 5-bp sequence (GAACT) that matches the 5-bp element consensus sequence (GAACA) in four of five positions (53). To determine if this C box functions in the same way as any of the C boxes mutated previously, eight mutations were made: a 7-bp change of the entire C box, and seven single-base-pair changes within the C box. These and all subsequent mutations reported here were made in the context of Ω4499 DNA from −218 to +50 bp.
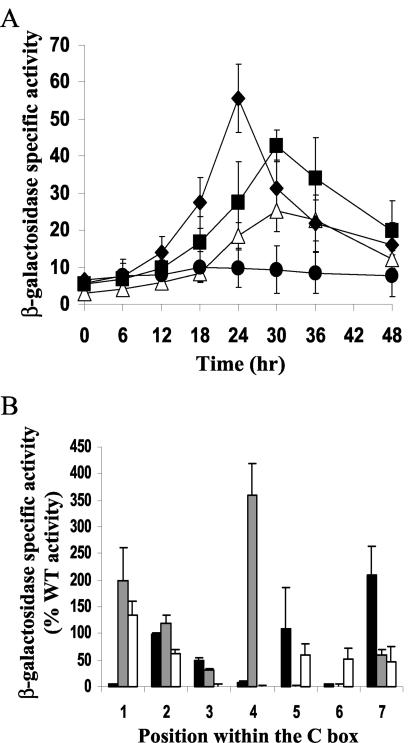
The 7-bp change of the entire C box caused a loss of promoter activity, as did single-base-pair mutations at −34 and −33 bp (Table 2). Single-base-pair mutations at −35, −32, −31, and −30 led to intermediate activity, and the mutation at −36 bp caused a slight increase in expression (Fig. 3A and Table 2).
FIG. 3.
Mutational analysis of the C box centered at −33 bp in the Ω4499 promoter. (A) Developmental lacZ expression was measured for M. xanthus strains with a T-to-G change at −30 bp (Δ) or a C-to-A change at −36 bp (⧫). The wild-type promoter region from −218 to +50 bp (▪) (seven determinations in three experiments) and the vector with no insert (•) were included as controls. The meaning of points and error bars is the same as described in the Fig. 1 legend. (B) Comparison of the effects of single-base-pair mutations in three different C boxes. The x axis represents the position in the C box corresponding to the consensus sequence 5′-CAYYCCY-3′, with the A being position 2, etc. The bars represent the average maximum developmental lacZ activity expressed as a percentage of the wild-type (WT) promoter activity for the C boxes centered at −49 bp in the Ω4400 (black) (56) and Ω4403 (gray) (53) promoter regions, or centered at −33 bp in the Ω4499 promoter region (white) (Table 2). Error bars show 1 standard deviation of the data.
The pattern of mutational effects observed was different than for any of the C boxes examined previously. Figure 3B compares the effects of single-base-pair mutations in the C box centered at −33 bp in the Ω4499 promoter with the effects of mutations in the C boxes centered at −49 bp in the Ω4400 (56) and Ω4403 (53) promoter regions. These C boxes have the sequence CATCCCT, which differs from the CATTCCT sequence centered at −33 bp in the Ω4499 promoter only at position 4. Except at this position, the single-base-pair changes compared in Fig. 3B are the same. Striking differences between the effects of mutations at positions 1, 4, 5, and 7 on Ω4400 and Ω4403 promoter activity indicated that the C boxes centered at −49 bp function differently. The effects of mutations in the C box centered at −33 bp in the Ω4499 promoter differed markedly from those in the Ω4400 C box at positions 1, 3, 6, and 7, and from those in the Ω4403 C box at positions 3, 5, and 6 (at position 4, a C-to-A change increases activity of the Ω4403 promoter, as indicated in Fig. 3B, but a C-to-G change abolishes activity [53], as does a T-to-G change in the Ω4499 promoter). A C box centered at −80 bp in the Ω4400 promoter has also been subjected to mutational analysis (56). Single-base-pair changes had less than a twofold effect on expression. We conclude that the Ω4499 C box centered at −33 bp functions differently than the other three C boxes that have been examined.
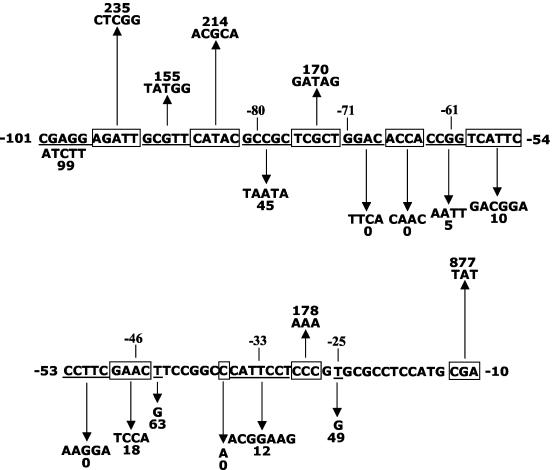
Two mutations were made in regions adjacent to the C box centered at −33 bp in the Ω4499 promoter. A C-to-A change at −37 bp led to a complete loss of promoter activity, while a CCC-to-AAA mutation from −29 to −27 bp increased activity nearly twofold (Fig. 4 and Table 2).
FIG. 4.
Summary of the effects of mutations in the Ω4499 promoter region. DNA subjected to mutagenesis is alternately underlined and boxed. Upward and downward arrows indicate that developmental lacZ expression was increased or decreased, respectively, by the given change in DNA sequence, and numbers indicate the maximum β-galactosidase specific activity observed for the mutant, expressed as a percentage of wild-type promoter activity measured in the same experiment (Table 2).
Effects of mutations in the 5-bp element.
The Ω4499 promoter region has a 5-bp element centered at −46 bp with the sequence GAACT, which matches the GAACA consensus sequence at four out of five positions (53). To determine if this 5-bp element is essential for expression, as are the 5-bp elements centered at −61 bp in the Ω4400 (56) and Ω4403 (53) promoters, two mutations were made. A 4-bp mutation, which converted GAAC at −48 to −45 bp to TCCA, resulted in a strong decrease (80%) in activity, demonstrating that this element is important for Ω4499 promoter expression (Fig. 4 and Table 2). A single-base-pair change from T to G at −44 bp retained 60% activity compared to the wild-type promoter (Fig. 4 and Table 2). This result is surprising because mutations at the corresponding position of the 5-bp elements centered at −61 bp in both the Ω4400 (56) and Ω4403 (53) promoter regions caused nearly complete loss of promoter activity. It appears that the 5-bp element, like the C box, functions somewhat differently in the Ω4499 promoter region than in the Ω4400 and Ω4403 promoter regions.
Effects of mutations between −71 and −49 bp.
Six mutations were made to investigate the role of DNA upstream of the 5-bp element centered at −46 bp, which our deletion analysis had indicated includes an element between −71 and −61 bp that is essential for Ω4499 promoter activity (Fig. 1B and Table 2). Five of these mutations are shown in Fig. 4. The sixth was a TCA-to-GAC mutation from −59 to −57 bp. All six mutations caused a dramatic decrease or loss of Ω4499 promoter activity. These results show that the entire region from approximately −70 to the 5-bp element centered at −46 bp is required for expression from the Ω4499 promoter.
Effects of mutations between −101 and −72 bp.
The region between −101 and −72 bp was divided into 5-bp sections that were mutated to attempt to define the element(s) that led to a 60% decrease in activity upon 5′ deletion to −71 bp (Fig. 1B and Table 2). Only one of the six mutations decreased Ω4499 promoter activity; changing GCCGC to TAATA from −81 to −77 bp lowered activity by 55% (Fig. 4 and Table 2), which is very similar to the decrease observed upon 5′ deletion to −71 bp. This shows that a small region approximately 29 bp upstream of the 5-bp element exerts a twofold positive effect on expression from the Ω4499 promoter.
C-signal dependence of the Ω4499 promoter.
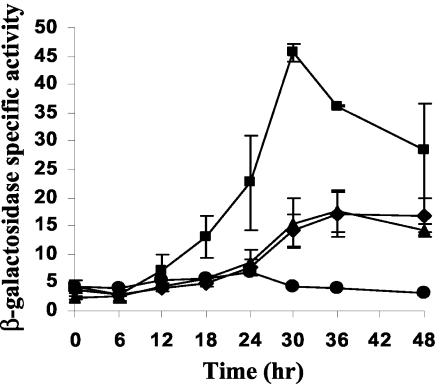
The Ω4499 promoter is partially dependent on C-signaling for expression (8, 31). In a csgA mutant defective in C-signaling, a twofold decrease in Ω4499 promoter activity has been observed. The loss in activity can be restored upon codevelopment with wild-type cells, which provide C-signal. Since a 5′ deletion to −71 bp resulted in about a twofold loss in expression (Fig. 1B and Table 2), we hypothesized that DNA upstream of −71 bp might be responsible for the partial C-signal dependence of the Ω4499 promoter, especially since DNA from −86 to −81 bp was shown previously to mediate, at least in part, the partial C-signal dependence of the Ω4400 promoter (56). We transformed pDY103, containing the Ω4499 promoter region from −71 to +50 bp, into csgA mutant M. xanthus DK5208 cells and measured developmental lacZ expression (Fig. 5). β-Galactosidase specific activity was lower in the csgA mutant than in the wild-type background, indicating that the 5′-deleted promoter region remains dependent on csgA. Addition of wild-type cells to the csgA mutant restored lacZ expression during development. This demonstrates that the promoter region remains responsive to extracellular C-signal despite the absence of DNA beyond −71 bp upstream. Similar results were observed for pDY134, which contains the GCCGC-to-TAATA mutation from −81 to −77 bp in the context of the Ω4499 promoter region from −218 to +50 bp (data not shown). Although this mutation causes a twofold decrease in expression in a wild-type background (Fig. 4 and Table 2), the mutant promoter region remains dependent on csgA and responsive to extracellular C-signal. We conclude that DNA upstream of −71 bp is not responsible for the partial C-signal dependence of the Ω4499 promoter.
FIG. 5.
C-signal dependence of a 5′-deleted Ω4499 promoter region. Developmental lacZ expression of pDY103 integrated at attB of wild-type DK1622 (⧫) or csgA mutant DK5208 in the absence (Δ) or presence (▴) of an equal number of wild-type DK1622 cells (lacking lacZ but capable of C signaling). The meaning of points and error bars is the same as described in the Fig. 1 legend.
Effects of sigD and sigE mutations.
Our mutational analysis suggests that the Ω4499 promoter is recognized by an σ factor in the σ70 family, rather than by σ54. σA RNA polymerase, the major form in growing cells (3), was unable to produce transcripts from the Ω4499 promoter in vitro (8). Also, a null mutation in sigB (encoding σB) or sigC (encoding σC) had no effect on Ω4499 expression (4). We tested the effect of a null mutation in sigD (51) or sigE (52) on expression from the wild-type Ω4499 promoter region (−218 to +50 bp). Both mutations led to decreased expression from the Ω4499 promoter, at about 30% of the wild-type level (Fig. 6). These results demonstrate that σD and σE directly or indirectly affect the activity of the Ω4499 promoter.
FIG. 6.
Effects of sigD and sigE mutations on expression from the Ω4499 promoter. Developmental β-galactosidase activity was determined for the wild-type Ω4499 promoter region from −218 to +50 bp fused to lacZ and integrated into the chromosome of M. xanthus sigD (⧫), sigE (▴), or wild-type DK1622 (▪) strains. The vector with no insert (•) served as a negative control. The meaning of points and error bars is the same as that described in the Fig. 1 legend.
DISCUSSION
Our characterization of the cis elements required for activity of the Ω4499 promoter provides further insight into C-signal-dependent gene regulation during M. xanthus development, especially when compared with previous mutational analyses of other promoter regions that depend on C-signaling for expression (53, 56). The other C-signal-dependent promoters examined so far do not resemble those thought to be recognized by σ54 RNA polymerase of M. xanthus. Our mutational analysis suggests that the Ω4499 promoter is not recognized by σ54 RNA polymerase either. The overall organization of the Ω4499 promoter region is much like that of the Ω4400 promoter region (56). Both include a large region spanning from about −30 to −60 or −70 bp with many sequence elements essential for promoter activity. Both also have a short (5- to 6-bp) region farther upstream (near −81 bp) that exerts a twofold positive effect on expression. Also, expression from both is reduced comparably in a sigE mutant. However, our results also reveal unique properties of Ω4499 promoter regulation. The effects of mutations in the C boxes are different than has been observed for other C-signal-dependent promoter regions. The short region near −81 bp does not appear to be necessary for C-signal dependence of the Ω4499 promoter, as it is for the Ω4400 promoter. Also, whereas a sigD mutation eliminates expression from the Ω4400 promoter, it does not completely abolish Ω4499 expression. We conclude that regulation of the Ω4499 operon exhibits both shared and unique properties in comparison with regulation of other C-signal-dependent genes.
Despite a resemblance between the Ω4499 promoter and M. xanthus promoters that are thought to be recognized by σ54 RNA polymerase, our mutational analysis did not support the idea that σ54 RNA polymerase is responsible for transcription form the Ω4499 promoter. In the alignment shown in Fig. 2A, none of the putative σ54-dependent promoters have a T at the position corresponding to the T at −25 bp in the Ω4499 promoter. Five out of seven have a G at that position, as does the E. coli σ54 consensus sequence (49). A mutation from T to G at −25 bp was expected to increase, or possibly not change, activity of the Ω4499 promoter, if it were recognized by σ54 RNA polymerase. However, the mutation led to a twofold loss in activity (Fig. 2B and Table 2). Conversely, mutating the perfectly conserved C at −12 bp in the Ω4499 promoter was expected to decrease activity. Instead, changing CGA to TAT at −12 to −10 bp led to an eightfold increase in activity (Fig. 2B and Table 2). Taken together, the two results suggest that the Ω4499 promoter is not recognized by σ54 RNA polymerase. These findings call into question whether all of the promoters shown in Fig. 2A really are σ54-dependent promoters. Only the spi promoter has been subjected to detailed mutational analysis, and the results support the idea that this promoter is recognized by σ54 RNA polymerase (26).
Why did the CGA-to-TAT change at −12 to −10 bp increase activity of the Ω4499 promoter? The change creates a perfect match in the −10 region of the mutant promoter to the consensus sequence recognized by E. coli σ70 RNA polymerase (38). Therefore, the high activity of the mutant promoter could reflect better recognition and/or initiation by RNA polymerase with a σ factor in the σ70 family. Its is noteworthy that the mutant promoter was no more active during growth than the wild-type promoter (Fig. 2B). Also, the time of maximum lacZ expression from the mutant promoter was similar to that for the wild-type promoter (Fig. 2B). Whether the mutant promoter is transcribed by RNA polymerase(s) with the same σ factor(s) as the wild-type Ω4499 promoter remains an open question.
The Ω4400 and Ω4403 promoters, which are the only other C-signal-dependent promoters so far characterized, do not resemble σ54 promoters (4, 9). Neither these promoters (D. Biran and L. Kroos, unpublished data) nor the Ω4499 promoter (8) directed transcription by M. xanthus σA RNA polymerase in vitro. σA RNA polymerase is the major form of RNA polymerase in growing M. xanthus cells (3). It was able to transcribe from the Ω4514 promoter in vitro, but this developmentally regulated promoter does not depend on C-signaling for expression, and its −35 region matches perfectly the consensus sequence (TTGACA) recognized by E. coli σ70 RNA polymerase (17). In contrast, the −35 regions of the three C-signal-dependent promoters do not match this consensus sequence (4, 8, 9). One or more transcription factors bound to upstream DNA elements in the C-signal-dependent promoter regions might enable σA RNA polymerase to transcribe from these promoters, or a different σ factor might be involved.
In addition to σA, six other σ factors in the σ70 family have been described in M. xanthus (1, 2, 5, 20, 51, 52, 54). Among these, σB and σC do not appear to be responsible for transcription of Ω4499, Ω4400, or Ω4403, since sigB and sigC mutants exhibited normal expression of lacZ reporters fused to these genes (4). On the other hand, sigD and sigE mutants showed reduced expression from the Ω4499 promoter (Fig. 6). Since mutations in sigD block aggregation (51), the effect on Ω4499 expression might be indirect. Interestingly, in the sigD mutant, the Ω4499 promoter retained 30% as much activity as in wild type (Fig. 6), whereas the Ω4400 promoter retained no activity (56). Apparently, one or more transcription factors essential for Ω4400 promoter activity is missing, or its level is insufficient, in the sigD mutant, but this does not prevent a low level of transcription from the Ω4499 promoter. Unlike the sigD mutant, the sigE mutant appears to aggregate normally (52). Yet, Ω4499 expression was reduced in the sigE mutant to a similar extent as in the sigD mutant (Fig. 6). The reduction in Ω4499 expression in the sigE mutant is comparable to that seen previously for expression from the Ω4400 promoter (56). This may imply that σE RNA polymerase is partially responsible for transcription from the Ω4499 and Ω4400 promoters. The proposed functional redundancy of σE with the highly similar σB and σC (52) may account for the residual transcription observed in the sigE mutant (Fig. 6). Alternatively, the effect of the sigE mutation on Ω4499 expression may be indirect.
The Ω4499 promoter region is unique among C-signal-dependent promoters examined thus far in terms of the positions of C boxes and 5-bp elements. It has C boxes centered at −33 and −55 bp (8) with 5-bp elements located 7 and 8 bp upstream, respectively (53). There is also a C box centered at −1 bp (8), but there is no apparent 5-bp element 5 to 10 bp upstream, and we did not test the effects of mutations in this C box. The Ω4400 and Ω4403 promoter regions have the identical C box (CATCCCT) centered at −49 bp, and in each case a 5-bp element is located 6 bp upstream, centered at −61 bp (53). Also, the Ω4400 promoter region has a C box centered at −80 bp, which is in the opposite orientation as the one centered at −49 bp (8), and has no apparent 5-bp element located 5 to 10 bp away in the 5′ direction.
We chose to perform detailed mutational analysis of the C box centered at −33 bp in the Ω4499 promoter region because it matches the C boxes centered at −49 bp in the Ω4400 and Ω4403 promoter regions more closely (six out of seven positions) than does the C box centered at −55 bp (five out of seven positions), and because its distance from the 5-bp element was more similar to that in the Ω4400 and Ω4403 promoter regions (7 bp versus 6 bp) than for the C box centered at −55 bp (8 bp versus 6 bp). However, we found that single-base-pair changes in the Ω4499 C box centered at −33 bp had a very different pattern of effects on lacZ expression than did changes in the Ω4400 or Ω4403 C box centered at −49 bp (Fig. 3B), or the Ω4400 C box centered at −80 bp (53, 56). Each C box appears to function somewhat differently. Conceivably, the Ω4499 C box centered at −55 bp might behave in a more similar fashion to one of the other C boxes, but that would be a break from the results observed so far, and it remains to be tested.
In keeping with the observation of different effects of mutations in similar sequences, each 5-bp element examined so far behaves differently with respect to single-base-pair changes, although the mutational analysis is much less complete than for C boxes. In this study, a T-to-G change at −44 bp had relatively little effect on Ω4499 promoter activity (Fig. 4 and Table 2) in comparison to changes at the corresponding position (−59 bp) of the 5-bp elements centered at −61 bp in the Ω4400 and Ω4403 promoter regions (53, 56). In prior studies, the effects of changing C to A at −60 bp in the Ω4400 and Ω4403 promoter regions were shown to be very different (53, 56).
Given that different effects of mutations in similar sequences is observed for both the 5-bp elements and the C boxes and given the similar distance between these cis-acting DNA elements in all three C-signal dependent promoters examined so far, we propose that a 5-bp element and a C box together constitute a recognition site for a transcription factor and that different transcription factors bind to these recognition sites in the Ω4499, Ω4400, and Ω4403 promoter regions.
The DNA between the C box and the 5-bp element may be part of a transcription factor recognition site in some cases, but not others. Changing CCGG to AATT between the C box centered at −55 bp in the Ω4499 promoter region and the 5-bp element that lies 8 bp upstream nearly abolished expression (Fig. 4 and Table 2). Likewise, changing the C at −37 bp, which is the first base pair upstream of the Ω4499 C box centered at −33 bp, abolished promoter activity (Fig. 4 and Table 2). A single-base-pair change at the position immediately upstream of the C box centered at −49 bp in the Ω4400 or Ω4403 promoter region also greatly reduced expression, as did a change from GTCCC to TGAAA between the Ω4400 C box centered at −49 bp and the 5-bp element centered at −61 bp (53, 56). On the other hand, changing CCGTC to AATGA at the corresponding position in the Ω4403 promoter region caused a 1.5-fold increase in expression and deleting the CCGTC segment abolished promoter activity (53), suggesting that the segment is an essential spacer between the C box and the 5-bp element but may not be part of a recognition site for a sequence-specific DNA-binding protein.
If our hypothesis that a 5-bp element and a C box (and in some cases the DNA in between) together constitute a recognition site for a transcription factor is correct, it is intriguing that the Ω4499 promoter regions has two such sites in tandem. The more upstream site is located upstream of the region typically occupied by RNA polymerase, while the downstream site overlaps the promoter −35 region. Hence, the upstream and downstream sites are located at positions occupied by the E. coli catabolite activator protein (CAP) in class I and class II CAP-dependent promoters (6). The basic features of transcription activation at class I and class II CAP-dependent promoters are understood and appear to be generalizable to other activators. Perhaps one or more transcription factors bind to the putative two sites in the Ω4499 promoter region and activate transcription by contacting RNA polymerase, facilitating formation of closed and open RNA polymerase-promoter complexes, as does CAP. According to this model, the C boxes centered at −49 bp and 5-bp elements centered at −61 bp in the Ω4400 and Ω4403 promoter regions would each constitute a single site located at a position analogous to that occupied by CAP in class I CAP-dependent promoters. Based on the different effects of mutations in these putative transcription factor recognition sites (Fig. 3B), we speculate that a family of sequence-specific DNA-binding proteins might interact in different ways with similar sequences in the three C-signal-dependent promoter regions. Alternatively, a single protein might bind differently to the putative recognition sites by adopting different conformations, possibly due to different states of posttranslational modification, interactions with other proteins, and/or the influence of DNA adjacent to the putative recognition sites.
The Ω4499 promoter region shares with Ω4400 and Ω4403 promoter regions the requirement for DNA farther upstream, beyond the 5-bp elements, for full promoter activity. In each case, these DNA elements are separated from the 5-bp elements by 5 to 17 bp of DNA in which transversion mutations have little effect on promoter activity (Fig. 4 and Table 2) (53, 56). Both the Ω4499 and Ω4400 promoter regions contain a small element near −81 bp that exerts a twofold to fourfold positive effect on expression. The boundaries of these elements are not well defined. In the Ω4499 promoter region, the element is defined by a mutation that changes GCCGC to TAATA at −81 to −77 bp, resulting in a twofold decrease in promoter activity (Fig. 4 and Table 2). In the Ω4400 promoter region, mutations that change GTC to TGA at −86 to −84 bp, and G to T at −81 bp, result in a fourfold and a twofold decrease in activity, respectively, defining an element with the sequence GTCGGG (56). This sequence is not strikingly similar to the GCCGC sequence in the Ω4499 promoter region. Both are GC rich, but such sequences are common in the M. xanthus genome with its high (near 70%) G+C content. In the Ω4403 promoter region, the sequence GGCATGTTCA from −79 to −70 bp has been called a 10-bp element (53). Single-base-pair transversions at any position in this element decrease expression more than twofold, and many abolish expression completely.
The element from −86 to −81 bp in the Ω4400 promoter region was shown to be responsible, at least in part, for the partial dependence of the promoter on C-signaling (56). It was attractive to think that the element from −81 to −77 bp in the Ω4499 promoter region might play the same role, since activity of this promoter also depends partially on C-signaling (8, 31). However, this does not appear to be the case. A segment lacking Ω4499 DNA upstream of −71 bp was still C-signal dependent (Fig. 5). Another candidate sequence to mediate C-signal dependence of the Ω4499 promoter was a 9-bp sequence centered at −65 bp, which matches a 9-bp sequence centered at −80 bp in the Ω4400 promoter region (8). However, transversion mutations at −80 to −76 bp had little effect on Ω4400 promoter activity (56) and, in contrast, mutations at −67 to −60 bp nearly abolished Ω4499 promoter activity (Fig. 4 and Table 2), and so despite their similarity, the 9-bp sequences function differently. Further studies of the Ω4499 promoter region will aim to identify and characterize the trans-acting factors that bind to the important cis-acting DNA elements defined by our mutational analysis. There do not appear to be binding sites in the Ω4499 promoter for an NtrC-like activator (42) such as ActB (14), or for the CAP-like activator MrpC (50), or for protein X (19). Of the putative M. xanthus transcription factors, FruA (7, 40) is the best candidate for a protein that binds to the Ω4499 regulatory region; however, FruA has not yet been reported to bind DNA.
Acknowledgments
We thank D. Oluwole for constructing pDO2 and pDO3 and S. Inouye for providing the sigD and sigE mutant M. xanthus strains.
This research was supported by NSF grant MCB-0090478 and by the Michigan Agricultural Experiment Station.
REFERENCES
- 1.Apelian, D., and S. Inouye. 1990. Development-specific σ-factor essential for late-stage differentiation of Myxococcus xanthus. Genes Dev. 4:1396-1403. [DOI] [PubMed] [Google Scholar]
- 2.Apelian, D., and S. Inouye. 1993. A new putative sigma factor of Myxococcus xanthus. J. Bacteriol. 175:3335-3342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Biran, D., and L. Kroos.1997. In vitro transcription of Myxococcus xanthus genes with RNA polymerase containing σA, the major sigma factor in growing cells. Mol. Microbiol. 25:463-472. [DOI] [PubMed] [Google Scholar]
- 4.Brandner, J. P., and L. Kroos. 1998. Identification of the Ω4400 regulatory region, a developmental promoter of Myxococcus xanthus. J. Bacteriol. 180:1995-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Browning, D. F., D. E. Whitworth, and D. A. Hodgson. 2003. Light-induced carotenogenesis in Myxococcus xanthus: functional characterization of the ECF sigma factor CarQ and antisigma factor CarR. Mol. Microbiol. 48:237-251. [DOI] [PubMed] [Google Scholar]
- 6.Busby, S., and R. H. Ebright. 1999. Transcription activation by catabolite activator protein (CAP). J. Mol. Biol. 293:199-213. [DOI] [PubMed] [Google Scholar]
- 7.Ellehauge, E., M. Norregaard-Madsen, and L. Sogaard-Andersen. 1998. The FruA signal transduction protein provides a checkpoint for the temporal co-ordination of intercellular signals in Myxococcus xanthus development. Mol. Microbiol. 30:807-817. [DOI] [PubMed] [Google Scholar]
- 8.Fisseha, M., D. Biran, and L. Kroos. 1999. Identification of the Ω4499 regulatory region controlling developmental expression of a Myxococcus xanthus cytochrome P-450 system. J. Bacteriol. 181:5467-5475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fisseha, M., M. Gloudemans, R. Gill, and L. Kroos. 1996. Characterization of the regulatory region of a cell interaction-dependent gene in Myxococcus xanthus. J. Bacteriol. 178:2539-2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garza, A. G., B. Z. Harris, B. M. Greenberg, and M. Singer. 2000. Control of asgE expression during growth and development of Myxococcus xanthus. J. Bacteriol. 182:6622-6629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garza, A. G., J. S. Pollack, B. Z. Harris, A. Lee, I. M. Keseler, E. F. Licking, and M. Singer. 1998. SdeK is required for early fruiting body development in Myxococcus xanthus. J. Bacteriol. 180:4628-4637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gill, R. E., and M. G. Cull. 1986. Control of developmental gene expression by cell-to-cell interactions in Myxococcus xanthus. J. Bacteriol. 168:341-347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gronewold, T. M., and D. Kaiser. 2002. act operon control of developmental gene expression in Myxococcus xanthus. J. Bacteriol. 184:1172-1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gronewold, T. M., and D. Kaiser. 2001. The act operon controls the level and time of C-signal production for Myxococcus xanthus development. Mol. Microbiol. 40:744-756. [DOI] [PubMed] [Google Scholar]
- 15.Gulati, P., D. Xu, and H. Kaplan. 1995. Identification of the minimum regulatory region of a Myxococcus xanthus A-signal-dependent developmental gene. J. Bacteriol. 177:4645-4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hanahan, D. 1983. Studies on transformation of Escherichia coli with plasmids. J. Mol. Biol. 166:557-580. [DOI] [PubMed] [Google Scholar]
- 17.Hao, T., D. Biran, G. J. Velicer, and L. Kroos. 2002. Identification of the Ω4514 regulatory region, a developmental promoter of Myxococcus xanthus that is transcribed in vitro by the major vegetative RNA polymerase. J. Bacteriol. 184:3348-3359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hodgkin, J., and D. Kaiser. 1977. Cell-to-cell stimulation of motility in nonmotile mutants of Myxococcus. Proc. Natl. Acad. Sci. USA 74:2938-2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Horiuchi, T., T. Akiyama, S. Inouye, and T. Komano. 2003. Regulation of FruA expression during vegetative growth and development of Myxococcus xanthus. J. Mol. Microbiol. Biotechnol. 5:87-96. [DOI] [PubMed] [Google Scholar]
- 20.Inouye, S. 1990. Cloning and DNA sequence of the gene coding for the major sigma factor from Myxococcus xanthus. J. Bacteriol. 172:80-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaiser, D. 2003. Coupling cell movement to multicellular development in myxobacteria. Nat. Rev. Microbiol. 1:45-54. [DOI] [PubMed] [Google Scholar]
- 22.Kaiser, D. 1979. Social gliding is correlated with the presence of pili in Myxococcus xanthus. Proc. Natl. Acad. Sci. USA 76:5952-5956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaplan, H. 2003. Multicellular development and gliding motility in Myxococcus xanthus. Curr. Opin. Microbiol. 6:572-577. [DOI] [PubMed] [Google Scholar]
- 24.Kaplan, H. B., and L. Plamann. 1996. A Myxococcus xanthus cell density-sensing system required for multicellular development. FEMS Microbiol. Lett. 139:89-95. [DOI] [PubMed] [Google Scholar]
- 25.Kashefi, K., and P. Hartzell. 1995. Genetic suppression and phenotypic masking of a Myxococcus xanthux frzF− defect. Mol. Microbiol. 15:483-494. [DOI] [PubMed] [Google Scholar]
- 26.Keseler, I., and D. Kaiser. 1995. An early A-signal-dependent gene in Myxococcus xanthus has a σ54-like promoter. J. Bacteriol. 177:4638-4644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Keseler, I., and D. Kaiser. 1997. σ54, a vital protein for Myxococcus xanthus. Proc. Natl. Acad. Sci. USA 94:1979-1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim, S. K., and D. Kaiser. 1991. C-factor has distinct aggregation and sporulation thresholds during Myxococcus development. J. Bacteriol. 173:1722-1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim, S. K., and D. Kaiser. 1990. C-factor: a cell-cell signaling protein required for fruiting body morphogenesis of M. xanthus. Cell 61:19-26. [DOI] [PubMed] [Google Scholar]
- 30.Kroos, L., and D. Kaiser. 1984. Construction of Tn5 lac, a transposon that fuses lacZ expression to exogenous promoters, and its introduction into Myxococcus xanthus. Proc. Natl. Acad. Sci. USA 81:5816-5820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kroos, L., and D. Kaiser. 1987. Expression of many developmentally regulated genes in Myxococcus depends on a sequence of cell interactions. Genes Dev. 1:840-854. [DOI] [PubMed] [Google Scholar]
- 32.Kroos, L., A. Kuspa, and D. Kaiser. 1986. A global analysis of developmentally regulated genes in Myxococcus xanthus. Dev. Biol. 117:252-266. [DOI] [PubMed] [Google Scholar]
- 33.Kuspa, A., L. Kroos, and D. Kaiser. 1986. Intercellular signaling is required for developmental gene expression in Myxococcus xanthus. Dev. Biol. 117:267-276. [DOI] [PubMed] [Google Scholar]
- 34.Kuspa, A., L. Plamann, and D. Kaiser. 1992. Identification of heat-stable A-factor from Myxococcus xanthus. J. Bacteriol. 174:3319-3326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kuspa, A., L. Plamann, and D. Kaiser. 1992. A-signalling and the cell density requirement for Myxococcus xanthus development. J. Bacteriol. 174:7360-7369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee, B.-U., K. Lee, J. Mendez, and L. Shimkets. 1995. A tactile sensory system of Myxococcus xanthus involves an extracellular NAD(P)+-containing protein. Genes Dev. 9:2964-2973. [DOI] [PubMed] [Google Scholar]
- 37.Li, S.-F., B. Lee, and L. J. Shimkets. 1992. csgA expression entrains Myxococcus xanthus development. Genes Dev. 6:401-410. [DOI] [PubMed] [Google Scholar]
- 38.Lisser, S., and H. Margalit. 1993. Compilation of E. coli mRNA promoter sequences. Nucleic Acids Res. 21:1507-1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lobedanz, S., and L. Sogaard-Andersen. 2003. Identification of the C-signal, a contact-dependent morphogen coordinating multiple developmental responses in Myxococcus xanthus. Genes Dev. 17:2151-2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ogawa, M., S. Fujitani, X. Mao, S. Inouye, and T. Komano. 1996. FruA, a putative transcription factor essential for the development of Myxococcus xanthus. Mol. Microbiol. 22:757-767. [DOI] [PubMed] [Google Scholar]
- 41.Plamann, L., A. Kuspa, and D. Kaiser. 1992. Proteins that rescue A-signal-defective mutants of Myxococcus xanthus. J. Bacteriol. 174:3311-3318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reitzer, L. J., and B. Magasanik. 1986. Transcription of glnA in E. coli is stimulated by activator bound to sites far from the promoter. Cell 45:785-792. [DOI] [PubMed] [Google Scholar]
- 43.Romeo, J. M., and D. R. Zusman. 1991. Transcription of the myxobacterial hemagglutinin gene is mediated by a F54-like promoter and a cis-acting upstream regulatory region of DNA. J. Bacteriol. 173:2969-2976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sambrook, J., E. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 45.Shimkets, L. J. 1999. Intercellular signaling during fruiting-body development of Myxococcus xanthus. Annu. Rev. Microbiol. 53:525-549. [DOI] [PubMed] [Google Scholar]
- 46.Shimkets, L. J., and S. J. Asher. 1988. Use of recombination techniques to examine the structure of the csg locus of Myxococcus xanthus. Mol. Gen. Genet. 211:63-71. [DOI] [PubMed] [Google Scholar]
- 47.Shimkets, L. J., R. E. Gill, and D. Kaiser. 1983. Developmental cell interactions in Myxococcus xanthus and the spoC locus. Proc. Natl. Acad. Sci. USA 80:1406-1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sogaard-Andersen, L., M. Overgaard, S. Lobedanz, E. Ellehauge, L. Jelsbak, and A. A. Rasmussen. 2003. Coupling gene expression and multicellular morphogenesis during fruiting body formation in Myxococcus xanthus. Mol. Microbiol. 48:1-8. [DOI] [PubMed] [Google Scholar]
- 49.Thony, B., and H. Hennecke. 1989. The −24/−12 promoter comes of age. FEMS Microbiol. Rev. 5:341-357. [DOI] [PubMed] [Google Scholar]
- 50.Ueki, T., and S. Inouye. 2003. Identification of an activator protein required for the induction of fruA, a gene essential for fruiting body development in Myxococcus xanthus. Proc. Natl. Acad. Sci. USA 100:8782-8787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ueki, T., and S. Inouye. 1998. A new sigma factor, SigD, essential for stationary phase is also required for multicellular differentiation in Myxococcus xanthus. Genes Cells 3:371-385. [DOI] [PubMed] [Google Scholar]
- 52.Ueki, T., and S. Inouye. 2001. SigB, SigC, and SigE from Myxococcus xanthus homologous to σ32 are not required for heat shock response but for multicellular differentiation. J. Mol. Microbiol. Biotechnol. 3:287-293. [PubMed] [Google Scholar]
- 53.Viswanathan, P., and L. Kroos. 2003. cis elements necessary for developmental expression of a Myxococcus xanthus gene that depends on C signaling. J. Bacteriol. 185:1405-1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ward, M., H. Lew, A. Treuner-Lange, and D. Zusman. 1998. Regulation of motility behavior in Myxococcus xanthus may require an extracytoplasmic-function sigma factor. J. Bacteriol. 180:5668-5675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wu, S. S., and D. Kaiser. 1997. Regulation of expression of the pilA gene in Myxococcus xanthus. J. Bacteriol. 179:7748-7758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yoder, D., and L. Kroos. 2004. Mutational analysis of the Myxococcus xanthus Ω4400 promoter region provides insight into developmental gene regulation by C signaling. J. Bacteriol. 186:661-671. [DOI] [PMC free article] [PubMed] [Google Scholar]