Abstract
Recent studies have revealed a novel mechanism of myosin regulation in which the actin-binding protein tropomyosin converts atypical type-V myosins into processive cargo transporters. To achieve this, tropomyosin's primary role appears to lie in its ability to influence myosin's enzyme kinetics, prolonging the strong actin-bound ADP/apo state to enable hand-over-hand walking of myosin-V dimers along actin tracks. Activation of myosin-V mediated transport by tropomyosin underscores its function in helping to direct cargos to specific actin tracks and subcellular destinations. This type of regulation supports the broader notion that tropomyosin plays a key role in actomyosin sorting.
Keywords: actin, tropomyosin, myosin-V, duty ratio, processivity, intracellular transport, actomyosin sorting
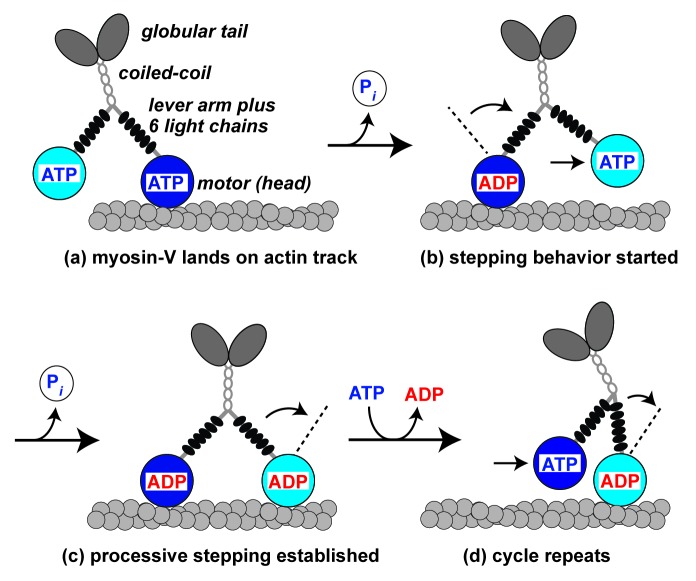
Class-V myosins are dimeric actin filament-based motors made up of four distinct domains (Fig. 1). The N-terminal heads or motor domains bind actin and hydrolyze ATP. Using the free energy generated from ATP hydrolysis, these motor domains propagate a conformational change that is amplified by the light chain-bound lever arm to generate the stepping action of the molecule (Fig. 1). A coiled coil region facilitates dimerization, followed by a C-terminal globular tail domain that attaches to cargo.
Figure 1. Actin filament-based motility of a processive myosin-V molecule. Myosin-V molecules are made up of four major domains: the ATPase and actin-binding motor (or head), the lever arm (stabilized through its association with six light chains), the coiled-coil region (for dimerization), and the C-terminal globular tail (or cargo-binding) domain. (a) The leading myosin-V head (dark blue) initially associates with actin in the weakly bound ATP (or ADP.Pi) state. (b) Pi release triggers movement into the strong actin-bound ADP state coupled with a conformational change that is amplified by a swinging motion of the lever arm. This in turns leads to further forward displacement of the molecule as the trailing head (light blue) moves into the lead position. (c) Processive myosin-Vs delay movement out of the strong actin-bound ADP/apo states, allowing sufficient time for the trailing head to move into the leading position, associate with actin, and establish the strong actin-bound ADP state. (d) The original leading head exchanges ADP for ATP, detaches from actin, and becomes the trailing head as repeated cycles of stepping propagate processive movement. Non-processive myosin-Vs fail to secure strong actin-binding of the trailing head before the ATP-induced dissociation of the leading head (and thus fail to move past stage b).
Myosin-Vs often function as transporters that traffic intracellular cargoes to different locations in the cell via actin filament-based motility. Processive motors, molecules which can take multiple steps along tracks without dissociating, represent efficient cargo transporters. In line with their function, most myosin-Vs examined to date use hand-over-hand walking of their two heads to move processively along actin tracks (Fig. 1). However, recent studies have shown that some myosin-Vs are intrinsically non-processive. These include HsMyosin V-C (Homo sapiens),1,2 DmMyosin V (Drosophila melanogaster),3 ScMyo2p (budding yeast; Saccharomyces cerevisiae),4,5 and SpMyo52p (fission yeast; Schizosaccharomyces pombe).6,7 Here, we highlight two studies which demonstrate how modifying the actin track can activate myosin-V processivity.
Non-processive myosin-Vs free in solution lack the ability to move along bare actin filaments in vitro. However, many of the actin tracks encountered by myosin in the cell are far from bare. Actin and myosin work together to perform a variety of functions which depend on a multitude of actin-binding proteins that regulate both the self-assembly and action of different actomyosin structures. Examples include proteins that nucleate, sever, cap, or crosslink actin filaments. Tropomyosins are a conserved family of proteins that bind along the length of unbranched actin filaments.8 They are homodimeric coiled-coil proteins that form strand-like molecules that polymerize along the actin filament via associations between their N and C termini.9 Mammals possess > 40 isoforms of tropomyosin generated by alternative splicing of four genes.10 This variability is thought to contribute to the specification of different types of actin structures owing to tropomyosin's ability to influence other downstream actin-binding proteins.11 The simple model systems provided by yeast offer reduced complexity in terms of tropomyosin expression: budding yeast expresses two tropomyosin isoforms, ScTpm1p and ScTpm2p, whereas fission yeast expresses just one, SpCdc8p. Interestingly, SpCdc8p is regulated by acetylation of its N-terminus, which differentiates this single isoform into two distinct populations. Acetylation of SpCdc8p is important for the assembly and constriction of contractile actomyosin rings, where the acetylated form predominates at cytokinesis.12 In contrast, unacetylated SpCdc8p decorates the actin cables that serve as tracks for SpMyo52p-mediated transport.12-14 ScTpm1p and ScTpm2p coat the actin cables that support myosin-V-mediated transport in budding yeast.15 Previous studies had shown that the major myosin-V transporters from each system were non-processive.4-6 More recent studies from Hodges et al.16 and Clayton et al.7 examined how tropomyosin influences cargo transport by the respective myosin-Vs (ScMyo2p or SpMyo52p).
Hodges et al.16 employed purified budding yeast components to reconstitute cargo transport in vitro and found that the processive movements of ScMyo2p were supported by actin decorated with tropomyosin.16 Tropomyosin-mediated regulation of ScMyo2p did not depend on the type of actin employed, with both skeletal muscle and budding yeast actin being capable of supporting processivity.16 Using the purified components from fission yeast, Clayton et al.7 found that SpMyo52p required decoration of actin filaments with SpCdc8p to move processively. Moreover, physiologically relevant, reconstituted cargos carrying multiple SpMyo52p molecules also relied on SpCdc8p to move along actin.7 Tropomyosin, rather than the motor number on a particular cargo, likely activates cargo transport in vivo given cargo-binding deficient SpMyo52p motors still exhibited actin-dependent transport in fission yeast.7 Given the large evolutionary distance between the two yeasts, the studies by Hodges et al.16 and Clayton et al.7 suggest a novel and conserved mechanism of myosin-V based regulation. We predict that tropomyosin isoforms may also regulate non-processive myosin-Vs (and potentially other classes of myosin) from higher eukaryotes.
Kinetic Mechanism Of Tropomyosin-Mediated Processivity
How does tropomyosin switch on myosin-V processivity? First we must consider that, like all myosins, myosin-V is an actin-dependent ATPase. Processivity depends on the myosin's duty ratio, which is the proportion of time each head of the myosin is strongly attached to actin in its ADP-bound or apo state per ATPase cycle. Duty ratio depends on the kinetics with which myosin enters and exits the strong- and weakly- bound (ATP) phases of its ATPase cycle. In order to increase the likelihood that at least one head remains attached to actin, a processive myosin must have an average duty ratio ≥ 50%. Based on actin-activated ATPase and other assays, Stark et al.17 demonstrated that SpCdc8p increased the actin affinity of the essential type-II myosin from fission yeast, SpMyo2p. Furthermore, SpCdc8p increased the sensitivity of SpMyo2p to ADP,17 which slows the transition out of the strong actin-binding state. This suggested that tropomyosin could increase SpMyo2p's affinity for ADP and favor the strong-bound state. While the estimated duty ratio of this type-II myosin was enhanced by tropomyosin, it was too low to support processivity.17 This is consistent with the physiological role of type-II myosins working in groups to provide tension at the contractile ring during cytokinesis.17 Tropomyosin also enhances the actin-activated ATPase activity and actin affinity of SpMyo52p.6 Additionally, in vitro motility assays were used to show that tropomyosin increases the duty ratio of SpMyo52p.6
Mechanistically, movement of myosin into the strong actin-binding ADP state is associated with inorganic phosphate (Pi) release (Fig. 1). Subsequently, ADP must be released and ATP must bind in order for the motor to dissociate from actin (Fig. 1). To ascertain differences in duty ratio, transients can be measured to assess the rates of these steps. Employing budding yeast proteins in transient kinetics experiments, Hodges et al.16 found that tropomyosin slowed the apparent ADP dissociation and the ATP-induced actomyosin dissociation rates of ScMyo2p. These data, combined with the positive tropomyosin-mediated changes in fission yeast myosin duty ratios (see above), suggest a mechanism where tropomyosin slows actomyosin dissociation steps leading to an increased duty ratio that can support myosin-V processivity (Fig. 2). Potentially, tropomyosin may also increase the Pi release rate of yeast myosin-Vs to promote time spent in the strong actin-bound state. While tropomyosin-dependent processivity may largely rely on duty ratio enhancement, other types of regulation may also contribute.

Figure 2. Tropomyosin-mediated myosin-V processivity. The non-processive yeast myosin-Vs have a low duty ratio, spending only a small proportion (< 50 %) of their ATPase cycle in the strong actin-bound ADP or apo states. This property prevents processive stepping of dimeric molecules along actin. However, the presence of tropomyosin on actin cables in the cell promotes an increase in the myosin-V duty ratio as motors now spend > 50 % of their ATPase cycle in the strong actin-bound state. This change in the kinetics facilitates processive stepping of myosin-V molecules along the actin track.
Additional Mechanisms Contributing to Tropomyosin-Mediated Processivity
A duty ratio-based mechanism does not fully explain some of the findings of Hodges et al.16 and Clayton et al.7 Both studies examined processive runs at low ATP concentration (10μM), where the duty ratio is artificially high (> 95%) due to limited ATP-driven actomyosin dissociation. Under these conditions, the presence of tropomyosin still enhanced the run-length and frequency of motile events. Hodges et al.16 proposed that increased run-length exhibited by myosin along actin-tropomyosin may be due to improved gating between the myosin heads (i.e. coordination between myosin heavy chains to facilitate efficient stepping). Specifically, tropomyosin may help to delay dissociation of the attached head to allow appropriate time for the trailing head to enter the strong bound state. This type of regulation could be an important feature of tropomyosin because poor gating would increase the frequency of both heads simultaneously dissociating from actin, regardless of duty ratio.
A model based on cooperative actomyosin binding may also provide insight into the mechanism of how tropomyosin mediates processivity. Such a mechanism takes its roots in the regulation of actomyosin in striated muscle. Here, the formation of an initial actomyosin cross-bridge changes the position of tropomyosin on the actin filament, facilitating movement from the closed state (where tropomyosin partially obscures myosin binding sites) to the open state (where tropomyosin shifts away from myosin-binding sites). These conformational changes propagate along the actin-tropomyosin filament allowing cooperative myosin binding.18 Electron microscopy studies revealed that SpCdc8p occupies the closed state when bound to actin.19 Thus, actin-tropomyosin complexes in yeast may utilize the closed and open states. This could favor the processivity of two-headed myosins where the actomyosin cross-bridge from one head favors association of the other head. Such cooperativity could also involve local recruitment of other myosin molecules surrounding an initial event and could explain why SpMyo52p molecules lacking their cargo-binding domain undergo actin-based motility clustered in groups.7 However, the allosteric changes associated with cooperative actin-binding are poorly understood. One study supported a mechanism in which tropomyosin may prevent salt from interfering with charged residues on the actin filament's surface and disrupting the transmission of allosteric interactions between actin subunits.20 Potentially, both actin and tropomyosin may work together in transmitting allostery within and between each filament. Tropomyosin may play a direct role in establishing an enhanced binding surface for myosin. While there is little evidence for direct physical interaction, one study identified potential electrostatic interactions that might occur at the myosin-tropomyosin interface.21 Given their close functional relationship, it is important to consider how the physical juxtaposition of actin-tropomyosin causes enhanced actomyosin binding.
In Vivo Ramifications of Tropomyosin-Mediated Processivity
Despite lacking intrinsic processivity, ScMyo2p and SpMyo52p transport cargo efficiently in vivo.22,23 Hodges et al.16 and Clayton et al.7 identified tropomyosin as a critical factor which enables these motors to move processively. These findings suggest a role for tropomyosin in sorting myosins-V activity to certain tracks, i.e., formin-nucleated unbranched actin cables (Fig. 2). Consistent with their enhanced activity on actin-tropomyosin tracks, SpMyo2p,17 SpMyo51p (a type-V myosin), and SpMyo52p6 are specific to tropomyosin-decorated actin structures in fission yeast. Additionally, budding yeast's ScMyo2p is only seen at tropomyosin-actin structures in vivo.24 Moreover, tropomyosin may help to exclude incorrect myosins from certain tracks, given that SpCdc8p inhibits fission yeast type-I myosin (SpMyo1p) in vitro.6 This makes sense physiologically because SpMyo1p only functions at branched actin networks in endocytic patches where tropomyosin is excluded by the actin cross-linker fimbrin.6,25 Tropomyosin-mediated sorting of actomyosin activity could represent a general mechanism for preventing inappropriate force production in addition to directing cargo transport.
Future Studies
ScTpm1p increases the duty ratio of ScMyo2p by slowing ADP release and ATP-induced dissociation.16 Additional transient kinetics studies could determine whether tropomyosin speeds up the Pi release rate as well. This would be important to flesh out tropomyosin's effect on ScMyo2p entering the strong actin-binding state. Similar analysis of SpMyo52p with SpCdc8p-actin could yield critical details about duty ratio enhancement in fission yeast and help establish a firmer basis for this mechanism. Moreover, probing into gating and cooperative models may help to advance understanding of tropomyosin regulation. In conjunction with these functional assays, high resolution electron microscopy of these myosin-Vs in complex with tropomyosin-actin would help to establish the fundamental nature of the intermolecular interactions. Moving forward, testing whether these mechanisms extend to higher eukaryotes will be imperative for understanding tropomyosin regulation in general.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank members of the Lord lab for comments on the manuscript. Work in the Lord lab is supported by a National Institutes of Health grant (GM097193).
Glossary
Abbreviations:
- ATP
adenosine triphosphate
- ADP
adenosine diphosphate
- Pi
inorganic phosphate
- Sc
Saccharomyces cerevisiae
- Sp
Schizosaccharomyces pombe
References
- 1.Takagi Y, Yang Y, Fujiwara I, Jacobs D, Cheney RE, Sellers JR, Kovács M. Human myosin Vc is a low duty ratio, nonprocessive molecular motor. J Biol Chem. 2008;283:8527–37. doi: 10.1074/jbc.M709150200. [DOI] [PubMed] [Google Scholar]
- 2.Watanabe S, Watanabe TM, Sato O, Awata J, Homma K, Umeki N, Higuchi H, Ikebe R, Ikebe M. Human myosin Vc is a low duty ratio nonprocessive motor. J Biol Chem. 2008;283:10581–92. doi: 10.1074/jbc.M707657200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tóth J, Kovács M, Wang F, Nyitray L, Sellers JR. Myosin V from Drosophila reveals diversity of motor mechanisms within the myosin V family. J Biol Chem. 2005;280:30594–603. doi: 10.1074/jbc.M505209200. [DOI] [PubMed] [Google Scholar]
- 4.Reck-Peterson SL, Tyska MJ, Novick PJ, Mooseker MS. The yeast class V myosins, Myo2p and Myo4p, are nonprocessive actin-based motors. J Cell Biol. 2001;153:1121–6. doi: 10.1083/jcb.153.5.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hodges AR, Bookwalter CS, Krementsova EB, Trybus KM. A nonprocessive class V myosin drives cargo processively when a kinesin- related protein is a passenger. Curr Biol. 2009;19:2121–5. doi: 10.1016/j.cub.2009.10.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clayton JE, Sammons MR, Stark BC, Hodges AR, Lord M. Differential regulation of unconventional fission yeast myosins via the actin track. Curr Biol. 2010;20:1423–31. doi: 10.1016/j.cub.2010.07.026. [DOI] [PubMed] [Google Scholar]
- 7.Clayton JE, Pollard LW, Sckolnick M, Bookwalter CS, Hodges AR, Trybus KM, Lord M. Fission yeast tropomyosin specifies directed transport of myosin-V along actin cables. Mol Biol Cell. 2014;25:66–75. doi: 10.1091/mbc.E13-04-0200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cooper JA. Actin dynamics: tropomyosin provides stability. Curr Biol. 2002;12:R523–5. doi: 10.1016/S0960-9822(02)01028-X. [DOI] [PubMed] [Google Scholar]
- 9.Gimona M. Dimerization of tropomyosins. Adv Exp Med Biol. 2008;644:73–84. doi: 10.1007/978-0-387-85766-4_6. [DOI] [PubMed] [Google Scholar]
- 10.Gunning PW, Schevzov G, Kee AJ, Hardeman EC. Tropomyosin isoforms: divining rods for actin cytoskeleton function. Trends Cell Biol. 2005;15:333–41. doi: 10.1016/j.tcb.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 11.Gunning P, O’Neill G, Hardeman E. Tropomyosin-based regulation of the actin cytoskeleton in time and space. Physiol Rev. 2008;88:1–35. doi: 10.1152/physrev.00001.2007. [DOI] [PubMed] [Google Scholar]
- 12.Coulton AT, East DA, Galinska-Rakoczy A, Lehman W, Mulvihill DP. The recruitment of acetylated and unacetylated tropomyosin to distinct actin polymers permits the discrete regulation of specific myosins in fission yeast. J Cell Sci. 2010;123:3235–43. doi: 10.1242/jcs.069971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Motegi F, Arai R, Mabuchi I. Identification of two type V myosins in fission yeast, one of which functions in polarized cell growth and moves rapidly in the cell. Mol Biol Cell. 2001;12:1367–80. doi: 10.1091/mbc.12.5.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Win TZ, Gachet Y, Mulvihill DP, May KM, Hyams JS. Two type V myosins with non-overlapping functions in the fission yeast Schizosaccharomyces pombe: Myo52 is concerned with growth polarity and cytokinesis, Myo51 is a component of the cytokinetic actin ring. J Cell Sci. 2001;114:69–79. doi: 10.1242/jcs.114.1.69. [DOI] [PubMed] [Google Scholar]
- 15.Pruyne DW, Schott DH, Bretscher A. Tropomyosin-containing actin cables direct the Myo2p-dependent polarized delivery of secretory vesicles in budding yeast. J Cell Biol. 1998;143:1931–45. doi: 10.1083/jcb.143.7.1931. [DOI] [PubMed] [Google Scholar]
- 16.Hodges AR, Krementsova EB, Bookwalter CS, Fagnant PM, Sladewski TE, Trybus KM. Tropomyosin is essential for processive movement of a class V myosin from budding yeast. Curr Biol. 2012;22:1410–6. doi: 10.1016/j.cub.2012.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stark BC, Sladewski TE, Pollard LW, Lord M. Tropomyosin and myosin-II cellular levels promote actomyosin ring assembly in fission yeast. Mol Biol Cell. 2010;21:989–1000. doi: 10.1091/mbc.E09-10-0852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gordon AM, Homsher E, Regnier M. Regulation of contraction in striated muscle. Physiol Rev. 2000;80:853–924. doi: 10.1152/physrev.2000.80.2.853. [DOI] [PubMed] [Google Scholar]
- 19.Skoumpla K, Coulton AT, Lehman W, Geeves MA, Mulvihill DP. Acetylation regulates tropomyosin function in the fission yeast Schizosaccharomyces pombe. J Cell Sci. 2007;120:1635–45. doi: 10.1242/jcs.001115. [DOI] [PubMed] [Google Scholar]
- 20.Ansari S, El-Mezgueldi M, Marston S. Cooperative inhibition of actin filaments in the absence of tropomyosin. J Muscle Res Cell Motil. 2003;24:513–20. doi: 10.1023/B:JURE.0000009812.74980.13. [DOI] [PubMed] [Google Scholar]
- 21.Behrmann E, Müller M, Penczek PA, Mannherz HG, Manstein DJ, Raunser S. Structure of the rigor actin-tropomyosin-myosin complex. Cell. 2012;150:327–38. doi: 10.1016/j.cell.2012.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karpova TS, Reck-Peterson SL, Elkind NB, Mooseker MS, Novick PJ, Cooper JA. Role of actin and Myo2p in polarized secretion and growth of Saccharomyces cerevisiae. Mol Biol Cell. 2000;11:1727–37. doi: 10.1091/mbc.11.5.1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lo Presti L, Chang F, Martin SG. Myosin Vs organize actin cables in fission yeast. Mol Biol Cell. 2012;23:4579–91. doi: 10.1091/mbc.E12-07-0499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pruyne DW, Schott DH, Bretscher A. Tropomyosin-containing actin cables direct the Myo2p-dependent polarized delivery of secretory vesicles in budding yeast. J Cell Biol. 1998;143:1931–45. doi: 10.1083/jcb.143.7.1931. [DOI] [PubMed] [Google Scholar]
- 25.Skau CT, Kovar DR. Fimbrin and tropomyosin competition regulates endocytosis and cytokinesis kinetics in fission yeast. Curr Biol. 2010;20:1415–22. doi: 10.1016/j.cub.2010.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]