Abstract
Background
People with chronic obstructive pulmonary disease lead sedentary lives and could benefit from increasing their physical activity. The purpose of this study was to determine if an exercise-specific self-efficacy enhancing intervention could increase physical activity and functional performance when delivered in the context of 4 months of upper body resistance training with a 12-month follow-up.
Methods
In this randomized controlled trial, subjects were assigned to: exercise-specific self-efficacy enhancing intervention with upper body resistance training (SE-UBR), health education with upper body resistance training (ED-UBR), or health education with gentle chair exercises (ED-Chair). Physical activity was measured with an accelerometer and functional performance was measured with the Functional Performance Inventory. Forty-nine people with moderate to severe chronic obstructive pulmonary disease completed 4 months of training and provided valid accelerometry data, and 34 also provided accelerometry data at 12 months of follow-up. The self-efficacy enhancing intervention emphasized meeting physical activity guidelines and increasing moderate-to-vigorous physical activity.
Results
Differences were observed in light physical activity (LPA) after 4 months of training, time by group interaction effect (P=0.045). The SE-UBR group increased time spent in LPA by +20.68±29.30 minutes/day and the other groups decreased time spent in LPA by −22.43±47.88 minutes/day and -25.73±51.76 minutes/day. Changes in LPA were not sustained at 12-month follow-up. There were no significant changes in moderate-to-vigorous physical activity, sedentary time, or functional performance. Subjects spent most of their waking hours sedentary: 72%±9% for SE-UBR, 68%±10% for ED-UBR, and 74%±9% for ED-Chair.
Conclusion
The self-efficacy enhancing intervention produced a modest short-term increase in LPA. Further work is needed to increase the magnitude and duration of effect, possibly by targeting LPA.
Keywords: behavioral intervention, physical activity, emphysema, chronic bronchitis
Introduction
Physical inactivity is a growing problem in the USA, especially for older people and for those with chronic diseases, such as chronic obstructive pulmonary disease (COPD). Sixty-three percent of older adults do not meet the 2008 Physical Activity Guidelines for Americans,1 and the problem is even greater for people with COPD.2 A recent systematic review reported that the duration (57%) and intensity (75%) of physical activity (PA) was lower for people with COPD than for age-matched healthy individuals.3 In another systematic review, it was noted that people with COPD took fewer steps/day than people with most other chronic diseases; in fact, people with COPD were among the least active groups.4
People with COPD could derive substantial health benefits by increasing PA, but exercise training and pulmonary rehabilitation have been unsuccessful in addressing the problem.5 Pulmonary rehabilitation is widely used, and is effective in increasing exercise capacity and health-related quality of life, but for the most part it has not been effective in increasing PA,6 possibly because it does not adequately address the behavioral issues associated with many years of habitual inactivity. While many pulmonary rehabilitation programs include health education and the benefits of PA, few have employed theory-based behavioral interventions to change PA behavior.
The primary aim of this research was to determine if an exercise-specific self-efficacy enhancing intervention could increase objectively measured PA and self-reported functional performance when given in the context of a 4-month upper body resistance training program. The secondary aim was to determine if observed treatment effects were maintained for 12 months with a booster session at 3, 6, and 9 months after training. The effects of the intervention on upper body strength and dyspnea were reported elsewhere.7
Materials and methods
This was a randomized controlled trial with subjects assigned to one of three groups and data collectors blinded to group assignment. All subjects were assigned to an active intervention without being told which intervention was the experimental intervention of interest. We explained that all three interventions were potentially beneficial and that we were comparing the effects of each. Randomization was stratified by sex and disease severity (Global Initiative on Obstructive Lung Disease stages II, III, and IV)8 using a customized computer program that blinded investigators to group assignment.
The three groups were an exercise-specific self-efficacy enhancing intervention with upper body resistance training (SE-UBR), health education with upper body resistance training (ED-UBR), and health education with gentle chair exercises (ED-Chair). Briefly, subjects performed resistance training with a cable crossover system twice a week in the laboratory and with hand weights, once a week at home. Gentle chair exercises were performed twice a week in the laboratory and once a week at home. Self-efficacy and educational components of the intervention were provided weekly during the laboratory-based training sessions. A single booster session was provided at 3, 6, and 9 months after the completion of training. For each booster, the SE-UBR group received a resistance training and self-efficacy enhancing session and the other groups received only health education to equalize the attention.
Subjects participated in 4 months of structured training with outcomes measured at baseline, after completing 4 months of training, and at 12 months after the end of the structured training. Outcomes of interest for this report include objectively measured PA and self-reported functional performance.
Sample
Subjects were eligible to participate if they had moderate to severe COPD (forced expiratory volume in one second [FEV1]/forced vital capacity <70 and FEV1 <80% predicted)8 and no other major health problems that limited PA, were aged ≥45 years, were currently in a stable clinical condition, and experienced dyspnea during upper body activity. People were excluded if they required an assistive device to walk. This research was approved by three institutional review boards. Subjects were recruited by letters and local radio advertisements. We used the International Classification of Diseases, Ninth Revision (ICD-9) codes from the medical records of a large urban health system and sent letters to people with codes that indicated a diagnosis of COPD. We then screened respondents to determine if they met the inclusion criteria as previously described.7
Intervention
The self-efficacy enhancing intervention was modeled after an intervention developed by McAuley et al for a seniors’ walking program.9 The self-efficacy enhancing sessions were designed to promote self-efficacy for upper body strength training and PA in daily life by maximizing all four sources of self-efficacy as described by Bandura.10 To optimize the mastery experience, staff provided feedback, graphed subjects’ progress, and assisted subjects to recall their progress. Vicarious experience was provided using video clips of other people doing upper body resistance training and talking about the new activities that they were doing and activities they were able to do with greater ease at home. We also mounted posters in the laboratory demonstrating older people exercising and having fun. Verbal and social persuasion experiences were provided by emphasizing the individual’s capacity to perform upper body resistance training early in the program and reinforcing observed improvements later in the program. We organized buddy groups of two to three people each, and instructed them to contact each other on the weekends and to call each other if they could not make it to class. We encouraged subjects to measure success as self-improvement and to avoid comparing themselves with others. We helped subjects interpret their physiological and affective states by explaining that it is normal to experience shortness of breath and an increase in heart rate while exercising, and it is normal to experience fatigue and initial muscle soreness after exercising. A detailed description of the self-efficacy enhancing intervention is available upon request from the first author of this paper.
The self-efficacy enhancing intervention included 16 sessions, 15 minutes a week for 4 months, and three booster sessions during the subsequent year at 3, 6, and 9 months. The self-efficacy enhancing sessions addressed the following topics: general principles of exercise (n=3 sessions), coping with respiratory infections by modifying PA (n=4 sessions), overcoming barriers to exercise (n=2 sessions), and establishing an active lifestyle (n=7 sessions).
Health education classes for the two control groups were performed 15 minutes a week and included topics of interest to people with COPD, such as basic lung physiology, pathophysiology of COPD, commonly used medications, breathing techniques, healthy eating, relaxation, travel considerations, and energy conservation. One general education session was administered as a booster at the 3-month, 6-month, and 9-month follow-up visits; specific topics were osteoarthritis, cardiovascular risk factors, and cholesterol.
The self-efficacy and health education components of the intervention were scripted to promote fidelity of the intervention. Research staff members were trained to deliver the intervention in a consistent manner, and intervention fidelity was monitored periodically during the study. Monitoring included the accuracy of content, the affect of staff, and general atmosphere during laboratory training to verify that the self-efficacy enhancing intervention was implemented in a consistent manner and that elements of the self-efficacy enhancing intervention were not accidentally introduced into the other two groups. Monitors provided a plausible rationale for their presence so that the monitoring could be done without the staff’s knowledge.
The upper body resistance training and gentle chair exercises are described elsewhere.7 Briefly, the upper body resistance training included three sets of eight lifts. Gentle chair exercises included stretching and toning exercises that were not aerobic.
Measures
PA data were collected before and after the completion of training, not during training, so did not include PA associated with exercise training sessions. Physical activity was measured using Actigraph (model 7164) accelerometers worn at the waist and wrist during waking hours for 7 consecutive days. Data from the waist-mounted accelerometers are reported here. Accelerometry data were considered valid and useable if the accelerometer was worn for a minimum of 3 days and for at least 10 hours a day.11,12 The accelerometry data were defined as sedentary activity at <100 counts per minute, light physical activity (LPA) at 100–1,951 counts per minute, and moderate to vigorous physical activity (MVPA) at ≥1,952 counts per minute.13–15 Sedentary time was reported as minutes per day and as the percentage of monitored time.
The accelerometry data were cleaned to remove nonwear time, defined as ≥60 minutes of zero counts. People with COPD are very sedentary, making it possible to label data as nonwear time when it was actually prolonged inactivity. A second accelerometer was worn on the wrist of the dominant hand, and for purposes of this report, the wrist data were used to validate wear time for the waist-mounted accelerometers. Subjects maintained a daily activity log that was used to facilitate interpretation of accelerometry data. We assumed that waist and wrist accelerometers were both worn together, and when the duration of zero counts was ≥60 minutes for data collected from the waist accelerometer, we checked the wrist data to determine if the wrist was active. If data from the wrist accelerometer and data from the activity log indicated that the subject was still being monitored, then the waist data were treated as valid and not removed as nonwear time.
The Functional Performance Inventory (FPI) was administered as a self-report measure of the effects of health on usual daily activities.16 The FPI is constructed of 65 items and multiple subscales, including body care, maintaining the household, physical exercise, recreation, spiritual activities, and social interaction with family and friends. Items range from activities that require a low level of exertion to activities that require a higher level of exertion. Subjects report the level of difficulty they experience in doing each activity on a four-point scale where 1 represents no difficulty, 2 some difficulty, 3 much difficulty, and 4 “don’t do” because of health. The data were transformed according to author guidelines, and the potential range for scores is 0–3 for the subscales and total FPI; higher scores reflect higher functional performance. The reliability and validity of the FPI has been established for people with COPD16,17 and it is sensitive to change, reflecting declines and increases in functional performance over time.18,19
The following measures were taken to describe the sample at baseline: spirometry,20 lung volumes,21 diffusing capacity of the lung for carbon monoxide (DLco),22 Charlson Comorbidity Index,23 body mass index (BMI), fat-free mass as measured by dual energy X-ray absorptiometry, peak work rate obtained from a symptom-limited incremental cycle ergometry test, and self-reported time spent in physical exercise each week prior to enrolling in this study.
Data analysis
The data were analyzed using Statistical Package for the Social Sciences version 21 software (IBM Corporation, Armonk, NY, USA). Exploratory analyses were used to determine if the data met the assumptions for statistical tests. Multivariate analysis of variance was used to compare the three groups at baseline and to compare the baseline characteristics for subjects who completed the study and those who withdrew or did not provide valid PA data. Repeated measures multivariate analysis of variance, controlling for age, was used to compare the three groups at baseline and after 4 months of intervention (n=49) and to compare the three groups at baseline, after 4 months of intervention, and at 12 months of follow-up (n=34). Significant interaction effects on the multivariate test were examined by univariate analysis of variance for repeated measures to determine differences between groups for individual variables. Data are presented as the mean ± standard deviation.
Results
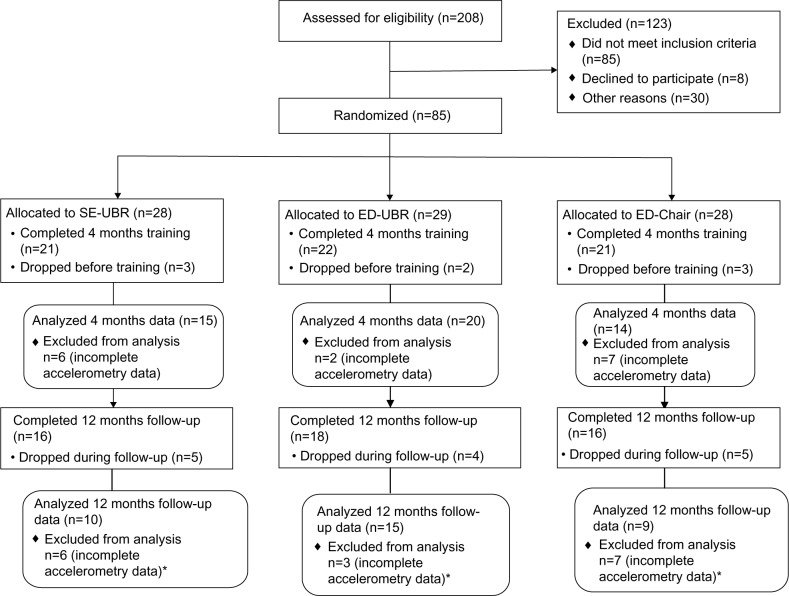
Eighty-five subjects with moderate to severe COPD (71 men, 14 women; 13 blacks, 72 whites) were randomized to one of three groups. Sixty-four completed 4 months of training, and of these, 50 returned for measurements at 12 months after the completion of training (Figure 1). Some accelerometry data were not valid/useable because subjects failed to wear the monitor for the minimal time required, ie, they did not wear it for three days at least 10 hours a day. They either forgot or chose not to wear the device; some applied the device late and/or removed it early, and some provided two valid measures but did not provide all three. Adherence to the monitoring protocol tended to decline over the course of participation in the study. Sixty-eight subjects provided valid/useable accelerometry data at baseline before training, 56 after the end of 4 months of training, and 37 at 12 months’ follow-up. Forty-nine provided valid accelerometry data at both baseline and after 4 months of training, and 34 provided data at all three measurement points.
Figure 1.
Flow diagram.
Note: *These subjects did not have useable accelerometry data for all three time points.
Abbreviations: SE-UBR, self-efficacy and upper body resistance; ED-UBR, health education and upper body resistance; ED-Chair, health education and chair aerobics.
Forty-nine subjects (41 men, eight women; seven blacks, 42 whites) completed 4 months of training and provided accelerometry data for SE-UBR (n=15), ED-UBR (n=20), and ED-Chair (n=14; Table 1). The mean BMI was 28±6, with 37% being overweight (BMI 25–29.9) and 27% being obese (BMI >30). The mean peak work rate was low for all three groups (46, 44, and 40% predicted normal value), suggesting substantially reduced exercise capacity.
Table 1.
Sample characteristics for subjects who completed 4 months of intervention and provided useable accelerometry data at baseline and end of training (n=49)
| Variable | SE-UBR (n=15) Mean ± SD |
ED-UBR (n=20) Mean ± SD |
ED-Chair (n=14) Mean ± SD |
|---|---|---|---|
| Age, years | 71±8 | 72±9 | 71±8 |
| FEV1, % predicted | 61±20 | 54±17 | 56±17 |
| DLco, % predicted | 70±22 | 64±20 | 62±24 |
| RV/TLC, ratio | 0.53±0.10 | 0.56±0.12 | 0.51±0.07 |
| Charlson Comorbidity Index | 1.4±0.6 | 1.8±1.1 | 1.1±0.5 |
| BMI, kg/m2 | 30±7 | 26±5 | 29±7 |
| Fat-free mass index, kg/m2 | 20±3 | 18±3 | 20±2 |
| Percent body fat | 33±8 | 30±6 | 20±8 |
| Peak work rate, % predicted | 46±14 | 44±20 | 40±14 |
| Self-reported physical exercise, minutes/week | 80±184 | 87±134 | 68±100 |
Abbreviations: SE-UBR, self-efficacy and upper body resistance; ED-UBR, health education and upper body resistance; ED-Chair; health education and chair aerobics; FEV1, forced expiratory volume in one second; DLco, diffusing capacity of the lung for carbon monoxide; RV/TLC, residual volume/total lung capacity; BMI, body mass index; SD, standard deviation.
Thirty-four subjects (28 men, six women; six blacks, 28 whites) completed 4 months of training and provided accelerometry data at all three measurement points for SE-UBR (n=10), ED-UBR (n=15), and ED-Chair (n=9). This group was similar to the larger group of 49 with respect to mean age (73±8 years), FEV1, % predicted (58±18), DLco % predicted (67±21), residual volume (RV)/total lung capacity (TLC; 0.54±0.11) and BMI (28±7). Twenty-four percent were overweight and 30% were obese.
The total group and subgroups as described above were similar with one exception. The 49 subjects who completed 4 months of training and provided accelerometry data had a significantly higher diffusion capacity than those who withdrew or failed to provide valid accelerometry data (DLco % predicted was 65±21 and 55±18, respectively; P=0.01). There were no other significant differences between subgroups.
The mean number of valid days of activity monitoring was 6.6±0.89 (n=49), 6.4±1.04 (n=49), and 6.4±0.71 (n=34) at baseline, end of the intervention, and 12 months after the end of the intervention, respectively, with no significant differences between the groups. The mean wear time was 14±2 (n=49), 14±2 (n=49), and 14±1 (n=34) hours a day at baseline, end of the intervention, and 12 months after the end of the intervention, respectively, with no significant differences in wear time between the groups.
Differences were observed in PA after 4 months of training (n=49), time by group interaction effect (P=0.045; Table 2). The SE-UBR group increased LPA and the other groups decreased LPA, post hoc comparisons SE-UBR versus ED-UBR (P=0.018) and SE-UBR versus ED-Chair (P=0.019). The change in the time spent in LPA was +20.68±29.30 (95% confidence interval [CI] −2.36, 43.72) minutes/day for SE-UBR; −22.43±47.88 (95% CI −42.38, −2.47) minutes/day for ED-UBR; and −25.73±51.76 (95% CI −49.58, −1.88) minutes/day for ED-Chair. There were no changes in time spent in MVPA or sedentary activity. The effect size (Cohen’s d) for change in the time spent in PA (SE-UBR versus the combined groups of ED-UBR and ED-Chair) was large for LPA (1.14) and very small for MVPA and sedentary activity (0.02 and 0.10, respectively).
Table 2.
Time spent in sedentary, light, and moderate to vigorous physical activity before and after 4 months of intervention and providing useable accelerometry data (n=49)
| Variable | SE-UBR (n=15) Mean ± SD |
ED-UBR (n=20) Mean ± SD |
ED-Chair (n=14) Mean ± SD |
Time × group interactionb P-value |
|---|---|---|---|---|
| MVPA (minutes/day)a | ||||
| Before | 7±7 | 6±5 | 3±4 | 0.961 |
| After | 6±6 | 4±3 | 3±2 | |
| LPA (minutes/day)a | ||||
| Before | 236±81 | 258±79 | 222±84 | 0.010 |
| After | 256±83 | 236±70 | 196±66 | |
| Sedentary time (minutes/day)a | ||||
| Before | 604±118 | 577±119 | 658±129 | 0.0552 |
| After | 602±112 | 577±107 | 634±114 | |
Notes: Sedentary activity ≤100 cpm, light physical activity =100–1,952 cpm, moderate to vigorous physical activity ≥1,952 cpm.
Multivariate analysis of variance with repeated measures was performed for the three levels of physical activity, group × time interaction effect was significant, P=0.045
univariate analysis of variance.
Abbreviations: cpm, counts per minute; SE-UBR, self-efficacy and upper body resistance; ED-UBR, health education and upper body resistance; ED-Chair, health education and chair aerobics; MVPA, moderate to vigorous physical activity; LPA, light physical activity; SD, standard deviation.
All three groups were highly sedentary. The percentage of monitored time spent in sedentary activities before and after training was 72%±9% and 70%±10% for SE-UBR, 68%±10% and 70%±9% for ED-UBR, and 74%±9% and 76%±9% for ED-Chair, respectively (group × time interaction; P=0.090).
There was no change in FPI total score for any of the groups (Table 3) and no change in individual subscale scores before and after training. The FPI Physical Exercise score was the lowest subscale (mean 1.3±0.65) and FPI Body Care was the highest (mean 2.8±0.29). At baseline, the FPI total and FPI Physical Exercise subscale scores were negatively and weakly correlated with sedentary minutes/day (r= −0.314, P=0.010 and r= −0.252, P=0.400), but not with LPA minutes/day or MVPA minutes/day. These correlations were not significant at the end of training.
Table 3.
FPI total score before and after 4 months of intervention (n=64)
| Variable | SE-UBR (n=21) Mean ± SD |
ED-UBR (n=22) Mean ± SD |
ED-Chair (n=21) Mean ± SD |
|---|---|---|---|
| FPI total | |||
| Before | 2.1±0.39 | 2.2±0.49 | 2.1±0.49 |
| After | 2.2±0.44 | 2.2±0.49 | 2.3±0.49 |
Notes: The FPI data reported here includes all subjects who completed 4 months of training and provided FPI data regardless of whether or not they provided useable accelerometry data. Univariate analysis of variance with repeated measures controlling for age was performed, group × time interaction effect was not significant, P=0.712.
Abbreviations: FPI, Functional Performance Inventory; SE-UBR, self-efficacy and upper body resistance; ED-UBR, health education and upper body resistance; ED-Chair, health education and chair aerobics; SD, standard deviation.
At 12 months, after the completion of training (n=34), there were no significant changes in the time spent in MVPA, LPA, and sedentary activity. All three groups had a nonsignificant decrease in LPA from baseline to 12 months of follow-up. The decrease was a mean of 9±76 minutes/day for the SE-UBR group and 41±73 minutes/day and 56±74 minutes/day, respectively, for the two health education groups (ED-UBR and ED-Chair). The effect size for change in LPA from baseline to 12 months of follow-up (SE-UBR versus combined ED-UBR and ED-Chair) was 0.472, which is just below Cohen’s definition of a medium-sized effect (d=0.5), but this analysis was not adequately powered. A sample size of 59 subjects per group would be required to detect an effect of the observed size. There were no significant changes in the FPI total score or subscale scores.
Post hoc analyses were conducted to determine if the obesity of subjects influenced response to the intervention. The groups were too small to examine group effects, so all three groups were combined for this analysis. There was a significant inverse relationship between BMI and LPA at baseline, controlling for age, FEV1 % predicted, and DLco % predicted (r=−0.319, P=0.031), but there was no relationship between BMI and change in PA following the intervention. There was no relationship between BMI and sedentary time or time spent in MVPA at baseline.
As noted above, the DLco % predicted was lower in subjects who withdrew from the study or did not provide valid accelerometer data. We explored this issue by post hoc examination of the relationship between DLco % predicted and PA. Combining all three groups, there was a significant inverse relationship between DLco % predicted and LPA at baseline, controlling for age and FEV1 % predicted (r=−0.313, P=0.032). There was a positive relationship between DLco % predicted at baseline and the change in time spent in LPA at the end of 4 months of training (r=0.408, P=0.004) and at 12 months of follow-up (r=0.418, P=0.017). There was no relationship between DLco % predicted and sedentary time or time spent in MVPA.
Discussion
We observed a significant increase in LPA at the end of the training in the SE-UBR group compared with the two control groups. In contrast, the control groups decreased LPA by 22 and 26 minutes/day each. Changes in LPA were not accompanied by self-reported changes in functional performance. The gains in LPA for the SE-UBR group (relative to the control groups) were not sustained at 12 months after the end of training. There was no change in the time spent in MVPA or in sedentary activity.
The observed gains in LPA are important as there is growing evidence to suggest that increasing LPA and decreasing sedentary time provides health benefits independent of the amount of MVPA performed.24,25 Potential benefits include reduced metabolic risk,26–28 attenuation of arterial stiffening in old age,29 a reduced risk of hospital admission for COPD,30,31 a delay in the onset of frailty for older adults,32 and decreased risk of mortality.33 The volume of LPA required to accrue health benefits is not known, but there is evidence to suggest that replacing 30 minutes of sedentary time with 30 minutes of LPA has the potential to improve physical health as indicated by body mass index, number of health conditions, number of medications, and general health rating.15 While the self-efficacy enhancing intervention was successful compared with health education, it is not clear that the observed increase in LPA was clinically meaningful even if it were sustained. The ultimate challenge is to change habits and establish an increase in LPA that is maintained over multiple years.
Physical activity declines with age for healthy older adults,34,35 and for people with COPD a change in the downward trajectory could be very important. At the end of training the observed increase in LPA for the SE-UBR group was not statistically significant when examined in isolation, but the two control groups experienced a decrease in LPA. The mean group differences (SE-UBR versus ED-UBR and ED-Chair) were much larger, and this illustrates the potential of this intervention to reverse or slow the rate of decline in PA. Others have observed comparable decreases in PA over relatively short periods of time (1236 and 3637 weeks). The 12-month follow-up demonstrated a similar trend, but at that point the study was underpowered and the variability in PA was high, suggesting that individuals responded differently. Ultimately, a much larger sample will be needed to examine subgroup differences and to identify those who benefit the most from this type of intervention.
It is difficult to compare the observed results with those of other studies because of differences in measures of PA. For people with COPD the time spent in LPA may be comparable with walking time because they tend to walk slowly. The number of steps/day is commonly used as a measure of PA, and 1,000 steps/day is estimated to be equivalent to 10 minutes of brisk walking.4,38 Neither of these methods provides a measure of the intensity of PA, but they do enable crude comparisons across studies. In addition, it is important to note that PA measures in this study reflect free-living PA and do not include the activity associated with the exercise sessions.
The self-efficacy enhancing intervention produced results that are consistent with other behavioral interventions designed to increase PA.6 People with COPD increased PA by 1,263 steps/day after 12 weeks of an Internet-based walking program,39 785 steps/day after 12 weeks of a customized exercise counseling program,36 and 609 steps/day after 36 weeks of cell phone supported self-monitoring intervention.37 In addition, our results were consistent with interventions that combined exercise and behavioral strategies. Effing et al40 demonstrated an increase of 876 steps/day after a 52-week self-management plus COPE-active intervention that included self-management and exercise. Pomidori et al41 demonstrated an increase in time spent walking at an intensity greater than 3 METs after 18 weeks of speed walking with a metronome to pace the walking and twice a month telephone calls.
It should be noted that subjects were encouraged to increase their MVPA to meet national PA guidelines, but instead they increased their LPA. The lack of an increase in MVPA is likely explained by two factors. It is possible that subjects overestimated the intensity of their PA and this is supported by their self-report of 68–87 minutes of exercise a week compared with objective measures of 5±5 minutes/day of MVPA at entry into the study. They thought they were exercising at a moderate-to-vigorous intensity, but in fact they were only exercising at a light intensity. Additionally, it could be explained by their pulmonary symptoms and an inability to sustain higher volumes of MVPA on a day-to-day basis.
Subjects in this study were very sedentary, spending less than 1% of their waking time in MVPA. This is consistent with other studies of objectively measured PA in this population. People with COPD from the National Health and Nutrition Examination Survey spent a mean of 6 minutes/day in MVPA, 249 minutes/day in LPA, and 676 minutes/day sedentary, equaling 1%, 27%, and 72% of waking hours, respectively.2 Eliason et al42 observed a mean of 7.5 and 7.0 minutes/day of MVPA and a mean of 478 and 483 minutes/day of sedentary time for people with moderate and severe COPD, respectively.
Recent evidence demonstrates the health benefits of reducing sedentary time,43,44 but this monitoring protocol was not designed to capture the full extent of sedentary time. The sedentary time is reported as a percentage of wear time, recognizing that the amount of sedentary time is influenced by the daily wear time of the accelerometer. Subjects were instructed to wear the accelerometer during waking hours and we compared accelerometry data with activity logs, but there is no way to establish the extent to which subjects actually adhered to these instructions. There was no change in sedentary time; this is not surprising because of the potential measurement issues, and the self-efficacy intervention did not emphasize the importance of reducing sedentary time.
The lack of observed changes in self-reported functional performance suggests that subjects did not perceive changes, ie, increases or decreases, in their day-to-day functioning. This is supported by the weak relationship observed between FPI total and FPI Physical Exercise scores and PA at baseline. It suggests that large changes in PA will be required before it is reflected in self-reported functional performance. This is useful information from a clinical perspective because it is important to avoid unrealistic expectations when prescribing an intervention of this nature.
Measurement issues could also influence the observed relationship between functional performance and PA. The FPI was developed for people with COPD. Its reliability and validity has been well established,16,17 and it has been demonstrated that the FPI scale is sensitive to decreases in functional performance in people with COPD over a 3-year period of time.18 In another study, the FPI demonstrated a small but significant increase in response to a 12-week yoga intervention.19 Therefore, the instrument appears to be responsive to change. It may be useful to examine the fundamental difference in the measures, given that one is an objective measure of PA and the other is a subjective measure of a related construct. People’s perceptions are not always consistent with reality, and this is illustrated in measures of PA. It is well established that self-reported and objectively measured PA can be very different.45,46 People overestimate their PA, and self-reported PA tends to be higher than objectively measured PA. This creates measurement error and makes it more difficult to detect a difference when it exists. A similar measurement error may exist for self-reported functional performance. Nevertheless, it is important to understand people’s perceptions of functional performance outcomes because it could influence their willingness to adhere to a more active lifestyle.
The self-efficacy enhancing intervention had two main thrusts, first to promote adherence to the resistance training protocol and second to promote a physically active lifestyle that meets the guidelines for PA. Seven of the 16 sessions were devoted to increasing lifestyle PA. Group work included strategies for overcoming barriers to exercise and increasing PA with an emphasis on PA guidelines. The feedback to subjects included strength gains and the importance of using the newly acquired strength to increase PA, not on setting specific goals for PA. This was a limitation of the intervention.
The subjects in this study were overweight or obese. This is consistent with the general population in the USA, where more than one third of adults are obese.47 It is also consistent with other clinical studies of pulmonary rehabilitation in the USA.48–50 Subjects were excluded if they reported difficulty with walking and/or required an assistive device to walk, thereby limiting some of the potential effects of joint problems associated with obesity. The small size in each group limited our ability to explore the relationship between obesity and PA response to the intervention, but it appears that BMI did not influence response to the intervention.
Limitations of this research include the relatively small sample size and the small proportion of women. Subjects were recruited from the community and two local Veterans Administration health systems, creating an imbalance in sex. The sample size was smaller than planned, in part because some subjects did not wear the accelerometer for the full 7 days. The percentage of subjects who provided valid/useable accelerometry data varied across the study (80% at baseline, 87.5% at the end of structured training, and 74% at the 12 months’ follow-up). This could be influenced by the burden associated with wearing the accelerometers for 7 days.
There was relatively high attrition in all three groups, and this is seen as a limitation of the research. Part of this can be explained by the substantial effort required for participation and for the long duration of the study, ie, 16 months. Dropouts were distributed throughout each phase; eight before starting training, 14 during training, and 16 during follow-up (Figure 1). During training and follow-up, the most common reasons for dropping out were other health problems (n=6), loss of interest (n=6), musculoskeletal problems (n=4), family/personal problems (n=3), and pulmonary health problems (n=2).7 An additional 31 subjects did not provide adequate accelerometry data for inclusion in the 12-month follow-up analysis. It is possible that subjects saw participation in the 12-month follow-up as too burdensome and/or they were less engaged in the research, and this influenced their willingness to adhere to the monitoring protocol.
Subjects who withdrew from the study and/or did not provide full accelerometry data had lower diffusion capacity and we cannot explain this. In our post hoc analysis, we found no clear relationship between diffusion capacity and PA. The observed negative relationship between baseline diffusion capacity and LPA is not consistent with clinical expectations. Still, the positive relationship between baseline diffusion capacity and change in LPA is reasonable since the combined groups had a decline in LPA. It suggests that subjects who started with a lower diffusion capacity experienced a greater decline in LPA.
Strengths of this research include the use of a theory-based intervention, attention to the fidelity of the intervention, use of objective measures of PA, and measurement of long-term effects. The self-efficacy enhancing intervention was designed so that it could be easily incorporated in a structured exercise or pulmonary rehabilitation program. Each session lasted only 15 minutes. The sessions were scripted, with structured activities to enhance treatment fidelity.
The self-efficacy enhancing intervention is promising because it resulted in short-term increases in LPA. However, we targeted the current activity guidelines, ie, 30 minutes of moderate PA 5 days a week or 150 minutes a week. Subjects increased their PA by a mean of 140 minutes a week, but with LPA instead of moderate PA. It is possible that this volume of moderate PA is too strenuous for people with COPD and a more realistic target would be LPA, possibly a larger increase in LPA. As noted earlier, an increase in LPA could produce multiple health benefits and be more sustainable over time.15,26–33
The fact that the increase in LPA was not sustained is disappointing, but not surprising as long-term maintenance is always challenging. We speculate that a single focused program of behavioral change may not be sufficient to change strongly entrenched habits such as low PA. This may be especially true for people with COPD because of symptoms that interrupt day-to-day PA patterns, including the experience of good days and bad days and acute exacerbations of COPD. To accomplish a sustained change in behavior may require the accumulation of multiple experiences with a variety of behavioral interventions over time, with the experience of each intervention the individual gains new knowledge and experience that will eventually be used to make a lasting change. This would be similar to the pattern observed in people who are trying to lose weight.
In summary the self-efficacy enhancing intervention is promising in that it produced a short-term increase in LPA, but the magnitude was limited and the duration of the effect was not sufficient. Further work is needed to increase the magnitude and duration of effect, possibly by specifically targeting LPA.
Acknowledgments
This research was funded by the National Institute of Nursing Research R01-NR08037 and the University of Illinois at Chicago General Clinical Research Center M01-RR-13987. The Clinical Trials Registration number is NCT01057797.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Schiller JS, Lucas JW, Ward BW, Peregoy JA. Summary health statistics for US adults: National Health Interview Survey, 2010. Vital Health Stat 10. 2012;252:1–207. [PubMed] [Google Scholar]
- 2.Park SK, Richardson CR, Holleman RG, Larson JL. Physical activity in people with COPD, using the National Health and Nutrition Evaluation Survey dataset. Heart Lung. 2013;42:235–240. doi: 10.1016/j.hrtlng.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vorrink SN, Kort HS, Troosters T, Lammers JW. Level of daily physical activity in individuals with COPD compared with healthy controls. Respir Res. 2011;12:33. doi: 10.1186/1465-9921-12-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tudor-Locke C, Washington TL, Hart TL. Expected values for steps/day in special populations. Prev Med. 2009;49:3–11. doi: 10.1016/j.ypmed.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 5.Ng CLW, Mackney J, Jenkins S, Hill K. Does exercise training change physical activity in people with COPD? A systematic review and meta-analysis. Chron Respir Dis. 2012;9:17–26. doi: 10.1177/1479972311430335. [DOI] [PubMed] [Google Scholar]
- 6.Larson JL, Vos CM, Fernandez D. Interventions to increase physical activity in people with COPD: systematic review. Ann Rev Nurs Res. 2013;31:297–326. doi: 10.1891/0739-6686.31.297. [DOI] [PubMed] [Google Scholar]
- 7.Covey MK, McAuley E, Kapella MC, et al. Upper-body resistance training and self-efficacy enhancement in COPD. J Pulm Respir Med. 2012;S9:001. doi: 10.4172/2161-105X.S9-001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.GOLD Science Committee Global Initiative for Chronic Obstructive Lung Disease, Global Strategy for the Diagnosis, Management and Prevention of Chronic Obstructive Lung Disease (updated 2007): Global Initiative for Chronic Obstructive Lung Disease. 2007. [Accessed August 8, 2014]. Available from: http://www.goldcopd.org/Guidelines/guidelines-global-strategy-for-diagnosis-management-2007.html.
- 9.McAuley E, Courneya KS, Rudolph DL, Lox CL. Enhancing exercise adherence in middle-aged males and females. Prev Med. 1994;23:498–506. doi: 10.1006/pmed.1994.1068. [DOI] [PubMed] [Google Scholar]
- 10.Bandura A. Self-Efficacy: The Exercise of Control. New York, NY, USA: WH Freeman & Co; 1997. [Google Scholar]
- 11.Matthews CE, Ainsworth BE, Thompson RW, Bassett DR. Sources of variance in daily physical activity levels as measured by an accelerometer. Med Sci Sports Exerc. 2002;34:1376–1381. doi: 10.1097/00005768-200208000-00021. [DOI] [PubMed] [Google Scholar]
- 12.Hart TL, Swartz AM, Cashin SE, Strath SJ. How many days of monitoring predict physical activity and sedentary behaviour in older adults? Int J Behav Nutr Phys Act. 2011;8:62. doi: 10.1186/1479-5868-8-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Freedson PS, Melanson E, Sirard J. Calibration of the Computer Science and Applications, Inc. accelerometer. Med Sci Sports Exerc. 1998;30:777–781. doi: 10.1097/00005768-199805000-00021. [DOI] [PubMed] [Google Scholar]
- 14.Lynch B, Dunstan D, Healy G, Winkler E, Eakin E, Owen N. Objectively measured physical activity and sedentary time of breast cancer survivors, and associations with adiposity: findings from NHANES (2003–2006) Cancer Causes Control. 2010;21:283–288. doi: 10.1007/s10552-009-9460-6. [DOI] [PubMed] [Google Scholar]
- 15.Buman MP, Hekler EB, Haskell WL, et al. Objective light-intensity physical activity associations with rated health in older adults. Am J Epidemiol. 2010;172:1155–1165. doi: 10.1093/aje/kwq249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leidy NK. Psychometric properties of the Functional Performance Inventory in patients which chronic obstructive pulmonary disease. Nurs Res. 1999;48:20–28. doi: 10.1097/00006199-199901000-00004. [DOI] [PubMed] [Google Scholar]
- 17.Larson JL, Kapella MC, Wirtz S, Covey MK, Berry J. Reliability and validity of the Functional Performance Inventory in patients with moderate to severe chronic obstructive pulmonary disease. J Nurs Meas. 1998;6:55–73. [PubMed] [Google Scholar]
- 18.Kapella MC, Larson JL, Covey MK, Alex CG. Functional performance in chronic obstructive pulmonary disease declines with time. Med Sci Sports Exerc. 2011;43:218–224. doi: 10.1249/MSS.0b013e3181eb6024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Donesky-Cuenco D, Nguyen H, Paul S, Carrieri-Kohlman V. Yoga therapy decreases dyspnea-related distress and improves functional performance in people with chronic obstructive pulmonary disease: a pilot study. J Altern Complement Med. 2009;15:225–234. doi: 10.1089/acm.2008.0389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 21.Wanger J, Clausen JL, Coates A, et al. Standardisation of the measurement of lung volumes. Eur Respir J. 2005;26:511–522. doi: 10.1183/09031936.05.00035005. [DOI] [PubMed] [Google Scholar]
- 22.MacIntyre N, Crapo RO, Viegi G, et al. Standardisation of the single-breath determination of carbon monoxide uptake in the lung. Eur Respir J. 2005;26:720–735. doi: 10.1183/09031936.05.00034905. [DOI] [PubMed] [Google Scholar]
- 23.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 24.Katzmarzyk PT, Church TS, Craig CL, Bouchard C. Sitting time and mortality from all causes, cardiovascular disease, and cancer. Med Sci Sports Exerc. 2009;41:998–1005. doi: 10.1249/MSS.0b013e3181930355. [DOI] [PubMed] [Google Scholar]
- 25.Woodcock J, Franco OH, Orsini N, Roberts I. Non-vigorous physical activity and all-cause mortality: systematic review and meta-analysis of cohort studies. Int J Epidemiol. 2011;40:121–138. doi: 10.1093/ije/dyq104. [DOI] [PubMed] [Google Scholar]
- 26.Healy G, Wijndaele K, Dunstan D, et al. Objectively measured sedentary time, physical activity, and metabolic risk: The Australian Diabetes, Obesity and Lifestyle Study (AusDiab) Diabetes Care. 2008;31:369–371. doi: 10.2337/dc07-1795. [DOI] [PubMed] [Google Scholar]
- 27.Watz H, Waschki B, Kirsten A, et al. The metabolic syndrome in patients with chronic bronchitis and COPD. Chest. 2009;136:1039–1046. doi: 10.1378/chest.09-0393. [DOI] [PubMed] [Google Scholar]
- 28.Healy GN, Matthews CE, Dunstan DW, Winkler EAH, Owen N. Sedentary time and cardio-metabolic biomarkers in US adults: NHANES 2003-06. Eur Heart J. 2011;32:590–597. doi: 10.1093/eurheartj/ehq451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gando Y, Yamamoto K, Murakami H, et al. Longer time spent in light physical activity is associated with reduced arterial stiffness in older adults. Hypertension. 2010;56:540–546. doi: 10.1161/HYPERTENSIONAHA.110.156331. [DOI] [PubMed] [Google Scholar]
- 30.Garcia-Aymerich J, Lange P, Benet M, Schnohr P, Anto JM. Regular physical activity reduces hospital admission and mortality in chronic obstructive pulmonary disease: a population based cohort study. Thorax. 2006;61:772–778. doi: 10.1136/thx.2006.060145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Benzo R, Chang C-CH, Farrell MH, et al. Physical activity, health status and risk of hospitalization in patients with severe chronic obstructive pulmonary disease. Respiration. 2010;80:10–18. doi: 10.1159/000296504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peterson MJ, Giuliani C, Morey MC, et al. Physical activity as a preventative factor for frailty: the Health, Aging, and Body Composition Study. J Gerontol A Biol Sci Med Sci. 2009;64A:61–68. doi: 10.1093/gerona/gln001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Katzmarzyk PT. Standing and mortality in a prospective cohort of Canadian adults. Med Sci Sports Exerc. 2014;46:940–946. doi: 10.1249/MSS.0000000000000198. [DOI] [PubMed] [Google Scholar]
- 34.Hawkins MS, Storti KL, Richardson CR, et al. Objectively measured physical activity of USA adults by sex, age, and racial/ethnic groups: a cross-sectional study. Int J Behav Nutr Phys Act. 2009;6:31. doi: 10.1186/1479-5868-6-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tucker JM, Welk GJ, Beyler NK. Physical activity in US adults compliance with the physical activity guidelines for Americans. Am J Prev Med. 2011;40:454–461. doi: 10.1016/j.amepre.2010.12.016. [DOI] [PubMed] [Google Scholar]
- 36.Hospes G, Bossenbroek L, Ten Hacken NH, van Hengel P, de Greef MH. Enhancement of daily physical activity increases physical fitness of outclinic COPD patients: results of an exercise counseling program. Patient Educ Couns. 2009;75:274–278. doi: 10.1016/j.pec.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 37.Nguyen HQ, Gill DP, Wolpin S, Steele BG, Benditt JO. Pilot study of a cell phone-based exercise persistence intervention post-rehabilitation for COPD. Int J Chron Obstruct Pulmon Dis. 2009;4:301–313. doi: 10.2147/copd.s6643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tudor-Locke C, Sisson SB, Collova T, Lee SM, Swan PD. Pedometer-determined step count guidelines for classifying walking intensity in a young ostensibly healthy population. Can J Appl Physiol. 2005;30:666–676. doi: 10.1139/h05-147. [DOI] [PubMed] [Google Scholar]
- 39.Moy ML, Weston NA, Wilson EJ, Hess ML, Richardson CR. A pilot study of an Internet walking program and pedometer in COPD. Respir Med. 2012;106:1342–1350. doi: 10.1016/j.rmed.2012.06.013. [DOI] [PubMed] [Google Scholar]
- 40.Effing T, Zielhuis G, Kerstjens H, van der Valk P, van der Palen J. Community based physiotherapeutic exercise in COPD self-management: a randomised controlled trial. Respir Med. 2011;105:418–426. doi: 10.1016/j.rmed.2010.09.017. [DOI] [PubMed] [Google Scholar]
- 41.Pomidori L, Contoli M, Mandolesi G, Cogo A. A simple method for home exercise training in patients with chronic obstructive pulmonary disease: one-year study. J Cardiopulm Rehabil Prev. 2012;32:53–57. doi: 10.1097/HCR.0b013e31823be0ce. [DOI] [PubMed] [Google Scholar]
- 42.Eliason G, Zakrisson A-B, Piehl-Aulin K, Hurtig-Wennlof A. Physical activity patterns in patients in different stages of chronic obstructive pulmonary disease. COPD. 2011;8:369–374. doi: 10.3109/15412555.2011.605403. [DOI] [PubMed] [Google Scholar]
- 43.Katzmarzyk PT, Lee I-M. Sedentary behavior and life expectancy in the USA: a cause-deleted life table analysis. BMJ Open. 2012;2:e000828. doi: 10.1136/bmjopen-2012-000828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dunstan DW, Kingwell BA, Larsen R, et al. Breaking up prolonged sitting reduces postprandial glucose and insulin responses. Diabetes Care. 2012;35:976–983. doi: 10.2337/dc11-1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Prince SA, Adamo KB, Hamel ME, Hardt J, Gorber SC, Tremblay M. A comparison of direct versus self-report measures for assessing physical activity in adults: a systematic review. Int J Behav Nutr Phys Act. 2008;5:56. doi: 10.1186/1479-5868-5-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Altschuler A, Picchi T, Nelson M, Rogers JD, Hart J, Sternfeld B. Physical activity questionnaire comprehension: lessons from cognitive interviews. Med Sci Sports Exerc. 2009;41:336–343. doi: 10.1249/MSS.0b013e318186b1b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Centers for Disease Control and Prevention Adult obesity facts. 2014. [Accessed June 14, 2014]. Available from: http://www.cdc.gov/obesity/data/adult.html.
- 48.Moy ML, Matthess K, Stolzmann K, Reilly J, Garshick E. Free-living physical activity in COPD: assessment with accelerometer and activity checklist. J Rehabil Res Dev. 2009;48:277–286. doi: 10.1682/jrrd.2008.07.0083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mador MJ, Patel AN, Nadler J. Effects of pulmonary rehabilitation on activity levels in patients with chronic obstructive pulmonary disease. J Cardiopulm Rehabil Prev. 2011;31:52–59. doi: 10.1097/HCR.0b013e3181ebf2ef. [DOI] [PubMed] [Google Scholar]
- 50.Steele B, Holt L, Belza B, Ferris S, Lakshminaryan S, Buchner D. Quantitating physical activity in COPD using a triaxial accelerometer. Chest. 2000;117:1359–1367. doi: 10.1378/chest.117.5.1359. [DOI] [PubMed] [Google Scholar]