Abstract
Objective
The essential role of platelet activation in haemostasis and thrombotic diseases focuses attention on unveiling the underlying intracellular signals of platelet activation. Disabled-2 (Dab2) has been implicated in platelet aggregation and in the control of clotting responses. However, there is not yet any in vivo study to provide direct evidence for the role of Dab2 in haemostasis and platelet activation.
Approach and Results
Megakaryocyte lineage-restricted Dab2 knockout (Dab2−/−) mice were generated to delineate in vivo functions of Dab2 in platelets. Dab2−/− mice appeared normal in size with prolonged bleeding time and impaired thrombus formation. Although normal in platelet production and granule biogenesis, Dab2−/− platelets elicited a selective defect in platelet aggregation and spreading on fibrinogen in response to low concentrations of thrombin, but not other soluble agonists. Investigation of the role of Dab2 in thrombin signaling revealed that Dab2 has no effect on the expression of thrombin receptors and the outside-in signaling. Dab2−/− platelets stimulated by low concentrations of thrombin were normal in Gαq-mediated calcium mobilization and PKC activation but were defective in Gα12/13-mediated RhoA-ROCKII activation. The attenuated Gα12/13 signaling led to impaired ADP release, Akt-mTOR and integrin αIIbβ3 activation, fibrinogen binding, and clot retraction. The defective responses of Dab2−/− platelets to low concentrations of thrombin stimulation may contribute to the impaired haemostasis and thrombosis of Dab2−/− mice.
Conclusions
This study sheds new insight in platelet biology and represents the first report demonstrating that Dab2 is a key regulator of haemostasis and thrombosis by functional interplay with Gα12/13-mediated thrombin signaling.
Keywords: Disabled-2, platelet activation, thrombin signaling
Introduction
Platelets are anucleated cells derived from megakaryocytes and represent the second most numerous blood cells in the peripheral blood.1 The essential role of platelet activation in haemostasis, myocardial infarction and other thrombotic diseases has prompted considerable focus on unveiling the underlying intracellular mechanisms of agonist-induced platelet activation.2,3 Thrombin binds to G-protein-linked protease-activated receptors (PARs) and causes Gαq-dependent increase in intracellular calcium and protein kinase C (PKC) activity, Gα12/13-dependent Rho activation, Gαi-dependent inhibition of adenylyl cyclase and Gβγ-mediated phosphoinositide 3-kinase-Akt activation.4,5 Collagen interacts with glycoprotein VI and recruits Syk to the plasma membrane followed by tyrosine phosphorylation of downstream substrates required for platelet activation.4 The agonist-induced inside-out signaling ultimately activates integrin αIIbβ3 and results in platelet activation, secretion and aggregation.
Cytoskeleton remodeling occurs at different stages of platelet activation.6 Following adhesion to surfaces coated with fibrinogen or collagen, platelets form broad, actin filament-containing lamellae and spread over an area of several μm2 in size. During platelet activation, the cytoplasmic domain of integrin αIIbβ3 associates with actin filaments and intracellular signaling molecules including talin, vinculin, zyxin, paxillin, filamin and α-actinin.7–10 Integrin αIIbβ3-mediated clot retraction then occurs through the action of non-muscle myosin IIA and IIB that are attached to actin and contract the actin filaments.11 Despite extensive studies, the underlying mechanisms of agonist-induced inside-out signaling and cytoskeleton reorganization still wait to be fully elucidated.
Disabled-2 (Dab2) is an adaptor protein with at least two isoforms p82 (p96) and p59 (p67) being generated through alternative splicing.12 p59-Dab2 lacks the ninth coding exon corresponding to the amino acids 230–445 of p82-Dab2, resulting in the deletion of several binding sites for endocytic proteins.12 By interactions with other cellular factors through the N-terminal phosphotyrosine binding domain, the aspartic acid-proline-phenylalanine motif and the C-terminal proline-rich region,7,13–16 Dab2 elicits functions in cytoskeleton reorganization, endocytosis, differentiation and cell signaling.17–20 Dab2 is known to regulate the signaling pathways of Ras-MAPK, Wnt, TGF-β and RhoA-ROCK14–16,21 and modulate cytoskeleton reorganization by binding to actin, myosin VI, non-muscle myosin heavy chain IIA and dynein.17,19,22
Dab2 is abundantly expressed in human platelets and is distributed mainly in the cytoplasm and α-granules.23 It is upregulated during megakaryocytic differentiation of human CD34+ hematopoietic pluripotent stem cells, murine embryonic stem cells and human leukemic K562 cells.13,20,24 In vitro studies have identified Dab2 as a regulator of platelet integrin activation, cell adhesion and fibrinogen uptake.13,25 Dab2 is released and binds to either integrin αIIb or phospholipid sulfatide in response to platelet activation, playing a role in platelet-fibrinogen and platelet-leukocyte adhesion and aggregation.23,26 The balance between sulfatide- and integrin receptor-bound states is involved in the control of the extent of clotting response.26,27 Nevertheless, there is not yet any in vivo study to provide direct evidence for the role of Dab2 in thrombosis and haemostasis.
In this study, megakaryocyte/platelet lineage-restricted Dab2 knockout mice were generated by using the PF4-Cre transgenic system.28 We report here that PF4-Cre-driven Dab2 knockout mice display a prolonged bleeding time and impaired thrombus formation. Dab2-deficient platelets are impaired in platelet aggregation, spreading on fibrinogen and clot retraction in response to low concentrations of thrombin. The functional defect of Dab2-deficient platelets is correlated with the lack of responsiveness to thrombin-induced RhoA-ROCKII and Akt-mTOR activation, ADP release and integrin αIIbβ3 activation. This study defines Dab2 as a key regulator of thrombosis and haemostasis by playing a selective role in thrombin-stimulated inside-out signaling in platelets.
Materials and Methods
Materials and Methods are available in the online-only Data Supplement.
Results
Generation of Megakaryocyte Lineage-Restricted Dab2 Knockout Mice
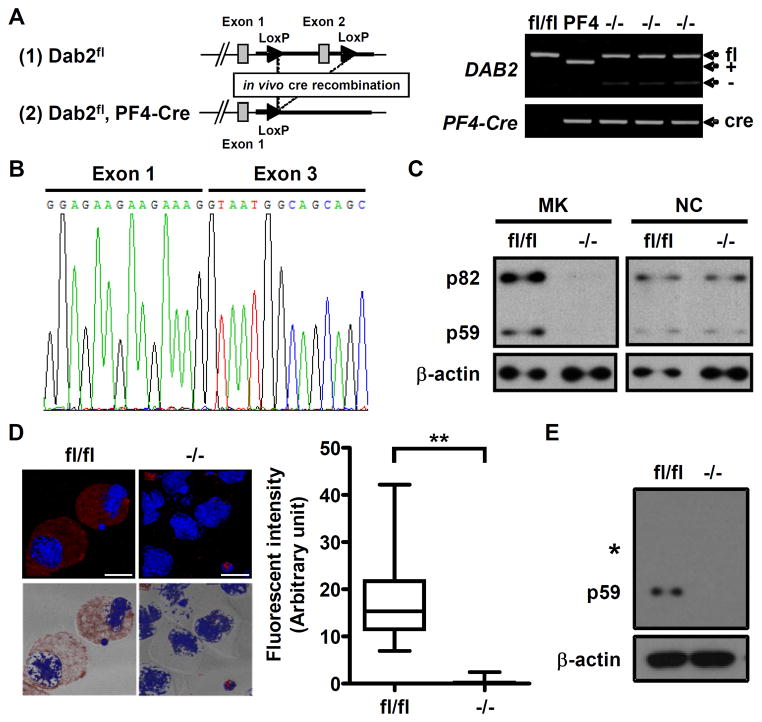
To determine the in vivo roles of Dab2 in platelet function, megakaryocyte lineage-restricted Dab2 knockout (Dab2−/−) mice were generated by cross-breeding Dab2fl/fl mice with PF4-Cre mice to ablate exon 2 of DAB2 gene. Genotyping of Dab2−/− tail genomic DNA by PCR revealed the PCR products of 530 and 450 bp corresponding to the alleles of DAB2 and PF4-Cre, respectively (Figure 1A). Sequencing of the RT-PCR product from a single Dab2−/− megakaryocyte revealed the presence of a truncated DAB2 transcript, indicating successful ablation of DAB2 gene by Cre-recombinase (Figure 1B).
Figure 1.
Generation of megakaryocyte lineage-restricted Dab2 knockout mice. A, Dab2 targeting strategy (left panel). The positions of the PF4-Cre (450 bp) and the wild-type (460 bp, +), null (250 bp, −) and floxed (530 bp, fl) alleles for DAB2 were indicated (right panel). B, The PCR product was sequenced and truncated DAB2 transcript was found to present in the −/− megakaryocyte. C, The lysates of the sorted megakaryocytes were collected to analyze Dab2 expression by Western blot using anti-Dab2 antibody. Cell lysates from the small cell fraction on the top of BSA gradient were also collected for the use as non-megakaryocytic small cells (NC) to demonstrate lineage-specific knockout of Dab2 protein. D, Dab2 expression in the fl/fl and −/− megakaryocytes was analyzed by immunofluorescence staining using anti-Dab2 antibody followed by Alexa Fluor 546 goat anti-rabbit secondary antibody (red) and counterstained with 4′,6-diamidino-2-phenylindole (DAPI, blue). The images were observed using fluorescence and differential interference contrast microscopy. Left panel, the upper images represent the fluorescent field of XY projection and the bottom images represent the fluorescent XY projection merged with its corresponding bright-field image (length of bar = 20 μm). The fluorescence intensity of fl/fl (n = 40) and −/− (n = 40) megakaryocytes was quantified by ImageJ software and was shown as Box and Whisker plot (right panel). The ends of the box are the upper and lower quartiles and the median is marked by a horizontal line inside the box. The whiskers are the two lines outside the box that extend to the highest and lowest values. **, p < 0.01. E, The lysates of fl/fl and −/− platelets were analyzed by Western blot using anti-Dab2 antibody. The position of membrane corresponding to the molecular weight of 82 kDa was marked. The expression of β-actin was used for the control of equal protein loading.
After BSA density gradient and cell sorting to enrich CD41+/Gr1− megakaryocytes for over 85% purity, both p82 and p59 Dab2 proteins were detectable in Dab2fl/fl but not in Dab2−/− megakaryocytes (Figure 1C). Dab2 expression in the non-megakaryocytic small cell population obtained from the upper layer of the BSA density gradient was not affected. Immunofluorescence staining using anti-Dab2 antibody further confirmed a lack of Dab2 expression in Dab2−/− megakaryocytes (Figure 1D).
Western blot analysis was then performed to characterize Dab2 expression in Dab2−/− platelets. p59 was the sole Dab2 isoform in Dab2fl/fl platelets and its expression was abrogated in Dab2−/− platelets (Figure 1E). These data confirm the generation of megakaryocyte/platelet-restricted Dab2 deficient mice.
Dab2−/− Mice Display Impaired Haemostasis and Thrombosis with No Defect in Platelet Production and Granule Biogenesis
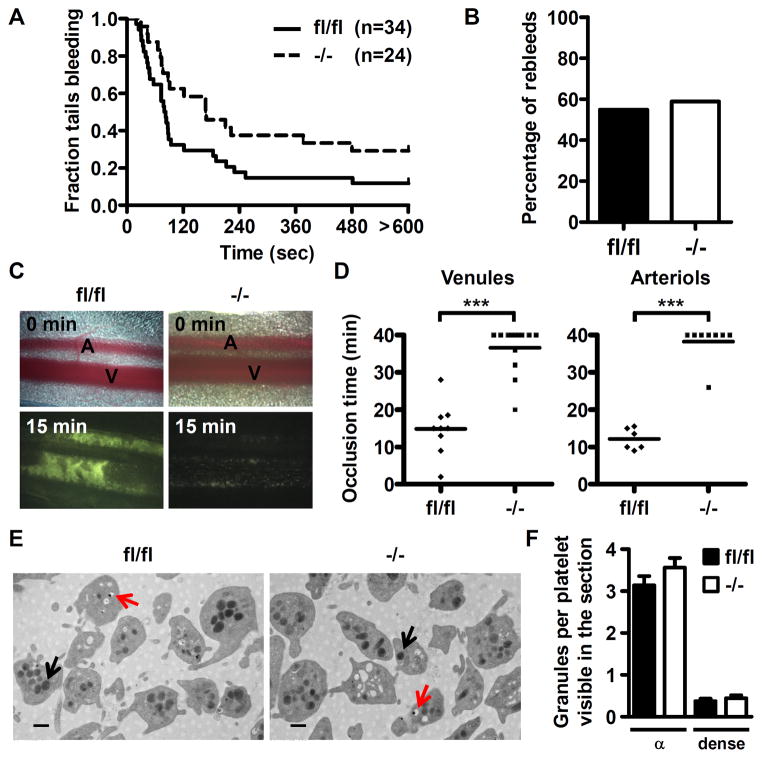
Dab2−/− mice were viable with no evidence of spontaneous bleeding or hemorrhage and no apparent differences in size and weight when compared to Dab2fl/fl mice. A bleeding time assay was performed to explore the primary haemostasis function of Dab2 following injury. Dab2−/− mice had an average bleeding time of 308.4 ± 56.4 sec that was significantly increased when compared to 171.5 ± 36.9 sec for Dab2fl/fl mice (p = 0.039 by log-rank test, Figure 2A). 29.2% of Dab2−/− mice had excessive bleeding (lasting for more than 10 min) when compared to 11.8% of Dab2fl/fl mice. In contrast, the rebleeding rate is similar for Dab2−/− and Dab2fl/fl mice (58.8% vs. 54.8%, Figure 2B).
Figure 2.
Dab2−/− mice display impaired haemostasis and thrombosis but are normal for platelet morphology and granule formation. A, Time from the excision to cessation of bleeding was recorded. The fraction of tails that are bleeding as a function of time after tail transection was shown. Genotypes and the number of mice of each genotype are indicated. The effect of Dab2 knockout on bleeding time was statistically significant (log-rank test, p = 0.039). B, The mice as described in A that were rebled within 2 min after blood flow stop were considered as having the tendency of rebleeding. The percentage of fl/fl and −/− mice with rebleeding tendency were plotted. C–D, Thrombus formation in the mesenteric arterioles and venules was induced by FeCl3. Mesentery was placed under a fluorescence microscope and thrombus formation was video recorded. Representative images for fl/fl and −/− mice at 0 and 15 min following injury were shown in C and the complete video was shown in Video 1. The dot plot shows occlusion time for venules and arterioles as a result of FeCl3-induced thrombosis in fl/fl (n = 9 for venules and n = 6 for arterioles) and −/− mice (n = 13 for venules and n = 8 for arterioles). Means are indicated by horizontal lines. ***, p < 0.001. E, Representative transmission electron microscopy images (30000 X, length of bar = 500 nm) of fl/fl and −/− platelets are shown. Black arrow: α-granule. Red arrow: dense granule. F, The number of α- and dense granules from a total of 12 fields of view in two independent experiments was counted. Data are shown as the mean ± SEM for the number of α- and dense granules per platelet visible in the section (n = 12).
Thrombus formation in Dab2−/− mice was evaluated using a FeCl3-induced mesenteric venules/arterioles thrombosis model. In control mice, the thrombus was compact and occupied the entire injured surface, as judged by the uniform intensity of fluorescence (Figure 2C). In contrast, the injured surface was not uniformly covered by thrombus in Dab2−/− mice, as observed by the lack of homogeneity of the fluorescence signal (Figure 2C and Video 1). The occlusion time was much slower in Dab2−/− compared with control venules (36.6 ± 1.7 min and 14.8 ± 2.4 min, respectively, p < 0.001) and arterioles (38.3 ± 1.8 min and 12.2 ± 1.2 min, respectively, p < 0.001, Figure 2D). These data indicate Dab2−/− mice elicit impaired primary haemostasis and thrombus formation.
To determine whether the changes in haemostasis and thrombus formation of Dab2−/− mice are due to the defect in hematopoiesis and platelet production, haematological profiles of Dab2−/− mice were determined. Blood counts were comparable between Dab2−/− and Dab2fl/fl mice (Table I in the online-only Data Supplement). In particular, the platelet numbers and mean volumes were not significantly different (910 ± 46/μl (x103) and 4.4 ± 0.1 fl for Dab2fl/fl and 872 ± 40/μl (x103) and 4.2 ± 0.1 fl for Dab2−/−, Table I in the online-only Data Supplement). Transmission electron microscopy analysis revealed that Dab2fl/fl and Dab2−/− platelets displayed similar morphology and granule biogenesis (Figure 2E and 2F). The number of α-granules per platelet in one single section was 3.1 ± 0.2 and 3.6 ± 0.2 for Dab2fl/fl and Dab2−/− platelets, respectively. The number of dense granules per platelet in one single section was 0.4 ± 0.1 and 0.5 ± 0.1 for Dab2fl/fl and Dab2−/− platelets, respectively. These data indicate that Dab2−/− mice are normal in blood cell production and platelet biogenesis.
Dab2−/− Platelets Are Selectively Defective in Thrombin-Induced Aggregation, Platelet Spreading on Fibrinogen and Clot Retraction
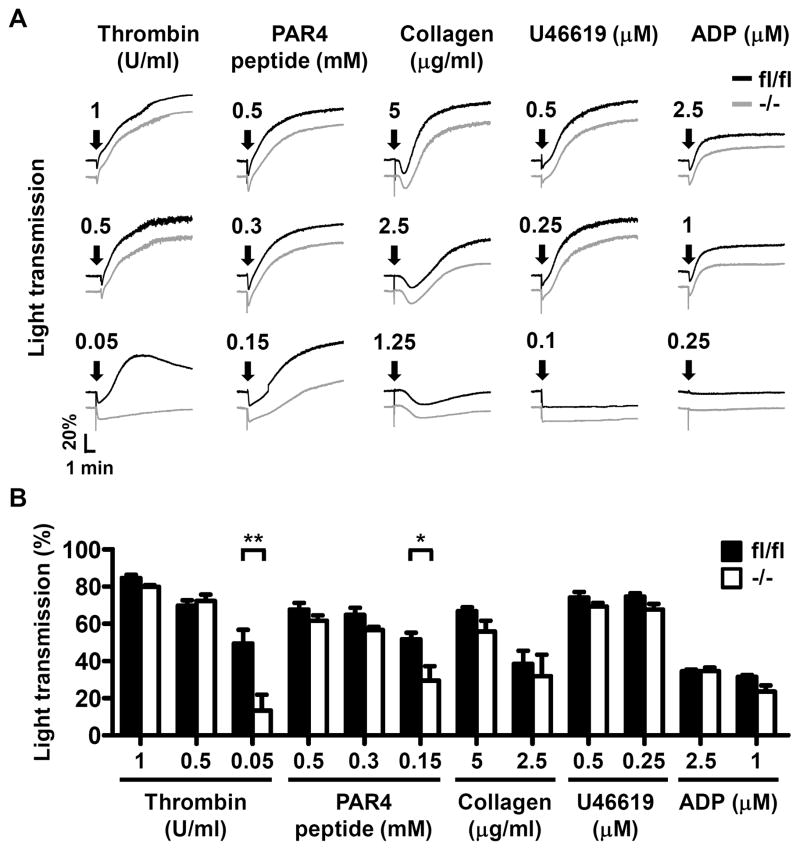
Agonist-induced platelet aggregation assays were performed to elucidate the effects of Dab2 deficiency on platelet function. Dab2−/− platelets responded normally to collagen, U46619, ADP, and high concentrations of thrombin and PAR4 peptide. However, the responsiveness of Dab2−/− platelets to low concentrations of thrombin (0.05 U/ml) and PAR4 peptide (0.15 mM) was significantly decreased (Figure 3A). At the end of the assays, the percentage of light transmission for thrombin (0.05 U/ml) treatment was 49.4 ± 7.4% and 13.3 ± 8.5% for Dab2fl/fl and Dab2−/− platelets, respectively (p < 0.01, Figure 3B). The percentage of light transmission for PAR4 peptide (0.15 mM) treatment was 51.6 ± 3.5% and 29.5 ± 7.7% for Dab2fl/fl and Dab2−/− platelets, respectively (p < 0.05, Figure 3B). These data indicate that Dab2-deficient platelets are defective in response to low concentrations of thrombin and PAR4 peptide.
Figure 3.
Selective defects in thrombin-induced aggregation of Dab2−/− platelets. A–B, The washed platelets of fl/fl and −/− mice were stimulated by soluble agonists and platelet aggregation was recorded by a platelet aggregometer (Chrono-Log). Representative aggregation curves in response to thrombin, PAR4 peptide (AYPGKF), collagen, U46619 and ADP at the indicated concentrations were shown in A. Arrows indicate the point of agonist addition. The percentage of light transmission at the end of aggregation assay was shown in B. The data represent the mean ± SEM of 3–9 independent experiments. *, p <0.05 and **, p <0.01.
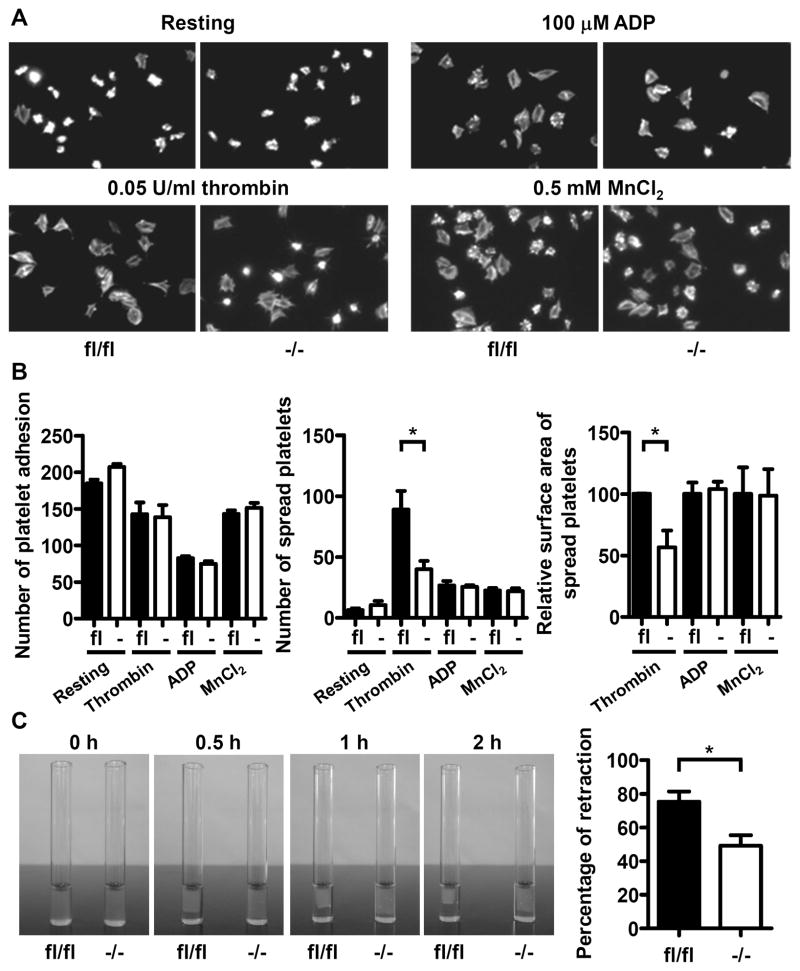
Platelet-fibrinogen interaction is important for the primary haemostatic plug and thrombus formation. The adhesion and spreading of Dab2−/− platelets on immobilized fibrinogen were analyzed (Figure 4A and 4B). The number of Dab2−/− platelet adhesions on fibrinogen was similar to the Dab2fl/fl platelets (Figure 4B, left panel). At the resting stage, Dab2fl/fl and Dab2−/− platelets did not spread well on fibrinogen, consistent with a previous report.29 Upon thrombin stimulation, a large portion of Dab2−/− platelets had rounded morphology with limited lamellipodia formation. The number of Dab2−/− platelets spread on fibrinogen (39.9 ± 6.9) was decreased when compared to the Dab2fl/fl platelets (88.9 ± 15.3, p < 0.05, Figure 4B, middle panel). The increase in surface area of spread of Dab2−/− platelets was 56.7 ± 13.7% of Dab2fl/fl platelets (p < 0.05, Figure 4B, right panel). In contrast, spreading of Dab2−/− and Dab2fl/fl platelets on fibrinogen stimulated by ADP was similar (Figure 4A and 4B), indicating that Dab2 acts on the thrombin- but not ADP-stimulated inside-out signaling. Dab2−/− and Dab2fl/fl platelets also spread equally on fibrinogen when stimulated by MnCl2 (an exogenous activator of the integrin, Figure 4A and 4B), revealing that Dab2 deficiency does not affect the outside-in signaling of integrin αIIbβ3.
Figure 4.
Reduced spreading on fibrinogen and impaired clot retraction of Dab2−/− platelets in response to low concentrations of thrombin. A–B, Platelet adhesion and spreading were performed. The platelets were then labeled with FITC-conjugated phalloidin and recorded by fluorescence microscopy under high power field (HPF) of 1,000 X magnification. The number of platelet adhesion/HPF was determined. The increase in surface area of spreading platelets by thrombin, ADP or MnCl2 stimulation was determined by subtraction of the surface area of resting platelets. The relative surface area of spreading platelets was shown. The data represent the mean ± SEM of 3–7 independent experiments. *, p < 0.05. C, The photographs corresponding to 0-, 0.5-, 1- and 2-h after initiation of thrombin-induced clot retraction were shown. The area of clot at 2 h after reaction was quantified by ImageJ and the percentage of retraction [(original area - clot area) X 100%] was calculated. The data represent the mean ± SEM of 5 independent experiments. *, p < 0.05.
Clot retraction is mediated by binding of fibrinogen to the activated integrin αIIbβ3 leading to contraction of platelets. Clot retraction assay revealed that Dab2fl/fl platelets started to retract at 30 min and were completely retracted by 2 h, while Dab2−/− platelets showed only partial retraction by 2 h (Figure 4C). After quantifying the clot area, the percentage of clot retraction was 73.5 ± 6.0% for Dab2fl/fl platelets, which was significantly different than 49.2 ± 6.3% for Dab2−/− platelets (p < 0.05, Figure 4C). These data indicate that Dab2 deficiency results in a defect in thrombin signaling leading to impaired platelet spreading on fibrinogen and clot retraction.
Dab2 Deficiency Causes a Decrease in Platelet Fibrinogen Storage and Thrombin-Stimulated ADP Release and Integrin αIIbβ3 Activation
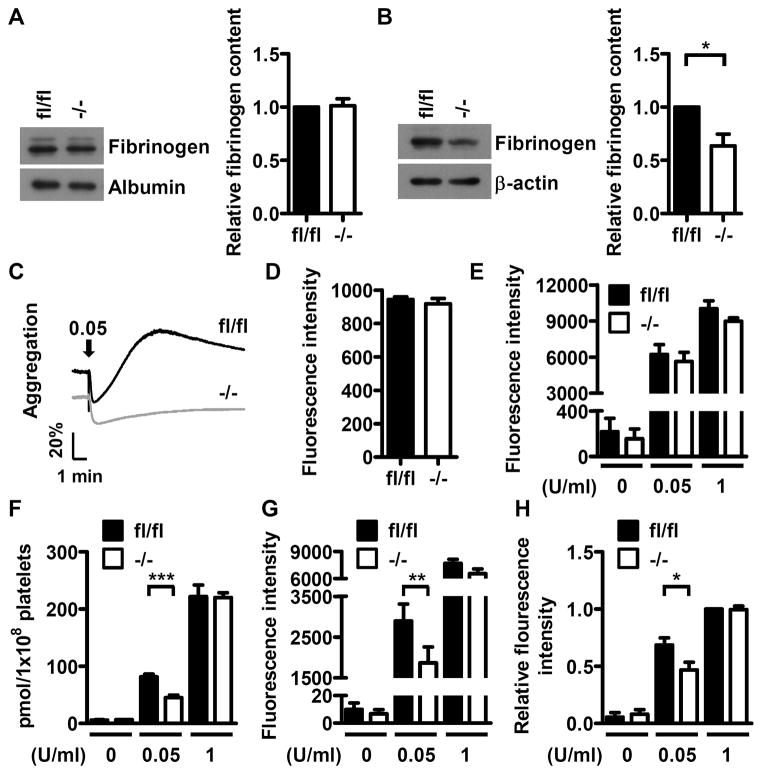
Dab2 has been shown to play a role in fibrinogen internalization in human K562 cells.25 The fibrinogen content in Dab2−/− platelets was analyzed to investigate whether Dab2 deficiency has any effect on fibrinogen storage in platelets. Western blot analysis revealed that Dab2fl/fl and Dab2−/− mice had similar amounts of plasma fibrinogen (Figure 5A). However, the fibrinogen content in Dab2−/− platelets was approximate 63% of the control (p < 0.05, Figure 5B), implicating a selective defect of Dab2−/− platelets in fibrinogen storage. Adding exogenous fibrinogen in the reaction mixtures was not able to restore thrombin-induced aggregation response of Dab2−/− platelets (Figure 5C). These data imply that the decrease in fibrinogen storage does not account for the attenuated response of Dab2−/− platelets to low concentrations of thrombin.
Figure 5.
Deficiency of platelet Dab2 causes a decrease in fibrinogen content and thrombin-induced ADP release and integrin αIIbβ3 activation. A, Platelet-poor-plasma (PPP) of fl/fl and −/− mice was collected and the fibrinogen content in PPP was determined by Western blotting using anti-fibrinogen antibody. The expression of albumin was used for the control of equal protein loading. The band intensity of fibrinogen normalized by albumin was quantified by ImageJ software. The data represent the mean ± SEM of 6 independent experiments. B, The platelets of fl/fl and −/− mice were collected and the fibrinogen contents were analyzed by Western blot using the anti-fibrinogen antibody. The expression of β-actin was used for the control of equal protein loading. The band intensity of fibrinogen normalized by β-actin was quantified by ImageJ software. The data represent the mean ± SEM of 4 independent experiments. *, p <0.05. C, 1 μg of fibrinogen was added to the fl/fl or −/− washed platelets from fl/fl or −/− mice followed by thrombin (0.05 U/ml) stimulation. Platelet aggregation was then recorded by a platelet aggregometer (Chrono-Log). Arrows indicate the point of agonist added. Essentially similar results were obtained in 3 independent experiments. D, The washed platelets from fl/fl and −/− mice were incubated with the FITC-conjugated anti-CD41 antibody and analyzed by flow cytometry. The data represent the mean ± SEM of 3 independent experiments E, Resting or thrombin-stimulated (0.05 U/ml and 1 U/ml) fl/fl and −/− platelets were incubated with the PE-conjugated anti-CD62P antibody and analyzed by flow cytometry. The corresponding isotype control antibody was used to define the background fluorescence signal. The data represent the mean ± SEM of 3–6 independent experiments. F, Supernatants from thrombin-stimulated fl/fl and −/− platelets were collected. ADP release assay was then performed and quantified using the GloMax 20/20 luminometer. The data represent the mean ± SEM of 5 independent experiments. ***, p < 0.001. G–H, Resting or thrombin-stimulated (0.05 U/ml and 1 U/ml) fl/fl and −/− platelets were incubated with the PE-conjugated JON/A antibody (panel G) or Alexa Fluor-488-conjugated fibrinogen (panel H) followed by flow cytometry. The corresponding isotype control antibody was used to define the background fluorescence signal. The data represent the mean ± SEM of 5–10 independent experiments. **, p < 0.01 and *, p < 0.05.
The effects of Dab2 deficiency on thrombin signaling was delineated further in the following experiments. Surface CD41 expression and thrombin-stimulated α-granule release as indicated by the surface expression of CD62P were normal in Dab2−/− platelets (Figure 5D and 5E). In contrast, low concentrations of thrombin induced less release of ADP, less integrin αIIbβ3 activation and less fibrinogen binding from Dab2−/− than from Dab2fl/fl platelets (Figures 5F, 5G and 5H). These data indicate that Dab2−/− platelets are defective in thrombin-stimulated ADP release and integrin αIIbβ3 activation.
Dab2 Regulates Thrombin-Stimulated Inside-Out Signaling
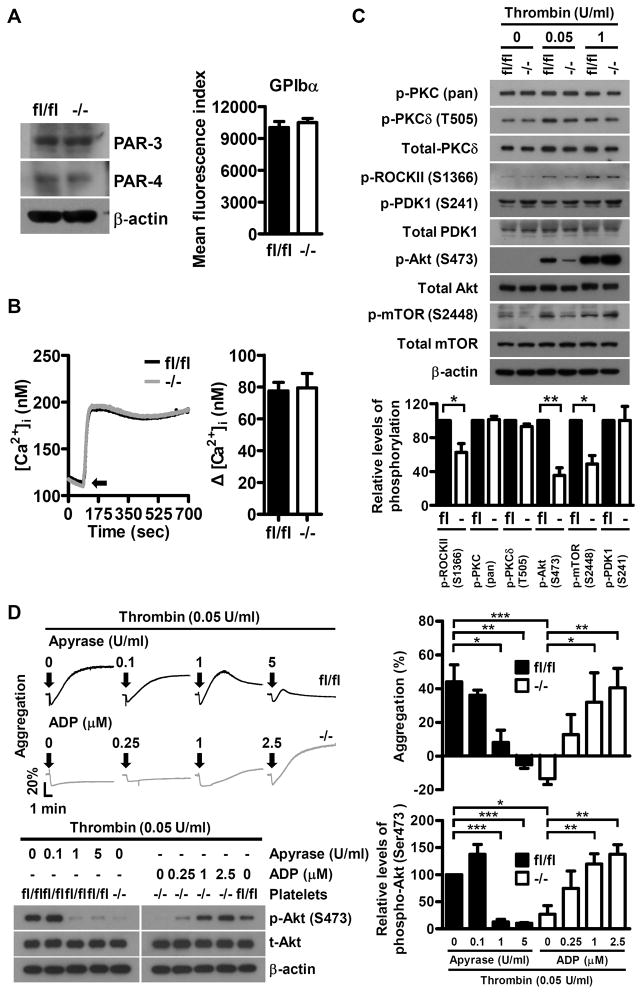
To elucidate the molecular insight for Dab2 function in thrombin signaling, we first analyzed whether the expression of thrombin receptors was altered in Dab2−/− platelets. Flow cytometry and Western blot analyses revealed that the expression levels of thrombin receptors GPIbα, PAR3 and PAR4 were comparable between Dab2fl/fl and Dab2−/− platelets (Figure 6A), ruling out aberrant thrombin receptor expression as the cause of impaired thrombin signaling in Dab2−/− platelets.
Figure 6.
Dab2 is involved in the regulation of low concentrations thrombin-stimulated ROCKII-Ser1366 and Akt-Ser473 phosphorylation. A, The expression of thrombin receptor GPIbα, PAR3 and PAR4 in fl/fl and −/− platelets was analyzed by flow cytometry or Western blot using FITC-conjugated anti-GPIbα, anti-PAR3 and anti-PAR4 antibodies, respectively. B, Fluo-3-loaded washed platelets from fl/fl and −/− mice were stimulated with 0.05 U/ml thrombin and the changes in intracellular Ca2+ concentration ([Ca2+]i) were calculated. Arrow indicates the starting point of thrombin stimulation (left panel). The data represent the mean ± SEM of 3 independent experiments (right panel). C, The fl/fl and −/− platelets were stimulated with the indicated concentrations of thrombin and the platelet lysates were collected for Western blot analysis using the indicated antibodies. The expression of β-actin was used for the control of equal protein loading (upper panel). The relative levels of phosphorylation for the indicated proteins stimulated with 0.05 U/ml of thrombin were quantified by ImageJ software (lower panel). The data represent the mean ± SEM of 3–4 independent experiments. **, p < 0.01 and *, p < 0.05. D, The fl/fl platelets were pre-incubated with the indicated concentrations of apyrase for 1 min and then stimulated with thrombin (0.05 U/ml). The −/− platelets were stimulated with thrombin (0.05 U/ml) and the indicated concentrations of ADP simultaneously. Platelet aggregation was recorded by a platelet aggregometer (Chrono-Log). Representative traces for platelet aggregation are shown (upper left panel). Arrows indicate the point of agonists addition. The lysates for the platelets with the indicated treatment were collected for Western blot analysis using the anti-Akt and anti-p-Akt (Ser473) antibodies. The expression of β-actin was used for the control of equal protein loading (lower left panel). The percentage of light transmission at the end of the aggregation assay (upper right panel) and the relative phosphorylation levels of p-Akt (Ser473) are shown (lower right panel). The data represent the mean ± SEM of 4 independent experiments. *, p <0.05, **, p <0.01 and ***, p < 0.001.
The inside-out signaling of thrombin is mainly transmitted through the G-protein-dependent pathways that cause an increase in intracellular calcium concentration and signaling proteins phosphorylation.5 Analyses of the molecular events downstream of thrombin receptors revealed that the intracellular calcium concentration, and the phosphorylation of PKC-pan, PKCδ-T505 and PDK1-Ser241 stimulated by low concentrations of thrombin were similar between Dab2fl/fl and Dab2−/− platelets (Figure 6B and 6C), while Gα12/13-dependent Rho activation, as represented by the phosphorylation of ROCKII (a downstream effector of RhoA) at Ser136630 in Dab2−/− platelets was 62.4 ± 10.5% of the control (p < 0.05, Figure 6C). The phosphorylation of Akt-Ser473 and mTOR-Ser2448 in Dab2−/− platelets were also significantly attenuated to 35.3 ± 8.9% and 48.8 ± 10.1% of the control, respectively, (p < 0.01 and p < 0.05, Figure 6C). These defects were not apparent if Dab2−/− platelets were stimulated with high concentrations of thrombin (0.5 U/ml or 1 U/ml) or other soluble agonists such as collagen, U46619 and ADP (Figure 6C and Figure I in the online-only Data Supplement). These data indicate that Dab2 mediates signaling by low doses of thrombin and acts upstream of RhoA-ROCK and Akt-mTOR. Platelet aggregation and Akt-Ser473 phosphorylation stimulated by low thrombin concentrations were inhibited by apyrase in Dab2fl/fl platelets and were restored by ADP in the Dab2−/− platelets in a dose-dependent manner (Figure 6D). These data indicate that impaired ADP release contributes to the defective platelet aggregation and Akt phosphorylation of Dab2−/− platelets.
Discussion
Our results define a key in vivo function for Dab2 in regulating haemostasis, thrombosis and platelet signaling in mice. Absence of Dab2 does not influence the process of platelet biogenesis, but selectively inhibits the phosphorylation of ROCKII-Ser1366, Akt-Ser473 and mTOR-Ser2448 under conditions of low dose thrombin stimulation. The impairment of the signaling response in Dab2−/− platelets causes functional changes in ADP release, integrin αIIbβ3 activation, fibrinogen binding, platelet aggregation, spreading on fibrinogen and clot retraction. In accord with the aforementioned functional defects of Dab2−/− platelets, Dab2−/− mice display a prolonged bleeding time and impaired thrombus formation. This study presents novel evidence demonstrating that Dab2 plays an important role in haemostasis, thrombosis and platelet signaling in vivo.
Various signaling pathways are activated when platelets are stimulated with different types of agonists. Collagen induces clustering of glycoprotein VI and causes an increase in glycoprotein VI-associated Src family kinase activity, leading to a rise in intracellular calcium and an increase in PKC activity.31,32 The receptors of thrombin and thromboxane A2 couple to both Gαq and Gα12/13 pathways, while ADP binds to P2Y1 and P2Y12 receptors that couple to Gαq and Gαi, respectively, for signal propagation.33 GPIbα is apparently another type of thrombin receptor.34,35 Since the expression levels of PAR3, PAR4 and GPIbα are similar between Dab2−/− and wild type platelets, the selective defects of Dab2−/− platelets in response to low thrombin concentrations are not attributed to the altered expression of thrombin receptors. Instead, Dab2−/− platelets are impaired in Gα12/13-mediated RhoA activation as shown by the decreased ROCKII-Ser1366 phosphorylation. This is consistent with the previous study showing that Dab2 modulates RhoA activity and inhibits nerve growth factor-induced neurite outgrowth.21 Dab2−/− and RhoA−/− mice are abnormal in haemostasis and thrombus formation, while the corresponding platelets are defective in integrin activation, clot retraction and dense granule/ADP secretion in response to low concentrations of thrombin. In contrast, Dab2−/− and RhoA−/− platelets response normally to ADP and collagen-stimulated platelet aggregation.36 These results imply that Dab2 and RhoA-ROCKII are in the same signaling axis.
Discrepancies between Dab2−/− and RhoA−/− platelets are also noted. For example, RhoA−/− but not Dab2−/− platelets are defective in P-selectin exposure in response to thrombin.36 This is explainable by the broad spectrum of RhoA function, which plays a central role in Gα12/13 signaling and also contributes to Gαq-mediated platelet activation.37 Several indirect studies indicate that Gαq may directly regulate RhoA activity by activating the Rho-guanine nucleotide exchange factors.38 In contrast, Dab2 appears to restrict its function in Gα12/13-associated RhoA-ROCK activation with no evidence for cross talk of Dab2 to Gαq; Dab2−/− platelets are normal in Gαq-mediated calcium mobilization and PKC phosphorylation in response to thrombin. This study reveals for the first time the functional interplay of Dab2 with Gα12/13-mediated RhoA-ROCK activation in murine platelets.
Because Src family kinase and Gαi/Gβγ/Gαq are the major signaling pathways mediating platelet activation by collagen and ADP, respectively, the restricted function of Dab2 in the Gα12/13 pathway also explains the normal aggregation patterns of Dab2−/− platelets in response to collagen and ADP. Despite Gα12/13 coupling to the thromboxane A2 receptors, Dab2−/− platelets are not defective in platelet aggregation stimulated by the thromboxane A2 mimetic U46619. Similar to our findings, Lyn-deficient platelets are defective in aggregation stimulated by low concentrations of thrombin and PAR4, but produce normal aggregation by U46619 and ADP.39 These studies highlight the presence of agonists-specific signaling downstream of Gα12/13 pathway.
Another important finding in this study is that Dab2−/− platelets are normal in PDK1-Ser241 phosphorylation, but are impaired in ADP release, platelet aggregation and the phosphorylations of Akt-Ser473 and mTOR-Ser2448. The defective platelet aggregation and Akt-Ser473 phosphorylation were rescued and restored by supplementation of ADP to the reaction. These data indicate that Akt-Ser473 phosphorylation is independent of PDK1 signaling, consistent with the notion that the mTORC2 complex is responsible for the phosphorylation of Akt-Ser473.40
Based on the findings in this study, a possible working model for the mechanisms of Dab2 action in thrombin-stimulated inside-out signaling is proposed. In response to low concentrations of thrombin, Dab2 regulates Gα12/13-mediated RhoA-ROCK activation and potentiates ADP release. The released ADP binds to its receptor P2Y12 and activates Gαi/Gβγ signaling leading to Akt-mTOR phosphorylation/activation, which potentiates integrin activation and platelet aggregation. This mode of Dab2 action is consistent with previous studies showing that Gα12/13-mediated RhoA-ROCK activation regulates cytoskeleton reorganization which is critical for degranulation,41 while ADP release is essential to stabilize platelet aggregates stimulated by concentrations of thrombin lower than 0.25 to 1 U/ml but is not required at high thrombin concentrations.42
Despite the fact that Dab2 elicits a restrictive function in response to low thrombin concentrations, but not other soluble agonists, Dab2 deficiency has profound effects on haemostasis and thrombosis in vivo, as evidenced by Dab2−/− mice having a prolonged bleeding time and impaired thrombus formation. Consistent with our findings, even a partial decrease in thrombin-induced platelet aggregation observed in PAR3-deficient mice impairs haemostasis and protects against thrombosis.43 Similarly, blocking thrombin receptor PAR4 on platelets extends bleeding time and protects against systemic platelet activation.44 This and other studies support the perception that thrombin signaling plays a pivotal role in haemostasis and thrombosis.
The defect in fibrinogen storage does not particularly contribute to the defective response of Dab2−/− platelets to low concentrations of thrombin. Nevertheless, this and other studies indicate that Dab2 positively regulates fibrinogen uptake. Fibrinogen uptake in K562 cells is a Dab2-dependent process.25 Expression of shDAB2 in rat megakaryocytes results in a moderate decrease in fibrinogen uptake (Figure II in the online-only Data Supplement). At the molecular level, Dab2 is known to bind the β3-NITY motif of the fibrinogen receptor integrin αIIbβ3 through the PTB domain7,13 and act as an NPXY sequence-specific clathrin adaptor protein in receptor endocytosis.17,45,46 With two-thirds of the normal fibrinogen content still present in Dab2−/− platelets, a Dab2-dependent and a Dab2-independent mechanism for fibrinogen internalization could occur in platelets.
Not all the observations obtained from Dab2−/− mice are consistent with our previous analyses using human cell lines and platelets. In K562 cells, Dab2 knockdown augments rather than weakens integrin αIIbβ3 activation and increases cell adhesion to fibrinogen.13 In human platelets, Dab2 negatively regulates platelet-fibrinogen interaction and platelet aggregation.13,23,26,47 Dab2 is required for murine platelet aggregation in response to low concentrations of thrombin. Several issues could be argued for the discrepancy among these studies. First, different Dab2 isoforms are present in human and murine platelets. Human platelets mainly expressed p82 whereas p59 is the sole Dab2 isoform in murine platelets (Figure III in the online-only Data Supplement). Distinct functions of p82 and p59 have been reported. p82 has been shown to function in receptor-mediated endocytosis, while p59 can act as a transcriptional regulator during the differentiation of F9 cells.48–51 In Dab2-knockout mice, expression of p59 only partially compensates for the absence of Dab2.12 Hence, species-specific expression of Dab2 isoforms may explain the distinct features of Dab2 in human and mouse megakaryocytes and platelets.
The discrepancy between this and previous studies could simply be due to the use of different experimental models. In the human platelet studies, the experiments were mainly performed in vitro using recombinant Dab2 protein as a tool to analyze Dab2 function and its effects on platelet response.23,26 No in vivo study was performed using Dab2 mutants or Dab2-deficient human platelets. In contrast, Dab2-deficient mouse platelets were used in the present study to analyze in vivo Dab2 function. Future study using an animal model system with expression of human platelet Dab2 protein may provide additional insight for the differential Dab2 function in human and mouse platelets.
In conclusion, the findings that Dab2 expression is required for (1) haemostasis and thrombosis, (2) fibrinogen storage, (3) thrombin-stimulated inside-out signaling, (4) optimal ADP release, integrin αIIbβ3 activation and fibrinogen binding leading to platelet aggregation, spreading on fibrinogen and clot retraction stimulated by low thrombin concentrations, are the first demonstration that Dab2 plays an important role in efficient haemostasis and platelet activation by thrombin in vivo.
Supplementary Material
Significance.
The essential role of platelet activation in haemostasis and thrombotic diseases focuses attention on unveiling the underlying intracellular signals of platelet activation. Dab2 has been implicated in platelet aggregation and in the control of clotting responses. By analyzing the phenotype and platelet functions using the megakaryocyte-lineage restricted Dab2 knock out mouse, we demonstrate in this study that Dab2 is a key regulator of haemostasis and thrombosis by playing a selective role in thrombin-stimulated inside-out signaling. This study sheds new insight in platelet biology and represents the first report demonstrating that Dab2 has a functional interplay with thrombin signaling.
Acknowledgments
The PF4-Cre mouse was a kind gift from Dr. Radek Skoda (University Hospital Basel, Basel, Switzerland). We would like to acknowledge the editorial help from Dr. Arnold Stern (New York University School of Medicine) and the technical advice from Drs. Shu-Wha Lin and Chung-Yang Kao (National Taiwan University) for FeCl3-induced thrombosis model.
Sources of Funding
This study was supported in part by the China Medical University Grant CMU97-153 to J.C.C., grant GM066257 from the U.S. Public Health Service to J.A.C., and National Science Council Grants NSC102-2628-B-182-009-MY3, and NSC102-2628-B-182-010-MY3, Chang Gung Memorial Hospital Grants CMRPD1C0552 and CMRPD1B0393, and Chang Gung Molecular Medicine Research Center Grant EMRPD1D1281 to C.P.T.
Abbreviations
- Dab2
Disabled-2
- PI3K
phosphoinositide 3-kinase
- PARs
protease-activated receptors
- PKC
protein kinase C
Footnotes
Disclosures: None.
References
- 1.George JN. Platelets. Lancet. 2000;355:1531–1539. doi: 10.1016/S0140-6736(00)02175-9. [DOI] [PubMed] [Google Scholar]
- 2.Chong AJ, Pohlman TH, Hampton CR, Shimamoto A, Mackman N, Verrier ED. Tissue factor and thrombin mediate myocardial ischemia-reperfusion injury. Ann Thorac Surg. 2003;75:S649–655. doi: 10.1016/s0003-4975(02)04691-x. [DOI] [PubMed] [Google Scholar]
- 3.Schonberger T, Ziegler M, Borst O, et al. The dimeric platelet collagen receptor GPVI-Fc reduces platelet adhesion to activated endothelium and preserves myocardial function after transient ischemia in mice. Am J Physiol Cell Physiol. 2012;303:C757–766. doi: 10.1152/ajpcell.00060.2012. [DOI] [PubMed] [Google Scholar]
- 4.Newman DK. PI3Kbeta goes to the head of its class. Blood. 2009;114:2011–2012. doi: 10.1182/blood-2009-06-228551. [DOI] [PubMed] [Google Scholar]
- 5.Angiolillo DJ, Capodanno D, Goto S. Platelet thrombin receptor antagonism and atherothrombosis. Eur Heart J. 2010;31:17–28. doi: 10.1093/eurheartj/ehp504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kamath S, Blann AD, Lip GY. Platelet activation: Assessment and quantification. Eur Heart J. 2001;22:1561–1571. doi: 10.1053/euhj.2000.2515. [DOI] [PubMed] [Google Scholar]
- 7.Calderwood DA, Fujioka Y, de Pereda JM, Garcia-Alvarez B, Nakamoto T, Margolis B, McGlade CJ, Liddington RC, Ginsberg MH. Integrin beta cytoplasmic domain interactions with phosphotyrosine-binding domains: A structural prototype for diversity in integrin signaling. Proc Natl Acad Sci USA. 2003;100:2272–2277. doi: 10.1073/pnas.262791999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wolfenson H, Lubelski A, Regev T, Klafter J, Henis YI, Geiger B. A role for the juxtamembrane cytoplasm in the molecular dynamics of focal adhesions. PLoS One. 2009;4:e4304. doi: 10.1371/journal.pone.0004304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Legate KR, Fassler R. Mechanisms that regulate adaptor binding to beta-integrin cytoplasmic tails. J Cell Sci. 2009;122:187–198. doi: 10.1242/jcs.041624. [DOI] [PubMed] [Google Scholar]
- 10.Roca-Cusachs P, Del Rio A, Puklin-Faucher E, Gauthier NC, Biais N, Sheetz MP. Integrin-dependent force transmission to the extracellular matrix by alpha-actinin triggers adhesion maturation. Proc Natl Acad Sci USA. 2013;110:E1361–1370. doi: 10.1073/pnas.1220723110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cohen I. The contractile system of blood platelets and its function. Methods Achiev Exp Pathol. 1979;9:40–86. [PubMed] [Google Scholar]
- 12.Maurer ME, Cooper JA. Endocytosis of megalin by visceral endoderm cells requires the Dab2 adaptor protein. J Cell Sci. 2005;118:5345–5355. doi: 10.1242/jcs.02650. [DOI] [PubMed] [Google Scholar]
- 13.Huang CL, Cheng JC, Liao CH, Stern A, Hsieh JT, Wang CH, Hsu HL, Tseng CP. Disabled-2 is a negative regulator of integrin alpha(IIb)beta(3)-mediated fibrinogen adhesion and cell signaling. J Biol Chem. 2004;279:42279–42289. doi: 10.1074/jbc.M402540200. [DOI] [PubMed] [Google Scholar]
- 14.Wang Z, Tseng CP, Pong RC, Chen H, McConnell JD, Navone N, Hsieh JT. The mechanism of growth-inhibitory effect of DOC-2/DAB2 in prostate cancer. Characterization of a novel GTPase-activating protein associated with N-terminal domain of DOC-2/DAB2. J Biol Chem. 2002;277:12622–12631. doi: 10.1074/jbc.M110568200. [DOI] [PubMed] [Google Scholar]
- 15.Hocevar BA, Smine A, Xu XX, Howe PH. The adaptor molecule Disabled-2 links the transforming growth factor beta receptors to the Smad pathway. EMBO J. 2001;20:2789–2801. doi: 10.1093/emboj/20.11.2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hocevar BA, Mou F, Rennolds JL, Morris SM, Cooper JA, Howe PH. Regulation of the Wnt signaling pathway by disabled-2 (Dab2) EMBO J. 2003;22:3084–3094. doi: 10.1093/emboj/cdg286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morris SM, Arden SD, Roberts RC, Kendrick-Jones J, Cooper JA, Luzio JP, Buss F. Myosin VI binds to and localises with Dab2, potentially linking receptor-mediated endocytosis and the actin cytoskeleton. Traffic. 2002;3:331–341. doi: 10.1034/j.1600-0854.2002.30503.x. [DOI] [PubMed] [Google Scholar]
- 18.Kowanetz K, Terzic J, Dikic I. Dab2 links CIN85 with clathrin-mediated receptor internalization. FEBS Lett. 2003;554:81–87. doi: 10.1016/s0014-5793(03)01111-6. [DOI] [PubMed] [Google Scholar]
- 19.Inoue A, Sato O, Homma K, Ikebe M. DOC-2/DAB2 is the binding partner of myosin VI. Biochem Biophys Res Commun. 2002;292:300–307. doi: 10.1006/bbrc.2002.6636. [DOI] [PubMed] [Google Scholar]
- 20.Huang CL, Cheng JC, Kitajima K, Nakano T, Yeh CF, Chong KY, Tseng CP. Disabled-2 is required for mesoderm differentiation of murine embryonic stem cells. J Cell Physiol. 2010;225:92–105. doi: 10.1002/jcp.22200. [DOI] [PubMed] [Google Scholar]
- 21.Huang CH, Cheng JC, Chen JC, Tseng CP. Evaluation of the role of Disabled-2 in nerve growth factor-mediated neurite outgrowth and cellular signalling. Cell Signal. 2007;19:1339–1347. doi: 10.1016/j.cellsig.2007.01.019. [DOI] [PubMed] [Google Scholar]
- 22.Hosaka K, Takeda T, Iino N, Hosojima M, Sato H, Kaseda R, Yamamoto K, Kobayashi A, Gejyo F, Saito A. Megalin and nonmuscle myosin heavy chain IIA interact with the adaptor protein Disabled-2 in proximal tubule cells. Kidney Int. 2009;75:1308–1315. doi: 10.1038/ki.2009.85. [DOI] [PubMed] [Google Scholar]
- 23.Huang CL, Cheng JC, Stern A, Hsieh JT, Liao CH, Tseng CP. Disabled-2 is a novel alphaIIb-integrin-binding protein that negatively regulates platelet-fibrinogen interactions and platelet aggregation. J Cell Sci. 2006;119:4420–4430. doi: 10.1242/jcs.03195. [DOI] [PubMed] [Google Scholar]
- 24.Tseng CP, Chang P, Huang CL, Cheng JC, Chang SS. Autocrine signaling of platelet-derived growth factor regulates disabled-2 expression during megakaryocytic differentiation of K562 cells. FEBS Lett. 2005;579:4395–4401. doi: 10.1016/j.febslet.2005.06.080. [DOI] [PubMed] [Google Scholar]
- 25.Hung WS, Huang CL, Fan JT, Huang DY, Yeh CF, Cheng JC, Tseng CP. The endocytic adaptor protein Disabled-2 is required for cellular uptake of fibrinogen. Biochim Biophys Acta. 2012;1823:1778–1788. doi: 10.1016/j.bbamcr.2012.06.008. [DOI] [PubMed] [Google Scholar]
- 26.Welsh JD, Charonko JJ, Salmanzadeh A, Drahos KE, Shafiee H, Stremler MA, Davalos RV, Capelluto DG, Vlachos PP, Finkielstein CV. Disabled-2 modulates homotypic and heterotypic platelet interactions by binding to sulfatides. Br J Haematol. 2011;154:122–133. doi: 10.1111/j.1365-2141.2011.08705.x. [DOI] [PubMed] [Google Scholar]
- 27.Xiao S, Charonko JJ, Fu X, Salmanzadeh A, Davalos RV, Vlachos PP, Finkielstein CV, Capelluto DG. Structure, sulfatide binding properties, and inhibition of platelet aggregation by a disabled-2 protein-derived peptide. J Biol Chem. 2012;287:37691–37702. doi: 10.1074/jbc.M112.385609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tiedt R, Schomber T, Hao-Shen H, Skoda RC. Pf4-Cre transgenic mice allow the generation of lineage-restricted gene knockouts for studying megakaryocyte and platelet function in vivo. Blood. 2007;109:1503–1506. doi: 10.1182/blood-2006-04-020362. [DOI] [PubMed] [Google Scholar]
- 29.Goschnick MW, Lau LM, Wee JL, Liu YS, Hogarth PM, Robb LM, Hickey MJ, Wright MD, Jackson DE. Impaired “outside-in” integrin alphaIIbbeta3 signaling and thrombus stability in TSSC6-deficient mice. Blood. 2006;108:1911–1918. doi: 10.1182/blood-2006-02-004267. [DOI] [PubMed] [Google Scholar]
- 30.Chuang HH, Yang CH, Tsay YG, Hsu CY, Tseng LM, Chang ZF, Lee HH. ROCKII Ser1366 phosphorylation reflects the activation status. Biochem J. 2012;443:145–151. doi: 10.1042/BJ20111839. [DOI] [PubMed] [Google Scholar]
- 31.Varga-Szabo D, Pleines I, Nieswandt B. Cell adhesion mechanisms in platelets. Arterioscler Thromb Vasc Biol. 2008;28:403–412. doi: 10.1161/ATVBAHA.107.150474. [DOI] [PubMed] [Google Scholar]
- 32.Yacoub D, Theoret JF, Villeneuve L, Abou-Saleh H, Mourad W, Allen BG, Merhi Y. Essential role of protein kinase C delta in platelet signaling, alpha IIb beta 3 activation, and thromboxane A2 release. J Biol Chem. 2006;281:30024–30035. doi: 10.1074/jbc.M604504200. [DOI] [PubMed] [Google Scholar]
- 33.Offermanns S. Activation of platelet function through G protein-coupled receptors. Circ Res. 2006;99:1293–1304. doi: 10.1161/01.RES.0000251742.71301.16. [DOI] [PubMed] [Google Scholar]
- 34.Dubois C, Steiner B, Kieffer N, Reigner SC. Thrombin binding to GPIbalpha induces platelet aggregation and fibrin clot retraction supported by resting alphaIIbbeta3 interaction with polymerized fibrin. Thromb Haemost. 2003;89:853–865. [PubMed] [Google Scholar]
- 35.Dubois C, Steiner B, Meyer Reigner SC. Contribution of PAR-1, PAR-4 and GPIbalpha in intracellular signaling leading to the cleavage of the beta3 cytoplasmic domain during thrombin-induced platelet aggregation. Thromb Haemost. 2004;91:733–742. doi: 10.1160/TH03-06-0391. [DOI] [PubMed] [Google Scholar]
- 36.Pleines I, Hagedorn I, Gupta S, May F, Chakarova L, van Hengel J, Offermanns S, Krohne G, Kleinschnitz C, Brakebusch C, Nieswandt B. Megakaryocyte-specific RhoA deficiency causes macrothrombocytopenia and defective platelet activation in hemostasis and thrombosis. Blood. 2012;119:1054–1063. doi: 10.1182/blood-2011-08-372193. [DOI] [PubMed] [Google Scholar]
- 37.Jin J, Mao Y, Thomas D, Kim S, Daniel JL, Kunapuli SP. RhoA downstream of G(q) and G(12/13) pathways regulates protease-activated receptor-mediated dense granule release in platelets. Biochem Pharmacol. 2009;77:835–844. doi: 10.1016/j.bcp.2008.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vogt S, Grosse R, Schultz G, Offermanns S. Receptor-dependent RhoA activation in G12/G13-deficient cells: genetic evidence for an involvement of Gq/G11. J Biol Chem. 2003;278:28743–28749. doi: 10.1074/jbc.M304570200. [DOI] [PubMed] [Google Scholar]
- 39.Cho MJ, Pestina TI, Steward SA, Lowell CA, Jackson CW, Gartner TK. Role of the Src family kinase Lyn in TxA2 production, adenosine diphosphate secretion, Akt phosphorylation, and irreversible aggregation in platelets stimulated with gamma-thrombin. Blood. 2002;99:2442–2447. doi: 10.1182/blood.v99.7.2442. [DOI] [PubMed] [Google Scholar]
- 40.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 41.Huang JS, Dong L, Kozasa T, Le Breton GC. Signaling through G(alpha)13 switch region I is essential for protease-activated receptor 1-mediated human platelet shape change, aggregation, and secretion. J Biol Chem. 2007;282:10210–10222. doi: 10.1074/jbc.M605678200. [DOI] [PubMed] [Google Scholar]
- 42.Cattaneo M, Canciani MT, Lecchi A, Kinlough-Rathbone RL, Packham MA, Mannucci PM, Mustard JF. Released adenosine diphosphate stabilizes thrombin-induced human platelet aggregates. Blood. 1990;75:1081–1086. [PubMed] [Google Scholar]
- 43.Weiss EJ, Hamilton JR, Lease KE, Coughlin SR. Protection against thrombosis in mice lacking PAR3. Blood. 2002;100:3240–3244. doi: 10.1182/blood-2002-05-1470. [DOI] [PubMed] [Google Scholar]
- 44.Covic L, Misra M, Badar J, Singh C, Kuliopulos A. Pepducin-based intervention of thrombin-receptor signaling and systemic platelet activation. Nat Med. 2002;8:1161–1165. doi: 10.1038/nm760. [DOI] [PubMed] [Google Scholar]
- 45.Morris SM, Cooper JA. Disabled-2 colocalizes with the LDLR in clathrin-coated pits and interacts with AP-2. Traffic. 2001;2:111–123. doi: 10.1034/j.1600-0854.2001.020206.x. [DOI] [PubMed] [Google Scholar]
- 46.Spudich G, Chibalina MV, Au JS, Arden SD, Buss F, Kendrick-Jones J. Myosin VI targeting to clathrin-coated structures and dimerization is mediated by binding to Disabled-2 and Ptdins(4,5)P2. Nat Cell Biol. 2007;9:176–183. doi: 10.1038/ncb1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Drahos KE, Welsh JD, Finkielstein CV, Capelluto DG. Sulfatides partition disabled-2 in response to platelet activation. PLoS One. 2009;4:e8007. doi: 10.1371/journal.pone.0008007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mishra SK, Keyel PA, Hawryluk MJ, Agostinelli NR, Watkins SC, Traub LM. Disabled-2 exhibits the properties of a cargo-selective endocytic clathrin adaptor. EMBO J. 2002;21:4915–4926. doi: 10.1093/emboj/cdf487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Keyel PA, Mishra SK, Roth R, Heuser JE, Watkins SC, Traub LM. A single common portal for clathrin-mediated endocytosis of distinct cargo governed by cargo-selective adaptors. Mol Biol Cell. 2006;17:4300–4317. doi: 10.1091/mbc.E06-05-0421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Teckchandani A, Toida N, Goodchild J, Henderson C, Watts J, Wollscheid B, Cooper JA. Quantitative proteomics identifies a Dab2/integrin module regulating cell migration. J Cell Biol. 2009;186:99–111. doi: 10.1083/jcb.200812160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cho SY, Jeon JW, Lee SH, Park SS. p67 isoform of mouse disabled 2 protein acts as a transcriptional activator during the differentiation of F9 cells. Biochem J. 2000;352(Pt 3):645–650. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.