Abstract
Atherosclerosis is a focal disease that develops preferentially where non-laminar, disturbed blood flow (d-flow) occurs such as branches, bifurcations, and curvatures of large arteries. Endothelial cells sense and respond differently to d-flow compared to steady laminar flow (s-flow). D-flow that occurs in so-called athero-prone areas activates pro-inflammatory and apoptotic signaling, and this results in endothelial dysfunction and leads to subsequent development of atherosclerosis. In contrast, s-flow as “athero-protective flow” promotes expression of many anti-inflammatory genes such as Kruppel-like factor 2 (KLF2) and endothelial nitric oxide synthase (eNOS) and inhibits endothelial inflammation and athrogenesis. Here we will discuss that d-flow and s-flow induce pro- and anti-atherogenic events via flow type-specific “mechanotransduction” pathways. We will focus on five mechano-sensitive pathways: MEK5 (MAPK/ERK kinase 5)-ERK5-KLF2 signaling, ERK5-PPAR (peroxisome proliferator-activated receptor) signaling, and mechano-signaling pathways involving SUMOylation, protein kinase C-ζ, (PKCζ), and p90 ribosomal S6 kinase (p90RSK). We believe that clarifying regulation mechanisms between these two flow types will provide new insights into therapeutic approaches for the prevention and treatment of atherosclerosis.
Keywords: flow, SUMOylation, ERK5, PKCζ, p90RSK
Introduction
The surface of the vasculature, which is comprised of a monolayer of endothelial cells (ECs), is constantly exposed to various forces as blood flows. It is well-established that atherosclerotic plaques localize to areas of disturbed flow (d-flow) found at regions where vessels curve and also at vessel bifurcations and branch points. Low endothelial nitric oxide synthase (eNOS) expression and increased adhesion molecule expression are observed in these particular areas1, 2. In addition, d-flow increases secretion of pro-inflammatory molecules such as MCP-1, PDGFs, and endothelin-1 from ECs, which promote leukocyte infiltration and smooth muscle proliferation, leading to the development of atherosclerosis3-5. In contrast, atherosclerosis is rare in areas exposed to steady laminar flow (s-flow). EC stimulated by s-flow have been shown to increase the secretion of NO, PGI2, and tPA, which down-regulate both thrombogenic and inflammatory cellular events6-9. The human coronary artery, especially at points of bifurcation, is exposed to d-flow and exhibits a susceptibility toward atherosclerosis. In essence, s-flow protects against atherosclerosis (athero-protective flow) while d-flow promotes atherosclerosis (athero-prone flow)10.
D-flow promotes inflammation and apoptosis in EC, and this effect of d-flow is critical for the pathogenesis of many chronic inflammatory conditions and endothelial dysfunction in epicardial blood vessels (coronary arteries in the heart) and peripheral blood vessels (such as the carotid artery and femoral artery). Blood flow in these vessels leads to activation of mechano-sensitive genes in EC and this process involves transcription factor regulation (e.g., KLF2/4, NF-κB, AP-1, early growth response-1, c-Jun, c-fos, and c-myc)11-13. Substantial evidence shows that these transcription factors are regulated by a family of mitogen activated protein kinases (MAPKs). Of note, athero-prone/d-flow-induced signaling in which PKCζ, p90RSK, and increased levels of SUMOylation are involved is not activated by athero-protective/s-flow14, suggesting that there must be specific mechano-sensing and signaling systems for each type of flow. In this brief review, we will discuss some of the recent findings unique to the EC mechano-transduction system with respect to both athero-prone/d-flow and athero-protective/s-flow.
S-flow activates ERK5 kinase
Mitogen-activated protein kinases (MAPKs) are highly conserved serine/threonine kinases. The MAPKs themselves require dual phosphorylation on a Thr-X-Tyr (TXY) motif to become active. Three major MAPK cascades have been extensively studied in blood vessels: extracellular signal–regulated kinases (ERK1 and ERK2), c-Jun N-terminal kinases (JNK1 and JNK2), and p38 kinases. A fourth MAPK member, ERK5, also known as big MAPK-1 (BMK1), has also been identified in EC15-17. MEK5 and ERK5 were first identified as two components of this new protein kinase signaling cascade18, 19. MEK5 is the only identified immediate upstream MAP kinase kinase of ERK5. The critical role of JNK activation in endothelial inflammation and apoptosis has been reported 20-22,23, 24. We found that s-flow decreases inflammation in EC induced by TNF-α-mediated JNK activation and subsequent VCAM1 expression. Although the exact mechanism remains unclear, the s-flow-induced inhibition of the JNK pathway is dependent upon activation of the MEK5-ERK5, but not MEK1-ERK1/2, pathway 25.
The unique aspect of ERK5 is that it is not only a kinase, but also a transcriptional co-activator with a unique C-terminus transactivation domain (Fig. 1)26, 27. Although both ERK1/2 and ERK5 contain the same TEY dual phosphorylation sites and are crucial for regulating proliferation of several different cell types, many unique functions of ERK5, which are different from other MAP kinases, have been reported. First, activation of ERK5 is documented to have an anti-apoptotic effect in cardiac, neuronal, and ECs through increasing Bad phosphorylation, but the detailed mechanism remains unclear25, 28,29, 30. Second, our studies have revealed that s-flow-induced ERK5 activation increases peroxisome proliferator-activated receptor (PPAR) γ transcriptional activity and KLF2/4 expression, with consequent anti-inflammatory and athero-protective effects26, 31.
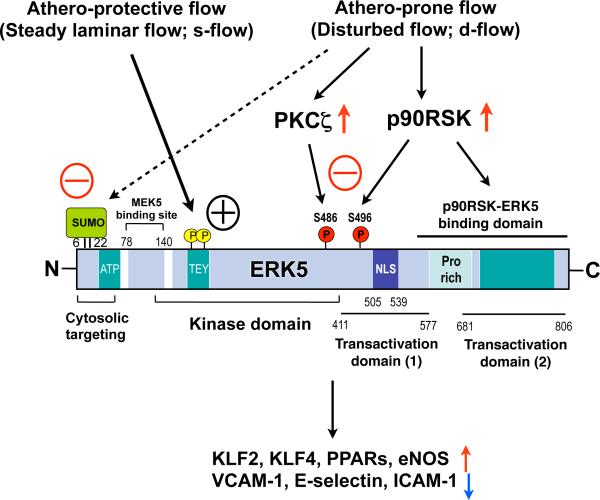
Figure 1. Primary structure of ERK5 and its regulation.
The N-terminus region with SUMO modification inhibits its own transactivation. After ERK5 kinase activation induced by MEK5 binding and TEY motif phosphorylation with de-SUMOylation of K6/K22 sites, ERK5 transcriptional activity at the C-terminus region is fully activated. In contrast athero-prone flow increases ERK5-SUMOylation and ERK5 S496 phosphorylation and inhibits ERK5 transcriptional activity.
S-flow activates PPARs transcriptional activity via ERK5
PPARs are ligand-activated transcription factors, which form a subfamily of the nuclear receptor gene family. PPARs contain two activation function (AF) domains residing in the NH2-terminus A/B domain (AF-1) and the COOH-terminus E domain (AF-2) (Fig. 2). Three related PPAR isotypes have been identified to date: PPARα, PPARβ/δ, and PPARγ. It is well-established that PPARs possess anti-inflammatory effects via ligand-dependent and ligand-independent mechanisms32-34. Phosphorylation of PPARγ Ser-82 by ERK1/2 significantly inhibits its transcriptional activation35. In contrast to ERK1/2, ERK5 does not phosphorylate PPARγ, but instead, its binding with PPARγ regulates PPARγ transcriptional activity. We have found that s-flow increases the association of ERK5 with the hinge-helix 1 region of PPARγ and up-regulates PPARγ transcriptional activity by releasing the co-repressor, SMRT (Fig. 2). Both PPARγ transcriptional activation and the release of its co-repressor (trans-repression) inhibit TNF- mediated NF-κB activation and subsequent inflammatory responses26, 36, 37. The detailed regulatory mechanism of trans-repression was discussed extensively in other reviews38-41.
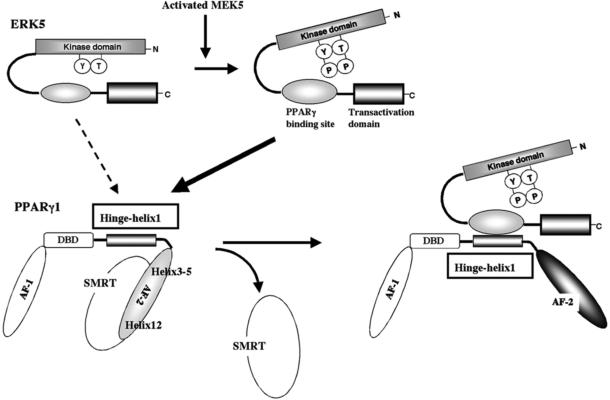
Figure 2. Model for the ERK5-PPARγ interaction-mediated PPARγ transactivation.
The position of Helix 12 is regulated by ligand binding. When the PPARγ ligand binds to the receptor, Helix 12 folds back to form a part of the co-activator binding surface, and inhibits corepressor (such as SMRT) binding to PPARγ94. The co-repressor interaction surface requires Helix 3-595. We found a critical role of the PPARγ hinge-helix 1 domain in ERK5-mediated PPARγ transactivation. The inactive N-terminus kinase domain of ERK5 inhibits its own transactivation and PPARγ binding. After ERK5 activation the inhibitory effect of the N-terminus domain decreases, and subsequently the middle region can fully interact with the hinge-helix 1 region of PPARγ. The association of ERK5 with the hinge-helix 1 region of PPARγ releases co-repressor of SMRT and induces full activation of PPARγ26. AF-1/2: Activating function (AF)-1/2 transactivation domain, DBD: DNA binding domain. Reprinted and modified from Akaike et al26.
In addition to PPARγ, ERK5 can also increase PPARδ transcriptional activation by its association with PPARδ, although the PPARδ binding site with ERK5 is not the hinge-helix 1 region unlike PPARγ42. ERK5-mediated PPARδ activation also contributes to anti-inflammatory responses induced by heme oxygenase 1. These data suggest that the ERK5-PPAR module play a crucial role in s-flow induced-anti-inflammatory processes.
ERK5, KLF2, and endothelial dysfunction
The KLF family is a group of zinc finger transcription factors with important biological roles in regulating blood vessel permeability, blood coagulation, and inflammation43. Dekker et al. first identified KLF2 as a gene regulated by s-flow in the endothelium, which is a key transcriptional regulator of EC inflammation44. NF-κB is a key transcriptional factor that regulates expression of pro-inflammatory mediators including cytokines, chemokines, and molecules which foster cell-to-cell adhesion45. KLF2 reduces NF-κB transcriptional activity and subsequent adhesion molecule expression via competing for the association of CBP/p300 co-factor with NF-κB46. Furthermore, Parmar et al. have reported that s-flow increases KLF2 expression via the MEK5-ERK5-MEF2 signaling pathway and impairs endothelial inflammation31. Another major endothelial function regulated transcriptionally by KLF2 is the control of vessel tone. KLF2 induces eNOS expression by direct association with the eNOS promoter with the recruitment of the coactivator CBP/p30047. A crucial role for KLF2 in inhibiting endothelial permeability by tight junction protein expression was also reported48.
Consistent with such key roles of ERK5 in EC physiology in vitro, EC apoptosis and inflammation are accelerated in endothelial-specific ERK5 knockout mice30, 49, and the deletion of ERK5 in ECs accelerates atherosclerosis formation in LDL receptor deficient (LDLR−/−) mice50. These data strongly suggest that both ERK5 kinase activity and transcriptional activity play key roles in ECs achieving athero-protective function. S-flow-induced ERK5 activation in ECs up-regulates PPARs and KLF2 transcriptional activity, elicits anti-inflammatory responses, and maintains normal vascular reactivity and endothelial barrier function.
SUMOylation as a mechano-signaling mediator
Small ubiquitin-like modifier (SUMO) proteins covalently modify certain residues of specific target substrates to alter their functions. A substantial amount of evidence indicates that SUMOylation plays roles in flow-induced signaling and the pathogenesis and development of cardiovascular complications51-53. SUMOylation is a dynamic and reversible process mediated by both conjugation and de-conjugation enzymes. It is analogous to ubiquitination, but SUMO conjugation involves a different set of enzymes (Fig. 3). First, the mature form of SUMO is activated by E1-activating enzymes, a SAE1-SAE2 heterodimer54. After this activation, SUMO is transferred to Ubc9, an E2 conjugase, forming a thioester bond between Ubc9 and SUMO55. Lastly, Ubc9 transfers SUMO to the target substrate containing the free e-amino group of a lysine residue, which is regulated by several SUMO E3 ligases including the family of protein inhibitors such as activated STAT (PIAS1-4), Polycomb-2 protein (Pc2), and RanBP2/Nup35856. Sentrin/SUMO-specific proteases (SENPs; SENP1-7) catalyze deconjugation of SUMOylated substrates, or edit SUMO precursor into a matured form, which terminates with a pair of glycine (Gly) residues (Fig. 3)57, 58. As described above, the number of SUMO E1 and E2 enzymes is small compared with SUMO E3 ligases and SENPs. Therefore, the coordination of different SUMO E3 ligases and SENPs may be crucial for a specific EC function in which flow-induced protein SUMOylation plays a role.
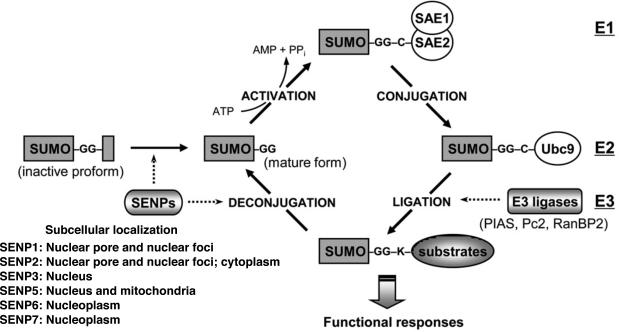
Figure 3. The regulation of SUMOylation pathway.
Protein SUMOylation is achieved by a recycle system consisting of conjugation and deconjugation pathway. SUMO conjugation to a target substrate requires an enzymatic cascade, which involves three classes of enzymes (E1→E2→E3). The sentrin/SUMO-specific proteases (SENPs) are responsible for the deconjugation pathway as well as the maturation process of newly synthesized SUMO protein. The primary subcellular localization of each SENP is also listed96. Reprinted and modified from Woo et al. with permission of the publisher53.
ERK5-SUMOylation and d-flow
It is clear that SUMO influences many different biological processes, but particularly important in the present context is the regulation of transcription and protein kinase activity of modified proteins53, 59. As explained above, s-flow has a vasoprotective effect via ERK5-mediated KLF2 and eNOS expression60, 61. Our studies showed that treatment of ECs with H2O2, advanced glycation end products (AGE), or d-flow significantly increased ERK5 SUMOylation at Lys6 and Lys22 residues and that this SUMOylation inhibited ERK5/MEF2 transcriptional activity, and subsequent KLF2 promoter activity and KLF2-mediated eNOS expression60. Of note, both H2O2 and AGE increased ERK5 TEY motif phosphorylation as well as its protein kinase activity, suggesting that the inhibition of ERK5 transcriptional activity by H2O2 and AGE is an event independent of its protein kinase activity. We also found that the reduction of eNOS and KLF2 expression by H2O2 and AGE treatment was abolished in ECs expressing ERK5 K6/22R SUMOylation mutant, suggesting that ERK5 SUMOylation may down-regulate the vaso-protective effects of s-flow60. Furthermore, we found that ERK5 SUMOylation was increased by d-flow, but it was decreased by s-flow62. These data strongly suggest that ERK5 SUMOylation plays an important role in regulating endothelial inflammation and vascular tone and that d- and s-flow have, respectively, yin and yang effects on ERK5 SUMOylation.
Role of p53 SUMOylation in d-flow-induced EC apoptosis
D-flow is able to increase both endothelial apoptosis and proliferation, which augments EC turnover and creates focal sites of increased endothelial permeability, inflammation, and dysfunction63. However, the mechanism by which d-flow regulates EC turnover, especially apoptosis, is unclear. To obtain some insights into this issue, we investigated the role of p53 in regulating d-flow-induced EC apoptosis (Fig. 4A). Acting as a sensor for DNA damage, the transcription factor p53 is a key molecule in determining cellular fate. p53 in the nucleus not only increases the expression of pro-apoptotic genes, but also is protective against cell death via up-regulating p21 expression64. In fact, Lin et al. reported that s-flow increased p53 expression and JNK-mediated p53 phosphorylation, which caused EC growth arrest via increasing GADD45 and p21cip1 expression65. These data suggest that the athero-protective effect exerted by s-flow increases p21 via p53, inducing growth arrest, and may inhibit simultaneously apoptosis. It is important to note here that most of p53 anti-apoptotic effects has been explained by its function in the nucleus, especially under the resting condition64. We found increased levels of nuclear p53 and reduced numbers of apoptotic ECs in the area exposed to s-flow51, which supports this general idea.
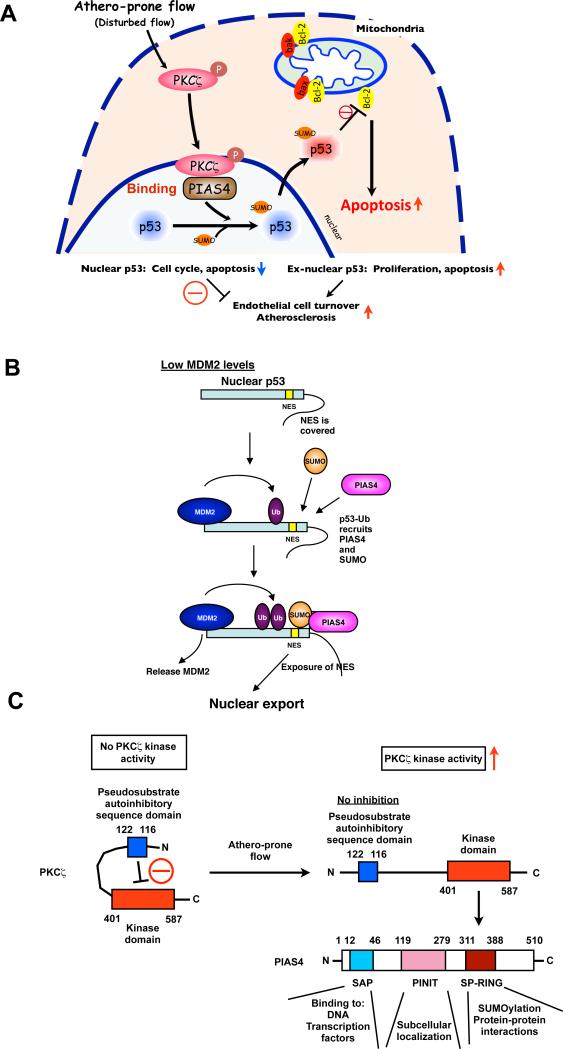
Figure 4. Athero-prone flow increases p53 SUMOylation via PKCζ-PIAS4 binding.
(A) Athero-prone flow uniquely activates PKCζ, which increases PKCζ-PIAS4 binding and PIAS4 SUMO E3 ligase activity, and subsequently increases p53 SUMOylation. SUMOylation causes p53 nuclear export and binds to Bcl-2, which inhibits anti-apoptotic function of Bcl-2 and increases apoptosis. (B) p53 nuclear export and stabilization. Masking of the C-terminus NES results in nuclear localization of unmodified p53, but a low level of ubiquitination by MDM2 exposes the NES, causing the p53-Ub fusion protein to come out of the nucleus. When MDM2 levels are low, ubiquitination promotes the interaction of p53 with PIAS4 and further modification of p53 by SUMOylation that causes the release of MDM2 and nuclear export, which may increase the cytoplasmic apoptotic function of p53. Under conditions of high MDM2, persistent binding and activity of MDM2 leads to polyubiquitination and degradation of p5366. These data suggest important roles of SUMOylation in p53 stabilization, localization, and subsequent apoptosis. Reprinted and modified from Carter et al with permission of the publisher66. (C) PKCζ-mediated p53 SUMOylation requires PKCζ-PIAS4 binding at SP-RING domain, not PKCζ-mediated phosphorylation of PIAS4. SAP: scaffold attachment factor-A/B, acinus and PIAS domain; SP-RING: Siz/PIAS-RING domain53.
In contrast to this, EC exposed to d-flow have decreased levels of nuclear p53 localization and become apoptosis. We have reported that d-flow induces EC apoptosis via p53 SUMOylation in a PKCζ-dependent manner51. Previously, Carter et al. reported a role of p53 SUMOylation in regulating p53 localization66. They showed that in its unmodified form, the p53 C-terminus nuclear export signal (NES) was masked by its own C-terminus region and that this caused persistent nuclear localization. A low level of ubiquitination by MDM2 (mouse double minute 2) exposed the NES, promoting p53 to interact with PIAS4 and causing further modification by SUMOylation which led to p53 nuclear export. These results show that p53 nuclear export is regulated by SUMOylation (Fig. 4B)66. Cytosolic p53 has non-transcriptional pro-apoptotic activities. It has been reported that cytoplasmic p53 directly interacts with Bcl-2 (B cell lymphoma/leukemia-2) family member proteins, Bcl-xL and Bcl-2, and blocks their well-known anti-apoptotic function67, 68. We have reported that d-flow induces p53 nuclear export, p53-Bcl-2 binding, and apoptosis in a p53 SUMOylation-dependent manner51.
The next question is how d-flow increases p53 SUMOylation. We found that athero-prone flow increased PKCζ binding to the E3 SUMO ligase PIAS4 and induced p53-SUMOylation69. Among the PKC family members, atypical PKCζ was recently reported to have an important function in EC70, 71. Magid and Davies reported that this PKC isoform was highly expressed in EC in the athero-prone areas of porcine aorta70. Frey et al. demonstrated involvement of PKCζ in oxidant generation in ECs via NADPH oxidase activation71. Consistent with these results, endothelial PKCζ activation was elevated in atherosclerotic lesions69, 72. Therefore we investigated the interactions of PKCζ with SUMO ligases and discovered that d-flow increased PKCζ binding to the E3 SUMO ligase PIAS4 and stimulated p53-SUMOylation69. It is likely that PIAS4 activation by PKCζ is likely to be phosphorylation-independent because we did not observe PIAS4 phosphorylation by PKCζ. It is noteworthy that active protein kinases may regulate signaling pathways and cell functions not only by phosphorylating substrates, but also by direct protein-protein interactions.
It has been reported that PKCζ contains a pseudosubstrate autoinhibitiry sequence (amino acids 116-122), and the release of the kinase domain (amino acids 268-587) from this auto-inhibitory domain leads to PKCζ activation73, 74 (Fig. 4C). We found that the C-terminus kinase domain of PKCζ (amino acids 401-587) was a PIAS4 binding site, and the deletion of the N-terminus autoinhibitory domain (amino acids 1-200) increased PKCζ-PIAS4 association51. Therefore, in addition to its protein kinase activation, the subsequent release of the PKCζ N-terminus auto-inhibitory domain is necessary for the PKCζ-PIAS4 association. PKCζ associates with the catalytic site, RING domain, of PIAS4, which recruits the cognate E2 conjugating enzyme into the PIAS4/substrate complex to facilitate SUMO conjugation. Therefore, the association of PKCζ with PIAS4 may alter the structure and enzymatic activity of PIAS4. Taken together, PKCζ activation and subsequent PKCζ-PIAS4 binding are crucial for d-flow-induced p53 SUMOylation and ECs apoptosis 51.
Other PKCζ that mediat e endothelial dysfunction
We have discussed the mechanisms by which PKCζ mediates d-flow-induced endothelial apoptosis in the previous section. Here, we discuss other PKCζ functions in ECs. PKCζ regulates not only endothelial apoptosis but also TNFα-induced endothelial dysfunction, particularly under s-flow conditions61. TNF-α promotes association between PKCζ and ERK5 and also increases ERK5 S486 phosphorylation. ERK5 S486 site, when phosphorylated, evokes eNOS protein degradation, leading to endothelial dysfunction. Although several mechanisms including calcium-dependent calpain-mediated degradation have been proposed for eNOS protein degradation75, 76, it remains unclear exactly how PKCζ-ERK5-pS486 mediates eNOS degradation.
In addition to ERK5, we also reported the importance of p62 on TNF-α-induced PKCζ activation77. p62 is a scaffold protein containing a Phox/Bem1p (PB1) domain in its NH2-terminus region, which can interact with other PB1 domain containing proteins via PB1-PB1 interaction78. PKCζ also contains a PB1 domain, and the p62-PKCζ association is critical for the activation of PKCζ downstream events such as JNK and caspase 3 activation77. The precise role of this p62-PKCζ module in s-flow and d-flow needs further investigation.
SENP2 and athero-prone d-flow
SENP2 is a de-SUMOylation enzyme, which is important for both processing new SUMO proteins for conjugation as well as deconjugation of SUMO from SUMOylated proteins. Six isoforms exist in human (SENP1-3 and 5-7)79. In contrast to the C-terminus that contains the well-conserved catalytic domain, the N-terminus is poorly conserved among different isoforms, suggesting that the enzyme is regulated by the N-terminus58, but it remains unclear how each SENP isoform recognizes its specific substrates and causes different functional consequences. Among the six isoforms, the functions of SENP1 and SENP2 have been relatively well studied. Li et al. showed that TNFα transiently induced SENP1 translocation from the cytosol to the nucleus and subsequently increased JNK activation and apoptosis via Homeodomain Interacting Protein Kinase 2 (HIPK2) de-SUMOylation in EC80. SENP1−/− embryos are severely anemic due to diminished erythropoietin production, and this leads to SUMOylation-induced HIF1α degradation81. The deletion of SENP2 in mouse causes defects in cardiac development by inhibiting Gata4 and Gata6 expression and accumulation of SUMOylated Pc2/CBX4 (a polycomb repressive complex 1 subunit). HIF1α stabilization is not affected in SENP2−/− mouse embryonic fibroblasts, demonstating the substrate specificity between SENP1 and SENP2.
As we explained above, we found that d-flow induced p53 and ERK5 SUMOylation, leading to EC apoptosis and inflammation, respectively51, 60. Interestingly, reduced expression of SENP2 increased both endothelial p53 and ERK5 SUMOylation, hence increased EC dysfunction and inflammation, and accelerated atherosclerotic plaque formation62. In addition, we found that d-flow-induced adhesion molecule expression and EC apoptosis were inhibited in cultured ECs overexpressing p53 or ERK5 SUMOylation mutant62. In contrast, s-flow inhibited ERK5 SUMOylation62. Taken together, we may conclude that SUMOylation of p53 and ERK5 is both necessary and sufficient to promote endothelial apoptosis and inflammation under the conditions of d-flow. One might expect SENP2 expression to be down-regulated by d-flow, but we did not observe this effect in EC exposed to d-flow62. We believe that d-flow likely regulates the de-SUMOylation activity of SENP2 or the cellular localization of SENP2, but further studies will be needed to clarify these points.
ERK5 and its inhibitory kinase, p90RSK, under d-flow
p90RSK is a serine/threonine kinase containing two functional kinase domains (Fig. 5)82. The N-terminus kinase belongs to the AGC group of kinases (i.e., protein kinase A [PKA] and protein kinase C [PKC]). Within this AGC group, p70S6K has the greatest sequence identity (~ 60%) within the p90RSK N-terminus kinase region. The C-terminus kinase belongs to the calcium/calmodulin-dependent kinase group. These two p90RSK kinase domains possess different functional properties. The N-terminus kinase has the most activity since it directly phosphorylates p90RSK substrates. The C-terminus kinase domain, conversely, plays only a minor direct role in phosphorylation, but its presence, together with the linker region, is required for full activation of the N-terminus kinase. The C-terminus tail contains a short docking motif for the specific association between p90RSK and ERK1/282-84. p90RSK is located downstream of the Raf-MEK-ERK1/2 signaling pathway85, and ERK1/2 activates the C-terminus kinase of p90RSK, leading to full activation of the N-terminus kinase and subsequent substrate phosphorylation. However, the involvement of an ERK1/2-independent pathway has also been suggested86.
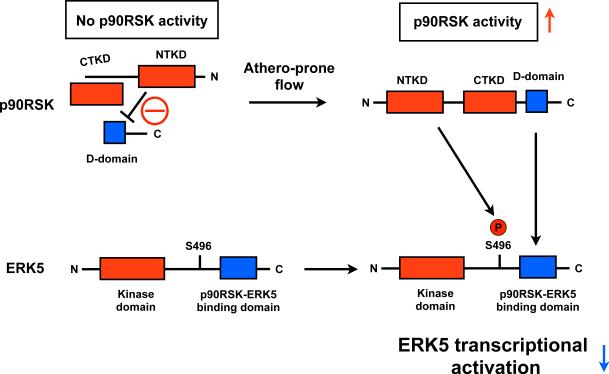
Figure 5. Athero-prone flow increases p90RSK activation, leading to p90RSK-ERK5 association and ERK5 S496 phosphorylation, and subsequently decreases in ERK5 transcriptional activity.
At the basal level, inactive p90RSK inhibits the D-domain to bind ERK5. Once p90RSK is activated, the inhibition of the kinase domain is released and the D-domain of p90RSK associates with the ERK5 C-terminus domain97 and increases ERK5 S496 phosphorylation, which inhibits ERK5 transcriptional activity98. NTKD: NH2-terminal kinase domain, CTKD: COOH-terminal kinase domain, D-domain: NH2-terminal docking domain.
The activation and nuclear translocation of p90RSK are concomitant with immediate early gene expression87, 88. p90RSK is also involved in the activation of NF-κB by phosphorylation of Iκ-B89 or phosphorylation of transcription factors including c-Fos90, Nur7791, and CREB92. Although ERK5 can regulate p90RSK kinase activation as an upstream kinase like ERK1/2 under certain conditions93, we have reported that p90RSK also directly phosphorylates ERK5 S496 and inhibits its transcriptional activity50. In this study we found that p90RSK associated with the ERK5 C-terminus transcriptional activation domain (amino acids 571-807). When we overexpressed this C-terminus fragment as a decoy, both p90RSK-ERK5 association and H2O2-induced reduction of ERK5 transcriptional activity were inhibited50. These data suggest that inhibition of ERK5 transcriptional activity depends on p90RSK-ERK5 binding. In addition, phosphorylation of ERK5 S496 by p90RSK inhibits ERK5 transcriptional activity as well as eNOS expression. Lastly, we also found increased p90RSK activation in regions of d-flow in the aortic arch, indicating that p90RSK activation and atherosclerosis are closely linked. The inhibition of 90RSK activation by FMK-MEA (a p90RSK specific inhibitor) significantly reduced atherosclerosis plaque formation50. Further studies are necessary to elucidate the precise mechanism by which d-flow regulates the function of p90RSK that leads to endothelial dysfunction.
Conclusion
It is apparent now from multiple studies that ECs sense and respond differently to s-flow and d-flow. Many studies have also sought to define molecular mechanisms responsible for mechanotrasduction initiated by different patterns of flow, but the exact nature of signaling that d-flow and s-flow initiate in ECs has so far evaded investigators. In this review we have discussed several molecules and signaling events, which appear to be differentially regulated by athero-prone and athero-protective blood flow patterns. Molecules that may be involved in flow pattern specific signaling include PKCζ and p90RSK for d-flow-initiated signaling and ERK5, KLF2/4, and PPARs for s-flow. Understanding the interplay among these molecules under the two different types of flow may be the final key needed to unlock the door which stands between endothelial cell dysfunction and atherosclerosis formation.
Acknowledgments
We thank the current and past members of our group who have contributed to the work in improving the understanding of shear stress-induced signal transduction pathways. We also thank Drs. Scott Cameron and Keigi Fujiwara, and Walter Knight for critical reading of this manuscript.
Sources of funding
This study was supported by a grant from National Institutes of Health to Drs Bradford C. Berk (HL-064839, HL 106158), Jun-ichi Abe (HL-064839, HL-108551, HL-102746).
Footnotes
Disclosures
None.
References
- 1.Won D, Zhu SN, Chen M, Teichert AM, Fish JE, Matouk CC, Bonert M, Ojha M, Marsden PA, Cybulsky MI. Relative reduction of endothelial nitric-oxide synthase expression and transcription in atherosclerosis-prone regions of the mouse aorta and in an in vitro model of disturbed flow. The American journal of pathology. 2007;171:1691–1704. doi: 10.2353/ajpath.2007.060860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jongstra-Bilen J, Haidari M, Zhu SN, Chen M, Guha D, Cybulsky MI. Low-grade chronic inflammation in regions of the normal mouse arterial intima predisposed to atherosclerosis. J Exp Med. 2006;203:2073–2083. doi: 10.1084/jem.20060245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bao X, Lu C, Frangos JA. Temporal gradient in shear but not steady shear stress induces pdgf-a and mcp-1 expression in endothelial cells: Role of no, nf kappa b, and egr-1. Arterioscler Thromb Vasc Biol. 1999;19:996–1003. doi: 10.1161/01.atv.19.4.996. [DOI] [PubMed] [Google Scholar]
- 4.Wang GX, Cai SX, Wang PQ, Ouyang KQ, Wang YL, Xu SR. Shear-induced changes in endothelin-1 secretion of microvascular endothelial cells. Microvasc Res. 2002;63:209–217. doi: 10.1006/mvre.2001.2387. [DOI] [PubMed] [Google Scholar]
- 5.Hsiai TK, Cho SK, Reddy S, Hama S, Navab M, Demer LL, Honda HM, Ho CM. Pulsatile flow regulates monocyte adhesion to oxidized lipid-induced endothelial cells. Arteriosclerosis, thrombosis, and vascular biology. 2001;21:1770–1776. doi: 10.1161/hq1001.097104. [DOI] [PubMed] [Google Scholar]
- 6.Frangos JA, Eskin SG, McIntire LV, Ives CL. Flow effects on prostacyclin production by cultured human endothelial cells. Science. 1985;227:1477–1479. doi: 10.1126/science.3883488. [DOI] [PubMed] [Google Scholar]
- 7.Di Francesco L, Totani L, Dovizio M, Piccoli A, Di Francesco A, Salvatore T, Pandolfi A, Evangelista V, Dercho RA, Seta F, Patrignani P. Induction of prostacyclin by steady laminar shear stress suppresses tumor necrosis factor-alpha biosynthesis via heme oxygenase-1 in human endothelial cells. Circ Res. 2009;104:506–513. doi: 10.1161/CIRCRESAHA.108.191114. [DOI] [PubMed] [Google Scholar]
- 8.Korenaga R, Ando J, Tsuboi H, Yang W, Sakuma I, Toyo oT, Kamiya A. Laminar flow stimulates atp- and shear stress-dependent nitric oxide production in cultured bovine endothelial cells. Biochem Biophys Res Commun. 1994;198:213–219. doi: 10.1006/bbrc.1994.1030. [DOI] [PubMed] [Google Scholar]
- 9.Diamond SL, Eskin SG, McIntire LV. Fluid flow stimulates tissue plasminogen activator secretion by cultured human endothelial cells. Science. 1989;243:1483–1485. doi: 10.1126/science.2467379. [DOI] [PubMed] [Google Scholar]
- 10.Gimbrone MA, Jr., Topper JN, Nagel T, Anderson KR, Garcia-Cardena G. Endothelial dysfunction, hemodynamic forces, and atherogenesis. Ann N Y Acad Sci. 2000;902:230–239. doi: 10.1111/j.1749-6632.2000.tb06318.x. discussion 239-240. [DOI] [PubMed] [Google Scholar]
- 11.Hsieh HJ, Li NQ, Frangos JA. Pulsatile and steady flow induces c-fos expression in human endothelial cells. J Cell Physiol. 1993;154:143–151. doi: 10.1002/jcp.1041540118. [DOI] [PubMed] [Google Scholar]
- 12.Khachigian LM, Resnick N, Gimbrone MA, Jr., Collins T. Nuclear factor-kappa b interacts functionally with the platelet-derived growth factor b-chain shear-stress response element in vascular endothelial cells exposed to fluid shear stress. J Clin Invest. 1995;96:1169–1175. doi: 10.1172/JCI118106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lan Q, Mercurius KO, Davies PF. Stimulation of transcription factors nf kappa b and ap1 in endothelial cells subjected to shear stress. Biochem Biophys Res Commun. 1994;201:950–956. doi: 10.1006/bbrc.1994.1794. [DOI] [PubMed] [Google Scholar]
- 14.Heo KS, Fujiwara K, Abe J. Disturbed-flow-mediated vascular reactive oxygen species induce endothelial dysfunction. Circ J. 2011;75:2722–2730. doi: 10.1253/circj.cj-11-1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abe J, Baines CP, Berk BC. Role of mitogen-activated protein kinases in ischemia and reperfusion injury : The good and the bad [editorial; comment]. Circ Res. 2000;86:607–609. doi: 10.1161/01.res.86.6.607. [DOI] [PubMed] [Google Scholar]
- 16.Abe J, Berk BC. Reactive oxygen species as mediators of signal transduction in cardiovascular disease. Trends Cardiovasc Med. 1998;8:59–64. doi: 10.1016/S1050-1738(97)00133-3. [DOI] [PubMed] [Google Scholar]
- 17.Yan C, Takahashi M, Okuda M, Lee JD, Berk BC. Fluid shear stress stimulates big mitogen-activated protein kinase 1 (bmk1) activity in endothelial cells. Dependence on tyrosine kinases and intracellular calcium. J Biol Chem. 1999;274:143–150. doi: 10.1074/jbc.274.1.143. [DOI] [PubMed] [Google Scholar]
- 18.Zhou G, Bao ZQ, Dixon JE. Components of a new human protein kinase signal transduction pathway. J Biol Chem. 1995;270:12665–12669. doi: 10.1074/jbc.270.21.12665. [DOI] [PubMed] [Google Scholar]
- 19.English JM, Vanderbilt CA, Xu S, Marcus S, Cobb MH. Isolation of mek5 and differential expression of alternatively spliced forms. J Biol Chem. 1995;270:28897–28902. doi: 10.1074/jbc.270.48.28897. [DOI] [PubMed] [Google Scholar]
- 20.De Cesaris P, Starace D, Starace G, Filippini A, Stefanini M, Ziparo E. Activation of jun n-terminal kinase/stress-activated protein kinase pathway by tumor necrosis factor alpha leads to intercellular adhesion molecule-1 expression. J Biol Chem. 1999;274:28978–28982. doi: 10.1074/jbc.274.41.28978. [DOI] [PubMed] [Google Scholar]
- 21.Ahmad M, Theofanidis P, Medford RM. Role of activating protein-1 in the regulation of the vascular cell adhesion molecule-1 gene expression by tumor necrosis factor-alpha. J Biol Chem. 1998;273:4616–4621. doi: 10.1074/jbc.273.8.4616. [DOI] [PubMed] [Google Scholar]
- 22.Min W, Pober JS. Tnf initiates e-selectin transcription in human endothelial cells through parallel traf-nf-kappa b and traf-rac/cdc42-jnk-c-jun/atf2 pathways. J Immunol. 1997;159:3508–3518. [PubMed] [Google Scholar]
- 23.Wong HK, Fricker M, Wyttenbach A, Villunger A, Michalak EM, Strasser A, Tolkovsky AM. Mutually exclusive subsets of bh3-only proteins are activated by the p53 and c-jun n-terminal kinase/c-jun signaling pathways during cortical neuron apoptosis induced by arsenite. Mol Cell Biol. 2005;25:8732–8747. doi: 10.1128/MCB.25.19.8732-8747.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fan G, Merritt SE, Kortenjann M, Shaw PE, Holzman LB. Dual leucine zipper-bearing kinase (dlk) activates p46sapk and p38mapk but not erk2. J Biol Chem. 1996;271:24788–24793. doi: 10.1074/jbc.271.40.24788. [DOI] [PubMed] [Google Scholar]
- 25.Li L, Tatake RJ, Natarajan K, Taba Y, Garin G, Tai C, Leung E, Surapisitchat J, Yoshizumi M, Yan C, Abe J, Berk BC. Fluid shear stress inhibits tnf-mediated jnk activation via mek5-bmk1 in endothelial cells. Biochem Biophys Res Commun. 2008;370:159–163. doi: 10.1016/j.bbrc.2008.03.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Akaike M, Che W, Marmarosh NL, Ohta S, Osawa M, Ding B, Berk BC, Yan C, Abe J. The hinge-helix 1 region of peroxisome proliferator-activated receptor gamma1 (ppargamma1) mediates interaction with extracellular signal-regulated kinase 5 and ppargamma1 transcriptional activation: Involvement in flow-induced ppargamma activation in endothelial cells. Mol Cell Biol. 2004;24:8691–8704. doi: 10.1128/MCB.24.19.8691-8704.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kasler HG, Victoria J, Duramad O, Winoto A. Erk5 is a novel type of mitogen-activated protein kinase containing a transcriptional activation domain. Mol Cell Biol. 2000;20:8382–8389. doi: 10.1128/mcb.20.22.8382-8389.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pi X, Garin G, Xie L, Zheng Q, Wei H, Abe J, Yan C, Berk BC. Bmk1/erk5 is a novel regulator of angiogenesis by destabilizing hypoxia inducible factor 1alpha. Circ Res. 2005;96:1145–1151. doi: 10.1161/01.RES.0000168802.43528.e1. [DOI] [PubMed] [Google Scholar]
- 29.Lee JD, Ulevitch RJ, Han J. Primary structure of bmk1: A new mammalian map kinase. Biochem Biophys Res Commun. 1995;213:715–724. doi: 10.1006/bbrc.1995.2189. [DOI] [PubMed] [Google Scholar]
- 30.Hayashi M, Kim SW, Imanaka-Yoshida K, Yoshida T, Abel ED, Eliceiri B, Yang Y, Ulevitch RJ, Lee JD. Targeted deletion of bmk1/erk5 in adult mice perturbs vascular integrity and leads to endothelial failure. J Clin Invest. 2004;113:1138–1148. doi: 10.1172/JCI19890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Parmar KM, Larman HB, Dai G, Zhang Y, Wang ET, Moorthy SN, Kratz JR, Lin Z, Jain MK, Gimbrone MA, Jr., Garcia-Cardena G. Integration of flow-dependent endothelial phenotypes by kruppel-like factor 2. J Clin Invest. 2006;116:49–58. doi: 10.1172/JCI24787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Piqueras L, Sanz MJ, Perretti M, Morcillo E, Norling L, Mitchell JA, Li Y, Bishop-Bailey D. Activation of pparbeta/delta inhibits leukocyte recruitment, cell adhesion molecule expression, and chemokine release. J Leukoc Biol. 2009;86:115–122. doi: 10.1189/jlb.0508284. [DOI] [PubMed] [Google Scholar]
- 33.Takata Y, Liu J, Yin F, Collins AR, Lyon CJ, Lee CH, Atkins AR, Downes M, Barish GD, Evans RM, Hsueh WA, Tangirala RK. Ppardelta-mediated antiinflammatory mechanisms inhibit angiotensin ii-accelerated atherosclerosis. Proc Natl Acad Sci U S A. 2008;105:4277–4282. doi: 10.1073/pnas.0708647105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang N, Verna L, Chen NG, Chen J, Li H, Forman BM, Stemerman MB. Constitutive activation of peroxisome proliferator-activated receptor-gamma suppresses pro-inflammatory adhesion molecules in human vascular endothelial cells. J Biol Chem. 2002;277:34176–34181. doi: 10.1074/jbc.M203436200. [DOI] [PubMed] [Google Scholar]
- 35.Camp HS, Tafuri SR. Regulation of peroxisome proliferator-activated receptor gamma activity by mitogen-activated protein kinase. J Biol Chem. 1997;272:10811–10816. doi: 10.1074/jbc.272.16.10811. [DOI] [PubMed] [Google Scholar]
- 36.Barish GD, Yu RT, Karunasiri MS, Becerra D, Kim J, Tseng TW, Tai LJ, Leblanc M, Diehl C, Cerchietti L, Miller YI, Witztum JL, Melnick AM, Dent AL, Tangirala RK, Evans RM. The bcl6-smrt/ncor cistrome represses inflammation to attenuate atherosclerosis. Cell Metab. 2012;15:554–562. doi: 10.1016/j.cmet.2012.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee CH, Chawla A, Urbiztondo N, Liao D, Boisvert WA, Evans RM, Curtiss LK. Transcriptional repression of atherogenic inflammation: Modulation by ppardelta. Science. 2003;302:453–457. doi: 10.1126/science.1087344. [DOI] [PubMed] [Google Scholar]
- 38.Glass CK, Saijo K. Nuclear receptor transrepression pathways that regulate inflammation in macrophages and t cells. Nat Rev Immunol. 2010;10:365–376. doi: 10.1038/nri2748. [DOI] [PubMed] [Google Scholar]
- 39.Straus DS, Glass CK. Anti-inflammatory actions of ppar ligands: New insights on cellular and molecular mechanisms. Trends Immunol. 2007;28:551–558. doi: 10.1016/j.it.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 40.Ricote M, Glass CK. Ppars and molecular mechanisms of transrepression. Biochim Biophys Acta. 2007;1771:926–935. doi: 10.1016/j.bbalip.2007.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pascual G, Glass CK. Nuclear receptors versus inflammation: Mechanisms of transrepression. Trends in endocrinology and metabolism: TEM. 2006;17:321–327. doi: 10.1016/j.tem.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 42.Woo CH, Massett MP, Shishido T, Itoh S, Ding B, McClain C, Che W, Vulapalli SR, Yan C, Abe J. Erk5 activation inhibits inflammatory responses via peroxisome proliferator-activated receptor delta (ppardelta) stimulation. J Biol Chem. 2006;281:32164–32174. doi: 10.1074/jbc.M602369200. [DOI] [PubMed] [Google Scholar]
- 43.Suzuki T, Aizawa K, Matsumura T, Nagai R. Vascular implications of the kruppel-like family of transcription factors. Arterioscler Thromb Vasc Biol. 2005;25:1135–1141. doi: 10.1161/01.ATV.0000165656.65359.23. [DOI] [PubMed] [Google Scholar]
- 44.Dekker RJ, van Soest S, Fontijn RD, Salamanca S, de Groot PG, VanBavel E, Pannekoek H, Horrevoets AJ. Prolonged fluid shear stress induces a distinct set of endothelial cell genes, most specifically lung kruppel-like factor (klf2). Blood. 2002;100:1689–1698. doi: 10.1182/blood-2002-01-0046. [DOI] [PubMed] [Google Scholar]
- 45.Rothwarf DM, Karin M. The nf-kappa b activation pathway: A paradigm in information transfer from membrane to nucleus. Sci STKE. 1999;1999:RE1. doi: 10.1126/stke.1999.5.re1. [DOI] [PubMed] [Google Scholar]
- 46.SenBanerjee S, Lin Z, Atkins GB, Greif DM, Rao RM, Kumar A, Feinberg MW, Chen Z, Simon DI, Luscinskas FW, Michel TM, Gimbrone MA, Jr., Garcia-Cardena G, Jain MK. Klf2 is a novel transcriptional regulator of endothelial proinflammatory activation. J Exp Med. 2004;199:1305–1315. doi: 10.1084/jem.20031132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sen-Banerjee S, Mir S, Lin Z, Hamik A, Atkins GB, Das H, Banerjee P, Kumar A, Jain MK. Kruppel-like factor 2 as a novel mediator of statin effects in endothelial cells. Circulation. 2005;112:720–726. doi: 10.1161/CIRCULATIONAHA.104.525774. [DOI] [PubMed] [Google Scholar]
- 48.Lin Z, Natesan V, Shi H, Dong F, Kawanami D, Mahabeleshwar GH, Atkins GB, Nayak L, Cui Y, Finigan JH, Jain MK. Kruppel-like factor 2 regulates endothelial barrier function. Arterioscler Thromb Vasc Biol. 2010;30:1952–1959. doi: 10.1161/ATVBAHA.110.211474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sohn SJ, Sarvis BK, Cado D, Winoto A. Erk5 mapk regulates embryonic angiogenesis and acts as a hypoxia- sensitive repressor of vascular endothelial growth factor expression. J Biol Chem. 2002;277:43344–43351. doi: 10.1074/jbc.M207573200. [DOI] [PubMed] [Google Scholar]
- 50.Le NT, Heo KS, Takei Y, Lee H, Woo CH, Chang E, McClain C, Hurley C, Wang X, Li F, Xu H, Morrell C, Sullivan MA, Cohen MS, Serafimova IM, Taunton J, Fujiwara K, Abe J. A crucial role for p90rsk-mediated reduction of erk5 transcriptional activity in endothelial dysfunction and atherosclerosis. Circulation. 2013;127:486–499. doi: 10.1161/CIRCULATIONAHA.112.116988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Heo KS, Lee H, Nigro P, Thomas T, Le NT, Chang E, McClain C, Reinhart-King CA, King MR, Berk BC, Fujiwara K, Woo CH, Abe J. Pkczeta mediates disturbed flow-induced endothelial apoptosis via p53 sumoylation. J Cell Biol. 2011;193:867–884. doi: 10.1083/jcb.201010051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Le NT, Corsetti JP, Dehoff-Sparks JL, Sparks CE, Fujiwara K, Abe J. Reactive oxygen species, sumoylation, and endothelial inflammation. International journal of inflammation. 2012;2012:678190. doi: 10.1155/2012/678190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Woo CH, Abe J. Sumo--a post-translational modification with therapeutic potential? Curr Opin Pharmacol. 2010;10:146–155. doi: 10.1016/j.coph.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Johnson ES, Schwienhorst I, Dohmen RJ, Blobel G. The ubiquitin-like protein smt3p is activated for conjugation to other proteins by an aos1p/uba2p heterodimer. Embo J. 1997;16:5509–5519. doi: 10.1093/emboj/16.18.5509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Johnson ES, Blobel G. Ubc9p is the conjugating enzyme for the ubiquitin-like protein smt3p. J Biol Chem. 1997;272:26799–26802. doi: 10.1074/jbc.272.43.26799. [DOI] [PubMed] [Google Scholar]
- 56.Johnson ES. Protein modification by sumo. Annu Rev Biochem. 2004;73:355–382. doi: 10.1146/annurev.biochem.73.011303.074118. [DOI] [PubMed] [Google Scholar]
- 57.Li SJ, Hochstrasser M. A new protease required for cell-cycle progression in yeast. Nature. 1999;398:246–251. doi: 10.1038/18457. [DOI] [PubMed] [Google Scholar]
- 58.Yeh ET. Sumoylation and de-sumoylation: Wrestling with life's processes. J Biol Chem. 2009;284:8223–8227. doi: 10.1074/jbc.R800050200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chang E, Heo KS, Woo CH, Lee H, Le NT, Thomas TN, Fujiwara K, Abe J. Mk2 sumoylation regulates actin filament remodeling and subsequent migration in endothelial cells by inhibiting mk2 kinase and hsp27 phosphorylation. Blood. 2011;117:2527–2537. doi: 10.1182/blood-2010-08-302281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Woo CH, Shishido T, McClain C, Lim JH, Li JD, Yang J, Yan C, Abe J. Extracellular signal-regulated kinase 5 sumoylation antagonizes shear stress-induced antiinflammatory response and endothelial nitric oxide synthase expression in endothelial cells. Circ Res. 2008;102:538–545. doi: 10.1161/CIRCRESAHA.107.156877. [DOI] [PubMed] [Google Scholar]
- 61.Nigro P, Abe J, Woo CH, Satoh K, McClain C, O'Dell MR, Lee H, Lim JH, Li JD, Heo KS, Fujiwara K, Berk BC. Pkczeta decreases enos protein stability via inhibitory phosphorylation of erk5. Blood. 2010;116:1971–1979. doi: 10.1182/blood-2010-02-269134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Heo KS, Chang E, Le NT, Cushman H, Yeh ET, Fujiwara K, Abe J. De-sumoylation enzyme of sentrin/sumo-specific protease 2 regulates disturbed flow-induced sumoylation of erk5 and p53 that leads to endothelial dysfunction and atherosclerosis. Circ Res. 2013;112:911–923. doi: 10.1161/CIRCRESAHA.111.300179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chiu YJ, Kusano K, Thomas TN, Fujiwara K. Endothelial cell-cell adhesion and mechanosignal transduction. Endothelium. 2004;11:59–73. doi: 10.1080/10623320490432489. [DOI] [PubMed] [Google Scholar]
- 64.Garner E, Raj K. Protective mechanisms of p53-p21-prb proteins against DNA damage-induced cell death. Cell Cycle. 2008;7:277–282. doi: 10.4161/cc.7.3.5328. [DOI] [PubMed] [Google Scholar]
- 65.Lin K, Hsu PP, Chen BP, Yuan S, Usami S, Shyy JY, Li YS, Chien S. Molecular mechanism of endothelial growth arrest by laminar shear stress. Proc Natl Acad Sci U S A. 2000;97:9385–9389. doi: 10.1073/pnas.170282597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Carter S, Bischof O, Dejean A, Vousden KH. C-terminal modifications regulate mdm2 dissociation and nuclear export of p53. Nat Cell Biol. 2007;9:428–435. doi: 10.1038/ncb1562. [DOI] [PubMed] [Google Scholar]
- 67.Mihara M, Erster S, Zaika A, Petrenko O, Chittenden T, Pancoska P, Moll UM. P53 has a direct apoptogenic role at the mitochondria. Mol Cell. 2003;11:577–590. doi: 10.1016/s1097-2765(03)00050-9. [DOI] [PubMed] [Google Scholar]
- 68.Miyashita T, Krajewski S, Krajewska M, Wang HG, Lin HK, Liebermann DA, Hoffman B, Reed JC. Tumor suppressor p53 is a regulator of bcl-2 and bax gene expression in vitro and in vivo. Oncogene. 1994;9:1799–1805. [PubMed] [Google Scholar]
- 69.Heo KS, Lee H, Nigro P, Thomas T, Le NT, Chang E, McClain C, Reinhart-King CA, King MR, Berk BC, Fujiwara K, Woo CH, Abe J. Pkczeta mediates disturbed flow-induced endothelial apoptosis via p53 sumoylation. The Journal of cell biology. 2011;193:867–884. doi: 10.1083/jcb.201010051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Magid R, Davies PF. Endothelial protein kinase c isoform identity and differential activity of pkczeta in an athero-susceptible region of porcine aorta. Circ Res. 2005;97:443–449. doi: 10.1161/01.RES.0000179767.37838.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Frey RS, Rahman A, Kefer JC, Minshall RD, Malik AB. Pkczeta regulates tnf-alpha-induced activation of nadph oxidase in endothelial cells. Circ Res. 2002;90:1012–1019. doi: 10.1161/01.res.0000017631.28815.8e. [DOI] [PubMed] [Google Scholar]
- 72.Nigro P, Abe JI, Woo CH, Satoh K, McClain C, O'Dell MR, Lee H, Lim JH, Li JD, Heo KS, Fujiwara K, Berk BC. Pkc{zeta} decreases enos protein stability via inhibitory phosphorylation of erk5. Blood. 2010 doi: 10.1182/blood-2010-02-269134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Newton AC. Protein kinase c: Structural and spatial regulation by phosphorylation, cofactors, and macromolecular interactions. Chem Rev. 2001;101:2353–2364. doi: 10.1021/cr0002801. [DOI] [PubMed] [Google Scholar]
- 74.Smith L, Wang Z, Smith JB. Caspase processing activates atypical protein kinase c zeta by relieving autoinhibition and destabilizes the protein. Biochem J. 2003;375:663–671. doi: 10.1042/BJ20030926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Averna M, Stifanese R, De Tullio R, Passalacqua M, Salamino F, Pontremoli S, Melloni E. Functional role of hsp90 complexes with endothelial nitric-oxide synthase (enos) and calpain on nitric oxide generation in endothelial cells. J Biol Chem. 2008;283:29069–29076. doi: 10.1074/jbc.M803638200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dong Y, Wu Y, Wu M, Wang S, Zhang J, Xie Z, Xu J, Song P, Wilson K, Zhao Z, Lyons T, Zou MH. Activation of protease calpain by oxidized and glycated ldl increases the degradation of endothelial nitric oxide synthase. Journal of cellular and molecular medicine. 2009;13:2899–2910. doi: 10.1111/j.1582-4934.2008.00416.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kim GY, Nigro P, Fujiwara K, Abe J, Berk BC. P62 binding to protein kinase c zeta regulates tumor necrosis factor alpha-induced apoptotic pathway in endothelial cells. Arterioscler Thromb Vasc Biol. 2012;32:2974–2980. doi: 10.1161/ATVBAHA.112.300054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Moscat J, Diaz-Meco MT, Wooten MW. Of the atypical pkcs, par-4 and p62: Recent understandings of the biology and pathology of a pb1-dominated complex. Cell Death Differ. 2009;16:1426–1437. doi: 10.1038/cdd.2009.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kang X, Qi Y, Zuo Y, Wang Q, Zou Y, Schwartz RJ, Cheng J, Yeh ET. Sumo-specific protease 2 is essential for suppression of polycomb group protein-mediated gene silencing during embryonic development. Mol Cell. 2010;38:191–201. doi: 10.1016/j.molcel.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Li X, Luo Y, Yu L, Lin Y, Luo D, Zhang H, He Y, Kim YO, Kim Y, Tang S, Min W. Senp1 mediates tnf-induced desumoylation and cytoplasmic translocation of hipk1 to enhance ask1-dependent apoptosis. Cell Death Differ. 2008;15:739–750. doi: 10.1038/sj.cdd.4402303. [DOI] [PubMed] [Google Scholar]
- 81.Cheng J, Kang X, Zhang S, Yeh ET. Sumo-specific protease 1 is essential for stabilization of hif1alpha during hypoxia. Cell. 2007;131:584–595. doi: 10.1016/j.cell.2007.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Frodin M, Gammeltoft S. Role and regulation of 90 kda ribosomal s6 kinase (rsk) in signal transduction. Mol Cell Endocrinol. 1999;151:65–77. doi: 10.1016/s0303-7207(99)00061-1. [DOI] [PubMed] [Google Scholar]
- 83.Zhao Y, Bjøbæk C, Moller DE. Regulation and interaction of pp90rsk isoforms with mitogen-activated protein kinases. J Biol Chem. 1996;271:29773–29779. doi: 10.1074/jbc.271.47.29773. [DOI] [PubMed] [Google Scholar]
- 84.Smith JA, Poteet-Smith CE, Malarkey K, Sturgill TW. Identification of an extracellular signal-regulated kinase (erk) docking site in ribosomal s6 kinase, a sequence critical for activation by erk in vivo. The Journal of biological chemistry. 1999;274:2893–2898. doi: 10.1074/jbc.274.5.2893. [DOI] [PubMed] [Google Scholar]
- 85.Blenis J. Signal transduction via the map kinases: Proceed at your own rsk. Proc. Natl. Acad. Sci. USA. 1993;90:5889–5892. doi: 10.1073/pnas.90.13.5889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Abe J, Okuda M, Huang Q, Yoshizumi M, Berk BC. Reactive oxygen species activate p90 ribosomal s6 kinase via fyn and ras. J Biol Chem. 2000;275:1739–1748. doi: 10.1074/jbc.275.3.1739. [DOI] [PubMed] [Google Scholar]
- 87.Chen RH, Sarnecki C, Blenis J. Nuclear localization and regulation of erk-and rsk-encoded protein kinases. Mol. Cell Biol. 1992;12:915–927. doi: 10.1128/mcb.12.3.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chen RH, Tung R, Abate C, Blenis J. Cytoplasmic to nuclear signal transduction by mitogen-activated protein kinase and 90 kda ribosomal s6 kinase. Biochem Soc Trans. 1993;21:895–900. doi: 10.1042/bst0210895. [DOI] [PubMed] [Google Scholar]
- 89.Ghoda L, Lin X, Greene WC. The 90-kda ribosomal s6 kinase (pp90rsk) phosphorylates the n-terminal regulatory domain of ikappabalpha and stimulates its degradation in vitro. J Biol Chem. 1997;272:21281–21288. doi: 10.1074/jbc.272.34.21281. [DOI] [PubMed] [Google Scholar]
- 90.Chen RH, Chung J, Blenis J. Regulation of pp90rsk phosphorylation and s6 phosphotransferase activity in swiss 3t3 cells by growth factor-, phorbol ester-, and cyclic amp-mediated signal transduction. Mol. Cell Biol. 1991;11:1861–1867. doi: 10.1128/mcb.11.4.1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fisher TL, Blenis J. Evidence for two catalytically active kinase domains in pp90rsk. Mol Cell Biol. 1996;16:1212–1219. doi: 10.1128/mcb.16.3.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Xing J, Ginty DD, Greenberg ME. Coupling of the ras-mapk pathway to gene activation by rsk2, a growth factor-regulated creb kinase. Science. 1996;273:959–963. doi: 10.1126/science.273.5277.959. [DOI] [PubMed] [Google Scholar]
- 93.Pearson G, English JM, White MA, Cobb MH. Erk5 and erk2 cooperate to regulate nf-{kappa}b and cell transformation. J Biol Chem. 2000;15:15. doi: 10.1074/jbc.M009764200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Schulman IG, Juguilon H, Evans RM. Activation and repression by nuclear hormone receptors: Hormone modulates an equilibrium between active and repressive states. Mol Cell Biol. 1996;16:3807–3813. doi: 10.1128/mcb.16.7.3807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hu X, Lazar MA. The cornr motif controls the recruitment of corepressors by nuclear hormone receptors. Nature. 1999;402:93–96. doi: 10.1038/47069. [DOI] [PubMed] [Google Scholar]
- 96.Hickey CM, Wilson NR, Hochstrasser M. Function and regulation of sumo proteases. Nat Rev Mol Cell Biol. 2012;13:755–766. doi: 10.1038/nrm3478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ranganathan A, Pearson GW, Chrestensen CA, Sturgill TW, Cobb MH. The map kinase erk5 binds to and phosphorylates p90 rsk. Arch Biochem Biophys. 2006;449:8–16. doi: 10.1016/j.abb.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 98.Le NT, Takei Y, Shishido T, Woo CH, Chang E, Heo KS, Lee H, Lu Y, Morrell C, Oikawa M, McClain C, Wang X, Tournier C, Molina CA, Taunton J, Yan C, Fujiwara K, Patterson C, Yang J, Abe J. P90rsk targets the erk5-chip ubiquitin e3 ligase activity in diabetic hearts and promotes cardiac apoptosis and dysfunction. Circ Res. 2012;110:536–550. doi: 10.1161/CIRCRESAHA.111.254730. [DOI] [PMC free article] [PubMed] [Google Scholar]