Abstract
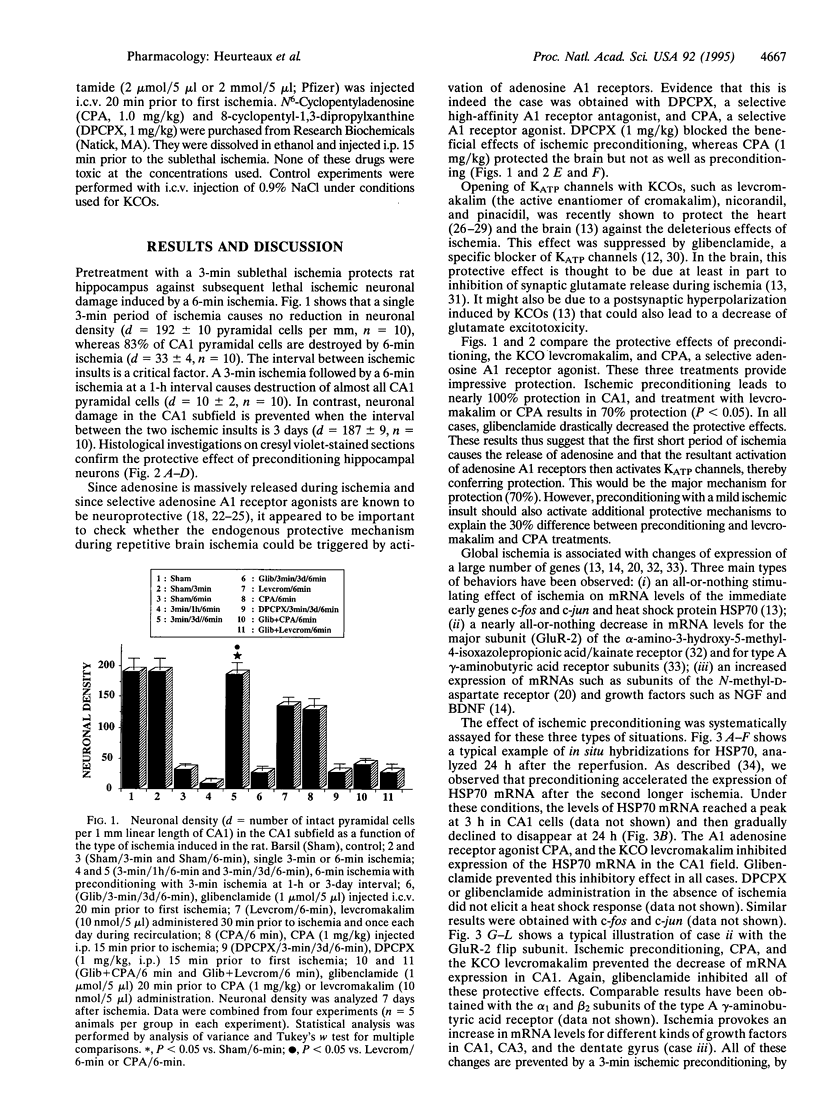
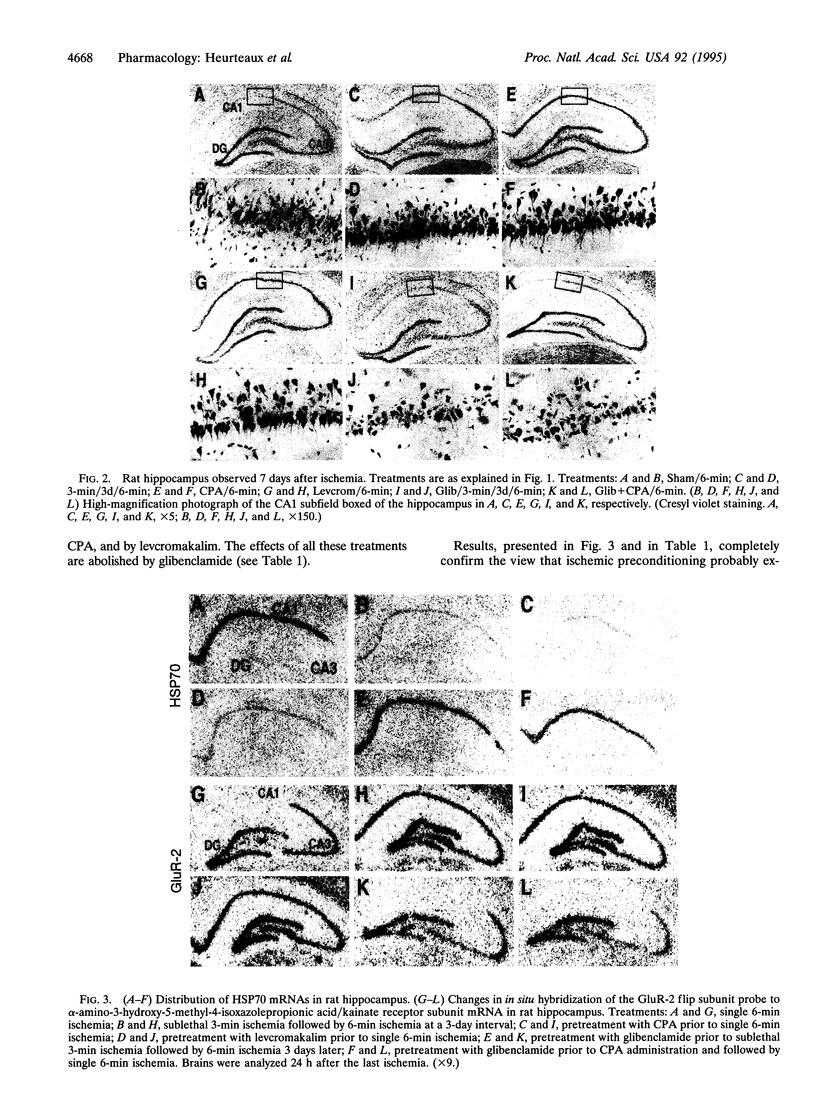
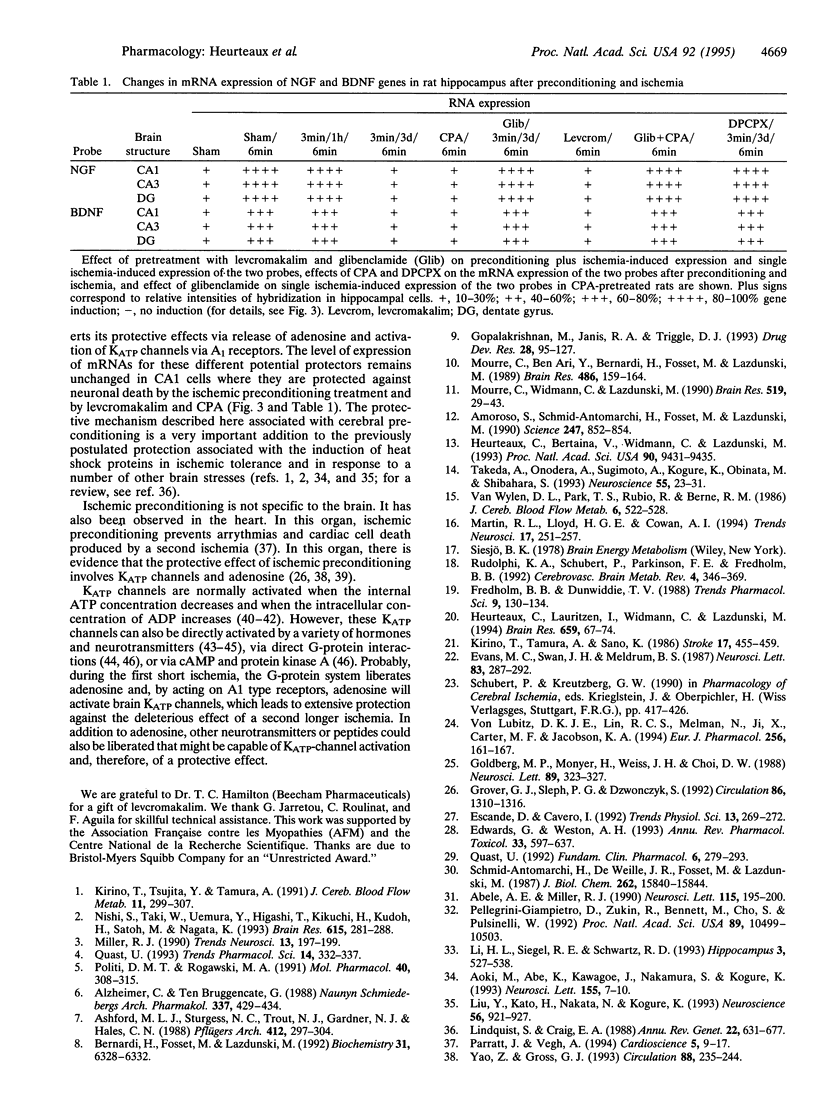
Preconditioning with sublethal ischemia protects against neuronal damage after subsequent lethal ischemic insults in hippocampal neurons. A pharmacological approach using agonists and antagonists at the adenosine A1 receptor as well as openers and blockers of ATP-sensitive K+ channels has been combined with an analysis of neuronal death and gene expression of subunits of glutamate and gamma-aminobutyric acid receptors, HSP70, c-fos, c-jun, and growth factors. It indicates that the mechanism of ischemic tolerance involves a cascade of events including liberation of adenosine, stimulation of adenosine A1 receptors, and, via these receptors, opening of sulfonylurea-sensitive ATP-sensitive K+ channels.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abele A. E., Miller R. J. Potassium channel activators abolish excitotoxicity in cultured hippocampal pyramidal neurons. Neurosci Lett. 1990 Jul 31;115(2-3):195–200. doi: 10.1016/0304-3940(90)90454-h. [DOI] [PubMed] [Google Scholar]
- Alzheimer C., ten Bruggencate G. Actions of BRL 34915 (Cromakalim) upon convulsive discharges in guinea pig hippocampal slices. Naunyn Schmiedebergs Arch Pharmacol. 1988 Apr;337(4):429–434. doi: 10.1007/BF00169535. [DOI] [PubMed] [Google Scholar]
- Amoroso S., Schmid-Antomarchi H., Fosset M., Lazdunski M. Glucose, sulfonylureas, and neurotransmitter release: role of ATP-sensitive K+ channels. Science. 1990 Feb 16;247(4944):852–854. doi: 10.1126/science.2305257. [DOI] [PubMed] [Google Scholar]
- Aoki M., Abe K., Kawagoe J., Nakamura S., Kogure K. The preconditioned hippocampus accelerates HSP70 heat shock gene expression following transient ischemia in the gerbil. Neurosci Lett. 1993 May 28;155(1):7–10. doi: 10.1016/0304-3940(93)90661-4. [DOI] [PubMed] [Google Scholar]
- Ashcroft F. M. Adenosine 5'-triphosphate-sensitive potassium channels. Annu Rev Neurosci. 1988;11:97–118. doi: 10.1146/annurev.ne.11.030188.000525. [DOI] [PubMed] [Google Scholar]
- Ashford M. L., Sturgess N. C., Trout N. J., Gardner N. J., Hales C. N. Adenosine-5'-triphosphate-sensitive ion channels in neonatal rat cultured central neurones. Pflugers Arch. 1988 Aug;412(3):297–304. doi: 10.1007/BF00582512. [DOI] [PubMed] [Google Scholar]
- Bernardi H., Fosset M., Lazdunski M. ATP/ADP binding sites are present in the sulfonylurea binding protein associated with brain ATP-sensitive K+ channels. Biochemistry. 1992 Jul 14;31(27):6328–6332. doi: 10.1021/bi00142a023. [DOI] [PubMed] [Google Scholar]
- Dunne M. J., Bullett M. J., Li G. D., Wollheim C. B., Petersen O. H. Galanin activates nucleotide-dependent K+ channels in insulin-secreting cells via a pertussis toxin-sensitive G-protein. EMBO J. 1989 Feb;8(2):413–420. doi: 10.1002/j.1460-2075.1989.tb03392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards G., Weston A. H. The pharmacology of ATP-sensitive potassium channels. Annu Rev Pharmacol Toxicol. 1993;33:597–637. doi: 10.1146/annurev.pa.33.040193.003121. [DOI] [PubMed] [Google Scholar]
- Escande D., Cavero I. K+ channel openers and 'natural' cardioprotection. Trends Pharmacol Sci. 1992 Jul;13(7):269–272. doi: 10.1016/0165-6147(92)90083-i. [DOI] [PubMed] [Google Scholar]
- Evans M. C., Swan J. H., Meldrum B. S. An adenosine analogue, 2-chloroadenosine, protects against long term development of ischaemic cell loss in the rat hippocampus. Neurosci Lett. 1987 Dec 29;83(3):287–292. doi: 10.1016/0304-3940(87)90101-7. [DOI] [PubMed] [Google Scholar]
- Fredholm B. B., Dunwiddie T. V. How does adenosine inhibit transmitter release? Trends Pharmacol Sci. 1988 Apr;9(4):130–134. doi: 10.1016/0165-6147(88)90194-0. [DOI] [PubMed] [Google Scholar]
- Goldberg M. P., Monyer H., Weiss J. H., Choi D. W. Adenosine reduces cortical neuronal injury induced by oxygen or glucose deprivation in vitro. Neurosci Lett. 1988 Jul 8;89(3):323–327. doi: 10.1016/0304-3940(88)90547-2. [DOI] [PubMed] [Google Scholar]
- Grover G. J., Sleph P. G., Dzwonczyk S. Role of myocardial ATP-sensitive potassium channels in mediating preconditioning in the dog heart and their possible interaction with adenosine A1-receptors. Circulation. 1992 Oct;86(4):1310–1316. doi: 10.1161/01.cir.86.4.1310. [DOI] [PubMed] [Google Scholar]
- Heurteaux C., Bertaina V., Widmann C., Lazdunski M. K+ channel openers prevent global ischemia-induced expression of c-fos, c-jun, heat shock protein, and amyloid beta-protein precursor genes and neuronal death in rat hippocampus. Proc Natl Acad Sci U S A. 1993 Oct 15;90(20):9431–9435. doi: 10.1073/pnas.90.20.9431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heurteaux C., Lauritzen I., Widmann C., Lazdunski M. Glutamate-induced overexpression of NMDA receptor messenger RNAs and protein triggered by activation of AMPA/kainate receptors in rat hippocampus following forebrain ischemia. Brain Res. 1994 Oct 3;659(1-2):67–74. doi: 10.1016/0006-8993(94)90864-8. [DOI] [PubMed] [Google Scholar]
- Honoré E., Lazdunski M. Single-channel properties and regulation of pinacidil/glibenclamide-sensitive K+ channels in follicular cells from Xenopus oocyte. Pflugers Arch. 1993 Jul;424(2):113–121. doi: 10.1007/BF00374601. [DOI] [PubMed] [Google Scholar]
- Kirino T., Tamura A., Sano K. A reversible type of neuronal injury following ischemia in the gerbil hippocampus. Stroke. 1986 May-Jun;17(3):455–459. doi: 10.1161/01.str.17.3.455. [DOI] [PubMed] [Google Scholar]
- Kirino T., Tsujita Y., Tamura A. Induced tolerance to ischemia in gerbil hippocampal neurons. J Cereb Blood Flow Metab. 1991 Mar;11(2):299–307. doi: 10.1038/jcbfm.1991.62. [DOI] [PubMed] [Google Scholar]
- Li H., Siegel R. E., Schwartz R. D. Rapid decline of GABAA receptor subunit mRNA expression in hippocampus following transient cerebral ischemia in the gerbil. Hippocampus. 1993 Oct;3(4):527–537. doi: 10.1002/hipo.450030412. [DOI] [PubMed] [Google Scholar]
- Lindquist S., Craig E. A. The heat-shock proteins. Annu Rev Genet. 1988;22:631–677. doi: 10.1146/annurev.ge.22.120188.003215. [DOI] [PubMed] [Google Scholar]
- Liu Y., Kato H., Nakata N., Kogure K. Temporal profile of heat shock protein 70 synthesis in ischemic tolerance induced by preconditioning ischemia in rat hippocampus. Neuroscience. 1993 Oct;56(4):921–927. doi: 10.1016/0306-4522(93)90138-6. [DOI] [PubMed] [Google Scholar]
- Martin R. L., Lloyd H. G., Cowan A. I. The early events of oxygen and glucose deprivation: setting the scene for neuronal death? Trends Neurosci. 1994 Jun;17(6):251–257. doi: 10.1016/0166-2236(94)90008-6. [DOI] [PubMed] [Google Scholar]
- Miller R. J. Glucose-regulated potassium channels are sweet news for neurobiologists. Trends Neurosci. 1990 Jun;13(6):197–199. doi: 10.1016/0166-2236(90)90158-7. [DOI] [PubMed] [Google Scholar]
- Mourre C., Ben Ari Y., Bernardi H., Fosset M., Lazdunski M. Antidiabetic sulfonylureas: localization of binding sites in the brain and effects on the hyperpolarization induced by anoxia in hippocampal slices. Brain Res. 1989 May 1;486(1):159–164. doi: 10.1016/0006-8993(89)91288-2. [DOI] [PubMed] [Google Scholar]
- Mourre C., Widmann C., Lazdunski M. Sulfonylurea binding sites associated with ATP-regulated K+ channels in the central nervous system: autoradiographic analysis of their distribution and ontogenesis, and of their localization in mutant mice cerebellum. Brain Res. 1990 Jun 11;519(1-2):29–43. doi: 10.1016/0006-8993(90)90057-i. [DOI] [PubMed] [Google Scholar]
- Nishi S., Taki W., Uemura Y., Higashi T., Kikuchi H., Kudoh H., Satoh M., Nagata K. Ischemic tolerance due to the induction of HSP70 in a rat ischemic recirculation model. Brain Res. 1993 Jul 2;615(2):281–288. doi: 10.1016/0006-8993(93)90039-p. [DOI] [PubMed] [Google Scholar]
- Parratt J., Vegh A. Pronounced antiarrhythmic effects of ischemic preconditioning. Cardioscience. 1994 Mar;5(1):9–18. [PubMed] [Google Scholar]
- Pellegrini-Giampietro D. E., Zukin R. S., Bennett M. V., Cho S., Pulsinelli W. A. Switch in glutamate receptor subunit gene expression in CA1 subfield of hippocampus following global ischemia in rats. Proc Natl Acad Sci U S A. 1992 Nov 1;89(21):10499–10503. doi: 10.1073/pnas.89.21.10499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen O. H., Dunne M. J. Regulation of K+ channels plays a crucial role in the control of insulin secretion. Pflugers Arch. 1989;414 (Suppl 1):S115–S120. doi: 10.1007/BF00582259. [DOI] [PubMed] [Google Scholar]
- Politi D. M., Rogawski M. A. Glyburide-sensitive K+ channels in cultured rat hippocampal neurons: activation by cromakalim and energy-depleting conditions. Mol Pharmacol. 1991 Aug;40(2):308–315. [PubMed] [Google Scholar]
- Quast U. Do the K+ channel openers relax smooth muscle by opening K+ channels? Trends Pharmacol Sci. 1993 Sep;14(9):332–337. doi: 10.1016/0165-6147(93)90006-6. [DOI] [PubMed] [Google Scholar]
- Quast U. Potassium channel openers: pharmacological and clinical aspects. Fundam Clin Pharmacol. 1992;6(7):279–293. doi: 10.1111/j.1472-8206.1992.tb00122.x. [DOI] [PubMed] [Google Scholar]
- Rudolphi K. A., Schubert P., Parkinson F. E., Fredholm B. B. Adenosine and brain ischemia. Cerebrovasc Brain Metab Rev. 1992 Winter;4(4):346–369. [PubMed] [Google Scholar]
- Schmid-Antomarchi H., De Weille J., Fosset M., Lazdunski M. The receptor for antidiabetic sulfonylureas controls the activity of the ATP-modulated K+ channel in insulin-secreting cells. J Biol Chem. 1987 Nov 25;262(33):15840–15844. [PubMed] [Google Scholar]
- Takeda A., Onodera H., Sugimoto A., Kogure K., Obinata M., Shibahara S. Coordinated expression of messenger RNAs for nerve growth factor, brain-derived neurotrophic factor and neurotrophin-3 in the rat hippocampus following transient forebrain ischemia. Neuroscience. 1993 Jul;55(1):23–31. doi: 10.1016/0306-4522(93)90451-k. [DOI] [PubMed] [Google Scholar]
- Van Wylen D. G., Park T. S., Rubio R., Berne R. M. Increases in cerebral interstitial fluid adenosine concentration during hypoxia, local potassium infusion, and ischemia. J Cereb Blood Flow Metab. 1986 Oct;6(5):522–528. doi: 10.1038/jcbfm.1986.97. [DOI] [PubMed] [Google Scholar]
- Von Lubitz D. K., Lin R. C., Melman N., Ji X. D., Carter M. F., Jacobson K. A. Chronic administration of selective adenosine A1 receptor agonist or antagonist in cerebral ischemia. Eur J Pharmacol. 1994 Apr 21;256(2):161–167. doi: 10.1016/0014-2999(94)90241-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Z., Gross G. J. Glibenclamide antagonizes adenosine A1 receptor-mediated cardioprotection in stunned canine myocardium. Circulation. 1993 Jul;88(1):235–244. doi: 10.1161/01.cir.88.1.235. [DOI] [PubMed] [Google Scholar]
- de Weille J. R., Lazdunski M. Regulation of the ATP-sensitive potassium channel. Ion Channels. 1990;2:205–222. doi: 10.1007/978-1-4615-7305-0_6. [DOI] [PubMed] [Google Scholar]
- de Weille J. R., Schmid-Antomarchi H., Fosset M., Lazdunski M. Regulation of ATP-sensitive K+ channels in insulinoma cells: activation by somatostatin and protein kinase C and the role of cAMP. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2971–2975. doi: 10.1073/pnas.86.8.2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Weille J., Schmid-Antomarchi H., Fosset M., Lazdunski M. ATP-sensitive K+ channels that are blocked by hypoglycemia-inducing sulfonylureas in insulin-secreting cells are activated by galanin, a hyperglycemia-inducing hormone. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1312–1316. doi: 10.1073/pnas.85.4.1312. [DOI] [PMC free article] [PubMed] [Google Scholar]




