Abstract
The neural microtubule-associated protein tau binds to and stabilizes microtubules. Because of alternative mRNA splicing, tau is expressed with either 3 or 4 C-terminal repeats. Two observations indicate that differences between these tau isoforms are functionally important. First, the pattern of tau isoform expression is tightly regulated during development. Second, mutation-induced changes in tau RNA splicing cause neuronal cell death and dementia simply by altering the isoform expression ratio. To investigate whether 3- and 4-repeat tau differentially regulate microtubule behavior in cells, we microinjected physiological levels of these two isoforms into EGFP-tubulin–expressing cultured MCF7 cells and measured the effects on the dynamic instability behavior of individual microtubules by time-lapse microscopy. Both isoforms suppressed microtubule dynamics, though to different extents. Specifically, 4-repeat tau reduced the rate and extent of both growing and shortening events. In contrast, 3-repeat tau stabilized most dynamic parameters about threefold less potently than 4-repeat tau and had only a minimal ability to suppress shortening events. These differences provide a mechanistic rationale for the developmental shift in tau isoform expression and are consistent with a loss-of-function model in which abnormal tau isoform expression results in the inability to properly regulate microtubule dynamics, leading to neuronal cell death and dementia.
INTRODUCTION
The microtubule-associated protein tau promotes neuronal cell polarization and axonal outgrowth; it is also necessary for maintaining axonal morphology and axonal transport (Caceres and Kosik, 1990; Esmaeli-Azad et al., 1994; Trinczek et al., 1999; Stamer et al., 2002). Alternatively, abnormal tau is the major component of neurofibrillary tangles, hallmark pathological features of Alzheimer's disease and related dementias.
Mechanistically, tau acts by binding to microtubules directly and regulating their growing, shortening, and treadmilling dynamics (Drechsel et al., 1992; Panda et al., 1995, 1999; Trinczek et al., 1995). Because tight regulation of microtubule dynamics and microtubule behavior can be critical to cell viability (Jordan et al., 1996; Goncalves et al., 2001), fine regulation of tau activity may be equally critical.
Tau activity is regulated in part by alternative mRNA splicing, which leads to the expression of two families of tau isoforms, those containing four C-terminal repeats and those with three such repeats (Figure 1A; Lee et al., 1988; Himmler, 1989). In vitro studies have shown that 4-repeat tau binds to, assembles, and stabilizes microtubules more effectively than 3-repeat tau (Butner and Kirschner, 1991; Gustke et al., 1994; Trinczek et al., 1995; Goode et al., 2000; Panda et al., 2003). However, there are limited data assessing the abilities of 3- or 4-repeat tau to modulate dynamics in a complex cellular environment.
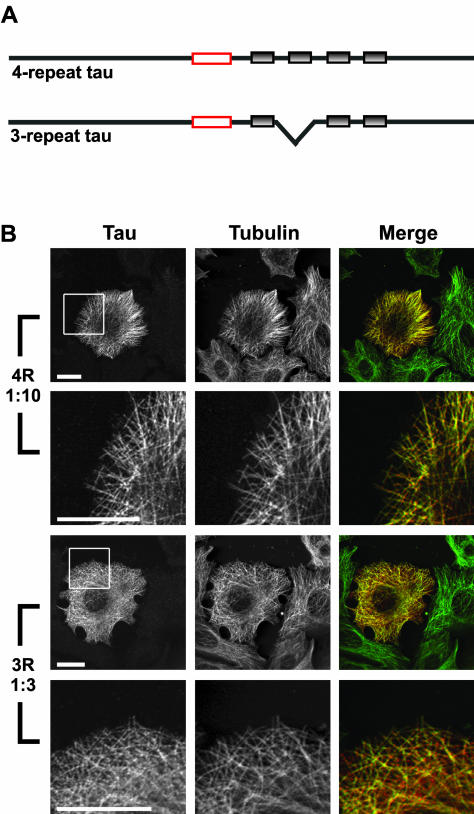
Figure 1.
Immunocytochemical analysis of MCF7 cells injected with 4- or 3-repeat tau. (A) Schematic of 4- and 3-repeat tau proteins. Gray shaded boxes represent the imperfect repeats. Red boxes indicate the epitope of the Tau 5 mAb used in B (LoPresti et al., 1995). (B) MCF7 cells injected with either 4-repeat tau (at a 1:10 tau:tubulin ratio) or 3-repeat tau (at a 1:3 tau:tubulin ratio). Cells were fixed and stained for tau (left column) and tubulin (center column) as described in MATERIALS AND METHODS. In the merged column, the tau staining is shown in red and the tubulin staining is shown in green; yellow indicates colocalization. The boxed areas in the first and third rows are enlarged in the second and fourth rows in order to visualize colocalization on individual microtubules. For both 4- and 3-repeat tau, tau colocalized with microtubules in injected cells, whereas uninjected cells (seen surrounding the injected cell at low magnification) did not stain positively for tau. Scale bars, 20 μm.
The importance of functional differences between 3- and 4-repeat tau is highlighted by two observations. First, although only 3-repeat tau is expressed in fetal brain, both 3- and 4-repeat tau are expressed in adult human brain at approximately equal levels (Goedert et al., 1989a, 1989b; Himmler, 1989; Himmler et al., 1989; Kosik et al., 1989). It has been hypothesized that these expression differences generate different microtubule behavior in fetal neurons undergoing axonal outgrowth, pathfinding, and synpatogenesis vs. relatively stable adult neurons. Second, genetic analyses demonstrate unequivocally that dominant mutations in the tau gene cause neuronal cell death and dementia in FTDP-17, a group of neurodegenerative disorders with many similarities to Alzheimer's (Clark et al., 1998; Hong et al., 1998; Hutton et al., 1998; Spillantini et al., 1998; Goedert et al., 1999). One class of FTDP-17 tau mutations generates amino acid substitutions. The second class of FTDP-17 mutations is regulatory, leading to altered ratios of 3- and 4-repeat tau. Because the majority of the regulatory mutations are intronic or silent, the encoded proteins are wild type in sequence. Thus, simply changing the ratio of otherwise wild-type tau isoforms is sufficient to cause neuronal cell death and dementia.
Whereas these genetic analyses have established a cause-and-effect relationship between tau mutations and disease, the underlying molecular mechanism(s) causing neuronal cell death are not understood. Several investigators have proposed a “gain-of-toxic-function” model in which mutations in tau increase its propensity to form abnormal cytotoxic fibers (Hong et al., 1998; Gamblin et al., 2000, 2003; Barghorn and Mandelkow, 2002). However, although this model easily accommodates amino acid substitution mutations, it is more difficult to apply to RNA splicing mutations, which produce all wild-type tau. Alternatively, dominant phenotypes could be achieved by a loss-of-function, haplo-insufficiency mechanism in which the mutations leave cells unable to maintain an appropriate level of tau activity necessary for normal microtubule and cellular function. Indeed, previous work has indicated that altered microtubule dynamic behavior may lead to cell death (Jordan et al., 1996; Goncalves et al., 2001). This mechanism could easily account for both the splicing and amino acid substitution classes of tau FTDP-17 mutations.
To better understand isoform-specific differences in normal and abnormal tau action, we compared the abilities of exogenously introduced 4- and 3-repeat tau to modulate the dynamic behavior of individual microtubules upon microinjection into living MCF7 cells. These analyses provide novel insights into the normal developmental transition in tau isoform expression and test the plausibility of a haplo-insufficiency model for tau-mediated neuronal cell death through aberrant regulation of microtubule dynamics.
MATERIALS AND METHODS
Tau Protein Purification
pRK expression vectors containing the human cDNA sequences for the shortest 4- and 3-repeat tau isoforms (encoding 383 and 352 amino acids, respectively) were kind gifts from Dr. Kenneth Kosik (Harvard University). Tau protein was expressed and purified by a modification of the procedures described in Goode et al. (1997). Briefly, tau expression was induced in Rosetta (DE3) pLacI cells (Novagen, Madison, WI) by adding isopropyl-β-d-thiogalactoside to a final concentration of 1 mM. Bacterial pellets were resuspended in BRB-80 buffer (80 mM Pipes, pH 6.8, 1 mM EGTA, 1 mM MgSO4) containing 0.1% β-mercaptoethanol and 1 mM PMSF. Cells were lysed by sonication and the lysate was clarified by centrifugation (12,000 × g, 15 min). Supernatants were boiled to precipitate heat-labile proteins and recentrifuged. The heat-stable proteins were bound to a phosphocellulose column and eluted with a salt gradient (0.2 to 1.0 M NaCl). Fractions containing tau protein were identified by SDS-PAGE followed by Coomassie blue staining. Tau-containing fractions were pooled and further purified using reverse-phase HPLC (DeltaPak-C18; Millipore, Billerica, MA). Tau elution occurred when the gradient was 74% 0.1% trifluoroacetic acid in water and 26% 0.1% trifluoroacetic acid in isopropanol. HPLC fractions containing tau were pooled, lyophilized, and resuspended in PBS. The concentration of each tau sample was determined by SDS-PAGE comparison with a “tau mass standard,” the concentration of which was established by mass spectrometry (Panda et al., 2003).
Cell Culture
The MCF7 human breast cancer cell line (ATCC, Manassas, VA) was stably transfected with the EGFP-tubulin plasmid pEGFP-Tub (CLONTECH, Palo Alto, CA) following the Superfect (Qiagen, Valencia, CA) protocol. Cells were maintained in DMEM (Life Technologies, Grand Island, NY) supplemented with nonessential amino acids, 10% FBS, antibiotic-antimycotic (Life Technologies), and geneticin (400 μg/ml; Life Technologies) at 37°C and 5.5% CO2. Cells were seeded 36–48 h before injection on 12 mm CelLocate coverslips (Eppendorf, Hamburg, Germany) coated with poly-d-lysine (100 μg/ml; Sigma, St. Louis, MO) followed by human fibronectin (20 μg/ml; Life Technologies) and laminin (10 μg/ml; Sigma). To induce a more flattened morphology, cells were serum-starved in media containing 2% FBS for 12 h before injection.
Microinjection
Seeded cells were transferred to DMEM lacking bicarbonate and containing 25 mM HEPES and 4.5 g/l glucose (recording media; Life Technologies). Tau was diluted to concentrations of 13.3 or 43.2 μM in PBS plus 1.4 mM β-mercaptoethanol and 0.45 mg/ml RITC-dextran as a marker for injected cells. Immediately before microinjection, the solution was centrifuged at 35,000 rpm in a Beckman TLA 100.3 ultracentrifuge rotor (15 min, 4°C) to remove any aggregates or debris. Pressure microinjection was performed using an Eppendorf Transjector 5246 and Injectman. The injection volume was ∼10% of the cell volume (Lamb and Fernandez, 1997), resulting in a ∼1.3 or 4.3 μM final tau concentration in the cell. Injected cells were returned to normal media and incubated 2–3 h at 37°C to allow equilibration of tau within the cells. In the same manner as Dhamodharan and Wadsworth (1995), we estimated the total tubulin in the cell to be 20 μM (Hiller and Weber, 1978) with 65% in polymer during interphase (Zhai and Borisy, 1994), resulting in 13 μM polymerized tubulin. Based on these estimates, the molar ratio of tau to polymerized tubulin in the cell was either 1:9.8 or 1:3.3.
Immunocytochemistry
Cells were rinsed once with PBS and fixed by the rapid addition of 100% methanol (4°C). Fixed cells were incubated overnight in blocking buffer (PBS containing 3% BSA, 0.1% Triton X-100, and 1% horse serum). Cells were incubated first with mouse monoclonal Tau 5 antibody (1:100; BioSource International, Camarillo, CA), then Cy3-conjugated donkey anti-mouse secondary antibody (1:100; Jackson ImmunoResearch Laboratories, West Grove, PA), followed by FITC-conjugated mouse monoclonal tubulin antibody DM1α (1:50; Sigma). All incubations were for 1 h at room temperature, followed by four 15-min washes in blocking buffer. Coverslips were mounted on glass slides using Prolong (Molecular Probes, Eugene, OR). Images were obtained using a laser scanning confocal microscope (MRC 1024; Bio-Rad, Hercules, CA). Images are 4 Kalman averages of the same plane in the z-axis. Images were processed using Adobe Photoshop 6.0 (San Jose, CA).
Time-Lapse Microscopy and Image Acquisition
Microinjected cells were mounted in a Rose chamber (Rose et al., 1958) in recording media supplemented with 40 μl Oxyrase (Oxyrase, Mansfield, OH) per ml of medium to reduce photobleaching. Injected cells were identified by the presence of RITC fluorescence. Images were captured with an inverted fluorescence microscope (Nikon Eclipse E800, Garden City, NY) with a Nikon plan apochromat 1.4 NA, 100× objective lens, maintained at 36.5 ± 1°C. Thirty-one images per cell were taken at 4-s intervals using a Hamamatsu Orca II (Middlesex, NJ) digital camera driven by MetaMorph software (Universal Imaging, Media, PA).
Analysis of Microtubule Dynamic Instability
Analysis of the growth and shortening dynamics of individual microtubules in the thin peripheral region of cells was performed as described by Goncalves et al. (2001). The positions of the plus-ends of microtubules were recorded over time using MetaMorph software, exported to Microsoft Excel, and analyzed using RTM software (Walker et al., 1988). The changes in length of individual microtubules were plotted as a function of time. Changes in length > 0.5 μm were designated as growth or shortening events. Periods in which changes in length were <0.5 μm were designated as phases of attenuated microtubule dynamics (or pause). A catastrophe was defined as a transition from either growth or attenuation to shortening. Frequencies of catastrophe were calculated either as the total number of catastrophes divided by the total time spent growing and attenuated or as the total number of catastrophes divided by the total length grown. A rescue was defined as a transition from shortening to either growth or attenuation. Frequencies of rescue were calculated either as the total number of rescues divided by the total amount of time spent shortening or as the total number of rescues divided by the total length shortened. The number of growth, shortening, and attenuation events was calculated by determining the probability of transitioning into a particular phase. Probabilities of each possible transition were calculated as the total number of a particular transition divided by the total number of transitions from that particular phase. Dynamicity was calculated as the total length grown and shortened divided by the total time measured.
It should be noted that a comparison of microtubule dynamics in control cells vs. tau-injected cells inherently underestimates the effects of tau on overall microtubule stabilization in the cells. This occurs because, as is standard for this procedure, we measure the dynamics of only those microtubules displaying dynamic instability behavior. However, in all cells there is a subpopulation of microtubules that is not undergoing detectable dynamic instability behavior. Because of tau's stabilization effects, the fraction of microtubules that are not displaying dynamic instability is increased in tau-injected cells. Although this is a difficult parameter to quantify, a rough observational estimation is that more than 50% of microtubules displayed dynamic instability behavior during a 2-min period in control cells, whereas as few as ∼10% of microtubules were dynamic upon stabilization with tau. Because we can only measure dynamic microtubules, this additional stabilizing effect of tau is not reflected in the measurements presented in this study. This does not affect measurements of growth and shortening, but it most likely results in an underestimation of the effect of tau on the amount of time that microtubules spent in an attenuated state.
Online Supplemental Material
Time-lapse movies of EGFP-microtubules undergoing microtubule dynamics in cells are available as supplemental material. Movie 1 shows microtubule dynamics in a control cell injected with buffer. Movie 2 shows microtubule dynamics in a cell injected with 4-repeat tau at a tau to tubulin ratio of 1:10. Movie 3 shows a cell similarly injected with 3-repeat tau. Each movie consists of 30 frames taken at 4-s intervals and is played 30 times faster than real time.
RESULTS
Before determining the effects of microinjected tau on microtubule dynamics in injected cells, we first sought to confirm that microinjected tau protein was not rapidly degraded and that it was associated with microtubules. Cells were injected with either 4- or 3-repeat tau and then incubated at 37°C for 2 h to allow sufficient time for the tau to equilibrate throughout the cells. Cells were fixed and double-stained for tau and tubulin. We found that tau was indeed present in injected cells and was bound to microtubules, whereas no tau staining could be detected in uninjected cells (Figure 1B). Tau staining was evenly distributed along the microtubules with little or no tau staining elsewhere in the cytoplasm. There was no visible difference in the intensity of 3- and 4-repeat tau staining on microtubules. Thus, any tau-dependent changes in the dynamic instability behavior of the microtubules most likely result from direct interactions between tau and microtubules.
To visualize microtubule dynamic instability in cells, we tracked changes in the length of individual microtubules in the flat peripheral region of living MCF7 cells stably transfected with EGFP-tubulin. As shown in Figure 2 and the online supplemental movies, the microtubules were well resolved and their ends were clearly visible. Cells were injected with buffer alone, 4- or 3-repeat tau, or a non–microtubule-associated protein (glutathione S-transferase [GST]) as a negative control. Although microtubules were visibly less dynamic in cells injected with tau, only a very small number of tau-injected cells (<1%) exhibited microtubule bundling, allowing for clear visualization of individual microtubules (see online movies). Next, we generated life history plots of the changes in length over time of individual microtubules. Representative examples are shown in Figure 3. From these plots, we identified each growth, shortening and attenuation event and measured its duration and rate (slope). Importantly, microtubules in the buffer-injected and GST-injected control cells behaved similarly to those in uninjected cells (Tables 1 and 2). For simplicity, therefore, our presentation of the data will focus on comparing tau-injected cells with buffer-injected cells.
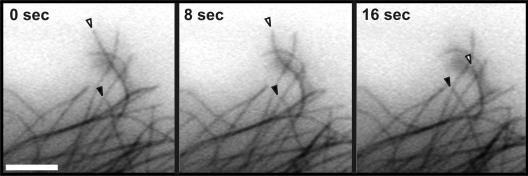
Figure 2.
Dynamic microtubules visualized after microinjection of buffer into an MCF7 cell expressing EGFP-tubulin. Time-lapse images of fluorescent (EGFP) microtubules in buffer-injected cells allow tracking of microtubule growth and shortening over time. One growing microtubule (closed arrowheads) and one shortening microtubule (open arrowheads) are indicated. Although microtubule ends are visible in these still images, it is much easier to discern changes in length in a time-lapse movie (online supplemental material). Scale bar, 5 μm.
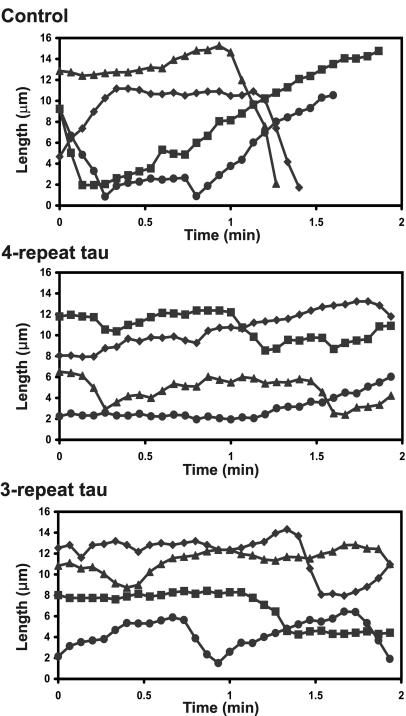
Figure 3.
Life history plots of the growth and shortening dynamics of individual microtubules. The positions of the ends of individual microtubules were tracked in cells injected with buffer or a 1:10 molar ratio of 4- or 3-repeat tau. Changes in the length of the microtubules were plotted vs. time. Each line represents a single microtubule. From these life history plots, individual growth, shortening, and attenuation events were identified and rates and durations of each event was measured.
Table 1.
Effects of 4- and 3-repeat tau on microtubule growth and shortening
| Uninjected | Buffer | GST | 4R (1:10) | 3R (1:10) | 3R (1:3) | |
|---|---|---|---|---|---|---|
| N (microtubules/cells) | 56/15 | 56/18 | 25/9 | 27/7 | 27/8 | 22/6 |
| Growth rate (μm/min) | 12.8 ± 0.4 | 13.3 ± 0.4 | 14.3 ± 0.7 | 9.4 ± 0.3a | 11.6 ± 0.5 | 9.3 ± 0.5a |
| Growth length (μm) | 2.8 ± 0.2 | 2.7 ± 0.2 | 2.8 ± 0.3 | 1.2 ± 0.1a | 1.8 ± 0.2a | 1.5 ± 0.2a |
| Shortening rate (μm/min) | 24.7 ± 1.3 | 27.1 ± 1.5 | 26.6 ± 1.8 | 16.5 ± 0.9a | 22.3 ± 1.5 | 22.6 ± 1.6 |
| Shortening length (μm) | 4.5 ± 0.4 | 5.2 ± 0.4 | 3.7 ± 0.4 | 2.9 ± 0.4a | 3.6 ± 0.4 | 3.8 ± 0.5 |
| Dynamicity (μm/min) | 11.2 | 11.7 | 11.5 | 5.2 | 7.3 | 5.2 |
Rate and length values are given as mean ± SEM. Dynamicity calculated as total length grown and shortened over total time measured.
Values significant at 99% confidence level compared to buffer control
Table 2.
Effects of 4- and 3-repeat tau on the percentage of time spent growing, shortening, and in an attenuated state
| Uninjected | Buffer | GST | 4R (1:10) | 3R (1:10) | 3R (1:3) | |
|---|---|---|---|---|---|---|
| % time spent growing | 39.6 | 39.0 | 36.8 | 24.4 | 31.4 | 24.3 |
| % time spent shortening | 24.9 | 24.1 | 23.4 | 17.9 | 16.4 | 13.0 |
| % time spent attenuated | 35.6 | 36.9 | 39.8 | 57.7 | 52.2 | 62.8 |
4-Repeat Tau Decreased the Microtubule Shortening Rate and the Average Length Shortened, Whereas 3-Repeat Tau Had Little Effect on These Parameters
To determine whether tau affects shortening parameters in cells, we compared the average rate of microtubule shortening in tau-injected vs. buffer-injected cells. Although 4-repeat tau had a strong suppressing effect on the shortening rate, 3-repeat tau exhibited much less of an effect on this parameter (Figure 4A, Table 1). A 1:10 molar ratio of 4-repeat tau to polymeric tubulin decreased the shortening rate by 39% from 27.1 to 16.5 μm/min. Injection of the same amount of 3-repeat tau decreased the shortening rate by only 18%, to 22.3 μm/min. To further assess this relatively minimal 3-repeat effect, we increased the molar ratio of 3-repeat tau to tubulin to 1:3. Interestingly, this increased level of 3-repeat tau did not further suppress the shortening rate. These data clearly indicate that there is a marked difference between the abilities of 4- and 3-repeat tau to affect microtubule shortening in cells, similar to what has been observed in vitro (Trinczek et al., 1995; Panda et al., 2003).
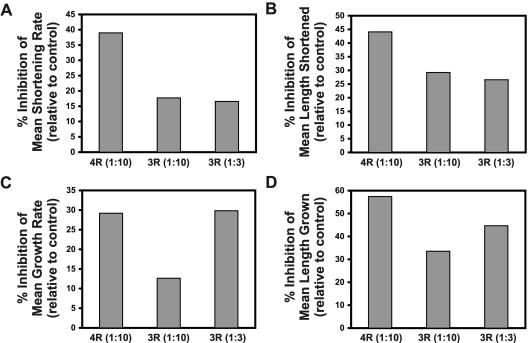
Figure 4.
Effects of 4- and 3-repeat tau on microtubule growth and shortening parameters. Microtubule growth and shortening parameters were measured in cells injected with 4- or 3-repeat tau. Presented are the mean rates (A) and lengths (B) of individual shortening events as well as the mean rates (C) and lengths (D) of individual growth events. Values are graphed as percent differences from buffer controls [(experimental value - buffer control value)/buffer control value × 100]. Tau-to-tubulin molar ratios are presented in parentheses.
Another measure of shortening is the average length shortened per shortening event. This parameter incorporates both the rate and the duration of a shortening event. When we compared the average length of a shortening event in tau-injected vs. buffer-injected cells, we again found a large difference between 4- and 3-repeat tau (Figure 4B, Table 1). A 1:10 molar ratio of 4-repeat tau had a strong stabilizing effect on the shortening length, reducing it by 44% from 5.2 to 2.9 μm. Injecting the same amount of 3-repeat tau reduced the average length shortened by 29% to 3.6 μm. Increasing the concentration of 3-repeat tau did not produce a greater decrease in the average length shortened, indicating again an important mechanistic difference between the effects on shortening of 4- and 3-repeat tau.
4- and 3-Repeat Tau Decreased the Microtubule Growth Rate and Average Length Grown
On comparing the average rate of growth in tau-injected cells vs. buffer-injected cells, we found that both 4- and 3-repeat wild-type tau suppressed the microtubule growth rate (Figure 4C, Table 1). Injection of 4-repeat tau at a 1:10 tau-to-tubulin ratio caused a 29% decrease in the average growth rate, from 13.3 to 9. 4 μm/min. Injection of 3-repeat tau at the same molar ratio caused a 13% decrease in the growth rate to 11.6 μm/min. As before, we assessed whether an increased amount of 3-repeat tau in the cell would produce effects similar to those seen with 4-repeat tau. In contrast to our observations related to shortening events, the increase compensated for the reduced activity of 3-repeat tau, causing a 30% decrease in the growth rate. These data indicate that there is an approximately threefold quantitative difference between the effects of 4- and 3-repeat tau on the growth rate.
When we compared the average length grown per growth event in tau-injected cells vs. buffer-injected cells, we found that tau strongly suppressed the average length that microtubules grew per growth event (Figure 4D, Table 1). 4-repeat tau caused a 57% decrease in the length grown at a 1:10 tau-to-tubulin molar ratio, reducing it from 2.8 μm per event to 1.2 μm. Injection of 3-repeat tau at the same molar ratio had a lesser but still significant effect, decreasing the average length grown by 34% to 1.8 μm per event. Increasing the level of 3-repeat tau to a tau-to-tubulin molar ratio of 1:3 resulted in a 45% decrease in average length grown. This increased effect with the injection of greater amounts of 3-repeat tau again indicates that the difference in effects on growth parameters between 3- and 4-repeat tau is quantitative. However, even at a 1:3 ratio of 3-repeat tau, we observed a smaller effect than was seen with 1:10 4-repeat tau, indicating that there is a greater than threefold difference in the effects of the two isoforms on the average length grown.
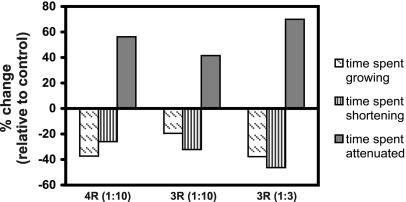
4- and 3-Repeat Tau Increased the Time Spent in an Attenuated State, Whereas Both Reduced the Time Spent Growing and Shortening
Microtubules divide their time among three phases: growth, shortening and attenuation (or pause). MAPs are thought to regulate microtubule stability in part by regulating how much time microtubules spend in each phase. To assess the effect of tau on the phase distribution, we calculated the percent time microtubules spent in each phase relative to the total time tracked. It is important to emphasize that these measurements are made only on the subset of microtubules that display growth and shortening dynamics. Since tau reduces the size of this subset, our assessment of the percentage of time spent in each phase under-estimates the stabilizing activity of tau. Not surprisingly, even among the subset of microtubules that were still dynamic, we found that tau had a dramatic stabilizing effect on the phase distribution (Figure 5 and Table 2). When we compared microtubules in cells injected with a 1:10 molar ratio of 4-repeat tau to buffer-injected control cells, we found that tau decreased the time spent growing by 37%, it decreased the time spent shortening by 26%, and it increased the time spent attenuated by 56%. The same ratio of 3-repeat tau had a lesser, but still impressive effect, decreasing the time spent growing and shortening by 19 and 32%, respectively, and increasing the time spent attenuated by 42%. Increasing the level of 3-repeat tau to a 1:3 molar ratio increased its effect on phase distribution beyond that of a 1:10 molar ratio of 4-repeat tau, indicating that there may be less than a threefold difference between 4- and 3-repeat tau for this parameter.
Figure 5.
Effects of 4- and 3-repeat tau on time spent growing, shortening and attenuated. The percentage of time microtubules spent growing (diagonal hatches), shortening (vertical stripes), or attenuated (solid gray) was measured in cells injected with 4- or 3-repeat tau. Values are graphed as percent differences from buffer controls [(experimental value - buffer control value)/buffer control value × 100]. Tau-to-tubulin molar ratios are presented in parentheses.
4- and 3-Repeat Tau Increased the Time Microtubules Spent Attenuated by Decreasing Growth and Shortening Rates, not by Changing Transition Frequencies
One mechanism by which the time spent attenuated could be increased is through reducing the catastrophe frequency or increasing the rescue frequency. In other words, tau might affect how long a microtubule grows or remains attenuated before it begins to shorten (defined as the catastrophe frequency) or conversely, tau might affect how long a microtubule shortens before it becomes attenuated or begins to grow (defined as the rescue frequency). We calculated the number of catastrophes and rescues over time and found that neither 4- nor 3-repeat tau had a significant effect on the frequency of catastrophe or rescue. We also calculated the number of catastrophes and rescues per length grown or shortened and found that the presence of tau induced small increases in the length-based frequencies of catastrophe and rescue (unpublished data). These increases are most likely a result of tau's effect on the growth and shortening rates; i.e., if rescues and catastrophes continue to occur at the same frequency over time in the presence of tau but microtubules grow and shorten more slowly, then it follows that a microtubule will grow or shorten less before the next event.
In addition, we found that the presence of tau induced only a small increase in the average attenuation duration, from 0.18 min in buffer control cells, to 0.22 and 0.21 min in cells injected with a 1:10 molar ratio of 4- or 3-repeat tau, respectively. However, these small effects on the attenuation duration are unlikely to explain the large effect tau has on the total time spent attenuated.
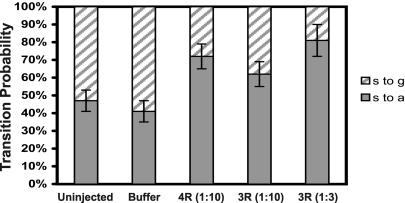
Another mechanism by which tau might increase the time microtubules spend attenuated is by decreasing growth and shortening rates to the extent that the microtubules appear attenuated, resulting in a shift from growth or shortening events to attenuation events. To examine this possibility, we calculated the number of growth, attenuation, and shortening events. We found that the number of attenuation events was increased by tau, whereas the number of growth events was greatly decreased. For example, in buffer-injected cells, a shortening microtubule transitioned to growth 59% of the time and became attenuated 41% of the time (Figure 6). Tau shifted this probability toward attenuation. In cells injected with a 1:10 molar ratio of 4-repeat tau, a shortening microtubule transitioned to a growth event only 28% of the time and became attenuated 72% of the time. Injection of 3-repeat tau at the same molar ratio had a similar but less marked effect, causing shortening microtubules to begin growing 38% of the time and to become attenuated 62% of the time. Increasing the amount of 3-repeat tau injected into the cells to a 1:3 molar ratio compensated for the difference between 4- and 3-repeat tau, indicating a threefold quantitative difference between the isoforms. These results agree well with our observations assessing the total time spent attenuated, indicating that the decrease in growth rate to the point where microtubules appear attenuated is likely to account for the majority of the increase in time spent attenuated observed in the presence of tau. Taken together, the data indicate that the primary effect of tau on dynamic instability is on the rate and extent of shortening and growth events. These, in turn, lead to increased attenuation, with little effect on the transition frequencies.
Figure 6.
Effects of 4- and 3-repeat tau on transitions from shortening to growth or attenuation. The probability that a shortening event would be followed by a growth event vs. an attenuation event was calculated from cells injected with buffer, GST, 4-repeat tau or 3-repeat tau. Probabilities of shortening to attenuation transitions (s to a; solid bars) and shortening to growth transitions (s to g; hatched bars) are presented ± SE. Tau to tubulin molar ratios are presented in parentheses.
4- and 3-Repeat Tau Decrease the Overall Dynamicity of Microtubules
Dynamicity is a general measure of the amount of dynamic instability occurring in a microtubule population. It is calculated from the total length grown and shortened over the time period observed and is an indicator of the amount of tubulin exchange taking place. We compared the dynamicity of dynamic microtubules in buffer-injected cells vs. tau-injected cells and found that tau greatly reduced the overall microtubule dynamicity (Table 1). A 1:10 molar ratio of 4-repeat tau reduced the dynamicity by 55%, from 11.7 to 5.2 μm/min. Injection of the same molar amount of 3-repeat tau decreased microtubule dynamicity by 38% to 7.3 μm/min. Injecting a 1:3 molar ratio of 3-repeat tau into cells compensated for the reduced effect of 3-repeat tau, decreasing dynamicity to a similar extent (57%) as seen with 4-repeat tau. These results indicate that there is an approximately three-fold quantitative difference between the effects of 4- and 3-repeat tau on dynamicity.
DISCUSSION
Using purified brain microtubules in vitro, we previously found that 4- and 3-repeat tau differentially regulate the dynamic instability behavior of microtubules (Panda et al., 2003). Although both isoforms suppressed microtubule growth to similar extents, 4-repeat but not 3-repeat tau strongly suppressed the rate and extent of microtubule shortening. Our goal here was to analyze the effects of these two tau isoforms on microtubule dynamic instability in living cells. The quantities of tau microinjected into cells in this study were designed to be physiologically relevant, based on previous quantification of tau levels in neuronal cells (Drubin et al., 1985). However, because it is technically extremely difficult at present to quantitatively assess the dynamic instability behavior of individual microtubules in neuronal cells, we turned to MCF7 breast tumor cells, an excellent living cell model in which robust dynamic instability behavior can be readily analyzed in the thin lamellar regions of the cells. An advantage of these cells is that they lack endogenous tau; thus, the effects of tau on dynamic instability can be determined in the absence of complications inherent to injecting exogenous tau upon a background of endogenous tau. Additionally, because phosphorylation of tau is believed to be an important regulatory mechanism, another advantage of this system is that we found that the injected, recombinant unphosphorylated tau has sufficient time to undergo phosphorylation by endogenous kinases (unpublished data). Regardless of cell type, our data demonstrate unequivocally that the two tau isoforms differentially affect microtubule stability within the complexity of a cellular environment. These results have implications both for the tau-induced neurodegeneration that occurs in FTDP-17 patients whose relative expression levels of 4- and 3-repeat tau differ from normal individuals and for normal neuronal development.
Tau Suppresses Microtubule Dynamics in Cells
We found that both 4- and 3-repeat tau produced strong stabilizing effects on microtubule dynamics, reducing the overall dynamicity by 55 and 38%, respectively (Table 1). Based on pharmacological investigations, changes of these magnitudes are very likely to significantly affect normal microtubule and cellular function. For frame-of-reference, a taxol-mediated reduction in microtubule dynamicity of only 30% severely inhibits normal microtubule function during mitosis (Yvon et al., 1999).
With respect to the effects of tau on individual parameters of microtubule dynamic instability, we found that 4-repeat but not 3-repeat tau strongly reduced the rate and extent of microtubule shortening. This difference is seen clearly at concentrations of 3- and 4-repeat tau that inhibit growth to the same extent; at these concentrations 3-repeat tau suppressed shortening much less strongly than 4-repeat tau. These results were similar to the in vitro results of Panda et al. (2003) and Trinczek et al. (1995). We also found that tau decreases the rate and extent of microtubule growth in cells. These data are in contrast to the widely recognized ability of tau to promote microtubule growth and assembly under non–steady state conditions, both in vitro (Cleveland et al., 1977; Fellous et al., 1977) and in cells (Caceres and Kosik, 1990; Knops et al., 1991; Esmaeli-Azad et al., 1994; reviewed in Buee et al., 2000). They are also in contrast to the tau-mediated enhancement of the rate and extent of microtubule growth observed in dynamics studies in vitro, also under non–steady state conditions of net microtubule assembly (Drechsel et al., 1992; Trinczek et al., 1995). However, consistent with our work, Panda et al. (1995, 2003) demonstrated that tau actually suppresses growth events in vitro under steady state conditions in which there is no net gain in microtubule mass. Arguably, it is these steady state conditions that are likely to most accurately reflect the conditions within cells that are not projecting processes or otherwise increasing their microtubule mass, including both our MCF-7 cells and mature neurons.
At steady state, the total number of tubulin subunits gained must equal the number of subunits lost. Therefore one possible mechanism underlying these changes in growth and shortening rates may be that a tau-mediated decrease in the microtubule-shortening rate indirectly leads to a decrease in microtubule growth. Another possibility is that the rates of microtubule shortening and growth are influenced by retrograde movement of microtubules resulting from association with actin filaments (Wittmann et al., 2003). However, because we are comparing the behavior of microtubules in buffer-injected control cells vs. tau-injected cells, we are only assaying tau-specific effects. If there were a tau-mediated increase in retrograde flow, it could explain our observed decrease in the microtubule growth rate, but it would also lead to an apparent increase in the microtubule shortening rate, which does not occur.
Our results and in vitro results differ in that tau had a much smaller effect on catastrophe and rescue frequencies in cells vs. in vitro. These differences are perhaps due to additional cellular regulatory factors that dampen the effect of tau on these parameters in cells but are absent from in vitro systems. Results similar to ours were also observed by Dhamodharan and Wadsworth (1995) using unpurified heat-stable MAPs.
In earlier work in transiently transfected cells, tau-mediated changes in microtubule growth and shortening rates were not observed (Kaech et al., 1996). However, dramatically different methodology (e.g., high levels of expression, the use of GFP-tau fusion protein constructs, days of exposure vs. 2 h of exposure to tau) makes comparison with our work difficult. In contrast, the cellular experiments of Dhamodharan and Wadsworth (1995), using unpurified heat-stable MAPs and similar methodology to our work, produced results similar to ours, including suppression of both growth and shortening rates and limited effects on transition frequencies.
4- and 3-Repeat Tau Differ in Their Abilities to Affect Dynamics
When comparing the effects of 4- and 3-repeat tau on microtubule dynamic instability, we found that 3-repeat tau had an approximately threefold weaker effect than 4-repeat tau on most parameters. At first glance, this quantitative difference seems to parallel the approximately threefold difference in their respective apparent binding affinities for microtubules (Gustke et al., 1994; Goode et al., 2000). However, using the estimated cellular concentrations of tau and tubulin (1.3 and 13 μM, respectively; see MATERIALS AND METHODS) and the binding constants of 4- and 3-repeat tau (0.15 and 0.46 μM, respectively; Goode et al., 2000), we calculate that >95% of both injected tau isoforms should be bound to microtubules. The data in Figure 1B are consistent with this prediction. Thus, approximately the same number of tau molecules are associated with microtubules in cells injected with either tau isoform. Therefore, the simplest interpretation of the data is that there are intrinsic difference(s) between the abilities of 3- and 4-repeat tau to modulate dynamics once bound to microtubules.
We also observed that injecting more 3-repeat tau compensated for its reduced effect on most parameters. This indicates that the microtubules are not saturated at a 1:10 tau-to-tubulin ratio and that higher levels of 3-repeat tau can suppress most parameters similarly to 4-repeat tau (a quantitative difference). The exception was the lack of effect of increased 3-repeat tau on shortening events. Although an unequivocal interpretation of this observation is not possible, it could be that 3-repeat tau actually has a negligible effect on the rate and length of shortening events. The observed effects, which are not significant at the 99% confidence level, may simply represent experimental scatter for this particular parameter. Indeed, Panda et al. (2003), found that 3-repeat tau had no effect on microtubule shortening at steady state in vitro. In any event, our data as well as complementary in vitro data (Trinczek et al., 1995; Panda et al., 2003) demonstrate that 3-repeat tau has a dramatically reduced ability to suppress microtubule shortening relative to 4-repeat tau.
Based on the foregoing observations, it follows that 3- and 4-repeat tau likely interact with microtubules in distinct manners, either at different sites or with different conformations. In light of this possibility, it is notable that the sequences encoded by exon 10, by which 3- and 4-repeat tau differ, contain the most potent microtubule binding and assembly sequences in the entire protein (Goode et al., 2000). Furthermore, it has been demonstrated that the carboxyl-terminus of tau plays a greater role in the binding of 3-repeat than 4-repeat tau to microtubules (Goode et al., 2000) and that a 4-repeat specific peptide competes with 4-repeat but not 3-repeat tau in microtubule binding (Goode and Feinstein, 1994). In addition, the fact that microtubules behave differently in the presence of the two isoforms indicates that tubulin may adopt different conformations upon binding different tau isoforms.
These structure-function differences have important implications for both normal development and the FTDP-17 RNA-splicing mutations. In fetal brain, only 3-repeat tau is expressed, suggesting that microtubules in fetal neurons may be less stable and more prone to shortening compared with microtubules in adult neurons. Increased shortening could be important during axonal outgrowth, pathfinding, and synaptogenesis. With respect to the FTDP-17 tau RNA-splicing mutations, which can either increase or decrease the ratio of 4- to 3-repeat tau (Clark et al., 1998; Hutton et al., 1998; Spillantini et al., 1998; D'Souza et al., 1999), our data indicate that these mutations should either overstabilize or understabilize microtubules, both of which can impede microtubule function (see below for more details, also Yvon et al., 1999; Goncalves et al., 2001; Jordan, 2002). Lu and Kosik (2001) demonstrated that 4-repeat tau may displace 3-repeat tau on microtubules when coexpressed at high levels in cells. If this displacement occurs in vivo, then the ratio of tau isoforms bound to microtubules could be further skewed, leading to even greater alterations in microtubule dynamics. This loss of normal microtubule regulation and function may be a mechanism through which the FTDP-17 tau RNA-splicing mutations result in neurodegeneration.
How Might Tau Dysfunction Cause Neurodegeneration?
On the basis of these data, we propose that tau-mediated cell death in the FTDP-17 tau splicing mutations correlates with altered microtubule dynamics. Consistent with this model, we have recently examined the effects of several FTDP-17 tau amino-acid substitution mutations on microtubule dynamics in cells and found that most of the tau mutants exhibit altered abilities to regulate microtubule dynamics, i.e., the microtubules are understabilized (Bunker, Wilson, Jordan, and Feinstein, unpublished data). Taken together with recent in vitro data (Panda et al., 2003), these data lead us to propose that there is a window of acceptable microtubule dynamic behavior in neurons, outside of which microtubules cannot function normally and cells cannot survive. In this loss-of-function, haplo-insufficiency model, the normal ability of tau to properly regulate microtubule dynamics is lost, leading to disrupted microtubule function and subsequent cell death.
Several pharmacological studies support our model. Treatment of cells with taxol at levels causing a modest overstabilization of microtubule dynamics leads to mitotic block and apoptosis (Jordan et al., 1996; Yvon et al., 1999). At the other extreme, taxol-resistant/dependent cell lines undergo mitotic block in the absence of taxol (Goncalves et al., 2001). Analysis of the dynamic behavior of the microtubules in these cells revealed overly dynamic behavior, correlating overactive microtubules with mitotic block, which in turn can result in cell death (Jordan et al., 1996; Goncalves et al., 2001). Taken together, these studies indicate that both overstabilization and understabilization of microtubules can lead to cell death.
What microtubule-dependent function, when disrupted, might lead to cell death in postmitotic neurons? One strong possibility is axonal transport. Disruption of axonal transport can lead to neuronal cell death in mice (LaMonte et al., 2002) and Drosophila (Gunawardena and Goldstein, 2001; Gunawardena et al., 2003). Although the underlying mechanism connecting microtubule dynamics to axonal transport is unclear, low concentrations of taxol have been shown to compromise axonal transport (Nakata and Hirokawa, 2003). At the same time, other work suggests that increased levels of tau may interfere with axonal transport via a competition between tau and motor proteins (Trinczek et al., 1999; Stamer et al., 2002; Mandelkow et al., 2003). Another mutually non-exclusive possibility is that tau dysfunction/deregulation causes altered microtubule dynamics in glial cells, leading to glial activation, which can be cytotoxic to neurons (Bamberger and Landreth, 2002). In fact, recent work has shown that overexpression of tau in astrocytes leads to disruption of the cytoskeleton, abnormal trafficking, and eventually cell death (Yoshiyama et al., 2003). From a more general perspective, several studies have linked changes in microtubule behavior to apoptotic signaling pathways (Srivastava et al., 1998; Giannakakou et al., 2001; Michaelis et al., 2002), suggesting that microtubules may serve as “biosensors” for cellular well being. Thus, loss of normal regulation of microtubule dynamics could have deleterious effects on cell viability via multiple possible mechanisms.
Supplementary Material
Acknowledgments
We are extremely grateful to Kathy Kamath and Herb Miller for technical assistance with microscopy and data analysis and to Herb Waite for performing the mass spectrometry. We also thank Allen Stewart-Oaten and Carol Vandenberg for valuable discussions as well as Dmitri Leonoudakis and Sasha Levy for comments on the manuscript. This research was supported by National Institutes of Health Grants NS35010 (S.C.F.), NS13560 (L.W.) and CA57291 (M.A.J.).
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E04-01-0062. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E04-01-0062.
Online version of this article contains supporting material. Online version is available at www.molbiolcell.org.
References
- Bamberger, M.E., and Landreth, G.E. (2002). Inflammation, apoptosis, and Alzheimer's disease. Neuroscientist 8, 276-283. [DOI] [PubMed] [Google Scholar]
- Barghorn, S., and Mandelkow, E. (2002). Toward a unified scheme for the aggregation of tau into Alzheimer paired helical filaments. Biochemistry 41, 14885-14896. [DOI] [PubMed] [Google Scholar]
- Buee, L., Bussiere, T., Buee-Scherrer, V., Delacourte, A., and Hof, P.R. (2000). Tau protein isoforms, phosphorylation and role in neurodegenerative disorders. Brain Res Brain Res Rev 33, 95-130. [DOI] [PubMed] [Google Scholar]
- Butner, K.A., and Kirschner, M.W. (1991). Tau protein binds to microtubules through a flexible array of distributed weak sites. J. Cell Biol. 115, 717-730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caceres, A., and Kosik, K.S. (1990). Inhibition of neurite polarity by tau antisense oligonucleotides in primary cerebellar neurons. Nature 343, 461-463. [DOI] [PubMed] [Google Scholar]
- Clark, L.N. et al. (1998). Pathogenic implications of mutations in the tau gene in pallido-ponto-nigral degeneration and related neurodegenerative disorders linked to chromosome 17. Proc. Natl. Acad. Sci. USA 95, 13103-13107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland, D.W., Hwo, S.Y., and Kirschner, M.W. (1977). Physical and chemical properties of purified tau factor and the role of tau in microtubule assembly. J. Mol. Biol. 116, 227-247. [DOI] [PubMed] [Google Scholar]
- Dhamodharan, R., and Wadsworth, P. (1995). Modulation of microtubule dynamic instability in vivo by brain microtubule associated proteins. J. Cell Sci. 108(Pt 4), 1679-1689. [DOI] [PubMed] [Google Scholar]
- Drechsel, D.N., Hyman, A.A., Cobb, M.H., and Kirschner, M.W. (1992). Modulation of the dynamic instability of tubulin assembly by the microtubule-associated protein tau. Mol. Biol. Cell 3, 1141-1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drubin, D.G., Feinstein, S.C., Shooter, E.M., and Kirschner, M.W. (1985). Nerve growth factor-induced neurite outgrowth in PC12 cells involves the coordinate induction of microtubule assembly and assembly-promoting factors. J. Mol. Biol. 101, 1799-1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Souza, I., Poorkaj, P., Hong, M., Nochlin, D., Lee, V.M., Bird, T.D., and Schellenberg, G.D. (1999). Missense and silent tau gene mutations cause frontotemporal dementia with parkinsonism-chromosome 17 type, by affecting multiple alternative RNA splicing regulatory elements. Proc. Natl. Acad. Sci. USA 96, 5598-5603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esmaeli-Azad, B., McCarty, J.H., and Feinstein, S.C. (1994). Sense and antisense transfection analysis of tau function: tau influences net microtubule assembly, neurite outgrowth and neuritic stability. J. Cell Sci. 107(Pt 4), 869-879. [DOI] [PubMed] [Google Scholar]
- Fellous, A., Francon, J., Lennon, A.M., and Nunez, J. (1977). Microtubule assembly in vitro. Purification of assembly-promoting factors. Eur. J. Biochem. 78, 167-174. [DOI] [PubMed] [Google Scholar]
- Gamblin, T.C., Berry, R.W., and Binder, L.I. (2003). Modeling tau polymerization in vitro: a review and synthesis. Biochemistry 42, 15009-15017. [DOI] [PubMed] [Google Scholar]
- Gamblin, T.C., King, M.E., Dawson, H., Vitek, M.P., Kuret, J., Berry, R.W., and Binder, L.I. (2000). In vitro polymerization of tau protein monitored by laser light scattering: method and application to the study of FTDP-17 mutants. Biochemistry 39, 6136-6144. [DOI] [PubMed] [Google Scholar]
- Giannakakou, P., Robey, R., Fojo, T., and Blagosklonny, M.V. (2001). Low concentrations of paclitaxel induce cell type-dependent p53, p21 and G1/G2 arrest instead of mitotic arrest: molecular determinants of paclitaxel-induced cytotoxicity. Oncogene 20, 3806-3813. [DOI] [PubMed] [Google Scholar]
- Goedert, M. et al. (1999). Tau gene mutation in familial progressive subcortical gliosis. Nat Med 5, 454-457. [DOI] [PubMed] [Google Scholar]
- Goedert, M., Spillantini, M.G., Jakes, R., Rutherford, D., and Crowther, R.A. (1989a). Multiple isoforms of human microtubule-associated protein tau: sequences and localization in neurofibrillary tangles of Alzheimer's disease. Neuron 3, 519-526. [DOI] [PubMed] [Google Scholar]
- Goedert, M., Spillantini, M.G., Potier, M.C., Ulrich, J., and Crowther, R.A. (1989b). Cloning and sequencing of the cDNA encoding an isoform of microtubule-associated protein tau containing four tandem repeats: differential expression of tau protein mRNAs in human brain. EMBO J. 8, 393-399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goncalves, A., Braguer, D., Kamath, K., Martello, L., Briand, C., Horwitz, S., Wilson, L., and Jordan, M.A. (2001). Resistance to Taxol in lung cancer cells associated with increased microtubule dynamics. Proc. Natl. Acad. Sci. USA 98, 11737-11742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goode, B.L., Chau, M., Denis, P.E., and Feinstein, S.C. (2000). Structural and functional differences between 3-repeat and 4-repeat tau isoforms. Implications for normal tau function and the onset of neurodegenetative disease. J. Biol. Chem. 275, 38182-38189. [DOI] [PubMed] [Google Scholar]
- Goode, B.L., Denis, P.E., Panda, D., Radeke, M.J., Miller, H.P., Wilson, L., and Feinstein, S.C. (1997). Functional interactions between the proline-rich and repeat regions of tau enhance microtubule binding and assembly. Mol. Biol. Cell 8, 353-365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goode, B.L., and Feinstein, S.C. (1994). Identification of a novel microtubule binding and assembly domain in the developmentally regulated inter-repeat region of tau. J. Cell Biol. 124, 769-782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunawardena, S., and Goldstein, L.S. (2001). Disruption of axonal transport and neuronal viability by amyloid precursor protein mutations in Drosophila. Neuron 32, 389-401. [DOI] [PubMed] [Google Scholar]
- Gunawardena, S., Her, L.S., Brusch, R.G., Laymon, R.A., Niesman, I.R., Gordesky-Gold, B., Sintasath, L., Bonini, N.M., and Goldstein, L.S. (2003). Disruption of axonal transport by loss of huntingtin or expression of pathogenic polyQ proteins in Drosophila. Neuron 40, 25-40. [DOI] [PubMed] [Google Scholar]
- Gustke, N., Trinczek, B., Biernat, J., Mandelkow, E.M., and Mandelkow, E. (1994). Domains of tau protein and interactions with microtubules. Biochemistry 33, 9511-9522. [DOI] [PubMed] [Google Scholar]
- Hiller, G., and Weber, K. (1978). Radioimmunoassay for tubulin: a quantitative comparison of the tubulin content of different established tissue culture cells and tissues. Cell 14, 795-804. [DOI] [PubMed] [Google Scholar]
- Himmler, A. (1989). Structure of the bovine tau gene: alternatively spliced transcripts generate a protein family. Mol. Cell. Biol. 9, 1389-1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himmler, A., Drechsel, D., Kirschner, M.W., and Martin, D.W., Jr. (1989). Tau consists of a set of proteins with repeated C-terminal microtubule-binding domains and variable N-terminal domains. Mol. Cell. Biol. 9, 1381-1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong, M. et al. (1998). Mutation-specific functional impairments in distinct tau isoforms of hereditary FTDP-17. Science 282, 1914-1917. [DOI] [PubMed] [Google Scholar]
- Hutton, M. et al. (1998). Association of missense and 5′-splice-site mutations in tau with the inherited dementia FTDP-17. Nature 393, 702-705. [DOI] [PubMed] [Google Scholar]
- Jordan, M.A. (2002). Mechanism of action of antitumor drugs that interact with microtubules and tubulin. Curr. Med. Chem. Anti-Cancer Agents 2, 1-17. [DOI] [PubMed] [Google Scholar]
- Jordan, M.A., Wendell, K., Gardiner, S., Derry, W.B., Copp, H., and Wilson, L. (1996). Mitotic block induced in HeLa cells by low concentrations of paclitaxel (Taxol) results in abnormal mitotic exit and apoptotic cell death. Cancer Res. 56, 816-825. [PubMed] [Google Scholar]
- Kaech, S., Ludin, B., and Matus, A. (1996). Cytoskeletal plasticity in cells expressing neuronal microtubule-associated proteins. Neuron 17, 1189-1199. [DOI] [PubMed] [Google Scholar]
- Knops, J., Kosik, K.S., Lee, G., Pardee, J.D., Cohen-Gould, L., and McConlogue, L. (1991). Overexpression of tau in a nonneuronal cell induces long cellular processes. J. Cell Biol. 114, 725-733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosik, K.S., Orecchio, L.D., Bakalis, S., and Neve, R.L. (1989). Developmentally regulated expression of specific tau sequences. Neuron 2, 1389-1397. [DOI] [PubMed] [Google Scholar]
- Lamb, N.J., and Fernandez, A. (1997). Microinjection of antibodies into mammalian cells. Methods Enzymol. 283, 72-83. [DOI] [PubMed] [Google Scholar]
- LaMonte, B.H., Wallace, K.E., Holloway, B.A., Shelly, S.S., Ascano, J., Tokito, M., Van Winkle, T., Howland, D.S., and Holzbaur, E.L. (2002). Disruption of dynein/dynactin inhibits axonal transport in motor neurons causing late-onset progressive degeneration. Neuron 34, 715-727. [DOI] [PubMed] [Google Scholar]
- Lee, G., Cowan, N., and Kirschner, M. (1988). The primary structure and heterogeneity of tau protein from mouse brain. Science 239, 285-288. [DOI] [PubMed] [Google Scholar]
- LoPresti, P., Szuchet, S., Papasozomenos, S.C., Zinkowski, R.P., and Binder, L.I. (1995). Functional implications for the microtubule-associated protein tau: localization in oligodendrocytes. Proc. Natl. Acad. Sci. USA 92, 10369-10373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandelkow, E.M., Stamer, K., Vogel, R., Thies, E., and Mandelkow, E. (2003). Clogging of axons by tau, inhibition of axonal traffic and starvation of synapses. Neurobiol. Aging 24, 1079-1085. [DOI] [PubMed] [Google Scholar]
- Michaelis, M.L., Dobrowsky, R.T., and Li, G. (2002). Tau neurofibrillary pathology and microtubule stability. J. Mol. Neurosci. 19, 289-293. [DOI] [PubMed] [Google Scholar]
- Nakata, T., and Hirokawa, N. (2003). Microtubules provide directional cues for polarized axonal transport through interaction with kinesin motor head. J. Cell Biol. 162, 1045-1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panda, D., Goode, B.L., Feinstein, S.C., and Wilson, L. (1995). Kinetic stabilization of microtubule dynamics at steady state by tau and microtubule-binding domains of tau. Biochemistry 34, 11117-11127. [DOI] [PubMed] [Google Scholar]
- Panda, D., Miller, H.P., and Wilson, L. (1999). Rapid treadmilling of brain microtubules free of microtubule-associated proteins in vitro and its suppression by tau. Proc. Natl. Acad. Sci. USA 96, 12459-12464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panda, D., Samuel, J.C., Massie, M., Feinstein, S.C., and Wilson, L. (2003). Differential regulation of microtubule dynamics by three- and four-repeat tau: implications for the onset of neurodegenerative disease. Proc. Natl. Acad. Sci. USA 100, 9548-9553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose, G.G., Pomerat, C.M., Shindler, T., and Trunnell, J. (1958). A cellophane strip technique for culturing tissue in multipurpose culture chambers. J. Biophys. Biochem. Cytol. 4, 761-764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spillantini, M.G., Crowther, R.A., Kamphorst, W., Heutink, P., and van Swieten, J.C. (1998). Tau pathology in two Dutch families with mutations in the microtubule-binding region of tau. Am. J. Pathol. 153, 1359-1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava, R.K., Srivastava, A.R., Korsmeyer, S.J., Nesterova, M., Cho-Chung, Y.S., and Longo, D.L. (1998). Involvement of microtubules in the regulation of Bcl2 phosphorylation and apoptosis through cyclic AMP-dependent protein kinase. Mol. Cell. Biol. 18, 3509-3517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamer, K., Vogel, R., Thies, E., Mandelkow, E., and Mandelkow, E.M. (2002). Tau blocks traffic of organelles, neurofilaments, and APP vesicles in neurons and enhances oxidative stress. J. Cell Biol. 156, 1051-1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinczek, B., Biernat, J., Baumann, K., Mandelkow, E.M., and Mandelkow, E. (1995). Domains of tau protein, differential phosphorylation, and dynamic instability of microtubules. Mol. Biol. Cell 6, 1887-1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinczek, B., Ebneth, A., Mandelkow, E.M., and Mandelkow, E. (1999). Tau regulates the attachment/detachment but not the speed of motors in microtubule-dependent transport of single vesicles and organelles. J. Cell Sci. 112(Pt 14), 2355-2367. [DOI] [PubMed] [Google Scholar]
- Walker, R.A., O'Brien, E.T., Pryer, N.K., Soboeiro, M.F., Voter, W.A., Erickson, H.P., and Salmon, E.D. (1988). Dynamic instability of individual microtubules analyzed by video light microscopy: rate constants and transition frequencies. J. Cell Biol. 107, 1437-1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittmann, T., Bokoch, G.M., and Waterman-Storer, C.M. (2003). Regulation of leading edge microtubule and actin dynamics downstream of Rac1. J. Cell Biol. 161, 845-851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshiyama, Y., Zhang, B., Bruce, J., Trojanowski, J.Q., and Lee, V.M. (2003). Reduction of detyrosinated microtubules and golgi fragmentation are linked to tau-induced degeneration in astrocytes. J. Neurosci. 23, 10662-10671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yvon, A.M., Wadsworth, P., and Jordan, M.A. (1999). Taxol suppresses dynamics of individual microtubules in living human tumor cells. Mol. Biol. Cell 10, 947-959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai, Y., and Borisy, G.G. (1994). Quantitative determination of the proportion of microtubule polymer present during the mitosis-interphase transition. J. Cell Sci. 107(Pt 4), 881-890. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.