Abstract
A series of sixteen bisphenylureas based on a 2,6-bis(2-anilinoethynyl)pyridine scaffold have been synthesized for use as potential anion sensors. Prior work with one of these receptors revealed a distinct fluorescence response in the presence of a suitable anion source with specificity towards chloride anion. This study demonstrates that the fluorescent properties of these receptors can be tuned through the systematic variation of the pendant functional groups.
Introduction
Supramolecular anion sensors have received considerable attention in recent years, specifically those displaying a colorimetric and/or fluorescence response in the presence of specific anions.1–7 A typical design motif for these sensors takes advantage of non-covalent interactions such as electrostatics,8–10 hydrogen bonding,11–13 anion-π,14–16 and hydrophilicity/hydrophobicity. 17–20 To obtain a colorimetric and/or fluorescent outcome, a fluorophore unit is often appended to the sensor scaffold, which changes its fluorescent response upon a binding event; however, the pendant fluorophores are often bulky and exhibit a limited range of responses.21 An alternative approach would be to functionalize a fluorophore backbone with typical anion binding motifs.22–24 This would eliminate the need for a receptor to have a pendant fluorophore and could allow for greater tunability of both fluorescence response and binding strength.
The 2,6-bis(arylethynyl)pyridine scaffold has found utility in numerous areas of chemistry. The inherent properties of such ethynylpyridines (conjugation, absorption/emission, pH-dependent speciation, metal binding capability, rigidity, etc.),25–27 have been exploited for applications such as liquid crystals,28 lightemitting materials,29 rotaxane-type structures,30 molecular magnets,31 antiangiogenic activity,32 polymer composites33 and coordination complexes.24,34,35 In contrast, the supramolecular chemistry of 2,6-bis(arylethynyl)pyridines has received little attention, perhaps in part due to the scarcity of uniting the fields of molecule/ion recognition with the synthesis of highly-conjugated, carbon-rich materials. Most of the host/guest studies reported have focused on exploiting the pyridine lone pair to bind metal ions22 or organoiodides.3 Alternatively, we hypothesized that the unique absorption/emission properties in tandem with the structural rigidity of (arylethynyl)pyridines aptly positions them to function as small molecule or ion receptors. Moreover, we envisioned that the 2,6-bis(arylethynyl)pyridine scaffold and its derivatives would serve as a versatile building block for the development of receptor molecules that target a variety of guests depending on the protonation state of the pyridine and the style of functional group appended to the arylethynyl unit.36
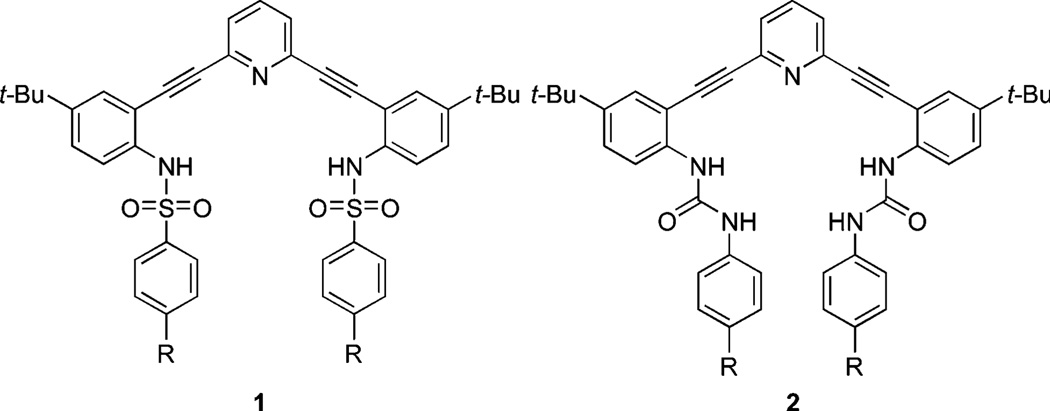
Our initial studies focused on sulfonamide derivatives of 2,6-bis(2-aniloethynyl)pyridine to form an anion binding cavity (e.g., 1, Fig. 1). To our delight, this class of receptors showed the remarkable propensity of forming 2 + 2 dimers with either water, halides or both depending on the protonation state of the receptor.37 Subsequent studies examined urea derivatives such as 2 (Fig. 1), which possessed much simpler solution speciation with halides, as the two urea binding groups obviate the need for higher order aggregates to form to satisfy the anion’s hydrogen bonding preference. Crystallographic data indeed showed that the urea receptors formed a 1 : 1 complex with chloride starting from TFA-protonated 2 (R = H).35 More importantly, the urea derivatives exhibited switchable fluorescent and colorimetric responses upon protonation. For example, the p-methoxy derivative of 2 displayed an “on-off” fluorescence behavior in the presence of chloride, while the p-nitro derivative exhibited an “off-on” fluorescence behavior in organic solvents.34 The magnitude of the fluorescence event was dictated by the anion, resulting in a rare, fully organic “turn-on” fluorescent sensor for chloride, which typically quenches fluorescence. While the cause of this behavior is still under investigation, it would be desirable to tune these systems so that the “turn on” behavior could be observed even in competitive solvents such as DMSO or water. To achieve this goal we describe herein the synthesis of a small library of sixteen different 2,6-bis(2-aniloethynyl)pyridine bisureas (2–5/a–d). We also report an exhaustive study of their absorption and emission profiles in CH3CN, and ultimately show that a similar “turn-on” behaviour can be observed even in relatively polar solvents.
Fig. 1.
Sulfonamide-based (1) and urea-based (2) anion sensors.
Results and discussion
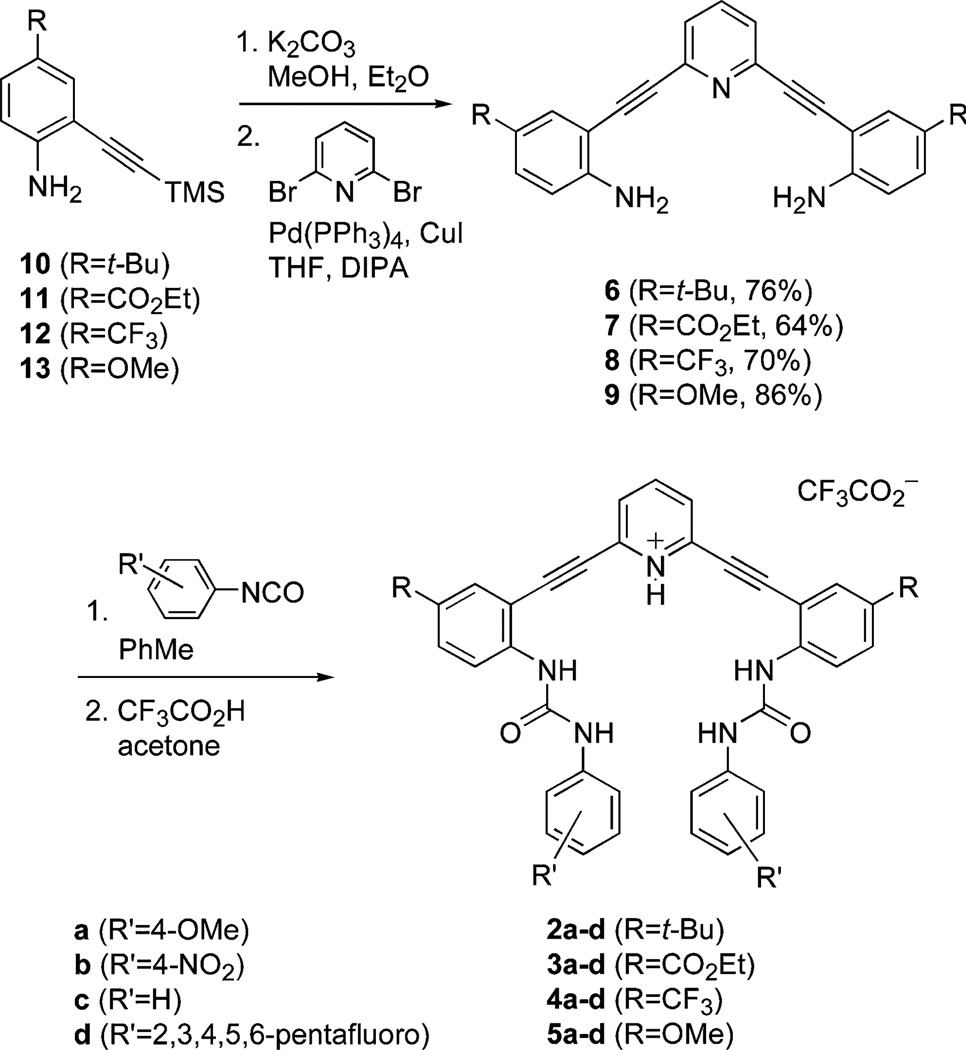
To obtain a full range of electron-poor and electron-rich scaffolds, we examined 2,6-bis(2-aniloethynyl)pyridine derivatives functionalized with tert-butyl (6), carboethoxy (7), trifluoromethyl (8), and methoxy (9) groups located at the 4-position on the aniline rings, to be consistent with our previous studies. Each derivative was synthesized via a twofold Sonogashira cross-coupling reaction between the respective 4-functionalized-2-ethynylanilines with 2,6-dibromopyridine as shown in Scheme 1; thus, protodesilylation of known anilines 10–1338,39 in basic MeOH (EtOH for 11) followed by cross-coupling afforded the 2,6-bis(2-aniloethynyl)pyridine cores 6–9 in good to excellent yield. Each of these derivatives was then reacted with 4-methoxyphenyl isocyanate (a), 4-nitrophenyl isocyanate (b), phenyl isocyanate (c) and pentafluorophenyl isocyanate (d) to furnish the sixteen bisureas 2–5/a–d.
Scheme 1.
Synthesis of sixteen differentially substituted 2,6-ethynylpyridine bisphenylurea scaffolds.
Interestingly, the purification and characterization of some of these compounds proved problematic. First, the yields for reactions with 7 and 8 were in general considerably lower than those of their electron-rich counterparts, 6 and 9 (Table 1). This was anticipated since the aniline nitrogens on 7 and 8 should be less nucleophilic due to the presence of the para-substituted electron-withdrawing groups. However, these bisureas were accompanied by myriad side products, as revealed by TLC analysis. In the case of 4d, a small amount of a 2-quinazolinone derivative was isolated along with the desired bisurea; the X-ray crystal structure of this unanticipated material and discussion of its formation are given in the Supporting Information†.
Table 1.
Yields for 2–5/a–d
| R | R′ | Yield | |
|---|---|---|---|
| 2a | t-Bu | 4-OMe | 93% |
| 2b | t-Bu | 4-NO2 | 93% |
| 2c | t-Bu | H | 92% |
| 2d | t-Bu | 2,3,4,5,6-pentafluoro | 81% |
| 3a | CO2Et | 4-OMe | 57% |
| 3b | CO2Et | 4-NO2 | 30% |
| 3c | CO2Et | H | 26% |
| 3d | CO2Et | 2,3,4,5,6-pentafluoro | 76% |
| 4a | CF3 | 4-OMe | 16% |
| 4b | CF3 | 4-NO2 | 34% |
| 4c | CF3 | H | 37% |
| 4d | CF3 | 2,3,4,5,6-pentafluoro | 75% |
| 5a | OMe | 4-OMe | 64% |
| 5b | OMe | 4-NO2 | 86% |
| 5c | OMe | H | 61% |
| 5d | OMe | 2,3,4,5,6-pentafluoro | 71% |
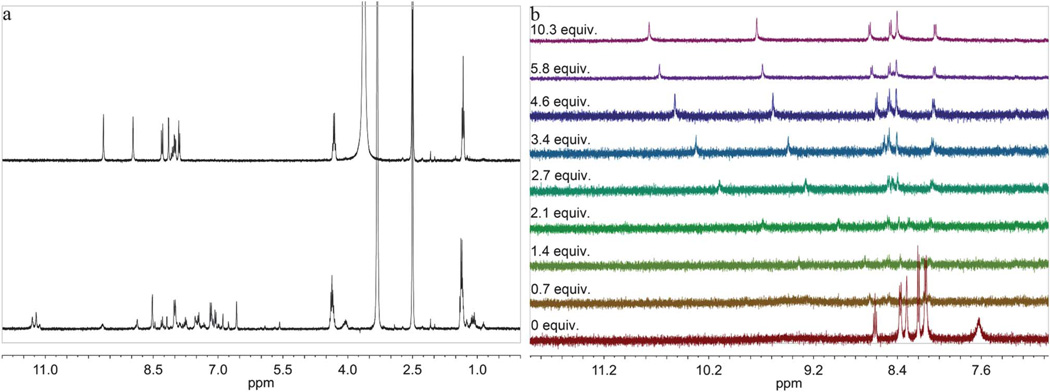
Second, the 1H NMR spectra of certain bisureas were unexpectedly complex. For example, the proton spectrum of 3d (which appears as one spot by thin layer chromatography) is shown on the bottom of Fig. 2a; however, when this same compound is prepared as the TFA salt, the spectrum simplifies to afford the anticipated set of resonances (Fig. 2a top). Interestingly, when TFA is added to 3d while in solution, the 1H NMR spectrum does not resolve, suggesting the presence of a kinetically stable aggregate. This behaviour was observed more often in scaffolds containing electron-withdrawing units at R and/or R′, which is an indication that the urea pKas are lowered, leading to increased hydrogen bond donor ability. Clearly, protonation and addition of an anionic guest to 3d breaks up the self-association/ aggregation, leading to the simplified 1H NMR spectrum; therefore, compounds 2–5/a–d were isolated as their corresponding trifluoroacetic acid (TFA) salts.
Fig. 2.
(a) 1H NMR spectrum of the 3d·TFA salt (top) in DMSO-d6 compared to the 1H NMR of 3d in its neutral state (bottom). When TFA is added to 3d while in solution the 1H NMR spectrum does not resolve, suggesting the presence of a kinetically stable aggregate. (b) 1H NMR titration of 3d with Bu4NCl in CD3CN. The 1H NMR titration shows resolved peaks prior to addition of Bu4NCl, but upon addition of ca. one equivalent Bu4NCl the sample forms insoluble aggregates. Upon addition of excess Bu4NCl the aggregation appears to dissipate and the 1H NMR spectra resolve.
Another unusual problem was encountered while attempting to determine binding constants and stoichiometries of some of the bisureas with various tetrabutylammonium salts. For example, the 1H NMR titration of 3d with tetrabutylammonium chloride is shown in Fig. 2b. During the course of the titration the 1H NMR spectra go from sharp and resolved prior to the addition of chloride to completely obscured at approximately one equiv. of chloride. At the half equivalent point visible aggregate is present in the NMR tube, but remarkably, upon addition of excess chloride the host goes back into solution and the 1H NMR spectra resolve.
The most likely explanation for the observed phenomena is an equilibrium involving aggregation in solution. This behavior was observed more often in scaffolds containing electron-withdrawing units at R and/or R′, which is an indication that when the urea pKas are lowered, hydrogen bond donor ability increases and aggregation increases. This phenomenon prevented the accurate determination of binding constants for this class of sensors, but it can clearly be seen that there is a strong propensity for these molecules to form complexes/aggregates in the presence of a suitable anionic guest.
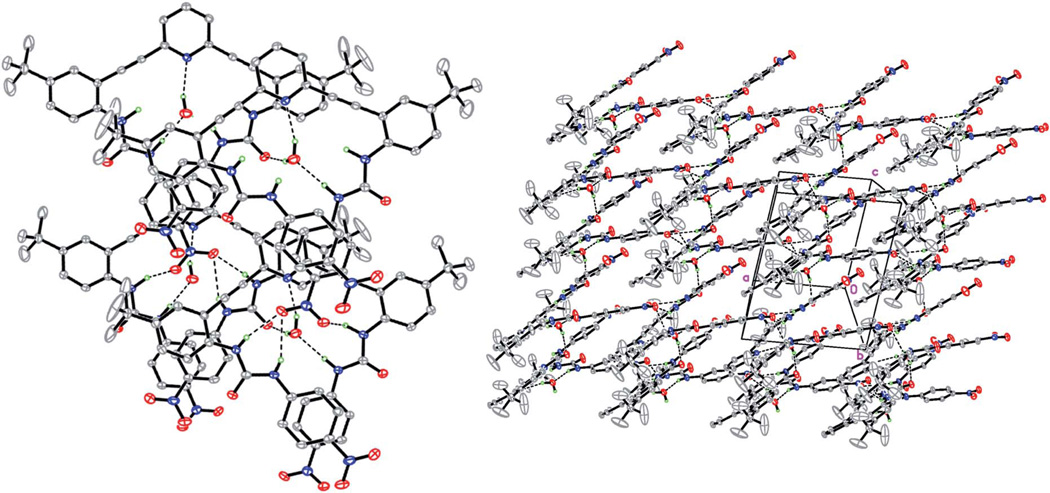
This hypothesized self-aggregation in solution can also be observed in the X-ray crystal structure of 2b.40 In the solid-state a solvent water molecule resides inside the molecular cavity of 2b, forming two solvent Ow–H…N, N–H…Ow hydrogen bonds and one bridging intermolecular Ow–H…O H-bond (Fig. 3 left). The molecules are also joined together by three N–H…O H-bonds between the −NO2 group in one molecule and NH groups in another one. In the crystal structure the molecules assemble into 1-D molecular strips that form layers perpendicular to the b axis (Fig. 3 right). Even in the case of such a complex molecular shape, all H atoms that can form H-bonds are involved in intra-and intermolecular H-bonds in the crystal structure, providing another example of the Hamilton rule.41
Fig. 3.
X-ray crystal structure of 2b–top view (left) and side view (right); ellipsoids drawn at the 30% probability level.
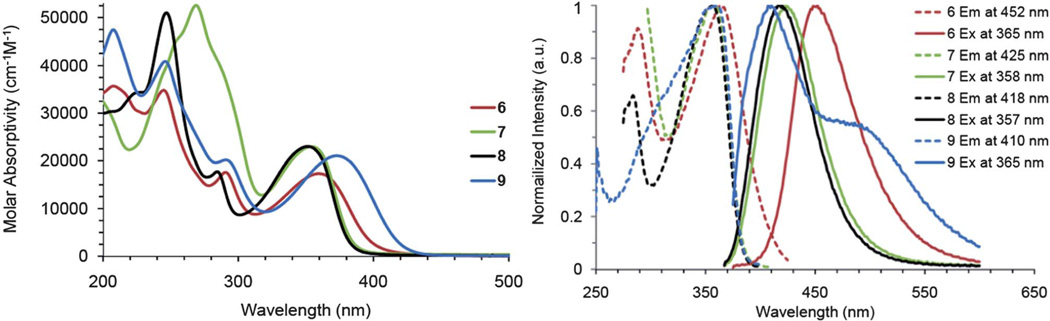
With the library of compounds now in hand, we examined the absorption and emission properties of the bisureas as well as those of the scaffold cores. Given that an ideal anion sensor is one that is capable of functioning in polar media, such as DMSO, H2O or CH3CN, all UV-vis and fluorescence studies were performed in CH3CN solutions. Fig. 4 shows the absorption spectra for the bis(arylethynyl)pyridines 6–9. Depending on the electronic nature of the substituents attached to these cores a red or blue shift in absorption is observed. For example, the trifluoromethyl substituted core 8 has a blue-shifted absorbance compared to the tert-butyl substituted core 6, while the methoxy substituted core 9 is red-shifted. This same red and blue shifting behavior is seen in the corresponding emission spectra (Fig. 4). The electron-withdrawn cores are blue shifted and the electron rich cores are red shifted. One interesting phenomenon occurs in the case of the methoxy-substituted core 9. It contains two distinct fluorescence bands, which are likely the result of dual emission from one species in solution.
Fig. 4.
Electronic absorption (left) and excitation/emission (right) spectra of ethynylpyridine cores 6–9.
The photoluminescence quantum yield (PLQY) for each of the four ethynylpyridines followed the trend in which the most electron rich cores had the lowest PLQY and the most electron poor cores had the highest PLQY (Table 2).
Table 2.
PLQY and Stokes Shifts for 6–9 in MeCN
| R | Stokes shift (cm−1) | Quantum yield | |
|---|---|---|---|
| 6 | t-Bu | 5270 | 0.6% |
| 7 | CO2Et | 4290 | 1.2% |
| 8 | CF3 | 4090 | 1.1% |
| 9 | OMe | 2890, 3150 | 0.1% |
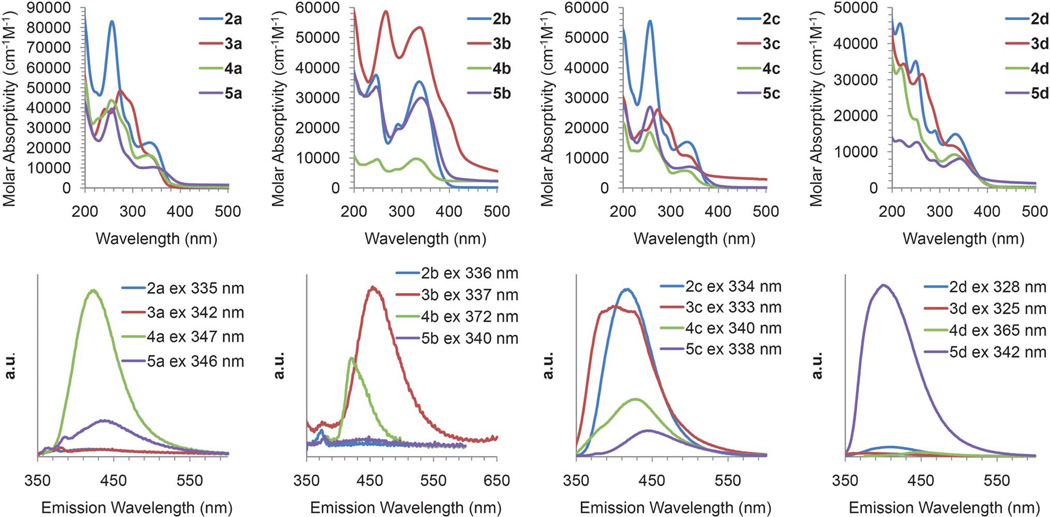
The neutral bisureas of each core were investigated in a similar manner. Fig. 5 shows the UV-vis spectra for the tert-butyl derivatives 2a–d. Interestingly, the substituted phenyl ureas had virtually no effect on the resulting UV-vis λmax indicating that the ethynylpyridine cores are primarily responsible for the observed adsorption behavior of the bisurea scaffolds.
Fig. 5.
Electronic absorption spectra of 2a–5a (top left), 2b–5b (top middle-left), 2c–5c (top middle-right), and 2d–5d (top right) followed by fluorescence spectra of 2a–5a (bottom left), 2b–5b (bottom middle-left), 2c–5c (bottom middle-right), and 2d–5d (bottom right).
The neutral bisurea scaffolds were also investigated with respect to their fluorescence, with careful attention paid to those scaffolds containing electron withdrawing R and R′ groups (e.g. 3b, 3d, 4b, and 4d). Given that an ideal sensor is one that would go from an “off” state to an “on” state, we were primarily interested in electron withdrawing scaffolds that were nonemissive in their unbound state. Based on these criteria, compound 3d was further scrutinized with respect to its ability to sense chloride, whereas 4d was not studied further due to its tendency to decompose into the 2-quinazolinone byproduct described in the Supporting Information†.
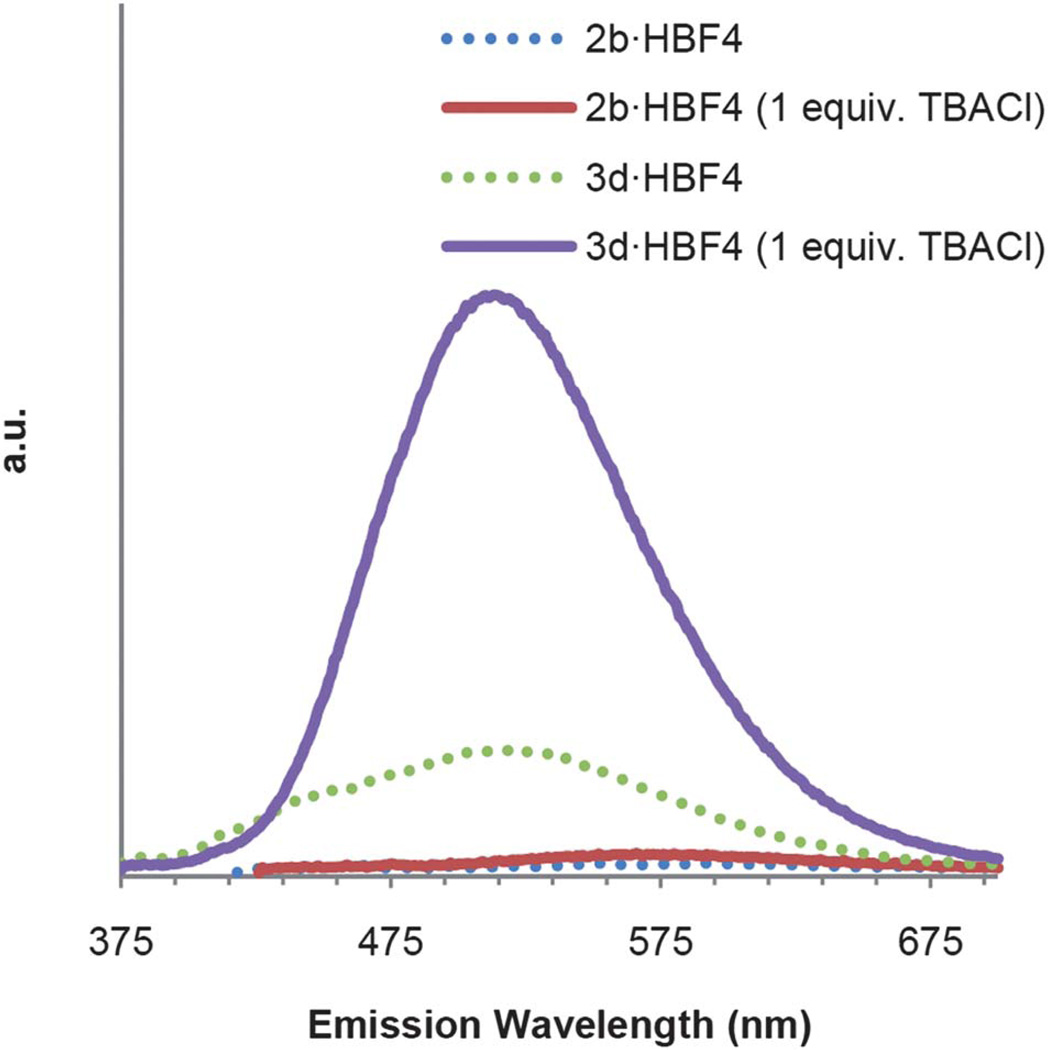
Gratifyingly, compound 3d exhibits a fluorescence “off to on” response in the presence of chloride despite being in a polar MeCN solvent (Fig. 6). Although compound 3d is not soluble in water, these results provide evidence that this particular ethynylpyridine scaffold can be tuned to exhibit a fluorescence response even in the presence of a highly competitive/polar solvent. Further studies will focus on determining the exact cause of this fluorescence and will aim to exhibit this same response in an aqueous solvent system.
Fig. 6.
Emission spectra of 2b·HBF4 and 3d·HBF4 before addition of TBACl (dotted lines) and after addition of TBACl. Compound 2b·HBF4 was excited at 416 nm, and compound 3d·HBF4 was excited at 365 nm.
Conclusion
The inherently fluorescent ethynylpyridine scaffolds presented herein can be tuned to exhibit either a red or blue shifted fluorescence response depending on the electron donating or withdrawing ability of the pendant functional groups. Similarly, the hydrogen bonding behavior of the bisurea scaffolds is highly dependent on the pendant functional groups. NMR spectroscopic and solid state structural studies indicate that without a suitable guest these receptors hydrogen bond strongly with themselves, suggesting that these sensors could allow for larger binding constants for anionic guests and potentially greater selectivity. The simple derivatization of this class of receptors allows for a “turn-on” fluorescence response to analytes in increasingly polar solvents, a feature often lacking in small molecule organic anion probes.24
Supplementary Material
Acknowledgements
This research was supported by the NIH (R01-GM087398). J.M.E. and C.N.C. acknowledge the NSF for GK-12 (DGE-0742540) and IGERT (DGE-0549503) fellowships, respectively. We also thank the NSF for support in the form of instrumentation grants (CHE-0639170 and CHE-0923589). HRMS were obtained at the Mass Spectrometry Facilities and Services Core of the Environmental Health Sciences Center, Oregon State University, supported by grant #P30-ES00210, National Institute of Environmental Health Sciences, National Institutes of Health.
Footnotes
Electronic supplementary information (ESI) available: synthetic procedures and spectral data for 2–9; discussion of and X-ray data for the 2-quinazolinone derivative. CCDC reference number 844848. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c2sc00975g
Notes and References
- 1.Gunnlaugsson T, Glynn M, Tocci GM, Kruger PE, Pfeffer FM. Coord. Chem. Rev. 2006;250:3094. [Google Scholar]
- 2.Amendola V, Esteban-Gomez D, Fabbrizzi L, Licchelli M. Acc. Chem. Res. 2006;39:343. doi: 10.1021/ar050195l. [DOI] [PubMed] [Google Scholar]
- 3.Beer PD, Gale PA. Angew. Chem., Int. Ed. 2001;40:486. [PubMed] [Google Scholar]
- 4.Holliday BJ, Mirkin CA. Angew. Chem., Int. Ed. 2001;40:2022. [PubMed] [Google Scholar]
- 5.Anslyn EV, Dougherty DA. Modern Physical Organic Chemistry. University Science; 2005. illustrated edition. [Google Scholar]
- 6.Bianchi A, Bowman-James K, García-Espana E. Supramolecular Chemistry of Anions. 1st edn. Wiley-VCH; 1997. [Google Scholar]
- 7.Sessler JL, Gale PA, Cho W-S. Anion Receptor Chemistry. Cambridge: Royal Society of Chemistry; pp. 1–26. [Google Scholar]
- 8.Savage PB, Holmgren SK, Gellman SH. J. Am. Chem. Soc. 1993;115:7900. [Google Scholar]
- 9.Savage PB, Holmgren SK, Gellman SH. J. Am. Chem. Soc. 1994;116:4069. [Google Scholar]
- 10.Schmidtchen FP. Angew. Chem., Int. Ed. Engl. 1977;16:720. [Google Scholar]
- 11.Kelly TR, Kim MH. J. Am. Chem. Soc. 1994;116:7072. [Google Scholar]
- 12.Bühlmann P, Nishizawa S, Xiao KP, Umezawa Y. Tetrahedron. 1997;53:1647. [Google Scholar]
- 13.Andrievsky A, Ahuis F, Sessler JL, Vögtle F, Gudat D, Moini M. J. Am. Chem. Soc. 1998;120:9712. [Google Scholar]
- 14.Schottel BL, Chifotides HT, Dunbar KR. Chem. Soc. Rev. 2008;37:68. doi: 10.1039/b614208g. [DOI] [PubMed] [Google Scholar]
- 15.Quiñonero D, Garau C, Rotger C, Frontera A, Ballester P, Costa A, Deyà PM. Angew. Chem., Int. Ed. 2002;41:3389. doi: 10.1002/1521-3773(20020916)41:18<3389::AID-ANIE3389>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 16.Berryman OB, Bryantsev VS, Stay DP, Johnson DW, Hay BP. J. Am. Chem. Soc. 2007;129:48. doi: 10.1021/ja063460m. [DOI] [PubMed] [Google Scholar]
- 17.Inoue Y, Hakushi T, Liu Y, Tong L, Shen B, Jin D. J. Am. Chem. Soc. 1993;115:475. [Google Scholar]
- 18.Steed JW, Juneja RK, Atwood JL. Angew. Chem., Int. Ed. Engl. 1995;33:2456. [Google Scholar]
- 19.Steed JW, Johnson CP, Juneja RK, Atwood JL, Burkhalter RS. Supramol. Chem. 1996;6:235. [Google Scholar]
- 20.Staffilani M, Hancock KSB, Steed JW, Holman KT, Atwood JL, Juneja RK, Burkhalter RS. J. Am. Chem. Soc. 1997;119:6324. [Google Scholar]
- 21.Lavis LD, Raines RT. ACS Chem. Biol. 2008;3:142. doi: 10.1021/cb700248m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Basabe-Desmonts L, Reinhoudt DN, Crego-Calama M. Chem. Soc. Rev. 2007;36:993. doi: 10.1039/b609548h. [DOI] [PubMed] [Google Scholar]
- 23.de Silva AP, Gunaratne HQN, Gunnlaugsson T, Huxley AJM, McCoy CP, Rademacher JT, Rice TE. Chem. Rev. 1997;97:1515. doi: 10.1021/cr960386p. [DOI] [PubMed] [Google Scholar]
- 24.Carroll CN, Naleway JJ, Haley MM, Johnson DW. Chem. Soc. Rev. 2010;39:3875. doi: 10.1039/b926231h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spitler EL, McClintock SP, Haley MM. J. Org. Chem. 2007;72:6692. doi: 10.1021/jo070827c. [DOI] [PubMed] [Google Scholar]
- 26.Spitler EL, Shirtcliff LD, Haley MM. J. Org. Chem. 2006;72:86. doi: 10.1021/jo061712w. [DOI] [PubMed] [Google Scholar]
- 27.Zucchero AJ, McGrier PL, Bunz UHF. Acc. Chem. Res. 2009;43:397. doi: 10.1021/ar900218d. [DOI] [PubMed] [Google Scholar]
- 28.Karchava AV, Maksimova FV, Yurovskaya MA. Chem. Heterocycl. Compd. 1994;30:1195. [Google Scholar]
- 29.Yamaguchi Y, Kobayashi S, Wakamiya T, Matsubara Y, Yoshida Z. Angew. Chem., Int. Ed. 2005;44:7040. doi: 10.1002/anie.200502214. [DOI] [PubMed] [Google Scholar]
- 30.Abe H, Takashima S, Yamamoto T, Inouye M. Chem. Commun. 2009:2121. doi: 10.1039/b902269d. [DOI] [PubMed] [Google Scholar]
- 31.Rajadurai C, Ivanova A, Enkelmann V, Baumgarten M. J. Org. Chem. 2003;68:9907. doi: 10.1021/jo034597n. [DOI] [PubMed] [Google Scholar]
- 32.Ahn CM, Shin W-S, Bum Woo H, Lee S, Lee H-W. Bioorg. Med. Chem. Lett. 2004;14:3893. doi: 10.1016/j.bmcl.2004.05.065. [DOI] [PubMed] [Google Scholar]
- 33.Holmes BT, Deb P, Pennington WT, Hanks TW. J. Polym. Res. 2005;13:133. [Google Scholar]
- 34.Carroll CN, Coombs BA, McClintock SP, Johnson CA, II, Berryman OB, Johnson DW, Haley MM. Chem. Commun. 2011;47:5539. doi: 10.1039/c1cc10947b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carroll CN, Berryman OB, Johnson CA, II, Zakharov LN, Haley MM, Johnson DW. Chem. Commun. 2009:2520. doi: 10.1039/b901643k. [DOI] [PubMed] [Google Scholar]
- 36.Engle JM, Lakshminarayanan PS, Carroll CN, Zakharov LN, Haley MM, Johnson DW. Cryst. Growth Des. 2011;11:5144. doi: 10.1021/cg201074v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Berryman OB, Johnson CA, II, Zakharov LN, Haley MM, Johnson DW. Angew. Chem., Int. Ed. 2008;47:117. doi: 10.1002/anie.200703971. [DOI] [PubMed] [Google Scholar]
- 38.Kimball DB, Weakley TJR, Haley MM. J. Org. Chem. 2002;67:6395. doi: 10.1021/jo020229s. [DOI] [PubMed] [Google Scholar]
- 39.McClintock SP, Forster N, Herges R, Haley MM. J. Org. Chem. 2009;74:6631. doi: 10.1021/jo9011283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Crystal data for 2b: C44H42Cl3N7O7, Mr = 887.20, 0.21 × 0.14 × 0.02 mm, monoclinic, P21/c, a = 9.5229(13) Å, b = 39.529(5) Å, c = 11.6647(16) Å, β = 98.282(3)°, V = 4345(1) Å3, Z = ρcalcd = 1.356 g cm−1, µ = 0.270 mm−1, 2θmax = 50.00°, T = 173(2) K, 41736 measured reflections, 7661 independent reflections [R int = 0.0927], 538 independent refined parameters, R 1 = 0.0808, wR 2 = 0.1968 (with I > 2σ (I)), R 1 = 0.1361, wR 2 = 0.2255 (all data), GOF = 0.996, max/min residual electron density +0.418/−0.316 e Å−3. CCDC 844848 contains the supplementary crystallographic data, which can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- 41.Hamilton W. Benjamin WA. Hydrogen bonding in solids: methods of molecular structure determination. New York: Amsterdam; 1968. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.