Abstract
In the phytopathogenic fungus Ustilago maydis, fusion of haploid cells is a prerequisite for infection. This process is controlled by a pheromone-receptor system. The receptors belong to the seven-transmembrane class that are coupled to heterotrimeric G proteins. Of four Gα subunits in U. maydis, only gpa3 has a function during mating and cyclic AMP (cAMP) signaling. Activation of the cAMP cascade induces pheromone gene expression; however, it does not lead to the induction of conjugation tubes seen after pheromone stimulation. To investigate the possibility that a Gβ subunit participates in pheromone signaling, we isolated the single β subunit gene, bpp1, from U. maydis. bpp1 deletion mutants grew filamentously and showed attenuated pheromone gene expression, phenotypes associated with Δgpa3 strains. In addition, a constitutively active allele of gpa3 suppressed the phenotype of the bpp1 deletion strains. We suggest that Bpp1 and Gpa3 are components of the same heterotrimeric G protein acting on adenylyl cyclase. Interestingly, while Δgpa3 strains are impaired in pathogenicity, Δbpp1 mutants are able to induce plant tumors. This could indicate that Gpa3 operates independently of Bpp1 during pathogenic development.
In a variety of pathogenic fungi, one of the key steps during pathogenesis is the switch from a unicellular yeast-like form to a multicellular filament. During this differentiation, sensing of the cellular environment plays a crucial role. Signal transduction cascades transmit the signals perceived at the cell surface to affect a variety of outputs. How these cascades are linked and mediate pathogenic development in fungi is not well defined. The phytopathogenic basidiomycete Ustilago maydis is one of the model organisms in which such issues are addressed (25). In this corn pathogen, sexual as well as pathogenic development requires recognition and fusion of haploid yeast-like sporidia. The fusion process is controlled by pheromones and receptors, both of which are encoded by the a mating type loci (7), while subsequent steps of development are promoted by the b locus (2). The b locus encodes two homeodomain proteins, bE and bW, which can form heterodimers when originating from different alleles (15, 26). Strains are designated compatible when they differ in both a and b mating type loci. During cell-cell recognition, pheromone elicits an increase in pheromone and receptor gene expression as well as the formation of conjugation tubes, which fuse at their tips (47). After fusion, the resulting dikaryon undergoes a morphological transition and switches to filamentous growth (3, 7). The HMG domain transcription factor Prf1 is essential for both basal and pheromone-responsive expression of the a and b genes and thus connects the action of the a and b loci (19).
In U. maydis, as in other fungi, the pheromone receptors belong to the family of seven-transmembrane receptors. Most members of this protein family are coupled to heterotrimeric G proteins (6, 7, 45). Heterotrimeric G proteins composed of α, β, and γ subunits are highly conserved and regulate transmission of the signal to cytoplasmic effector proteins. In most cases, it is the Gα subunit that actively transmits the signal; however, there are an increasing number of examples where Gβγ dimers also play a functional role. In U. maydis, four different genes encoding Gα subunits (gpa1, gpa2, gpa3, and gpa4) have been cloned and characterized. Of these, only gpa3 deletion strains have a severe mating defect (39). In particular, Δgpa3 mutants are affected in basal as well as pheromone-responsive gene expression and exhibit filamentous growth (39). Since these phenotypes can be reverted to the wild type by exogenous cyclic AMP (cAMP), gpa3 was subsequently placed upstream of adenylyl cyclase uac1 (5), where it is postulated to activate cAMP signaling (29).
The major effector of intracellular cAMP content is the cAMP-dependent protein kinase protein kinase A (PKA), composed of the regulatory subunit Ubc1 and the catalytic subunit Adr1 (12, 17). A constitutively active allele of gpa3 leads to induced pheromone gene expression. Recently, it has been shown that cAMP signaling acts through Prf1 (24). However, as this kind of activation does not result in conjugation tube formation, another not yet identified G protein might be involved in triggering this morphological transition in response to pheromone. Since it was shown that a mitogen-activated protein (MAP) kinase cascade composed of Ubc4/Kpp4 (MEKK), Fuz7 (MEK), and Kpp2/Ubc3 (MAPK) is required for conjugation tube formation as well as pheromone-responsive gene expression, this hypothetical G protein is likely to act upstream of this module (1, 4, 34, 35, 36). In Saccharomyces cerevisiae and Cryptococcus neoformans, the pheromone signal is transmitted to a MAP kinase cascade by the Gβ subunits Ste4p and Gpb1, respectively. On these grounds, we considered the possibility that a Gβ subunit might also be involved in regulation of the MAP kinase cascade in U. maydis (53, 55). Thus, we isolated and characterized the U. maydis bpp1 gene coding for a Gβ subunit.
MATERIALS AND METHODS
Isolation of bpp1.
Degenerate primers (single-letter code follows International Union of Pure and Applied Chemistry format; I stands for inosine) Beta#1f (AARATHTAYGCIATGCAYTGG) and Beta#8r (SWRTCCCAISWICCNGTRC) were used for amplification of U. maydis DNA from strain FB1. Reactions contained 10 mM Tris-HCl (pH 8.3), 6 mM MgCl2, 50 mM KCl, 25 pmol of primers, and 1 U of Taq polymerase (Roche). The same conditions were used for nested PCR amplifications with the following additional primers: Beta#2f (WSIGCIWSICARGAYGG), Beta#5f (GGIGGIYTIGAYAAYATHTG), Beta#9r (GCRTCRCAIGCICCNSWDAT), Beta#10r (GCRCANGTCATRTCNCC), and Beta#12r (CADATRTTRTCNARNCCNCC). PCR products were cloned directly into pCR2.1-TOPO (Invitrogen) according to the manufacturer's protocol. The bpp1 fragment amplified with Beta#5f and Beta#9r was used to screen a genomic cosmid library (9). From hybridizing cosmid 6D6, bpp1 was subcloned as 5.4-kb BamHI-BamHI and 5.6-kb PstI-PstI fragments in pTZ19R. The resulting plasmids were designated pBK1 and pPK1, respectively.
Plasmids and plasmid constructions.
Plasmids pTZ19R (Pharmacia), pTZ18R (Pharmacia), pUC18 (Pharmacia), and pBS-SKII(+) (Stratagene) were used for cloning, subcloning, and sequencing. pSLNat(+), pSLHyg(−), and pSLHyg(+) contain the nourseothricin and hygromycin resistance cassettes, respectively (11, 35). For the bpp1 gene disruption plasmid pBPP1-1::Hyg, the hygromycin resistance cassette was cloned as a 2.9-kb NotI-NotI fragment from pSLHyg(−) into the NotI site of pBK1, thereby disrupting the open reading frame of bpp1 at position +404. In the deletion construct pΔBPP1-2, the region between +74 and +1046 of the bpp1 open reading frame is deleted and replaced by a nourseothricin resistance cassette. For construction of pΔBPP1-2, we ligated a 1.4-kb PstI-BstYI fragment from pBK1, a 1.5-kb BamHI-AflII fragment encoding a nourseothricin resistance cassette isolated from pSLNat(−), and a 2.6-kb AflII-EcoRI fragment derived from pBK1 into pTZ18R. The complementation plasmid pNEBUH-bpp1 is a pNEBUH (35) derivative containing the 5.6-kb PstI fragment from pPK1 in its SbfI site.
Strains and strain constructions.
For cloning purposes, the Escherichia coli K-12 derivative DH5α was used. Haploid U. maydis strains FB1 (a1 b1), FB2 (a2 b2), FB6a (a2 b1), RK1786 (a1 b1 pra1:Tn5H), FB2Δgpa1, FB2Δgpa2, FB1Δgpa3, FB1gpa3QL, FB2Δgpa3, FB2Δgpa4, FB1Δubc1, and FB2Δfuz7 have been described before (3, 7, 20, 36, 39). The haploid solopathogenic strains CL13 (a1 bE1bW2), and the derivative SG200 (a1::mfa2 bE1bW2) differ only with respect to the mfa2 gene, which was introduced in the resident a1 locus of SG200 and provides autocrine pheromone stimulation, resulting in filamentous growth (8, 9). HA103 (a1 bcon), expressing bE1bW2 constitutively, and CL13prf1con, expressing prf1 constitutively, are both CL13 derivatives (19, 35).
The strains constructed in this study are listed in Table 1. With the exception of the Δgpa3 Δbpp1-2 double mutants, the Δbpp1-2 deletion strains were constructed by transformation of the respective progenitor strains with pΔBPP1-2 digested with DraI. Homologous recombination at the bpp1 locus was verified by Southern analysis after cleaving the DNA with BamHI and PstI. The 5-kb BamHI-PstI fragment of pBK1 was used as probe. The Δgpa3 Δbpp1-2 double mutants were segregants isolated from crosses between FB2Δgpa3 and FB1Δbpp1-2 and were verified by Southern analysis.
TABLE 1.
U. maydis strains constructed in this study
| Strain or genotype | Plasmid transformed or description | Integration site | Progenitor strain (reference) |
|---|---|---|---|
| FB1Δbpp1-2 | pΔBPP1-2 | bpp1 locus | FB1 (3) |
| FB2Δbpp1-2 | pΔBPP1-2 | bpp1 locus | FB2 (3) |
| FB1Δbpp1-2/pNEBUH-bpp1 | pNEBUH-bpp1 | Self-replicating | FB1Δbpp1-2 (this study) |
| FB2Δbpp1-2/pNEBUH-bpp1 | pNEBUH-bpp1 | Self-replicating | FB2Δbpp1-2 (this study) |
| SG200Δbpp1-2 | pΔBPP1-2 | bpp1 locus | SG200 (9) |
| CL13Δbpp1-2 | pΔBPP1-2 | bpp1 locus | CL13 (8) |
| HA103Δbpp1-2 | pΔBPP1-2 | bpp1 locus | HA103 (19) |
| CL13prf1conΔbpp1-2 | pΔBPP1-2 | bpp1 locus | CL13prf1con (35) |
| FB2Δgpa1Δbpp1-2 | pΔBPP1-2 | bpp1 locus | FB2Δgpa1 (39) |
| FB2Δgpa2Δbpp1-2 | pΔBPP1-2 | bpp1 locus | FB2Δgpa2 (39) |
| FB2Δgpa4Δbpp1-2 | pΔBPP1-2 | bpp1 locus | FB2Δgpa4 (39) |
| FB1gpa3QLΔbpp1-2 | pΔBPP1-2 | bpp1 locus | FB1gpa3QL (39) |
| FB1Δbpp1-1 | pΔBPP1-1::Hyg | bpp1 locus | FB1 (3) |
| FB2Δbpp1-1 | pΔBPP1-1::Hyg | bpp1 locus | FB2 (3) |
| FB1Δubc1Δbpp1-1 | pΔBPP1-1::Hyg | bpp1 locus | FB1Δubc1 (20) |
| FB1Hygr | pSL-Hyg(+) | Ectopic | FB1 (3) |
| FB6aHygr | pSL-Hyg(+) | Ectopic | FB6a (3) |
| FB6aOligor | Spontaneous mutation | FB6a (3) | |
| a1 b1 Δgpa3 Δbpp2-1 | Progeny of crosses between FB2Δgpa3 and FB1Δbpp2-1 | ||
| a2 b2 Δgpa3 Δbpp2-1 | Progeny of crosses between FB2Δgpa3 and FB1Δbpp2-1 | ||
| a1 b2 Δgpa3 Δbpp2-1 | Progeny of crosses between FB2Δgpa3 and FB1Δbpp2-1 | ||
| a2 b1 Δgpa3 Δbpp2-1 | Progeny of crosses between FB2Δgpa3 and FB1Δbpp2-1 |
To generate the double mutant FB1Δubc1Δbpp1-1, FB1Δubc1 was transformed with plasmid pBPP1-1::Hyg digested with DraI. DNA from individual transformants was cleaved with BamHI, and homologous recombination was detected by Southern analysis. As controls, FB1 and FB2 were also transformed with pBPP1-1::Hyg. The resulting bpp1-1 disruption mutants grew filamentously, and filamentous growth could be suppressed by cAMP. Thus, they were indistinguishable from the Δbpp1-2 deletion strains.
The complemented Δbpp1-2 deletion strains FB1Δbpp1-2/pNEBUH-bpp1 and FB2Δbpp1-2/pNEBUH-bpp1 were generated by transforming the respective Δbpp1-2 deletion strains with the self-replicating plasmid pNEBUH-bpp1.
FB1Hygr and FB6aHygr, carrying a hygromycin cassette ectopically, are derived form FB1 and FB6a, respectively. FB6aOligor is a spontaneous oligomycin-resistant mutant which was generated by plating 3 × 108 FB6a cells on CM plates containing 1% glucose and 5 μg of oligomycin per ml.
Growth conditions for U. maydis.
Strains were grown at 28°C in modified YEPS medium (51), potato dextrose medium (PD), or complete medium (CM) (22). Liquid medium, which contains cAMP (Sigma), was filter sterilized prior to use. Oligomycin (Sigma) was used at a concentration of 5 μg/ml. Hygromycin B (final concentration of 200 μg/ml) was purchased from Roche, and nourseothricin (final concentration of 150 μg/ml) was purchased from the Hans Knöll Institute (Jena, Gemany). Synthetic a1 and a2 pheromones were kindly supplied by M. Tönnis and H. Kessler (Technical University, Munich, Germany). All chemicals used were of analytical grade and were obtained from Sigma or Merck.
The mating reaction was observed after cospotting strains on charcoal-containing plates supplemented with 6 mM cAMP after incubation at 28°C for 48 h.
For pheromone stimulation experiments, strains were grown for 16 h to an optical density at 600 nm (OD600) of 0.6 in CM containing 1% glucose supplemented with 6 mM cAMP when indicated. Pheromone dissolved in dimethyl sulfoxide was added to a final concentration of 2.5 μg/ml, and cells were harvested for microscopic observations after 5 h of incubation in a 15-ml plastic tube on a tissue culture roller at 28°C. Quantification was done with microscopic pictures by manual counting.
Cytoduction assays (50) were conducted with the following modifications. Strains were grown for 16 h to an OD600 of 0.6 in CM containing 1% glucose and supplemented with 6 mM cAMP when indicated. Cells were washed in sterile water twice and resuspended in CM containing 1% glucose (and 6 mM cAMP when indicated) to a density of 109 cells/ml; 1 ml of the a1 b1 strains tested was mixed with 1 ml of 109 cells/ml of FB6aOligor (a2 b1) in a 15-ml plastic tube and incubated for 16 h on a slowly turning tissue culture roller at 28°C. Serial dilutions of these mixtures were spread on selection plates.
Plant infections of the variety Early Golden Bantam (Olds Seeds, Madison, Wis.) were performed essentially as described previously (15) with the following modification. Strains were grown overnight at 28°C in YEPS liquid medium to an OD600 of 0.8, harvested, and resuspended in water to an OD600 of 3 prior to inoculation.
DNA and RNA procedures.
Standard molecular techniques were followed for DNA and RNA procedures (40). Transformation of U. maydis was performed as described previously (43). U. maydis DNA was isolated as described previously (21). RNA was isolated from strains grown on charcoal-containing CM plates as described previously (42). To assay the effects of cAMP on gene expression and morphology, strains were grown in PD liquid medium containing the indicated amount of cAMP, and RNA was prepared as described previously (29).
The following probes were used for Northern analysis: a 675-bp EcoRV-EcoRV fragment spanning the mfa1 gene isolated from pUMa1 (52), a 2.2-kb EcoRI-EcoRV fragment of pCBX122 (28) that detects the succinate dehydrogenase message (cbx), and a 5′-end-labeled oligonucleotide complementary to the 18s rRNA (10); the last two probes served as loading controls. Radioactive labeling of the DNA was performed with random primers from New England Biolabs. A Storm PhosphorImager (Molecular Dynamics) was used for signal detection. The intensity of the signals was calculated and compared with ImageQuant.
The sequence of bpp1 was derived from pBK1 with an ABI 373 automated sequencer. Sequence analysis was performed with standard bioinformatic tools.
Light microscopy.
For microscopic observation, we used a Zeiss Axiophot with differential interference contrast optics. The pictures were taken with a charge-coupled device camera (C4742-95; Hamamatsu, Herrsching, Germany). Image processing was done with Image Pro, Adobe Photoshop 6.0, and Canvas 6.0 (Deneba Systems). For documentation, colonies were magnified with an Olympus Binocular.
RESULTS
Identification of bpp1, coding for a G protein β subunit.
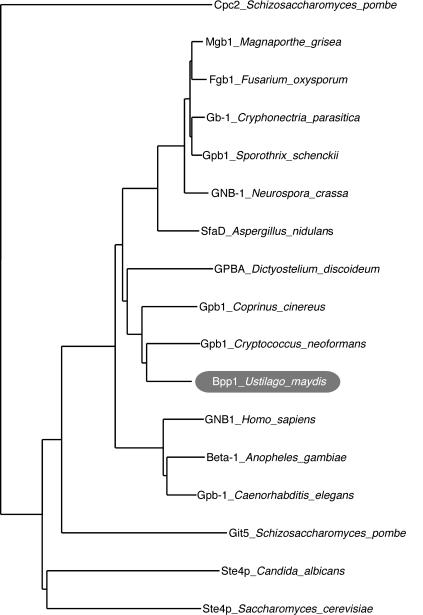
G protein β subunits of several organisms show a high degree of amino acid identity. Therefore, a PCR-based strategy was used to identify a Gβ subunit-encoding gene in U. maydis. Sequence analysis confirmed that all amplicons generated were derived from the same genomic template encoding a putative G protein β subunit. Further analysis led to the characterization of an open reading frame of 1,050 bp with no evidence of introns. This gene was designated bpp1 (accession no. AF536325). The deduced polypeptide of Bpp1 consists of 349 amino acids, harbors all seven WD-40 repeats characteristic of Gβ subunits (14, 37, 46), and shows high similarity to known fungal Gβ subunits, ranging in amino acid identity from 79% to GPB1 of Cryptococcus neoformans (53) to 30% with Ste4p of Saccharomyces cerevisiae (55) (Fig. 1). No additional genes showing the classical features of Gβ subunits were found in the genome sequence of U. maydis (http://www-genome.wi.mit.edu/annotation/fungi/ustilago_maydis/"). This is in accordance with other fungal genomes that have been analyzed, which also contain only one Gβ subunit gene (Fig. 1).
FIG. 1.
Phylogenetic comparison of Bpp1 of U. maydis to various Gβ subunits of fungi and higher eukaryotes. The bootstrap analysis was performed with Clustal W1.8 (49) with the seven-WD40-repeat-containing protein Cpc2 from S. pombe as an outgroup. Accession numbers are CAB11079.1 for Cpc2 of Schizosaccharomyces pombe, BAC01165 for Mgb1 of Magnaporthe grisea, BAB69489 for Fgb1 of Fusarium oxysporum, O14435 for Gb-1 of Cryphonectria parasitica, AAL90861 for Gpb1 of Sporothrix schenckii, AAM53552 for GNB-1 of Neurospora crassa, AAC33436 for SfaD of Aspergillus nidulans, P36408 for GPBA of Dictyostelium discoideum, and Gpb1 of Coprinus cinereus on Contig 1.28 (http://www-genome.wi.mit.edu/annotation/fungi/coprinus_cinereus/); AAD03596 for Gpb1 of Cryptococcus neoformans, AAN33051 for Bpp1 of U. maydis, NP_002065.1 for GNB1 of Homo sapiens, XP_315941 for Beta-1 of Anopheles gambiae, NP_496508 for Gpb-1 of Caenorhabditis elegans, NP_595613 for Git5p of Schizosaccharomyces pombe, and Ste4p of Candida albicans on Contig6-1885 (http://www.ncbi.nlm.nih.gov/genomes/framik.cgi?taxid=5476); and NP_014855 for Ste4p of Saccharomyces cerevisiae.
Δbpp1-2 deletion mutants are filamentous.
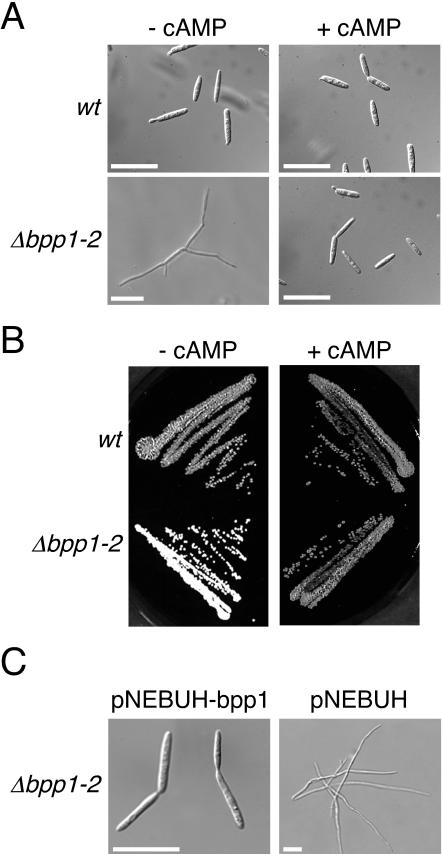
To analyze the function of bpp1, we generated Δbpp1-2 deletion mutants in strains FB1 (a1 b1) and FB2 (a2 b2) by gene replacement (see Materials and Methods). Morphological analysis of FB1Δbpp1-2 and FB2Δbpp1-2 revealed that deletion of bpp1 causes filamentous growth in liquid as well as on solid media (Fig. 2A and B, left panels). Filaments developed by the Δbpp1-2 mutants were branched and filled with cytoplasm. Budding growth in Δbpp1-2 mutant strains was fully restored by introducing the bpp1 gene on an autonomously replicating plasmid (Fig. 2C). This demonstrates that the filamentous phenotype observed in Δbpp1-2 mutants is due only to the absence of bpp1.
FIG. 2.
Exogenous cAMP restores budding growth and mating in Δbpp1-2 deletion mutants. (A) Strains as indicated on the left were grown in PD liquid medium without cAMP (left panels) or with 6 mM cAMP (right panels) for 16 h. Both strains are in the a2 b2 background. Bars, 20 μm. (B) Wild-type strain FB2 (a2 b2) and FB2Δbpp1-2 were incubated for 36 h on a PD-charcoal plate in the absence (left panel) or presence (right panel) of 6 mM cAMP. (C) FB2Δbpp1-2 carrying either pNEBUH-bpp1 (left panel) or the empty vector pNEBUH (right panel) was grown in PD liquid medium containing hygromycin. Bars, 20 μm.
With respect to filament formation, bpp1-2 deletion mutants resemble gpa3 (encoding a Gα subunit) and uac1 (encoding adenylyl cyclase) mutants, which are defective in cAMP signaling. Since the filamentous phenotype of gpa3 as well as uac1 deletion mutants is suppressed in the presence of exogenous cAMP (16, 29), cAMP was added to Δbpp1-2 strains to a final concentration of 6 mM. Upon cAMP treatment, Δbpp1-2 cells switched to budding growth on plates as well as in liquid media (Fig. 2A and B, right panels), suggesting that bpp1 is involved in cAMP signaling.
Constitutively active allele of gpa3 can activate cAMP signaling independently of bpp1.
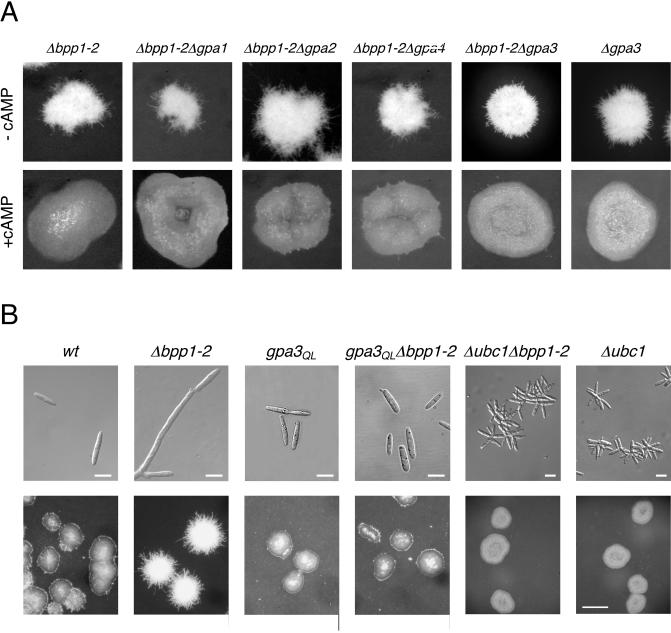
Since bpp1 encodes a Gβ subunit, we investigated the genetic relationship between bpp1 and the genes encoding Gα subunits. First, we constructed double mutants by deleting bpp1 in FB2Δgpa1, FB2Δgpa2, and FB2Δgpa4. All resulting double mutants exhibited filamentous growth and were indistinguishable from the Δbpp1-2 single mutant strain (Fig. 3A, upper panel). In addition, filamentous growth of the double mutants was suppressed by addition of cAMP (Fig. 3A, lower panel). This result rules out the possibility that the filamentous phenotype observed in Δbpp1-2 strains is mediated through one of these Gα subunits.
FIG. 3.
Relationship between bpp1 and the four different genes encoding Gα subunits. (A) Strains were all in the a2 b2 background and were grown on charcoal-containing CM plates in the absence (upper panel) or presence (lower panel) of 6 mM cAMP for 36 h. Pictures were taken with an Olympus Binocular. (B) Strains (all in a1 b1 background) were grown in liquid CM (upper panel) for 16 h or on charcoal-containing CM plates (lower panel) for 36 h. Bars, 10 μm (upper panels) or 500 μm (lower panels).
Next, we analyzed the genetic relationship between bpp1 and gpa3. We generated Δbpp1-2 Δgpa3 double mutants by isolating segregants from crosses between FB1Δbpp1-2 and FB2Δgpa3. All double mutants obtained grew filamentously, and this phenotype could be suppressed by 6 mM cAMP (Fig. 3A). With respect to colony morphology, we could not observe a difference between the Δbpp1-2 Δgpa3 strain and the progenitor single mutants. This genetic relationship suggests that the β subunit Bpp1 and the α subunit Gpa3 are in the same cascade. To examine this further, we introduced the Δbpp1-2 mutation into strain FB1gpa3QL, which carries the constitutively active gpa3Q206L allele of this Gα subunit. Strains expressing gpa3Q206L grow by budding and display a glossy colony phenotype (30, 39). With respect to these traits, FB1gpa3QLΔbpp1-2 was indistinguishable from the progenitor strain FB1gpa3QL (Fig. 3B). This indicates that constitutively active Gpa3 can suppress the filamentous phenotype of the bpp1-2 mutant and suggests that Bpp1 is epistatic to Gpa3.
bpp1 regulates pheromone gene expression by acting upstream of PKA.
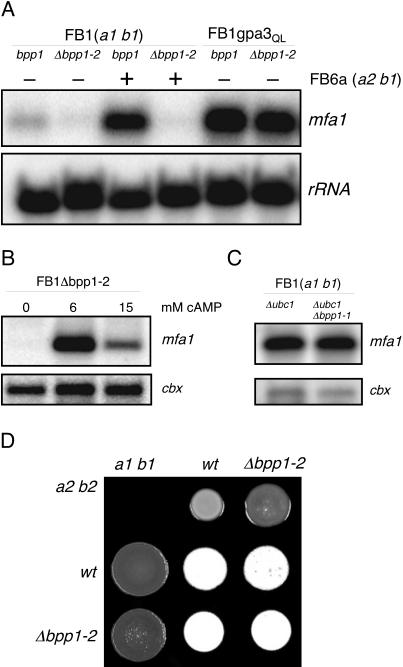
Besides regulating filamentous growth, cAMP signaling is essential for pheromone-induced gene expression, and therefore this signaling pathway plays a crucial role during mating. Since bpp1 appears to be a component of the cAMP cascade, we examined the ability of Δbpp1-2 strains to be stimulated by pheromone of the opposite (a) mating type. The deletion of bpp1 strongly attenuated basal as well as pheromone-induced expression of mfa1 compared to the wild-type situation (Fig. 4A). Addition of 6 mM cAMP, which should activate the cAMP-dependent protein kinase PKA, stimulated mfa1 transcription in FB1Δbpp1-2, while the addition of 15 mM cAMP resulted in only moderate mfa1 induction (Fig. 4B). Such cAMP concentration-dependent expression of mfa1 has been described for wild-type and gpa3 deletion strains (29). This suggests that bpp1 also functions upstream of PKA.
FIG. 4.
Pheromone gene expression in Δbpp1-2 strains. We loaded 15 μg of total RNA per lane and subjected it to Northern analysis. The same filter was hybridized in succession with probes for mfa1 and cbx or rRNA as loading controls. (A) Strains and strain mixtures, indicated at the top, were grown on charcoal-containing CM plates for 48 h. (B) The FB1Δbpp1-2 deletion mutant was grown in liquid PD medium without and with cAMP at the concentrations indicated. (C) Epistatic relationship of bpp1 and ubc1. Strains were grown on charcoal-containing CM plates for 48 h. (D) Mating reactions of bpp1 deletion strains on PD-charcoal plates supplemented with 6 mM cAMP. Dikaryon formation can be observed as white fuzzy filaments. The strain combinations indicated were spotted alone or in combination and incubated for 48 h at 28°C.
To strengthen this assertion, we analyzed the epistatic relationship between bpp1, gpa3QL, and ubc1, encoding the regulatory subunit of PKA. Deletion of ubc1 resulted in constitutive PKA activity, and as a consequence, the mutant cells exhibited a multiple budding phenotype (Fig. 3B). This phenotype was also observed with cells of the Δubc1 Δbpp1-1 double mutant (Fig. 3B). In addition, FB1Δubc1Δbpp1-1 as well as FB1gpa3QLΔbpp1-2 displayed high levels of mfa1 expression, as observed in the progenitor strains FB1Δubc1 and FB1gpa3QL, respectively (Fig. 4A and B). These findings indicate that the function of bpp1 in pheromone gene expression is upstream of PKA and can be bypassed by the active Gα subunit Gpa3QL.
Since pheromone perception and signaling are important for cell-cell fusion during mating, we examined the fusion capacity of Δbpp1-2 mutant cells in a cytoduction assay (50). Wild-type cells fused with a frequency of 2.3 × 10−5, but the fusion ability of Δbpp1-2 cells was reduced to 11% of that value (Table 2). Interestingly, the fusion frequencies of Δgpa3 and Δbpp1-2 Δgpa3 cells were even lower (1.3 and 2.3% of wild-type frequency, respectively; Table 2). Thus, gpa3 seems to be more important for the fusion process than bpp1. However, the presence of 6 mM cAMP restored the fusion ability of all mutants tested, although it did not induce fusion in mixtures of strains carrying the same a locus (Table 2). These results strengthen the conclusion that bpp1 is part of the cAMP signaling pathway which is critical for cell-cell fusion. In addition, Δbpp1-2 mutants formed dikaryotic filaments as effectively as combinations of the respective wild-type strains on CM-charcoal plates supplemented with 6 mM cAMP (Fig. 4D).
TABLE 2.
Cytoduction assays
| Genotype of strain mixed with the a2 b1 oligomycin- resistant strain | cAMP added (mM) | Cytoduction frequencya | % of wild-type frequency |
|---|---|---|---|
| a1 b1 Hygr (positive control) | None | 2.3 × 10−5 | 100 |
| a2 b1 Hygr (negative control) | None | < 6.1 × 10−8 | <0.27b |
| a1 b1 Δgpa3 | None | 3 × 10−7 | 1.3 |
| a1 b1 Δbpp1-2 | None | 2.5 × 10−6 | 11 |
| a1 b1 Δgpa3 Δbpp1-2 | None | 5.4 × 10−7 | 2.3 |
| a1 b1 Hygr | 6 | 3.4 × 10−5 | 100 |
| a2 b1 Hygr | 6 | < 9 × 10−8 | <0.26b |
| a1 b1 Δgpa3 | 6 | 4.0 × 10−5 | 118 |
| a1 b1 Δbpp1-2 | 6 | 4.3 × 10−5 | 126 |
| a1 b1 Δgpa3 Δbpp1-2 | 6 | 3.1 × 10−5 | 91 |
Proportion of hygromycin- and nourseothricin-resistant colonies that were also oligomycin resistant.
No cytoductants detected.
bpp1 appears to be dispensable for conjugation tube formation.
In U. maydis, pheromone induces the mating response through triggering cAMP and MAP kinase signaling (24, 29). The formation of conjugation tubes, which is an integral part of this process, is induced by the MAP kinase cascade and cannot be induced by elevated cAMP levels (29, 36).
To examine whether bpp1 has a role during conjugation tube formation, we exposed FB2Δbpp1-2 to synthetic a1 pheromone. In this analysis, budding growth as well as a gene expression of FB2Δbpp1-2 was restored by addition of 6 mM cAMP. FB2 (a2 b2) without cAMP and FB2 and FB2Δgpa3, both treated with 6 mM cAMP, were used as controls. After 5 h of exposure to pheromone, 82% of FB2 cells had formed conjugation tubes, while in all cAMP-treated strains conjugation tube formation was reduced to 17 to 18% (Table 3). Even after more than 18 h of exposure to pheromone, no increase in the percentage of cells that formed conjugation tubes was observed (not shown). In contrast, a Δpra1 strain, lacking the pheromone receptor, and Δfuz7 cells, in which the MAP kinase module is disrupted, were unable to form conjugation tubes irrespective of the presence or absence of cAMP (Table 3).
TABLE 3.
Conjugation tube formationa
| Strain stimulated with pheromone | cAMP added (mM) | Total no. of cells counted | % with conjuga- tion tubes |
|---|---|---|---|
| FB2 | 0 | 320 | 82 |
| FB2 | 6 | 351 | 17 |
| FB2Δgpa3 | 6 | 319 | 18 |
| FB2Δbpp1-2 | 6 | 316 | 17 |
| FB2Δfuz7 | 6 | 371 | 0 |
| RK1786b | 6 | 382 | 0 |
| FB1 | 0 | 361 | 74 |
| FB1gpa3QL | 0 | 1,017 | 17.5 |
| FB1gpa3QLΔbpp1-2 | 0 | 709 | 18 |
Cells were treated for 5 h with synthetic a1 pheromone (FB2 and derivatives) or a2 pheromone (FB1 and derivatives). Conjugation tube formation was assessed microscopically.
RK1786 is derived from FB1 and carries a Tn5H insertion in the pra1 gene.
To explain the reduction in conjugation tube formation induced by exogenous cAMP, we also performed the assay with strains carrying gpa3QL, which have an activated cAMP signaling pathway. In FB1gpa3QL and FB1gpa3QLΔbpp1-2, conjugation tube formation was reduced to 17.5 and 18%, respectively (Table 3). This shows that activated cAMP signaling reduces the morphological response to pheromone. However, neither bpp1 nor gpa3 appeared to be necessary for conjugation tube formation despite the fact that both genes are required for the transcriptional response to pheromone. Collectively, these observations make it unlikely that the Gβ subunit Bpp1 of U. maydis acts on the MAP kinase module during pheromone signaling.
Role of bpp1 in pathogenic development.
In U. maydis, disruption of the cAMP signaling cascade impairs pathogenic development even in the presence of an active b heterodimer (12, 17, 39). Therefore, we expected bpp1 to be necessary for pathogenesis. However, when corn plants were inoculated with mixtures of compatible Δbpp1-2 deletion strains, tumor formation was only slightly reduced in comparison to inoculations with the respective wild-type strains (Table 4). In addition, tumors induced by infection with Δbpp1-2 strains were filled with spores, which were able to germinate (not shown), but mixtures of compatible Δbpp1-2 Δgpa3 double mutants were nonpathogenic (Table 4), as was observed for the gpa3 single mutants (39). These results demonstrate that bpp1 is dispensable for pathogenesis in the wild-type situation, suggesting different signaling functions for bpp1 and gpa3 during pathogenic development.
TABLE 4.
Plant infection assaysa
| Inoculum | No. of infected plants | No. of plants with tumors | Avg % tumor formation |
|---|---|---|---|
| FB1 (a1 b1) × FB2 (a2 b2)b | 152 | 146 | 96 ± 5 |
| FB1Δbpp1-2 × FB2Δbpp1-2b | 195 | 151 | 79 ± 2 |
| CL13 (a1 bE1 bW2)b | 185 | 161 | 85 ± 5 |
| CL13Δbpp1-2b | 325 | 4 | 1 ± 1 |
| CL13prf1con | 38 | 36 | 95 |
| CL13prf1conΔbpp1-2b | 78 | 0 | 0 ± 0 |
| HA103 (a1 bcon)b | 84 | 46 | 55 ± 9 |
| HA103Δbpp1-2b | 181 | 74 | 41 ± 8 |
| SG200 (a1::mfa2 bE1 bW2)b | 132 | 127 | 94 ± 5 |
| SG200Δbpp1-2b | 135 | 103 | 72 ± 13 |
| a1 b1 Δbpp1-2 Δgpa3 | 82 | 0 | 0 |
| a2 b2 Δbpp1-2 Δgpa3 | |||
| a2 b1 Δbpp1-2 Δgpa3 | 76 | 0 | 0 |
| a1 b2 Δbpp1-2 Δgpa3 |
Data from two to four separate experiments were collected and combined.
Infections were performed with at least two independently generated mutant strains.
Surprisingly, plants infected with the solopathogenic haploid strain CL13 (a1 bE1bW2) with a deletion of bpp1 were severely attenuated in tumor formation (Table 4). The haploid strain CL13 is deficient in autocrine pheromone stimulation because it harbors only one a allele. Nevertheless, CL13 is solopathogenic because it express an active bE1/bW2 gene combination (8). For CL13, it has been postulated that sensing of putative plant signals triggers b gene expression during pathogenesis (19). We considered the possibility that the defect in pathogenesis of CL13Δbpp1-2 is due to insufficient b gene transcription during infection. As b gene expression is dependent on the transcription factor prf1 (19), we first examined whether constitutive expression of prf1 rescues pathogenicity in CL13Δbpp1-2. The generated strain, CL13prf1conΔbpp1-2, was unable to induce tumors in any of the 78 plants infected (Table 4). Thus, the defect in pathogenicity of CL13Δbpp1-2 is not a result of insufficient prf1 transcription.
Next, we introduced the Δbpp1-2 allele into strains HA103 (a1 bcon) and SG200 (a1:mfa2 bE1bW2). HA103 expresses the bE1 and bW2 genes under the control of constitutive promoters, and SG200 differs from CL13 only in its ability to carry out autocrine pheromone stimulation (9, 19). Plant infection assays showed that HA103Δbpp1-2 was only slightly affected in pathogenic development and SG200Δbpp1-2 was as pathogenic as the progenitor strain SG200 (Table 4). These results suggest that bpp1 plays an important function in b gene expression during pathogenic development, and this becomes evident when autocrine pheromone stimulation is prevented.
DISCUSSION
In this article, we describe the isolation and characterization of the U. maydis bpp1 gene, encoding a G protein β subunit. bpp1 appears to be the only Gβ subunit gene in this organism, a finding that is in line with the situation in all other fungi for which total genome sequences are available.
U. maydis strains lacking the Gβ subunit Bpp1 exhibit a set of phenotypes that are characteristic of cells deficient in cAMP signaling, suggesting that Bpp1 acts upstream of the cAMP-dependent protein kinase PKA. Surprisingly, while other mutants disrupted in cAMP signaling are nonpathogenic, compatible Δbpp1-2 deletion mutants are not affected in pathogenic development.
Bpp1 is a component of the cAMP signaling pathway.
In U. maydis, adenylyl cyclase activity is positively controlled by the Gα subunit Gpa3 (29). Here, we show that the identified Gβ subunit Bpp1 influences cAMP signaling. First, bpp1 deletion strains show filamentous growth and are severely attenuated in cell fusion; both defects can be suppressed by exogenous cAMP. Second, bpp1 deletion mutants are affected in basal as well as pheromone-induced pheromone gene (mfa) expression, while induction of mfa transcription by exogenous cAMP is still possible. Third, epistatic analysis places Bpp1 upstream of PKA. Bpp1 is thus most likely involved in regulating the activity of adenylyl cyclase Uac1.
How does Bpp1 regulate adenylyl cyclase?
In several systems, the Gα subunit as well as the Gβ subunit in complex with the Gγ subunit have been shown to function positively in signal transduction, e.g., mammalian adenylyl cyclases of types II and IV are directly activated by Gα and Gβ/γ subunits in a synergistic manner (13, 48). The Gβγ dimer simply requires its release from the interacting Gα subunit to act on its effector. In U. maydis deletion of either gpa1, gpa2, gpa3, or gpa4 (39) does not lead to elevated pheromone gene expression, indicating that cAMP signaling is not activated. This makes it unlikely that Bpp1 regulates adenylyl cyclase activity directly.
Alternatively, Bpp1 could exert its function through the Gα subunit with which it is associated. The analysis of double mutants in which bpp1 and gpa1, gpa2, or gpa4 were deleted showed that none of these Gα subunit genes was required for filamentous growth of the Δbpp1-2 strains. Thus, decreased cAMP signaling in Δbpp1-2 strains (which is indicated by the filamentous phenotype) is not mediated by one of these Gα subunits.
Instead, genetic data from this study support a model in which Bpp1 (or the complex with a Gγ subunit) appears to be required for proper function of the heterotrimeric G protein containing Gpa3 during axenic growth: Δbpp1-2 Δgpa3 double mutants display filamentous growth and are thus indistinguishable from Δbpp1-2 and Δgpa3 single mutants. However, Δbpp1-2 Δgpa3 and Δgpa3 cells are severely impaired in cell-cell fusion, whereas this process is less severely affected by deleting bpp1. Furthermore, mixtures of Δbpp1-2 mutants are pathogenic, while compatible Δbpp1-2 Δgpa3 mutants are nonpathogenic, as described for Δgpa3 strains (39). Analysis with gpa3Q206L, which codes for a constitutively active Gα mutant protein with decreased GTPase activity, revealed that bpp1 is epistatic to gpa3. gpa3Q206L Δbpp1-2 strains grow by budding and show elevated pheromone gene expression, as do gpa3Q206L single mutant strains. Thus, it is conceivable that Bpp1, Gpa3, and a Gγ subunit form a heterotrimeric G protein.
Bpp1 may be necessary for the proper coupling of Gpa3 to its receptor, and weakening this interaction may result in diminished adenylyl cyclase activity. Interestingly, similar regulation of adenylyl cyclase was observed in Schizosaccharomyces pombe. In Gα (git8), Gβ (git5), and Gγ (git1) deletion mutants of S. pombe, glucose-repressed gene expression and nutrient regulation of sexual development are affected (31, 32, 54). In addition, it has been shown that a constitutively active allele of git8 suppresses the loss of either git5 or git11. On these grounds, the Gα subunit Git8p has been considered the primary regulator of adenylyl cyclase in S. pombe (32).
Another possible explanation for the phenotype of Δbpp1-2 mutants is that deletion of the Gβ subunit Bpp1 affects the protein stability of the Gα subunit Gpa3, as has been described for Neurospora crassa and Cryphonectria parasitica (44, 56). Unfortunately, we were unable to visualize Gpa3 protein in U. maydis by using antibodies raised against GNA-3 of N. crassa (kindly provided by K. Borkovich) or antibodies raised against specific peptides of Gpa3. Nevertheless, we consider this option unlikely because Δbpp1-2 mutants differ from Δbpp1-2 Δgpa3 double mutants in fusion frequency and pathogenic development.
Gβ subunit does not act upstream of the MAP kinase module during mating in U. maydis.
In this communication, we have shown that a bpp1 deletion strain is able to develop conjugation tubes when its defect in cAMP signaling is suppressed by cAMP addition or when the constitutively active allele gpa3Q206L is expressed. In addition, conjugation tube formation was also observed in gpa3 mutant strains in the presence of cAMP and pheromone but was abolished in fuz7 (encoding a MAP kinase kinase) mutants irrespective of cAMP addition. These findings indicate that neither gpa3 nor bpp1 is necessary for this pheromone-induced morphological transition. Therefore, we consider it unlikely that Bpp1 acts on the MAP kinase cascade in U. maydis. This contrasts with the situation in S. cerevisiae and C. neoformans, where the Gβ/γ complex Ste4p/Ste18 and the Gβ subunit Gbp1, respectively, actively transmit the pheromone stimulus to a MAP kinase cascade (53, 55).
The observation that the fraction of cells developing conjugation tubes was reduced in the presence of cAMP is in accordance with observations that formation of dikaryotic filaments is also sensitive to high cAMP levels (17). Thus, constitutively elevated cAMP signaling appears to inhibit morphological transitions during mating. In wild-type cells, the situation may be more balanced through transient activation of cAMP signaling during mating (24, 41).
bpp1 and pathogenic development.
Signal transduction via the cAMP pathway is one of the critical parameters in pathogenesis in a number of pathogenic fungi (33). In U. maydis, deletion of either gpa3, uac1, or adr1 abolishes pathogenesis (12, 39). Therefore, it was an unexpected result that deletion of bpp1 in haploid strains, which abolishes cAMP signaling during saprophytic growth, did not affect the ability of compatible mutants to induce tumors in corn. Thus, the function of the Gβ subunit in the basidiomycete U. maydis differs from that of the Gβ subunits in ascomycetes fungi such as Magnaporthe grisea, Cryphonectria parasitica, and Fusarium oxysporum, where gene deletion severely interferes with pathogenic development (23, 27, 38).
The analyses of solopathogenic strains with deletions of bpp1 revealed an unexpected behavior with respect to pathogenicity: derivatives of CL13 were strongly reduced in their ability to induce tumors in corn plants, while SG200 derivatives, which differ from CL13 in having an autocrine pheromone response, were efficient in tumor induction. In addition, overexpressing an active bE/W heterodimer in CL13 with a deletion of bpp1 (HA103Δbpp1-2) restored pathogenicity. Thus, bpp1 appears to be involved in regulating b gene expression during pathogenesis, when pheromone signaling is turned off. It would be interesting to analyze whether this function of Bpp1 is linked to Gpa1 and/or Gpa2.
Since we observed that Δbpp1-2 strains which are pathogenic require gpa3 to successfully infect corn plants, it is likely that Gpa3 is active in the absence of Bpp1. There is no evidence for another gene encoding a classical Gβ subunit in U. maydis. It is therefore tempting to speculate on the existence of alternative receptors that activate cAMP signaling. One could be coupled to Gpa3, Bpp1, and a not-yet-identified Gγ subunit and might sense environmental cues in axenic culture. The other one would be stimulated only during infectious growth and would activate Gpa3 independently of Bpp1. The idea that Gα subunits can function with nonclassical Gβ/γ dimers is supported by observations in S. cerevisiae. In this organism, the seven-Kelch-domain-containing proteins Gpb1p and Gpb2p function as Gβ proteins and are associated with the Gα subunit Gpa2p (18). Based on the genome sequence, we were unable to detect homologues of either Gpb1p or Gpb2p in U. maydis. However, this does not rule out the existence of another type of nonclassical Gβ subunit in U. maydis. To identify these components, which may be of crucial importance for the interaction of U. maydis with its host, will be a future challenge.
ADDENDUM IN PROOF
After the manuscript was accepted, we were able to demonstrate with the help of affinity-purified Gpa3 antibodies that Gpa3 protein levels in bpp1 deletion strains were comparable to levels detected in wild-type strains.
Acknowledgments
We thank Nicole Rössel for technical assistance in strain construction and Manuel Tönnis as well as Horst Kessler for providing synthetic pheromone. We thank Jan Schirawski for critical comments on the manuscript and K. Borkovich for discussion and providing antibodies.
This work was supported by the DFG through SFB369.
REFERENCES
- 1.Andrews, D. L., J. D. Egan, M. E. Mayorga, and S. E. Gold. 2000. The Ustilago maydis ubc4 and ubc5 genes encode members of a MAP kinase cascade required for filamentous growth. Mol. Plant-Microbe Interact. 13:781-786. [DOI] [PubMed] [Google Scholar]
- 2.Banuett, F. 1992. Ustilago maydis, the delightful blight. Trends Genet. 8:174-180. [DOI] [PubMed] [Google Scholar]
- 3.Banuett, F., and I. Herskowitz. 1989. Different a alleles of Ustilago maydis are necessary for maintenance of filamentous growth but not for meiosis. Proc. Natl. Acad. Sci. USA 86:5878-5882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Banuett, F., and I. Herskowitz. 1994. Identification of fuz7, a Ustilago maydis MEK/MAPKK homolog required for a-locus-dependent and -independent steps in the fungal life cycle. Genes Dev. 8:1367-1378. [DOI] [PubMed] [Google Scholar]
- 5.Barrett, K. J., S. E. Gold, and J. W. Kronstad. 1993. Identification and complementation of a mutation to constitutive filamentous growth in Ustilago maydis. Mol. Plant-Microbe Interact. 6:274-283. [DOI] [PubMed] [Google Scholar]
- 6.Birnbaumer, L., J. Abramowitz, and A. Brown. 1990. Receptor-effector coupling by G proteins. Biochim. Biophys. Acta 1031:163-224. [DOI] [PubMed] [Google Scholar]
- 7.Bolker, M., M. Urban, and R. Kahmann. 1992. The a mating type locus of U. maydis specifies cell signaling components. Cell 68:441-450. [DOI] [PubMed] [Google Scholar]
- 8.Bolker, M., H. U. Bohnert, K. H. Braun, J. Gorl, and R. Kahmann. 1995. Tagging pathogenicity genes in Ustilago maydis by restriction enzyme-mediated integration (REMI). Mol. Gen. Genet. 248:547-552. [DOI] [PubMed] [Google Scholar]
- 9.Bolker, M., S. Genin, C. Lehmler, and R. Kahmann. 1995. Genetic regulation of mating, and dimorphism in Ustilago maydis. Can. J. Bot. 73:320-325. [Google Scholar]
- 10.Bottin, A., J. Kamper, and R. Kahmann. 1996. Isolation of a carbon source-regulated gene from Ustilago maydis. Mol. Gen. Genet. 253:342-352. [DOI] [PubMed] [Google Scholar]
- 11.Brachmann, A., G. Weinzierl, J. Kamper, and R. Kahmann. 2001. Identification of genes in the bW/bE regulatory cascade in Ustilago maydis. Mol. Microbiol. 42:1047-1063. [DOI] [PubMed] [Google Scholar]
- 12.Durrenberger, F., K. Wong, and J. W. Kronstad. 1998. Identification of a cAMP-dependent protein kinase catalytic subunit required for virulence and morphogenesis in Ustilago maydis. Proc. Natl. Acad. Sci. USA 95:5684-5689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gao, B. N., and A. G. Gilman. 1991. Cloning and expression of a widely distributed (type IV) adenylyl cyclase. Proc. Natl. Acad. Sci. USA 88:10178-10182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garcia-Higuera, I., J. Fenoglio, Y. Li, C. Lewis, M. P. Panchenko, O. Reiner, T. F. Smith, and E. J. Neer. 1996. Folding of proteins with WD-repeats: comparison of six members of the WD-repeat superfamily to the G protein beta subunit. Biochemistry 35:13985-13994. [DOI] [PubMed] [Google Scholar]
- 15.Gillissen, B., J. Bergemann, C. Sandmann, B. Schroeer, M. Bolker, and R. Kahmann. 1992. A two-component regulatory system for self/non-self recognition in Ustilago maydis. Cell 68:647-657. [DOI] [PubMed] [Google Scholar]
- 16.Gold, S. E., G. Duncan, K. J. Barrett, and J. Kronstad. 1994. cAMP regulates morphogenesis in the fungal pathogen Ustilago maydis. Genes Dev. 8:2805-2816. [DOI] [PubMed] [Google Scholar]
- 17.Gold, S. E., S. M. Brogdon, M. E. Mayorga, and J. W. Kronstad. 1997. The Ustilago maydis regulatory subunit of a cAMP-dependent protein kinase is required for gall formation in maize. Plant Cell 9:1585-1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harashima, T., and J. Heitman. 2002. The Gα protein Gpa2 controls yeast differentiation by interacting with kelch repeat proteins that mimic Gβ subunits. Mol. Cell 10:163-173. [DOI] [PubMed] [Google Scholar]
- 19.Hartmann, H. A., R. Kahmann, and M. Bolker. 1996. The pheromone response factor coordinates filamentous growth and pathogenicity in Ustilago maydis. EMBO J. 15:1632-1641. [PMC free article] [PubMed] [Google Scholar]
- 20.Hartmann, H. A., J. Kruger, F. Lottspeich, and R. Kahmann. 1999. Environmental signals controlling sexual development of the corn smut fungus Ustilago maydis through the transcriptional regulator Prf1. Plant Cell 11:1293-1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoffmann, C. S., and F. Winston. 1987. A ten minute DNA preparation from yeast efficiently releases autonomous plasmids for transformation in E. coli. Gene 57:267-272. [DOI] [PubMed] [Google Scholar]
- 22.Holliday, R. 1974. Ustilago maydis, p. 575-595. In R. C. King (ed.), Handbook of genetics, vol. 1. Plenum Press, New York, N.Y. [Google Scholar]
- 23.Jain, S., K. Akiyama, T. Kan, T. Ohguchi, and R. Takata. 2003. The G protein beta subunit FGB1 regulates development and pathogenicity in Fusarium oxysporum. Curr. Genet. 43:79-86. [DOI] [PubMed] [Google Scholar]
- 24.Kaffarnik, F., P. Muller, M. Leibundgut, R. Kahmann, and M. Feldbrugge. 2003. PKA and MAPK phosphorylation of Prf1 allows promoter discrimination in Ustilago maydis. EMBO J. 22:5817-5826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kahmann, R., G. Steinberg, C. Basse, M. Feldbrugge, and J. Kamper. 2000. Ustilago maydis, the causative agent of corn smut disease, p. 347-371. In J. W. Kronstad (ed.), Fungal pathology. Kluwer Academic Publishers, Dordrecht, The Netherlands.
- 26.Kamper, J., M. Reichmann, T. Romeis, M. Bolker, and R. Kahmann. 1995. Multiallelic recognition: non-self-dependent dimerization of the bE and bW homeodomain proteins in Ustilago maydis. Cell 81:73-83. [DOI] [PubMed] [Google Scholar]
- 27.Kasahara, S., and D. L. Nuss. 1997. Targeted disruption of a fungal G-protein beta subunit gene results in increased vegetative growth but reduced virulence. Mol. Plant-Microbe Interact. 10:984-993. [DOI] [PubMed] [Google Scholar]
- 28.Koen, J. P. R., G. A. White, and J. A. Hargreaves. 1991. Isolation, characterization and sequence of a gene conferring resistance to the systemic fungicide carboxin from the maize smut pathogen Ustilago maydis. Curr. Genet. 19:475-481. [DOI] [PubMed] [Google Scholar]
- 29.Kruger, J., G. Loubradou, E. Regenfelder, A. Hartmann, and R. Kahmann. 1998. Crosstalk between cAMP and pheromone signalling pathways in Ustilago maydis. Mol. Gen. Genet. 260:193-198. [DOI] [PubMed] [Google Scholar]
- 30.Kruger, J., G. Loubradou, G. Wanner, E. Regenfelder, M. Feldbrugge, and R. Kahmann. 2000. Activation of the cAMP pathway in Ustilago maydis reduces fungal proliferation and teliospore formation in plant tumors. Mol. Plant-Microbe Interact. 13:1034-1040. [DOI] [PubMed] [Google Scholar]
- 31.Landry, S., M. T. Pettit, E. Apolinario, and C. S. Hoffman. 2000. The fission yeast git5 gene encodes a Gβ subunit required for glucose-triggered adenylate cyclase activation. Genetics 154:1463-1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Landry, S., and C. S. Hoffman. 2001. The git5 Gβ and git11 Gγ form an atypical Gβγ dimer acting in the fission yeast glucose/cAMP pathway. Genetics 157:1159-1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lengeler, K. B., R. C. Davidson, C. D'Souza, T. Harashima, W. C. Shen, P. Wang, X. Pan, M. Waugh, and J. Heitman. 2000. Signal transduction cascades regulating fungal development and virulence. Microbiol. Mol. Biol. Rev. 64:746-785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mayorga, M. E., and S. E. Gold. 1999. A MAP kinase encoded by the ubc3 gene of Ustilago maydis is required for filamentous growth and full virulence. Mol. Microbiol. 34:485-497. [DOI] [PubMed] [Google Scholar]
- 35.Muller, P., C. Aichinger, M. Feldbrugge, and R. Kahmann. 1999. The MAP kinase kpp2 regulates mating and pathogenic development in Ustilago maydis. Mol. Microbiol. 34:1007-1017. [DOI] [PubMed] [Google Scholar]
- 36.Muller, P., G. Weinzierl, A. Brachmann, M. Feldbrugge, and R. Kahmann. 2003. Both mating and pathogenic development of the smut fungus Ustilago maydis are regulated by one mitogen-activated protein kinase cascade. Eukaryot. Cell 2:1187-1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Neer, E. J., C. J. Schmidt, R. Nambudripad, and T. F. Smith. 1994. WD repeat proteins: an ancient family of regulatory proteins. Nature 371:297-300. [DOI] [PubMed] [Google Scholar]
- 38.Nishimura, M., G. Park, and J. R. Xu. 2003. The G-beta subunit MGB1 is involved in regulating multiple steps of infection-related morphogenesis in Magnaporthe grisea. Mol. Microbiol. 50:231-243. [DOI] [PubMed] [Google Scholar]
- 39.Regenfelder, E., T. Spellig, A. Hartmann, S. Lauenstein, M. Bolker, and R. Kahmann. 1997. G proteins in Ustilago maydis: transmission of multiple signals? EMBO J. 16:1934-1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 41.Sanchez-Martinez, C., and J. Perez-Martin. 2001. Dimorphism in fungal pathogens: Candida albicans and Ustilago maydis—similar inputs, different outputs. Curr. Opin. Microbiol. 4:214-221. [DOI] [PubMed] [Google Scholar]
- 42.Schauwecker, F., G. Wanner, and R. Kahmann. 1995. Filament-specific expression of a cellulase gene in the dimorphic fungus Ustilago maydis. Biol. Chem. Hoppe Seyler 376:617-625. [DOI] [PubMed] [Google Scholar]
- 43.Schulz, B., F. Banuett, M. Dahl, R. Schlesinger, W. Schafer, T. Martin, I. Herskowitz, and R. Kahmann. 1990. The b alleles of U. maydis, whose combinations program pathogenic development, code for polypeptides containing a homeodomain-related motif. Cell 60:295-306. [DOI] [PubMed] [Google Scholar]
- 44.Segers, G. C., and D. L. Nuss. 2003. Constitutively activated Galpha negatively regulates virulence, reproduction and hydrophobin gene expression in the chestnut blight fungus Cryphonectria parasitica. Fungal Genet. Biol. 38:198-208. [DOI] [PubMed] [Google Scholar]
- 45.Simon, M. I., M. P. Strathmann, and N. Gautam. 1991. Diversity of G proteins in signal transduction. Science 252:802-808. [DOI] [PubMed] [Google Scholar]
- 46.Smith, T. F., C. Gaitatzes, K. Saxena, and E. J. Neer. 1999. The WD repeat: a common architecture for diverse functions. Trends Biochem. Sci. 24:181-185. [DOI] [PubMed] [Google Scholar]
- 47.Snetselaar, K. M., M. Bolker, and R. Kahmann. 1996. Ustilago maydis mating hyphae orient their growth toward pheromone sources. Fungal Genet. Biol. 20:299-312. [DOI] [PubMed] [Google Scholar]
- 48.Tang, W. J., and A. G. Gilman. 1991. Type-specific regulation of adenylyl cyclase by G protein βγ subunits. Science 254:1500-1503. [DOI] [PubMed] [Google Scholar]
- 49.Thompson, J. D., D. G. Higgins, and T. J. Gibson. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Trueheart, J., and I. Herskowitz. 1992. The a locus governs cytoduction in Ustilago maydis. J. Bacteriol. 174:7831-7833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tsukuda, T., S. Carleton, S. Fotheringham, and W. K. Holloman. 1988. Isolation and characterization of an autonomously replicating sequence from Ustilago maydis. Mol. Cell. Biol. 8:3703-3709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Urban, M., R. Kahmann, and M. Bolker. 1996. Identification of the pheromone response element in Ustilago maydis. Mol. Gen. Genet. 251:31-37. [DOI] [PubMed] [Google Scholar]
- 53.Wang, P., J. R. Perfect, and J. Heitman. 2000. The G-protein beta subunit GPB1 is required for mating and haploid fruiting in Cryptococcus neoformans. Mol. Cell. Biol. 20:352-362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Welton, R. M., and C. S. Hoffman. 2000. Glucose monitoring in fission yeast via the Gpa2 galpha, the git5 Gbeta and the git3 putative glucose receptor. Genetics 156:513-521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Whiteway, M., L. Hougan, D. Dignard, D. Y. Thomas, L. Bell, G. C. Saari, F. G. Grant, P. O'Hara, and V. L. MacKay. 1989. The STE4 and STE18 genes of yeast encode potential beta and gamma subunits of the mating factor receptor-coupled G protein. Cell 56:467-477. [DOI] [PubMed] [Google Scholar]
- 56.Yang, Q., S. I. Poole, and K. A. Borkovich. 2002. A G-protein beta subunit required for sexual and vegetative development and maintenance of normal Gα protein levels in Neurospora crassa. Eukaryot. Cell 1:378-390. [DOI] [PMC free article] [PubMed] [Google Scholar]