Abstract
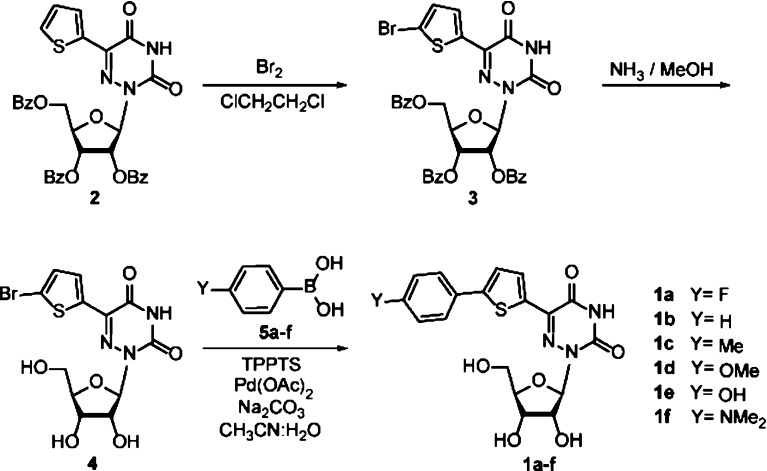
A family of extended 5-modified-6-aza-uridines was obtained via Suzuki coupling reactions with a common brominated precursor. Extending the conjugated-6-aza-uridines with substituted aryl rings increases the push–pull interactions yielding enhanced bathochromic shifts and solvatochromism compared to the parent nucleosides. For example, the methoxy substituted derivative 1d displays λmax abs around 375 nm, with visible emission maxima at 486 nm (Φ = 0.74) and 525 nm (Φ = 0.02) in dioxane and water, respectively.
Diverse approaches have been devised for modifying the nonemissive pyrimidine and purine nucleobases in DNA and RNA into fluorescent surrogates.1−4 The fundamental challenges result from both structural and electronic dilemmas, where any modification aimed at enhancing the electronic features favoring fluorescence can hamper the WC face and its tautomeric preferences, as well as the hybridization and folding features of the resulting oligomers. This issue is particularly challenging when one aims at shifting the emission bands further into the visible and red spectral domains. Such low energy emission is frequently associated with relatively large chromophores with physical footprints, which are much larger than the native nucleobases.5
Nature exploits various mechanisms to tune the photophysics of its small and environmentally sensitive visibly emitting chromophores, such as oxyluciferin6−8 and arylideneimidazolidones (in fluorescent proteins).9−11 Strong charge transfer transitions and proton transfer processes in the excited state typically yield low energy emission, which is dependent on the compactness and polarity of the chromophore’s environment.12−15 Applying these motifs to visibly emitting nucleosides presents additional challenges. In particular, the electron-withdrawing ability of the native nucleobases needs to be augmented to promote effective CT bands. Diverse efforts have resulted in numerous motifs; most, however, either electronically decouple the native pyrimidine or purine from the actual chromophore16 or significantly alter the native structure resulting in less than favorable hybridization features.17−19
Here we combine several key features to advance a family of visibly emitting pyrimidine analogs. Figure 1 depicts the evolution of our design principles, illustrating the transformation of uridine into visibly emissive nucleosides. Conjugation of a 5-membered heterocycle, such as thiophene, at the 5 position of uridine, a “dark” nonemissive native nucleoside (Φ ∼10–4), yielded our first-generation fluorescent nucleosides (A, Figure 1). Depending on the conjugated heterocycle, such 5-modified pyrimidines emit in the visible range (390–443 nm) and have very large Stokes shifts (8400–9700 cm–1), while their quantum efficiency is relatively low (Φ = 0.01–0.035).20,21 Further enhancing the polarization of this conjugated electron-poor/electron-rich biaryl system by introducing the electronegative nitrogen at the pyrimidine’s 6-position (B, Figure 1) resulted in red-shifted absorption and emission maxima and substantially augmented quantum yields (Φ = 0.2–0.8).22 To further shift the emission into the red region of the spectrum, we have advanced the family shown here by directly conjugating a donor group through an extended aromatic system to the electron-deficient 6-aza U (C, Figure 1). Here we disclose the synthesis, as well as structural and photophysical features of this advanced visibly emitting motif.
Figure 1.
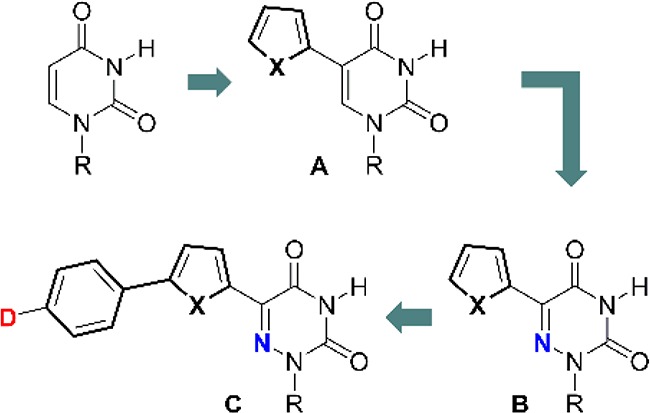
Evolution of the design elements leading to visibly emitting nucleosides.
Although multiple synthetic approaches are conceivable, we have selected Suzuki coupling reactions as the key step in constructing all derivatives (1a–f) from one common precursor (Scheme 1). Bromination of the protected nucleoside 2, which was synthesized using a previously published procedure,22 gave 3 in very good yields (Scheme 1, Figure 2a). Deprotection with methanolic ammonia at 60 °C, followed by recrystallization, yielded 4. All extended nucleosides 1a–f were obtained via a Suzuki coupling reaction between 4 and boronic acids 5a–f,23,24 using a combination of a water-soluble ligand, tris(3-sulfophenyl)phosphine trisodium salt (TPPTS), and palladium acetate as a catalyst.25 Trituration with water and recrystallization from methanol afforded pure 1a–f (49–74%). All nucleosides were thoroughly characterized by 1H and 13C NMR spectroscopy as well as by HRMS and crystallography.25
Scheme 1. Syntheses of Nucleosides 1a–f.
See Supporting Information for synthetic procedures and analytical data.
Figure 2.
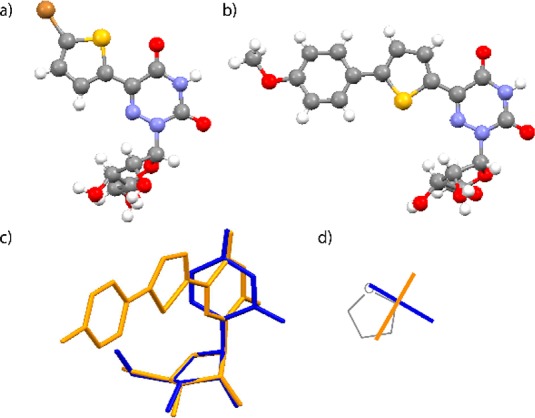
(a, b) X-ray crystal structures of 4 and 1d, respectively; (c) overlay of X-ray the crystal structure of 1c (orange) with uridine (blue); overlaying the ribose rings shows minimal impact on the sugar pucker (rmsd = 0.04 Å); (d) schematic top view illustrating the relative conformation of the nucleobases in uridine (blue) and 1c (orange).
The crystal structure of 1d (Figure 2b) illustrates the common structural features of these extended nucleosides (see also Figures S2.2–S2.6). Overlaying the structures of uridine and 1c (Figure 2c) shows the minimal impact on the sugar pucker, although with a noticeable difference in the dihedral angle χ (−164.41° and −89.0° for uridine and 1c, respectively; Figure 2d). Such differences are likely a result of crystal packing forces, as the extended derivatives frequently show extensive aromatic–aromatic interactions in the solid state (see Figure S3.1).
To evaluate their basic features and assess the influence of the remote substituents on the photophysical properties of 1a–f, absorption and emission spectra were recorded in dioxane and water (Figure 3, Table 1). The absorption maxima of all nucleosides are red-shifted compared to the parent conjugated aza-uridine22 and found in the low energy range of the UV spectrum (350–400 nm). All nucleosides 1a–f are visibly fluorescent, covering a wide window of emission energies ranging from ca. 450 to 600 nm. All display rather large Stokes shifts (>5000 cm–1), which for all derivatives but one (1f) become even more pronounced as polarity increases (>7000 cm–1). Their emission quantum yields in dioxane are moderate to high (0.2–0.75), but drop in a substitution-dependent manner, as discussed below, when taken in more polar solvents.
Figure 3.
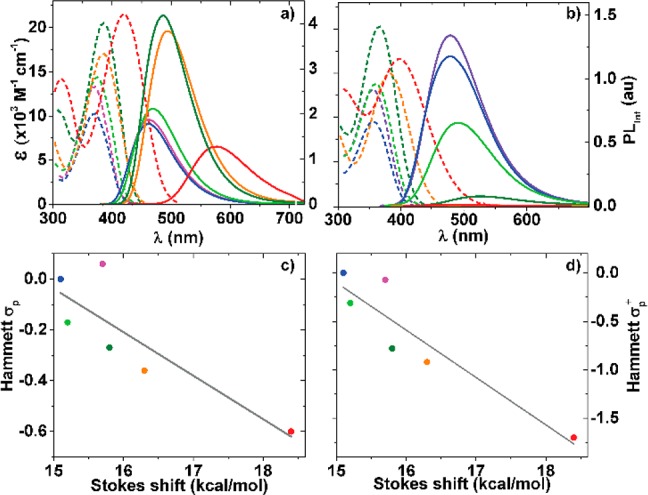
Molar absorptivity (dotted line) and emission (solid line) spectra for 1a (purple), 1b (blue), 1c (green), 1d (dark green), 1e (orange), and 1f (red) in dioxane (a) and water (b). Emission was recorded after excitation at λabs max for each derivative (Table 1). Calculated Stokes shift in kcal/mol for spectra taken in dioxane are correlated with Hammett σpara (c) and Hammett σ+para (d).
Table 1. Photophysical Properties of Nucleosides 1a–fa.
| |
|
Stokes
shift |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| substituent | absorption |
emission |
brightness | νabs –
νem |
|||||
| compound | Y | solvent | λmax [nm] | εb | λmax [nm] | Φc | Φ × ε × 103 | [cm–1] | [kcal/mol] |
| B(22) | (R = ribose) (X = S) | dioxane | 335 | 13 | 415 | 0.80 | 10.4 | 6025 | 16.4 |
| methanol | 334 | 11 | 433 | 0.50 | 5.5 | 7332 | 19.6 | ||
| water | 332 | 11 | 455 | 0.20 | 2.2 | 8492 | 23.3 | ||
| 1a | F | dioxane | 368 | 15.0 | 462 | 0.32 | 4.8 | 5504 | 15.7 |
| methanol | 365 | 14.0 | 488 | 0.11 | 1.5 | 6867 | 19.6 | ||
| water | 357 | 12.6 | 478 | 0.24 | 3.0 | 7140 | 20.4 | ||
| 1b | H | dioxane | 371 | 9.7 | 461 | 0.30 | 2.9 | 5286 | 15.1 |
| methanol | 367 | 8.9 | 488 | 0.16 | 1.4 | 6767 | 19.3 | ||
| water | 356 | 9.6 | 478 | 0.21 | 2.0 | 7184 | 20.5 | ||
| 1c | Me | dioxane | 375 | 14.6 | 468 | 0.38 | 5.6 | 5308 | 15.2 |
| methanol | 372 | 14.0 | 499 | 0.07 | 1.0 | 6855 | 19.6 | ||
| water | 360 | 13.9 | 490 | 0.12 | 1.7 | 7370 | 21.1 | ||
| 1d | MeO | dioxane | 383 | 20.8 | 486 | 0.74 | 15.4 | 5534 | 15.8 |
| water | 366 | 19.7 | 525 | 0.02 | 0.4 | 8275 | 23.7 | ||
| 1e | HO | dioxane | 385 | 16.9 | 494 | 0.71 | 12.0 | 5695 | 16.3 |
| water | 381 | 15.0 | 517 | <0.01 | <0.1 | 6944 | 19.8 | ||
| 1f | Me2N | dioxane | 420 | 21.4 | 575 | 0.20 | 4.3 | 6428 | 18.4 |
| water | 398 | 16.8 | 484 | <0.01 | <0.1 | 4464 | 12.8 | ||
Absorption and steady-state emission spectroscopy studies were performed using samples prepared from a concentrated DMSO stock solution.25
ε in [× 103 M–1 cm–1].
Fluorescence standards: Coumarin 102 was used for 1a–e, and Coumarin 153 was used for 1f.
Increasing the electron-rich character of the substituent on the phenyl ring results in a bathochromic shift for both absorption and emission maxima in the following general order: 1a ≈ 1b < 1c < 1d < 1e < 1f.26 This illustrates the impact of the substituent on both the ground and excited state. In apolar media the highest quantum yield is observed for derivatives with oxygen-containing substituents 1d and 1e (0.74 and 0.71, respectively). The opposite is observed in polar protic media where the less electron-rich substitutions show a higher fluorescent intensity while strong fluorescent quenching is observed for the most electron-rich derivatives 1e and 1f compared to 1a–d. Such facilitation of nonradiative decay pathways for fluorophores capable of H-bonding is not uncommon.13,27,28 Correlating the calculated Stokes shifts observed in dioxane against Hammett σpara and σ+para parameters shows a reliable trend (Figure 3c,d). This provides a useful design tool enabling the use of established linear free energy parameters to confidentially anticipate select photophysical properties.29,30
To evaluate the influence of polarity on the nucleosides’ photophysical properties and hence their responsiveness, spectra were measured in dioxane [ET(30) = 36.0 kcal/mol], methanol [ET(30) = 55.4 kcal/mol], and mixtures thereof.31 For each solution the ET(30) value was experimentally determined using Reichardt’s dye.32 While absorption spectra show little to no variation as polarity is systematically varied,33 significant changes are seen in both emission wavelengths and intensity (Figure 4a,b).34 With increasing polarity a bathochromic shift of λem max was seen for all extended nucleosides 1a–f. For example, the emission maximum of 1c is 468 in dioxane and 499 nm in methanol. Similar trends were reported for the parent 5-thiopheno-6-aza-uridine,22 and 5-thiopheno-uridine.35 To better quantify this effect, Stokes shifts were calculated for each sample containing the dioxane/methanol mixture and plotted against the experimentally determined ET(30) values. As with related emissive nucleosides, a linear fit is observed (Figure 4c,d). Additionally, a steady decrease in the integrated emission was observed for 1c and 1d (as well as the parent 5-thiopheno-6-aza-uridine,22 and 5-thiophene-uridine35) as the content of the protic solvent (e.g., H2O, MeOH) increases. This phenomenon is rather common in fluorophores that display significant charge transfer character in their excited state.13,36
Figure 4.
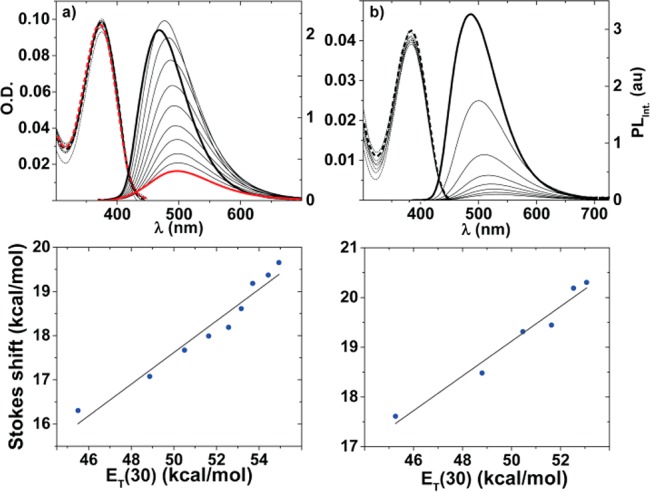
(a,b) Assessing the effect of solvent polarity on absorption (dotted line) and emission (solid line), in dioxane (bold black line), methanol (bold red line), and their mixtures (black lines) for 1c (6.7 × 10–6 M) and 1d (2.0 × 10–6 M); respectively.25 (c, d) Correlating Stokes shift vs ET(30) values obtained from dioxane–methanol mixtures for 1c (90%:10% → 10%:90%; slope: 0.36 and R2 = 0.95) and 1d (90%:10% → 40%:60%; slope: 0.35 and R2 = 0.98).25 Experimental errors are smaller than the data symbols; see SI for enlarged correlations (Figures S8.1 and S8.2).
In summary, visibly emitting, bright, and responsive nucleosides have been obtained by implementing an enhanced charge transfer character in 5-substituted 6-aza-uridines. The photophysical features of these synthetically accessible analogs can be tuned by judiciously introducing substituents of distinct electronic character at a remote but conjugated position. In general, the extended analogs reported here display higher emission quantum yields in apolar solvents but remain sufficiently bright in polar media. This trend is, however, dependent on the nature of the substituent with highly electron-rich derivatives suffering the highest loss in emission quantum yield. Nevertheless, the highly desirable and tunable photophysical properties, including pronounced solvatochromism, make this 6-aza-uridine motif a very attractive scaffold for the design and development of useful biophysical probes.
Acknowledgments
This work was supported by a grant from the NIH (GM 069773). We are grateful to Drs. Curtis Moore and Arnold Rheingold (UCSD Chemistry and Biochemistry X-ray Facility) for their help.
Supporting Information Available
Experimental procedures and full characterization details including, 1H, 13C, and 19F NMR, as well as crystal structures. This material is available free of charge via the Internet at http://pubs.acs.org.
The authors declare no competing financial interest.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- Sinkeldam R. W.; Greco N. J.; Tor Y. Chem. Rev. 2010, 110, 2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelmsson L. M. Q. Rev. Biophys. 2010, 43, 159. [DOI] [PubMed] [Google Scholar]
- a Hawkins M. E. Cell Biochem. Biophys. 2001, 34, 257. [DOI] [PubMed] [Google Scholar]; b Okamoto A.; Saito Y.; Saito I. J. Photochem. Photobiol., C 2005, 6, 108. [Google Scholar]; c Dodd D. W.; Hudson R. H. E. Mini-Rev. Org. Chem. 2009, 6, 378. [Google Scholar]
- a Rist M. J.; Marino J. P. Curr. Org. Chem. 2002, 6, 775. [Google Scholar]; b Wilson J. N.; Kool E. T. Org. Biomol. Chem. 2006, 4, 4265. [DOI] [PubMed] [Google Scholar]; c Kimoto M.; Cox R. S. III; Hirao I. Expert Rev. Mol. Diagn. 2011, 11, 321. [DOI] [PubMed] [Google Scholar]
- Lavis L. D.; Raines R. T. ACS Chem. Biol. 2008, 3, 142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood K. V.; Lam Y. A.; Seliger H. H.; Mcelroy W. D. Science 1989, 244, 700. [DOI] [PubMed] [Google Scholar]
- Branchini B. R.; Magyar R. A.; Murtiashaw M. H.; Anderson S. M.; Helgerson L. C.; Zimmer M. Biochemistry 1999, 38, 13223. [DOI] [PubMed] [Google Scholar]
- Branchini B. R.; Southworth T. L.; Murtiashaw M. H.; Magyar R. A.; Gonzalez S. A.; Ruggiero M. C.; Stroh J. G. Biochemistry 2004, 43, 7255. [DOI] [PubMed] [Google Scholar]
- Tsien R. Y. Annu. Rev. Biochem. 1998, 67, 509. [DOI] [PubMed] [Google Scholar]
- a Wachter R. M. Acc. Chem. Res. 2007, 40, 120. [DOI] [PubMed] [Google Scholar]; b Tolbert L. M.; Baldridge A.; Kowaloik J.; Solntsev K. M. Acc. Chem. Res. 2012, 45, 171. [DOI] [PubMed] [Google Scholar]
- Wachter R. M. Photochem. Photobiol. 2006, 82, 339. [DOI] [PubMed] [Google Scholar]
- Nakatsu T.; Ichiyama S.; Hiratake J.; Saldanha A.; Kobashi N.; Sakata K.; Kato H. Nature 2006, 440, 372. [DOI] [PubMed] [Google Scholar]
- a Strong donor/acceptor interactions are also found in synthetic fluorophores such as PRODAN and cyanine dyes. See:Lakowicz J. R.Principles of fluorescence spectroscopy, 3rd ed.; Springer: New York, 2006. [Google Scholar]
- Raymond S. B.; Skoch J.; Hills I. D.; Nesterov E. E.; Swager T. M.; Bacskai B. J. Eur. J. Nucl. Med. Mol. Imaging 2008, 35Suppl 1S93. [DOI] [PubMed] [Google Scholar]
- Meek S. T.; Nesterov E. E.; Swager T. M. Org. Lett. 2008, 10, 2991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedl J.; Menova P.; Pohl R.; Orsag P.; Fojta M.; Hocek M. J. Org. Chem. 2012, 77, 8287. [DOI] [PubMed] [Google Scholar]
- Gaballah S. T.; Collier G.; Netzel T. L. J. Phys. Chem. B 2005, 109, 12175. [DOI] [PubMed] [Google Scholar]
- Grunwald C.; Kwon T.; Piton N.; Forster U.; Wachtveitl J.; Engels J. W. Bioorg. Med. Chem. 2008, 16, 19. [DOI] [PubMed] [Google Scholar]
- Okamoto A.; Tainaka K.; Fujiwara Y. J. Org. Chem. 2006, 71, 3592. [DOI] [PubMed] [Google Scholar]
- Greco N. J.; Tor Y. J. Am. Chem. Soc. 2005, 127, 10784. [DOI] [PubMed] [Google Scholar]
- a Greco N. J.; Tor Y. Tetrahedron 2007, 63, 3515. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Sinkeldam R. W.; Wheat A. J.; Boyaci H.; Tor Y. ChemPhysChem 2011, 12, 567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinkeldam R. W.; Hopkins P. A.; Tor Y. ChemPhysChem 2012, 13, 3350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovaliov M.; Segal M.; Fischer B. Tetrahedron 2013, 69, 3698. [Google Scholar]
- Capek P.; Pohl R.; Hocek M. Org. Biomol. Chem. 2006, 4, 2278. [DOI] [PubMed] [Google Scholar]
- See Supporting Information for additional details.
- This is true for nucleosides 1a–f, with the exception of 1e and 1f when taken in water.
- Zhao G. J.; Han K. L. Acc. Chem. Res. 2012, 45, 404. [DOI] [PubMed] [Google Scholar]
- Liu Y. H.; Zhao G. J.; Li G. Y.; Han K. L. J. Photochem. Photobiol., A 2010, 209, 181. [Google Scholar]
- a Tzalis D.; Tor Y. Tetrahedron Lett. 1995, 36, 6017. [Google Scholar]; b Joshi H. S.; Jamshidi R.; Tor Y. Angew. Chem., Int. Ed. 1999, 38, 2722. [DOI] [PubMed] [Google Scholar]
- a Mizuta M.; Seio K.; Ohkubo A.; Sekine M. J. Phys. Chem. B 2009, 113, 9562. [DOI] [PubMed] [Google Scholar]; b Wahba A. S.; Azizi F.; Deleavey G. F.; Brown C.; Robert F.; Carrier M.; Kalota A.; Gewirtz A. M.; Pelletier J.; Hudson R. H. E.; Damha M. J. ACS Chem. Biol. 2011, 6, 912. [DOI] [PubMed] [Google Scholar]; c Kovaliov M.; Weitman M.; Major D. T.; Fischer B. J. Org. Chem. 2014, 79, 7051. [DOI] [PubMed] [Google Scholar]
- As apparent from their structures, the photophysical features of the azauridine-based nucleosides are inherently sensitive to changes in pH. Ionizable groups (e.g., OH) further complicate their acid/base features. Titration of 1c between pH 2 and 12, however, gives a single pKa value of 6.7 (Figure S7.1, S7.2), similar to the value obtained for the unmodified parent system (ref (22)).
- Reichardt C. Chem. Rev. 1994, 94, 2319. [Google Scholar]
- This statement is true for methanol, dioxane, and their mixtures. When comparing the differences between absorption maxima in dioxane and water, however, the shifts are more significant.
- Although low quantum yield values were observed for 1e and 1f in water, it is important to note that all modified nucleosides besides 1b have higher extinction coefficients compared to the parent compound B.
- Srivatsan S. G.; Tor Y. Chem.—Asian J. 2009, 4, 419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhuguru J.; Liu W. J.; Gonzalez W. G.; Babinchak W. M.; Miksovska J.; Landgraf R.; Wilson J. N. J. Org. Chem. 2014, 79, 4940. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.