Abstract
The planum temporale is a region on the posterior surface of the temporal lobe that exhibits robust leftward structural asymmetry that has been linked to verbal ability in children and adults. Traditionally, structural asymmetry has been quantified with manual assessment of high resolution MRI scans. Such measures require subjective and frequently unreliable determination of highly variable anatomical boundaries. Methodological developments in automated image processing (voxel based morphometry -VBM) offer the opportunity to obtain objective and reliable measures of structural variation. This study examined the extent to which a VBM measure of gray matter asymmetry in the posterior superior temporal gyrus (pSTG) characterized the same individual variation as a manual measure of planum temporale asymmetry in 99 healthy adults and 39 typically developing children. Planum temporale asymmetry was significantly correlated with pSTG gray matter asymmetry in the samples of adults and children. As a measure of validity we examined the extent to which the VBM measure of pSTG gray matter asymmetry predicted measures of verbal ability that were associated with the manual measure of planum temporale asymmetry in the same children. The two asymmetry measures predicted the same variance in verbal ability. The automated measure of pSTG gray matter asymmetry predicted additional significant variance in verbal ability, however. In addition, a posterior STS region was also identified that significantly predicted verbal ability. These results demonstrate significant advantages of an automated voxel-based measure over a manual measure of planum temporale asymmetry.
Introduction
Automated methods for brain structure measurement at volumetric, sulcal, and voxel levels of analysis provide fast and consistent information about brain morphology (Ashburner and Friston, 2005; Fischl and Dale, 2000; Mangin et al., 2004; Van Essen et al., 2001). Automated methods have been increasingly adopted because manual methods of brain structure measurement can be time consuming, require considerable anatomical expertise, and are limited to brain regions with visible and constant anatomical boundaries. Few studies have compared the information provided by the two types of methods. One relatively neglected question is whether automated and manual measures characterize the same or complementary anatomical features (Eckert et al., 2005; Luders et al., 2004; Tisserand et al., 2002). Validation studies examining the association between automated and manual methods are critical for understanding how findings from studies employing automated measures might relate to findings from studies employing manual measures of brain structure. Moreover, it is important to understand if these different methods yield unique or similar biological information relevant in the investigation of structural substrates of brain function. In this study, we examined whether an automated measure of VBM gray matter asymmetry in the pSTG could replace a manual measure of planum temporale asymmetry.
The planum temporale is a triangular shaped region on the surface of the pSTG. Because the pSTG is engaged in complex auditory processing (Griffiths and Warren, 2002) and lesions in this region can produce receptive aphasia (Wernicke, 1874), the exaggerated leftward structural asymmetry of the planum temporale (Geschwind and Levitsky, 1968; Pfeifer, 1936; Steinmetz et al., 1991; Teszner et al., 1972; Witelson and Pallie, 1973) has excited scientists interested in language lateralization. Two large studies have now provided clear evidence that the structural asymmetry of the planum temporale is largely independent of language dominance (Dorsaint-Pierre et al., 2006; Eckert et al., 2006a). There is, however, copious evidence that manual measures of planum temporale asymmetry are associated with verbal ability (Eckert et al., 2001; Galaburda et al., 1985; Gauger et al., 1997; Leonard et al., 1996; Rumsey et al., 1997), musical ability (Schlaug et al., 1995), neurogenetic disorders (Eckert et al., 2006b; Leonard et al., 1993), and handedness (Dos Santos Sequeira et al., 2006; Zetzsche et al., 2001). In one relatively large sample, for example, Eckert et al. (2001) demonstrated that leftward planum temporale asymmetry was associated with superior verbal IQ and phonological skill in a community sample of strongly right-handed sixth-grade children. Surprisingly, the non-right-handed children with superior verbal IQ had rightward planum temporale asymmetry, raising methodological questions as to whether similar results would have been observed with a different measure of anatomical asymmetry.
The planum temporale is bounded anteriorly by Heschl’s sulcus and posteriorly by the ascending branch of the Sylvian fissure. The variability of these boundaries leads to difficulty in obtaining reliable manual measurements of the planum temporale. For this reason, an automated measure of planum temporale asymmetry is preferable. VBM has been used to examine cerebral asymmetries in gray matter and to demonstrate asymmetries at pSTG regions corresponding in location to the planum temporale (Good et al., 2001; Watkins et al., 2001; Luders et al. 2004). The present study was designed to examine the extent to which a manual measure of planum temporale asymmetry is associated with VBM gray matter asymmetry in the pSTG. We examined the concordance of these measures in a sample of adults and a sample of children, as well as the extent to which each measure was influenced by sulcal/gyral features. In this sample of children it was also possible to examine the extent to which the automated and manual asymmetry measures predicted the same individual variability in cognitive ability among the children. Specifically, we sought to determine whether the automated measure would provide a more localized explanation for the association between planum temporale asymmetry and verbal ability or whether each measure provided unique biological information that explained individual variability in verbal ability.
Methods
Participants
Adults
Ninety-nine adults (51 females, 48 males; mean age 28.05 years, range-18.6 to 72.4), recruited from Milwaukee, WI, gave written informed consent according to the institutional guidelines of the Medical College of Wisconsin Human Research Review Committee. These participants were included in fMRI studies of language organization (Springer et al., 1999; Szaflarski et al., 2002), as well as a study examining the association between language laterality and planum temporale asymmetry (Eckert et al., 2006b).
Children
Thirty-nine children (20 females, 19 males; mean age 11.4 years, range-10.8 to 12.0) were recruited from the Alachua County, FL area. Informed consent and assent were obtained from the parents and children according to the institutional guidelines of the University of Florida Institutional Review Board. This sample was representative of sixth grade children enrolled in the Alachua County public school system according to gender, race and ethnicity, scholastic achievement based on the Iowa Tests of Basic Skills, and socioeconomic status (Eckert et al., 2001). Socioeconomic status (SES), or family income, was estimated from participation in the public schools lunch subsidy program. Eligibility for this program requires that families earn below a federally defined level of yearly income. Twelve of the children in this study were receiving partial or full lunch subsidy and were classified as coming from low income families (Barddoulle-Crema et al., 1986). These children demonstrated lower verbal ability compared to children who were not receiving lunch subsidy and they had parents who spent significantly less time helping with homework (Eckert et al., 2001). SES explains a large percentage of individual variability on psychometric measures of cognition that could be independent of biological events that occur in early development. For example, planum temporale asymmetry is established early in development (Witelson and Pallie, 1973) and has been observed to predict verbal ability within lunch subsidy groups (Eckert et al., 2001).
Image Acquisition
Adults
The volumetric and gapless spoiled gradient-recalled sequence (SPGR) structural images (matrix size = 256 × 128 and FOV = 24 cm) used in this study were acquired on a 1.5 T GE Signa scanner (GE Medical Systems, Milwaukee, WI) at the Medical College of Wisconsin. This study included 1 image, 73 images, and 25 images with 1.1, 1.2, and 1.3 mm thick sections, respectively. As noted in the Results section, there were no differences in gray matter asymmetry between groups of images with different section thickness in a region of the pSTG that is shown to exhibit a significant leftward asymmetry (F(1,98)=.38, ns) or for planum temporale asymmetry (F(1,98)=.78, ns).
Children
The volumetric and gapless MP-Rage structural images used in this study (matrix size = 256 × 130 and FOV = 25 cm; 1.25 mm thick) were acquired on a 1.5 T Siemens Magnetom scanner (Siemens Medical Solutions USA, Malvern, PA) at the University of Florida.
VBM Asymmetry Processing
The structural images were normalized, bias field corrected, and segmented using an integrated generative model (unified segmentation; (Ashburner and Friston, 2005)). Unified segmentation involves alternating between segmentation, bias field correction, and normalization to obtain local optimal solutions for each process. The adult ICBM/MNI a priori templates and the pediatric CCHMC a priori templates (Wilke, 2002) were used to segment and normalize (affine and 16 iteration non-linear transformations) the adult’s and children’s images, respectively. The resulting images were modulated to correct voxel signal intensities for the amount of volume displacement during normalization. The normalized and segmented images were averaged across the adult and children’s datasets to produce gray matter, white matter, and CSF sample specific a priori templates. Each a priori template was flipped and averaged with the unflipped template to create symmetrical gray matter, white matter, and CSF templates for each sample. Unified segmentation was then performed again for each sample using the respective symmetrical templates. The images were modulated to correct voxel signal intensity for volume displacement during normalization and reflect the volume of gray matter. The normalized, segmented, and modulated images were then smoothed using a 12 mm kernel to ensure the data are normally distributed and to limit the number of false positive findings (Salmond et al., 2002). A gray matter asymmetry image was created using the following formula where i1 refers to each subject’s smoothed and symmetrically normalized image: (i1-flipped(i1))/(i1+flipped(i1)/2). These preprocessing methods are consistent with methods used to produce asymmetry images in other VBM studies (Good et al., 2001; Luders et al., 2004; Watkins et al., 2001). The statistics section below describes how these asymmetry images were used to create a variable representing gray matter asymmetry within the pSTG.
Manual Planum Temporale Measure
Each image was rigidly aligned to the anterior commissure - posterior commissure plane, but not warped into a standard space as in the VBM pre-processing steps. These different alignment approaches were used so that comparisons could be made using methods that are generally employed in the extant literature. The surface area of the planum temporale was measured between 46 and 56 mm lateral to the midline in sagittal sections that exhibit the most exaggerated planum temporale asymmetry (Best and Demb, 1999). Heschl’s sulcus and the intersection between the horizontal and vertical banks of the Sylvian fissure served as the respective anterior and posterior boundaries for the planum temporale measurement. The left and right planum temporale were measured blindly by randomly ordering the display of the left or right hemisphere planum temporale across subjects in order to ensure that raters were not biased by expectations of planum size and Sylvian fissure shape. Planum temporale asymmetry was defined using the same formula used to obtain gray matter asymmetry (surface area of the left planum — surface area of the right planum / average of the left and right planum). The planum temporale asymmetry data used for this study were included in a study of language laterality involving the adults (Eckert et al., 2006) and reading skill performance involving the children (Eckert et al., 2001). Additional details regarding the planum temporale measurement methods can be found in Eckert et al. (2001; 2006).
The anterior boundary of the planum temporale measurement is dependent on the shape of Heschl’s gyrus. The shape of Heschl’s gyrus was classified into 3 distinct types: 1) a single Heschl’s gyrus; 2) a Heschl’s gyrus that is divided into 2 gyri by a sulcus intermedius; and 3) a duplicated Heschl’s gyrus in which a second gyrus appears posterior to Heschl’s sulcus (Leonard et al., 1998). In cases of a sulcus intermedius and a duplicated Heschl’s gyrus, Heschl’s sulcus is used to define the anterior boundary of the planum temporale. This operational definition of the anterior boundary is consistent with “Pfeifer’s criterion” (von Economo and Horn, 1930) for defining the anterior boundary of the planum temporale as the sulcus appearing with Heschl’s gyrus at the most medial appearance of the gyrus where it abuts the retroinsular region (Pfeifer, 1920; Steinmetz et al. 1989). The Results section demonstrates that this classification approach influences the size of the planum temporale. The posterior boundary of the planum temporale is dependent on the shape of the Sylvian fissure and can influence the planum temporale measure. The shape of the Sylvian fissure was classified into 3 distinct types (Witelson and Kigar, 1992): 1) an L shape in which horizontal and vertical banks of the Sylvian fissure are present; 2) a V shape in which only a vertical bank was present; and 3) an H shape in which only the horizontal bank was present. The frequencies of these sulcal/gyral features are reported in the Results section.
Behavioral Measures
The children in this study were administered a battery of tests selected to assess their verbal ability for spoken and written language. To assess reading abililty, the children were administered three tests from the Woodcock-Johnson Reading Mastery Tests-Revised (WJRMT-R), Word Attack, Word Identification, and Passage Comprehension. To assess spoken language and to obtain an estimate of intelligence, the children were administered three tests from the Wechsler Intelligence Scales for Children-III (WISC), Information, Similarities, and Block Design tests (Sattler, 1992). Additional details regarding this assessment battery are presented in Eckert et al. (2001). The Information test, which assesses a child’s knowledge of events, objects, places, and people, was a particularly important measure because performance on this test was strongly correlated with performance on the other verbal tasks, indicating that Information was a good representation of general verbal ability. Specifically, Information performance was significantly related to Similarities (r=.62, p<.001), Passage Comprehension (r=.53, p<.001), Word Attack (r=.56, p<.001), Word Identification (r=.57, p<.001), after controlling for high or low family income. In addition, Information performance was strongly associated with planum temporale asymmetry, making it a good test variable for determining the extent to which the VBM asymmetry measure could predict the same individual variation in a measure of verbal ability.
The adults and children were administered the Edinburgh Handedness questionnaire to collect an index of hand preference. Witelson & Kigar (1992) demonstrated differences in planum temporale size in strongly right-handed compared to non-right-handed men. The participants in this study were classified as right-handed if they demonstrated a handedness score of 75 or greater (scale: −100 to 100), and this classification was used to specifically examine associations within groups of right-handed (n=27) and non-right-handed (n=12) children. The same classification was used in Eckert et al. (2001), in which leftward planum temporale asymmetry positively predicted verbal ability in right-handed children and negatively predicted verbal ability in non-right-handed-children.
Statistics
Manual and Automated Asymmetry Associations
To determine the extent to which the VBM gray matter asymmetry and planum temporale measures were related, the following analyses were performed: 1) SPM5 was used to identify areas of significant gray matter asymmetry in the adults and children according to the results of a one-sample t-test (relative to test value = 0) examining the VBM asymmetry images; and 2) SPM5 was used to perform regression analyses within the adults and children to examine the association between the manual measure of planum temporale asymmetry and voxel-wise gray matter asymmetry. Statistical significance thresholds of p<.001 were used for the VBM 1-sample t-test and correlation analyses within the pSTG, which was predicted to exhibit significant laterality and correlate with the planum temporale asymmetry measure. These uncorrected thresholds were used because of our a priori predictions about the association between measures from the same anatomical region and previous evidence linking VBM asymmetry and planum temporale measures (Luders et al., 2004). MarsBar (Brett et al., 2002) was used to obtain an average voxel-wise gray matter asymmetry value from the pSTG voxels that exhibited significant leftward asymmetry in the adults and children, which we have labeled the VBM pSTG asymmetry.
Sulcal/Gyral Features & Asymmetry
To determine the extent to which sulcal/gyral features were related to the asymmetry measures, Analysis of Variance was performed. For each sample, the planum temporale and VBM pSTG asymmetry measures were compared between groups of subjects according to their Heschl’s gyrus and Sylvian fissure classifications. Bonferroni post-hoc comparisons were performed to determine the extent to which group differences could be attributed to specific sulcal/gyral classifications.
Structure-Function Associations: Children
To determine the extent to which VBM gray matter asymmetry and planum temporale asymmetry predicted shared variance in verbal ability, multiple regression analyses were performed. First, SPM5 analyses were performed to identify pSTG gray matter voxels that predicted verbal ability in the children. This analysis was restricted to the 27 right-handed children, given previous evidence that leftward planum temporale asymmetry is positively correlated with verbal ability only in this subgroup (Eckert et al., 2001), to localize the pSTG region that contributed to the association between verbal ability and planum termporale asymmetry. The estimate of SES was included as a covariate in this analysis. The average gray matter asymmetry within a cluster of significantly predicting pSTG voxels (restricted to the region exhibiting significant asymmetry across the group) was created by: 1) multiplying the gray matter asymmetry results (p<.001) and the verbal ability correlation results (p<.001) using IMCALC in SPM5; and 2) collecting the average asymmetry from each subject’s image within this overlapping region using MarsBar. This more focused pSTG gray matter asymmetry value, which we have labeled the predictive VBM pSTG asymmetry, was used in a regression analysis to compare the predictive power of the voxel-based and manual asymmetry measures. We asked whether the voxel-based measure would predict significantly more variance than the manual measure of planum temporale asymmetry.
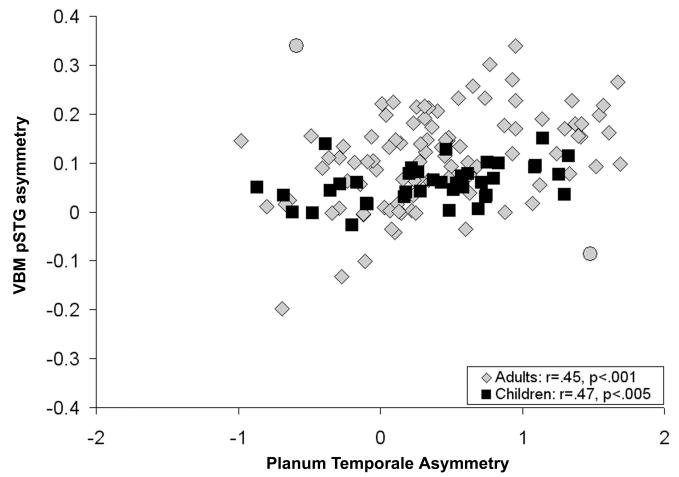
Two outliers (Cohen & Cohen, 1983) in the sample of adults were observed when examining a scatter-plot of planum temporale asymmetry and VBM pSTG asymmetry (subject 1: planum temporale asymmetry = -.60, VBM pSTG asymmetry = .34, standardized residual error = 3.06; subject 2: planum temporale asymmetry = 1.48, VBM pSTG asymmetry = -.09, standardized residual error = 2.75). Visual inspection of the planum temporale measures indicated that the planum measures were correct, and visual inspection of the VBM pre-processed images indicated that there were not pre-processing errors affecting the gray matter asymmetry measures. These outliers are presented in Figure 1 and been have included in the statistical analyses, except for analyses directly comparing the manual and VBM measures.
Figure 1.
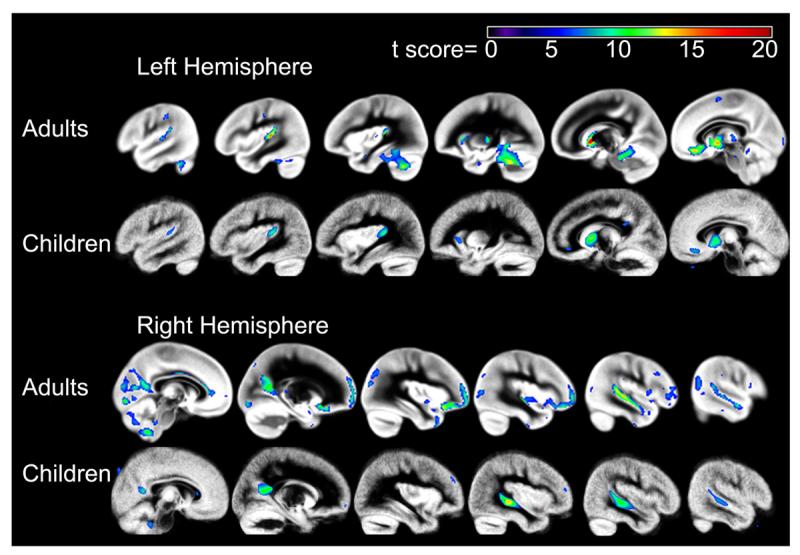
One-sample t-test gray matter asymmetry results across the brain for the adults (top) and children (bottom). The color scale represents the t score at each voxel, for a peak threshold of p<.05, family wise error corrected.
Results
Manual and Automated Asymmetry Associations
A one-sample t-test revealed regions of significant leftward gray matter asymmetry in the samples of adults and children. The whole brain gray matter asymmetry results are presented in Figure 1 (p<.05, family-wise error corrected). Significant leftward pSTG asymmetry, in particular, is presented in Figure 2 (p<.001, uncorrected). Figure 2 also shows that manually defined planum temporale asymmetry was significantly correlated with pSTG gray matter asymmetry in regions that exhibited significant leftward gray matter asymmetry in the one-sample t-test. These associations were present within anterior and posterior pSTG regions in the adults and focused within anterior pSTG regions in the children. Twenty percent of the variance in manually measured planum temporale asymmetry could be explained by the average gray matter asymmetry in the pSTG cluster from the one-sample t-tests (VBM pSTG asymmetry) in the adults (r(97)=.45, p<.001). In the sample of children, 22 percent of the variance in planum temporale asymmetry could be explained by the VBM pSTG asymmetry (r(39)=.47, p<.005). Scatter-plots of the association between the anatomical measures are presented in Figure 3. Correlations between planum temporale asymmetry and VBM gray matter asymmetry outside of the pSTG region were treated as post-hoc analyses for both samples, and there were no correlations that survived a family-wise error threshold of p<.05.
Figure 2.
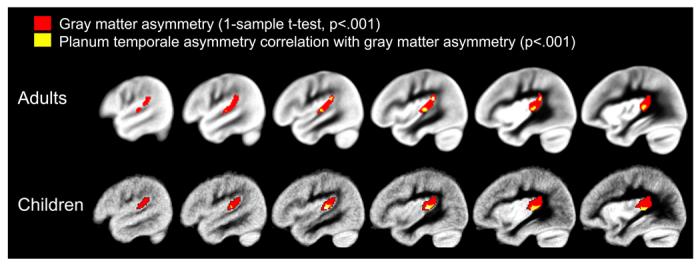
The one-sample t-test pSTG results and correlation results between gray matter asymmetry and planum temporale asymmetry are presented for the adults and children. The red clusters represent areas exhibiting leftward gray matter asymmetry in the pSTG (VBM pSTG asymmetry). The yellow clusters represent areas exhibiting significant correlations between gray matter asymmetry and manually defined planum temporale asymmetry. Note that the areas showing a correlation between planum temporale asymmetry and gray matter asymmetry overlap with areas of significant gray matter asymmetry from the one-sample t-test. In addition, the overlap in results occurs at the boundaries of the planum temporale, and in a region corresponding to Heschl’s sulcus in particular. The maps are thresholded at p<.001, uncorrected for multiple comparisons.
Figure 3.
Scatter-plot of the association between manually defined planum temporale asymmetry and the VBM pSTG asymmetry measure (Figure 2) in the samples of adults (gray diamonds) and children (black squares). Two outliers (gray circles) in the adult sample were not included in the correlation between the two anatomical measures.
Sulcal/Gyral Features & Asymmetry
Individual variability in the sulcal/gyral features of Heschl’s gyrus and the Sylvian fissure influence the anterior and posterior boundaries of the manual planum temporale measurement. The morphology of Heschl’s gyrus and the Sylvian fissure was examined to determine the extent to which these sulcal/gyral features contributed to variability in the manual planum temporale asymmetry and automated voxel-based asymmetry measures. There was considerable variability in Heschl’s gyrus morphology across the two samples (Adults: left hemisphere frequencies, single: 54; divided=26; duplicated=19; right hemisphere frequencies: single: 43; divided=39; duplicated=17; Children: left hemisphere frequencies, single: 19; divided=12; duplicated=8; right hemisphere frequencies: single: 17; divided=10; duplicated=12). For each sample, the presence of a divided Heschl’s gyrus in the right hemisphere was associated with greater leftward planum temporale asymmetry than a single Heschl’s gyrus in the right hemisphere (Adults: F(2,98)=2.42, p<.10; Children F(2,38)=6.49, p<.005). Bonferroni post-hoc comparisons revealed a significant difference in planum temporale asymmetry (mean difference = .70, std error =.20; p<.005) for the divided compared to single Heschl’s gyrus. A similar trend was observed in the adult sample. The VBM pSTG asymmetry measure from the one-sample t-test was then used to examine the influence of Heschl’s gyrus features on voxel-based asymmetry. The presence of a divided Heschl’s gyrus in the right hemisphere was not associated with greater leftward VBM pSTG asymmetry in the samples of adults (F(1,80)=0.21, ns) or in the sample of children (F(2,26)=0.02, ns).
Similar results were observed for Sylvian fissure morphology. There was also considerable variability in Sylvian fissure morphology across the two samples (Adults: left hemisphere frequencies, L-type: 91; H-type=7; V-type=1; right hemisphere frequencies: L-type: 86; H-type=1; V-type=12; Children: left hemisphere frequencies, L-type: 32; H-type=7; V-type=0; right hemisphere frequencies: L-type: 33; H-type=1; V-type=5). For each sample, a right hemisphere V-type Sylvian fissure was associated with significantly greater leftward planum temporale asymmetry than a right hemisphere L-type Sylvian fissure (Adults: F(2,98)=5.72, p<.001; Children F(2,38)=7.29, p<.005). The VBM pSTG asymmetry from the one-sample t-test was used to examine the influence of Sylvian fissure morphology on voxel-based asymmetry. The presence of a V-type Sylvian fissure was not related to greater VBM pSTG asymmetry compared to a right hemisphere L-type Sylvian fissure in the sample of adults (F(1,95)=1.16, ns) or in the children (F(1,37)=2.03, ns). These results show that the manual planum temporale asymmetry measure, but not the VBM pSTG measure, is strongly influenced by sulcal/gyral features of Heschl’s gyrus and the Sylvian fissure.
Structure-Function Associations: Children
Pearson correlation demonstrated that planum temporale asymmetry significantly predicted Information performance in right-handed children from both high (r=.53, p<.05) and low income families (r=.68, p<.05). SPM5 regression analyses demonstrated that voxel-based estimates of pSTG gray matter asymmetry also significantly predicted Information performance in the right-handed children from these two subgroups. Significant voxel-wise associations with Information were not observed across the entire asymmetric pSTG region (VBM pSTG asymmetry from the one-sample t-test and Information: r=.32, ns), but were localized to regions within (predictive VBM pSTG asymmetry, 4.7% of the asymmetric pSTG voxels) and outside of the asymmetric pSTG region as shown in Figure 4. These latter regions included two areas in the posterior STS (pSTS), one immediately ventral to the pSTG area of significant asymmetry, and the other more posterior, in the inferior parietal lobe. Supplementary Table 1 presents structure-function correlation results for the additional behavioral variables, which demonstrate consistently strong associations between the structural measures and verbal measures.
Figure 4.
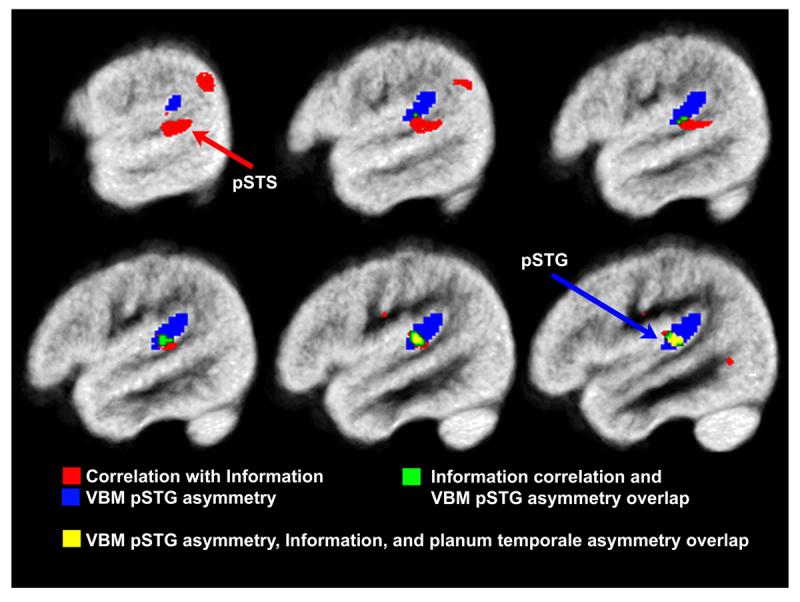
pSTG and pSTS gray matter asymmetry was significantly correlated with WISC Information performance. The red clusters represent regions correlated with Information performance, overlaid with the blue cluster showing regions of significant pSTG gray matter asymmetry across the group (VBM pSTG asymmetry), the green clusters represent regions that exhibited significant leftward VBM pSTG asymmetry and were correlated with Information performance, and the yellow cluster represents regions that exhibited significant leftward VBM pSTG asymmetry, were correlated with Information performance, and were correlated with planum temporale asymmetry. Note the overlap between the three clusters, which is consistent with the regression results demonstrating redundant predictive power of the planum temporale asymmetry and pSTG gray matter asymmetry variables. The maps are thresholded at p<.001, uncorrected for multiple comparisons. The most lateral pSTS region (red arrow) was also significantly correlated with Information performance after controlling for family wise error p<.05.
A hierarchical multiple regression was performed to determine the extent to which planum temporale asymmetry and predictive VBM pSTG asymmetry accounted for the same variance in Information performance. The index of family income was entered in the first level of the regression (R=.59, p<.001). Planum temporale asymmetry was entered in the second level of the regression, which resulted in a significant R2 change (R=.73, p<.005; R2 change=.20, p<.005). When the predictive VBM pSTG asymmetry, from the voxels in the Information correlation cluster that also showed a significant leftward gray matter asymmetry, was entered in the third level of the regression there was a significant R2 change (R=.81, p<.001; R2 change=.12, p<.01). The standardized beta weights for this regression, given in Table 1, show that planum temporale asymmetry predicted the same variance in Information as the predictive VBM pSTG asymmetry measure. In addition, the predictive VBM pSTG asymmetry measure accounted for additional significant variance in Information performance.
Table 1.
Hierarchical regression results showing the unique and common predictive power of the anatomical measures for WISC Information performance.
| Variables | Standardized Beta |
t | Sig. | |
|---|---|---|---|---|
| Level 1; R2=0.34 | Lunch Subsidy (yes, no) | -0.59 | -3.62 | ** |
|
| ||||
| Level 2; R2=0.54 | Lunch Subsidy (yes, no) | -0.59 | -4.22 | *** |
| Planum Temporale Asymmetry | 0.44 | 3.18 | ** | |
|
| ||||
| Level 3; R2=0.66 | Lunch Subsidy (yes, no) | -0.68 | -5.38 | *** |
| Planum Temporale Asymmetry | 0.23 | 1.61 | ns | |
| pSTG Gray Matter Asymmetry | 0.42 | 2.87 | ** | |
p<.05
p<.01
p<.001
ns=non-significant
The predictive VBM pSTG asymmetry measure was weakly related to Information performance in the non-right-handed children. Consistent with the planum temporale asymmetry results, this association was in the opposite direction (r=-.57, p<.10). Rightward asymmetry in non-right-handed children was associated with superior performance. Supplementary Table 1 presents correlation coefficients of the asymmetry measures with the additional measures of verbal ability described in the Methods section for the right-handed and non-right-handed children.
Discussion
The increasing reliance on automated measures of brain morphology raises questions about how findings obtained with those measures relate to earlier findings obtained with manual measures of brain morphology. The goal of this study was to determine the extent of concordance between a manual measure of planum temporale asymmetry and a voxel-based measure of gray matter asymmetry in pSTG regions in the vicinity of the planum temporale. The two measures were significantly correlated, but a large amount of variance was left unexplained. These results indicate regionally specific concordance between the two measures of asymmetry and support previous studies suggesting that manual and automated measures provide complementary information about brain morphology (Eckert et al., 2005). Interestingly, the shared variance between the two measures predicted the same individual variability in children’s verbal ability. The voxel-based measure explained additional significant variance in verbal ability, suggesting that the automated measure may be preferable to the manual measure of planum temporale asymmetry given the challenges associated with manual measurements of brain morphology.
The association between the gray matter asymmetry and planum temporale asymmetry measures was particularly robust in areas corresponding to Heschl’s sulcus and the posterior end of the pSTG. While this may seem surprising given that the Heschl’s gyrus and Sylvian fissure morphology did not predict gray matter asymmetry, the sulcal/gyral classifications do not capture all the variance in the anterior to posterior coordinate positions of the planum temporale boundaries, which can vary considerably even within classifications of Heschl’s gyrus and Sylvian fissure (Leonard et al., 1998). This observation is important because variation in the sulcal/gyral features can create difficulty in measurement and has led to debate about appropriate boundary criteria (Westbury et al., 1999). As demonstrated by our results, qualitative features of Heschl’s gyrus and Sylvian fissure morphology influence the planum temporale measure. Use of the automated gray matter asymmetry method avoids some of these challenges to data collection and the interpretation of results. Sulcal/gyral features appear to have biological and behavioral significance, however. Sulcal/gyral features have been associated with cognitive abilities (Chiarello et al., 2006; Craggs et al., 2006; Clark and Plante, 1998), genetics (Eckert et al., 2006b; Miller et al., 2007) and may reflect patterns of neural connectivity and brain organization (Van Essen 1997; Binder et al., 1996). Measures of sulcal/gyral morphology may provide complementary information to voxel-based measures of brain morphology (Molko et al., 2003).
The spatial extent of the association between the two asymmetry measures varied somewhat between the adults and children. These associations were observed in both anterior and posterior pSTG regions in the adults but only in anterior pSTG regions in the children. These differences could be due to technical differences in image acquisition parameters, sample size differences, or more interestingly, developmental changes in temporal lobe cortex. Although there appears to be some change in planum temporale asymmetry with age (Sowell et al., 2002), these changes are modest in comparison to the robust developmental changes in gray matter and white matter proportions (Giedd, 2004; Giedd et al., 2006; Sowell et al., 2003; Wilke et al., 2007). Any developmental changes in the relation between gray matter asymmetry and planum temporale asymmetry probably reflect these more profound changes in gray matter proportion.
One other study has examined the association between a measure of planum temporale asymmetry and voxel based gray matter asymmetry in adults. Luders et al. (2004) also demonstrated that planum temporale asymmetry predicted gray matter asymmetry in a lateral STG region. In addition, they found that planum temporale asymmetry was related to gray matter asymmetry in the caudate, which was not observed in the present study. In contrast to our planum temporale measurement, which focused on the most asymmetric region of the planum temporale (Best and Demb, 1999), the planum temporale measurement used in the Luders et al. (2004) study was obtained by tracing the entire medial to lateral extent of the planum temporale and using a triangular mesh to connect the tracings across the topography of the planum temporale in order to estimate the entire planum temporale surface area (Keenan et al., 2001). The fact that the correlation between manual and automated measures survives profound differences in manual measurement technique provides confidence that the VBM asymmetry reflects a robust feature of brain organization.
The planum temporale measurement method used in this study captures individual variability that predicts verbal ability (Eckert et al., 2001; Leonard et al., 1996). We observed that both planum temporale asymmetry and pSTG gray matter asymmetry predicted WISC Information performance. This result provides initial validation that the automated gray matter asymmetry measure can be used in research studies instead of the manual planum temporale asymmetry measure, which is prone to measurement error. Each measure predicted verbal ability within family income and hand preference groups. The voxel-based analyses identified additional lateral pSTG and pSTS regions that also strongly predicted verbal ability. This result demonstrates how voxel-based processing can provide increased regional specificity for structure-function associations and identify anatomical regions that are not included in an arbitrarily selected set of ROIs.
The fact that verbal ability was related to anatomical variation in pSTG and pSTS regions rather than the anterior STS or middle temporal gyrus regions associated with speech comprehension (Binder et al., 1996; Scott et al., 2000; Dronkers et al., 2004) requires rethinking of the premises relating asymmetry and verbal behavior. The original hypothesis relating language behavior and temporal lobe asymmetry is that of Galaburda and Geschwind (1985a,b,c). They hypothesized that reduced asymmetry was associated with reduced left hemisphere dominance for language, increased frequency of left-handedness, and increased risk for the language disorder dyslexia. Later studies suggested that reduced asymmetry was associated with severe oral language problems rather than the milder disorder of dyslexia (Eckert, 2004). Although the present results provide further evidence that pSTG asymmetry is associated with oral language ability, this association was observed within a group of strongly right-handed children. These children were likely to have leftward asymmetry for language organization based on functional imaging evidence that nearly all strongly right-handed subjects have leftward asymmetry for language representations (Knecht et al., 2000; Pujol et al., 1999; Springer et al., 1999). In addition, two imaging studies have failed to demonstrate an association between asymmetry of the planum temporale and estimates of language laterality (Dorsaint-Pierre et al. 2006; Eckert et al., 2006a). For these reasons, it seems unlikely that atypical language laterality accounts for the association between anatomical asymmetry and verbal ability in this study.
The most robust association between anatomical asymmetry and verbal ability was in a pSTS region that exhibited a trend towards rightward asymmetry based on the average asymmetry across the pSTS cluster. This region overlapped with and included lateral cortex just posterior to pSTS voxels exhibiting significant rightward asymmetry (Figure 1). Right-handed children with superior verbal ability had leftward gray matter asymmetry across the pSTS cluster compared to right-handed children with average verbal ability who exhibited symmetry and modest rightward asymmetry and compared to right-handed children with poor verbal ability and the most rightward asymmetry (Supplementary Figure 1). This observation points to an alternative explanation for associations between temporal lobe asymmetry and verbal ability. The pSTS region exhibiting the strongest association with verbal ability in this study is similar in location to cortex engaged during phonological working memory and speech production tasks (Buchsbaum et al., 2005; Hickock et al., 2000; Indefrey and Levelt, 2004; Wise et al., 2001). Individual morphological variability in this region may be related to the ability to perform verbal tasks while holding phonological information in working memory. Performing the Information test requires that subjects hold a spoken question in memory while formulating an appropriate response. In support of this premise, previous observations of associations between planum temporale asymmetry and verbal ability involved behavioral tasks that required working memory to perform the task (Eckert et al., 2001; Kibby et al., 2004; Leonard et al., 1996). Taken as a whole, the evidence suggests the following conclusions: 1) language lateralization is not strongly linked to morphological asymmetries in the pSTG, 2) regions of the pSTG that exhibit the strongest morphological asymmetry are not strongly linked to verbal ability, 3) verbal ability is more strongly linked to asymmetry in the pSTS than to more dorsal regions of the pSTG, and 4) posterior temporal asymmetries linked to verbal ability likely reflect individual differences in phonological working memory.
We were surprised by the consistency in pattern of structure-function correlations between the manual and automated measures. These results may have implications for structure-function studies, which frequently yield results that are unconfirmed in subsequent studies (Eckert et al., 2003). The results of this study demonstrated that right-handed children exhibited the opposite relation with Information performance relative to the non-right-handed subjects, and that brain asymmetry and Information performance associations were strongest within socioeconomic status groups. Performing analyses without controlling for these factors would not have yielded significant results. Many functional imaging studies have included only right-handed participants because of concern that non-right-handed subjects exhibit different patterns of brain activity than right-handed subjects, particularly for language-related studies (Pujol et al., 1999; Szaflarski et al., 2002). These observations, as well as our findings, emphasize the methodological advantage of considering hand preference and socioeconomic status for studies designed to integrate neuroimaging measures with behavioral or cognitive measures.
The results of this study support replacing manual structural asymmetry measures with automated voxel-based measures based on the significant association between the measures and the greater power of the automated voxel-based measure than the manual measure for predicting verbal ability. The differences between the measures may be explained by manual measurement error, automated normalization and segmentation error, or sulcal/gyral boundaries that strongly affect the manual measure of the planum temporale but are diminished in voxel-based data due to the smoothing of the images. We suggest that structure-function studies employing voxel-based measures consider additional measures of sulcal/gyral features that may relate to brain organization and individual variability in behavior.
Supplementary Material
Acknowledgements
We would like to thank the families who participated in these studies, Edward Possing, Laurie Gauger, Marina Santarpia, Angela Bollich, and Wendy Swearingen for their assistance, the anonymous reviewers whose comments strengthened this manuscript, and the private and public organizations that supported this research (International Dyslexia Association, McKnight Brain Institute of the University of Florida, NIDCD ROI 2922, NIMH T32 MH 15737, NSF BCS 0224260, NINDS R01 NS/DC33576-06). This investigation was conducted in a facility constructed with support from Research Facilities Improvement Program Grant Number C06 RR14516 from the National Center for Research Resources, NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26:839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Bardoulle-Crema K, Black J, Ledhusen P. Performance on Piagetian tasks of black children of different socioeconomic levels. Dev Psych. 1986;22:841–844. [Google Scholar]
- Best M, Demb JB. Normal planum temporale asymmetry in dyslexics with a magnocellular pathway deficit. Neuroreport. 1999;10:607–612. doi: 10.1097/00001756-199902250-00030. [DOI] [PubMed] [Google Scholar]
- Binder JR, Frost JA, Hammeke TA, Rao SM, Cox RW. Function of the left planum temporale in auditory and linguistic processing. Brain. 1996;119(Pt 4):1239–1247. doi: 10.1093/brain/119.4.1239. [DOI] [PubMed] [Google Scholar]
- Brett M, Anton J, Valabregue R, Poline J. Region of interest analysis using an SPM toolbox. 8th International Conference on Functional Mapping of the Human Brain; Sendai, Japan. NeuroImage; 2002. [Google Scholar]
- Buchsbaum BR, Olsen RK, Koch P, Berman KF. Human dorsal and ventral auditory streams subserve rehearsal-based and echoic processes during verbal working memory. Neuron. 2005;48:687–697. doi: 10.1016/j.neuron.2005.09.029. [DOI] [PubMed] [Google Scholar]
- Chiarello C, Lombardino LJ, Kacinik NA, Otto R, Leonard CM. Neuroanatomical and behavioral asymmetry in an adult compensated dyslexic. Brain Lang. 2006;98:169–181. doi: 10.1016/j.bandl.2006.04.012. [DOI] [PubMed] [Google Scholar]
- Clark MM, Plante E. Morphology of the inferior frontal gyrus in developmentally language-disordered adults. Brain Lang. 1998;61:288–303. doi: 10.1006/brln.1997.1864. [DOI] [PubMed] [Google Scholar]
- Cohen J, Cohen P. Applied multiple regression/correlation analysis for the behavioral sciences. Erlbaum; Hillsdale, NJ: 1983. [Google Scholar]
- Craggs JG, Sanchez J, Kibby MY, Gilger JW, Hynd GW. Brain morphology and neuropsychological profiles in a family displaying dyslexia and superior nonverbal intelligence. Cortex. 2006;42:1107–1118. doi: 10.1016/s0010-9452(08)70222-3. [DOI] [PubMed] [Google Scholar]
- Dorsaint-Pierre R, Penhune VB, Watkins KE, Neelin P, Lerch JP, Bouffard M, Zatorre RJ. Asymmetries of the planum temporale and Heschl’s gyrus: relationship to language lateralization. Brain. 2006;129:1164–1176. doi: 10.1093/brain/awl055. [DOI] [PubMed] [Google Scholar]
- Sequeira S Dos Santos, Woerner W, Walter C, Kreuder F, Lueken U, Westerhausen R, Wittling RA, Schweiger E, Wittling W. Handedness, dichotic-listening ear advantage, and gender effects on planum temporale asymmetry--a volumetric investigation using structural magnetic resonance imaging. Neuropsychologia. 2006;44:622–636. doi: 10.1016/j.neuropsychologia.2005.06.014. [DOI] [PubMed] [Google Scholar]
- Dronkers NF, Wilkins DP, Van Valin RD, Redfern BB, Jaeger JJ. Lesion analysis of the brain areas involved in language comprehension. Cognition. 2004;92:145–177. doi: 10.1016/j.cognition.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Eckert MA, Lombardino LJ, Leonard CM. Planar asymmetry tips the phonological playground and environment raises the bar. Child Development. 2001;72:988–1002. doi: 10.1111/1467-8624.00330. [DOI] [PubMed] [Google Scholar]
- Eckert MA, Leonard CM, Richards TL, Aylward EH, Thomson J, Berninger VW. Anatomical correlates of dyslexia: frontal and cerebellar findings. Brain. 2003;126:482–494. doi: 10.1093/brain/awg026. [DOI] [PubMed] [Google Scholar]
- Eckert MA. Neuroanatomical markers for dyslexia: a review of dyslexia structural imaging studies. Neuroscientist. 2004;10:362–371. doi: 10.1177/1073858404263596. [DOI] [PubMed] [Google Scholar]
- Eckert MA, Leonard CM, Wilke M, Eckert M, Richards T, Richards A, Berninger V. Anatomical signatures of dyslexia in children: unique information from manual and voxel based morphometry brain measures. Cortex. 2005;41:304–315. doi: 10.1016/s0010-9452(08)70268-5. [DOI] [PubMed] [Google Scholar]
- Eckert MA, Leonard CM, Possing ET, Binder JR. Uncoupled leftward asymmetries for planum morphology and functional language processing. Brain Lang. 2006a;98:102–111. doi: 10.1016/j.bandl.2006.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert MA, Galaburda AM, Karchemskiy A, Liang A, Thompson P, Dutton RA, Lee AD, Bellugi U, Korenberg JR, Mills D, Rose FE, Reiss AL. Anomalous sylvian fissure morphology in Williams syndrome. Neuroimage. 2006b;33:39–45. doi: 10.1016/j.neuroimage.2006.05.062. [DOI] [PubMed] [Google Scholar]
- Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci U S A. 2000;97:11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galaburda AM, Sherman GF, Rosen GD, Aboitiz F, Geschwind N. Developmental dyslexia: four consecutive patients with cortical anomalies. Ann Neurol. 1985;18:222–233. doi: 10.1002/ana.410180210. [DOI] [PubMed] [Google Scholar]
- Gauger LM, Lombardino LJ, Leonard CM. Brain morphology in children with specific language impairment. J Speech Lang Hear Res. 1997;40:1272–1284. doi: 10.1044/jslhr.4006.1272. [DOI] [PubMed] [Google Scholar]
- Geschwind N, Galaburda AM. Cerebral lateralization. Biological mechanisms, associations, and pathology: III. A hypothesis and a program for research. Arch Neurol. 1985a;42:634–654. doi: 10.1001/archneur.1985.04060070024012. [DOI] [PubMed] [Google Scholar]
- Geschwind N, Galaburda AM. Cerebral lateralization. Biological mechanisms, associations, and pathology: II. A hypothesis and a program for research. Arch Neurol. 1985b;42:521–552. doi: 10.1001/archneur.1985.04060060019009. [DOI] [PubMed] [Google Scholar]
- Geschwind N, Galaburda AM. Cerebral lateralization. Biological mechanisms, associations, and pathology: I. A hypothesis and a program for research. Arch Neurol. 1985c;42:428–459. doi: 10.1001/archneur.1985.04060050026008. [DOI] [PubMed] [Google Scholar]
- Geschwind N, Levitsky W. Human brain: left-right asymmetries in temporal speech region. Science. 1968;161:186–187. doi: 10.1126/science.161.3837.186. [DOI] [PubMed] [Google Scholar]
- Giedd JN. Structural magnetic resonance imaging of the adolescent brain. Ann N Y Acad Sci. 2004;1021:77–85. doi: 10.1196/annals.1308.009. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Clasen LS, Lenroot R, Greenstein D, Wallace GL, Ordaz S, Molloy EA, Blumenthal JD, Tossell JW, Stayer C, Samango-Sprouse CA, Shen D, Davatzikos C, Merke D, Chrousos GP. Puberty-related influences on brain development. Mol Cell Endocrinol. 2006;254-255:154–162. doi: 10.1016/j.mce.2006.04.016. [DOI] [PubMed] [Google Scholar]
- Good CD, Johnsrude I, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. Cerebral asymmetry and the effects of sex and handedness on brain structure: a voxel-based morphometric analysis of 465 normal adult human brains. Neuroimage. 2001;14:685–700. doi: 10.1006/nimg.2001.0857. [DOI] [PubMed] [Google Scholar]
- Griffiths TD, Warren JD. The planum temporale as a computational hub. Trends Neurosci. 2002;25:348–353. doi: 10.1016/s0166-2236(02)02191-4. [DOI] [PubMed] [Google Scholar]
- Hickok G, Erhard P, Kassubek J, Helms-Tillery AK, Naeve-Velguth S, Strupp JP, Strick PL, Ugurbil K. A functional magnetic resonance imaging study of the role of left posterior superior temporal gyrus in speech production: implications for the explanation of conduction aphasia. Neuroscience Letters. 2000;287:156–160. doi: 10.1016/s0304-3940(00)01143-5. [DOI] [PubMed] [Google Scholar]
- Indefrey P, Levelt WMJ. The spatial and temporal signatures of word production components. Cognition. 2004;92:101–144. doi: 10.1016/j.cognition.2002.06.001. [DOI] [PubMed] [Google Scholar]
- Keenan JP, Thangaraj V, Halpern AR, Schlaug G. Absolute pitch and planum temporale. Neuroimage. 2001;14:1402–1408. doi: 10.1006/nimg.2001.0925. [DOI] [PubMed] [Google Scholar]
- Kibby MY, Kroese JM, Morgan AE, Hiemenz JR, Cohen MJ, Hynd GW. The relationship between perisylvian morphology and verbal short-term memory functioning in children with neurodevelopmental disorders. Brain Lang. 2004;89:122–135. doi: 10.1016/S0093-934X(03)00310-9. [DOI] [PubMed] [Google Scholar]
- Knecht S, Drager B, Deppe M, Bobe L, Lohmann H, Floel A, Ringelstein EB, Henningsen H. Handedness and hemispheric language dominance in healthy humans. Brain. 2000;123(Pt 12):2512–2518. doi: 10.1093/brain/123.12.2512. [DOI] [PubMed] [Google Scholar]
- Leonard CM, Williams CA, Nicholls RD, Agee OF, Voeller KK, Honeyman JC, Staab EV. Angelman and Prader-Willi syndrome: a magnetic resonance imaging study of differences in cerebral structure. Am J Med Genet. 1993;46:26–33. doi: 10.1002/ajmg.1320460107. [DOI] [PubMed] [Google Scholar]
- Leonard CM, Lombardino LJ, Mercado LR, Browd SR, Breier JI, Agee OF. Cerebral asymmetry and cognitive development in children: A magnetic resonance imaging study. Psychological Science. 1996;7:79–85. [Google Scholar]
- Leonard CM, Puranik C, Kuldau JM, Lombardino LJ. Normal variation in the frequency and location of human auditory cortex landmarks. Heschl’s gyrus: where is it? Cereb Cortex. 1998;8:397–406. doi: 10.1093/cercor/8.5.397. [DOI] [PubMed] [Google Scholar]
- Luders E, Gaser C, Jancke L, Schlaug G. A voxel-based approach to gray matter asymmetries. Neuroimage. 2004;22:656–664. doi: 10.1016/j.neuroimage.2004.01.032. [DOI] [PubMed] [Google Scholar]
- Mangin JF, Riviere D, Cachia A, Duchesnay E, Cointepas Y, Papadopoulos-Orfanos D, Scifo P, Ochiai T, Brunelle F, Regis J. A framework to study the cortical folding patterns. Neuroimage. 2004;23(Suppl 1):S129–138. doi: 10.1016/j.neuroimage.2004.07.019. [DOI] [PubMed] [Google Scholar]
- Miller JL, Couch JA, Leonard CM, Schwenk K, Towler SD, Shuster J, Goldstone AP, He G, Driscoll DJ, Liu Y. Sylvian fissure morphology in Prader-Willi syndrome and early-onset morbid obesity. Genet Med. 2007;9:536–543. doi: 10.1097/gim.0b013e31812f720d. [DOI] [PubMed] [Google Scholar]
- Molko N, Cachia A, Riviere D, Mangin JF, Bruandet M, Le Bihan D, Cohen L, Dehaene S. Functional and structural alterations of the intraparietal sulcus in a developmental dyscalculia of genetic origin. Neuron. 2003;40:847–858. doi: 10.1016/s0896-6273(03)00670-6. [DOI] [PubMed] [Google Scholar]
- Pfeifer RA. Mylogenetisch—anatomische untersuchungen uber das korticale ende untersuchungen end der heorleitung. Acad. Wiss. 1920;37:1–54. [Google Scholar]
- Pfeifer RA. Pathologie der Hörstrahlung und der corticalen Hörsphäre. Springer-Verlag; Berlin: 1936. [Google Scholar]
- Pujol J, Deus J, Losilla JM, Capdevila A. Cerebral lateralization of language in normal left-handed people studied by functional MRI. Neurology. 1999;52:1038–1043. doi: 10.1212/wnl.52.5.1038. [DOI] [PubMed] [Google Scholar]
- Rumsey JM, Donohue BC, Brady DR, Nace K, Giedd JN, Andreason P. A magnetic resonance imaging study of planum temporale asymmetry in men with developmental dyslexia. Arch Neurol. 1997;54:1481–1489. doi: 10.1001/archneur.1997.00550240035010. [DOI] [PubMed] [Google Scholar]
- Salmond CH, Ashburner J, Vargha-Khadem F, Connelly A, Gadian DG, Friston KJ. Distributional assumptions in voxel-based morphometry. Neuroimage. 2002;17:1027–1030. [PubMed] [Google Scholar]
- Sattler J. Assessment of children. 3rd La Mesa, CA: 1992. [Google Scholar]
- Schlaug G, Jancke L, Huang Y, Steinmetz H. In vivo evidence of structural brain asymmetry in musicians. Science. 1995;267:699–701. doi: 10.1126/science.7839149. [DOI] [PubMed] [Google Scholar]
- Scott SKC, Blank C, Rosen S, Wise RJS. Identification of a pathway for intelligible speech in the left temporal lobe. Brain. 2000;123:2400–2406. doi: 10.1093/brain/123.12.2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Rex D, Kornsand D, Tessner KD, Jernigan TL, Toga AW. Mapping sulcal pattern asymmetry and local cortical surface gray matter distribution in vivo: maturation in perisylvian cortices. Cereb Cortex. 2002;12:17–26. doi: 10.1093/cercor/12.1.17. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Peterson BS, Thompson PM, Welcome SE, Henkenius AL, Toga AW. Mapping cortical change across the human life span. Nat Neurosci. 2003;6:309–315. doi: 10.1038/nn1008. [DOI] [PubMed] [Google Scholar]
- Springer JA, Binder JR, Hammeke TA, Swanson SJ, Frost JA, Bellgowan PS, Brewer CC, Perry HM, Morris GL, Mueller WM. Language dominance in neurologically normal and epilepsy subjects: a functional MRI study. Brain. 1999;122(Pt 11):2033–2046. doi: 10.1093/brain/122.11.2033. [DOI] [PubMed] [Google Scholar]
- Steinmetz H, Rademacher J, Huang YX, Hefter K, Zilles K, Thron A, Freund HJ. Cerebral asymmetry: MR planimetry of the human planum temporale. J. Comput. Assisted Tomogr. 1989;13:996–1005. [PubMed] [Google Scholar]
- Steinmetz H, Volkmann J, Jancke L, Freund HJ. Anatomical left-right asymmetry of language-related temporal cortex is different in left- and right-handers. Ann Neurol. 1991;29:315–319. doi: 10.1002/ana.410290314. [DOI] [PubMed] [Google Scholar]
- Szaflarski JP, Binder JR, Possing ET, McKiernan KA, Ward BD, Hammeke TA. Language lateralization in left-handed and ambidextrous people: fMRI data. Neurology. 2002;59:238–244. doi: 10.1212/wnl.59.2.238. [DOI] [PubMed] [Google Scholar]
- Teszner D, Tzavaras A, Gruner J, Hecaen H. [Right-left asymmetry of the planum temporale; apropos of the anatomical study of 100 brains] Rev Neurol (Paris) 1972;126:444–449. [PubMed] [Google Scholar]
- Tisserand DJ, Pruessner JC, Arigita EJ Sanz, van Boxtel MP, Evans AC, Jolles J, Uylings HB. Regional frontal cortical volumes decrease differentially in aging: an MRI study to compare volumetric approaches and voxel-based morphometry. Neuroimage. 2002;17:657–669. [PubMed] [Google Scholar]
- Van Essen DC, Drury HA, Dickson J, Harwell J, Hanlon D, Anderson CH. An integrated software suite for surface-based analyses of cerebral cortex. J Am Med Inform Assoc. 2001;8:443–459. doi: 10.1136/jamia.2001.0080443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigneau M, Beaucousin V, Herve PY, Duffau H, Crivello F, Houde O, Mazoyer B, Tzourio-Mazoyer N. Meta-analyzing left hemisphere language areas: phonology, semantics, and sentence processing. Neuroimage. 2006;30:1414–1432. doi: 10.1016/j.neuroimage.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Von Economo C, Horn L. Uber windungsrelief mabe und Rindenarchitektonic der supratemparalflache, ihre individuellen und seitenunterschiede. Zeitschrift für die gesamte Neurologie und Psychiatrie. 1930;130:678–757. [Google Scholar]
- Watkins KE, Paus T, Lerch JP, Zijdenbos A, Collins DL, Neelin P, Taylor J, Worsley KJ, Evans AC. Structural asymmetries in the human brain: a voxel-based statistical analysis of 142 MRI scans. Cereb Cortex. 2001;11:868–877. doi: 10.1093/cercor/11.9.868. [DOI] [PubMed] [Google Scholar]
- Wernicke C. Des Aphasiche Symptomenkomplex. Cohn and Weigart; Breslau: 1874. [Google Scholar]
- Westbury CF, Zatorre RJ, Evans AC. Quantifying variability in the planum temporale: a probability map. Cereb Cortex. 1999;9:392–405. doi: 10.1093/cercor/9.4.392. [DOI] [PubMed] [Google Scholar]
- Wilke M, Schmithorst V, Holland S.Construction of a pediatric template for use within SPM99 Child’s Nervous System 2002London: Euro. Soc. Mag. Res. Neurol. [Google Scholar]
- Wilke M, Krageloh-Mann I, Holland SK. Global and local development of gray and white matter volume in normal children and adolescents. Exp Brain Res. 2007;178:296–307. doi: 10.1007/s00221-006-0732-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RSJ, Scott SK, Blank SC, Mummery CJ, Murphy K, Warburton EA. Separate neural subsystems within ‘Wernicke’s area’. Brain. 2001;124:83–95. doi: 10.1093/brain/124.1.83. [DOI] [PubMed] [Google Scholar]
- Witelson SF, Pallie W. Left hemisphere specialization for language in the newborn. Neuroanatomical evidence of asymmetry. Brain. 1973;96:641–646. doi: 10.1093/brain/96.3.641. [DOI] [PubMed] [Google Scholar]
- Witelson SF, Kigar DL. Sylvian fissure morphology and asymmetry in men and women: bilateral differences in relation to handedness in men. J Comp Neurol. 1992;323:326–340. doi: 10.1002/cne.903230303. [DOI] [PubMed] [Google Scholar]
- Zetzsche T, Meisenzahl EM, Preuss UW, Holder JJ, Kathmann N, Leinsinger G, Hahn K, Hegerl U, Moller HJ. In-vivo analysis of the human planum temporale (PT): does the definition of PT borders influence the results with regard to cerebral asymmetry and correlation with handedness? Psychiatry Res. 2001;107:99–115. doi: 10.1016/s0925-4927(01)00087-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.