Abstract
Neutrophils (PMN) and macrophages are crucial contributors to neovascularization serving as a source of chemokines, growth factors and proteases. αMβ2(CD11b/CD18) and αLβ2(CD11a/CD18) are expressed prominently and have been implicated in various responses of these cell types. Thus, we investigated the role of these β2 integrins in angiogenesis. Angiogenesis was analyzed in wild-type (WT), αM-knockout (αM−/−) and αL-deficient (αL−/−) mice using B16F10 melanoma, RM1 prostate cancer and matrigel implants. In all models, vascular area was decreased by 50–70% in αM−/− mice resulting in stunted tumor growth as compared to WT mice. In contrast, αL deficiency did not impair angiogenesis and tumor growth. The neovessels in αM−/− mice were leaky and immature as they lacked smooth muscle cell and pericytes. Defective angiogenesis in the αM−/− mice was associated with attenuated neutrophil (PMN) and macrophage recruitment into tumors. In contrast to WT or the αL−/− leukocytes, the αM−/− myeloid cells showed impaired plasmin (Plm)-dependent ECM invasion, resulting from 50–75% decrease in plasminogen (Plg) binding and pericellular Plm activity. Surface plasmon resonance verified direct interaction of the αMI-domain, the major ligand binding site in the β2 integrins, with Plg. However, the αLI-domain failed to bind Plg. Also, endothelial cells failed to form tubes in the presence of conditioned medium collected from TNF-α-stimulated PMNs derived from the αM−/− mice due to severely impaired degranulation and secretion of VEGF. Thus, αMβ2 plays a dual role in angiogenesis, supporting not only Plm-dependent recruitment of myeloid cells to angiogenic niches, but also secretion of VEGF by these cells.
Introduction
Bone marrow derived myeloid cells, particularly neutrophils (PMNs) and macrophages, are key regulators of tumor progression and metastasis. One of the major tumor promoting functions of these cells is their facilitation of angiogenesis (reviewed in (1, 2)). PMNs and macrophages contribute to angiogenesis via a variety of well-established mechanisms. One example is their capacity to produce and secrete a variety of pro-angiogenic factors such as VEGF-A, FGF, IL-8, IL-10, CXCL1/GRO and COX-2 (3, 4). In addition, PMNs and macrophages are a rich source of numerous proteases including neutrophil elastase, cathepsin G and several metalloproteinases, which are crucial not only for ECM degradation and remodeling, but also regulate bioavailability of various proangiogenic stimuli (5), all requisite events in angiogenesis (reviewed in (4, 6–8)). In addition, both PMNs and macrophages secrete urokinase-type plasminogen activator (uPA), which converts plasminogen (Plg) to plasmin (Plm). There are diverse Plg receptors on leukocyte surface (reviewed in (9)) and bound Plm facilitates leukocyte migration/invasion by directly degrading ECM, and encourages leukocyte recruitment in a variety of in vivo models of inflammation (10–12).
αMβ2 (CD11b/CD18) and αLβ2 (CD11a/CD18), two the most broadly studied members of the β2 integrin subfamily, are particularly enriched in PMNs and macrophages, where they regulate diverse cell functions, including migration, adhesion, the respiratory burst and cytokine production (13). In addition, we have previously demonstrated that αMβ2 enhances uPA-dependent Plg activation on the PMN surface (14, 15), which has the potential to influence their recruitment to inflammatory/angiogenic sites in vivo. Based on these observations, we hypothesized that αMβ2 and αLβ2, as key regulators of leukocyte functions, might be implicated in angiogenesis. Here, we used αM−/− and αL−/− mice and 3 distinct in vivo angiogenesis models to show that αMβ2, but not αLβ2, is a critical contributor to angiogenesis. This function of αMβ2 is mediated by two distinct mechanisms: 1) support of Plm-dependent PMN/macrophage recruitment to angiogenic niches; and 2) enhancement of leukocyte production and secretion of the primary angiogenic growth factor, VEGF-A.
Materials and Methods
Materials
Mouse VEGF165 and KC were from Biosource International (Camarillo, CA), heparin was from Sigma (St. Louis, MO), biotin-conjugated anti-mouse CD31 mAb was from BD Pharmigen (San Jose, CA), rabbit anti-Smooth Muscle Actin (SMA, Abcam, Cambridge, MA), rabbit anti-NG2 (Millipore, Temecula, CA), rabbit anti-mouse laminin (Serotec, Oxford, UK), goat anti-Fibrin II (Accurate Chemical, Westbury, NY), purified or FITC-labeled rat anti-Ly6G, clone 1A8, specific for mouse PMNs were from BD Pharmigen (San Jose, CA), anti-mouse macrophages/monocytes mAb (MOMA-2) was from Chemicon (Temecula, CA), rat LEAF TM purified anti-mouse αM integrin (clone M1/70) was from Biolegend (San Diego, CA). Glu-Plg was isolated from normal human plasma by affinity chromatography on lysine-Sepharose followed by gel filtration. Growth Factor-reduced Matrigel matrix was from BD Bioscience (San Diego, CA). Murine recombinant TNFα was from R&D Systems, cycloheximide and pentoxifylline were from Sigma.
Mice
The αM−/− mice were generated as previously described (16), and αL−/− mice were purchased from the Jackson Laboratory. Both strains were backcrossed for 12 generations into a C57BL/J6 background. The study was conducted under protocols approved by the IACUC of the Cleveland Clinic.
Angiogenesis models in vivo
8–12 week-old mice were injected subcutaneously with 106 murine B16F10 melanoma or RM1 prostate cancer cells. Tumors were collected 8–14 days after injection and were weighted, photographed and processed for immunohistochemical staining. EC were identified using a biotinylated mouse CD31 mAb, Smooth Muscle Cells with anti-SMA Ab, pericytes with anti-NG2 Ab, fibrin with anti-Fibrin II Ab, basement membrane with anti-laminin Ab, PMNs with rat anti-Ly6G (clone 1A8) and monocytes/macrophages with MOMA-2 mAbs. Stained sections were analyzed using fluorescent imaging microscopy (Leica, Germany) and ImagePro Plus Capture and Analysis software (Media Cybernetics). CD31, fibrin, Ly6G- or MOMA2-positive area was quantified in 5–10 independent fields. The average area per field was determined from duplicate measurements of each of the fields analyzed. Matrigel plug assay has been performed as described (17). Mice were injected with 500μl of growth factor-reduced Matrigel was mixed at 4°C with heparin (26U/ml) alone or with KC (Keratinocyte-derived cytokine) (500ng/ml) or VEGF 165 (100ng/ml) (R&D Systems). Matrigel plugs were harvested 8 days after injection, snap-frozen and 8 μm sections were processed for immunohistochemical analyses as described above. In αM integrin blocking experiments, WT mice were injected intravenously with rat LEAF™ anti-mouse αM integrin (clone M1/70) (Biolegend, San Diego, CA) or isotype matched normal rat IgG2b (3.5 mg/kg), 4h before and then 2, 4 and 6 days after Matrigel-KC implantation. The Matrigel plugs were collected 8 days after injection, sectioned and stained with anti-CD31 to examine vascular formation.
Bone marrow transplantation
Two months old recipient mice were lethally X-irradiated with a total dose of 9Gy and reconstituted with intravenous injection of 107 BM cells isolated from the femurs of donor mice. Mice were used 6–8 weeks after BMT. Engraftment efficiency was examined 6 weeks after BMT in chimeric αM−/− → WT and WT → αM−/− mice using WT and αM−/− mice, which did not undergo BMT as controls. Single cell suspensions from spleens and thymuses from these mice were prepared and percentages of individual leukocyte subsets were measured by flow cytometry using FITC-labeled Abs to cell specific markers (Ly-6G for PMN, F4/80 for macrophages, CD19 for B lymphocytes, CD3 for T lymphocytes) and FITC-labeled isotype-matched Abs as controls.
Plg binding to αM and αL-I domains
GST-fused αMI and αLI domains were purified by glutathione chromatography. Real-time protein-protein interactions were analyzed using a Biacore 3000 instrument (BIAcore AB). Plg was immobilized on CM5 biosensor chips by amine coupling. Experiments were performed at 22°C in 10mM HEPES buffer, pH 7.4, containing 150mM NaCl and 0.005% surfactant P20 (flow rate, 25μL/minute). Surface plasmon resonance (SPR) sensograms were obtained by injecting various concentrations of GST-tagged αMI or αLI domain over immobilized Plg and reference flow cells. Surfaces were regenerated by 30s pulses of 10 mM NaOH. Association/dissociation curves were determined after the subtraction of the reference surface values and buffer binding at 6 selected concentrations. Sensograms were analyzed using BIAevaluation software (version 4.01; GE Healthcare).
PMN and macrophage isolation
Mouse PMN for use in MAEC tube formation and Plg activation assays (see below), were isolated from blood drawn from hearts of anesthetized animals into sterile acid-citrate-dextrose (1:7 volume 145mM sodium citrate, pH 4.6, and 2% dextrose). Blood was transferred to 1.25% dextran T500 solution (1:9) to sediment erythrocytes for 30 min at RT (14, 18). Leukocyte-rich supernatants were washed with PBS once, and PMNs were isolated by magnetic positive selection using mouse anti-Ly6G MicroBead Isolation Kit (Miltenyi Biotec, Auburn, CA) according to manufacturer’s instructions. Eluted cells were 98% granulocytes, of which more than 96% were neutrophils and 1–2% were eosinophils. Contaminating lymphocytes and monocytes were less than 2% as determined by Wright staining. PMN viability was >98% as determined by trypan blue staining. The PMN yield was usually ~0.5×106 per mouse, and blood pooled from 10–15 mice was used. For Plg activation assays lymphocytes and monocytes were collected from buffy coats, and washed twice with the HBSS buffer. Leukocytes were obtained from blood pooled from 7–10 mice. For matrix invasion and Plg binding assays, macrophages and PMN were isolated from peritoneal lavages, PMN at 6h and macrophages at 72h after intraperitoneal thioglycolate injection, when their recruitment was at highest levels for each cell type (10). PMNs constitute 92% and macrophages 90% of all cells in the 6h and 72h peritoneal lavages, respectively. PMN and macrophages harvested from lavages are referred to as peritoneal PMN or peritoneal macrophages in the manuscript to distinguish them from blood cells.
Matrix Invasion Assays ex Vivo
Prechilled tissue culture inserts with porous (8μm pore size) polyester membrane (Costar, Corning, NY) were coated with 50μl/insert of Matrigel (0.5mg/ml) overnight at 4°C until dry. Next, matrices were rehydrated with 600μL DMEM F-12 for 1h. Peritoneal PMNs or macrophages were suspended in serum-free DMEM F-12 medium (Gibco, Carlsbad, CA), and added to matrix-coated inserts (1×105/insert), which were placed in a 24-well plate containing serum-free DMEM F-12 supplemented with or without 100ng/ml KC. Plg (90μg/ml) was added to appropriate inserts, and cells were incubated for 18h at 37°C. Assays were stopped by removing the inserts and wiping the inside with a cotton swab to remove non-migrated cells. The migrated cells were quantified using the Cyquant Cell Proliferation Kit (Molecular Probes, Eugene, OR) according to manufacturer’s instructions.
Plg Activation on the Leukocyte Surface
Peripheral blood PMNs or lymphocyte/monocyte mixtures were incubated with KC (100ng/ml) for 1h at 37°C in the presence of 25nM sc-uPA in 10mM Tris-Cl buffer, pH 7.4, containing 0.14mM NaCl, 0.1%BSA, 1mM CaCl2 and 1mM MgCl2. The cells were washed three times, and 50μl of the cell suspension (1×106cells/well) were added to each well of the microtiter plates. Peritoneal macrophages, which had not been incubated with KC or sc-uPA, were also added to the plates. Then, 100μl of a Glu-Plg (1μM) and Plm-specific fluorescent substrate H-D-Val-Leu-Lys-7-amido-4-methylcoumarin (2mM) mixture was added, and Plm formation was monitored over 45min at 37°C at αem=370nm and αex=470nm using a fluorescence plate reader (SpectraMax GeminiXS, Molecular Devices).
Regulation of VEGF by PMNs
Peripheral blood WT, αM−/− or αL−/− PMNs were incubated in 24-well tissue culture plates (Costar) (3×106 cells/well) in 250 μl of DMEM F-12 medium in the absence or presence of TNFα (20ng/ml) for 2h at 37°C. The inhibitors of protein synthesis (cycloheximide-10μg/ml) or PMN degranulation (pentoxifylline, 3,7-dimethyl-1-(5-oxohexyl)-xantine-300 μM) were added to PMNs 60 min before addition of TNFα. Supernatants were collected, centrifuged at 1500 rpm in a Beckman GS-6 centrifuge for 10 min and VEGF was measured using mouse VEGF Quantikine Elisa Kit (R&D Systems). In parallel, lactoferrin concentration was measured in PMN supernatants using mouse Lactoferrin LTF/LF Elisa Kit (Cusabio).
Quantitative Real Time PCR
Total RNA was isolated from peripheral blood mouse PMNs, either resting or stimulated with TNFα (20ng/ml) for 2h at 37 °C, and from WT and αM−/− mouse CD117+ bone marrow progenitor cells with Trizol reagent according to the manufacturer’s protocol. Total RNA (4 μg) was reverse transcribed using the SuperScript III First-Strand Synthesis System for RT-PCR (Invitrogen) and random hexamers, according to the manufacturer’s protocol. Real time quantitative PCR of the cDNA template was performed in an iCycler (Bio-Rad). VEGF and GAPDH primers were from SABiosciences (Frederick, MD). The PCR contained 150 ng of cDNA, 10 μM forward and reverse primers, and 12.5 μl of 2× RT2 Real Time SYBR Green PCR Master mix (SABiosciences) in a total volume of 25 μl. All PCR were performed in triplicate. Results were calculated as expression of the target gene relative to expression of the reference gene (GAPDH).
MAEC tube formation assay
24-well tissue culture plates were coated with 250μl of Growth Factor-Reduced Matrigel (BD Bioscience, San Diego, CA) and incubated at 37°C for 30min. When the Matrigel solidified, 1.25×105 of WT mouse aortic endothelial cells (MAECs) were added to each well in 250 μl of chosen PMN-conditioned DMEMF-12 medium obtained as described in “Regulation of VEGF in PMNs” and supplemented with 10% FBS, 90μg/ml heparin. Inhibitors of VEGF, neutralizing LEAF™ rat anti-mouse VEGF mAb (Biolegend), isotype matched rat IgG2a (100 μg/ml) (Biolegend) and recombinant mouse sFLT-1 (R&D Systems) (100ng/ml), were preincubated for 60 min with conditioned media of WT TNFα-stimulated PMNs before its addition to MAECs. Live time-lapse photography was performed for 12h, using 5min intervals on Leica DMIRB Inverted Microscope equipped with Roper Scientific CoolSNAP HQ Cooled CCD camera, temperature Controller and CO2 incubation Chamber. Snapshots were taken using MetaMorph Software. Tube formation was analyzed and quantified using ImageJ 1.34 software.
Statistical analysis
Data are expressed as means ± SEM. To determine significance, a one way ANOVA test was performed to compare angiogenic responses between the three mouse genotypes and a two-tailed Student’s t-test was performed for comparisons between WT and the αM−/− mice using the Sigma-Plot software program (SPSS). P<0.05 was considered to be statistically significant.
Results
Integrin αMβ2 is critical in angiogenesis in vivo
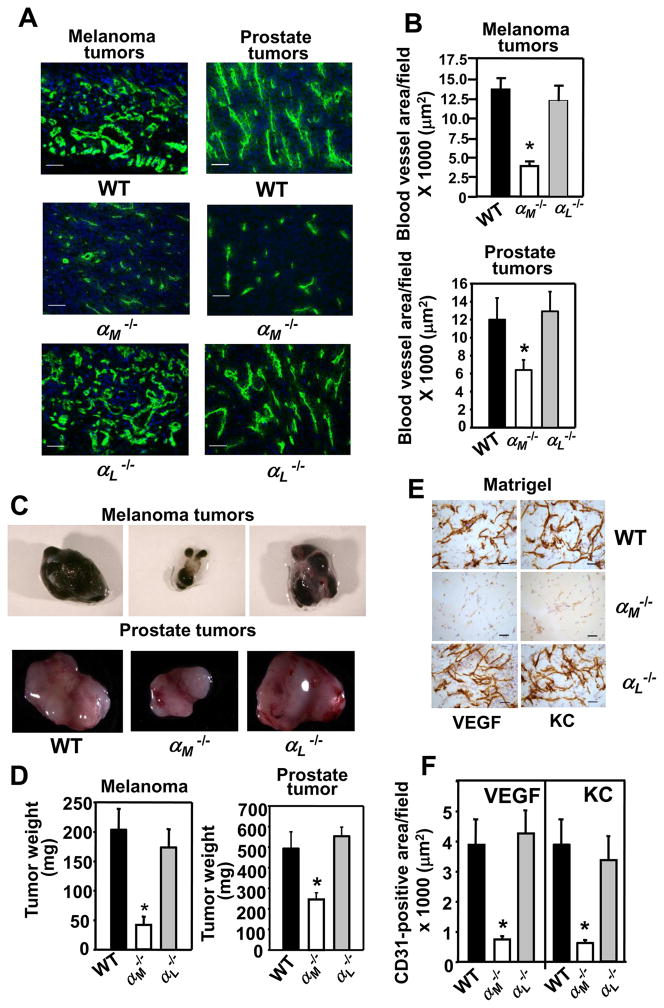
Leukocytes, particularly PMNs and macrophages, are important supporters of angiogenesis as a source of proteases and angiogenic factors (reviewed in (1, 2)). The β2 integrins are crucial in regulation of a variety of leukocyte responses including adhesion, migration and cytokine production (reviewed in (13)). Accordingly, we examined the role of two prominent members of the β2 integrin subfamily, αMβ2 and αLβ2, in angiogenesis. These two integrins share the same β2 subunit and their α-subunits are 49% identical (19). Angiogenesis was analyzed in the αM−/−, αL−/− and control WT mice using two tumor models, murine B16F10 melanoma and RM1 prostate cancer. These tumors are known to be highly vascularized, and their growth is heavily dependent upon an angiogenic response (20, 21). Staining of tumor sections for endothelial cells with CD31 (green fluorescence) revealed normal, well-developed, thick vasculature in tumors grown in the αL−/− and WT mice. In contrast, angiogenesis was significantly impaired in αM−/− mice as only a few short and thin vessel-like structures were observed in melanomas and prostate tumors from these mice (Fig. 1A). Blood vessel area of the melanoma sections was attenuated by ~70% in the αM−/− compared to tumors in the αL−/− and WT mice (P<0.001, n=8 per group) (Fig. 1B, upper panel). Also, significantly reduced vascular area was observed in RM1 prostate tumors grown in αM−/− mice compared to αL−/− and WT mice: 6200 μm2 ± 1000 vs 12800 ± 2000 in αL−/− and 12000 ± 2150 μm2 in WT mice (P<0.01, n=8 per group) (Fig. 1B, lower panel). Consistent with the blunted angiogenic response in the αM−/− mice, melanomas grown in these mice were 70–80% smaller (P<0.01, n=7 per group) than those derived in the αL−/− and WT mice: 40±10 mg in αM−/− vs 202±42 mg in WT and 178±34 mg in αL−/− mice. Additionally, the average weight of RM1 prostate tumors recovered from the αM−/− mice was ~40–50% lower than from the αL−/− and WT mice (P<0.05, n=8 per group): 295±30 mg in αM−/− vs 497±80 mg in WT and 570±100 mg in αL−/− mice (Fig. 1C&D).
FIGURE 1.
Angiogenesis is impaired in the αM−/− mice. (A) Representative images of melanoma (left panel) and prostate (right panel) tumor sections stained with an EC marker, CD31 antibody (green), Scale bars, 50 μm. (B) Decreased area of CD31-stained vasculature in tumors grown in αM−/− mice. Data are representative of two independent experiments with 8 mice per group. (C&D). Average weight of melanoma and prostate tumors grown in αM−/− is lower than in WT mice and representative tumors are shown in C. Data are means ± SEM, (n= 8 mice per group) (E) Representative images of Matrigel implant sections stained with CD31 antibody. Scale bars, 50 μm. (F) Reduced area of CD31-positive vasculature in the VEGF- or KC containing Matrigel implants from the αM−/− mice. The data are representative of 3 independent experiments with 8 mice per group.
Impaired angiogenesis in the αM−/− mice was corroborated using Matrigel as a third angiogenic model. Mice were injected with Matrigel alone or with Matrigel supplemented with VEGF or KC (keratinocyte-derived factor) to stimulate angiogenesis. CD31 staining of Matrigel plugs containing VEGF or KC, revealed well-formed vasculature in the implants from WT and αL−/− mice while the Matrigel plugs from αM−/− mice showed no distinct vascular formations although a few CD31-positive ECs were discerned within the plugs (Fig. 1E). Also, regardless of the angiogenic factor used, blood vessel area in the Matrigel implants in the αM−/− mice was reduced by ~75% compared to the implants from the αL−/− and WT animals (P<0.01, n=8 per group) (Fig. 1F). In control Matrigel plugs without proangiogenic cytokines, no blood vessels were detected in any of the three mouse genotypes tested (data not shown).
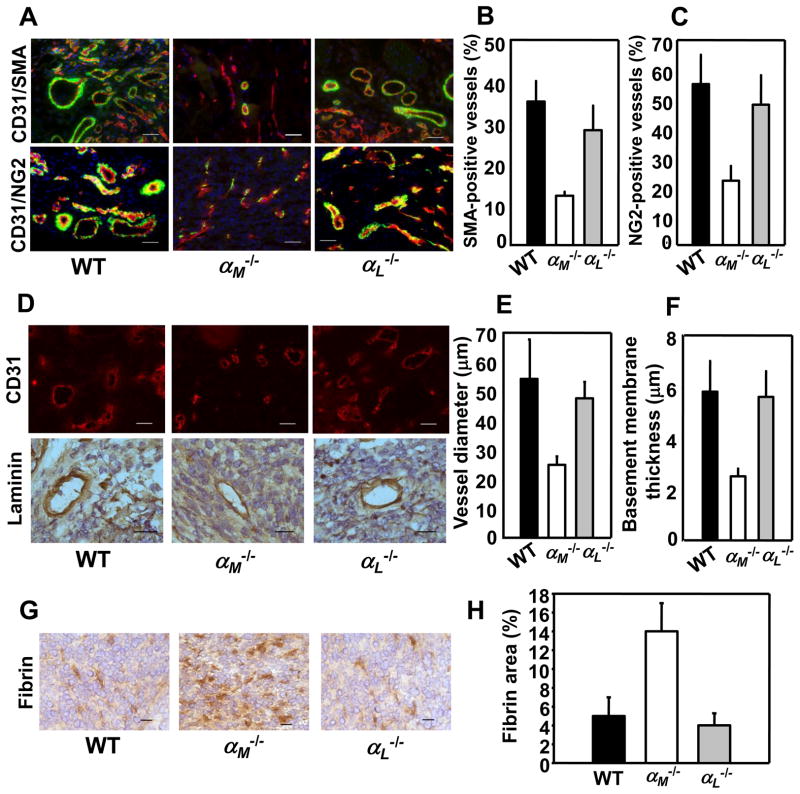
The neovasculature in αM−/− mice is immature
The presence of smooth muscle cells and pericytes within vasculature is a key indicator of its maturity since they stabilize blood vessels. We double-stained melanoma and prostate tumor sections with Abs to CD31 and to smooth muscle cell actin (SMA, Fig. 2A top panel) or to NG2 chondroitin sulfate proteoglycan, a marker of pericytes (Fig, 2A bottom panel). In prostate tumors grown in WT and αL−/− mice 30–37% of total CD31+ blood vessels stained for SMA, whereas only 15% of blood vessels formed in αM−/− mice expressed this marker (P<0.02, n=8) (Fig. 2B). Costaining for CD31 and NG2 revealed reduced pericytes interacting with blood vessels in prostate tumors in αM−/− mice as compared to WT and αL−/− mice (25% NG2-positive blood vessels in αM−/− vs 50–58% in αL−/− and WT mice, (P<0.05, n=8) (Fig. 2C). Measurement of blood vessel diameter revealed that they were substantially smaller in prostate tumors of αM−/− mice as compared to WT and αL−/− mice (25±8μm vs 50–55±10m, P<0.05, n=60, 4 mice per group) (Fig. 2D, top panel & E). Also, immunostaining for laminin showed 50% reduction basement membrane thickness of blood vessel in αM−/− mice (2.7±0.3μm) compared to WT and αL−/− mice (5.8–6 ±1.3 μm, P<0.03, n=60, 4 mice per group; Fig. 2D lower panel & F). With decreased maturation and laminin deposition, we considered that vasculature in αM−/− mice might be leaky. Plasma leakage measured as an area positive for plasma-derived fibrin was enhanced by 2.5-fold in tumors grown in αM−/− mice compared with WT and αL−/− mice (13.8 ±1.8% vs 5.2 ± 1.1% and 3.8± 0.7% respectively, P<0.03, n=20, 4 mice per group; Fig. 2G&H). The vasculature in melanoma tumors grown in αM−/− mice also showed reduced maturity and leakiness (data not shown). In addition, the permeability of preexisting blood vessels in dorsal skin of αM−/− and WT mice was examined using Evans Blue dye injected intravenously. Baseline permeability upon injection of control PBS as well as VEGF-A-induced vascular permeability were similar in αM−/− and WT mice indicating that preexisting vasculature in αM−/− mice was normal (Supplemental Fig. 1).
FIGURE 2.
Neovasculature in prostate tumors in αM−/− mice is immature and leaky. Immunohistochemistry and image analyses of RM1 prostate tumors implanted into WT, αM−/− and αL−/− mice. (A) Costaining for SMA (green) and CD31 (red; top panel) and for NG2 (green) and CD31 (red; bottom panel) in RM1 prostate tumors. Nuclei are stained with DAPI. Scale bars, 50 μm. (B&C) Quantification of the data presented in A top and bottom panel, respectively. Data are expressed as mean ± SEM and are representative of 3 independent experiments (n=8 mice/group). (D) CD31-stained (top panel) and laminin-stained (bottom panel) blood vessels in tumors grown in WT, αM−/− and αL−/− mice. Scale bars, 50 μm (top) and 25 μm (lower panel). (E) Vessel diameter was measured in 60 tumor vessels cut perpendicular to their longitudinal axis in 8 μm-thick sections, stained for CD31 from each mouse (4 mice/group). Data are expressed as mean ± SEM, n=60. (F) Thickness of laminin-positive basement membrane in blood vessels formed in WT, αM−/− and αL−/− mice. Data are mean ± SEM, n=60 and are representative of 3 independent experiments including 4 mice per group. (G) Representative photograph of fibrin content (brown) in tumors grown in WT, αM−/− and αL−/− mice. Scale bars, 25 μm. (H) Quantification of fibrin-positive areas in tumor sections stained with anti-fibrin Ab. Data are means ± SEM, n=8 and are representative of 3 independent experiments including 8 mice per group.
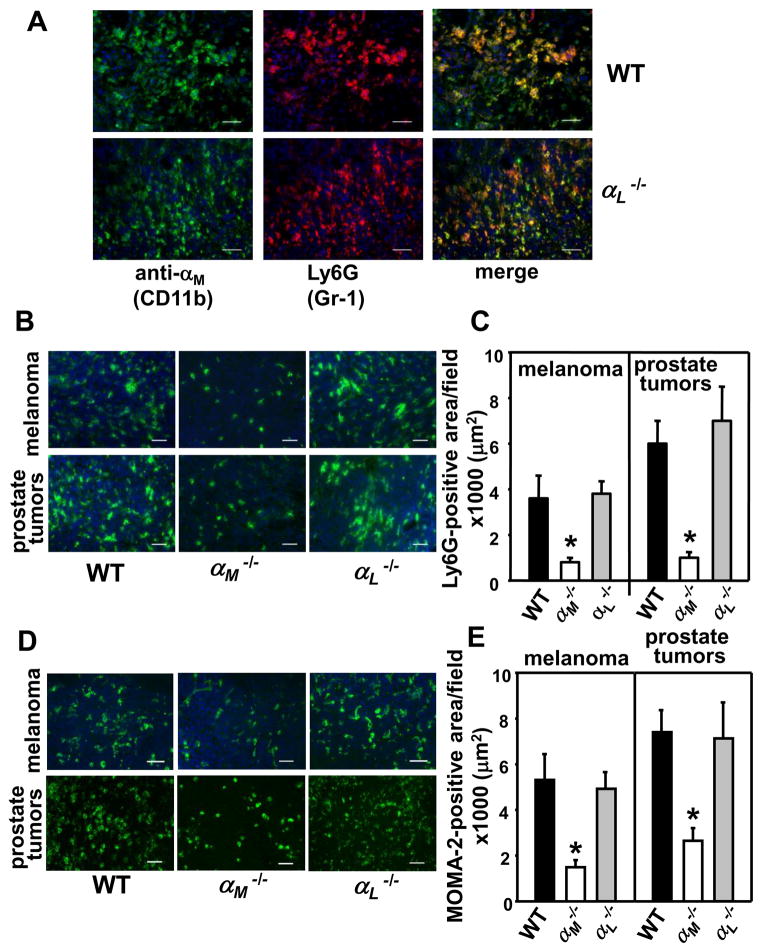
Impaired PMN and macrophage recruitment to angiogenic sites in αM−/− mice
The CD11b+/Gr-1+ myeloid cells consisting primarily of PMNs are crucial enhancers of angiogenesis and contribute to angiogenic switch in many tumors (4, 5, 22). Since tumor growth and angiogenesis were impaired in the αM−/− (CD11b−/−) mice, we considered the possibility that this integrin may regulate recruitment of these cells to growing tumors. First, we examined infiltration of CD11b+/Gr-1+ cells in prostate and melanoma tumors in WT and αL−/− mice by double staining with anti-CD11b (green) and anti-Ly6G (Gr-1) mAbs (red). As shown in Fig. 3A, 70–80% of CD11b+ cells were also positive for Gr-1, and numerous CD11b+/Gr-1+ cells were detected in prostate tumors in WT and αL−/− mice with no differences in their recruitment observed in the two mouse strains. Similar results were obtained with melanoma tumors (data not shown). Since assessment of CD11b+/Gr-1+ cell recruitment to tumors in αM−/− (CD11b−/−) mice was not feasible due to the absence of the αM integrin subunit on these cells, we stained tumor sections only with PMN-specific anti-Ly6G (Gr-1) mAb. Indeed, PMN infiltration into both melanoma and prostate tumors was significantly reduced in the αM−/− mice compared to WT and αL−/− littermates (Fig. 3B). Quantification of Ly6G-positive areas in tumor sections verified these observations: 900μm2 ± 210 in αM−/− vs 3600μm2 ± 1120 in WT and 3950μm2 ± 350 in αL−/− mice in melanomas (P<0.02, n=8) and 1200μm2 ± 265 in αM−/− vs 6020μm2 ± 840 in WT and 7135μm2 ± 1780 in αL−/− mice in prostate tumors (P< 0.01, n=8 per group) (Fig. 3C). Next, tumor sections were stained with the monocyte/macrophage-specific mAb (MOMA-2). Macrophage infiltration into both tumors was decreased by 50–60 % in the αM−/− mice as compared to WT and αL−/− littermates (Fig. 3D&E). In melanomas grown in the αM−/− mice: macrophage-positive area was 1820μm2 ± 160 compared to 5610μm2 ± 985 in WT and 5240μm2 ± 450 in αL−/− mice, whereas in prostate tumors it was 3600μm2 ± 750 in αM−/− mice vs 7500μm2 ± 1100 in WT and 7280μm2 ± 1300 in αL−/− littermates (P<0.05, n=8 per group) (Fig. 3E). In addition, staining of Matrigel implant sections with anti-PMN and MOMA-2 Abs revealed robust VEGF- and KC-dependent leukocyte infiltration into the centers of the implants in WT and αL−/− mice while it was inhibited in the αM−/− mice (data not shown).
FIGURE 3.
Reduced leukocyte infiltration of tumors in the αM−/− mice. (A) Prostate tumor sections from WT and αL−/− mice were double stained with FITC-labeled rat anti-CD11b (M1/70) mAb (green) and rat anti-Ly6G (Gr-1) (clone 1A8-red). Numerous CD11b+/Gr-1+ cells are present on merged images (yellow/orange) in both mouse strains. Scale bars, 50 μm. Representative images of the melanoma and prostate tumor sections stained with PMN-specific anti-Ly6G (B) and the monocyte/macrophage-specific MOMA-2 (D) antibodies, Scale bar, 50 μm. (C&E) Image analysis shows reduced Ly6G-positive (C) and MOMA-2-positive (E) area in melanoma and prostate tumors in the αM−/− mice. Data are means ± SEM, (n=8 mice per group) and are representative of 3 independent experiments.
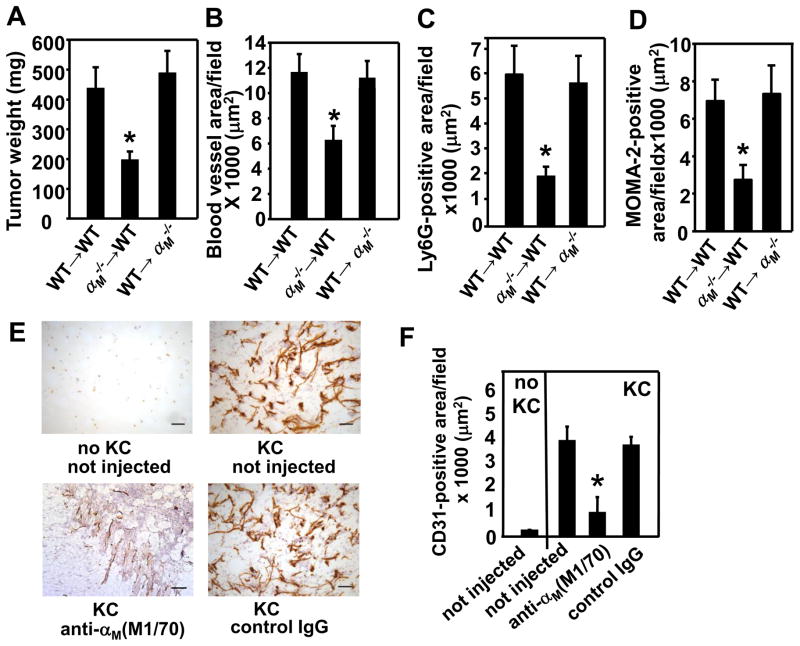
Bone marrow transplantation (BMT) and suppression with blocking antibodies confirm the importance of αMβ2 in angiogenesis
Next, we sought to confirm that reduced tumor growth and angiogenesis in the αM−/− mice are due to impaired functions of bone marrow-derived cells (BMDC) including leukocytes, and are not caused by defective vascular cells. Thus, we performed bone marrow transplant (BMT) experiments and examined growth and angiogenesis in RM1 tumors. Transplantation of αM−/− BM into WT hosts (αM− − → WT) resulted in reduced RM1 tumor growth and angiogenesis (tumor weight: P=0.0169, vascular area: P=0.028 for αM−/− → WT vs WT → WT, n=5) (Fig. 4A&B). Alternatively, transplantation of WT BM into the αM−/− hosts (WT → M−/−) restored growth and angiogenesis of prostate tumors to these of control WT mice receiving WT bone marrow (RM1 weight: P=0.0183, vascular area: P=0.012 for WT → αM−/− vs αM−/−; → WT, n=5) (Fig. 4A&B). These results suggest that blunted tumor growth and angiogenesis in the αM−/− mice is a consequence of altered BMDC functions. In addition, image analysis of RM1 tumor sections stained with PMN-specific anti-Ly6G and anti-monocyte/macrophage MOMA-2 antibodies revealed impaired recruitment of these cells to tumors grown in WT recipients receiving αM−/− BM as compared to control WT → WT mice, indicating a crucial role of αMβ2 in this process (P=0.0184 for PMNs (Ly6G) and P=0.0167 for MOMA-2, n=5) (Fig. 4C&D). In contrast, transplantation of WT BM to αM−/− recipients restored not only angiogenesis, but also PMN and macrophage migration into tumors (PMNs: P=0.021, MOMA-2: P=0.0454 for WT → αM−/− vs αM−/− → WT, n=5) (Fig. 4C&D). To exclude the possibility that αM deficiency might impair recovery of the immune system upon BMT, we assessed engraftment efficiency 6 weeks after BMT in chimeric αM−/− → WT and WT → αM−/− mice using WT and αM−/− mice not undergoing BMT as controls. We have collected spleens and thymuses from these mice, prepared single cell suspensions and measured percentages of PMN, macrophage, B and T cells by flow cytometry using FITC-labeled Abs to cell specific markers (Ly-6G, F4/80, CD19, CD3, respectively) and FITC-labeled isotype-matched Abs as controls. We found that the content of individual leukocyte subsets was similar in respective organs in both chimeric mouse lines as well as in control mice (no BMT), indicating that αM deficiency did not impact BMT engraftment efficiency (supplemental Table 1). In addition, flow cytometry of circulating total leukocytes with anti-mouse αM mAb confirmed its absence in the αM−/− → WT chimeras and its presence in the WT → M−/− mice (data not shown). Also, to confirm the importance of the αM integrin in angiogenesis, WT mice were injected with a rat anti-αM blocking antibody (clone M1/70), that had been shown to specifically inhibit neointima formation in rabbits (23). These neutralizing antibodies bind to the ligand binding site within the αM subunit and inhibit its interactions with ligands thereby mimicking the gene ablation. Matrigel implants were harvested after 8 days and the antibody was injected before Matrigel injection and then every 2 days. Vasculature was analyzed in Matrigel sections stained with anti-CD31 mAb (Fig. 4E). The anti-αM antibody reduced KC-induced angiogenesis in Matrigel implants by~60–80% as compared to not injected mice, whereas isotype-matched normal rat IgG2b had no effect indicating specificity (P<0.05 mice injected with anti-αM vs not injected, n=4 mice per group) (Fig. 4E&F). Taken together, these experiments verify the importance of αMβ2 on myeloid cells in tumor-induced angiogenesis via regulation of leukocyte recruitment to sites of neovascularization.
FIGURE 4.
Defective hematopoietic cells contribute to reduced tumor growth and angiogenesis in the αM−/− mice. (A) Average weight and (B) CD31-positive vascular area in RM1 prostate tumors in mice undergoing BMT with WT or αM−/− donor marrow. Data represent mean ± SEM, (n=5 mice per group) (C) Ly6G-positive and monocyte/macrophage-positive (D) area in RM1 tumors in mice undergoing BMT with WT or αM−/− donor marrow. Data represent mean ± SEM, (n=5 mice per group) (E&F) Intravenous administration of blocking mAb (M1/70) to α M−/− inhibits KC-dependent angiogenesis in Matrigel plug model in WT mice. M1/70 and normal rat IgG2b (3.5 mg/kg) were injected before Matrigel injection and on days 2, 4 and 6. Matrigel implants were harvested on day 8, sectioned and stained with anti-CD31 mAb. (E) Representative images of Matrigel sections stained with anti-CD31 (brown), Scale bars, 50 μm (F) Quantification of the CD31-positive area in Matrigel implants. Data are means ± SEM, n=4 and are representative of 2 independent experiments.
αMβ2 facilitates leukocyte recruitment to angiogenic sites via interaction with Plg and enhancement of Plg activation
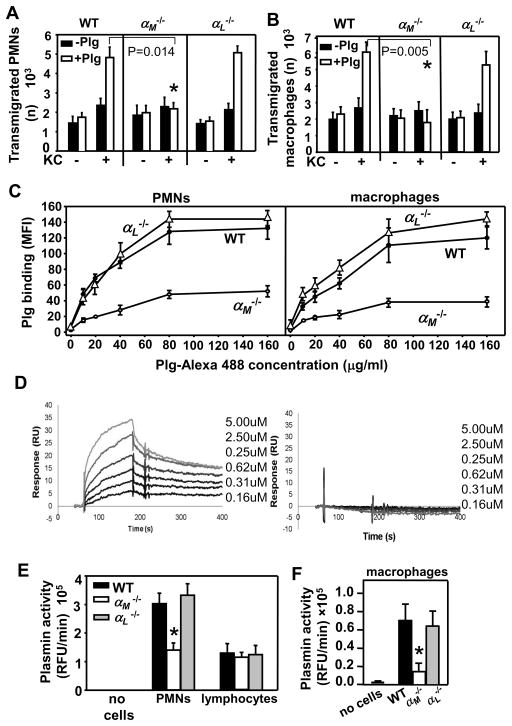
As Plm-dependent ECM proteolysis greatly facilitates leukocyte invasion (10–12), we hypothesized that the inability of αM−/− leukocytes to invade tumors could be caused by impaired Plg activation on the leukocyte surface. Since αMβ2 and αLβ2 are the most abundant β2-integrins on PMNs and macrophages, we used a modified Boyden chamber system to elucidate the role of αMβ2 and αLβ2 in mouse peritoneal PMN and macrophage migration through a Matrigel matrix barrier (ECM extracted from Engelbreth-Holm-Swarm (EHS) mouse sarcoma) in response to KC (Fig. 5A&B). In the absence of KC, there was minimal migration of leukocytes into the lower chambers in the presence or absence of added Plg. However, although KC-induced migration of PMNs and macrophages was significantly impeded by Matrigel, this barrier effect was overcome by addition of Plg to the αL−/− and WT leukocytes (Figs. 5A&B). In contrast, addition of Plg to the αM−/− cells failed to improve their migration through the Matrigel barrier, suggesting that the capability of these cells to bind and activate Plg was limited (P=0.014 αM−/− vs WT PMNs and P=0.005 αM−/− vs WT macrophages, n=3) (Fig. 5A&B). Indeed, flow cytometry showed a 50–60% reduction in binding of Alexa488-conjugated soluble Plg to αM−/− peritoneal PMNs and macrophages as compared to WT and αL−/− leukocytes (Fig. 5C).
FIGURE 5.
αMβ2 directly interacts with Plg, enhances its activation and facilitates Plm-dependent leukocyte recruitment. KC-directed transmigration of peritoneal PMNs (A) and macrophages (B) through Matrigel-coated inserts is Plg-dependent and is impaired in αM−/− leukocytes. The data are means ± SEM of triplicate samples and are representative of two independent experiments including 3 mice per group. (C) Reduced binding of soluble Alexa488-labeled Plg to peritoneal αM−/− PMNs and macrophages. The cells were incubated with increasing concentrations of Plg as indicated for 30 min at 37°C. After two washings, Plg binding to cell surface was analyzed using a FACSCalibur flow cytometer and CellQuest software. The cells incubated without Plg were set as negative controls. The data are means ± SEM of triplicate samples, 3 mice per group and are representative of two independent experiments. (D) Plg was immobilized on the CM5 sensor chip surfaces (500 RU). Sensograms obtained for a concentration series of GST-αM-I (left panel) and GST-αL–I domain (right panel). (E&F) Reduced Plg activation on the surface of peripheral blood αM−/− PMNs and peritoneal macrophages. The results are means ± SEM of triplicate samples, n=5 mice per group and are representative of two independent experiments.
To determine whether Plg interacts directly with the αMI- and αLI- domain, the major site of ligand binding in β2 integrins (13), we performed SPR experiments. GST-tagged αMI-domain interacted with Plg immobilized on biosensor chips in a concentration-dependent manner while the GST-tagged αLI-domain did not bind Plg (Fig. 5D). GST alone also did not interact with Plg. From the progress curves of the Plg:αMI-domain interaction, we estimated a Kd =1.76±0.9×10−7. This value was derived by fitting the kinetic data to a 1:1 global Langmuir model, and the stoichiometry observed at ligand saturation was 1:1.
Next, we compared Plg activation on the surface of WT, αL−/− and αM−/− leukocytes using a fluorogenic plasmin-specific peptide substrate. Peripheral blood PMNs and lymphocytes were stimulated with KC and pretreated with sc-uPA to activate the integrin and enable sc-uPA binding to leukocyte surface, respectively. No plasmin activity was detected in the absence of leukocytes. The αM−/− PMNs showed a 50% reduction in Plm generation as compared to WT and αL−/− PMNs (P<0.03, n=5) (Fig. 5E). In contrast, Plg activation was similar on WT, αM−/− and αL−/− lymphocytes (Fig. 5E). As with PMNs, peritoneal αM-deficient macrophages also exhibited severely reduced (by 75%) Plg activation compared to the αL−/− and WT macrophages (P<0.05, n=5) (Fig. 5F). In control experiments, we examined αL expression on αM-deficient PMN, αM levels on αL-null and control WT PMNs (both peritoneal and circulating) by flow cytometry. Neither αL- nor αM-deficiency altered expression of its counterpart β2-integrin on PMNs (data not shown), confirming that Plg recognition and activation is αMβ2-specific. Taken together, αMβ2 functions as a Plg receptor and this function is critical in Plm-dependent leukocyte recruitment to angiogenic sites.
αMβ2 regulates secretion of VEGF-A by PMNs
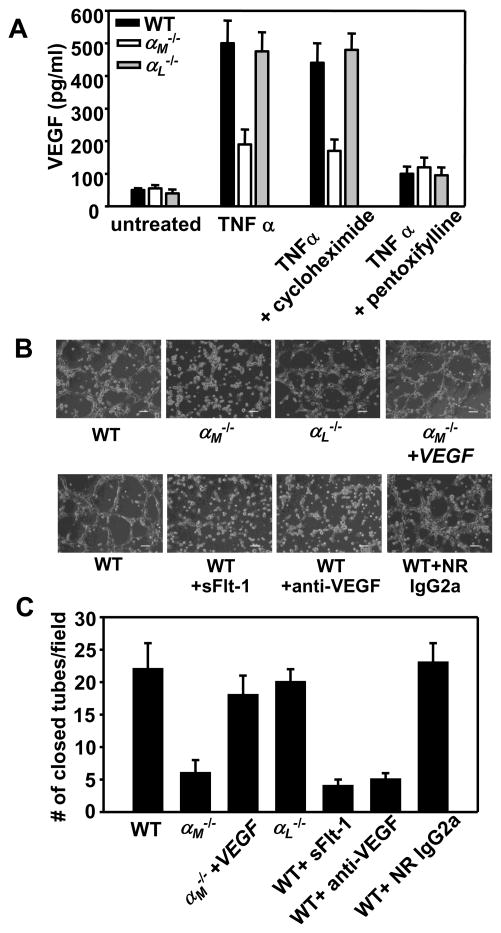
Our data demonstrate a critical role of αMβ2 in leukocyte recruitment to angiogenic sites. However, we considered a possibility that αMβ2 might also regulate other proangiogenic leukocyte functions such as production/secretion of angiogenic stimulators. The CD11b+/Gr-1+ cells, that primarily constitute PMNs, are of particular interest as they secrete high levels of MMP-9 and VEGF leading to “angiogenic switch” in many tumors and to a failure of anti-VEGF therapies (5, 22). Thus, we compared the VEGF-A content of supernatants released from peripheral blood PMN of WT and αM−/− and αL−/− mice. In the absence of TNFα stimulation, VEGF-A levels were low and similar in PMNs of all three genotypes. Notably, upon stimulation with TNFα, VEGF-A content was substantially lower (by~60%) in the αM−/− PMN-conditioned medium compared to medium harvested from WT or αL−/− PMNs (P<0.05, n=6) (Fig. 6A). VEGF is stored in specific granules in human PMNs (24). To determine whether αMβ2 regulates de novo synthesis of VEGF or/and its secretion, we utilized cycloheximide, an inhibitor of protein synthesis, and pentoxifylline, an inhibitor of PMN degranulation. Cycloheximide did not affect VEGF content in supernatants of TNFα-stimulated PMNs of any mouse strains tested, suggesting that TNFα did not stimulate de novo VEGF synthesis (Fig. 6A). This interpretation was corroborated by qRT-PCR assays, which revealed that VEGF mRNA levels were similar not only in resting and TNFα-treated PMNs but also in WT, αM−/− and αL−/− PMNs (Table 1). In contrast, pentoxifylline inhibited secretion of VEGF from PMNs and reduced its concentration by 80–85% in supernatants from WT and αL−/− PMNs and by additional 20% from αM−/− PMNs (Fig. 6A). Taken together, these data suggest that αMβ2 integrin does not regulate VEGF synthesis but rather its secretion via control of PMN degranulation, consistent with prior data implicating αMβ2 in degranulation of human PMNs ex vivo (25). To corroborate this conclusion, we measured the concentration of lactoferrin, a marker of PMN specific granules, in PMN supernatants. The relative changes of lactoferrin and VEGF in the PMN-conditioned media were very similar. Importantly, the αM−/− PMNs, but not the αL−/− PMNs, showed severely impaired release of lactoferrin into the supernatants of TNFα-stimulated αM−/− PMNs, which were almost as low as in supernatants of resting PMNs from each mouse strain (Supplemental Fig. 2). We also sought to examine VEGF production/secretion in CD117+ progenitor cells isolated from bone marrow of WT, αM−/− and αL−/− mice. However, we failed to detect mRNA for VEGF in these immature cells.
FIGURE 6.
αMβ2 supports angiogenesis via regulation of VEGF secretion by PMNs. (A) Peripheral blood WT, αM−/− or αL−/− PMNs (3×106 cells/well) were incubated in 24-well TC plates in the absence or presence of TNFα (20ng/ml) for 2h at 37°C. Cycloheximide (10μg/ml) or pentoxifylline (300 μM) were added 60 min before addition of TNFα. VEGF concentration was measured in supernatants using mouse VEGF Quantikine Elisa Kit. Data are means ± SEM of triplicate samples and are representative of three independent experiments. (B) Bright field microscopy of tube formation by WT MAECs in the presence of conditioned media collected from WT, αM−/− or αL−/− peripheral blood PMNs stimulated with TNFα (upper panels). Inhibitors of VEGF: neutralizing anti-VEGF mAb, isotype matched rat IgG2a (100 μg/ml) and recombinant mouse sFLT-1 (100ng/ml) were preincubated for 60 min with conditioned media of WT TNFα-stimulated PMNs before its addition to MAECs (lower panels). The images were taken after 6h incubation in 37°C, 5% CO2. Scale bars, 75 μm. (C) Quantification of tube formation. Number of closed tubes was counted in 20 different fields of each treatment and plotted as mean ± SEM and are representative of two independent experiments.
Table 1.
Comparison of VEGF-A mRNA content in WT, αM−/− and αL−/− peripheral blood PMNs. Values are expressed relative to GAPDH mRNA levels
| Treatment | WT | αM−/− | αL−/− |
|---|---|---|---|
| Untreated | 0.22 | 0.19 | 0.2 |
| TNFα (20 ng/ml) | 0.24 | 0.20 | 0.24 |
PMNs were incubated in the presence or absence of TNFα (20 ng/ml) for 2h at 37°C, total RNA was isolated using Trizol reagent and RT-PCR has been performed as described in Materials and Methods.
Next, we analyzed the capacity of WT mouse aortic endothelial cells (MAEC) to form tubes in the presence of the conditioned media derived from WT, αM−/− or αL−/− TNFα-stimulated PMNs. MAEC in the presence of media collected from WT or αL−/− PMNs formed well-organized tube-like networks. In contrast, tubes formed by MAECs in the presence of the αM−/− PMN-conditioned media were incomplete (P<0.05, n=20) (Fig. 6 B&C). In control samples, TNFα alone did not support tube formation by ECs. In order to confirm that tube formation by MAEC in the presence of WT or αL−/− PMN supernatants is VEGF-dependent, we added neutralizing rat anti-mouse VEGF mAb (clone 2G11-2A05), or its isotype control rat IgG2a, or soluble VEGF receptor-1 (sFLT-1). The effectiveness of both of these VEGF inhibitors has been previously established (e.g. (26, 27)). These inhibitors of VEGF almost completely inhibited (by 75–80%) tube formation by MAEC (P<0.05, n=20). In contrast, the isotype control antibody did not have any effect (Fig. 6B bottom panel, & C). Although other pro-angiogenic factors are likely to be present in PMN supernatants, VEGF does appear to be the key stimulator of this process in our experimental system. Finally, supplementation of αM−/− PMN conditioned medium with recombinant mouse VEGF to the same concentration as in medium from WT PMNs (520 pg/ml) enhanced the numbers of closed tubes by 2.5-fold and almost completely restored MAEC tube formation (Fig. 6B, top panel). Taken together, αMβ2 does not directly regulate VEGF-A de novo synthesis. However, it enhances VEGF-A secretion from PMN intracellular stores via its enhancement of PMN degranulation.
Discussion
The goal of this study was to examine involvement of the two major leukocyte β2 integrins, αMβ2 and αLβ2, in angiogenesis. Using the αM- and αL-deficient mice, we demonstrate that αMβ2 promotes angiogenesis in model melanoma and prostate tumors, as well as in Matrigel implants, while the αLβ2 integrin does not. Blood vessel formation and tumor growth were impaired in α M−/− mice as compared to the α L−/− or WT mice. Impaired angiogenesis in αM−/− mice was due to dramatic reduction in recruitment of PMNs and macrophages into the tumors and Matrigel implants. Furthermore, we showed that Plg binding and activation on the surface of αM−/− PMNs and macrophages as well as their Plm-dependent invasion through Matrigel were significantly attenuated as compared to the αL−/− and WT cells. These data were consistent with the SPR sensograms showing that recombinant αMI-domain directly interacts with Plg, but the αLI-domain does not. These findings are in concord with prior studies showing that αMβ2 recognizes urokinase (uPA) and Plg enhancing their reciprocal activation on PMN surface (14, 15, 28). To our knowledge this is the first report implicating αMβ2 in angiogenesis and demonstrating its intimate interplay with Plg in vivo. Although, the β2-deficient mice showed slowed angiogenesis in healing wounds (29), none of the individual β2 integrin family members was shown to contribute to this process. The implication of αMβ2 in angiogenesis and Plg binding/activation is quite specific as αLβ2 did not show any impairment in these responses. This distinction may be explained, at least in part, by the relatively low sequence identity between the ligand binding αMI- and αLI-domains resulting in the αMβ2 promiscuity for many structurally unrelated ligands, while αLβ2 shows a very limited ligand repertoire with little overlap in ligand recognition with αMβ2 (19). With regard to ligand repertoire, the two β2 integrins, αXβ2 and αDβ2, are more similar to αMβ2 than αLβ2, and it would be interesting to examine their role in angiogenesis. The critical role of αMβ2-dependent Plg activation in PMN and macrophage recruitment to angiogenic niches is in accord with previous studies demonstrating the importance of cell-bound plasmin in leukocyte recruitment in a variety of in vivo models of inflammation (10–12), and also with crucial role of the Plg system in angiogenesis (30–36). Pericellular proteolysis is critical for initiation of angiogenesis as evidenced by suppression of neovascularization in mice deficient in various proteases (31, 37–39) and by administration of a variety of protease inhibitors (40, 41). Among the proteases implicated in angiogenesis, in addition to plasmin, are its activators and metalloproteinases (reviewed in (4, 8)). Plasmin is one of pro-MMP-9 activators (42) and PMN-derived MMP-9 is responsible for angiogenic switch in some tumors (5). Consistent with decreased Plm activity and the capacity of αMβ2 to bind and activate MMP-9 (43), we observed reduced MMP-9 activity in tumor extracts from the αM−/− mice (data not shown).
As a key source of proteases and proangiogenic factors, PMNs and macrophages are essential for angiogenesis. Angiogenesis is severely blunted in neutropenic mice (5, 29, 44, 45) or in mice in which macrophages have been eliminated (2). Robust PMN and macrophage recruitment is observed into ischemic tissues including tumors (46), and in many tumors recruitment of these cells tumors correlates with poor host survival (reviewed in (2)). With such evidence, the virtual absence of PMNs and macrophages in the angiogenic tissue of the αM−/− mice provides a mechanism to account for profound reduction in neovascularization and tumor growth in these animals. In addition to defective recruitment, the αM−/− PMNs also exhibited severely attenuated secretion of VEGF-A due to impaired degranulation, and supernatants collected from these cells did not support EC tube formation in in vitro assays. As enhanced VEGF is the hallmark and a key contributor to CD11b+/Gr-1+ (mostly PMNs)-dependent resistance of many tumors to VEGF targeting anti-cancer therapies, we have focused our investigations on the regulation of this pivotal cytokine. However, we can not exclude, that other pro-angiogenic factors, particularly those stored in PMN granules, might also be regulated by αMβ2 via its influence on degranulation. This important issue is open for further investigation. Even if αM−/− PMNs had been able to migrate, they would likely to have failed to promote angiogenesis due to an inability to supply of the requisite amounts of the major pro-angiogenic stimulus, VEGF. The neovasculature in growing tumors, but not preexisting blood vessels, in αM−/− mice showed immature and leaky phenotype. This might be caused by insufficient infiltration of CD11b+/Ly6G+ PMNs known to support vascular maturation via elevated levels of proangiogenic factors VEGF and MMP-9 (22).
Taken together, our studies demonstrate that integrin αMβ2 promotes angiogenesis in vivo via dual mechanism: first, as a Plg receptor, αMβ2 supports Plm-dependent recruitment of myeloid cells to angiogenic niches; second, αMβ2 enhances VEGF-A secretion by PMN degranulation. Based on our findings, selective antagonists of αMβ2 may be considered as a new target to inhibit tumor angiogenesis.
Supplementary Material
Acknowledgments
We thank Dr. Tatiana Byzova for expertise and advice regarding the in vivo angiogenesis models.
This work was supported by funding from NIAID (AIO 80596 to D.A.S.) AHA SDG 0335088N to E.P., NIH grants from the Heart, Lung and Blood Institute (R01 HL17964 and P01 HL07331 to E.F.P.).
Nonstandard abbreviations used
- MAEC
mouse aortic endothelial cells
- KC
keratinocyte-derived cytokine
- VEGF-A
vascular endothelial growth factor A
- Plg
plasminogen
- Plm
plasmin
- PMNs
neutrophils
- sc-uPA
single-chain urokinase-type plasminogen activator
- SMA
smooth muscle cell actin
- NG2
neural/glial antigen 2
- BMT
bone marrow transplantation
Footnotes
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Tazzyman S, Niaz H, Murdoch C. Neutrophil-mediated tumour angiogenesis: subversion of immune responses to promote tumour growth. Semin Cancer Biol. 2013;23:149–158. doi: 10.1016/j.semcancer.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 2.Qian B-Z, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141:39–51. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kusumanto YH, Dam WA, Hospers GA, Meijer C, Mulder NH. Platelets and granulocytes, in particular the neutrophils, form important compartments for circulating vascular endothelial growth factor. Angiogenesis. 2003;6:283–287. doi: 10.1023/B:AGEN.0000029415.62384.ba. [DOI] [PubMed] [Google Scholar]
- 4.Noonan DM, De Lerma Barbaro A, Vannini N, Mortara L, Albini A. Inflammation, inflammatory cells and angiogenesis: decisions and indecisions. Cancer Metastasis Rev. 2008;27:31–40. doi: 10.1007/s10555-007-9108-5. [DOI] [PubMed] [Google Scholar]
- 5.Nozawa H, Chiu C, Hanahan D. Infiltrating neutrophils mediate the initial angiogenic switch in a mouse model of multistage carcinogenesis. Proc Natl Acad Sci U S A. 2006;103:12493–12498. doi: 10.1073/pnas.0601807103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Van Hinsbergh VWM, Engelse MA, Quax PHA. Pericellular Proteases in Angiogenesis and Vasculogenesis. Arterioscler Thromb Vasc Biol. 2006;26:716–728. doi: 10.1161/01.ATV.0000209518.58252.17. [DOI] [PubMed] [Google Scholar]
- 7.Lee S, Zheng M, Kim B, Rouse BT. Role of matrix metalloproteinase-9 in angiogenesis caused by ocular infection with herpes simplex virus. J Clin Invest. 2002;110:1105–1111. doi: 10.1172/JCI15755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shamamian P, Schwartz JD, Pocock BJ, Monea S, Whiting D, Marcus SG, Mignatti P. Activation of progelatinase A (MMP-2) by neutrophil elastase, cathepsin G, and proteinase-3: arole for inflammatory cells in tumor invasion and angiogenesis. J Cell Physiol. 2001;189:197–206. doi: 10.1002/jcp.10014. [DOI] [PubMed] [Google Scholar]
- 9.Das R, Pluskota E, Plow EF. Plasminogen and its receptors as regulators of cardiovascular inflammatory responses. Trends Cardiovasc Med. 2010;20:120–124. doi: 10.1016/j.tcm.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ploplis VA, French EL, Carmeliet P, Collen D, Plow EF. Plasminogen deficiency differentially affects recruitment of inflammatory cell populations in mice. Blood. 1998;91:2005–2009. [PubMed] [Google Scholar]
- 11.Busuttil SJ, Ploplis VA, Castellino FJ, Tang L, Eaton JW, Plow EF. A central role for plasminogen in the inflammatory response to biomaterials. J Thromb Haemost. 2004;2:1798–1805. doi: 10.1111/j.1538-7836.2004.00916.x. [DOI] [PubMed] [Google Scholar]
- 12.Swaisgood CM, Aronica MA, Swaidani S, Plow EF. Plasminogen is an important regulator in the pathogenesis of a murine model of asthma. Am J Respir Crit Care Med. 2007;176:333–342. doi: 10.1164/rccm.200609-1345OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tan SM. The leucocyte beta2 (CD18) integrins: the structure, functional regulation and signalling properties. Biosci Rep. 2012;32:241–269. doi: 10.1042/BSR20110101. [DOI] [PubMed] [Google Scholar]
- 14.Pluskota E, Solovjov DA, Plow EF. Convergence of the adhesive and fibrinolytic systems: recognition of urokinase by integrin αMβ2 as well as by the urokinase receptor regulates cell adhesion and migration. Blood. 2003;101:1582–1590. doi: 10.1182/blood-2002-06-1842. [DOI] [PubMed] [Google Scholar]
- 15.Pluskota E, Soloviev DA, Bdeir K, Cines DB, Plow EF. Integrin αMβ2 orchestrates and accelerates plasminogen activation and fibrinolysis by neutrophils. J Biol Chem. 2004;279:18063–18072. doi: 10.1074/jbc.M310462200. [DOI] [PubMed] [Google Scholar]
- 16.Lu H, Smith CW, Perrard J, Bullard D, Tang L, Entman ML, Beaudet AL, Ballantyne CM. LFA-1 is sufficient in mediating neutrophil emigration in Mac-1 deficient mice. J Clin Invest. 1997;99:1340–1350. doi: 10.1172/JCI119293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Passaniti A, Taylor RM, Pili R, Guo Y, Long PV, Haney JA, Pauly RR, Grant DS, Martin GR. A simple, quantitative method for assessing angiogenesis and antiangiogenic agents using reconstituted basement membrane, heparin, and fibroblast growth factor. Lab Invest. 1992;67:519–528. [PubMed] [Google Scholar]
- 18.Cotter MJ, Norman KE, Hellewell PG, Ridger VC. A novel method for isolation of neutrophils from murine blood using negative immunomagnetic separation. Am J Pathol. 2001;159:473–481. doi: 10.1016/S0002-9440(10)61719-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ustinov VA, Plow EF. Identity of the amino acid residues involved in C3bi binding to the I-domain supports a mosaic model to explain the broad ligand repertoire of integrin alpha M beta 2. Biochemistry. 2005;44:4357–4364. doi: 10.1021/bi047807e. [DOI] [PubMed] [Google Scholar]
- 20.Huang X, Raskovalova T, Lokshin A, Krasinskas A, Devlin J, Watkins S, Wolf SF, Gorelik E. Combined antiangiogenic and immune therapy of prostate cancer. Angiogenesis. 2005;8:13–23. doi: 10.1007/s10456-005-2893-y. [DOI] [PubMed] [Google Scholar]
- 21.van der Schaft DW, Dings RP, de Lussanet QG, van Eijk LI, Nap AW, Beets-Tan RG, Bouma-Ter Steege JC, Wagstaff J, Mayo KH, Griffioen AW. The designer anti-angiogenic peptide anginex targets tumor endothelial cells and inhibits tumor growth in animal models. FASEB J. 2002;16:1991–1993. doi: 10.1096/fj.02-0509fje. [DOI] [PubMed] [Google Scholar]
- 22.Jablonska J, Leschner S, Westphal K, Lienenklaus S, Weiss S. Neutrophils responsive to endogenous IFN-beta regulate tumor angiogenesis and growth in a mouse tumor model. J Clin Invest. 2010;120:1151–1164. doi: 10.1172/JCI37223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rogers C, Edelman ER, Simon DI. A mAb to the β2-leukocyte integrin Mac-1 (CD11b/CD18) reduces intimal thickening after angioplasty or stent implantation in rabbits. Proc Natl Acad Sci USA. 1998;95:10134–10139. doi: 10.1073/pnas.95.17.10134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gaudry M, Bregerie O, Andrieu V, El BJ, Pocidalo MA, Hakim J. Intracellular pool of vascular endothelial growth factor in human neutrophils. Blood. 1997;90:4153–4161. [PubMed] [Google Scholar]
- 25.Richter J, Ng-Sikorski J, Olsson I, Andersson T. Tumor necrosis factor-induced degranulation in adherent human neutrophils is dependent on CD11b/CD18-integrin-triggered oscillations of cytosolic free Ca2+ Proc Natl Acad Sci U S A. 1990;87:9472–9476. doi: 10.1073/pnas.87.23.9472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Basu A, Contreras AG, Datta D, Flynn E, Zeng L, Cohen HT, Briscoe DM, Pal S. Overexpression of vascular endothelial growth factor and the development of post-transplantation cancer. Cancer Res. 2008;68:5689–5698. doi: 10.1158/0008-5472.CAN-07-6603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sossey-Alaoui K, Pluskota E, Davuluri G, Bialkowska K, Das M, Szpak D, Lindner DJ, Downs-Kelly E, Thompson CL, Plow EF. Kindlin-3 enhances breast cancer progression and metastasis by activating Twist-mediated angiogenesis. FASEB J. 2014;28:2260–2271. doi: 10.1096/fj.13-244004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lishko VK, Novokhatny VV, Yakubenko VP, Skomorovska-Prokvolit HV, Ugarova TP. Characterization of plasminogen as an adhesive ligand for integrins αMβ2 (Mac-1) and 51 (VLA-5) Blood. 2004;104:719–726. doi: 10.1182/blood-2003-09-3016. [DOI] [PubMed] [Google Scholar]
- 29.Schruefer R, Sulyok S, Schymeinsky J, Peters T, Scharffetter-Kochanek K, Walzog B. The proangiogenic capacity of polymorphonuclear neutrophils delineated by microarray technique and by measurement of neovascularization in wounded skin of CD18-deficient mice. J Vasc Res. 2006;43:1–11. doi: 10.1159/000088975. [DOI] [PubMed] [Google Scholar]
- 30.Oh C-W, Hoover-Plow J, Plow EF. The role of plasminogen in angiogenesis in vivo. J Thromb Haemost. 2003;1:1683–1687. doi: 10.1046/j.1538-7836.2003.00182.x. [DOI] [PubMed] [Google Scholar]
- 31.Heymans S, Luttun A, Nuyens D, Theilmeier G, Creemers E, Moons L, Dyspersin GD, Cleutjens JPM, Shipley M, Angellilo A, Levi M, Nübe O, Baker A, Keshet E, Lupu F, Herbert JM, Smits JFM, Shapiro SD, Baes M, Borgers M, Collen D, Daemen MJAP, Carmeliet P. Inhibition of plasminogen activators or matrix metalloproteinases prevents cardiac rupture but impairs therapeutic angiogenesis and causes cardiac failure. Nature Med. 1999;5:1135–1142. doi: 10.1038/13459. [DOI] [PubMed] [Google Scholar]
- 32.Vogten JM, Reijerkerk A, Meijers JC, Voest EE, Borel RI, Gebbink MF. The role of the fibrinolytic system in corneal angiogenesis. Angiogenesis. 2003;6:311–316. doi: 10.1023/B:AGEN.0000029414.24060.fe. [DOI] [PubMed] [Google Scholar]
- 33.Drixler TA, Vogten JM, Gebbink MF, Carmeliet P, Voest EE, Borel RIH. Plasminogen mediates liver regeneration and angiogenesis after experimental partial hepatectomy. Br J Surg. 2003;90:1384–1390. doi: 10.1002/bjs.4275. [DOI] [PubMed] [Google Scholar]
- 34.Creemers E, Cleutjens J, Smits J, Heymans S, Moons L, Collen D, Daemen M, Carmeliet P. Disruption of the plasminogen gene in mice abolishes wound healing after myocardial infarction. Am J Pathol. 2000;156:1865–1873. doi: 10.1016/S0002-9440(10)65060-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ling Q, Jacovina AT, Deora A, Febbraio M, Simantov R, Silverstein RL, Hempstead B, Mark WH, Hajjar KA. Annexin II regulates fibrin homeostasis and neoangiogenesis in vivo. J Clin Invest. 2004;113:38–48. doi: 10.1172/JCI200419684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen PK, Chang BI, Kuo CH, Chen PS, Cho CF, Chang CF, Shi GY, Wu HL. Thrombomodulin functions as a plasminogen receptor to modulate angiogenesis. FASEB J. 2013;27:4520–4531. doi: 10.1096/fj.13-227561. [DOI] [PubMed] [Google Scholar]
- 37.Johnson C, Sung HI, Lessner SM, Fini ME, Galis ZS. Matrix metalloproteinase-9 is required for adequate angiogenic revascularization of ischemic tissues: potential role in capillary branching. Circ Res. 2004;94:262–268. doi: 10.1161/01.RES.0000111527.42357.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Masson V, de la Ballina LR, Munaut C, Wielockx B, Jost M, Maillard C, Blacher S, Bajou K, Itoh T, Itohara S, Werb Z, Libert C, Foidart JM, Noel A. Contribution of host MMP-2 and MMP-9 to promote tumor vascularization and invasion of malignant keratinocytes. FASEB J. 2005;19:234–236. doi: 10.1096/fj.04-2140fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ahn GO, Brown JM. Matrix metalloproteinase-9 is required for tumor vasculogenesis but not for angiogenesis:role of bone-marrow-derived myelomonocytic cells. Cancer Cell. 2008;13:181–183. doi: 10.1016/j.ccr.2007.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pepper MS. Role of the Matrix Metalloproteinase and Plasminogen Activator-Plasmin Systems in Angiogenesis. Arterioscler Thromb Vasc Biol. 2001;21:1104–1117. doi: 10.1161/hq0701.093685. [DOI] [PubMed] [Google Scholar]
- 41.Stefanidakis M, Koivunen E. Cell-surface association between matrix metalloproteinases and integrins: role of the complexes in leukocyte migration and cancer progression. Blood. 2006;108:1441–1450. doi: 10.1182/blood-2006-02-005363. [DOI] [PubMed] [Google Scholar]
- 42.Gong Y, Hart E, Shchurin A, Hoover-Plow J. Inflammatory macrophage migration requires MMP-9 activation by plasminogen in mice. J Clin Invest. 2008 doi: 10.1172/JCI32750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stefanidakis M, Ruohtula T, Borregaard N, Gahmberg CG, Koivunen E. Intacellular and cell surface localization of a complex between alphaMbeta2 integrin and promatrix metalloproteinase-9 progelatinase in neutrophils. J Immunol. 2004;172:7060–7068. doi: 10.4049/jimmunol.172.11.7060. [DOI] [PubMed] [Google Scholar]
- 44.Benelli R, Morini M, Carrozzino F, Ferrari N, Minghelli S, Santi L, Cassatella M, Noonan DM, Albini A. Neutrophils as a key cellular target for angiostatin: implications for regulation of angiogenesis and inflammation. FASEB J. 2002;16:267–269. doi: 10.1096/fj.01-0651fje. [DOI] [PubMed] [Google Scholar]
- 45.Shaw JP, Chuang N, Yee H, Shamamian P. Polymorphonuclear neutrophils promote rFGF-2-induced angiogenesis in vivo. J Surg Res. 2003;109:37–42. doi: 10.1016/s0022-4804(02)00020-3. [DOI] [PubMed] [Google Scholar]
- 46.Cursiefen C, Chen L, Borges LP, Jackson D, Cao J, Radziejewski C, D’Amore PA, Dana MR, Wiegand SJ, Streilein JW. VEGF-A stimulates lymphangiogenesis and hemangiogenesis in inflammatory neovascularization via macrophage recruitment. J Clin Invest. 2004;113:1040–1050. doi: 10.1172/JCI20465. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.