Abstract
8-Oxoguanine-DNA glycosylase-1 (OGG1) is the primary enzyme for repairing 7,8-dihydro-8-oxoguanine (8-oxoG) via the DNA base excision repair pathway (OGG1-BER). Accumulation of 8-oxoG in the genomic DNA leads to genetic instability and carcinogenesis, and is thought to contribute to the worsening of various inflammatory and disease processes. However, the disease mechanism is unknown. Here we proposed that the mechanistic link between OGG1-BER and pro-inflammatory gene expression is OGG1’s guanine nucleotide exchange factor activity, acquired after release of the 8-oxoG base and consequent activation of the small GTPase RAS. To test this hypothesis, we utilized BALB/c mice expressing or deficient in OGG1 in their airway epithelium and various molecular biological approaches, including active RAS pull-down, reporter and Comet assays, siRNA-mediated depletion of gene expression, quantitative RT-PCR, and immunoblotting. We report that the OGG1-intiated repair of oxidatively damaged DNA is a prerequisite for GDP→GTP exchange, KRAS-GTP-driven signaling via MAP-, PI3-, and MS kinases for NF-κB activation, pro-inflammatory chemokine/cytokine expression, and inflammatory cell recruitment to the airways. Mice deficient in OGG1-BER showed significantly decreased immune responses, while a lack of other Nei-like DNA glycosylases, i.e., NEIL1 and NEIL2, had no significant effect. These data unveil a previously unidentified role of OGG1-driven DNA BER in the generation of endogenous signals for inflammation in the innate signaling pathway.
Introduction
Reactive oxygen species (ROS) generated by biological, physical, and chemical agents, ligand-receptor interactions, and as a byproduct of metabolic processes induce oxidative modifications to lipids, proteins and DNA, and generate cell activation signals – all of which are thought to be associated with inflammatory disease processes (1). The primary target of ROS in the DNA is guanine, because it has the lowest redox potential of the four nucleobases (2, 3). Although guanine base lesions vary according to the nature of the oxidants (4), 7,8-dihydro-8-oxoguanine (8-oxoG) is the most frequent oxidation product in both DNA and RNA. Moreover, the accumulation of 8-oxoG along with other oxidatively modified bases in the mammalian genome has been associated with a loss of cellular/tissue homeostasis, and is believed to contribute to various inflammatory processes and aging-related diseases (5, 6). In mammalian cells, oxidatively modified base lesions are repaired by the base-specific DNA glycosylases OGG1, endonuclease III-like protein 1 (NTH1), and Nei-like (NEIL1, NEIL2 and NEIL3) (7, 8). OGG1 and NTH1 incise the DNA strand via β-lyase activity, thereby generating 3′-phospho-α,β-unsaturated aldehyde terminus (3′dRP) and 5′-phosphate, while NEIL1,2 and 3 have βδ-lyase activity, thus generating 3′- and 5′ phosphate (3′P and 5′P) at the strand gaps. The 3′dRP and 5′P are then removed by AP endonuclease 1 (APE1) and polynucleotide kinase 3′ phosphatase (PNKP), respectively, to generate a polymerase-ready 3′OH residue. Gap filling can involve 1-nt incorporation by DNA polymerase β (Polβ) in the short-patch repair sub-pathway, or displacement synthesis of 2–8 nts by either Polβ or replicative DNA polymerase (Polδ) in the long-patch repair sub-pathway (7–9).
To address the role of 8-oxoG in the genome, knockout mice lacking OGG1 activity have been generated (10, 11). Surprisingly, OGG1-null mice did not show impaired embryonic development or phenotypic changes, including altered lifespan, despite supraphysiological levels of 8-oxoG in their genomes. In other reports, Ogg1-null mice were shown to be found resistant to innate and allergic inflammation (14), and when placed on a high fat diet, showed increased susceptibility to obesity and metabolic dysfunctions (15). Mabley and colleagues showed that that Ogg1-null mice are resistant to endotoxin (LPS)-induced organ dysfunction, neutrophil infiltration, and oxidative stress compared to the response seen in wild-type controls (12). In another study, sensitized Ogg1-null mice exhibited lower IL-4, IL-6, IL-10, and IL-17 production and decreased levels of inflammatory cell infiltration and oxidative stress in the lungs after ovalbumin challenge compared to levels in wild-type controls (13). Bacsi and colleagues showed that supraphysiological levels of 8-oxoG in the genome of the airway epithelium (AE) due to decreased OGG1 expression evoked a lower inflammatory response in challenged-sensitized mice, as determined by expression of Th2 cytokines, eosinophilia, epithelial metaplasia, and airway hyperresponsiveness (AHR) (14). Taken together, these observations strongly imply the presence of yet-to-be-defined function(s) of OGG1 protein and/or its repair product, 8-oxoG base, in inflammatory processes. Intriguing recent studies have also shown that OGG1 binds the 8-oxoG base with a high affinity, and that the resulting complex (OGG1•8-oxoG) acts as a guanine nucleotide exchange factor that activates RAS family GTPases (16–18).
In the present study, we test the hypothesis that the repair of 8-oxoG and not the accumulation of 8-oxoG in the genome is etiologically associated with an innate immune response. Utilizing BALB/c mice expressing or lacking OGG1 in the airways, we demonstrated that the repair of oxidatively damaged DNA by OGG1, but not by other lesion-specific DNA glycosylases (NEIL1, NEIL2), induces the activation of wild-type KRAS, MAP-, PI3- and MS kinases and NF-κB, resulting in proinflammatory gene expression and inflammatory cell accumulation.
Materials and Methods
Reagents
Inhibitors
Trans-farnesylthiosalicylic acid (FTS, Cayman Chemical, Ann Arbor, Michigan ASU); GW5074 (5-iodo-3-[(3,5-dibromo-4-hydroxyphenyl)methylene]-2-indolinone (Sigma-Aldrich, St. Louis, MO, USA); PD98059 (2-(2-Amino-3-methoxyphenyl)-4H-1-benzopyran-4-one (EMD Millipore Chemicals, Gibbstown, NJ); LY294002 (2-morpholin-4-yl-8-phenylchromen-4-one; EMD Millipore Chemicals, Gibbstown, NJ). 4,6-diamidino-2-phenylindole dihydrochloride (DAPI, Sigma-Aldrich, St. Louis, MO, USA).
Nucleotides and nucleosides
8-oxoguanine (Cayman Chemical, Ann Arbor, MI, USA). 2,6-diamino-4-hydroxy-5-formamidopyrimidine (FapyG) was kindly provided by Miral Dizdaroglu (National Institute of Standards and Technology, Gaithersburg, MD). 7,8-dihydro-8-oxoadenine (8-oxoA) was purchased from BioLog Life Science Institute (Axxora, LLC, San Diego, CA, USA). Guanine and 8-oxo-2′-deoxyguanosine (8-oxodG) were from Sigma-Aldrich (St. Louis, MO). 8-OxoG was provided as a hydroacetate salt and dissolved as recommended by the manufacturer (Cayman Chemical, Ann Arbor, MI, USA). Briefly, 8-oxoG was dissolved in 10 mM NaOH (pH 12). Stock solution was diluted to 0.001 to 10 μM concentrations in saline (w/o Ca2+, Mg2+; pH 7.4) and used immediately. For experiments, filter-sterilized, pH-balanced 8-oxoG or solutions of control reagents were used. All reagents were free of lipopolysaccharides as determined using an LAL Chromogenic Endotoxin Quantitation Kit (Cat # 88282; Pierce Biotechnology (Rockford, IL, USA). Glucose oxidase (Cat# G7141) was purchased from Sigma-Aldrich (St. Louis, MO, USA). The dose of GO was set (in preliminary studies) to 1 mU, which caused an ~2-fold increase in ROS levels in primary airway epithelial cells, resulting in a 2.5-fold increase in 8-oxoG levels in OGG1-expressing airways.
Antibodies
Primary antibodies (Abs) -- OGG1 Ab (Cat # ab124741) and subtype-specific HRAS Ab (Cat# 1521–1) were purchased from Epitomics (Burlingame, CA, USA). pan-RAS Ab (Cat# 05–1072) was from Millipore (Billerica, MA) and KRAS (Cat # sc-30), NRAS (Cat# sc-3), RelA (Cat# sc-372), and p-RelA (Ser276, Cat # sc-101749) Abs from Santa Cruz Biotechnology (Dallas, TX, USA). The following Abs were purchased from Cell Signaling Technologies (Danvers MA, USA): RAF-1 (Cat# 9422), phosphorylated (p)-RAF-1 (Ser338 Cat# 9427), ERK 1,2 (Cat # 4695), p-ERK 1,2 (Thr202/Tyr204, Cat# 9101), MEK 1,2 (Cat# 9122), p-MEK1,2 (Ser217/221 Cat# 9154), MSK1 (Cat# 3489), p-MSK1 (Ser376) Cat# 9591), PI3K(p85, Cat #4292), p-PI3K (p85/Tyr458; p55/Tyr199, Cat# 4228), AKT (Cat #9272), p-AKT (Ser473, Cat# 4058), IKKα (Cat#2682), IKKβ (Cat#2370), p-IKKα/β (Ser176/180, Cat #2697), IκBα (Cat# 4814), and p-IκBα (Ser32/36 Cat# 9246). Secondary Abs, e.g., goat anti-rabbit, rabbit anti mouse and donkey anti-mouse, were from GE Healthcare (Pittsburgh, PA, USA).
Cell cultures
Human diploid fibroblasts (MRC-5), murine embryonic fibroblasts (Ogg1−/− MEF, and Ogg1+/+ MEF) and the mouse lung epithelial cell line (MLE-12) were maintained respectively in Eagle’s minimum essential medium, Dulbecco’s modified Eagle’s low glucose, and Iscove’s modified Dulbecco’s medium, RPMI 1640, supplemented with 10% fetal bovine serum (FBS). Ogg1−/− and Ogg1+/+ MEF cells were kindly provided by Dr. Barnes (Imperial Cancer Research Fund, Clare Hall Laboratories, South Mims, Hertfordshire EN6 3LD, United Kingdom). All media contained glutamine, penicillin, and streptomycin, and cells were grown at 37 °C, in a 5% CO2 atmosphere. Primary mouse lung fibroblasts (Cat # C57–6013) and primary mouse tracheal epithelial cells (Cat # BALB-5033) were obtained from Cell Biologics Inc (Chicago IL, USA), and cells were maintained as recommended by the provider (in Complete Fibroblast Medium, Cat # M2267 and Complete Epithelial Cell Medium, Cat # M6621). A549 cells expressing GFP-RelA fusion protein were established by Dr. AR Brasier’s laboratory and maintained in Dulbecco’s modified Eagle’s medium containing 10% FBS, penicillin (100 units/ml), streptomycin (100 μg/ml) and hygromycin B (100 μg/ml) at 37 °C in a 5% CO2 incubator. The human telomerase (hTERT) and cyclin-dependent kinase (CDK)-4- immortalized human normal bronchial epithelial cell (hNBECs) were kindly provided by Dr. JD Minna (Hamon Center for Therapeutic Oncology Research, Department of Internal Medicine Pharmacology, University of Texas Southwestern Medical Center, Dallas, TX, USA), and were grown in bronchial airway epithelial cell growth medium (Lonza, Walkersville, MD) in a humidified atmosphere of 5% CO2 (19).
Animals and treatments
Animal experiments were performed according to the NIH Guide for Care and Use of Experimental Animals and approved by the UTMB Animal Care and Use Committee (approval no. 0807044A). Eight- to ten-week-old female BALB/c mice (The Jackson Laboratory) were used for these studies. Mice (n =5–6) were challenged via the nasal route with 60 μl of pH balanced saline containing 8-oxoG (pH: 7.4; 1 μM), glucose oxidase (GOx; 1 mU) or other nucleotides or nucleosides under mild anesthesia. Animals were sacrificed at various time points, and their lungs subjected to lavage to obtain bronchoalveolar lavage fluid (BALF). The lungs were then excised and portions sectioned or homogenized for RNA extraction and preparation of lung extracts for Western blotting (WB) or to determine changes in Ras-GTP levels (16). Stealth RNAi for depletion of target proteins was undertaken as we described under “Depletion of gen expression.” Intraperitoneal (i.p.) inhibitor treatments: FTS (trans-farnesylthiosalicylic acid, RAS inhibitor) 2 mg/kg i.p.; GW5074 (Raf1 inhibitor) 10 mg/kg i.p.; PD98059 (MEK1/2 inhibitor) 10 mg/kg i.p. or LY294002 (pan-PI3K inhibitor) 5 mg/kg i.p. per mice were added as described in previous studies (20–22).
Evaluation of airway inflammation
Bronchoalveolar lavage fluids were collected, total cell numbers were determined, and cytospin preparations were made for cell typing by Abs using immunohistochemistry or staining with Wright-Giemsa for differential cell counts as we previously described (23, 24). Randomly selected fields were photographed, and differential cell counts were evaluated using ImageJ v1.47 (NIH) with the built-in cell counter plugin. Immunofluorescence staining was carried out (see Immunohistochemistry) on non-permeabilized cells using an Ab to neutrophil elastase (Cat# SC-9518, Santa Cruz Biotechnology). Fluorescent images were captured at a magnification of x144 using A CoolSNAP HQ2 camera mounted on a Nikon Eclipse Ti microscope. The microscope was operated via NIS-Elements AR Software Ver3.22.09 for 64 bit.
Depletion of gene expression in lung
Stealth RNAi™ was obtained from Invitrogen Life Technologies. Under mild anesthesia, Stealth™ RNAi to target gene (or control RNAi) in transfection reagent (Polyplus-transfection, New York, NY) (Cat# 201–10G) was introduced into the airways i.n. at 0 and 24 h (14, 25). Stealth™ RNAi duplex for Ogg1 (Cat # MSS237431) Sense (S) was: 5′-GAUGUCACUUAUCAUGGCUUCCCAA-3′; Antisense (AS): 5′-UUGGGAAGCCAUGAUAAGUGACAUC-3′; Stealth™ RNAi duplex for Kras (Cat # MSS236996_F1N), Sense: 5′-CACUUUGUGGAUGAGUACGACCCUA-3′, Antisense: 5′-UAGGGUCGUACUCAUCCACAAAGUG-3′; Stealth™ RNAi duplex for Nras (Cat# NM_010937.2_stealth_244), Sense: 5′-GAACCACUUUGUGGAUGAAUAUGAU-3′, Antisense: 5′-AUCAUAUUCAUCCACAAAGUGGUUC-3′; Stealth™ RNAi duplex for Hras (Cat# NM_008284.2_stealth_279), Sense: 5′-CAGAACCACUUUGUGGACGAGUAUG-3′, Antisense: 5′-CAUACUCGUCCACAAAGUGGUUCUG-3′. Neil1 Stealth™ RNAi (Cat #: 1320001; ID #: MSS231767), Neil2 Stealth™ RNAi (Cat #: 1320001; ID #: MSS283982).
Ablation of gene expression in cultured cells
siRNAs (20 to 40 nM; optimal final concentrations were determined in preliminary studies) in transfection reagent (INTERFERin™; Polyplus-transfection Inc., NY) were added to dishes along with 5 × 106 cells in serum-free media for 3 hr. Cells were further incubated in growth media for 48 h. Control siRNA and siRNAs to target genes were obtained from Dharmacon, Thermo Fisher Scientific Inc. (Rockford, IL, USA) siGENOME Smart pool; Hras Cat # M-004142, Kras Cat # M-005069, Nras Cat # M-003919. siRNA to deplete mouse OGG1 (Cat # M-048121-01-005) and human OGG1 (Cat# M-005147-03-0005) were purchased from Dharmacon (Pittsburgh, PA, USA). OGG1 was depleted via a simultaneous siRNA transfection and plating method (14, 18). siRNAs to PI3K (Cat# M-041079-01-0005); MEK1-2, (MAP2K1 Cat# M-040605-01-0005, MAP2K2 Cat# M-040606-01-0005); and MSK1 (Cat# M-040751-01-0005) were from Dharmacon (Pittsburgh, PA, USA). Depletion of the target at the mRNA / protein levels was determined by qRT-PCR and WB analysis, respectively.
Assessment of genomic 8-oxoG levels
Genomic 8-oxoG levels were determined by assessing OGG1-sensitive sites using a FLARE™ comet assay kit (Trevigen, Gaithersburg, MD USA) (14, 26, 27). Briefly, freshly isolated, exfoliated airway epithelial cells were suspended in 320 μL of 0.6% low melting-point agarose and placed onto pre-coated microscope slides (Trevigen Inc). Slides with solidified agarose were placed in lysis buffer (2.5 M NaCl, 100 mM EDTA, 10 mM Trish-HCl (pH 10), 1% sodium sarcosinate and 1% Triton X-100) for 24 h. Slides were gently rinsed three times for 10 min with an enzyme reaction buffer (40 mM HEPES, 0.1 M KCl, 0.5 mM EDTA, 1 mM DTT; 0.2 mg ml BSA). Slides were than immersed in enzyme reaction buffer ± 10 μg/mL OGG1 [generated locally (28)]. DNA was digested for 180 min at 37°C. Slides were placed in a pre-cooled (4 °C) electrophoresis tank, and electrophoresis was carried out at pH 11.5 in a buffer provided by the manufacturer (Trevigen Inc). Before electrophoresis, DNA was allowed to unwind for 10 min. After electrophoresis at 1.25 V per cm) at 4 °C, preparations were neutralized (0.4 M Trish-HCl, pH 7.4) 3 times (15 min). The agarose was air-dried and the DNA stained with SYBR®Green. Comets were analyzed using the Comet Assay IV v4.2 system image analysis software (Perceptive Instruments, Suffolk, UK) (14). Depending on cell availability, 120–160 comets were scored. The fold changes in tail intensities are expressed as the mean (± SD) from three to five mice.
Immunohistochemistry
Cells on microscope coverslips were fixed in 4% paraformaldehyde at 4°C and then permeabilized with Triton X100 for 30 min at 37°C. The cells were then incubated for overnight at 4°C with primary Ab to OGG1 (1:1000), 8-oxodezoxyguanosine (1:400). After washing (PBS-Tween 20: PBS-T) cells were incubated for 1 h at room temperature with Alexa 488-conjugated secondary Abs. Nuclei of cells were stained for 15 min with DAPI (4′6-diamidino-2-phenylindole dihydrochloride; 10 ng/mL; blue). Cells were then mounted in anti-fade medium (Dako Inc. Carpinteria, CA) on a microscope slide. Microscopy was performed on a NIKON Eclipse Ti System operated via NIS-Elements Software AR Ver3.22.09 for 64 bit (NIKON Instruments, Tokyo, Japan).
Assessment of RAS-GTP levels
Changes in RAS-GTP levels were quantified using an Active RAS Pull-Down and Detection Kit (Pierce Biotechnology, Thermo Fisher Scientific) as described previously (16, 17). In brief, RAS-GTP from lung extracts and cell lysates (25 mM Tris-HCl, pH 7.5, 150 mM NaCl, 60 mM MgCl2, 1% NP40 and 5% glycerol) was captured by the RAS-binding domain of Raf1 (RBD) (29) immobilized on Glutathione Resin GST-RAF1-RBD. After washing with binding buffer, captured RAS was eluted in Laemmli buffer and detected by WB. Total RAS proteins were detected using pan-RAS Ab (Millipore Inc). RAS family proteins were identified using subtype-specific Abs for HRAS (Epitomics), KRAS and NRAS Abs (Santa Cruz Biotechnology).
Immunoblotting
Cell and lung extracts were fractionated on 4% to 20% gradient SDS polyacrylamide gels (NuSep Ltd, Frenchs Forest, Australia), and proteins were subsequently transferred onto nitrocellulose membranes (Amersham Biosciences). Primary Abs (see Reagents) against OGG1, RAF-1, pRAF-1, ERK1/2, pERK1/2, MEK1/2, pMEK1/2, MSK1, pMSK1; IκB, pIκB, IKK, pIKK, RelA and pRelA were used. Ras proteins were detected using pan-RAS Ab or subtype-specific HRAS, KRAS, NRAS Abs. Binding of primary Abs was detected with horseradish peroxidase–conjugated secondary (goat anti-rabbit and donkey anti-mouse) Ab(s). The chemiluminescence signals were detected using the ECL Plus detection system (GE Life Sciences, Pittsburgh, PA). Band intensities were quantitated by densitometry using ImageJ software (ver. 1.44, NIH). In selected studies, the percentage changes in protein levels were calculated using MS Excel.
Luciferase assays
Expression vectors were introduced using Lipofectamine 2000 (Invitrogen) per the manufacturer’s instructions. Briefly, cells were seeded on plates for 24 h and incubated in growth medium without antibiotics overnight, then co-transfected with pGL4.32 luc2P/NF-κBRE/Hygro (Promega, Madison, WI) and pRL-TK Renilla Luciferase plasmid (Promega). After a 24h transfection, cells were exposed to 8-oxoG (10 μM), and triplicate samples were lysed with 100 μL of lysis buffer. Cellular debris was removed by centrifugation. Aliquots of the supernatants (20 μL) were added to the luciferase substrate solution and evaluated on a Glomax® Luminometer (Promega). Firefly luciferase values were normalized against Renilla luciferase activity and expressed as relative light units (RLU).
Quantitation of chemokines and cytokines
To determine the levels of proinflammatory mediators, we used the Bio-Plex Pro™ Mouse Cytokine 23-plex Assay (Cat#M60-009RDPD; Bio-Rad Laboratories, Inc) (30). Briefly, BALF was centrifuged at 14,000 g (at 4 °C) to remove cell debris, and 25 μL of supernatant (diluted when needed) was mixed with magnetic beads covalently bound to capture Abs against cytokines, chemokines, or growth factors. After washing, a biotinylated detection Ab specific to an epitope different from that of the capture Ab was added to the reaction mixture. A streptavidin-phycoerythrin reporter complex was then added to the biotinylated Abs on the bead surface. Plates were evaluated on a Bio-Plex system using a dual-laser, flow-based microplate reader system. Bio-Plex Manager™ software (version 4.0) was used to calculate the results.
Quantitative real-time (qRT-PCR) analysis
RNA was extracted using an RNeasy kit per the manufacturer’s instructions (Qiagen, Valencia, CA). Total pooled (n=5) RNA (1 μg) was reverse-transcribed into cDNA using a SuperScript® III First-Strand Synthesis System (Invitrogen, Carlsbad, CA) and analyzed using a Mouse Inflammatory Cytokines & Receptors PCR Array (Cat # PAMM-011A, SABiosciences, Valencia, CA, USA). Briefly, cDNA in water was mixed with an equal amount of 2x SYBR® Green Supermix (Qiagen), and 20-μL of reaction mixture was added to each well containing forward and reverse gene-specific primers; reactions were run for 40 cycles in an ABI7000 thermal cycler. Data were analyzed using RT2 profiler PCR data analysis template version 2.0. The results are reported as mean fold increases for all experimental groups, and data sets were deposited in the National Center for Biotechnology Information Gene Expression Omnibus accession number GSE60711 (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE60711).
To confirm expression levels we utilized primer pairs purchased from Integrated DNA Technologies: Cxcl-1: F: 5′-GCGAAAAGAAGTGCAGAGAG-3′, R: 5′-CACAAAATGTCCAAGGGAAG-3′; Cxcl-2: F: 5′-CTCCTTTCCAGGTCAGTTAGC-3′, R: 5′-CAGAAGTCATAGCCACTCTCAA-3′; Hras F: 5′-TGTCTCTGGCTGGAAGTAGG-3′, R: 5′-TGCAGCAAACACAAGACAGT-3′; Kras F: 5′-TTCTTCCCATCTTTGCTCATCT-3′, R: 5′-ATTCCGTTCATTGAGACCTCAG-3′ Nras F: 5′-TCCCCATCACCTTGAAACTA-3′, R: 5′-AGACCAAGTGCTGGTCTCAG-3′, Gapdh F: 5′-GTGGAGTCATACTGGAACATGTAG-3′, R: 5′-AATGGTGAAGGTCGGTGTG-3′. Quantitation of changes in gene expression was calculated using the ΔΔCt method and unstimulated cells as the calibrator, and then normalized to GAPDH (25).
Statistical analysis
Statistical analysis was performed using Student’s t-test to analyze changes at the mRNA and protein levels. Data from mouse treatment groups were analyzed using ANOVA, followed by Bonferroni post-hoc analyses for least significant difference. The data are presented as the means ± the standard error of the mean. Differences were considered to be statistically significant at P < 0.05.
Results
Repair of oxidatively damaged DNA via OGG1-BER is a prerequisite to neutrophil accumulation in the airways
Given our previously published data showing that when in complex with free 8-oxoG, OGG1 activates RAS GTPases (16), and the role of RAS in inflammatory signaling (31, 32), we proposed that OGG1-BER in the AE is a regulator of an innate immune response. Our experimental strategy to test this hypothesis is shown in Fig. 1A. We generated mice that were OGG1-deficient in their airway epithelia (Fig. 1A; Materials and Methods), as shown by decreased Ogg1 mRNA levels (Fig. 1B) and the lack of OGG1 protein in the nuclei of airway epithelial cells by immunohistochemistry (IHC; Fig. 1C). OGG1-expressing mice were used as a control. Upon a mild oxidative burst (Materials and Methods), there was a multiple-fold increase in genomic 8-oxoG levels in the OGG1-deficient AE, while OGG1-expressing epithelia showed only a transient change (Fig. 1D), as determined by Flare Comet assays (14) and IHC (Fig. 1E). The multiple-fold differences in genomic 8-oxoG levels between OGG1-deficient and OGG1-expressing mice imply the repair of 8-oxoG by OGG1-BER.
FIGURE 1.
Experimental strategy and characterization of site-specific OGG1 depletion. (A) Experimental design. (B) Stealth RNAi decreased both Ogg1 mRNA and (C) protein levels in airway epithelial cells, as shown by qRT-PCR and immunohistochemistry, respectively. Green fluorescence mediated by Alexa-488 shows subcellular localization of OGG1 (left panels). Nuclei of cells were stained with DAPI (blue, right panels). (D) Changes in genomic 8-oxoG levels in OGG1-expressing (control RNAi; blue columns) and OGG1-depleted (Stealth RNAi) airway epithelial cells (red columns) as shown by FLARE™ Comet assays (Material and Methods). (E) Genomic 8-oxoG levels in the airway epithelium of OGG1-expressing mice at time 0 (upper panel) and 1h after oxidative challenge (lower panel) as shown by IHC (magnification x216). DAPI, 4′6-diamidino-2-phenylindole dihydrochloride; AE RNA, RNA isolated from exfoliated airway epithelial cells; RAS, mammalian homolog of viral H-, K-, N-RAS protein; MAPK(s), mitogen activated protein kinases; MSK1, mitogen- and stress-activated protein kinase1; PI3K, phosphoinositide 3-kinase; OGG1, 8-oxoguanine DNA glycosylase-1; 8-oxoG, 7,8-dihydro-8-oxoguanine; Trf-R, transfection reagent for in vivo use (Materials and Methods). *** = P<0.001
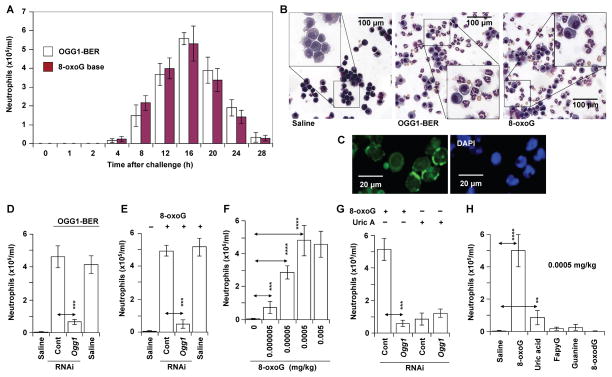
Our results show that 8-oxoG repair by OGG1-BER resulted in inflammatory cell recruitment into the lungs from 4h on, reaching a maximum at 16 h (Fig. 2A). Differential staining (Fig. 2B, middle panel) and IHC (Fig. 2C) showed that the recruited cells were neutrophils. By contrast, in the absence of OGG1-BER (OGG1-deficient airways), significantly fewer (~8-times) inflammatory cells were found in the BALF (Fig. 2D; the 16 h time point is shown). This low level of inflammation occurred despite supraphysiological genomic 8-oxoG levels (Fig. 1D). Oxidative DNA base lesions were generated by identical doses of oxidative stress, so the data imply that the rate-limiting event in inflammatory responses is OGG1-BER and the resulting generation of free 8-oxoG base.
FIGURE 2.
OGG1-initiated DNA base excision repair results in the recruitment of neutrophils. (A) Kinetics of neutrophil recruitment to the airways after OGG1-BER (empty columns) or challenge with 8-oxoG base (filled columns, 0.0005 mg/kg). Each time point represents the average number of neutrophils from 5 to 6 mice. (B) Representative images of differentially stained cells derived from the BALF of saline- (left panel), OGG1-BER (middle panel) and 8-oxoG (right panel)-challenged airways. (C) Immunohistochemical identification of neutrophils based on neutrophil elastase localized to cell membrane (Materials and Methods). Magnification x144. (D) OGG1 deficiency in airway epithelia decreases the accumulation of neutrophils. (E) The 8-oxoG base challenge-induced recruitment of neutrophils is OGG1-dependent. (F) 8-oxoG base induces dose-dependent increases in the recruitment of neutrophils to airways. (G) OGG1-independent changes in neutrophil numbers upon uric acid (0.0005 mg/kg) challenge. (H) 8-oxoG, but not 2,6-diamino-4-hydroxy-5-formamidopyrimidine (FapyG), 8-oxoguanine deoxynucleoside (8-oxodG) or guanine induces neutrophil recruitment. In A,D,F,G and H, BALF was obtained at 16 h post-challenge, total cell numbers were determined, and cyto-spin preparations made for differential staining with Wright-Giemsa to determine neutrophil counts. 8-oxoG, FapyG, 8-oxodG, guanine base or uric acid was introduced via the intranasal route in 60 μL saline. 8-oxoG, 7,8-dihydro-8-oxoguanine; OGG1-BER, OGG1-initiated DNA base excision repair; Uric A, uric acid; ** = P<0.01; *** = P<0.001; **** = P<0.0001.
To mimic OGG1-mediated generation of 8-oxoG (and avoid ROS-induced signaling), we challenged mice with 8-oxoG base, the specific product of OGG1-BER (33). Intriguingly, 8-oxoG base (at 0.00005 mg/kg, chosen based on preliminary studies) is a potent inducer of neutrophils (Fig. 2A and B, right panel) in OGG1-expressing airways. In OGG1-deficient airways, an identical dose of 8-oxoG induced a low level of neutrophils (~10-fold less, Fig. 2E). We have also established a dose-response relationship (Fig. 2F). The lowest dose that induced an immune response was 0.000005 mg/kg, which can be generated by OGG1-BER in vivo (34). In controls, depletion of NEIL1 had no effect, while the lack of NEIL2 somewhat increased neutrophil accumulation after 8-oxoG (0.00005 mg per kg) challenge to the airways (data not shown). The possible role of NEIL2 in immune responses is currently being investigated and will be the subject of a future manuscript.
To demonstrate the specificity of the 8-oxoG challenge-induced inflammatory response, we showed that in OGG1-expressing mice uric acid (0.0005 mg/kg), a deaminated form of 8-oxoG, a danger-associated molecular pattern molecule (35), induced considerably fewer neutrophils than did 8-oxoG, and the uric acid-induced accumulation of inflammatory cells was not OGG1-dependent (Fig. 2G). Moreover, the 8-oxoG base was unique in inducing OGG1-dependent inflammation, as 8-oxo-deoxyguanosine, 2,6-diamino-4-hydroxy-5-formo-midopyrimidine (substrates of OGG1 in the genome) (33) and the intact guanine base elicited little or no neutrophil recruitment (Fig. 2H).
OGG1-BER and RAS activation are prerequisites for the expression of proinflammatory mediators
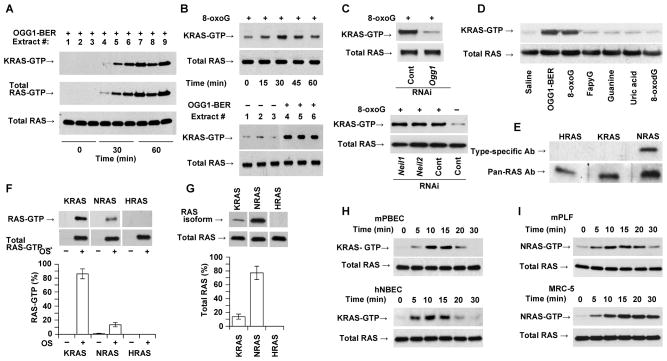
Next we determined whether the immune response in airways is coupled to OGG1-BER-associated activation of RAS-GTPases. OGG1-BER was initiated by a mild oxidative burst in the airways of OGG1-expressing mice. Lung extracts were then prepared and active RAS levels determined. GTP-bound RAS levels were increased from 30 min on (Fig. 3A). Use of subtype-specific Abs revealed that nearly all this RAS-GTP was KRAS (a homolog of the Kirsten sarcoma virus oncogene; Fig. 3A, upper and middle panels). Challenging OGG1-expressing airways with 8-oxoG caused rapid (from 15 min on) increases in KRAS-GTP levels (Fig. 3B; the upper panel shows the time course, the lower panel, the response of individual animals). In the absence of OGG1 expression, 8-oxoG challenge failed to activate KRAS (Fig. 3C, upper panel), while depletion of NEIL1 or NEIL2 had no effect (Fig. 3C, lower panel). FapyG [repaired via both OGG1- and NEIL1-BER (36)], 8-oxodG, uric acid, and the intact guanine base failed to activate RAS (Fig. 3D).
FIGURE 3.
OGG1-BER and its product 8-oxoG induce activation of KRAS. (A) OGG1-BER-induced activation of KRAS in the lungs as a function of time. At indicated times, lung extracts were prepared and changes in GTP-bound RAS were determined by active RAS pull-down assays. Subtype specific Ab to K-RAS and pan-RAS Ab was used to determine KRAS-GTP and total RAS levels, respectively (Materials and Methods). (B) KRAS activation after 8-oxoG challenge (time course, upper panels) and upon initiation of OGG1-BER (lower panels, 30 min time point is shown) in individual lungs. GTP-bound and total RAS levels were determined as described in legend to 3A. (C) Activation of KRAS is OGG1- (upper panel), but not NEIL1- or NEIL2-expression dependent (lower panel) in 8-oxoG-challenged airways. GTP-bound RAS levels were determined at 30 min post-challenge. (D), OGG1-BER and 8-oxoG challenge, but not FapyG, guanine, uric acid, or 8-oxodG activates RAS (KRAS) in the lungs. The 30 min time point is shown. GTP-bound RAS was determined as described in the legend to 3A. (E) Expression of NRAS, but not KRAS or HRAS in resident alveolar macrophages. Extracts of macrophages from unchallenged lungs (n = 6) were tested by WB using subtype-specific Abs. (F) GTP-bound RAS protein levels in pooled lung extracts (n = 5) after 8-oxoG challenge, and graphical depiction of the percentages of K-, N- and HRAS-GTP in total RAS-GTP. (G) Expression of RAS subtypes in lung. K-, N- and HRAS levels (upper panels) are shown by subtype-specific Abs. Lower panel shows graphical depiction of their percentages. Total RAS level is shown by pan-RAS Ab. In F and G, band intensities were quantified using Image J (ver. 1.44) software. Expression percentages were calculated using MS Excel. (H) KRAS activation in mouse (mPBEC) and human (hNBEC) bronchial airway epithelial cells upon 8-oxoG exposure. (I) Activation of N-RAS upon 8-oxoG exposure in mouse (mPLF) and human (MRC5) lung fibroblast cells. In A–I, 250 μg extract was utilized for assessing RAS-GTP levels; 20 μg for total RAS. In B (upper panel),C,D,F, and G, lungs were challenged with 60 μL saline containing 0.0005 mg per kg 8-oxoG. In H and I, cells were challenged with 10 μM of 8-oxoG solution in medium. In A and B (lower panels) oxidative DNA damage was generated by challenging the airways with glucose oxidase (1 mU in 60 μl saline). HRAS and KRAS, mammalian homolog of Harvey and Kirsten sarcoma virus oncogen; NRAS neuroblastoma RAS viral oncogene homolog; NEIL1 and 2, Nei (endonuclease VIII)-like DNA glycosylase-1 and -2; mPBEC, mouse primary bronchial epithelial cells; mPLF, mouse primary fibroblasts; hNBEC, human normal bronchial epithelial cells; MRC5, human diploid lung fibroblasts. FapyG, 2,6-diamino-4-hydroxy-5-formamidopyrimidine; 8-oxodG, 8-oxoguanine deoxynucleoside; 8-oxoG, 7,8-dihydro-8-oxoguanine.
To pursue these observations, we examined the levels of H- (homolog of the Harvey sarcoma virus oncogene), N- (neuroblastoma RAS viral oncogene homolog), and KRAS in lung extracts and the percentages of their GTP-bound forms. Upon quantifying the band intensities, we unexpectedly observed that 85 ± 7% of RAS-GTP was KRAS (Fig. 3F, lower panel), which made up only ~12 ± 5% of total RAS (Fig. 3G, lower panel); NRAS represented 78 ± 11% of total RAS (Fig. 3G, lower panel), but only 15 ± 3% was GTP-bound (Fig. 3F, lower panel). HRAS protein was not detectable in 20 μg of lung extracts (Fig. 3G). In freshly isolated alveolar macrophages, there were no increases in RAS-GTP levels after 8-oxoG (or oxidative stress) challenge, although NRAS was abundant. HRAS and KRAS were undetectable in 20 μg of macrophage extract (Fig. 3E).
Rapid KRAS activation after 8-oxoG challenge to the airways (Fig. 3B), and its dependence on OGG1, suggested that the AE is the site of GDP→GTP exchange. To support this hypothesis, we utilized primary cultured mouse bronchial epithelial cells (mPBECs) and human normal bronchial epithelial cells (hNBEC); KRAS-GTP levels were increased in response to 8-oxoG (10 μM) base in both (Fig. 3H). By contrast, NRAS was activated in primary mouse (mPLFs) and diploid human (MRC-5) lung fibroblasts after challenge with an identical dose of 8-oxoG (Fig. 3I). These results, along with those shown in Fig. 3A–D,F, strongly imply that KRAS is activated at the site of exposure, the airway epithelium.
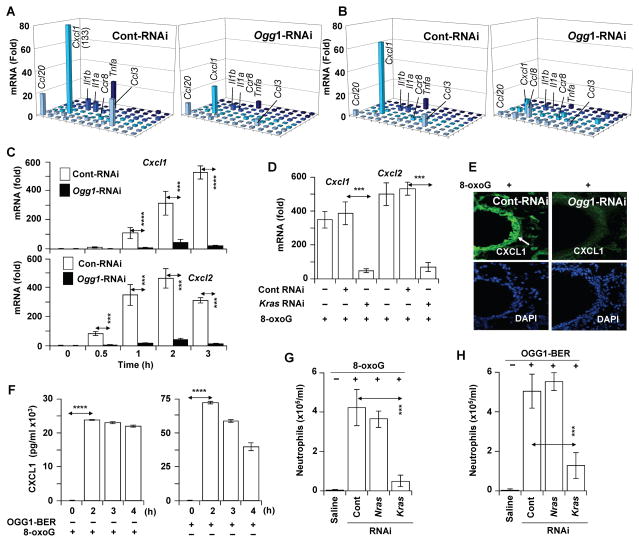
Next, we examined whether KRAS activation and downstream signaling result in the expression of proinflammatory chemokines/cytokines. The airways were challenged for 0, 30, 60, 120 and 180 min, and the mRNA levels of chemokines/cytokines were examined using Mouse Inflammatory Cytokines & Receptors PCR Arrays. Intriguingly, OGG1-BER (Fig. 4A, left panel), or a challenge with 8-oxoG (Fig. 4B, left panel), induced similar expression profiles of proinflammatory mediators (e.g., Cxcl1, Tnfα, Ccl20, Ccl3, Il1a, Il1b). The expression of these chemokines/cytokines was greatly decreased in OGG1-deficient compared to OGG1-expressing airways (the 2 h time point is shown, Fig. 4A and B, right panels). We expanded these observations by determining the kinetic changes in the mRNA levels in individual airways for Cxcl1 and Cxcl2, mediators of neutrophil recruitment (37). Cxcl1 and Cxcl2 mRNA levels were increased from 30 min on, peaking between 2 and 3h (Fig. 4C). Later time points were not investigated, as neutrophils were recruited into the lungs from 4h on (Fig. 2A). Importantly, the expression of Cxcl1 and 2 was significantly lower in OGG1- (Fig 4C) and KRAS-deficient airways (Fig. 4D). IHC showed that CXCL1 is not detectable in AE cells of OGG1-deficient mice (Ogg1-RNAi; Fig. 4E), while it is highly expressed in OGG1-proficient airways (Cont-RNAi; right panel; Fig. 4E). CXCL1 was present at high levels from 2h on in the BALF from 8-oxoG-exposed AE (Fig. 4F, left panel) and in those airways repairing DNA via OGG1-BER after an oxidative burst (Fig. 4F, right panel). These data are consistent with OGG1-driven activation of KRAS (Fig. 3A–C) and KRAS-expression-dependent accumulation of neutrophils (Fig. 4G,H). In controls, NRAS depletion had no impact on OGG1-BER and 8-oxoG challenge-induced neutrophil levels (Fig. 4G,H). These results imply that OGG1-BER-dependent KRAS activation in the AE is a prerequisite for the expression of proinflammatory mediators and for inflammatory cell recruitment.
FIGURE 4.
OGG1-BER-dependent expression of proinflammatory chemokines and cytokines. (A,B) Expression of proinflammatory mediators upon OGG1-initiated DNA base excision repair (A) and after 8-oxoG (B) challenge of OGG1-expressing (Cont-RNAi, left panel) or OGG1-deficient (Ogg1-RNAi, right panel) lungs. RNAs were isolated at from five lungs and pooled, and the synthesized cDNAs were analyzed by Mouse Inflammatory Cytokines & Receptors PCR Array (2h time point is shown). (C) Time course of OGG1-dependent expression of Cxcl1 (upper panel) and Cxcl2 (lower panel) mRNAs upon 8-oxoG challenge (n=5–6; □, Cont-RNAi; ■, Ogg1-RNAi). (D) KRAS-dependent expression of Cxcl1 and Cxcl2 after 8-oxoG challenge to the airways. KRas was depleted from the airways by RNAi. RNAs isolated after 8-oxoG challenge were analyzed by qRT-PCR (n=5–6, 2 h time point is shown). (E) Expression of CXCL1 in the airway epithelia (white arrow) of OGG1-proficient (Cont-RNAi) and OGG1-deficient airways (Ogg1-RNAi), as shown by IHC. Representative lung sections (upper panels) are shown after a 2h 8-oxoG challenge (DAPI-staining of sections is shown on lower panel). (F) Levels of CXCL1 in the BALF of mice (n=5) challenged with 8-oxoG (left panel) or in response to OGG1-BER (n=6) as determined by BioPlex assays. (G) KRAS-, but not NRAS-expression-dependent recruitment of neutrophils to airways. RAS was depleted by RNAi to Kras or Nras, and airways (n=5–6) were challenged with 8-oxoG. Neutrophil numbers were determined 16 h post-challenge. (H) OGG1-BER-induced recruitment of neutrophils to airways is KRAS-, but not NRAS-expression dependent. RAS were depleted in airways (n=5–6) as in the legend to G, and neutrophil numbers were determined 16 h post-challenge. (B,C,D,F,G) Lungs were challenged with 60 μL saline containing 0.0005 mg per kg 8-oxoG. (A,F,H) Oxidative DNA damage was generated by challenging the airways with glucose oxidase (Materials and Methods). *** = P<0.001). Cxcl1, chemokine (C-X-C motif) ligand 1 transcript; Cxcl2, chemokine (C-X-C motif) ligand 2 transcript; Ccl20, C-C motif chemokine 20; Ccl3, chemokine (C-C motif) ligand 3; Il1a, Interleukin-1 alpha; Il1b, Interleukin-1 beta; Tnfa, tumor necrosis factor; CXCL1, chemokine (C-X-C motif) ligand 1. *** = P<0.001; **** = P<0.0001.
OGG1-BER induces MAP-, PI3-, MS kinases for NF-κB activation
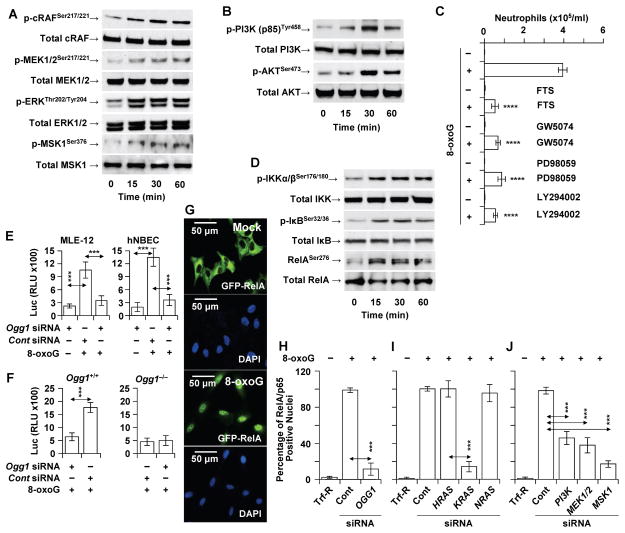
To examine activation by post-translational modifications of OGG1-BER-RAS’ downstream effectors, OGG1-expressing airways were 8-oxoG-challenged (in these studies an oxidative burst was not used to exclude the role of ROS in the activation of redox-sensitive kinases), and lung extracts were prepared. WB analysis showed increased levels of phosphorylated (p)-RAF1 (v-raf1 murine leukemia viral oncogene homolog 1), p-MEK1/2, p-ERK1/2 and p-MSK1 (mitogen-stress related kinase-1) (Fig. 5A). Moreover, we observed phosphorylation of another RAS target, phosphatidylinositol-4,5-bisphosphate 3-kinase [(p-PI3K(p85)], which is an activator of the serine/threonine protein kinase B (AKT; Fig. 5B). To demonstrate the relevance of activated KRAS (shown in Fig. 3A,B) and its targets (MAPKs and PI3K) to an immune response, we showed that treating mice with FTS [trans-farnesylthiosalicylic acid, an inhibitor of RAS membrane interactions (38)], inhibitors of RAF1 (GW5074) and MEK1/2 (PD98059), or the pan-PI3K inhibitor LY294002 (20–22) decreased the recruitment of neutrophils to the airways (Fig. 5C).
FIGURE 5.
OGG1-BER-dependent activation of MAP, PI3 and MS kinases and NF-κB. (A,B) 8-oxoG challenge to the airways increased the phosphorylated levels of RAS targets, (A) MAP kinases (RAF1, MEK1/2, ERK1/2, and MSK1) and (B) PI3K, and AKT. In A,B, mice were challenged via the nasal route with 8-oxoG (0,0005 mg per kg), and the lungs were harvested after 0, 15, 30 and 60 min. Lung extracts were prepared from 5 animals per time points. Then 40 μg protein extract was fractionated by 5 to 20% SDS PAGE, and proteins transferred to membranes were identified by phopspho-specific and pan Abs (Materials and Methods). (C) Pharmacological inhibitors of RAS, RAF-1, MEK1/2 and PI3K decreased the recruitment of neutrophils to the airways. BALF was derived at 16 h post-challenge (n=6) and cell types determined by differential staining of cells. (D) Phosphorylation of IKK, I-κB and Rel/p65 in 8-oxoG-challenged airways. Mice were challenged, lung extracts were made and changes in phosphorylated and total IKK, I-κB and Rel/p65 was detected by immune-blotting using phopspho-specific and pan Abs as in legend to 5A,B. (E) OGG1-dependent activation from an NF-κB-driven promoter. MLE-12 and hNBEC were transfected with control or siRNA to OGG1, and 36 h later a luc2P reporter driven by NF-κB response elements was introduced and the cells exposed to 8-oxoG (10 μM). Luc activities were assessed 6 h later. (F) Ogg1+/+ and Ogg1−/− murine fibroblasts were transfected with control or siRNA to OGG1, and 36 h later, a luc2P reporter driven by NF-κB response elements was introduced and the cells exposed to 8-oxoG (10 μM). Luc activities were assessed 6 h later. (G) Nuclear translocation of GFP-RelA in A549 cells after exposure to 8-oxoG (10 μM). (H) Ablation of OGG1 or (I) KRAS by siRNA from GFP-RelA-expressing A549 cells decreased the 8-oxoG-induced nuclear accumulation of GFP-RelA. Ablation of H- and NRAS, had no effect. (J) siRNA depletion of PI3 or MAP kinases from GFP-RelA-expressing cells decreased the nuclear accumulation of GFP-RelA. RAF1, v-raf1 murine leukemia viral oncogene homolog 1; ERK1/2, extracellular signal-regulated kinase ½; MSK1, mitogen-stress related kinase-1; PI3K, phosphatidylinositol-4,5-bisphosphate 3-kinase; AKT, serine/threonine kinase (protein kinase B); KRAS, Kirsten rat sarcoma viral oncogene homolog; HRAS, Harvey rat sarcoma viral oncogene homolog; NRAS, neuroblastoma RAS viral oncogene homolog; GW5074, RAF-1 kinase inhibitor; PD98059, MAP kinase kinase (MEK1,2); LY294002, phosphatidylinositol 3-kinase inhibitor; FTS, trans-farnesyl-thiosalicylic acid (RAS membrane anchorage inhibitor). *** = P<0.01; **** = P< 0.001.
Signaling by MAP and PI3 kinases have been linked to activation of the trans-acting factor NF-κB, which is rate-limiting for transcription from pro-inflammatory genes, including Cxcl1 and Cxcl2 (39, 40). Upon challenging OGG1-expressing airways with 8-oxoG, we observed a rapid increase in the levels of the p-IκB kinase complex (p-IKKα/β), p-IκB, and p-RelA (at Serine 276) (Fig. 5D). To validate these observations, we transfected MLE-12 cells and hNBECs with a luciferase reporter construct (luc2P) driven by NF-κB promoter elements. We found that 8-oxoG challenge of these cells increased luc2P activity in control siRNA-, but not Ogg1 siRNA-transfected mouse (MLE-12) and human (hNBEC) cells (Fig. 5E). Consistent with these data, the luc2P reporter activity was increased in 8-oxoG-challenged Ogg1+/+, but not Ogg1−/− fibroblasts (Fig. 5F).
To gain further insights into the role of OGG1-BER-driven MAPK and PI3K pathways in NF-κB/RelA activation, we utilized human AE cells (A549) stably expressing a RelA-fused green fluorescent protein (GFP-RelA) (40) localized to the cytoplasm in non-stimulated cells (Fig. 5G, lower panels). In response to 8-oxoG, GFP-RelA was imported into the cell nuclei (Fig. 5G, lower panels). siRNA depletion of OGG1 (Fig. 5H) or KRAS (but not NRAS or HRAS) decreased the percentage of GFP-RelA-positive nuclei (Fig. 5I). Depleting PI3K, MEK1, 2 or MSK1 with siRNAs significantly decreased NF-κB/RelA’s nuclear accumulation (Fig. 5J). Together these results show that OGG1-BER-KRAS induces the canonical NF-κB activation pathway for pro-inflammatory chemokine/cytokine expression and the innate immune response.
Discussion
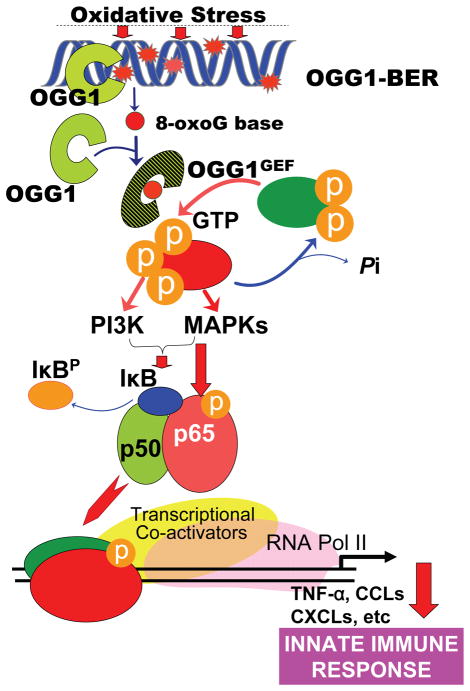
The mechanism by which DNA damage causes inflammation is complex; it often, if not in all documented cases, involves kinases activated by DNA single- or double-strand breaks, or DNA fragments that were recognized by pattern-recognition receptors inducing downstream inflammatory signaling (41, 42). For the first time, our data show that the release of the 8-oxoG base from the genome by OGG1-BER is a prerequisite for signaling increased expression of pro-inflammatory mediators and an innate immune response. Downstream of OGG1-BER, KRAS, PI3, MAP and MS kinases activate the canonical NF-κB pathway, a central mediator of airway mucosal inflammation, by inducing RelA activation by its phosphorylation at Ser 276 and nuclear translocation (Fig. 6). These two events are essential for the full activation of NF-κB-dependent inflammation; Ser 276 phosphorylation is a post-translational modification required for its interactions with histone acetyl transferases and transcriptional elongation complexes for activation of the proinflammatory innate sub-network, including CXCLs (40).
Figure 6.
Schematic depiction of the role of OGG1-initiated DNA base excision repair in an innate immune response.
OGG1 is the key enzyme in removing 8-oxoG and FapyG from DNA with equal specificity and kinetics during BER to prevent mutations and maintain genomic integrity (33, 43). Traditionally, accumulation of oxidatively damaged DNA base lesions, including 8-oxoG, has been linked to various inflammatory diseases, as well as aging processes (6, 7, 44). Here we document that OGG1-BER or challenging the airways with 8-oxoG base, a product of OGG1-BER, induced proinflammatory gene expression and inflammatory cell recruitment to airways. By contrast, a lack of OGG1 activity in airway epithelia resulted in poor inflammation. In light of earlier studies (14) these results are not entirely surprising, but the involvement of OGG1-BER-dependent signaling in NF-κB activation and proinflammatory gene expression is a novel observation.
Oxidative DNA damage was generated by challenging airways with mild oxidative stress using glucose oxidase (Materials and Methods) that we have previously characterized (45). ROS generated in the AE both induce damage to molecules and signal transduction via phosphorylation (at tyrosine or serine residues) or cysteine modification of proteins (46). Moreover, small GTPases, including RAS, have redox-sensitive motifs adjacent to their guanine nucleotide-binding sites, so guanine nucleotide exchange can be activated by reactive species (47), and ROS signaling is also etiologically linked to inflammatory processes (48). OGG1-expressing and OGG1-deficient lungs were exposed to identical doses of an oxidative burst, but only the OGG1-expressing ones showed activation of RAS, increased chemokine/cytokine expression and robust inflammation. Although these observations require further investigation, they imply that OGG1-BER appears to “override” the effect of ROS on RAS activation and downstream signaling. Another possibility is that OGG1 deficiency mediates a resistance to oxidative stress, as suggested previously (12, 13). To exclude this possibility, we determined GSSG levels after oxidative challenge using BALF of OGG1-expressing and deficient mice; there were no differences.
An unexpected observation was that both OGG1-BER and 8-oxoG challenge selectively activated KRAS in lungs, although the level of NRAS was approximately 6-times higher in total lung extracts. These results imply that KRAS activation occurs at the site of exposure. To confirm, we utilized primary epithelial and fibroblast cells. In epithelial cells the GTP-bound level of KRAS was increased, while in fibroblast cells NRAS-GTP levels were increased upon 8-oxoG challenge, even though the abundance of KRAS and NRAS was similar in these cells (HRAS was undetectable). We speculate that OGG1, via its 8-oxoG base-dependent GEF activity, increased GTP-bound RAS levels in a cell type-specific manner whose mechanism is being investigated.
We also considered the possibility that the proinflammatory response due to OGG1-BER induced single-strand gap/break signaling, as shown in a previous study (14, 41). Via its glycosylase activity OGG1 generates abasic sites at which it cleaves the DNA backbone via its AP lyase activity, transiently generating single-strand gaps (33, 49, 50). When not repaired in a timely fashion, these could generate signaling for proinflammatory gene expression, as shown previously (14, 41). To exclude the role of these DNA repair intermediates, and also ROS-induced signaling we utilized OGG1-BER’s product, the 8-oxoG base.
8-OxoG challenge of the airways caused rapid KRAS GDP→GTP exchange, leading to pro-inflammatory gene expression, and recruitment of neutrophils. We propose that 8-oxoG penetrated the airway lining fluid, was internalized by airway epithelium, similarly to those occur in cultured cells (16). The internalized 8-oxoG then mediates the GEF activity of OGG1, which, in turn, activates KRAS in a cell type-dependent manner. Indeed, the innate immune response occurred only in OGG1-expressing airways. Moreover, 8-oxoG was unique in activating RAS in the airways and inducing an immune response, as the FapyG base, like other nucleotides and nucleosides, failed to do so. Uric acid, a deaminated form of 8-oxoG and a major alarmin inflammatory molecule (35, 51), induced low levels of inflammation independent of OGG1.
8-oxoG exposure and OGG1-BER (induced by an oxidative burst) increased chemokine and cytokine expression in an identical pattern. The only differences we observed were the precise fold changes. Expression of these proinflammatory mediators was dependent on OGG1 in both 8-oxoG and oxidatively challenged airways. These results are consistent with OGG1-dependent activation of KRAS GTPases after oxidative burst, raising the possibility that ROS-generated signals are transmitted via KRAS. In this scenario, 8-oxoG is first formed in DNA due to guanine’s susceptibility to oxidation. Because it is a highly mutagenic 8-oxoG is promptly repaired via OGG1-BER. The released 8-oxoG base forms OGG1•8-oxoG complex [a GEF; (16)] activate KRAS and down-stream signaling.
In addition to the epithelium, oxidative (and 8-oxoG) challenge impacts other cell types including macrophages in the airways. To address the role of macrophages in an inflammatory response, we showed that freshly isolated alveolar macrophages express NRAS, while expression of KRAS and HRAS was not at detectable levels. Interestingly, there were no increases in NRAS-GTP levels after oxidative stress (or 8-oxoG) challenge and downregulation of NRAS (expressed in macrophages and epithelium) had no effect on the inflammatory response. Together, these data suggest that pro-inflammatory cytokines and chemokines are produced primarily by airway epithelial cells, resulting in neutrophil recruitment to the airways. 8-oxoG challenge of OGG1-expressing airways resulted in both MAP (RAF1, MEK1,2, ERK1,2 and MSK-1) and PI3 kinase activation, leading to activation of IκB kinase (IKK). Activated IKK phosphorylates IκB, which then becomes a target for ubiquitination and proteasome-mediated degradation, leading to liberation of NF-κB homo- and heterodimers, including RelA-p65 (52). In addition to its release, RelA-p65 is subject to phosphorylation at multiple sites, including Ser residues 210, 276, 529, 536, and others (39). However, among the Ser phosphorylation, that at amino acid 276 appears to be one of the most important, which is mediated by the catalytic subunit of protein kinase A and MSK1 (39, 53–55). These events can be explained by the results from previous studies showing that OGG1 binds the 8-oxoG base at a substrate-independent site, and that 8-oxoG-induced conformational changes in the OGG1 molecule have allowed its physical interaction with and activation of RAS GTPases (16, 17), whose targets are RAF/MAPK and IP3Ks.
To further verify the significance of OGG1-KRAS/MAPK/PI3K signaling for nuclear translocation of RelA-p65, a critical step in gene activation, we show that depletion of OGG1, KRAS, MEK1,2, MSK1 or PI3Ks by siRNA decreased RelA-p65 nuclear accumulation. Activation of NF-κB via OGG1-BER-KRAS signaling was further confirmed by reporter assays. Moreover, to support OGG1-KRAS/MAPK/PI3K signaling in the immune response, we showed that inhibitors of RAS membrane anchorage (FTS), as well as RAF1, MEK1/2 or a pan-PI3K inhibitor, decreased neutrophilia in the lungs. Combining the results from cultured cells and those from the lungs strongly support the relevance of OGG1-BER signaling to an immune response via KRAS, MAPKs, PI3K, MSK1 and the NF-κB pathway.
In conclusion, here we document a novel mechanism by which the repair of oxidatively damaged DNA via OGG1-inititated DNA BER and KRAS signaling lead to activation of NF-κB (and possibly other transacting factors) in the airway epithelium to mount an immune response in the lungs. These data also show that epithelial cells are the primary cell type in orchestrating an inflammatory response, in agreement with previous studies (56–58). Importantly, the evolutionarily conserved 8-oxoguanine DNA glycosylase-1 is not only a key repair enzyme charged with maintaining genomic integrity and preventing mutations, but it also plays an essential role in the host’s innate immune response. Genomic DNA is continuously challenged by ROS, and its integrity should be continually maintained by DNA repair. It is thus plausible to speculate that OGG1-initiated DNA BER is “fueling” the chronic inflammation that is a major underlying process associated with asthma and COPD. Moreover OGG1-BER could also be etiologically linked to other inflammatory diseases such as atherosclerosis, arthritis, cancer, osteoporosis, dementia, vascular diseases, obesity, the metabolic syndrome, and diabetes. Taken together, our results point to a novel discovery – the role of OGG1-dependent repair of damaged DNA in pro-inflammatory signaling, which may be one among many mechanistic explanations for OGG1’s previously proposed link to disease and aging processes. These data also imply that modulating OGG1’s guanine nucleotide exchange factor activity and/or cellular 8-oxoG base levels could benefit human health.
Acknowledgments
Funding: This work was supported by grants NIEHS RO1 ES018948 (IB), NIAID/AI062885 (IB, SS, ARB), NIEHS T32 ES007254 (LA) and the NHLBI Proteomic Center, N01HV00245 (IB, SS, ARB, Director: Dr. A. Kurosky); TAMOP 4.2.2.A-11/1/KONV-2012-2023 project, which is co-financed by the European Union and the European Social Fund (A.B).
We greatly appreciate the critical editing of the manuscript by Dr. David Konkel (Institute for Translational Sciences, UTMB) and Mardelle Susman (Department of Microbiology and Immunology, UTMB). We also thank Drs Miral Dizdaroglu and Pawel Jaruga (Chemical Science and Technology Laboratory, National Institute of Standards and Technology, Gaithersburg, MD USA) for providing FapyG.
Abbreviations used in this article
- 8-oxoG
7,8-dihydro-8-oxoguanine
- AE
airway epithelium
- AEC
airway epithelial cells
- BALF
bronchoalveolar lavage fluid
- CA
comet assay
- FLARE CA
Fragment Length Analysis by Repair Enzymes CA
- CXCL-1,-2
C-X-C motif ligand-1 and -2 chemokines
- FTS
trans-farnesylthiosalicylic acid
- GSH
GSSG, reduced and oxidized glutathione, respectively
- IIR
innate immune response
- i.n
intranasal
- HRAS and KRAS
mammalian homolog of Harvey and Kirsten sarcoma virus oncogen
- hNBEC
human normal bronchial epithelial cells
- mPBEC
mouse primary bronchial epithelial cells
- MRC5
human diploid lung fibroblast
- MLE-12
a mouse lung epithelial cell line
- NRAS
neuroblastoma RAS viral oncogene homolog
- mPLF
primary mouse lung fibroblast
- NEIL1 and 2
Nei (endonuclease VIII)-like DNA glycosylase-1 and -2
- OGG1
8-oxoguanine DNA glycosylase-1
- OGG1-BER
OGG1-initiated DNA base excision repair
- OS
oxidative stress
- ROS
reactive oxygen species
Footnotes
The data sets presented in this article have been submitted to the National Center for Biotechnology Information (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE60711) under accession number GSE60711.
Conflict of interest: The authors declare that no conflict of interest exists.
REFRENCES
- 1.D’Autreaux B, Toledano MB. ROS as signalling molecules: mechanisms that generate specificity in ROS homeostasis. Nat Rev Mol Cell Biol. 2007;8:813–824. doi: 10.1038/nrm2256. [DOI] [PubMed] [Google Scholar]
- 2.Dizdaroglu M. Oxidative damage to DNA in mammalian chromatin. Mutat Res. 1992;275:331–342. doi: 10.1016/0921-8734(92)90036-o. [DOI] [PubMed] [Google Scholar]
- 3.Steenken S, Jovanovic SV. How easily oxidizable is DNA? One-electron reduction potentials of adenosine and guanosine radicals in aqueous solution. J Am Chem Soc. 1997;119:617–618. [Google Scholar]
- 4.Chan SW, Dedon PC. The biological and metabolic fates of endogenous DNA damage products. J Nucleic Acids. 2010;2010:929047. doi: 10.4061/2010/929047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shigenaga MK, Hagen TM, Ames BN. Oxidative damage and mitochondrial decay in aging. Proc Natl Acad Sci U S A. 1994;91:10771–10778. doi: 10.1073/pnas.91.23.10771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.David SS, O’Shea VL, Kundu S. Base-excision repair of oxidative DNA damage. Nature. 2007;447:941–950. doi: 10.1038/nature05978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hegde MLHP, Rao KS, Mitra S. Oxidative genome damage and its repair in neurodegenerative diseases: function of transition metals as a double-edged sword. J Alzheimers Dis. 2011;24:183–198. doi: 10.3233/JAD-2011-110281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hazra TK, Hill JW, Izumi T, Mitra S. Multiple DNA glycosylases for repair of 8-oxoguanine and their potential in vivo functions. Prog Nucleic Acid Res Mol Biol. 2001;68:193–205. doi: 10.1016/s0079-6603(01)68100-5. [DOI] [PubMed] [Google Scholar]
- 9.Mitra S, Izumi T, Boldogh I, Bhakat KK, Hill JW, Hazra TK. Choreography of oxidative damage repair in mammalian genomes. Free Radic Biol Med. 2002;33:15–28. doi: 10.1016/s0891-5849(02)00819-5. [DOI] [PubMed] [Google Scholar]
- 10.Klungland A, Rosewell I, Hollenbach S, Larsen E, Daly G, Epe B, Seeberg E, Lindahl T, Barnes DE. Accumulation of premutagenic DNA lesions in mice defective in removal of oxidative base damage. Proc Natl Acad Sci U S A. 1999;96:13300–13305. doi: 10.1073/pnas.96.23.13300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Minowa O, Arai T, Hirano M, Monden Y, Nakai S, Fukuda M, Itoh M, Takano H, Hippou Y, Aburatani H, Masumura K, Nohmi T, Nishimura S, Noda T. Mmh/Ogg1 gene inactivation results in accumulation of 8-hydroxyguanine in mice. Proc Natl Acad Sci U S A. 2000;97:4156–4161. doi: 10.1073/pnas.050404497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mabley JG, Pacher P, Deb A, Wallace R, Elder RH, Szabo C. Potential role for 8-oxoguanine DNA glycosylase in regulating inflammation. Faseb J. 2005;19:290–292. doi: 10.1096/fj.04-2278fje. [DOI] [PubMed] [Google Scholar]
- 13.Li G, Yuan K, Yan C, Fox J, 3rd, Gaid M, Breitwieser W, Bansal AK, Zeng H, Gao H, Wu M. 8-Oxoguanine-DNA glycosylase 1 deficiency modifies allergic airway inflammation by regulating STAT6 and IL-4 in cells and in mice. Free Radic Biol Med. 2012;52:392–401. doi: 10.1016/j.freeradbiomed.2011.10.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bacsi A, Aguilera-Aguirre L, Szczesny B, Radak Z, Hazra TK, Sur S, Ba X, Boldogh I. Down-regulation of 8-oxoguanine DNA glycosylase 1 expression in the airway epithelium ameliorates allergic lung inflammation. DNA Repair (Amst) 2013;12:18–26. doi: 10.1016/j.dnarep.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sampath H, Vartanian V, Rollins MR, Sakumi K, Nakabeppu Y, Lloyd RS. 8-Oxoguanine DNA glycosylase (OGG1) deficiency increases susceptibility to obesity and metabolic dysfunction. PLoS One. 2012;7:e51697. doi: 10.1371/journal.pone.0051697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boldogh I, Hajas G, Aguilera-Aguirre L, Hegde ML, Radak Z, Bacsi A, Sur S, Hazra TK, Mitra S. Activation of ras signaling pathway by 8-oxoguanine DNA glycosylase bound to its excision product, 8-oxoguanine. J Biol Chem. 2012;287:20769–20773. doi: 10.1074/jbc.C112.364620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.German P, Szaniszlo P, Hajas G, Radak Z, Bacsi A, Hazra TK, Hegde ML, Ba X, Boldogh I. Activation of cellular signaling by 8-oxoguanine DNA glycosylase-1-initiated DNA base excision repair. DNA Repair (Amst) 2013;12:856–863. doi: 10.1016/j.dnarep.2013.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hajas G, Bacsi A, Aguilera-Aguirre L, Hegde ML, Tapas KH, Sur S, Radak Z, Ba X, Boldogh I. 8-Oxoguanine DNA glycosylase-1 links DNA repair to cellular signaling via the activation of the small GTPase Rac1. Free Radic Biol Med. 2013;61:384–394. doi: 10.1016/j.freeradbiomed.2013.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ramirez RD, Sheridan S, Girard L, Sato M, Kim Y, Pollack J, Peyton M, Zou Y, Kurie JM, Dimaio JM, Milchgrub S, Smith AL, Souza RF, Gilbey L, Zhang X, Gandia K, Vaughan MB, Wright WE, Gazdar AF, Shay JW, Minna JD. Immortalization of human bronchial epithelial cells in the absence of viral oncoproteins. Cancer Res. 2004;64:9027–9034. doi: 10.1158/0008-5472.CAN-04-3703. [DOI] [PubMed] [Google Scholar]
- 20.Katzav A, Kloog Y, Korczyn AD, Niv H, Karussis DM, Wang N, Rabinowitz R, Blank M, Shoenfeld Y, Chapman J. Treatment of MRL/lpr mice, a genetic autoimmune model, with the Ras inhibitor, farnesylthiosalicylate (FTS) Clin Exp Immunol. 2001;126:570–577. doi: 10.1046/j.1365-2249.2001.01674.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Di Paola R, Crisafulli C, Mazzon E, Genovese T, Paterniti I, Bramanti P, Cuzzocrea S. Effect of PD98059, a selective MAPK3/MAPK1 inhibitor, on acute lung injury in mice. Int J Immunopathol Pharmacol. 2009;22:937–950. doi: 10.1177/039463200902200409. [DOI] [PubMed] [Google Scholar]
- 22.Duan W, Aguinaldo Datiles AM, Leung BP, Vlahos CJ, Wong WS. An anti-inflammatory role for a phosphoinositide 3-kinase inhibitor LY294002 in a mouse asthma model. Int Immunopharmacol. 2005;5:495–502. doi: 10.1016/j.intimp.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 23.Boldogh I, Bacsi A, Choudhury BK, Dharajiya N, Alam R, Hazra TK, Mitra S, Goldblum RM, Sur S. ROS generated by pollen NADPH oxidase provide a signal that augments antigen-induced allergic airway inflammation. J Clin Invest. 2005;115:2169–2179. doi: 10.1172/JCI24422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bacsi A, Choudhury BK, Dharajiya N, Sur S, Boldogh I. Subpollen particles: carriers of allergenic proteins and oxidases. J Allergy Clin Immunol. 2006;118:844–850. doi: 10.1016/j.jaci.2006.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aguilera-Aguirre L, Bacsi A, Saavedra-Molina A, Kurosky A, Sur S, Boldogh I. Mitochondrial dysfunction increases allergic airway inflammation. Journal of Immunology. 2009;183:5379–5387. doi: 10.4049/jimmunol.0900228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Olive PL, Wlodek D, Banath JP. DNA double-strand breaks measured in individual cells subjected to gel electrophoresis. Cancer Res. 1991;51:4671–4676. [PubMed] [Google Scholar]
- 27.Bacsi A, Chodaczek G, Hazra TK, Konkel D, Boldogh I. Increased ROS generation in subsets of OGG1 knockout fibroblast cells. Mech Ageing Dev. 2007;128:637–649. doi: 10.1016/j.mad.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hill JW, Hazra TK, Izumi T, Mitra S. Stimulation of human 8-oxoguanine-DNA glycosylase by AP-endonuclease: potential coordination of the initial steps in base excision repair. Nucleic Acids Res. 2001;29:430–438. doi: 10.1093/nar/29.2.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Taylor SJ, Resnick RJ, Shalloway D. Nonradioactive determination of Ras-GTP levels using activated ras interaction assay. Methods Enzymol. 2001;333:333–342. doi: 10.1016/s0076-6879(01)33067-7. [DOI] [PubMed] [Google Scholar]
- 30.Yadav UC, Naura AS, Aguilera-Aguirre L, Ramana KV, Boldogh I, Sur S, Boulares HA, Srivastava SK. Aldose reductase inhibition suppresses the expression of Th2 cytokines and airway inflammation in ovalbumin-induced asthma in mice. J Immunol. 2009;183:4723–4732. doi: 10.4049/jimmunol.0901177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ji H, Houghton AM, Mariani TJ, Perera S, Kim CB, Padera R, Tonon G, McNamara K, Marconcini LA, Hezel A, El-Bardeesy N, Bronson RT, Sugarbaker D, Maser RS, Shapiro SD, Wong KK. K-ras activation generates an inflammatory response in lung tumors. Oncogene. 2006;25:2105–2112. doi: 10.1038/sj.onc.1209237. [DOI] [PubMed] [Google Scholar]
- 32.Sparmann A, Bar-Sagi D. Ras-induced interleukin-8 expression plays a critical role in tumor growth and angiogenesis. Cancer Cell. 2004;6:447–458. doi: 10.1016/j.ccr.2004.09.028. [DOI] [PubMed] [Google Scholar]
- 33.Mitra S, Hazra TK, Roy R, Ikeda S, Biswas T, Lock J, Boldogh I, Izumi T. Complexities of DNA base excision repair in mammalian cells. Mol Cells. 1997;7:305–312. [PubMed] [Google Scholar]
- 34.Svoboda P, Maekawa M, Kawai K, Tominaga T, Savela K, Kasai H. Urinary 8-hydroxyguanine may be a better marker of oxidative stress than 8-hydroxydeoxyguanosine in relation to the life spans of various species. Antioxid Redox Signal. 2006;8:985–992. doi: 10.1089/ars.2006.8.985. [DOI] [PubMed] [Google Scholar]
- 35.Seong SY, Matzinger P. Hydrophobicity: an ancient damage-associated molecular pattern that initiates innate immune responses. Nat Rev Immunol. 2004;4:469–478. doi: 10.1038/nri1372. [DOI] [PubMed] [Google Scholar]
- 36.Hu J, de Souza-Pinto NC, Haraguchi K, Hogue BA, Jaruga P, Greenberg MM, Dizdaroglu M, Bohr VA. Repair of formamidopyrimidines in DNA involves different glycosylases: role of the OGG1, NTH1, and NEIL1 enzymes. J Biol Chem. 2005;280:40544–40551. doi: 10.1074/jbc.M508772200. [DOI] [PubMed] [Google Scholar]
- 37.Mantovani A, Cassatella MA, Costantini C, Jaillon S. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat Rev Immunol. 11:519–531. doi: 10.1038/nri3024. [DOI] [PubMed] [Google Scholar]
- 38.Kraitzer A, Kloog Y, Zilberman M. Novel farnesylthiosalicylate (FTS)-eluting composite structures. Eur J Pharm Sci. 2009;37:351–362. doi: 10.1016/j.ejps.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 39.Brasier AR. The nuclear factor-kappaB-interleukin-6 signalling pathway mediating vascular inflammation. Cardiovasc Res. 2010;86:211–218. doi: 10.1093/cvr/cvq076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nowak DE, Tian B, Jamaluddin M, Boldogh I, Vergara LA, Choudhary S, Brasier AR. RelA Ser276 phosphorylation is required for activation of a subset of NF-kappaB-dependent genes by recruiting cyclin-dependent kinase 9/cyclin T1 complexes. Mol Cell Biol. 2008;28:3623–3638. doi: 10.1128/MCB.01152-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen GY, Nunez G. Sterile inflammation: sensing and reacting to damage. Nat Rev Immunol. 2010;10:826–837. doi: 10.1038/nri2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Polo SE, Jackson SP. Dynamics of DNA damage response proteins at DNA breaks: a focus on protein modifications. Genes Dev. 2011;25:409–433. doi: 10.1101/gad.2021311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nishimura S. Involvement of mammalian OGG1(MMH) in excision of the 8-hydroxyguanine residue in DNA. Free Radic Biol Med. 2002;32:813–821. doi: 10.1016/s0891-5849(02)00778-5. [DOI] [PubMed] [Google Scholar]
- 44.Radak Z, Boldogh I. 8-Oxo-7,8-dihydroguanine: links to gene expression, aging, and defense against oxidative stress. Free Radic Biol Med. 2010;49:587–596. doi: 10.1016/j.freeradbiomed.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Das A, Hazra TK, Boldogh I, Mitra S, Bhakat KK. Induction of the human oxidized base-specific DNA glycosylase NEIL1 by reactive oxygen species. J Biol Chem. 2005;280:35272–35280. doi: 10.1074/jbc.M505526200. [DOI] [PubMed] [Google Scholar]
- 46.Holmstrom KM, Finkel T. Cellular mechanisms and physiological consequences of redox-dependent signalling. Nat Rev Mol Cell Biol. 2014;15:411–421. doi: 10.1038/nrm3801. [DOI] [PubMed] [Google Scholar]
- 47.Heo J. Redox control of GTPases: from molecular mechanisms to functional significance in health and disease. Antioxid Redox Signal. 2011;14:689–724. doi: 10.1089/ars.2009.2984. [DOI] [PubMed] [Google Scholar]
- 48.Mittal M, Siddiqui MR, Tran K, Reddy SP, Malik AB. Reactive oxygen species in inflammation and tissue injury. Antioxid Redox Signal. 2014;20:1126–1167. doi: 10.1089/ars.2012.5149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fromme JC, Bruner SD, Yang W, Karplus M, Verdine GL. Product-assisted catalysis in base-excision DNA repair. Nat Struct Biol. 2003;10:204–211. doi: 10.1038/nsb902. [DOI] [PubMed] [Google Scholar]
- 50.Izumi T, Schein CH, Oezguen N, Feng Y, Braun W. Effects of backbone contacts 3′ to the abasic site on the cleavage and the product binding by human apurinic/apyrimidinic endonuclease (APE1) Biochemistry. 2004;43:684–689. doi: 10.1021/bi0346190. [DOI] [PubMed] [Google Scholar]
- 51.Shi Y, Evans JE, Rock KL. Molecular identification of a danger signal that alerts the immune system to dying cells. Nature. 2003;425:516–521. doi: 10.1038/nature01991. [DOI] [PubMed] [Google Scholar]
- 52.Brasier AR. The NF-kappaB regulatory network. Cardiovasc Toxicol. 2006;6:111–130. doi: 10.1385/ct:6:2:111. [DOI] [PubMed] [Google Scholar]
- 53.Jamaluddin M, Wang S, Boldogh I, Tian B, Brasier AR. TNF-alpha-induced NF-kappaB/RelA Ser(276) phosphorylation and enhanceosome formation is mediated by an ROS-dependent PKAc pathway. Cell Signal. 2007;19:1419–1433. doi: 10.1016/j.cellsig.2007.01.020. [DOI] [PubMed] [Google Scholar]
- 54.Jamaluddin M, Tian B, Boldogh I, Garofalo RP, Brasier AR. Respiratory syncytial virus infection induces a reactive oxygen species-MSK1-phospho-Ser-276 RelA pathway required for cytokine expression. J Virol. 2009;83:10605–10615. doi: 10.1128/JVI.01090-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen LF, Williams SA, Mu Y, Nakano H, Duerr JM, Buckbinder L, Greene WC. NF-kappaB RelA phosphorylation regulates RelA acetylation. Mol Cell Biol. 2005;25:7966–7975. doi: 10.1128/MCB.25.18.7966-7975.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Swamy M, Jamora C, Havran W, Hayday A. Epithelial decision makers: in search of the ‘epimmunome’. Nat Immunol. 2010;11:656–665. doi: 10.1038/ni.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Holgate ST. Epithelium dysfunction in asthma. J Allergy Clin Immunol. 2007;120:1233–1244. doi: 10.1016/j.jaci.2007.10.025. quiz 1245–1236. [DOI] [PubMed] [Google Scholar]
- 58.Holgate ST. The sentinel role of the airway epithelium in asthma pathogenesis. Immunol Rev. 2011;242:205–219. doi: 10.1111/j.1600-065X.2011.01030.x. [DOI] [PubMed] [Google Scholar]