Highlight text
QTL-based parameters of an ecophysiological model, calibrated on an association genetics panel of 210 genotypes, allowed prediction of heading dates of 80 independent genotypes in six independent experiments with a median prediction error of 5.6 days.
Key words: Association genetics, ecophysiological model, gene-based modelling, heading date, phenology, wheat.
Abstract
Prediction of wheat phenology facilitates the selection of cultivars with specific adaptations to a particular environment. However, while QTL analysis for heading date can identify major genes controlling phenology, the results are limited to the environments and genotypes tested. Moreover, while ecophysiological models allow accurate predictions in new environments, they may require substantial phenotypic data to parameterize each genotype. Also, the model parameters are rarely related to all underlying genes, and all the possible allelic combinations that could be obtained by breeding cannot be tested with models. In this study, a QTL-based model is proposed to predict heading date in bread wheat (Triticum aestivum L.). Two parameters of an ecophysiological model (V sat and P base, representing genotype vernalization requirements and photoperiod sensitivity, respectively) were optimized for 210 genotypes grown in 10 contrasting location × sowing date combinations. Multiple linear regression models predicting V sat and P base with 11 and 12 associated genetic markers accounted for 71 and 68% of the variance of these parameters, respectively. QTL-based V sat and P base estimates were able to predict heading date of an independent validation data set (88 genotypes in six location × sowing date combinations) with a root mean square error of prediction of 5 to 8.6 days, explaining 48 to 63% of the variation for heading date. The QTL-based model proposed in this study may be used for agronomic purposes and to assist breeders in suggesting locally adapted ideotypes for wheat phenology.
Introduction
Hexaploid wheat is native to the Middle East and now occupies 22% of the total cultivated area in the world (Leff et al., 2004). Much of this expansion in cultivation area has been facilitated by developing adapted wheat cultivars through the use of genetic variation for the timing of plant development or phenology (Cockram et al., 2007). Short-cycle spring wheat cultivars have been selected for regions with a highly continental climate that require late planting in the spring to avoid frost damage and early harvesting to avoid drought or heat stress in summer. In more temperate climatic regions, long-cycle cultivars allow planting in autumn to benefit from a longer growing season. These adaptations have resulted from both historical selection of seed by farmers over centuries of local production, and over the last century or so, from breeder selections for phenological variation, particularly for heading date
By influencing the time that a crop has to use resources, phenology accounts for substantial genetic control of grain yield in cultivated plant species. In wheat, the fine tuning of cultivar earliness is a prerequisite for matching climatic conditions and realising yield potential in a given environment (Snape et al., 2001; Reynolds et al., 2009; Foulkes et al., 2011). In the context of changes in global climate that are associated with an increase in the occurrence and intensity of drought and heat stress in the main areas of wheat cultivation (Lobell et al., 2011; Semenov and Shewry, 2011), strategies based on adaptation through earliness are often proposed to avoid these constraints (Gouache et al., 2012; Zheng et al., 2012). In cultivated conditions, wheat susceptibility to biotic and abiotic stress (Slafer and Rawson, 1995; Porter and Semenov, 2005; Del Ponte et al., 2007) and fertilizer requirements (Hirel et al., 2007; Pask et al., 2012) varies during the life cycle. Reciprocally, the efficiency of cultural management varies during the crop life cycle. For example, heading date is usually taken as a reference point for the last nitrogen fertilization application as this increases grain protein concentration without decreasing grain yield (Gooding and Davies, 1992; Woolfolk et al., 2002). Therefore, predicting phenology of wheat cultivars is essential for choosing the best-adapted cultivar for a specific growing region and to plan cultural management at the right time.
Wheat phenology depends on vernalization requirements, photoperiod sensitivity, and earliness per se (Worland, 1996). Substantial progress has been made in understanding the genetic control of wheat phenology, and several major genes have been cloned. The Ppd-1 genes, located on the homoeologous group 2, are involved in the circadian clock (Beales et al., 2007). Mutations at these loci cause semi-dominant photoperiod insensitivity which results in early flowering. Photoperiod-insensitive alleles have been identified for each of the three homoeologous copies (Mohler et al., 2004; Beales et al., 2007; Wilhelm et al., 2009), conferring different degrees of photoperiod insensitivity. The allele denoted Ppd-D1a creates the earliest-flowering phenotype, followed by Ppd-A1a and Ppd-B1a (Bentley et al., 2010). The major effects of vernalization requirements are determined by the Vrn genes (Worland, 1996; Snape et al., 2001). The Vrn-1 series (on homoeologous group 5) are involved in the perception of the vernalization signal and the induction of the floral transition at the shoot apex (Yan et al., 2003). Mutations (insertions or deletions) in the regulatory regions of at least one of the three homoeologous Vrn-1 copies are associated with dominant spring growth alleles (Fu et al., 2005). The Vrn-2 series are located on homoeologous group 4 (Vrn-B2 and Vrn-D2) and 5 (Vrn-A2) (Yan et al., 2004), and act as flowering repressors which are downregulated by vernalization (Yan et al., 2004). The Vrn-3 series, located on homoeologous group 7, has been identified as the ‘florigen’ signal moving from leaves to promote the floral transition at the shoot apex (Yan et al., 2006; Li and Dubcovsky, 2008). Mutations (SNP, insertions-deletions) on the Vrn-D3 series have been shown to induce variations for heading date (Bonnin et al., 2008). A Vrn-D4 gene, located in the centromeric region of chromosome 5D, has also been identified (Yoshida et al., 2010). Molecular studies have unravelled interactions among these major genes, and gene networks showing their inter-relationship have been proposed (Trevaskis et al., 2007; Distelfeld et al., 2009; Shimada et al., 2009; Trevaskis, 2010). No major functional genes have yet been identified with earliness per se, the characteristic whereby an ‘early’ line will flower more quickly than a ‘late’ line if both are first vernalized and then grown under an extended photoperiod regime.
Numerous QTL studies for heading date have been carried out in wheat (e.g. Sourdille et al., 2000; Börner et al., 2002; Hanocq et al., 2004; Bogard et al., 2011; Carter et al., 2011). This growth stage is relatively easier to measure compared to other critical growth stages such as the beginning of stem elongation or anthesis. Although heading date correlates with anthesis date, genetic variability for the heading to anthesis date period exists in wheat (unpublished results) and would probably require specific studies. Apart from the major genes described above, the combined analysis of these QTL studies or meta-QTL analysis have revealed small-effect loci on most of the wheat chromosomes (Hanocq et al., 2007; Griffiths et al., 2009). However, while these studies identify novel loci, their utility is limited in terms of prediction. Indeed, QTL analysis of heading date in multiple environments leads to an ‘environment-specific’ QTL model as significant QTLs and estimated additive effects differ with the environments. For example, in a study of a wheat population grown in 10 environments, the estimated additive effect of the Ppd-D1 gene varied from 5 to 9 days depending on the environment (Bogard et al. 2011).
Ecophysiological models account for both environment and genetic effects through a design process that aims to dissect traits into ‘environment-independent’ components. Genotype × environment interactions then become emergent properties of the models (Chapman et al., 2003; Trewavas, 2006; Bertin et al., 2010; Yin and Struik, 2010). The use of ecophysiological models allows prediction of wheat with a good accuracy with root mean square error of prediction (RMSEP) for heading date being as low as 4 to 7 days (Weir et al., 1984; Asseng et al., 1998; White et al., 2008; He et al., 2011; Zheng et al., 2013). Ecophysiological models that predict wheat phenology include: (1) Empirical models based on accumulation of thermal time modified by vernalization and photoperiod (Weir et al., 1984; Ritchie and Otter, 1985; Asseng et al., 1998) and (2) mechanistic models simulating the emission of leaves and spike primordia at the shoot apex (Jamieson et al., 1998). Empirical models rely on the daily calculation of vernalization and photoperiod factors reducing daily thermal time accumulation; once the total accumulated thermal time passes a defined value, a given stage is reached. In the mechanistic model Sirius (Jamieson et al., 1998), photoperiod affects the final leaf number in response to the daylength occurring at the two-leaf stage after the flag-leaf primordium has formed [see details in Brooking et al. (1995)]. The vernalization submodel in Sirius was described in Robertson et al. (1996). Briefly, a vernalization index is calculated daily depending on daily mean temperature and cardinal vernalizing temperatures (vernalizing effects increasing linearly from 0 to 15°C and decreasing linearly to 0 from 15 to 17°C). Vernalization is satisfied either when the vernalization index reaches one or when the number of primordia on the apex exceeds a maximal attainable final leaf number corresponding to the number of leaves produced by a winter cultivar grown in long days at temperatures above 17°C. Although these models differ in form, their performances in terms of prediction are comparable (Jamieson et al., 2007). In addition to agro-climatic inputs (e.g. temperature, photoperiod, sowing date, and soil type), response parameters are required to initialize these models. Some of these, termed ‘genetic parameters’, reflect genetic variations in the model and may differ among cultivars. An important property of these genetic parameters is their assumed de facto independence from the environment. Unfortunately, determining these parameters’ values requires extensive and time-consuming experiments for each cultivar either for them to be measured (when this is feasible) or to be optimized.
The concept of a gene-based model has been proposed to describe the possible future direction of crop modelling to capture the large amount of data generated by molecular techniques (White and Hoogenboom, 1996; Chapman et al., 2002a; Chapman et al., 2002b; White and Hoogenboom, 2003; White, 2006; Chapman, 2008; Letort et al., 2008; White, 2009). Gene-based modelling aims to fill the gap between ecophysiological modelling and genetics by integrating knowledge from each field into a holistic framework. The approach consists of replacing empirically determined genetic parameters by genetic coefficients explicitly reflecting the allelic composition of the genotypes. For crop improvement, one output of gene-based ecophysiological models would be the in silico identification of the best allelic combination for a given set of environments, so-called ‘ideotypes’ (Reymond et al., 2003; Letort et al., 2008; Chenu et al., 2009). Examples of gene-based or QTL-based models include: leaf elongation rate in Zea mays (Reymond et al., 2003; Chenu et al., 2008); Prunus quality (Quilot et al., 2005); phenology in Glycine max (Messina et al., 2006); flowering date in Oryza sativa (Nakagawa et al., 2005); barley (Yin et al., 2005); Phaseolus vulgaris (White and Hoogenboom, 2003); wheat (White et al., 2008); Brassica oleracea (Uptmoor et al., 2011); and using knowledge on the gene network regulating flowering in wheat (Brown et al., 2013). Particularly relevant to the research domain here, White et al. (2008) proposed a gene-based model to predict the genetic parameters of the CSM-CROPSIM-CERES model for wheat cultivars using multiple linear regressions with genetic markers for Ppd-D1 and Vrn1 genes as predictors. They concluded that, if gene-based prediction of wheat phenology appeared feasible, more genetic information should be included into the model. More recently, Zheng et al. (2013) estimated the effect of Ppd-D1 and Vrn1 genes on two phenological parameters of the APSIM model (Keating et al., 2003) and obtained high predictive ability for spring wheats grown across the Australian wheat belt. However, their method still requires that experiments be carried out under artificial conditions (artificial vernalization and extended photoperiod) to estimate a third genotype-specific parameter related to the duration in modified thermal time between emergence and heading, and is therefore not a method that can be applied using only genetic information.
The objective of this study is to propose a QTL-based ecophysiological model to predict heading date in bread wheat (Triticum aestivum L.). In contrast with the approaches of White et al. (2008) and Zheng et al. (2013), no assumptions were made about which genes determined the model parameters. The strategy consisted in optimizing two genetic parameters of an ecophysiological model (V sat and P base, representing vernalization requirement and photoperiod sensitivity, respectively) for each of the 210 genotypes of an association genetics panel grown under contrasting conditions. Genetic markers associated with model parameters were then identified and used to obtain multiple linear models predicting V sat and P base by stepwise regression. Finally, predictions of heading dates using QTL-based parameters were tested on an independent set of 88 genotypes grown in six environments.
Materials and methods
Phenotypic data
Heading dates were recorded in various contrasting conditions for a large panel of bread wheats comprising different combinations of spring/winter and photoperiod sensitive/insensitive accessions. Association genetics analyses have been published using this panel to study earliness components (Bonnin et al., 2008; Rousset et al., 2011; Le Gouis et al., 2012). The panel used here is a subset of 210 accessions from the INRA bread wheat core collection of 372 accessions which aims to represent worldwide wheat diversity (Balfourier et al., 2007). The subset aimed to maintain the diversity originally present in the entire panel (Rousset et al., 2011). Experiments were carried out in France in different location × sowing date combinations. The association genetics panel was grown for three autumn sowings and one spring sowing at Clermont-Ferrand (45°46’N, 03°09’E, 401m a.s.l.) and three autumn sowings and three spring sowings at Le Moulon (48°42’ N, 02°08’ E, 156m a.s.l.) as described by Bonnin et al. (2008), Rousset et al. (2011) and Le Gouis et al. (2012). Autumn-sown experiments in Le Moulon (2003 to 2005) were sown in two randomized complete blocks where 20 seeds of each genotype were sown in two 1.2-m-long single rows. In the other experiments, 10 seeds of each genotype were sown in a single-row. Ear emergence day of the main tiller of five to six individual plants was recorded when half of the ear had emerged from the flag leaf. Numbers were averaged to obtain heading date for each genotype, reported here as day of the year (DOY).
Ecophysiological model
The ecophysiological model used in this study is based on the accumulation of thermal time modified by vernalization and photoperiod factors. The model works on a daily time-step and calculates, for each day, the thermal time accumulated as a function of daily temperature and daily photoperiod. Wheat development is split into different phases, and three factors limited to vary between 0 and 1 reducing thermal time accumulation are calculated based on response curves for temperature, accumulated vernalizing days, and photoperiod (FT, FV, and FP, respectively). Daily accumulated thermal time (Tt) is modified by vernalization and photoperiod factors (FV and FP) during the phase from emergence to heading and leads to the calculation of a modified thermal time (PVTt):
| (1) |
A growth stage is reached when the accumulated modified thermal time exceeds the duration of the corresponding phase. The duration of the sowing to emergence phase was 148 modified degree days, and the duration from emergence to heading (TT emhe) was either fixed at 500 modified degree days or allowed to vary between genotypes depending on the optimization strategy (see ‘Optimization of model parameters’ section).
The model is a modified version of that proposed by Weir et al. (1984). The original form relied on the simulation of a cosinusoidal variation of temperature across the day based on daily minimal and maximal temperature data. The day is divided into eight three-hours periods, and the contribution of temperature across the day is then integrated by averaging the contributions of the eight periods [equation 1 in Weir et al. (1984)]. In contrast, the present model used daily mean temperature (TM) only:
| (2) |
with being the daily minimum temperature, and the daily maximum temperature. FT is calculated from TM and a bilinear function with three cardinal temperatures (T base, T opt, and T max):
| (3) |
| (4) |
| (5) |
T base, T opt, and T max were set to 1°C, 26°C, and 37°C, respectively, as in Weir et al. (1984). FT allows calculation of the contribution of TM to the daily accumulated thermal time (Tt):
| (6) |
The vernalization factor FV is calculated depending on the number of vernalizing days (VDD) accumulated by the crop. VDD accumulates from germination, although FV modifies the daily accumulated thermal time only from plant emergence with the effect continuing until heading is reached. This is a simplification of the model proposed by Weir et al. (1984) in which the vernalization effect stops at floral initiation. Each day, the efficiency of vernalization (V eff) is calculated from TM and a function with four cardinal temperatures (T 1, T 2, T 3, and T 4):
| (7) |
| (8) |
| (9) |
| (10) |
T 1, T 2, T 3, and T 4 were set to –4°C, 3°C, 10°C, and 17°C, respectively, as in Weir et al. (1984). VDD is then calculated by summing V eff from day 1 to day i:
| (11) |
FV varies between 0 and 1 and is calculated from VDD and a function with two parameters, V base and V sat:
| (12) |
V base was set to zero days so that spring wheats with V sat close to zero reach full vernalization (FV = 1) very early. V sat was considered as a genetic parameter, thus varying between genotypes.
Daily photoperiod was calculated as in Weir et al. (1984) by considering that photoperiod-effective radiation starts and ends when the sun is 6° below the horizon. The photoperiod factor (FP) is calculated from daily photoperiod (Ph) and a function with two parameters, P opt and P base. FP is limited to vary between 0 and 1 and is calculated as:
| (13) |
P opt was set to 20h while P base was considered as a genetic parameter. The model was coded in S language and run using R (R Development Core Team, 2013).
Optimization of model parameters
Either two (V sat and P base, 2p strategy) or three (V sat, P base, and TT emhe , 3p strategy) parameters representing different earliness components were optimized. Regarding photoperiod sensitivity, P base or P opt could have been optimized indifferently (equation 13). The choice of P base was therefore arbitrary. Regarding vernalization requirement, cardinal vernalizing temperatures could have been optimized instead of V sat, but this would probably have required optimizing at least two temperatures (T1 and T2 in equation 7 to 9) and therefore increased the number of parameters to optimize. In the same way, it appeared more relevant to optimize TT emhe, representing earliness per se, instead of cardinal temperatures T base, T opt, or T max.
P base was varied between 0 and 10h with a 0.1h step (101 values); V sat, between 0 and 130 days with a 1 day step (131 values); and TT emhe, between 400 and 800 with a 10 modified degree days step (41 values). In the 2p strategy, TT emhe was fixed at 500 modified degree days. All the V sat × P base (2p strategy) or V sat × P base × TT emhe (3p strategy) combinations were generated leading to 101×131 = 13 231 or 101×131×41 = 542 471 parameter combinations (or ‘parameters vectors), for 2p and 3p, respectively. Results obtained with the 2p and 3p strategies were compared. Additionally, V sat and P base were optimized for the original and the modified version of the Weir et al. (1984) model, and results of predictions obtained for the calibration data set were compared to check if our modified version for temperature did not reduce model predictive ability.
For each genotype, parameter optimization was carried out by simulating predicted heading dates for all the V sat × P base (2p strategy) or V sat × P base × TT emhe (3p strategy) combinations, for all experiments of the calibration data set where the genotype was tested, and then calculating the RMSEP between observed and predicted heading dates:
| (14) |
where and are the observed and predicted heading dates of genotype i in DOY for experiment j, and n is the number of experiments where genotype i was tested. For each genotype, the vector(s) of parameters that resulted in the minimum RMSEP across environments was (were) selected.
In order to link parameters to genetic markers, it is necessary to unambiguously identify parameter vectors that not only allow accurate prediction of heading date but also reflect the genetic architecture of the accessions making up the panel used in the study. The panel used in this study may be split into 53 winter and 157 spring genotypes, the former being genotypes that did not head in the spring-sown experiments and apparently had an obligate requirement for vernalisation. Parameter optimization was conducted differently for these two groups. Optimizations of spring genotypes were carried out using autumn- and spring-sown experiments, while optimizations of winter genotypes were carried out using autumn-sown experiments only. As results of optimization were ambiguous in this latter case, with multiple parameter vectors minimizing the RMSEP, the information that winter genotypes did not head under spring sowing was used to filter parameter vectors. This filtering consisted in removing sets of parameters that predicted heading dates which were earlier than the last heading date recorded plus 10 days in each of the spring-sown experiments. When multiple parameter vectors led to the minimum RMSEP, a vector of parameters was arbitrarily chosen so that P base and V sat were close to the median values of the different parameter vectors minimizing the RMSEP.
An additional analysis was performed to test the robustness of P base and V sat optimization (2p strategy). The procedure consisted in optimizing P base and V sat for each genotype using different sets of experiments. Each set of experiments was obtained for each genotype by sampling at random nspring–1 of the spring-sown experiments and nautumn–1 of the autumn-sown experiments (nspring and nautumn being the total number of available spring or autumn experiments for a given genotype). For each genotype, optimizations were carried out separately for each random set of experiments, and results were compared to assess the robustness of the V sat and P base parameters.
Model sensitivity analysis
A basic sensitivity analysis was carried out by running simulations of heading date for all the 542 471 P base × V sat × TT emhe parameter combinations for each autumn- and spring-sown experiment of the calibration data set. Variations of heading date for each experiment were modelled as a multiple linear model with V sat, P base, and TT emhe as predictors:
The variation of heading date due to each parameter was assessed using standardized regression coefficients (SRC) calculated as follows:
with
where Y represents simulated heading dates; Xi, values of parameter i (P base, V sat, or TT emhe); β0, the intercept; and βi, regression coefficient for parameter i. This showed (as a percentage) how much variation of heading date was due to V sat, P base, or TT emhe. The SRC were then aggregated for the autumn-and spring-sown experiments to show differences in model sensitivity.
Genotype data
Genotype imputation was carried out for 1797 (SSR, DArT, and SNP) unmapped markers by random forest regression for all the genotypes (Stekhoven and Bühlmann, 2012). Genotype imputation allows missing genotype data to be filled by using genetic information from relatives. Aside from methods requiring physical or genetic map information, random forest regression allows imputation of genotype data without prior knowledge of marker positions. Analysis of imputed data should lead to increased statistical power for association genetics, but in this study is essential to allow fitting of statistical models linking parameters to genetic markers on the whole set of genotypes (otherwise, genotypes with missing data for any of the markers involved in the model would have been discarded). Genotype imputation of the unordered markers was carried out in R using the missForest package (Stekhoven and Bühlmann, 2012). The missForest function provides the proportion of falsely classified (PFC) entries for each marker. Markers for which genotype imputation resulted in a PFC > 0.2 were discarded before subsequent regression analysis. This resulted in 1603 polymorphic genetic markers including 53 SSR, 451 DArT, and 1099 SNP.
Association genetic analysis
Association analysis was carried out in order to identify genetic markers associated to variation of V sat, P base, and TT emhe for the 2p and 3p strategies. For each marker, rare genotypes (frequency less than 5%) were considered as missing data. This led to a missing data rate ranging from 0 to 9% with only 5% of the markers showing some missing data. The structure was obtained by Rousset et al. (2011) with 82 SSR markers and was further used by Le Gouis et al. (2012). The structure of the panel was taken into account by using the relative contribution of each genotype to four ancestral groups as covariates in the model. Association genetics was carried out for each marker by fitting a linear model as follow:
| (15) |
where yij is the value of trait j (V sat , P base, or TT emhe) for genotype i, mik is the allele of genotype i at marker k, and are the contributions of genotype i to each of four ancestral groups. Association analysis was carried out using TASSEL v2.1 (Bradbury et al., 2007). Adjusted P-values were obtained after 1000 permutations.
Statistical models to predict V sat, P base, and TT emhe
Only genetic markers associated (P < 0.05) with one of the model parameters (V sat, P base, and TT emhe) were considered. First, blocks of collinear markers were identified by calculating the correlation coefficient (rLD) between all pairs of loci. Markers were considered collinear when rLD ≥ 0.8. Among markers included in a collinear block, the marker with the fewest missing data before genotype imputation was chosen in order to minimize possible errors coming from genotype imputation. Linear models for V sat, P base, and TT emhe were obtained using multiple linear regressions with backward elimination of the markers: all the markers associated with V sat, P base, or TT emhe and previously filtered for collinearity were included in the model and markers with P-values > 0.05 were iteratively removed one at a time. The same approach was used to model V sat and P base using only associated major genes. This analysis quantified the proportion of variation of model parameters that could be explained by additional minor-effect QTLs, compared to known major gene effects.
Validation of the QTL-based model
The QTL-based model was validated on a set of 88 independent genotypes grown for two years (2006 and 2007) in Estrées-Mons (49°53’N, 3°00’E, 85m a.s.l.), Le Moulon (48°40’N, 2°10’E, 156m a.s.l.), and Joze (45°86’N, 3°30’E, 300m a.s.l.) as described in Bordes et al. (2013). These six location × sowing date combinations were not used for model calibration. For each genotype, predicted V sat and P base (2p strategy) or V sat, P base, and TT emhe (3p strategy) were obtained from the corresponding models linking genetic markers to ecophysiological parameters. The ecophysiological model was then used to predict heading dates for each genotype using parameters as estimated by the genetic markers. Quality criteria used to assess the QTL-based model included the RMSEP between observed and predicted heading dates and the coefficient of determination (R2).
Results
Model sensitivity analysis
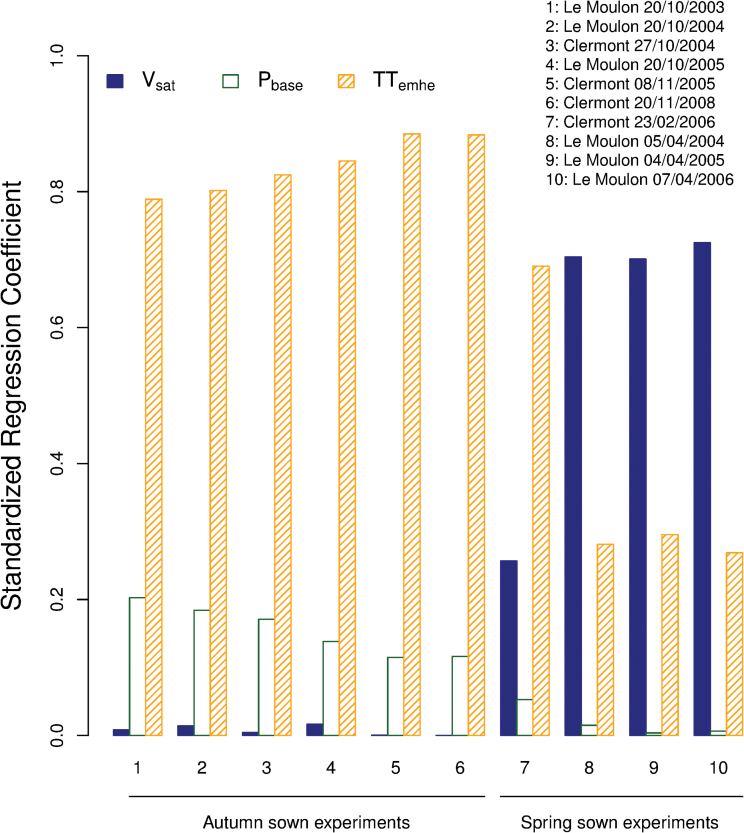
Standardized regression coefficients (SRC) ranged from 0 to 72%, 0 to 20%, and 27 to 88% for the V sat, P base,and TT emhe parameters, respectively (Fig. 1). In autumn-sown experiments, the model was much more sensitive to TT emhe and P base (SRC ranging from 79 to 88% and 11 to 20%, respectively) than to V sat (SRC ranging from 0 to 0.01%) (Fig. 1). In contrast, in spring-sown experiments, the model was highly sensitive to V sat (SRC ranging from 70 to 72%) and less so to TT emhe (SRC ranging from 27 to 29%) or P base (SRC ranging from 0 to 0.01%; Fig. 1). These trends were expected as cold temperatures experienced by the crop in autumn-sown experiments generally allows full vernalization, while development of wheat sown under long days in the spring is not expected to be limited by photoperiod.
Fig. 1.
Sensitivity analysis of a modified version of the Weir et al. (1984) phenological model. Standardized regression coefficients showing the percentage of variation of heading date explained by each model parameter (V sat, P base, and TT emhe) were calculated in each autumn- and spring-sown experiment used to optimize the model for the genotypes of the calibration data set. This figure is available in colour at JXB online.
Optimization of the ecophysiological model parameters
For each of the 53 winter genotypes (lines that did not head in srping-sown experiments and were parameterized using only autumn-sown experiments), the number of parameter vectors leading to the minimum RMSEP with the 2p strategy ranged from 1 to 86 and half of the genotypes showed more than eight unique parameter vectors that minimized the RMSEP. Depending on the genotype, standard deviations ranged from 0 to 42 days and 0 to 0.6h for V sat and P base, respectively. After filtering of these parameter vectors, the number of vectors ranged from 1 to 39 with half of these genotypes having <5 parameter vectors selected. Standard deviations for V sat and P base decreased, ranging from 0 to 8 days and 0 to 0.2h for V sat and P base, respectively. Regarding the 157 spring genotypes for which optimization was carried out using autumn- and spring-sown experiments, the number of parameter vectors selected for each genotype ranged from 1 to 16 and 75% of genotypes had only one unambiguous parameter vector. Standard deviations ranged from 0 to 2.9 days and from 0 to 0.28h for V sat and P base, respectively. When multiple parameters vectors were found minimizing RMSEP, a vector of parameters was arbitrarily chosen so that P base and V sat were close to the median values of the parameter vectors minimizing the RMSEP. As shown by standard deviations of V sat and P base, for the spring genotypes and after filtering for the winter genotypes, all the possible vectors had relatively similar values for V sat and P base. Similar results were obtained with the 3p strategy. Similarly close values of V sat and P base were obtained for each genotype when multiple optimizations were carried out using different random sets of experiments (ESM; Fig. 1). Across genotypes, standard deviations of V sat and P base ranged from 0 to 12 days and from 0 to 0.4h, respectively. This demonstrated that the optimization procedure was sufficiently robust to reliably parameterize each genotype.
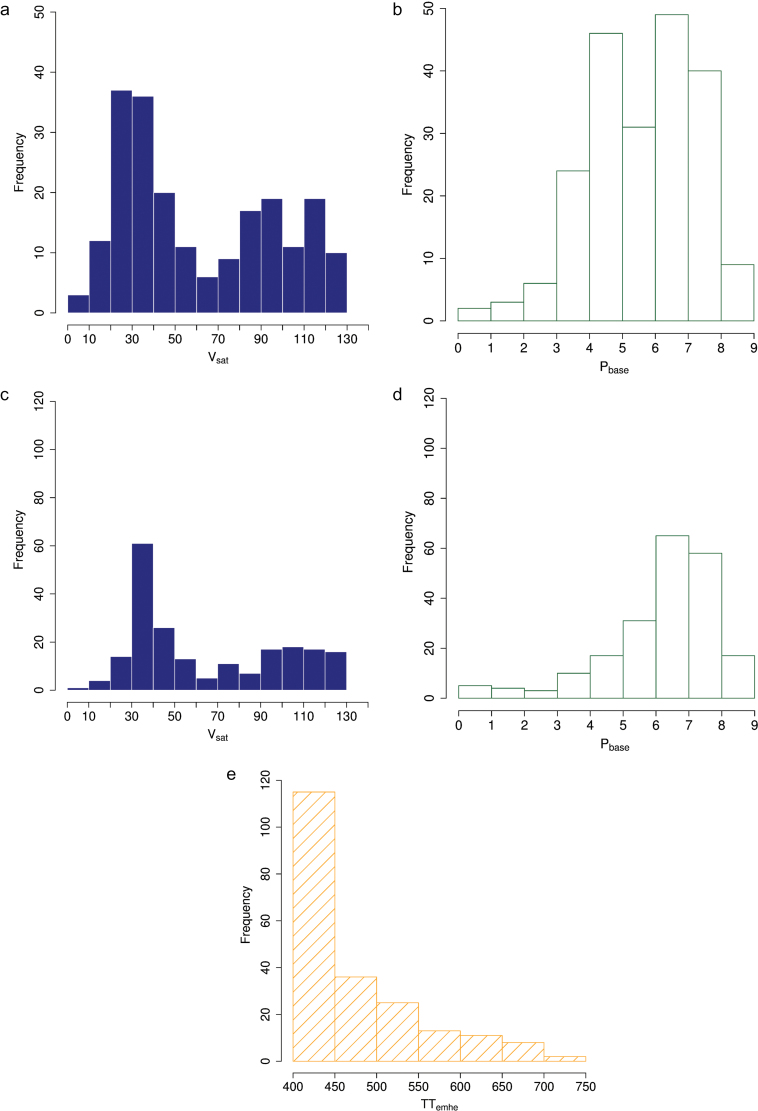
In the 2p analysis, V sat varied from 6 to 127 days and P base from 0 to 9h among genotypes. Based on Shapiro-Wilk tests, distributions of V sat and P base could not be considered as Gaussian (P-values <0.001 and <0.01, respectively). Both parameters showed multimodal distributions suggesting the presence of major genes with large effects segregating in this material (Fig. 2A and 2B). A significant but small positive correlation coefficient (r) was found between V sat and P base (r = 0.32, P < 0.001, data not shown).
Fig. 2.
Distributions of the V sat (a, c), P base (b, d) and TT emhe (e) parameters of a modified version of the Weir et al. (1984) phenological model optimized for the 210 genotypes of a wheat-association genetics panel when two (V sat and P base; a, b) or three (V sat, P base, and TT emh; c, d, and e) parameters were optimized. This figure is available in colour at JXB online.
In the 3p analysis, V sat varied from 7 to 130 days among genotypes; P base, from 0.1 to 8.8h; and TT emhe, from 400 to 740 modified degree days. The distributions of optimized parameters P base and TT emhe were skewed towards extreme values (7h and 400°C days, respectively; Fig. 2D and 2E). Moreover, the correlation between these two parameters was highly negative (r = –0.70, P < 0.001, data not shown), indicating possible compensations between these parameters and making it difficult to identify parameter vectors that independently reflect genotype photoperiod sensitivity and earliness per se.
Prediction of heading date using the ecophysiological model with optimized parameters
Depending on the environment, the difference between the latest and the earliest heading date varied from 24 to 66 days, thus reflecting the large genotypic variability for heading date in this germplasm (Table 1). In autumn-sown experiments, this difference varied from 24 to 36 days while it was higher in spring-sown experiments, ranging from 60 to 66 days (Table 1).
Table 1.
RMSEP and percentages of variance explained (R2) of the relationships between observed and predicted heading dates simulated with a modified version of the Weir et al. (1984) phenological model for the calibration and validation data sets grown in contrasting location × sowing date combinations using optimized (calibration data set only) or QTL-based parameters (calibration and validation data sets)a
| Data set | Location | Sowing date | n | Mean (min.; max.) | Optimized parameters | QTL-based parameters | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2p | 3p | 2p | 3p | |||||||||
| RMSEP | R2 | RMSEP | R2 | RMSEP | R2 | RMSEP | R2 | |||||
| Calibration | Clermont- Ferrand | 27/10/2004 | 206 | 139 (126; 154) | 2.3 (5.2) | 0.93 (0.87) | 2.0 | 0.94 | 3.6 | 0.68 | 5.0 | 0.34 |
| 08/11/2005 | 62 | 143 (133; 157) | 3.7 (2.4) | 0.93 (0.89) | 3.0 | 0.94 | 4.5 | 0.68 | 5.3 | 0.31 | ||
| 23/02/2006 | 75 | 149 (140; 165) | 5.5 (8.1) | 0.73 (0.56) | 3.5 | 0.84 | 8.2 | 0.41 | 8.7 | 0.27 | ||
| 20/11/2008 | 210 | 143 (130; 159) | 2.4 (3.6) | 0.92 (0.84) | 1.9 | 0.94 | 4.3 | 0.62 | 5.5 | 0.32 | ||
| Le Moulon | 20/10/2003 | 171 | 146 (125; 161) | 2.0 (10.0) | 0.97 (0.95) | 1.4 | 0.98 | 4.2 | 0.76 | 6.3 | 0.42 | |
| 05/04/2004 | 114 | 181 (160; 226) | 5.4 (7.9) | 0.95 (0.91) | 5.0 | 0.95 | 17.6 | 0.63 | 14.6 | 0.68 | ||
| 20/10/2004 | 164 | 143 (121; 156) | 1.9 (8.1) | 0.96 (0.90) | 1.5 | 0.97 | 4.1 | 0.73 | 5.9 | 0.41 | ||
| 04/04/2005 | 122 | 178 (160; 223) | 4.0 (6.2) | 0.94 (0.93) | 4.1 | 0.94 | 13.4 | 0.56 | 10.6 | 0.62 | ||
| 20/10/2005 | 165 | 146 (132; 162) | 1.5 (8.6) | 0.96 (0.91) | 1.6 | 0.96 | 4.3 | 0.69 | 6.5 | 0.34 | ||
| 07/04/2006 | 131 | 179 (160; 220) | 2.6 (4.8) | 0.97 (0.90) | 2.5 | 0.97 | 15.0 | 0.54 | 15.4 | 0.48 | ||
| Validation | Le Moulon | 26/10/2006 | 88 | 127 (100; 141) | - | - | - | - | 5.6 | 0.59 | 6.6 | 0.38 |
| 23/10/2007 | 88 | 142 (115; 157) | - | - | - | - | 5.0 | 0.61 | 7.0 | 0.37 | ||
| Joze | 29/10/2006 | 88 | 133 (112; 146) | - | - | - | - | 5.7 | 0.57 | 7.7 | 0.31 | |
| 25/10/2007 | 88 | 147 (134; 160) | - | - | - | - | 8.6 | 0.48 | 10.9 | 0.30 | ||
| Estrées- Mons | 17/10/2006 | 88 | 135 (104; 151) | - | - | - | - | 5.6 | 0.63 | 8.0 | 0.41 | |
| 22/10/2007 | 88 | 148 [129; 160) | - | - | - | - | 6.7 | 0.58 | 9.0 | 0.31 | ||
a Unusual spring sowings are highlighted in bold. Results obtained with optimized parameters on the calibration data set with the original model from Weir et al. (1984) are shown in parentheses. Results obtained after optimization of two (2p) or three (3p) parameters are shown. The number of wheat genotypes (n), the mean and the range of variation of heading dates (in days) are indicated. Genotypes and location × sowing date combinations of the validation data set were not used to optimize parameters or calibrate the QTL-based model and are therefore totally independent.
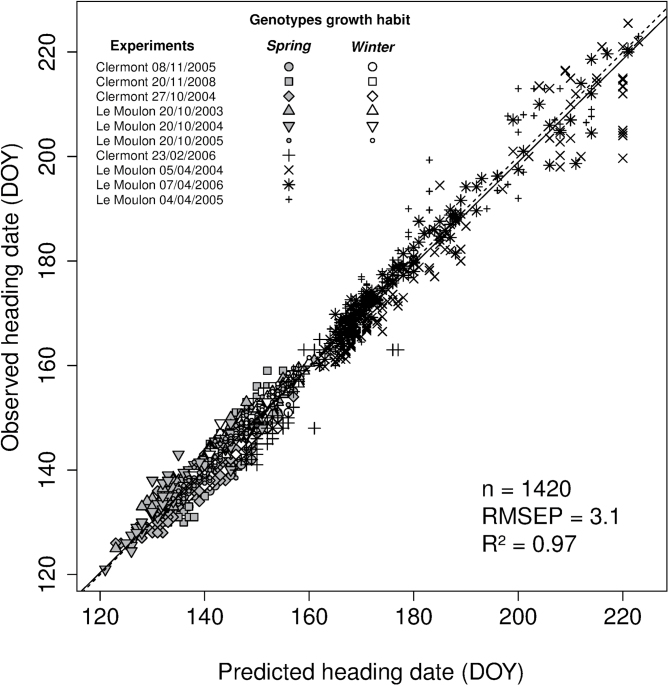
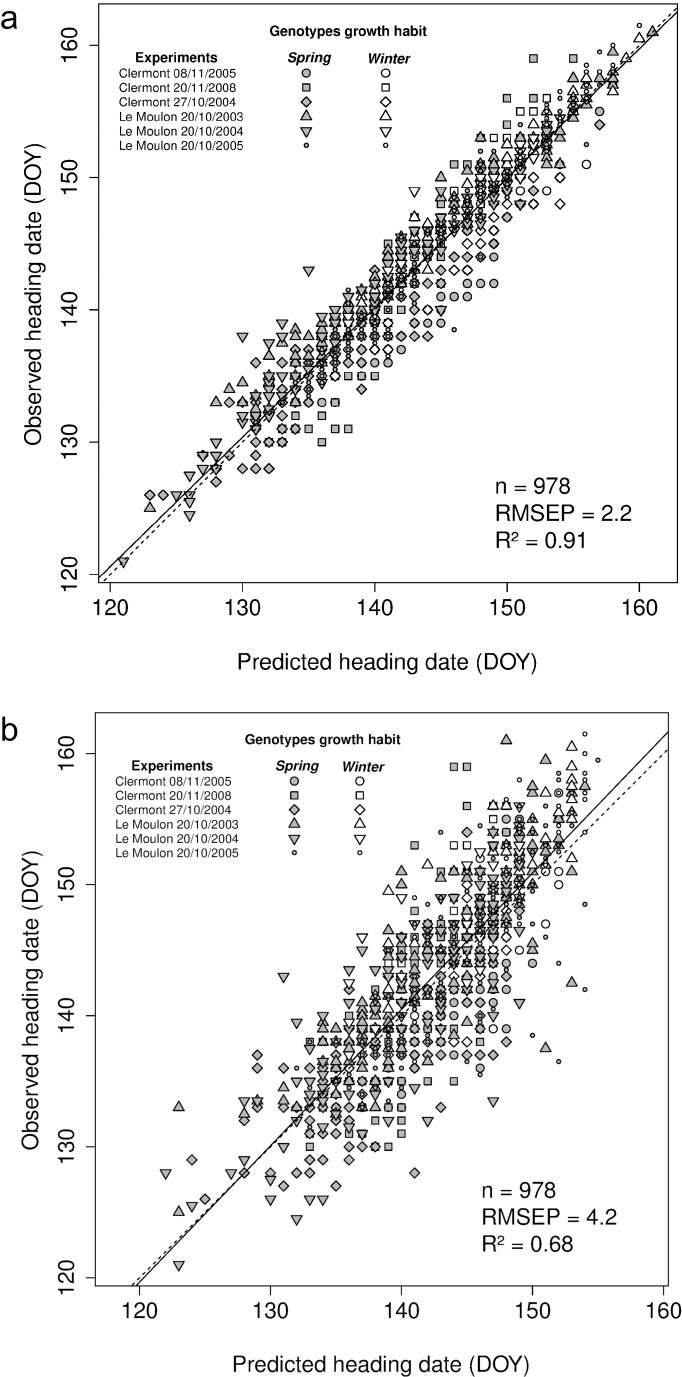
Across the 10 location × sowing date combinations, predicted heading dates obtained using the modified Weir et al. (1984) model with V sat and P base optimized parameters (2p strategy), explained 97% of the variation with an RMSEP of 3.1 days (Fig. 3). Separate analyses for the spring and winter genotypes showed RMSEP of 3.2 and 2.1, and R2 of 0.98 and 0.89, for these two groups of genotypes, respectively (data not shown). Considering each location × sowing date combination separately, RMSEP and R2 ranged from 1.5 to 5.5 days and 0.73 to 0.97, respectively (Table 1), thus showing that the model reproduced genotypic variability for heading date in the set of environments used for model calibration.
Fig. 3.
Relationship between observed and predicted heading dates obtained with two optimized parameters (2p strategy) of a modified version of the Weir et al. (1984) phenological model for a wheat calibration data set grown in 10 location × sowing date combinations. Autumn- and spring-sown experiments are shown with closed symbols and stars, respectively. Winter genotypes headed only in autumn-sown experiments while spring genotypes headed in autumn- and spring-sown experiments. Symbols for autumn-sown experiments are filled in white and grey for winter and spring genotypes, respectively. Linear regression (solid) and bisecting (dashed) lines are shown. The number of data points (n), the percentage of variance explained (R2) and RMSEP are indicated.
The modified 2p strategy described above was also compared to a 2p strategy using the original Weir et al. (1984) model, i.e. where thermal time was calculated using a three-hourly time step. The results with the original model showed an RMSEP ranging from 2.4 to 10 days with percentage of variance explained from 56 to 95% depending on the experiment (Table 1, numbers in parentheses), i.e. the modified model using thermal time based on daily mean temperature performed better than the original model, at least on this data set.
The results obtained with the 3p strategy applied to the modified Weir model (optimization of V sat, P base, and TT emhe) were slightly better than those for the 2p strategy modified Weir model, showing an RMSEP of 2.6 days and an R2 of 0.98 across all the 10 experiments of the calibration data set (data not shown). For the 3p model, a separate analysis for the different experiments showed RMSEP ranging from 1.4 to 5.0 days and R2 from 0.84 to 0.98 depending on the experiment (Table 1).
Association genetics analysis
For the 2p strategy using the modified Weir et al. (1984) model, the genetic analysis identified 43 and 66 markers associated with V sat and P base (P < 0.05), respectively. The percentage of variance explained by each associated marker varied from 4 to 21% and 3 to 17% for V sat and P base, respectively. Most of the markers (90%) associated with either V sat or P base explained less than 10% of the variation. Only markers that were co-located at known major gene loci (Ppd-D1, Vrn-A1 and Vrn-B1) explained greater than 10% of parameter variations. No marker was associated with both V sat and P base. All genomic regions associated to variations of V sat and P base had been previously detected as associated to variations of heading date in this panel (Bonnin et al., 2008; Rousset et al., 2011; Le Gouis et al., 2012).
The results for the 3p strategy identified 37, 2, and 3 markers associated with V sat, P base, and TT emhe (P < 0.05), respectively. While the number of markers associated with V sat appeared consistent between the 2p (40 markers) and 3p (37 markers) strategies, the number of genetic markers associated with P base dropped between the 2p (66 markers) and 3p (two markers) strategies. The marker for the photoperiod-sensitivity gene Ppd-D1, which was previously associated with P base in the 2p strategy, was associated to TT emhe in the 3p strategy.
QTL-based prediction of ecophysiological model parameters
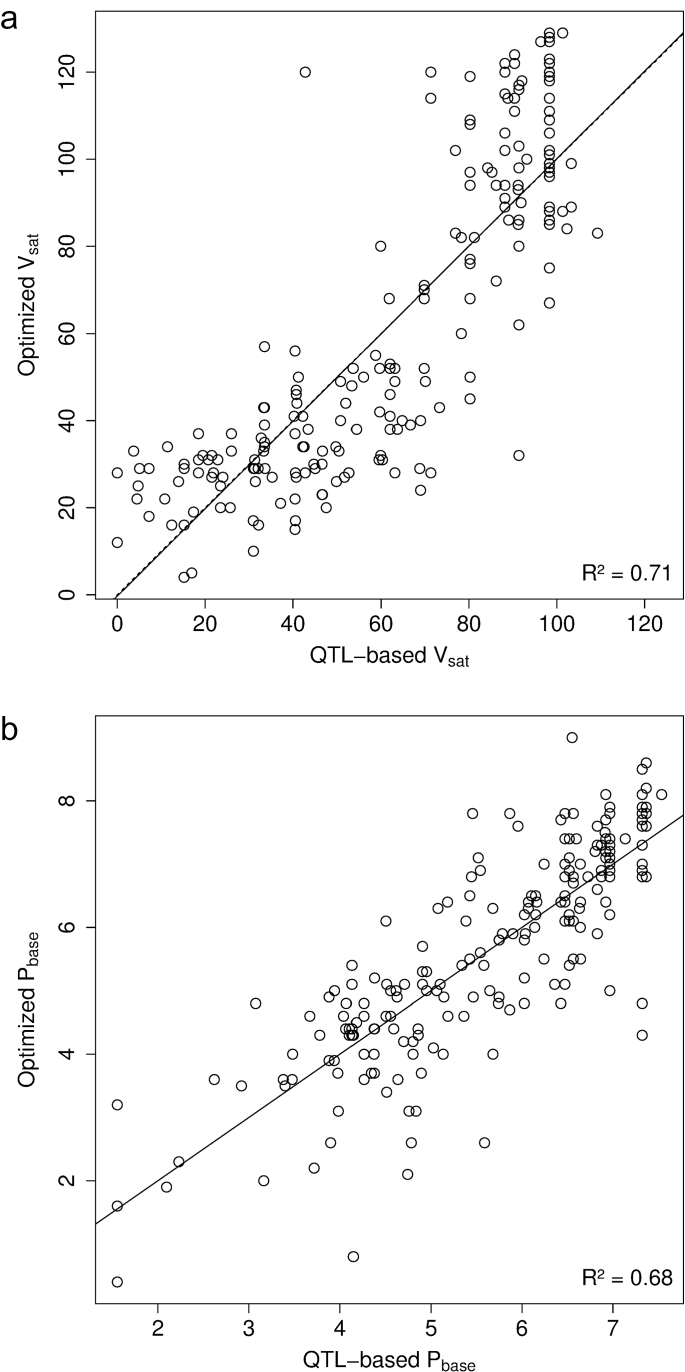
After filtering markers obtained with the 2p strategy for collinearity, 28 and 36 markers were available to model V sat and P base, respectively. The statistical model predicting V sat comprised 11 markers located on chromosomes 2D, 4A, 4B, 5A, 5B, and 7A (Table 2) and explained 71% of the genotypic variation for V sat (Fig. 4A). The most prominent markers in this model were those associated with Vrn-A1 (markers Vrn.A1ex7 and Vrn.A1pr) and Vrn-B1 genes (markers vern.5B.Sins.8761 and Vrn.B1int1) and one marker located on chromosome 2D (FdGogat.2D.Y.545). Markers Vrn.A1ex7, vern.5B.Sins.8761, Vrn.A1pr, and FdGogat.2D.Y.545 explained 39.5, 16.1, 4.6, and 5.3% of the variation of V sat (Table 2). Two markers for each of the Vrn-A1 and Vrn-B1 genes were selected in the model, suggesting that there may be different polymorphic regions in these genes responsible for variations of vernalization requirement. Allelic effects (in absolute value) on V sat ranged from 2.1 to 36.6 days (Table 2). Coefficients of the model for the alleles of the Vrn.A1ex7 marker agreed with the expectation that spring allele ‘22’ decreased the vernalization requirement (Table 2) (Rousset et al., 2011). The statistical model predicting P base comprised 12 markers located on chromosomes 1A, 2B, 2D, 3B, 5A, 5B, 6A, 6B, 7A, and 7B (Table 2) and explained 68% of the genotypic variation for P base (Fig. 4B). The main explanatory markers were for the Ppd-D1 gene (PpdD1.PromDel), an SNP (cfn5828), and an SSR (gpw1107), which explained 27.4, 15.3, and 12.2% of the variation of P base, respectively (Table 2). Allelic effects (in absolute value) on P base ranged from 0.1 to 1.5h (Table 2). Coefficients of the model for the alleles of the PpdD1.PromDel marker agreed with the expectation that insensitive allele ‘2’ decreased the value of P base.
Table 2.
Chromosome locations, allelic effects, standard errors (SE), P-values, and individual percentage of variance explained (R 2) of the markers used to predict the V sat and P base parameters of a modified version of the Weir et al. (1984) phenological modela
| Parameter | Marker (reference allele) | Chromosome location | Allele | Allelic effects | SE | P-value | R 2 (%) |
|---|---|---|---|---|---|---|---|
| V sat | Intercept | - | - | 53.9 | 9.2 | <0.001 | - |
| Vrn.A1ex7 (11) | 5A | 12 | –24.7 | 10 | <0.05 | 39.5 | |
| 22 | –28.3 | 4 | <0.001 | ||||
| vern.5B.Sins.8761 (del) | 5B | ins | 13.3 | 5.7 | <0.05 | 16.1 | |
| Vrn.A1pr (11) | 5A | 22 | 7.0 | 5.3 | 0.19 | 4.6 | |
| 25 | –29.9 | 15.1 | <0.05 | ||||
| 33 | 17.9 | 8.6 | <0.05 | ||||
| 44 | 36.6 | 11.1 | <0.01 | ||||
| 55 | –1.7 | 6.5 | 0.80 | ||||
| FdGogat.2D.Y.545 (C) | 2D | T | –9.4 | 3.9 | <0.05 | 5.3 | |
| Vrn.B1int1 (11) | 5B | 22 | –13.1 | 5.8 | <0.05 | 0.6 | |
| cfn5274 (T) | 2D | G | –6.6 | 4.0 | 0.10 | 1.4 | |
| cfn5187 (T) | 4A | C | 2.1 | 4.3 | 0.62 | 1.1 | |
| cfn5114 (T) | 4A | C | 8.0 | 4.2 | 0.06 | 0.6 | |
| cfn5680 (A) | 7A | G | 7.0 | 3.5 | <0.05 | 0.6 | |
| wPt-7062 (0) | 4B | 1 | 10.0 | 4.2 | <0.05 | 0.7 | |
| cfn5405 (T) | na | C | –6.7 | 3.3 | <0.05 | 0.6 | |
| P base | Intercept | - | - | 4.85 | 0.4 | <0.001 | - |
| PpdD1.PromDel (1) | 2D | 2 | –1.5 | 0.2 | <0.001 | 27.4 | |
| cfn5828 (A) | 2D | G | –0.5 | 0.2 | <0.01 | 15.3 | |
| gpw1107 (149) | 3B | 153 | 0.4 | 0.3 | 0.23 | 12.2 | |
| 169 | 0.5 | 0.3 | 0.15 | ||||
| 171 | 0.7 | 0.4 | 0.12 | ||||
| 173 | 0.1 | 0.3 | 0.83 | ||||
| 175 | –0.4 | 0.5 | 0.41 | ||||
| 177 | –0.1 | 1.1 | 0.94 | ||||
| 179 | –0.5 | 1.1 | 0.66 | ||||
| wPt-7063 (0) | 6A | 1 | 0.3 | 0.2 | 0.13 | 2.4 | |
| cfn5539 (A) | 2B | G | 0.4 | 0.2 | 0.07 | 3.5 | |
| cfn4818 (T) | 7A | G | 0.6 | 0.3 | 0.08 | 1.5 | |
| cfn4764 (A) | 5A | C | –0.5 | 0.2 | <0.05 | 1 | |
| cfn5634 (T) | 6B | C | –0.4 | 0.2 | <0.05 | 1 | |
| cfn5055 (T) | 5B | C | –0.4 | 0.2 | 0.05 | 0.9 | |
| wPt-5346 (0) | 5B | 1 | 0.3 | 0.2 | 0.06 | 0.9 | |
| gluA1.1.Y.1667 (C) | 1A | T | 0.4 | 0.2 | <0.05 | 1.1 | |
| wPt-7108 (0) | 7B | 1 | –0.4 | 0.2 | <0.01 | 1.2 |
a Markers were identified by association genetics and chosen to be in low-linkage disequilibrium. Models were obtained using multiple linear regressions with iterative backward elimination of markers with P-values < 0.05. For each marker, the allele used as a reference to calculate the coefficient is indicated in brackets.na, not available.
Fig. 4.
Relationships between optimized and QTL-based predicted V sat (a) and P base (b) parameters of a modified version of the Weir et al. (1984) phenological model for the 210 genotypes of the calibration data set. Solid lines are regression lines and R2 is the percentage of variance explained.
Models obtained using only the known associated major genes for V sat (Vrn-A1, Vrn-B1, and LUMINIDEPENDENS) and P base (Ppd-D1 and Vrn-A3) were calculated. Clearly, it appeared that prediction of V sat was already reasonable when using only Vrn-A1, Vrn-B1, and LUMINIDEPENDENS as predictors (R2 = 0.61, data not shown) although the use of additional loci allowed reaching R2 = 0.71 (Fig. 4A). However, prediction of P base using Ppd-D1 and Vrn-A3 was poor (R2 = 0.36, data not shown) and benefited from the inclusion of additional genetic markers (Fig. 4B).
For the 3p strategy, 16 markers were identified for V sat, explaining 73% of the variation (data not shown). The model and QTL-based predictions of V sat were very similar for the 2p and 3p strategies. However, the model for P base comprised only one marker (wPt-1664, not associated to P base in the 2p strategy) and explained only 5% of P base (data not shown). In the same way, model TT emhe comprised only three markers (wPt-1664, Ppd-D1, and wPt-7108 associated to P base in the 2p strategy) and explained 25% of the variation for this parameter (data not shown).
Predictions of heading date and model validation
Observed heading dates were compared to heading dates predicted by optimized or QTL-based parameters obtained with the 2p strategy for the autumn-sown experiments of the calibration data set. Across autumn-sown experiments of the calibration data set, predictions with optimized parameters explained 91% of the variation with an RMSEP of 2.2 days (Fig. 5A) while predictions with QTL-based parameters explained 68 % of the variation with an RMSEP of 4.2 days (Fig. 5B). Relationships between observed and predicted heading dates with optimized parameters across the autumn sown experiments showed RMSEP of 2.3 and 2.1 days and R2 of 0.90 and 0.89 for the spring and winter genotypes, respectively. In comparison, when QTL-based parameters were used, spring and winter genotypes showed RMSEP of 4.1 and 4.3 days and R2 of 0.66 and 0.67, respectively. This separate analysis did not show any prediction bias between the spring and winter genotypes. When considering individual experiments, R2 ranged from 0.92 to 0.97 (median equal to 0.94) and RMSEP from 1.5 to 3.7 days (median equal to 2.2) when heading dates were predicted with optimized parameters (Table 1) while R2 ranged from 0.62 to 0.76 (median equal to 0.68) and RMSEP from 3.6 to 4.5 days (median equal to 4.3) when heading dates were predicted with QTL-based parameters (Table 1). Compared to results obtained using optimized parameters, RMSEP was increased by 0.8 to 2.8 days (median increase of 2.0 days) and R2 decreased by 0.21 to 0.30 (median decrease of 0.25) when heading date was predicted using QTL-based parameters.
Fig. 5.
Relationships between observed heading dates and heading dates predicted with two optimized (a) or two QTL-based (b) parameters (2p strategy) for the calibration data set across the autumn-sown experiments using a modified version of the Weir et al. (1984) phenological model. Symbols are filled in white and grey for winter and spring genotypes, respectively. Linear regression (solid) and bisecting (dashed) lines are shown. The number of data points (n), the percentage of variance explained (R2), and RMSEP are indicated.
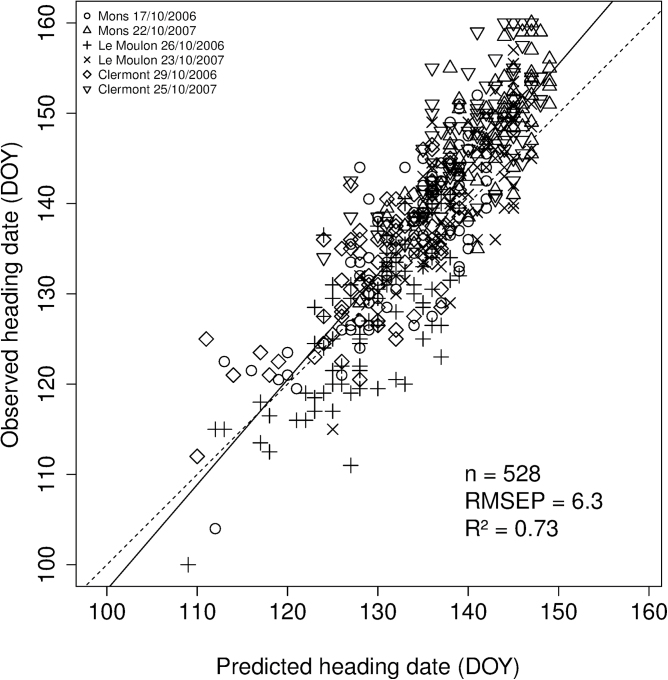
The model was next tested on a set of 88 independent genotypes grown in six independent location × sowing date combinations. Differences between the earliest and the latest genotypes varied from 26 to 47 days in this validation data set (Table 1). This largely covered the range of variation observed in the calibration data set for autumn sowings which varied between 24 and 36 days (Table 1). Across all of the validation data set, heading dates predicted with QTL-based parameters explained 73% of the variation with an RMSEP of 6.3 days (Fig. 6). When looking at each location × sowing date combination of the validation data set separately (Table 1), RMSEP ranged from 5.0 to 8.6 (median equal to 5.6 days) and R2 from 0.48 to 0.63 (median equal to 0.58).
Fig. 6.
Relationships between observed heading dates and heading dates predicted with two QTL-based parameters (2p strategy) using a modified version of the Weir et al. (1984) phenological model for the validation data set comprising 88 independent wheat genotypes grown in six independent location × sowing date combinations. Linear regression (solid) and bisecting (dashed) lines are shown. The number of datapoints (n), the percentage of variance explained (R2), and the RMSEP are indicated.
In comparison to the 2p strategy, QTL-based prediction results obtained with the 3p strategy showed reduced predictive ability for both the calibration and validation data sets. Separate analyses for each autumn-sown experiment of the calibration data set showed RMSEP ranging from 5 to 6.5 days and R2 ranging from 0.31 to 0.42 (Table 1). Considering experiments of the validation data set separately, RMSEP ranged from 6.6 to 10.9 days and R2 from 0.30 to 0.41 (Table 1).
Discussion
Gene-based modelling assists in integrating knowledge from plant ecophysiology and genetics (Chapman et al., 2002a; Chapman et al., 2002b; White and Hoogenboom, 2003; White, 2006; Letort et al., 2008; White, 2009). One outcome of this is to improve ecophysiological modelling and the prediction of cultivar performance. Another outcome is to assist in quantitatively assessing the effect of genes by explicitly accounting for genotype × environment interactions. In terms of targeting breeding, these outcomes can open the way to the identification of ideotypes for current and/or future climate environments (Chenu et al., 2009). In this study, we proposed a QTL-based ecophysiological model to predict heading date in bread wheat. Two parameters (V sat and P base) of an ecophysiological model were optimized for each genotype of an association genetics panel representative of the wheat germplasm (Balfourier et al., 2007; Rousset et al., 2011). Multiple linear models predicting V sat and P base using associated genetic markers were obtained by stepwise regression. Predictions of heading dates using QTL-based parameters were tested on a data set with independent genotypes and environments. The conditions required to identify parameter vectors which reflect genotypic differences and the ability to predict heading date using QTL-based parameters are discussed.
Identifying parameter vectors reflecting genetic differences
The first condition for a successful gene-based modelling approach is to use an ecophysiological model with adequate predictive capabilities. In particular, the ability of the model to deal with complex genotype × environment interactions relevant to the studied process is a key feature. The ecophysiological model used in this study was a modified version of a model proposed by Weir et al. (1984), with a simplification of the calculation of accumulated daily temperature. The original formulation relies on the simulation of a cosinusoidal variation of temperature across the day from daily minimal and maximal temperature data. Temperature across the day is then integrated by averaging the contributions of the eight three-hour temperatures each day [equation 1 in Weir et al. (1984)]. In the present study, as in other major wheat crop models Sirius (Jamieson et al., 1998) and APSIM (Keating et al., 2003), we used daily mean temperature as an input and therefore ignored temperature variation across the day. The two formulations may give different results because of the non-linearity of the response curves used. However, the formulations used by Weir et al. (1984) and the one used in the present study are extremely empirical and do not reflect the current knowledge on the underlying physiological processes (Brown et al., 2013). Hence, the use of the original or the modified model can only be justified by comparing their predictive power. Results obtained with the present model and the original Weir et al. (1984) model indicated that calculation of thermal time accumulation using daily mean temperature performed better than using the original formulation (Weir et al. 1984), at least on this data set (Table 1). We did not investigate further why our degraded version of the model performed better with our data set as this was not the primary objective of our study.
We also modified the original model by suppressing the intermediate phase from emergence to double-ridge and by assuming that thermal time accumulation from emergence to heading was affected by vernalization and photoperiod factors throughout the phase. This is an oversimplification as vernalization mainly affects the phase from emergence to double-ridge (Ritchie, 1991), although it may also have minor effects from double-ridge to heading (Griffiths et al., 1985). These modifications were applied because no observations of double-ridge were available, and the model could not be parameterized for this growth stage. Again, while still quite empirical, the model showed good capability in predicting heading dates of an extremely diverse genetic panel with contrasting sowing dates (Table 1). Prediction errors were comparable to the ones reported by previous studies, which varied from 4 to 7 days (Weir et al., 1984; Asseng et al., 1998; White et al., 2008; He et al., 2011).
The second condition for a successful gene-modelling approach relies on an ability to unambiguously parameterize the model for a large number of genotypes. Most of the studies linking genetic markers and parameters of an ecophysiological model were carried out after measuring parameters on a mapping population (Reymond et al., 2003; Nakagawa et al., 2005; Quilot et al., 2005; Yin et al., 2005; Uptmoor et al., 2011). Direct measurement of parameters allows unambiguous identification of parameter vectors but is feasible only in the case of mechanistic models for which parameters have a clear biological meaning. Only few studies have been carried out using optimized parameters (Messina et al., 2006; White et al., 2008), which is less demanding on phenotyping. However, parameter optimization is generally not straightforward and sometimes depends on subjective decisions (Mavromatis et al., 2001) as different parameter combinations may equally fit the relationships between observations and model predictions. Although this is not an issue when the objective is only to predict heading dates, this becomes an obstacle when the objective is to link parameters to genes.
The dimension of parameter space, which depends on the number of parameters to be optimized and the range of variation of each parameter, contributes to both accurate and unambiguous parameterization. Indeed, in the 3p strategy, compensations between P base and TT emhe led to multiple solutions and parameter vectors could not be unambiguously identified. This was not the case when only V sat and P base were optimized (2p strategy). Moreover, the restricted parameter space used in this study allowed optimizing parameters by calculating RMSEP for all the parameter combinations (brute-force optimization), an approach that allows a full exploration of parameter space. More-complex traits depending on a higher number of model parameters would probably need to use optimization algorithms, ideally independent of starting values and able to avoid local minima (He et al., 2011).
The use of phenotypic data recorded under various experimental conditions for which model sensitivity varied greatly (Fig. 1) also contributed to unambiguous identification of parameter vectors. In this study, wheat genotypes were tested under contrasting sowing dates ranging from the usual autumn sowings corresponding to local agronomic practices under these latitudes to the most extreme spring sowings under which winter genotypes did not head due to incomplete vernalization. The use of srping-sown experiments, either to reveal the strong winter nature of some genotypes that did not head or more generally to quantify accurately genetic variation for vernalization requirements, assisted in accurately optimizing genotype parameters. Other experiments carried out in different latitudes or under controlled photoperiodic or vernalizing conditions could assist in optimizing genotype parameters if the simple ecophysiological model used in this study proved valid for these experiments where non-natural rapid changes in photoperiod and temperature regimes are created.
A third condition for a successful gene-based modelling approach is to develop a clear and robust genetic determinism to optimized parameter values. Reducing the number of parameters to calibrate the model may have led to a dilution of the genetic effects by increasing the number of genes involved in the determinism of each parameter. Among the markers associated with V sat and P base, none were common to the two parameters, reflecting the fact that the model was able to separate the two individual physiological processes linked to vernalization and photoperiod. This was not the case when Le Gouis et al. (2012) estimated vernalization requirement and photoperiod sensitivity based on differences of heading dates under specific experiments designed to separate earliness components, but without using an ecophysiological model. In the gene network proposed by Trevaskis et al. (2007), vernalization and photoperiod perception are indeed independent: exposure to cold temperatures (vernalization) induces VRN1 in the leaves, but VRN1 expression at the apex remains low until Ppd-D1 is induced by long days. It may also be hypothesized that as many genes will be involved in their determinism if the number of parameters is low, a larger proportion of parameter variation may be explained by interactions between markers rather than by marker additive effect. In our case, however, bi-locus marker × marker interactions were either non-significant or small (approximately 1% of V sat variation explained for the most significant epistatic interaction between Vrn-A1 and Vrn-B1; data not shown).
QTL-based predictions of heading date
From a biological point of view, the values of V sat and P base may appear unrealistic, particularly where V sat exceeds 80 days for some genotypes. Two possible explanations are the reduced number of parameters used to calibrate the model for different genotypes and the formulation of the model itself.
In the 2p strategy, the absence of a parameter representing genetic variation for earliness per se implied that genetic variation for this earliness component would be included into V sat and P base. The same approach with three parameters (3p strategy) appeared attractive since the three classical components of wheat earliness would have been represented by three different model parameters. However, comparison of the QTL-based predictions obtained with the 2p and 3p strategies clearly showed that the 2p strategy performed better. The main objective of the present study was to maximize the predictive power of the QTL-based model using temperature and photoperiod environmental information, rather than to create a model formulation that would specifically coincide with actual knowledge of wheat earliness.
A final consideration is that the effect of the V sat parameter may have been distorted due to the model itself. In the original model of Weir et al. (1984), the accumulated thermal time from emergence to double-ridge is modified by vernalization and photoperiod, but the following period from double-ridge to heading is only affected by photoperiod. As we could not calibrate this intermediate phase (due to absence of measurements), vernalization affects wheat development from emergence to heading in our model. However, despite these unrealistic values and its high empiricism, our QTL-based model was able to predict genotype variations for heading date across contrasting autumn sowings in different locations of France (Table 1, Fig. 6).
Association genetics did not reveal any novel loci compared to studies on heading date carried out with this plant material (Rousset et al., 2011; Le Gouis et al., 2012; Bordes et al., 2013), i.e. in this case and contrary to other studies which investigated more complex traits (Reymond et al., 2003; Quilot et al., 2005), working on model parameters did not identify new chromosomal regions. However, contrary to studies carried out on heading date, this approach developed estimated effects that were independent of the environment and therefore more useful in prediction of performance in environments other than those originally tested. Indeed, contrary to classical QTL-analysis studies in which the effect of a QTL detected in different environments sometimes varies considerably, even for major genes like Ppd-D1 (Bogard et al., 2011), this approach led to a single value for V sat and P base taking into account all the environments. Given results obtained on a set of independent genotypes and environments (Table 1, Fig. 6), the heading dates of any allelic combination can be simulated with the QTL-based ecophysiological model with a median prediction error of 5.6 days, with the expectation that predicted heading dates would explain 60% of the variation for this trait.
However, as the multiple linear regression models for V sat and P base explained only 71 and 68% of the variation of these parameters, about one third of their genetic variation remains unexplained. The use of genetic markers in linkage disequilibrium as proxies instead of markers in the causal polymorphism may have reduced the ability to predict V sat and P base due to possible recombinations. Moreover, the presence of multiple alleles for Ppd-B1 and Ppd-D1 could be taken into account to improve our estimation of the genotype P base value, i.e. one improvement would be to use diagnostic markers for these genes (Guo et al., 2010; Díaz et al., 2012; Shaw et al., 2012; Cane et al., 2013). In addition to errors coming from the model itself, which is quite simple, the remaining unexplained genetic variation may also be attributed to the fact that small-effect loci could not be detected by association genetics due to insufficient size of the panel or insufficient coverage of the wheat genome. Contrary to maize, where no major genes but approximately 50 small-effect additive QTLs with large polymorphism regulate flowering (Buckler et al., 2009), the genetic architecture of wheat flowering appears to comprise relatively few major genes which have, as in Arabidopsis (Salomé et al., 2011), a large effect. However, our results show that small-effect loci are likely to be important in terms of prediction. This was shown when using only major genes to model V sat (Vrn-A1, Vrn-B1, and LUMINIDEPENDENS, R2 = 0.61) and in particular for P base (Ppd-D1, Vrn-A3, R2 = 0.36). Identification of additional minor loci would probably require a larger association panel and/or higher-resolution coverage of the genome. In a study of the same germplasm, Le Gouis et al. (2012) estimated genome coverage of about 60% with 760 markers. Although the number of markers used in our study was much higher (1603) and genome coverage was therefore expected to be >60%, it is not possible to guarantee full genome coverage as the total number of markers may have increased without a proportional increase in information. High-throughput DNA chips for wheat containing thousands of SNPs derived from ISBP would probably assist in resolving this issue (Paux et al., 2010).
Conclusions
Prediction of the parameters V sat and P base using 11 and 12 genetic markers, respectively, allowed simulation of heading date with a median RMSEP of 5.6 days and accounted for approximately 60% of the genotypic variation for heading date in an independent data set of 88 genotypes grown in six location × sowing date combinations. Contrary to a study carried out on heading date, the use of an ecophysiological model allows an estimation of the allelic effects of genes independently of the considered environments as long as temperature data are available. This represents added value for breeders as simulations of heading date carried out for different allelic combinations in different environments can assist in determining the most suitable allelic combination for a given targeted population of environments (i.e. using a historical temperature record) and represents a first step in the in silico identification of ideotypes (Tardieu, 2003; Chenu et al., 2009).
Supplementary material
Supplementary data can be found at JXB online.
Supplementary Figure S1. Values of the Vsat (A) and Pbase (B) parameters of a modified version of the Weir et al. (1984) phenological model obtained for 210 genotypes after optimization using 10 different sets of experiments.
Funding
This work was funded by the EU Framework 7 ADAPTAWHEAT project (FP7-KBBE-2011–5, led by Simon Griffiths, JIC, UK).
Supplementary Material
Acknowledgements
The authors thank Séverine Rougeol and Florence Exbrayat-Vinson (UMR 1095 INRA/UBP GDEC, France) for technical assistance in genotyping of the germplasm and Sébastien Praud (Biogemma, France) and Michel Rousset (UMR GMQ Le Moulon, France) for genotyping of Ppd genes developed during the ANR WheatPerformance project (ANR-07-GPLA-023).
Glossary
Abbreviations:
- DOY
day of the year
- RMSEP
root mean square error of prediction.
References
- Asseng S, Keating B, Fillery IR, Gregory P, Bowden J, Turner N, Palta J, Abrecht D. 1998. Performance of the APSIM-wheat model in Western Australia. Field Crops Research 57, 163–179 [Google Scholar]
- Balfourier F, Roussel V, Strelchenko P, Exbrayat-Vinson F, Sourdille P, Boutet G, Koenig J, Ravel C, Mitrofanova O, Beckert M. 2007. A worldwide bread wheat core collection arrayed in a 384-well plate. Theoretical and Applied Genetics 114, 1265–1275 [DOI] [PubMed] [Google Scholar]
- Beales J, Turner A, Griffiths S, Snape JW, Laurie DA. 2007. A pseudo-response regulator is misexpressed in the photoperiod insensitive Ppd-D1a mutant of wheat (Triticum aestivum L.). Theoretical and Applied Genetics 115, 721–733 [DOI] [PubMed] [Google Scholar]
- Bentley AR, Turner AS, Gosman N, Leigh FJ, Maccaferri M, Dreisigacker S, Greenland A, Laurie DA. 2010. Frequency of photoperiod-insensitive Ppd-A1a alleles in tetraploid, hexaploid and synthetic hexaploid wheat germplasm. Plant Breeding 130, 10–15 [Google Scholar]
- Bertin N, Martre P, Génard M, Quilot B, Salon C. 2010. Under what circumstances can process-based simulation models link genotype to phenotype for complex traits? Case-study of fruit and grain quality traits. Journal of Experimental Botany 61, 955–967 [DOI] [PubMed] [Google Scholar]
- Bogard M, Jourdan M, Allard V, et al. 2011. Anthesis date mainly explained correlations between post-anthesis leaf senescence, grain yield, and grain protein concentration in a winter wheat population segregating for flowering time QTLs. Journal of Experimental Botany 62, 3621–3636 [DOI] [PubMed] [Google Scholar]
- Bonnin I, Rousset M, Madur D, Sourdille P, Dupuits C, Brunel D, Goldringer I. 2008. FT genome A and D polymorphisms are associated with the variation of earliness components in hexaploid wheat. Theoretical and Applied Genetics 116, 383–394 [DOI] [PubMed] [Google Scholar]
- Bordes J, Ravel C, Jaubertie JP, Duperrier B, Gardet O, Heumez E, Pissavy AL, Charmet G, Le Gouis J, Balfourier F. 2013. Genomic regions associated with the nitrogen limitation response revealed in a global wheat core collection. Theoretical and Applied Genetics 126, 805–822 [DOI] [PubMed] [Google Scholar]
- Börner A, Schumann E, Fürste A, Cöster H, Leithold B, Röder M, Weber W. 2002. Mapping of quantitative trait loci determining agronomic important characters in hexaploid wheat (Triticum aestivum L.). Theoretical and Applied Genetics 105, 921–936 [DOI] [PubMed] [Google Scholar]
- Bradbury PJ, Zhang Z, Kroon DE, Casstevens TM, Ramdoss Y, Buckler ES. 2007. TASSEL: software for association mapping of complex traits in diverse samples. Bioinformatics 23, 2633–2635 [DOI] [PubMed] [Google Scholar]
- Brooking IR, Jamieson PD, Porter JR. 1995. The influence of daylength on final leaf number in spring wheat. Field Crops Research 41, 155–165 [Google Scholar]
- Brown HE, Jamieson PD, Brooking IR, Moot DJ, Huth NI. 2013. Integration of molecular and physiological models to explain time of anthesis in wheat. Annals of Botany 112, 1683–1703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckler ES, Holland JB, Bradbury PJ, et al. 2009. The genetic architecture of maize flowering time. Science 325, 714–718 [DOI] [PubMed] [Google Scholar]
- Cane K, Eagles HA, Laurie DA, Trevaskis B, Vallance N, Eastwood RF, Gororo NN, Kuchel H, Martin PJ. (2013). Ppd-B1 and Ppd-D1 and their effects in southern Australian wheat. Crop and Pasture Science 64, 100–114 [Google Scholar]
- Carter AH, Garland-Campbell K, Kidwell KK. 2011. Genetic mapping of quantitative trait loci associated with important agronomic traits in the spring wheat (L.) cross “Louise” × “Penawawa”. Crop Science 51, 84 [Google Scholar]
- Chapman SC. 2008. Use of crop models to understand genotype by environment interactions for drought in real-world and simulated plant breeding trials. Euphytica 161, 195–208 [Google Scholar]
- Chapman SC, Cooper M, Hammer GL. 2002a. Using crop simulation to generate genotype by environment interaction effects for sorghum in water-limited environments. Crop and Pasture Science 53, 379–389 [Google Scholar]
- Chapman SC, Hammer GL, Podlich DW, Cooper M. 2002b. Linking bio-physical and genetic models to integrate physiology, molecular biology and plant breeding. In: Kang M, ed. Quantitative genetics, genomics, and plant breeding. Wallingford, UK: CAB International, 167–187 [Google Scholar]
- Chapman S, Cooper M, Podlich D, Hammer G. 2003. Evaluating plant breeding strategies by simulating gene action and dryland environment effects. Agronomy Journal 95, 99–113 [Google Scholar]
- Chenu K, Chapman SC, Hammer GL, Mclean G, Salah HBH, Tardieu F. 2008. Short-term responses of leaf growth rate to water deficit scale up to whole-plant and crop levels: an integrated modelling approach in maize. Plant, Cell and Environment 31, 378–391 [DOI] [PubMed] [Google Scholar]
- Chenu K, Chapman SC, Tardieu F, McLean G, Welcker C, Hammer GL. 2009. Simulating the yield impacts of organ-level quantitative trait loci associated with drought response in maize: a “gene-to-phenotype” modeling approach. Genetics 183, 1507–1523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockram J, Jones H, Leigh FJ, O’Sullivan D, Powell W, Laurie DA, Greenland AJ. 2007. Control of flowering time in temperate cereals: genes, domestication, and sustainable productivity. Journal of Experimental Botany 58, 1231–1244 [DOI] [PubMed] [Google Scholar]
- Del Ponte EM, Fernandes JMC, Bergstrom GC. 2007. Influence of growth stage on Fusarium Head Blight and deoxynivalenol production in wheat. Journal of Phytopathology 155, 577–581 [Google Scholar]
- Díaz A, Zikhali M, Turner AS, Isaac P, Laurie DA. (2012). Copy number variation affecting the Photoperiod-B1 and Vernalization-A1 genes is associated with altered flowering time in wheat (Triticum aestivum). PLoS One 7, e33234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Distelfeld A, Li C, Dubcovsky J. 2009. Regulation of flowering in temperate cereals. Current Opinion in Plant Biology 12, 178–184 [DOI] [PubMed] [Google Scholar]
- Foulkes MJ, Slafer GA, Davies WJ, Berry PM, Sylvester-Bradley R, Martre P, Calderini DF, Griffiths S, Reynolds MP. 2011. Raising yield potential of wheat. III. Optimizing partitioning to grain while maintaining lodging resistance. Journal of Experimental Botany 62, 469–486 [DOI] [PubMed] [Google Scholar]
- Fu D, Szucs P, Yan L, Helguera M, Skinner JS, Von Zitzewitz J, Hayes PM, Dubcovsky J. 2005. Large deletions within the first intron in VRN-1 are associated with spring growth habit in barley and wheat. Molecular Genetics and Genomics 273, 54–65 [DOI] [PubMed] [Google Scholar]
- Gooding MJ, Davies WP. 1992. Foliar urea fertilization of cereals: A review. Fertilizer Research 32, 209–222 [Google Scholar]
- Gouache D, Le Bris X, Bogard M, Deudon O, Pagé C, Gate P. 2012. Evaluating agronomic adaptation options to increasing heat stress under climate change during wheat grain filling in France. European Journal of Agronomy 39, 62–70 [Google Scholar]
- Griffiths FE, Lyndon RF, Bennett MD. 1985. The effects of vernalization on the growth of the wheat shoot apex. Annals of Botany 56, 501–511 [Google Scholar]
- Griffiths S, Simmonds J, Leverington M, et al. 2009. Meta-QTL analysis of the genetic control of ear emergence in elite European winter wheat germplasm. Theoretical and Applied Genetics 119, 383–395 [DOI] [PubMed] [Google Scholar]
- Guo Z, Song Y, Zhou R, Ren Z, Jia J. (2010). Discovery, evaluation and distribution of haplotypes of the wheat Ppd–D1 gene. New Phytologist 185, 841–851 [DOI] [PubMed] [Google Scholar]
- Hanocq E, Laperche A, Jaminon O, Lainé A-L, Le Gouis J. 2007. Most significant genome regions involved in the control of earliness traits in bread wheat, as revealed by QTL meta-analysis. Theoretical and Applied Genetics 114, 569–584 [DOI] [PubMed] [Google Scholar]
- Hanocq E, Niarquin M, Heumez E, Rousset M, Le Gouis J. 2004. Detection and mapping of QTL for earliness components in a bread wheat recombinant inbred lines population. Theoretical and Applied Genetics 110, 106–115 [DOI] [PubMed] [Google Scholar]
- He J, Le Gouis J, Stratonovitch P, Allard V, Gaju O, Heumez E, Orford S, Griffiths S, Snape JW, Foulkes MJ. 2011. Simulation of environmental and genotypic variations of final leaf number and anthesis date for wheat. European Journal of Agronomy 42, 22–33 [Google Scholar]
- Hirel B, Gouis JL, Ney B, Gallais A. 2007. The challenge of improving nitrogen use efficiency in crop plants: towards a more central role for genetic variability and quantitative genetics within integrated approaches. Journal of Experimental Botany 58, 2369–2387 [DOI] [PubMed] [Google Scholar]
- Jamieson PD, Brooking IR, Semenov MA, McMaster GS, White JW, Porter JR. 2007. Reconciling alternative models of phenological development in winter wheat. Field Crops Research 103, 36–41 [Google Scholar]
- Jamieson PD, Semenov MA, Brooking IR, Francis GS. 1998. Sirius: a mechanistic model of wheat response to environmental variation. European Journal of Agronomy 8, 161–179 [Google Scholar]
- Keating BA, Carberry PS, Hammer GL, Probert ME, Robertson MJ, Holzworth D, Huth NI, Hargreaves JNG, Meinke H, Hochman Z. 2003. An overview of APSIM, a model designed for farming systems simulation. European Journal of Agronomy 18, 267–288 [Google Scholar]
- Leff B, Ramankutty N, Foley JA. 2004. Geographic distribution of major crops across the world. Global Biogeochemical Cycles 18, GB1009 [Google Scholar]
- Le Gouis J, Bordes J, Ravel C, Heumez E, Faure S, Praud S, Galic N, Remoué C, Balfourier F, Allard V. 2012. Genome-wide association analysis to identify chromosomal regions determining components of earliness in wheat. Theoretical and Applied Genetics 124, 597–611 [DOI] [PubMed] [Google Scholar]
- Letort V, Mahe P, Cournède P-H, Reffye P de, Courtois B. 2008. Quantitative genetics and functional–structural plant growth models: simulation of quantitative trait loci detection for model parameters and application to potential yield optimization. Annals of Botany 101, 1243–1254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Dubcovsky J. 2008. Wheat FT protein regulates VRN1 transcription through interactions with FDL2. The Plant Journal 55, 543–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobell DB, Schlenker W, Costa-Roberts J. 2011. Climate trends and global crop production since 1980. Science 333, 616–620 [DOI] [PubMed] [Google Scholar]
- Mavromatis T, Boote KJ, Jones JW, Irmak A, Shinde D, Hoogenboom G. 2001. Developing genetic coefficients for crop simulation models with data from crop performance trials. Crop Science 41, 40 [Google Scholar]
- Messina CD, Jones JW, Boote KJ, Vallejos CE. 2006. A gene-based model to simulate soybean development and yield responses to environment. Crop Science 46, 456–466 [Google Scholar]
- Mohler V, Lukman R, Ortiz-Islas S, William M, Worland AJ, van Beem J, Wenzel G. 2004. Genetic and physical mapping of photoperiod insensitive gene Ppd-B1 in common wheat. Euphytica 138, 33–40 [Google Scholar]
- Nakagawa H, Yamagishi J, Miyamoto N, Motoyama M, Yano M, Nemoto K. 2005. Flowering response of rice to photoperiod and temperature: a QTL analysis using a phenological model. Theoretical and Applied Genetics 110, 778–786 [DOI] [PubMed] [Google Scholar]
- Pask AJD, Sylvester-Bradley R, Jamieson PD, Foulkes MJ. 2012. Quantifying how winter wheat crops accumulate and use nitrogen reserves during growth. Field Crops Research 126, 104–118 [Google Scholar]
- Paux E, Faure S, Choulet F, Roger D, et al. 2010. Insertion site-based polymorphism markers open new perspectives for genome saturation and marker-assisted selection in wheat. Plant Biotechnology Journal 8, 196–210 [DOI] [PubMed] [Google Scholar]
- Porter JR, Semenov MA. 2005. Crop responses to climatic variation. Philosophical Transactions of the Royal Society B 360, 2021–2035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quilot B, Génard M, Lescourret F, Kervella J. 2005. Simulating genotypic variation of fruit quality in an advanced peach x Prunus davidiana cross. Journal of Experimental Botany 56, 3071–3081 [DOI] [PubMed] [Google Scholar]
- R Development Core Team. 2013. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing [Google Scholar]
- Reymond M, Muller B, Leonardi A, Charcosset A, Tardieu F. 2003. Combining quantitative trait loci analysis and an ecophysiological model to analyze the genetic variability of the responses of maize leaf growth to temperature and water deficit. Plant Physiology 131, 664–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds M, Foulkes MJ, Slafer GA, Berry P, Parry MAJ, Snape JW, Angus WJ. 2009. Raising yield potential in wheat. Journal of Experimental Botany 60, 1899–1918 [DOI] [PubMed] [Google Scholar]
- Ritchie JR, Otter S. 1985. Description and performance of CERES-Wheat: a user-oriented wheat yield model. ARS - United States Department of Agriculture, Agricultural Research Service 38, 159–175 [Google Scholar]
- Ritchie JT. 1991. Wheat phasic development. In Hanks J, Ritchie JT, eds. Modeling plant and soil systems. Madison, Wisconsin: American Society of Agronomy, Crop Science Society of America, Soil Science Society of America; 31–54 [Google Scholar]
- Robertson MJ, Brooking IR, Ritchie JT. 1996. Temperature response of vernalization in wheat: modelling the effect on the final number of mainstem leaves. Annals of Botany 78, 371–381 [Google Scholar]
- Rousset M, Bonnin I, Remoué C, Falque M, Rhoné B, Veyrieras JB, Madur D, Murigneux A, Balfourier F, Le Gouis J. 2011. Deciphering the genetics of flowering time by an association study on candidate genes in bread wheat (Triticum aestivum L.). Theoretical and Applied Genetics 123, 907–926 [DOI] [PubMed] [Google Scholar]
- Salomé PA, Bomblies K, Laitinen RA, Yant L, Mott R, Weigel D. 2011. Genetic architecture of flowering-time variation in Arabidopsis thaliana. Genetics 188, 421–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenov MA, Shewry PR. 2011. Modelling predicts that heat stress, not drought, will increase vulnerability of wheat in Europe. Scientific Reports 1, 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw LM, Turner AS, Laurie DA. (2012). The impact of photoperiod insensitive Ppd–1a mutations on the photoperiod pathway across the three genomes of hexaploid wheat (Triticum aestivum). The Plant Journal 71, 71–84 [DOI] [PubMed] [Google Scholar]
- Shimada S, Ogawa T, Kitagawa S, Suzuki T, Ikari C, Shitsukawa N, Abe T, Kawahigashi H, Kikuchi R, Handa H. 2009. A genetic network of flowering-time genes in wheat leaves, in which an APETALA1/FRUITFULL-like gene, VRN1, is upstream of FLOWERING LOCUS T. The Plant Journal 58, 668–681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slafer GA, Rawson HM. 1995. Base and optimum temperatures vary with genotype and stage of development in wheat. Plant, Cell and Environment 18, 671–679 [Google Scholar]
- Snape JW, Butterworth K, Whitechurch E, Worland AJ. 2001. Waiting for fine times: genetics of flowering time in wheat. Euphytica 119, 185–190 [Google Scholar]
- Sourdille P, Snape JW, Cadalen T, Charmet G, Nakata N, Bernard S, Bernard M. 2000. Detection of QTLs for heading time and photoperiod response in wheat using a doubled-haploid population. Genome 43, 487–494 [PubMed] [Google Scholar]
- Stekhoven DJ, Bühlmann P. 2012. MissForest—non-parametric missing value imputation for mixed-type data. Bioinformatics 28, 112–118 [DOI] [PubMed] [Google Scholar]
- Tardieu F. 2003. Virtual plants: modelling as a tool for the genomics of tolerance to water deficit. Trends in Plant Science 8, 9–14 [DOI] [PubMed] [Google Scholar]
- Trevaskis B. 2010. Goldacre Paper: The central role of the VERNALIZATION1 gene in the vernalization response of cereals. Functional Plant Biology 37, 479–487 [Google Scholar]
- Trevaskis B, Hemming MN, Dennis ES, Peacock WJ. 2007. The molecular basis of vernalization-induced flowering in cereals. Trends in Plant Science 12, 352–357 [DOI] [PubMed] [Google Scholar]
- Trewavas A. 2006. A brief history of systems biology “Every object that biology studies is a system of systems.” Francois Jacob (1974). The Plant Cell 18, 2420–2430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uptmoor R, Li J, Schrag T, Stützel H. 2011. Prediction of flowering time in Brassica oleracea using a quantitative trait loci-based phenology model. Plant Biology 14, 179–189 [DOI] [PubMed] [Google Scholar]
- Weir AH, Bragg PL, Porter JR, Rayner JH. 1984. A winter wheat crop simulation model without water or nutrient limitations. Journal of Agricultural Science 102, 371–382 [Google Scholar]
- White JW. 2009. Combining ecophysiological models and genomics to decipher the GEM-to-P problem. NJAS-Wageningen Journal of Life Sciences 57, 53–58 [Google Scholar]
- White JW. 2006. From genome to wheat: Emerging opportunities for modelling wheat growth and development. European Journal of Agronomy 25, 79–88 [Google Scholar]
- White JW, Herndl M, Hunt LA, Payne TS, Hoogenboom G. 2008. Simulation-based analysis of effects of and loci on flowering in wheat. Crop Science 48, 678 [Google Scholar]
- White JW, Hoogenboom G. 2003. Gene-based approaches to crop simulation. Agronomy Journal 95, 52–64 [Google Scholar]
- White JW, Hoogenboom G. 1996. Simulating effects of genes for physiological traits in a process-oriented crop model. Agronomy Journal 88, 416–422 [Google Scholar]
- Wilhelm EP, Turner AS, Laurie DA. 2009. Photoperiod insensitive Ppd-A1a mutations in tetraploid wheat (Triticum durum Desf.). Theoretical and Applied Genetics 118, 285–294 [DOI] [PubMed] [Google Scholar]
- Woolfolk CW, Raun WR, Johnson GV, Thomason WE, Mullen RW, Wynn KJ, Freeman KW. 2002. Influence of late-season foliar nitrogen applications on yield and grain nitrogen in winter wheat. Agronomy Journal 94, 429–434 [Google Scholar]
- Worland AJ. 1996. The influence of flowering time genes on environmental adaptability in European wheats. Euphytica 89, 49–57 [Google Scholar]
- Yan L, Fu D, Li C, Blechl A, Tranquilli G, Bonafede M, Sanchez A, Valarik M, Yasuda S, Dubcovsky J. 2006. The wheat and barley vernalization gene VRN3 is an orthologue of FT. Proceedings of the National Academy of Sciences, USA 103, 19581–19586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan L, Helguera M, Kato K, Fukuyama S, Sherman J, Dubcovsky J. 2004. Allelic variation at the VRN-1 promoter region in polyploid wheat. Theoretical and Applied Genetics 109, 1677–1686 [DOI] [PubMed] [Google Scholar]
- Yan L, Loukoianov A, Tranquilli G, Helguera M, Fahima T, Dubcovsky J. 2003. Positional cloning of the wheat vernalization gene VRN1. Proceedings of the National Academy of Sciences, USA 100, 6263–6268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin X, Struik PC. 2010. Modelling the crop: from system dynamics to systems biology. Journal of Experimental Botany 61, 2171–2183 [DOI] [PubMed] [Google Scholar]
- Yin X, Struik PC, Van Eeuwijk FA, Stam P, Tang J. 2005. QTL analysis and QTL-based prediction of flowering phenology in recombinant inbred lines of barley. Journal of Experimental Botany 56, 967–976 [DOI] [PubMed] [Google Scholar]
- Yoshida T, Nishida H, Zhu J, Nitcher R, Distelfeld A, Akashi Y, Kato K, Dubcovsky J. 2010. Vrn-D4 is a vernalization gene located on the centromeric region of chromosome 5D in hexaploid wheat. Theoretical and Applied Genetics 120, 543–552 [DOI] [PubMed] [Google Scholar]
- Zheng B, Biddulph B, Li D, Kuchel H, Chapman S. 2013. Quantification of the effects of VRN1 and Ppd-D1 to predict spring wheat (Triticum aestivum) heading time across diverse environments. Journal of Experimental Botany 64, 3747–3761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng B, Chenu K, Fernanda Dreccer M, Chapman SC. 2012. Breeding for the future: what are the potential impacts of future frost and heat events on sowing and flowering time requirements for Australian bread wheat (Triticum aestivium) varieties? Global Change Biology 18, 2899–2914 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.