Abstract
Purpose.
Chronic inflammation is a key factor contributing to the progression of age-related macular degeneration (AMD). The goals of the current study were to develop an improved mouse model with retinal pathologic features similar to those of AMD and to characterize the immunoreactive cells in the outer retina and choroid during degeneration of the retinal pigment epithelium (RPE).
Methods.
Mice deficient in nuclear erythroid 2-related factor 2 (Nrf2) at 12 months of age were fed a high-fat, cholesterol-rich diet for up to 16 weeks. Ocular phenotype was monitored by optical coherence tomography (OCT) and scanning laser ophthalmoscopy (SLO) in live animals, and was further validated by retinal histopathology. Immunofluorescence staining of either cryosections or RPE flat mounts was used to define immunoreactive cells. Flow cytometry analyses were further performed to define the subsets of intraocular T lymphocytes.
Results.
After 16 weeks on a high-fat (HF) diet, 58% of the eyes from Nrf2−/− mice had progression of retinal lesions. Major histocompatibility complex class II (MHC II)-positive microglia, FoxP3+ regulatory T cells (Tregs), and CD3+ IL-17-producing T cells were detected in either the retina or sub-RPE space. Flow cytometry analyses further revealed that most of the IL-17-producing cells were CD3+ CD4− TCRγδ+ cells.
Conclusions.
The results suggest that the T cell-mediated immune responses played important roles in controlling the progression of AMD-like phenotype in Nrf2-deficient mice.
Keywords: age-related macular degeneration, IL-17, inflammation, T lymphocyte
γδ T lymphocytes infiltrated in degenerating RPE and produced IL-17.
Introduction
The incidence of age-related macular degeneration (AMD) and the prevalence of patients at late stages of the disease increase exponentially with aging.1–4 The majority of AMD patients have the atrophic (dry) form of the disease, which results from degeneration of the RPE and loss of photoreceptor cells in the central retina. The molecular mechanisms of disease progression from initial RPE degeneration to the more severe and vision-threatening conditions of geographic atrophy and choroidal neovascularization (CNV) are largely unknown. Chronic inflammation and immune responses in the outer retina can be key mechanisms that amplify the initial pathologic lesions and accelerate the otherwise linear decline of visual function with age.5,6 Genetic variations in complement gene are major risk factors, and the innate immune responses contribute to wet AMD.7,8 Whether adaptive immune responses play a role in AMD has not been clearly established.
The back of the eye has immune privilege. The RPE not only provides a barrier to shield the retinal antigens from entering systemic circulation, but also actively eliminates infiltrated T cells by Fas-mediated apoptosis9,10 or triggers their anergy.11 However, the immune suppressive functions of the RPE become compromised in AMD; and retinal infiltrating T lymphocytes12,13 and autoantibodies against retinal antigens14 have been identified in AMD patients at different stages. A recent study by Cruz-Guilloty et al.15 reported that CD8+ T cells caused RPE degeneration induced by active immunization with carboxyethylpyrrole (CEP)-modified albumin in Freund's adjuvant. Carboxyethylpyrrole-protein adducts are generated from oxidation of docosahexaenoate (DHA)-containing lipid and are potential biomarkers of AMD.16 Immunizing and boosting mice with CEP-modified albumin resulted in degenerative pathology of the RPE,17 indicating that when the acquired immune responses persist, particularly with adjuvant, activated T lymphocytes can penetrate the blood–retinal barrier and attack cells bearing the immunogenic epitope of DHA oxidation product. In addition to the cytotoxic T cells, IL-17-producing T cells have gained significant interest recently.
Interleukin-17 is a proinflammatory cytokine that plays causal roles in a number of age-related or autoimmune diseases.18,19 Mice deficient in IL-17A were less susceptible to developing experimental autoimmune encephalomyelitis and collagen-induced arthritis,20,21 and had smaller lesions of laser-induced CNV.22 The receptor for IL-17A is a heterodimer composed of IL-17 receptor A and C (RA and RC). The mRNA and protein levels of IL-17 RC were found to be elevated in macular tissues from AMD patients.23,24 The source of IL-17 in the outer retina has not been defined. Th17 cells, a subtype of activated CD4+ T cells, are often deemed the major producers of IL-17. On the other hand, γδ T cells can produce a large amount of IL-17 in tissue-specific pathogen defense and autoimmune responses.25,26
The γδ T cells are characterized by their unique T cell receptor (TCR), a dimer constituted by γ and δ chains. Compared to the more commonly studied αβT cells, γδ T cells respond to antigens with lower specificity but much faster kinetics, with minimal requirement for clonal expansion.25 Activated γδ T cells can produce effector cytokines, such as IL-17 and IFN-γ, independent of TCR ligation, and they form a primary line of defense against invading pathogens.27–34 The unique properties of γδ T cells make them ideal candidates to mediate immune responses in ocular tissue, which is otherwise immune privileged.
A main goal of the current study was to define the identity of IL-17-producing cells in the posterior segment during RPE degeneration. We previously reported that nuclear erythroid 2-related factor 2 (Nrf2) knockout mice had age-dependent retinal pathology.35 In the current study we further optimized the model by feeding the mice a high-fat (HF) diet. Our results showed that the HF diet promoted the development and progression of AMD-like pathology in Nrf2-deficient mice. During RPE degeneration, IL-17-positive T cells were identified as infiltrating the RPE layer. Using combined immunofluroscence staining and flow cytometry approaches, we concluded that tissue-specific γδ T cells were the main source of IL-17 in the outer retina.
Methods
Animals
Protocols for animal breeding, housing, and handling were approved by the University of Texas Medical Branch (UTMB) Institutional Animal Care and Use Committee (IACUC). All procedures were conducted in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. The Nrf2−/− mice of C57BL6/SV129 background have been described previously,36 and were housed at a pathogen-free vivarium of the UTMB Animal Resource Center. Animals were fed normal rodent chow for the first 12 months and were then changed to either HF diet or control diet for up to an additional 4 months before being euthanized. The HF Brelsow Western diet (TestDiet, St. Louis, MO, USA) contained 20% fat and 0.15% cholesterol, while control rodent chow had 5% fat. The absence of rd8 mutation was verified by retinal histopathology, gene-specific PCR amplification,37 and sequencing of the PCR products.
Clinical Examination of Mouse Fundus Pathology
Optical coherence tomography (OCT) and scanning laser ophthalmoscopy (SLO) were performed on a Spectralis spectral-domain (SD)-OCT multimodality imaging system (Heidelberg Engineering, Carlsbad, CA, USA). A 20-diopter (D) lens (Edmund Optics, Inc., Barrington, NJ, USA) was used to adapt for the refraction of rodent eye. Color fundus images and fundus fluorescein angiography were taken by a Micron III mouse fundus imaging system (Phoenix Research Labs, Pleasanton, CA, USA). During image acquisition, animals were anesthetized by isoflurane inhalation from a precision vaporizer (Harvard Apparatus, Holliston, MA, USA), with cornea kept moisturized and pupil fully dilated by Tropicamide Ophthalmic Solution (Bausch & Lomb, Rochester, NY, USA).
Animals were examined by fundus imaging before the start of the HF diet to create a baseline record for each mouse. Optical coherence tomography and SLO images were routinely taken every 4 to 6 weeks. On SLO images, areas of 1 × 1 mm2 centered on the optic disc were used to count the autofluorescent spots. Data for each mouse were maintained and analyzed with Heidelberg Eye Explorer software (Heidelberg Engineering). Images were reviewed retrospectively for lesion grading and progression. A total of 50 mice were included in the in vivo imaging studies, and the details are elaborated in the Table.
Table.
Summary of Phenotypic Findings
|
WT ND |
WT HF |
KO ND |
KO HF |
HF vs. ND, Nrf2−/− |
|
| Total eyes, n | 28 | 36 | 34 | 64 | |
| OCT | |||||
| n | 18 | 18 | 26 | 38 | |
| Subretinal drusenoid deposits | 1 | 3 | 9 | 22 | *P < 0.05 |
| Lesion progression | 1 | 0 | 2 | 12 | *P < 0.05 |
| Fundus examination | |||||
| n | 8 | 6 | 12 | 16 | |
| RPE mottling | 2 | 4 | 7 | 14 | |
| Drusen-like deposits | 1 | 5 | 8 | 13 | |
| Patchy lesions | 1 | 2 | 7 | 12 | |
| Histology | |||||
| n | 7 | 10 | 8 | 20 | |
| Pigmentary changes | 2 | 8 | 6 | 18 | |
| Vacuoles | 1 | 6 | 5 | 19 | |
| Drusen-like deposits | 0 | 1 | 5 | 10 | |
| CNV | 0 | 0 | 1 | 3 | |
WT, wild type; ND, normal diet; KO, knockout.
χ2 test between Nrf2 knockout mice on either control or high-fat diet.
Histology and Immunofluorescence Staining
For paraffin sections, eyes from euthanized mice in each experimental group were enucleated and postfixed in 4% paraformaldehyde for 24 hours before processing and embedding. For each eye, approximately 250 sagittal sections of 4-μm thickness were cut from cornea to the optic nerve and stained with either hematoxylin and eosin (H&E) or periodic acid–Schiff (PAS). Every section was reviewed for RPE and retina pathology; and 10 slides from the perioptic nerve area spanning a distance of approximately 200 μm were picked from every eye for pathology scoring and quantification of subretinal/sub-RPE cell infiltration.
A pathology scoring system with a scale of 0 to 4, similar to the one reported by Cruz-Guilloty et al.,15 was used to document the progression of histology findings (Supplementary Table S1). For subretinal cell infiltration, only cells located underneath the photoreceptor outer segment in direct contact with the apical side of the RPE were included for quantification. Cells within a distance of 60 μm from the Bruch's membrane (BrM) were quantified for sub-RPE cellularity. Endothelial cells with a spindle or crescent shape were excluded.
For cryosections, mice were terminally anesthetized and subjected to whole-body perfusion with 4% paraformaldehyde (PFA) in phosphate-buffered saline (PBS). Enucleated eyes were postfixed in the same fixative overnight before being embedded in Tissue-TeK Cryomold (Electron Microscopy Sciences, Hatfield, PA, USA). Sagittal cryosections of 8-μm thickness were prepared from cornea to optic nerve and stained for various antigens of interest. To block nonspecific binding, tissue sections were incubated with normal serum appropriate to the species of secondary antibodies diluted in 0.5% Triton X-100/PBS. They were then incubated with primary antibodies followed by Alexa Fluor-conjugated secondary antibodies (Life Technologies, Grand Island, NY, USA).
For RPE flat mounts, enucleated eyeballs were briefly fixed in 4% PFA for 30 minutes before dissection. After separating the retina and RPE, tissues were further fixed in the same fixative for 3 hours before blocking with 5% goat serum/0.1% Triton X-100/1× PBS overnight at 4°C. Primary antibodies were diluted with blocking buffer and incubated overnight at 4°C, followed by appropriate secondary antibodies. Nuclei were stained by Hoechst 33342 (Life Technologies), and tissues were mounted with mounting medium (Electron Microscopy Sciences). Alexa Fluor 647 phalloidin (Life Technologies) was used to delineate boundaries of the RPE. Fluorescence images were acquired by confocal microscopy (LSM510; Carl Zeiss, Thornwood, NY, USA). Detailed information on primary antibodies is presented in Supplementary Table S2.
Flow Cytometry Analyses of Lymphocytes in the Posterior Segment
Lymphocytes were enriched by discontinuous gradient centrifugation.38 Briefly, eyes were enucleated and dissected to discard the anterior segment and the lens. Retina and RPE–choroid complex were isolated and digested with 0.05% collagenase D (Roche Applied Science, Indianapolis, IN, USA) in RPMI 1640 medium. Tissues were incubated at 37°C for 30 minutes and were dislodged into single cell suspension by repeated pipetting. After passing through a 40-μm nylon cell strainer, cells were collected by centrifugation at 300g for 3 minutes. The cell pellet was resuspended in 30% Percoll/RPMI 1640 solution and laid over a 30%/70% discontinuous Percoll gradient (Sigma-Aldrich Corp., St. Louis, MO, USA), then centrifuged at 400g for 30 minutes. The enriched lymphocytes were visualized as a single band located at the interphase of the lower one-third of the gradient and were carefully collected for further analyses.
Staining for cell surface markers and intracellular antigens was performed according to our previously published methods.38,39 The enriched lymphocytes were first treated for 4 hours with phorbol 12-myristate 13-acetate (50 ng/mL) and ionomycin (750 ng/mL) in the presence of GolgiStop (BD Bioscience, San Jose, CA, USA). Cells were then collected and blocked with FcγR blocker (anti-mouse CD16/32; eBioscience, San Diego, CA, USA) and stained for specific surface molecules. Following surface staining, cells were processed with a fixation/permeabilization kit (eBioscience) and stained for intracellular cytokines. After stringent washes, samples were analyzed on an LSRII FACSFortessa flow cytometer (Becton Dickinson, San Jose, CA, USA), and the data were processed with FlowJo software (TreeStar, Ashland, OR, USA).
RT-PCR Analyses of γδ T Cell Subset
Mouse lymphocytes were enriched from either RPE/choroid preparations or spleen as described above. Total RNA was extracted with Trizol reagent (Life Technologies) and reverse transcribed for PCR amplification with subset-specific primers.40,41 The primers used were Vγ1, 5′-CCGGCAAAAAGCAAAAAAGT-3′ and 5′-AAGGAGACAAAGGTAGGTCCCAGC-3′; Vγ2, 5′-TTGGTACCGGCAAAAAACAAATCA-3′ and 5′-CAATACACCCTTATGACATCG-3′; Vγ4, 5′-CTTGCAACCCCTACCCATAT-3′; Vγ5, 5′-GAGGATCCCGCTTGGAAATGGAT GAGA-3′; Vγ6, 5′-GATCCAAGAGGAAAGGAAAGACGGC-3′; Vγ7, GATCCAACTTCGTCAGTTCCACAAC-3′. The reverse primer for Vγ4 to Vγ7 was 5′- CCACCACTCGTTTCTTTAGG-3′. Polymerase chain reaction products were analyzed by 1% agarose gel electrophoresis and imaged with an Ultralum Omega fluorescence gel analysis system (Biovision Technologies, Claremont, CA, USA).
Results
Accelerated RPE Degeneration in Nrf2−/− Mice Fed High-Fat, Cholesterol-Rich Diet
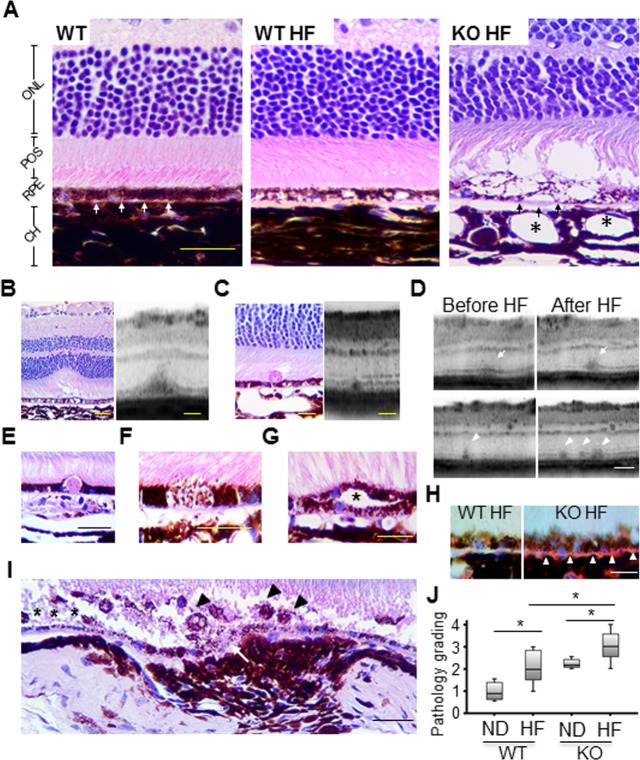
Age-dependent RPE pathology occurred in Nrf2 knockout mice around 12 months, which is approximately 50% of their expected life span.35 To further optimize this in vivo model of RPE aging and degeneration, we fed the 12-month-old Nrf2−/− mice the HF diet and monitored the progression of their ocular pathology for additional 4 months. At 16 months, Nrf2−/− mice on regular rodent chow showed RPE hypertrophy, vacuolation, and pigmentary changes, which are consistent with our previous observations.35 The HF diet led to deposition of Oil Red O-positive particles in samples from both wild-type and Nrf2−/− mice (Fig. 1H). Wild-type mice on the HF diet showed moderate RPE pathology such as vacuoles and pigmentary changes (Fig. 1A). In Nrf2−/− mice on the HF diet, histology and OCT examinations identified severe RPE and retinal pathology, including RPE hyper- and hypopigmentation (Fig. 1F), RPE hyperplasia (Fig. 1G), and occasionally eosinophilic, PAS-positive deposits in sub-RPE (Fig. 1E) and subretinal space (Figs. 1B, 1C). Choroidal neovascularization was found in 3 of the 20 eyes examined in the Nrf2−/− HF group, but not in the wild-type HF group. The vascular lesions presented a hypercellular structure with RPE proliferation and infiltration of melanin-ingested macrophages and cell debris (Fig. 1I), similar to the structure of CNV membranes excised from AMD eyes.42,43 The retinal pathology data are summarized in the Table. Overall, the HF-fed Nrf2−/− mice had the most significant RPE pathology; and both wild-type and knockout mice treated with the HF diet had an exacerbated RPE pathology as compared to their normal chow–fed counterparts (Fig. 1J). Other pathologic features related to AMD, such as increased RPE autofluorescent granules, deposition of complement protein, and accumulation of oxidative-damaged protein in the RPE, were also observed in Nrf2−/− mice (Supplementary Figs. S1–S3).
Figure 1.
Accelerated RPE degeneration in Nrf2−/− mice fed HF diet at advanced age. (A) Representative histology section from a 16-month-old wild-type mouse, showing normal outer retinal structure with intact RPE and BrM (white arrows). RPE vacuoles and pigmentary changes were observed in an age-matched wild-type mouse on HF diet, whereas photoreceptor layer was spared. Severe degenerative changes were observed in HF diet–treated Nrf2−/− mouse with large area of RPE lysis, thickening of BrM (black arrows), dilation of choroidal vessels (asterisks), and loss of photoreceptors. Stage 2 (C) and stage 3 (B) SDDs were found on both OCT and histology sections in HF Nrf2−/− mice. (D) Follow-up OCT scans of the same Nrf2−/− mouse before and after HF diet, showing lesion progression in both size (arrows) and number (arrowheads). Other AMD-like pathologies included PAS-positive deposit (E), RPE hypopigmentation (F), and double-layer RPE proliferation (asterisk in [G]) in the knockout HF group. (H) Oil Red O-stained lipid droplets were accumulated in the BrM area in knockout HF mice, whereas only little staining was observed in wild-type counterparts. (I) CNV lesion was observed in one Nrf2−/− HF mouse, which showed discontinuous BrM (arrow), RPE loss (asterisk), and subretinal cell infiltration (arrowheads). (J) Histology grading on paraffin sections showed that HF diet aggravated the retinal pathology in both wild-type and Nrf2−/− mice, and that Nrf2−/− HF mice had the most significant RPE pathology among all four groups (*P < 0.05, one-way ANOVA and Bonferroni post hoc test). Scale bars: 100 μm (A); 50 μm (B–H).
Subretinal drusen-like deposit (SDD) was recently reported as closely correlated to different stages of RPE degeneration in AMD patients.44–46 In addition to histopathology findings, OCT scan identified that 58% (22 out of 38) eyes in the HF Nrf2−/− group developed SDD, versus 35% (9 out of 26 eyes) in normal chow–fed Nrf2−/− mice (Table, P < 0.05, χ2 test). By analyzing the OCT data from the same animal at the same region of the retina (Fig. 1D), progression in both size and number of SDD lesions was noted in Nrf2 knockout mice. The HF diet increased the risk of progression by 5.5-fold (95% confidence interval ranging from 1.1 to 27.4) (Table). Subretinal drusen-like deposits of the Nrf2−/− model were highly similar to various-stage SDDs as reported in previous human clinical studies.47 Thus, by combining aging, oxidative stress, and dietary manipulation, we have further optimized our animal model and achieved markedly accelerated RPE degeneration in Nrf2-deficient mice at an advanced age.
Subretinal Accumulation of Microglia/Macrophages
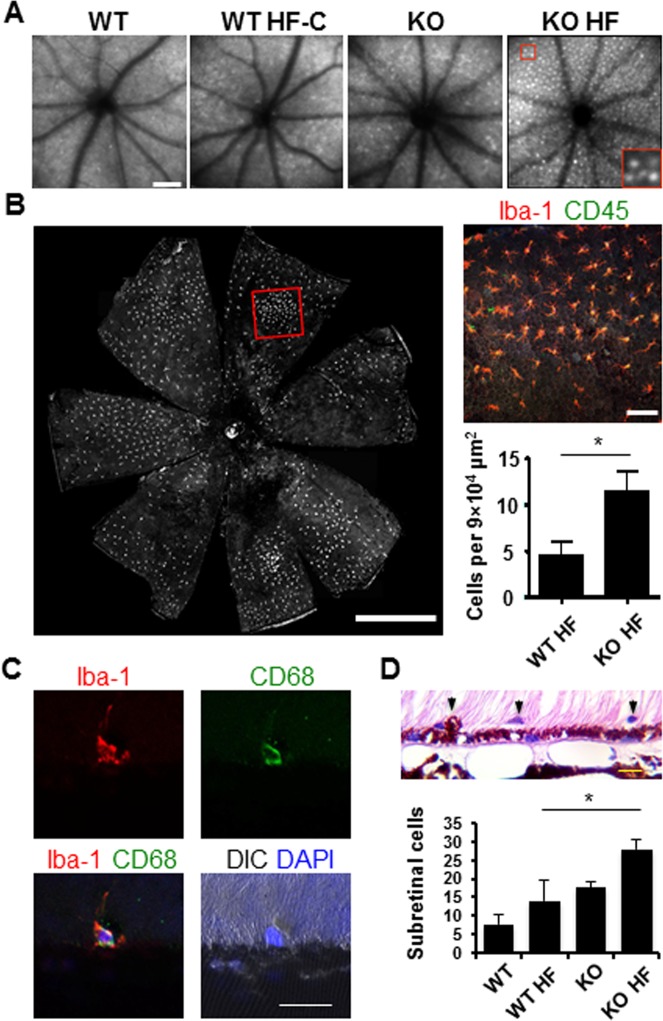
Under SLO, a significant increase of autofluorescent spots was detected in Nrf2−/− mice after the HF diet (Fig. 2A) (67 ± 11.4 spots/graph) as compared to knockout mice on normal chow (35 ± 6.4 spots/graph, P < 0.001, Student's t-test). Because the imaging focal plane was on the RPE, the RPE autofluorescence could contribute to the signals. On the other hand, in human AMD eyes, retinal microglia and infiltrated macrophages are frequently found at the sites of lesion.42,48 The dome shape along with the lattice distribution of the autofluorescent spots on SLO was suggestive of subretinal lipid-ingested microglia/macrophages.49,50 To test this possibility, we stained retinal and RPE whole mounts with microglial/macrophage markers Iba-1 and CD45.51,52 The number of double-positive cells in the subretinal space was significantly higher in Nrf2−/− mice on the HF diet (Fig. 2B) compared to HF wild-type mice. The result was also confirmed by the histology approach, which identified significantly more subretinal cell infiltration in Nrf2 knockouts on the HF diet (Fig. 2D).
Figure 2.
Fundus autofluorescence and subretinal microglia accumulation in Nrf2−/− mice on HF diet. (A) SLO images showing fundus autofluorescence in mice fed either control or HF diet. Digitally magnified area shown in the red box. (B) Immunofluorescent staining of Iba-1 and CD45 on RPE/choroid flat mount prepared from Nrf2−/− mouse on HF diet. Boxed area in (B) is magnified on the right. (C) Immunostaining of cryosections, showing Iba-1 and CD68 double-positive microglia in the subretinal space. (D) Quantification of subretinal cells on histology sections (*P < 0.05, one-way ANOVA and Bonferroni post hoc test). Scale bars: 200 μm (A); 1000 μm (B); 20 μm (C, D).
Further scrutiny of these subretinal cells in Nrf2−/− HF mice revealed heterogeneous morphology varying from those with thin ramified processes to those in ameboid shapes, representing microglia at different functional stages (Fig. 2B, inset). Moreover, CD68 (also known as ED1), a lysosomal membrane protein in myeloid cells, was found in some of the subretinal microglia/macrophages (Fig. 2C), indicating their activation with increased phagocytic activity.53,54
IL-17-Producing γδ T Cells in Posterior Segment
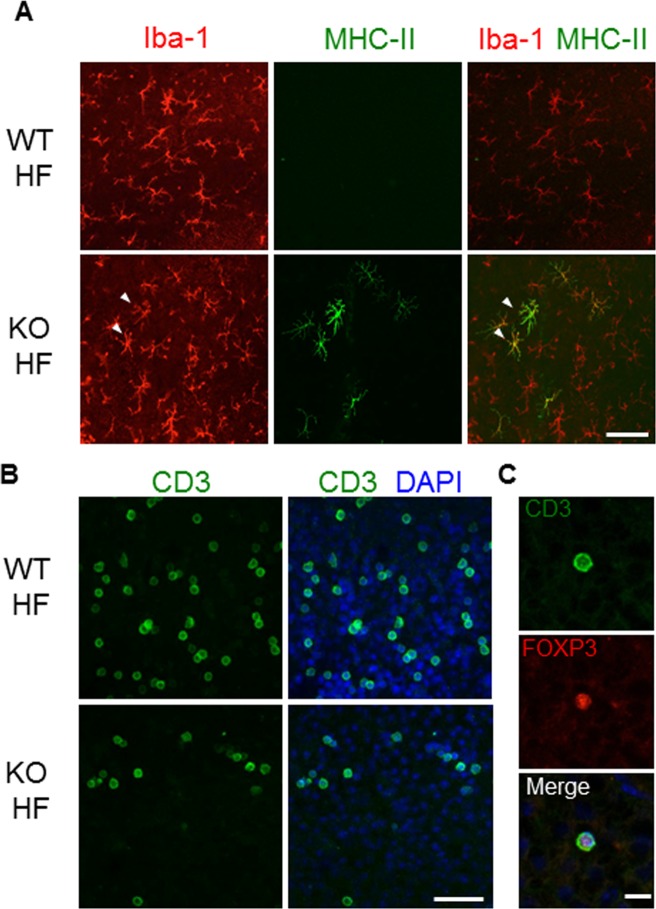
Increased expression of major histocompatibility complex class II (MHC II) molecule on residential retinal microglia has been reported to occur in aging retina as well as in AMD eyes.48,55,56 In the retina of HF diet–treated mice, MHC II+ microglial cells were detected exclusively in the Nrf2−/− mice (Fig. 3A). The presence of antigen-presenting microglia prompted us to search for other immunocompetent cells in the eye. By staining retinal flat mounts, CD3+ lymphocytes were detected in HF diet–treated mice, both wild type and Nrf2 deficient (Fig. 3B). The CD3+ cells displayed large round nuclei and scanty cytoplasm, with an average diameter of 12.9 ± 2.1 μm. Most of the retinal T cells were located in the outer plexiform layer of the retina (data not shown). Further characterization of these cells revealed a subset of forkhead box P3 (FoxP3) and CD3 double-positive lymphocytes (Fig. 3C), indicating the presence of regulatory T cells (Treg). CD3+ lymphocytes were barely detectable in the retina of mice on normal chow.
Figure 3.
Activated retinal microglia and lymphocyte infiltration in Nrf2−/− mice on HF diet. (A) Immunofluorescent staining of Iba-1 and MHC II on retina flat mounts prepared from either wild-type or Nrf2−/− mouse on HF diet. MHC II-positive microglia (arrowheads) were found exclusively in retina of Nrf2−/− mice. (B) CD3 staining of retina flat mounts. T lymphocytes were found in retina of both wild-type and knockout mice on the HF diet. (C) CD3 and FoxP3 double staining of retina flat mount for identifying Tregs. Scale bars: 100 μm (A, B); 20 μm (C).
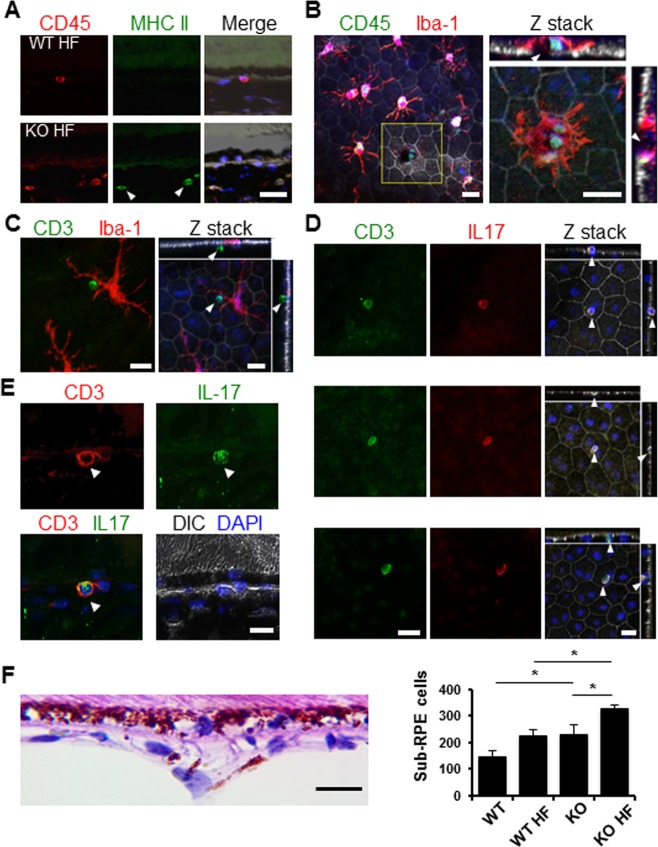
During the histopathologic examination of paraffin sections, we frequently spotted cells in the choriocapillaris underneath the BrM (Figs. 4F, 1F). By quantifying the cells within 60-μm distance from the BrM, we found a significant increase of sub-RPE cellularity in eyes from HF diet–treated Nrf2−/− mice (Fig. 4F). On cryosections, we detected CD3+ lymphocytes in the sub-RPE space, often aggregated near RPE cells with signs of degeneration (Fig. 4E). By immunostaining of RPE flat mounts and three-dimensional reconstruction of confocal optic sections, we identified CD3+ cells intercalating in the RPE layer at different depth (Fig. 4D). Some of the infiltrated lymphocytes were close to retinal microglia, suggesting possible antigen presentation to T cells (Figs. 4A–C). Furthermore, in Nrf2 knockout mice on the HF diet, some of the sub-RPE lymphocytes were positively stained for IL-17 (Figs. 4D, 4E).
Figure 4.
Infiltration of IL-17-producing lymphocytes in the RPE/choroid complex of Nrf2-deficient mice on the HF diet. (A) Immunostaining of cryosections for MHC II and CD45. CD45 and MHC II double-positive cells (arrowheads) were mostly observed in Nrf2 knockout mice. (B–D) Immunostaining of RPE flat mounts from Nrf2−/− mice for lymphocyte markers. (B) CD45+ cells observed close to Iba-1+ microglia. Reconstruction of Z stack of the yellow boxed area showed CD45+ cells near the RPE defect as indicated by the discontinued tight junction (arrowheads in sidebars), surrounded by a microglia cell (red signal). (C) Adjacent CD3+ lymphocytes (arrowheads) and Iba-1+ microglia (red) separated by the RPE. (D, E) CD3 and IL-17 staining of RPE flat mount and cryosections, respectively. IL-17-producing lymphocytes (arrowheads) were identified on apical, in middle, or on basal side of the RPE layer (D). (F) Quantification of sub-RPE cells within 60-μm distance to the BrM on histology slides, showing significant increase of cells in knockout HF group compared to either normal chow–fed Nrf2−/− mice or wild-type mice on HF diet (*P < 0.05, one-way ANOVA and Bonferroni post hoc test). Scale bars: 20 μm.
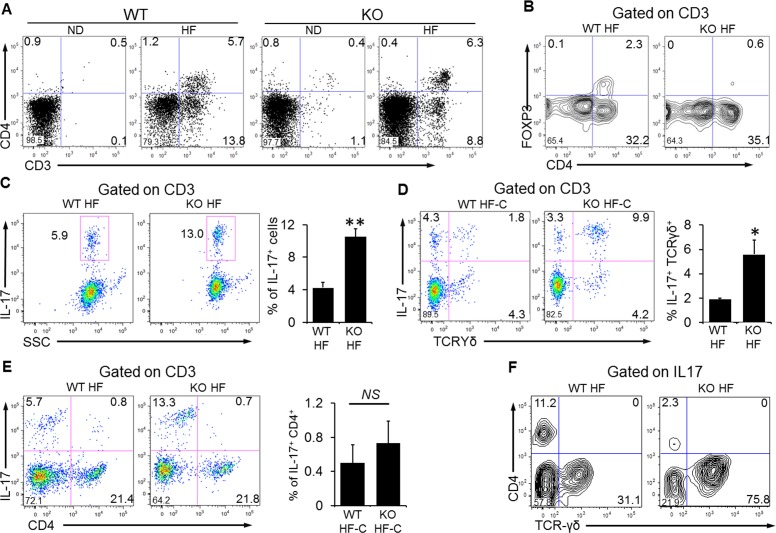
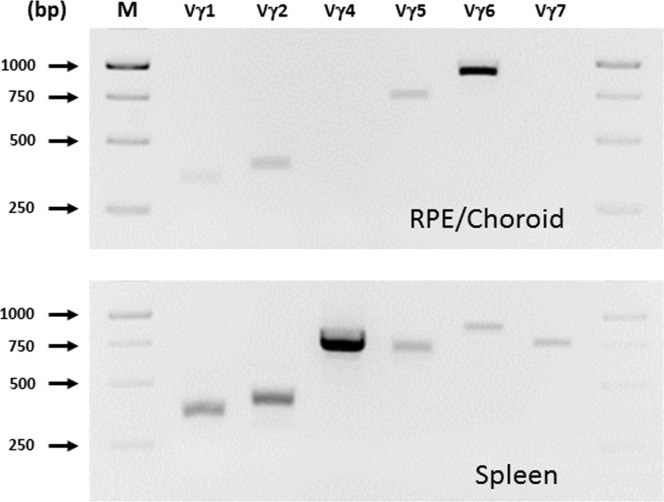
To provide an independent measure of infiltrated ocular lymphocytes, we next performed flow cytometry analyses of these cells. Consistent with our findings on tissue immunostaining, CD3+ lymphocytes were noted in both wild-type and Nrf2 knockout mice fed the HF diet (Fig. 5A). Among those cells there were two subpopulations, CD3+ CD4− cells and CD3+ CD4+ cells (8.8% and 6.3%, respectively, in HF Nrf2−/− mice). However, those two groups of cells were either diminished or absent in eyes from Nrf2−/− mice on the normal diet (1.1% and 0.4%, respectively). CD4+ FoxP3+ Tregs were identified in both wild-type and Nrf2 knockout mice on the HF diet (Fig. 5B), and the percentage of Tregs in the total CD3+ population was higher in wild-type mice. In contrast, the number of IL-17-producing lymphocytes was significantly higher in Nrf2−/− mice (Fig. 5C, 10.5% ± 0.9 vs. 4.1% ± 0.6, mean ± SEM, P < 0.01, Student's t-test). Further analyses revealed that the majority of IL-17 producers were γδ T cells (Fig. 5F); and there was a significant increase of IL-17+ and TCRγδ+ cells in HF-fed Nrf2−/− mice (Fig. 5D) (5.6 ± 1.5 vs. 1.9 ± 0.1, mean ± SEM, P < 0.05, Student's t-test). The number of Th17 cells (CD4+ IL-17+) was minimal in the eyes from either wild-type or Nrf2−/− mice (Fig. 5E). Thus, γδ T cells are the major source of IL-17 production in the degenerating retina/RPE. We further characterized the subsets of γδ T cells in the RPE/choroid in HF diet–fed Nrf2−/− mice. As shown in Figure 6, the profile was different between spleen and RPE/choroid, and Vγ6 was the major subset in the eye.
Figure 5.
Flow cytometry analyses of retinal and choroidal lymphocytes. (A) CD3+ and CD3+/CD4+ lymphocytes in both wild-type and knockout mice fed the HF diet. (B) CD4+/FoxP3+ Tregs in wild-type and knockout mice on the HF diet. (C) IL-17-producing T cells (CD3+) in wild-type and knockout mice on the HF diet. (D) Increased percentage of IL17+ TCRγδ+ cells in Nrf2−/− mice on the HF diet. (E) No significant difference in Th17 cells (CD3+ IL-17+) between two strains of mice on the HF diet. (F) Percentage of TCRγδ+ cells in IL-17+ T cell population. The majority of IL-17-producing cells in Nrf2−/− HF mice were TCR-γδ+. Data presented are representative of four to six separate experiments (mean ± SEM, *P < 0.05; **P < 0.01, Student's t-test). Each plot contains pooled intraocular lymphocytes from three animals.
Figure 6.
Characterization of γδT cell subset of intraocular lymphocytes. Enriched lymphocytes from RPE/choroid or spleen were used for RNA extraction and RT-PCR analyses. PCR products were analyzed by agarose gel electrophoresis. bp, base pair; M, 100-bp molecular weight marker.
Discussion
We previously reported that mice deficient in Nrf2 developed age-dependent RPE degeneration.35 In the present study we showed that Nrf2−/− mice fed high-fat, cholesterol-rich chow displayed more severe RPE pathology and accelerated progression of retinal lesions (Fig. 1). By monitoring the subretinal drusenoid deposits with SD-OCT, accelerated disease progression was observed in 58% of eyes (Supplementary Table S1) in mice aged between 12 and 16 months. The pathologic features of the retinal and RPE lesions highly resemble those of human AMD.45–47 Thus, with noninvasive imaging technique and dietary control, we were able to show the dynamics of the disease progression within a narrowed time frame. While it should be kept in mind that rodents do not have macula and that the clinical manifestations of human AMD are not limited to the pathologic features presented by the Nrf2-deficient mice, this improved animal model of age-dependent RPE degeneration can provide a new tool for future interventional studies designed to control the disease progression in early AMD.
Strategies that increase dietary fat intake and/or disruption of lipid metabolism have often been employed to produce animal models related to AMD.57–63 In Nrf2−/− mice on the HF diet, RPE cells were positively stained by Oil Red O (Fig. 1H) and showed signs of degeneration such as increased vacuolation, disturbance of pigment, sub-RPE deposit formation, and RPE atrophy (Figs. 1A–F). It is likely that the chronic oxidative stress in Nrf2−/− mice had additive effects with the hyperlipidemia, which changed the lipid composition of the BrM and retina. Although the underlying mechanisms remain to be explored, they are likely related to the increased lipid peroxidation products, such as 4-hydroxynonenal (4-HNE) and malondialdehyde (MDA) equivalents,64,65 that are implicated in the etiology of AMD.66,67
Activation of multiple lines of immune cells within the otherwise immunoprivileged intraocular environment (Figs. 3, 4) was observed in HF diet–fed Nrf2−/− mice. Infiltrated T cells were detected in areas with RPE degeneration (Fig. 4B); and microglia activation and transition to antigen-presenting cells can be a key immune modulating event (Fig. 4B). Intraocular microglia can be divided into three populations: perivascular, parenchymal, and subretinal. Previous studies by Xu et al.68 and Luhmann et al.69 demonstrated that during aging and pathologic conditions in the outer retina, the parenchymal microglia migrated into the subretinal space to facilitate the removal of subretinal debris derived from shedding photoreceptor outer segments. Ingestion of these lipid-rich components rendered the microglia autofluorescent. The findings are consistent with our data showing the dome-shape look and autofluorescent cells on SLO (Fig. 2A). The subretinal microglia in HF-fed Nrf2−/− mice can have in situ effects to promote the formation of SDD and drusen70 and may secrete complement regulatory proteins, such as complement factor H (CFH) and complement factor B (CFB), which have been described in human SDD.71,72
In this study, we observed a population of Iba-1+ microglia that expressed MHC II exclusively in the retina of HF diet–fed Nrf2−/− mice (Fig. 3). In light of the finding that aberrant intraocular lymphocyte infiltration was discovered in this model, it is reasonable to suggest that the appearance of these cells was involved in T cell recruitment and activation. Under physiological conditions, retinal microglia are MHC IIlow and are immunosuppressive with limited function to active T lymphocytes.3,74 However, under extensive aging56 or disease conditions, MHC IIhi microglia are observed in rodent retina correlating to RPE structural changes.75 These cells are capable of activating T lymphocytes and are necessary for developing autoimmunity in the eye.73,76 The origin of these cells still remains elusive. While our current study is in favor of activated residential microglia,77,78 it can also be infiltrating macrophages from the breakdown of the blood–retinal barrier.75
One of the novel findings of our study is the discovery of γδ T cells in the sub-RPE space during age-dependent RPE degeneration (Fig. 4). The retina and choroid do not have well-developed lymphatic drainage.79,80 Conventional clonal expansion of αβT cells in local lymph nodes may have only limited efficiency during adaptive immune response of the outer retina and choroid. The tissue-specific γδ T cells can effectively recognize a wide range of antigens and respond quickly in a way similar to the innate immune cells. Upon pathogen infection, γδ T cells produce a large amount of effector cytokines including IL-17.27–34 Similarly, when RPE cells degenerate, they may release either antigens or cytokines that can activate γδ T cells. Future studies will be needed to further determine how the local microenvironment and cytokines produced by retinal cells control the differentiation of T cell lineages. In mice, IL-17+ γδ T cells are restricted to the Vγ4 and Vγ6 subsets, and Vγ6 was confirmed in the Nrf2 model (Fig. 6). It should be noted that the approach of staining RPE flat mounts gives an underestimate of infiltrating T cells, as the RPE pigments interfere with the fluorescence imaging. And our flow cytometry analyses used pooled samples and did not distinguish between retinal and choroidal lymphocytes. Consistent with our findings, in laser-induced CNV, γδ T cells were recruited to the site of laser lesion and were responsible for the production of IL-17.22
Increased IL-17 production and upregulation of its receptor have been reported in macular tissue prepared from human AMD eyes.23,24 Interleukin-17 was cytotoxic to cultured RPE cells.24,81 A recent study by Ardeljan and colleagues24 showed that overexpression of a soluble decoy receptor of IL-17 prevented retinal and RPE degeneration in Ccl2 and Cx3cr1 double-knockout mice with Crb1rd8mutation. The results from their preclinical study suggest that intervening IL-17-mediated signaling pathways can be a novel therapeutic approach to treat retinal and/or RPE diseases including AMD.
In summary, in the current study we have developed an improved model of age-dependent RPE degeneration with pathologic features similar to those of human AMD. We have identified the activation and infiltration of multiple lines of immunoreactive cells in the degenerating retina. Lymphocytes infiltrated in the sub-RPE space were mainly γδ T cells, which produced IL-17 as a proinflammatory signal. Deciphering mechanisms bridging between innate and acquired immune responses elicited by RPE degeneration can reveal new insights into the pathobiology of AMD.
Acknowledgments
Supported by National Institutes of Health Grants EY 021937, EY 019706, and AI 109100; BrightFocus Foundation; International Retina Research Foundation; and Ted Nash Long Life Foundation.
Disclosure: Z. Zhao, None; P. Xu, None; Z. Jie, None; Y. Zuo, None; B. Yu, None; L. Soong, None; J. Sun, None; Y. Chen, None; J. Cai, None
References
- 1. Owen CG, Jarrar Z, Wormald R, Cook DG, Fletcher AE, Rudnicka AR. The estimated prevalence and incidence of late stage age related macular degeneration in the UK. Br J Ophthalmol. 2012; 96: 752–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pauleikhoff D, Koch JM. Prevalence of age-related macular degeneration. Curr Opin Ophthalmol. 1995; 6: 51–56 [DOI] [PubMed] [Google Scholar]
- 3. Klein R. Overview of progress in the epidemiology of age-related macular degeneration. Ophthalmic Epidemiol. 2007; 14: 184–187 [DOI] [PubMed] [Google Scholar]
- 4. Klein R, Peto T, Bird A, Vannewkirk MR. The epidemiology of age-related macular degeneration. Am J Ophthalmol. 2004; 137: 486–495 [DOI] [PubMed] [Google Scholar]
- 5. Donoso LA, Kim D, Frost A, Callahan A, Hageman G. The role of inflammation in the pathogenesis of age-related macular degeneration. Surv Ophthalmol. 2006; 51: 137–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jager RD, Mieler WF, Miller JW. Age-related macular degeneration. N Engl J Med. 2008; 358: 2606–2617 [DOI] [PubMed] [Google Scholar]
- 7. Ambati J, Atkinson JP, Gelfand BD. Immunology of age-related macular degeneration. Nat Rev Immunol. 2013; 13: 438–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nussenblatt RB, Liu B, Li Z. Age-related macular degeneration: an immunologically driven disease. Curr Opin Investig Drugs. 2009; 10: 434–442 [PubMed] [Google Scholar]
- 9. Jorgensen A, Wiencke AK, la Cour M, et al. Human retinal pigment epithelial cell-induced apoptosis in activated T cells. Invest Ophthalmol Vis Sci. 1998; 39: 1590–1599 [PubMed] [Google Scholar]
- 10. Griffith TS, Brunner T, Fletcher SM, Green DR, Ferguson TA. Fas ligand-induced apoptosis as a mechanism of immune privilege. Science. 1995; 270: 1189–1192 [DOI] [PubMed] [Google Scholar]
- 11. Gregerson DS, Heuss ND, Lew KL, McPherson SW, Ferrington DA. Interaction of retinal pigmented epithelial cells and CD4 T cells leads to T-cell anergy. Invest Ophthalmol Vis Sci. 2007; 48: 4654–4663 [DOI] [PubMed] [Google Scholar]
- 12. Ezzat MK, Hann CR, Vuk-Pavlovic S, Pulido JS. Immune cells in the human choroid. Br J Ophthalmol. 2008; 92: 976–980 [DOI] [PubMed] [Google Scholar]
- 13. Penfold P, Killingsworth M, Sarks S. An ultrastructural study of the role of leucocytes and fibroblasts in the breakdown of Bruch's membrane. Aust J Ophthalmol. 1984; 12: 23–31 [PubMed] [Google Scholar]
- 14. Patel N, Ohbayashi M, Nugent AK, et al. Circulating anti-retinal antibodies as immune markers in age-related macular degeneration. Immunology. 2005; 115: 422–430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cruz-Guilloty F, Saeed AM, Duffort S, et al. T cells and macrophages responding to oxidative damage cooperate in pathogenesis of a mouse model of age-related macular degeneration. PLoS One. 2014; 9: e88201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gu X, Meer SG, Miyagi M, et al. Carboxyethylpyrrole protein adducts and autoantibodies, biomarkers for age-related macular degeneration. J Biol Chem. 2003; 278: 42027–42035 [DOI] [PubMed] [Google Scholar]
- 17. Hollyfield JG, Bonilha VL, Rayborn ME, et al. Oxidative damage-induced inflammation initiates age-related macular degeneration. Nat Med. 2008; 14: 194–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Miossec P, Kolls JK. Targeting IL-17 and TH17 cells in chronic inflammation. Nat Rev Drug Discov. 2012; 11: 763–776 [DOI] [PubMed] [Google Scholar]
- 19. Iwakura Y, Ishigame H, Saijo S, Nakae S. Functional specialization of interleukin-17 family members. Immunity. 2011; 34: 149–162 [DOI] [PubMed] [Google Scholar]
- 20. Yang XO, Chang SH, Park H, et al. Regulation of inflammatory responses by IL-17F. J Exp Med. 2008; 205: 1063–1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ishigame H, Kakuta S, Nagai T, et al. Differential roles of interleukin-17A and -17F in host defense against mucoepithelial bacterial infection and allergic responses. Immunity. 2009; 30: 108–119 [DOI] [PubMed] [Google Scholar]
- 22. Hasegawa E, Sonoda KH, Shichita T, et al. IL-23-independent induction of IL-17 from gammadeltaT cells and innate lymphoid cells promotes experimental intraocular neovascularization. J Immunol. 2013; 190: 1778–1787 [DOI] [PubMed] [Google Scholar]
- 23. Wei L, Liu B, Tuo J, et al. Hypomethylation of the IL17RC promoter associates with age-related macular degeneration. Cell Rep. 2012; 2: 1151–1158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ardeljan D, Wang Y, Park S, et al. Interleukin-17 retinotoxicity is prevented by gene transfer of a soluble interleukin-17 receptor acting as a cytokine blocker: implications for age-related macular degeneration. PLoS One. 2014; 9: e95900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Vantourout P, Hayday A. Six-of-the-best: unique contributions of gammadelta T cells to immunology. Nat Rev Immunol. 2013; 13: 88–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bonneville M, O'Brien RL, Born WK. Gammadelta T cell effector functions: a blend of innate programming and acquired plasticity. Nat Rev Immunol. 2010; 10: 467–478 [DOI] [PubMed] [Google Scholar]
- 27. Cui Y, Shao H, Lan C, et al. Major role of gamma delta T cells in the generation of IL-17+ uveitogenic T cells. J Immunol. 2009; 183: 560–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Roark CL, French JD, Taylor MA, Bendele AM, Born WK, O'Brien RL. Exacerbation of collagen-induced arthritis by oligoclonal, IL-17-producing gamma delta T cells. J Immunol. 2007; 179: 5576–5583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Blink SE, Miller SD. The contribution of gammadelta T cells to the pathogenesis of EAE and MS. Curr Mol Med. 2009; 9: 15–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hamada S, Umemura M, Shiono T, et al. IL-17A Produced by gammadelta T cells plays a critical role in innate immunity against Listeria monocytogenes infection in the liver. J Immunol. 2008; 181: 3456–3463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Shibata K, Yamada H, Hara H, Kishihara K, Yoshikai Y. Resident Vdelta1+ gammadelta T cells control early infiltration of neutrophils after Escherichia coli infection via IL-17 production. J Immunol. 2007; 178: 4466–4472 [DOI] [PubMed] [Google Scholar]
- 32. Lockhart E, Green AM, Flynn JL. IL-17 production is dominated by gammadelta T cells rather than CD4 T cells during Mycobacterium tuberculosis infection. J Immunol. 2006; 177: 4662–4669 [DOI] [PubMed] [Google Scholar]
- 33. Cai Y, Shen X, Ding C, et al. Pivotal role of dermal IL-17-producing gd T cells in skin inflammation. Immunity. 2011; 35: 596–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Shichita T, Sugiyama Y, Ooboshi H, et al. Pivotal role of cerebral interleukin-17-producing gammadeltaT cells in the delayed phase of ischemic brain injury. Nat Med. 2009; 15: 946–950 [DOI] [PubMed] [Google Scholar]
- 35. Zhao Z, Chen Y, Wang J, et al. Age-related retinopathy in NRF2-deficient mice. PLoS One. 2011; 6: e19456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chan K, Lu R, Chang JC, Kan YW. NRF2, a member of the NFE2 family of transcription factors, is not essential for murine erythropoiesis, growth, and development. Proc Natl Acad Sci U S A. 1996; 93: 13943–13948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mattapallil MJ, Wawrousek EF, Chan CC, et al. The Rd8 mutation of the Crb1 gene is present in vendor lines of C57BL/6N mice and embryonic stem cells, and confounds ocular induced mutant phenotypes. Invest Ophthalmol Vis Sci. 2012; 53: 2921–2927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hou L, Jie Z, Desai M, et al. Early IL-17 production by intrahepatic T cells is important for adaptive immune responses in viral hepatitis. J Immunol. 2013; 190: 621–629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jie Z, Liang Y, Hou L, et al. Intrahepatic innate lymphoid cells secrete IL-17A and IL-17F that are crucial for T cell priming in viral infection. J Immunol. 2014; 192: 3289–3300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Andrew EM, Newton DJ, Dalton JE, et al. Delineation of the function of a major gamma delta T cell subset during infection. J Immunol. 2005; 175: 1741–1750 [DOI] [PubMed] [Google Scholar]
- 41. Heilig JS, Tonegawa S. Diversity of murine gamma genes and expression in fetal and adult T lymphocytes. Nature. 1986; 322: 836–840 [DOI] [PubMed] [Google Scholar]
- 42. Grossniklaus HE, Green WR. Histopathologic and ultrastructural findings of surgically excised choroidal neovascularization. Submacular Surgery Trials Research Group. Arch Ophthalmol. 1998; 116: 745–749 [DOI] [PubMed] [Google Scholar]
- 43. Grossniklaus HE, Hutchinson AK, Capone A Jr, Woolfson J, Lambert HM. Clinicopathologic features of surgically excised choroidal neovascular membranes. Ophthalmology. 1994; 101: 1099–1111 [DOI] [PubMed] [Google Scholar]
- 44. Alten F, Clemens CR, Milojcic C, Eter N. Subretinal drusenoid deposits associated with pigment epithelium detachment in age-related macular degeneration. Retina. 2012; 32: 1727–1732 [DOI] [PubMed] [Google Scholar]
- 45. Curcio CA, Messinger JD, Sloan KR, McGwin G, Medeiros NE, Spaide RF. Subretinal drusenoid deposits in non-neovascular age-related macular degeneration: morphology, prevalence, topography, and biogenesis model. Retina. 2013; 33: 265–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zweifel SA, Imamura Y, Spaide TC, Fujiwara T, Spaide RF. Prevalence and significance of subretinal drusenoid deposits (reticular pseudodrusen) in age-related macular degeneration. Ophthalmology. 2010; 117: 1775–1781 [DOI] [PubMed] [Google Scholar]
- 47. Zweifel SA, Spaide RF, Curcio CA, Malek G, Imamura Y. Reticular pseudodrusen are subretinal drusenoid deposits. Ophthalmology. 2010; 117: 303–312, e301 [DOI] [PubMed] [Google Scholar]
- 48. Xu H, Forrester J. Inflammation in age-related macular degeneration: what is the evidence? In: Pleyer U, Forrester JV, eds. Uveitis and Immunological Disorders. Berlin, Heidelberg: Springer; 2009: 61–71 [Google Scholar]
- 49. Raoul W, Feumi C, Keller N, et al. Lipid-bloated subretinal microglial cells are at the origin of drusen appearance in CX3CR1-deficient mice. Ophthalmic Res. 2008; 40: 115–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Luhmann UF, Robbie S, Munro PM, et al. The drusenlike phenotype in aging Ccl2-knockout mice is caused by an accelerated accumulation of swollen autofluorescent subretinal macrophages. Invest Ophthalmol Vis Sci. 2009; 50: 5934–5943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Provis JM, Diaz CM, Penfold PL. Microglia in human retina: a heterogeneous population with distinct ontogenies. Perspect Dev Neurobiol. 1996; 3: 213–222 [PubMed] [Google Scholar]
- 52. Ito D, Imai Y, Ohsawa K, Nakajima K, Fukuuchi Y, Kohsaka S. Microglia-specific localisation of a novel calcium binding protein, Iba1. Brain Res Mol Brain Res. 1998; 57: 1–9 [DOI] [PubMed] [Google Scholar]
- 53. Santos AM, Martin-Oliva D, Ferrer-Martin RM, et al. Microglial response to light-induced photoreceptor degeneration in the mouse retina. J Comp Neurol. 2010; 518: 477–492 [DOI] [PubMed] [Google Scholar]
- 54. Kullberg S, Aldskogius H, Ulfhake B. Microglial activation, emergence of ED1-expressing cells and clusterin upregulation in the aging rat CNS, with special reference to the spinal cord. Brain Res. 2001; 899: 169–186 [DOI] [PubMed] [Google Scholar]
- 55. Penfold PL, Liew SC, Madigan MC, Provis JM. Modulation of major histocompatibility complex class II expression in retinas with age-related macular degeneration. Invest Ophthalmol Vis Sci. 1997; 38: 2125–2133 [PubMed] [Google Scholar]
- 56. Chan-Ling T, Hughes S, Baxter L, et al. Inflammation and breakdown of the blood-retinal barrier during “physiological aging” in the rat retina: a model for CNS aging. Microcirculation. 2007; 14: 63–76 [DOI] [PubMed] [Google Scholar]
- 57. Dithmar S, Curcio CA, Le NA, Brown S, Grossniklaus HE. Ultrastructural changes in Bruch's membrane of apolipoprotein E-deficient mice. Invest Ophthalmol Vis Sci. 2000; 41: 2035–2042 [PubMed] [Google Scholar]
- 58. Kliffen M, Lutgens E, Daemen MJ, de Muinck ED, Mooy CM, de Jong PT. The APO(*)E3-Leiden mouse as an animal model for basal laminar deposit. Br J Ophthalmol. 2000; 84: 1415–1419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ong JM, Zorapapel NC, Rich KA, et al. Effects of cholesterol and apolipoprotein E on retinal abnormalities in ApoE-deficient mice. Invest Ophthalmol Vis Sci. 2001; 42: 1891–1900 [PubMed] [Google Scholar]
- 60. Cousins SW, Espinosa-Heidmann DG, Alexandridou A, Sall J, Dubovy S, Csaky K. The role of aging, high fat diet and blue light exposure in an experimental mouse model for basal laminar deposit formation. Exp Eye Res. 2002; 75: 543–553 [DOI] [PubMed] [Google Scholar]
- 61. Malek G, Johnson LV, Mace BE, et al. Apolipoprotein E allele-dependent pathogenesis: a model for age-related retinal degeneration. Proc Natl Acad Sci U S A. 2005; 102: 11900–11905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Dasari B, Prasanthi JR, Marwarha G, Singh BB, Ghribi O. Cholesterol-enriched diet causes age-related macular degeneration-like pathology in rabbit retina. BMC Ophthalmol. 2011; 11: 22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Heckenlively JR, Hawes NL, Friedlander M, et al. Mouse model of subretinal neovascularization with choroidal anastomosis. Retina. 2003; 23: 518–522 [DOI] [PubMed] [Google Scholar]
- 64. Tanaka Y, Aleksunes LM, Yeager RL, et al. NF-E2-related factor 2 inhibits lipid accumulation and oxidative stress in mice fed a high-fat diet. J Pharmacol Exp Ther. 2008; 325: 655–664 [DOI] [PubMed] [Google Scholar]
- 65. Okada K, Warabi E, Sugimoto H, et al. Deletion of Nrf2 leads to rapid progression of steatohepatitis in mice fed atherogenic plus high-fat diet. J Gastroenterol. 2013; 48: 620–632 [DOI] [PubMed] [Google Scholar]
- 66. Schutt F, Bergmann M, Holz FG, Kopitz J. Proteins modified by malondialdehyde, 4-hydroxynonenal, or advanced glycation end products in lipofuscin of human retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2003; 44: 3663–3668 [DOI] [PubMed] [Google Scholar]
- 67. Totan Y, Cekic O, Borazan M, Uz E, Sogut S, Akyol O. Plasma malondialdehyde and nitric oxide levels in age related macular degeneration. Br J Ophthalmol. 2001; 85: 1426–1428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Xu H, Chen M, Manivannan A, Lois N, Forrester JV. Age-dependent accumulation of lipofuscin in perivascular and subretinal microglia in experimental mice. Aging Cell. 2008; 7: 58–68 [DOI] [PubMed] [Google Scholar]
- 69. Luhmann UF, Robbie S, Munro PM, et al. The drusen-like phenotype in aging Ccl2 knockout mice is caused by an accelerated accumulation of swollen autofluorescent subretinal macrophages. Invest Ophthalmol Vis Sci. 2009; 50: 5934–5943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Combadiere C, Feumi C, Raoul W, et al. CX3CR1-dependent subretinal microglia cell accumulation is associated with cardinal features of age-related macular degeneration. J Clin Invest. 2007; 117: 2920–2928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Rudolf M, Malek G, Messinger JD, Clark ME, Wang L, Curcio CA. Sub-retinal drusenoid deposits in human retina: organization and composition. Exp Eye Res. 2008; 87: 402–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Ma W, Coon S, Zhao L, Fariss RN, Wong WT. A2E accumulation influences retinal microglial activation and complement regulation. Neurobiol Aging. 2013; 34: 943–960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Gregerson DS, Sam TN, McPherson SW. The antigen-presenting activity of fresh, adult parenchymal microglia and perivascular cells from retina. J Immunol. 2004; 172: 6587–6597 [DOI] [PubMed] [Google Scholar]
- 74. Gregerson DS, Yang J. CD45-positive cells of the retina and their responsiveness to in vivo and in vitro treatment with IFN-gamma or anti-CD40. Invest Ophthalmol Vis Sci. 2003; 44: 3083–3093 [DOI] [PubMed] [Google Scholar]
- 75. Xu H, Dawson R, Forrester JV, Liversidge J. Identification of novel dendritic cell populations in normal mouse retina. Invest Ophthalmol Vis Sci. 2007; 48: 1701–1710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Ishimoto S, Zhang J, Gullapalli VK, Pararajasegaram G, Rao NA. Antigen-presenting cells in experimental autoimmune uveoretinitis. Exp Eye Res. 1998; 67: 539–548 [DOI] [PubMed] [Google Scholar]
- 77. Ebneter A, Casson RJ, Wood JP, Chidlow G. Microglial activation in the visual pathway in experimental glaucoma: spatiotemporal characterization and correlation with axonal injury. Invest Ophthalmol Vis Sci. 2010; 51: 6448–6460 [DOI] [PubMed] [Google Scholar]
- 78. de Hoz R, Gallego BI, Ramirez AI, et al. Rod-like microglia are restricted to eyes with laser-induced ocular hypertension but absent from the microglial changes in the contralateral untreated eye. PLoS One. 2013; 8: e83733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Xu H, Chen M, Reid DM, Forrester JV. LYVE-1-positive macrophages are present in normal murine eyes. Invest Ophthalmol Vis Sci. 2007; 48: 2162–2171 [DOI] [PubMed] [Google Scholar]
- 80. Schroedl F, Brehmer A, Neuhuber WL, Kruse FE, May CA, Cursiefen C. The normal human choroid is endowed with a significant number of lymphatic vessel endothelial hyaluronate receptor 1 (LYVE-1)-positive macrophages. Invest Ophthalmol Vis Sci. 2008; 49: 5222–5229 [DOI] [PubMed] [Google Scholar]
- 81. Chen Y, Yang P, Li F, Kijlstra A. The effects of Th17 cytokines on the inflammatory mediator production and barrier function of ARPE-19 cells. PLoS One. 2011; 6: e18139 [DOI] [PMC free article] [PubMed] [Google Scholar]