Abstract
The glutamate system is involved in many aspects of neuronal synaptic strength and function during development and throughout life. Synapse formation in early brain development, synapse maintenance, and synaptic plasticity are all influenced by the glutamate system. The number of neurons and the number of their connections are determined by the activity of the glutamate system and its receptors. Malfunctions of the glutamate system affect neuroplasticity and can cause neuronal toxicity. In schizophrenia, many glutamate-regulated processes seem to be perturbed. Abnormal neuronal development, abnormal synaptic plasticity, and neurodegeneration have been proposed to be causal or contributing factors in schizophrenia. Interestingly, it seems that the glutamate system is dysregulated and that N-methyl-d-aspartate receptors operate at reduced activity. Here we discuss how the molecular aspects of glutamate malfunction can explain some of the neuropathology observed in schizophrenia, and how the available treatment intervenes through the glutamate system.
Keywords: Schizophrenia, Glutamate, Neuroplasticity, Neurotoxicity, Antipsychotic drugs, Neuropathology
1. Introduction
Schizophrenia is a severe neuropsychiatric disorder that afflicts 1% of the world population (Andreasen, 1996; Bromet & Fennig, 1999; Carpenter & Buchanan, 1994). Although it is believed that multiple pathological processes can lead to schizophrenia, we have neither identified them nor linked them to the various clinical manifestations of the disorder (Carpenter & Buchanan, 1994). Unlike other neuropsychiatric disorders such as Parkinson's disease, no single anatomical abnormality is consistently observed in schizophrenia, nor are there any biochemical tests that can confirm the clinical diagnosis.
An accurate clinical diagnosis of schizophrenia is imperative for the research effort since it provides the highest likelihood to separate multiple disease-causing processes. Based on the clinical presentation, schizophrenia must be distinguished from several schizophrenia-like psychoses, including atypical, brief reactive, schizoaffective, and schizophreniform psychosis (Carpenter & Buchanan, 1994). After this first step, schizophrenia can be further subdivided into paranoid-hallucinatory, catatonic, and disorganized subtypes.
A variety of experimental approaches have been used to formulate testable hypotheses about disease mechanisms, such as neurochemistry, neuropathology, structural brain imaging, functional neuroimaging, and pharmacology (Heckers, 1997, 2001; Lewis & Lieberman, 2000). The serendipitous discovery of antipsychotic drugs has been particularly fruitful in providing insight into the pathological processes of schizophrenia.
The observation that conventional antipsychotic drugs inhibit D2 receptors (Creese et al., 1976; Snyder, 1976) has provided one of the first testable hypotheses about the etiology of schizophrenia as a malfunction of the dopaminergic system. The ‘dopamine hypothesis’ (Matthysse, 1974), which initiated the search for abnormalities of the dopaminergic system, was supported by two major clinical observation: (1) schizophrenia-like symptoms occur in amphetamine abusers, due to excessive dopamine release, and (2) D2 antagonists are efficacious in the treatment of schizophrenia (Snyder, 1973). However, the hypothesis is weakened by the lack of antipsychotic properties of some potent D2 receptor antagonists such as eticlopride. Moreover, a newer group of drugs, atypical antipsychotic drugs (Andersson et al., 1998), have behavioral benefits similar to conventional antipsychotic drugs, yet have a lower affinity for the D2 receptor (Seeman et al., 1997). Thus, while the dopaminergic system may be one factor involved in schizophrenia, the search for the disease-causing mechanisms needs to include additional candidates. Because the dopaminergic system is modulating other neurotransmitter systems in the brain, the performance of these systems in schizophrenia needs to be carefully examined. The glutamatergic system, in particular, interacts closely with the dopaminergic system, both, on the neuronal-circuitry level and on the intracellular level. Inhibition of D2 receptors by conventional antipsychotic drugs affects the glutamate system (Leveque et al., 2000), and the glutamate system has been directly implicated in schizophrenia (Goff & Coyle, 2001; Olney & Farber, 1995). For instance, N-methyl-d- aspartate (NMDA) receptor antagonists exacerbate psychotic symptoms in schizophrenics (Jentsch & Roth, 1999) and they produce cognitive deficits and psychotic symptoms in healthy volunteers, strikingly reminiscent of schizophrenia (Krystal et al., 1994). These observations gave rise to the ‘glutamate hypothesis of schizophrenia.’
The dopamine hypothesis of schizophrenia predicts abnormally increased activity within the dopamine neuro-transmitter system as the primary deficit, while the gluta-mate hypothesis of schizophrenia emphasizes abnormally decreased activity within the glutamate neurotransmitter system, particularly of NMDA receptors. Since there may be multiple forms of schizophrenia, patients may have malfunctions in just one or both systems. Moreover, both systems interact in a manner that allows dopamine D2 inhibitors to modulate an abnormally low glutamate system. As will be outlined in Section 3.3, overstimulation of D2 receptors can influence NMDA receptor activity. This interdependence of the glutamate and dopamine neurotransmitter systems could reflect an abnormality of either system in the other.
This review will illustrate how the molecular aspects of glutamate receptor activity and pharmacology support the glutamate hypothesis of schizophrenia. A dysregulated glutamate system, as proposed for schizophrenia, can affect brain development in a way that is consistent with the pathology of schizophrenia. Since excellent reviews have been published on the role of glutamate in the schizophrenic brain (Goff & Coyle, 2001; Olney & Farber, 1995; Sherman et al., 1991), we will focus here on the consequences of glutamate dysfunction on neuronal development and performance. In particular, we will discuss the molecular and the pharmacologic aspects of how the glutamate and dopamine systems influence each other.
2. The various roles of glutamate neurotransmission in schizophrenia
The first indication of an altered glutamate system in schizophrenia was provided by a report of significantly reduced glutamate levels in patients (Kim et al., 1980). Since then, a variety of studies have supported a primarily hypoactive glutamate system in schizophrenia (Coyle, 1996; Goff & Coyle, 2001; Goff & Wine, 1997; Jentsch & Roth, 1999; Meador-Woodruff & Healy, 2000; Olney & Farber, 1995), with secondary increased glutamate release in selected brain areas (Olney et al., 1999). A malfunction of the NMDA type of glutamate receptors has gained particular attention in light of two observations: (1) inhibition of the NMDA receptors with phencyclidine (PCP) or ketamine causes schizophrenia-like psychoses in normal subjects and worsens the symptoms of schizophrenia in patients (Javitt & Zukin, 1991; Jentsch & Roth, 1999) and (2) facilitation of the glycine site of the NMDA receptor improves the outcome of the treatment in schizophrenia (Dall'Olio & Gandolfi, 1993; Goff et al., 1999b; Heresco-Levy et al., 1996b, 1999).
This section will deal with the role of glutamate and its receptors in brain development, brain function, neuroplasticity, and neurotoxicity, and how a malfunction of the glutamate system could explain some of the neuropathology observed in schizophrenia.
2.1. Pharmacology of glutamate receptors
Glutamate controls the excitation of neurons and glia through the activation of various glutamate receptors. Glutamate receptors include ion channels (ionotropic glutamate receptors) and G-protein-coupled receptors [metabotropic glutamate receptors (mGluRs)] (Hollmann & Heinemann, 1994; Ozawa et al., 1998).
2.1.1. Ionotropic glutamate receptors
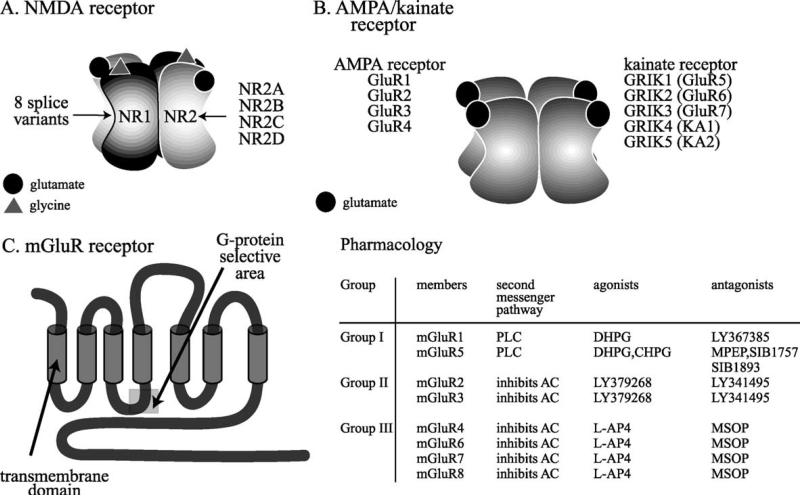
Ionotropic glutamate receptors are named after their distinguishing ligands and classified into NMDA receptors, α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA) receptors, and kainate receptors (Hollmann & Heinemann, 1994; Ozawa et al., 1998). Molecular cloning has shown that all ionotropic glutamate receptors consist of four subunits, which form a functional receptor with an ion permeable pore (Clements & Westbrook, 1991; Rosenmund et al., 1998; Safferling et al., 2001) (Figs. 1A, 1B). Each of the four subunits binds one ligand molecule (Clements & Westbrook, 1991). NMDA receptors require both l-glutamate and glycine for efficient channel activation (Fig. 1A). The NMDA receptor is assembled from two NR1 subunits, expressed in eight splice variants, and two of a family of four NR2 subunits (NR2A–D) (Hollmann & Heinemann, 1994). The NR1 subunit binds to glycine, while the NR2 subunits bind glutamate, enabling the receptor to bind to a maximum of two glutamate and two glycine molecules (Laube et al., 1997). Unlike the glycine receptor, glycine binding to the NMDA receptor is strychnine-insensitive (Johnson & Ascher, 1987). The facilitation by glycine distinguishes the NMDA receptor from other ionotropic receptors, a feature that has been employed in treatment trials of schizophrenia. Following the observation that the noncompetitive NMDA receptor antagonist PCP can induce a psychotomimetic state akin to schizophrenia (Javitt & Zukin, 1991), it was proposed that facilitation of NMDA receptor function might provide a therapeutic benefit in schizophrenia. Thus, agonists at the glycine site of the NMDA receptor, such as glycine, serine, or d-cycloserine (Henderson et al., 1990; Hood et al., 1989; Watson et al., 1990), have been tested in clinical studies and have shown promise as adjuvants in the treatment of schizophrenia (Dall'Olio & Gandolfi, 1993; Goff et al., 1995b, 1999b; Heresco-Levy et al., 1996a, 1999; Javitt et al., 1994; Tsai et al., 1998b).
Fig. 1.
Glutamate receptor families. A: NMDA receptors are assembled in tetramers from two NR1 subunits and two NR2 subunits. The NR1 subunit is encoded by one gene and has eight different splice variants. The NR1 subunit is an essential component of the NMDA receptor. The NR2 subunit family is encoded by four genes, which give rise to NR2A–NR2D. A third, novel family of NMDA receptor subunits, the NR3 family, assembles with NR1 for surface expression (Perez-Otano et al., 2001). NR3 is not shown. B: AMPA receptors are assembled in tetramers from a family of four GluR subtypes, GluR1–GluR4. All four subtypes bind glutamate, AMPA, and kainate. Kainate receptors are assembled in tetramers from a family of three GluR subtypes, GluR5–GluR7, also termed GRIK1-3, and two kainate subtypes, KA1 and KA2, also termed GRIK4–5. GRIK1–3 have a lower affinity to kainate than GRIK4 and GRIK5. C: Metabotropic glutamate receptors are encoded by eight genes, mGluR1–8. These G-protein-coupled receptors have seven transmembrane domains, and are divided into three groups according to sequence similarity, the signal transduction pathways to which they are linked, and the specificity for agonists and antagonists (Bortolotto et al., 1999; De Blasi et al., 2001). A gray box marks the G-protein selective area. The pharmacological agents that bind with the highest specificity to the three groups of receptors are shown on the right. AC, adenylate cyclase; CHPG, (R,S)-2-chloro-5-hydroxyphenylglycine; DHPG, 3,5-dihydroxyphenylglycine; L-AP4, L-2-amino-4-phosphonobutanoate; MSOP, (R,S)-α-methylserine-O-phosphate; PLC, phospholipase C. Pharmacology from Bortolotto et al. (1999) and De Blasi et al. (2001).
A novel family of NMDA receptor subunits, consisting of NR3A and NR3B, has been described recently (Nishi et al., 2001; Sucher et al., 1995). NR3A has at least two splice variants (Sun et al., 1998). NR3 subunits form functional channels with NR1 (Perez-Otano et al., 2001), and, unlike other subunits, work in a dominant-negative fashion, i.e., they assemble with NR1 and suppress glutamate-induced currents (Nishi et al., 2001; Sucher et al., 1995).
AMPA and kainate receptors are assembled in tetramers from a family of receptor subunits, GluR1–4 for AMPA and GluR5–7 (GRIK1–3; low-affinity subtypes) and KA1–2 (GRIK4 and -5, high-affinity subtypes) for kainate (Hollmann & Heinemann, 1994; Ozawa et al., 1998) (Fig. 1B). AMPA receptors are predominantly postsynaptically located, whereas kainate receptors may have additional presynaptic function in the regulation of glutamate release (Frerking & Nicoll, 2000; Lerma et al., 2001).
AMPA receptors are functional in homomeric and heteromeric assemblies of all four subunits. Kainate receptors cannot form functional channels with homomeric assemblies of GluR7, KA1, and KA2 (GRIK3–5) (Ozawa et al., 1998). AMPA and kainate receptors require only glutamate for activation. For the AMPA receptors, it has been shown that each subunit can bind one molecule of glutamate. Activation of the AMPA receptor does not require the occupation of all four subunits with glutamate, but the number of subunits occupied determines the conductance of the receptor (Rosenmund et al., 1998). While the molecular pharmacology of the kainate receptor has not been studied as thoroughly as the AMPA receptor, it appears to be similar (Lerma et al., 2001).
Upon ligand binding, Na + and K + pass through the pore of ionotropic glutamate receptors and cause depolarization of the neuronal membrane (Ascher & Nowak, 1987; Kandel et al., 1991). In addition to depolarizing membranes, iono-tropic glutamate receptors can initiate long-term molecular adaptations in neurons. These adaptations are mediated by Ca2 + influx through the channel pore. Ca2 + can pass through NMDA receptors (Connor et al., 1988; MacDermott et al., 1986), kainate receptors (Egebjerg & Heinemann, 1993; Kohler et al., 1993), and, under some circumstances, AMPA receptors (Hume et al., 1991; Nakanishi, 1992; Pellegrini-Giampietro et al., 1997). The Ca2 + permeability of AMPA receptors is determined by the GluR2 subunit. Co-expression of GluR2 subunits with GluR1, GluR3, or GluR4 results in the formation of receptors with little Ca2 + permeability, while the absence of GluR2 renders AMPA receptors Ca2 + permeable (Hollmann & Heinemann, 1994; Ozawa et al., 1998; Seeburg, 1993). Whereas native AMPA receptors are frequently heteromers with GluR2 subunits and little Ca2 + permeability, some AMPA receptors with high Ca2 + permeability have been identified in subpopulations of neurons in the hippocampus, cerebellum, cortex, and other brain areas (Ozawa et al., 1998).
Kainate receptors have a higher Ca2 + permeability than AMPA receptors. In particular, the GluR6 subunit of the kainate receptor forms channels with high Ca2 + permeability (Egebjerg & Heinemann, 1993; Kohler et al., 1993). The RNA sequences of GluR2 (AMPA) and GluR6 (kai-nate) can be modified, whereby the code for the amino acid glutamine (which is encoded by the DNA sequence) is edited to arginine (Egebjerg & Heinemann, 1993; Kohler et al., 1993; Sommer et al., 1991). In GluR2, this replacement is important to prevent Ca2 + flow through the AMPA receptor, whereas in GluR6 the replacement seems to be of lesser significance (Ozawa et al., 1998).
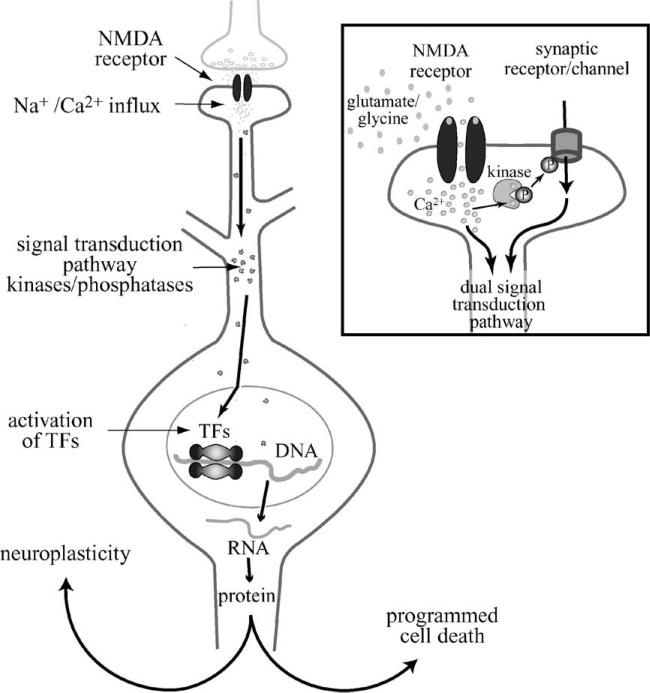
The influx of Ca2 + through ionotropic glutamate receptors has been the focus of much attention, because Ca2 + stimulates intraneuronal signal transduction cascades that determine cellular plasticity and cell death (Fig. 2). Signal transduction cascades activated by Ca2 + stimulate kinases and phosphatases, which affect the phosphor-ylation state of receptors, ion channels, signaling proteins, and transcription factors (TFs). The consequences of these phosphorylation events are widespread and range from altered neuronal excitability to the expression of new genes and proteins. Depending on which signal transduction pathways are activated, the proteins synthesized are involved in the construction of new synapses and reinforcement of existing synapses, or the controlled disassembly of the neuron in a cell death program (Fig. 2). Thus, glutamate has a critical role in neuroplasticity and neurotoxicity, neurodevelopment, and neuronal death. The NMDA receptor is particularly important for the initiation of cellular programs because of its Ca2 + permeability and its physiological properties. A distinguishing feature of this receptor is a voltage-dependent block by Mg2 + (Mayer et al., 1984), which prevents channel opening in response to ligand binding. To relieve the block and to open the channel, the NMDA receptor needs simultaneous depolarization and ligand binding. The need for a coincidence of presynaptic neurotransmitter release and postsynaptic depolarization is thought to provide an important safeguard for the regulation of neuroplasticity (Herron et al., 1986).
Fig. 2.
Intracellular mechanism by which NMDA receptors mediate neuroplasticity and programmed cell death. Upon interaction with glutamate and glycine, NMDA receptor channels open and pass Na + and Ca2 + ions. The influx of Ca2 + activates signal transduction molecules, such as kinases and phosphatases, which proceed to phosphorylate cellular proteins at the synapse (see insert) and in the nucleus. Among the proteins phosphorylated are TFs that are activated by phosphorylation. These TFs bring RNA polymerase to DNA, which transcribes genes encoded by the DNA into RNA. RNA is shuttled out of the nucleus and translated into protein. The signal transduction pathways activated are specific to the strength of ion influx and the route(s) of ion influx (see insert). The genes induced by these pathways can be involved in either neuroplastic or cell death mechanisms. Insert: At the synapse, Ca2 + entering through NMDA receptors activates local kinases. These kinases phosphorylate/activate neighboring receptors and ion channels, which initiate signal transduction pathways that can combine with the NMDA receptor signal transduction pathway. The combination of receptors and channels activated lends further specificity to the signal transduction pathway, and helps to determine the type of molecular response the neuron will exhibit. P, phosphate residue.
2.1.2. Metabotropic glutamate receptors
mGluRs are localized at presynaptic and/or postsynaptic sites on glia and neurons (De Blasi et al., 2001). Like all G-protein-coupled receptors, mGluRs have seven transmembrane domains (De Blasi et al., 2001; Schoepp, 1993), yet they share little sequence homology with the other known G-protein-coupled receptors (Fig. 1C) and form a unique superfamily of G-protein-coupled receptors (Ozawa et al., 1998).
Eight mGluR subtypes have been cloned. They are divided into three groups, depending on their amino acid sequence and the signal transduction pathways that they activate (De Blasi et al., 2001) (Fig. 1C). Group I mGluRs (mGluR1, mGluR5) stimulate phospholipase C activity, cyclic AMP formation, and arachidonic acid (AA) release (Aramori & Nakanishi, 1992), whereas Groups II (mGluR2, mGluR3) and III (mGluR4, mGluR6, mGluR7, mGluR8) inhibit adenylate cyclase activity (Fagni et al., 2000; Pellicciari & Costantino, 1999). mGluRs affect neuroplasticity and gene expression via their modulation of these signal transduction pathways. mGluR2, mGluR4, mGluR7, and mGluR8 are also located at presynaptic terminals (Schoepp, 2001), and it has been shown that mGluRs reduce neuro-transmitter outputs of excitatory and inhibitory amino acids, monoamines, and neuropeptides (Cartmell & Schoepp, 2000). mGluRs regulate neuronal presynaptic activity by modulating the activity of Ca2 + or K + channels and by interfering with release processes downstream of Ca2+ entry (Cartmell & Schoepp, 2000).
Postsynaptic mGluRs increase the intracellular Ca2+ concentration by activating ryanodine-sensitive and inositol 1,4,5-trisphosphate-sensitive Ca2 + stores (Fagni et al., 2000; Murphy & Miller, 1988). In concordance with the notion that the mobilization of Ca2 + promotes neuroplasticity, mGluRs have been shown to contribute to synaptic plasticity, learning, and memory (De Blasi et al., 2001). They support the induction of long-term potentiation (LTP) in the hippocampus, and they play a role in hippocampal and cerebellar long-term depression (LTD) (Bortolotto et al., 1999; Fagni et al., 2000).
2.2. Physiological role of the glutamatergic system
Glutamate neurotransmission is critically involved in neuronal development, neuroplasticity, and neurotoxicity. Abnormal development, abnormal neuroplasticity, and neurotoxicity have also been implicated in the pathology of schizophrenia (Goff & Coyle, 2001; Olney & Farber, 1995). A detailed evaluation of the normal role of glutamate in brain development, adult brain function, and neurotoxicity is presented to help develop the potential contribution of abnormal glutamate receptor activity in schizophrenia.
2.2.1. The role of glutamate in brain development
In early neuronal development, neurons extend filopodia that search the environment for presynaptic partners with which to form synapses (Lee & Sheng, 2000; Wong & Wong, 2000) (Fig. 3A). As neurons mature, synapses replace filopodia (Harris, 1999). Whereas motility is important in very early development to maximize the chance to find partners, stability is needed in later development to maintain and strengthen synapses. The glutamate system is critically involved in both.
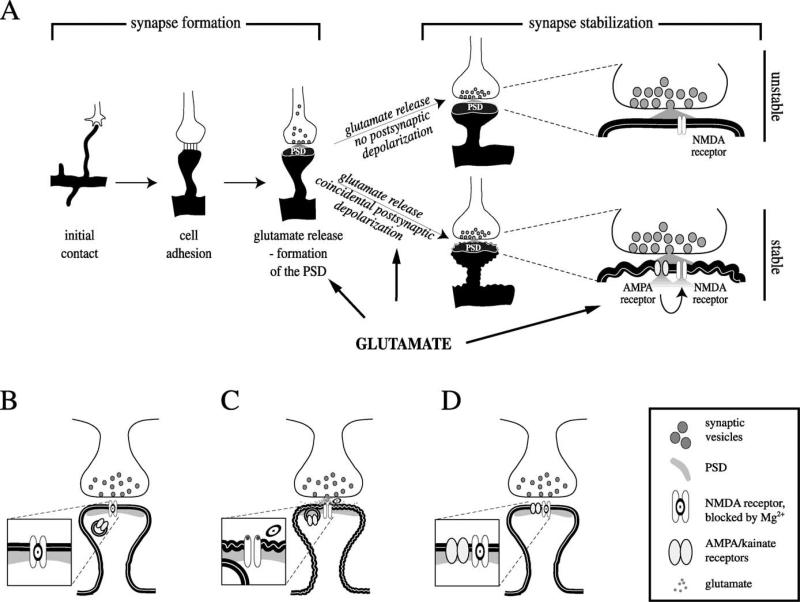
Fig. 3.
Glutamate is involved in early synapse formation and synapse stabilization. A: Synapse formation: after contact of filopodium and growth cone, cell adhesion molecules provide a bond between pre- and postsynaptic membranes. Prefabricated release packages accumulate and release glutamate, which stimulates the formation of the postsynaptic density (PSD). Synapse stabilization: pre- and postsynaptic activities need to be synchronized, i.e., the postsynaptic membrane needs to depolarize in response to presynaptic glutamate release. Initially, a coincidence of presynaptic glutamate release and postsynaptic depolarization is needed to promote an incorporation of AMPA/kainate receptors into the postsynaptic membrane, which will stabilize the synapse. A more active glutamate system has a higher likelihood of coincidental glutamate release and postsynaptic depolarization. B, C, D: Details of synapse stabilization. B: In the immature synapse, the postsynaptic site initially has only NMDA receptors. NMDA receptors are blocked by Mg + , which is removed by the activation of the receptor with glutamate and simultaneous depolarization of the cell membrane. In the mature synapse, activation of AMPA/kainate receptors depolarizes the neuron in response to glutamate release and provides the depolarization needed to remove the Mg + from NMDA receptors. This allows ion flux through the NMDA receptor. The developing synapse has no AMPA/kainate receptors, and an initial postsynaptic depolarization during presynaptic glutamate release is either coincidental or can be carried over from mature, neighboring synapses. C: A coincidence of presynaptic glutamate release and postsynaptic depolarization removes the Mg2 + block and opens NMDA receptors. D: The initial activation of NMDA receptors prompts the incorporation of AMPA/kainate receptors into the postsynaptic membrane. Subsequent glutamate release will trigger depolarization via AMPA/kainate receptors, which assist the opening of NMDA receptors. Activation of postsynaptic CaM kinase II by the NMDA signal transduction pathway is responsible for the incorporation of AMPA receptors (Wu et al., 1996).
After a filopodium makes contact with a potential partner, cell adhesion molecules provide the initial stimulus for the formation of a synapse (Doherty et al., 1995; Murase & Schuman, 1999) (Fig. 3A). Following the adhesion of the membranes, prefabricated protein packages, which are able to release neurotransmitter, get trapped at the presynaptic site of contact (Ahmari et al., 2000; Haas, 2000). The release of glutamate stimulates the postsynaptic membrane to recruit anchoring proteins that can secure all the necessary elements for a functional postsynaptic site (Fig. 3A) (Friedman et al., 2000). The ability of a neuron to synthesize and release adequate amounts of glutamate is crucial for the formation of a sufficient number of synapses. Presynaptic neurons that cannot provide enough glutamate may prevent the formation of a postsynaptic site, which decreases the likelihood of synapse formation.
Once the initial synapse is established, activities of the pre- and postsynaptic membrane need to be synchronized, i.e., the postsynaptic membrane needs to respond to pre-synaptic neurotransmitter release. Synchronization stabilizes the synapse. Failure to synchronize leaves the postsynaptic membrane unstable, which leads to a retraction and elimination of the synapse (Frank, 1997).
The glutamate system and its various receptors play a crucial role in the stabilization of synapses. Although early synapses contain NMDA receptors, they are unresponsive to glutamate unless AMPA/kainate receptors are present in the postsynaptic membrane (Fig. 3) (Isaac et al., 1995; Petralia et al., 1999; Wu et al., 1996). Mg2 + , which is blocking the NMDA receptor pore (Mayer et al., 1984), needs to be dislodged to allow ion influx. In order to dislodge the Mg2 + and to depolarize the neuron, a coincidence of presynaptic glutamate release and postsynaptic depolarization is needed. This ‘coincidence’ seems to initially happen by chance, although it may be facilitated through a spillover of depolarization of more mature, neighboring synapses (Fig. 3) (Wu et al., 1996). As development progresses, more and more synapses incorporate AMPA receptors (Isaac et al., 1995; Petralia et al., 1999; Wu et al., 1996), which appear in the postsynaptic membrane as a consequence of NMDA receptor depolarization and activation of Ca2 + /calmodulin-dependent protein kinase (CaM kinase) II (Figs. 3C, 3D) (Wu et al., 1996). Once both types of receptors are in the membrane, the release of glutamate activates AMPA and NMDA receptors. AMPA receptors provide a short, postsynaptic depolarization that removes the Mg2 + block and allows ion flux through NMDA receptors. At this point, synchronization between presynaptic glutamate release and post-synaptic depolarization response is established.
Synapses that contain only NMDA receptors, but not AMPA/kainate receptors, are termed ‘silent synapses’ for their lack of electrophysiological response to glutamate under normal resting potentials (Gomperts et al., 1998; Isaac et al., 1995; Liao et al., 1995; Wu et al., 1996). They are also found in adult rats, where, for example, only 85% of the hippocampal synapses made by Schaffer collaterals onto CA1 pyramidal cell spines have detectable levels of AMPA receptors, whereas 100% have NMDA receptors (Racca et al., 2000).
The initial fate of a synapse is thus determined by presynaptic glutamate release and the incorporation of sufficient numbers of postsynaptic NMDA and AMPA/ kainate receptors. Reduced levels of glutamate or reduced activity of NMDA receptors impact the number of synapses established. If any of these abnormalities are present early in brain development, they should have crucial consequences for the establishment of proper brain circuitry and synaptic connectivity. Indeed, schizophrenia is thought to be a neurodevelopmental disorder, with defective connectivity and decreased synaptic density (Lewis & Lieberman, 2000). Among the abnormalities found in the adult neocortex in schizophrenia are a reduction in the density of spines (Garey et al., 1998; Glantz & Lewis, 2000) and a reduction in neuropil (Selemon & Goldman-Rakic, 1999). The functional consequences of the anatomical abnormalities are a disconnection of circuits involving prefrontal-temporolimbic areas (Friston & Frith, 1995). These abnormalities are thought to be the result of a subtle disease process that affects the formation of critical circuits in the brain during early neuronal development. This disease process becomes clinically apparent during adolescence or early adulthood. Because the infant brain has an overabundance of connections, which are pruned throughout childhood and adolescence (Huttenlocher, 1984; Huttenlocher et al., 1982), schizophrenia presents itself at a time when a critical number of synaptic connections is lost, which, in many cases, occurs in late adolescence or early adulthood.
While the evidence is circumstantial, there are strong indications that early malfunctions in the glutamate system lead to defective connectivity. A primary hypoactive glutamate system, as proposed for schizophrenia (Goff & Coyle, 2001), therefore, could affect the formation of neuronal connections early in life, which fits well with the anatomical abnormalities found in the adult schizophrenic brain.
2.2.2. The role of glutamate in neuroplasticity throughout life
The ability of neurons to adapt to changes in the environment is crucial for survival and an important aspect of brain function. This adaptive feature is referred to as ‘neuroplasticity,’ and its ultimate manifestations are learning and memory. The interaction between signaling molecules, such as glutamate and its receptors, has short-term consequences (depolarization of the membrane) and long-term consequences (e.g., altered gene and protein synthesis) that affect the properties and shape of the neuron. New gene expression is needed for the long-lasting changes of the efficacy of synaptic communication, which is crucial for learning and memory. For example, membrane depolarization and activation of NMDA receptors enables Ca2+ influx, which induces LTP and gene expression (Malenka & Nicoll, 1999). The functional consequences of Ca2 + influx include increased sensitivity to glutamate (demonstrated by a decrease in synaptic failures) (Nicoll & Malenka, 1999) and a promotion of neurite outgrowth, cell adhesion, and cellular interactions, all of which represent the structural substrate for neuroplasticity (Segal, 2001).
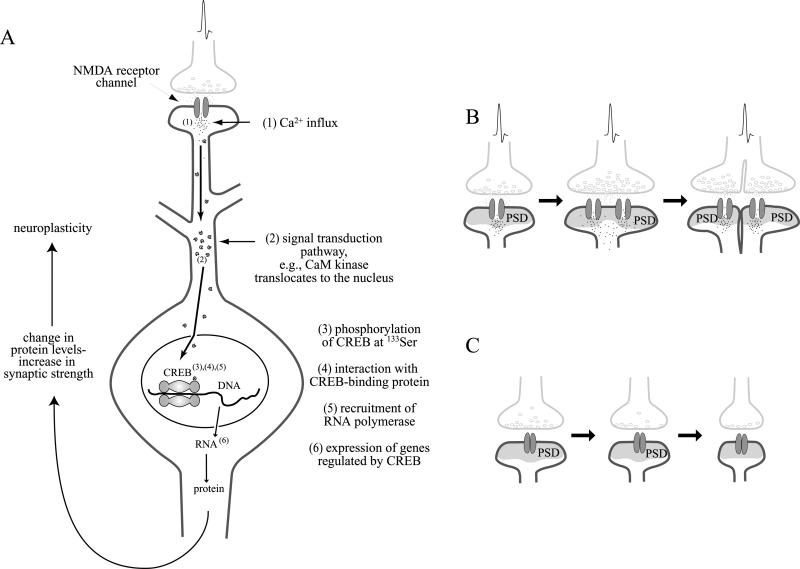
How does electrical activity mediate Ca2 + influx and gene expression? Studies of the TF cyclic AMP response element-binding protein (CREB) provide some interesting insights into this question (Fig. 4). Presynaptic glutamate release in combination with postsynaptic depolarization causes the influx of Ca2 + ions through the NMDA receptor channel (MacDermott et al., 1986) and through Ca2+ channels (Mermelstein et al., 2000; Rajadhyaksha et al., 1999). Free intracellular Ca2 + activates Ca2 + -dependent enzymes such as CaM kinases and the phosphatase calcineurin (Thompson, 2000). These enzymes modulate glutamate receptor function in a feed-back-loop, and they activate TFs. For instance, phosphorylation of ionotropic glutamate receptors by CaM kinases potentiates the conductance of these channels to glutamate (Carvalho et al., 1999; Derkach et al., 1999; Mammen et al., 1997; Omkumar et al., 1996; Soderling, 1993; Swope et al., 1999; Tan et al., 1994; Wang & Salter, 1994; Yakel et al., 1995). Moreover, phosphor-ylation affects the transport and incorporation of glutamate receptors into the postsynaptic membrane (Nicoll & Malenka, 1999; Rongo & Kaplan, 1999). The phosphatase calcineurin shortens the channel opening time of ionotropic glutamate receptors and promotes AMPA receptor endocytosis (Beattie et al., 2000; Ghetti & Heinemann, 2000; Lieberman & Mody, 1994; Shi et al., 2000; Tong et al., 1995). Thus, Ca2 +-activated kinases and phosphatases influence glutamate receptor activity and the neuronal response to glutamate.
Fig. 4.
Activation of the transcription factor CREB by the NMDA signal transduction pathway. Activation of NMDA receptors causes Ca2 + influx (A). Ca2 + interacts with kinases and phosphatases that act as messengers in signal transduction pathways. Kinases and phosphatases alter the phosphorylation patterns of TFs and, thus, influence their ability to stimulate the synthesis of mRNA. For example, CaM kinases, activated by Ca2 +, can translocate to the nucleus and phosphorylate the TF CREB (Bito et al., 1996; Sheng et al., 1991; Sun et al., 1994). Phosphorylated CREB stimulates the synthesis of many different mRNAs, which are translated into proteins. These proteins alter neuronal properties and contribute to memory formation and synaptic strength. For instance, the newly synthesized proteins are incorporated into synapses. An active synapse can increase in size, and may even split into two synapses (B). An inactive synapse can decrease in size and may be even disassembled (C). PSD, postsynaptic density.
In addition, Ca2 +-dependent kinases translocate to the nucleus, where they phosphorylate/activate TFs such as CREB (Fig. 4A) (Rajadhyaksha et al., 1999; Schulman, 1995). CREB has been shown to be involved in the formation of long-term memory (Davis et al., 1996; Frank & Greenberg, 1994). In Drosophila, long-term memory was blocked by a dominant-negative CREB transgene (Yin et al., 1994). Conversely, long-term memory was enhanced after expression of an activator of CREB (Yin et al., 1995). Similarly, mice with a targeted disruption of the a and d isoforms of CREB were deficient in long-term memory (Bourtchuladze et al., 1994), whereas on the other hand, contextual learning causes a significant increase in CREB-dependent gene expression in the hippocampus of mice (Impey et al., 1998).
Protein synthesis is an important component of CREB-mediated synaptic plasticity, and some of the genes that are needed for synapse formation seem to be under the control of CREB. In Aplysia neurons, it has been shown that CREB-dependent synaptic facilitation requires protein synthesis, and that it involves the growth of new synaptic connections (Martin et al., 1997). Many effects of Ca2 + on gene expression, synaptic plasticity, and processes of learning can be explained by the Ca2 + -dependent activation of the TF CREB.
It has been demonstrated that glutamate, Ca2 +, gene expression, and neuroplasticity are intricately linked. LTP and LTD, two features of neuroplasticity, depend on NMDA receptor function (Bear & Malenka, 1994; Malenka, 1991; Nicoll & Malenka, 1999). Similarly, learning and memory depend on gene expression and NMDA receptors (Bailey et al., 1996). The activation of intraneuronal signal transduction pathways, TFs, and gene expression results in increased protein expression, leading to a remodeling of the structure and function of the neuron. Thus, throughout life, glutamate can initiate changes in synapse morphology and can influence synaptic strength (Figs. 4B, 4C). These are important qualities needed for proper cognitive function. Many of these processes are disturbed in schizophrenia, and it is likely that malfunctions of the glutamate system, at least to some extent, are responsible for the observed cognitive deficits.
2.2.3. The role of glutamate in neurotoxicity
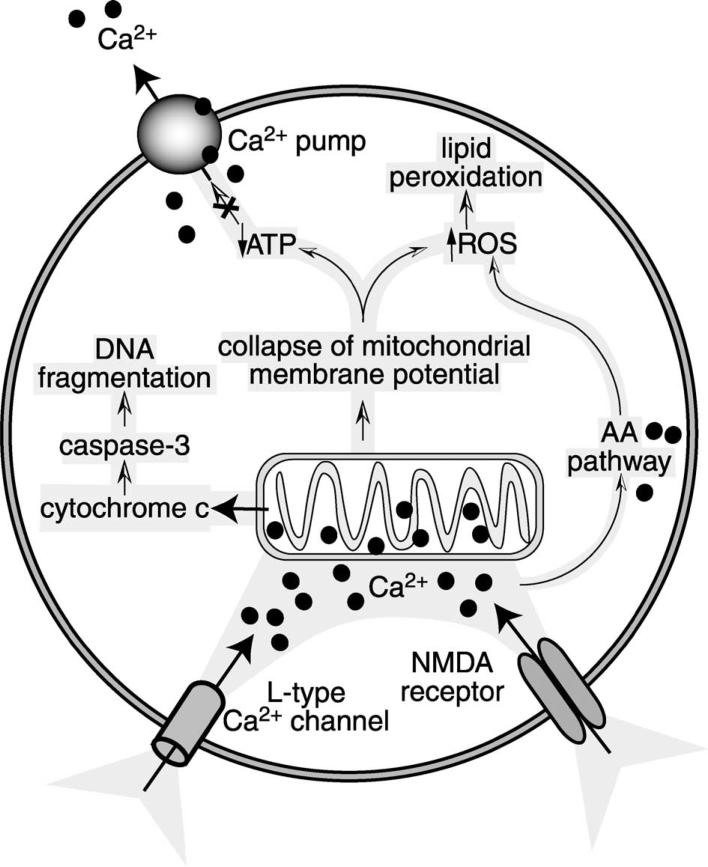
Whereas the physiologic stimulation of glutamate recep tors is generally beneficial for neurons, excessive stimulation of glutamate receptors can compromise neuronal viability by impeding structural and functional integrity. This process is known as ‘excitotoxicity.’ Under extreme circumstances, excessive activation of NMDA receptors can cause unmanageable Na + and Cl − influx, which can build up osmotic pressure to the point where the cell ruptures (Olney et al., 1986; Rothman, 1985). If the cell manages to avoid this necrotic death, a delayed, apoptotic, Ca2 + -dependent death can be initiated (Choi, 1987). Apoptotic cell death is caused by irreversible intracellular Ca2+ elevation, a Ca2 + overload (Randall & Thayer, 1992). Mitochondrial damage seems to play an important role in Ca2 + -mediated apoptosis (Fig. 5) (Schinder et al., 1996). Due to the Ca2 + accumulation, the mitochondrial membrane potential collapses, cellular levels of ATP drop, and reactive oxygen species (ROS) build up (Luetjens et al., 2000; Montal, 1998; Sengpiel et al., 1998). Since ATP is needed to shuttle Ca2 + out of the cells, the vicious cycle is potentiated (Sattler & Tymianski, 2000). In addition, among the second messenger pathways activated by Ca2 + is the AA pathway (Lazarewicz et al., 1990) that contributes to the generation of free radicals (Keyser & Alger, 1990; Rao et al., 1982). Free radicals compromise cellular function and membrane integrity by attacking proteins and nucleic acids, and by mediating lipid peroxidation of cell membranes (Farooqui & Horrocks, 1998; Halliwell & Chirico, 1993; Khodorov, 2000).
Fig. 5.
Ca2 + overload damages mitochondria and causes neurotoxicity. Prolonged opening of NMDA receptors leads to the influx of Ca2 + through NMDA receptor channels, as well as through L-type Ca2 + channels. Ca2 + accumulation inside the cell causes mitochondrial damage. Leakage of cytochrome c from mitochondria activates the caspase-3 pathway, which leads to DNA fragmentation, a hallmark of apoptosis. Because of the collapse of the mitochondrial membrane potential, the production of ATP is compromised. This causes further accumulation of Ca2 + , since Ca2 + pumps need ATP to shuttle excess Ca2 + out of the cell. ROS accumulate in response to mitochondrial damage and in response to the activation of the AA pathway by Ca2 + . ROS damage proteins and nucleic acids, and cause lipid peroxidation of membranes.
Apoptosis is an active process, a ‘suicide’ program of cells designed to avoid an inflammatory response (Bredesen, 1995). Molecular mechanisms leading to apoptosis have been most systematically identified in Caenorhabditis elegans (Metzstein et al., 1998), and homologous genes that regulate apoptosis have been identified in vertebrates (Alnemri, 1997; Bergeron & Yuan, 1998; Driscoll, 1996; Martinou & Sadoul, 1996; Merry & Korsmeyer, 1997; Schwartz & Milligan, 1996). A group of cysteine proteases, called caspases, are centrally involved in apoptosis (Bergeron & Yuan, 1998). Caspases are constitutively expressed as proenzymes that undergo proteolytic cleavage. Once cleaved, they become active and proceed to cleave-activate other enzymes, and to cause enzymatic breakdown of proteins and structural elements inside the cell. Caspase-3 activates a protein that triggers fragmentation of DNA (Liu et al., 1997). NMDA-mediated neurotoxicity activates caspases inside neurons (Fig. 5) (Du et al., 1997; Hirashima et al., 1999; Martin et al., 1998; Nath et al., 1998; Tenneti et al., 1998; Tenneti & Lipton, 2000). The damage to the mitochondria by Ca2 + causes a release of cytochrome c (Luetjens et al., 2000); cytochrome c, in turn, activates caspase-3 (Zou et al., 1997). Given the relationship of caspase-3 and DNA fragmentation, it is not surprising that excitotoxicity activates endonucleases and causes internucleosomal DNA fragmentation in neurons (Ankarcrona et al., 1995; Kure et al., 1991; Nath et al., 2000; Simonian et al., 1996).
Some of the deleterious side effects of conventional antipsychotic drugs may be derived from their facilitation of NMDA receptor activity and Ca2 + influx, which controls a delicate equilibrium between neuroplasticity and neurotoxicity. These mechanisms will be discussed in Section 3.
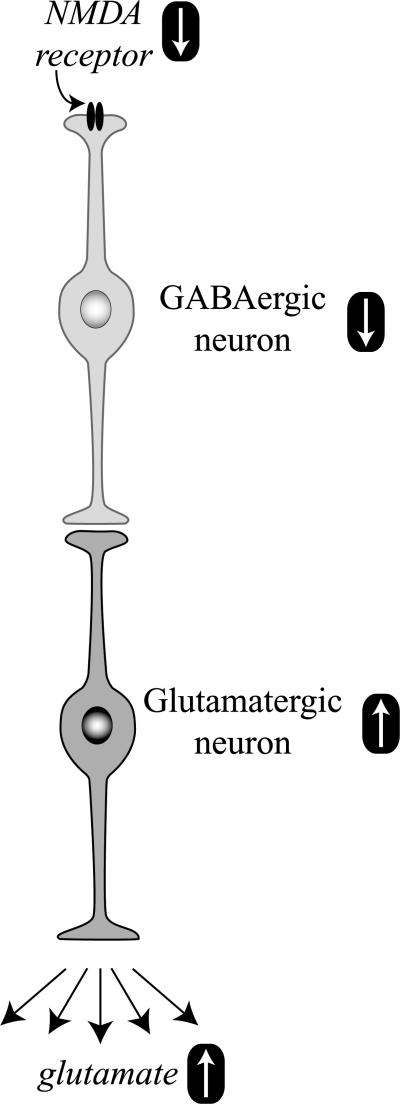
Interestingly, a decrease in the activity of NMDA receptors, as achieved with NMDA antagonists and as suspected in schizophrenia, also causes excitotoxicity (Farber et al., 1995; Olney et al., 1989). This paradoxical phenomenon is explained by a reduced activity of gaminobutyric acid (GABA)ergic neurons under the control of hypoactive NMDA receptors (Fig. 6) (Olney et al., 1991). The decreased stimulation of NMDA receptors on γ-aminobutyric acid (GABA)ergic neurons leads to a dis-inhibition of postsynaptic glutamate neurons, excessive glutamate release, and neurotoxicity.
Fig. 6.
NMDA receptor hypoactivity and glutamate neurotoxicity. Hypoactive NMDA receptors on GABAergic neurons are responsible for decreased neuronal activity. The inhibitory tone of GABA neurons on glutamate neurons is attenuated, and the activity of glutamate neurons is increased. More glutamate gets released, causing excitotoxic stress and damage.
2.3. Pathology of the glutamate system in schizophrenia
2.3.1. The glutamate system is dysregulated in schizophrenia
The most striking evidence for an involvement of the glutamate system in schizophrenia comes from the clinical observation that inhibition of NMDA receptors by the noncompetitive antagonists PCP or ketamine causes schizophrenia-like psychoses in normal people and exacerbates psychotic symptoms in schizophrenics (Jentsch & Roth, 1999; Krystal et al., 1994). PCP psychosis in normal people mimics the positive, as well as the negative, symptoms observed in schizophrenia, and is regarded by many as the best pharmacological model of schizophrenia (Javitt & Zukin, 1991; Petersen & Stillman, 1978). Thus, animals treated with moderate doses of NMDA receptor antagonists, such as PCP, ketamine, or MK801, are used to model various aspects of schizophrenia. In rodents and monkeys, subanesthetic doses of NMDA antagonists produce behaviors analogous to those observed in schizophrenics. They include hyperlocomotion, enhanced stereotypic behaviors, cognitive and sensorimotor gating deficits, and impaired social interactions (Lipska & Weinberger, 2000). Like in human healthy controls, NMDA receptor antagonist models of schizophrenia in animals mimic not only positive symptoms, such as hyperlocomotion and stereotypy, but also negative symptoms such as deficits in social interaction (Gainetdinov et al., 2001). Importantly, the behavioral effects of subanesthetic doses of NMDA antagonists in rats and monkeys can be antagonized by the administration of antipsychotic drugs (Kilts, 2001).
Abnormalities of glutamatergic neurotransmission have also been linked to one of the perceptual/cognitive deficits seen in schizophrenia, i.e., abnormal sensorimotor gating. One of the experimental paradigms to study sensorimotor gating involves the examination of the startle reflex. The startle response to a strong stimulus is measured in the presence and absence of a weak stimulus immediately before the startle stimulus. In healthy animals and humans, a preceding weaker stimulus will blunt the response to the startle stimulus, a behavior referred to as ‘prepulse inhibition’ (PPI). Schizophrenics have impaired PPI of startle, i.e., their startle response is less blunted by a weaker stimulus immediately before the startle stimulus (Swerdlow & Geyer, 1998). Similar deficits in PPI are produced in rats under NMDA receptor inhibition (Geyer et al., 2001). Although the experimentally induced PPI deficits in rats may not represent an animal model of schizophrenia per se, they do provide a valid model of sensorimotor gating deficits seen in schizophrenia (Geyer et al., 2001). The effects of non-competitive NMDA antagonists on PPI are quite potent, as the doses that disrupt PPI are below the doses that affect locomotor activity (Mansbach & Geyer, 1989). Ketamine-disrupted PPI in rats is reversed with the antipsychotic drugs clozapine, seroquel, and chlorpromazine (Swerdlow et al., 1998), thus providing a link between the deficits in PPI, NMDA receptors, and schizophrenia.
Genetic animal models that use knockdown of NR1 receptors and knockout of the mouse NR2A receptor, also support a model in which reduced NMDA receptor activity results in schizophrenia-like behavior. Mice that express only 5% of the normal levels of the NR1 subunit display behavioral abnormalities similar to those observed in pharmacologically induced animal models of schizophrenia (Mohn et al., 1999). These abnormalities, which include increased motor activity, stereotypy, and deficits in social and sexual interactions, can be ameliorated by treatment with haloperidol or clozapine (Mohn et al., 1999). NR2A knockout mice, another example of malfunction of NMDA receptors, exhibited increased locomotor activity in a novel environment and an impairment of latent learning (which is associated with selective attention) in a water source-finding task (Miyamoto et al., 2001). Hyperlocomotion in NR2A mutant mice was attenuated by treatment with the antipsychotic drugs haloperidol and risperidone (Miyamoto et al., 2001).
In light of the implication of altered glutamate receptor function in schizophrenia, investigators have examined levels of glutamate receptors and found a dysregulation (Meador-Woodruff & Healy, 2000). However, it is difficult to draw inferences about receptor activity by studying receptor density. A decrease in the number of receptors may be interpreted as a molecular response to increased receptor activity or, conversely, as a cause of decreased receptor function. Keeping this explanatory dilemma in mind, it is worthwhile to review the studies of glutamate receptors in schizophrenia. Several investigators have reported that the expression of AMPA and kainate receptor subtypes is reduced in the hippocampus, an area that is thought to play an important role in schizophrenia (Gao et al., 2000; Meador-Woodruff & Healy, 2000). In a number of studies, the AMPA subunits GluR1 and GluR2 were decreased in the hippocampus and the parahippocampal gyrus (Eastwood et al., 1995b, 1997a, 1997b; Harrison et al., 1991). In concordance, ligand binding to AMPA receptors was decreased (Kerwin et al., 1990). The kainate receptor subtypes GluR6 and KA2 were also significantly reduced in the schizophrenic hippocampus (Porter et al., 1997). Studies on kainate receptor density, conducted with radiolabeled kainate, demonstrated a decrease in the hippocampus, as well as an increase in the cortex (Deakin et al., 1989; Kerwin et al., 1990; Nishikawa et al., 1983). Data for the NMDA receptor point to an abnormal expression in the cortex and putamen in schizophrenia (Meador-Woodruff & Healy, 2000). In the putamen, NMDA receptor numbers are elevated (Aparicio-Legarza et al., 1998; Kornhuber et al., 1989), and in the cortex, the number is increased and the composition of the NMDA receptor subunits is altered (Akbarian et al., 1996; Grimwood et al., 1999; Ishimaru et al., 1994; Nudmamud & Reynolds, 2001; Simpson et al., 1991). However, as might be expected from human post mortem studies, not all investigators found similar changes in the expression of ionotropic glutamate receptors in schizophrenia (Breese et al., 1995; Noga et al., 1997; Weissman et al., 1991). Nevertheless, several observations appear to be fairly consistent among different research groups. Among them is the abnormally low expression of AMPA/kainate receptors in the hippocampus and the increase in NMDA receptor numbers in putamen and cortex (Meador-Woodruff & Healy, 2000). This increase in NMDA receptor levels could be a consequence of decreased NMDA receptor function, whereas AMPA receptors may be reduced in brain areas with secondary glutamate elevation (see Fig. 6). Thus, while schizophrenia is accompanied by a dysregulation of ionotropic glutamate receptors, the pathological basis and consequences vary across brain areas, and are still subject to interpretation.
The expression of the inhibitory subunit NR3 has not been analyzed yet in post mortem schizophrenic brain. However, in the light of NMDA receptor hypofunction, the expression of these receptor subtypes will be important to explore in schizophrenia. Levels of expression of the NR3A subunit are particularly interesting, as NR3A has been found in brain areas of significance for schizophrenia, such as cortical areas and the thalamus (Sucher et al., 1995). mGluRs are of interest for schizophrenia research as well. Animal experiments suggested that Group II mGluRs (mGluR2/3) may have a functional role in schizophrenia. In rats, Group II mGluRs reverse the behavioral, locomotor, and cognitive effects of PCP (Cartmell et al., 2000; Moghaddam & Adams, 1998), and they seem to interfere with PPI (Grauer & Marquis, 1999). However, little evidence has been found for structural or quantitative differences of any mGluRs in schizophrenia. The expression of mGluRs (mGluR1-5, -7, and -8) was examined in various brain areas [prefrontal cortex (PFC), hippocampus, thalamus], but was found unaltered in schizophrenia (Crook et al., 2002; Ohnuma et al., 1998, 2000; Richardson-Burns et al., 2000). An association of a genetic polymorphism with schizophrenia has been reported for mGluR5 (Devon et al., 2001), whereas polymorphisms for mGluR2, -4, -7, and -8 did not demonstrate any significant association with schizophrenia (Bolonna et al., 2001; Bray et al., 2000; Joo et al., 2001; Ohtsuki et al., 2001). Interestingly, mGluR5 potentiates NMDA receptor activity, which could argue for a therapeutic potential of mGluR5 agonists in schizophrenia (Jia et al., 1998; Pisani et al., 2001).
Abnormal function of the glutamate system has consequences for other neurotransmitter systems in the brain, such as the GABAergic system, which may be reduced in its activity because it is under control of the glutamate system (Fig. 6) (Olney et al., 1999). Recent studies have provided evidence that hippocampal function during memory retrieval is abnormal in schizophrenia (Heckers et al., 1998). A pattern of hippocampal hyperactivity was observed (Heckers, 2001), which may be caused by a decrease of inhibitory, GABAergic activity of interneurons and a secondary increase in glutamate release (Fig. 6) (Benes, 1999; Benes & Berretta, 2001; Heckers et al., 2001). The hippocampal hyperactivity data fit well with the observed decrease in AMPA receptors (Meador-Woodruff & Healy, 2000) as a consequence of the secondary increase in glutamate release. While it may be speculative to assume that the primary defect is in the glutamate system rather than the GABA system, it is noteworthy that in experiments with NR2A knockout mice, [3H]GABA release was markedly diminished (Miyamoto et al., 2001). In these mice, the primary defect is in the glutamate system, which causes a secondary effect in the GABA system. In line with these data, it has been demonstrated that a decrease in the activity of NMDA receptors decreases GABA activity (Olney et al., 1989). The decreased stimulation of NMDA receptors on GABAergic neurons leads to a decreased release of GABA and a disinhibition of glutamate neurons under the control of GABA receptors. These disinhibited neurons proceed to release abnormally high levels of glutamate (Fig. 6) (Olney et al., 1991).
Taken together, the abnormalities found in schizophrenia and the various models of schizophrenia in humans and animals all point to an important contribution of the glutamate system to the disease. The central pathology seems to be caused by a hypofunction of NMDA receptors, followed by an overstimulation of non-NMDA receptors (e.g., AMPA/kainate). It will be important to find out which neurons express altered ionotropic glutamate receptor sub-types, whether these neurons are inhibitory or excitatory, and how the circuitries in which these neurons take part are affected.
2.3.2. Schizophrenia as a neurodevelopmental disorder: role of glutamate
Epidemiological evidence suggests that schizophrenia is established in early prenatal development. Obstetric complications, low birth weight, intrauterine malnutrition, smaller head circumference, congenital malformations, and maternal influenza during the second trimester are all positively correlated with the development of schizophrenia (Jones et al., 1998; Kunugi et al., 1996; McNeil et al., 1994; O'Callaghan et al., 1991; Wahlbeck et al., 2001; Watson et al., 1984; Willinger et al., 2001; Wright et al., 1995). Moreover, during childhood and early adolescence, neuro-motor dysfunction, psychological abnormalities, social mal-adjustment, and cognitive problems are observed long before schizophrenia is diagnosed (Done et al., 1994; Jones et al., 1994; Rosso et al., 2000; Walker et al., 1993, 1994). These early clinical and psychological observations suggest that schizophrenia is a neurodevelopmental disorder that starts prenatally and progresses throughout childhood and adolescence.
Anatomical studies also point to a neurodevelopmental etiology of abnormal neuronal circuits (Lewis & Lieberman, 2000; Selemon & Goldman-Rakic, 1999) and decreased density of dendritic spines (Garey et al., 1998; Glantz & Lewis, 2000). In concordance, synapse-related proteins are altered in the brains of schizophrenics (Eastwood et al., 1995a). The epidemiologic evidence, the abnormalities in cytoarchitecture, and the altered expression of synapse-related proteins provide support for a model of abnormal neurodevelopment in schizophrenia that affects neuronal migration, synaptogenesis, and synaptic pruning.
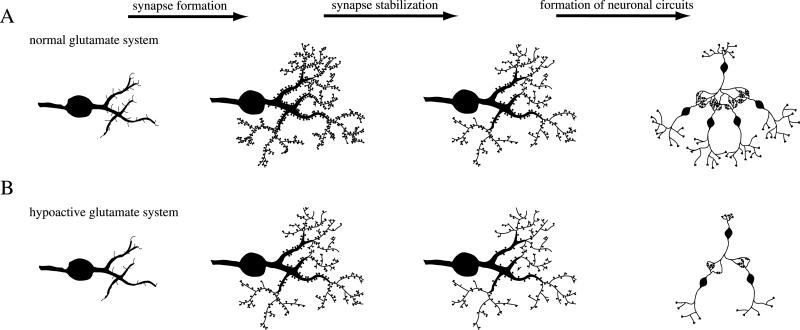
Since glutamate and its receptors play an important role in the establishment and maintenance of synaptic connections (Fig. 3), the anomaly of synaptic connections in schizophrenia might be a consequence of a hypoactive glutamate system during early development (Fig. 7). In a normal glutamate system, the formation of synapses and the retention of synapses during the period of synapse stabilization will provide well-functioning neuronal circuits with ample potential for proper communication between the neuron partners (Fig. 7A). If levels of glutamate or its receptors are low during early development, synapse formation and synapse stabilization will be affected (Fig. 7B). The consequence is a lower synapse number and sparser neuronal networks with inferior communication abilities, as has been hypothesized for schizophrenia (Friston & Frith, 1995).
Fig. 7.
A hypoactive glutamate system leads to sparse neuronal circuits. In a normal functioning glutamate system (A), an excess amount of synapses is formed in the cortex. During synapse stabilization, many of these synapses are retracted, leaving an optimal number of synapses to form neuronal circuits (Huttenlocher, 1984; Huttenlocher et al., 1982). With a weak glutamate system (B), less synapses are formed and less synapses are retained (see Fig. 3). The neuronal circuits in such a brain are insufficient to sustain proper function throughout life. Because initially an abundance of synapses is built, the problems with this circuit will be uncovered during the time of pruning when the number of connections falls below a critical threshold, which would be expected during late adolescence or early adulthood, coinciding with the time of onset of schizophrenia.
2.3.3. Neurotoxicity in schizophrenia
There is not much evidence for neurotoxicity as the cause of schizophrenia. Schizophrenia appears, by most accounts, to be a neurodevelopmental disorder (Bunney et al., 1997; Harrison, 1997; Woods, 1998). However, excitotoxic neurodegeneration may contribute to the course of the disease. Certain features of schizophrenia suggest a limited neurodegenerative process; e.g., a correlation between the progression of symptoms, including behavioral and cognitive deterioration (Lieberman, 1999), with ventricular enlargement (DeLisi et al., 1997; Flaum et al., 1995; Kelsoe et al., 1988; Pfefferbaum et al., 1988; Weinberger et al., 1979), hippocampal volume reduction (Becker et al., 1996; Heckers, 2001; Marsh et al., 1994), and a reduction in cortical gray matter (Lim et al., 1996; Zipursky et al., 1992). Some of these abnormalities may be present early on, and they may not progress much during the course of the illness (Davies et al., 1998; Illowsky et al., 1988; Nasrallah et al., 1986; Vita et al., 1997), while others are consistent with the notion that abnormal glutamatergic mechanisms and excitotoxicity contribute to schizophrenia (Olney et al., 1999). The most prominent example is the increased prevalence of spontaneous dyskinesias in untreated schizophrenia. Recent studies have estimated that between 5 and 15% of never-medicated schizophrenics experience spontaneous dyskinesias (Cassady et al., 1998; Fenton, 2000; Puri et al., 1998). Although the pathophysiology of spontaneous dyskinesias is not known (Casey, 2000), it is presumed that an abnormally low glutamatergic tone (NMDA receptors) causes an insufficient activation of GABAergic neurons and a disinhibition of glutamate neurons under the control of GABA (Fig. 6) (Coyle, 1996; Olney & Farber, 1995). The lack of histologic evidence of neurodegeneration such as gliosis in schizophrenia (Falke et al., 2000; Heckers, 1997; Roberts et al., 1986) does not rule out excitotoxicity through NMDA receptor hypofunction. Indeed, rats treated with NMDA antagonists had only a transient increase in gliosis, despite neuronal loss in the cortex (Fix et al., 1995). Thus, gliosis may occur in schizophrenia transiently, and may not be present any more at the time of the pathological examination.
Taken together, abnormal glutamatergic function could contribute to a primary neurodevelopmental, as well as secondary neurodegenerative/excitotoxic component, in schizophrenia.
3. Implications for the treatment of schizophrenia
Antipsychotic drugs are used to treat primarily psychosis in schizophrenia. However, they may also have beneficial effects on the progression of the disease. Epidemiological studies suggest that early intervention in schizophrenia with antipsychotic drugs may help to reduce psychotic symptoms in subsequent episodes and to reduce relapse (Lewis & Lieberman, 2000; Lieberman et al., 2001; Robinson et al., 1999).
Antipsychotic drugs are commonly grouped into ‘conventional antipsychotic drugs’ and ‘atypical antipsychotic drugs.’ The differences between both groups are defined in clinical, as well as in pharmacological, terms. Clinically, atypical antipsychotic drugs cause less extrapyramidal side effects, less tardive dyskinesia (TD), and are more effective than conventional antipsychotic drugs in treating negative symptoms (e.g., social withdrawal, flattened affect). Pharmacologically, conventional antipsychotic drugs such as haloperidol have a high affinity for D2 receptors (Levinson, 1991), whereas atypical antipsychotic drugs such as cloza-pine have affinities to multiple receptor systems, including D2 receptors (Remington & Chong, 1999).
Antipsychotic drugs act primarily on the dopamine and serotonin (5-HT) systems, and although they have direct effects on the glutamate system, in general, these are small. However, through their interaction with monoaminergic systems, antipsychotic drugs can modulate glutamatergic function via a powerful, indirect mechanism (Leveque et al., 2000).
3.1. Pharmacology of antipsychotic drugs
It has been reported that the clinical potency of conventional antipsychotic drugs is directly correlated with their affinity for the dopamine D2 receptor (Creese et al., 1976; Seeman & Lee, 1975; Seeman & Van Tol, 1993). Conventional antipsychotic drugs inhibit D2 receptors and dopa-mine release (Creese et al., 1976; Seeman & Lee, 1975). Optimal therapeutic benefits are observed in the range of 70–80% D2 receptor occupancy (Kapur et al., 1999). This has led to the hypothesis that schizophrenia is caused by an overactive dopaminergic system (Meltzer & Stahl, 1976). However, conventional antipsychotic drugs are not just D2 antagonists, but are also effectors of other neurotransmitter systems (Andersson et al., 1998; Stockmeier et al., 1993) and, to some part, of the glutamate system (see Section 3.3) (Arvanov et al., 1997; Brimecombe et al., 1998; Fletcher & MacDonald, 1993; Gallagher et al., 1998; Ilyin et al., 1996; Lidsky et al., 1997; Micheletti et al., 1993).
Whereas all conventional antipsychotic drugs are D2 antagonists (Creese et al., 1976; Seeman & Lee, 1975), the mechanism of action of atypical antipsychotic drugs is less homogenous (Andersson et al., 1998). Clozapine is the prototypical atypical antipsychotic drug; the list of atypical antipsychotic drugs also includes risperidone, olanzapine, ziprasidone, quetiapine, and sertindole. Atypical antipsychotic drugs are grouped together for their lack of extra-pyramidal side effects typical for conventional antipsychotic drugs, but as a group, they have different pharmacological profiles and different side effects. More than one pharmacological feature seems to contribute to the therapeutic properties of atypical antipsychotic drugs. There is some evidence that they interact with dopamine D2, D4, and D1 receptors, 5-HT2 and 5-HT1A receptors, muscarinic m4 receptors, adrenergic a1 receptors, and histamine H1 receptors (Bymaster et al., 1996, 1999; Canton et al., 1990; Kapur et al., 1998, 1999; Lavalaye et al., 1999; Meltzer, 1994, 1999; Nordstrom et al., 1995; Nyberg et al., 1997; Pilowsky et al., 1996; Prinssen et al., 1994; Raedler et al., 1999; Richelson, 1999; Seeman, 1992; Stockmeier et al., 1993; Travis et al., 1998; Trichard et al., 1998; Zorn et al., 1994), but none of these effects predominate or can explain satisfactorily the mechanism of action of atypical antipsychotic drugs. Although it is possible that different antipsychotic drugs are atypical for different reasons, one prevailing theory suggests that the relationship of the inhibitory potency at 5-HT2A receptors and D2 receptors may be important for the ‘atypical’ profile (Meltzer, 1999; Remington & Kapur, 1999; Richelson, 1999).
An interesting clinical difference between conventional and atypical antipsychotic drugs is that the latter are more effective in reducing the negative symptoms and in improving the cognitive deficits of schizophrenia, whereas the former have more motor side effects (Tandon et al., 1999). This feature is explained by the anatomical differences in their sites of action. Atypical antipsychotic drugs have a stronger effect in the mesolimbic dopamine system [ventral tegmental area (VTA), PFC, nucleus accumbens], whereas conventional antipsychotic drugs have a stronger effect in the nigrostriatal dopamine system (substantia nigra, striatum). Analysis of Fos expression in rats after treatment with conventional and atypical antipsychotic drugs demonstrates that conventionals induce Fos expression primarily in the striatum and nucleus accumbens, whereas atypical antipsychotic drugs induce Fos expression in the PFC, nucleus accumbens, and the striatum (MacGibbon et al., 1994; Nguyen et al., 1992; Robertson & Fibiger, 1992; Robertson et al., 1994; Sebens et al., 1995; Wan et al., 1995). Studies that measure dopamine-cell depolarization block, the delayed inactivation of dopamine-neuron firing in the mid-brain after treatment with antipsychotic drugs, show a stronger effect in the VTA by atypical antipsychotic drugs and in the substantia nigra by conventional antipsychotic drugs (Grace et al., 1997). Finally, in schizophrenics, striatal volume is increased after treatment with conventional anti-psychotic drugs, but not with atypical antipsychotic drugs (Chakos et al., 1994; Gur et al., 1998; Keshavan et al., 1994). In rats treated chronically with haloperidol, it was confirmed that chronic neuroleptic treatment causes striatal enlargement (Chakos et al., 1998). Thus, conventional antipsychotic drugs have a much stronger influence on striatal plasticity than atypical antipsychotic drugs.
3.2. Direct interaction of antipsychotic drugs with the glutamatergic neurotransmitter system
An ideal pharmacologic support of the glutamate hypothesis of schizophrenia would be the demonstration that glutamate receptor agonists have antipsychotic properties. Unfortunately, glutamate agonists cannot be used for therapeutic purposes, as unphysiologic excitation of the glutamatergic system will inevitably cause neurotoxicity and neuronal death (Choi, 1988; Choi & Rothman, 1990). However, facilitation of NMDA receptor function, when combined with antipsychotic drugs, has been linked to an improved treatment outcome in schizophrenia (Goff et al., 1999b; Heresco-Levy et al., 1996a, 1999). Interestingly, many of the known antipsychotic drugs interact directly with the glutamate system. At concentrations similar to those found in the cerebrospinal fluid of schizophrenics, antipsychotic drugs bind to NMDA receptors, augment NMDA activity, and increase the expression of AMPA and NMDA receptor subtypes (Banerjee et al., 1995; Brene et al., 1998; Eastwood et al., 1996; Fitzgerald et al., 1995; Lidsky et al., 1997; Meshul et al., 1996). Chronic treatment with conventional antipsychotic drugs inhibits the glutamate transporter (De Souza et al., 1999) and increases the basal concentration of extracellular glutamate (Meshul et al., 1996; Yamamoto & Cooperman, 1994). Likewise, atypical antipsychotic drugs increase the release of glutamate (Arvanov et al., 1997; Daly & Moghaddam, 1993; Evins et al., 1997) and of glycine (Chapman & See, 1996). However, the direct effects of antipsychotic drugs on the glutamate system are far less robust than their effects on other systems such as the dopamine system.
3.3. Antipsychotic drugs modulate N-methyl-d-aspartate receptor activity via the dopamine system
Conventional antipsychotic drugs are potent dopamine D2 receptor inhibitors (Creese et al., 1976; Seeman & Lee, 1975). Inhibition of D2 receptors can cause activation of the glutamate system. Indeed, many properties of haloperidol seem to originate in its facilitation of the glutamate system and of the NMDA receptor. Behaviorally, NMDA antagonists prevent haloperidol-induced catalepsy in rats, suggesting that neuroleptic-induced catalepsy is caused by an activation of NMDA receptors (Kaur et al., 1997; Moore et al., 1993; Yoshida et al., 1991). On the molecular level, NMDA antagonists block haloperidol-mediated striatal gene expression, which indicates that haloperidol leads to gene expression through an activation of the NMDA receptor (Boegman & Vincent, 1996; Dragunow et al., 1990; Leveque et al., 2000; Ziolkowska & Hollt, 1993). These findings cannot be explained by a direct interaction of haloperidol with the NMDA receptor, since haloperidol is a weak inhibitor of the NMDA receptor (Coughenour & Cordon, 1997; Gallagher et al., 1998; Hayashi et al., 1995; Ilyin et al., 1996; Lynch & Gallagher, 1996), an action that is contrary to the behavioral and molecular findings. However, the glutamate and dopamine systems interact in a way that enables D2 antagonists to increase the activity of the glutamate system. Dopamine and glutamate are involved in the same neuronal circuits and influence each other's activity and release of neurotransmitter (Morari et al., 1998). Generally, D2 receptor agonists decrease the activity of the glutamate system, whereas D2 antagonists increase the activity (Cepeda et al., 2001; Koga & Momiyama, 2000; Yamamoto & Davy, 1992).
Dopamine neurons emanate from two areas in the mid-brain, the substantia nigra and the VTA. Neurons of the substantia nigra are predominantly involved in the nigralstriatal motor circuits, whereas neurons from the VTA are involved in corticolimbic circuits, which include the nucleus accumbens and the PFC. Dopaminergic neurons in the substantia nigra and the VTA receive glutamatergic inputs (Carr et al., 1999; Carr & Sesack, 2000; Counihan et al., 1998; Gauchy et al., 1994; Smith et al., 1996), and gluta-mate stimulates dopaminergic activity (Meltzer et al., 1997). Conversely, the glutamate system is inhibited by dopamine and facilitated by the inhibition of D2 receptors. D2 receptors on glutamatergic fibers in the VTA inhibit glutamate release, while D2 antagonists reverse this inhibition (Koga & Momiyama, 2000). D2 receptors also reduce glutamate receptor-mediated activity in the corticostriatal and thalamostriatal pathways, while D2 antagonists increase the activity (Cepeda et al., 2001). These data indicate that modulation of the dopamine system by antipsychotic drugs influences the performance of the glutamate system. Conversely, malfunction of the glutamate system affects the performance of the dopamine system (Meador-Woodruff & Healy, 2000).
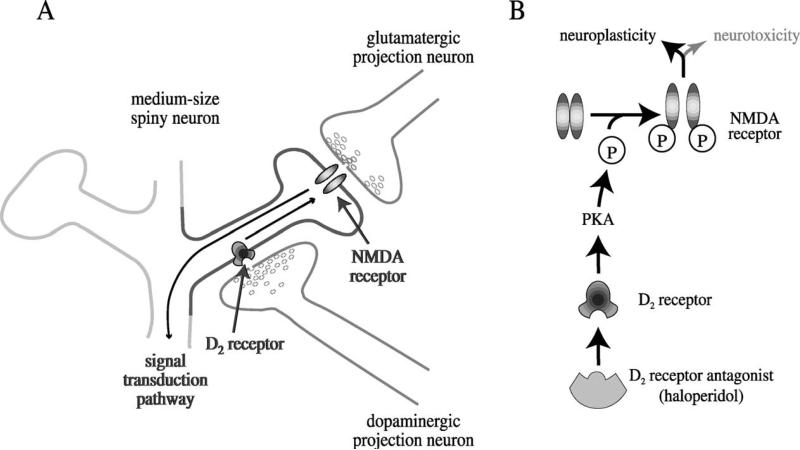
Aside from the systemic interaction, an intracellular interaction between D2 receptors and NMDA receptors in the striatum was reported (Fig. 8) (Cepeda et al., 1993; Leveque et al., 2000). D2 receptor antagonists activate an intraneuronal signal transduction pathway that causes phosphorylation of the NMDA receptor (Fig. 8B). This phosphorylation enhances the activity of the NMDA receptor, which leads to the activation of the NMDA signal transduction pathway and to gene expression (Leveque et al., 2000). Haloperidol depends on NMDA receptors to increase gene expression, and cannot do so when NMDA receptors are blocked (Boegman & Vincent, 1996; Dragunow et al., 1990; Leveque et al., 2000; Ziolkowska & Hollt, 1993).
Fig. 8.
D2 antagonists such as haloperidol facilitate NMDA receptor activity in the striatum by an intracellular mechanism. A: The medium-size spiny neurons in the striatum receive glutamate inputs from the cortex and dopamine inputs from the midbrain. Inhibition of D2 receptors on the spiny neurons facilitates NMDA receptor activity and promotes a signal transduction pathway to the nucleus. B: D2 antagonists activate an intracellular signal transduction pathway, including protein kinase A (PKA), which leads to the phosphorylation of the NR1 subtype of the NMDA receptor (Leveque et al., 2000). This phosphorylation increases the sensitivity of the NMDA receptor to glutamate and stimulates a signal transduction pathway that reaches the cell body. Gene expression enables the neuron to adjust its structure and function. Strong and persistent inhibition of D2 receptors can overstimulate NMDA receptors and cause neurotoxicity. P, phosphate residue.
The monoamine 5-HT may modulate the glutamate system in a fashion similar to the monoamine dopamine (Aghajanian & Marek, 2000). In brain areas innervated by 5-HT and dopamine, a two-pronged approach of an antipsychotic drug, modulation of the glutamate system via dopamine and via 5-HT, can work with a low affinity of the drug to either monoaminergic system. Moreover, because of anatomical differences in the target areas of 5-HT and dopamine, anti-psychotic drugs that affect both systems such as clozapine will have a wider impact across brain areas. For a ‘disease of neuronal connectivity’ that affects multiple brain areas (Andreasen, 2000), this seems a fitting treatment approach.
While many of the effects of antipsychotic drugs on the glutamatergic system may be elicited indirectly via other neurotransmitter systems, the glutamate system could be essential for the therapeutic properties of these drugs (Konradi & Heckers, 2001).
3.4. Facilitation of glutamate receptor activity as treatment for schizophrenia
Glycine, the agonist at the glycine site of the NMDA receptor, was tested as adjuvant to antipsychotic drugs. It improved negative symptoms when combined with conventional antipsychotic drugs (Heresco-Levy et al., 1996a, 1999; Javitt et al., 1994), but not when combined with clozapine (Evins et al., 2000; Potkin et al., 1999). Unfortunately, glycine does not enter the brain freely and had to be administered at uncomfortably high doses (30–60 g per day) (Heresco-Levy et al., 1999; Javitt et al., 1994). Thus, two agents that readily cross the blood-brain barrier were used in clinical trials: D-cycloserine, a partial agonist at the glycine site of the NMDA receptor (Emmett et al., 1991; Henderson et al., 1990; Johnson & Ascher, 1987), and D-serine, a full agonist (Tsai et al., 1998b). Both improved negative symptoms and cognitive function when added to conventional neuroleptics (Goff et al., 1995b, 1999b; Tsai et al., 1998b), but not when added to clozapine (Goff et al., 1996, 1999a; Tsai et al., 1999). The co-administration of clozapine and D-cycloserine even worsened the negative symptoms in schizophrenia (Goff et al., 1999a). How is the different effect of glycine site agonists on clozapine versus conventional antipsychotic drugs explained? The rationale for clinical trials of glycinergic agents is that the glycine sites of the NMDA receptor complex are not fully saturated, and thus, NMDA receptor activity is restrained. Indeed, the glycine transporter may keep glycine concentrations in the synaptic cleft at levels that prevent maximal NMDA receptor currents (Supplisson & Bergman, 1997). Clozapine has been shown to elevate extracellular levels of glycine in the brain of rats (Chapman & See, 1996), whereas haloperidol has no effect or may even lower glycine levels (Baruah et al., 1993; Chapman & See, 1996). Because clozapine already increases the levels of glycine, the administration of glycine agonists will not further benefit NMDA activity. The partial agonist D-cycloserine acts as an agonist at the glycine site when endogenous levels of ligand are low, and as an antagonist when endogenous levels of ligand are high. This explains why D-cycloserine decreases the therapeutic effect of clozapine.
Recently, AMPA receptors have been considered as targets for the treatment of schizophrenia. Ampakines, novel compounds that enhance synaptic currents mediated by AMPA, have shown promise as adjuvants to clozapine treatment (Goff et al., 2001; Johnson et al., 1999).
3.5. Role of glutamate in neuroplasticity by antipsychotic drugs
The delayed effect of antipsychotic drugs suggests that neuroplasticity is an important component of their therapeutic benefits (Hyman & Nestler, 1996; Konradi & Heckers, 2001). In fact, antipsychotic drugs are known to induce neuroplasticity in the striatum and in brain areas relevant for the cognitive deficits seen in schizophrenia (Robertson & Fibiger, 1992; Robertson et al., 1994). Haloperidol, for instance, has been shown to induce TFs in the striatum and to affect the expression of many proteins (Konradi & Heckers, 2001). Ultrastructural alterations of synaptic structures (Benes et al., 1985; Kerns et al., 1992; Uranova et al., 1991) and increases in striatal volumes in human brain (Chakos et al., 1994; Heckers et al., 1991; Jernigan et al., 1991; Shihabuddin et al., 1998) have also been described. Interestingly, the number of glutamate-containing synapses is increased in the rat striatum after chronic haloperidol treatment (Kerns et al., 1992; Meshul & Casey, 1989; See et al., 1992; Uranova et al., 1991), and an increase in the number of perforated synapses and double synapses has been reported (Kerns et al., 1992). Thus, haloperidol seems to promote synapse splitting, a process by which synapses multiply in the mature brain (Jones & Harris, 1995; Kirov et al., 1999; Toni et al., 1999) (Fig. 4B). While neuroanatomic changes by conventional antipsychotic drugs have been studied predominantly in the striatum, it is assumed that other brain areas with more relevance for schizophrenia show similar adaptations, albeit not as strongly as the striatum (Konradi & Heckers, 2001). Atypical antipsychotic drugs affect neuroplasticity in brain areas more relevant for schizophrenia. C-Fos expression, for example, is induced by atypical antipsychotic drugs in many brain areas relevant for schizophrenia, such as the PFC and nucleus accumbens (Deutch & Duman, 1996; Lidow & Goldman-Rakic, 1997; Nguyen et al., 1992; Robertson & Fibiger, 1992).
Glutamate and NMDA receptors are involved in many aspects of neuroplasticity. Antipsychotic drugs induce gene expression via a stimulation of NMDA receptors (Leveque et al., 2000). Thus, neuroplasticity by antipsychotic drugs depends on a functioning glutamate system, which puts glutamate at the center of consideration for the delayed therapeutic benefits of antipsychotic drugs.
3.6. Role of glutamate in neurotoxicity by antipsychotic drugs
The facilitation of the glutamate system by conventional antipsychotic drugs in the striatum could be a risk factor for TD (Hamid et al., 1998; Hauber, 1996; Hauber & Schmidt, 1990; Kaur et al., 1997). Indeed, patients who develop TD in response to neuroleptic drug treatment have higher markers for glutamatergic neurotransmission than patients who do not develop TD (Goff et al., 1995a; Tsai et al., 1998a). TD is thought to be a consequence of oxidative stress and excitotoxicity by glutamate (Andreassen & Jorgensen, 2000). These data suggest that in a subgroup of patients, antipsychotic drugs up-regulate the glutamate system to excitotoxic levels. In addition, episodic peak releases of glutamate could be excitotoxic and could progressively damage neurons. There is little protection for these neurons against an overactive glutamate system triggered by a pharmacologic agent for which endogenous defense systems are not readily available.
Atypical antipsychotic drugs have a much lower probability to cause TD (Casey, 1999; Glazer, 2000a, 2000b; Littrell et al., 1998). This is consistent with the finding that they are less effective than conventional antipsychotic drugs in facilitating gene expression and NMDA receptor activity in the striatum (Leveque et al., 2000; Nguyen et al., 1992; Robertson et al., 1994). While the pharmacological mechanism of action of atypical antipsychotic drugs is less clear than that of conventionals, their lower affinity to D2 receptors could avert an excitotoxic activation of NMDA receptors in motor areas.
The combination neuroplasticity-neurotoxicity observed with conventional antipsychotic drugs reflects the complex nature of the glutamate system (Bear & Malenka, 1994; Choi, 1988; Malenka & Nicoll, 1993; Mattson, 1988; Olney, 1994; Platenik et al., 2000; Rothman & Olney, 1995). While the induction of neuroplasticity by antipsychotic drugs seems important for the therapeutic benefits, the generation of neurotoxicity is detrimental. Therefore, it is important that we find physiologic ways to stabilize and facilitate the glutamate system in schizophrenia by means that provide ‘brakes’ only when the glutamate system is overactive. Partial NMDA receptor agonists (which allow normal levels of glutamatergic neurotransmission, but block high levels) and Ampakines could fulfill some of these requirements, as could effectors of neuromodulatory systems, such as monoaminergic systems or mGluRs.
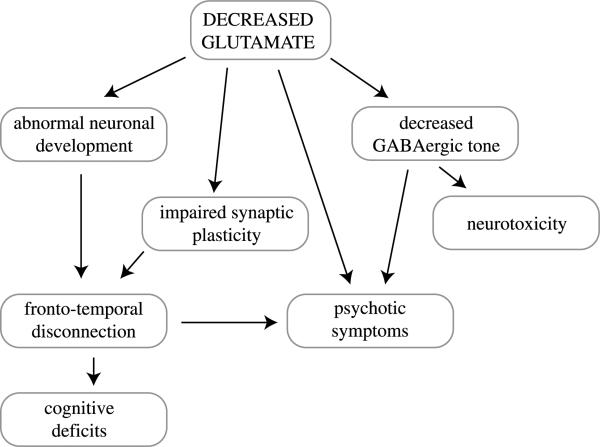
4. Conclusion
A hypoactive glutamate system can severely impede the proper formation of neural circuits during brain devel opment. Even if connections are formed properly and in abundance during early development, the hypoactive glutamate system can cause excessive pruning and can affect the number of synapses retained during adolescence. Moreover, the failure to properly and consistently activate GABA neurons may result in neurotoxicity. Thus, a hypoactive glutamate system has a negative influence on neuroplasticity and it facilitates neurotoxicity.
There is much biochemical, pharmacological, and clinical evidence that the glutamate system is abnormal in schizophrenia. Several models of schizophrenia, e.g., the neuro-developmental and the progressive neurodegeneration models, the overactive dopamine or the hypoactive GABA system models, can be explained by a primary episodic malfunctioning of the glutamate system (Fig. 9). This malfunction could be based on genetic abnormalities and could be exacerbated by stress and environmental factors. In light of the role of glutamate in the pathology of schizophrenia, a pharmacologic stabilization of the glutamate system may allow us to prevent psychotic episodes and neurotoxicity.
Fig. 9.
Implications of glutamate hypofunction for schizophrenia. Decreased glutamate levels cause abnormal neuronal development, impaired synaptic plasticity, psychotic symptoms, decreased GABAergic tone, and neurotoxicity. Inhibition of D2 receptors increases the activity of glutamate receptors.
Acknowledgements
This work was supported by the National Alliance for Research on Schizophrenia and Depression (C.K., S.H.) and a National Institute of Drug Abuse grant DA07134 (C.K.).
Abbreviations
- AA
arachidonic acid
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- CaM kinase
Ca2+/calmodulin-dependent protein kinase
- CREB
cyclic AMP response element-binding protein
- GABA
γ-aminobutyric acid
- mGluR
metabotropic glutamate receptor
- 5-HT
serotonin
- LTD
long-term depression
- LTP
long-term potentiation
- NMDA
N-methyl-d-aspartate
- PCP
phencyclidine
- PFC
prefrontal cortex
- PPI
prepulse inhibition
- ROS
reactive oxygen species
- TD
tardive dyskinesia
- TF
transcription factor
- VTA
ventral tegmental area
References
- Aghajanian GK, Marek GJ. Serotonin model of schizophrenia: emerging role of glutamate mechanisms. Brain Res Brain Res Rev. 2000;31:302–312. doi: 10.1016/s0165-0173(99)00046-6. [DOI] [PubMed] [Google Scholar]
- Ahmari SE, Buchanan J, Smith SJ. Assembly of pre-synaptic active zones from cytoplasmic transport packets. Nat Neurosci. 2000;3:445–451. doi: 10.1038/74814. [DOI] [PubMed] [Google Scholar]
- Akbarian S, Sucher NJ, Bradley D, Tafazzoli A, Trinh D, Hetrick WP, Potkin SG, Sandman CA, Bunney WE, Jr., Jones EG. Selective alterations in gene expression for NMDA receptor subunits in prefrontal cortex of schizophrenics. J Neurosci. 1996;16:19–30. doi: 10.1523/JNEUROSCI.16-01-00019.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alnemri ES. Mammalian cell death proteases: a family of highly conserved aspartate specific cysteine proteases. J Cell Biochem. 1997;64:33–42. doi: 10.1002/(sici)1097-4644(199701)64:1<33::aid-jcb6>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Andersson C, Chakos M, Mailman R, Lieberman J. Emerging roles for novel antipsychotic medications in the treatment of schizophrenia. Psychiatr Clin North Am. 1998;21:151–179. doi: 10.1016/s0193-953x(05)70365-8. [DOI] [PubMed] [Google Scholar]
- Andreasen NC. Pieces of the schizophrenia puzzle fall into place. Neuron. 1996;16:697–700. doi: 10.1016/s0896-6273(00)80090-2. [DOI] [PubMed] [Google Scholar]
- Andreasen NC. Schizophrenia: the fundamental questions. Brain Res Brain Res Rev. 2000;31:106–112. doi: 10.1016/s0165-0173(99)00027-2. [DOI] [PubMed] [Google Scholar]
- Andreassen OA, Jorgensen HA. Neurotoxicity associated with neuroleptic-induced oral dyskinesias in rats. Implications for tardive dyskinesia? Prog Neurobiol. 2000;61:525–541. doi: 10.1016/s0301-0082(99)00064-7. [DOI] [PubMed] [Google Scholar]
- Ankarcrona M, Dypbukt JM, Bonfoco E, Zhivotovsky B, Orrenius S, Lipton SA, Nicotera P. Glutamate-induced neuronal death: a succession of necrosis or apoptosis depending on mitochondrial function. Neuron. 1995;15:961–973. doi: 10.1016/0896-6273(95)90186-8. [DOI] [PubMed] [Google Scholar]
- Aparicio-Legarza MI, Davis B, Hutson PH, Reynolds GP. Increased density of glutamate/N-methyl-d-aspartate receptors in putamen from schizophrenic patients. Neurosci Lett. 1998;241:143–146. doi: 10.1016/s0304-3940(98)00017-2. [DOI] [PubMed] [Google Scholar]
- Aramori I, Nakanishi S. Signal transduction and pharmacological characteristics of a metabotropic glutamate receptor, mGluR1, in transfected CHO cells. Neuron. 1992;8:757–765. doi: 10.1016/0896-6273(92)90096-v. [DOI] [PubMed] [Google Scholar]
- Arvanov VL, Liang X, Schwartz J, Grossman S, Wang RY. Clozapine and haloperidol modulate N-methyl-d-aspartate-and non-N-methyl-d-aspartate receptor-mediated neurotransmission in rat prefrontal cortical neurons in vitro. J Pharmacol Exp Ther. 1997;283:226–234. [PubMed] [Google Scholar]
- Ascher P, Nowak L. Electrophysiological studies of NMDA receptors. Trends Neurosci. 1987;10:284–287. [Google Scholar]
- Bailey CH, Bartsch D, Kandel ER. Toward a molecular definition of long-term memory storage. Proc Natl Acad Sci USA. 1996;93:13445–13452. doi: 10.1073/pnas.93.24.13445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee SP, Zuck LG, Yablonsky-Alter E, Lidsky TI. Glutamate agonist activity: implications for antipsychotic drug action and schizophrenia. Neuroreport. 1995;6:2500–2504. [PubMed] [Google Scholar]
- Baruah S, Waziri R, Sherman A. Neuroleptic effects on serine and glycine metabolism. Biol Psychiatry. 1993;34:544–550. doi: 10.1016/0006-3223(93)90197-l. [DOI] [PubMed] [Google Scholar]
- Bear MF, Malenka RC. Synaptic plasticity: LTP and LTD. Curr Opin Neurobiol. 1994;4:389–399. doi: 10.1016/0959-4388(94)90101-5. [DOI] [PubMed] [Google Scholar]
- Beattie EC, Carroll RC, Yu X, Morishita W, Yasuda H, von Zastrow M, Malenka RC. Regulation of AMPA receptor endocytosis by a signaling mechanism shared with LTD. Nat Neurosci. 2000;3:1291–1300. doi: 10.1038/81823. [DOI] [PubMed] [Google Scholar]
- Becker T, Elmer K, Schneider F, Schneider M, Grodd W, Bartels M, Heckers S, Beckmann H. Confirmation of reduced temporal limbic structure volume on magnetic resonance imaging in male patients with schizophrenia. Psychiatry Res. 1996;67:135–143. doi: 10.1016/0925-4927(96)03002-8. [DOI] [PubMed] [Google Scholar]
- Benes FM. Evidence for altered trisynaptic circuitry in schizophrenic hippocampus. Biol Psychiatry. 1999;46:589–599. doi: 10.1016/s0006-3223(99)00136-5. [DOI] [PubMed] [Google Scholar]
- Benes FM, Berretta S. GABAergic interneurons: implications for understanding schizophrenia and bipolar disorder. Neuropsychopharmacology. 2001;25:1–27. doi: 10.1016/S0893-133X(01)00225-1. [DOI] [PubMed] [Google Scholar]
- Benes FM, Paskevich PA, Davidson J, Domesick VB. The effects of haloperidol on synaptic patterns in the rat striatum. Brain Res. 1985;329:265–273. doi: 10.1016/0006-8993(85)90532-3. [DOI] [PubMed] [Google Scholar]
- Bergeron L, Yuan J. Sealing one's fate: control of cell death in neurons. Curr Opin Neurobiol. 1998;8:55–63. doi: 10.1016/s0959-4388(98)80008-1. [DOI] [PubMed] [Google Scholar]
- Bito H, Deisseroth K, Tsien RW. CREB phosphorylation and dephosphorylation: a Ca2+ - and stimulus duration-dependent switch for hippocampal gene expression. Cell. 1996;87:1203–1214. doi: 10.1016/s0092-8674(00)81816-4. [DOI] [PubMed] [Google Scholar]
- Boegman RJ, Vincent SR. Involvement of adenosine and glutamate receptors in the induction of c-fos in the striatum by haloperidol. Synapse. 1996;22:70–77. doi: 10.1002/(SICI)1098-2396(199601)22:1<70::AID-SYN8>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Bolonna AA, Kerwin RW, Munro J, Arranz MJ, Makoff AJ. Polymorphisms in the genes for mGluR types 7 and 8: association studies with schizophrenia. Schizophr Res. 2001;47:99–103. doi: 10.1016/s0920-9964(99)00235-2. [DOI] [PubMed] [Google Scholar]
- Bortolotto ZA, Fitzjohn SM, Collingridge GL. Roles of metabotropic glutamate receptors in LTP and LTD in the hippocampus. Curr Opin Neurobiol. 1999;9:299–304. doi: 10.1016/s0959-4388(99)80044-0. [DOI] [PubMed] [Google Scholar]
- Bourtchuladze R, Frenguelli B, Blendy J, Cioffi D, Schutz G, Silva AJ. Deficient long-term memory in mice with a targeted mutation of the cAMP-responsive element-binding protein. Cell. 1994;79:59–68. doi: 10.1016/0092-8674(94)90400-6. [DOI] [PubMed] [Google Scholar]
- Bray NJ, Williams NM, Bowen T, Cardno AG, Gray M, Jones LA, Murphy KC, Sanders RD, Spurlock G, Odonovan MC, Owen MJ. No evidence for association between a non-synonymous polymorphism in the gene encoding human metabotropic glutamate receptor 7 and schizophrenia. Psychiatr Genet. 2000;10:83–86. doi: 10.1097/00041444-200010020-00005. [DOI] [PubMed] [Google Scholar]
- Bredesen DE. Neural apoptosis. Ann Neurol. 1995;38:839–851. doi: 10.1002/ana.410380604. [DOI] [PubMed] [Google Scholar]
- Breese CR, Freedman R, Leonard SS. Glutamate receptor subtype expression in human postmortem brain tissue from schizophrenics and alcohol abusers. Brain Res. 1995;674:82–90. doi: 10.1016/0006-8993(94)01384-t. [DOI] [PubMed] [Google Scholar]
- Brene S, Messer C, Nestler EJ. Expression of messenger RNAs encoding ionotropic glutamate receptors in rat brain: regulation by haloperidol. Neuroscience. 1998;84:813–823. doi: 10.1016/s0306-4522(97)00490-9. [DOI] [PubMed] [Google Scholar]
- Brimecombe JC, Gallagher MJ, Lynch DR, Aizenman E. An NR2B point mutation affecting haloperidol and CP101,606 sensitivity of single recombinant N-methyl-d-aspartate receptors. J Pharmacol Exp Ther. 1998;286:627–634. [PubMed] [Google Scholar]
- Bromet EJ, Fennig S. Epidemiology and natural history of schizophrenia. Biol Psychiatry. 1999;46:871–881. doi: 10.1016/s0006-3223(99)00153-5. [DOI] [PubMed] [Google Scholar]
- Bunney BG, Potkin SG, Bunney WE. Neuropathological studies of brain tissue in schizophrenia. J Psychiatr Res. 1997;31:159–173. doi: 10.1016/s0022-3956(96)00038-6. [DOI] [PubMed] [Google Scholar]
- Bymaster FP, Hemrick-Luecke SK, Perry KW, Fuller RW. Neurochemical evidence for antagonism by olanzapine of dopamine, serotonin, α1-adrenergic and muscarinic receptors in vivo in rats. Psychopharmacology. 1996;124:87–94. doi: 10.1007/BF02245608. [DOI] [PubMed] [Google Scholar]
- Bymaster FP, Nelson DL, DeLapp NW, Falcone JF, Eckols K, Truex LL, Foreman MM, Lucaites VL, Calligaro DO. Antagonism by olanzapine of dopamine D1, serotonin2, muscarinic, histamine H1 and α1-adrenergic receptors in vitro. Schizophr Res. 1999;37:107–122. doi: 10.1016/s0920-9964(98)00146-7. [DOI] [PubMed] [Google Scholar]
- Canton H, Verriele L, Colpaert FC. Binding of typical and atypical antipsychotics to 5-HT1C and 5-HT2 sites: clozapine potently interacts with 5-HT1C sites. Eur J Pharmacol. 1990;191:93–96. doi: 10.1016/0014-2999(90)94100-c. [DOI] [PubMed] [Google Scholar]
- Carpenter WT, Jr., Buchanan RW. Schizophrenia. New Engl J Med. 1994;330:681–690. doi: 10.1056/NEJM199403103301006. [DOI] [PubMed] [Google Scholar]
- Carr DB, Sesack SR. Projections from the rat prefrontal cortex to the ventral tegmental area: target specificity in the synaptic associations with mesoaccumbens and mesocortical neurons. J Neurosci. 2000;20:3864–3873. doi: 10.1523/JNEUROSCI.20-10-03864.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr DB, O'Donnell P, Card JP, Sesack SR. Dopamine terminals in the rat prefrontal cortex synapse on pyramidal cells that project to the nucleus accumbens. J Neurosci. 1999;19:11049–11060. doi: 10.1523/JNEUROSCI.19-24-11049.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartmell J, Schoepp DD. Regulation of neurotransmitter release by metabotropic glutamate receptors. J Neurochem. 2000;75:889–907. doi: 10.1046/j.1471-4159.2000.0750889.x. [DOI] [PubMed] [Google Scholar]
- Cartmell J, Monn JA, Schoepp DD. Attenuation of specific PCP-evoked behaviors by the potent mGlu2/3 receptor agonist, LY379268 and comparison with the atypical antipsychotic, clozapine. Psychopharmacology. 2000;148:423–429. doi: 10.1007/s002130050072. [DOI] [PubMed] [Google Scholar]
- Carvalho AL, Kameyama K, Huganir RL. Characterization of phosphorylation sites on the glutamate receptor 4 subunit of the AMPA receptors. J Neurosci. 1999;19:4748–4754. doi: 10.1523/JNEUROSCI.19-12-04748.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey DE. Tardive dyskinesia and atypical antipsychotic drugs. Schizophr Res. 1999;35:S61–S66. doi: 10.1016/s0920-9964(98)00160-1. [DOI] [PubMed] [Google Scholar]
- Casey DE. Tardive dyskinesia: pathophysiology and animal models. J Clin Psychiatry. 2000;61(suppl. 4):5–9. [PubMed] [Google Scholar]
- Cassady SL, Adami H, Moran M, Kunkel R, Thaker GK. Spontaneous dyskinesia in schizophrenia spectrum personality. Am J Psychiatry. 1998;155:70–75. doi: 10.1176/ajp.155.1.70. [DOI] [PubMed] [Google Scholar]
- Cepeda C, Buchwald NA, Levine MS. Neuromodulatory actions of dopamine in the neostriatum are dependent upon the excitatory amino acid receptor subtypes activated. Proc Natl Acad Sci USA. 1993;90:9576–9580. doi: 10.1073/pnas.90.20.9576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cepeda C, Hurst RS, Altemus KL, Flores-Hernandez J, Calvert CR, Jokel ES, Grandy DK, Low MJ, Rubinstein M, Ariano MA, Levine MS. Facilitated glutamatergic transmission in the striatum of D2 dopamine receptor-deficient mice. J Neurophysiol. 2001;85:659–670. doi: 10.1152/jn.2001.85.2.659. [DOI] [PubMed] [Google Scholar]
- Chakos MH, Lieberman JA, Bilder RM, Borenstein M, Lerner G, Bogerts B, Wu H, Kinon B, Ashtari M. Increase in caudate nuclei volumes of first-episode schizophrenic patients taking antipsychotic drugs. Am J Psychiatry. 1994;151:1430–1436. doi: 10.1176/ajp.151.10.1430. [DOI] [PubMed] [Google Scholar]
- Chakos MH, Shirakawa O, Lieberman J, Lee H, Bilder R, Tamminga CA. Striatal enlargement in rats chronically treated with neuroleptic. Biol Psychiatry. 1998;44:675–684. doi: 10.1016/s0006-3223(98)00029-8. [DOI] [PubMed] [Google Scholar]
- Chapman MA, See RE. Differential effects of unique profile antipsychotic drugs on extracellular amino acids in the ventral pallidum and globus pallidus of rats. J Pharmacol Exp Ther. 1996;277:1586–1594. [PubMed] [Google Scholar]
- Choi DW. Ionic dependence of glutamate neurotoxicity. J Neurosci. 1987;7:369–379. doi: 10.1523/JNEUROSCI.07-02-00369.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi DW. Glutamate neurotoxicity and diseases of the nervous system. Neuron. 1988;1:623–634. doi: 10.1016/0896-6273(88)90162-6. [DOI] [PubMed] [Google Scholar]
- Choi DW, Rothman SM. The role of glutamate neurotoxicity in hypoxic-ischemic neuronal death. Annu Rev Neurosci. 1990;13:171–182. doi: 10.1146/annurev.ne.13.030190.001131. [DOI] [PubMed] [Google Scholar]
- Clements JD, Westbrook GL. Activation kinetics reveal the number of glutamate and glycine binding sites on the N-methyl-d-aspartate receptor. Neuron. 1991;7:605–613. doi: 10.1016/0896-6273(91)90373-8. [DOI] [PubMed] [Google Scholar]
- Connor JA, Wadman WJ, Hockberger PE, Wong RK. Sustained dendritic gradients of Ca2+ induced by excitatory amino acids in CA1 hippocampal neurons. Science. 1988;240:649–653. doi: 10.1126/science.2452481. [DOI] [PubMed] [Google Scholar]
- Coughenour LL, Cordon JJ. Characterization of haloperidol and trifluperidol as subtype-selective N-methyl-d-aspartate (NMDA) receptor antagonists using [3H]TCP and [3H]ifenprodil binding in rat brain membranes. J Pharmacol Exp Ther. 1997;280:584–592. [PubMed] [Google Scholar]
- Counihan TJ, Landwehrmeyer GB, Standaert DG, Kosinski CM, Scherzer CR, Daggett LP, Velicelebi G, Young AB, Penney JB., Jr. Expression of N-methyl-d-aspartate receptor subunit mRNA in the human brain: mesencephalic dopaminergic neurons. J Comp Neurol. 1998;390:91–101. [PubMed] [Google Scholar]
- Coyle JT. The glutamatergic dysfunction hypothesis for schizophrenia. Harv Rev Psychiatry. 1996;3:241–253. doi: 10.3109/10673229609017192. [DOI] [PubMed] [Google Scholar]
- Creese I, Burt DR, Snyder SH. Dopamine receptor binding predicts clinical and pharmacological potencies of antischizophrenic drugs. Science. 1976;192:481–483. doi: 10.1126/science.3854. [DOI] [PubMed] [Google Scholar]
- Crook JM, Akil M, Law BC, Hyde TM, Kleinman JE. Comparative analysis of group II metabotropic glutamate receptor immunoreactivity in Brodmann's area 46 of the dorsolateral prefrontal cortex from patients with schizophrenia and normal subjects. Mol Psychiatry. 2002;7:157–164. doi: 10.1038/sj.mp.4000966. [DOI] [PubMed] [Google Scholar]
- Dall'Olio R, Gandolfi O. The NMDA positive modulator D-cycloserine potentiates the neuroleptic activity of D1 and D2 dopamine receptor blockers in the rat. Psychopharmacology (Berl.) 1993;110:165–168. doi: 10.1007/BF02246967. [DOI] [PubMed] [Google Scholar]
- Daly DA, Moghaddam B. Actions of clozapine and haloperidol on the extracellular levels of excitatory amino acids in the prefrontal cortex and striatum of conscious rats. Neurosci Lett. 1993;152:61–64. doi: 10.1016/0304-3940(93)90483-2. [DOI] [PubMed] [Google Scholar]
- Davies N, Russell A, Jones P, Murray RM. Which characteristics of schizophrenia predate psychosis? J Psychiatr Res. 1998;32:121–131. doi: 10.1016/s0022-3956(97)00027-7. [DOI] [PubMed] [Google Scholar]
- Davis GW, Schuster CM, Goodman CS. Genetic dissection of structural and functional components of synaptic plasticity. III. CREB is necessary for presynaptic functional plasticity. Neuron. 1996;17:669–679. doi: 10.1016/s0896-6273(00)80199-3. [DOI] [PubMed] [Google Scholar]
- Deakin JF, Slater P, Simpson MD, Gilchrist AC, Skan WJ, Royston MC, Reynolds GP, Cross AJ. Frontal cortical and left temporal glutamatergic dysfunction in schizophrenia. J Neurochem. 1989;52:1781–1786. doi: 10.1111/j.1471-4159.1989.tb07257.x. [DOI] [PubMed] [Google Scholar]
- De Blasi A, Conn PJ, Pin J, Nicoletti F. Molecular determinants of metabotropic glutamate receptor signaling. Trends Pharmacol Sci. 2001;22:114–120. doi: 10.1016/s0165-6147(00)01635-7. [DOI] [PubMed] [Google Scholar]
- DeLisi LE, Sakuma M, Tew W, Kushner M, Hoff AL, Grimson R. Schizophrenia as a chronic active brain process: a study of progressive brain structural change subsequent to the onset of schizophrenia. Psychiatry Res. 1997;74:129–140. doi: 10.1016/s0925-4927(97)00012-7. [DOI] [PubMed] [Google Scholar]
- Derkach V, Barria A, Soderling TR. Ca2+ /calmodulin-kinase II enhances channel conductance of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionate type glutamate receptors. Proc Natl Acad Sci USA. 1999;96:3269–3274. doi: 10.1073/pnas.96.6.3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Souza IE, McBean GJ, Meredith GE. Chronic haloperidol treatment impairs glutamate transport in the rat striatum. Eur J Pharmacol. 1999;382:139–142. doi: 10.1016/s0014-2999(99)00589-0. [DOI] [PubMed] [Google Scholar]
- Deutch AY, Duman RS. The effects of antipsychotic drugs on Fos protein expression in the prefrontal cortex: cellular localization and pharmacological characterization. Neuroscience. 1996;70:377–389. doi: 10.1016/0306-4522(95)00357-6. [DOI] [PubMed] [Google Scholar]
- Devon RS, Anderson S, Teague PW, Muir WJ, Murray V, Pelosi AJ, Blackwood DH, Porteous DJ. The genomic organisation of the metabotropic glutamate receptor subtype 5 gene, and its association with schizophrenia. Mol Psychiatry. 2001;6:311–314. doi: 10.1038/sj.mp.4000848. [DOI] [PubMed] [Google Scholar]
- Doherty P, Fazeli MS, Walsh FS. The neural cell adhesion molecule and synaptic plasticity. J Neurobiol. 1995;26:437–446. doi: 10.1002/neu.480260315. [DOI] [PubMed] [Google Scholar]
- Done DJ, Crow TJ, Johnstone EC, Sacker A. Childhood antecedents of schizophrenia and affective illness: social adjustment at ages 7 and 11. Br Med J. 1994;309:699–703. doi: 10.1136/bmj.309.6956.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragunow M, Robertson GS, Faull RL, Robertson HA, Jansen K. D2 dopamine receptor antagonists induce fos and related proteins in rat striatal neurons. Neuroscience. 1990;37:287–294. doi: 10.1016/0306-4522(90)90399-o. [DOI] [PubMed] [Google Scholar]
- Driscoll M. Cell death in C. elegans: molecular insights into mechanisms conserved between nematodes and mammals. Brain Pathol. 1996;6:411–425. doi: 10.1111/j.1750-3639.1996.tb00873.x. [DOI] [PubMed] [Google Scholar]
- Du Y, Bales KR, Dodel RC, Hamilton-Byrd E, Horn JW, Czilli DL, Simmons LK, Ni B, Paul SM. Activation of a caspase 3-related cysteine protease is required for glutamate-mediated apoptosis of cultured cerebellar granule neurons. Proc Natl Acad Sci USA. 1997;94:11657–11662. doi: 10.1073/pnas.94.21.11657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastwood SL, Burnet PW, Harrison PJ. Altered synaptophysin expression as a marker of synaptic pathology in schizophrenia. Neuroscience. 1995a;66:309–319. doi: 10.1016/0306-4522(94)00586-t. [DOI] [PubMed] [Google Scholar]
- Eastwood SL, McDonald B, Burnet PW, Beckwith JP, Kerwin RW, Harrison PJ. Decreased expression of mRNAs encoding non-NMDA glutamate receptors GluR1 and GluR2 in medial temporal lobe neurons in schizophrenia. Brain Res Mol Brain Res. 1995b;29:211–223. doi: 10.1016/0169-328x(94)00247-c. [DOI] [PubMed] [Google Scholar]
- Eastwood SL, Porter RH, Harrison PJ. The effect of chronic haloperidol treatment on glutamate receptor subunit (GluR1, GluR2, KA1, KA2, NR1) mRNAs and glutamate binding protein mRNA in rat forebrain. Neurosci Lett. 1996;212:163–166. doi: 10.1016/0304-3940(96)12801-9. [DOI] [PubMed] [Google Scholar]
- Eastwood SL, Burnet PW, Harrison PJ. GluR2 glutamate receptor subunit flip and flop isoforms are decreased in the hippocampal formation in schizophrenia: a reverse transcriptase-polymerase chain reaction (RT-PCR) study. Brain Res Mol Brain Res. 1997a;44:92–98. doi: 10.1016/s0169-328x(96)00195-7. [DOI] [PubMed] [Google Scholar]
- Eastwood SL, Kerwin RW, Harrison PJ. Immunoauto-radiographic evidence for a loss of alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionate-preferring non-N-methyl-d-aspartate glutamate receptors within the medial temporal lobe in schizophrenia. Biol Psychiatry. 1997b;41:636–643. doi: 10.1016/S0006-3223(96)00220-X. [DOI] [PubMed] [Google Scholar]
- Egebjerg J, Heinemann SF. Ca2+ permeability of unedited and edited versions of the kainate selective glutamate receptor GluR6. Proc Natl Acad Sci USA. 1993;90:755–759. doi: 10.1073/pnas.90.2.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmett MR, Mick SJ, Cler JA, Rao TS, Iyengar S, Wood PL. Actions of D-cycloserine at the N-methyl-d-aspartate-associated glycine receptor site in vivo. Neuropharmacology. 1991;30:1167–1171. doi: 10.1016/0028-3908(91)90161-4. [DOI] [PubMed] [Google Scholar]
- Evins AE, Amico ET, Shih V, Goff DC. Clozapine treatment increases serum glutamate and aspartate compared to conventional neuroleptics. J Neural Transm. 1997;104:761–766. doi: 10.1007/BF01291892. [DOI] [PubMed] [Google Scholar]
- Evins AE, Fitzgerald SM, Wine L, Rosselli R, Goff DC. Placebo-controlled trial of glycine added to clozapine in schizophrenia. Am J Psychiatry. 2000;157:826–828. doi: 10.1176/appi.ajp.157.5.826. [DOI] [PubMed] [Google Scholar]
- Fagni L, Chavis P, Ango F, Bockaert J. Complex interactions between mGluRs, intracellular Ca2+ stores and ion channels in neurons. Trends Neurosci. 2000;23:80–88. doi: 10.1016/s0166-2236(99)01492-7. [DOI] [PubMed] [Google Scholar]
- Falke E, Han LY, Arnold SE. Absence of neurodegeneration in the thalamus and caudate of elderly patients with schizophrenia. Psychiatry Res. 2000;93:103–110. doi: 10.1016/s0165-1781(00)00104-9. [DOI] [PubMed] [Google Scholar]
- Farber NB, Wozniak DF, Price MT, Labruyere J, Huss J, St Peter H, Olney JW. Age-specific neurotoxicity in the rat associated with NMDA receptor blockade: potential relevance to schizophrenia. Biol Psychiatry. 1995;38:788–796. doi: 10.1016/0006-3223(95)00046-1. [DOI] [PubMed] [Google Scholar]
- Farooqui AA, Horrocks LA. Lipid peroxides in the free radical pathophysiology of brain diseases. Cell Mol Neurobiol. 1998;18:599–608. doi: 10.1023/a:1020625717298. [DOI] [PubMed] [Google Scholar]
- Fenton WS. Prevalence of spontaneous dyskinesia in schizophrenia. J Clin Psychiatry. 2000;61(suppl. 4):10–14. [PubMed] [Google Scholar]
- Fitzgerald LW, Deutch AY, Gasic G, Heinemann SF, Nestler EJ. Regulation of cortical and subcortical glutamate receptor subunit expression by antipsychotic drugs. J Neurosci. 1995;15:2453–2461. doi: 10.1523/JNEUROSCI.15-03-02453.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fix AS, Wightman KA, O'Callaghan JP. Reactive gliosis induced by MK-801 in the rat posterior cingulate/retrosplenial cortex: GFAP evaluation by sandwich ELISA and immunocytochemistry. Neurotoxicology. 1995;16:229–237. [PubMed] [Google Scholar]
- Flaum M, O'Leary DS, Swayze VW, 2nd, Miller DD, Arndt S, Andreasen NC. Symptom dimensions and brain morphology in schizophrenia and related psychotic disorders. J Psychiatr Res. 1995;29:261–276. doi: 10.1016/0022-3956(94)00046-t. [DOI] [PubMed] [Google Scholar]
- Fletcher EJ, MacDonald JF. Haloperidol interacts with the strychnine-insensitive glycine site at the NMDA receptor in cultured mouse hippocampal neurones. Eur J Pharmacol. 1993;235:291–295. doi: 10.1016/0014-2999(93)90148-b. [DOI] [PubMed] [Google Scholar]
- Frank DA, Greenberg ME. CREB: a mediator of long-term memory from mollusks to mammals. Cell. 1994;79:5–8. doi: 10.1016/0092-8674(94)90394-8. [DOI] [PubMed] [Google Scholar]
- Frank E. Synapse elimination: for nerves it's all or nothing. Science. 1997;275:324–325. doi: 10.1126/science.275.5298.324. [DOI] [PubMed] [Google Scholar]
- Frerking M, Nicoll RA. Synaptic kainate receptors. Curr Opin Neurobiol. 2000;10:342–351. doi: 10.1016/s0959-4388(00)00094-5. [DOI] [PubMed] [Google Scholar]
- Friedman HV, Bresler T, Garner CC, Ziv NE. Assembly of new individual excitatory synapses: time course and temporal order of synaptic molecular recruitment. Neuron. 2000;27:57–69. doi: 10.1016/s0896-6273(00)00009-x. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Frith CD. Schizophrenia: a disconnection syndrome? Clin Neurosci. 1995;3:89–97. [PubMed] [Google Scholar]
- Gainetdinov RR, Mohn AR, Caron MG. Genetic animal models: focus on schizophrenia. Trends Neurosci. 2001;24:527–533. doi: 10.1016/s0166-2236(00)01886-5. [DOI] [PubMed] [Google Scholar]
- Gallagher MJ, Huang H, Lynch DR. Modulation of the N-methyl-d-aspartate receptor by haloperidol: NR2B-specific interactions. J Neurochem. 1998;70:2120–2128. doi: 10.1046/j.1471-4159.1998.70052120.x. [DOI] [PubMed] [Google Scholar]
- Gao X-M, Sakai K, Roberts RC, Conley RR, Dean B, Tamminga CA. Ionotropic glutamate receptors and expression of N-methyl-d-aspartate receptor subunits in subregions of human hippocampus: effects of schizophrenia. Am J Psychiatry. 2000;157:1141–1149. doi: 10.1176/appi.ajp.157.7.1141. [DOI] [PubMed] [Google Scholar]
- Garey LJ, Ong WY, Patel TS, Kanani M, Davis A, Mortimer AM, Barnes TR, Hirsch SR. Reduced dendritic spine density on cerebral cortical pyramidal neurons in schizophrenia. J Neurol Neurosurg Psychiatry. 1998;65:446–453. doi: 10.1136/jnnp.65.4.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauchy C, Desban M, Glowinski J, Kemel ML. NMDA regulation of dopamine release from proximal and distal dendrites in the cat substantia nigra. Brain Res. 1994;635:249–256. doi: 10.1016/0006-8993(94)91446-x. [DOI] [PubMed] [Google Scholar]
- Geyer MA, Krebs-Thomson K, Braff DL, Swerdlow NR. Pharmacological studies of prepulse inhibition models of sensorimotor gating deficits in schizophrenia: a decade in review. Psychopharmacology. 2001;156:117–154. doi: 10.1007/s002130100811. [DOI] [PubMed] [Google Scholar]
- Ghetti A, Heinemann SF. NMDA-dependent modulation of hippocampal kainate receptors by calcineurin and Ca2+/calmodulin-dependent protein kinase. J Neurosci. 2000;20:2766–2773. doi: 10.1523/JNEUROSCI.20-08-02766.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glantz LA, Lewis DA. Decreased dendritic spine density on prefrontal cortical pyramidal neurons in schizophrenia. Arch Gen Psychiatry. 2000;57:65–73. doi: 10.1001/archpsyc.57.1.65. [DOI] [PubMed] [Google Scholar]
- Glazer WM. Extrapyramidal side effects, tardive dyskinesia, and the concept of atypicality. J Clin Psychiatry. 2000a;61:16–21. [PubMed] [Google Scholar]
- Glazer WM. Expected incidence of tardive dyskinesia associated with atypical antipsychotics. J Clin Psychiatry. 2000b;61:21–26. [PubMed] [Google Scholar]
- Goff DC, Coyle JT. The emerging role of glutamate in the pathophysiology and treatment of schizophrenia. Am J Psychiatry. 2001;158:1367–1377. doi: 10.1176/appi.ajp.158.9.1367. [DOI] [PubMed] [Google Scholar]
- Goff DC, Wine L. Glutamate in schizophrenia: clinical and research implications. Schizophr Res. 1997;27:157–168. doi: 10.1016/S0920-9964(97)00079-0. [DOI] [PubMed] [Google Scholar]
- Goff DC, Tsai G, Beal MF, Coyle JT. Tardive dyskinesia and substrates of energy metabolism in CSF. Am J Psychiatry. 1995a;152:1730–1736. doi: 10.1176/ajp.152.12.1730. [DOI] [PubMed] [Google Scholar]
- Goff DC, Tsai G, Manoach DS, Coyle JT. Dose-finding trial of D-cycloserine added to neuroleptics for negative symptoms in schizophrenia. Am J Psychiatry. 1995b;152:1213–1215. doi: 10.1176/ajp.152.8.1213. [DOI] [PubMed] [Google Scholar]
- Goff DC, Tsai G, Manoach DS, Flood J, Darby DG, Coyle JT. D-cycloserine added to clozapine for patients with schizophrenia. Am J Psychiatry. 1996;153:1628–1630. doi: 10.1176/ajp.153.12.1628. [DOI] [PubMed] [Google Scholar]
- Goff DC, Henderson DC, Evins AE, Amico E. A placebo-controlled crossover trial of D-cycloserine added to clozapine in patients with schizophrenia. Biol Psychiatry. 1999a;45:512–514. doi: 10.1016/s0006-3223(98)00367-9. [DOI] [PubMed] [Google Scholar]
- Goff DC, Tsai G, Levitt J, Amico E, Manoach D, Schoenfeld DA, Hayden DL, McCarley R, Coyle JT. A placebo-controlled trial of D-cycloserine added to conventional neuroleptics in patients with schizophrenia. Arch Gen Psychiatry. 1999b;56:21–27. doi: 10.1001/archpsyc.56.1.21. [DOI] [PubMed] [Google Scholar]
- Goff DC, Leahy L, Berman I, Posever T, Herz L, Leon AC, Johnson SA, Lynch G. A placebo-controlled pilot study of the ampakine CX516 added to clozapine in schizophrenia. J Clin Psychopharmacol. 2001;21:484–487. doi: 10.1097/00004714-200110000-00005. [DOI] [PubMed] [Google Scholar]
- Gomperts SN, Rao A, Craig AM, Malenka RC, Nicoll RA. Postsynaptically silent synapses in single neuron cultures. Neuron. 1998;21:1443–1451. doi: 10.1016/s0896-6273(00)80662-5. [DOI] [PubMed] [Google Scholar]
- Grace AA, Bunney BS, Moore H, Todd CL. Dopaminecell depolarization block as a model for the therapeutic actions of anti-psychotic drugs. Trends Neurosci. 1997;20:31–37. doi: 10.1016/S0166-2236(96)10064-3. [DOI] [PubMed] [Google Scholar]
- Grauer SM, Marquis KL. Intracerebral administration of metabotropic glutamate receptor agonists disrupts prepulse inhibition of acoustic startle in Sprague-Dawley rats. Psychopharmacology. 1999;141:405–412. doi: 10.1007/s002130050850. [DOI] [PubMed] [Google Scholar]
- Grimwood S, Slater P, Deakin JF, Hutson PH. NR2B-containing NMDA receptors are up-regulated in temporal cortex in schizophrenia. Neuroreport. 1999;10:461–465. doi: 10.1097/00001756-199902250-00004. [DOI] [PubMed] [Google Scholar]
- Gur RE, Maany V, Mozley PD, Swanson C, Bilker W, Gur RC. Subcortical MRI volumes in neuroleptic-naive and treated patients with schizophrenia. Am J Psychiatry. 1998;155:1711–1717. doi: 10.1176/ajp.155.12.1711. [DOI] [PubMed] [Google Scholar]
- Haas K. Prefabrication provides synapses on demand. Trends Neurosci. 2000;23:449. doi: 10.1016/s0166-2236(00)01678-7. [DOI] [PubMed] [Google Scholar]
- Halliwell B, Chirico S. Lipid peroxidation: its mechanism, measurement, and significance. Am J Clin Nutr. 1993;57:715S–725S. doi: 10.1093/ajcn/57.5.715S. [DOI] [PubMed] [Google Scholar]
- Hamid EH, Hyde TM, Baca SM, Egan MF. Failure to down regulate NMDA receptors in the striatum and nucleus accumbens associated with neuroleptic-induced dyskinesia. Brain Res. 1998;796:291–295. doi: 10.1016/s0006-8993(98)00196-6. [DOI] [PubMed] [Google Scholar]
- Harris KM. Structure, development, and plasticity of dendritic spines. Curr Opin Neurobiol. 1999;9:343–348. doi: 10.1016/s0959-4388(99)80050-6. [DOI] [PubMed] [Google Scholar]
- Harrison PJ. Schizophrenia: a disorder of neurodevelopment? Curr Opin Neurobiol. 1997;7:285–289. doi: 10.1016/s0959-4388(97)80018-9. [DOI] [PubMed] [Google Scholar]
- Harrison PJ, McLaughlin D, Kerwin RW. Decreased hippocampal expression of a glutamate receptor gene in schizophrenia. Lancet. 1991;337:450–452. doi: 10.1016/0140-6736(91)93392-m. [DOI] [PubMed] [Google Scholar]
- Hauber W. Impairments of movement initiation and execution induced by a blockade of dopamine D1 or D2 receptors are reversed by a blockade of N-methyl-d-aspartate receptors. Neuroscience. 1996;73:121–130. doi: 10.1016/0306-4522(96)00036-x. [DOI] [PubMed] [Google Scholar]
- Hauber W, Schmidt WJ. The NMDA antagonist dizocilpine (MK-801) reverses haloperidol-induced movement initiation deficits. Behav Brain Res. 1990;41:161–166. doi: 10.1016/0166-4328(90)90151-4. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Kagaya A, Takebayashi M, Shimizu M, Uchitomi Y, Motohashi N, Yamawaki S. Modulation by sigma ligands of intracellular free Ca++ mobilization by N-methyl-d-aspartate in primary culture of rat frontal cortical neurons. J Pharmacol Exp Ther. 1995;275:207–214. [PubMed] [Google Scholar]
- Heckers S. Neuropathology of schizophrenia: cortex, thalamus, basal ganglia, and neurotransmitter-specific projection systems. Schizophr Bull. 1997;23:403–421. doi: 10.1093/schbul/23.3.403. [DOI] [PubMed] [Google Scholar]
- Heckers S. Neuroimaging studies of the hippocampus in schizophrenia. Hippocampus. 2001;11:520–528. doi: 10.1002/hipo.1068. [DOI] [PubMed] [Google Scholar]
- Heckers S, Heinsen H, Heinsen Y, Beckmann H. Cortex, white matter, and basal ganglia in schizophrenia: a volumetric postmortem study. Biol Psychiatry. 1991;29:556–566. doi: 10.1016/0006-3223(91)90091-y. [DOI] [PubMed] [Google Scholar]
- Heckers S, Rauch SL, Goff D, Savage CR, Schacter DL, Fischman AJ, Alpert NM. Impaired recruitment of the hippocampus during conscious recollection in schizophrenia. Nat Neurosci. 1998;1:318–323. doi: 10.1038/1137. [DOI] [PubMed] [Google Scholar]
- Heckers S, Stone D, Walsh J, Shick J, Koul P, Benes FM. Differential hippocampal expression of glutamic acid decarboxylase 65 and 67 messenger RNA in bipolar disorder and schizophrenia. Arch Gen Psychiatry. 2001;59:521–529. doi: 10.1001/archpsyc.59.6.521. [DOI] [PubMed] [Google Scholar]
- Henderson G, Johnson JW, Ascher P. Competitive antagonists and partial agonists at the glycine modulatory site of the mouse N-methyl-d-aspartate receptor. J Physiol (Lond.) 1990;430:189–212. doi: 10.1113/jphysiol.1990.sp018288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heresco-Levy U, Javitt DC, Ermilov M, Mordel C, Horowitz A, Kelly D. Double-blind, placebo-controlled, crossover trial of glycine adjuvant therapy for treatment-resistant schizophrenia. Br J Psychiatry. 1996a;169:610–617. doi: 10.1192/bjp.169.5.610. [DOI] [PubMed] [Google Scholar]
- Heresco-Levy U, Silipo G, Javitt DC. Glycinergic augmentation of NMDA receptor-mediated neurotransmission in the treatment of schizophrenia. Psychopharmacol Bull. 1996b;32:731–740. [PubMed] [Google Scholar]
- Heresco-Levy U, Javitt DC, Ermilov M, Mordel C, Silipo G, Lichtenstein M. Efficacy of high-dose glycine in the treatment of enduring negative symptoms of schizophrenia. Arch Gen Psychiatry. 1999;56:29–36. doi: 10.1001/archpsyc.56.1.29. [DOI] [PubMed] [Google Scholar]
- Herron CE, Lester RA, Coan EJ, Collingridge GL. Frequency-dependent involvement of NMDA receptors in the hippocampus: a novel synaptic mechanism. Nature. 1986;322:265–268. doi: 10.1038/322265a0. [DOI] [PubMed] [Google Scholar]
- Hirashima Y, Kurimoto M, Nogami K, Endo S, Saitoh M, Ohtani O, Nagata T, Muraguchi A, Takaku A. Correlation of glutamate-induced apoptosis with caspase activities in cultured rat cerebral cortical neurons. Brain Res. 1999;849:109–118. doi: 10.1016/s0006-8993(99)02009-0. [DOI] [PubMed] [Google Scholar]
- Hollmann M, Heinemann S. Cloned glutamate receptors. Annu Rev Neurosci. 1994;17:31–108. doi: 10.1146/annurev.ne.17.030194.000335. [DOI] [PubMed] [Google Scholar]
- Hood WF, Compton RP, Monahan JB. D-cycloserine: a ligand for the N-methyl-d-aspartate coupled glycine receptor has partial agonist characteristics. Neurosci Lett. 1989;98:91–95. doi: 10.1016/0304-3940(89)90379-0. [DOI] [PubMed] [Google Scholar]
- Hume RI, Dingledine R, Heinemann SF. Identification of a site in glutamate receptor subunits that controls calcium permeability. Science. 1991;253:1028–1031. doi: 10.1126/science.1653450. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR. Synapse elimination and plasticity in developing human cerebral cortex. Am J Ment Defic. 1984;88:488–496. [PubMed] [Google Scholar]
- Huttenlocher PR, de Courten C, Garey LJ, Van der Loos H. Synaptogenesis in human visual cortex—evidence for synapse elimination during normal development. Neurosci Lett. 1982;33:247–252. doi: 10.1016/0304-3940(82)90379-2. [DOI] [PubMed] [Google Scholar]
- Hyman SE, Nestler EJ. Initiation and adaptation: a paradigm for understanding psychotropic drug action. Am J Psychiatry. 1996;153:151–162. doi: 10.1176/ajp.153.2.151. [DOI] [PubMed] [Google Scholar]
- Illowsky BP, Juliano DM, Bigelow LB, Weinberger DR. Stability of CT scan findings in schizophrenia: results of an 8 year follow-up study. J Neurol Neurosurg Psychiatry. 1988;51:209–213. doi: 10.1136/jnnp.51.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilyin VI, Whittemore ER, Guastella J, Weber E, Woodward RM. Subtype-selective inhibition of N-methyl-d-aspartate receptors by haloperidol. Mol Pharmacol. 1996;50:1541–1550. [PubMed] [Google Scholar]
- Impey S, Smith DM, Obrietan K, Donahue R, Wade C, Storm DR. Stimulation of cAMP response element (CRE)-mediated transcription during contextual learning. Nat Neurosci. 1998;1:595–601. doi: 10.1038/2830. [DOI] [PubMed] [Google Scholar]
- Isaac JT, Nicoll RA, Malenka RC. Evidence for silent synapses: implications for the expression of LTP. Neuron. 1995;15:427–434. doi: 10.1016/0896-6273(95)90046-2. [DOI] [PubMed] [Google Scholar]
- Ishimaru M, Kurumaji A, Toru M. Increases in strychnine-insensitive glycine binding sites in cerebral cortex of chronic schizophrenics: evidence for glutamate hypothesis. Biol Psychiatry. 1994;35:84–95. doi: 10.1016/0006-3223(94)91197-5. [DOI] [PubMed] [Google Scholar]
- Javitt DC, Zukin SR. Recent advances in the phencyclidine model of schizophrenia. Am J Psychiatry. 1991;148:1301–1308. doi: 10.1176/ajp.148.10.1301. [DOI] [PubMed] [Google Scholar]
- Javitt DC, Zylberman I, Zukin SR, Heresco-Levy U, Linden-mayer JP. Amelioration of negative symptoms in schizophrenia by glycine. Am J Psychiatry. 1994;151:1234–1236. doi: 10.1176/ajp.151.8.1234. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Roth RH. The neuropsychopharmacology of phencyclidine: from NMDA receptor hypofunction to the dopamine hypothesis of schizophrenia. Neuropsychopharmacology. 1999;20:201–225. doi: 10.1016/S0893-133X(98)00060-8. [DOI] [PubMed] [Google Scholar]
- Jernigan TL, Zisook S, Heaton RK, Moranville JT, Hesselink JR, Braff DL. Magnetic resonance imaging abnormalities in lenticular nuclei and cerebral cortex in schizophrenia. Arch Gen Psychiatry. 1991;48:881–890. doi: 10.1001/archpsyc.1991.01810340013002. [DOI] [PubMed] [Google Scholar]
- Jia Z, Lu Y, Henderson J, Taverna F, Romano C, Abramow-Newerly W, Wojtowicz JM, Roder J. Selective abolition of the NMDA component of long-term potentiation in mice lacking mGluR5. Learn Mem. 1998;5:331–343. [PMC free article] [PubMed] [Google Scholar]
- Johnson JW, Ascher P. Glycine potentiates the NMDA response in cultured mouse brain neurons. Nature. 1987;325:529–531. doi: 10.1038/325529a0. [DOI] [PubMed] [Google Scholar]
- Johnson SA, Luu NT, Herbst TA, Knapp R, Lutz D, Arai A, Rogers GA, Lynch G. Synergistic interactions between ampakines and antipsychotic drugs. J Pharmacol Exp Ther. 1999;289:392–397. [PubMed] [Google Scholar]
- Jones DG, Harris RJ. An analysis of contemporary morphological concepts of synaptic remodelling in the CNS: perforated synapses revisited. Rev Neurosci. 1995;6:177–219. doi: 10.1515/revneuro.1995.6.3.177. [DOI] [PubMed] [Google Scholar]
- Jones P, Rodgers B, Murray R, Marmot M. Child development risk factors for adult schizophrenia in the British 1946 birth cohort. Lancet. 1994;344:1398–1402. doi: 10.1016/s0140-6736(94)90569-x. [DOI] [PubMed] [Google Scholar]
- Jones PB, Rantakallio P, Hartikainen AL, Isohanni M, Sipila P. Schizophrenia as a long-term outcome of pregnancy, delivery, and perinatal complications: a 28-year follow-up of the 1966 north Finland general population birth cohort. Am J Psychiatry. 1998;155:355–364. doi: 10.1176/ajp.155.3.355. [DOI] [PubMed] [Google Scholar]
- Joo A, Shibata H, Ninomiya H, Kawasaki H, Tashiro N, Fukumaki Y. Structure and polymorphisms of the human metabotropic glutamate receptor type 2 gene (GRM2): analysis of association with schizophrenia. Mol Psychiatry. 2001;6:186–192. doi: 10.1038/sj.mp.4000841. [DOI] [PubMed] [Google Scholar]
- Kandel ER, Schwartz JH, Jessell TM. Principles of Neural Science. 3rd edn. Elsevier; New York: 1991. [Google Scholar]
- Kapur S, Zipursky RB, Remington G, Jones C, DaSilva J, Wilson AA, Houle S. 5-HT2 and D2 receptor occupancy of olanzapine in schizophrenia: a PET investigation. Am J Psychiatry. 1998;155:921–928. doi: 10.1176/ajp.155.7.921. [DOI] [PubMed] [Google Scholar]
- Kapur S, Zipursky RB, Remington G. Clinical and theoretical implications of 5-HT2 and D2 receptor occupancy of clozapine, risperidone, and olanzapine in schizophrenia. Am J Psychiatry. 1999;156:286–293. doi: 10.1176/ajp.156.2.286. [DOI] [PubMed] [Google Scholar]
- Kaur S, Ozer H, Starr M. MK 801 reverses haloperidol-induced catalepsy from both striatal and extrastriatal sites in the rat brain. Eur J Pharmacol. 1997;332:153–160. doi: 10.1016/s0014-2999(97)01078-9. [DOI] [PubMed] [Google Scholar]
- Kelsoe JR, Cadet JL, Pickar D, Weinberger DR. Quantitative neuroanatomy in schizophrenia. A controlled magnetic resonance imaging study. Arch Gen Psychiatry. 1988;45:533–541. doi: 10.1001/archpsyc.1988.01800300029003. [DOI] [PubMed] [Google Scholar]
- Kerns JM, Sierens DK, Kao LC, Klawans HL, Carvey PM. Synaptic plasticity in the rat striatum following chronic haloperidol treatment. Clin Neuropharmacol. 1992;15:488–500. doi: 10.1097/00002826-199212000-00006. [DOI] [PubMed] [Google Scholar]
- Kerwin R, Patel S, Meldrum B. Quantitative autoradiographic analysis of glutamate binding sites in the hippocampal formation in normal and schizophrenic brain post mortem. Neuroscience. 1990;39:25–32. doi: 10.1016/0306-4522(90)90219-t. [DOI] [PubMed] [Google Scholar]
- Keshavan MS, Bagwell WW, Haas GL, Sweeney JA, Schooler NR, Pettegrew JW. Changes in caudate volume with neuroleptic treatment. Lancet. 1994;344:1434. doi: 10.1016/s0140-6736(94)90599-1. [DOI] [PubMed] [Google Scholar]
- Keyser DO, Alger BE. Arachidonic acid modulates hippocampal calcium current via protein kinase C and oxygen radicals. Neuron. 1990;5:545–553. doi: 10.1016/0896-6273(90)90092-t. [DOI] [PubMed] [Google Scholar]
- Khodorov BI. Mechanisms of destabilization of Ca2+-homeostasis of brain neurons caused by toxic glutamate challenge. Membr Cell Biol. 2000;14:149–162. [PubMed] [Google Scholar]
- Kilts CD. The changing roles and targets for animal models of schizophrenia. Biol Psychiatry. 2001;50:845–855. doi: 10.1016/s0006-3223(01)01286-0. [DOI] [PubMed] [Google Scholar]
- Kim JS, Kornhuber HH, Schmid-Burgk W, Holzmuller B. Low cerebrospinal fluid glutamate in schizophrenic patients and a new hypothesis on schizophrenia. Neurosci Lett. 1980;20:379–382. doi: 10.1016/0304-3940(80)90178-0. [DOI] [PubMed] [Google Scholar]
- Kirov SA, Sorra KE, Harris KM. Slices have more synapses than perfusion-fixed hippocampus from both young and mature rats. J Neurosci. 1999;19:2876–2886. doi: 10.1523/JNEUROSCI.19-08-02876.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koga E, Momiyama T. Presynaptic dopamine D2-like receptors inhibit excitatory transmission onto rat ventral tegmental dopaminergic neurones. J Physiol. 2000;523:163–173. doi: 10.1111/j.1469-7793.2000.t01-2-00163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler M, Burnashev N, Sakmann B, Seeburg PH. Determinants of Ca2+ permeability in both TM1 and TM2 of high affinity kainate receptor channels: diversity by RNA editing. Neuron. 1993;10:491–500. doi: 10.1016/0896-6273(93)90336-p. [DOI] [PubMed] [Google Scholar]
- Konradi C, Heckers S. Antipsychotic drugs and neuroplasticity: insights into the treatment and neurobiology of schizophrenia. Biol Psychiatry. 2001;50:729–742. doi: 10.1016/s0006-3223(01)01267-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornhuber J, Mack-Burkhardt F, Riederer P, Hebenstreit GF, Reynolds GP, Andrews HB, Beckmann H. [3H]MK-801 binding sites in postmortem brain regions of schizophrenic patients. J Neural Transm. 1989;77:231–236. doi: 10.1007/BF01248936. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD, Heninger GR, Bowers MB, Jr., Charney DS. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry. 1994;51:199–214. doi: 10.1001/archpsyc.1994.03950030035004. [DOI] [PubMed] [Google Scholar]
- Kunugi H, Takei N, Murray RM, Saito K, Nanko S. Small head circumference at birth in schizophrenia. Schizophr Res. 1996;20:165–170. doi: 10.1016/0920-9964(96)00007-2. [DOI] [PubMed] [Google Scholar]
- Kure S, Tominaga T, Yoshimoto T, Tada K, Narisawa K. Glutamate triggers internucleosomal DNA cleavage in neuronal cells. Biochem Biophys Res Commun. 1991;179:39–45. doi: 10.1016/0006-291x(91)91330-f. [DOI] [PubMed] [Google Scholar]
- Laube B, Hirai H, Sturgess M, Betz H, Kuhse J. Molecular determinants of agonist discrimination by NMDA receptor subunits: analysis of the glutamate binding site on the NR2B subunit. Neuron. 1997;18:493–503. doi: 10.1016/s0896-6273(00)81249-0. [DOI] [PubMed] [Google Scholar]
- Lavalaye J, Linszen DH, Booij J, Reneman L, Gersons BP, van Royen EA. Dopamine D2 receptor occupancy by olanzapine or risperidone in young patients with schizophrenia. Psychiatry Res. 1999;92:33–44. doi: 10.1016/s0925-4927(99)00032-3. [DOI] [PubMed] [Google Scholar]
- Lazarewicz JW, Wroblewski JT, Costa E. N-methyl-d-aspartate-sensitive glutamate receptors induce calcium-mediated arachidonic acid release in primary cultures of cerebellar granule cells. J Neurochem. 1990;55:1875–1881. doi: 10.1111/j.1471-4159.1990.tb05771.x. [DOI] [PubMed] [Google Scholar]
- Lee SH, Sheng M. Development of neuron-neuron synapses. Curr Opin Neurobiol. 2000;10:125–131. doi: 10.1016/s0959-4388(99)00046-x. [DOI] [PubMed] [Google Scholar]
- Lerma J, Paternain AV, Rodriguez-Moreno A, Lopez-Garcia JC. Molecular physiology of kainate receptors. Physiol Rev. 2001;81:971–998. doi: 10.1152/physrev.2001.81.3.971. [DOI] [PubMed] [Google Scholar]
- Leveque J-C, Macías W, Rajadhyaksha A, Carlson RR, Barczak A, Kang S, Li X-M, Coyle JT, Huganir RL, Heckers S, Konradi C. Intracellular modulation of NMDA receptor function by antipsychotic drugs. J Neurosci. 2000;20:4011–4020. doi: 10.1523/JNEUROSCI.20-11-04011.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levinson DF. Pharmacologic treatment of schizophrenia. Clin Ther. 1991;13:326–352. [PubMed] [Google Scholar]
- Lewis DA, Lieberman JA. Catching up on schizophrenia. Natural history and neurobiology. Neuron. 2000;28:325–334. doi: 10.1016/s0896-6273(00)00111-2. [DOI] [PubMed] [Google Scholar]
- Liao D, Hessler NA, Malinow R. Activation of postsynaptically silent synapses during pairing-induced LTP in CA1 region of hippocampal slice. Nature. 1995;375:400–404. doi: 10.1038/375400a0. [DOI] [PubMed] [Google Scholar]
- Lidow MS, Goldman-Rakic PS. Differential regulation of D2 and D4 dopamine receptor mRNAs in the primate cerebral cortex vs. neostriatum: effects of chronic treatment with typical and atypical anti-psychotic drugs. J Pharmacol Exp Ther. 1997;283:939–946. [PubMed] [Google Scholar]
- Lidsky TI, Yablonsky-Alter E, Zuck LG, Banerjee SP. Antipsychotic drug effects on glutamatergic activity. Brain Res. 1997;764:46–52. doi: 10.1016/s0006-8993(97)00423-x. [DOI] [PubMed] [Google Scholar]
- Lieberman DN, Mody I. Regulation of NMDA channel function by endogenous Ca2+-dependent phosphatase. Nature. 1994;369:235–239. doi: 10.1038/369235a0. [DOI] [PubMed] [Google Scholar]
- Lieberman JA. Is schizophrenia a neurodegenerative disorder? A clinical and neurobiological perspective. Biol Psychiatry. 1999;46:729–739. doi: 10.1016/s0006-3223(99)00147-x. [DOI] [PubMed] [Google Scholar]
- Lieberman JA, Perkins D, Belger A, Chakos M, Jarskog F, Boteva K, Gilmore J. The early stages of schizophrenia: speculations on pathogenesis, pathophysiology, and therapeutic approaches. Biol Psychiatry. 2001;50:884–897. doi: 10.1016/s0006-3223(01)01303-8. [DOI] [PubMed] [Google Scholar]
- Lim KO, Tew W, Kushner M, Chow K, Matsumoto B, DeLisi LE. Cortical gray matter volume deficit in patients with firstepisode schizophrenia. Am J Psychiatry. 1996;153:1548–1553. doi: 10.1176/ajp.153.12.1548. [DOI] [PubMed] [Google Scholar]
- Lipska BK, Weinberger DR. To model a psychiatric disorder in animals: schizophrenia as a reality test. Neuropsychopharmacology. 2000;23:223–239. doi: 10.1016/S0893-133X(00)00137-8. [DOI] [PubMed] [Google Scholar]
- Littrell KH, Johnson CG, Littrell S, Peabody CD. Marked reduction of tardive dyskinesia with olanzapine. Arch Gen Psychiatry. 1998;55:279–280. doi: 10.1001/archpsyc.55.3.279. [DOI] [PubMed] [Google Scholar]
- Liu X, Zou H, Slaughter C, Wang X. DFF, a heterodimeric protein that functions downstream of caspase-3 to trigger DNA fragmentation during apoptosis. Cell. 1997;89:175–184. doi: 10.1016/s0092-8674(00)80197-x. [DOI] [PubMed] [Google Scholar]
- Luetjens CM, Bui NT, Sengpiel B, Munstermann G, Poppe M, Krohn AJ, Bauerbach E, Krieglstein J, Prehn JH. Delayed mitochondrial dysfunction in excitotoxic neuron death: cytochrome c release and a secondary increase in superoxide production. J Neurosci. 2000;20:5715–5723. doi: 10.1523/JNEUROSCI.20-15-05715.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch DR, Gallagher MJ. Inhibition of N-methyl-d-aspartate receptors by haloperidol: developmental and pharmacological characterization in native and recombinant receptors. J Pharmacol Exp Ther. 1996;279:154–161. [PubMed] [Google Scholar]
- MacDermott AB, Mayer ML, Westbrook GL, Smith SJ, Barker JL. NMDA-receptor activation increases cytoplasmic calcium concentration in cultured spinal cord neurones. Nature. 1986;321:519–522. doi: 10.1038/321519a0. [DOI] [PubMed] [Google Scholar]
- MacGibbon GA, Lawlor PA, Bravo R, Dragunow M. Clozapine and haloperidol produce a differential pattern of immediate early gene expression in rat caudate-putamen, nucleus accumbens, lateral septum and islands of Calleja. Brain Res Mol Brain Res. 1994;23:21–32. doi: 10.1016/0169-328x(94)90207-0. [DOI] [PubMed] [Google Scholar]
- Malenka RC. The role of postsynaptic calcium in the induction of long-term potentiation. Mol Neurobiol. 1991;5:289–295. doi: 10.1007/BF02935552. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Nicoll RA. NMDA-receptor-dependent synaptic plasticity: multiple forms and mechanisms. Trends Neurosci. 1993;16:521–527. doi: 10.1016/0166-2236(93)90197-t. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Nicoll RA. Long-term potentiation—a decade of progress? Science. 1999;285:1870–1874. doi: 10.1126/science.285.5435.1870. [DOI] [PubMed] [Google Scholar]
- Mammen AL, Kameyama K, Roche KW, Huganir RL. Phosphorylation of the α-amino-3-hydroxy-5-methylisoxazole4-propionic acid receptor GluR1 subunit by calcium/calmodulin-dependent kinase II. J Biol Chem. 1997;272:32528–32533. doi: 10.1074/jbc.272.51.32528. [DOI] [PubMed] [Google Scholar]
- Mansbach RS, Geyer MA. Effects of phencyclidine and phencyclidine biology on sensorimotor gating in the rat. Neuropsychopharmacology. 1989;2:299–308. doi: 10.1016/0893-133x(89)90035-3. [DOI] [PubMed] [Google Scholar]
- Marsh L, Suddath RL, Higgins N, Weinberger DR. Medial temporal lobe structures in schizophrenia: relationship of size to duration of illness. Schizophr Res. 1994;11:225–238. doi: 10.1016/0920-9964(94)90016-7. [DOI] [PubMed] [Google Scholar]
- Martin KC, Casadio A, Zhu HEY, Rose JC, Chen M, Bailey CH, Kandel ER. Synapse-specific, long-term facilitation of aplysia sensory to motor synapses: a function for local protein synthesis in memory storage. Cell. 1997;91:927–938. doi: 10.1016/s0092-8674(00)80484-5. [DOI] [PubMed] [Google Scholar]
- Martin LJ, Al-Abdulla NA, Brambrink AM, Kirsch JR, Sieber FE, Portera-Cailliau C. Neurodegeneration in excitotoxicity, global cerebral ischemia, and target deprivation: a perspective on the contributions of apoptosis and necrosis. Brain Res Bull. 1998;46:281–309. doi: 10.1016/s0361-9230(98)00024-0. [DOI] [PubMed] [Google Scholar]
- Martinou JC, Sadoul R. ICE-like proteases execute the neuronal death program. Curr Opin Neurobiol. 1996;6:609–614. doi: 10.1016/s0959-4388(96)80092-4. [DOI] [PubMed] [Google Scholar]
- Matthysse S. Dopamine and the pharmacology of schizophrenia: the state of the evidence. J Psychiatr Res. 1974;11:107–113. doi: 10.1016/0022-3956(74)90081-8. [DOI] [PubMed] [Google Scholar]
- Mattson MP. Neurotransmitters in the regulation of neuronal cytoarchitecture. Brain Res. 1988;472:179–212. doi: 10.1016/0165-0173(88)90020-3. [DOI] [PubMed] [Google Scholar]
- Mayer ML, Westbrook GL, Guthrie PB. Voltage-dependent block by Mg2+ of NMDA responses in spinal cord neurones. Nature. 1984;309:261–263. doi: 10.1038/309261a0. [DOI] [PubMed] [Google Scholar]
- McNeil TF, Cantor-Graae E, Torrey EF, Sjostrom K, Bowler A, Taylor E, Rawlings R, Higgins ES. Obstetric complications in histories of monozygotic twins discordant and concordant for schizophrenia. Acta Psychiatr Scand. 1994;89:196–204. doi: 10.1111/j.1600-0447.1994.tb08092.x. [DOI] [PubMed] [Google Scholar]
- Meador-Woodruff JH, Healy DJ. Glutamate receptor expression in schizophrenic brain. Brain Res Brain Res Rev. 2000;31:288–294. doi: 10.1016/s0165-0173(99)00044-2. [DOI] [PubMed] [Google Scholar]
- Meltzer HY. An overview of the mechanism of action of clozapine. J Clin Psychiatry. 1994;55(suppl. B):47–52. [PubMed] [Google Scholar]
- Meltzer HY. The role of serotonin in antipsychotic drug action. Neuropsychopharmacology. 1999;21:106S–115S. doi: 10.1016/S0893-133X(99)00046-9. [DOI] [PubMed] [Google Scholar]
- Meltzer HY, Stahl SM. The dopamine hypothesis of schizophrenia: a review. Schizophr Bull. 1976;2:19–76. doi: 10.1093/schbul/2.1.19. [DOI] [PubMed] [Google Scholar]
- Meltzer LT, Christoffersen CL, Serpa KA. Modulation of dopamine neuronal activity by glutamate receptor subtypes. Neurosci Biobehav Rev. 1997;21:511–518. doi: 10.1016/s0149-7634(96)00030-9. [DOI] [PubMed] [Google Scholar]
- Mermelstein PG, Bito H, Deisseroth K, Tsien RW. Critical dependence of cAMP response element-binding protein phosphorylation on L-type calcium channels supports a selective response to EPSPs in preference to action potentials. J Neurosci. 2000;20:266–273. doi: 10.1523/JNEUROSCI.20-01-00266.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merry DE, Korsmeyer SJ. Bcl-2 gene family in the nervous system. Annu Rev Neurosci. 1997;20:245–267. doi: 10.1146/annurev.neuro.20.1.245. [DOI] [PubMed] [Google Scholar]
- Meshul CK, Casey DE. Regional, reversible ultrastructural changes in rat brain with chronic neuroleptic treatment. Brain Res. 1989;489:338–346. doi: 10.1016/0006-8993(89)90867-6. [DOI] [PubMed] [Google Scholar]
- Meshul CK, Bunker GL, Mason JN, Allen C, Janowsky A. Effects of subchronic clozapine and haloperidol on striatal glutamatergic synapses. J Neurochem. 1996;67:1965–1973. doi: 10.1046/j.1471-4159.1996.67051965.x. [DOI] [PubMed] [Google Scholar]
- Metzstein MM, Stanfield GM, Horvitz HR. Genetics of programmed cell death in C. elegans: past, present and future. Trends Genet. 1998;14:410–416. doi: 10.1016/s0168-9525(98)01573-x. [DOI] [PubMed] [Google Scholar]
- Micheletti G, Lannes B, Warter JM, Zwiller J. Evidence for NMDA/D2 receptor-receptor interactions in the rat striatum. Adv Neurol. 1993;60:128–132. [PubMed] [Google Scholar]
- Miyamoto Y, Yamada K, Noda Y, Mori H, Mishina M, Nabeshima T. Hyperfunction of dopaminergic and serotonergic neuronal systems in mice lacking the NMDA receptor epsilon1 subunit. J Neurosci. 2001;21:750–757. doi: 10.1523/JNEUROSCI.21-02-00750.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghaddam B, Adams BW. Reversal of phencyclidine effects by a group II metabotropic glutamate receptor agonist in rats. Science. 1998;281:1349–1352. doi: 10.1126/science.281.5381.1349. [DOI] [PubMed] [Google Scholar]
- Mohn AR, Gainetdinov RR, Caron MG, Koller BH. Mice with reduced NMDA receptor expression display behaviors related to schizophrenia. Cell. 1999;98:427–436. doi: 10.1016/s0092-8674(00)81972-8. [DOI] [PubMed] [Google Scholar]
- Montal M. Mitochondria, glutamate neurotoxicity and the death cascade. Biochim Biophys Acta. 1998;1366:113–126. doi: 10.1016/s0005-2728(98)00124-8. [DOI] [PubMed] [Google Scholar]
- Moore NA, Blackman A, Awere S, Leander JD. NMDA receptor antagonists inhibit catalepsy induced by either dopamine D1 or D2 receptor antagonists. Eur J Pharmacol. 1993;237:1–7. doi: 10.1016/0014-2999(93)90085-v. [DOI] [PubMed] [Google Scholar]
- Morari M, Marti M, Sbrenna S, Fuxe K, Bianchi C, Beani L. Reciprocal dopamine-glutamate modulation of release in the basal ganglia. Neurochem Int. 1998;33:383–397. doi: 10.1016/s0197-0186(98)00052-7. [DOI] [PubMed] [Google Scholar]
- Murase S, Schuman EM. The role of cell adhesion molecules in synaptic plasticity and memory. Curr Opin Cell Biol. 1999;11:549–553. doi: 10.1016/s0955-0674(99)00019-8. [DOI] [PubMed] [Google Scholar]
- Murphy SN, Miller RJ. A glutamate receptor regulates Ca2+ mobilization in hippocampal neurons. Proc Natl Acad Sci USA. 1988;85:8737–8741. doi: 10.1073/pnas.85.22.8737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi S. Molecular diversity of glutamate receptors and implications for brain function. Science. 1992;258:597–603. doi: 10.1126/science.1329206. [DOI] [PubMed] [Google Scholar]
- Nasrallah HA, Olson SC, McCalley-Whitters M, Chapman S, Jacoby CG. Cerebral ventricular enlargement in schizophrenia. A preliminary follow-up study. Arch Gen Psychiatry. 1986;43:157–159. doi: 10.1001/archpsyc.1986.01800020067008. [DOI] [PubMed] [Google Scholar]
- Nath R, Probert A, Jr., McGinnis KM, Wang KK. Evidence for activation of caspase-3-like protease in excitotoxin- and hypoxia/hypoglycemia-injured neurons. J Neurochem. 1998;71:186–195. doi: 10.1046/j.1471-4159.1998.71010186.x. [DOI] [PubMed] [Google Scholar]
- Nath R, Scott M, Nadimpalli R, Gupta R, Wang KK. Activation of apoptosis-linked caspase(s) in NMDA-injured brains in neonatal rats. Neurochem Int. 2000;36:119–126. doi: 10.1016/s0197-0186(99)00112-6. [DOI] [PubMed] [Google Scholar]
- Nguyen TV, Kosofsky BE, Birnbaum R, Cohen BM, Hyman SE. Differential expression of c-fos and zif268 in rat striatum after haloperidol, clozapine, and amphetamine. Proc Natl Acad Sci USA. 1992;89:4270–4274. doi: 10.1073/pnas.89.10.4270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoll RA, Malenka RC. Expression mechanisms underlying NMDA receptor-dependent long-term potentiation. Ann NY Acad Sci. 1999;868:515–525. doi: 10.1111/j.1749-6632.1999.tb11320.x. [DOI] [PubMed] [Google Scholar]
- Nishi M, Hinds H, Lu HP, Kawata M, Hayashi Y. Moto-neuron-specific expression of NR3B, a novel NMDA-type glutamate receptor subunit that works in a dominant-negative manner. J Neurosci. 2001;21:RC185. doi: 10.1523/JNEUROSCI.21-23-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikawa T, Takashima M, Toru M. Increased [3H]kainic acid binding in the prefrontal cortex in schizophrenia. Neurosci Lett. 1983;40:245–250. doi: 10.1016/0304-3940(83)90046-0. [DOI] [PubMed] [Google Scholar]
- Noga JT, Hyde TM, Herman MM, Spurney CF, Bigelow LB, Weinberger DR, Kleinman JE. Glutamate receptors in the postmortem striatum of schizophrenic, suicide, and control brains. Synapse. 1997;27:168–176. doi: 10.1002/(SICI)1098-2396(199711)27:3<168::AID-SYN2>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Nordstrom AL, Farde L, Nyberg S, Karlsson P, Halldin C, Sedvall G. D1, D2, and 5-HT2 receptor occupancy in relation to clozapine serum concentration: a PET study of schizophrenic patients. Am J Psychiatry. 1995;152:1444–1449. doi: 10.1176/ajp.152.10.1444. [DOI] [PubMed] [Google Scholar]
- Nudmamud S, Reynolds GP. Increased density of glutamate/N-methyl-d-aspartate receptors in superior temporal cortex in schizophrenia. Neurosci Lett. 2001;304:9–12. doi: 10.1016/s0304-3940(01)01727-x. [DOI] [PubMed] [Google Scholar]
- Nyberg S, Farde L, Halldin C. A PET study of 5-HT2 and D2 dopamine receptor occupancy induced by olanzapine in healthy subjects. Neuropsychopharmacology. 1997;16:1–7. doi: 10.1016/S0893-133X(96)00218-7. [DOI] [PubMed] [Google Scholar]
- O'Callaghan E, Larkin C, Kinsella A, Waddington JL. Familial, obstetric, and other clinical correlates of minor physical anomalies in schizophrenia. Am J Psychiatry. 1991;148:479–483. doi: 10.1176/ajp.148.4.479. [DOI] [PubMed] [Google Scholar]
- Ohnuma T, Augood SJ, Arai H, McKenna PJ, Emson PC. Expression of the human excitatory amino acid transporter 2 and metabotropic glutamate receptors 3 and 5 in the prefrontal cortex from normal individuals and patients with schizophrenia. Brain Res Mol Brain Res. 1998;56:207–217. doi: 10.1016/s0169-328x(98)00063-1. [DOI] [PubMed] [Google Scholar]
- Ohnuma T, Tessler S, Arai H, Faull RL, McKenna PJ, Emson PC. Gene expression of metabotropic glutamate receptor 5 and excitatory amino acid transporter 2 in the schizophrenic hippocampus. Brain Res Mol Brain Res. 2000;85:24–31. doi: 10.1016/s0169-328x(00)00222-9. [DOI] [PubMed] [Google Scholar]
- Ohtsuki T, Toru M, Arinami T. Mutation screening of the metabotropic glutamate receptor mGluR4 (GRM4) gene in patients with schizophrenia. Psychiatr Genet. 2001;11:79–83. doi: 10.1097/00041444-200106000-00004. [DOI] [PubMed] [Google Scholar]
- Olney JW. Excitatory transmitter neurotoxicity. Neurobiol Aging. 1994;15:259–260. doi: 10.1016/0197-4580(94)90127-9. [DOI] [PubMed] [Google Scholar]
- Olney JW, Farber NB. Glutamate receptor dysfunction and schizophrenia. Arch Gen Psychiatry. 1995;52:998–1007. doi: 10.1001/archpsyc.1995.03950240016004. [DOI] [PubMed] [Google Scholar]
- Olney JW, Price MT, Samson L, Labruyere J. The role of specific ions in glutamate neurotoxicity. Neurosci Lett. 1986;65:65–71. doi: 10.1016/0304-3940(86)90121-7. [DOI] [PubMed] [Google Scholar]
- Olney JW, Labruyere J, Price MT. Pathological changes induced in cerebrocortical neurons by phencyclidine and related drugs. Science. 1989;244:1360–1362. doi: 10.1126/science.2660263. [DOI] [PubMed] [Google Scholar]
- Olney JW, Labruyere J, Wang G, Wozniak DF, Price MT, Sesma MA. NMDA antagonist neurotoxicity: mechanism and prevention. Science. 1991;254:1515–1518. doi: 10.1126/science.1835799. [DOI] [PubMed] [Google Scholar]
- Olney JW, Newcomer JW, Farber NB. NMDA receptor hypofunction model of schizophrenia. J Psychiatr Res. 1999;33:523–533. doi: 10.1016/s0022-3956(99)00029-1. [DOI] [PubMed] [Google Scholar]
- Omkumar RV, Kiely MJ, Rosenstein AJ, Min KT, Kennedy MB. Identification of a phosphorylation site for calcium/calmodulin-dependent protein kinase II in the NR2B subunit of the N-methyl-d-aspartate receptor. J Biol Chem. 1996;271:31670–31678. doi: 10.1074/jbc.271.49.31670. [DOI] [PubMed] [Google Scholar]
- Ozawa S, Kamiya H, Tsuzuki K. Glutamate receptors in the mammalian central nervous system. Prog Neurobiol. 1998;54:581–618. doi: 10.1016/s0301-0082(97)00085-3. [DOI] [PubMed] [Google Scholar]
- Pellegrini-Giampietro DE, Gorter JA, Bennett MV, Zukin RS. The GluR2 (GluR-B) hypothesis: Ca2+-permeable AMPA receptors in neurological disorders. Trends Neurosci. 1997;20:464–470. doi: 10.1016/s0166-2236(97)01100-4. [DOI] [PubMed] [Google Scholar]
- Pellicciari R, Costantino G. Metabotropic G-protein-coupled glutamate receptors as therapeutic targets. Curr Opin Chem Biol. 1999;3:433–440. doi: 10.1016/S1367-5931(99)80064-7. [DOI] [PubMed] [Google Scholar]
- Perez-Otano I, Schulteis CT, Contractor A, Lipton SA, Trimmer JS, Sucher NJ, Heinemann SF. Assembly with the NR1 subunit is required for surface expression of NR3A-containing NMDA receptors. J Neurosci. 2001;21:1228–1237. doi: 10.1523/JNEUROSCI.21-04-01228.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen RC, Stillman RC. Phencyclidine: an overview. NIDA Res Monogr. 1978;21:1–17. [PubMed] [Google Scholar]
- Petralia RS, Esteban JA, Wang YX, Partridge JG, Zhao HM, Wenthold RJ, Malinow R. Selective acquisition of AMPA receptors over postnatal development suggests a molecular basis for silent synapses. Nat Neurosci. 1999;2:31–36. doi: 10.1038/4532. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Zipursky RB, Lim KO, Zatz LM, Stahl SM, Jernigan TL. Computed tomographic evidence for generalized sulcal and ventricular enlargement in schizophrenia. Arch Gen Psychiatry. 1988;45:633–640. doi: 10.1001/archpsyc.1988.01800310037005. [DOI] [PubMed] [Google Scholar]
- Pilowsky LS, Busatto GF, Taylor M, Costa DC, Sharma T, Sigmundsson T, Ell PJ, Nohria V, Kerwin RW. Dopamine D2 receptor occupancy in vivo by the novel atypical antipsychotic olanzapine—a 123I IBZM single photon emission tomography (SPET) study. Psychopharmacology. 1996;124:148–153. doi: 10.1007/BF02245615. [DOI] [PubMed] [Google Scholar]
- Pisani A, Gubellini P, Bonsi P, Conquet F, Picconi B, Centonze D, Bernardi G, Calabresi P. Metabotropic glutamate receptor 5 mediates the potentiation of N-methyl-d-aspartate responses in medium spiny striatal neurons. Neuroscience. 2001;106:579–587. doi: 10.1016/s0306-4522(01)00297-4. [DOI] [PubMed] [Google Scholar]
- Platenik J, Kuramoto N, Yoneda Y. Molecular mechanisms associated with long-term consolidation of the NMDA signals. Life Sci. 2000;67:335–364. doi: 10.1016/s0024-3205(00)00632-9. [DOI] [PubMed] [Google Scholar]
- Porter RH, Eastwood SL, Harrison PJ. Distribution of kainate receptor subunit mRNAs in human hippocampus, neocortex and cerebellum, and bilateral reduction of hippocampal GluR6 and KA2 transcripts in schizophrenia. Brain Res. 1997;751:217–231. doi: 10.1016/s0006-8993(96)01404-7. [DOI] [PubMed] [Google Scholar]
- Potkin SG, Jin Y, Bunney BG, Costa J, Gulasekaram B. Effect of clozapine and adjunctive high-dose glycine in treatment-resistant schizophrenia. Am J Psychiatry. 1999;156:145–147. doi: 10.1176/ajp.156.1.145. [DOI] [PubMed] [Google Scholar]
- Prinssen EP, Ellenbroek BA, Cools AR. Combined antagonism of adrenoceptors and dopamine and 5-HT receptors underlies the atypical profile of clozapine. Eur J Pharmacol. 1994;262:167–170. doi: 10.1016/0014-2999(94)90041-8. [DOI] [PubMed] [Google Scholar]
- Puri BK, Barnes TRE, Chapman MJ, Hutton SB, Joyce EM. Spontaneous dyskinesia in first episode schizophrenia. J Neurol Neurosurg Psychiatry. 1998;66:76–78. doi: 10.1136/jnnp.66.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racca C, Stephenson FA, Streit P, Roberts JD, Somogyi P. NMDA receptor content of synapses in stratum radiatum of the hippocampal CA1 area. J Neurosci. 2000;20:2512–2522. doi: 10.1523/JNEUROSCI.20-07-02512.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raedler TJ, Knable MB, Lafargue T, Urbina RA, Egan MF, Pickar D, Weinberger DR. In vivo determination of striatal dopamine D2 receptor occupancy in patients treated with olanzapine. Psychiatry Res. 1999;90:81–90. doi: 10.1016/s0925-4927(99)00010-4. [DOI] [PubMed] [Google Scholar]
- Rajadhyaksha A, Barczak A, Macías W, Leveque J-C, Lewis SE, Konradi C. L-type Ca2+ channels are essential for glutamate-mediated CREB phosphorylation and c-fos gene expression in striatal neurons. J Neurosci. 1999;19:6348–6359. doi: 10.1523/JNEUROSCI.19-15-06348.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall RD, Thayer SA. Glutamate-induced calcium transient triggers delayed calcium overload and neurotoxicity in rat hippocampal neurons. J Neurosci. 1992;12:1882–1895. doi: 10.1523/JNEUROSCI.12-05-01882.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao PS, Ayres SM, Mueller HS. Identity of peroxy radicals produced from arachidonic acid in oxygenated solutions as studied by pulse radiolysis technique. Biochem Biophys Res Commun. 1982;104:1532–1536. doi: 10.1016/0006-291x(82)91425-5. [DOI] [PubMed] [Google Scholar]
- Remington G, Chong SA. Conventional versus novel anti-psychotics: changing concepts and clinical implications. J Psychiatry Neurosci. 1999;24:431–441. [PMC free article] [PubMed] [Google Scholar]
- Remington G, Kapur S. D2 and 5-HT2 receptor effects of antipsychotics: bridging basic and clinical findings using PET. J Clin Psychiatry. 1999;60:15–19. [PubMed] [Google Scholar]
- Richardson-Burns SM, Haroutunian V, Davis KL, Watson SJ, Meador-Woodruff JH. Metabotropic glutamate receptor mRNA expression in the schizophrenic thalamus. Biol Psychiatry. 2000;47:22–28. doi: 10.1016/s0006-3223(99)00207-3. [DOI] [PubMed] [Google Scholar]
- Richelson E. Receptor pharmacology of neuroleptics: relation to clinical effects. J Clin Psychiatry. 1999;60:5–14. [PubMed] [Google Scholar]
- Roberts GW, Colter N, Lofthouse R, Bogerts B, Zech M, Crow TJ. Gliosis in schizophrenia: a survey. Biol Psychiatry. 1986;21:1043–1050. doi: 10.1016/0006-3223(86)90285-4. [DOI] [PubMed] [Google Scholar]
- Robertson GS, Fibiger HC. Neuroleptics increase c-fos expression in the forebrain: contrasting effects of haloperidol and clozapine. Neuroscience. 1992;46:315–328. doi: 10.1016/0306-4522(92)90054-6. [DOI] [PubMed] [Google Scholar]
- Robertson GS, Matsumura H, Fibiger HC. Induction patterns of fos-like immunoreactivity in the forebrain as predictors of atypical antipsychotic activity. J Pharmacol Exp Ther. 1994;271:1058–1066. [PubMed] [Google Scholar]
- Robinson D, Woerner MG, Alvir JM, Bilder R, Goldman R, Geisler S, Koreen A, Sheitman B, Chakos M, Mayerhoff D, Lieberman JA. Predictors of relapse following response from a first episode of schizophrenia or schizoaffective disorder. Arch Gen Psychiatry. 1999;56:241–247. doi: 10.1001/archpsyc.56.3.241. [DOI] [PubMed] [Google Scholar]
- Rongo C, Kaplan JM. CaMKII regulates the density of central glutamatergic synapses in vivo. Nature. 1999;402:195–199. doi: 10.1038/46065. [DOI] [PubMed] [Google Scholar]
- Rosenmund C, Stern-Bach Y, Stevens CF. The tetrameric structure of a glutamate receptor channel. Science. 1998;280:1596–1599. doi: 10.1126/science.280.5369.1596. [DOI] [PubMed] [Google Scholar]
- Rosso IM, Bearden CE, Hollister JM, Gasperoni TL, Sanchez LE, Hadley T, Cannon TD. Childhood neuromotor dysfunction in schizophrenia patients and their unaffected siblings: a prospective cohort study. Schizophr Bull. 2000;26:367–378. doi: 10.1093/oxfordjournals.schbul.a033459. [DOI] [PubMed] [Google Scholar]
- Rothman SM. The neurotoxicity of excitatory amino acids is produced by passive chloride influx. J Neurosci. 1985;5:1483–1489. doi: 10.1523/JNEUROSCI.05-06-01483.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman SM, Olney JW. Excitotoxicity and the NMDA receptor—still lethal after eight years. Trends Neurosci. 1995;18:57–58. doi: 10.1016/0166-2236(95)93869-y. [DOI] [PubMed] [Google Scholar]
- Safferling M, Tichelaar W, Kummerle G, Jouppila A, Kuusinen A, Keinanen K, Madden DR. First images of a glutamate receptor ion channel: oligomeric state and molecular dimensions of GluRB homomers. Biochemistry. 2001;40:13948–13953. doi: 10.1021/bi011143g. [DOI] [PubMed] [Google Scholar]
- Sattler R, Tymianski M. Molecular mechanisms of calcium-dependent excitotoxicity. J Mol Med. 2000;78:3–13. doi: 10.1007/s001090000077. [DOI] [PubMed] [Google Scholar]
- Schinder AF, Olson EC, Spitzer NC, Montal M. Mitochondrial dysfunction is a primary event in glutamate neurotoxicity. J Neurosci. 1996;16:6125–6133. doi: 10.1523/JNEUROSCI.16-19-06125.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoepp DD. The biochemical pharmacology of metabotropic glutamate receptors. Biochem Soc Trans. 1993;21:97–102. doi: 10.1042/bst0210097. [DOI] [PubMed] [Google Scholar]
- Schoepp DD. Unveiling the functions of presynaptic metabotropic glutamate receptors in the central nervous system. J Pharmacol Exp Ther. 2001;299:12–20. [PubMed] [Google Scholar]
- Schulman H. Protein phosphorylation in neuronal plasticity and gene expression. Curr Opin Neurobiol. 1995;5:375–381. doi: 10.1016/0959-4388(95)80051-4. [DOI] [PubMed] [Google Scholar]
- Schwartz LM, Milligan CE. Cold thoughts of death: the role of ICE proteases in neuronal cell death. Trends Neurosci. 1996;19:555–562. doi: 10.1016/s0166-2236(96)10067-9. [DOI] [PubMed] [Google Scholar]
- Sebens JB, Koch T, Ter Horst GJ, Korf J. Differential Fosprotein induction in rat forebrain regions after acute and long-term haloperidol and clozapine treatment. Eur J Pharmacol. 1995;273:175–182. doi: 10.1016/0014-2999(94)00692-z. [DOI] [PubMed] [Google Scholar]
- See RE, Chapman MA, Meshul CK. Comparison of chronic intermittent haloperidol and raclopride effects on striatal dopamine release and synaptic ultrastructure in rats. Synapse. 1992;12:147–154. doi: 10.1002/syn.890120208. [DOI] [PubMed] [Google Scholar]
- Seeburg PH. The molecular biology of mammalian glutamate receptor channels. Trends Neurosci. 1993;16:359–365. doi: 10.1016/0166-2236(93)90093-2. [DOI] [PubMed] [Google Scholar]
- Seeman P. Dopamine receptor sequences. Therapeutic levels of neuroleptics occupy D2 receptors, clozapine occupies D4. Neuropsychopharmacology. 1992;7:261–284. [PubMed] [Google Scholar]
- Seeman P, Lee T. Antipsychotic drugs: direct correlation between clinical potency and presynaptic action on dopamine neurons. Science. 1975;188:1217–1219. doi: 10.1126/science.1145194. [DOI] [PubMed] [Google Scholar]
- Seeman P, Van Tol HHM. Dopamine receptor pharmacology. Curr Opin Neurol Neurosurg. 1993;6:602–608. [PubMed] [Google Scholar]
- Seeman P, Corbett R, Van Tol HH. Atypical neuroleptics have low affinity for dopamine D2 receptors or are selective for D4 receptors. Neuropsychopharmacology. 1997;16:93–110. doi: 10.1016/S0893-133X(96)00187-X. [DOI] [PubMed] [Google Scholar]
- Segal M. Rapid plasticity of dendritic spine: hints to possible functions? Prog Neurobiol. 2001;63:61–70. doi: 10.1016/s0301-0082(00)00021-6. [DOI] [PubMed] [Google Scholar]
- Selemon LD, Goldman-Rakic PS. The reduced neuropil hypothesis: a circuit based model of schizophrenia. Biol Psychiatry. 1999;45:17–25. doi: 10.1016/s0006-3223(98)00281-9. [DOI] [PubMed] [Google Scholar]
- Sengpiel B, Preis E, Krieglstein J, Prehn JH. NMDA-induced superoxide production and neurotoxicity in cultured rat hippocampal neurons: role of mitochondria. Eur J Neurosci. 1998;10:1903–1910. doi: 10.1046/j.1460-9568.1998.00202.x. [DOI] [PubMed] [Google Scholar]
- Sheng M, Thompson MA, Greenberg ME. CREB: a Ca2+-regulated transcription factor phosphorylated by calmodulin-dependent kinases. Science. 1991;252:1427–1430. doi: 10.1126/science.1646483. [DOI] [PubMed] [Google Scholar]
- Sherman AD, Davidson AT, Baruah S, Hegwood TS, Waziri R. Evidence of glutamatergic deficiency in schizophrenia. Neurosci Lett. 1991;121:77–80. doi: 10.1016/0304-3940(91)90653-b. [DOI] [PubMed] [Google Scholar]
- Shi J, Townsend M, Constantine-Paton M. Activity-dependent induction of tonic calcineurin activity mediates a rapid developmental downregulation of NMDA receptor currents. Neuron. 2000;28:103–114. doi: 10.1016/s0896-6273(00)00089-1. [DOI] [PubMed] [Google Scholar]
- Shihabuddin L, Buchsbaum MS, Hazlett EA, Haznedar MM, Harvey PD, Newman A, Schnur DB, Spiegel-Cohen J, Wei T, Machac J, Knesaurek K, Vallabhajosula S, Biren MA, Ciaravolo TM, Luu-Hsia C. Dorsal striatal size, shape, and metabolic rate in never-medicated and previously medicated schizophrenics performing a verbal learning task. Arch Gen Psychiatry. 1998;55:235–243. doi: 10.1001/archpsyc.55.3.235. [DOI] [PubMed] [Google Scholar]
- Simonian NA, Getz RL, Leveque JC, Konradi C, Coyle JT. Kainic acid induces apoptosis in neurons. Neuroscience. 1996;75:1047–1055. doi: 10.1016/0306-4522(96)00326-0. [DOI] [PubMed] [Google Scholar]
- Simpson MD, Slater P, Royston MC, Deakin JF. Alterations in phencyclidine and sigma binding sites in schizophrenic brains. Effects of disease process and neuroleptic medication. Schizophr Res. 1991;6:41–48. doi: 10.1016/0920-9964(91)90019-n. [DOI] [PubMed] [Google Scholar]
- Smith Y, Charara A, Parent A. Synaptic innervation of midbrain dopaminergic neurons by glutamate-enriched terminals in the squirrel monkey. J Comp Neurol. 1996;364:231–253. doi: 10.1002/(SICI)1096-9861(19960108)364:2<231::AID-CNE4>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Snyder SH. Amphetamine psychosis: a “model” schizophrenia mediated by catecholamines. Am J Psychiatry. 1973;130:61–67. doi: 10.1176/ajp.130.1.61. [DOI] [PubMed] [Google Scholar]
- Snyder SH. The dopamine hypothesis of schizophrenia: focus on the dopamine receptor. Am J Psychiatry. 1976;133:197–202. doi: 10.1176/ajp.133.2.197. [DOI] [PubMed] [Google Scholar]
- Soderling TR. Calcium/calmodulin-dependent protein kinase II: role in learning and memory. Mol Cell Biochem. 1993;127-128:93–101. doi: 10.1007/BF01076760. [DOI] [PubMed] [Google Scholar]
- Sommer B, Kohler M, Sprengel R, Seeburg PH. RNA editing in brain controls a determinant of ion flow in glutamate-gated channels. Cell. 1991;67:11–19. doi: 10.1016/0092-8674(91)90568-j. [DOI] [PubMed] [Google Scholar]
- Stockmeier CA, DiCarlo JJ, Zhang Y, Thompson P, Meltzer HY. Characterization of typical and atypical antipsychotic drugs based on in vivo occupancy of serotonin2 and dopamine2 receptors. J Pharmacol Exp Ther. 1993;266:1374–1384. [PubMed] [Google Scholar]
- Sucher NJ, Akbarian S, Chi CL, Leclerc CL, Awobuluyi M, Deitcher DL, Wu MK, Yuan JP, Jones EG, Lipton SA. Developmental and regional expression pattern of a novel NMDA receptor-like subunit (NMDAR-L) in the rodent brain. J Neurosci. 1995;15:6509–6520. doi: 10.1523/JNEUROSCI.15-10-06509.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L, Margolis FL, Shipley MT, Lidow MS. Identification of a long variant of mRNA encoding the NR3 subunit of the NMDA receptor: its regional distribution and developmental expression in the rat brain. FEBS Lett. 1998;441:392–396. doi: 10.1016/s0014-5793(98)01590-7. [DOI] [PubMed] [Google Scholar]
- Sun P, Enslen H, Myung PS, Maurer RA. Differential activation of CREB by Ca2+/calmodulin-dependent protein kinases type II and type IV involves phosphorylation of a site that negatively regulates activity. Genes Dev. 1994;8:2527–2539. doi: 10.1101/gad.8.21.2527. [DOI] [PubMed] [Google Scholar]
- Supplisson S, Bergman C. Control of NMDA receptor activation by a glycine transporter co-expressed in Xenopus oocytes. J Neurosci. 1997;17:4580–4590. doi: 10.1523/JNEUROSCI.17-12-04580.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow NR, Geyer MA. Using an animal model of deficient sensorimotor gating to study the pathophysiology and new treatments of schizophrenia. Schizophr Bull. 1998;24:285–301. doi: 10.1093/oxfordjournals.schbul.a033326. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Bakshi V, Waikar M, Taaid N, Geyer MA. Seroquel, clozapine and chlorpromazine restore sensorimotor gating in ketamine-treated rats. Psychopharmacologia. 1998;140:75–80. doi: 10.1007/s002130050741. [DOI] [PubMed] [Google Scholar]
- Swope SL, Moss SI, Raymond LA, Huganir RL. Regulation of ligand-gated ion channels by protein phosphorylation. Adv Second Messenger Phosphoprot Res. 1999;33:49–78. doi: 10.1016/s1040-7952(99)80005-6. [DOI] [PubMed] [Google Scholar]
- Tan SE, Wenthold RJ, Soderling TR. Phosphorylation of AMPA-type glutamate receptors by calcium/calmodulin-dependent protein kinase II and protein kinase C in cultured hippocampal neurons. J Neurosci. 1994;14:1123–1129. doi: 10.1523/JNEUROSCI.14-03-01123.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tandon R, Milner K, Jibson MD. Antipsychotics from theory to practice: integrating clinical and basic data. J Clin Psychiatry. 1999;60:21–28. [PubMed] [Google Scholar]
- Tenneti L, Lipton SA. Involvement of activated caspase-3-like proteases in N-methyl-d-aspartate-induced apoptosis in cerebrocortical neurons. J Neurochem. 2000;74:134–142. doi: 10.1046/j.1471-4159.2000.0740134.x. [DOI] [PubMed] [Google Scholar]
- Tenneti L, D'Emilia DM, Troy CM, Lipton SA. Role of caspases in N-methyl-d-aspartate-induced apoptosis in cerebrocortical neurons. J Neurochem. 1998;71:946–959. doi: 10.1046/j.1471-4159.1998.71030946.x. [DOI] [PubMed] [Google Scholar]
- Thompson SM. Synaptic plasticity: building memories to last. Curr Biol. 2000;10:R218–R221. doi: 10.1016/s0960-9822(00)00370-5. [DOI] [PubMed] [Google Scholar]
- Tong G, Shepherd D, Jahr CE. Synaptic desensitization of NMDA receptors by calcineurin. Science. 1995;267:1510–1512. doi: 10.1126/science.7878472. [DOI] [PubMed] [Google Scholar]
- Toni N, Buchs P-A, Nikonenko I, Bron CR, Muller D. LTP promotes formation of multiple spine synapses between a single axon terminal and a dendrite. Nature. 1999;402:421–425. doi: 10.1038/46574. [DOI] [PubMed] [Google Scholar]
- Travis MJ, Busatto GF, Pilowsky LS, Mulligan R, Acton PD, Gacinovic S, Mertens J, Terriere D, Costa DC, Ell PJ, Kerwin RW. 5-HT2A receptor blockade in patients with schizophrenia treated with risperidone or clozapine. A SPET study using the novel 5-HT2A ligand 123I-5-I-R-91150. Br J Psychiatry. 1998;173:236–241. doi: 10.1192/bjp.173.3.236. [DOI] [PubMed] [Google Scholar]
- Trichard C, Paillere-Martinot ML, Attar-Levy D, Recassens C, Monnet F, Martinot JL. Binding of antipsychotic drugs to cortical 5-HT2A receptors: a PET study of chlorpromazine, clozapine, and amisulpride in schizophrenic patients. Am J Psychiatry. 1998;155:505–508. doi: 10.1176/ajp.155.4.505. [DOI] [PubMed] [Google Scholar]
- Tsai G, Goff DC, Chang RW, Flood J, Baer L, Coyle JT. Markers of glutamatergic neurotransmission and oxidative stress associated with tardive dyskinesia. Am J Psychiatry. 1998a;155:1207–1213. doi: 10.1176/ajp.155.9.1207. [DOI] [PubMed] [Google Scholar]
- Tsai G, Yang P, Chung LC, Lange N, Coyle JT. D-serine added to antipsychotics for the treatment of schizophrenia. Biol Psychiatry. 1998b;44:1081–1089. doi: 10.1016/s0006-3223(98)00279-0. [DOI] [PubMed] [Google Scholar]
- Tsai GE, Yang P, Chung LC, Tsai IC, Tsai CW, Coyle JT. D-serine added to clozapine for the treatment of schizophrenia. Am J Psychiatry. 1999;156:1822–1825. doi: 10.1176/ajp.156.11.1822. [DOI] [PubMed] [Google Scholar]
- Uranova NA, Orlovskaya DD, Apel K, Klintsova AJ, Haselhorst U, Schenk H. Morphometric study of synaptic patterns in the rat caudate nucleus and hippocampus under haloperidol treatment. Synapse. 1991;7:253–259. doi: 10.1002/syn.890070402. [DOI] [PubMed] [Google Scholar]
- Vita A, Dieci M, Giobbio GM, Tenconi F, Invernizzi G. Time course of cerebral ventricular enlargement in schizophrenia supports the hypothesis of its neurodevelopmental nature. Schizophr Res. 1997;23:25–30. doi: 10.1016/s0920-9964(96)00085-0. [DOI] [PubMed] [Google Scholar]
- Wahlbeck K, Forsen T, Osmond C, Barker DJ, Eriksson JG. Association of schizophrenia with low maternal body mass index, small size at birth, and thinness during childhood. Arch Gen Psychiatry. 2001;58:48–52. doi: 10.1001/archpsyc.58.1.48. [DOI] [PubMed] [Google Scholar]
- Walker EF, Grimes KE, Davis DM, Smith AJ. Childhood precursors of schizophrenia: facial expressions of emotion. Am J Psychiatry. 1993;150:1654–1660. doi: 10.1176/ajp.150.11.1654. [DOI] [PubMed] [Google Scholar]
- Walker EF, Savoie T, Davis D. Neuromotor precursors of schizophrenia. Schizophr Bull. 1994;20:441–451. doi: 10.1093/schbul/20.3.441. [DOI] [PubMed] [Google Scholar]
- Wan W, Ennulat DJ, Cohen BM. Acute administration of typical and atypical antipsychotic drugs induces distinctive patterns of Fos expression in the rat forebrain. Brain Res. 1995;688:95–104. doi: 10.1016/0006-8993(95)00544-z. [DOI] [PubMed] [Google Scholar]
- Wang YT, Salter MW. Regulation of NMDA receptors by tyrosine kinases and phosphatases. Nature. 1994;369:233–235. doi: 10.1038/369233a0. [DOI] [PubMed] [Google Scholar]
- Watson CG, Kucala T, Tilleskjor C, Jacobs L. Schizophrenic birth seasonality in relation to the incidence of infectious diseases and temperature extremes. Arch Gen Psychiatry. 1984;41:85–90. doi: 10.1001/archpsyc.1984.01790120089011. [DOI] [PubMed] [Google Scholar]
- Watson GB, Bolanowski MA, Baganoff MP, Deppeler CL, Lanthorn TH. D-cycloserine acts as a partial agonist at the glycine modulatory site of the NMDA receptor expressed in Xenopus oocytes. Brain Res. 1990;510:158–160. doi: 10.1016/0006-8993(90)90745-w. [DOI] [PubMed] [Google Scholar]
- Weinberger DR, Torrey EF, Neophytides AN, Wyatt RJ. Lateral cerebral ventricular enlargement in chronic schizophrenia. Arch Gen Psychiatry. 1979;36:735–739. doi: 10.1001/archpsyc.1979.01780070013001. [DOI] [PubMed] [Google Scholar]
- Weissman AD, Casanova MF, Kleinman JE, London ED, De Souza EB. Selective loss of cerebral cortical sigma, but not PCP binding sites in schizophrenia. Biol Psychiatry. 1991;29:41–54. doi: 10.1016/0006-3223(91)90209-5. [DOI] [PubMed] [Google Scholar]
- Willinger U, Heiden AM, Meszaros K, Formann AK, Aschauer HN. Neurodevelopmental schizophrenia: obstetric complications, birth weight, premorbid social withdrawal and learning disabilities. Neuropsychobiology. 2001;43:163–169. doi: 10.1159/000054885. [DOI] [PubMed] [Google Scholar]
- Wong WT, Wong RO. Rapid dendritic movements during synapse formation and rearrangement. Curr Opin Neurobiol. 2000;10:118–124. doi: 10.1016/s0959-4388(99)00059-8. [DOI] [PubMed] [Google Scholar]
- Woods BT. Is schizophrenia a progressive neurodevelopmental disorder? Toward a unitary pathogenetic mechanism. Am J Psychiatry. 1998;155:1661–1670. doi: 10.1176/ajp.155.12.1661. [DOI] [PubMed] [Google Scholar]
- Wright P, Takei N, Rifkin L, Murray RM. Maternal influenza, obstetric complications, and schizophrenia. Am J Psychiatry. 1995;152:1714–1720. doi: 10.1176/ajp.152.12.1714. [DOI] [PubMed] [Google Scholar]
- Wu G, Malinow R, Cline HT. Maturation of a central glutamatergic synapse. Science. 1996;274:972–976. doi: 10.1126/science.274.5289.972. [DOI] [PubMed] [Google Scholar]
- Yakel JL, Vissavajjhala P, Derkach VA, Brickey DA, Soderling TR. Identification of a Ca2+/calmodulin-dependent protein kinase II regulatory phosphorylation site in non-N-methyl-d-aspartate glutamate receptors. Proc Natl Acad Sci USA. 1995;92:1376–1380. doi: 10.1073/pnas.92.5.1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto BK, Cooperman MA. Differential effects of chronic antipsychotic drug treatment on extracellular glutamate and dopamine concentrations. J Neurosci. 1994;14:4159–4166. doi: 10.1523/JNEUROSCI.14-07-04159.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto BK, Davy S. Dopaminergic modulation of glutamate release in striatum as measured by microdialysis. J Neurochem. 1992;58:1736–1742. doi: 10.1111/j.1471-4159.1992.tb10048.x. [DOI] [PubMed] [Google Scholar]
- Yin JC, Wallach JS, Del Vecchio M, Wilder EL, Zhou H, Quinn WG, Tully T. Induction of a dominant negative CREB transgene specifically blocks long-term memory in Drosophila. Cell. 1994;79:49–58. doi: 10.1016/0092-8674(94)90399-9. [DOI] [PubMed] [Google Scholar]
- Yin JC, Del Vecchio M, Zhou H, Tully T. CREB as a memory modulator: induced expression of a dCREB2 activator isoform enhances long-term memory in Drosophila. Cell. 1995;81:107–115. doi: 10.1016/0092-8674(95)90375-5. [DOI] [PubMed] [Google Scholar]
- Yoshida Y, Ono T, Kizu A, Fukushima R, Miyagishi T. Striatal N-methyl-d-aspartate receptors in haloperidol-induced catalepsy. Eur J Pharmacol. 1991;203:173–180. doi: 10.1016/0014-2999(91)90712-y. [DOI] [PubMed] [Google Scholar]
- Ziolkowska B, Hollt V. The NMDA receptor antagonist MK-801 markedly reduces the induction of c-fos gene by haloperidol in the mouse striatum. Neurosci Lett. 1993;156:39–42. doi: 10.1016/0304-3940(93)90434-m. [DOI] [PubMed] [Google Scholar]
- Zipursky RB, Lim KO, Sullivan EV, Brown BW, Pfefferbaum A. Widespread cerebral gray matter volume deficits in schizophrenia. Arch Gen Psychiatry. 1992;49:195–205. doi: 10.1001/archpsyc.1992.01820030027004. [DOI] [PubMed] [Google Scholar]
- Zorn SH, Jones SB, Ward KM, Liston DR. Clozapine is a potent and selective muscarinic M4 receptor agonist. Eur J Pharmacol. 1994;269:R1–R2. doi: 10.1016/0922-4106(94)90047-7. [DOI] [PubMed] [Google Scholar]
- Zou H, Henzel WJ, Liu X, Lutschg A, Wang X. Apaf-1, a human protein homologous to C. elegans CED-4, participates in cytochrome c-dependent activation of caspase-3. Cell. 1997;90:405–413. doi: 10.1016/s0092-8674(00)80501-2. [DOI] [PubMed] [Google Scholar]