Abstract
The plant hormone jasmonic acid (JA) has a crucial role in defense responses against pathogens in rice. We recently reported that some volatile compounds accumulate in response to JA treatment, and that the monoterpene linalool plays an important role in JA-induced resistance to rice bacterial blight caused by Xanthomonas oryzae pv oryzae (Xoo) in rice. One of the JA-responsive volatiles, (E,E)-2,4-heptadienal, has both antibacterial and antifungal activity against Xoo, and the rice fungal pathogen Magnaporthe oryzae. In addition, (E,E)-2,4-heptadienal was toxic to rice plants. These phenomena were not observed when linalool was treated. These results indicate that accumulation of the (E,E)-2,4-heptadienal in response to JA is a double-edged sword, but it is essential for survival against pathogen attacks in rice.
Keywords: (E,E)-2,4-heptadienal; jasmonic acid; Magnaporthe oryzae; plant volatile; Xanthomonas oryzae
Rice is not only a model plant for molecular studies of other monocots, but is also one of the most important crops worldwide. Jasmonic acid (JA) and its derivatives such as amino acid conjugates and methyl ester of JA function as plant signaling molecules involved in the regulation of many stress responses and developments.1-3 It is also well known that JA plays an important role in rice defense responses.4-8 In addition, it has been revealed that JA treatment causes the accumulation of plant volatile compounds in rice.9-11
Plant volatile compounds, such as terpenoids and C6 volatiles, are emitted from microbe-infected or wounded plant sites, and are potential signal compounds that induce defenses against tissue damage from herbivores and plant pathogens.12-16 In addition to the function as a signaling molecule, some volatile compounds have antimicrobial activities against plant pathogens.17-21 When hydroperoxide lyase, which is a key enzyme to produce the C6 and C9 volatiles, was overexpressed in rice and Arabidopsis, these plants exhibited increased resistance against pathogens.21,22 Furthermore, some defense-related genes were upregulated by vapor treatment with a C6 volatile, (E)-2-hexenal, in rice and Arabidopsis.21,23 Among terpenoids, monoterpenes and sesquiterpenes are one of the universal volatile components of plant. These volatile terpenoids are emitted from damaged plant tissues by attack of herbivores.24 Some of these volatile terpenoids are also emitted from plant tissues after treatment with elicitors and inoculation with fungal pathogen in rice.9 We recently demonstrated that the JA-responsive monoterpene linalool functions as a signal molecule involved in the JA-induced resistance against Xanthomonas oryzae pv oryzae (Xoo) in rice.11 Taken together, it is indicated that plant volatile compounds have an important role in stress and defense responses in plants. However, the function of individual volatiles in plant defense remains unclear. We recently identified 7 JA-responsive volatile compounds, 2,4-heptadienal, linalool, β-cyclocitral, β-elemene, β-caryophyllene, methyl salicylate, and β-ionone, in rice leaf blades.11 These volatile compounds reproducibly accumulated in response to treatment of 100 µM JA for 24 h in rice leaf blades.11 Although there was no statistical difference between WT and JA-treated rice, the levels of 2,4-heptadienal tended to accumulate more in JA-treated rice than in the WT.11 In addition, further study revealed that 2,4-heptadienal was (E,E) type of 2,4-heptadienal (data not shown). Here, we focus on these volatile compounds and discuss the role of a volatile compound, (E,E)-2,4-heptadienal, in the defense response of rice.
When these compounds were treated to Xoo, (E,E)-2,4-heptadienal showed a significant antibacterial activity against Xoo (Fig. 1). Linalool and β-elemene have been reported to have no effect against Xoo.11,25 In this study, we focused on (E,E)-2,4-heptadienal and characterized its properties in detail because there is currently no information available on its physiological function in the JA-responsive defense response in rice. In addition to negative effect to Xoo growth, treatment with (E,E)-2,4-heptadienal showed significant inhibitory activity toward spore germination of the rice fungal pathogen Magnaporthe oryzae (Fig. 2). However, linalool had no significant effect on spore germination of M. oryzae (Fig. 2). Treatment with linalool to rice upregulates the expression of defense-related genes.11 We investigated expression of 14 defense-related genes, which were previously identified as JA-responsive PR genes by microarray analysis,7 after treatment with 10 µM (E,E)-2,4-heptadienal for 24 h by qRT-PCR. As a result, treatment with (E,E)-2,4-heptadienal caused no upregulation of expression of these genes (data not shown). It has recently been reported that JA enhances M. oryzae resistance in rice.5,26 Transgenic rice plants overexpressing allene oxide synthase 2 (OsAOS2), which is a key enzyme to produce JA, exhibited resistance against rice blast disease caused by M. oryzae.5 Furthermore, 2 jasmonate-deficient rice mutants, cpm2 and hebiba, exhibited increased susceptibility to an incompatible strain of M. oryzae.26 These results suggest that (E,E)-2,4-heptadienal has both antibacterial and antifungal activity against rice pathogens and its accumulation in response to JA is important for protection against invasion by pathogens in rice.
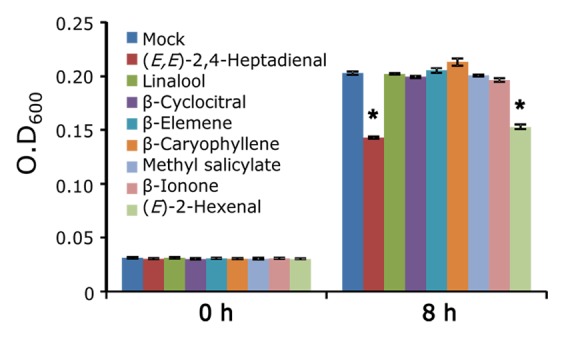
Figure 1. Effect of JA-responsive volatiles on Xoo. Evaluation of the antibacterial activity of JA-responsive volatiles was performed according to Taniguchi et al.25 Increases in O.D600 are presented for cultures treated with JA-responsive volatile compounds at 50 µM for 8 h. Values are the means ± SE of 4 independent experiments. (E)-2-Hexenal was used as a positive control.21 Asterisks represent statistically significant difference from a mock-treated control at P < 0.05 (the Student t-test).
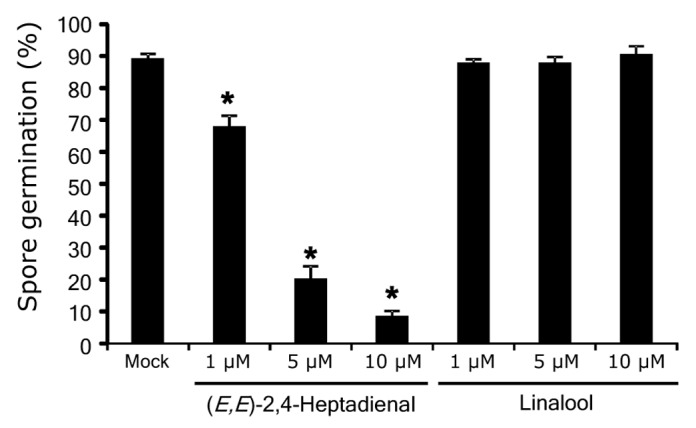
Figure 2. Effect of (E,E)-2,4-heptadienal on M. oryzae. Assay of the antifungal activity of volatile compounds was performed according to Taniguchi et al.25 Rate of spore germination after treatment with (E,E)-2,4-heptadienal and linalool for 6 h is indicated. Values are the means ± SE of 4 independent experiments (n = 100 spores per one experiment). Asterisks represent statistically significant difference from a mock-treated control at P < 0.05 (the Student t-test).
There is no information on the biosynthesis of (E,E)-2,4-heptadienal in rice, although some studies have reported the emission of (E,E)-2,4-heptadienal in rice.11,27,28 (E,E)-2,4-Heptadienal is a C7 volatile compound produced from linolenic acid via lipoxygenase (LOX) pathway in leek.29 Our previous study revealed the upregulation of 2 LOX genes in response to JA,7 suggesting that these genes may be involved in biosynthesis of the JA-responsive (E,E)-2,4-heptadienal. Further analyses are needed to determine the detailed biosynthetic pathway of (E,E)-2,4-heptadienal in rice.
(E,E)-2,4-Heptadienal has an inhibitory activity on seed germination in some plants,30 indicating that it is a toxic compound not only to pathogens but also to plants themselves. We confirmed its inhibitory activity on seed germination in rice (data not shown). Rice plants died when exposed to (E,E)-2,4-heptadienal for 24 h (Fig. 3). In contrast, linalool had no significant effect on rice plants (Fig. 3). Treatment with (E,E)-2,4-heptadienal induced reduction in growth rate and cell membrane disruption, along with chlorophyll degradation, and changes in cell size and granulosity in some phytoplanktons.31 There is no information regarding the mechanism of the cell death induced by (E,E)-2,4-heptadienal in rice. Thus, further studies are needed. In summary, (E,E)-2,4-heptadienal is a toxic but essential compound in JA-induced disease resistance in rice.

Figure 3. Effect of (E,E)-2,4-heptadienal on rice plants. Rice plants were grown to the 5-leaf stage, and treatment with volatile compounds was performed according to Taniguchi et al.11 Rice plants were exposed to 10 µM of (E,E)-2,4-heptadienal and linalool for 24 h at 25 °C and then photographed.
We have revealed a new role for some plant volatile compounds in defense systems in rice. In addition to these volatile compounds, we found other volatile compounds accumulated by treatment with JA in rice.11 However, the functions of these volatile compounds remain ambiguous. Functional analyses of these volatiles may reveal novel molecular mechanisms in JA-induced disease resistance against rice pathogens.
Acknowledgments
We thank Drs Y Nishizawa (National Institute of Agrobiological Sciences, NIAS) and H Kaku (NIAS) for providing the M. oryzae and Xoo strain, respectively. We also thank K Sano (Soda Aromatic Co, Ltd), M Satoh (National Agricultural Research Center for Kyushu Okinawa Region, NARO), and Dr H Kanno (NARO) for laying the foundations for parts of this study. This work was supported in part by a Funding Program for Next Generation World-Leading Researchers from the Japan Society for Promotion of Science (No. GS022).
References
- 1.Turner JG, Ellis C, Devoto A. The jasmonate signal pathway. Plant Cell. 2002;14:S153–64. doi: 10.1105/tpc.000679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Avanci NC, Luche DD, Goldman GH, Goldman MH. Jasmonates are phytohormones with multiple functions, including plant defense and reproduction. Genet Mol Res. 2010;9:484–505. doi: 10.4238/vol9-1gmr754. [DOI] [PubMed] [Google Scholar]
- 3.Pauwels L, Goossens A. The JAZ proteins: a crucial interface in the jasmonate signaling cascade. Plant Cell. 2011;23:3089–100. doi: 10.1105/tpc.111.089300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tamogami S, Rakwal R, Kodama O. Phytoalexin production elicited by exogenously applied jasmonic acid in rice leaves (Oryza sativa L.) is under the control of cytokinins and ascorbic acid. FEBS Lett. 1997;412:61–4. doi: 10.1016/S0014-5793(97)00743-6. [DOI] [PubMed] [Google Scholar]
- 5.Mei C, Qi M, Sheng G, Yang Y. Inducible overexpression of a rice allene oxide synthase gene increases the endogenous jasmonic acid level, PR gene expression, and host resistance to fungal infection. Mol Plant Microbe Interact. 2006;19:1127–37. doi: 10.1094/MPMI-19-1127. [DOI] [PubMed] [Google Scholar]
- 6.Kanno H, Hasegawa M, Kodama O. Accumulation of salicylic acid, jasmonic acid and phytoalexins in rice, Oryza sativa, infested by the white-backed planthopper, Sogatella furcifera (Hemiptera: Delphacidae) Appl Entomol Zool (Jpn) 2012;47:27–34. doi: 10.1007/s13355-011-0085-3. [DOI] [Google Scholar]
- 7.Yamada S, Kano A, Tamaoki D, Miyamoto A, Shishido H, Miyoshi S, Taniguchi S, Akimitsu K, Gomi K. Involvement of OsJAZ8 in jasmonate-induced resistance to bacterial blight in rice. Plant Cell Physiol. 2012;53:2060–72. doi: 10.1093/pcp/pcs145. [DOI] [PubMed] [Google Scholar]
- 8.Tamaoki D, Seo S, Yamada S, Kano A, Miyamoto A, Shishido H, Miyoshi S, Taniguchi S, Akimitsu K, Gomi K. Jasmonic acid and salicylic acid activate a common defense system in rice. Plant Signal Behav. 2013;8:e24260. doi: 10.4161/psb.24260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Obara N, Hasegawa M, Kodama O. Induced volatiles in elicitor-treated and rice blast fungus-inoculated rice leaves. Biosci Biotechnol Biochem. 2002;66:2549–59. doi: 10.1271/bbb.66.2549. [DOI] [PubMed] [Google Scholar]
- 10.Cheng AX, Xiang CY, Li JX, Yang CQ, Hu WL, Wang LJ, Lou YG, Chen XY. The rice (E)-beta-caryophyllene synthase (OsTPS3) accounts for the major inducible volatile sesquiterpenes. Phytochemistry. 2007;68:1632–41. doi: 10.1016/j.phytochem.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 11.Taniguchi S, Hosokawa-Shinonaga Y, Tamaoki D, Yamada S, Akimitsu K, Gomi K. Jasmonate induction of the monoterpene linalool confers resistance to rice bacterial blight and its biosynthesis is regulated by JAZ protein in rice. Plant Cell Environ. 2014;37:451–61. doi: 10.1111/pce.12169. [DOI] [PubMed] [Google Scholar]
- 12.Arimura G, Ozawa R, Shimoda T, Nishioka T, Boland W, Takabayashi J. Herbivory-induced volatiles elicit defence genes in lima bean leaves. Nature. 2000;406:512–5. doi: 10.1038/35020072. [DOI] [PubMed] [Google Scholar]
- 13.Pichersky E, Gershenzon J. The formation and function of plant volatiles: perfumes for pollinator attraction and defense. Curr Opin Plant Biol. 2002;5:237–43. doi: 10.1016/S1369-5266(02)00251-0. [DOI] [PubMed] [Google Scholar]
- 14.Gomi K, Yamasaki Y, Yamamoto H, Akimitsu K. Characterization of a hydroperoxide lyase gene and effect of C6-volatiles on expression of genes of the oxylipin metabolism in Citrus. J Plant Physiol. 2003;160:1219–31. doi: 10.1078/0176-1617-01177. [DOI] [PubMed] [Google Scholar]
- 15.Lou YG, Ma B, Cheng JA. Attraction of the parasitoid Anagrus nilaparvatae to rice volatiles induced by the rice brown planthopper Nilaparvata lugens. J Chem Ecol. 2005;31:2357–72. doi: 10.1007/s10886-005-7106-z. [DOI] [PubMed] [Google Scholar]
- 16.Kohzaki K, Gomi K, Yamasaki-Kokudo Y, Ozawa R, Takabayashi J, Akimitsu K. Characterization of a sabinene synthase gene from rough lemon (Citrus jambhiri) J Plant Physiol. 2009;166:1700–4. doi: 10.1016/j.jplph.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 17.Hamilton-Kemp TR, McCracken CTJ, Jr., Loughrin JH, Andersen RA, Hildebrand DF. Effects of some natural volatile compounds on the pathogenic fungiAlternaria alternata andBotrytis cinerea. J Chem Ecol. 1992;18:1083–91. doi: 10.1007/BF00980064. [DOI] [PubMed] [Google Scholar]
- 18.Nakamura S, Hatanaka A. Green-leaf-derived C6-aroma compounds with potent antibacterial action that act on both Gram-negative and Gram-positive bacteria. J Agric Food Chem. 2002;50:7639–44. doi: 10.1021/jf025808c. [DOI] [PubMed] [Google Scholar]
- 19.Kotan R, Kordali S, Cakir A. Screening of antibacterial activities of twenty-one oxygenated monoterpenes. Z Naturforsch C. 2007;62:507–13. doi: 10.1515/znc-2007-7-808. [DOI] [PubMed] [Google Scholar]
- 20.Kishimoto K, Matsui K, Ozawa R, Takabayashi J. Direct fungicidal activities of C6-aldehydes are important constituents for defense responses in Arabidopsis against Botrytis cinerea. Phytochemistry. 2008;69:2127–32. doi: 10.1016/j.phytochem.2008.04.023. [DOI] [PubMed] [Google Scholar]
- 21.Gomi K, Satoh M, Ozawa R, Shinonaga Y, Sanada S, Sasaki K, Matsumura M, Ohashi Y, Kanno H, Akimitsu K, et al. Role of hydroperoxide lyase in white-backed planthopper (Sogatella furcifera Horváth)-induced resistance to bacterial blight in rice, Oryza sativa L. Plant J. 2010;61:46–57. doi: 10.1111/j.1365-313X.2009.04031.x. [DOI] [PubMed] [Google Scholar]
- 22.Shiojiri K, Kishimoto K, Ozawa R, Kugimiya S, Urashimo S, Arimura G, Horiuchi J, Nishioka T, Matsui K, Takabayashi J. Changing green leaf volatile biosynthesis in plants: an approach for improving plant resistance against both herbivores and pathogens. Proc Natl Acad Sci U S A. 2006;103:16672–6. doi: 10.1073/pnas.0607780103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kishimoto K, Matsui K, Ozawa R, Takabayashi J. Volatile C6-aldehydes and Allo-ocimene activate defense genes and induce resistance against Botrytis cinerea in Arabidopsis thaliana. Plant Cell Physiol. 2005;46:1093–102. doi: 10.1093/pcp/pci122. [DOI] [PubMed] [Google Scholar]
- 24.Pare PW, Tumlinson JH. Plant volatiles as a defense against insect herbivores. Plant Physiol. 1999;121:325–32. doi: 10.1104/pp.121.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taniguchi S, Miyoshi S, Tamaoki D, Yamada S, Tanaka K, Uji Y, Tanaka S, Akimitsu K, Gomi K. Isolation of jasmonate-induced sesquiterpene synthase of rice: product of which has an antifungal activity against Magnaporthe oryzae. J Plant Physiol. 2014;171:625–32. doi: 10.1016/j.jplph.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 26.Riemann M, Haga K, Shimizu T, Okada K, Ando S, Mochizuki S, Nishizawa Y, Yamanouchi U, Nick P, Yano M, et al. Identification of rice Allene Oxide Cyclase mutants and the function of jasmonate for defence against Magnaporthe oryzae. Plant J. 2013;74:226–38. doi: 10.1111/tpj.12115. [DOI] [PubMed] [Google Scholar]
- 27.Bullard RW, Holguin G. Volatile components of unprocessed rice (Oryza sativa L.) J Agric Food Chem. 1976;25:99–103. doi: 10.1021/jf60209a050. [DOI] [PubMed] [Google Scholar]
- 28.Hernandez HP, Hsieh TCY, Smith CM, Fischer NH. Foliage volatiles of two rice cultivars. Phytochemistry. 1989;28:2959–62. doi: 10.1016/0031-9422(89)80261-4. [DOI] [Google Scholar]
- 29.Nielsen GS, Larsen LM, Poll L. Formation of volatile compounds in model experiments with crude leek (Allium ampeloprasum Var. Lancelot) enzyme extract and linoleic acid or linolenic acid. J Agric Food Chem. 2004;52:2315–21. doi: 10.1021/jf030600s. [DOI] [PubMed] [Google Scholar]
- 30.Bradow JM. Relationships between chemical structure and inhibitory activity of C6 through C 9 volatiles emitted by plant residues. J Chem Ecol. 1991;17:2193–212. doi: 10.1007/BF00988001. [DOI] [PubMed] [Google Scholar]
- 31.Ribalet F, Berges JA, Ianora A, Casotti R. Growth inhibition of cultured marine phytoplankton by toxic algal-derived polyunsaturated aldehydes. Aquat Toxicol. 2007;85:219–27. doi: 10.1016/j.aquatox.2007.09.006. [DOI] [PubMed] [Google Scholar]


