Abstract
Great progress has been made in the elucidation of the function of proteins in membrane traffic. Less is known about the regulatory role of lipids in membrane dynamics. Studies of nerve terminals, compartments highly specialized for the recycling of synaptic vesicles, have converged with studies from other systems to reveal mechanisms in protein-lipid interactions that affect membrane shape as well as the fusion and fission of vesicles. Phosphoinositides have emerged as major regulators of the binding of cytosolic proteins to the bilayer. Phosphorylation on different positions of the inositol ring generates different isomers that are heterogeneously distributed on cell membranes and that together with membrane proteins generate a “dual keys” code for the recruitment of cytosolic proteins. This code helps controlling vectoriality of membrane transport. Powerful methods for the detection of lipids are rapidly advancing this field, thus complementing the broad range of information about biological systems that can be obtained from genomic and proteomic approaches.
Keywords: Review
Synapses are junctions between a neuron and its target cells and are the sites of neurotransmission. Depolarization of the presynaptic membrane triggers Ca2+ entry, leading to exocytosis of synaptic vesicles and release of their contents into the synaptic cleft. After fusion, synaptic vesicle membranes are rapidly retrieved and reutilized to generate new neurotransmitter-filled vesicles. The temporal and spatial coordination of this exoendocytic recycling requires a complex molecular machinery that integrates cell signaling, dynamic changes within membranes, and cytoskeletal rearrangements. Over the past 25 years, many proteins of this machinery have been identified and characterized. Less is known about the function of lipids. Here we review current knowledge on the role of lipids in the physiology of neurotransmitter release and vesicle traffic in presynaptic nerve terminals with emphasis on the regulatory role of phosphoinositides (PIs).
The Hydrophobic Core of the Bilayer and Exo-Endocytosis
Generally, membrane lipids are amphipathic. As a consequence, lipids may exert their functions at the membrane interface or via their hydrophobic portion in the bilayer interior. We will first briefly discuss issues relating to a potential role of the bilayer interior in vesicle fusion and fission, and then review in more detail current knowledge about the important regulatory function of PIs, primarily mediated by interfacial chemistry.
Synaptic Vesicle Exocytosis. Exocytosis is a specific case of membrane fusion, a process that involves lipids by definition. However, the relative contribution of lipids and proteins to the regulation of membrane fusion has been the object of a long-standing debate (1). The identification of an evolutionary conserved protein machinery critically required for fusion reactions along the endocytic and secretory pathways has conclusively established the essential role of proteins. This machinery, based on the zippering of soluble N-ethylmaleimide-sensitive factor attachment protein receptors (SNARE) proteins, is sufficient to mediate membrane fusion in vitro (2). However, the importance of lipid chemistry and organization in the regulation of membrane fusion remains open for discussion.
A strikingly simple view of the molecular rearrangements within the two bilayers during fusion is based on the “shape model” originally proposed in the 1970s (3, 4). Lipid molecules are considered building blocks with different basic shapes (cones, cylinders, and inverted cones) depending on their molecular architecture. At sites of fusion, the bilayer arrangement of lipids must undergo distortion, and nonbilayer intermediates, facilitated by lipids with noncylindrical shapes (e.g., lysoplipids, fatty acids, and phosphatidic acid), may promote bilayer merging (1, 4-6). Typically, the cellular levels of these lipids are relatively low in resting conditions, but can rise significantly upon stimulation and activation of lipases (7). In addition, high concentrations of lysolipids were reported in membranes of dense core vesicles of neuroendocrine cells (8). A role of lysolipids in fusion is supported by the powerful stimulatory action on synaptic vesicle exocytosis of several neurotoxins with phospholipase A2 activity (9).
A unique characteristic of synaptic vesicle membranes is the high abundance of polyunsaturated fatty acids (PUFAs) (10). These lipids function as precursors for second messengers but may have additional direct roles in vesicle traffic. In Caenorhabditis elegans, mutation of the FAT-3 gene, which encodes a fatty acid desaturase essential for the production of PUFAs (11), displays a variety of developmental and behavioral phenotypes including a reduced number of synaptic vesicles and defects in neurosecretion (12). In Saccharomyces cerevisiae, mutants that affect fatty acid and lipid metabolism bypass the requirement for Snc v-SNARE proteins in secretion (13, 14).
Lipids of synaptic vesicles and of the plasma membrane may also affect fusion indirectly, via interfacial (see below) and hydrophobic core interactions with proteins. The latter interactions may occur not only with intrinsic membrane proteins, but also with cytosolic proteins that expose hydrophobic regions upon membrane binding (for example, see refs. 15-17). An important open question is whether local lipid heterogeneity in the presynaptic plasma membrane may play a role in defining sites of exocytosis, possibly in the spatial segregation of exo- and endocytosis. Syntaxins, SNARE proteins involved in membrane fusion, were reported to cluster in large cholesterol-dependent patches in the plasma membrane of PC12 cells, and cholesterol removal disrupts these patches and impairs exocytosis (18, 19).
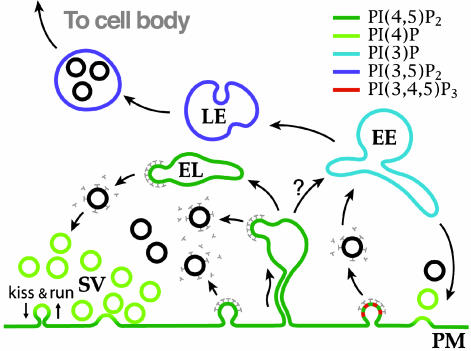
Synaptic Vesicle Endocytosis. Although the first systematic description of synaptic vesicle recycling was made >30 years ago, the precise pathway of recycling remains a matter of debate (20-23). Three main mechanisms of synaptic vesicle membrane endocytosis have been proposed: (i) clathrin-mediated endocytosis (20), (ii) direct reformation of synaptic vesicles via the rapid closure of a transient fusion pore (kiss-and-run) (22), and (iii) bulk endocytosis (23, 24). In this review, we will refer primarily to the model of synaptic vesicle endocytosis proposed by Takei et al. (24) (Fig. 1) and based on the following concepts: (i) vesicle recycling is mediated by clathrin-mediated budding followed by uncoating without an intermediate sorting station, (ii) bulk endocytosis represents a way through which excess membrane is rapidly internalized from deep plasma membrane invaginations after a strong exocytic burst, and (iii) clathrin-coated vesicles bud in parallel from the plasma membrane and from endosome-like intermediates generated by bulk endocytosis.
Fig. 1.
Vesicle traffic in nerve terminals and putative relationship to phosphoinositide metabolism. Membranes are color-coded based on their putative content in specific phosphoinositide species. The synaptic vesicle traffic is shown at left. PI(4)P is thought to be present on synaptic vesicles (SVs), whereas PI(4,5)P2 (dark green) is selectively concentrated in the plasma membrane (PM). During intense activity, deep plasma membrane invaginations and endosome-like (EL) structures generated form their fission retrieve excess membrane before clathrin-mediated budding (24). At right, the classical clathrin-mediated recycling pathway, which in nerve terminals may participate in receptors, transporters and channels endocytosis, is illustrated. Based on work in other systems, presence of PI(3)P (light blue) and PI(3,5)P2 (dark blue) on early (EE) and late (LE) endosomes, respectively, is proposed. PI(3,4,5)P3, which is generated by PI3-kinases associated with growth factor receptor signaling, is shown in red. The relationship of classical early endosomes to endosome-like structures that participate in synaptic vesicle recycling (a compartment that undergoes transient expansion during intense stimulation) remains to be elucidated.
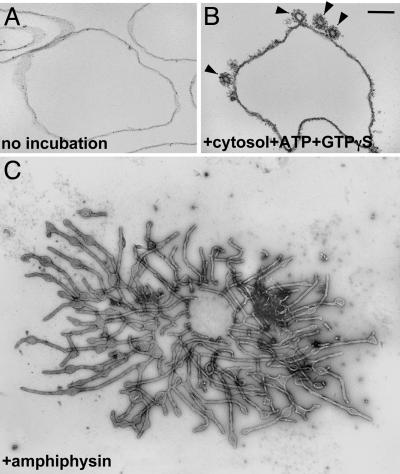
Irrespective of its mechanism, an endocytic reaction implies either the generation of bilayer curvature (for example, clathrin-mediated endocytosis) or retention of bilayer curvature to prevent postfusion membrane collapse (for example, kiss-and- run). Clathrin has long been thought to be the primary determinant of the curvature of clathrin coated vesicles. Its anchoring to the membrane, in turn, was attributed to its link to intrinsic proteins of the membrane via the clathrin adaptors. Recent findings, however, have revealed an unexpected central role of lipids in clathrin coat dynamics. First, it was found that protein-free liposomes are able to support the formation of clathrincoated pits, indicating a direct binding of the adaptors to lipids (25, 26) (Fig. 2B). Similar observations were made for other vesicle coats, such as COPI and COPII (27, 28). Second, studies of a variety of clathrin accessory factors revealed their unexpected property to bind lipids and to generate bilayer curvature when incubated with liposomes or when overexpressed in cells. Endocytic proteins with potent bilayer deforming properties include dynamin (a GTPase involved in vesicle fission) (25, 29), epsin (a clathrin adaptor) (26, 30), and BAR domain-containing proteins such as amphiphysin (Fig. 2C) and endophilin (adaptors between the membrane and other endocytic factors) (31-34). Structural studies have provided an insight about the different mechanisms through which these proteins may increase membrane curvature, thus generating the dome and the stalk of the vesicle bud (26, 34).
Fig. 2.
Morphological changes of lipid vesicles caused by incubation with cytosolic proteins. (A and B) Unilamellar liposomes before and after incubation with rat brain cytosol and nucleotides. Clathrin-coated profiles (arrowheads) that closely resemble those observed in situ are visible in B. (C) Incubation of liposomes with purified amphiphysin, a clathrin and dynamin interacting protein, leads to massive tubulation. Liposomes were analyzed by electron microscopy after plastic embedding and thin sectioning (A and B)or negative staining (C). (Scale bar = 100 nm in A, 200 nm in B, and 250 nm in C.) [B reproduced with permission from ref. 25 (Copyright 1998, Elsevier, Amsterdam).] [C reproduced with permission from ref. 152 (Copyright 2003, Elsevier, Amsterdam).]
Several studies have implicated cholesterol in the acquisition of curvature by endocytic vesicles, including those generated by caveolin and by clathrin (35, 36). Cholesterol intercalates among phospholipid acyl chains. Thus, it disrupts the order of the bilayer, but it also reduces the motion of phospholipid acyl chains. Cholesterol also binds to intrinsic proteins of the bilayer, and photoaffinity-labeling experiments identified synaptophysin, an integral membrane protein enriched in synaptic vesicles, as a specific binding partner of cholesterol (37). However, no major neurological or cell biological defects were observed in mice that lack the expression of synaptophysin (38). Furthermore, cholesterol does not appear to have an essential structural role in C. elegans (39), despite the high evolutionary conservation of mechanisms in membrane traffic.
A characteristic of synaptic vesicles, shared by all small vesicles, is a high degree of curvature, implying a quantitative asymmetry of phospholipids in the two leaflets. Whether lipid flippases, including aminophospholipid translocases, are implicated in their biogenesis remains unclear (40).
Interfacial Interactions of the Bilayer
Many of the functions of the bilayer are mediated by interactions of the polar head groups of the lipids with proteins of the cytoplasmic and luminal/extracellular milieu. Interfacial interactions may account entirely for the binding of a protein to the membrane. In other cases, a first interfacial interaction is followed by a partial and reversible penetration of the protein into the bilayer (26, 41). The cytoplasmic leaflets of membranes are enriched in negatively charged phospholipids that interact with positively charged protein surfaces. Phosphoinositides play a particularly important role in the regulation of protein binding, because the number and location of negative charges on the inositol ring is controlled by a variety of kinases and phosphatases. The noncytoplasmic leaflet contains a variety of glycolipids that, together with their interacting proteins, may contribute to the generation of lipid microdomains. In the case of synaptic vesicles, some of these interactions may help cluster sets of proteins, thus preventing dispersion after fusion, or promoting incorporation into nascent vesicles.
PIs as Membrane-Signaling Molecules
An important role for PIs in cellular function was first suggested in the 1950s by the observation that stimulation of a variety of tissues, including brain slices, led to increased incorporation of phosphate into inositol phospholipids (42). In synaptosomes, this turnover was found to be particularly high (43). A first explanation for the signaling role of PIs came from the discovery of the second messenger function of its metabolites, primarily inositolpolyphosphate 3 (IP3), diacylglycerol (DAG), and arachidonic acid, which derive from the degradation of phosphatidylinositol-4,5-biphosphate [PI(4,5)P2] by phospholipase C and phospholipase A2, respectively (44, 45). Subsequently, as new phosphoinositide species were identified, it became clear that inositol phospholipids themselves, including PI(4,5)P2, have signaling functions of their own, because of the properties of their head groups to bind with variable affinities and specificities to a variety of protein modules. These include PH, C2, Phox, Fyve, Dix, and ENTH domains, as well as short basic amino acid-rich sequences (41, 46). The reversible phosphorylation of inositol phospholipids may rival in importance the reversible tyrosine phosphorylation of membrane proteins as a mechanism to control recruitment and regulation of proteins at the membrane interface.
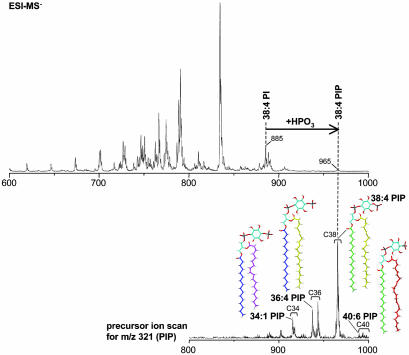
Analytic methods for phosphoinositide analysis have greatly expanded over the last few years, although each method has intrinsic limitations. HPLC-based methods allow discrimination of each of the seven stereoisomer species, but generally require metabolic labeling because of low sensitivity (47). Mass spectrometry methods offer much greater sensitivity, as well as the possibility to profile the fatty acid chain composition of phospholipids (48, 49) (Fig. 3). However, they do not allow discriminating among PI stereoisomers. A growing repertoire of protein modules that bind specific PIs allows for their visualization in living cells by using fluorescent fusion proteins (50, 51). These morphological methods bypass problems represented by the lability of phosphoinositide species in subcellular fractionation. However, one should consider that membrane recruitment of phosphoinositide binding modules is driven in part by protein-protein interactions (52). PIs are not distributed at random in the cell (53). Each stereoisomer has a unique distribution, and its localization is spatially and temporally controlled. For example, PI(4)P, which together with PI(4,5)P2 is the most abundant phosphoinositide species, is the predominant phosphoinositide in membranes of the Golgi complex (52, 54, 55) and of outbound vesicles originating from this organelle (53), PI(3)P and PI(3,5)P2 are selectively concentrated on early endosomes and late endosomes, respectively (56, 57), PI(4,5)P2 is primarily localized in the plasma membrane (50, 51) (Fig. 1). PI(3,4,5)P3 is also mainly found at the plasma membrane, where it accumulates transiently in response to stimuli (58). In the following, we will summarize evidence for an important role of PI(4,5)P2 and its lipid and soluble metabolites in the control of synaptic vesicle traffic.
Fig. 3.
Profiling of PIs in a brain lipid extract by electrospray ionization mass spectrometry. (Upper) Negative ion single stage mass spectrum of a total rat brain lipid extract. A large number of ions are detected in the mass range of m/z 700-900, where the majority of phospholipids species cluster. Phosphorylation of the major PI species, 38:4 PI, shifts the corresponding PIP (38:4 PI, m/z 965) by 80 units, the mass of a phosphate moiety. Less abundant PIP species are detected by precursor ion scanning (Lower). A precursor ion scan for m/z 321 (the inositol headgroup of PIP) yields clusters of PIP species with 34, 36, 38, and 40 fatty acid carbons (Lower), whose structures are shown in color (blue, C16:0, palmitic acid; green, C18:0, stearic acid; magenta, C18:1, oleic acid; yellow, C20:4, arachidonic acid; red, C22:6, docosahexaenoic acid) (49).
PI(4,5)P2 and Exocytosis. The first evidence for a requirement of PI(4,5)P2 in regulated neuroendocrine secretion came from studies of the exocytosis of dense core granules from lysed chromaffin cells (59, 60). A model was proposed in which phosphatidylinositol is delivered to granule membranes via a phosphatidylinositol-transfer protein, then phosphorylated to PI(4)P by a type II PI 4-kinase activity tightly associated with secretory granule membranes, and finally converted to PI(4,5)P2 via the recruitment of a cytosolic PI(4)P 5-kinase (60). More recent studies provided evidence for the importance of PI(4,5)P2 in neuroendocrine secretion and showed that, both in chromaffin cells and in other cells, the bulk of PI(4,5)P2 is localized in the plasma membrane (61, 62). Thus, PI(4,5)P2 may act “in trans” in dense core granule exocytosis. Given the many mechanistic similarities between dense core vesicle and synaptic vesicle exocytosis, it is likely that a requirement for PI(4,5)P2 may also apply to synaptic vesicle-mediated secretion. Accordingly, a PI(4)P 5-kinase (PIP kinase type 1γ) is concentrated in nerve terminals, where it acts on the plasma membrane (63). Additionally, a type II PI 4-kinase activity was detected on synaptic vesicles (64, 65) and another PI 4-kinase (type IIIβ), which is regulated by a small Ca2+ binding protein, was implicated in the regulation of neurotransmitter release (66). However, the precise location in nerve terminals of PI(4)P pools that serve as PI(4,5)P2 precursors, and the role of specific PI 4-kinases in the synthesis of these pools deserve further investigation.
Additional evidence for a role of PI(4,5)P2 in neurosecretion comes from the presence of phosphoinositide-binding domains, primarily C2 domains, in many proteins that play a critical role in the docking and stimulus-secretion coupling of both synaptic vesicles and dense core vesicles. C2 domains are present, for example, in the vesicle proteins synaptotagmin, Doc2, and rabphilin, in the active zone proteins Piccolo and Rim, as well as in critical other “exocytic factors” such as Munc13 (see below) (67). A PTB domain that specifically interacts with PI(4,5)P2 is present in Mint (68), which is part of a complex containing Munc18-1 and syntaxin, two critical players in exocytosis (1). Binding of these proteins to PI(4,5)P2 may contribute to the specificity of fusion of secretory vesicles with the plasma membrane, but may also contribute directly to the exocytotic reaction. For example, studies of the C2 domain of synaptotagmin, a putative Ca2+ sensor in the regulation of SNARE-dependent fusion, have suggested that a Ca2+-dependent interaction of its C2B domain with plasma membrane PI(4,5)P2 may help bridging the two lipid bilayers in a critical step leading to fusion (17, 67)
DAG in the Priming Reaction of Exocytosis. Strong genetic evidence links one of the lipid metabolites of PI(4,5)P2, DAG, to the maturation from a docked vesicle to a ready-to-fuse vesicle, a process collectively called “priming.” DAG has long been known to be a regulator of protein kinase C function, via its binding to its C1 domain (69). DAG-binding C1 domains are also present in other proteins, including the presynaptically enriched proteins Unc13/Munc13 (69). Unc13 C. elegans mutants have striking neurotransmitter release defects (70). In mice, genetic disruption of Munc13 function completely abolishes neurotransmitter release (71), but not vesicle docking, thus suggesting a “priming” defect. Such a defect is phenocopied in knock-in mice harboring a single amino acid substitution in the C1 domain of Munc13, pointing to an essential role of its binding to DAG (72). This interaction may represent the critical end-point of an important regulatory network at active zones because (i) the Unc-13 defect in worms can be partially bypassed by an “open” form of the T-SNARE syntaxin (73), and (ii) disruption of RIM, a Rab3 effector that binds Munc13, also results in priming defects (74, 75). Munc13 was shown to interact with mSec7, a guanyl nucleotide exchange factor (GEF) for Arf6 that is recruited to membranes by a phosphoinositide binding PH domain (76). Arf6, in turn, is a potent activator of PIP kinase type Iγ, the main PI(4,5)P2 generating enzymes at synapses (63, 77). Thus, Munc13, mSec7, Arf6, and PIP kinase type Iγ may be involved in a positive feedback mechanism leading to the generation of PI(4,5)P2 at sites of release. A pool of this PI(4,5)P2 may function as precursor for DAG. At the neuromuscular junction of nematodes, availability of DAG was proposed to be regulated by the antagonistic actions of a phospholipase Cβ (EGL-8) and a DAG kinase (DGK-1), respectively (70).
PI(4,5)P2 in Clathrin-Mediated Endocytosis. Initially, the search of membrane factors leading to the recruitment and assembly of clathrin coats focused exclusively on intrinsic membrane proteins. However, investigations of binding partners for IPs led, surprisingly, to the identification of the clathrin adaptors AP-2 and AP180. Follow up investigations suggested that adaptors could bind membrane PIs as well (78-80). The subsequent demonstration that clathrin coats can assemble on liposomes (25) and the identification of a polyphosphoinositide phosphatase, synaptojanin, which is concentrated at endocytic sites (81), provided evidence for a physiological function of these interactions and generated an impetus for their characterization.
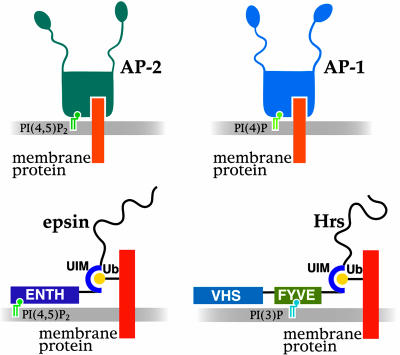
Endocytic Proteins Bind Phosphoinositides. In addition to AP-2 and AP-180, which are the main adaptors found on synaptic clathrincoated vesicles, many other proteins that fit the broad definition of endocytic clathrin adaptors, including epsin, Hip1/Hip1R and ARH/Dab, have recently been identified within the rapidly expanding families of endocytic accessory factors (82, 83). Strikingly, a general property of most endocytic adaptors is the binding to the head group of PI(4,5)P2, and at least in some cases PI(3,4,5)P3 (83-86). Interestingly, clathrin adaptors that do not function at the plasma membrane bind other PIs (54, 87). For example, AP-1 adaptors bind PI(4)P, which is concentrated on Golgi membranes (54) (Fig. 4). Dynamin, which plays a key role in the fission reaction of endocytosis, also binds to the lipid bilayer. It does so at least in part via a PI(4,5)P2 binding PH domain (88).
Fig. 4.
PIs as part of a coincidence detection mechanism for the recruitment of cytosolic proteins. PI(4)P and PI(4,5)P2 function as coreceptors together with intrinsic membrane proteins in the recruitment of AP-1 and AP-2 clathrin adaptors at the Golgi complex and on the plasma membrane, respectively. (Lower) Schematic representation of two clathrin adaptors, epsin and Hrs, that contain a phosphoinositide-binding domain and ubiquitin-interacting domains (UIM) (143). These proteins are thought to participate in the sorting of monoubiquitinated membrane proteins. Hrs, which is localized on endosomes, binds PI(3)P via a Fyve domain, whereas epsin, localized primarily at the plasma membrane, binds PI(4,5)P2 via an ENTH domain. Additional interactions with components of the membrane may be mediated by the ENTH and VHS domains, respectively. Binding sites for clathrin coat components are located in unfolded low-complexity COOH-terminal half of epsin and Hrs (black line).
PI(4,5)P2 Metabolism and Endocytosis. The importance of PI(4,5)P2 in the recruitment of endocytic proteins to the plasma membrane is confirmed by a variety of functional studies. Manipulations that stimulate endocytic clathrin coat nucleation on membranes in vitro, for example ATP and guanosine 5′-[γ-thio]triphosphate (GTPγS), act, at least in part, by stimulating PI(4,5)P2 production (77, 89, 90). Accordingly, electron microscopy immunogold analysis of synaptic membranes incubated in the presence of these nucleotides and brain cytosol revealed a concentration of PIP kinase type 1γ in close proximity of clathrin-coated pits (63). Conversely, masking of PI(4,5)P2 by PH domains or neomycin (91), or dephosphorylation of PI(4,5)P2 by overexpression of a membrane-targeted inositol 5-phosphatase, inhibits clathrinmediated endocytosis (77). Likewise, AP-2 recruitment to membranes is blocked by mutation of its main PI(4,5)P2-binding site (92).
Genetic evidence for a role of PI(4,5)P2 in clathrin coat dynamics at the synapse came from studies of synaptojanin 1 (81). Synaptojanin 1, which dephosphorylates PI(4,5)P2 [but can also dephosphorylate PI(3,4,5)P3], is highly concentrated at synapses, is further concentrated on endocytic intermediates, and interacts via its COOH-terminal targeting domain with several proteins with direct or indirect roles in endocytosis that also bind dynamin (81, 82). The COOH terminus of a splice variant of synaptojanin 1 binds directly clathrin and AP-2 (93).
Synaptojanin 1 knockout (-/-) mice die shortly after birth, have severe neurological deficits and elevated levels of PI(4,5)P2 in brain (89). Synaptojanin 1-/- neurons exhibit defects in synaptic vesicle recycling that were attributed to a delay in clathrin-coated vesicle uncoating and to the trapping of endocytic vesicles in a cytoskeletal matrix (see below) at endocytic zones. Impaired PI(4,5)P2 hydrolysis could delay adaptor shedding but allow partial clathrin uncoating, thus explaining why the thick clathrin coat is visible on some, but not all, vesicles that accumulate at endocytic zones (89, 94). Similar results were observed in synaptojanin mutants of Drosophila (95) and C. elegans (96) as well as in giant synapses of the lamprey after microinjection of anti-synaptojanin 1 antibodies (97).
The defects produced by synaptojanin disruption are mimicked by the functional (lamprey axons) or genetic (Drosophila and C. elegans) disruption of endophilin, the main synaptojanin 1 binding partner (95-97). Endophilin was reported to have lysophosphatidic acid acyl transferase activity (98). However, this reported activity was very low. Furthermore, the putative enzymatic module, a BAR domain, appears to have instead a structural role in endocytosis (32, 34). Thus, a main role of endophilin may be to recruit synaptojanin. Synaptojanin 1 binding to endophilin and enzymatic activity are inhibited by cyclin-dependent kinase 5 (Cdk5)-dependent phosphorylation in resting nerve terminals but stimulated by calcineurin dependent dephosphorylation after nerve terminal depolarization, when endocytosis is up-regulated (99). Endophilin also binds dynamin (100). The proximity of synaptojanin 1 to dynamin may restrict the localization of PI(4,5)P2 to the plasma membrane by promoting its dephosphorylation on endocytic membranes. One should note, however, that synaptojanin 2, another synaptojanin isoform that is much less abundant, can be recruited by Rac to the plasma membrane (101, 102). Thus, additional functions of synaptojanin family proteins both at synapses and elsewhere should be considered.
3-Phosphoinositides and Endosomal Traffic. PIs with a phosphate in the 3-position of the inositol ring (3-PIs) play an important role in the endocytic pathway and in signaling (58, 103). 3-PIs were found to enhance clathrin coat recruitment in vitro (104), and PI(3,4,5)P3, a low abundance phosphoinositide whose levels can be drastically stimulated by growth factor receptor activation, may cooperate with PI(4,5)P2 in the recruitment of endocytic proteins. In one study, treatment of the frog neuromuscular junction with a PI3-kinase inhibitor resulted in the inhibition of synaptic vesicle cycling (105). Synapsin (a synaptic vesicle associated protein), dynamin and synaptojanin 1 can interact with PI3-kinases in vitro and, in the case of synapsin, this interaction is supported by studies in living neurons (106, 107). However, little is known about upstream regulators and downstream effectors of PI(3,4,5)P3 in presynaptic function. A type II PI3-kinase whose binding to clathrin in vitro changes its substrate preference from PI to PI(4,5)P2 is concentrated in clathrin-coated vesicles, but, so far, this protein has been found only at the trans-Golgi region (108-110).
PI(3)P, which is enriched on endosomal membranes, recruits to these organelles a variety of factors required for their interactions, shape, motility, and sorting functions (53, 57). Based on yeast studies, PI(3,5)P2 plays a critical role in the biogenesis of late endosomes and multivesicular bodies (53) (Fig. 1). In neurons, multivesicular bodies are involved in retrograde transport to the cell body. Accordingly, PI3-kinase signaling has been implicated in this process (111).
IPs
IP3 has an important modulatory role in synaptic physiology via its actions on Ca2+ dynamics (44). Several other IPs, which can be generated from the reversible phosphorylation-dephosphorylation of IP3, have regulatory functions both in the nucleus and in the cytosol (112-114). Roles in membrane traffic have also been suggested. Inositol high-polyphosphates bind synaptotagmin (115) and clathrin adaptors (78-80, 84), and their microinjection in the squid giant synapse produced a potent block of synaptic vesicle recycling (116). IP6 was shown to stimulate insulin secretion and dynamin mediated endocytosis in pancreatic β cells (117) and regulate interactions of dynamin via an IP6-regulated kinase (118). Because IPs can compete with PIs in their binding to a variety of protein modules, an important open question is the potential cross-talk between the two forms of inositol metabolites. IP metabolism has long been connected to neuronal function by the therapeutic effect of Li+ in bipolar disorders. One of the putative targets of Li+ is IP-1-phosphatase, which studies in Drosophila have implicated in synaptic vesicle traffic (119).
Recently, a class of IPs that carry diphosphate groups has been identified. They were proposed to participate in membrane traffic and/or in energy storage (120, 121). IP6K1, one of the two kinases involved in their synthesis, associates with a Rab3 GEF in nerve terminals, suggesting a potential role in the regulation of synaptic vesicle exocytosis (122).
PI(4,5)P2 and the Actin Cytoskeleton
Synapses represent focal specialization of the cortical cytoplasm of neurons and are enriched in actin. Postsynaptic actin scaffolds have been extensively characterized. Less is known about presynaptic actin. The analysis of large model synapses have revealed an actin rich zone surrounding synaptic vesicle clusters (123, 124), where clathrin-mediated endocytosis occurs (97, 124). A similar organization may occur at most synapses. Talin, an adaptor protein between integrins and actin at focal adhesion sites, where it binds and potently activates PIP kinase type Iγ, is also present at synapses (125, 126).
PIs, PI(4,5)P2 in particular, but also PI(3,4,5)P3, are key regulators of actin nucleation (127, 128). For example, PI(4,5)P2 binds proteins of the WASP family. It further synergizes in the activation of these proteins via its binding to PH domains of GEFs responsible for the GTP loading of Rho and Arf family GTPases and to a variety of other actin regulatory proteins (128-130). Rho family GTPases, in turn, together with Arf6, bind and stimulate type I PIP kinases (77, 131), thus stimulating PI(4,5)P2 production. In nerve terminals with defective synaptojanin 1 function, and therefore impaired PI(4,5)P2 hydrolysis, a more abundant cytoskeletal matrix is observed around synaptic vesicle clusters (89, 94, 95, 97). Thus, the PI(4,5)P2 pool present at endocytic zones of synapses may play a dual role in actin nucleation and clathrin-mediated endocytosis.
This potential dual role is of special interest. A close, yet mechanistically still unclear, link has emerged between actin and endocytosis (132, 133). Particularly compelling evidence for a close relationship between actin and endocytosis was obtained from genetic and cell biological studies in yeast (134, 135). Bursts of actin polymerization and depolymerization were found to accompany K+-induced depolarization in synaptosomes, and stimulation-dependent changes in presynaptic actin were recently observed in nerve terminals of intact neurons (136, 137). Thus, activation of the synaptic vesicle cycle may correlate with actin changes, and PI(4,5)P2 may participate in the coordination of these reactions.
General Considerations
A Phosphoinositide Cycle in Synaptic Vesicle Recycling. The collective evidence discussed above points to important roles of PI(4,5)P2 in the traffic of synaptic vesicles beyond its classical role as a precursor of second messengers. During exocytosis, PI(4,5)P2, which is concentrated in the plasma membrane, helps marking this membrane as the appropriate target for vesicle fusion, and regulates membrane components of the exocytic machinery. Further roles of PI(4,5)P2 in exocytosis are mediated by the action of its metabolite DAG on vesicle priming. In endocytosis, PI(4,5)P2 helps recruiting clathrin adaptors and other endocytic factors, including dynamin and actin regulatory proteins. Neither endocytic motifs of vesicle proteins nor PI(4,5)P2 may be sufficient to recruit the clathrin adaptors unless they are present together in the same membrane (Fig. 4). This “coincidence detection mechanism” would ensure clathrin coat nucleation onto synaptic vesicle membranes only after exocytosis. After endocytosis, dephosphorylation of PI(4,5)P2 helps clathrin coat shedding, thus allowing uncoated vesicle to reenter the vesicle cycle. New acquisition of PI(4,5)P2 by these membranes would happen only after the next round of exocytosis. In summary, the model proposes the occurrence of a cycle of PI(4,5)P2 synthesis and dephosphorylation that is nested within the vesicle cycle and that accounts, at least in part, for its vectoriality (Fig. 1).
Other pathways of synaptic vesicle endocytosis may also capitalize on this PI(4,5)P2 cycle. For example, bulk endocytosis was shown to depend on actin in other systems (130). Thus, the rapid excess-membrane retrieval that is produced by strong synapse stimulation (Fig. 1) may be regulated by PI(4,5)P2-triggered actin polymerization. Likewise, PI(4,5)P2 may play a role in the closure of the “kiss-and-run” fusion pore, and in the reverse exocytosis of dense-core vesicles, because of the importance of dynamin in these pathways (138).
It should be noted, however, that the cycling of monoester phosphates on PIP and PIP2 is very rapid in synaptic membranes even under resting conditions, implying a cycle of phosphorylation-dephosphorylation also in the plasma membrane, irrespective of the occurrence of exo-endocytosis. What was once defined as a “futile cycle” (139) might be a very well controllable and versatile mechanism to allow rapid changes in absolute mass levels of PIs in a given membrane. Synthesis and dephosphorylation of PI(4,5)P2 in nerve terminals must be tightly coordinated with PI(4,5)P2 consumption by phospholipase C (possibly also by PI3-kinases) to preserve a PI(4,5)P2 pool required for both exocytosis and endocytosis. Synaptic stimulation triggers a peak of incorporation of 32P into PI(4,5)P2 to compensate, possibly offset, a decrease in PI(4,5)P2 (G. Di Paolo, M.R.W., and P.D.C., unpublished observations). A retrograde signal by NO was suggested to stimulate PI(4,5)P2 synthesis in the presynapse (140), which may be accounted for by the recruitment and stimulation of PIP kinase type Iγ.
Some General Principles in Membrane Traffic. Studies on membrane traffic at synapses have converged with studies in other systems to help defining some general principles concerning the role of lipids in vesicular transport. Some facts and hypotheses are listed below.
Membranes of different subcellular compartments vary in lipid composition. PIs are important determinants of this variability, which can be further enhanced by focal heterogeneity within a given membrane. The heterogeneous lipid composition accounts in part for the selective recruitment of cytosolic proteins to specific membranes. It may also account for the optimal functioning of integral membrane proteins in a specific membrane, as they travel through a variety of precursor or recycling compartments (141).
Progression of membranes along the secretory and endocytic pathways correlates with the sequential modification of their predominant phosphoinositide content (53). Thus, specific phosphoinositide species signal arrival in a given compartment and, together with GTPases (see below), are powerful regulators of compartment-specific functions.
Some cytosolic proteins contain both lipid binding modules and binding sites for membrane proteins. An interaction of sufficient affinity occurs only when both sites are engaged. This “dual keys” code may explain why both AP-1 and AP-2 clathrin adaptors can recognize the same cytosolic domains of intrinsic membrane proteins (142), yet, AP-1 adaptors bind to Golgi membranes (54), whereas AP-2 adaptors bind to the plasma membrane (77, 92) (Fig. 4). A similar dual code may account for the differential localization of adaptor proteins that link ubiquitinated membrane cargo to clathrin coats. Epsin, which binds PI(4,5)P2 via its ENTH domain, functions at the plasma membrane, whereas Hrs, which binds PI(3)P via a Fyve domain, functions on endosomes (143) (Fig. 4).
When a vesicle undergoes fusion, its lipids are rapidly diluted into the lipids of the target membrane. Conversely, vesicle fission results in a “closed” membrane compartment whose lipid composition can be globally modified by lipid metabolizing enzymes recruited to the vesicle. Many such enzymes function by interfacial catalysis (i.e., act processively through multiple catalytic cycles), thus enhancing the speed and efficiency with which these changes can occur. The role of synaptojanin in the dephosphorylation of PI(4,5)P2 on endocytic vesicles of the synapse was described above. Similar mechanisms may contribute to the “catastrophic” loss of PI(4,5)P2 and PI(3,4,5)P3 from micropinocytotic (144) and phagocytic vesicles (145), respectively, after they lose contact with the plasma membrane.
The signaling properties of PIs are enhanced by the multiple levels at which their synthesis and degradation is regulated by feedback loops. An example of positive feedback is the regulation of enzymes leading to PI(4,5)P2 and PI(3,4,5)P3 synthesis by small GTPases whose activation, in turn, depends on the phosphoinositide-mediated recruitment of their GEFs (131, 146, 147).
The role of a phosphoinositide cycle in controlling vectoriality of synaptic vesicle traffic is likely to be a special example of a more general role of PIs as regulators of vectorial transport. Another class of molecules that control vectoriality are GTPases (148). It is therefore of interest that many PI metabolizing enzymes are both downstream and upstream to Rho and Arf family GTPases. Relationship between Rabs and PI kinases have also been identified (149). Thus, these two systems function synergistically in the control of membrane traffic.
Concluding Remarks
Our understanding of the regulatory role of lipids and lipidprotein interactions in membrane traffic is still in the early stage. The synaptic vesicle cycle will continue to represent a very powerful model system to address fundamental questions in this area. Much of this information may be directly applicable to the field of postsynaptic receptor recycling, because growing evidence indicates that this process is very similar mechanistically to presynaptic vesicle traffic. Open questions include a precise elucidation of the key determinants of vesicle curvature and size, the role of lipids or lipid metabolism in the fusion and fission reactions, the potential function of heterogeneous lipid domains in the biogenesis of vesicles, and a potential differential role of pools of membrane lipids characterized by distinct fatty acid composition (150). Furthermore, specific lipids may contribute to anchor membranes to molecular motors, thus helping to direct long-distance traffic (151). It is surprising that despite the multiplicity of the phosphoinositide-metabolizing enzymes expressed in the nervous system, so little is known about their precise function in neuronal and synaptic physiology. After the advances of genomics and proteomics, the rapid development of methods for the systemic analysis of lipids (lipidomics) makes this field a most promising area of research.
Acknowledgments
We thank Gilbert Di Paolo, Toshiki Itoh, and Michele Solimena for discussion and critical reading of this manuscript.
This contribution is part of the special series of Inaugural Articles by members of the National Academy of Sciences elected on May 1, 2001.
Abbreviations: PIs, phosphoinositides; SNARE, soluble N-ethylmaleimide-sensitive factor attachment protein receptors; IP, inositolpolyphosphate; DAG, diacylglycerol; PI(4,5,)P2, phosphatidylinositol-4,5-biphosphate; GEF, guanyl nucleotide exchange factor.
See accompanying Biography on page 8259.
References
- 1.Jahn, R., Lang, T. & Sudhof, T. C. (2003) Cell 112, 519-533. [DOI] [PubMed] [Google Scholar]
- 2.Rothman, J. E. (2002) Nat. Med. 8, 1059-1062. [DOI] [PubMed] [Google Scholar]
- 3.Israelachvili, J. N. & Mitchell, D. J. (1975) Biochim. Biophys. Acta 389, 13-19. [DOI] [PubMed] [Google Scholar]
- 4.Chernomordik, L., Kozlov, M. M. & Zimmerberg, J. (1995) J. Membr. Biol. 146, 1-14. [DOI] [PubMed] [Google Scholar]
- 5.Burger, K. N. (2000) Traffic 1, 605-613. [DOI] [PubMed] [Google Scholar]
- 6.Tamm, L. K., Crane, J. & Kiessling, V. (2003) Curr. Opin. Struct. Biol. 13, 453-466. [DOI] [PubMed] [Google Scholar]
- 7.Ivanova, P. T., Cerda, B. A., Horn, D. M., Cohen, J. S., McLafferty, F. W. & Brown, H. A. (2001) Proc. Natl. Acad. Sci. USA 98, 7152-7157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blaschko, H., Firemark, H., Smith, A. D. & Winkler, H. (1967) Biochem. J. 104, 545-549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Montecucco, C. & Rossetto, O. (2000) Trends Biochem. Sci. 25, 266-270. [DOI] [PubMed] [Google Scholar]
- 10.Deutsch, J. W. & Kelly, R. B. (1981) Biochemistry 20, 378-385. [DOI] [PubMed] [Google Scholar]
- 11.Watts, J. L. & Browse, J. (2002) Proc. Natl. Acad. Sci. USA 99, 5854-5859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lesa, G. M., Palfreyman, M., Hall, D. H., Clandinin, M. T., Rudolph, C., Jorgensen, E. M. & Schiavo, G. (2003) J. Cell Sci. 116, 4965-4975. [DOI] [PubMed] [Google Scholar]
- 13.David, D., Sundarababu, S. & Gerst, J. E. (1998) J. Cell Biol. 143, 1167-1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grote, E., Vlacich, G., Pypaert, M. & Novick, P. J. (2000) Mol. Biol. Cell 11, 4051-4065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Benfenati, F., Greengard, P., Brunner, J. & Bahler, M. (1989) J. Cell Biol. 108, 1851-1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fernandez, I., Arac, D., Ubach, J., Gerber, S. H., Shin, O., Gao, Y., Anderson, R. G., Sudhof, T. C. & Rizo, J. (2001) Neuron 32, 1057-1069. [DOI] [PubMed] [Google Scholar]
- 17.Bai, J., Tucker, W. C. & Chapman, E. R. (2004) Nat. Struct. Mol. Biol. 11, 36-44. [DOI] [PubMed] [Google Scholar]
- 18.Lang, T., Bruns, D., Wenzel, D., Riedel, D., Holroyd, P., Thiele, C. & Jahn, R. (2001) EMBO J. 20, 2202-2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chamberlain, L. H., Burgoyne, R. D. & Gould, G. W. (2001) Proc. Natl. Acad. Sci. USA 98, 5619-5624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heuser, J. E. & Reese, T. S. (1973) J. Cell Biol. 57, 315-344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ceccarelli, B., Hurlbut, W. P. & Mauro, A. (1973) J. Cell Biol. 57, 499-524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Valtorta, F., Meldolesi, J. & Fesce, R. (2001) Trends Cell Biol. 11, 324-328. [DOI] [PubMed] [Google Scholar]
- 23.Royle, S. J. & Lagnado, L. (2003) J. Physiol. (London) 553, 345-355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takei, K., Mundigl, O., Daniell, L. & De Camilli, P. (1996) J. Cell Biol. 133, 1237-1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takei, K., Haucke, V., Slepnev, V., Farsad, K., Salazar, M., Chen, H. & De Camilli, P. (1998) Cell 94, 131-141. [DOI] [PubMed] [Google Scholar]
- 26.Ford, M. G., Mills, I. G., Peter, B. J., Vallis, Y., Praefcke, G. J., Evans, P. R. & McMahon, H. T. (2002) Nature 419, 361-366. [DOI] [PubMed] [Google Scholar]
- 27.Matsuoka, K., Orci, L., Amherdt, M., Bednarek, S. Y., Hamamoto, S., Schekman, R. & Yeung, T. (1998) Cell 93, 263-275. [DOI] [PubMed] [Google Scholar]
- 28.Spang, A., Matsuoka, K., Hamamoto, S., Schekman, R. & Orci, L. (1998) Proc. Natl. Acad. Sci. USA 95, 11199-11204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sweitzer, S. M. & Hinshaw, J. E. (1998) Cell 93, 1021-1029. [DOI] [PubMed] [Google Scholar]
- 30.De Camilli, P., Chen, H., Hyman, J., Panepucci, E., Bateman, A. & Brunger, A. T. (2002) FEBS Lett. 513, 11-18. [DOI] [PubMed] [Google Scholar]
- 31.Takei, K., Slepnev, V. I., Haucke, V. & De Camilli, P. (1999) Nat. Cell Biol. 1, 33-39. [DOI] [PubMed] [Google Scholar]
- 32.Farsad, K., Ringstad, N., Takei, K., Floyd, S. R., Rose, K. & De Camilli, P. (2001) J. Cell Biol. 155, 193-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee, E., Marcucci, M., Daniell, L., Pypaert, M., Weisz, O. A., Ochoa, G. C., Farsad, K., Wenk, M. R. & De Camilli, P. (2002) Science 297, 1193-1196. [DOI] [PubMed] [Google Scholar]
- 34.Peter, B. J., Kent, H. M., Mills, I. G., Vallis, Y., Butler, P. J., Evans, P. R. & McMahon, H. T. (2003) Science 303, 495-499. [DOI] [PubMed] [Google Scholar]
- 35.Kurzchalia, T. V. & Parton, R. G. (1999) Curr. Opin. Cell Biol. 11, 424-431. [DOI] [PubMed] [Google Scholar]
- 36.Rodal, S. K., Skretting, G., Garred, O., Vilhardt, F., van Deurs, B. & Sandvig, K. (1999) Mol. Biol. Cell 10, 961-974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thiele, C., Hannah, M. J., Fahrenholz, F. & Huttner, W. B. (2000) Nat. Cell Biol. 2, 42-49. [DOI] [PubMed] [Google Scholar]
- 38.McMahon, H. T., Bolshakov, V. Y., Janz, R., Hammer, R. E., Siegelbaum, S. A. & Sudhof, T. C. (1996) Proc. Natl. Acad. Sci. USA 93, 4760-4764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kurzchalia, T. V. & Ward, S. (2003) Nat. Cell Biol. 5, 684-688. [DOI] [PubMed] [Google Scholar]
- 40.Pomorski, T., Hrafnsdottir, S., Devaux, P. F. & van Meer, G. (2001) Semin. Cell Dev. Biol. 12, 139-148. [DOI] [PubMed] [Google Scholar]
- 41.Hurley, J. H. & Meyer, T. (2001) Curr. Opin. Cell Biol. 13, 146-152. [DOI] [PubMed] [Google Scholar]
- 42.Hokin, L. E. & Hokin, M. R. (1955) Biochim. Biophys. Acta 18, 102-110. [DOI] [PubMed] [Google Scholar]
- 43.Harwood, J. L. & Hawthorne, J. N. (1969) J. Neurochem. 16, 1377-1387. [DOI] [PubMed] [Google Scholar]
- 44.Berridge, M. J. (1993) Nature 361, 315-325. [DOI] [PubMed] [Google Scholar]
- 45.Tanaka, C. & Nishizuka, Y. (1994) Annu. Rev. Neurosci. 17, 551-567. [DOI] [PubMed] [Google Scholar]
- 46.Lemmon, M. A. (2003) Traffic 4, 201-213. [DOI] [PubMed] [Google Scholar]
- 47.Serunian, L. A., Auger, K. R. & Cantley, L. C. (1991) Methods Enzymol. 198, 78-87. [DOI] [PubMed] [Google Scholar]
- 48.Pulfer, M. & Murphy, R. C. (2003) Mass Spectrom. Rev. 22, 332-364. [DOI] [PubMed] [Google Scholar]
- 49.Wenk, M. R., Lucast, L., Di Paolo, G., Romanelli, A. J., Suchy, S. F., Nussbaum, R. L., Cline, G. W., Shulman, G. I., McMurray, W. & De Camilli, P. (2003) Nat. Biotechnol. 21, 813-817. [DOI] [PubMed] [Google Scholar]
- 50.Balla, T. & Varnai, P. (2002) Sci STKE 2002, L3. [DOI] [PubMed] [Google Scholar]
- 51.Meyer, T. & Teruel, M. N. (2003) Trends Cell Biol. 13, 101-106. [DOI] [PubMed] [Google Scholar]
- 52.Levine, T. P. & Munro, S. (2002) Curr. Biol. 12, 695-704. [DOI] [PubMed] [Google Scholar]
- 53.Odorizzi, G., Babst, M. & Emr, S. D. (2000) Trends Biochem. Sci. 25, 229-235. [DOI] [PubMed] [Google Scholar]
- 54.Wang, Y. J., Wang, J., Sun, H. Q., Martinez, M., Sun, Y. X., Macia, E., Kirchhausen, T., Albanesi, J. P., Roth, M. G. & Yin, H. L. (2003) Cell 114, 299-310. [DOI] [PubMed] [Google Scholar]
- 55.Cockcroft, S. & De Matteis, M. A. (2001) J. Membr. Biol. 180, 187-194. [DOI] [PubMed] [Google Scholar]
- 56.Odorizzi, G., Babst, M. & Emr, S. D. (1998) Cell 95, 847-858. [DOI] [PubMed] [Google Scholar]
- 57.Birkeland, H. C. & Stenmark, H. (2004) Curr. Top. Microbiol. Immunol. 282, 89-115. [DOI] [PubMed] [Google Scholar]
- 58.Cantley, L. C. (2002) Science 296, 1655-1657. [DOI] [PubMed] [Google Scholar]
- 59.Eberhard, D. A., Cooper, C. L., Low, M. G. & Holz, R. W. (1990) Biochem. J. 268, 15-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hay, J. C., Fisette, P. L., Jenkins, G. H., Fukami, K., Takenawa, T., Anderson, R. A. & Martin, T. F. (1995) Nature 374, 173-177. [DOI] [PubMed] [Google Scholar]
- 61.Micheva, K. D., Holz, R. W. & Smith, S. J. (2001) J. Cell Biol. 154, 355-368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Aikawa, Y. & Martin, T. F. (2003) J. Cell Biol. 162, 647-659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wenk, M. R., Pellegrini, L., Klenchin, V. A., Di Paolo, G., Chang, S., Daniell, L., Arioka, M., Martin, T. F. & De Camilli, P. (2001) Neuron 32, 79-88. [DOI] [PubMed] [Google Scholar]
- 64.Wiedemann, C., Schafer, T., Burger, M. M. & Sihra, T. S. (1998) J. Neurosci. 18, 5594-5602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Guo, J., Wenk, M. R., Pellegrini, L., Onofri, F., Benfenati, F. & De Camilli, P. (2003) Proc. Natl. Acad. Sci. USA 100, 3995-4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Strahl, T., Grafelmann, B., Dannenberg, J., Thorner, J. & Pongs, O. (2003) J. Biol. Chem. 278, 49589-49599. [DOI] [PubMed] [Google Scholar]
- 67.Rizo, J. & Sudhof, T. C. (1998) J. Biol. Chem. 273, 15879-15882. [DOI] [PubMed] [Google Scholar]
- 68.Okamoto, M. & Sudhof, T. C. (1997) J. Biol. Chem. 272, 31459-31464. [DOI] [PubMed] [Google Scholar]
- 69.Brose, N. & Rosenmund, C. (2002) J. Cell Sci. 115, 4399-4411. [DOI] [PubMed] [Google Scholar]
- 70.Lackner, M. R., Nurrish, S. J. & Kaplan, J. M. (1999) Neuron 24, 335-346. [DOI] [PubMed] [Google Scholar]
- 71.Varoqueaux, F., Sigler, A., Rhee, J. S., Brose, N., Enk, C., Reim, K. & Rosenmund, C. (2002) Proc. Natl. Acad. Sci. USA 99, 9037-9042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rhee, J. S., Betz, A., Pyott, S., Reim, K., Varoqueaux, F., Augustin, I., Hesse, D., Sudhof, T. C., Takahashi, M., Rosenmund, C. & Brose, N. (2002) Cell 108, 121-133. [DOI] [PubMed] [Google Scholar]
- 73.Richmond, J. E., Weimer, R. M. & Jorgensen, E. M. (2001) Nature 412, 338-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Koushika, S. P., Richmond, J. E., Hadwiger, G., Weimer, R. M., Jorgensen, E. M. & Nonet, M. L. (2001) Nat. Neurosci. 4, 997-1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schoch, S., Castillo, P. E., Jo, T., Mukherjee, K., Geppert, M., Wang, Y., Schmitz, F., Malenka, R. C. & Sudhof, T. C. (2002) Nature 415, 321-326. [DOI] [PubMed] [Google Scholar]
- 76.Neeb, A., Koch, H., Schurmann, A. & Brose, N. (1999) Eur. J. Cell Biol. 78, 533-538. [DOI] [PubMed] [Google Scholar]
- 77.Krauss, M., Kinuta, M., Wenk, M. R., De Camilli, P., Takei, K. & Haucke, V. (2003) J. Cell Biol. 162, 113-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Beck, K. A. & Keen, J. H. (1991) J. Biol. Chem. 266, 4442-4447. [PubMed] [Google Scholar]
- 79.Voglmaier, S. M., Keen, J. H., Murphy, J. E., Ferris, C. D., Prestwich, G. D., Snyder, S. H. & Theibert, A. B. (1992) Biochem. Biophys. Res. Commun. 187, 158-163. [DOI] [PubMed] [Google Scholar]
- 80.Hao, W., Tan, Z., Prasad, K., Reddy, K. K., Chen, J., Prestwich, G. D., Falck, J. R., Shears, S. B. & Lafer, E. M. (1997) J. Biol. Chem. 272, 6393-6398. [DOI] [PubMed] [Google Scholar]
- 81.McPherson, P. S., Garcia, E. P., Slepnev, V. I., David, C., Zhang, X., Grabs, D., Sossin, W. S., Bauerfeind, R., Nemoto, Y. & De Camilli, P. (1996) Nature 379, 353-357. [DOI] [PubMed] [Google Scholar]
- 82.Slepnev, V. I. & De Camilli, P. (2000) Nat. Rev. 1, 161-172. [DOI] [PubMed] [Google Scholar]
- 83.Traub, L. M. (2003) J. Cell Biol. 163, 203-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Collins, B. M., McCoy, A. J., Kent, H. M., Evans, P. R. & Owen, D. J. (2002) Cell 109, 523-535. [DOI] [PubMed] [Google Scholar]
- 85.Rohde, G., Wenzel, D. & Haucke, V. (2002) J. Cell Biol. 158, 209-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Itoh, T. & Takenawa, T. (2004) Curr. Top. Microbiol. Immunol. 282, 31-47. [DOI] [PubMed] [Google Scholar]
- 87.Friant, S., Pecheur, E. I., Eugster, A., Michel, F., Lefkir, Y., Nourrisson, D. & Letourneur, F. (2003) Dev. Cell 5, 499-511. [DOI] [PubMed] [Google Scholar]
- 88.Danino, D. & Hinshaw, J. E. (2001) Curr. Opin. Cell Biol. 13, 454-460. [DOI] [PubMed] [Google Scholar]
- 89.Cremona, O., Di Paolo, G., Wenk, M. R., Luthi, A., Kim, W. T., Takei, K., Daniell, L., Nemoto, Y., Shears, S. B., Flavell, R. A., et al. (1999) Cell 99, 179-188. [DOI] [PubMed] [Google Scholar]
- 90.Ford, M. G., Pearse, B. M., Higgins, M. K., Vallis, Y., Owen, D. J., Gibson, A., Hopkins, C. R., Evans, P. R. & McMahon, H. T. (2001) Science 291, 1051-1055. [DOI] [PubMed] [Google Scholar]
- 91.Jost, M., Simpson, F., Kavran, J. M., Lemmon, M. A. & Schmid, S. L. (1998) Curr. Biol. 8, 1399-1402. [DOI] [PubMed] [Google Scholar]
- 92.Gaidarov, I. & Keen, J. H. (1999) J. Cell Biol. 146, 755-764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Haffner, C., Paolo, G. D., Rosenthal, J. A. & de Camilli, P. (2000) Curr. Biol. 10, 471-474. [DOI] [PubMed] [Google Scholar]
- 94.Kim, W. T., Chang, S., Daniell, L., Cremona, O., Di Paolo, G. & De Camilli, P. (2002) Proc. Natl. Acad. Sci. USA 99, 17143-17148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Verstreken, P., Koh, T. W., Schulze, K. L., Zhai, R. G., Hiesinger, P. R., Zhou, Y., Mehta, S. Q., Cao, Y., Roos, J. & Bellen, H. J. (2003) Neuron 40, 733-748. [DOI] [PubMed] [Google Scholar]
- 96.Schuske, K. R., Richmond, J. E., Matthies, D. S., Davis, W. S., Runz, S., Rube, D. A., van der Bliek, A. M. & Jorgensen, E. M. (2003) Neuron 40, 749-762. [DOI] [PubMed] [Google Scholar]
- 97.Gad, H., Ringstad, N., Low, P., Kjaerulff, O., Gustafsson, J., Wenk, M., Di Paolo, G., Nemoto, Y., Crun, J., Ellisman, M. H., et al. (2000) Neuron 27, 301-312. [DOI] [PubMed] [Google Scholar]
- 98.Schmidt, A., Wolde, M., Thiele, C., Fest, W., Kratzin, H., Podtelejnikov, A. V., Witke, W., Huttner, W. B. & Soling, H. D. (1999) Nature 401, 133-141. [DOI] [PubMed] [Google Scholar]
- 99.Lee, S. Y., Wenk, M. R., Kim, Y., Nairn, A. C. & De Camilli, P. (2004) Proc. Natl. Acad. Sci. USA 101, 546-551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ringstad, N., Nemoto, Y. & De Camilli, P. (1997) Proc. Natl. Acad. Sci. USA 94, 8569-8574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nemoto, Y., Wenk, M. R., Watanabe, M., Daniell, L., Murakami, T., Ringstad, N., Yamada, H., Takei, K. & De Camilli, P. (2001) J. Biol. Chem. 276, 41133-41142. [DOI] [PubMed] [Google Scholar]
- 102.Rusk, N., Le, P. U., Mariggio, S., Guay, G., Lurisci, C., Nabi, I. R., Corda, D. & Symons, M. (2003) Curr. Biol. 13, 659-663, and erratum (2003) 13, 1746. [DOI] [PubMed] [Google Scholar]
- 103.Czech, M. P. (2003) Annu. Rev. Physiol. 65, 791-815. [DOI] [PubMed] [Google Scholar]
- 104.Rapoport, I., Miyazaki, M., Boll, W., Duckworth, B., Cantley, L. C., Shoelson, S. & Kirchhausen, T. (1997) EMBO J. 16, 2240-2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rizzoli, S. O. & Betz, W. J. (2002) J. Neurosci. 22, 10680-10689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Onofri, F., Giovedi, S., Kao, H. T., Valtorta, F., Borbone, L. B., De Camilli, P., Greengard, P. & Benfenati, F. (2000) J. Biol. Chem. 275, 29857-29867. [DOI] [PubMed] [Google Scholar]
- 107.Cousin, M. A., Malladi, C. S., Tan, T. C., Raymond, C. R., Smillie, K. J. & Robinson, P. J. (2003) J. Biol. Chem. 278, 29065-29071. [DOI] [PubMed] [Google Scholar]
- 108.Prior, I. A. & Clague, M. J. (1999) Mol. Cell. Biol. Res. Commun. 1, 162-166. [DOI] [PubMed] [Google Scholar]
- 109.Domin, J., Gaidarov, I., Smith, M. E., Keen, J. H. & Waterfield, M. D. (2000) J. Biol. Chem. 275, 11943-11950. [DOI] [PubMed] [Google Scholar]
- 110.Gaidarov, I., Smith, M. E., Domin, J. & Keen, J. H. (2001) Mol. Cell 7, 443-449. [DOI] [PubMed] [Google Scholar]
- 111.Kuruvilla, R., Ye, H. & Ginty, D. D. (2000) Neuron 27, 499-512. [DOI] [PubMed] [Google Scholar]
- 112.Loomis-Husselbee, J. W., Walker, C. D., Bottomley, J. R., Cullen, P. J., Irvine, R. F. & Dawson, A. P. (1998) Biochem. J. 331, 947-952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.York, J. D. (2003) in Handbook of Cell Signaling (Elsevier Science, Amsterdam), Vol. 2, pp. 229-232. [Google Scholar]
- 114.Hammond, G., Thomas, C. L. & Schiavo, G. (2004) Curr. Top. Microbiol. Immunol. 282, 177-206. [DOI] [PubMed] [Google Scholar]
- 115.Fukuda, M., Aruga, J., Niinobe, M., Aimoto, S. & Mikoshiba, K. (1994) J. Biol. Chem. 269, 29206-29211. [PubMed] [Google Scholar]
- 116.Llinas, R., Sugimori, M., Lang, E. J., Morita, M., Fukuda, M., Niinobe, M. & Mikoshiba, K. (1994) Proc. Natl. Acad. Sci. USA 91, 12990-12293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hoy, M., Efanov, A. M., Bertorello, A. M., Zaitsev, S. V., Olsen, H. L., Bokvist, K., Leibiger, B., Leibiger, I. B., Zwiller, J., Berggren, P. O. & Gromada, J. (2002) Proc. Natl. Acad. Sci. USA 99, 6773-6777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hilton, J. M., Plomann, M., Ritter, B., Modregger, J., Freeman, H. N., Falck, J. R., Krishna, U. M. & Tobin, A. B. (2001) J. Biol. Chem. 276, 16341-16347. [DOI] [PubMed] [Google Scholar]
- 119.Acharya, J. K., Labarca, P., Delgado, R., Jalink, K. & Zuker, C. S. (1998) Neuron 20, 1219-1229. [DOI] [PubMed] [Google Scholar]
- 120.Safrany, S. T., Caffrey, J. J., Yang, X. & Shears, S. B. (1999) J. Biol. Chem. 380, 945-951. [DOI] [PubMed] [Google Scholar]
- 121.Saiardi, A., Sciambi, C., McCaffery, J. M., Wendland, B. & Snyder, S. H. (2002) Proc. Natl. Acad. Sci. USA 99, 14206-14211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Luo, H. R., Saiardi, A., Nagata, E., Ye, K., Yu, H., Jung, T. S., Luo, X., Jain, S., Sawa, A. & Snyder, S. H. (2001) Neuron 31, 439-451. [DOI] [PubMed] [Google Scholar]
- 123.Dunaevsky, A. & Connor, E. A. (2000) J. Neurosci. 20, 6007-6012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Shupliakov, O., Bloom, O., Gustafsson, J. S., Kjaerulff, O., Low, P., Tomilin, N., Pieribone, V. A., Greengard, P. & Brodin, L. (2002) Proc. Natl. Acad. Sci. USA 99, 14476-14481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Di Paolo, G., Pellegrini, L., Letinic, K., Cestra, G., Zoncu, R., Voronov, S., Chang, S., Guo, J., Wenk, M. R. & De Camilli, P. (2002) Nature 420, 85-89. [DOI] [PubMed] [Google Scholar]
- 126.Ling, K., Doughman, R. L., Firestone, A. J., Bunce, M. W. & Anderson, R. A. (2002) Nature 420, 89-93. [DOI] [PubMed] [Google Scholar]
- 127.Takenawa, T. & Itoh, T. (2001) Biochim. Biophys. Acta 1533, 190-206. [DOI] [PubMed] [Google Scholar]
- 128.Yin, H. L. & Janmey, P. A. (2003) Annu. Rev. Physiol. 65, 761-789. [DOI] [PubMed] [Google Scholar]
- 129.Miki, H. & Takenawa, T. (2003) J. Biochem. (Tokyo) 134, 309-313. [DOI] [PubMed] [Google Scholar]
- 130.Donaldson, J. G. (2003) J. Biol. Chem. 278, 41573-41576. [DOI] [PubMed] [Google Scholar]
- 131.Weernink, P. A., Meletiadis, K., Hommeltenberg, S., Hinz, M., Ishihara, H., Schmidt, M. & Jakobs, K. H. (2004) J. Biol. Chem. 279, 7840-7849. [DOI] [PubMed] [Google Scholar]
- 132.Qualmann, B., Kessels, M. M. & Kelly, R. B. (2000) J. Cell Biol. 150, F111-F116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kaksonen, M., Sun, Y. & Drubin, D. G. (2003) Cell 115, 475-487. [DOI] [PubMed] [Google Scholar]
- 134.D'Hondt, K., Heese-Peck, A. & Riezman, H. (2000) Annu. Rev. Genet. 34, 255-295. [DOI] [PubMed] [Google Scholar]
- 135.Engqvist-Goldstein, A. E., Zhang, C. X., Carreno, S., Barroso, C., Heuser, J. E. & Drubin, D. G. (2004) Mol. Biol. Cell 15, 1666-1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Colicos, M. A., Collins, B. E., Sailor, M. J. & Goda, Y. (2001) Cell 107, 605-616. [DOI] [PubMed] [Google Scholar]
- 137.Sankaranarayanan, S., Atluri, P. P. & Ryan, T. A. (2003) Nat. Neurosci. 6, 127-135. [DOI] [PubMed] [Google Scholar]
- 138.Holroyd, P., Lang, T., Wenzel, D., De Camilli, P. & Jahn, R. (2002) Proc. Natl. Acad. Sci. USA 99, 16806-16811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Dale, G. L. (1985) Blood 66, 1133-1137. [PubMed] [Google Scholar]
- 140.Micheva, K. D., Buchanan, J., Holz, R. W. & Smith, S. J. (2003) Nat. Neurosci. 6, 925-932. [DOI] [PubMed] [Google Scholar]
- 141.Hilgemann, D. W., Feng, S. & Nasuhoglu, C. (2001) Sci. STKE 2001, RE19. [DOI] [PubMed] [Google Scholar]
- 142.Bonifacino, J. S. & Dell'Angelica, E. C. (1999) J. Cell Biol. 145, 923-926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Polo, S., Confalonieri, S., Salcini, A. E. & Di Fiore, P. P. (2003) Sci. STKE 2003, re17. [DOI] [PubMed] [Google Scholar]
- 144.Brown, F. D., Rozelle, A. L., Yin, H. L., Balla, T. & Donaldson, J. G. (2001) J. Cell Biol. 154, 1007-1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Marshall, J. G., Booth, J. W., Stambolic, V., Mak, T., Balla, T., Schreiber, A. D., Meyer, T. & Grinstein, S. (2001) J. Cell Biol. 153, 1369-1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Tolias, K. F., Cantley, L. C. & Carpenter, C. L. (1995) J. Biol. Chem. 270, 17656-17659. [DOI] [PubMed] [Google Scholar]
- 147.Rossman, K. L., Cheng, L., Mahon, G. M., Rojas, R. J., Snyder, J. T., Whitehead, I. P. & Sondek, J. (2003) J. Biol. Chem. 278, 18393-18400. [DOI] [PubMed] [Google Scholar]
- 148.Springer, S., Spang, A. & Schekman, R. (1999) Cell 97, 145-148. [DOI] [PubMed] [Google Scholar]
- 149.Christoforidis, S., Miaczynska, M., Ashman, K., Wilm, M., Zhao, L., Yip, S. C., Waterfield, M. D., Backer, J. M. & Zerial, M. (1999) Nat. Cell Biol. 1, 249-252. [DOI] [PubMed] [Google Scholar]
- 150.Wakelam, M. J. (1998) Biochim. Biophys. Acta 1436, 117-126. [DOI] [PubMed] [Google Scholar]
- 151.Klopfenstein, D. R., Tomishige, M., Stuurman, N. & Vale, R. D. (2002) Cell 109, 347-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Farsad, K. & De Camilli, P. (2003) Curr. Opin. Cell Biol. 15, 372-381. [DOI] [PubMed] [Google Scholar]