Abstract
Recent advances in understanding the role of neurotrophins on activity-dependent plasticity have provided insight into how behavior can affect specific aspects of neuronal biology. We present evidence that voluntary exercise can prime adult dorsal root ganglion neurons for increased axonal regeneration through a neurotrophin-dependent mechanism. Dorsal root ganglion neurons showed an increase in neurite outgrowth when cultured from animals that had undergone 3 or 7 days of exercise compared with sedentary animals. Neurite length over 18–22 h in culture correlated directly with the distance that animals ran. The exercise-conditioned animals also showed enhanced regrowth of axons after an in vivo nerve crush injury. Sensory ganglia from the 3- and 7-day-exercised animals contained higher brain-derived neurotrophic factor, neurotrophin 3, synapsin I, and GAP43 mRNA levels than those from sedentary animals. Consistent with the rise in brain-derived neurotrophic factor and neurotrophin 3 during exercise, the increased growth potential of the exercise-conditioned animals required activation of the neurotrophin signaling in vivo during the exercise period but did not require new mRNA synthesis in culture.
Keywords: neurotrophin, gene expression, plasticity, neural activity, experience
Alterations in neuronal activity can lead to lasting changes in the ability of the nervous system to transduce information (1). Such synaptic plasticity is well documented in the developing nervous system where the levels of neuronal activity can influence the eventual organization of cortical circuits (2). More recent studies (3) indicate that activity-dependent plasticity is retained into adulthood. The morphological basis of lasting forms of activity-dependent synaptic plasticity is manifest as an overall change in the number and/or area of synaptic contacts (1–3). In the developed organism, both of these processes require remodeling of synaptic structures, either through retraction of existing neuronal processes or by growth of new neuronal processes.
Formation of synaptic contacts and growth is a dynamic process that is largely affected through interactions with the environment, such that experience can imprint the nervous system by regulating these events (3). Neurotrophins, originally described for their role on growth and differentiation of neurons, are becoming recognized as regulators of synaptic plasticity (4, 5). The levels of the neurotrophins and/or their receptors can be altered by neuronal activity, thus providing a potential means to perpetuate changes in synaptic transmission (6, 7). Brain-derived neurotrophic factor (BDNF) and neurotrophin 3 (NT3) are important for the regulation of sensorimotor function taking place at the muscle–dorsal root ganglion (DRG)–spinal cord interface (8–10). We previously showed that the expression of BDNF and NT-3 is increased in the spinal cord and skeletal muscle after voluntary exercise and this alters expression of synapsin I mRNA in motor neurons (11–13). Here, we have asked how voluntary exercise affects the structural plasticity of the DRG. Exercise increased the capacity for axonal outgrowth from cultured DRG neurons and for nerve regeneration in vivo. Pharmacological inhibition of Trk neurotrophin receptors before exercise indicates that activation of neurotrophin signaling is required for the priming effect of exercise on axonal regeneration.
Materials and Methods
Exercise Conditioning. For exercise conditioning, animals were housed in individual cages with voluntary access to a running wheel (11). Sedentary animals were housed in standard rodent cages. Animals were treated with K252A before exercise to evaluate the role of neurotrophin signaling during exercise. For this purpose, latex microspheres were absorbed with a solution of K252A or cytochrome C (CytC) as described (14). Briefly, fluorescent latex microspheres (Lumafluor) were incubated in a 1:5 ratio with 46.8 ng/μl solution of K252A (Biomol) or CytC overnight at 4°C. Pelleted microspheres were resuspended in 1:10 in sterile water. A total of 3 μl of coated microspheres were injected into the space surrounding the L4 and L5 DRGs by injecting through the intervertebral foramina. The right side was injected with K252A and the left with CytC. Approximately 12 h after surgery, six of the animals were provided access to a running wheel and the other six were housed in standard cages.
DRG Culture. L4–5 DRGs were dissociated and plated onto polylysine/laminin-coated coverslips as described (15). To evaluate the role of transcription during the culture period, 80 μM 5,6-dichlorobenzimidazole 1-β-d-ribofuranoside (DRB; Sigma) was included in the culture medium (16). After 20–22 h in vitro, cultures were fixed in 4% paraformaldehyde and processed for immunofluorescence (see below). For quantification of neurite growth, digital images were captured, and the longest process of ≥70 neurons for each condition was measured as described (17).
Nerve Crush and Analysis of Regeneration in Vivo. To evaluate the effect of exercise on nerve regeneration, 7-day-exercised or sedentary animals were subjected to unilateral sciatic nerve crush at mid-thigh level as described (15). Three days after crush, the sciatic nerve was transected at 8 mm distal to the crush site. The nerve stump was placed in a blind-ended silicon cuff containing FluoroGold (Fluorochrome, Denver) solution such that nerve 5–8 mm distal to the crush site was contained within the cuff. Two days after transection, the animals were killed, and sciatic nerves and L4–5 DRGs were dissected and fixed in 4% paraformaldehyde. Cryostat sections of the DRGs and sciatic nerve were used for immunofluorescence as described below.
Immunofluorescence. Immunofluorescence for DRG cultures and tissue sections was performed as described (18). The following antibodies were used for indirect immunofluorescence: mouse antineurofilament (1:1,000; Zymed), mouse anti-tubulin βIII (1:1,000; Chemicon), rabbit anti-FluoroGold antibodies (1:100) and Texas red-conjugated donkey anti-mouse or FITC-conjugated donkey anti-rabbit (1:400; Jackson Immuno-Research).
Isolation of Total RNA and Real-Time Quantitative RT-PCR. For analysis of mRNA levels, total RNA was isolated from DRGs immediately after dissection by using the RNA STAT-60 kit (Tel-Test, Friendswood, TX). Quantitative real-time RT-PCR was used determine the relative levels of BDNF, NT-3, synapsin I, and GAP43 mRNAs. Amplification of GAPDH mRNA used as a control. One hundred nanograms of total RNA was reverse-transcribed by using TaqMan EZ RT-PCR core reagents (Perkin–Elmer). Fluorescently tagged probes and forward and reverse PCR primers, designed by Integrated DNA Technologies (Coralville, IA), consisted of the following: BDNF, probe 5′-AGTCATTTGCGCACAACTTTAAAAGTCTGCATT-3′, forward primer 5′-GGACATATCCATGACCAGAAAGAAA-3′, reverse primer 5′-GCAACAAACCACAACATTATCGAG3′; synapsin I, probe 5′-CATGGCACGTAATGGAGACTACCGCA-3′, forward primer 5′-CCGCCAGCTGCCTTC-3′, reverse primer 5′-TGCAGCCCAATGACCAAA-3′; and GA P43, probe 5′-CTCATAAGGCTGCAACCAAAATTCAGGCT-3′, forward primer 5′-GATGGTGTCAAACCGGAGGAT-3′, reverse primer 5′-CTTGTTATGTGTCCACGGAAGC-3′. Proprietary TaqMan probe and forward and reverse primers were used for rat GAPDH (Applied Biosystems). PCR was performed with abi prism 7700 software. RT reaction conditions were as follows: 2 min at 50°C to activate uracil glycosylase, 30 min at 60°C for reverse transcription, and 95°C for 5 min to deactivate uracil glycosylase. Two-step PCR consisted of 20 s at 94°C and 1 min at 62°C for 40 cycles. sequence detector 1.6.3 software (Applied Biosystems) was used to determine the threshold cycle, where the first significant increase of fluorescence occurred in individual RT-PCR amplifications. The threshold cycle values for BDNF, NT3, GAP43, and synapsin I mRNAs were then normalized to the GAPDH control amplification for each RNA template. The normalized values were used to make comparisons across experimental groups.
Statistical Analyses. Neurite lengths are displayed as mean ± SEM. Significance for mean neurite length between different conditions was calculated by using Student's t test (unpaired with unequal variation). Correlation between neurite length and distance run (i.e., revolution number) was assessed by calculation of Spearman's rank correlation coefficient. Potential significance of differences RT-PCR signals were determined by using one-way ANOVA.
Results
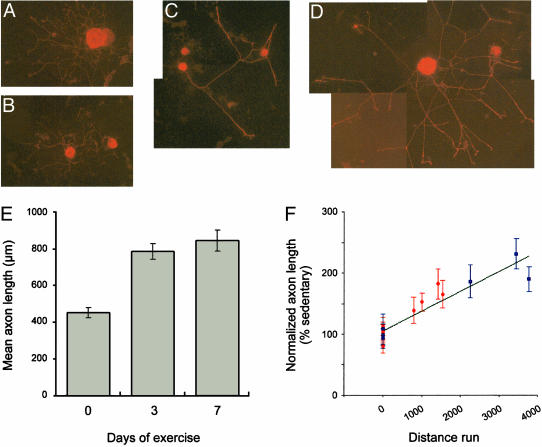
Exercise Conditioning Increases DRG Growth Potential in Vitro. Previous studies (16, 19, 20) have shown that axonal injury can prime DRG neurons for increased axonal regeneration both in vitro and in vivo. To investigate the possibility that physiological stimuli could also contribute to regenerative capacity, dissociated cultures were prepared from L4–5 DRGs of animals that had been allowed access to running wheels for 0, 3, or 7 days. The 0-day time point constitutes the control sedentary animals. The pattern and magnitude of neurite outgrowth was analyzed after 20 h in culture. Neurons from the exercised animals (Fig. 1 C and D) showed overall more robust neurite extension when compared with neurons from the sedentary animals (Fig. 1 A and B). Analogous to DRGs cultured after nerve crush (16, 19), the neurites of the 3-day exercise-conditioned DRGs were less branched than the sedentary DRG cultures. The cultures from 7-day exercise-conditioned animals showed similarly longer and less branched neurites compared with the sedentary cultures (data not shown). The length of the longest neurite was measured in 20-h DRG cultures prepared from each condition. Mean neurite length in cultures from the 3-day-exercised animals was statistically greater than that of the cultures from the sedentary animals (Fig. 1E). Cultures from the 7-day-exercised animals showed statistically longer neurites than those from the 3-day-exercised animals (Fig. 1E), suggesting that capacity for axonal outgrowth increased with longer periods of exercise. Comparing of the mean neurite length with the number of wheel revolutions in individual animals showed statistically significant correlation between neurite outgrowth and distance run (Fig. 1F).
Fig. 1.
DRG neurons from exercised animals have increased neurite outgrowth in culture. (A–D) Representative images of DRG cultures (20 h duration) from sedentary (A and B) or 3-day-exercised animals (C and D) that were immunostained for tubulin βIII. The increased neurite length in the 3-day-exercised DRGs required a montage to include the entire neuritic arbor of these neurons. Mean neurite length in DRG cultures from 0-, 3-, and 7-day exercise-conditioned animals is shown in E (error bars = 2 × SEM). Student's t test showed that differences in length of 3 and 7 days vs. 0 and 7 days vs. 3 days were statistically significant (P < 0.0001 and P < 0.01, respectively). Mean neurite length in individual cultures vs. the distance run for individual animals (number of wheel revolutions) is shown in F. Matched sedentary and 3-day-exercised animals are displayed as red circles and sedentary and 7-day-exercised animals as blue squares with neurite length expressed as the percent of average sedentary length for each sample set (error bars = 2 × SEM). Linear regression revealed R2 of 0.855. Spearman's rho correlation coefficient calculation showed a highly significant correlation between distance run and neurite length (rho coefficient = 0.626, P < 0.001).
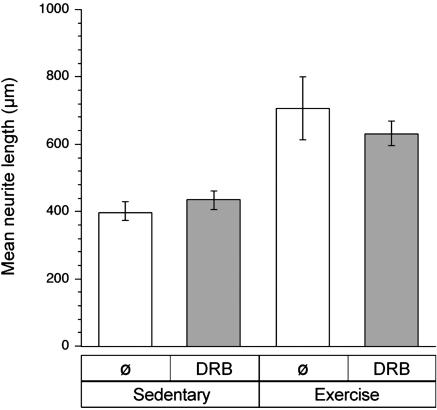
Transcription-Independent Growth from Exercise-Conditioned Neurons. The increased neurite growth after exercise is reminiscent of the accelerated axonal outgrowth seen in neurons cultured after axonal crush injury (16, 17, 19, 21). In injury-conditioned DRGs, this enhanced outgrowth does not require new RNA synthesis in culture (16). To determine whether the increased outgrowth from the exercise-conditioned neurons required new mRNA synthesis in vitro, DRG cultures were treated with the RNA synthesis inhibitor DRB. At 80 μM, DRB inhibits ≥95% of new RNA synthesis in rat DRG cultures (16). Similar to injury-conditioned neurons, inhibition of new mRNA synthesis throughout the culture period did not affect axonal outgrowth from the exercise-conditioned DRG cultures (Fig. 2).
Fig. 2.
Enhanced neurite outgrowth from exercise-conditioned neurons does not require new mRNA synthesis in vitro. L4–5 DRGs were isolated from sedentary and 7-day-exercised animals and dissociated for culture. The RNA polymerase inhibitor DRB (80 μM) was included in the culture medium to evaluate the requirement for new mRNA synthesis by the exercise conditioned DRGs. After 22 h in vitro, cultures were fixed and stained for neurofilament. Results are displayed as mean neurite length (error bars = 2 × SEM). Student's t test revealed P < 0.0001 for exercise plus DRB cultures vs. sedentary cultures. Comparing exercised neurons cultured with vs. without DRB and sedentary neurons cultured with vs. without DRG showed no statistically significant differences (P > 0.05).
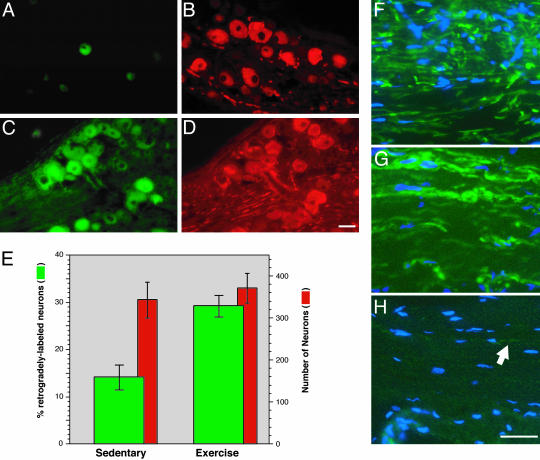
Exercise-Conditioned Animals Show Increased Nerve Regeneration in Vivo. To address the effect of exercise on axonal regeneration in vivo, a peripheral nerve crush was performed on animals that had been allowed access to a running wheel for 7 days. The sciatic nerve of exercised and sedentary animals was crushed at the mid-thigh level as described (18). The sciatic nerve was then transected at 0.8 cm distal to the crush site and proximal end of the transected nerve stump was placed into a 0.5-cm blind-ended cuff containing the retrograde tracer FluoroGold at 72 h after the initial crush injury. Animals were killed 48 h later and then the number of L4–5 DRG neuronal cell bodies that contained FluoroGold was determined. The exercise-conditioned animals had approximately twice as many FluoroGold-positive neurons than sedentary animals that received identical nerve crush lesions (Fig. 3 A–D). Sections of sciatic nerve distal to the crush site showed more axons in the nerves from the exercised animals than in those from the sedentary animals (Fig. 3 F and G). Sections of uninjured sciatic nerve are shown for comparison (Fig. 3E).
Fig. 3.
Exercise-conditioned animals show increased nerve regeneration after crush injury. Sedentary and 7-day-exercised animals were subjected to sciatic nerve crush. Three days after crush, the nerve was transected at 8 mm distal to the crush site and was exposed to FluoroGold for additional 2 days. FluoroGold-positive neurons were more abundant in the L4–5 DRGs from the exercised animals (B) than in those from the sedentary animals (A). Costaining for neurofilament antibody showed similar numbers of neurons in these sections (C and D). Mean number of retrogradedly labeled neurons (FluoroGold-positive, left axis) and total neurons (neurofilament-positive, right axis) are presented of three serial sections from three animals for each condition in E (error bars = SD). (G and H) Representative sections of sciatic nerve distal to the crush site that were stained for neurofilament (green) and counterstained with 4′,6-diamidino-2-phenylindole (blue). Sections of uninjured sciatic nerve taken at a distal thigh level are shown for comparison (F). Only a few axons were discernable in the sections of crushed nerve from the sedentary animals (arrow, H). (Scale bars: 50 μmin A–D and 20 μmin F–H).
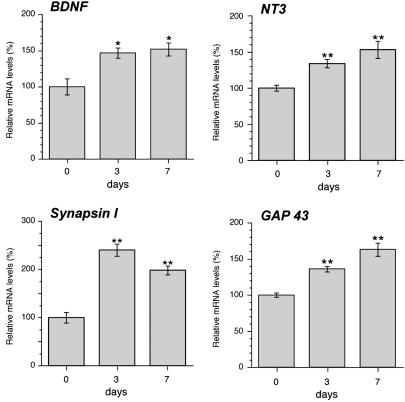
Exercise Induces Changes in DRG Gene Expression. In previous studies (11–13), we showed that voluntary exercise increased gene expression in motor neurons. Because the sensory nervous system would also be stimulated by this physiological activity, we asked whether exercise affects gene expression in the L4–5 DRGs. For this purpose, we used real-time RT-PCR to first determine whether neurotrophin mRNA levels changed during voluntary exercise. Both BDNF and NT3 mRNA levels were increased by 3 days of exercise (Fig. 4). After 7 days of exercise, BDNF and NT3 mRNA levels were ≈1.5-fold greater in the DRGs of exercised animals than in those of sedentary animals. In other systems, BDNF has been shown to regulate the transcription of synapsin I mRNA (22). Thus, we asked whether the increased BDNF levels during exercise might affect expression of synapsin I mRNA in the DRG. Levels of synapsin I mRNA increased by >2-fold in the DRG after 3 days of exercise and remained elevated above sedentary levels at 7 days (Fig. 4). To determine whether activity alters levels of gene products that are known to be up-regulated during axonal growth, we analyzed the GAP43 mRNA levels in DRGs of 0-, 3-, and 7-day-exercised animals. High GAP43 mRNA expression is seen during periods of developmental and regenerative axonal growth (23). GAP43 mRNA was higher in DRGs from the 3- and 7-day-exercised compared with sedentary animals (Fig. 4).
Fig. 4.
Exercise increases mRNA levels in the DRG. Levels of BDNF, NT3, synapsin I, and GAP43 mRNAs in the L4–5 DRG were measured by real-time RT-PCR. All values were normalized for the amplification of GAPDH. BDNF, NT3, synapsin I, and GAP43 mRNA levels are expressed as a percentage relative to the 0-day-exercised animals (error bars = standard SD for ≥5 animals per condition). BDNF, NT3, synapsin I, and GAP43 each showed statistically significant differences when comparing the 3- and 7-day with the 0-day samples with P < 0.05 (*)or <0.01 (**) by ANOVA.
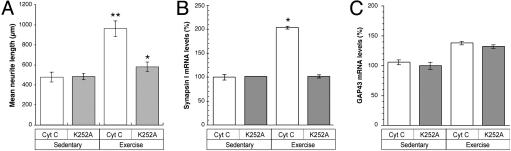
Inhibition of Trk Tyrosine Kinase Blocks the Conditioning Effect of Exercise. To address the potential activation and effect of neurotrophin receptors during exercise, we used a pharmacological inhibitor of the Trk tyrosine kinase, K252A (24), in an attempt to block potential intracellular effects of neurotrophins. For this experiment, latex microspheres were used as an in vivo reservoir for release of K252A over a period of 3 days of exercise. Previous studies (14, 25, 26) have used this method for sustained release of neurotrophins or inhibitors in CNS tissues and have proven the specificity of such microspheres in vivo by using CytC-absorbed microspheres as a control. In this series of experiments, equal volumes of K252A- or CytC-coated microspheres were injected into the space surrounding L4 and L5 DRGs. Animals were allowed to recover for 12 h after injection and were then provided running wheel access for 3 days. Dissociated DRG cultures were then prepared from the L4 DRGs and the L5 DRGs were used for isolation of RNA and RT-PCR. K252A near completely blocked the conditioning effect of exercise on neurite outgrowth when DRGs were cultured after 3 days of exercise (Fig. 5A). Real-time RT-PCR was used to evaluate the potential changes in mRNA levels after exercise in the animals that had been injected with K252A and CytC. In the CytC-injected DRGs, exercised animals had statistically higher synapsin I and GAP43 mRNA levels than did those from sedentary animals (Fig. 5B). This increase in synapsin I mRNA after exercise is identical to that seen in animals that had not been manipulated before exercise (see Fig. 4). The exercise-induced increase in synapsin I mRNA was effectively blocked by K252A pretreatment (Fig. 5C). Thus, activation of Trk during exercise contributes to the increase in synapsin I mRNA in the DRG. Surprisingly, there was no statistically significant difference in GAP43 mRNA levels comparing the K252A- and CytC-injected ganglia (Fig. 5C). This finding suggests that Trk activity does not contribute to the moderate increase in GAP43 mRNA levels that are seen after 3 days of voluntary exercise (see Fig. 4).
Fig. 5.
Inhibition of Trk receptors before exercise blocks exercise-induced growth and synapsin I mRNA increase. (A)L4–5 DRGs were pretreated with K252A (gray columns) or CytC (white columns) before exercise as described in Materials and Methods. After a 3-day exercise period, L5 DRGs were cultured for 20 h. Mean neurite length is presented (error bars = 2 × SEM). The difference between CytC-exercised DRG vs. K252A-exercised DRG is statistically significant (**, P < 0.0001 by Student's t test). Although mean neurite length in the K252A exercised DRG is closer to that of the sedentary conditions, the difference between exercise and sedentary K252A-treated DRGs is statistically significant (*, P < 0.01 by Student's t test). (B and C) RNAs from the L5 DRGs of the sedentary and exercised animals from A were used as a template for real-time RT-PCR to evaluate levels of synapsin I (B) and GAP43 (C) mRNAs. Signals were normalized to GAPDH levels and are expressed as the relative to the CytC-injected, sedentary signals (error bars = SD for six animals per condition). Synapsin I showed statistically significant differences when comparing the exercise CytC with the exercise K252A samples (*, P < 0.01 by ANOVA). Differences in GAP43 mRNA levels between CytC and K252A exercise samples were not statistically significant (P > 0.05).
Discussion
The results presented here indicate that voluntary physical activity can prime adult sensory neurons for enhanced axonal regeneration after subsequent axotomy. Both early neurite extension in vitro and nerve regeneration in vivo were enhanced in sensory neurons of exercised animals compared with those of sedentary animals. Inhibition of RNA synthesis in cultures of exercise-conditioned neurons did not affect neurite outgrowth in vitro, arguing that exercise increases the expression of genes needed for rapid neurite growth. Consistent with this notion, L4–5 DRGs from exercised animals had higher levels of BDNF, NT3, synapsin I, and GAP43 mRNAs than did DRGs from sedentary animals. Injection of the K252A inhibitor of Trk neurotrophin receptor activity into the DRGs before exercise blocked the effects of exercise on synapsin I transcription and attenuated the exercise-dependent increase in neurite growth. This finding indicates that activation of neurotrophin signal transduction pathways during exercise plays a critical role promoting the growth potential of DRG neurons. In previous work (11, 13), we showed that exercise regulates gene expression in the spinal cord. The increased rate of axonal outgrowth after exercise in the current study now demonstrates a direct functional outcome of exercise-dependent changes in gene expression.
The increased axonal outgrowth from the exercise-conditioned neurons closely resembles the conditioning effect of peripheral axotomy, where distal nerve injury primes neurons for more rapid axonal regeneration after a second injury placed proximal to the “conditioning” injury (27–29). In an analogous fashion, our results show enhanced nerve regeneration in animals that had exercised for 7 days compared with sedentary animals. In DRG cultures from 3- and 7-day-exercised animals, the pattern of neurite growth was also similar to that of injury-conditioned neurons. Cultures prepared from L4–5 DRG of animals subjected to peripheral nerve crush 7 days before culture show processes >5-fold longer than those from uninjured or naive DRGs over the first day in vitro (16). The processes of injury-conditioned DRG neurons have fewer branches when compared with naive cultures that show highly branched processes (referred to as “elongating” vs. “arborizing” growth patterns, respectively; refs. 16 and 19). Neurite growth from the 3- and 7-day-exercised DRGs was comparable with this elongating growth. Smith and Skene (16) argued that retrogradedly transported signals reduce the capacity for elongating growth in the intact nerve, and axotomy alleviates this inhibitory effect. Cultures of naive DRG cultures transition to elongating growth over 2–4 days in culture but this growth requires a period of at least 12 h for new RNA synthesis in culture; elongating growth from the injury-conditioned DRGs does not require new RNA synthesis in vitro (16). This result suggests that injury induces transcription of new gene products in vivo that can enhance axonal growth capacity. The increased growth from the exercised-conditioned neurons and elevations in neurotrophin and GAP43 mRNAs in our study argue that exercise activates expression of mRNAs encoding proteins needed for rapid elongating axonal growth. This idea is further supported by the fact that inhibiting mRNA synthesis in culture did not affect elongating growth from the exercise-conditioned neurons. However, our studies do not distinguish between exercise directly activating a growth program vs. exercise alleviating an inhibitory signal in the sedentary animals.
The conditioning effect of exercise on axonal outgrowth from the DRG neurons suggests that neural activity initiates molecular changes similar to those seen in axotomized neurons. Studies in other systems also support the notion that activity-dependent plasticity and neurite regeneration share some mechanisms. Serotonin stimuli that generate long-term facilitation, a form of synaptic plasticity, also enhance regrowth of severed Aplysia axons (30). Similar retrograde messengers, which transmit signals from axons to the cell body, are activated by axonal injury and stimuli that generate long-term facilitation (31). Recent work (32, 33) suggests that analogous retrograde messengers are activated in vertebrate axons after injury to signal the nucleus to initiate a regenerative response. Increase in synapsin I and GAP43 mRNAs during exercise likely illustrates an overlap between retrograde signals induced by activity and injury. Synapsin I is a synaptic vesicle protein that is up-regulated after nerve injury in some systems, and this fact has been suggested to provide the growing axon with a source of axoplasmic membranes (34). The inhibitory effect of K252A pretreatment on the exercise-dependent increase in synapsin I mRNA indicates that activation of neurotrophin receptors during exercise positively regulates synapsin I expression. In hippocampal neurons, BDNF regulates synthesis of synapsin I to increase neurotransmitter release (22). GAP43, on the other hand, has a more direct tie with injury. High GAP43 mRNA levels, both during development and after injury, correlate with periods of axonal growth and GAP43 protein localizes to growth cones (23). However, there is also evidence that GAP43 can play a role in synaptic function, because overexpression of GAP43 enhances learning in transgenic mice (35), and neural activity leads increased GAP43 expression in wild-type mice (35, 36). The lack of any effect of K252A treatment on exercise-induced GAP43 expression in our system suggests that GAP43 mRNA, in contrast to synapsin I mRNA, may be more directly regulated by neural activity than by Trk signaling in our experimental system. Nonetheless, we cannot exclude the possibility that differences in GAP43 levels with K252A vs. CytC treatment may be more apparent with longer duration of exercise where differences in GAP43 levels reached higher statistical significance.
Activity-dependent increase in BDNF and NT3 is one means by which exercise could trigger the increased axonal growth potential in the DRG neurons. The decrease in neurite outgrowth with K252A pretreatment in exercised animals argues that neurotrophins play an essential role in regulating DRG growth potential. Growth-promoting effects of the neurotrophins are well characterized (37). In recent years, it has become clear that neurotrophins can also play a role in synaptic function or plasticity (4, 5). BDNF, NT3, and NT4 have been shown to exert rapid, local effects on neuronal excitability and synaptic efficacy (38). In addition to such immediate effects, neurotrophins can exert more lasting effects on synaptic function by altering gene expression and structure of synaptic contacts (39–42). Activity-dependent release of and synthesis of neurotrophins have been shown to alter dendritic and axonal formations in the developing nervous system (43). Enhanced dendritic sprouting seen in cultured hippocampal neurons after depolarization requires BDNF, but sprouting in response to exogenous BDNF does not require neuronal activity (44). This finding suggests a chronological sequence where activity triggers synthesis/release of neurotrophins that can then regulate expression of genes needed for growth. The inhibitory effect of K252A pretreatment on transcription of synapsin I mRNA in the DRG after exercise is consistent with this notion (see Fig. 5B).
Our study has focused on the effects of physical activity on the DRG neurons before axonal injury. In addition to our findings, there is also experimental evidence showing that neural activity after injury may have positive effects on axonal growth. Application of electrical fields after optic nerve crush enhances axonal regeneration (45) and electrical activity can enhance recovery after experimental spinal cord hemisection (46, 47). Electrical stimulation facilitates peripheral nervous system motor nerve regeneration allowing early extension of axons across an injury site (48, 49). Similar to the effects of exercise before injury that we show here, increased expression of BDNF temporally correlates with this effect of exogenous electrical activity (7). Taken together with our data, these observations suggest that physiological forms of activity may also have beneficial effects on regenerative potential after nerve injury through neurotrophin-dependent mechanisms.
Acknowledgments
We thank Drs. Reggie Edgerton, Michael Sofroniew, and Carolyn Schanen for helpful suggestions over the course of this work and Dr. Joseph Glutting for assistance with statistical analyses. This work was supported by Roman Reed Funds from the State of California, and in part by National Institutes of Health Grants NS41596 (to J.L.T.) and NS39522 (to F.G.-P.).
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: BDNF, brain-derived neurotrophic factor; NT3, neurotrophin 3; DRG, dorsal root ganglion; CytC, cytochrome c; DRB, 5,6-dichlorobenzimidazole 1-β-d-ribofuranoside.
References
- 1.Kandel, E. R. (2001) Science 294, 1030-1038. [DOI] [PubMed] [Google Scholar]
- 2.Katz, L. C. & Shatz, C. J. (1996) Science 274, 1133-1138. [DOI] [PubMed] [Google Scholar]
- 3.Zito, K. & Svoboda, K. (2002) Neuron 35, 1015-1017. [DOI] [PubMed] [Google Scholar]
- 4.McAllister, A., Katz, L. C. & Lo, D. (1999) Annu. Rev. Neurosci. 22, 295-318. [DOI] [PubMed] [Google Scholar]
- 5.Klintsova, A. Y. & Greenough, W. T. (1999) Cur. Opin. Neurobiol. 9, 203-208. [DOI] [PubMed] [Google Scholar]
- 6.Canossa, M., Gartner, A., Campana, G., Inagaki, N. & Thoenen, H. (2001) EMBO J. 20, 1640-1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Al-Majed, A., Brushart, T. & Gordon, T. (2000) Eur. J. Neurosci. 12, 4381-4390. [PubMed] [Google Scholar]
- 8.Thompson, S. W. N., Bennet, D. L. H., Kerr, B. J., Bradbury, E. J. & McMahon, S. B. (1999) Proc. Natl. Acad. Sci. USA 96, 7714-7718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carrol, P., Lewin, G., Koltzenburg, M., Toyka, K. & Thoenen, H. (1998) Nat. Neurosci. 1, 42-46. [DOI] [PubMed] [Google Scholar]
- 10.Mannion, R. J., Costigan, M., Decosterd, I., Amaya, F., Ma, Q.-P., Holstege, J. C., Ji, R.-R., Acheson, A., Lindsay, R., Wilkinson, G. & Woolf, C. J. (1999) Proc. Natl. Acad. Sci. USA 96, 9385-9390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gomez-Pinilla, F., Ying, Z., Opazo, P., Roy, R. R. & Edgerton, V. R. (2001) Eur. J. Neurosci. 13, 1078-1084. [DOI] [PubMed] [Google Scholar]
- 12.Gomez-Pinilla, F., Ying, Z., Roy, R., Molteni, R. & Edgerton, V. (2002) J. Neurophysiol. 88, 2187-2195. [DOI] [PubMed] [Google Scholar]
- 13.Ying, Z., Roy, R. R., Edgerton, V. R. & Gomez-Pinilla, F. (2003) Brain Res. 987, 93-99. [DOI] [PubMed] [Google Scholar]
- 14.Vaynman, S., Ying, Z. & Gomez-Pinilla, F. (2003) Neuroscience 3, 647-657. [DOI] [PubMed] [Google Scholar]
- 15.Twiss, J., Smith, D., Chang, B. & Shooter, E. (2000) Neurobiol. Dis. 7, 416-428. [DOI] [PubMed] [Google Scholar]
- 16.Smith, D. S. & Skene, P. (1997) J. Neurosci. 17, 646-658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu, D.-Y., Zheng, J.-Q., McDonald, M. A., Chang, B. & Twiss, J. L. (2003) Invest. Ophthalmol. Visual Sci. 44, 2783-2790. [DOI] [PubMed] [Google Scholar]
- 18.Zheng, J.-Q., Kelly, T., Chang, B., Ryazantsev, S., Rajasekaran, A., Martin, K. & Twiss, J. (2001) J. Neurosci. 21, 9291-9303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lankford, K., Waxman, S. & Kocsis, J. (1998) J. Comp. Neurol. 391, 11-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sjoberg, J. & Kanje, M. (1990) Brain Res. 529, 79-84. [DOI] [PubMed] [Google Scholar]
- 21.Landreth, G. E. & Agranoff, B. W. (1979) Brain Res. 161, 39-53. [DOI] [PubMed] [Google Scholar]
- 22.Jovanovic, J., Czernik, A., Fienberg, A., Greenberg, P. & Sihra, T. (2000) Nat. Neurosci. 3, 323-329. [DOI] [PubMed] [Google Scholar]
- 23.Benowitz, L. I. & Routtenberg, A. (1997) Trends Neurosci. 20, 84-91. [DOI] [PubMed] [Google Scholar]
- 24.Berg, M., Sternberg, D., Parada, L. & Chao, M. (1992) J. Biol. Chem 267, 13-16. [PubMed] [Google Scholar]
- 25.Lom, B. & Cohen-Cory, S. (1999) J. Neurosci. 19, 9928-9938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Riddle, D. R., Katz, L. C. & Lo, D. C. (1997) BioTechniques 23, 928-937. [DOI] [PubMed] [Google Scholar]
- 27.McQuarrie, I. G. (1978) Brain Res. 152, 597-602. [DOI] [PubMed] [Google Scholar]
- 28.Forman, D. S., McQuarrie, I. G., Labore, F. W., Wood, D. K., Stone, L. S., Braddock, C. H. & Fuchs, D. A. (1980) Brain Res. 182, 180-185. [DOI] [PubMed] [Google Scholar]
- 29.Sjoberg, J. & Kanje, M. (1990) Brain Res. 530, 167-169. [DOI] [PubMed] [Google Scholar]
- 30.Bedi, S. & Glanzman, D. (2001) J. Neurosci. 21, 9667-9677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ambron, R. T. & Walters, E. T. (1996) Mol. Neurobiol. 13, 61-79. [DOI] [PubMed] [Google Scholar]
- 32.Lin, H., Bao, J., Ying, J. S., Walters, E. T. & Ambron, R. T. (2003) J. Neurobiol. 57, 204-220. [DOI] [PubMed] [Google Scholar]
- 33.Hanz, S., Perlson, E., Willis, D., Zheng, J. Q., Massarwa, R., Huerta, J. J., Koltzenburg, M., Kohler, M., van-Minnen, J., Twiss, J. L. & Fainzilber, M. (2003) Neuron 40, 1095-1104. [DOI] [PubMed] [Google Scholar]
- 34.Akagi, S., Mizoguchi, A., Sobue, K., Nakamura, H. & Ide, C. (1996) Histochem. Cell Biol. 105, 365-373. [DOI] [PubMed] [Google Scholar]
- 35.Routtenberg, A., Cantallops, I., Zaffuto, S., Serrano, P. & Namgung, U. (2000) Proc. Natl. Acad. Sci. USA 97, 7657-7662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Namgung, U., Matsuyama, S. & Routtenberg, A. (1997) Proc. Natl. Acad. Sci. USA 94, 11675-11680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Markus, A., Patel, T. & Snider, W. (2002) Curr. Opin. Neurobiol. 12, 523-531. [DOI] [PubMed] [Google Scholar]
- 38.Berninger, B. & Poo, M. (1996) Curr. Opin. Neurobiol. 6, 324-330. [DOI] [PubMed] [Google Scholar]
- 39.Messaoudi, M., Ying, S.-W., Kanhema, T., Croll, S. & Bramham, C. (2002) J. Neurosci. 22, 7453-7461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vicario-Abejon, C., Collin, C., McKay, R. D. G. & Segal, M. (1998) J. Neurosci. 18, 7256-7271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Causing, C., Gloster, A., Aloyz, R., Bamji, S., Chang, E., Fawcett, J., Kuchel, G. & Miller, F. D. (1997) Neuron 18, 257-267. [DOI] [PubMed] [Google Scholar]
- 42.Lom, B., Cogen, J., Sanchez, A., Vu, T. & Cohen-Cory, S. (2002) J. Neurosci. 22, 7639-7649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Horch, H. & Katz, L. C. (2002) Nat. Neurosci. 5, 1177-1184. [DOI] [PubMed] [Google Scholar]
- 44.Kohara, K., Kitamura, A., Adachi, N., Nishida, M., Itami, C., Nakamura, S. & Tsumoto, T. (2003) J. Neurosci. 23, 6123-6131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Politis, M., Zanakis, M. & Albala, B. (1988) J. Trauma 28, 1548-1552. [DOI] [PubMed] [Google Scholar]
- 46.Borgens, R., Roederer, E. & Cohen, M. (1981) Science 213, 611-617. [DOI] [PubMed] [Google Scholar]
- 47.Borgens, R., Blight, A. & McGinnis, M. (1987) Science 238, 366-369. [DOI] [PubMed] [Google Scholar]
- 48.Brushart, T. M., Hoffman, P. N., Royall, R. M., Murinson, B. B., Witzel, C. & Gordon, T. (2002) J. Neurosci. 22, 6631-6638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Al-Majed, A., Neumann, C., Brushart, T. & Gordon, T. (2000) J. Neurosci. 20, 2602-2608. [DOI] [PMC free article] [PubMed] [Google Scholar]