Abstract
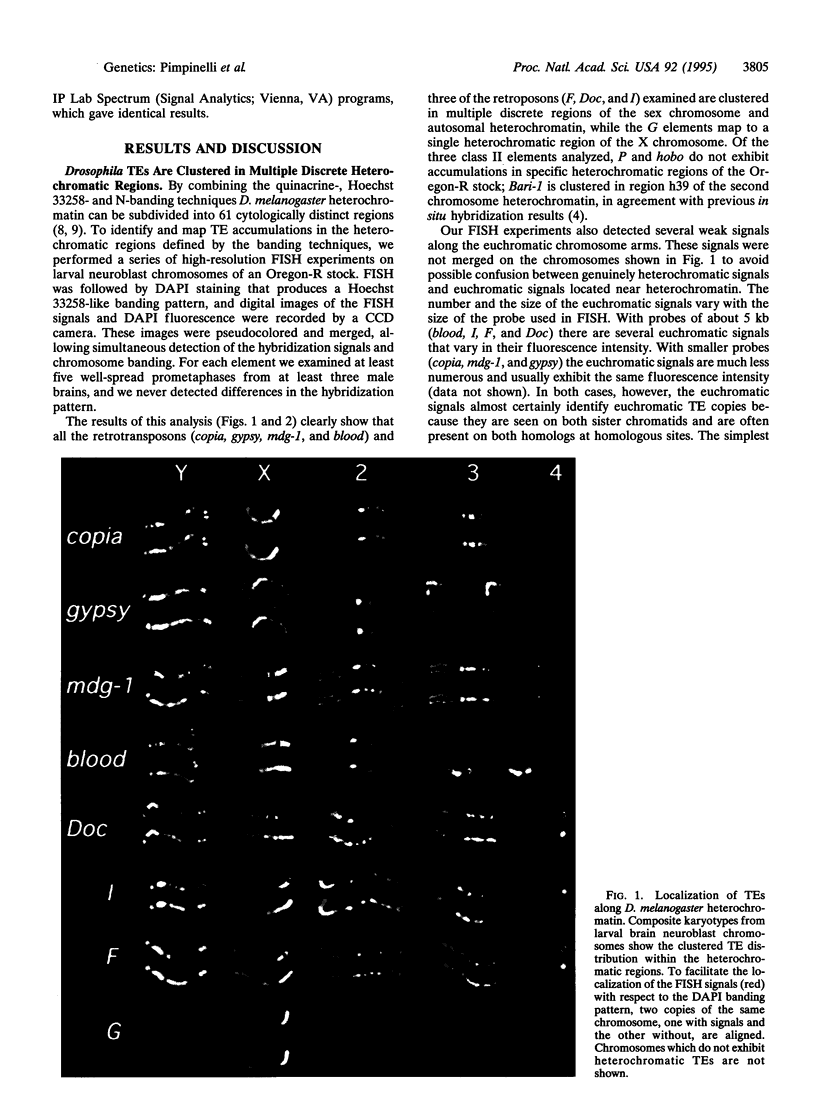
We determined the distribution of 11 different transposable elements on Drosophila melanogaster mitotic chromosomes by using high-resolution fluorescent in situ hybridization (FISH) coupled with charge-coupled device camera analysis. Nine of these transposable elements (copia, gypsy, mdg-1, blood, Doc, I, F, G, and Bari-1) are preferentially clustered into one or more discrete heterochromatic regions in chromosomes of the Oregon-R laboratory stock. Moreover, FISH analysis of geographically distant strains revealed that the locations of these heterochromatic transposable element clusters are highly conserved. The P and hobo elements, which are likely to have invaded the D. melanogaster genome at the beginning of this century, are absent from Oregon-R heterochromatin but clearly exhibit heterochromatic clusters in certain natural populations. Together these data indicate that transposable elements are major structural components of Drosophila heterochromatin, and they change the current views on the role of transposable elements in host genome evolution.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonaccorsi S., Lohe A. Fine mapping of satellite DNA sequences along the Y chromosome of Drosophila melanogaster: relationships between satellite sequences and fertility factors. Genetics. 1991 Sep;129(1):177–189. doi: 10.1093/genetics/129.1.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonaccorsi S., Pisano C., Puoti F., Gatti M. Y chromosome loops in Drosophila melanogaster. Genetics. 1988 Dec;120(4):1015–1034. doi: 10.1093/genetics/120.4.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucheton A., Paro R., Sang H. M., Pelisson A., Finnegan D. J. The molecular basis of I-R hybrid dysgenesis in Drosophila melanogaster: identification, cloning, and properties of the I factor. Cell. 1984 Aug;38(1):153–163. doi: 10.1016/0092-8674(84)90536-1. [DOI] [PubMed] [Google Scholar]
- Bucheton A., Paro R., Sang H. M., Pelisson A., Finnegan D. J. The molecular basis of I-R hybrid dysgenesis in Drosophila melanogaster: identification, cloning, and properties of the I factor. Cell. 1984 Aug;38(1):153–163. doi: 10.1016/0092-8674(84)90536-1. [DOI] [PubMed] [Google Scholar]
- Bucheton A., Vaury C., Chaboissier M. C., Abad P., Pélisson A., Simonelig M. I elements and the Drosophila genome. Genetica. 1992;86(1-3):175–190. doi: 10.1007/BF00133719. [DOI] [PubMed] [Google Scholar]
- Caizzi R., Caggese C., Pimpinelli S. Bari-1, a new transposon-like family in Drosophila melanogaster with a unique heterochromatic organization. Genetics. 1993 Feb;133(2):335–345. doi: 10.1093/genetics/133.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlesworth B., Langley C. H. The population genetics of Drosophila transposable elements. Annu Rev Genet. 1989;23:251–287. doi: 10.1146/annurev.ge.23.120189.001343. [DOI] [PubMed] [Google Scholar]
- Dawid I. B., Long E. O., DiNocera P. P., Pardue M. L. Ribosomal insertion-like elements in Drosophila melanogaster are interspersed with mobile sequences. Cell. 1981 Aug;25(2):399–408. doi: 10.1016/0092-8674(81)90058-1. [DOI] [PubMed] [Google Scholar]
- Di Nocera P. P., Dawid I. B. Interdigitated arrangement of two oligo(A)-terminated DNA sequences in Drosophila. Nucleic Acids Res. 1983 Aug 25;11(16):5475–5482. doi: 10.1093/nar/11.16.5475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Nocera P. P., Graziani F., Lavorgna G. Genomic and structural organization of Drosophila melanogaster G elements. Nucleic Acids Res. 1986 Jan 24;14(2):675–691. doi: 10.1093/nar/14.2.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Nocera P. P., Graziani F., Lavorgna G. Genomic and structural organization of Drosophila melanogaster G elements. Nucleic Acids Res. 1986 Jan 24;14(2):675–691. doi: 10.1093/nar/14.2.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engels W. R. The origin of P elements in Drosophila melanogaster. Bioessays. 1992 Oct;14(10):681–686. doi: 10.1002/bies.950141007. [DOI] [PubMed] [Google Scholar]
- Finnegan D. J. Eukaryotic transposable elements and genome evolution. Trends Genet. 1989 Apr;5(4):103–107. doi: 10.1016/0168-9525(89)90039-5. [DOI] [PubMed] [Google Scholar]
- Finnegan D. J. Eukaryotic transposable elements and genome evolution. Trends Genet. 1989 Apr;5(4):103–107. doi: 10.1016/0168-9525(89)90039-5. [DOI] [PubMed] [Google Scholar]
- Gatti M., Bonaccorsi S., Pimpinelli S. Looking at Drosophila mitotic chromosomes. Methods Cell Biol. 1994;44:371–391. doi: 10.1016/s0091-679x(08)60924-3. [DOI] [PubMed] [Google Scholar]
- Gatti M., Pimpinelli S. Functional elements in Drosophila melanogaster heterochromatin. Annu Rev Genet. 1992;26:239–275. doi: 10.1146/annurev.ge.26.120192.001323. [DOI] [PubMed] [Google Scholar]
- Hiraoka Y., Sedat J. W., Agard D. A. The use of a charge-coupled device for quantitative optical microscopy of biological structures. Science. 1987 Oct 2;238(4823):36–41. doi: 10.1126/science.3116667. [DOI] [PubMed] [Google Scholar]
- Hochstenbach R., Pötgens A., Meijer H., Dijkhof R., Knops M., Schouren K., Hennig W. Partial reconstruction of the lampbrush loop pair Nooses on the Y chromosome of Drosophila hydei. Chromosoma. 1993 Sep;102(8):526–545. doi: 10.1007/BF00368346. [DOI] [PubMed] [Google Scholar]
- Huijser P., Kirchhoff C., Lankenau D. H., Hennig W. Retrotransposon-like sequences are expressed in Y chromosomal lampbrush loops of Drosophila hydei. J Mol Biol. 1988 Oct 5;203(3):689–697. doi: 10.1016/0022-2836(88)90202-1. [DOI] [PubMed] [Google Scholar]
- Karpen G. H., Spradling A. C. Analysis of subtelomeric heterochromatin in the Drosophila minichromosome Dp1187 by single P element insertional mutagenesis. Genetics. 1992 Nov;132(3):737–753. doi: 10.1093/genetics/132.3.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidwell M. G. Lateral transfer in natural populations of eukaryotes. Annu Rev Genet. 1993;27:235–256. doi: 10.1146/annurev.ge.27.120193.001315. [DOI] [PubMed] [Google Scholar]
- Lohe A. R., Brutlag D. L. Multiplicity of satellite DNA sequences in Drosophila melanogaster. Proc Natl Acad Sci U S A. 1986 Feb;83(3):696–700. doi: 10.1073/pnas.83.3.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohe A. R., Brutlag D. L. Multiplicity of satellite DNA sequences in Drosophila melanogaster. Proc Natl Acad Sci U S A. 1986 Feb;83(3):696–700. doi: 10.1073/pnas.83.3.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohe A. R., Hilliker A. J., Roberts P. A. Mapping simple repeated DNA sequences in heterochromatin of Drosophila melanogaster. Genetics. 1993 Aug;134(4):1149–1174. doi: 10.1093/genetics/134.4.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miklos G. L., Cotsell J. N. Chromosome structure at interfaces between major chromatin types: alpha- and beta-heterochromatin. Bioessays. 1990 Jan;12(1):1–6. doi: 10.1002/bies.950120102. [DOI] [PubMed] [Google Scholar]
- Miller W. J., Hagemann S., Reiter E., Pinsker W. P-element homologous sequences are tandemly repeated in the genome of Drosophila guanche. Proc Natl Acad Sci U S A. 1992 May 1;89(9):4018–4022. doi: 10.1073/pnas.89.9.4018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller W. J., Hagemann S., Reiter E., Pinsker W. P-element homologous sequences are tandemly repeated in the genome of Drosophila guanche. Proc Natl Acad Sci U S A. 1992 May 1;89(9):4018–4022. doi: 10.1073/pnas.89.9.4018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery E., Charlesworth B., Langley C. H. A test for the role of natural selection in the stabilization of transposable element copy number in a population of Drosophila melanogaster. Genet Res. 1987 Feb;49(1):31–41. doi: 10.1017/s0016672300026707. [DOI] [PubMed] [Google Scholar]
- Montgomery E., Charlesworth B., Langley C. H. A test for the role of natural selection in the stabilization of transposable element copy number in a population of Drosophila melanogaster. Genet Res. 1987 Feb;49(1):31–41. doi: 10.1017/s0016672300026707. [DOI] [PubMed] [Google Scholar]
- Pascual L., Periquet G. Distribution of hobo transposable elements in natural populations of Drosophila melanogaster. Mol Biol Evol. 1991 May;8(3):282–296. doi: 10.1093/oxfordjournals.molbev.a040649. [DOI] [PubMed] [Google Scholar]
- Sandmeyer S. B., Hansen L. J., Chalker D. L. Integration specificity of retrotransposons and retroviruses. Annu Rev Genet. 1990;24:491–518. doi: 10.1146/annurev.ge.24.120190.002423. [DOI] [PubMed] [Google Scholar]
- Sandmeyer S. B., Hansen L. J., Chalker D. L. Integration specificity of retrotransposons and retroviruses. Annu Rev Genet. 1990;24:491–518. doi: 10.1146/annurev.ge.24.120190.002423. [DOI] [PubMed] [Google Scholar]
- Shevelyov YuYa, Balakireva M. D., Gvozdev V. A. Heterochromatic regions in different Drosophila melanogaster stocks contain similar arrangements of moderate repeats with inserted copia-like elements (MDG1). Chromosoma. 1989 Aug;98(2):117–122. doi: 10.1007/BF00291047. [DOI] [PubMed] [Google Scholar]
- Shevelyov YuYa, Balakireva M. D., Gvozdev V. A. Heterochromatic regions in different Drosophila melanogaster stocks contain similar arrangements of moderate repeats with inserted copia-like elements (MDG1). Chromosoma. 1989 Aug;98(2):117–122. doi: 10.1007/BF00291047. [DOI] [PubMed] [Google Scholar]
- Traverse K. L., Pardue M. L. Studies of He-T DNA sequences in the pericentric regions of Drosophila chromosomes. Chromosoma. 1989 Jan;97(4):261–271. doi: 10.1007/BF00371965. [DOI] [PubMed] [Google Scholar]
- Vaury C., Bucheton A., Pelisson A. The beta heterochromatic sequences flanking the I elements are themselves defective transposable elements. Chromosoma. 1989 Sep;98(3):215–224. doi: 10.1007/BF00329686. [DOI] [PubMed] [Google Scholar]
- Zhang P., Spradling A. C. Insertional mutagenesis of Drosophila heterochromatin with single P elements. Proc Natl Acad Sci U S A. 1994 Apr 26;91(9):3539–3543. doi: 10.1073/pnas.91.9.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P., Spradling A. C. Insertional mutagenesis of Drosophila heterochromatin with single P elements. Proc Natl Acad Sci U S A. 1994 Apr 26;91(9):3539–3543. doi: 10.1073/pnas.91.9.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]




