Abstract
A photoactivated, site-selective conjugation of poly(ethylene glycol) (PEG) to the glutathione (GSH) binding pocket of glutathione S-transferase (GST) is described. To achieve this, a GSH analogue (GSH-BP) was designed and chemically synthesized with three functionalities: (1) the binding affinity of GSH to GST, (2) a free thiol for polymer functionalization, and (3) a photoreactive benzophenone (BP) component. Different molecular weights (2 kDa, 5 kDa, and 20 kDa) of GSH-BP modified PEGs (GSBP-PEGs) were synthesized and showed conjugation efficiencies between 52% and 76% to GST. Diazirine (DA) PEG were also prepared but gave conjugation yields lower than for GSBP-PEGs. PEGs with different end-groups were also synthesized to validate the importance of each component in the end-group design. End-groups included glutathione (GS-PEG) and benzophenone (BP-PEG). Results showed that both GSH and BP were crucial for successful conjugation to GST. In addition, conjugations of 5 kDa GSBP-PEG to different proteins were investigated, including bovine serum albumin (BSA), lysozyme (Lyz), ubiquitin (Ubq), and GST-fused ubiquitin (GST-Ubq) to ensure specific binding to GST. By combining noncovalent and covalent interactions, we have developed a new phototriggered protein–polymer conjugation method that is generally applicable to GST-fusion proteins.
Introduction
Protein–polymer conjugates are of great interest due to their applications in drug delivery, biomaterials, and nanotechnology.1−5 The role of the attached polymer is to enhance existing functions or introduce new properties to the unmodified protein. For example, the covalent attachment of poly(ethylene glycol) (PEG), also known as PEGylation, is commonly used to improve the pharmacological properties of therapeutic proteins.6 Site-specific conjugation is important for retaining bioactivity of the protein, and therefore development of new conjugation approaches are of high interest in the field of bioconjugation.7 Typically site-specific conjugation is achieved by targeting rare natural amino acids, genetic modifications, or chemically installed functionalities.8−14
Other methods have also been used for protein–polymer conjugation, such as noncovalent interactions including ligand protein affinity. Using ligand–protein binding affinity, ligand-modified polymers can specifically bind to the corresponding protein. This type of conjugation method has been most commonly used with the biotin–streptavidin systems.15−18 Another example is cofactor reconstitution, which has been employed to couple polystyrene to heme-dependent proteins, such as horseradish peroxidase (HRP) and myoglobin, to form bioactive giant amphiphiles.19,20 Recently, our group and others have also utilized the interaction between glutathione (GSH) and gluthathione S-transeferase (GST) to form protein–polymer conjugates.18,21 However, the major drawback of noncovalent interactions is that the conjugates are prone to dissociate under charge disruption, heat, or other denaturing conditions.
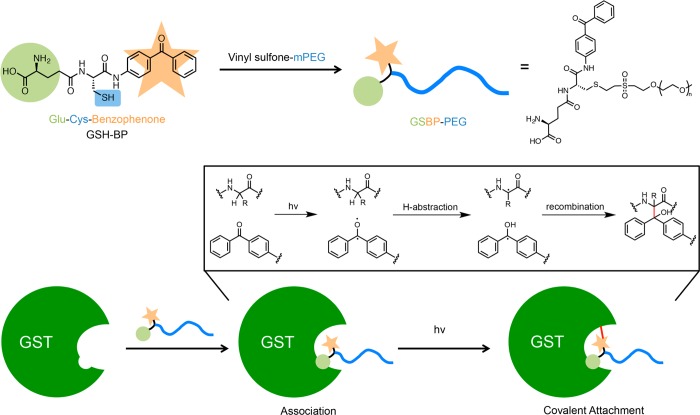
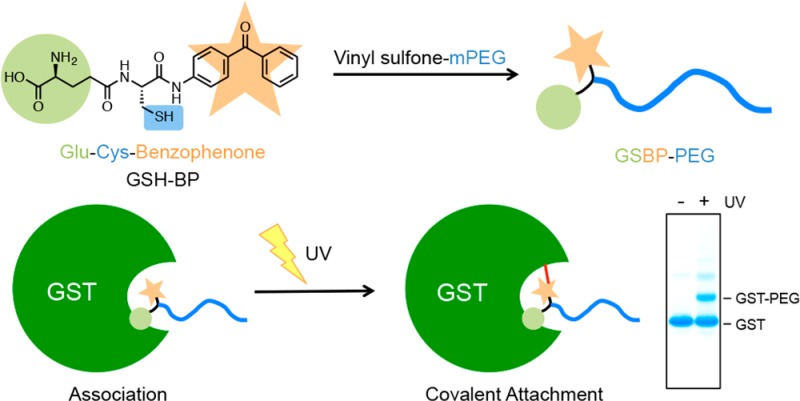
GSTs (EC 2.5.1.18) are a family of enzymes that catalyze the nucleophilic addition of the thiol of GSH to electrophilic centers in organic compounds for means of cellular detoxification.22,23 There are several classes of GSTs, but all exhibit similar monomer sizes (23–28 kDa), amino acid sequences, and substrate specificity. They function as homodimeric or heterodimeric species, with heterodimers only forming between different subunits belonging to the same class. GSTs have two active sites per dimer, with each active site separated into two distinct functional regions: a hydrophilic “G-site” for recognition of GSH, and an adjacent “H-site” for binding hydrophobic electrophiles. Due to its specificity to GSH (with a dissociation constant of kd ∼ 10–4 M–1),24 GST has been commonly integrated into recombinant proteins to allow for affinity chromatography purification with immobilized GSH.25,26 Herein, we describe an approach to prepare site selective PEG-GST proteins by light activation of photoreactive GSH-PEG (Figure 1).
Figure 1.
Schematic overview of the photoaffinity PEGylation using GSH-BP.
Photoaffinity labeling has been widely used in mapping the active sites of enzymes and studying protein interactions.27,28 A photoaffinity probe contains at least two parts: an affinity unit and a photoreactive unit. Benzophenone, diazirine, and azide are widely used photoreactive groups that can generate highly reactive species, namely, triplet state carbonyls, carbenes, and nitrenes, respectively. Benzophenone (BP) was selected for study because it has an advantage in that it is chemically more stable than the other groups or species, can be manipulated in ambient light, and inserts into unreactive C–H bonds even in the presence of nucleophilic solvents such as water and alcohols.29 Recently, affinity association followed by photoexcitation of benzophenone has been utilized by Aida and co-workers to develop “photoclickable molecular glue” that labels proteins with a fluorescent dendron.30 Yet there have been no examples of using benzophenone to form protein–polymer conjugates. We also prepared a GSH analogue containing a diazirine (DA) moiety. Upon irradiation, diazirine generates a highly active carbene intermediate that can insert into C–H bonds within close proximity.31,32 In one earlier report, PEG has been functionalized with diazopyruvate and nitroaryl azide for protein conjugation, which after photolysis generated carbene and nitrene, respectively. The reactive species then underwent rearrangements followed by amine insertion of protein lysine residues or N-terminal to give PEGylated proteins.33 However, the PEGs did not contain a specific affinity tag and highly reactive carbene and nitrene could also insert into C–H and N–H bonds, especially without close proximity of the reactive groups. Described herein is the first example, to our knowledge, of PEGylation through photoaffinity conjugation.
Results and Discussion
Synthesis of GSH-BP
To form an irreversibly bound, covalent protein–polymer conjugate, a GSH analogue containing a photoreactive probe was designed to act as the cross-linking moiety between GST and the polymer. We hypothesized that the modified polymer end-group could be directed into the GSH binding pocket of GST through ligand affinity, and then activated by UV irradiation to form a stable and irreversible covalent bond. The overall approach was to prepare a BP labeled GSH and react vinyl sulfone PEG (2K mPEG, 5K mPEG, and 20K mPEG) with the GSH via thiol–ene chemistry. The modified polymers could then conjugate to GST and a GST fusion protein through photoactivation (Figure 1).
It has been reported that the γ-glutamyl residue of the GSH tripeptide is crucial for binding to GST, whereas the C-terminal glycyl carboxylate is nonessential.34 Therefore, the design of our GSH analogue (GSH-BP) replaces the glycine with amino-benzophenone. The free thiol of cysteine was used as a handle for PEG modification, and the glutamyl group remained free for GST binding. The GSH-BP was synthesized by solution phase peptide synthesis. To incorporate the benzophenone moiety, 4-aminobenzophenone was coupled to Fmoc-Cys(Trt)-OH. Following Fmoc deprotection and subsequently coupling Boc-Glu-OtBu, fully protected GSH-BP was obtained. Global deprotection yielded GSH-BP in 28% overall yield. UV–vis showed λmax = 295 nm; ESI-MS gave the mass +H+ 430.1450 (calculated 430.1392). (The synthetic scheme and NMR spectrum of each step are provided in Supporting Information Scheme S1 and Figure S1 through Figure S6.)
Conjugation of GSH-BP to GST
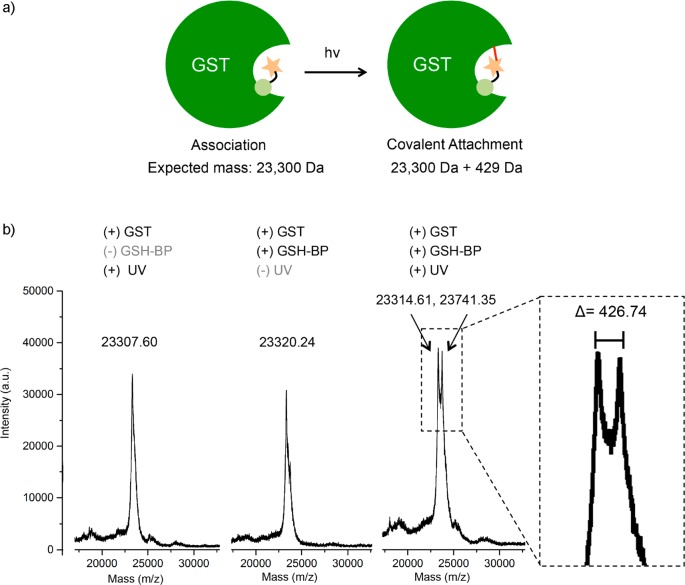
To confirm GSH-BP attachment to GST after irradiation, 200 mM GSH-BP was added to 4 mM GST, incubated on ice for 2 h to allow association, and subsequently irradiated for 30 min with a mercury arc lamp. At the same time, two control samples were prepared: one without the addition of GSH-BP substrate but with 30 min of UV irradiation, to confirm the integrity of GST after the irradiation condition. The other was with GSH-BP, but without UV irradiation, to confirm that irradiation is necessary for GSH-BP binding. The GST samples were analyzed by MALDI-TOF (Figure 2). The peak corresponding to GST without GSH-BP or UV irradiation showed up as the GST monomer subunit with m/z around 23 300. An increase of mass was observed only when both GSH-BP and UV irradiation were present. The difference in mass between the new peak and unmodified GST was 426.74, which closely corresponded to the mass of GSH-BP (429.14). This confirmed the covalent attachment of a single GSH-BP to GST after irradiation.
Figure 2.
(a) Photoconjugation of GSH-BP to GST and (b) MALDI-TOF MS results with or without the presence of GSH-BP or UV irradiation. Only in the case of addition of GSH-BP and application of UV irradiation was an increase in mass observed.
The peak intensities of the unmodified GST and modified GST were very similar, which could be explained if only one subunit of the GST dimer was modified. Wang et al. have synthesized a photoaffinity probe consisting of glutathione and benzophenone, in which benzophenone acts as the photoreactive group as well as the xenobiotic substrate.35 They demonstrated that the probe binds to both the glutathione binding site (G-site) and hydrophobic substrate binding site (H-site), and reacts specifically with Met-112 of the μ class GST, isoenzyme 4–4. From experimental and docking results, they concluded that modification of one subunit prevents the modification of the other dimer subunit. This would explain the results observed in the MALDI spectrum, namely, that the GSH-BP covalently binds to one of the two GSTs in the dimer and subsequently the dimer dissociates during the mass spectrometry experiment.
Synthesis of GSH-DA
We also synthesized a GSH analogue containing a diazirine group to compare its photoconjugation efficiency to GSH-BP. Diazirine was incorporated into the peptide sequence as an amino acid side chain using commercially available l-photo-leucine (see Supporting Information Figure S7 and S8 for NMR spectra of Fmoc-photo-Leucine). The peptide (GSH-DA) was synthesized by solid phase synthesis and verified by ESI-MS (see Supporting Information for Scheme S2). GSH-DA has a smaller photoreactive group than GSH-BP, and conserves the carboxylic acid on the original glycine position.
PEG Functionalization with GSH-BP, GSH-DA, and Controls
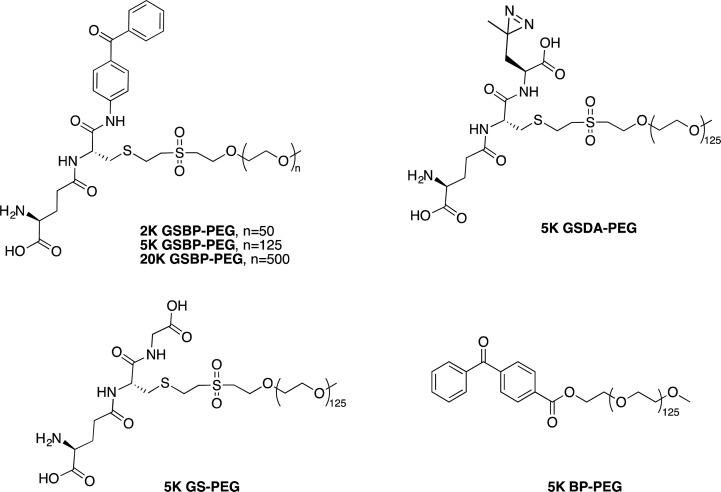
Vinyl sulfone-PEG (VS-PEG) was prepared from three sizes of mPEG, modifying reported procedures (see Supporting Information for Scheme S3).36 Michael addition of the PEG hydroxyl group to divinyl sulfone yielded monofunctionalized VS-PEGs (see Supporting Information Figures S9–S11 for 1H NMR spectra of 2K, 5K, and 20K VS-PEG). By comparing the integration of the vinyl peaks to that of the ethylene glycol peak in 1H NMR, the vinyl sulfone conversion of 2K, 5K, and 20K VS-PEG, was calculated to be 89%, 85%, and 90%, respectively. GSH-BP was then added to each size of VS-PEG to form the corresponding size of GSBP-PEG (see Supporting Information for Scheme S4). GSH-BP is insoluble in water, so it was dissolved in methanol before addition to a phosphate buffer solution of VS-PEG. The pH of the reaction mixture was kept at pH 8.0 to avoid side reactions of vinyl sulfone with the free amine of GSH-BP.37 The disappearance of vinyl protons was confirmed by 1H NMR after 48 h. GSBP-PEGs were purified by HPLC with gradient elution of MeOH/H2O or dialysis against water followed by filtration to remove excess GSH-BP (see Supporting Information Figures S12–S14 for 1H NMR spectra of 2K, 5K, and 20K GSBP-PEG). By comparing the integration of the aromatic protons of benzophenone to the ethylene glycol protons in 1H NMR, the modification ratio of 2K, 5K, and 20K GSBP-PEG, was calculated to be 94%, 100%, and 95%, respectively. 5K GSDA-PEG was synthesized in a similar fashion (see Supporting Information Scheme S5), and the conversion was calculated as 75% (see Supporting Information Figure S15 for 1H NMR spectrum).
To confirm the selectivity and ability of GSBP-PEG to covalently attach to GST, we prepared PEG with different end-groups, including GSH and BP, as controls (Figure 3). 5K GS-PEG, with the affinity probe (GSH), but not the photoreactive component (BP), was synthesized by conjugating GSH to VS-PEG through the free thiol of the cysteine moiety, and the conversion was 86%. 5K BP-PEG, with the photoreactive probe (BP) but no affinity moiety (GSH), was synthesized via 1-ethyl-3-(3-(dimethylamino)propyl)carbodiimide (EDC) coupling of mPEG and 4-benzoylbenzoic acid. The end-group conversion was calculated to be 100% (see Supporting Information Schemes S6 and S7 for 5K GS-PEG and 5K BP-PEG synthesis, and Figure S16 and S17 for their 1H NMR spectra).
Figure 3.
Chemical structures of GSBP-PEG, GSDA-PEG, GS-PEG, and BP-PEG.
Conjugation of GSBP-PEG to GST
To investigate the photoinduced reactivity of GSBP-PEG to GST, a mixture of GSBP-PEG (20 mM) and GST (0.4 mM) in pH 7.4 D-PBS was irradiated in an ice bath over a period of 15 min. All subsequent conjugation experiments used the same protocol. SDS-PAGE analysis demonstrated that the synthesized GSBP-PEGs could be covalently conjugated to GST, appearing as an additional band with higher molecular weight above the unconjugated GST band (Figure 4a). Importantly, no conjugates were observed in samples that were not exposed to UV irradiation. The conjugation yields of 2K, 5K, and 20K GSBP-PEG were estimated by quantification of the band intensities taking into account the dimeric form of GST, and were calculated to be 52%, 56%, and 76%, respectively (Table 1). Various concentrations of protein and polymer equivalents were then investigated for 5K GSBP-PEG (Table 2) and it was found that by increasing the polymer concentration to 50 equiv and the irradiation time to 30 min keeping the polymer concentration the same, the yield was increased from 56% to 74%. Photoaffinity labeling approaches are often limited from their low efficiencies. Photolysis of benzophenone in protein–protein cross-linking events or the labeling of peptide binding sites generally resulted in efficiencies lower than 40%.38,39 Recent reports that have utilized benzophenone to covalently conjugate immunoglobin-binding domains to antibodies showed conjugation yields of 70% at maximum.40,41 Thus, the conjugation yields of GSBP-PEGs to GST ranging from 52% to 76% are fairly high.
Figure 4.
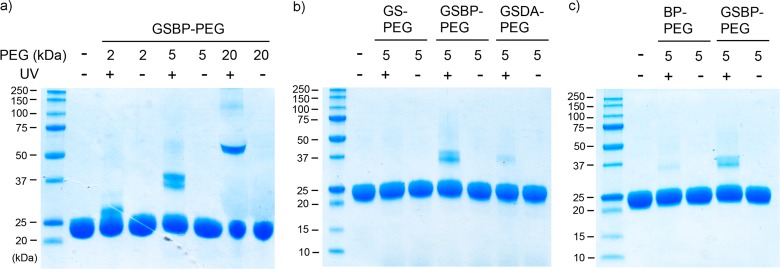
SDS-PAGE of (a) GSBP-PEG conjugation to GST; (b) comparison between GS-PEG, 5K GSBP-PEG, and GSDA-PEG; (c) comparison between BP-PEG and 5K GSBP-PEG.
Table 1. Conjugation Yields for the Different PEGs to GST.
| size | 2K | 5K | 20K | 5K | 5K | 5K |
|---|---|---|---|---|---|---|
| polymer | GSBP-PEG | GSBP-PEG | GSBP-PEG | GS-PEG | BP-PEG | GSDA-PEG |
| conjugation yielda | 52% | 56%b | 76% | 0% | 14% | 18% |
GST is a protein dimer, and showed on SDS-PAGE gels as broken subunits. The yield was obtained by dividing the conjugate percentage on gel by the theoretical maximum yield because of the dimer (50%).
The conjugation yield is presented as an average from three separate SDS-PAGE gels.
Table 2. Conjugation Yields for 5K GSBP-PEG to GST, BSA, Lyz, Ubq, and GST-Ubq at Various Conditionsa.
| protein | GST | Ubq | GST-Ubq | ||||||
|---|---|---|---|---|---|---|---|---|---|
| protein conc. (mM) | 0.4 | 0.4 | 0.4 | 0.1 | 0.05 | 0.05 | 0.05 | 0.4 | 0.4 |
| polymer (eq) | 50 | 10 | 5 | 1 | 1 | 5 | 10 | 10 | 10 |
| UV time (min) | 30 | 30 | 30 | 15 | 15 | 15 | 15 | 15 | 15 |
| conj. yield (%) | 74a | 62 | 54 | 26 | 20 | 54 | 52 | 9 | 36 |
| protein | BSA | Lyz | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| protein conc. (mM) | 0.4 | 0.4 | 0.1 | 0.05 | 0.05 | 0.05 | 0.4 | 0.4 | 0.1 | 0.05 | 0.05 | 0.05 |
| polymer (eq) | 10 | 5 | 1 | 1 | 5 | 10 | 10 | 5 | 1 | 1 | 5 | 10 |
| UV time (min) | 30 | 30 | 15 | 15 | 15 | 15 | 30 | 30 | 15 | 15 | 15 | 15 |
| conj. yield (%) | 37 | 38 | 16 | 13 | 28 | 24 | 24 | 12 | 4 | 0 | 8 | 12 |
The conjugation efficiency is presented as an average from two separate SDS-PAGE gels.
While the naturally existing GST dimer is denatured and unfolded during SDS-PAGE analysis releasing free GST, the band we observe is based on the molecular weight of one monomer subunit. Therefore, we also analyzed unmodified GST and its conjugates by native PAGE, because native gels are run under nondenaturing conditions, and the mobility of the proteins is based on its charge and hydrodynamic size. However, the conjugates and protein itself appear as a smear, which makes the results unsuitable for determination of the conjugation yield (see Supporting Information Figure S18a for native PAGE results).
To compare the conjugation efficiency of GS-PEG and GSDA-PEG to GSBP-PEG, a mixture of each polymer (20 mM) and GST (0.4 mM) was irradiated following the previously mentioned protocol. SDS-PAGE analysis demonstrated that, as expected, GS-PEG does not form any covalent conjugates with GST after UV irradiation due to the lack of photoreactive group (Figure 4b). This is exactly what we would expect, since SDS would disrupt any noncovalent interactions. Surprisingly, GSDA-PEG only showed a small conjugation yield of 18%, compared to the 56% conjugation yield of 5K GSBP-PEG (Table 1). Native PAGE analysis was also conducted, and no obvious difference in the bands was observed in either GS-PEG or GSDA-PEG conjugate after irradiation (see Supporting Information Figure S18b for native PAGE). However, the conjugation of GSBP-PEG was observed as an intense high molecular weight smear. We originally anticipated that the GSH with the smaller DA group would bind better than the larger BP group. However, given the results, we propose that the larger, hydrophobic benzophenone may play an important role in binding the hydrophobic “H-site” of the binding pocket, which further increases the binding affinity of GSH-BP to GST.
To confirm that covalent conjugation was not exclusively due to hydrophobic binding of benzophenone, the photoinduced conjugation of GSBP-PEG and BP-PEG to GST were compared under the same conditions. From SDS-PAGE analysis, the conjugation yield of BP-PEG to GST was calculated as 14%, which is much lower than the 56% yield of GSBP-PEG (Figure 4c and Table 1). This demonstrates that the GSH binding component is crucial to the design of the GSH-BP probe. The conjugation results were also analyzed by native PAGE, and the higher molecular weight smear of GSBP-PEG conjugate was observed to be significantly more intense than BP-PEG, which further demonstrates the higher conjugation efficiency of GSBP-PEG (see Supporting Information Figure S18c for native PAGE). The conjugation efficiencies of different sizes of GSBP-PEG, as well as different end-functionalized 5K PEGs, are summarized in Table 1.
GSBP-PEG Conjugation to Other Proteins—BSA, Lyz, Ubq, and GST-Ubq
In order to investigate the further applicability of this conjugation method, several proteins including GST were tested under various conjugation conditions (including protein concentration, polymer ratio, and irradiation time). First, bovine serum albumin (BSA, 66.5 kDa) was chosen as a model protein. A mixture of GSBP-PEG (2K or 5K, 20 mM) and BSA (or GST as a control, 0.4 mM) was irradiated on ice over a period of 30 min and analyzed by SDS-PAGE. Similar conjugation efficiency of GSBP-PEG to BSA as to GST was observed, which can be explained by the known binding effect of benzophenone to the subdomain IIA hydrophobic cavities of BSA (Figure 5a).42 This further strengthens our hypothesis that GSH-BP is interacting with the hydrophobic binding site (H-site) in GST. To eliminate this factor, lysozyme (Lyz, 14.4 kDa) was also tested as a general nonbinding protein (Figure 5b). Under conditions of 0.4 mM protein concentration and 30 min of irradiation, 12% and 24% of nonspecific conjugation to Lyz were observed after adding 5 equiv and 10 equiv of 5K GSBP-PEG, respectively. When protein concentration, irradiation time, and the polymer ratio were decreased, the nonspecific conjugation was significantly reduced compared to GST and BSA under the same conditions down to 0% (Table 2 and Supporting Information Figure S19). These results convey that after photoexcitation, benzophenone nonspecifically attaches to nonbinding proteins (Lyz) at high protein concentrations and large excess of GSBP-PEG. Decreasing concentration, stoichiometry, and irradiation time can reduce the nonspecificity. The results indicate that polymer concentrations and polymer equivalents may be identified for each fusion protein to yield conjugation to the GST tag component only. For example, at 0.05 mM GST and 5 equiv of polymer the conjugation yield to GST was 54%, while to Lys it was 8%, and 1 equiv of polymer resulted in yields of 20% and 0%, respectively.
Figure 5.
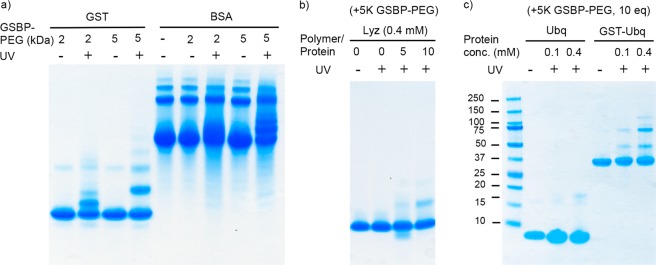
SDS-PAGE of the conjugation of (a) 2K and 5K GSBP-PEG to GST and BSA, (b) 5K GSBP-PEG to Lyz with different polymer to protein ratios, and (c) 5K GSBP-PEG to Ubq and GST-Ubq.
To further study the applicability of the approach, the conjugations of GSBP-PEG to Ubiquitin (Ubq, 8.5 kDa) and GST-tagged Ubq (GST-Ubq, 38.5 kDa) were compared side-by-side, with higher conjugation efficiency of the fusion protein expected. The important role of the GST tag in the conjugation of GSBP-PEG to Ubq was demonstrated by SDS-PAGE analysis (Figure 5c). The conjugation efficiencies calculated by the band intensities were 9% for Ubq and 36% for GST-Ubq (Table 2).
From the investigation of various end-functionalized PEGs to GST, we have determined that GSH (affinity) and BP (labeling) were both crucial to the irreversible conjugation of GSH-BP to GST. By the tuning the concentration, stoichiometry, and irradiation time, the conjugation yields of 5K GSBP-PEG to GST ranged from 20% to 74%. However, at higher concentrations, larger excess of polymer, and longer irradiation time, nonspecific proton abstraction and radical recombination would be more prone to occur. Investigations of the interactions between GSH-BP and several other proteins, including BSA, Lyz, Ubq, and GST-Ubq, were also conducted. Under each condition, the conjugation yields of GST were clearly higher than that of Ubq and Lyz. As a result, the described conjugation method should be generally applicable to other GST-fusion proteins for applications such as therapeutic protein delivery or construction of macromolecule architectures, provided the protein does not bind to BP (as demonstrated for BSA). PEGylation should exclusively occur at the binding site of GST, leaving the major protein unaffected when the photoconjugation condition is optimized. Other polymers, macromolecules, or small molecule probes that allow GSH-BP functionalization are also applicable for site-selective protein modifications. This would be convenient since many GST-tagged proteins are produced for purification purposes and then the GST group is removed post expression and purification prior to PEG conjugation. The approach reported herein would allow for the GST tag to remain and serve as the site for polymer conjugation, reducing steps, and cost in the construction of the protein–polymer conjugates.
Conclusions
Herein, we reported the synthesis of a new GSH analogue containing a photoreactive benzophenone and analyzed its use for photoinduced GST fusion protein–PEG conjugation. To test our hypothesis, different end-functionalized PEGs were synthesized as controls, including GS-PEG and BP-PEG. Without the addition of both GSH and benzophenone moieties, conjugation efficiency was significantly reduced. The conjugation yields of different proteins, including BSA, Lyz, Ubq, and GST-Ubq, were analyzed. GSBP-PEG nonspecifically conjugates to BSA through the hydrophobic binding effect with benzophenone, but has less than 10% conjugation efficiency to Lyz and Ubq under controlled conditions. In other cases, the GST moiety is crucial for the conjugation effect to occur. The conjugation efficiency of 5K GSBP-PEG ranges from 20% to 74% for GST. As a result, this system is a light-responsive and site-selective protein–polymer conjugation that occurs only after photoexcitation. It is a versatile method, for the GSH analogue could be attached to any thiol-reactive polymers or substrates, and other GST-fusion proteins could serve as conjugation targets.
Experimental Section
Materials
All the reagents and solvents were purchased from Sigma-Aldrich and Fisher Scientific and used without purification unless noted otherwise. 4-Aminobenzophenone was recrystallized from DCM/MeOH = 1/5. GST from equine liver was purchased from Sigma-Aldrich as a lyophilized powder containing salts, and the protein concentration was determined by its extinction coefficient (ε280 = 47 000 M–1 cm–1) at 280 nm. Polyacrylamide gels for electrophoresis were purchased from Invitrogen and Bio-Rad. l-Photoleucine was purchased from Thermo Scientific. Ubiquitin (human, recombinant) GST-tag was purchased from Enzo Life Sciences.
Instrumentation
NMR spectra were obtained on Bruker AV 500 and DRX 500 MHz spectrometers. 1H NMR spectra were acquired with a relaxation delay of 2 s for small molecules and 30 s for polymers. UV–vis spectroscopy was performed using a Biomate 5 Thermo Spectronic spectrometer or Thermo Scientific NanoDrop 2000 (for small quantities). ESI-MS data were gathered on a Waters LCT premier with ACQUITY LC. Infrared absorption spectra were recorded using a PerkinElmer FT-IR equipped with an ATR accessory. Photoreactions were carried out in a photochemical safety cabinet equipped with a broadband, medium pressure Ace-Hanocia 7825–34 quartz mercury arc lamp and ACE Glass Inc. power supply. Matrix-assisted laser desorption/ionization time-of-flight mass (MALDI-TOF MS) spectrometry data was gathered using sinapic acid (SA) as a matrix on an Applied Biosystems Voyager-DE-STR spectrometer.
Methods
Reaction of GSH-BP with GST
GST (0.94 μL, 100 mg/mL in Milli-Q H2O) and GSH-BP (1 μL, 86 mg/mL in MeOH) were added to Milli-Q H2O (8.06 μL) to prepare a mixture with final concentration of 0.4 mM GST and 20 mM GSH-BP, containing 10% MeOH for GSH-BP solubility. Upon addition of the GSH-BP solution to water, a precipitate was observed. The sample was kept in the dark and incubated on ice for 2 h and then irradiated in an ice bath for 30 min in the photochemical safety cabinet with a mercury arc lamp. The crude mixture was purified by centrifugal filtration (0.5 mL, MWCO 3000) and three washes of H2O. The solution was then collected and analyzed by MALDI-TOF MS using sinapic acid as the matrix. Controls were prepared following the same procedure, but without adding GSH-BP, or without UV irradiation.
General Procedure of Irradiation Experiments of PEG
With GSBP-PEG as an example, GST (10 μL, 0.8 mM, 18.64 mg/mL in pH 7.4 D-PBS) and GSBP-PEG (10 μL, 8 mM, 40 mg/mL in Milli-Q H2O) were combined to prepare a 20 μL mixture with final concentration of 0.4 mM GST and 4 mM GSBP-PEG. The concentration of GST was ascertained by UV–vis measurement of A280 using ε = 47 000 M–1 cm–1. The mixture was kept in the dark and incubated on ice for 2 h to allow polymer association. An aliquot (10 μL) was transferred into a disposable UV cuvette and irradiated on ice bath for 15 min in the photochemical safety cabinet, while the other 10 μL was kept in the dark on ice as a control without UV irradiation. The samples were then directly analyzed by SDS-PAGE (200 V, 35 min) and/or native PAGE (100 V, 3.5 h) with Bio-Rad Any kD precast polyacrylamide gels (or Invitrogen 4–12% precast polyacrylamide gels). Coomassie was used to stain the protein, and iodine was used to stain PEG.43 All control experiments with other proteins (BSA and Lyz) and different PEGs (GS-PEG, GSDA-PEG, and BP-PEG) were conducted following the same procedure.
Analysis of Conjugation Yield
The SDS-PAGE gels were scanned with an Epson Perfection 2480 scanner as tiff images, and analyzed with the ImageJ software. Each lane was selected by the rectangular selection tool, and plotted with the gel analysis function. The percentage peak area of the conjugate divided by the sum of the conjugate and unmodified protein is the conjugation efficiency (conjugation yield). In the cases of GST and GST-Ubq, the conjugate percentage was divided by the theoretical maximum yield (50%) considering the dissociation of the protein dimer.
Acknowledgments
This work was funded by the National Science Foundation (CHE-1112550). E.-W. L. thanks the 2014 Cram Fellowship for funding. N.B. thanks the NIH Biotechnology Training Fellowship (5T32 GM06755509) for funding. AV 500 NMR was supported by National Science Foundation under equipment grant number CHE-1049904.
Glossary
Abbreviations
- BP
benzophenone
- BSA
bovine serum albumin
- Cys
cysteine
- DA
diazirine
- DCM
dichloromethane
- D-PBS
Dulbecco’s phosphate buffer saline
- EDC
1-ethyl-3-(3-(dimethylamino)propyl)carbodiimide
- ESI-MS
electrospray ionization mass spectrometry
- Glu
glutamic acid
- GSH
glutathione
- GST
glutathione S-transferase
- HPLC
high performance liquid chromatography
- HRP
horseradish peroxidase
- Lyz
lysozyme
- MALDI-TOF
matrix-assisted laser desorption/ionization time-of-flight
- MeOH
methanol
- NMR
nuclear magnetic resonance
- PAGE
polyacrylamide gel electrophoresis
- PEG
poly(ethylene glycol)
- SDS
sodium dodecyl sulfate
- SA
sinapic acid
- Ubq
ubiquitin
- VS
vinyl sulfone
Supporting Information Available
Synthesis and characterization of the photoreactive probes (GSH-BP and GSH-DA) and all modified polymers, and results of additional photoconjugation experiments. This material is available free of charge via the Internet at http://pubs.acs.org.
The authors declare no competing financial interest.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- Duncan R. (2003) The dawning era of polymer therapeutics. Nat. Rev. Drug Discovery 2, 347–360. [DOI] [PubMed] [Google Scholar]
- Heredia K. L.; Maynard H. D. (2007) Synthesis of protein-polymer conjugates. Org. Biomol. Chem. 5, 45–53. [DOI] [PubMed] [Google Scholar]
- Gauthier M. A.; Klok H. A. (2008) Peptide/protein-polymer conjugates: synthetic strategies and design concepts. Chem. Commun. 2591–2611. [DOI] [PubMed] [Google Scholar]
- Canalle L. A.; Lowik D.; van Hest J. C. M. (2010) Polypeptide-polymer bioconjugates. Chem. Soc. Rev. 39, 329–353. [DOI] [PubMed] [Google Scholar]
- Shu J. Y.; Panganiban B.; Xu T. (2013) Peptide-polymer conjugates: from fundamental science to application. Annual Review of Physical Chemistry (Johnson M. A., Martinez T. J., Eds.) pp 631–657, Vol 64, Annual Reviews, Palo Alto. [DOI] [PubMed] [Google Scholar]
- Alconcel S. N. S.; Baas A. S.; Maynard H. D. (2011) FDA-approved poly(ethylene glycol)-protein conjugate drugs. Polym. Chem. 2, 1442–1448. [Google Scholar]
- Kochendoerfer G. G. (2005) Site-specific polymer modification of therapeutic proteins. Curr. Opin. Chem. Biol. 9, 555–560. [DOI] [PubMed] [Google Scholar]
- Li N.; Lim R. K. V.; Edwardraja S.; Lin Q. (2011) Copper-free sonogashira cross-coupling for functionalization of alkyne-encoded proteins in aqueous medium and in bacterial cells. J. Am. Chem. Soc. 133, 15316–15319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumas A.; Spicer C. D.; Gao Z.; Takehana T.; Lin Y. A.; Yasukohchi T.; Davis B. G. (2013) Self-liganded Suzuki-Miyaura coupling for site-selective protein PEGylation. Angew. Chem., Int. Ed. 52, 3916–3921. [DOI] [PubMed] [Google Scholar]
- Toda N.; Asano S.; Barbas C. F. (2013) Rapid, stable, chemoselective labeling of thiols with Julia-Kocienski-like reagents: a serum-stable alternative to maleimide-based protein conjugation. Angew. Chem., Int. Ed. 52, 12592–12596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendeler M.; Grinberg L.; Wang X. Y.; Dawson P. E.; Baca M. (2014) Enhanced catalysis of oxime-based bioconjugations by substituted anilines. Bioconjugate Chem. 25, 93–101. [DOI] [PubMed] [Google Scholar]
- Zhou Z.; Zhang J.; Sun L. J.; Ma G. H.; Su Z. G. (2014) Comparison of site-specific PEGylations of the N-terminus of interferon beta-1b: selectivity, efficiency, and in vivo/vitro activity. Bioconjugate Chem. 25, 138–146. [DOI] [PubMed] [Google Scholar]
- Li Y. M.; Li Y. T.; Pan M.; Kong X. Q.; Huang Y. C.; Hong Z. Y.; Liu L. (2014) Irreversible site-specific hydrazinolysis of proteins by use of sortase. Angew. Chem., Int. Ed. 53, 2198–2202. [DOI] [PubMed] [Google Scholar]
- Levine P. M.; Craven T. W.; Bonneau R.; Kirshenbaum K. (2014) Intrinsic bioconjugation for site-specific protein PEGylation at N-terminal serine. Chem. Commun. 50, 6909–6912. [DOI] [PubMed] [Google Scholar]
- Hannink J. M.; Cornelissen J.; Farrera J. A.; Foubert P.; De Schryver F. C.; Sommerdijk N.; Nolte R. J. M. (2001) Protein-polymer hybrid amphiphiles. Angew. Chem., Int. Ed. 40, 4868–4870. [PubMed] [Google Scholar]
- Hou S. J.; Sun X. L.; Dong C. M.; Chaikof E. L. (2004) Facile synthesis of chain-end functionalized glycopolymers for site-specific bioconjugation. Bioconjugate Chem. 15, 954–959. [DOI] [PubMed] [Google Scholar]
- Bontempo D.; Li R. C.; Ly T.; Brubaker C. E.; Maynard H. D. (2005) One-step synthesis of low polydispersity, biotinylated poly(N-isopropylacrylamide) by ATRP. Chem. Commun. 4702–4704. [DOI] [PubMed] [Google Scholar]
- Huang X.; Boyer C.; Davis T. P.; Bulmus V. (2011) Synthesis of heterotelechelic polymers with affinity to glutathione-S-transferase and biotin-tagged proteins by RAFT polymerization and thiol-ene reactions. Polym. Chem. 2, 1505–1512. [Google Scholar]
- Boerakker M. J.; Hannink J. M.; Bomans P. H. H.; Frederik P. M.; Nolte R. J. M.; Meijer E. M.; Sommerdijk N. (2002) Giant amphiphiles by cofactor reconstitution. Angew. Chem., Int. Ed. 41, 4239–4241. [DOI] [PubMed] [Google Scholar]
- Boerakker M. J.; Botterhuis N. E.; Bomans P. H. H.; Frederik P. M.; Meijer E. M.; Nolte R. J. M.; Sommerdijk N. (2006) Aggregation behavior of giant amphiphiles prepared by cofactor reconstitution. Chem.—Eur. J. 12, 6071–6080. [DOI] [PubMed] [Google Scholar]
- Chang C. W.; Nguyen T. H.; Maynard H. D. (2010) Thermoprecipitation of glutathione S-transferase by glutathione-poly(N-isopropylacrylamide) prepared by RAFT polymerization. Macromol. Rapid Commun. 31, 1691–1695. [DOI] [PubMed] [Google Scholar]
- Wilce M. C. J.; Parker M. W. (1994) Structure and function of glutahion S-transferases. Biochim. Biophys. Acta, Protein Struct. Mol. Enzymol. 1205, 1–18. [DOI] [PubMed] [Google Scholar]
- Dirr H.; Reinemer P.; Huber R. (1994) X-ray crystal-structures of cytosolic glutathione S-transferases - implications for protein architecture, substrate recogniction and catalytic function. Eur. J. Biochem. 220, 645–661. [DOI] [PubMed] [Google Scholar]
- Yan F.; Yang W. K.; Li X. Y.; Lin T. T.; Lun Y. N.; Lin F.; Lv S. W.; Yan G. L.; Liu J. Q.; Shen J. C.; Mu Y.; Luo G. M. (2008) A trifunctional enzyme with glutathione S-transferase, glutathione peroxidase and superoxide dismutase activity. Biochim. Biophys. Acta, Gen. Subj. 1780, 869–872. [DOI] [PubMed] [Google Scholar]
- Simons P. C.; Vanderjagt D. L. (1977) Purification of glutathione S-transferases from human liver by glutathione-affinity chromatography. Anal. Biochem. 82, 334–341. [DOI] [PubMed] [Google Scholar]
- Smith D. B.; Johnson K. S. (1988) Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene 67, 31–40. [DOI] [PubMed] [Google Scholar]
- Bayley H.; Knowles J. R. (1977) Photoaffinity labeling. Methods Enzymol. 46, 69–114. [DOI] [PubMed] [Google Scholar]
- Kotzybahibert F.; Kapfer I.; Goeldner M. (1995) Recent trends in photoaffinity-labeling. Angew. Chem., Int. Ed. Engl. 34, 1296–1312. [Google Scholar]
- Dorman G.; Prestwich G. D. (1994) Benzophenone photophores in biochemistry. Biochemistry 33, 5661–5673. [DOI] [PubMed] [Google Scholar]
- Uchida N.; Okuro K.; Niitani Y.; Ling X.; Ariga T.; Tomishige M.; Aida T. (2013) Photoclickable dendritic molecular glue: noncovalent-to-covalent photochemical transformation of protein hybrids. J. Am. Chem. Soc. 135, 4684–4687. [DOI] [PubMed] [Google Scholar]
- Das J. (2011) Aliphatic diazirines as photoaffinity probes for proteins: recent developments. Chem. Rev. 111, 4405–4417. [DOI] [PubMed] [Google Scholar]
- Dubinsky L.; Krom B. P.; Meijler M. M. (2012) Diazirine based photoaffinity labeling. Bioorg. Med. Chem. 20, 554–570. [DOI] [PubMed] [Google Scholar]
- Pedone E.; Brocchini S. (2006) Synthesis of two photolabile poly(ethylene glycol) derivatives for protein conjugation. React. Funct. Polym. 66, 167–176. [Google Scholar]
- Adang A. E. P.; Brussee J.; Vandergen A.; Mulder G. J. (1990) The glutathione-binding site in glutathione S-transferases - investigation of the cysteinyl, glycyl, and gamma-glutamyl domains. Biochem. J. 269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. B.; Bauman S.; Colman R. F. (1998) Photoaffinity labeling of rat liver glutathione S-transferase, 4–4, by glutathionyl S-4-(succinimidyl)-benzophenone. Biochemistry 37, 15671–15679. [DOI] [PubMed] [Google Scholar]
- Ladd D. L.; Snow R. A.. Sterling Winthrop Inc. (1995) Vinyl sulfone coupling of polyoxylalkylenes to proteins, US Patent No US5414135-A.
- Morpurgo M.; Veronese F. M.; Kachensky D.; Harris J. M. (1996) Preparation and characterization of poly(ethylene glycol) vinyl sulfone. Bioconjugate Chem. 7, 363–368. [DOI] [PubMed] [Google Scholar]
- Guo L. W.; Hajipour A. R.; Gavala M. L.; Arbabian M.; Martemyanov K. A.; Arshavsky V. Y.; Ruoho A. E. (2005) Sulfhydryl-reactive, cleavable, and radioiodinatable benzophenone photoprobes for study of protein-protein interaction. Bioconjugate Chem. 16, 685–693. [DOI] [PubMed] [Google Scholar]
- Galardy R. E.; Craig L. C.; Jamieson J. D.; Printz M. P. (1974) Photoaffinity labeling of peptide hormone binding-sites. J. Biol. Chem. 249, 3510–3518. [PubMed] [Google Scholar]
- Perols A.; Karlstrom A. E. (2014) Site-specific photoconjugation of antibodies using chemically synthesized IgG-binding domains. Bioconjugate Chem. 25, 481–488. [DOI] [PubMed] [Google Scholar]
- Yu F. F.; Jarver P.; Nygren P. A. (2013) Tailor-making a protein A-derived domain for efficient site-specific photocoupling to Fc of mouse IgG(1). PLoS One, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y. Z.; Zhang J.; Li F. F.; Xiang X.; Ren A. Q.; Liu Y. (2011) Studies on the interaction between benzophenone and bovine serum albumin by spectroscopic methods. Mol. Biol. Rep. 38, 2445–2453. [DOI] [PubMed] [Google Scholar]
- Childs C. E. (1975) Determination of polyethylene-glycol in gamma-globulin solutions. Microchem. J. 20, 190–192. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.