Abstract
The goal of the study was to elucidate the cellular and molecular mechanisms by which a clinically applicable immune tolerance regimen of combined bone marrow and heart transplants in mice results in mixed chimerism and graft acceptance. The conditioning regimen of lymphoid irradiation and anti-T cell antibodies changed the balance of cells in the lymphoid tissues to create a tolerogenic microenvironment favoring the increase of natural killer T (NKT) cells, CD4+CD25+ Tregs, and Gr-1+CD11b+ myeloid derived suppressor cells (MDSCs), over conventional T cells. The depletion of MDSCs abrogated chimerism and tolerance, and add back of these purified cells was restorative. The conditioning regimen activated the MDSCs as judged by the increased expression of arginase-1, IL-4Rα, and PDL1, and the activated cells gained the capacity to suppress the proliferation of conventional T cells to alloantigens in the mixed leukocyte reaction. MDSC activation was dependent on the presence of host invariant NKT cells. The conditioning regimen polarized the host invariant NKT cells toward IL-4 secretion, and MDSC activation was dependent on IL-4. In conclusion, there was a requirement for MDSCs for chimerism and tolerance, and their suppressive function was dependent on their interactions with NKT cells and IL-4.
Introduction
A continuing important goal in the field of organ transplantation is the induction of immune tolerance in order to eliminate the lifelong need for anti-rejection drugs and their attendant side effects. Although many tolerance induction protocols have been successfully developed in laboratory animals, only a few, using hematopoietic cell transplantation to induce chimerism and tolerance have been applied to clinical trials (1–6). The conditioning regimen of total lymphoid irradiation (TLI) used with the T cell depletive reagent, anti-thymocyte globulin/serum (ATG/ATS), has been shown to induce tolerance in mice and humans after the development of persistent mixed chimerism (3, 4, 7–10). The conditioning creates an imbalance of T cell subsets favoring immunosuppressive invariant natural killer T (NKT) cells and Treg cells over conventional T cells (8–10). The NKT and Treg cells are required for chimerism and tolerance induction, and the lack of NKT cells in Jα18−/− mice or the depletion of Treg cells in wild type mice by a single pretransplant injection of anti-CD25 mAb abrogated tolerance (9, 10). Add back of wild type purified NKT cells to Jα18−/− mice or purified wild type CD4+CD25+ T cells to Treg depleted wild type mice restored tolerance (9, 10). Furthermore, the added back host NKT cells must produce IL-4 in order to activate the host Treg cells to produce IL-10 that is also necessary for tolerance (9, 10). The TLI and ATS conditioning biases the NKT cells and Treg cells as well as Tcon cells toward secretion of these Th2 cytokines (9–11). Interestingly, after TLI based conditioning the host NKT cells can also interact with donor Treg cells in an IL-4 dependent manner to protect recipients against graft versus host disease (GVHD)(12). In the latter model, the NKT cells must first interact with host CD11c+MHCII+Gr-1lo dendritic like cells that subsequently interact with donor Tregs in a Stat-6 dependent manner (13).
In the current work, we determined whether host myeloid derived suppressor cells (MDSCs), myeloid cells identified by the Gr-1hiCD11b+ phenotype in mice (14–17) play, a required role in the development of chimerism and tolerance using the TLI and ATS conditioning regimen. A previous study of tolerance induction using a non-chimeric model of co-stimulation blockade showed that MDSCs were required for heart graft acceptance, and that the MDSCs were generated in the bone marrow and needed to traffic to the heart graft in a CCR2 dependent manner (18). In the present study, we determined whether the MDSCs must interact with NKT cells that are activated during the conditioning regimen to achieve tolerance, since previous studies of immunity to the CT26 colon tumor reported that MDSCs suppress tumor immunity after interaction with non-invariant (type II) NKT cells (19–22).
The results of the current study show that MDSCs are required for the induction of chimerism and tolerance in the TLI and ATS protocol, and that the MDSCs are activated to suppress alloreactivity by the direct or indirect interaction with host invariant (type I) NKT cells and IL-4.
MATERIALS AND METHODS
Mice
Adult 8–10-week old male BALB/c (H-2Kd), C57BL/6 (H-2Kb) and IL-4−/− (BALB/c) mice were purchased from the Jackson Laboratory (Maine). C57BL/6 neonates were obtained from Charles River Laboratories. CD1d−/− (BALB/c; H-2Kd)(23) and Ja18−/− (BALB/c; H-2Kd) (24) were bred in the Department of Comparative Medicine, Stanford University. All the animals were maintained in the department according to institutional guidelines approved by the National Institutes of Health (NIH).
Cardiac transplantation and monitoring for graft survival
Heterotopic heart transplantation was performed on day 0 using C57BL/6 neonatal hearts (one day old) transplanted into the ear pinna of anesthetized BALB/c hosts as previously described (25). Graft survival was assessed by daily palpation, and rejection by cessation of heartbeat. Heart grafts that failed within 72h were excluded from the experimental groups as “technical failures”.
TLI conditioning and Rabbit antithymocyte serum treatment
Mice were conditioned with 10 doses of 240cGy total lymphoid irradiation (TLI) using an X-ray machine (Polaris MC-500, KIMTRON inc. Woodbury, CT, USA) as described before (7–10). In addition, BALB/c hosts were also injected intraperitoneally with 5 doses of 0.05 mL of anti-thymocyte serum (ATS) in 0.5mL saline (Accurate chemical and Scientific, Westbury, NY).
Bone marrow transplantation and assessment of graft tolerance
Bone marrow harvesting and transplantation was performed as previously described (26, 27). TLI and ATS conditioned hosts (10 – 12 mice per group per experiment) were injected intravenously with an inoculum of donor C57BL/6 BM (50 × 106). Bone marrow graft acceptance was assessed by chimerism (donor - derived cells in blood) by staining PBMC with anti-H-2Kb mAb.
Reagents and immunophenotypic analysis by flow cytometry
The antibodies used for phenotypic analysis of the cells were purchased from ebiosciences: anti-PD-1 (clone: J43), anti- CD25 (clone: PC61.5), anti- CD4 (GK1.5), anti- MHCII (IA/IE), anti-H-2Kb and anti- PDL1 (clone: MIH5) or Biolegend: anti- Gr-1 (clone: RB6-8C5), CD11b (clone: M1/70), anti- TCRβ (clone: H57-597), anti- CD8, anti- CD4, ant- Tim-3 (clone: B8.2C12), and anti- mouse F4/80 (clone: BM8); and anti-Ly6C (clone: AL-21), anti- Ly6-G (clone: 1A8), anti-CD124 (IL-4Rα), or anti- arginase-1 from BD biosciences. The fluorescent conjugates used were as follows: phycoerithrin (PE), PE– CY7, FITC, allophycocyannin (APC), APC-CY7, pacific blue, peridinin-chlorophyll-cy5.5 (PerCP Cy5.5), and aqua amine (Molecular Probe, Invitrogen) for exclusion of dead cells. NKT cells were stained using CD1d tetramer (tet) obtained from the NIH Tetramer facility, Rockville, MD.
Anti-Gr-1 monoclonal antibody treatment
For the depletion studies, MDSCs (CD11b+Gr-1+) were depleted with a single intraperitoneal injection of anti-Gr-1mAb (clone: RB6-8C5)(280μg/ml) on day 13 after heart transplant and 2 days before bone marrow cell infusion on day 15. The efficacy of depletion was verified 24 and 72 hrs post injection by FACs.
Intracellular staining for the detection of arginase-1
For intracellular staining of arginase-1, cells were fixed and permeabilized with Fix/perm buffer (eBioscience), washed and then stained with anti-arginase-1 (BD biosciences), as described before (28).
Sorting of MDSCs for adoptive transfer experiments and in vitro assays
Splenocytes were enriched using anti- CD11b microbeads and MACS columns (Miltenyi), and stained with anti- CD11b (Mac-1) and Gr-1 mAbs, sorted using the FACS Aria cell sorter.
Sorting of NKT cells for adoptive transfer into IL-4−/− conditioned mice
NKT cells were enriched from the spleen of wild type untreated BALB/c mice after incubation with CD1d tetramers and purification on MACS columns before FACS sorting as described before (10).
In vitro MDSC suppression assays, (Mixed leukocyte reactions)
Single cell suspensions of enriched and sorted MDSCs cells obtained from untreated wild type (UNT WT), or TLI/ATS conditioned wild type, or Jα18−/− or CD1d−/−, or IL-4−/− hosts, 5 days after completion of conditioning were cultured in RPMI medium with 10% fetal calf serum in flat-bottomed 96-well plates (Corning, B.V.) at a density of 1 × 105 cells per well. Stimulator cells (splenocytes) were obtained from C57BL/6 or BALB/c male mice, irradiated with 3,000 rad from a 137Cs source (Shephard), and cultured at a density of 2 × 105 cells/well.
Preparations of responder cells obtained from BALB/c or C57BL/6 splenocytes were pulsed with 2.5μM CFSE (Molecular Probe, Invitrogen) and cultured at a density of 1 × 105 cells/well. The responders, stimulators and respective MDSCs were cultured at 37°C and 5% CO2 in Complete (10% FBS) RPMI medium for 5 days. To assess MDSCs mediated suppression of responder cell proliferation, cells were harvested, and stained with TCRαβ, CD4, and CD8 T cell markers versus CFSE (FITC) using FACS LSRII analyzer (BD). Data was reported as percentage of CFSEdull cells among gated CD4+ and CD8+ T cells.
In vitro culture and (IL-4, IL- 13 and IFNγ) Cytokine analysis
Invariant splenic NKT cells were purified and stimulated in vitro using polyclonal activators, phorbol myristate acetate (PMA) and ionomycin as previously described (9). The culture supernatants from the wells were harvested after 48 hours, and analyzed. The quantity of IL-4, IFNγ and IL-13 production was measured using a luminex machine (Bioplex, BioRAD).
Statistical analysis
The data were analyzed using the Mann-whitney test, and expressed as mean ± SE. The p-values of <0.05 were considered significantly different. The survival curves were analyzed using Logrank (mantel-cox) test.
Results
An imbalance favoring NKT cells, Tregs, and MDSCs over Tcon cells creates a tolerogenic microenvironment
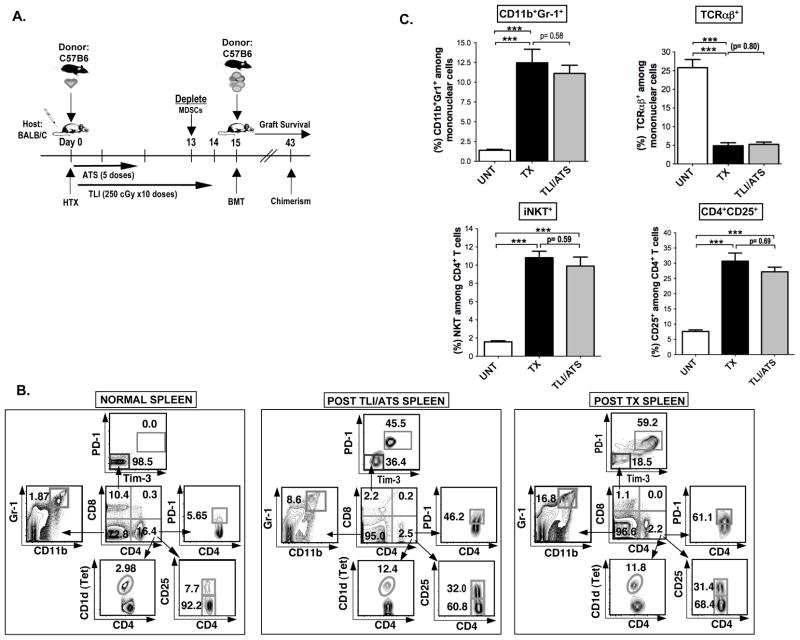
In the current study, we conditioned BALB/c mice with 10 doses of TLI of 240cGy each and 5 doses of ATS given during the first 2 weeks after C57BL/6 heart transplantation as shown in Figure 1A. Donor bone marrow cells (50×106) were injected intravenously immediately after the completion of conditioning on day 15. Figure 1B shows the changes in the composition of spleen cells in untreated control BALB/c mice as compared to experimental mice given the full transplantation regimen including infusion of bone marrow cells or experimental mice given only TLI/ATS conditioning regimen without heart or bone marrow transplantation. As shown in representative flow cytometric 2 color profiles in Figure 1B gated on all mononuclear cells, the spleen of untreated mice contained about 10% of CD8+ T cells and about 16% of CD4+ T cells. Among the CD8+ T cells almost all (98.5%) were PD-1−Tim-3−, and among the CD4+ T cells about 8% were CD25+ Treg cells, about 3% were CD1d tetramer+ NKT cells, and about 6% were PD-1+. Among the gated cells that did not stain for CD4 or CD8, about 2% were Gr-1hiCD11b+ cells that expressed the phenotype of MDSCs (14–18).
Figure 1. An imbalance favoring NKT cells, Tregs and MDSCs over Tcon cells and upregulation of negative costimulatory receptors creates a tolerogenic microenvironment after TLI/ATS conditioning.
(A) Experimental scheme: BALB/c hosts were given donor C57BL/6 neonatal heart transplants (HTX) on day 0, and ATS was injected i.p. on days 0, 2, 6, 8, and 10. Hosts were conditioned over 14 days with 10 doses of 240cGy each. Some hosts were depleted of CD11b+Gr-1+ cells (MDSCs) with a single dose of anti- Gr-1 mAb (280 μg i.p. on day13). On day 15, 50 × 106 C57BL/6 donor bone marrow cells were injected intravenously (BMT), and chimerism and heart graft survival were monitored thereafter. (B) Representative FACS patterns of showing changes in the phenotypes of CD4+ and CD8+ T cell subsets, CD4+CD25+Tregs, CD4+CD1dtetramer+NKT cells, and Gr-1+CD11b+ MDSCs in the spleen of untreated normal wild type mice, hosts conditioned with TLI and ATS without transplantation or with combined bone marrow and heart allografts (TX, SPLEEN) on day 5 after the completion of TLI. Boxes show percentages of each cell type. Arrows shows gating of cells from a box. (C) Mean (± SEM) percentages of CD11b+Gr-1+ cells, TCR αβ +, CD4+CD1dtetramer+NKT, and CD4+CD25+ cells in the spleen of untreated (UNT) controls, of TLI and ATS conditioned BALB/c (TLI/ATS), and of TLI and ATS conditioned and transplanted (TX) hosts (day 5 after injection of donor bone marrow cells) (Upper panels and lower panels respectively) (UNT, N= 8; TX, N= 10; TLI/ATS, N= 8).
After transplantation, the balance of cells changed dramatically such that the percentage of CD8+ and CD4+ T cells fell at least 5 fold to about 1 to 2% each, and the percentage of MDSCs increased about 8 fold to about 17% Figure 1B (right panel). As shown in Figure 1C, this changed the balance of T cells to MDSCs in the spleen such that the mean percentage of T cells (26%) was about 15 fold higher than MDSCs (1.5%) before transplantation, and about 2 to 3 fold lower (5% vs 12.5%) after transplantation. The changed ratios were explained by the increase in the absolute number of MDSCs in the spleen and a decrease in the absolute numbers of T cells (Supplemental Figure 1). The changed balance of MDSCs and T cells after transplantation was mainly accounted for by the TLI/ATS conditioning regimen, since the mean percentage of T cells fell to about 5% and the mean percentage of MDSCs rose to about 11% after conditioning alone (Figure 1B; middle panel; and Figure 1C). The mean percentages of Tregs, and NKT cells among CD4+ T cells rose about 5 fold to about 30% and 10% respectively after conditioning alone or transplantation (Figure 1B, and 1C) as reported before (9, 10). As expected, CD4+CD25+ Tregs expressed high levels of intracellular Foxp3 (Supplementary Figure 1C).
Additional changes after conditioning and transplantation or conditioning alone were observed for the expression of the negative co-stimulator receptors, PD-1 and Tim-3, on CD8+ T cells. Our previous study showed an increase in the mean percentage of PD-1+Tim-3+ cells among CD8+ T cells from about 1% in untreated mice to about 50% after conditioning alone or after transplantation (10). Similarly, the mean percentage of PD-1+ cells among CD4+ T cells increased from about 5% in untreated mice to about 60% in conditioned or transplanted mice (10). These changes were confirmed in the present study (Figure 1B).
In order to determine the contribution of ATS alone and TLI alone as compared to the combination in promoting an increase in the percentage of MDSCs in the spleen, PBMC, and bone marrow, each tissue was analyzed at the same time point after initiation of treatment as shown in Supplemental Figure 2. TLI alone but not ATS alone induced a significant increase in the mean percentage of MDSCs in the spleen, and there was a significant difference between the combination of TLI and ATS as compared to TLI alone. Addition of transplantation to the TLI/ATS conditioning did not result in a significant increase. The pattern observed in the spleen was also observed in the PBMC and bone marrow with the largest increase observed after TLI/ATS conditioning and transplantation, and no significant increase with ATS alone (Supplemental Figure 2).
Depletion of MDSCs abrogates tolerance to combined bone marrow and heart transplants
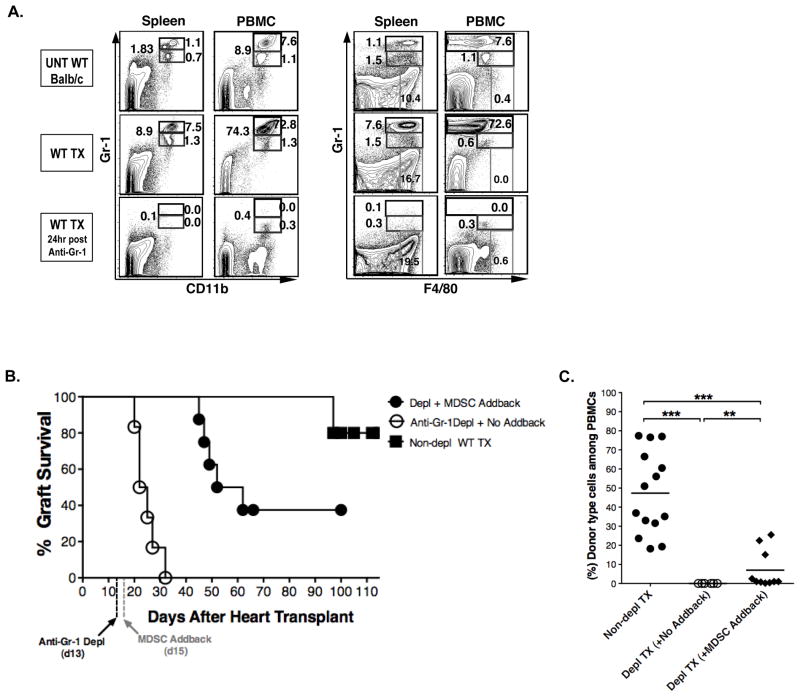
Since MDSCs have not been studied previously in our model system, we determined whether tolerance induction required MDSCs. Mice given the conditioning regimen and combined transplants were treated with an anti-Gr-1 mAb that has been reported previously to deplete MDSCs and abrogate tolerance in a non-chimeric model using co-stimulatory blockade to achieve organ graft acceptance (18). Accordingly, mice given heart transplants and postoperative TLI/ATS conditioning were given anti-Gr-1 mAb on day 13 (2 days before the injection of donor marrow cells on day 15). Figure 2A shows that the marked increase in the Gr-1hiCD11b+ MDSCs in the spleen and PBMC after heart transplantation and conditioning was markedly reduced 24 hour after the injection of mAb and less than 1% of cells were detected by 2 color staining with Gr-1 versus CD11b or Gr-1 versus F4/80.
Figure 2. Depletion of MDSCs abrogates tolerance to combined bone marrow and heart transplants.
(A) Representative spleen FACS patterns of Gr-1 versus CD11b and F4/80 staining are shown in untreated wild type mice, transplanted wild type hosts without anti- Gr-1 mAb treatment (WT TX), and transplanted wild type hosts with anti- Gr-1 mAb treatment. Patterns are also shown for peripheral blood mononuclear cells (PBMCs). Two days prior to end of TLI conditioning (day 13), BALB/c hosts were given a single injection of anti- Gr-1 mAb antibody (280 μg/ml/mouse) intraperitoneally, and spleen cells were obtained for analysis 24 hours after the injection. (B) Heart allograft survival in untreated (Non depl, WT TX, N= 10), or treated with anti- Gr-1 mAb (day 13) (Anti- Gr-1 depl + No add back, N= 8) or treated with anti- Gr-1 mAb and given an add back of sorted (1 × 106) MDSCs (CD11b+Gr-1+) cells (Depl+MDSCs add back, N= 8) from TLI/ATS conditioned wild type mice at the time of donor bone marrow infusion. (C) Shows the mean percentage of donor type cells (H-2Kb+) among peripheral blood mononuclear cells (PBMC) at day 28 after injection of donor bone marrow cells in non depleted, anti- Gr-1 depleted alone, or anti- Gr-1 depleted hosts given an add back of sorted (1 × 106) MDSCs cells. Data are from 3 independent experiments. * p< 0.05, ** p< 0.01, *** p< 0.001
The depletion of these MDSCs was also confirmed by the reduction of F4/80+Ly6G+ or F4/80+Ly6C+ cells to about 1% or less (Supplemental Figure 3) of spleen cells, since the MDSCs express the latter markers as shown in Figure 3A. The depletion of MDSCs to 1% or less persisted for 48 hours after injection of mAb, and then a gradual increase to pretreatment levels was observed starting at about 72 hours (data not shown). Figure 2B shows that the depletion of MDSCs abrogated tolerance in mice after conditioning and transplantation, since the heart grafts were uniformly rejected by day 35. None of the hosts developed chimerism by day 28 (Figure 2C). The reduction of graft survival and the percentage of donor type cells among PBMCs were significantly reduced as compared to the non-depleted controls (p<0.001).
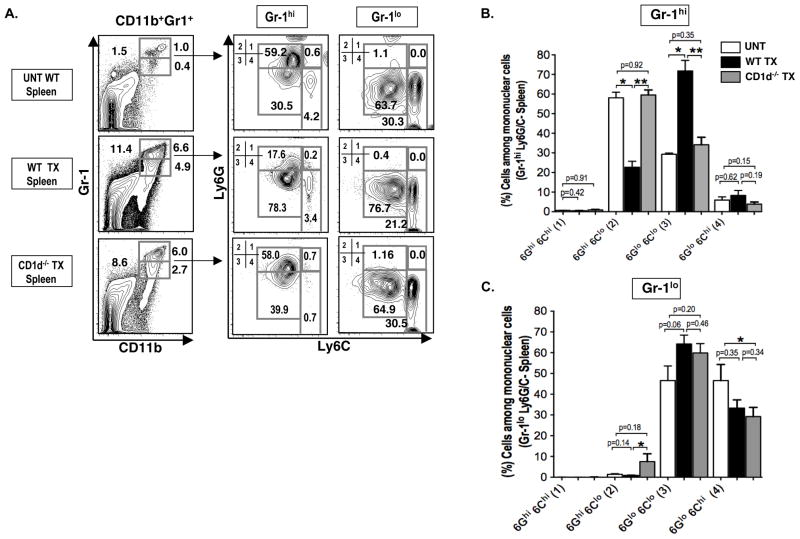
Figure 3. NKT cells regulate Ly6G expression on MDSCs after transplantation.
(A) Representative FACs staining of Ly6G versus Ly6C among CD11b+Gr-1+ cell subsets (Gr-1 hi vs Gr-1lo) in the spleen of untreated hosts (UNT WT, N= 12), or in wild type (WT TX, N= 10) or CD1d−/− hosts (CD1d−/− TX, N= 12) after conditioning and transplant. Four subgroups of Gr-1hi or Gr-1lo CD11b+ cells were enclosed in boxes labeled 1, 2, 3, and 4 with percentages shown; Ly6GhiLy6Chi (1), Ly6GhiLy6Clo (2), Ly6GloLy6Clo (3), and Ly6GloLy6Chi (4). (B) Bar graphs showing mean (± SEM) percentages of Ly6G+ Ly6C+ cell subgroups in boxes 1–4 among Gr-1hi cells. (C) Bar graphs show among Gr-1lo cells.
In further experiments, purified BALB/c MDSCs obtained from TLI/ATS conditioned mice without transplants (see Materials and Methods, and enrichment scheme in Figure 6) were added back to the depleted hosts at the time of the bone marrow cells infusion. Figure 2B shows that the add back significantly increased the heart graft survival as compared to the depleted hosts (p<0.01), and about 40% of grafts survived at least 100 days. Of 8 hosts given the added back MDSCs, 3 had donor cell chimerism of at least 10% in the PBMC as compared to the uniform failure to detect chimerism in the depleted mice without the add back (Figure 2C).
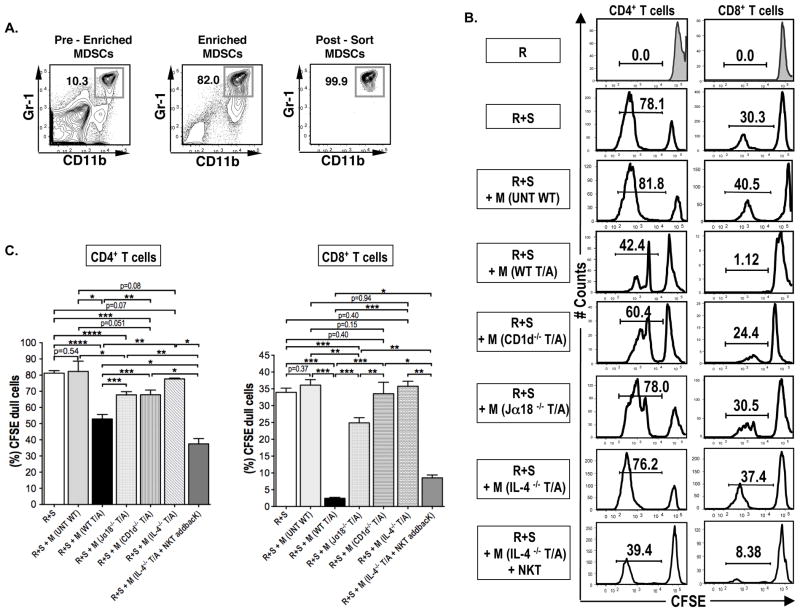
Figure 6. MDSC suppression of alloreactivity in vitro is dependent on NKT cells and IL-4.
(A) Representative FACS patterns showing the CD11b+Gr-1+ enrichment and sorting for assay of in vitro suppression in the mixed leukocyte reactions (MLR). (B) Representative CFSE histogram plots showing CD4+ and CD8+ T cell proliferation in the presence or absence of sorted BALB/c MDSCs (2 × 105) from untreated wild type mice, N= 8 (WT T/A), TLI and ATS conditioned wild type mice N=8 (UNT WT), or TLI and ATS conditioned CD1d−/− mice, N= 10 (CD1d−/− T/A), or TLI and ATS conditioned Jα18−/− mice, N= 8 (Jα18−/− T/A), or TLI and ATS conditioned IL-4−/− mice, N= 5 (IL-4−/− T/A) or TLI and ATS conditioned IL-4−/− mice given an intravenous injection of 0.5 × 106 wild type NKT cells on day 15 after completion of TLI, N= 5 (IL-4−/− T/A + NKT) in day 5 cultures. There were 2×105 C57BL/6 responders (R) cells and 4×105 BALB/c stimulator (S) cells in MLR cultures. The percentages of dull CFSE cells are shown. (C) Mean (± SEM) percentages of CFSE+ dull cells among gated CD4+ and CD8+ T cells after in vitro culture.
Activation of MDSCs after transplantation is regulated by NKT cells
In order to study the changes in surface receptors that define subsets of MDSCs during tolerance induction, the expression of Ly6G and Ly6C on Gr-1+CD11b+ MDSCs in untreated wild type BALB/c mice was compared to wild type or CD1d−/− BALB/c mice given TLI/ATS conditioning and transplants. Ly6G is highly expressed on granulocytes and Ly6C is highly expressed on monocytes (15, 29–31). Figure 3A shows that staining for Gr-1 versus CD11b in untreated mice detected a population of CD11b+ cells that could be divided into Gr-1hi and Gr-1lo subsets as shown in the boxes in the 2 color panels. The percentage of Gr-1hiCD11b+ cells increased from 1% in untreated mice to 6.6% and 6.0 % in the WT and CD1−/− treated mice respectively, and the percentage of Gr-1loCD11b+ cells increased from 0.4% to 4.9 and 2.7%. Thus, the increase in Gr-1hi and Gr-1loCD11b+ cells after transplantation occurred in the presence or absence of NKT cells. The Gr-1hi and Gr-1loCD11b+ subsets were gated and examined for expression of Ly6G and Ly6C. Among the Gr-1hiCD11b+ cells the percentage of Ly6GhiLy6Clo cells was reduced from 59% in untreated mice to about 18% after transplantation in WT but remained at 58% in CD1d−/− mice. The mean values for these cells were significantly different in the WT transplanted mice versus either the untreated or CD1d−/− treated mice (Figure 3B). There were opposite significant changes in the mean percentages of Ly6GloLy6Clo cells (Figure 3B). In contrast, there were no significant changes in the staining patterns for Ly6G or Ly6C in the spleen of untreated versus transplanted mice in the gated Gr-1loCD11b+ cells (Figure 3C). In summary, there was a down regulation of Ly6G expression among splenic MDSCs after transplantation that occured in wild type, but not in NKT cell deficient mice.
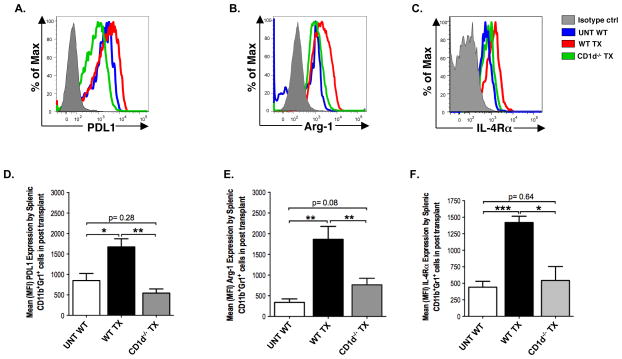
The immunosuppressive molecules PDL1 and arginase-1 are expressed by activated MDSCs in tumor infiltrates or after immune stress, and contribute to their regulatory function (32, 33). In addition, signaling via the IL-4Rα and IL-13R has been reported to activate MDSCs to secrete TGFβ and to upregulate the IL-4Rα receptor expression (22, 34). Accordingly, we compared the intensity of staining of these Gr-1+CD11b+ MDSC activation molecules in untreated WT mice versus WT and CD1d−/− transplanted mice. Figure 4A–C shows that PDL1, arginase-1, and IL-4Rα molecules were all upregulated after transplantation as judged by the increase in staining intensity in WT transplanted mice as compared to untreated mice. The mean intensity of staining was significantly increased for all 3 molecules after transplantation (Figure 4D–F). However, when CD1d−/− transplanted mice were studied instead of WT transplanted mice, then there were no significant increases (p>0.05)(Figure 4D–F).
Figure 4. Upregulation of PDL1, Arginase-1, and IL-4Rα on MDSCs after transplantation is dependent on NKT cells.
(A–C) Representative histogram plots showing the expression of PDL1, arg-1 (arginase-1), and IL-4Rα (interleukin- 4 receptor alpha) on gated CD11b+Gr-1+ cells from untreated wild type mice (UNT WT, N= 8), or transplanted wild type mice (WT TX, N= 10), or transplanted CD1d−/− mice (CD1d−/− TX, N= 12) 12 days after bone marrow infusion. (D –F) Bar graphs showing means (± SEM) of mean fluorescence intensity (MFI) measurements of PDL-1, arg-1, and IL-4Rα on CD11b+Gr-1+ cells in spleen.
The increase in MDSCs after transplantation is transient
We examined the kinetics of the increase in Gr-1hi and Gr-lloCD11b+ cells in the spleen and blood at serial time points before and after combined bone marrow and heart transplantation. Figure 5A shows that the Gr-1hiCD11b+ spleen cells increased sharply to a peak of about 12% about 10 days after the infusion of donor bone marrow cells, and then declined slowly thereafter, such that their percentage returned to the pre-treatment levels by 100 days after heart transplantation. Donor cells were not detected among the MDSCs at the peak, but were easily detected by day 28 after marrow infusion (data not shown). The Gr-llo cells followed the kinetics of the Gr-1hi cells. Interestingly, the sharp rise in the Gr-1hi cells in the blood preceded the infusion of the donor bone marrow cells, peaked at about 5 days after the infusion, and gradually declined to pretreatment levels about 100 days after heart transplantation (Figure 5B). Interestingly, the upregulation of PDL-1, and IL-4Rα declined to baseline by day 43 on CD11b+Gr-1hi cells in concert with the decline in the number of CD11b+Gr-1hi cells in the blood (Supplementary Figure 6).
Figure 5. The increase in MDSCs after transplantation is transient.
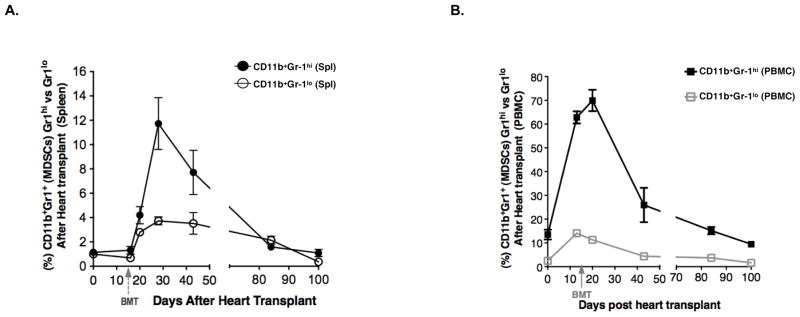
(A–B) The mean (± SEM) percentages of CD11b+Gr-1hi and CD11b+Gr-1lo in the spleen (A) and among PBMCs (B) of TLI/ATS conditioned and transplanted wild type hosts at different time points (CD11b+Gr-1hi, N= 15; CD11b+Gr-1lo, N= 15). Data represents 5 independent experiments.
MDSC suppression of alloreactivity in vitro is dependent on NKT cells
In order to confirm that the cells expressing the Gr-1hiCD11b+ phenotype in the spleen after transplantation are able to suppress alloreactivity, the Gr-1hiCD11b+ splenic cells were purified as shown in Figure 6A, and added to responder and irradiated allogeneic stimulator spleen cells in the mixed leukocyte reaction. Responder cells were labeled with CFSE, and the dilution of this dye was used as an indicator of cell division. MDSCs were obtained from untreated mice, and from mice conditioned with TLI/ATS without transplants in order to determine whether the conditioning regimen alone induces MDSCs with alloreactive suppressor function. In additional experiments, MDSCs were harvested from either conditioned BALB/c CD1d−/− or Jα18−/− mice. Whereas the CD1d−/− mice lacked both invariant (type I) NKT cells and non-invariant (type II) NKT cells, the Jα18−/− mice were lacking only invariant NKT cells (23, 24, 35, 36). Experiments were performed with BALB/c responder cells in Supplemental Figure 4 or C57BL/6 responder cells in Figure 6B, since MDSCs have been reported to suppress alloreactivity without MHC restriction (17).
The bright staining of CFSE in BALB/c or C57BL/6 WT responders cells cultured alone was markedly reduced, as expected in representative histograms, after the addition of allogeneic stimulator cells due to proliferation of responder cells that diluted the CFSE content per cell (Supplemental Figure 4 and Figure 6B). Whereas less than 1% of C57BL/6 responder cells alone expressed dull CFSE staining, the addition of stimulator cells resulted in about 78% and 30% of dull staining cells among gated CD4+ and CD8+ T cells respectively (Figure 6B). The addition of MDSCs from untreated mice to the cultures did not result in significant reduction in the percent of dull CFSE cells (Figure 6B and 6C). Addition of MDSCs from conditioned WT mice resulted in a reduction in dull staining CD8+ T cells to about 1%, and a reduction of CD4+ dull staining cells to about 42% (Figure 6B). The reduction in the mean percentages of dull staining cells after the addition of the latter MDSCs was statistically significant, and more profound with CD8+ T cells than with CD4+ T cells (Figure 6C). Similar results were observed with C57BL/6 or BALB/c responder cells (Supplemental Figure 4).
The in vitro experiments were repeated adding MDSCs from conditioned CD1d−/− and Jα18−/− mice to the cultures of responder and stimulator cells. As shown in staining profiles in Figure 6B, and in comparisons of means in Figure 6C, the potency of suppression of proliferation was significantly reduced when MDSCs were obtained from CD1d−/− or Jα18−/− NKT cell deficient mice instead of from WT mice. In summary, the MDSC suppression of proliferation of responder cells to alloantigen stimulation that developed after conditioning was dependent on the presence of invariant NKT cells in the conditioned mice, and was more profound with CD8+ responders.
Since the suppressive capacity of MDSCs in the current study was dependent on the contribution of invariant NKT cells, we determined whether the invariant NKT cells from conditioned hosts secrete IL-13, IFNγ and/or IL-4 after in vitro stimulation. Invariant NKT cells from conditioned mice were sorted after staining positively with CD1d tetramers, stimulated in vitro with PMA and ionomycin, and supernatants were assayed for the concentrations of IL-4, IFNγ, and IL-13. As shown in Supplemental Figure 4, the mean concentration of IL-4 in the supernatants from stimulated NKT cells was about 10,000 pg/ml. The mean concentrations for IL-13 and for IFNγ were less than 500 pg/ml and similar to concentrations from non-stimulated NKT cells. Accordingly, we tested the ability of MDSCs from IL-4−/− conditioned mice to reduce proliferation in the mixed leukocyte reaction. As shown in Figure 6B and C, the addition of the IL-4−/− MDSCs failed to significantly suppress proliferation as compared to cultures without MDSCs. In further experiments, purified NKT cells from wild type mice were injected into IL-4−/− conditioned mice in order to determine whether the injected NKT cells can restore the suppressive function of the MDSCs. Figure 6B and C shows that the MDSCs from the latter mice had significantly increased suppressive function as compared to IL-4−/− mice without the cell injection. It is of interest that sorted wild type MDSCs that suppressed the MLR expressed low levels of MHC Class II receptors (Supplementary Figure 4C), and did not show the dendritic cell characteristics of the myeloid cells that interacted with donor Tregs in the study by Van der Merwe et al. (13)
Discussion
The goal of the current study was to determine whether a direct or indirect interaction between host NKT cells and MDSCs was a requirement to induce tolerance to combined bone marrow and heart transplants using posttransplant conditioning with TLI and ATS. The conditioning created an imbalance favoring immune suppressive cells and established a “tolerogenic microenvironment” that had features similar to that reported in tumor infiltrating immune cells (11, 37–40). Hosts depleted of MDSCs could no longer be tolerized, and instead failed to achieve chimerism and heart graft acceptance. Tolerance could be restored to a portion of hosts depleted of MDSCs by treatment with anti-Gr-1 mAb by adding back-purified Gr-1+CD11b+ MDSCs from the spleen of syngeneic BALB/c mice that had been conditioned with TLI and ATS.
A previous study showed that MDSCs are required for the induction of tolerance to allografts in mice treated with co-stimulatory blockade using anti-CD40L mAb to promote heart graft acceptance in a non-chimeric model (18). The role of NKT cells was not investigated. The current study is consistent with the latter study, and suggests that the TLI alone or in combination with ATS conditioning regimen stimulated the generation and efflux of MDSCs from the marrow, and that the host MDSCs contributed to the engraftment of the donor hematopoietic progenitors during the transient release of MDSCs from the host marrow. Tolerance and chimerism were maintained after the percentage of MDSC returned to baseline about 3 months after transplantation. This suggests that the induction but not the persistence of tolerance was dependent on MDSCs. Local tumor irradiation also induces the rapid efflux of myeloid cells from the bone marrow into the tumors (41).
After the TLI and ATS conditioning regimen, the MDSCs developed an activated phenotype with increased expression of PDL-1, arginase-1, and IL-4Rα. The PDL-1 can suppress immune responses of activated conventional T cells by inducing negative co-stimulation via PD-1, and arginase-1 can suppress by increasing superoxide production and by reducing the availability of arginine that is required for T cell metabolism (34, 42–45). The increased expression of activation markers on the MDSCs after conditioning was dependent upon the presence of NKT cells, and these changes were markedly attenuated in CD1d−/− as compared to wild type mice. This attenuation was associated with a failure of MDSCs from CD1d−/− or Jα18−/− mice lacking invariant NKT cells to suppress the ability of conventional CD4+ and CD8+ T cells to proliferate in response to alloantigenic stimulation in the MLR assay. In view of the reported role of IL-13 in the activation of MDSCs (19, 20, 46), and the requirement for invariant NKT cells in the current study of tolerance, the invariant NKT cells obtained from the spleen of TLI and ATS conditioned mice were stimulated in vitro to induce cytokine secretion. Whereas a large quantity of IL-4 was secreted, IL-13 and IFNγ were not easily detected. Accordingly, we compared the suppressive function of the MDSCs from conditioned wild type and IL-4−/− mice in the MLR assay, and found that the IL-4−/− MDSCs failed to suppress. Injection of wild type NKT cells into IL-4−/− conditioned mice restored the MDSC suppressive function. Although we did not study the role of MDSCs in the injected donor bone marrow cells, it is possible that the host NKT cells could also activate donor MDSCs in addition to activating donor Tregs (12, 14) such that the activated donor MDSCs contribute to the induction of tolerance. In conclusion, the establishment of chimerism and tolerance required activated host MDSCs, and their activation and suppressive function was dependent on a direct or indirect interaction with NKT cells and IL-4.
Supplementary Material
Supplementary Figure 1: TLI/ATS conditioning changes the absolute numbers of CD11b+Gr-1+, and TCRαβ+cells, and increases the percentage of CD4+CD25+FoxP3+ Tregs in the spleen. (A–B) Absolute numbers (horizontal lines show means) of CD11b+Gr-1+, and TCRαβ+ cells in the spleens of untreated (UNT) controls, of TLI and ATS conditioned BALB/c mice (TLI/ATS), and of TLI and ATS conditioned and transplanted (TX) BALB/c mice (day 5 after injection of donor bone marrow cells) (UNT, N= 8; TX, N= 10; TLI/ATS, N= 8). (C) Representative FACS patterns showing the expression of intracellular Foxp3 by the increased percentage of CD4+CD25+ cells in the spleen of hosts conditioned with TLI and ATS on day 5 after completion of TLI. Arrow shows gating of cells from a box.
Supplementary Figure 2: TLI synergizes with ATS treatment to increase the percentage of MDSCs in lymphoid tissues. Data shows the relative contribution of each or combination of conditioning treatments on the percentage of MDSCs in the spleen, PBMCs and bone marrow (BM) of untreated (UNT WT, N= 10), and or ATS (ATS, N= 8), or TLI (TLI, N= 12), or ATS and TLI (TLI/ATS, N= 12), or ATS and TLI treated hosts given heart and BM transplants (TLI/ATS/TX, N= 12). (A). Representative FACS plots of percent CD11b+Gr-1hi and CD11b+Gr-1lo cells among spleen, PBMC and BM cells given conditioning regimens shown, 5 days after each treatment. (B) Mean (± SEM) percentages of CD11b+Gr-1+ (combined Gr-1hi and Gr-1lo) cells among spleen, PBMCs, and BM in groups shown in (A).
Supplementary Figure 3: Depletion of Ly6G+ and Ly6C+F4/80+ cells after a single injection of anti-Gr-1 mAb. Representative FACS plots show marked increases in the percentage of Ly6G+F4/80+ and Ly6C+F4/80+ cells in the spleen and PBMC 5 days after completion of conditioning and transplantation as compared to untreated WT BALB/c mice. Depletion of the (Ly6G+), and (Ly6C+) cells is shown 24 hours after the transplant hosts were given a single injection of anti-Gr-1 mAb.
Supplementary Figure 4: Sorted Gr-1hiCD11b+ cells from TLI/ATS conditioned BALB/c mice but not from untreated mice suppress proliferation in mixed lymphocyte cultures with BALB/c responder cells and C57BL/6 stimulator cells. (A) Representative CFSE histogram plots showing CD4+ and CD8+ T cell proliferation in the presence or absence of sorted BALB/c MDSCs (2 × 105) from untreated BALB/c (UNT WT, N= 7) or TLI and ATS conditioned wild type mice (WT-T/A, N= 8) in day 5 cultures. There were 2×105 BALB/c responders (R) cells and 4×105 C57BL/6 stimulator (S) cells in MLR cultures. The percentages of dull CFSE cells are shown. (B) Mean (± SEM) percentages of CFSE+ dull cells among gated CD4+ and CD8+ T cells after in vitro culture. (C) Representative FACS pattern showing the percentage of gated Gr-1hiCD11b+ cells that are MHC Class IIhi cells in the spleen of TLI/ATS conditioned mice 5 days after completion of TLI. Arrow shows gating of Gr-1hiCD11b+ cells.
Supplementary Figure 5: Sorted NKT cells from TLI/ATS conditioned BALB/c mice secrete high levels of IL-4 but not IL-13 or IFNγ after in vitro stimulation. (A) Representative FACS patterns showing staining of TCRαβ versus CD4 and CD1dtetramer of enriched and sorted NKT cells. (B) Data showing the concentrations of IL-4, IL-13, and IFNγ by CD4+NKT cells stimulated in vitro. Cells were harvested from TLI and ATS conditioned BALB/c mice 5 days after completion of TLI and ATS conditioning. Sorted CD4+NKT cells were stimulated in vitro using PMA/ionomycin and cultured at 37°C and 5% CO2 in Complete (10% FBS) RPMI medium for 5 days. Analysis of supernatants obtained 48 hours after cell culture using Luminex showed significant levels of IL-4 but not IL-13, and lower levels of IFNγ production (N= 8).
Supplementary Figure 6: The upregulation of PDL-1, and IL-4Rα declines significantly on CD11b+Gr-1hi cells in concert with the decline in the number of CD11b+Gr-1hi cells in the blood. (A–B) Representative histogram plots showing the expression of PDL1, and IL-4Rα (interleukin-4 receptor alpha) on gated CD11b+Gr-1hi cells from untreated wild type mice (UNT WT, N= 8), or transplanted wild type mice (WT TX, N= 10), on days 20 and 43 after heart transplantation. (C–D) Bar graphs showing means (± SEM) of mean fluorescence intensity (MFI) measurements of PDL-1 and IL-4Rα on CD11b+Gr-1hi cells in spleen.
Acknowledgments
Grant Support: This work was supported by grants from the National Institutes of Allergy and Infectious Diseases (RO1AI-037683), National Cancer Institute (PO1CA-4960523), and National Heart, Lung, and Blood Institute (PO1HL- 075462)
We thank Glenna Letsinger for assistance in the submission of the manuscript. This work was supported by grants from the National Institutes of Allergy and Infectious Diseases (RO1- AI-037683), National Cancer Institute (PO1- CA-4960523), and National Heart, Lung, and Blood Institute (PO1HL- 075462). In addition, we also thank the NIH Tetramer Facility, Rockville, MD, for providing the CD1d-tetramer, as well as Jeanette Baker (BMT Division), and Bianca Gomez (Stanford FACS Facility) for their assistance. We thank Glenna Letsinger for assistance in the submission of the manuscript. In addition, we also thank the NIH tetramer facility, Rockville Md., for providing CD1d-tetramer.
Abbreviations
- TLI
Total lymphoid irradiation
- NKT
Natural Killer T cells
- ATS
Anti-thymocyte serum
- MDSCs
Myeloid-derived suppressor cells
Footnotes
Author Contributions
D. Hongo designed and performed research, contributed vital analytical methods, collected, analyzed and interpreted data, and wrote the manuscript; X. Tang purified MDSCs cells and helped perform adoptive transfer experiments; J. Baker helped perform in vitro experiments; E. G. Engleman helped with research design; S. Strober provided overall research supervision and wrote the manuscript.
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
Additional Supporting Information may be found in the online version of this article.
References
- 1.Kawai T, Cosimi AB, Spitzer TR, Tolkoff-Rubin N, Suthanthiran M, Saidman SL, et al. HLA-mismatched renal transplantation without maintenance immunosuppression. N Engl J Med. 2008;358(4):353–361. doi: 10.1056/NEJMoa071074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Farris AB, Taheri D, Kawai T, Fazlollahi L, Wong W, Tolkoff-Rubin N, et al. Acute renal endothelial injury during marrow recovery in a cohort of combined kidney and bone marrow allografts. Am J Transplant. 2011;11(7):1464–1477. doi: 10.1111/j.1600-6143.2011.03572.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scandling JD, Busque S, Dejbakhsh-Jones S, Benike C, Millan MT, Shizuru JA, et al. Tolerance and chimerism after renal and hematopoietic-cell transplantation. N Engl J Med. 2008;358(4):362–368. doi: 10.1056/NEJMoa074191. [DOI] [PubMed] [Google Scholar]
- 4.Scandling JD, Busque S, Dejbakhsh-Jones S, Benike C, Sarwal M, Millan MT, et al. Tolerance and withdrawal of immunosuppressive drugs in patients given kidney and hematopoietic cell transplants. Am J Transplant. 2012;12(5):1133–1145. doi: 10.1111/j.1600-6143.2012.03992.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leventhal J, Abecassis M, Miller J, Gallon L, Ravindra K, Tollerud DJ, et al. Chimerism and tolerance without GVHD or engraftment syndrome in HLA-mismatched combined kidney and hematopoietic stem cell transplantation. Sci Transl Med. 2012;4(124):124ra128. doi: 10.1126/scitranslmed.3003509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leventhal J, Abecassis M, Miller J, Gallon L, Tollerud D, Elliott MJ, et al. Tolerance induction in HLA disparate living donor kidney transplantation by donor stem cell infusion: durable chimerism predicts outcome. Transplantation. 2013;95(1):169–176. doi: 10.1097/TP.0b013e3182782fc1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hayamizu K, Lan F, Huie P, Sibley RK, Strober S. Comparison of chimeric and non-chimeric tolerance using posttransplant total lymphoid irradiation: cytokine expression and chronic rejection. Transplantation. 1999;68(7):1036–1044. doi: 10.1097/00007890-199910150-00023. [DOI] [PubMed] [Google Scholar]
- 8.Lan F, Hayamizu K, Strober S. Cyclosporine facilitates chimeric and inhibits nonchimeric tolerance after posttransplant total lymphoid irradiation. Transplantation. 2000;69(4):649–655. doi: 10.1097/00007890-200002270-00029. [DOI] [PubMed] [Google Scholar]
- 9.Nador RG, Hongo D, Baker J, Yao Z, Strober S. The changed balance of regulatory and naive T cells promotes tolerance after TLI and anti-T-cell antibody conditioning. Am J Transplant. 2010;10(2):262–272. doi: 10.1111/j.1600-6143.2009.02942.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hongo D, Tang X, Dutt S, Nador RG, Strober S. Interactions between NKT cells and Tregs are required for tolerance to combined bone marrow and organ transplants. Blood. 2012;119(6):1581–1589. doi: 10.1182/blood-2011-08-371948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lan F, Zeng D, Higuchi M, Huie P, Higgins JP, Strober S. Predominance of NK1. 1+TCR alpha beta+ or DX5+TCR alpha beta+ T cells in mice conditioned with fractionated lymphoid irradiation protects against graft-versus-host disease: “natural suppressor” cells. J Immunol. 2001;167(4):2087–2096. doi: 10.4049/jimmunol.167.4.2087. [DOI] [PubMed] [Google Scholar]
- 12.Pillai AB, George TI, Dutt S, Strober S. Host natural killer T cells induce an interleukin-4-dependent expansion of donor CD4+CD25+Foxp3+ T regulatory cells that protects against graft-versus-host disease. Blood. 2009;113(18):4458–4467. doi: 10.1182/blood-2008-06-165506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van der Merwe M, Abdelsamed HA, Seth A, Ong T, Vogel P, Pillai AB. Recipient myeloid-derived immunomodulatory cells induce PD-1 ligand-dependent donor CD4+Foxp3+ regulatory T cell proliferation and donor-recipient immune tolerance after murine nonmyeloablative bone marrow transplantation. J Immunol. 2013;191(11):5764–5776. doi: 10.4049/jimmunol.1302191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gallina G, Dolcetti L, Serafini P, De Santo C, Marigo I, Colombo MP, et al. Tumors induce a subset of inflammatory monocytes with immunosuppressive activity on CD8+ T cells. J Clin Invest. 2006;116(10):2777–2790. doi: 10.1172/JCI28828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang B, Pan PY, Li Q, Sato AI, Levy DE, Bromberg J, et al. Gr-1+CD115+ immature myeloid suppressor cells mediate the development of tumor-induced T regulatory cells and T-cell anergy in tumor-bearing host. Cancer Res. 2006;66(2):1123–1131. doi: 10.1158/0008-5472.CAN-05-1299. [DOI] [PubMed] [Google Scholar]
- 16.Dolcetti L, Peranzoni E, Ugel S, Marigo I, Fernandez Gomez A, Mesa C, et al. Hierarchy of immunosuppressive strength among myeloid-derived suppressor cell subsets is determined by GM-CSF. Eur J Immunol. 2010;40(1):22–35. doi: 10.1002/eji.200939903. [DOI] [PubMed] [Google Scholar]
- 17.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9(3):162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garcia MR, Ledgerwood L, Yang Y, Xu J, Lal G, Burrell B, et al. Monocytic suppressive cells mediate cardiovascular transplantation tolerance in mice. J Clin Invest. 2010;120(7):2486–2496. doi: 10.1172/JCI41628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Terabe M, Matsui S, Noben-Trauth N, Chen H, Watson C, Donaldson DD, et al. NKT cell-mediated repression of tumor immunosurveillance by IL-13 and the IL-4R-STAT6 pathway. Nat Immunol. 2000;1(6):515–520. doi: 10.1038/82771. [DOI] [PubMed] [Google Scholar]
- 20.Terabe M, Matsui S, Park JM, Mamura M, Noben-Trauth N, Donaldson DD, et al. Transforming growth factor-beta production and myeloid cells are an effector mechanism through which CD1d-restricted T cells block cytotoxic T lymphocyte-mediated tumor immunosurveillance: abrogation prevents tumor recurrence. J Exp Med. 2003;198(11):1741–1752. doi: 10.1084/jem.20022227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Terabe M, Swann J, Ambrosino E, Sinha P, Takaku S, Hayakawa Y, et al. A nonclassical non-Valpha14Jalpha18 CD1d-restricted (type II) NKT cell is sufficient for down-regulation of tumor immunosurveillance. J Exp Med. 2005;202(12):1627–1633. doi: 10.1084/jem.20051381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Izhak L, Ambrosino E, Kato S, Parish ST, O’Konek JJ, Weber H, et al. Delicate balance among three types of T cells in concurrent regulation of tumor immunity. Cancer Res. 2013;73(5):1514–1523. doi: 10.1158/0008-5472.CAN-12-2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smiley ST, Kaplan MH, Grusby MJ. Immunoglobulin E production in the absence of interleukin-4-secreting CD1-dependent cells. Science. 1997;275(5302):977–979. doi: 10.1126/science.275.5302.977. [DOI] [PubMed] [Google Scholar]
- 24.Cui J, Shin T, Kawano T, Sato H, Kondo E, Toura I, et al. Requirement for Valpha14 NKT cells in IL-12-mediated rejection of tumors. Science. 1997;278(5343):1623–1626. doi: 10.1126/science.278.5343.1623. [DOI] [PubMed] [Google Scholar]
- 25.Trager DK, Banks BA, Rosenbaum GE, Holm BI, Shizuru JA, Strober S, et al. Cardiac allograft prolongation in mice treated with combined posttransplantation total-lymphoid irradiation and anti-L3T4 antibody therapy. Transplantation. 1989;47(4):587–591. doi: 10.1097/00007890-198904000-00004. [DOI] [PubMed] [Google Scholar]
- 26.Lan F, Zeng D, Higuchi M, Higgins JP, Strober S. Host conditioning with total lymphoid irradiation and antithymocyte globulin prevents graft-versus-host disease: the role of CD1-reactive natural killer T cells. Biol Blood Marrow Transplant. 2003;9(6):355–363. doi: 10.1016/s1083-8791(03)00108-3. [DOI] [PubMed] [Google Scholar]
- 27.Higuchi M, Zeng D, Shizuru J, Gworek J, Dejbakhsh-Jones S, Taniguchi M, et al. Immune tolerance to combined organ and bone marrow transplants after fractionated lymphoid irradiation involves regulatory NK T cells and clonal deletion. J Immunol. 2002;169(10):5564–5570. doi: 10.4049/jimmunol.169.10.5564. [DOI] [PubMed] [Google Scholar]
- 28.Shirota Y, Shirota H, Klinman DM. Intratumoral injection of CpG oligonucleotides induces the differentiation and reduces the immunosuppressive activity of myeloid-derived suppressor cells. J Immunol. 2012;188(4):1592–1599. doi: 10.4049/jimmunol.1101304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gumley TP, McKenzie IF, Sandrin MS. Tissue expression, structure and function of the murine Ly-6 family of molecules. Immunol Cell Biol. 1995;73(4):277–296. doi: 10.1038/icb.1995.45. [DOI] [PubMed] [Google Scholar]
- 30.Youn JI, Nagaraj S, Collazo M, Gabrilovich DI. Subsets of myeloid-derived suppressor cells in tumor-bearing mice. J Immunol. 2008;181(8):5791–5802. doi: 10.4049/jimmunol.181.8.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ochando JC, Chen SH. Myeloid-derived suppressor cells in transplantation and cancer. Immunol Res. 2012;54(1–3):275–285. doi: 10.1007/s12026-012-8335-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Basso D, Fogar P, Falconi M, Fadi E, Sperti C, Frasson C, et al. Pancreatic tumors and immature immunosuppressive myeloid cells in blood and spleen: role of inhibitory co-stimulatory molecules PDL1 and CTLA4. An in vivo and in vitro study. PLoS One. 2013;8(1):e54824. doi: 10.1371/journal.pone.0054824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rodriguez PC, Ochoa AC. Arginine regulation by myeloid derived suppressor cells and tolerance in cancer: mechanisms and therapeutic perspectives. Immunol Rev. 2008;222:180–191. doi: 10.1111/j.1600-065X.2008.00608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bronte V, Serafini P, De Santo C, Marigo I, Tosello V, Mazzoni A, et al. IL-4-induced arginase 1 suppresses alloreactive T cells in tumor-bearing mice. J Immunol. 2003;170(1):270–278. doi: 10.4049/jimmunol.170.1.270. [DOI] [PubMed] [Google Scholar]
- 35.Brigl M, Brenner MB. CD1: antigen presentation and T cell function. Annu Rev Immunol. 2004;22:817–890. doi: 10.1146/annurev.immunol.22.012703.104608. [DOI] [PubMed] [Google Scholar]
- 36.Kronenberg M. Toward an understanding of NKT cell biology: progress and paradoxes. Annu Rev Immunol. 2005;23:877–900. doi: 10.1146/annurev.immunol.23.021704.115742. [DOI] [PubMed] [Google Scholar]
- 37.Blank C, Brown I, Marks R, Nishimura H, Honjo T, Gajewski TF. Absence of programmed death receptor 1 alters thymic development and enhances generation of CD4/CD8 double-negative TCR-transgenic T cells. J Immunol. 2003;171(9):4574–4581. doi: 10.4049/jimmunol.171.9.4574. [DOI] [PubMed] [Google Scholar]
- 38.Hamanishi J, Mandai M, Iwasaki M, Okazaki T, Tanaka Y, Yamaguchi K, et al. Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proc Natl Acad Sci U S A. 2007;104(9):3360–3365. doi: 10.1073/pnas.0611533104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Konishi J, Yamazaki K, Azuma M, Kinoshita I, Dosaka-Akita H, Nishimura M. B7-H1 expression on non-small cell lung cancer cells and its relationship with tumor-infiltrating lymphocytes and their PD-1 expression. Clin Cancer Res. 2004;10(15):5094–5100. doi: 10.1158/1078-0432.CCR-04-0428. [DOI] [PubMed] [Google Scholar]
- 40.Sakuishi K, Apetoh L, Sullivan JM, Blazar BR, Kuchroo VK, Anderson AC. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J Exp Med. 2010;207(10):2187–2194. doi: 10.1084/jem.20100643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ahn GO, Brown JM. Matrix metalloproteinase-9 is required for tumor vasculogenesis but not for angiogenesis: role of bone marrow-derived myelomonocytic cells. Cancer Cell. 2008;13(3):193–205. doi: 10.1016/j.ccr.2007.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Keir ME, Liang SC, Guleria I, Latchman YE, Qipo A, Albacker LA, et al. Tissue expression of PD-L1 mediates peripheral T cell tolerance. J Exp Med. 2006;203(4):883–895. doi: 10.1084/jem.20051776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bronte V, Zanovello P. Regulation of immune responses by L-arginine metabolism. Nat Rev Immunol. 2005;5(8):641–654. doi: 10.1038/nri1668. [DOI] [PubMed] [Google Scholar]
- 45.Wells AD, Li XC, Li Y, Walsh MC, Zheng XX, Wu Z, et al. Requirement for T-cell apoptosis in the induction of peripheral transplantation tolerance. Nat Med. 1999;5(11):1303–1307. doi: 10.1038/15260. [DOI] [PubMed] [Google Scholar]
- 46.Highfill SL, Rodriguez PC, Zhou Q, Goetz CA, Koehn BH, Veenstra R, et al. Bone marrow myeloid-derived suppressor cells (MDSCs) inhibit graft-versus-host disease (GVHD) via an arginase-1-dependent mechanism that is up-regulated by interleukin-13. Blood. 2010;116(25):5738–5747. doi: 10.1182/blood-2010-06-287839. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1: TLI/ATS conditioning changes the absolute numbers of CD11b+Gr-1+, and TCRαβ+cells, and increases the percentage of CD4+CD25+FoxP3+ Tregs in the spleen. (A–B) Absolute numbers (horizontal lines show means) of CD11b+Gr-1+, and TCRαβ+ cells in the spleens of untreated (UNT) controls, of TLI and ATS conditioned BALB/c mice (TLI/ATS), and of TLI and ATS conditioned and transplanted (TX) BALB/c mice (day 5 after injection of donor bone marrow cells) (UNT, N= 8; TX, N= 10; TLI/ATS, N= 8). (C) Representative FACS patterns showing the expression of intracellular Foxp3 by the increased percentage of CD4+CD25+ cells in the spleen of hosts conditioned with TLI and ATS on day 5 after completion of TLI. Arrow shows gating of cells from a box.
Supplementary Figure 2: TLI synergizes with ATS treatment to increase the percentage of MDSCs in lymphoid tissues. Data shows the relative contribution of each or combination of conditioning treatments on the percentage of MDSCs in the spleen, PBMCs and bone marrow (BM) of untreated (UNT WT, N= 10), and or ATS (ATS, N= 8), or TLI (TLI, N= 12), or ATS and TLI (TLI/ATS, N= 12), or ATS and TLI treated hosts given heart and BM transplants (TLI/ATS/TX, N= 12). (A). Representative FACS plots of percent CD11b+Gr-1hi and CD11b+Gr-1lo cells among spleen, PBMC and BM cells given conditioning regimens shown, 5 days after each treatment. (B) Mean (± SEM) percentages of CD11b+Gr-1+ (combined Gr-1hi and Gr-1lo) cells among spleen, PBMCs, and BM in groups shown in (A).
Supplementary Figure 3: Depletion of Ly6G+ and Ly6C+F4/80+ cells after a single injection of anti-Gr-1 mAb. Representative FACS plots show marked increases in the percentage of Ly6G+F4/80+ and Ly6C+F4/80+ cells in the spleen and PBMC 5 days after completion of conditioning and transplantation as compared to untreated WT BALB/c mice. Depletion of the (Ly6G+), and (Ly6C+) cells is shown 24 hours after the transplant hosts were given a single injection of anti-Gr-1 mAb.
Supplementary Figure 4: Sorted Gr-1hiCD11b+ cells from TLI/ATS conditioned BALB/c mice but not from untreated mice suppress proliferation in mixed lymphocyte cultures with BALB/c responder cells and C57BL/6 stimulator cells. (A) Representative CFSE histogram plots showing CD4+ and CD8+ T cell proliferation in the presence or absence of sorted BALB/c MDSCs (2 × 105) from untreated BALB/c (UNT WT, N= 7) or TLI and ATS conditioned wild type mice (WT-T/A, N= 8) in day 5 cultures. There were 2×105 BALB/c responders (R) cells and 4×105 C57BL/6 stimulator (S) cells in MLR cultures. The percentages of dull CFSE cells are shown. (B) Mean (± SEM) percentages of CFSE+ dull cells among gated CD4+ and CD8+ T cells after in vitro culture. (C) Representative FACS pattern showing the percentage of gated Gr-1hiCD11b+ cells that are MHC Class IIhi cells in the spleen of TLI/ATS conditioned mice 5 days after completion of TLI. Arrow shows gating of Gr-1hiCD11b+ cells.
Supplementary Figure 5: Sorted NKT cells from TLI/ATS conditioned BALB/c mice secrete high levels of IL-4 but not IL-13 or IFNγ after in vitro stimulation. (A) Representative FACS patterns showing staining of TCRαβ versus CD4 and CD1dtetramer of enriched and sorted NKT cells. (B) Data showing the concentrations of IL-4, IL-13, and IFNγ by CD4+NKT cells stimulated in vitro. Cells were harvested from TLI and ATS conditioned BALB/c mice 5 days after completion of TLI and ATS conditioning. Sorted CD4+NKT cells were stimulated in vitro using PMA/ionomycin and cultured at 37°C and 5% CO2 in Complete (10% FBS) RPMI medium for 5 days. Analysis of supernatants obtained 48 hours after cell culture using Luminex showed significant levels of IL-4 but not IL-13, and lower levels of IFNγ production (N= 8).
Supplementary Figure 6: The upregulation of PDL-1, and IL-4Rα declines significantly on CD11b+Gr-1hi cells in concert with the decline in the number of CD11b+Gr-1hi cells in the blood. (A–B) Representative histogram plots showing the expression of PDL1, and IL-4Rα (interleukin-4 receptor alpha) on gated CD11b+Gr-1hi cells from untreated wild type mice (UNT WT, N= 8), or transplanted wild type mice (WT TX, N= 10), on days 20 and 43 after heart transplantation. (C–D) Bar graphs showing means (± SEM) of mean fluorescence intensity (MFI) measurements of PDL-1 and IL-4Rα on CD11b+Gr-1hi cells in spleen.