Abstract
Aims
Disability, an individual’s reduced capacity to perform physical tasks encountered in daily routine, is associated with urinary incontinence in the elderly. Our objective was to determine if urinary incontinence is associated with disability in community-dwelling women 40 years and older.
Methods
Cross-sectional study among US women ≥ 40 years (n=4,458) from National Health and Nutrition Examination Surveys 2005–2010. We estimated the age-stratified weighted prevalence and factors independently associated with disability (Activities of Daily Living (ADLs), Instrumental Activities of Daily Living (IADLs), mobility, and functional limitations) in women with and without urinary incontinence while controlling for confounders of the association between disability and urinary incontinence.
Results
The weighted prevalence of all disabilities was higher in women with urinary incontinence than women without urinary incontinence across most decades of life with the greatest difference in the prevalence of mobility disabilities: 40–49 years (12.1% versus 7.0%), 50–59 years (17.0% versus 9.2%), 60–69 years (28.3% versus 19.8%), and 70+ years (43.8% versus 33.0%, all P < .05). On multivariable analysis, after controlling for the confounding effect of age, co-morbidities, and income-poverty ratio, urinary incontinence was weakly associated with disabilities. The adjusted odds ratio (95% confidence interval) of disabilities for urinary incontinence was ADL 1.96 (1.07, 3.58), IADL 1.18 (0.78, 1.78), mobility 1.26 (1.01, 1.56) and functional limitations 1.36 (1.07, 1.73).
Conclusions
Urinary incontinence is weakly associated with disabilities and cannot be implicated as a cause of disability in community dwelling women.
Keywords: Centers for Disease Control and Prevention, disability, National Health and Nutrition Examination Survey, urinary incontinence, women
Introduction
Disability represents an individual’s reduced capacity to perform discrete physical tasks encountered in daily routine such as walking, reaching, and handling everyday objects.(1) Types of disabilities include disabilities in activities of daily living (ADL), instrumental activities of daily living (IADL), mobility and functional limitations.(2) In the elderly, disabilities are both a cause and result of urinary incontinence. Tinetti et al. showed that in elderly adults with a mean age of 79 years, functional decline and disability as measured by worsening ADL, IADL, and physical performance measures were prospectively associated with an increased risk of developing incident urinary incontinence.(3) Conversely, worsening urinary incontinence has been shown to be an independent predictor of falls, fractures and functional dependence in the elderly over age of 65 years.(4)
Most studies that have examined the relationship between urinary incontinence and disability have been performed in the elderly over age 65 years in the context of late life geriatric syndromes such as delirium, falls, and frailty.(3) Younger community-dwelling women with urinary incontinence (both stress and urge) are less likely to be physically active than continent controls.(5, 6) Though low levels of physical activity are an important risk factor for disability (3), relatively little data is available on the prevalence of disability in ambulatory community-dwelling women with urinary incontinence or on the relationship between urinary incontinence and disability in these women.
The aim of the present study is to investigate if urinary incontinence is associated with disability in community-dwelling women age 40 years and older. Our hypothesis was that urinary incontinence is associated with disability in ambulatory community-dwelling women.
Materials and Methods
2005–2010 NHANES Program
The National Health and Nutrition Examination Survey (NHANES) is a program of studies designed to assess the health and nutritional status of adults and children in the United States (http://www.cdc.gov/nchs/nhanes.htm). The survey examines a nationally representative sample of approximately 5,000 persons each year. The NHANES survey oversamples low income persons, the elderly, Blacks and Mexican Americans to obtain more precise estimates of these groups. The NHANES interview, conducted in the subject’s home, includes demographic, socioeconomic, dietary, and health-related questions.
This analysis of the NHANES data met criteria for exemption of human subjects research review by the University of Pennsylvania Institutional Review Board.
Study Population
We chose to limit our population to women aged 40 years and older due to the low prevalence of urinary incontinence in the group of women aged 20–39 years.(7) Of 7976 women aged 40 years or older originally selected through a probability sampling for the 2005–2010 NHANES survey, 5684 women completed the household interview and 5474 women participated in the mobile examination center. The questions on reproductive health and urinary incontinence were asked via Computer-Assisted Personal Interview methodology by trained examiners at the mobile examination center. After eliminating women with missing data on urinary incontinence, women who were currently pregnant, and the women who reported Other Hispanic race, based on NHANES analytic recommendations, our final analytic dataset comprised 4458 women (Figure 1).
Figure 1. Flow Diagram NHANES 2005.
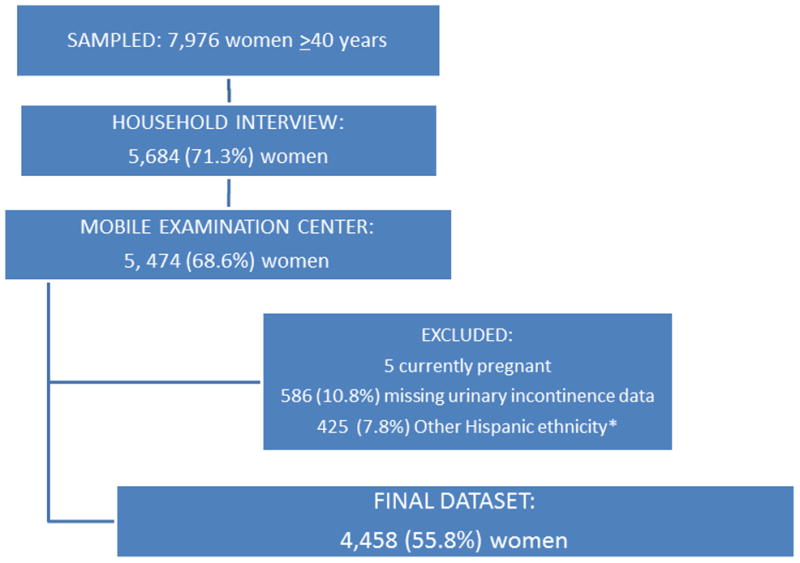
–2010
*Other Hispanics excluded as NHANES stated that this ethnicity could not be grouped due to changes in sampling methodology over study period.
Urinary incontinence was defined using the validated 2-item Incontinence Severity Index (included on the NHANES Kidney and Incontinence Questionnaire), which correlates well with incontinence volume based on pad weights and incontinence frequency obtained on bladder diaries.(8) The Incontinence Severity Index is based on response to frequency (<once per month, a few times a month, a few times a week, or every day and/or night) and amount of leakage (drops, splashes or more). By multiplying the responses to frequency and leakage amount, an index value of 1–12 is reached. We limited our case definition to moderate to severe incontinence defined as a severity score of at least 3 on the Incontinence Severity Index as reported by Nygaard and colleagues.(7) This corresponds to at least weekly leakage or monthly leakage of volumes more than just drops. We considered women to be continent when the severity score was less than 3.(7)
Age was stratified into decades: 40–49, 50–59, 60–69, and 70+. Participants self-reported their race/ethnicity (7) based on lists that included an open response. In this analysis, we assessed differences in urinary incontinence among 4 racial/ethnic groups: 1) non-Hispanic white; 2) non-Hispanic black; 3) Mexican American; 4) Other which did not include Other Hispanics due to specific changes in NHANES sampling methodology. Education (7) was dichotomized into less than or equal to high school diploma including General Education Development, or more than high school. The income-poverty ratio, an indicator of socioeconomic status that uses the ratio of income to the family’s poverty threshold set by the US Census Bureau, was included. A ratio of higher than 2 indicates income more than twice the poverty threshold.
We measured co-morbidities that are included in the Functional Co-morbidity Index (9) and have previously been reported to be highly correlated to the physical function component of the SF-36.(10) Co-morbidities measured included hypertension, depression, diabetes, obesity, visual difficulty, arthritis, congestive heart failure, coronary heart disease, angina, heart attack, emphysema, stroke, upper gastrointestinal problems - liver conditions, and osteoporosis. Co-morbidities were defined as the presence of two or more disease processes (1, 11) and dichotomized into less than two or two or more disease processes. This method of classifying co-morbidities has been previously used in epidemiologic studies.(11)
Parity (total number of vaginal and cesarean deliveries) was categorized as 0, 1, 2, and 3 or more.(7) Symptomatic pelvic organ prolapse is defined as a positive response to the question, “Do you experience bulging or something falling out that you can see or feel in the vaginal area?” A positive response correlates with the presence of a vaginal bulge on examination(12) however the question has higher specificity than sensitivity for pelvic organ prolapse based on exam.(12) Prolapse estimates using this question underreport the true prevalence of prolapse on examination.(13)
Disability
Disability was measured using The Physical Function Questionnaire and classified into four categories per Seeman et al for the NHANES population.(2) These four types of disability included: 1) basic activities of daily living (ADL), 2) instrumental activities of daily living (IADL), 3) mobility disability (walking a quarter of a mile and walking up 10 steps without rest), and 4) functional limitations (difficulty stooping, crouching, or kneeling; lifting or carrying 10 pounds; and standing from an armless chair).(2) The ADLs specifically queried in NHANES included difficulty eating, dressing, getting in and out of bed, and walking from room to room on the same level. The IADLS specifically queried in NHANES included difficulty preparing meals, doing household chores, and managing money. The specific mobility limitations queried were difficulty walking a quarter of a mile and difficulty walking up 10 steps without rest. The itemized functional limitations queried were 1) difficulty stooping, crouching, or kneeling; 2) difficulty lifting or carrying 10 pounds; and 3) difficulty standing from an armless chair. For each activity, responses ranged from no difficulty, some difficulty, much difficulty, or unable to do. Similar to Seeman et al. (2), for each outcome, presence of a disability was defined as the reports of some or greater difficulty on 1 or more relevant items for that disability. Individuals that reported that they did not do a queried activity were classified as having a missing response for that question as the level of disability could not be ascertained.
Statistical Analysis
Analyses were performed using STATA 12.0 (Statacorp, College Station, TX), incorporating the appropriate sampling weights of the complex NHANES sample design into all analyses. The sample weights adjust for unequal probabilities of selection and non-response. Comparisons of demographic and medical measures between the two urinary incontinence groups used Student’s t-tests for continuous measures and chi-squared test for binary outcomes. We compared the weighted prevalence of each type of disability, stratified by age, in women with and without urinary incontinence using the chi-square test. We performed logistic regression to determine the odds of each type of disability for urinary incontinence while controlling for potential confounding variables. We first estimated the odds of each kind of disability (ADL, IADL, mobility and functional limitations) in women with urinary incontinence. Since pelvic organ prolapse commonly co-exists with urinary incontinence, we then estimated the odds for urinary incontinence for each kind of disability while adjusting for pelvic organ prolapse. Finally, we performed multivariable regression with all possible confounders for the relationship between disability and urinary incontinence in the model. A variable was retained in the final model if it was associated with disability with P<0.2.(14) The rate of missing data for each demographic variable and type of disability was calculated and compared between the urinary incontinence and no urinary incontinence groups. Bilateral oophorectomy was the only variable not included in the regression model as the rate of missing data was greater than 10%. P<0.05 was considered statistically significant.
Results
In our cohort of 4,458 women, we noted significant differences in demographic variables in women with and without urinary incontinence (Table I). Women with urinary incontinence were significantly more likely to have a higher BMI and two or more co-morbidities than women without urinary incontinence.
Table I.
Demographic Dataa of Community-Dwelling Women with and without Urinary Incontinence
| N | No Urinary Incontinence (N=3370) | Urinary Incontinence (N=1088) | P-value | |
|---|---|---|---|---|
|
| ||||
| Age, y mean (SE) | 4458 | 56.7 (0.3) | 61.4 (0.5) | <0.001 |
|
| ||||
| Race | ||||
| Mexican American | 797 | 5.6 % (4.2%, 7.4%) | 6.0 % (4.2%, 8.5%) | 0.01 |
| Non-Hispanic White | 2506 | 77.4 % (73.8%, 80.6%) | 81.1 % (76.9%, 84.6%) | |
| Non-Hispanic Black | 985 | 12.0 % (9.8%, 14.6%) | 8.7 % (6.7%, 11.1%) | |
| Other including multi-racial | 170 | 5.1 % (4.1%, 6.3%) | 4.3 % (2.8%, 6.4%) | |
|
| ||||
| Education > High Schoolb | 4449 | 57.3 % (54.6%, 59.9%) | 49.5 % (45.5%, 53.5%) | <0.001 |
|
| ||||
| Income-Poverty Ratiob, mean (SE) | 4136 | 3.2 (0.1) | 2.9 (0.1) | <0.001 |
|
| ||||
| Body Mass Indexb, mean (SE) | 4398 | 28.7 (0.2) | 30.5 (0.2) | <0.001 |
|
| ||||
| Co-morbiditiesb | 4440 | 35.2 % (33.2%, 37.3%) | 54.0 % (48.9%, 59.0%) | <0.001 |
|
| ||||
| Parity | 4458 | |||
| 0 | 14.2 % (12.6%, 15.9%) | 10.7 % (8.6%, 13.3%) | <0.001 | |
| 1 | 8.3 % (7.1%, 9.7%) | 4.9 % (3.6%, 6.6%) | ||
| 2 | 15.5 % (14.0%, 17.2%) | 12.5 % (10.2%, 15.1%) | ||
| 3+ | 62.0 % (59.2%, 64.7%) | 71.9 % (68.5%, 75.1%) | ||
|
| ||||
| Hysterectomyb | 4161 | 31.9 % (30.0%, 34.0%) | 45.2 % (41.9%, 48.6%) | <0.001 |
|
| ||||
| Bilateral Oophorectomyb | 3121 | 14.3 % (12.6%, 16.2%) | 24.6 % (20.8%, 28.9%) | <0.001 |
|
| ||||
| Pelvic Organ Prolapseb | 4378 | 2.8% (2.2%, 3.6%) | 6.6% (5.1%, 8.6%) | <0.001 |
SE= standard error
Data is listed as weighted prevalences (95% confidence interval) except where noted.
Of the 4458 women, 9 were missing education, 322 were missing income-poverty ratio, 60 were missing body mass index (calculated as weight in kilograms divided by height in meters squared), 8 were missing co-morbidities, 297 were missing hysterectomy, 1342 women were missing oophorectomy, and 80 were missing prolapse. The missing data was not significantly different between the urinary incontinence groups.
Table II compares the weighted prevalence of disabilities in women with and without UI stratified by 10-year increments in age. The weighted prevalence of disabilities increased with each decade of life both in women with and without UI. The weighted prevalence of disabilities was higher in women with UI than women without UI across most age groups (with the exception of disabilities in ADL and IADL in the 60–69 year age group). The greatest difference in the prevalence of disabilities was noted for mobility disability; the weighted prevalence of mobility disability was significantly higher for women with UI than women without UI across all decades of life. The prevalence of disabilities in IADL was not significantly different between women with and without UI for any decade of life.
Table II.
Weighted Prevalences (95% Confidence Intervals) of Disabilities in Women with and without Urinary Incontinence (Stratified by Age)
| Disability Type | N | No Urinary Incontinence | Urinary Incontinence | P-value |
|---|---|---|---|---|
| ADL | ||||
| 40–49 | 1193 | 0.2% (0.04, 0.5) | 0.7% (0.09, 0.6) | 0.06 |
| 50–59 | 984 | 0.4% (0.1, 1.4) | 2.2% (0.8, 5.9) | 0.01 |
| 60–69 | 1061 | 1.4% (0.7, 2.7) | 1.4% (0.4, 4.5) | 0.95 |
| 70+ | 1220 | 1.2% (0.6, 2.2) | 3.9% (2.4, 6.1) | 0.002 |
| IADL | ||||
| 40–49 | 1193 | 0.8% (0.4, 1.9) | 2.5% (1.1, 5.5) | 0.08 |
| 50–59 | 984 | 1.6% (0.8, 3.2) | 1.8% (0.9, 3.9) | 0.85 |
| 60–69 | 1061 | 2.6% (1.5, 4.7) | 2.0% (0.9, 4.3) | 0.51 |
| 70+ | 1220 | 4.7% (3.2, 6.9) | 7.1% (5.0, 10.0) | 0.06 |
| Mobility | ||||
| 40–49 | 1193 | 7.0% (5.0, 9.6) | 12.1% (8.1, 17.8) | 0.01 |
| 50–59 | 984 | 9.2% (7.1, 11.8) | 17.0% (12.7, 22.5) | 0.001 |
| 60–69 | 1061 | 19.8% (16.5, 23.6) | 28.3% (21.5, 36.3) | 0.05 |
| 70+ | 1220 | 33.0% (29.4, 36.8) | 43.8% (37.6, 50.2) | 0.004 |
| Functional Limitations | ||||
| 40–49 | 1193 | 4.5% (3.2, 6.2) | 6.8% (4.4, 10.6) | 0.12 |
| 50–59 | 984 | 4.6% (3.0, 6.8) | 9.8% (6.8, 13.8) | 0.003 |
| 60–69 | 1061 | 9.8% (7.8, 12.3) | 11.9% (8.5, 16.4) | 0.31 |
| 70+ | 1220 | 14.3% (12.2, 16.6) | 25.6% (21.2, 30.5) | <0.001 |
ADL=Activities of Daily Living; IADL=Instrumental Activities of Daily Living
On univariable analysis, the odds (95% CI) of disability in ADLs, IADLs, mobility, and functional limitations for women with urinary incontinence were 3.71 (1.95, 7.05), 1.75 (1.21, 2.53), 2.13 (1.76, 2.57), and 2.13 (1.76, 2.58), respectively. After controlling for confounding variables including age, co-morbidities, race, BMI, pelvic organ prolapse, hysterectomy, and income-poverty ratio, the association between UI and disability decreased with odds ratios ranging from 1.12 to 1.90 (Table III). UI was not significantly associated with mobility disability or IADL disability and the strength of the association between UI and ADL disabilities and functional limitations was weak. Variables that remained significantly associated with disability were increasing age and number of co-morbidities (increased odds of disabilities), and higher income-poverty ratio (protective effect).
Table III.
Association between Urinary Incontinence and Disability in Community-Dwelling Women 40 Years of Age and Older: Results of Multivariable Regression Analysis (N=4,458)
| Variable | ADL OR (95% CI) |
IADL OR (95% CI) |
Mobility OR (95% CI) |
Functional Limitations OR (95% CI) |
|---|---|---|---|---|
| Urinary Incontinence | 1.90 (1.03, 3.50) | 1.12 (0.75, 1.68) | 1. 17 (0.94, 1.46) | 1.30 (1.02, 1.66) |
| Pelvic Organ Prolapse | 1.13 (0.26, 4.94) | 1.04 (0.39, 2.76) | 1.28 (0.93, 1.75) | 0.96 (0.55, 1.67) |
| Age a | 1.03 (1.00, 1.05) | 1.04 (1.01, 1.07) | 1. 06 (1.05, 1.07) | 1.04 (1.02, 1.05) |
| Raceb | ||||
| Mexican American | 1.51 (0.71, 3.21) | 1.67 (0.82, 3.41) | 0.62 (0.44, 0.86) | 1.15 (0.78, 1.71) |
| Non-Hispanic Black | 1.54 (0.71, 3.36) | 1.65 (1.03, 2.65) | 1.01 (0.77, 1.33) | 1.58 (1.09, 2.27) |
| Other including multi-racial | 4.33 (1.33, 14.12) | 2.51 (1.23, 5.13) | 1.18 (0.57, 2.42) | 1.88 (1.12, 3.14) |
| Parous | 1.54 (0.35, 6.72) | 0.75 (0.38, 1.50) | 0.87 (0.63, 1.20) | 1.20 (0.76, 1.89) |
| Body Mass Indexc | 1.04 (1.00, 1.08) | 0.99 (0.95, 1.03) | 1.07 (1.05, 1.09) | 1.02 (1.01, 1.04) |
| Co-morbidities | 3.41 (1.31, 8.86) | 7.09 (3.34, 15.07) | 4.20 (3.39, 5.19) | 4.84 (3.61, 6.48) |
| Income-Poverty Ratiod | 0.70 (0.51, 0.97) | 0.78 (0.66, 0.93) | 0.66 (0.61, 0.71) | 0.65 (0.60, 0.71) |
| Hysterectomye | 1.55 (0.76, 3.19) | 0.81 (0.47, 1.40) | 1.30 (1.02, 1.63) | 1.15 (0.86, 1.53) |
ADL=Activities of Daily Living; IADL=Instrumental Activities of Daily Living; OR=odds ratio; CI=confidence interval
OR for every 1 year increase in age
Non-Hispanic White group is baseline race comparator
calculated as weight in kilograms divided by height in meters squared
OR for every 1 point increase in the income-poverty ratio
OR for hysterectomy controlled for missing hysterectomy data.
Discussion
Though urinary incontinence has been directly implicated in late life disability in the elderly (15), our study shows that in younger community-dwelling women, urinary incontinence is not significantly associated with IADL disability or mobility disability and only weakly associated with ADL disability and functional limitations (OR < 2.0, Table III). The odds ratio of the association between co-morbidities and disabilities ranged from 3.41 to 7.09 while the odds ratios of disabilities and urinary incontinence ranged from 1.12 and 1.90. These findings suggest that the presence of co-morbidities rather than urinary incontinence explains the observed high prevalence of disabilities in younger ambulatory community-dwelling women and urinary incontinence cannot be implicated as a cause of disability in these women.
Prior studies have examined the relationship between UI and specific co-morbidities such as obesity, depression, diabetes, hypertension, heart disease, and asthma.(16, 17) These studies show that while there is lack of consistency across studies as to which specific co-morbidities are associated with urinary incontinence, increasing number of co-morbidities is associated with worsening incontinence. In our study also, the number of co-morbidities emerged as an important variable that was associated with both urinary incontinence and disabilities.
The overall prevalence of disabilities in women age 60 and older in our study is lower than previously reported by Seeman et al. in the NHANES dataset.(2) This is likely because the urinary incontinence survey in NHANES was conducted at a health assessment center that required women to travel to the examination center and women with more severe disabilities are likely under-represented in our population.
In our study, mobility disability and functional limitations are much more prevalent than disabilities in ADL and IADL. Additionally, the prevalence of mobility disability is higher in women with urinary incontinence than in women without urinary incontinence across all age groups. Mobility disability and functional limitations represent inability to perform physical activities that require high level of functioning, muscle strength, and endurance such as walking a quarter of a mile and walking up 10 steps without rest. Kafri et al. (18) have previously shown that limitations in lower limb function, such as walking long distances and climbing, are associated with the presence of urinary incontinence, but whether urinary incontinence is the cause or the result of such disability in community dwelling women is not known. We report a similar prevalence of IADL disability in women with and without urinary incontinence; this is not surprising because IADL disability measures difficulty in fine motor skills and cognitive functions such as difficulty preparing meals and managing money. Similar to mobility and functional limitations, ADL disabilities (such as difficulty walking across a room) require lower limb muscle strength and their prevalence, though low, is higher in women with urinary incontinence than in women without incontinence.
In the elderly, especially those over age 70, a direct relationship between urinary incontinence and disability has been elucidated. Poor physical function, with increasing frailty and disability has been prospectively shown to be a risk factor for urinary incontinence, likely through decreased ability to reach the toilet.(3) Conversely, urgency to reach the toilet can result in increased falls and fractures with subsequent reduced physical activity, functional decline and disability.(4, 15) The relationship between urinary incontinence and disability in younger ambulatory community-dwelling women appears to be more complex. Prior studies suggest that women with stress and urge incontinence are less likely to participate in recreational exercise (5) and everyday physical activities such as walking, shopping, and house-cleaning than continent women.(6) Disability is reached when restriction of physical activity is severe enough to result in inability to carry out activities of everyday living. Our study shows that the prevalence of disability in younger ambulatory community-dwelling women with UI is high but not directly related to urinary incontinence. Longitudinal studies will be required to determine if a continuum of decreased physical activity and disability exists as younger ambulatory community-dwelling women with urinary incontinence get older.
Even though urinary incontinence is likely not a cause of disability in community dwelling women, our study does show that the prevalence of disabilities in mobility and functional limitations is high in women with urinary incontinence especially in the age group of 40 to 59 years. Surgical methods are commonly used to treat urinary incontinence in this age group. Recent studies suggest that the presence of coexisting disabilities is associated with worse functional outcomes following surgery.(19, 20) When planning treatment options for women with urinary incontinence, surgeons need to be cognizant of the possibility of worse outcomes in women with urinary incontinence when co-existing disabilities are present. Our study also shows that coexisting co-morbidities are common in women with urinary incontinence. Coexisting co-morbidities, also common in women with urinary incontinence, can lead to worse treatment outcomes (19–21) and will require appropriate management by primary care physicians and or other specialists for optimal results.
Strengths of our study include the analysis of a large national database of community-dwelling women in the United States. Disability was self-reported using questionnaires, and prior studies support the reliability of self-reported instruments to measure disability.(22) Analyses of large databases can also increase the probability to finding an association by chance. In accordance with recommendations of Grimes and Schultz (23), we considered odds ratios to be clinically significant only if they were greater than 3.5 or 4. Our study is limited by its cross-sectional design that does not allow us to determine a causal relationship between urinary incontinence and disability or the effect of treatment of urinary incontinence. We did not measure the individual effect of specific co-morbid disease processes on disability, however, the focus of this study was the relationship of urinary incontinence with disabilities with co-morbidities as a possible confounding factor; furthermore, prior studies have already shown that co-morbidities increase the risk of disability.(1, 24, 25)
Conclusions
In summary, urinary incontinence is weakly associated with disabilities and cannot be implicated as a cause of disability in community dwelling women. Disabilities, however, are commonly present in women with urinary incontinence and have been associated with worse outcomes following surgery. Therefore, specialists taking care of women with urinary incontinence should be should be aware that women with urinary incontinence are likely to have one or more type of disability and plan therapy appropriately.
Acknowledgments
Kathleen J. Propert, ScD was partially supported by grant number UL1TR000003 from the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health.
Footnotes
Institution at which the work was performed:
Perelman School of Medicine at the University of Pennsylvania, Philadelphia, PA
Research data derived from an approved University of Pennsylvania, Philadelphia, PA IRB protocol.
Conflict of Interest: NONE
Disclaimer: Joy A. Greer, MD, CDR, MC, USN is a military service member. This work was prepared as part of her official duties. Title 17, USC, ¶105 provides that ‘Copyright protection under this title is not available for any work of the United States Government.’ Title 17, USC, ¶ 101 defines a U.S. Government work as a work prepared by a military service member or employee of the U.S. Government as part of that person’s official duties. The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Department of the Navy, Department of Defense, nor the U.S. Government.
References
- 1.Fried LP, Ferrucci L, Darer J, Williamson JD, Anderson G. Untangling the concepts of disability, frailty, and comorbidity: Implications for improved targeting and care. J Gerontol A Biol Sci Med Sci. 2004;59:255–63. doi: 10.1093/gerona/59.3.m255. [DOI] [PubMed] [Google Scholar]
- 2.Seeman TE, Merkin SS, Crimmins EM, Karlamangla AS. Disability trends among older Americans: National Health and Nutrition Examination Surveys, 1988–1994 and 1999–2004. American Journal of Public Health. 2010;100:100–7. doi: 10.2105/AJPH.2008.157388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tinetti ME, Inouye SK, Gill TM, Doucette JT. Shared risk factors for falls, incontinence, and functional dependence. Unifying the approach to geriatric syndromes. JAMA. 1995;273:1348–53. [PubMed] [Google Scholar]
- 4.Brown JS, Vittinghoff E, Wyman JF, Stone KL, Nevitt MC, Ensrud KE, et al. Urinary incontinence: Does it increase risk for falls and fractures? Study of Osteoporotic Fractures Research Group. J Am Geriatr Soc. 2000;48:721–5. doi: 10.1111/j.1532-5415.2000.tb04744.x. [DOI] [PubMed] [Google Scholar]
- 5.Nygaard I, Girts T, Fultz NH, Kinchen K, Phol G, Sternfield B. Is urinary incontinence a barrier to exercise in women? Obstet Gynecol. 2005;106:307–14. doi: 10.1097/01.AOG.0000168455.39156.0f. [DOI] [PubMed] [Google Scholar]
- 6.Fultz NH, Fisher GG, Jenkins KR. Does urinary incontinence affect middle-aged and older women’s time use and activity patterns? Obstet Gynecol. 2004;104(6):1327–34. doi: 10.1097/01.AOG.0000143829.21758.3c. [DOI] [PubMed] [Google Scholar]
- 7.Nygaard I, Barber MD, Burgio KL, Kenton K, Meikle S, Schaffer J, et al. Prevalence of symptomatic pelvic floor disorders in US women. JAMA. 2008;300:1311–6. doi: 10.1001/jama.300.11.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sandvik H, Seim A, Vanvik A, Hunskaar S. A severity index for epidemiological surveys of female urinary incontinence: Comparison with 48-hour pad-weighing tests. Neurourol Urodyn. 2000;19:137–45. doi: 10.1002/(sici)1520-6777(2000)19:2<137::aid-nau4>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 9.Groll DL, To T, Bombardier C, Wright JG. The development of a comorbidity index with physical function as the outcome. J Clin Epidemiol. 2005;58:595–602. doi: 10.1016/j.jclinepi.2004.10.018. [DOI] [PubMed] [Google Scholar]
- 10.Fortin M, Hudon C, Dubois MF, Almirall J, Lapointe L, Soubhi H. Comparative assessment of three different indices of multimorbidity for studies on health-related quality of life. Health Qual Life Outcomes. 2005;3:74. doi: 10.1186/1477-7525-3-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guralnick J, LaCroix A, Everett D, Kovar M. Advance Data from Vital and Health Statistics. Hyattsville, MD: National Center for Health Statistics; 1989. Aging in the Eighties: The prevalence of comorbidity and its association with disability. [Google Scholar]
- 12.Barber MD, Walters MD, Cundiff GW PESSRI Trial Group . Responsiveness of the pelvic floor distress inventory (PFDI) and pelvic floor impact questionnaire (PFIQ) in women undergoing vaginal surgery and pessary treatment for pelvic organ prolapse. Am J Obstet Gynecol. 2006;194:1492–8. doi: 10.1016/j.ajog.2006.01.076. [DOI] [PubMed] [Google Scholar]
- 13.Handa VL, Blomquist JL, Knoepp LR, Hoskey KA, McDermott KC, Munoz A. Pelvic floor disorders 5–10 years after vaginal or cesarean childbirth. Obstet Gynecol. 2011;118:777–84. doi: 10.1097/AOG.0b013e3182267f2f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maldonado G, Greenland S. Simulation study of confounder-selection strategies. Am J Epidemiol. 1993;138:923–36. doi: 10.1093/oxfordjournals.aje.a116813. [DOI] [PubMed] [Google Scholar]
- 15.Miles TP, Palmer RF, Espino DV, Mouton CP, Lichtenstein MJ, Markides KS. New-onset incontinence and markers of frailty: Data from the Hispanic Established Populations for Epidemiologic Studies of the Elderly. J Gerontol A Biol Sci Med Sci. 2001;56:M19–24. doi: 10.1093/gerona/56.1.m19. [DOI] [PubMed] [Google Scholar]
- 16.Melville JL, Katon W, Delaney K, Newton K. Urinary incontinence in US women: A population-based study. Arch Intern Med. 2005;165:537–42. doi: 10.1001/archinte.165.5.537. [DOI] [PubMed] [Google Scholar]
- 17.Tennstedt SL, Link CL, Steers WD, McKinlay JB. Prevalence of and risk factors for urine leakage in a racially and ethnically diverse population of adults. Am J Epidemiol. 2008;167:390–9. doi: 10.1093/aje/kwm356. [DOI] [PubMed] [Google Scholar]
- 18.Kafri R, Shames J, Golomb J, Melzer I. Self-report function and disability: a comparison between women with and without urinary incontinence. Disability & Rehabilitation. 2012;34(20):1699–1705. doi: 10.3109/09638288.2012.660597. [DOI] [PubMed] [Google Scholar]
- 19.Robinson TN, Eiseman B, Wallace JI, Church SD, McFann KK, Pfister SM, et al. Redefining geriatric preoperative assessment using frailty, disability and co-morbidity. Ann Surg. 2009;250:449–55. doi: 10.1097/SLA.0b013e3181b45598. [DOI] [PubMed] [Google Scholar]
- 20.Greer JA, Northington GN, Harvie HS, Segal S, Johnson JC, Arya LA. Functional status and post-operative morbidity in older women with prolapse. J Urol. 2013;190:948–52. doi: 10.1016/j.juro.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 21.Sung VW, Weitzen S, Sokol ER, Rardin CR, Myers DL. Effect of patient age on increasing morbidity and mortality following urogynecologic surgery. Am J Obstet Gynecol. 2006;194(5):1411–7. doi: 10.1016/j.ajog.2006.01.050. [DOI] [PubMed] [Google Scholar]
- 22.Kehoe R, Wu SY, Leske MC, Chylack LT., Jr Comparing self-reported and physician-reported medical history. Am J Epidemiol. 1994;139:813–8. doi: 10.1093/oxfordjournals.aje.a117078. [DOI] [PubMed] [Google Scholar]
- 23.Grimes DA, Schulz KF. False alarms and pseudo-epidemics: The limitations of observational epidemiology. Obstet Gynecol. 2012;120(4):920–7. doi: 10.1097/AOG.0b013e31826af61a. [DOI] [PubMed] [Google Scholar]
- 24.Fried LP, Kronmal RA, Newman AB, Bild DE, Mittelmark MB, Polak JF, et al. Risk factors for 5-year mortality in older adults: The Cardiovascular Health Study. JAMA. 1998;279:585–92. doi: 10.1001/jama.279.8.585. [DOI] [PubMed] [Google Scholar]
- 25.Verbrugge LM, Lepkowski JM, Imanaka Y. Comorbidity and its impact on disability. Milbank Q. 1989;67:450–84. [PubMed] [Google Scholar]


