Abstract
Carbonic anhydrases (CAs) catalyse the hydration of CO2 to bicarbonate at physiological pH. This chemical interconversion is crucial since HCO3- is the substrate for several biosynthetic reactions. This review is focused on the distribution and role of CA isoenzymes in both normal and pathological gastrointestinal (GI) tract tissues. It has been known for many years that CAs are widely present in the GI tract and play important roles in several physiological functions such as production of saliva, gastric acid, bile, and pancreatic juice as well as in absorption of salt and water in intestine. New information suggests that these enzymes participate in several processes that were not envisioned earlier. Especially, the recent reports on plasma membrane-bound isoenzymes IX and XII have raised considerable interest since they were reported to participate in cancer invasion and spread. They are induced by tumour hypoxia and may also play a role in von Hippel-Lindau (VHL)-mediated carcinogenesis.
Keywords: Gastrointestinal tract, Gastrointestinal tumour, Carbonic anhydrases
CARBONIC ANHYDRASES - GENERAL ASPECTS
Carbonic anhydrases are a group of zinc-containing metalloenzymes that catalyse the reversible hydration of carbon dioxide, CO2+H2O <=> HCO3- + H+. Up to now, 12 enzymatically active alpha carbonic anhydrases have been identified and characterized in mammals including 5 cytoplasmic (CA I, CA II, CA III, CA VII, and CA XIII), 2 mitochondrial (CA VA and CA VB), 1 secreted (CA VI), and 4 membrane-associated (CA IV, CA IX, CA XII, and CA XIV) forms.
CAs are virtually ubiquitous in living systems, having varied functions in animal, plant and bacterial cells. The great diversity in both cellular distribution (Figure 1) and biological functions is remarkable and the catalytic activity of CAs, found in almost all organisms, is extremely high, placing the high-activity isoenzymes among the most efficient enzymes known[1]. In addition to its role in the regulation of pH homeostasis, CA activity facilitates biosynthetic processes, which involve an early carboxylation step requiring bicarbonate. These processes include gluconeogenesis and synthesis of certain amino acids (pyruvate carboxylase), lipogenesis (pyruvate carboxylase and acetyl CoA carboxylase), ureagenesis (carbamyl phosphate synthetase I), and pyrimide synthesis (carbamyl phosphate synthetase II)[2].
Figure 1.
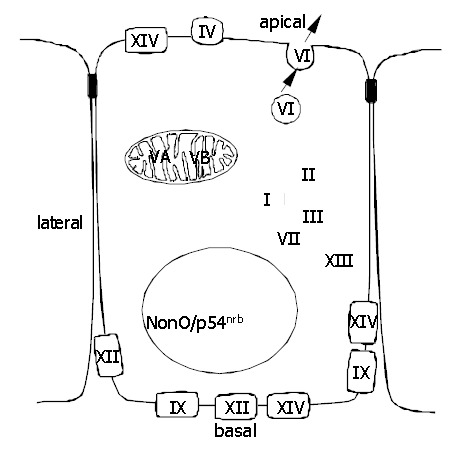
Subcellular localization of active CA isoenzymes and a novel nonclassical form of CA (nonO/p54nrb).
Carbonic anhydrase isoenzymes
CA I is a well characterized cytoplasmic low-activity isoenzyme with a molecular weight of about 30 kDa[3,4]. It is one of the most abundant proteins in mammalian red cells[5,6]. Interestingly, it is not expressed in red cells of certain species, e.g., ruminants and felids, and no haematological abnormalities have emerged in its absence as the result of a mutation. Thus, the assignment of the physiological role of CA I is problematic[7-9].
CA II is the most widely distributed member of the CA family, being present in virtually every human tissue or organ[9]. The gene for human CA II is 17 kb long and located on chromosome 8, like those for CA I and III[1,10]. The high-active, cytoplasmic isoenzyme CA II has a turnover number of 1.3-1.9×106/s under physiological conditions, and thus it is one of the most efficient enzymes known[11-13]. CA II participates in the control of bone resorption. Osteopetrosis, involving renal acidosis and cerebral calcification, is a human recessive inherited syndrome with a deficiency of CA II as a primary defect[14-17]. Even though CA II is the most abundant isoenzyme in the alimentary tract, no gastrointestinal symptoms have been associated with CA II-deficient patients.
Hormonally regulated cytoplasmic CA III has very low CA catalytic activity. On the other hand, it seems to protect from hydrogen peroxide-induced apoptosis and induce cell proliferation, whereas CA II does not have these effects[18]. These results suggest that CA III may provide protection against oxidative damage, and the lower levels of free radicals in cells overexpressing CA III may also affect growth signalling pathways.
To date four membrane-associated CA isoenzymes have been identified. CA IV is a glycosylphosphatidylinositol-anchored enzyme being expressed in the apical plasma membrane of epithelial cells (Figures 1 and 2). The gene for CA IV is located on chromosome 17[19,20] and the molecular weight of the human protein is 35 kDa[21]. Human CA IV is present in the subepithelial capillary endothelium in all segments of the gastrointestinal canal as well as in epithelial cells of colon and gallbladder[22,23].
Figure 2.
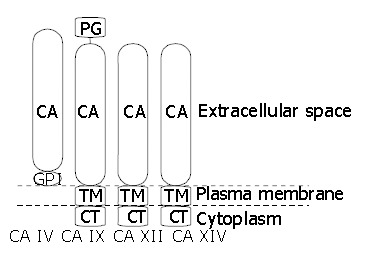
Topography of membrane-associated CA isoenzymes. CA: carbonic anhydrase domain, GPI: glycosylphosphatidylinositol link, TM: transmembrane part, CT: cytoplasmic C-terminal tail, PG: proteoglycan domain.
CA V is a low-activity isoenzyme located in the mitochondrial matrix[24]. cDNA for human mitochondrial CA V was originally cloned from a human liver cDNA library, and its gene was localized to chromosome 16[24]. Recently, two laboratories independently characterized another mitochondrial CA and thereafter the two isoenzymes have been termed CA VA and CA VB[25,26]. The expression of human CA5A mRNA has been demonstrated only in liver[26]. In the alimentary tract, CA5B mRNA has been shown in pancreas and salivary glands by reverse transcription-PCR (RT-PCR)[26].
CA VI is to date the only known secretory isoenzyme of the CA gene family. This isoenzyme with a molecular weight of 39-46 kDa has several properties that distinguish it from the well-characterized cytoplasmic isoenzymes[27-33]. On the other hand, the catalytic domain of CA VI is highly homologous to four other “extracellular” CAs (CA IV, CA IX, CA XII, and CA XIV), which are in fact transmembrane proteins with an extracellularly exposed CA domain[34,35].
CA VII is a cytoplasmic isoenzyme, the gene of which has been isolated from a human genomic library[9,36]. The gene is about 10 kb long and located on chromosome 16, and the predicted amino acid sequence of CA VII has shown that it is the most highly conserved isoenzyme in mammals[37]. Recombinant CA VII has shown high enzyme activity, but the expression of the protein in tissues has not yet been described[38]. Its mRNA has been detected in the human salivary gland[36], rat and mouse lung[39] and mouse brain neurons[37,40].
CA IX was first recognized as a novel tumour-associated antigen, MN, in several human carcinomas and normal gastric mucosae[41,42]. When the full-length cDNA for MN protein was cloned, it was found to contain a central part with sequence homology to the CAs[42,43], on which basis the MN protein was named CA IX[44]. Human CA9 gene has been mapped to chromosome 17[45]. CA IX is a glycoprotein of 54 and 58 kDa mass expressed at the basolateral plasma membrane of epithelial cells (Figure 1) and, in some cases, also in nuclei[46]. The mature CA IX molecule consists of a proteoglycan-like domain, CA domain, transmembrane segment, and a short intracellular tail (Figure 2)[42].
Expression of CA9 gene is subject to complex regulation both via promoter/enhancer elements at the level of transcription initiation[47] and via VHL tumour suppressor protein, possibly at the level of transcription elongation[45,48]. CA IX has been linked to oncogenesis, and its overexpression has been observed in malignant tumour cells. CA IX expression was found to be regulated by cell density in HeLa cells and to correlate with tumourigenicity in HeLa cell/fibroblast cell hybrids[49]. Moreover, transfection of NIH3T3 fibroblasts with CA IX caused changes in both morphology and growth parameters that were indicative of transformation[42].
Another active transmembrane CA isoenzyme is CA XII. CA12 gene has been mapped to chromosome 15[50]. The cDNA sequence predicts a 354-amino acid polypeptide with a molecular mass of 39448 Da[45,50]. The amino acid sequence includes a 29-amino acid signal peptide, 261-amino acid CA domain, an additional short extracellular segment, a 26-amino acid hydrophobic transmembrane domain, and a 29-amino acid C-terminal cytoplasmic tail containing two potential phosphorylation sites. The extracellular CA domain has three zinc-binding histidine residues found in active CAs and two potential sites for asparagine glycosylation[50]. It has a sequence identity of 30-42% to other CAs. The reported molecular weight of CA XII produced in transfected COS cells is 43-44 kDa. It is reduced to 39 kDa by PNGase F digestion, which is consistent with the removal of two oligosaccharide chains[50]. Recombinant CA XII protein is an active isoenzyme and its catalytic properties are similar to those of the high-activity membrane-associated CA IV[51].
Recent analyses of human and mouse databases provided evidence that human and mouse genomes contain genes for still another cytosolic CA isoenzyme named CA XIII. It is a globular molecule with high structural similarity to CAs I, II, and III[52]. Recombinant mouse CA XIII has catalytic activity similar to those of mitochondrial CA V and cytosolic CA I. Immunohistochemical staining and RT-PCR have shown a unique and widespread distribution pattern for CA XIII protein and mRNA compared to the other cytosolic CA isoenzymes. It is present in several gastrointestinal organs including salivary glands, small and large intestine.
CA XIV is a recently described transmembrane isoenzyme[35], consisting of a putative amino-terminal signal sequence, a CA domain with high homology with other extracellular CAs, a transmembrane domain and a short intracellular C-terminal tail. A cDNA coding for human CA XIV has been reported and the CA14 gene has been mapped to chromosome 1q21[53]. The Northern blot method showed CA14 mRNA expression in human heart, brain, liver and skeletal muscle. RT-PCR analysis pointed to an intense signal in the normal human liver and spinal cord. RNA dot blot analysis showed weak signals in the small intestine, colon, kidney, and urinary bladder[53]. By immunohistochemistry, CA XIV protein has been demonstrated in the mouse brain, liver, and kidney[54-56]. In the brain, high signal has been detected in the neuronal membranes and axons[54]. In the liver, CA XIV is confined to the plasma membrane of hepatocytes[55]. Interestingly, it is located in both the apical and basolateral membranes. In contrast, the other transmembrane isoenzymes, CA IX and XII, are clearly restricted to the basolateral membranes. The location in apical and basolateral membrane domains has been described for CA XIV also in the kidney, where the enzyme is expressed in the proximal tubules and thin descending limb of Henle[56].
In addition to the members of the classical CA gene family, a 66-kDa polypeptide was recently purified from several rat tissues on CA inhibitor affinity chromatography[57]. Its amino acid sequence has revealed that it represents the previously cloned and characterized nuclear protein, nonO/p54nrb, a non-POU (Pit-Oct-Unc) domain-containing octamer-binding protein, which is homologous to the nuclear 54 kDa RNA-binding protein[58,59]. CA activity of nonO is interesting because no other classes of mammalian proteins except CAs have been shown to bind specifically to CA inhibitor affinity chromatography matrix and to contain CA catalytic activity. CA catalytic activity in nonO could explain at least part of the nuclear CA activity seen in histochemical CA staining[57]. It also suggests that CA activity is an important factor in transcriptional regulation, which would be a novel function for CA.
CARBONIC ANHYDRASES IN THE GASTROINTESTINAL TRACT
Carbonic anhydrases are evolutionarily ancient enzymes, which can be found along the entire GI tract, from the mouth and salivary glands to the rectum (Table 1)[60-62]. However, there is a considerable heterogeneity between different organs with respect to CA enzymatic activity, isoenzyme content, and their cellular localization. It is also notable that CAs of the GI tract can serve several functions such as regulation of acid-base homeostasis, salt absorption and cell volume[63].
Table 1.
Immunohistochemical distribution of CAs in human alimentary tract.
| Isoenzyme | Molecular weight (ku) | Positive cell types | Main references |
| CA I | 30 | Epithelial cells of | [5,60,61,86] |
| oesophagus | |||
| jejunum | |||
| ileum | |||
| colon | |||
| Subepithelial capillary endothelium | |||
| a-cells of Langerhans islets | |||
| CA II | 30 | Epithelial cells of | [5,60,64,75,86] |
| salivary glands | |||
| oesophagus | |||
| stomach | |||
| duodenum | |||
| jejunum | |||
| ileum | |||
| caecum | |||
| colon | |||
| rectum | |||
| biliary tract | |||
| pancreatic duct | |||
| Brunner's glands | |||
| Hepatocytes | |||
| CA III | 30 | Epithelial cells of oesophagus | [74,86] |
| Hepatocytes | |||
| CA IV | 35 | Epithelial cells of | [22,23,61,86] |
| oesophagus | |||
| duodenum | |||
| colon | |||
| rectum | |||
| biliary tract | |||
| Subepithelial capillary endothelium | |||
| CA V | 30 | Hepatocytes | [14,24,79] |
| b-cells of Langerhans islets | |||
| CA VI | 42 | Epithelial cells of | [62,64] |
| salivary glands | |||
| von Ebner's glands | |||
| CA VII | |||
| CA IX | 54/58 | Epithelial cells of | [61,78,88,117] |
| salivary glands | |||
| oesophagus | |||
| stomach | |||
| duodenum | |||
| jejunum | |||
| ileum | |||
| colon | |||
| rectum | |||
| biliary tract | |||
| pancreatic duct | |||
| CA XII | 44 | Epithelial cells of | [117] |
| salivary glands | |||
| stomach | |||
| colon | |||
| pancreatic acini | |||
| CA XIII | 30 | Epithelial cells of | [52] |
| salivary glands | |||
| intestine | |||
| colon | |||
| CA XIV | 37.5 |
Major digestive glands
Three CA isoenzymes, CAs II, VI, and XIIII, are known to be expressed in the mammalian salivary glands[52,64]. A study by Leinonen et al[65] showed that salivary CA VI was associated with the enamel pellicle, a thin layer of proteins covering the dental enamel. It has also been detected in the gastric mucus, but the failure to find any expression of it in the gastric epithelial cells[60] implies that gastric CA VI must be of salivary origin. CAs II, VI, and XIII may together form a complementary system regulating the acid-base balance in the mouth and upper alimentary tract[63,64,66,67]. CAs II and XIII in the salivary glands may supply the saliva with HCO3- and the CA VI secreted into the saliva would then accelerate the removal of bacterially produced acid from the local microenvironment of the tooth surface in the form of CO2. This hypothetical model of CA VI functions is supported by Kivelä et al[68], who showed that low salivary CA VI concentrations were associated with increased caries prevalence, particularly in subjects with neglected oral hygiene. In the gastric mucus, CA VI may contribute to the maintenance of pH gradient on the surface epithelial cells by catalyzing the conversion of bicarbonate produced by these cells and protons of the gastric juice to water and carbon dioxide.
Recent studies have shown that CA VI is also present in human and rat milk[69]. The high concentration of CA VI in colostrum suggests that it may play an important developmental role in the morphogenesis of the gastrointestinal canal during the early postnatal period.
There are two other findings pointing to novel physiological roles for CA VI. First, gustin, a salivary factor involved in taste perception, was shown to be identical to CA VI[70], and second, identification of a novel stress-inducible intracellular form of CA VI (type B) suggests that the enzyme could also participate in intracellular pH changes induced by stress, including apoptosis[71].
Compared with other secretory organs, the mammalian liver contains relatively low levels of total CA activity. A basic physiological function of CA II in the liver is to produce HCO3- for the alkalization of the bile[72]. The mammalian liver expresses high levels of mitochondrial CA V[14,24,26], which has been implicated in two metabolic processes: ureagenesis and gluconeogenesis, supplying bicarbonate for the first urea cycle enzyme, carbamyl phosphate synthetase I in ureagenesis and for pyruvate carboxylase in gluconeogenesis[73].
The presence of low activity, hormonally regulated CA III in hepatocytes[74,75] has aroused interest in its specific function. Cabiscol and Levine[76] have demonstrated that it functions in an oxidizing environment and that it is the most oxidatively modified protein in the liver known so far. These and other results[18] suggest that CA III may provide protection against oxidative damage and CA III may serve as a useful marker protein to investigate the in vivo mechanisms, which contribute to oxidative damage in the liver.
In addition to CA II, CA III, and CA VA, hepatocytes contain CA XIV[55]. Positive signal for CA XIV was detected in both domains of the plasma membrane, i.e., basolateral and apical (canalicular), but the apical membrane was more strongly labelled by immunohistochemical staining[55]. CA XIV is the only membrane-bound CA isoform which most probably regulates pH and ion transport between hepatocytes, bile canaliculi and hepatic sinusoids. CA II is expressed in epithelial cells of the hepatic bile ducts and gallbladder[60,77]. Immunohistochemical studies have also shown that two membrane associated CA isoenzymes, CA IV and CA IX, are expressed in the biliary epithelial cells, whereas hepatocytes are negative[23,78]. It is notable that both isoenzymes show high expression in the human gallbladder - CA IV at the apical and CA IX at the basolateral plasma membrane. Based on the localization, CAs might be involved in acidification and concentration of bile, even though the exact functional mechanisms have not been described.
Pancreas contains two morphologically and functionally different elements: endocrine and exocrine compartments. CA I is expressed in α-cells of the endocrine Langerhans islets[60]. However, the physiological role of CA I in α-cell function has remained unclear. CA V is another isoenzyme described in the endocrine pancreas where its expression is solely confined to β-cells[79]. The suggestion that CA V may have a role in the regulation of insulin secretion was based on its cellular distribution and the observation that the CA inhibitor acetazolamide inhibited glucose-stimulated insulin secretion[79].
In the exocrine pancreas, immunohistochemical staining has shown an intense positive signal for CA II in epithelial duct cells[80-82], where its role has been linked to secretion of bicarbonate into the pancreatic juice[72]. In addition to CA II, both CA IX and XII have been detected in the pancreatic epithelium, the expression being confined to the basolateral plasma membranes of acinar and ductal cells[83]. The mechanisms involved in the pancreatic duct cell ion transport have been recently reviewed by Ishiguro et al[84]. They presented a model where the basolateral plasma membrane contained Na+-HCO3- cotransporter and Cl-/HCO3- exchanger. The bicarbonate secretion across the apical plasma membrane was proposed to occur by exchange for Cl- ions on Cl-/HCO3- exchanger working in parallel with the cystic fibrosis transmembrane conductance regulator (CFTR) Cl- channel. Solving the question of how different CAs can function in pancreatic duct and acinar cells will be a challenging task, not least technically. The knockout mouse models will hopefully provide invaluable information on the role of each isoenzyme. It is noteworthy, however, that recent studies on CA IX-deficient mice did not reveal any obvious pancreatic phenotype[85].
Gastrointestinal canal
Squamous epithelial cells of the human oesophagus express several CA isoenzymes. The cytoplasmic low-activity isoenzymes CAs I and III are confined to the basal cell layer, where they have been suggested to facilitate the efflux of CO2 and H2O from metabolically active basal cells to capillaries of lamina propria[86]. CA II has been located in the suprabasal cell layers, where it may contribute to HCO3- secretion[60,86]. The presence of this high-activity isoenzyme in the oesophagus is physiologically important, because endogenous HCO3- secretion is capable of raising the pH of the gastro-oesophageal reflux-derived residual acid from 2.5 almost to neutrality[87]. The immunohistochemical evidence for the presence of CA II in the human oesophagus is thus in accordance with the biochemical evidence that the oesophagus disposes of an endogenous mechanism for protecting the mucosa against acidity, but suggests that the stratified oesophageal epithelium rather than the submucous glands is responsible for HCO3- secretion.
In addition to cytoplasmic isoenzymes, two membrane-associated CAs are expressed in the oesophageal epithelium. Immunohistochemical studies have revealed a positive signal for the membrane-linked CA IV in suprabasal cell layers[86]. Similarly, weak immunostaining for CA IX has been found in the basal layer of the oesophageal epithelium[88].
Immunohistochemical techniques have revealed cytosolic CA II in parietal cells of the gastric glands, where it regulates the acidity of the gastric juice by proton secretion[5,60,63,81]. On the other hand, in gastric surface epithelial cells CA II is involved in the secretion of mucus and HCO3- to form a bicarbonate containing mucous gel layer covering the epithelium and protecting it from digestion. This gastroduodenal HCO3- secreted by the surface epithelial cells neutralizes the gastric acid[89].
Membrane-associated CA IX is another major CA isoenzyme expressed in gastric epithelium. Both parietal and surface epithelial cells contain CA IX at the basolateral plasma membrane[78]. Evolutionary conservation in vertebrates and the abundant expression of CA IX in normal human gastric mucosa have indicated its physiological importance. CA IX may participate in physiological processes via the activity of its CA-like domain. On the other hand, basolateral localization of CA IX suggests its possible involvement in intercellular communication and/or cell proliferation. Recent study on CA IX knockout mice demonstrated that this enzyme deficiency could result in a clear gastric phenotype[85]. These mice showed gastric hyperplasia and numerous cysts in gastric mucosa. The number of proliferative cells in gastric mucosa was increased, whereas the indices of gastric secretion showed normal values.
In the small intestine, CA I has been found in cryptal enterocytes[61] and CA II in surface epithelial cells[60]. High expression of CA IX has been reported in the epithelium of intestinal mucosa, where CA IX is confined to the basolateral cell surface of enterocytes and the cellular distribution is restricted to the proliferative cryptal enterocytes[61,78]. Interestingly, its regional expression is distinctive compared with other CAs, being most intense in the duodenum and jejunum and decreasing distally to only weak and sporadic expression in the distal large intestine[61].
It is known that cytosolic CAs I, II, and XIII are expressed in non-goblet epithelial cells of the mammalian colon[5,52,60,61], in which these isoenzymes may participate in the regulation of electroneutral NaCl reabsorption via the synchronous operation of apical Na+-H+ and Cl--HCO3- exchange[90]. In addition to cytosolic CAs I, II, and XIII, and mitochondrial CA V, intestinal enterocytes express at least four membrane-associated isoenzymes, CA IV[22], CA IX[61,78], CA XII[91], and CA XIV[55]. In the colon, CA IX is restricted to the colonic glands[61]. CA XII is highly expressed in the caecum, ascending colon, transverse colon, descending colon, sigmoid colon, and rectum. The expression is confined to the basolateral plasma membranes in enterocytes and is most prominent in the surface epithelial cuff region of the large intestine[91].
The new evidence that at least eight enzymatically active CA isoenzymes (CAs I, II, IV, V, IX, XII, XIII, and XIV) are expressed in the mammalian large intestine indicates the complexity of the physiological processes occurring in the GI tract. In addition, CA isoenzymes are differentially expressed along the intestinal segments and also between cryptal and villal enterocytes. Finally, it is a matter of debate whether the regulatory mechanisms could be different depending on the species studied. Even though some of these isoenzymes have heretofore been demonstrated only in rodents or humans, it is conceivable that general transport and pH regulation mechanisms are qualitatively similar in various species[92,93].
CA function is ultimately linked to certain transport proteins in both apical and basolateral cell membranes. In a recent article, Kunzelmann and Mall[94] have thoroughly reviewed these ion transport mechanisms in both colonic absorption and secretion. Identification of various ion transport proteins in the colon has made it possible to build schematic models for ion movement in different segments of the colon. Figure 3 represents a model for colonic ion and water transport that incorporates current knowledge regarding membrane transport processes[92,94-97]. It is important to emphasize that it is still a matter of discussion whether some of the proposed ion transport proteins are functional in the human colon. The absorption of NaCl can be electrogenic or electroneutral depending on the site of the process. In the proximal colon the absorption is primarily due to an electroneutral process via parallel luminal Na+/H+ and Cl-/HCO3- exchange[98]. Electroneutral luminal Na+ uptake is driven by the basolateral Na+-K+-ATPase lowering intracellular Na+ concentration. Na+ and Cl- transport is coupled via changes in intracellular pH and Cl- concentration[99]. Electroneutral NaCl absorption is also stimulated by short-chain fatty acids (SCFA), which are produced by colonic bacteria. The absorbed SCFA can stimulate apical Na+/H+ and Cl-/HCO3- exchangers, thus having a significant impact also on the regulation of colonic fluid balance and luminal as well as cytosolic pH[94]. The electrogenic absorption of NaCl mainly occurs in the distal colon via the luminal epithelial Na+ channels (ENaC) and transcellular/paracellular absorption of Cl-[100].
Figure 3.
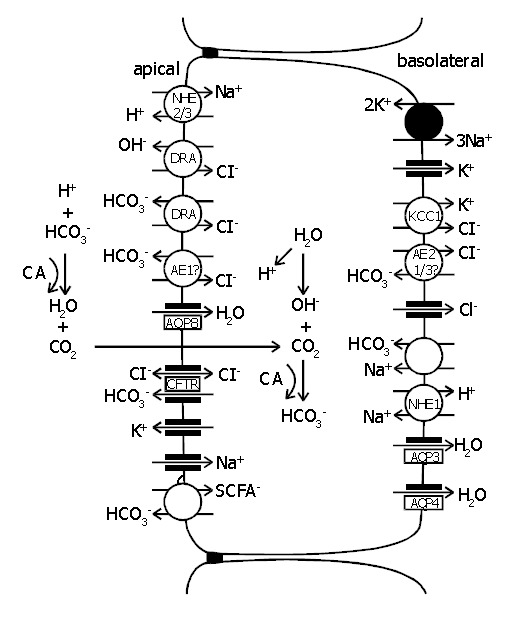
Schematic model of the cellular absorption and secretion of electrolytes and water in mammalian colon. Examples of ion transport mechanisms were demonstrated in a single enterocyte. Na+/H+ exchangers types 1, 2, and 3 (NHE1/2/3), proteins downregulated in adenoma (DRA), anion exchangers types 1, 2, and 3 (AE1/2/3), aquaporins 3, 4, and 8 (AQP3/4/8), cystic fibrosis transmembrane conductance regulator (CFTR), short-chain fatty acids (SCFA-), KCl cotransporter (KCC1), carbonic anhydrase (CA) [92,94-97] .
The participation of colonic CA in the electroneutral NaCl reabsorption is well established. Studies with acetazolamide suggest that CA activity is involved in the absorption of NaCl via the synchronous operation of apical Na+/H+ and Cl-/HCO3- exchange processes. CAs are also shown to participate in the alkalization of the luminal contents by generating HCO3- for apical Cl-/HCO3- exchange[101]. Recent exciting results from Casey’s laboratory have indicated that CAs can physically and functionally associate with Cl-/HCO3- (AE1) and Na+/H+ exchangers[102-104]. So far, CA IV is the only membrane-bound CA isoenzyme known to interact with AE1 protein[103]. It would be tempting to hypothesize that CAs IX, XII, and XIV could also drive ion exchange across the plasma membrane via direct links with transport proteins. The locations of membrane-associated isoenzymes CAs IV, IX, XII, and XIV in the surface epithelium of the colon, place them in a strategic position for the regulation of luminal and extracellular acid-base homeostasis and microclimate[22,55,61,91].
The net movement of water in colon is driven osmotically. Water is transported via both paracellular and transepithelial routes[105]. Although the proteins involved in NaCl absorption have been studied for decades, the exact molecular mechanisms of water absorption have remained largely unresolved. Recently, new information on water channel proteins, aquaporins (AQP), has opened new avenues to explore this understudied area of physiology. From different isoforms of AQPs, at least AQP3, AQP4, and AQP8 are expressed in colonic epithelial cells[106-109]. AQP3 and AQP4 are expressed at the basolateral plasma membrane like CA XII. AQP8 is confined to the apical plasma membrane like CA IV and CA XIV. Interestingly, Nakhoul et al[110] have shown that in Xenopus oocytes CO2 flux can be mediated by AQP1, and therefore, AQPs could act as a gas channel even though CO2 has earlier been believed to diffuse freely across the cell membranes. Despite that there is no experimental evidence yet available, one could speculate that AQPs would be an interesting target when searching protein interactions of membrane-bound CAs. By analogy to ion exchangers, AQPs are typically expressed in tissues expressing high levels of CAs such as colon, liver, pancreas, kidney, salivary gland, and stomach tissues[111].
CARBONIC ANHYDRASES IN NEOPLASTIC GASTROINTESTINAL TISSUES
To date, CA expression has been studied most extensively in colorectal tumors[91,112,113]. Scattered reports have also been published on hepatobiliary[114], pancreatic[83,115], esophageal[88], and gastric tumours[116]. In view of the recent experimental data, it appears inevitable that some CA isoenzymes may play a role in carcinogenic processes such as uncontrolled cell proliferation and malignant cell invasion. Studies have shown an important causal link between hypoxia, extracellular acidification, and induction or enhanced expression of these enzymes in human tumours[117]. A first direct link has been obtained by the discovery that isoenzymes CA IX and XII are down-regulated by wild-type VHL tumour suppressor protein, and a mutation in VHL gene can lead to overexpression of these enzymes[45].
CA IX is a predominant isoenzyme in various tumours including those arising from the GI tract. It has been demonstrated that CA IX is expressed in proliferating enterocytes of the normal human colon and its expression is increased in most adenomas and primary colorectal carcinomas[61,113]. The co-occurrence of CA IX and Ki-67 at the site of rapid cell proliferation indicates that CA IX could be used as a biomarker of increased cell proliferation in colorectal mucosa. Furthermore, its high expression in premalignant lesions such as adenomas suggests that it might be an especially useful biomarker in early stages of adenoma-carcinoma sequence.
Recent results have demonstrated that in colorectal tumours the expression of CA XII is up-regulated in the deep parts of the mucosa where it is weakly expressed[91]. In contrast, CAs I and II have shown maximal immunoreactions in normal colorectal mucosa, and the reactions decline in adenomas and carcinomas[118]. The reciprocal changes in the expression of soluble cytoplasmic isoenzymes (CAs I and II) and membrane-associated forms (CAs IX and XII) observed in colorectal tumours are probably due to different mechanisms. Loss of expression of the closely linked CA1 and 2 genes could result from loss of alleles specifying these genes. As a second option, the down-regulation of CAs I and II could also result from reduced levels of a common transcription factor. The latter is supported by a recent finding that a reduced expression of CA II in colorectal cancer is due to disruption of β-catenin/TCF-4 activity[119].
Up-regulation of CAs IX and XII could also be explained by two different mechanisms. First, contribution of microenvironmental stress to development of tumour phenotype has become an increasingly accepted explanation. Indeed, Wykoff et al[120] have demonstrated a close connection between tumour hypoxia and the expression of CAs IX and XII. They showed that up-regulation of CA IX occurred at the level of transcriptional activation via HIF-1 transcriptional complex, the critical mediator of hypoxic responses. The transcriptional complex hypoxia-inducible factor-1 (HIF-1) has emerged as an important mediator of gene expression patterns in tumours[121]. HIF-1 is regulated by ubiquitin-mediated proteolysis and targeted for destruction by the pVHL in normoxia and stabilized under hypoxia[122]. Both CA9 and CA12 are strongly induced by hypoxia in a range of tumour cell lines. They define a new class of HIF-1-responsive genes, the activation of which has implications for the understanding of hypoxic tumour metabolism and which may provide endogenous markers for tumour hypoxia[120].
Second, overexpression of CAs IX and XII has been associated with the loss of expression of the VHL tumour suppressor gene. In 1998, Ivanov and his group[45] used renal cell carcinoma cell lines stably transfected with wild-type VHL-expressing transgenes to discover genes involved in VHL-mediated carcinogenesis. The involvement of CAs IX and XII was indirectly supported by the findings that normal von Hippel-Lindau protein down-regulated the expression of both CA IX and CA XII, while the mutations in the VHL gene found in renal cell carcinomas could lead to overexpression of these isoenzymes.
The VHL gene is a tumour suppressor gene. This means that its role in a normal cell is to stop uncontrolled growth and proliferation. If the gene is deleted or mutated, its inhibitory effect on cell growth can be consequently lost or diminished, which in combination with defects in other regulatory proteins, can lead to cancerous growth. VHL seems to act as a ‘gatekeeper’ to the multistep process of tumourigenesis. The molecular mechanisms by which the product of VHL (pVHL) modulates the expression of target genes are not well understood. The original hypothesis based on the discovery of elongin B bound to pVHL assumed that VHL could negatively regulate transcription elongation of target genes, CA9 and CA12, by inhibiting the elongin function. Although this expectation has been confirmed in vitro, there is no compelling evidence to date that pVHL can exert the same effect in vivo[45,123,124].
Both CA IX and CA XII are transmembrane proteins with catalytic domain on the cell exterior, suggesting that they might participate in acid-base regulation of the extracellular space. There is substantial evidence that extracellular pH of human tumours is generally more acidic than that of normal tissues[125] and that this acidic pH may enhance both the migration and the invasive behaviour of some tumour cell types[126]. Ivanov et al[45] hypothesized that CA XII might be involved in tumour invasion by acidifying the extracellular milieu surrounding the cancer cells. This acidification would, in turn, contribute to the activation of enzymes involved in extracellular matrix degradation. This hypothesis is supported by more recent findings that acetazolamide, a potent inhibitor of CA activity, could reduce the invasive capacity of renal cancer cells[127]. Even more interesting from this point of view is the finding that a similar effect was described in a number of other malignant cell types including those derived from colorectal cancer[128].
The mechanism by which CA inhibitors affect tumour growth is not known at present, but several hypotheses have been proposed. These compounds might reduce the provision of bicarbonate for the synthesis of nucleotides and other cell components. On the other hand, CA inhibitors could prevent the acidification of extracellular milieu. A combination of several mechanisms is also possible. E7070, a member of recently reported class of antitumour sulfonamides, blocks cell cycle progression in the G1 phase. It has been suggested that E7070, possessing a free SO2NH2 moiety, probably acts as a strong CA inhibitor[129]. This compound demonstrates significant antitumour activity both in vitro and in vivo against different human tumours, e.g., human colon carcinoma. E7070 produces not only growth suppression but also reduction in tumour size. The clinical trials with E7070 may turn out to be successful, since CA inhibitor (acetazolamide) has already been shown to be beneficial in anticancer therapies in combination with cytotoxic agents[130].
Footnotes
Supported by the Grants from Sigrid Juselius Foundation, The Academy of Finland (SP), Finnish Cultural Foundation and Finnish Dental Society (JK)
Edited by Wang XL Proofread by Xu FM
References
- 1.Tashian RE. The carbonic anhydrases: widening perspectives on their evolution, expression and function. Bioessays. 1989;10:186–192. doi: 10.1002/bies.950100603. [DOI] [PubMed] [Google Scholar]
- 2.Chegwidden WR, Dodgson SJ, Spencer IM. The roles of carbonic anhydrase in metabolism, cell growth and cancer in animals. EXS. 2000:343–363. doi: 10.1007/978-3-0348-8446-4_16. [DOI] [PubMed] [Google Scholar]
- 3.Bundy HF. Carbonic anhydrase. Comp Biochem Physiol B. 1977;57:1–7. doi: 10.1016/0305-0491(77)90072-4. [DOI] [PubMed] [Google Scholar]
- 4.Lindskog S, Engberg P, Forsman C, Ibrahim SA, Jonsson BH, Simonsson I, Tibell L. Kinetics and mechanism of carbonic anhydrase isoenzymes. Ann N Y Acad Sci. 1984;429:61–75. doi: 10.1111/j.1749-6632.1984.tb12315.x. [DOI] [PubMed] [Google Scholar]
- 5.Lönnerholm G, Selking O, Wistrand PJ. Amount and distribution of carbonic anhydrases CA I and CA II in the gastrointestinal tract. Gastroenterology. 1985;88:1151–1161. doi: 10.1016/s0016-5085(85)80074-3. [DOI] [PubMed] [Google Scholar]
- 6.Venta PJ, Montgomery JC, Tashian RE. Molecular genetics of carbonic anhydrase isozymes. Isozymes Curr Top Biol Med Res. 1987;14:59–72. [PubMed] [Google Scholar]
- 7.Tashian RE, Goodman M, Headings VE, Ward RH, DeSimone J. Genetic variation and evolution in the red cell carbonic anhydrase isozymes of macaque monkeys. Biochem Genet. 1971;5:183–200. doi: 10.1007/BF00485644. [DOI] [PubMed] [Google Scholar]
- 8.Tashian RE, Kendall AG, Carter ND. Inherited variants of human red cell carbonic anhydrases. Hemoglobin. 1980;4:635–651. doi: 10.3109/03630268008997733. [DOI] [PubMed] [Google Scholar]
- 9.Tashian RE. Genetics of the mammalian carbonic anhydrases. Adv Genet. 1992;30:321–356. doi: 10.1016/s0065-2660(08)60323-5. [DOI] [PubMed] [Google Scholar]
- 10.Nakai H, Byers MG, Venta PJ, Tashian RE, Shows TB. The gene for human carbonic anhydrase II (CA2) is located at chromosome 8q22. Cytogenet Cell Genet. 1987;44:234–235. doi: 10.1159/000132378. [DOI] [PubMed] [Google Scholar]
- 11.Khalifah RG. The carbon dioxide hydration activity of carbonic anhydrase. I. Stop-flow kinetic studies on the native human isoenzymes B and C. J Biol Chem. 1971;246:2561–2573. [PubMed] [Google Scholar]
- 12.Sanyal G, Maren TH. Thermodynamics of carbonic anhydrase catalysis. A comparison between human isoenzymes B and C. J Biol Chem. 1981;256:608–612. [PubMed] [Google Scholar]
- 13.Wistrand PJ. The importance of carbonic anhydrase B and C for the unloading of CO2 by the human erythrocyte. Acta Physiol Scand. 1981;113:417–426. doi: 10.1111/j.1748-1716.1981.tb06918.x. [DOI] [PubMed] [Google Scholar]
- 14.Sly WS, Hu PY. Human carbonic anhydrases and carbonic anhydrase deficiencies. Annu Rev Biochem. 1995;64:375–401. doi: 10.1146/annurev.bi.64.070195.002111. [DOI] [PubMed] [Google Scholar]
- 15.Sly WS, Hewett-Emmett D, Whyte MP, Yu YS, Tashian RE. Carbonic anhydrase II deficiency identified as the primary defect in the autosomal recessive syndrome of osteopetrosis with renal tubular acidosis and cerebral calcification. Proc Natl Acad Sci USA. 1983;80:2752–2756. doi: 10.1073/pnas.80.9.2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sly WS, Whyte MP, Sundaram V, Tashian RE, Hewett-Emmett D, Guibaud P, Vainsel M, Baluarte HJ, Gruskin A, Al-Mosawi M. Carbonic anhydrase II deficiency in 12 families with the autosomal recessive syndrome of osteopetrosis with renal tubular acidosis and cerebral calcification. N Engl J Med. 1985;313:139–145. doi: 10.1056/NEJM198507183130302. [DOI] [PubMed] [Google Scholar]
- 17.Sly WS, Hu PY. The carbonic anhydrase II deficiency syndrome: osteopetrosis with renal tubular acidosis and cerebral calcification. In: Scriver CR, Beaudet AL, Sly WS, Valle D, eds , et al., editors. The Molecular and Metabolic Bases of Inherited Disease. New York: McGraw-Hill; 1995. pp. 4113–4124. [Google Scholar]
- 18.Räisänen SR, Lehenkari P, Tasanen M, Rahkila P, Härkönen PL, Väänänen HK. Carbonic anhydrase III protects cells from hydrogen peroxide-induced apoptosis. FASEB J. 1999;13:513–522. doi: 10.1096/fasebj.13.3.513. [DOI] [PubMed] [Google Scholar]
- 19.Okuyama T, Sato S, Zhu XL, Waheed A, Sly WS. Human carbonic anhydrase IV: cDNA cloning, sequence comparison, and expression in COS cell membranes. Proc Natl Acad Sci USA. 1992;89:1315–1319. doi: 10.1073/pnas.89.4.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Okuyama T, Batanian JR, Sly WS. Genomic organization and localization of gene for human carbonic anhydrase IV to chromosome 17q. Genomics. 1993;16:678–684. doi: 10.1006/geno.1993.1247. [DOI] [PubMed] [Google Scholar]
- 21.Zhu XL, Sly WS. Carbonic anhydrase IV from human lung. Purification, characterization, and comparison with membrane carbonic anhydrase from human kidney. J Biol Chem. 1990;265:8795–8801. [PubMed] [Google Scholar]
- 22.Fleming RE, Parkkila S, Parkkila AK, Rajaniemi H, Waheed A, Sly WS. Carbonic anhydrase IV expression in rat and human gastrointestinal tract regional, cellular, and subcellular localization. J Clin Invest. 1995;96:2907–2913. doi: 10.1172/JCI118362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parkkila S, Parkkila AK, Juvonen T, Waheed A, Sly WS, Saarnio J, Kaunisto K, Kellokumpu S, Rajaniemi H. Membrane-bound carbonic anhydrase IV is expressed in the luminal plasma membrane of the human gallbladder epithelium. Hepatology. 1996;24:1104–1108. doi: 10.1002/hep.510240521. [DOI] [PubMed] [Google Scholar]
- 24.Nagao Y, Platero JS, Waheed A, Sly WS. Human mitochondrial carbonic anhydrase: cDNA cloning, expression, subcellular localization, and mapping to chromosome 16. Proc Natl Acad Sci USA. 1993;90:7623–7627. doi: 10.1073/pnas.90.16.7623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shah GN, Hewett-Emmett D, Grubb JH, Migas MC, Fleming RE, Waheed A, Sly WS. Mitochondrial carbonic anhydrase CA VB: differences in tissue distribution and pattern of evolution from those of CA VA suggest distinct physiological roles. Proc Natl Acad Sci USA. 2000;97:1677–1682. doi: 10.1073/pnas.97.4.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fujikawa-Adachi K, Nishimori I, Taguchi T, Onishi S. Human mitochondrial carbonic anhydrase VB. cDNA cloning, mRNA expression, subcellular localization, and mapping to chromosome x. J Biol Chem. 1999;274:21228–21233. doi: 10.1074/jbc.274.30.21228. [DOI] [PubMed] [Google Scholar]
- 27.Kadoya Y, Kuwahara H, Shimazaki M, Ogawa Y, Yagi T. Isolation of a novel carbonic anhydrase from human saliva and immunohistochemical demonstration of its related isozymes in salivary gland. Osaka City Med J. 1987;33:99–109. [PubMed] [Google Scholar]
- 28.Murakami H, Sly WS. Purification and characterization of human salivary carbonic anhydrase. J Biol Chem. 1987;262:1382–1388. [PubMed] [Google Scholar]
- 29.Feldstein JB, Silverman DN. Purification and characterization of carbonic anhydrase from the saliva of the rat. J Biol Chem. 1984;259:5447–5453. [PubMed] [Google Scholar]
- 30.Ogawa Y, Chang CK, Kuwahara H, Hong SS, Toyosawa S, Yagi T. Immunoelectron microscopy of carbonic anhydrase isozyme VI in rat submandibular gland: comparison with isozymes I and II. J Histochem Cytochem. 1992;40:807–817. doi: 10.1177/40.6.1588027. [DOI] [PubMed] [Google Scholar]
- 31.Fernley RT. Carbonic anhydrases secreted in the saliva. In: Dodgson SJ, Tashian RE, Gros G, Carter ND, eds , et al., editors. The Carbonic Anhydrases. Cellular physiology and molecular genetics. New York: Plenum Press; 1991. pp. 365–373. [Google Scholar]
- 32.Fernley RT. Secreted carbonic anhydrases. In: Botré F, Gros G, Storey BT, eds , et al., editors. Carbonic anhydrase. From biochemistry and genetics to physiology and clinical medicine. Weinheim: VCH Verlagsgesellschaft; 1991. pp. 178–185. [Google Scholar]
- 33.Parkkila S, Kaunisto K, Rajaniemi H. Location of the carbonic anhydrase isoenzymes VI and II in human salivary glands by immunohistochemistry. In: Botré F, Gros G, Storey BT, eds , et al., editors. Carbonic anhydrase. From Biochemistry and Genetics to Physiology and Clinical Medicine. Weinheim: VCH Verlagsgesellschaft; 1991. pp. 254–257. [Google Scholar]
- 34.Fujikawa-Adachi K, Nishimori I, Sakamoto S, Morita M, Onishi S, Yonezawa S, Hollingsworth MA. Identification of carbonic anhydrase IV and VI mRNA expression in human pancreas and salivary glands. Pancreas. 1999;18:329–335. doi: 10.1097/00006676-199905000-00001. [DOI] [PubMed] [Google Scholar]
- 35.Mori K, Ogawa Y, Ebihara K, Tamura N, Tashiro K, Kuwahara T, Mukoyama M, Sugawara A, Ozaki S, Tanaka I, et al. Isolation and characterization of CA XIV, a novel membrane-bound carbonic anhydrase from mouse kidney. J Biol Chem. 1999;274:15701–15705. doi: 10.1074/jbc.274.22.15701. [DOI] [PubMed] [Google Scholar]
- 36.Montgomery JC, Venta PJ, Eddy RL, Fukushima YS, Shows TB, Tashian RE. Characterization of the human gene for a newly discovered carbonic anhydrase, CA VII, and its localization to chromosome 16. Genomics. 1991;11:835–848. doi: 10.1016/0888-7543(91)90006-z. [DOI] [PubMed] [Google Scholar]
- 37.Lakkis MM, O'Shea KS, Tashian RE. Differential expression of the carbonic anhydrase genes for CA VII (Car7) and CA-RP VIII (Car8) in mouse brain. J Histochem Cytochem. 1997;45:657–662. doi: 10.1177/002215549704500503. [DOI] [PubMed] [Google Scholar]
- 38.Earnhardt JN, Qian M, Tu C, Lakkis MM, Bergenhem NC, Laipis PJ, Tashian RE, Silverman DN. The catalytic properties of murine carbonic anhydrase VII. Biochemistry. 1998;37:10837–10845. doi: 10.1021/bi980046t. [DOI] [PubMed] [Google Scholar]
- 39.Ling B, Bergenhem NCH, Dodgson SJ, Forster RE, Tashian RE. Determination of mRNA levels for six carbonic anhydrase isozymes in rat lung. Isozyme Bull. 1994;28:32. [Google Scholar]
- 40.Lakkis MM, Bergenhem NC, Tashian RE. Expression of mouse carbonic anhydrase VII in E. coli and demonstration of its CO2 hydrase activity. Biochem Biophys Res Commun. 1996;226:268–272. doi: 10.1006/bbrc.1996.1344. [DOI] [PubMed] [Google Scholar]
- 41.Liao SY, Brewer C, Závada J, Pastorek J, Pastorekova S, Manetta A, Berman ML, DiSaia PJ, Stanbridge EJ. Identification of the MN antigen as a diagnostic biomarker of cervical intraepithelial squamous and glandular neoplasia and cervical carcinomas. Am J Pathol. 1994;145:598–609. [PMC free article] [PubMed] [Google Scholar]
- 42.Pastorek J, Pastoreková S, Callebaut I, Mornon JP, Zelník V, Opavský R, Zat'ovicová M, Liao S, Portetelle D, Stanbridge EJ. Cloning and characterization of MN, a human tumor-associated protein with a domain homologous to carbonic anhydrase and a putative helix-loop-helix DNA binding segment. Oncogene. 1994;9:2877–2888. [PubMed] [Google Scholar]
- 43.Opavský R, Pastoreková S, Zelník V, Gibadulinová A, Stanbridge EJ, Závada J, Kettmann R, Pastorek J. Human MN/CA9 gene, a novel member of the carbonic anhydrase family: structure and exon to protein domain relationships. Genomics. 1996;33:480–487. doi: 10.1006/geno.1996.0223. [DOI] [PubMed] [Google Scholar]
- 44.Hewett-Emmett D, Tashian RE. Functional diversity, conservation, and convergence in the evolution of the alpha-, beta-, and gamma-carbonic anhydrase gene families. Mol Phylogenet Evol. 1996;5:50–77. doi: 10.1006/mpev.1996.0006. [DOI] [PubMed] [Google Scholar]
- 45.Ivanov SV, Kuzmin I, Wei MH, Pack S, Geil L, Johnson BE, Stanbridge EJ, Lerman MI. Down-regulation of transmembrane carbonic anhydrases in renal cell carcinoma cell lines by wild-type von Hippel-Lindau transgenes. Proc Natl Acad Sci USA. 1998;95:12596–12601. doi: 10.1073/pnas.95.21.12596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pastoreková S, Závadová Z, Kostál M, Babusíková O, Závada J. A novel quasi-viral agent, MaTu, is a two-component system. Virology. 1992;187:620–626. doi: 10.1016/0042-6822(92)90464-z. [DOI] [PubMed] [Google Scholar]
- 47.Kaluz S, Kaluzová M, Opavský R, Pastoreková S, Gibadulinová A, Dequiedt F, Kettmann R, Pastorek J. Transcriptional regulation of the MN/CA 9 gene coding for the tumor-associated carbonic anhydrase IX. Identification and characterization of a proximal silencer element. J Biol Chem. 1999;274:32588–32595. doi: 10.1074/jbc.274.46.32588. [DOI] [PubMed] [Google Scholar]
- 48.Chen F, Kishida T, Duh FM, Renbaum P, Orcutt ML, Schmidt L, Zbar B. Suppression of growth of renal carcinoma cells by the von Hippel-Lindau tumor suppressor gene. Cancer Res. 1995;55:4804–4807. [PubMed] [Google Scholar]
- 49.Závada J, Závadová Z, Pastoreková S, Ciampor F, Pastorek J, Zelník V. Expression of MaTu-MN protein in human tumor cultures and in clinical specimens. Int J Cancer. 1993;54:268–274. doi: 10.1002/ijc.2910540218. [DOI] [PubMed] [Google Scholar]
- 50.Türeci O, Sahin U, Vollmar E, Siemer S, Göttert E, Seitz G, Parkkila AK, Shah GN, Grubb JH, Pfreundschuh M, et al. Human carbonic anhydrase XII: cDNA cloning, expression, and chromosomal localization of a carbonic anhydrase gene that is overexpressed in some renal cell cancers. Proc Natl Acad Sci USA. 1998;95:7608–7613. doi: 10.1073/pnas.95.13.7608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ulmasov B, Waheed A, Shah GN, Grubb JH, Sly WS, Tu C, Silverman DN. Purification and kinetic analysis of recombinant CA XII, a membrane carbonic anhydrase overexpressed in certain cancers. Proc Natl Acad Sci USA. 2000;97:14212–14217. doi: 10.1073/pnas.97.26.14212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lehtonen J, Shen B, Vihinen M, Casini A, Scozzafava A, Supuran CT, Parkkila AK, Saarnio J, Kivelä AJ, Waheed A, et al. Characterization of CA XIII, a novel member of the carbonic anhydrase isozyme family. J Biol Chem. 2004;279:2719–2727. doi: 10.1074/jbc.M308984200. [DOI] [PubMed] [Google Scholar]
- 53.Fujikawa-Adachi K, Nishimori I, Taguchi T, Onishi S. Human carbonic anhydrase XIV (CA14): cDNA cloning, mRNA expression, and mapping to chromosome 1. Genomics. 1999;61:74–81. doi: 10.1006/geno.1999.5938. [DOI] [PubMed] [Google Scholar]
- 54.Parkkila S, Parkkila AK, Rajaniemi H, Shah GN, Grubb JH, Waheed A, Sly WS. Expression of membrane-associated carbonic anhydrase XIV on neurons and axons in mouse and human brain. Proc Natl Acad Sci USA. 2001;98:1918–1923. doi: 10.1073/pnas.98.4.1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Parkkila S, Kivelä AJ, Kaunisto K, Parkkila AK, Hakkola J, Rajaniemi H, Waheed A, Sly WS. The plasma membrane carbonic anhydrase in murine hepatocytes identified as isozyme XIV. BMC Gastroenterol. 2002;2:13. doi: 10.1186/1471-230X-2-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kaunisto K, Parkkila S, Rajaniemi H, Waheed A, Grubb J, Sly WS. Carbonic anhydrase XIV: luminal expression suggests key role in renal acidification. Kidney Int. 2002;61:2111–2118. doi: 10.1046/j.1523-1755.2002.00371.x. [DOI] [PubMed] [Google Scholar]
- 57.Karhumaa P, Parkkila S, Waheed A, Parkkila AK, Kaunisto K, Tucker PW, Huang CJ, Sly WS, Rajaniemi H. Nuclear NonO/p54(nrb) protein is a nonclassical carbonic anhydrase. J Biol Chem. 2000;275:16044–16049. doi: 10.1074/jbc.275.21.16044. [DOI] [PubMed] [Google Scholar]
- 58.Yang YS, Hanke JH, Carayannopoulos L, Craft CM, Capra JD, Tucker PW. NonO, a non-POU-domain-containing, octamer-binding protein, is the mammalian homolog of Drosophila nonAdiss. Mol Cell Biol. 1993;13:5593–5603. doi: 10.1128/mcb.13.9.5593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dong B, Horowitz DS, Kobayashi R, Krainer AR. Purification and cDNA cloning of HeLa cell p54nrb, a nuclear protein with two RNA recognition motifs and extensive homology to human splicing factor PSF and Drosophila NONA/BJ6. Nucleic Acids Res. 1993;21:4085–4092. doi: 10.1093/nar/21.17.4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Parkkila S, Parkkila AK, Juvonen T, Rajaniemi H. Distribution of the carbonic anhydrase isoenzymes I, II, and VI in the human alimentary tract. Gut. 1994;35:646–650. doi: 10.1136/gut.35.5.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Saarnio J, Parkkila S, Parkkila AK, Waheed A, Casey MC, Zhou XY, Pastoreková S, Pastorek J, Karttunen T, Haukipuro K, et al. Immunohistochemistry of carbonic anhydrase isozyme IX (MN/CA IX) in human gut reveals polarized expression in the epithelial cells with the highest proliferative capacity. J Histochem Cytochem. 1998;46:497–504. doi: 10.1177/002215549804600409. [DOI] [PubMed] [Google Scholar]
- 62.Leinonen J, Parkkila S, Kaunisto K, Koivunen P, Rajaniemi H. Secretion of carbonic anhydrase isoenzyme VI (CA VI) from human and rat lingual serous von Ebner's glands. J Histochem Cytochem. 2001;49:657–662. doi: 10.1177/002215540104900513. [DOI] [PubMed] [Google Scholar]
- 63.Parkkila S, Parkkila AK. Carbonic anhydrase in the alimentary tract. Roles of the different isozymes and salivary factors in the maintenance of optimal conditions in the gastrointestinal canal. Scand J Gastroenterol. 1996;31:305–317. doi: 10.3109/00365529609006403. [DOI] [PubMed] [Google Scholar]
- 64.Parkkila S, Kaunisto K, Rajaniemi L, Kumpulainen T, Jokinen K, Rajaniemi H. Immunohistochemical localization of carbonic anhydrase isoenzymes VI, II, and I in human parotid and submandibular glands. J Histochem Cytochem. 1990;38:941–947. doi: 10.1177/38.7.2113069. [DOI] [PubMed] [Google Scholar]
- 65.Leinonen J, Kivelä J, Parkkila S, Parkkila AK, Rajaniemi H. Salivary carbonic anhydrase isoenzyme VI is located in the human enamel pellicle. Caries Res. 1999;33:185–190. doi: 10.1159/000016515. [DOI] [PubMed] [Google Scholar]
- 66.Kivela J, Parkkila S, Parkkila AK, Leinonen J, Rajaniemi H. Salivary carbonic anhydrase isoenzyme VI. J Physiol. 1999;520 Pt 2:315–320. doi: 10.1111/j.1469-7793.1999.t01-1-00315.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Parkkila S, Parkkila AK, Lehtola J, Reinilä A, Södervik HJ, Rannisto M, Rajaniemi H. Salivary carbonic anhydrase protects gastroesophageal mucosa from acid injury. Dig Dis Sci. 1997;42:1013–1019. doi: 10.1023/a:1018889120034. [DOI] [PubMed] [Google Scholar]
- 68.Kivelä J, Parkkila S, Parkkila AK, Rajaniemi H. A low concentration of carbonic anhydrase isoenzyme VI in whole saliva is associated with caries prevalence. Caries Res. 1999;33:178–184. doi: 10.1159/000016514. [DOI] [PubMed] [Google Scholar]
- 69.Karhumaa P, Leinonen J, Parkkila S, Kaunisto K, Tapanainen J, Rajaniemi H. The identification of secreted carbonic anhydrase VI as a constitutive glycoprotein of human and rat milk. Proc Natl Acad Sci USA. 2001;98:11604–11608. doi: 10.1073/pnas.121172598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Thatcher BJ, Doherty AE, Orvisky E, Martin BM, Henkin RI. Gustin from human parotid saliva is carbonic anhydrase VI. Biochem Biophys Res Commun. 1998;250:635–641. doi: 10.1006/bbrc.1998.9356. [DOI] [PubMed] [Google Scholar]
- 71.Sok J, Wang XZ, Batchvarova N, Kuroda M, Harding H, Ron D. CHOP-Dependent stress-inducible expression of a novel form of carbonic anhydrase VI. Mol Cell Biol. 1999;19:495–504. doi: 10.1128/mcb.19.1.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Swenson ER. Distribution and functions of carbonic anhydrase in the gastrointestinal tract. In: Dodgson SJ, Tashian RE, Gros G, Carter ND, eds , et al., editors. The Carbonic Anhydrases. Cellular Physiology and Molecular Genetics. New York: Plenum Press; 1991. pp. 265–287. [Google Scholar]
- 73.Dodgson SJ. Liver mitochondrial carbonic anhydrase (CA V), gluconeogenesis, and ureagenesis in the hepatocyte. In: Dodgson SJ, Tashian RE, Gros G, Carter ND, eds , et al., editors. The Carbonic Anhydrases. Cellular Physiology and Molecular Genetics. New York: Plenum Press; 1991. pp. 297–306. [Google Scholar]
- 74.Jeffery S, Edwards Y, Carter N. Distribution of CAIII in fetal and adult human tissue. Biochem Genet. 1980;18:843–849. doi: 10.1007/BF00500117. [DOI] [PubMed] [Google Scholar]
- 75.Carter N, Wistrand PJ, Lönnerholm G. Carbonic anhydrase localization to perivenous hepatocytes. Acta Physiol Scand. 1989;135:163–167. doi: 10.1111/j.1748-1716.1989.tb08563.x. [DOI] [PubMed] [Google Scholar]
- 76.Cabiscol E, Levine RL. Carbonic anhydrase III. Oxidative modification in vivo and loss of phosphatase activity during aging. J Biol Chem. 1995;270:14742–14747. doi: 10.1074/jbc.270.24.14742. [DOI] [PubMed] [Google Scholar]
- 77.Juvonen T, Parkkila S, Parkkila AK, Niemelä O, Lajunen LH, Kairaluoma MI, Perämäki P, Rajaniemi H. High-activity carbonic anhydrase isoenzyme (CA II) in human gallbladder epithelium. J Histochem Cytochem. 1994;42:1393–1397. doi: 10.1177/42.10.7930522. [DOI] [PubMed] [Google Scholar]
- 78.Pastoreková S, Parkkila S, Parkkila AK, Opavský R, Zelník V, Saarnio J, Pastorek J. Carbonic anhydrase IX, MN/CA IX: analysis of stomach complementary DNA sequence and expression in human and rat alimentary tracts. Gastroenterology. 1997;112:398–408. doi: 10.1053/gast.1997.v112.pm9024293. [DOI] [PubMed] [Google Scholar]
- 79.Parkkila AK, Scarim AL, Parkkila S, Waheed A, Corbett JA, Sly WS. Expression of carbonic anhydrase V in pancreatic beta cells suggests role for mitochondrial carbonic anhydrase in insulin secretion. J Biol Chem. 1998;273:24620–24623. doi: 10.1074/jbc.273.38.24620. [DOI] [PubMed] [Google Scholar]
- 80.Kumpulainen T, Jalovaara P. Immunohistochemical localization of carbonic anhydrase isoenzymes in the human pancreas. Gastroenterology. 1981;80:796–799. [PubMed] [Google Scholar]
- 81.Kumpulainen T. Immunohistochemical localization of human carbonic anhydrase isozymes. Ann N Y Acad Sci. 1984;429:359–368. doi: 10.1111/j.1749-6632.1984.tb12360.x. [DOI] [PubMed] [Google Scholar]
- 82.Spicer SS, Sens MA, Tashian RE. Immunocytochemical demonstration of carbonic anhydrase in human epithelial cells. J Histochem Cytochem. 1982;30:864–873. doi: 10.1177/30.9.6813372. [DOI] [PubMed] [Google Scholar]
- 83.Kivelä AJ, Parkkila S, Saarnio J, Karttunen TJ, Kivelä J, Parkkila AK, Pastoreková S, Pastorek J, Waheed A, Sly WS, et al. Expression of transmembrane carbonic anhydrase isoenzymes IX and XII in normal human pancreas and pancreatic tumours. Histochem Cell Biol. 2000;114:197–204. doi: 10.1007/s004180000181. [DOI] [PubMed] [Google Scholar]
- 84.Ishiguro H, Naruse S, San Román JI, Case M, Steward MC. Pancreatic ductal bicarbonate secretion: past, present and future. JOP. 2001;2:192–197. [PubMed] [Google Scholar]
- 85.Gut MO, Parkkila S, Vernerová Z, Rohde E, Závada J, Höcker M, Pastorek J, Karttunen T, Gibadulinová A, Závadová Z, et al. Gastric hyperplasia in mice with targeted disruption of the carbonic anhydrase gene Car9. Gastroenterology. 2002;123:1889–1903. doi: 10.1053/gast.2002.37052. [DOI] [PubMed] [Google Scholar]
- 86.Christie KN, Thomson C, Xue L, Lucocq JM, Hopwood D. Carbonic anhydrase isoenzymes I, II, III, and IV are present in human esophageal epithelium. J Histochem Cytochem. 1997;45:35–40. doi: 10.1177/002215549704500105. [DOI] [PubMed] [Google Scholar]
- 87.Meyers RL, Orlando RC. In vivo bicarbonate secretion by human esophagus. Gastroenterology. 1992;103:1174–1178. doi: 10.1016/0016-5085(92)91501-t. [DOI] [PubMed] [Google Scholar]
- 88.Turner JR, Odze RD, Crum CP, Resnick MB. MN antigen expression in normal, preneoplastic, and neoplastic esophagus: a clinicopathological study of a new cancer-associated biomarker. Hum Pathol. 1997;28:740–744. doi: 10.1016/s0046-8177(97)90185-4. [DOI] [PubMed] [Google Scholar]
- 89.Richardson CT. Pathogenetic factors in peptic ulcer disease. Am J Med. 1985;79:1–7. doi: 10.1016/0002-9343(85)90564-9. [DOI] [PubMed] [Google Scholar]
- 90.Charney AN, Egnor RW. Membrane site of action of CO2 on colonic sodium absorption. Am J Physiol. 1989;256:C584–C590. doi: 10.1152/ajpcell.1989.256.3.C584. [DOI] [PubMed] [Google Scholar]
- 91.Kivelä A, Parkkila S, Saarnio J, Karttunen TJ, Kivelä J, Parkkila AK, Waheed A, Sly WS, Grubb JH, Shah G, et al. Expression of a novel transmembrane carbonic anhydrase isozyme XII in normal human gut and colorectal tumors. Am J Pathol. 2000;156:577–584. doi: 10.1016/S0002-9440(10)64762-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dawson DC. Ion channels and colonic salt transport. Annu Rev Physiol. 1991;53:321–339. doi: 10.1146/annurev.ph.53.030191.001541. [DOI] [PubMed] [Google Scholar]
- 93.Wills NK. Apical membrane potassium and chloride permeabilities in surface cells of rabbit descending colon epithelium. J Physiol. 1985;358:433–445. doi: 10.1113/jphysiol.1985.sp015560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kunzelmann K, Mall M. Electrolyte transport in the mammalian colon: mechanisms and implications for disease. Physiol Rev. 2002;82:245–289. doi: 10.1152/physrev.00026.2001. [DOI] [PubMed] [Google Scholar]
- 95.Flemström G, Isenberg JI. Gastroduodenal mucosal alkaline secretion and mucosal protection. News Physiol Sci. 2001;16:23–28. doi: 10.1152/physiologyonline.2001.16.1.23. [DOI] [PubMed] [Google Scholar]
- 96.Alrefai WA, Tyagi S, Nazir TM, Barakat J, Anwar SS, Hadjiagapiou C, Bavishi D, Sahi J, Malik P, Goldstein J, et al. Human intestinal anion exchanger isoforms: expression, distribution, and membrane localization. Biochim Biophys Acta. 2001;1511:17–27. doi: 10.1016/s0005-2736(00)00366-7. [DOI] [PubMed] [Google Scholar]
- 97.Seidler U, Bachmann O, Jacob P, Christiani S, Blumenstein I, Rossmann H. Na+/HCO3- cotransport in normal and cystic fibrosis intestine. JOP. 2001;2:247–256. [PubMed] [Google Scholar]
- 98.Rajendran VM, Binder HJ. Characterization and molecular localization of anion transporters in colonic epithelial cells. Ann N Y Acad Sci. 2000;915:15–29. doi: 10.1111/j.1749-6632.2000.tb05220.x. [DOI] [PubMed] [Google Scholar]
- 99.Geibel JP, Rajendran VM, Binder HJ. Na(+)-dependent fluid absorption in intact perfused rat colonic crypts. Gastroenterology. 2001;120:144–150. doi: 10.1053/gast.2001.20890. [DOI] [PubMed] [Google Scholar]
- 100.Greger R, Bleich M, Leipziger J, Ecke D, Mall M, Kunzelmann K. Regulation of ion transport in colonic crypts. News Physiol Sci. 1997;12:62–66. [Google Scholar]
- 101.Feldman GM. HCO3- secretion by rat distal colon: effects of inhibitors and extracellular Na+ Gastroenterology. 1994;107:329–338. doi: 10.1016/0016-5085(94)90156-2. [DOI] [PubMed] [Google Scholar]
- 102.Sterling D, Reithmeier RA, Casey JR. A transport metabolon. Functional interaction of carbonic anhydrase II and chloride/bicarbonate exchangers. J Biol Chem. 2001;276:47886–47894. doi: 10.1074/jbc.M105959200. [DOI] [PubMed] [Google Scholar]
- 103.Sterling D, Alvarez BV, Casey JR. The extracellular component of a transport metabolon. Extracellular loop 4 of the human AE1 Cl-/HCO3- exchanger binds carbonic anhydrase IV. J Biol Chem. 2002;277:25239–25246. doi: 10.1074/jbc.M202562200. [DOI] [PubMed] [Google Scholar]
- 104.Li X, Alvarez B, Casey JR, Reithmeier RA, Fliegel L. Carbonic anhydrase II binds to and enhances activity of the Na+/H+ exchanger. J Biol Chem. 2002;277:36085–36091. doi: 10.1074/jbc.M111952200. [DOI] [PubMed] [Google Scholar]
- 105.Spring KR. Routes and mechanism of fluid transport by epithelia. Annu Rev Physiol. 1998;60:105–119. doi: 10.1146/annurev.physiol.60.1.105. [DOI] [PubMed] [Google Scholar]
- 106.Hasegawa H, Lian SC, Finkbeiner WE, Verkman AS. Extrarenal tissue distribution of CHIP28 water channels by in situ hybridization and antibody staining. Am J Physiol. 1994;266:C893–C903. doi: 10.1152/ajpcell.1994.266.4.C893. [DOI] [PubMed] [Google Scholar]
- 107.Ishibashi K, Sasaki S, Saito F, Ikeuchi T, Marumo F. Structure and chromosomal localization of a human water channel (AQP3) gene. Genomics. 1995;27:352–354. doi: 10.1006/geno.1995.1055. [DOI] [PubMed] [Google Scholar]
- 108.Frigeri A, Gropper MA, Turck CW, Verkman AS. Immunolocalization of the mercurial-insensitive water channel and glycerol intrinsic protein in epithelial cell plasma membranes. Proc Natl Acad Sci USA. 1995;92:4328–4331. doi: 10.1073/pnas.92.10.4328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Koyama Y, Yamamoto T, Kondo D, Funaki H, Yaoita E, Kawasaki K, Sato N, Hatakeyama K, Kihara I. Molecular cloning of a new aquaporin from rat pancreas and liver. J Biol Chem. 1997;272:30329–30333. doi: 10.1074/jbc.272.48.30329. [DOI] [PubMed] [Google Scholar]
- 110.Nakhoul NL, Davis BA, Romero MF, Boron WF. Effect of expressing the water channel aquaporin-1 on the CO2 permeability of Xenopus oocytes. Am J Physiol. 1998;274:C543–C548. doi: 10.1152/ajpcell.1998.274.2.C543. [DOI] [PubMed] [Google Scholar]
- 111.Ma T, Verkman AS. Aquaporin water channels in gastrointestinal physiology. J Physiol. 1999;517(Pt 2):317–326. doi: 10.1111/j.1469-7793.1999.0317t.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mori M, Staniunas RJ, Barnard GF, Jessup JM, Steele GD, Chen LB. The significance of carbonic anhydrase expression in human colorectal cancer. Gastroenterology. 1993;105:820–826. doi: 10.1016/0016-5085(93)90900-w. [DOI] [PubMed] [Google Scholar]
- 113.Saarnio J, Parkkila S, Parkkila AK, Haukipuro K, Pastoreková S, Pastorek J, Kairaluoma MI, Karttunen TJ. Immunohistochemical study of colorectal tumors for expression of a novel transmembrane carbonic anhydrase, MN/CA IX, with potential value as a marker of cell proliferation. Am J Pathol. 1998;153:279–285. doi: 10.1016/S0002-9440(10)65569-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Saarnio J, Parkkila S, Parkkila AK, Pastoreková S, Haukipuro K, Pastorek J, Juvonen T, Karttunen TJ. Transmembrane carbonic anhydrase, MN/CA IX, is a potential biomarker for biliary tumours. J Hepatol. 2001;35:643–649. doi: 10.1016/s0168-8278(01)00193-3. [DOI] [PubMed] [Google Scholar]
- 115.Parkkila S, Parkkila AK, Juvonen T, Lehto VP, Rajaniemi H. Immunohistochemical demonstration of the carbonic anhydrase isoenzymes I and II in pancreatic tumours. Histochem J. 1995;27:133–138. doi: 10.1007/BF00243908. [DOI] [PubMed] [Google Scholar]
- 116.Leppilampi M, Saarnio J, Karttunen TJ, Kivelä J, Pastoreková S, Pastorek J, Waheed A, Sly WS, Parkkila S. Carbonic anhydrase isozymes IX and XII in gastric tumors. World J Gastroenterol. 2003;9:1398–1403. doi: 10.3748/wjg.v9.i7.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ivanov S, Liao SY, Ivanova A, Danilkovitch-Miagkova A, Tarasova N, Weirich G, Merrill MJ, Proescholdt MA, Oldfield EH, Lee J, et al. Expression of hypoxia-inducible cell-surface transmembrane carbonic anhydrases in human cancer. Am J Pathol. 2001;158:905–919. doi: 10.1016/S0002-9440(10)64038-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kivela AJ, Saarnio J, Karttunen TJ, Kivelä J, Parkkila AK, Pastorekova S, Pastorek J, Waheed A, Sly WS, Parkkila TS, et al. Differential expression of cytoplasmic carbonic anhydrases, CA I and II, and membrane-associated isozymes, CA IX and XII, in normal mucosa of large intestine and in colorectal tumors. Dig Dis Sci. 2001;46:2179–2186. doi: 10.1023/a:1011910931210. [DOI] [PubMed] [Google Scholar]
- 119.van de Wetering M, Sancho E, Verweij C, de Lau W, Oving I, Hurlstone A, van der Horn K, Batlle E, Coudreuse D, Haramis AP, et al. The beta-catenin/TCF-4 complex imposes a crypt progenitor phenotype on colorectal cancer cells. Cell. 2002;111:241–250. doi: 10.1016/s0092-8674(02)01014-0. [DOI] [PubMed] [Google Scholar]
- 120.Wykoff CC, Beasley NJ, Watson PH, Turner KJ, Pastorek J, Sibtain A, Wilson GD, Turley H, Talks KL, Maxwell PH, et al. Hypoxia-inducible expression of tumor-associated carbonic anhydrases. Cancer Res. 2000;60:7075–7083. [PubMed] [Google Scholar]
- 121.Wenger RH, Gassmann M. Oxygen(es) and the hypoxia-inducible factor-1. Biol Chem. 1997;378:609–616. [PubMed] [Google Scholar]
- 122.Maxwell PH, Wiesener MS, Chang GW, Clifford SC, Vaux EC, Cockman ME, Wykoff CC, Pugh CW, Maher ER, Ratcliffe PJ. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature. 1999;399:271–275. doi: 10.1038/20459. [DOI] [PubMed] [Google Scholar]
- 123.Duan DR, Pause A, Burgess WH, Aso T, Chen DY, Garrett KP, Conaway RC, Conaway JW, Linehan WM, Klausner RD. Inhibition of transcription elongation by the VHL tumor suppressor protein. Science. 1995;269:1402–1406. doi: 10.1126/science.7660122. [DOI] [PubMed] [Google Scholar]
- 124.Zbar B, Kaelin W, Maher E, Richard S. Third International Meeting on von Hippel-Lindau disease. Cancer Res. 1999;59:2251–2253. [PubMed] [Google Scholar]
- 125.Griffiths JR. Are cancer cells acidic? Br J Cancer. 1991;64:425–427. doi: 10.1038/bjc.1991.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Martínez-Zaguilán R, Seftor EA, Seftor RE, Chu YW, Gillies RJ, Hendrix MJ. Acidic pH enhances the invasive behavior of human melanoma cells. Clin Exp Metastasis. 1996;14:176–186. doi: 10.1007/BF00121214. [DOI] [PubMed] [Google Scholar]
- 127.Parkkila S, Rajaniemi H, Parkkila AK, Kivela J, Waheed A, Pastorekova S, Pastorek J, Sly WS. Carbonic anhydrase inhibitor suppresses invasion of renal cancer cells in vitro. Proc Natl Acad Sci USA. 2000;97:2220–2224. doi: 10.1073/pnas.040554897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Supuran CT, Briganti F, Tilli S, Chegwidden WR, Scozzafava A. Carbonic anhydrase inhibitors: sulfonamides as antitumor agents? Bioorg Med Chem. 2001;9:703–714. doi: 10.1016/s0968-0896(00)00288-1. [DOI] [PubMed] [Google Scholar]
- 129.Casini A, Scozzafava A, Mastrolorenzo A, Supuran LT. Sulfonamides and sulfonylated derivatives as anticancer agents. Curr Cancer Drug Targets. 2002;2:55–75. doi: 10.2174/1568009023334060. [DOI] [PubMed] [Google Scholar]
- 130.Teicher BA, Liu SD, Liu JT, Holden SA, Herman TS. A carbonic anhydrase inhibitor as a potential modulator of cancer therapies. Anticancer Res. 1993;13:1549–1556. [PubMed] [Google Scholar]


