Abstract
The International Diabetes Federation predicts that by 2035 10% of the population of the world will have been diagnosed with diabetes, raising serious concerns over the resulting elevated morbidity and mortality as well as the impact on health care budgets. It is also well recognized that cardiovascular disease is the primary cause of the high morbidity and mortality associated with diabetes, raising the concern that appropriate drug therapy should not only correct metabolic dysfunction, but also protect the cardiovascular system from the effects of, in particular, the epigenetic changes that result from hyperglycaemia. A number of new classes of drugs for the treatment of diabetes have been introduced in the past decade, providing the opportunity to optimize treatment; however, comparative information of the cardiovascular benefits, or risks, of the newer drugs versus older therapies such as metformin is variable. This review, in addition to summarizing the cellular basis for the therapeutic action of these drugs, addresses the evidence for their cardiovascular benefits and risks. A particular focus is provided on metformin as it is the first choice drug for most patients with type 2 diabetes.
Keywords: cardiovascular disease, diabetes, endothelial dysfunction, hyperglycaemia, hypoglycaemia, insulin, insulin resistance, metformin, sulfonylureas, thiazolidinediones
Introduction
The global impact of obesity and diabetes continues to increase and negatively affect morbidity, mortality and health care budgets. Reports in 2011 from the International Diabetes Federation [IDF, 2013] stated that there was an estimated 285 million people worldwide who had already been diagnosed with diabetes and that the worldwide prevalence of diabetes has truly reached pandemic levels [Chen et al. 2011; Zimmet, 2011]. Furthermore, increases in the prevalence of diabetes for all age groups worldwide are predicted, with total numbers to reach approximately 450 million in 2030 amounting to 7% of the population of the world. The most recent estimate predicts 592 million in 2035 or approximately 10% of the total population [Chen et al. 2011; Zimmet, 2011; IDF, 2013]. A serious concern is for developing countries where it is predicted there will be a 69% increase in the number of adults with diabetes compared with so-called developed countries where a 20% increase is anticipated [Shaw et al. 2010]. Type 2 diabetes (T2D) accounts for approximately 90% of all cases of adult-onset diabetes and the increase in the prevalence of T2D and associated insulin resistance can be linked to a rise in obesity resulting from a combination of lifestyle changes and genetic susceptibility [Daousi et al. 2006].
A natural consequence of an increased incidence in diabetes is the high likelihood that this will be accompanied by a marked rise in the occurrence of cardiovascular disease [Nolan et al. 2011]. Although the aetiology of vascular dysfunction in diabetes has been extensively investigated we still have not optimized the therapeutic management of diabetes such that the cardiovascular system is appropriately protected. It is also very important to emphasize that diabetes-associated vascular complications result in 75% of the deaths linked to diabetes [Grundy et al. 2002]. In addition there is a two- to fourfold increase in the incidence of coronary artery disease, a 10-fold increase in peripheral vascular diseases and, overall, a three- to fourfold higher mortality rate when the data are compared with those from people without diabetes [Grundy et al. 2002]. Essentially diabetes is a disease that has a profound negative effect on the health of the human vascular system and clearly a better understanding of the cellular basis of diabetes-associated cardiovascular disease is an important goal. To date, diabetes management has largely focused on the control of hyperglycaemia; however, since the rising burden of this disease is mainly correlated to its vascular complications, it is also important to consider how best to protect cardiovascular function and, therefore, not only focus on the reduction of plasma glucose [Schalkwijk and Stehouwer, 2005; Fowler, 2008; Defronzo, 2009]. We now recognize that advances in our understanding of the pathophysiology of diabetic cardiovascular disease will facilitate the discovery of potential new treatment regimens. Since the use of drugs for the treatment of diabetes is frequently a major component of the management of diabetes, it is also important that both the beneficial and potentially negative cardiovascular effects of these same drugs are fully recognized [Ledford, 2013; Singh et al. 2013].
Diabetes, hyperglycaemia, glucose toxicity and cardiovascular disease
The results from the 1991 United Kingdom Prospective Diabetes Study (UKPDS), with 5102 subjects with T2D, and the 1993 Diabetes Control and Complications Trial (DCCT), with 1441 subjects with type 1 diabetes (T1D) helped establish the dogma that the most beneficial approach to the management for patients with diabetes is tight glycaemic control [DCCT, 1993; UKPDS, 1991, 1998]. Such conclusions have been reemphasized as reflected by a report by Standl and colleagues indicating that the incidence of cardiovascular events associated with diabetes is reduced by 10–15% per 1% reduction in absolute glycated haemoglobin (HbA1c) [Standl et al. 2009]; the level of HBA1c being a measure of the average impact of plasma glucose over the previous 4–12 weeks. In addition, the Emerging Risk Factors Collaboration (ERFC) study, a collaborative meta-analysis of 102 prospective studies, made the observation that the preexistence of diabetes more than doubled the risk of vascular disease [ERFC et al. 2010]. Furthermore, the ERFC study of 820,900 participants also concluded that a 50 year old with diabetes died approximately 6 years earlier than a person without diabetes and that diabetes was moderately associated (but not necessarily causality) with death from certain cancers, such as liver, pancreas, bladder, breast, colorectal, lung and ovary as well as infectious diseases, degenerative disorders and other diseases [ERFC, 2010]. Nonetheless, a ‘glucocentric’ approach to the treatment of diabetes has been delivered a setback with the results, released in 2007, from the Action to Control Cardiovascular Risk in Diabetes (ACCORD) study of 10,251 patients. The intensified glucose lowering arm of the ACCORD study with 5128 patients with a targeted HBA1c of less than 6% was discontinued when it became apparent that such an intensive regimen resulted in a significantly higher all-cause risk of death of 22% and a 35% increase in cardiovascular mortality [ACCORD, 2008]. A contribution to the increase in mortality associated with the intensified glucose-lowering arm of the ACCORD study may be linked to an increase in the frequency of hypoglycaemia-related events and associated endothelial-vascular dysfunction [Wang et al. 2012]. Clearly, in order to improve the clinical management of diabetes, a better understanding of the impact of both hypoglycaemia and hyperglycaemia on the progression of endothelial dysfunction and vascular disease is required [Ding and Triggle, 2010; Basha et al. 2012; Triggle and Ding, 2010, 2011; Triggle et al. 2012].
Insulin, insulin resistance, hyperglycaemia, oxidative stress and endothelial dysfunction
Insulin was first discovered in 1921 by Banting, Best and McLeod [Best and Scott, 1923] and the importance of insulin in mediating multiple cellular effects is now well recognized. Insulin, released from pancreatic β cells, mediates its anabolic and metabolic cellular effects via trans-phosphorylation activation of its plasma membrane heterotetramer receptor. Activation of the insulin receptor catalyzes the tyrosine phosphorylation of a number of substrates, including the insulin receptor substrate (IRS1-4) proteins, initiates activation of the phosphatidylinositol 3 (PI-3) kinase pathway that, in turn, activates protein kinases, notably Akt (protein kinase B) and atypical protein kinase C. Via the phosphorylation of an adapter protein, APS, insulin also regulates Glut4 translocation and the regulation of glucose transport into skeletal, cardiac muscle and adipose tissue with approximately 80% of glucose disposal going into skeletal muscle [Saltiel and Kahn, 2001]. Once released from pancreatic β cells, the rate-limiting step for the blood-borne insulin to reach its target tissues and mediate its hormonal effects is transport across the endothelium. King and Johnson demonstrated that insulin can be transported into endothelial cells by an insulin receptor-mediated process [King and Johnson, 1985]. Insulin is internalized into capillary and arterial endothelial cells following the activation of insulin receptors on the endothelial cell by a nitric oxide (NO)-independent process that requires PI-3-kinase and mitogen-activated protein kinase (MAPK)/ERK signalling [Jialal et al. 1984; Hachiya et al. 1988; Brunner and Wascher, 1998; Genders et al. 2013]. Insulin uptake is dependent on the expression of caveolin 1 and is reduced by the inflammatory cytokines, interleukin 6 (IL-6) and tumour necrosis factor α (TNFα) [Wang et al. 2011]. Insulin resistance is associated with endothelial dysfunction and with a reduced delivery of insulin across the endothelium (Mather et al. 2013).
Insulin plays an important role in the regulation of vascular tone and, within the physiological concentration range, promotes vasodilatation [Baron, 1994; Steinberg et al. 1994]. Insulin-mediated regulation of vascular function is endothelium dependent via PI-3-kinase/Akt activation of endothelial NO synthase (NOS) and the generation of NO [Dimmeler et al. 1999; Montagnani et al. 2002; Clark et al. 2003]. NO facilitates capillary recruitment and increased blood flow in the microcirculation [Clark et al. 2003; Vincent et al. 2003]. Mice in which the IRS-2 has been genetically ablated (IRS-2−/−) and become insulin resistant and T2D after about 10 weeks of age demonstrated impaired endothelium-dependent, but not endothelium-independent, vasodilatation and are prone to injury-induced atherosclerosis [Kubota et al. 2003]. Furthermore, insulin-mediated eNOS phosphorylation is deficient in endothelial cells from IRS-2−/− mice and insulin-induced capillary recruitment, insulin delivery and glucose uptake by skeletal muscle are all reduced [Kubota et al. 2011]. Interestingly, this vasodilator action of insulin is also blunted in obesity as well as in the presence of insulin resistance [Laakso et al. 1990; Del Turco et al. 2007]. Insulin resistance is also associated with the reduction in the availability of endothelium-derived NO and with an increase in the synthesis of the highly potent and endothelium-derived vasoconstrictor peptide, endothelin 1 [Boulanger and Lüscher, 1990; Irving et al. 2001]. Potenza and colleagues demonstrated the link between insulin resistance, activation of the Ras/MAPK pathway and impairment of the PI-3-kinase-dependent signalling, resulting in an imbalance in the production of the potent vasoconstrictor, endothelin, versus the generation of the vasodilator NO [Potenza et al. 2005]. Insulin signalling in the heart is also altered in insulin resistance with hyperinsulinaemia and diabetes and is associated with cardiac hypertrophy, possibly indirectly via the angiotensin system, angiotensin II expression and activation of the MEK-ERK1/2 pathway [Samuelsson et al. 2006; Bertrand et al. 2008].
Endothelial dysfunction, as reflected by a reduction in endothelium-dependent vasodilatation (EDV), is an early indicator of the development of cardiovascular disease and is closely associated with hyperglycaemia and the development of microvascular disease in both T1D and T2D. Endothelial dysfunction contributes to the reduction in blood flow and capillary recruitment and, in the presence of insulin resistance, both insulin and glucose delivery to tissues are exacerbated [Clark et al. 2003; Kim et al. 2006]. In addition, obesity may, independent of insulin resistance, contribute to the development of endothelial dysfunction as has been reported from studies of the effects of direct measurements of leg blood flow induced by the injection of the endothelium-dependent vasodilator, methacholine, into the femoral artery [Steinberg et al. 1996]. Of significance was that the response to sodium nitroprusside, an endothelium-independent vasodilator, was normal, supporting the argument that endothelial dysfunction precedes vascular smooth muscle dysfunction [Steinberg et al. 1996]. In the study by Steinberg and colleagues EDV was reduced by about 40–50% in patients with obesity compared with lean patients regardless of whether they were diabetic. Of additional importance was the finding that although low physiologic levels of insulin enhanced blow flow in the control lean group, higher levels of insulin were ineffective in the group with obesity (×2 insulin dose) and diabetic (×30) [Steinberg et al. 1996].
Under normoglycaemic conditions endothelial cells are predominantly glycolytic [Dobrina and Rossi, 1983]; however, during periods of hyperglycaemia the increase in intracellular glucose results in mitochondria generating excessive amounts of superoxide with the result that glyceraldehyde-3-phosphate dehydrogenase is inhibited and glucose is shunted into other pathways: the polyol and the hexosamine pathways, increased formation of advanced glycation end products (AGEs) and their receptors (RAGEs), and activation of protein kinase C isoforms [Du et al. 2000; Giacco and Brownlee, 2010]. Oxidative stress is also responsible for the uncoupling of eNOS from the dimeric NO-generating enzyme to the monomeric superoxide generating form [Aljofan and Ding, 2010; Triggle et al. 2012]. A rapid increase in blood levels of glucose, as triggered by a 75 g oral glucose load, is sufficient to reduce blood flow, as determined by flow-mediated vasodilatation, even in subjects without diabetes, but the impact is greater in those with impaired glucose tolerance or in those with diabetes [Kawano et al. 1999]. In addition, it has been reported that the effects of an oral glucose load on blood flow reduction can be prevented by the active form of the key cofactor for eNOS, 6R-tetrahydrobiopterin (BH4), but not the inactive 6S-BH4 [Ihlemann et al. 2003]. The reduction in the bioavailability of NO has profound negative effects on vascular health, resulting in the development of atherogenesis [Li and Förstermann, 2013]. Furthermore, frequent fluctuations, or transients, in plasma glucose levels may have a greater long-term effect on endothelial function and the development of vascular disease than sustained hyperglycaemia [Mah and Bruno, 2012]. Glucose transients have also been linked to epigenetic changes and the development of hyperglycaemic memory that has been linked to changes in promoter function of, for instance, nuclear factor κB [El-Osta et al. 2008]. Hyperglycaemia, possibly linked to vascular dysfunction, has also been associated with impaired memory and may therefore contribute to the development of dementia [Kerti et al. 2013]. Elevated glucose and its subsequent metabolism and the generation of superoxide inhibits vascular potassium channels and thus promotes vasoconstriction [Rainbow et al. 2006]. Acute hyperglycaemia, most likely via mitochondria-derived superoxide, results in an increase in the release of inflammatory cytokines, such as the interleukins, IL-6, IL-8, IL-18, as well as TNFα, which have been linked to the development of insulin resistance and monocyte adhesion (IL-8) [Esposito et al. 2002; Marette, 2002; Srinivasan et al. 2003; Zhou et al. 2011]. In conclusion, in the treatment of diabetes the minimal goal of drug therapy should be to maintain normoglycaemia and avoid frequent fluctuations in blood glucose, thus protecting the cardiovascular system from glucose toxicity.
The risk of hypoglycaemia
As reflected by the results of the ACCORD study, hypoglycaemia is a potential risk in the treatment of T2D. Severe hypoglycaemia is most frequently associated with TID, but the incidence increases in T2D as the use of insulin therapy increases. Apart from insulin, hypoglycaemia is particularly associated with the use of sulfonylurea and meglitinide oral hypoglycaemic drugs, but not with metformin. Severe hypoglycaemia is also a very serious issue and can lead to an increased risk of dementia, cardiovascular events and death [Brunton, 2012].
Biomarkers for cardiovascular disease and diabetes
The availability of reliable and readily obtainable biomarkers, preferably a panel of biomarkers, for the assessment of the progression, or regression, of cardiovascular disease in patients with diabetes would be an obvious benefit. Advances have been made, but not to the extent that there is agreement [Herder et al. 2014]. Since the early stages of cardiovascular disease are associated with the development of changes in the endothelium, it is tempting to use measures of endothelial function as surrogate markers. Thus, measurements of flow-mediated dilatation, C-reactive protein, vascular cell adhesion molecules, the endogenous NOS inhibitor, asymmetric dimethylarginine (ADMA), von Willebrand factor, microRNAs and other putative biomarkers have all been discussed [Tousoulis et al. 2013]. Levels of adiponectin, an adipose tissue-derived hormone, are inversely correlated with inflammation, obesity, insulin resistance, diabetes and cardiovascular risk as well as mortality and thus represents another potentially useful biomarker of metabolic disease burden [Mather and Goldberg, 2014]
Diabetes drug therapy and cardiovascular complications
The use of drugs for the treatment of diabetes may itself increase the risk from cardiovascular complications as a result of direct side effects related to the drug’s molecular actions or indirect effects that result from changes in the metabolic profile of the patient. For the latter, for instance, the risk of hypoglycaemia is potentially serious. Such risks may not have been detected, or fully appreciated, during drug development, but were realized after meta-analysis of multiple clinical trial data. Thus, it is very important that health care workers weigh such risks versus established benefits when recommending the most appropriate therapeutic regimen. As a result of postmarketing data revealing cardiovascular problems that became evident with the thiazolidinedione (TZD) rosiglitazone, the US Food and Drug Administration (FDA), since 2008, has required clinical trial evidence attesting to the cardiovascular risk of new drugs.
A summary of the cardiovascular benefits as well as major side effects and cardiovascular risks of the drugs used for the treatment of diabetes are summarised in Table 1.
Table 1.
Drugs used for the treatment of diabetes: cardiovascular benefits and significant side effects.
| Drug | Cardiovascular benefits | Significant side effects |
|---|---|---|
| Metformin | Indirect via maintaining glycaemic control without hypoglycaemia (does not promote insulin release); improved lipoprotein metabolism; direct/indirect protection of endothelial/vascular function; weight neutral/weight reduction | Lactic acidosis, very rareGI, minor |
| Sulfonylureas | Enhanced insulin release; reduction of hyperglycaemia | Hypoglycaemia |
| Increased cardiovascular risk | ||
| Thiazolidinediones | Enhanced sensitivity to insulin, reduced hepatic gluconeogenesis, pleiotropic actions to reduce inflammation, fat redistribution to subcutaneous sites and reduction in lipotoxicity; reduction in BP | Weight gain, oedema, bone fractures, cardiovascular risk? Cancer? |
| Glitazars | Insufficient data regarding relative risk | Cardiovascular risk (drugs withdrawn) |
| Incretins | Promote insulin release, pleiotropic protective effects on pancreatic β cells, improved endothelial function | GI, nausea, pancreatitis, pancreatic cancer? |
| DPP-4 inhibitors | Enhance insulin release via GLP-1 thereby reduce blood glucose and glucagon, direct and indirect effects on endothelium | Headache, nausea, hypersensitivity,Unknown effects due to nonspecificity of DPP-4 inhibition. |
| α-Glucosidase inhibitors | Slow postprandial increases in blood glucose, weight loss, lower triglycerides | GI |
| SGLT2 inhibitors | Improved insulin sensitivity, reduced blood pressure | Polyuria, restrict use in renal insufficiency; lack of information as only recently introduced |
| Insulin | Improved glucose disposal, improved blood flow; anti-inflammatory action (?) | Hypoglycaemia; promotes cardiac hypertrophy, increase in all-cause mortality? Cancer? |
BP, blood pressure; DPP-4, dipeptidyl peptidase 4; GI, gastrointestinal; GLP-1, glucagon-like peptide 1; SGLT2, sodium glucose cotransporter 2.
Drug classes and drugs currently in use for the treatment of T1D and T2D
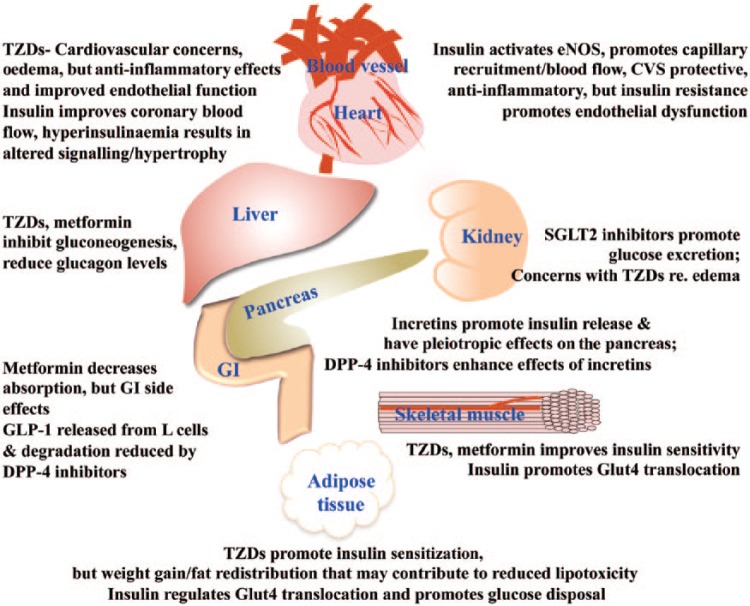
Figure 1 summarizes the site of action, cardiovascular effects and significant side effects of the drugs used for the treatment of diabetes.
Figure 1.
Summary of important sites of action and side effects and cardiovascular actions of drugs used in the treatment of diabetes.
DPP-4, dipeptidyl peptidase 4; eNOS, endothelial nitric oxide synthase; GI, gastrointestinal; GLP-1, glucagon-like peptide 1; SGLT2, sodium glucose cotransporter 2; TZD, thiazolidinedione.
The biguanide, metformin
The biguanide, metformin (dimethylbiguanide), was first introduced for use in the treatment of T2D in the UK in the late 1950s and today metformin is considered to be the first-choice drug and the ‘gold standard’ for most people with T2D [Halimi, 2006; Bosi, 2009]. Metformin is also the most widely prescribed as well as one of only two oral hypoglycaemic agents listed on the World Health Organization (WHO) Model List of Essential Medicines [WHO, 2013]. For this reason, a particular focus is based on metformin in this review article.
It has been estimated that the worldwide annual number of people receiving prescriptions for metformin is in excess of 120 million [Bosi, 2009]. Metformin is a synthetic oral hypoglycaemic drug with a chemistry linked to the guanidines, such as isoamylene guanidine, found in French lilac, Galega officinalis (or Italian fitch also known as Goat’s rue), a remedy known since medieval times for the relief of the excessive urination associated with diabetes [Witters, 2001]. The benefits of metformin include not only its hypoglycaemic efficacy with minimal side effects other than gastrointestinal-related issues, but also its ability to improve insulin sensitivity, thus improving glucose utilization as well as lipid metabolism. In addition, the use of metformin, unlike a number of drugs used in the treatment of diabetes, is either weight neutral or associated with weight loss [Glueck et al. 2001].
Metformin has been the subject of a number of recent reviews but, despite being in clinical use for over 50 years, the precise cellular modes of action remain unclear [Kirpichnikov et al. 2002; Halimi, 2006; Viollet et al. 2012; Bosi, 2009; Rena et al. 2013; Rojas and Gomes, 2013; Pernicova and Korbonits, 2014].
Pharmacokinetic properties of metformin
An appreciation of the pharmacokinetic properties of metformin is important in order to better understand the cellular basis for the therapeutic effects of metformin. Metformin has a bioavailability of between 30% and 60%, a plasma half life of approximately 2 h and an elimination half life of between 4 and 8.7 h; is not measurably metabolized, it is not bound to plasma protein and it is excreted unchanged in the urine with elimination characteristics consistent with a three-compartment model [Pentikäinen et al. 1979; Tucker et al. 1981]. Although a strong base and highly ionized molecule at physiological pH, metformin has a volume of distribution, VD, considerably greater than total body water, indicating that some accumulation into tissues must occur. The gastrointestinal tract serves as a major site of accumulation and this may explain the frequency of gastrointestinal side effects that are associated with the use of metformin [Wilcock and Bailey, 1994]. Erythrocytes may also serve as a potential deep compartment for metformin [Lalau and Lacroix, 2003]. Nonetheless, the very brief plasma and elimination half lives of metformin make it very unlikely that significant tissue accumulation of the drug will occur with a standard three times daily therapeutic regimen [Sirtori et al. 1978].
Based on the pharmacokinetic properties of metformin and assuming 50% bioavailability and a VD of 100 liters, the peak concentration of approximately 10 µM metformin should theoretically be achieved following an oral dose of 500 mg. Frid and colleagues also indicate that, not surprisingly given that metformin is excreted unchanged, the glomerular filtration rate (GFR) will also influence serum metformin levels [Frid et al. 2010]. Tucker and colleagues report the peak plasma concentration determined in normal controls following an oral dose of 500 mg is approximately 15 µM and for an intravenous dose 80 µM [Tucker et al. 1981]. It is important to keep these peak concentrations in mind when critiquing the published literature describing the in vitro effects of metformin. The discrepancy between the blood levels obtained for intravenous versus oral can be explained by slow absorption from the gastrointestinal tract, which has been estimated at approximately 6 h to achieve total maximal absorption from one dose. Absorption is thus the rate-limiting step for determining the therapeutic efficacy of metformin. Metformin can be transported into cells via organic cation transporters (OCTs) also known as solute carriers, of which there are 22 members, as well as the plasma membrane monoamine transporter, also known as hENT4 [Nies et al. 2011]. The accumulation of metformin into tissue, notably the gastrointestinal tract enterocytes, erythrocytes, hepatic and endothelial cells, via OCTs may also account for the comparatively short plasma half life of metformin [Tucker et al. 1981]. OCTs, specifically the organic cation transporter, OCT1, abundant in the liver and in the intestine, and OCT2, which is abundant in the kidney, are involved in the rapid renal clearance of metformin via tubular secretion [Shu et al. 2008]. Polymorphisms in OCTs may also affect the absorption from the gastrointestinal tract, hepatic uptake as well as the renal clearance of metformin, and thus result in patient-to-patient variations in the therapeutic efficacy of metformin [Wang et al. 2002; Shu et al. 2008; Graham et al. 2011; Yue et al. 2013].
Beneficial effects of metformin on cardiovascular function
In addition to its hepatic-mediated hypoglycaemic actions in human subjects, the cardiovascular benefits of metformin are also demonstrated in the prospective, randomized, multicentre UKPDS study of patients with T2DM [UKPDS, 1998]. The reduced cardiovascular morbidity and mortality benefits of treatment of patients with T2D with metformin has been confirmed in other reports; for instance Johnson and colleagues [Johnson et al. 2005]. Metformin also has a positive effect on the lipid profile [Glueck et al. 2001; Eleftheriadou et al. 2008]. In addition, metformin also improves endothelial function via an action that seems to be unrelated to its hypoglycaemic actions and is the only antidiabetic drug with a proven therapeutic efficacy to reduce the cardiovascular complications of diabetes [Ajjan and Grant, 2006; de Jager et al. 2005; Johnson et al. 2005; Arunachalam et al. 2014]. Metformin has also been shown to improve endothelial function in patients with T2DM [Mather et al. 2001; Vitale et al. 2005].
Side effects associated with use of metformin
The side effects associated with metformin use are usually minor with gastrointestinal problems the most common and, as noted, gastrointestinal side effects may reflect the accumulation of the drug in the small intestine [Wilcock and Bailey, 1994]. An association with lactic acidosis, possibly linked to putative effects of metformin in mitochondria to inhibit Complex 1, limit use in patients with kidney and liver dysfunction, although the Cochrane reports indicate that the risk of developing lactic acidosis with metformin treatment is exceedingly low and less than that in nonmetformin-treated patients with diabetes: 4.3 versus 5.4 cases per 100,000 patient-years respectively [Halimi, 2006; Salpeter et al. 2010]. Frid and colleagues make the valid observation that at GFRs greater than 30 ml/min/1.73 m2 serum metformin levels are unlikely to rise above 20 µM, whereas lactic acidosis has been associated with metformin concentrations greater than 200 µM [Frid et al. 2010].
The gastrointestinal side effects of metformin, although considered fairly minor, can also result in folate malabsorption problems and vitamin B deficiency. Such deficiencies can result in elevated homocysteine levels and an elevated risk of cardiovascular disease secondary to endothelial dysfunction. Elevated homocysteine is an established risk factor for cardiovascular disease [Tousoulis et al. 2012]. However, folate supplementation can offset increases in homocysteine levels and, furthermore, the effects of metformin, at least over a 16-week treatment period, have been reported to be quite modest [Wulffelé et al. 2003; Aghamohammadi et al. 2011]. Furthermore, the gastrointestinal side effects are dose related and usually more evident at initiation of therapy with metformin.
Cellular basis for the oral hypoglycaemic action of metformin
Metformin reduces hepatic gluconeogenesis, decreases glycogenolysis, facilitates glucose disposal into peripheral tissues, reduces fatty acid oxidation and also protects the cardiovascular system from the negative effects of hyperglycaemia [Kirpichnikov et al. 2002]. Metformin may also delay or decrease glucose absorption from the gastrointestinal tract; however, this has been disputed [Czyzyk et al. 1968; Cuber et al. 1994]. Despite over 50 years of research, the precise mode of action of metformin remains controversial and, in fact, there may be several sites of action that are determined by concentration/dose-dependent effects as well as tissue selectivity and the relative expression levels of drug transporters. It is thus very important, particularly with respect to analyzing data from ex vivo studies, to determine whether the concentrations of the drug that were studied can be related to therapeutic levels of metformin.
Metformin has been shown to inhibit gene expression of the rate-limiting enzymes for hepatic gluconeogenesis, namely enzyme phosphenolpyruvate carboxykinase and glucose-6-phosphatase [Minassian et al. 1998; Yuan et al. 2002]. Adenosine monophosphate (AMP)-activated protein kinase (AMPK) is the frequently cited cellular target for metformin with the primary effects mediated by AMPK being the inhibition of hepatic gluconeogenesis and lipogenesis, and also the enhancement of insulin-regulated GLUT-4-mediated glucose transport into striated muscle and adipocytes [Owen et al. 2000; Zhou et al. 2001; Yang and Holman, 2006; Viollet et al. 2012; Fullerton et al. 2013]. AMPK, a heterotrimeric enzyme comprising α subunit and regulatory β and γ subunits with at least 12 potential combinations, serves as a metabolic energy sensor and is activated when adenosine triphosphate (ATP) levels decrease and AMP rises as a result of, for instance, exercise or cellular stress, with subsequent allosteric activation as a result of AMP binding to the γ subunit. AMPK can also be activated by the upstream tumour suppressor kinase, liver kinase B1 (LKB1), as well as calcium/calmodulin-dependent protein kinase β [Hardie et al. 2012; Hardie, 2013].
It has also been reported that metformin inhibits mitochondria respiration via an action on complex 1 [El-Mir et al. 2000; Owen et al. 2000]. In the study by El-Mir and colleagues a concentration-dependent effect of metformin on mitochondria respiration in rat hepatocytes, with a half maximal inhibitory concentration of 1 mM, was reported, but only in intact and not permeabilized cells. Furthermore, the inhibitory action of metformin was observed in functionally isolated complex 1 [nicotinamide adenine dinucleotide (NADH), quinone oxidoreductase]. Collectively, these data led to the conclusion that metformin inhibited mitochondria respiration via a cell membrane receptor-mediated signalling pathway [El-Mir et al. 2000]. A direct mitochondrial action may also explain the ability of metformin to inhibit glucose-induced endothelial cell death via inhibiting the opening of permeability transition pore; in this regard the effects of metformin are comparable to those of the antioxidant N-acetyl-cysteine [Detaille et al. 2005]. The increase in the AMP:ATP ratio that would be predicted to result from this ‘mild inhibitory’ effect of metformin might also be the basis of the role of AMPK in mediating the cellular effects of metformin [Hardie, 2007; Hawley et al. 2010]. However, caution is required as the inhibitory effects of metformin require a minimal concentration of 100 µM and, in some protocols up to 10 mM metformin was used [Owen et al. 2000]. In addition, the ability of metformin to bind to mitochondria is rather poor and only about 2% of that reported for another biguanide, phenformin, which was withdrawn from clinical use due to a high incidence of lactic acidosis [Schäfer, 1980]. Furthermore, Wollen and Bailey reported that whereas 10 µM metformin, together with 10 nM insulin, did not lower mitochondrial ATP in rat hepatocytes, evidence of decreased oxidative phosphorylation (an increased NADH/NAD+) was observed with 10 mM metformin [Wollen and Bailey, 1988]. The data from Wilcock and colleagues detailing the distribution of [14C]metformin in mice also indicated that 78% of the accumulation of [14C]metformin in the liver was associated with the cytosolic fraction and only 7–10% with mitochondria [Wilcock et al. 1991]. These data are clearly not supportive of a hypothesis that the therapeutic effects of metformin can be explained by a mitochondrial action that requires high micromolar concentrations unless, of course, metformin can accumulate to appropriately high concentrations in mitochondria. However, the latter has not been reported in the literature.
The cellular basis for the cardiovascular protective effects of metformin
As already noted, the cellular basis for the therapeutic hypoglycaemic actions of metformin has been attributed to the enhancement of AMPK-mediated suppression of hepatic gluconeogenesis and enhancement of insulin-regulated GLUT-4-mediated glucose transport into striated muscle and adipocytes [Zhou et al. 2001]. Improving glycaemic control would reduce the impact of hyperglycaemia on endothelial dysfunction and the development of microvascular disease.
Activation of AMPK might also be a contributing factor to the beneficial effects of metformin on the endothelium as AMPK also phosphorylates and activates eNOS [Chen et al. 2009]. However, Isoda and colleagues reported that phosphorylation of AMPK by metformin in human saphenous vein smooth muscle and endothelial cells required concentrations greater than 200 µM [Isoda et al. 2006]. Furthermore, in cell-free assays, AMPK is activated by the AMP analogue, 5-aminoimidazole-4-carboxamide riboside, a known activator of AMPK, but is not activated by metformin, thus suggesting that the effects of metformin on AMPK are indirect [Hawley et al. 2002, 2003]. Another apparent paradox is that although the kinase upstream of AMPK, LKB1, seems to be required for the activation of AMPK, it is not itself activated by biguanides such as phenformin, which is a close relative of metformin [Lizcano et al. 2004; Sakamoto et al. 2004]. Another question concerning the role of AMPK also arises because mice that are genetically deficient in AMPKα1 and also carry a liver-specific deletion of AMPKα2 still demonstrate a hypoglycaemic response to metformin [Foretz et al. 2010]. Thus, it seems unlikely that either AMPK or LKB1 are directly activated by metformin, at least in the therapeutic concentration range. Arunachalaum and colleagues have reported that metformin, via an insulin-independent mechanism that requires the ‘longevity gene’, SIRT1, protects microvascular endothelial cells against hyperglycaemia-induced oxidative stress and promotion of senescence, apoptosis and dysfunction [Arunachalaum et al. 2014]. Of interest is that high doses of metformin, with serum concentrations of approximately 500 μM that provide anti-inflammatory effects and protection against oxidative stress, have been reported to extend the lifespan and improve health parameters, ‘healthspan’, of middle-age mice by approximately 6% [Martin-Montalvo et al. 2013].
Another cell signalling pathway that might also be involved in mediating the cellular effects of metformin is through changes in cyclic AMP as an increase in AMP will inhibit adenylate cyclase and therefore suppress glucagon-mediated gluconeogenesis in the liver [Miller et al. 2013].
In summary, further studies of the cellular sites of action of metformin are required, particularly to determine the basis for its cardiovascular protective action, as although activation of AMPK may play a role in mediating the effects of metformin it is unlikely to be the direct target, or at least, not the sole target and other targets should be considered [Hardie, 2013].
The sulfonyureas
The discovery of sulfonylureas as hypoglycaemic drugs came about by serendipity as result of the use of a new sulphonamide antibiotic, p-amino-benzene-sulfamido-isopropyl-thiodiazole (2254 RP), by Dr Marcel Janbon at the Clinic of the Montpellier Medical School during an outbreak of typhoid in 1942. One of the adverse reactions was a large drop in blood glucose. This observation was pursued by a physiologist, Dr Auguste-Louis Loubatières, and ultimately resulted in the development of the sulfonylurea oral hypoglycaemic drugs for T2D [Loubatières-Mariani, 2007]. Carbutamide, shortly followed by tolbutamide, was the first analogue to be used for its hypoglycaemic actions with apparent early controversy as to who first described its synthesis [Kleinsorge, 1998].
The sulfonylureas inhibit the opening of KATP channels resulting in the depolarization of pancreatic β cells, a subsequent increase in calcium influx and intracellular free calcium levels and, thus, the promotion of the release of insulin. KATP channels are ubiquitous and are expressed in mitochondria (mitoKATP) as well as the plasma membrane (sarcoKATP). KATP channels also play an important function in protecting against myocardial ischemia, ‘ischaemic preconditioning’, and hence there are concerns over an increase in cardiovascular risk with the use of this class of oral hypoglycaemic drug.
The sulfonylureas inhibit the KATP channel by binding to the sulfonylurea receptor, SUR, component of the channel that, in turn, regulates the associated Kir6.x (either Kir6.1 or Kir6.2) potassium channel. The KATP channel is a hetero-octomeric complex composed of two types of subunits: four inward-rectifying potassium channel pore-forming (Kir6.x) subunits and four high-affinity SUR1 subunits. The SUR protein is a member of the ATP-binding cassette (ABC) gene family homologues. The SUR protein senses the intracellular ATP:ADP ratio and hence the metabolic status of the cell that reflects glucose metabolism; higher ATP closes the channel and enhances insulin release. There are three isoforms of SUR: SUR1, encoded by the ABCC8 gene, and SUR2A and SUR2B, which are splice variants of the ABCC9 gene. The β-cell KATP channel is composed of four Kir6.2 subunits and four SUR1 subunits. Cardiac KATP channels are also composed of four Kir6.2, but the SUR protein is represented by four SUR2A subunits. Vascular KATP channels, which are also regulated by cell metabolism, are composed of Kir6.1 and SUR2B. Thus, ideally an oral hypoglycaemic sulfonylurea drug should selectively target only the SUR1 protein and the pancreatic β cell KATP channel. Although absolute selectivity does not exist, glibenclamide does show relatively good selectivity, but can inhibit cardiac KATP channels within the anticipated therapeutic concentration range; selectivity for repaglinide, a non-sulonylurea KATP channel inhibitor and so-called meglitinide, is less [Nagashima et al. 2004; Stephan et al. 2006].
Cardiovascular safety of the sulfonylureas
Serious concerns have been expressed about the cardiovascular safety of the short-acting sulfonylurea, tolbutamide, and the following statement made: ‘With no evidence of efficacy and a definite possibility of toxicity, the investigators concluded that the safety of the patients still receiving tolbutamide therapy required its discontinuance and that the factual basis for this decision needed to be communicated to the biomedical scientific community’ [Cornfield, 1971: 1676]. See also The University Group Diabetes Program (UGDP, 1970)].
In 1971 the existence of the KATP channel was not known, nor, of course, the role of the KATP channel in preconditioning. Concerns regarding the link between hypoglycaemia and cardiovascular risk were also not apparent until the report of the UKPDS in 1998. Recurrent hypoglycaemia is another major morbidity that is linked to sulfonylureas and the risk with glibenclamide is higher than with other sulfonylureas [Gangji et al. 2007]. A large retrospective analysis of over 27,000 patients treated with sulfonylureas versus metformin has also stressed an increase in cardiovascular risk for those treated with sulfonylureas versus metformin alone [Pantalone et al. 2012]. The addition of a sulfonylurea to a drug regimen initially based on monotherapy with metformin is common practice. A MEDLINE database analysis for the period January 1966 through July 2007 with data reviewed from almost 300 sources for the combination therapy metformin plus sulfonylurea significantly increased the relative risk (RR) of the composite endpoint of cardiovascular hospitalization or mortality (RR 1.29) versus control group therapy with diet, or monotherapy with metformin or a sulfonylurea [Rao et al. 2008]. It should be noted that the combination of a sulfonylurea with metformin has been the accepted second step in the management of patients with T2D and such patients may therefore have a more severe form of T2D. The recent availability of agents such as the incretins and gliptins is changing prescribing practice [Cakirca et al. 2014]
The cardiovascular safety of sulfonylureas continues to be debated, particularly with respect to monotherapy with sulfonylureas versus other drugs [Hemmingsen et al. 2013; Monami et al. 2013].
As for metformin, retrospective analysis of clinical data suggests that treatment with at least some sulfonylureas, namely gliciazide and tolbutamide, may reduce mortality associated with cancer [Bo et al. 2013]. The putative protective actions of drugs used for the treatment of diabetes against cancer will not be further discussed in this review.
The meglitinides (glinides)
The meglitinides, first introduced in 1997 with repaglinide and followed by nateglinide and mitaglinides, are oral hypoglycaemic agents that inhibit KATP channels and thus serve as insulin secretalogues. They share comparable side effects, notably hypoglycaemia and weight gain, with the sulfonylurea drugs. They differ from the sulfonylureas in having a faster onset and shorter duration of action, thus making them suitable for postprandial control of blood glucose.
The TZDs or glitazones
The glitazones are activators of the nuclear peroxisome proliferator-activated receptor γ (PPARγ). PPARγ is expressed in adipose tissue, pancreatic β cells, muscle, liver, endothelial cells and also macrophages. Activation of PPARγ results in increased GLUT4 expression, enhanced sensitivity to insulin, reduced hepatic gluconeogenesis and therefore reduce blood glucose and HbA1c levels. However, the glitazones do not enhance insulin secretion. The glitazones also reduce lipotoxicity and redistribute fat from muscle, liver and pancreatic β cells to subcutaneous depots. Glitazones also enhance the expression of PPARγ cofactor 1, an important regulator of mitochondria biogenesis, and thus modulate energy homeostasis [Puigserver and Spiegelman, 2003]. In addition, likely secondary to their effects on improved insulin sensitivity and glucose homeostasis, the glitazones also reduce blood pressure and microalbuminuria [de Rivas et al. 2005; Defronzo et al. 2013a]. Thus, the beneficial effects of glitazones can be linked to their effects on insulin sensitivity and the reduction of lipotoxicity and glucotoxicity with improved β cell function. The ACT NOW Study (Actos Now for the prevention of diabetes) was designed to determine if pioglitazone can prevent/delay progression to diabetes in high-risk impaired glucose tolerance subjects and also to define the mechanisms that mediate the beneficial effects. The study concluded that the primary mechanism whereby pioglitazone prevents diabetes and improves glucose tolerance is via the preservation and improvement of pancreatic β cell function [Defronzo et al. 2013b]. Rosiglitazone and pioglitazone are the two glitazones currently remaining in therapeutic use. They are orally effective and have half lives from 3 h up to 24 h respectively, with pioglitazone having active metabolites.
Beneficial effects of glitazones on cardiovascular function
Data from the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events) indicate that patients with diabetes who are at high cardiovascular risk and are treated with pioglitazone show improvement in insulin sensitivity, a reduction in the need for insulin, and improved cardiovascular outcome [Dormandy et al. 2005]. The beneficial effects of the glitazones, notably pioglitazone, on cardiovascular function have also been linked to their pleiotropic effects, such as the reduction of inflammatory markers and, on the basis of apparent differing effects on cardiovascular outcomes, pioglitazone is the preferred choice over rosiglitazone, particularly when low doses are administered [Defronzo et al. 2013a; Zou and Hu, 2013]. Glitazones, like metformin, do not promote insulin release and have been described as ‘insulin secretion decelerators’ and, together with their actions to reduce energy production, as already discussed for metformin, may be the basis for their beneficial effects in the treatment of T2D [Lamontagne et al. 2013].
Cardiovascular and other safety issues with the glitazones
Troglitazone was withdrawn from clinical use in the UK in 1997 due to hepatotoxicity and elsewhere shortly after. However, studies with rosiglitazone and pioglitazone indicate that hepatotoxicity is not a class effect [Lebovitz, 2002]. Despite the positive effects of the glitazones to lower plasma triglycerides and raise high-density lipoprotein, low-density lipoprotein levels are also raised. Furthermore, glitazones reduce leptin levels and thus promote weight gain in the form of redistribution of fat to subcutaneous depots. The glitazones also have negative effects on cardiac function due to oedema, which is seen in about 5% of patients and therefore may precipitate heart failure. Therefore, glitazones are contraindicated for use in patients with New York Heart Association class III and IV heart failure. There is a greater concern with rosiglitazone than with pioglitazone and a retrospective analysis of data from more than 225,000 patients [Graham et al. 2010] concluded that the use of rosiglitazone was associated with an increased risk of stroke, heart failure and all-cause mortality, as well as an increased risk of the combination of acute myocardial infarction, stroke, heart failure or all-cause mortality, in patients aged 65 years or older. On the basis that the risks outweigh the benefits, rosiglitazone use was restricted in the USA to patients with T2D who were not effectively treated with other medications. Rosiglitazone was suspended in the European market and in some other countries such as New Zealand [Bourg and Phillips, 2012]. In late 2013 the FDA lifted this restriction on the basis of the results from the RECORD clinical trial (Rosiglitazone Evaluated for Cardiovascular Outcomes and Regulation of Glycemia in Diabetes), which failed to reproduce the results from the 2007 meta-analysis and indicated no elevated risk of heart attack or death in patients being treated with rosiglitazone versus diabetes medications [Mahaffey et al. 2013]. Nonetheless vigilance is needed as concerns over cardiovascular safety issues with pioglitazone were raised as a result of the PROactive study of patients with diabetes, which indicated an 11% increased risk of congestive heart failure (CHF) [Dorkhan et al. 2009]. However, an earlier retrospective cohort analysis of more than 16,000 patients concluded that despite the increase in peripheral oedema the use of glitazones decreased mortality [Masoudi et al. 2005].
There are also differences in the safety profiles of the two glitazones in use with respect to the potential for drug interactions as the oxidative metabolism for each drug occurs by distinct cytochrome pathways: pioglitazone involves cytochrome P450 (CYP) 3A4 and CYP 2C8, whereas rosiglitazone is principally metabolized by CYP 2C8. Since CYP 3A4 is involved in the metabolism of well over 150 drugs, the potential for drug interactions with pioglitazone is far greater than with rosiglitazone. Furthermore, pioglitazone use has been associated with an increased risk of bladder cancer and this has resulted in 2011 in the withdrawal from use in France and restricted use elsewhere (Germany) or appropriate warnings (FDA). Tumours of the bladder were noted in the PROactive study [Dormandy et al. 2005], but at that time were not considered to be a result of treatment with pioglitazone. A number of more recent reports, including a large retrospective cohort study by Mamatami and colleagues [Mamtani et al. 2012], support a link, particularly with long-term treatment of more than 5 years. To further add to the risk benefits for the use of glitazones is their association with doubling the risk of bone fractures, particularly hip and wrist, notably in postmenopausal women with diabetes who are treated with either pioglitazone or rosiglitazone, compared with other oral glycaemic agents [Meier et al. 2008; Betteridge, 2011]. The risk of bone fractures is most likely a class effect and related to PPARγ regulation to reduce osteoblast activity and bone formation.
Glitazars
Glitizars, a combination of a PPARα agonist and a PPARγ agonist, have a pharmacological profile similar to combining a fibrate with a glitazone. Two agents, muraglitazar and tesaglitazar, were introduced into clinical trials, but discontinued in 2006 due to elevated risk of CHF and reduced renal function.
The incretins: glucagon-like peptide 1
The development of exenatide, approved in 2005, and liraglutide, approved in 2009, and other analogues more recently, was based on the discovery of two gut hormones that were found to have an ‘incretin effect’. The ‘incretin effect’ defined as the ability to elicit a higher insulin release to an oral glucose load (as, for instance, would be administered as a test for impaired glucose tolerance) compared with that resulting from an equivalent intravenous glucose load. Glucose-dependent insulinotropic polypeptide (GIP) is secreted from the K cells in the duodenum and jejunum, and glucagon-like peptide 1 (GLP-1) is secreted from the L cells of the distal ileum and colon. It was the pioneering work of the Canadian scientist, John Brown, and his team that first discovered and described GIP that stimulated further research into the role of incretins in the regulation of glucose homeostasis [Brown and Dryburgh, 1971; Kim and Egan, 2008]. Not only do the incretins promote insulin release via the activation of their specific G-protein coupled receptors (GPCRs) to enhance cyclic AMP in the β cell and, in turn, enhance insulin release, but activation of the GLP-1 GPCR also results in the inhibition of glucagon release most likely via paracrine actions of insulin [Komatsu et al. 1989; Kim and Egan, 2008]. In T2D the insulinotropic actions of GIP are deficient and thus only GLP-1 has been pursued as a pharmacological target [Kim and Egan, 2008].
Cardiovascular benefits of GLP-1 mimetics
A significant advantage of the incretin analogues relates to their pleiotropic actions on the pancreas where they help maintain β cell function and the biphasic release of insulin. In 2005 exenatide, the first GLP-1 mimetic approved for the treatment of T2D, is a synthetic version of exendin 4 isolated from the saliva of the Gila monster lizard. Exenatide and liraglutide slow the emptying of the stomach and decrease body weight, but also may have a positive inotropic action. GLP-1 has been shown to have a beneficial effect on endothelial function that is negated by cotreatment with the sulfonylurea, glyburide (USAN) (glibenclamide, INN) [Basu et al. 2007]. GLP-1 infusion improved left ventricular function in patients with severe systolic dysfunction [Nikolaidis et al. 2004]. Since GLP-1 receptors are also found on vascular cells, monocytes and macrophages it has been suggested that the protective effects of GLP-1 analogues and dipeptidyl peptidase 4 (DPP-4) inhibitors on cardiovascular function involves direct actions to reduce oxidative stress, vascular inflammation and improve endothelial function [Nikolaidis et al. 2004; Oeseburg et al. 2010; Mita and Watada, 2012]. The ability of GLP-1 to directly affect vascular function may, however, be vessel and species dependent. The rat aorta responds to GLP-1 with a dose-dependent relaxation at a threshold of 10 picomolar via an endothelium-independent and NOS inhibitor (L-NAME), cyclooxygenase (indomethacin) and hydrogen peroxide (catalase)-insensitive mechanism involving an elevation of cyclic AMP and the opening of KATP channels [Green et al. 2008]. The response to GLP-1 receptor activation and the resultant vasodilatation was sensitive to the GLP-1 receptor antagonist, exendin(9-39) [Green et al. 2008]. Although, rat pulmonary and femoral arteries also relax in response to GLP-1, the maximum vasorelaxation response is no more than 30% and is endothelium dependent in the rat pulmonary artery, but endothelium independent and L-NAME insensitive in the rat femoral artery [Golpon et al. 2001; Nyström et al. 2005].
GLP-1 has a very short half life of less than 30 min, but synthetic derivatives have longer half lives. Nonetheless a disadvantage in the use of GLP-1 peptide analogues is that they require frequent subcutaneous injection. Exenatide has a relatively short half life of approximately 3 h, but longer for liraglutide, which has a half life of 11–15 h. The requirement for subcutaneous injection raises the issue of patient compliance; however, a once weekly injection formulation has been developed and was approved in 2012. An orally effective formulation of liraglutide, NN9924, has also been developed and is currently undergoing clinical evaluation. Concerns have recently been raised over a link between the use of incretins and pancreatitis; however, based on a joint evaluation by the FDA and their European equivalent, the European Medicines Agency (EMA), the data were not conclusive, but the need is recognized for an ongoing evaluation [Egan et al. 2014].
DPP-4 inhibitors
The FDA approved the first DPP-4 inhibitor, sitagliptin, in 2006. The primary therapeutic benefit is assumed to be via the increase in incretin levels (the glucagon-like peptides, GLP and GLP-1) subsequent to the inhibition of DPP-4. DPP-4, also known as CD26, is a 766-amino-acid cell surface membrane-associated, serine-type protease enzyme which is widely, if not ubiquitously, distributed in numerous tissues, including adipocytes, endothelial cells, kidney, intestine, liver, spleen, placenta, adrenal glands and lymphocytes [Abbott et al. 1994]. DPP-4 is a multifunctional protein and thus inhibition of DPP-4 may have many diverse effects [Boonacker and Van Noorden, 2003]. DPP-4 expression is increased in adipocytes from subjects with obesity and the release of DPP-4 is correlated with adipocyte size and, furthermore, DPP-4 has been shown to impair insulin signalling [Lamers et al. 2011].
Potential cardiovascular benefits of DPP-4 inhibitors
DPP-4 is not specific for GLP-1 and also plays a role in the degradation of other peptides wherein the penultimate amino acid is either a proline or alanine. Peptides that are substrates for DPP-4 include a number of chemokines and the neuropeptide and endothelium-dependent vasodilator, substance P [Mentlein and Struckhoff, 1989]. Thus, DPP-4 inhibitors, via the inhibition of the breakdown of vasodilator neuropeptides, may play an important role in enhancing blood flow and reducing the negative impact of hyperglycaemia on vascular function. In addition, DPP-4 inhibition also has been shown both in vitro and in vivo to enhance endothelial cell growth, thus suggesting that DPP-4 inhibitors, in addition to their action to enhance the effects of GLP-1, may also have direct actions to reverse the deleterious effects of diabetes on vascular function [Takasawa et al. 2010]. Van Poppel and colleagues reported that 4 weeks of treatment with the DPP-4 inhibitor, vildagliptin, improved acetylcholine-mediated EDV [Van Poppel et al. 2011]. Matsubara and colleagues reported that the DPP-4 inhibitor, des-fluoro-sitagliptin, improved acetylcholine-mediated EDV and reduced atherosclerotic lesion development in apolipoprotein E deficient mice via the augmentation of GLP-1-mediated effects in endothelial cells and macrophages [Matsubara et al. 2012]. DPP-4, a serine proteinase, will also activate proteinase-activated receptors (PARs) via the cleavage of the extracellular amino terminus of the PAR, thus exposing the amino-terminal ‘tethered ligand’ domain that binds to and activates the cleaved PAR. PAR1, 2, 3 and 4 are targets for thrombin, trypsin, matrix metalloproteinases and other proteinases, and are involved in a multitude of physiological functions. They have also been linked to inflammation and pathophysiological cellular processes [Ramachandran and Holleneberg, 2008; Gieseler et al. 2013]. Activation of PAR2 in endothelial cells promotes EDV via NO and non-NO-mediated cell signalling [McGuire et al. 2002]. Thus, inhibition of DPP-4 could, indirectly, impair EDV. The acute exposure of endothelial cells in culture to alogliptin enhances eNOS and Akt phosphorylation (Ser1177 and Thr473 respectively) [Shah et al. 2011]. Thus, although stated as ‘cardiovascular neutral’, there are reports of favourable effects in animal models of ischaemia and reperfusion, and they may also modulate blood pressure. Thus, pharmacological inhibition and also genetic ablation of DPP-4 are cardiovascular protective in ischaemia/reperfusion protocols and post myocardial infarction in mice [Ku et al. 2011; Sauvé et al. 2010]. In part, the beneficial effects of DPP-4 inhibitors may involve the inhibition of AGE-mediated increase in oxidative stress via the direct inhibition of NADPH-oxidase or xanthine oxidase [Kröller-Schön et al. 2012; Ishibashi et al. 2013]; however, this may not be a class effect, but limited to linagliptin [Kröller-Schön et al. 2012]. Based on studies with Zucker diabetic fatty rat and human umbilical vein endothelial cells (HUVECs) it has been argued that in vivo DPP-4 inhibition will enhance GLP-1-mediated increases in cellular cyclic AMP levels and, via cyclic AMP response element-binding protein transcription of antioxidant genes, such as HO-1 [Oesburg et al. 2010]. An anti-inflammatory action of DPP-4 inhibitors can also be linked to a reduction in the production of pro-inflammatory cytokines [Reinhold et al. 1993].
In conclusion, an accumulation of data indicates that inhibition of DPP-4, in addition to enhancing the effects of both endogenously released GLP-1 and exogenously administered GLP-1, has actions unrelated to inhibition of the enzymatic breakdown of GLP-1 [Fadini and Avogaro, 2011; Zhong et al. 2013]. However, negative effects on endothelium function have also been noted. In addition to the potential reduction of PAR2-mediated vasodilatation, other negative effects have been described. Thus, Ayaori et al. [2013] reported that a 6-week treatment of men with T2D with either sitagliptin or voglibose resulted in an attenuation of flow-mediated vasodilatation in the brachial artery. Furthermore, DPP-4 inhibits the polymerization of fibrin monomers and thus inhibition of DPP-4 may be prothrombotic [Mentlein and Heymann, 1982]. The antithrombotic properties of DPP-4 and the fact that the protease serves as an immobilized anticoagulant on endothelial cells have also been emphasized in a study by Krijnen and colleagues. They reported that the expression and activity of DPP-4 are decreased following myocardial infarction in humans and this results in a prothrombogenic phenotype [Krijnen et al. 2012]. A number of clinical trials are underway with DPP-4 inhibitors and the results should shed further light on their effects on cardiovascular function and cardiovascular risk factors [Scheen 2013a, 2013b].
The DPP-4 inhibitor, vildagliptin, as an add-on therapy with metformin administered to a group of 68 patients with T2D has been shown to result in a favourable cardiovascular response as measured by a reduction in serum ADMA levels. However, no difference was noted in levels of C-reactive protein or fibrinogen [Cakirca et al. 2014].
α-Glucosidase inhibitors
Acarbose was the first such inhibitor approved by the FDA in 1995 and miglitol in 1996. Acarbose and related drugs are orally effective and should be taken together with meals. Predictably the most frequent side effects are gastrointestinal related, such as flatulence and diarrhoea; however, hypoglycaemia can occur and requires treatment with a monosaccharide such as glucose. There have been rare cases of liver toxicity reported following several months of therapy with acarbose. The primary action of these compounds is to inhibit the α-glucosidase enzymes (maltase, isomaltase, sucrase, glucoamylase) that hydrolyze oligosaccharides and disaccharides to glucose. These enzymes are associated with the small intestine and thus, when inhibited, there is a reduction of glucose availability and absorption.
Cardiovascular benefits of α-glucosidase inhibitors
By slowing glucose absorption and reducing plasma glucose levels, particularly postprandial glucose, α-glucosidase inhibitors should reduce cardiovascular risk in patients with diabetes and this has been shown for the inhibitor, acarbose, with additional benefits in terms of weight loss, blood pressure reduction and lowering of triglycerides [Hanefeld and Schaper, 2008; Hanefeld et al. 2008].
Sodium glucose cotransporter 2 inhibitors
Sodium glucose cotransporter 2 (SGLT2) inhibitors are the newest group of drugs to be used for the treatment of diabetes and, by binding to the SGLT2 in the proximal tubule of the kidney, block glucose reabsorption. The development of SGLT2 inhibitors was based on studies with the flavinoid, phlorizen, that occurs in several fruit trees, including apple and cherry, and the bark of the pear tree. Phlorizen is an inhibitor of the widely expressed SGLT1 as well as SGLT2. Phlorizen has been shown to correct hyperglycaemia and improve insulin sensitivity in an animal model of diabetes [Rossetti et al. 1987]. Phlorizen has not been developed for clinical use, presumably because of assumed toxicity and nonspecificity of action on the SGLT transporter family [Ehrenkranz et al. 2005]. The advantage of SGLT2 inhibitors is that expression of SGLT2 is highly specific for the kidney and thus, theoretically, should not affect glucose transport in other tissues [Chen et al. 2010]. Canagliflozin was approved in the USA in 2013 and dapagliflozin was approved in Europe in 2012 and in the USA in early 2014. Other ‘flozins’ are under development. The use of SGLT2 inhibitors is associated with improved insulin sensitivity, reduction in blood pressure and weight loss; however, inhibiting SGLT2 can, potentially, result in dehydration and, it has also been reported, enhance endogenous glucose production; the latter, presumably, as a compensatory effect to the excretion of glucose in the urine [Merovci et al. 2014].
Cardiovascular impact of SGLT2 inhibitors
Correction of hyperglycaemia, weight loss and reduction in blood pressure suggest a positive impact on cardiovascular health; however, effects on other cardiovascular risk factors such as lipid profile are less clear and insufficient data are available to make a conclusion at this time [Guthrie, 2013]. The effectiveness of the flozins depends on adequate renal function and since chronic kidney disease is a feature of the advanced stages of T2D, caution in their use in patients with renal impairment is required.
Insulin
Treatment with insulin is essential for patients with T1D and, with the progression of β-cell dysfunction in T2D, is ultimately required for many subjects with T2D. Insulin facilitates the storage and synthesis of carbohydrates, lipids and protein. From a physiological perspective and the regulation of glycaemia, insulin functions as a physiological antagonist of glucagon; however, insulin has multiple effects in the body, including effects on gene expression. Today there are a variety of insulin preparations available from the ultra-rapid-acting lispro, with onset within 15 min and duration of up to 5 h, to ultra-long-acting degludec (approved in Europe but not yet by FDA) with a duration of action of 40 h. The major side effects of insulin use are hypoglycaemia and weight gain. As reflected by the results of the ACCORD study, hypoglycaemia is a potential risk in the treatment of T2D. Severe hypoglycaemia is most frequently associated with T1D, but the incidence increases in T2D as the use of insulin therapy increases. Hypoglycaemia, as already discussed, is also associated with the use of sulfonylurea and meglitinide oral hypoglycaemic drugs, but not with metformin. Severe hypoglycaemia is a very serious issue and can lead to an increased risk of dementia, cardiovascular events and death [Brunton, 2012].
An important question regarding insulin therapy in patients with T2D with insulin resistance is whether high levels of insulin, or the use of long-acting insulins, contributes to cardiovascular morbidity? In this regard it is of interest to note that the FDA has suspended approval of the ultra-long-acting insulin, degludec, subject to provision of cardiovascular safety data. Based on the data from the DCCT and UKPDS studies, we know that maintaining near normal HbA1c decreases mortality in patients with T1D and T2D. The recently completed 6-year duration ORIGIN trial with the long-acting insulin, glargine, enrolled over 12,000 patients and was designed to maintain basal insulin and help normalize plasma glucose levels [ORIGIN et al. 2012]. The results demonstrated a neutral effect on cardiovascular outcomes and also cancers, a reduction in the incidence of diabetes, but a positive link to weight gain and hypoglycaemia. An association of insulin use with cancer has also been reported. In one study, a meta-analysis of over 40 studies indicated an increased risk for some forms of cancer (breast), but not others (colon) [Karlstad et al. 2013]. Others have suggested that the risk of cancer associated with exogenous insulin use increases in a cumulative fashion whereas monotherapy with metformin, compared with a sulfonylurea, lowered risk [Bowker et al. 2010]. The cardiovascular and neurological risk potential of hypoglycaemia resulting from insulin use are well recognized, but the longer-term effect of hyperinsulinaemia on cardiovascular function remains unclear. The proproliferative effect of insulin on vascular smooth cells in culture has been known for almost 45 years and a positive link between hyperinsulinaemia and lipid abnormalities, atherosclerosis and hypertension reported [Stout and Vallance-Owen, 1969; Stout et al. 1975; Stout 1990; Muis et al. 2005]. For instance, the results from a study of the effects of hyperinsulinaemia in the absence and presence of high concentrations of the free fatty acid, palmitate, on primary cell cultures of human coronary arteries demonstrated that palmitate inhibited the effects of insulin on Akt signalling, but promoted the mitogenic actions of insulin, thus suggesting that in a metabolic syndrome and diabetic milieu, hyperinsulinaemia would be proatherogenic [Cersosimo et al. 2012]. However, a retrospective study of 70 patients with insulinomas failed to link hyperinsulinaemia to lipid abnormalities and hypertension was only present in five of the patients who remained hypertensive after removal of the insulinoma [Leonetti et al. 1993]. In addition, Sawicki and colleagues failed to link hyperinsulinaemia to hypertension in patients with insulinomas and a meta-analysis of 12 published studies of patients with hyperinsulinaemia reported only a weak positive link to cardiovascular disease [Ruige et al. 1998]. Furthermore, there is a substantial body of data indicating that insulin has an anti-inflammatory action that might therefore offset proatherogenic effects [Hyan et al. 2011]. In its physiological concentration range, insulin is anti-inflammatory, but at supraphysiological concentrations insulin is pro-inflammatory [Krieger-Brauer et al. 1992, 1997]. However, intensive insulin therapy administered to patients in intensive care units has also been shown to normalize hyperglycaemia, lower IL-6 and raise levels of the antioxidant enzyme superoxide dismutase that dismutases superoxide anions into oxygen and hydrogen peroxide [Wiryana, 2009]. For the latter study it is, of course, important to recognize that the benefits of intensive insulin use that were described were in an acute setting and the effects of high insulin levels and insulin resistance in the chronic setting may have a different end result. Thus, it is argued that insulin resistance serves a protective role and guards insulin-sensitive tissues against glucose overload, glucose toxicity and, by enhancing glucose uptake in the presence of high lipid concentrations, glucolipotoxicity [Nolan et al. 2011, 2013]. Recent reports also raise concern, linking insulin use with an increase in mortality. Thus, in a retrospective analysis of over 84,000 patients with T2D, treatment with exogenous insulin was associated with an increased risk of diabetes-related complications and all-cause mortality, as well as cancer [Currie et al. 2013].
Summary
There is an increasing number of drugs available for the treatment of diabetes and therapeutic choices can be made on the basis of an expanding knowledge base of the cardiovascular benefits versus potential cardiovascular risks of the available drugs. Recognition that treatment should not only address blood glucose levels and HbA1c is important for the reduction of the morbidity and mortality associated with diabetes.
Footnotes
Conflict of interest statement: Drs Ding and Triggle are funded by the Qatar Foundation National Priorities Research Program, NPRP.
Funding: Drs Ding and Triggle are in receipt of research funding from the Qatar Foundation National Priorities Research Program, NPRP 4-910-3-244, NPRP 09-413-3-104, NPRP 5-149-3-040 and NPRP 6-428-3-113 that have provided support for the data reported in this review article that has emanated from their research programs.
Contributor Information
Chris R. Triggle, Departments of Pharmacology and Medical Education, Weill Cornell Medical College in Qatar, PO Box 24144, Education City, Doha, Qatar
Hong Ding, Departments of Pharmacology and Medical Education, Weill Cornell Medical College in Qatar, Education City, Doha, Qatar.
References
- Abbott C., Baker E., Sutherland G., McCaughan G. (1994) Genomic organization, exact localization, and tissue expression of the human CD26 (dipeptidyl peptidase IV) gene. Immunogenetics 40: 331–338 [DOI] [PubMed] [Google Scholar]
- ACCORD (2008) The Action to Control Cardiovascular Risk in Diabetes Study Group. Effects of intensive glucose lowering in type-2 diabetes. New Engl J Med 358: 2560–2572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aghamohammadi V., Gargari B., Aliasgharzadeh A. (2011) Effect of folic acid supplementation on homocysteine, serum total antioxidant capacity, and malondialdehyde in patients with type 2 diabetes mellitus. J Am Coll Nutr 30: 210–215 [DOI] [PubMed] [Google Scholar]
- Ajjan R., Grant P. (2006) Cardiovascular disease prevention in patients with type 2 diabetes: the role of oral anti-diabetic agents. Diab Vasc Dis Res 3: 147–158 [DOI] [PubMed] [Google Scholar]
- Aljofan M., Ding H. (2010) High glucose increases expression of cyclooxygenase-2, increases oxidative stress and decreases the generation of nitric oxide in mouse microvessel endothelial cells. J Cell Physiol 222: 669–675 [DOI] [PubMed] [Google Scholar]
- Arunachalam G., Samuel S., Marei I., Ding H., Triggle C. (2014) Metformin modulates hyperglycaemia-induced endothelial senescence and apoptosis through SIRT1. Br J Pharmacol 171: 523–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayaori M., Iwakami N., Uto-Kondo H., Sato H., Sasaki M., Komatsu T., et al. (2013) Dipeptidyl peptidase-4 inhibitors attenuate endothelial function as evaluated by flow-mediated vasodilatation in type 2 diabetic patients. J Am Heart Assoc 2: e003277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron A. (1994) Hemodynamic actions of insulin. Am J Physiol 267: E187–E202 [DOI] [PubMed] [Google Scholar]
- Basha B., Samuel S., Triggle C., Ding H. (2012) Endothelial dysfunction in diabetes mellitus: possible involvement of endoplasmic reticulum stress? Exp Diabetes Res 2012: 481840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu A., Charkoudian N., Schrage W., Rizza R., Basu R., Joyner M. (2007) Beneficial effects of GLP-1 on endothelial function in humans: dampening by glyburide but not by glimepiride. Am J Physiol Endocrinol Metab 293: E1289–E1295 [DOI] [PubMed] [Google Scholar]
- Bertrand L., Horman S., Beauloye C., Vanoverschelde J. (2008) Insulin signalling in the heart. Cardiovasc Res. 79: 238–248 [DOI] [PubMed] [Google Scholar]
- Best C., Scott D. (1923) The preparation of insulin. J Biol Chem 57: 709–723 [Google Scholar]
- Betteridge DJ. (2011) Thiazolidinediones and fracture risk in patients with type 2 diabetes. Diabet Med 28: 759–771 [DOI] [PubMed] [Google Scholar]
- Bo S., Castiglione A., Ghigo E., Gentile L., Durazzo M., Cavallo-Perin P., et al. (2013) Mortality outcomes of different sulphonylurea drugs: the results of a 14-year cohort study of type 2 diabetic patients. Eur J Endocrinol 169: 117–126 [DOI] [PubMed] [Google Scholar]
- Boonacker E., Van Noorden C. (2003) The multifunctional or moonlighting protein CD26/DPPIV. Eur J Cell Biol 82: 53–73 [DOI] [PubMed] [Google Scholar]
- Bosi E. (2009) Metformin – the gold standard in type 2 diabetes: what does the evidence tell us? Diabetes Obes Metab 11(Suppl. 2): 3–8 [DOI] [PubMed] [Google Scholar]
- Boulanger C., Lüscher T. (1990) Release of endothelin from the porcine aorta. Inhibition by endothelium-derived nitric oxide. J Clin Invest 85: 587–590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourg C., Phillips B. (2012) Rosiglitazone, myocardial ischemic risk, and recent regulatory actions. Ann Pharmacother 46: 282–289 [DOI] [PubMed] [Google Scholar]
- Bowker S., Yasui Y., Veugelers P., Johnson J. (2010) Glucose-lowering agents and cancer mortality rates in type 2 diabetes: assessing effects of time-varying exposure. Diabetologia 53:1631–1637 [DOI] [PubMed] [Google Scholar]
- Brown J., Dryburgh J. (1971) A gastric inhibitory polypeptide. II. The complete amino acid sequence. Can J Biochem 49: 867–872 [DOI] [PubMed] [Google Scholar]
- Brunner F., Wascher T. (1998) Contribution of the endothelium to transcapillary insulin transport in rat isolated perfused hearts. Diabetes 47: 1127–1134 [DOI] [PubMed] [Google Scholar]
- Brunton S. (2012) Hypoglycemic potential of current and emerging pharmacotherapies in type 2 diabetes mellitus. Postgrad Med 124: 74–83 [DOI] [PubMed] [Google Scholar]
- Cakirca M., Karatoprak C., Zorlu M., Kiskac M., Kanat M., Cikrikcioglu M., et al. (2014) Effect of vildagliptin add-on treatment to metformin on plasma asymmetric dimethylarginine in type 2 diabetes mellitus patients. Drug Des Devel Ther 8: 239–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cersosimo E., Xu X., Musi N. (2012) Potential role of insulin signaling on vascular smooth muscle cell migration, proliferation, and inflammation pathways. Am J Physiol Cell Physiol 302: C652–C657 [DOI] [PubMed] [Google Scholar]
- Chen J., Williams S., Ho S., Loraine H., Hagan D., Whaley J., et al. (2010) Quantitative PCR tissue expression profiling of the human SGLT2 gene and related family members. Diabetes Ther 1: 57–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Magliano D., Zimmet P. (2011) The worldwide epidemiology of type 2 diabetes mellitus–present and future perspectives. Nat Rev Endocrinol 8: 228–236 [DOI] [PubMed] [Google Scholar]
- Chen Z., Peng I., Sun W., Su. M., Hsu P.H., Fu Y., et al. (2009) AMP-activated protein kinase functionally phosphorylates endothelial nitric oxide synthase Ser633. Circ Res 104: 496–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark M., Wallis M., Barrett E., Vincent M., Richards S., Clerk L., et al. (2003) Blood flow and muscle metabolism: a focus on insulin action. Am J Physiol Endocrinol Metab 284: E241–E258 [DOI] [PubMed] [Google Scholar]
- Cornfield J. (1971) The University Group Diabetes Program: a further statistical analysis of the mortality findings JAMA 217: 1676–1687 [PubMed] [Google Scholar]
- Cuber J. C., Bosshard A., Vidal H., Vega F., Wiernsperger N., Rapin J. R. (1994) Metabolic and drug distribution studies do not support direct inhibitory effects of metformin on intestinal glucose absorption. Diabete Metab 20: 532–539 [PubMed] [Google Scholar]
- Currie C., Poole C., Evans M., Peters J., Morgan C. (2013) Mortality and other important diabetes-related outcomes with insulin vs other antihyperglycemic therapies in type 2 diabetes. J Clin Endocrinol Metab 98: 668–677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czyzyk A., Tawecki J., Sadowski J., Ponikowska I., Szczepanik Z. (1968) Effect of biguanides on intestinal absorption of glucose. Diabetes 17: 492–498 [DOI] [PubMed] [Google Scholar]
- Daousi C., Casson I., Gill G., MacFarlane I., Wilding J., Pinkney J. (2006) Prevalence of obesity in type 2 diabetes in secondary care: association with cardiovascular risk factors. Postgrad Med J 82(966): 280–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DCCT (1993) The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med 329: 977–986 [DOI] [PubMed] [Google Scholar]
- Defronzo R. (2009) Banting Lecture. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes 58: 773–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Defronzo R., Mehta R., Schnure J. (2013a) Pleiotropic effects of thiazolidinediones: implications for the treatment of patients with type 2 diabetes mellitus. Hosp Pract (1995) 41: 132–147 [DOI] [PubMed] [Google Scholar]
- Defronzo R., Tripathy D., Schwenke D., Banerji M., Bray G., Buchanan T., et al. ACT NOW Study (2013b) Prevention of diabetes with pioglitazone in ACT NOW: physiologic correlates. Diabetes 62: 3920–3926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Jager J., Kooy A., Lehert P., Bets D., Wulffelé M., Teerlink T., et al. (2005) Effects of short-term treatment with metformin on markers of endothelial function and inflammatory activity in type 2 diabetes mellitus: a randomized, placebo-controlled trial. J Intern Med 257: 100–109 [DOI] [PubMed] [Google Scholar]
- Del Turco S., Gaggini M., Daniele G., Basta G., Folli F., Sicari R., et al. (2007) Insulin resistance and endothelial dysfunction: a mutual relationship in cardiometabolic risk. Curr Pharm Des 19: 2420–2431 [DOI] [PubMed] [Google Scholar]
- de Rivas B., Luque M., Martell N., Fernández C., Fernández-Cruz A. (2007) Pioglitazone decreases ambulatory blood pressure in type 2 diabetics with difficult-to-control hypertension. J Clin Hypertens (Greenwich) 9: 530–537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detaille D., Guigas B., Chauvin C., Batandier C., Fontaine E., Wiernsperger N., et al. (2005) Metformin prevents high-glucose-induced endothelial cell death through a mitochondrial permeability transition-dependent process. Diabetes 54: 2179–2187 [DOI] [PubMed] [Google Scholar]
- Dimmeler S., Fleming I., Fisslthaler B., Hermann C., Busse R., Zeiher A. (1999) Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature 399: 601–605 [DOI] [PubMed] [Google Scholar]
- Ding H., Triggle C. (2010) Endothelial dysfunction in diabetes: multiple targets for treatment. Pflugers Archiv 459: 977–994 [DOI] [PubMed] [Google Scholar]
- Dobrina A., Rossi F. (1983) Metabolic properties of freshly isolated bovine endothelial cells. Biochim Biophys Acta 762: 295–301 [DOI] [PubMed] [Google Scholar]
- Dorkhan M., Dencker M., Stagmo M., Groop L. (2009) Effect of pioglitazone versus insulin glargine on cardiac size, function, and measures of fluid retention in patients with type 2 diabetes. Cardiovasc Diabetol 8: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dormandy J., Charbonnel B., Eckland D., Erdmann E., Massi-Benedetti M., Moules I., et al.; PROactive Investigators (2005) Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet 366: 1279–1289 [DOI] [PubMed] [Google Scholar]
- Du X., Edelstein D., Rossetti L., Fantus I., Goldberg H., Ziyadeh F., et al. (2000) Hyperglycemia-induced mitochondrial superoxide overproduction activates the hexosamine pathway and induces plasminogen activator inhibitor-1 expression by increasing Sp1 glycosylation. Proc Natl Acad Sci U S A 97: 12222–12226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan A., Blind E., Dunder K., de Graeff P., Hummer B., Bourcier T., et al. (2014) Pancreatic safety of incretin-based drugs – FDA and EMA assessment. N Engl J Med 370: 794–797 [DOI] [PubMed] [Google Scholar]
- Ehrenkranz J., Lewis N., Kahn C., Roth J. (2005) Phlorizen: a review. Diabetes Metab Res Rev 21: 31–38 [DOI] [PubMed] [Google Scholar]
- Eleftheriadou I., Grigoropoulou P., Katsilambros N., Tentolouris N. (2008) The effects of medications used for the management of diabetes and obesity on postprandial lipid metabolism. Curr Diabetes Rev 4: 340–356 [DOI] [PubMed] [Google Scholar]
- El-Mir M., Nogueira V., Fontaine E., Avéret N., Rigoulet M., Leverve X. (2000) Dimethylbiguanide inhibits cell respiration via an indirect effect targeted on the respiratory chain complex I. J Biol Chem 275: 223–238 [DOI] [PubMed] [Google Scholar]
- El-Osta A., Brasacchio D., Yao D., Pocai A., Jones P., Roeder R., et al. (2008) Transient high glucose causes persistent epigenetic changes and altered gene expression during subsequent normoglycemia. J Exp Med 205: 2409–2417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerging Risk Factors Collaboration (ERFC), Sarwar N., Gao P., Seshasai S., Gobin R., Kaptoge S., Di Angelantonio E., et al. (2010) Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet 375: 2215–2222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito K., Nappo F., Marfella R., Giugliano G., Giugliano F., Ciotola M., et al. (2002) Inflammatory cytokine concentrations are acutely increased by hyperglycemia in humans: role of oxidative stress. Circulation 106: 2067–2072 [DOI] [PubMed] [Google Scholar]
- Fadini G., Avogaro A. (2011) Cardiovascular effects of DPP-4 inhibition: beyond GLP-1. Vascul Pharmacol 55: 10–16 [DOI] [PubMed] [Google Scholar]
- Foretz M., Hébrard S., Leclerc J., Zarrinpashneh E., Soty M., Mithieux G., et al. (2010) Metformin inhibits hepatic gluconeogenesis in mice independently of the LKB1/AMPK pathway via a decrease in hepatic energy state. J Clin Invest 120: 2355–2369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler M. (2008) Microvascular and macrovascular complications of diabetes. Clin Diabetes 26: 77–82 [Google Scholar]
- Frid A., Sterner G., Löndahl M., Wiklander C, Cato A, Vinge E., et al. (2010) Novel assay of metformin levels in patients with type 2 diabetes and varying levels of renal function: clinical recommendations. Diabetes Care 33: 1291–1293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fullerton M., Galic S., Marcinko K., Sikkema S., Pulinikunnil T., Chen Z., et al. (2013) Single phosphorylation sites in Acc1 and Acc2 regulate lipid homeostasis and the insulin-sensitizing effects of metformin. Nat Med 19: 1649–1654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangji A., Cukierman T., Gerstein H., Goldsmith C., Clase C. (2007) A systematic review and meta-analysis of hypoglycemia and cardiovascular events. Diabetes Care 30: 389–394 [DOI] [PubMed] [Google Scholar]
- Genders A., Frison V., Abramson S., Barrett E. (2013) Endothelial cells actively concentrate insulin during its transendothelial transport. Microcirculation 20: 434–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacco F., Brownlee M. (2010) Oxidative stress and diabetic complications. Circ Res 107: 1058–1070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gieseler F., Ungefroren H., Settmacher U., Hollenberg M., Kaufmann R. (2013) Proteinase-activated receptors (PARs) – focus on receptor-receptor-interactions and their physiological and pathophysiological impact. Cell Commun Signal 11: 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glueck C., Fontaine R., Wang P., Subbiah M., Weber K., Illig E., et al. (2001) Metformin reduces weight, centripetal obesity, insulin, leptin, and low-density lipoprotein cholesterol in nondiabetic, morbidly obese subjects with body mass index greater than 30. Metabolism 50: 856–861 [DOI] [PubMed] [Google Scholar]
- Golpon H., Puechner A., Welte T., Wichert P., Feddersen C. (2001) Vasorelaxant effect of glucagon-like peptide-(7–36)amide and amylin on the pulmonary circulation of the rat. Regul Pept 102: 81–86 [DOI] [PubMed] [Google Scholar]
- Graham D., Ouellet-Hellstrom R., MaCurdy T., Ali F., Sholley C., Worrall C., et al. (2010) Risk of acute myocardial infarction, stroke, heart failure, and death in elderly Medicare patients treated with rosiglitazone or pioglitazone. JAMA 304: 411–418 [DOI] [PubMed] [Google Scholar]
- Graham G., Punt J., Arora M., Day R., Doogue M., Duong J., et al. (2011) Clinical pharmacokinetics of metformin. Clin Pharmacokinet 50: 81–98 [DOI] [PubMed] [Google Scholar]
- Green B., Hand K., Dougan J., McDonnell B., Cassidy R., Grieve D. (2008) GLP-1 and related peptides cause concentration-dependent relaxation of rat aorta through a pathway involving KATP and cAMP. Arch Biochem Biophys 478: 136–142 [DOI] [PubMed] [Google Scholar]
- Grundy S., Howard B., Smith S., Jr, Eckel R., Redberg R., Bonow R. (2002) Prevention Conference VI: Diabetes and Cardiovascular Disease: executive summary: conference proceeding for healthcare professionals from a special writing group of the American Heart Association. Circulation 105: 2231–2239 [DOI] [PubMed] [Google Scholar]
- Guthrie R. (2013) Sodium-glucose co-transporter 2 inhibitors and the potential for cardiovascular risk reduction in patients with type 2 diabetes mellitus. Postgrad Med 125: 21–32 [DOI] [PubMed] [Google Scholar]
- Hachiya H., Halban P., King G. (1988) Intracellular pathways of insulin transport across vascular endothelial cells. Am J Physiol 255: C459-C464 [DOI] [PubMed] [Google Scholar]
- Halimi S. (2006) Metformin: 50 years old, fit as a fiddle, and indispensable for its pivotal role in type 2 diabetes management. Diabetes Metab 32: 555–556 [DOI] [PubMed] [Google Scholar]
- Hanefeld M., Schaper F. (2008) Acarbose: oral anti-diabetes drug with additional cardiovascular benefits. Expert Rev Cardiovasc Ther 6: 153–163 [DOI] [PubMed] [Google Scholar]
- Hanefeld M., Schaper F., Koehler C. (2008) Effect of acarbose on vascular disease in patients with abnormal glucose tolerance. Cardiovasc Drugs Ther 22: 225–231 [DOI] [PubMed] [Google Scholar]
- Hardie D. (2007) AMP-activated protein kinase as a drug target. Annu Rev Pharmacol Toxicol 47: 185–210 [DOI] [PubMed] [Google Scholar]
- Hardie D. (2013) AMPK: a target for drugs and natural products with effects on both diabetes and cancer. Diabetes 62: 2164–2172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie D., Ross F., Hawley S. (2012) AMPK: a nutrient and energy sensor that maintains energy homeostasis Nat Rev Mol Cell Biol 13: 251–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley S., Boudeau J., Reid J., Mustard K., Udd L., Mäkelä T., et al. (2003) Complexes between the LKB1 tumor suppressor, STRAD alpha/beta and MO25 alpha/beta are upstream kinases in the AMP-activated protein kinase cascade. J Biol 2: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley S., Gadalla A., Olsen G., Hardie D. (2002) The antidiabetic drug metformin activates the AMP-activated protein kinase cascade via an adenine nucleotide-independent mechanism. Diabetes 51: 2420–2425 [DOI] [PubMed] [Google Scholar]
- Hawley S., Ross F., Chevtzoff C., Green K., Evans A., Fogarty S., et al. (2010) Use of cells expressing gamma subunit variants to identify diverse mechanisms of AMPK activation. Cell Metab 11: 554–565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmingsen B., Schroll J., Lund S., Wetterslev J., Gluud C., Vaag A., et al. (2013) Sulphonylurea monotherapy for patients with type 2 diabetes mellitus. Cochrane Database Syst Rev 4: CD009008. [DOI] [PubMed] [Google Scholar]
- Herder C., Kowall B., Tabak A., Rathmann W. (2014) The potential of novel biomarkers to improve risk prediction of type 2 diabetes. Diabetologia 57: 16–29 [DOI] [PubMed] [Google Scholar]
- Hyun E., Ramachandran R., Hollenberg M., Vergnolle N. (2011) Mechanisms behind the anti-inflammatory actions of insulin Crit Rev Immunol 31: 307–340 [DOI] [PubMed] [Google Scholar]
- Ihlemann N., Rask-Madsen C., Perner A., Dominguez H., Hermann T., Køber L., et al. (2003) Tetrahydrobiopterin restores endothelial dysfunction induced by an oral glucose challenge in healthy subjects. Am J Physiol Heart Circ Physiol 285: H875-H882 [DOI] [PubMed] [Google Scholar]
- International Diabetes Federation (2013) IDF Diabetes Atlas, 6th edition. Available at: http://www.idf.org/diabetesatlas/introduction (accessed March 2014).
- Irving R., Noon J., Watt G., Webb D., Walker B. (2001) Activation of the endothelin system in insulin resistance. QJM 94: 321–326 [DOI] [PubMed] [Google Scholar]
- Ishibashi Y., Matsui T., Maeda S., Higashimoto Y., Yamagishi S. (2013) Advanced glycation end products evoke endothelial cell damage by stimulating soluble dipeptidyl peptidase-4 production and its interaction with mannose 6-phosphate/insulin-like growth factor II receptor. Cardiovasc Diabetol 12: 125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isoda K., Young J., Zirlik A., MacFarlane L., Tsuboi N., Gerdes N., et al. (2006) Metformin inhibits proinflammatory responses and nuclear factor-kappaB in human vascular wall cells. Arterioscler Thromb Vasc Biol 26: 611–617 [DOI] [PubMed] [Google Scholar]
- Jialal I., Jing G., Buchwald S., Kahn C., Crettaz M. (1984) Processing of insulin by bovine endothelial cells in culture. Internalization without degradation. Diabetes 33: 794–800 [DOI] [PubMed] [Google Scholar]
- Johnson J., Simpson S., Toth E., Majumdar S. (2005) Reduced cardiovascular morbidity and mortality associated with metformin use in subjects with type 2 diabetes. Diabet Med 22: 497–502 [DOI] [PubMed] [Google Scholar]
- Karlstad O., Starup-Linde J., Vestergaard P., Hjellvik V., Bazelier M., Schmidt M., et al. (2013) Use of insulin and insulin analogs and risk of cancer – systematic review and meta analysis of observational studies. Curr Drug Saf 8: 333–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano H., Motoyama T., Hirashima O., Hirai N., Miyao Y., Sakamoto T., et al. (1999) Hyperglycemia rapidly suppresses flow-mediated endothelium-dependent vasodilation of brachial artery. J Am Coll Cardiol 34: 146–154 [DOI] [PubMed] [Google Scholar]
- Kerti L., Witte A., Winkler A., Grittner U., Rujescu D., Flöel A. (2013) Higher glucose levels associated with lower memory and reduced hippocampal microstructure. Neurology 81: 1746–1752 [DOI] [PubMed] [Google Scholar]
- Kim J., Montagnani M., Koh K., Quon M. (2006) Reciprocal relationships between insulin resistance and endothelial dysfunction: molecular and pathophysiological mechanisms. Circulation 113: 1888–1904 [DOI] [PubMed] [Google Scholar]
- Kim W., Egan J. (2008) The role of incretins in glucose homeostasis and diabetes treatment. Pharmacol Rev 60: 470–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King G., Johnson S. (1985) Receptor-mediated transport of insulin across endothelial cells. Science 227: 1583–1586 [DOI] [PubMed] [Google Scholar]
- Kirpichnikov D., McFarlane S., Sowers J. (2002) Metformin: an update. Ann Intern Med 137: 25–33 [DOI] [PubMed] [Google Scholar]
- Kleinsorge H. (1998) Carbutamide – the first oral antidiabetic. A retrospect. Exp Clin Endocrinol Diabetes 106: 149–151 [DOI] [PubMed] [Google Scholar]
- Komatsu R., Matsuyama T., Namba M., Watanabe N., Itoh H., Kono N., et al. (1989) Glucagonostatic and insulinotropic action of glucagonlike peptide I-(7–36)-amide. Diabetes 38: 902–905 [DOI] [PubMed] [Google Scholar]
- Krieger-Brauer H., Kather H. (1992) Human fat cells possess a plasma membrane H2O2-generating system that is activated via a mechanism bypassing the receptor kinase J Clin Invest 89: 1006–1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieger-Brauer H., Medda P., Kather H. (1997) Insulin-induced activation of NADPH-dependent H2O2 generation in human adipocyte plasma membranes is mediated by Galphai2. J Biol Chem 272: 10135–10143 [DOI] [PubMed] [Google Scholar]
- Krijnen P., Hahn N., Kholová I., Baylan U., Sipkens J., van Alphen F., et al. (2012) Loss of DPP4 activity is related to a prothrombogenic status of endothelial cells: implications for the coronary microvasculature of myocardial infarction patients. Basic Res Cardiol 107: 233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kröller-Schön S., Knorr M., Hausding M., Oelze M., Schuff A., Schell R., et al. (2012) Glucose-independent improvement of vascular dysfunction in experimental sepsis by dipeptidyl-peptidase 4 inhibition. Cardiovasc Res 96: 140–149 [DOI] [PubMed] [Google Scholar]
- Ku H., Chen W., Su M. (2011) DPP4 deficiency preserves cardiac function via GLP-1 signaling in rats subjected to myocardial ischemia/reperfusion. Naunyn Schmiedebergs Arch Pharmacol 384: 197–207 [DOI] [PubMed] [Google Scholar]
- Kubota T., Kubota N., Kumagai H., Yamaguchi S., Kozono H., Takahashi T., et al. (2011) Impaired insulin signaling in endothelial cells reduces insulin-induced glucose uptake by skeletal muscle. Cell Metab 13: 294–307 [DOI] [PubMed] [Google Scholar]
- Kubota T., Kubota N., Moroi M., Terauchi Y., Kobayashi T., Kamata K., et al. (2003) Lack of insulin receptor substrate-2 causes progressive neointima formation in response to vessel injury. Circulation 107: 3073–3080 [DOI] [PubMed] [Google Scholar]
- Laakso M., Edelman S., Brechtel G., Baron A. (1990) Decreased effect of insulin to stimulate skeletal muscle blood flow in obese man. A novel mechanism for insulin resistance. J Clin Invest 85: 1844–1852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalau J., Lacroix C. (2003) Measurement of metformin concentration in erythrocytes: clinical implications. Diabetes Obes Metab 5: 93–98 [DOI] [PubMed] [Google Scholar]
- Lamers D., Famulla S., Wronkowitz N., Hartwig S., Lehr S., Ouwens D., et al. (2011) Dipeptidyl peptidase 4 is a novel adipokine potentially linking obesity to the metabolic syndrome. Diabetes 60: 1917–1925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamontagne J., Jalbert-Arsenault E., Pepin E., Peyot M., Ruderman N., Nolan C., et al. (2013) Pioglitazone acutely reduces energy metabolism and insulin secretion in rats. Diabetes 62(6): 2122–2129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebovitz H. (2002) Differentiating members of the thiazolidinedione class: a focus on safety. Diabetes Metab Res Rev 18(Suppl. 2): S23–S29 [DOI] [PubMed] [Google Scholar]
- Ledford H. (2013) Diabetes drugs ride a bumpy road. Nature 504: 198. [DOI] [PubMed] [Google Scholar]
- Leonetti F., Iozzo P., Giaccari A., Sbraccia P., Buongiorno A., Tamburrano G., et al. (1993) Absence of clinically overt atherosclerotic vascular disease and adverse changes in cardiovascular risk factors in 70 patients with insulinoma. J Endocrinol Invest 16: 875–880 [DOI] [PubMed] [Google Scholar]
- Li H., Förstermann U. (2013) Uncoupling of endothelial NO synthase in atherosclerosis and vascular disease. Curr Opin Pharmacol 13: 161–167 [DOI] [PubMed] [Google Scholar]
- Lizcano J., Göransson O., Toth R., Deak M., Morrice N., Boudeau J., et al. (2004) LKB1 is a master kinase that activates 13 kinases of the AMPK subfamily, including MARK/PAR-1. EMBO J 23: 833–843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loubatières-Mariani M. (2007) The discovery of hypoglycemic sulfonamides. J Soc Biol 201: 121–125 [DOI] [PubMed] [Google Scholar]
- Mah E., Bruno R. (2012) hyperglycemia on vascular endothelial function: mechanisms and consequences. Nutr Res 32: 727–740 [DOI] [PubMed] [Google Scholar]
- Mahaffey K., Hafley G., Dickerson S., Burns S., Tourt-Uhlig S., White J., et al. (2013) Results of a reevaluation of cardiovascular outcomes in the RECORD trial. Am Heart J 166: 240–249 [DOI] [PubMed] [Google Scholar]
- Mamtani R., Haynes K., Bilker W., Vaughn D., Strom B., Glanz K., et al. (2012) Association between longer therapy with thiazolidinediones and risk of bladder cancer: a cohort study. J Natl Cancer Inst 104: 1141–1421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marette A. (2002) Mediators of cytokine-induced insulin resistance in obesity and other inflammatory settings. Curr Opin Clin Nutr Metab Care 5: 377–383 [DOI] [PubMed] [Google Scholar]
- Martin-Montalvo A., Mercken E., Mitchell S., Palacios H., Mote P., Scheibye-Knudsen M. (2013) Metformin improves healthspan and lifespan in mice. Nat Commun 4: 2192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masoudi F., Inzucchi S., Wang Y., Havranek E., Foody J., Krumholz H. (2005) Thiazolidinediones, metformin, and outcomes in older patients with diabetes and heart failure: an observational study. Circulation 111: 583–590 [DOI] [PubMed] [Google Scholar]
- Mather K., Goldberg R. (2014) Clinical use of adiponectin as a marker of metabolic dysregulation. Best Pract Res Clin Endocrinol Metab 28: 107–117 [DOI] [PubMed] [Google Scholar]
- Mather K., Steinberg H., Baron A. (2013) Insulin resistance in the vasculature. J Clin Invest 123: 1003–1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mather K., Verma S., Anderson T. (2001) Improved endothelial function with metformin in type 2 diabetes mellitus. J Am Coll Cardiol 37: 1344–1350 [DOI] [PubMed] [Google Scholar]
- Matsubara J., Sugiyama S., Sugamura K., Nakamura T., Fujiwara Y., Akiyama E., et al. (2012) A dipeptidyl peptidase-4 inhibitor, des-fluoro-sitagliptin, improves endothelial function and reduces atherosclerotic lesion formation in apolipoprotein E-deficient mice. J Am Coll Cardiol 59: 265–276 [DOI] [PubMed] [Google Scholar]
- McGuire J., Hollenberg M., Andrade-Gordon P., Triggle C. (2002) Multiple mechanisms of vascular smooth muscle relaxation by the activation of proteinase-activated receptor 2 in mouse mesenteric arterioles. Br J Pharmacol 135: 155–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier C., Kraenzlin M., Bodmer M., Jick S., Jick H., Meier C. (2008) Use of thiazolidinediones and fracture risk. Arch Intern Med 168: 820–825 [DOI] [PubMed] [Google Scholar]
- Mentlein R., Heymann E. (1982) Dipeptidyl peptidase IV inhibits the polymerization of fibrin monomers. Arch Biochem Biophys 217: 748–750 [DOI] [PubMed] [Google Scholar]
- Mentlein R., Struckhoff G. (1989). Purification of two dipeptidyl aminopeptidases II from rat brain and their action on proline-containing neuropeptides. J Neurochem 52: 1284–1293 [DOI] [PubMed] [Google Scholar]
- Merovci A., Solis-Herrera C., Daniele G., Eldor R., Fiorentino T., Tripathy D., et al. (2014) Dapagliflozin improves muscle insulin sensitivity but enhances endogenous glucose production. J Clin Invest 124: 509–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R., Chu Q., Xie J., Foretz M., Viollet B., Birnbaum M. (2013) Biguanides suppress hepatic glucagon signaling by decreasing production of cyclic AMP. Nature 494: 256–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minassian C., Tarpin S., Mithieux G. (1998) Role of glucose-6 phosphatase, glucokinase, and glucose-6 phosphate in liver insulin resistance and its correction by metformin. Biochem Pharmacol 55: 1213–1219 [DOI] [PubMed] [Google Scholar]
- Mita T., Watada H. (2012) Glucagon-like peptide-1 and atherosclerosis. Cardiovasc Hematol Agents Med Chem 10: 309–318 [DOI] [PubMed] [Google Scholar]
- Monami M., Genovese S., Mannucci E. (2013) Cardiovascular safety of sulfonylureas: a meta-analysis of randomized clinical trials. Diabetes Obes Metab 15: 938–953 [DOI] [PubMed] [Google Scholar]
- Montagnani M., Ravichandran L., Chen H., Esposito D., Quon M. (2002) Insulin receptor substrate-1 and phosphoinositide-dependent kinase-1 are required for insulin-stimulated production of nitric oxide in endothelial cells. Mol Endocrinol 16: 1931–1942 [DOI] [PubMed] [Google Scholar]
- Muis M., Bots M., Grobbee D., Stolk R. (2005) Insulin treatment and cardiovascular disease: friend or foe? A point of view. Diab Med 22: 118–126 [DOI] [PubMed] [Google Scholar]
- Nagashima K., Takahashi A., Ikeda H., Hamasaki A., Kuwamura N., Yamada Y., et al. (2004) Sulfonylurea and non-sulfonylurea hypoglycemic agents: pharmacological properties and tissue selectivity. Diabetes Res Clin Pract 66(Suppl. 1): S75–S78 [DOI] [PubMed] [Google Scholar]
- Nies A., Koepsell H., Damme K., Schwab M. (2011) Organic cation transporters (OCTs, MATEs), in vitro and in vivo evidence for the importance in drug therapy. Handb Exp Pharmacol 201: 105–167 [DOI] [PubMed] [Google Scholar]
- Nikolaidis L., Mankad S., Sokos G., Miske G., Shah A., Elahi D., et al. (2004) Effects of glucagon-like peptide-1 in patients with acute myocardial infarction and left ventricular dysfunction after successful reperfusion. Circulation 109: 962–965 [DOI] [PubMed] [Google Scholar]
- Nolan C., Damm P., Prentki M. (2011) Type 2 diabetes across generations: from pathophysiology to prevention and management. Lancet 378: 169–181 [DOI] [PubMed] [Google Scholar]
- Nolan C., Ruderman N., Prentki M. (2013) Intensive insulin for type 2 diabetes: the risk of causing harm. Lancet Diabetes Endocrinol 1: 9–10 [DOI] [PubMed] [Google Scholar]
- Nyström T., Gonon A., Sjöholm A., Pernow J. (2005) Glucagon-like peptide-1 relaxes rat conduit arteries via an endothelium-independent mechanism. Regul Pept 125: 173–177 [DOI] [PubMed] [Google Scholar]
- Oeseburg H., de Boer R, Buikema H., van der Harst P., van Gilst W., Silljé H. (2010) Glucagon-like peptide 1 prevents reactive oxygen species-induced endothelial cell senescence through the activation of protein kinase A. Arterioscler Thromb Vasc Biol 30: 1407–1414 [DOI] [PubMed] [Google Scholar]
- ORIGIN Trial Investigators, Gerstein H., Bosch J., Dagenais G., Díaz R., Jung H., Maggioni A., et al. (2012) Basal insulin and cardiovascular and other outcomes in dysglycaemia. N Engl J Med 367: 319–328 [DOI] [PubMed] [Google Scholar]
- Owen M., Doran E., Halestrap A. (2000) Evidence that metformin exerts its anti-diabetic effects through inhibition of complex 1 of the mitochondrial respiratory chain. Biochem J 348: 607–614 [PMC free article] [PubMed] [Google Scholar]
- Pantalone K., Kattan M., Yu C., Wells B., Arrigain S., Jain A., et al. (2012) Increase in overall mortality risk in patients with type 2 diabetes receiving glipizide, glyburide or glimepiride monotherapy versus metformin: a retrospective analysis. Diabetes Obes Metab 14: 803–809 [DOI] [PubMed] [Google Scholar]
- Pentikäinen P., Neuvonen P., Penttilä A. (1979) Pharmacokinetics of metformin after intravenous and oral administration to man. Eur J Clin Pharmacol 16: 195–202 [DOI] [PubMed] [Google Scholar]
- Pernicova I., Korbonits M. (2014) Metformin-mode of action and clinical implications for diabetes and cancer. Nature Rev Endocrinol 10: 143–156 [DOI] [PubMed] [Google Scholar]
- Potenza M., Marasciulo F., Chieppa D., Brigiani G., Formoso G., Quon M., et al. (2005) Insulin resistance in spontaneously hypertensive rats is associated with endothelial dysfunction characterized by imbalance between NO and ET-1 production. Am J Physiol Heart Circ Physiol 289: H813–H822 [DOI] [PubMed] [Google Scholar]
- Puigserver P., Spiegelman B. (2003) Peroxisome proliferator-activated receptor-gamma coactivator 1 alpha (PGC-1 alpha): transcriptional coactivator and metabolic regulator. Endocr Rev 24: 78–90 [DOI] [PubMed] [Google Scholar]
- Rainbow R., Hardy M., Standen N., Davies N. (2006) Glucose reduces endothelin inhibition of voltage-gated potassium channels in rat arterial smooth muscle cells. J Physiol 575: 833–844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran R., Hollenberg M. (2008) Proteinases and signalling: pathophysiological and therapeutic implications via PARs and more. Br J Pharmacol 153(Suppl. 1): S263–S282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao A., Kuhadiya N., Reynolds K., Fonseca V. (2008) Is the combination of sulfonylureas and metformin associated with an increased risk of cardiovascular disease or all-cause mortality? A meta-analysis of observational studies. Diabetes Care 31: 1672–1678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhold D., Bank U., Bühling F., Neubert K., Mattern T., Ulmer A., et al. (1993) Dipeptidyl peptidase IV (CD26) on human lymphocytes Synthetic inhibitors of and antibodies against dipeptidyl peptidase IV suppress the proliferation of pokeweed mitogen-stimulated peripheral blood mononuclear cells, and IL-2 and IL-6 production. Immunobiology 188: 403–414 [DOI] [PubMed] [Google Scholar]
- Rena G., Pearson E., Sakamoto K. (2013) Molecular mechanism of action of metformin: old or new insights? Diabetologia 56: 1898–1906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas L., Gomes M. (2013) Metformin: an old but still the best treatment for type 2 diabetes. Diab Metab Syndromes 5: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossetti L., Smith D., Shulman G., Papachristou D., Defronzo R. (1987) Correction of hyperglycemia with phlorizin normalizes tissue sensitivity to insulin in diabetic rats. J Clin Invest 79: 1510–1515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruige J., Assendelft W., Dekker J., Kostense P., Heine R., Bouter L. (1998) Insulin and risk of cardiovascular disease: a meta-analysis Circulation 97: 996–1001 [DOI] [PubMed] [Google Scholar]
- Sakamoto K., Göransson O., Hardie D., Alessi D. (2004) Activity of LKB1 and AMPK-related kinases in skeletal muscle: effects of contraction, phenformin, and AICAR. Am J Physiol Endocrinol Metab 287: E310–E317 [DOI] [PubMed] [Google Scholar]
- Salpeter S., Greyber E., Pasternak G., Salpeter E. (2010) Risk of fatal and nonfatal lactic acidosis with metformin use in type 2 diabetes mellitus. Cochrane Database Syst Rev 14: CD002967. [DOI] [PubMed] [Google Scholar]
- Saltiel A., Kahn C. (2001) Insulin signaling and the regulation of glucose and lipid metabolism. Nature 414: 799–806 [DOI] [PubMed] [Google Scholar]
- Samuelsson A., Bollano E., Mobini R., Larsson B., Omerovic E., Fu M., et al. (2006) Hyperinsulinemia: effect on cardiac mass/function, angiotensin II receptor expression, and insulin signaling pathways. Am J Physiol Heart Circ Physiol 291: H787–H796 [DOI] [PubMed] [Google Scholar]
- Sauvé M., Ban K., Momen M., Zhou Y., Henkelman R., Husain M., et al. (2010) Genetic depletion or pharmacological inhibition of dipeptidyl peptidase-4 improves cardiovascular outcomes after myocardial infarction in mice. Diabetes 59: 1063–1073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawicki P., Heinemann L., Starke A., Berger M. (1992) Hyperinsulinaemia is not linked with blood pressure elevation in patients with insulinoma. Diabetologia 35: 649–652 [DOI] [PubMed] [Google Scholar]
- Schäfer G. (1980) Guanidines and biguanides. Pharmacol Ther 8: 275–295 [DOI] [PubMed] [Google Scholar]
- Schalkwijk C., Stehouwer C. (2005) Vascular complications in diabetes mellitus: the role of endothelial dysfunction. Clin Sci (Lond) 109: 143–159 [DOI] [PubMed] [Google Scholar]
- Scheen A. (2013a) Cardiovascular effects of dipeptidyl peptidase-4 inhibitors: from risk factors to clinical outcomes. Postgrad Med 125: 7–20 [DOI] [PubMed] [Google Scholar]
- Scheen A. (2013b) Cardiovascular effects of gliptins. Nat Rev Cardiol 10: 73–84 [DOI] [PubMed] [Google Scholar]
- Shah Z., Pineda C., Kampfrath T., Maiseyeu A., Ying Z., Racoma I., et al. (2011) Acute DPP-4 inhibition modulates vascular tone through GLP-1 independent pathways. Vascul Pharmacol 55: 2–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw J., Sicree R., Zimmet P. (2010) Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract 87: 4–14 [DOI] [PubMed] [Google Scholar]
- Shu Y., Brown C., Castro R., Shi R., Lin E., Owen R., et al. (2008) Effect of genetic variation in the organic cation transporter 1, OCT1, on metformin pharmacokinetics. Clin Pharmacol Ther 83: 273–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S., Bhat J., Wang P. (2013) Cardiovascular effects of anti-diabetic medications in type 2 diabetes mellitus. Curr Cardiol Rep 15: 327. [DOI] [PubMed] [Google Scholar]
- Sirtori C., Franceschini G., Galli-Kienle M., Cighetti G., Galli G., Bondioli A., et al. (1978) Disposition of metformin (N,N-dimethylbiguanide) in man. Clin Pharmacol Ther 24: 683–693 [DOI] [PubMed] [Google Scholar]
- Srinavasan S., Yeh M., Danziger E., Hatley M., Riggan A., Leitinger N., et al. (2003) Glucose regulates monocyte adhesion through endothelial production of interleukin-8. Circ Res 92: 371–377 [DOI] [PubMed] [Google Scholar]
- Standl E., Müller M., Schnell O. (2009) The impact of glucose-lowering therapy on cardiovascular outcomes. Best Pract Res Clin Endocrinol Metab 23: 401–411 [DOI] [PubMed] [Google Scholar]
- Steinberg H., Brechtel G., Johnson A., Fineberg N., Baron A. (1994) Insulin-mediated skeletal muscle vasodilation is nitric oxide dependent. A novel action of insulin to increase nitric oxide release. J Clin Invest 94: 1172–1179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg H., Chaker H., Leaming R., Johnson A., Brechtel G., Baron A. (1996) Obesity/insulin resistance is associated with endothelial dysfunction. Implications for the syndrome of insulin resistance. J Clin Invest 97: 2601–2610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan D., Winkler M., Kühner P., Russ U., Quast U. (2006) Selectivity of repaglinide and glibenclamide for the pancreatic over the cardiovascular K(ATP) channels. Diabetologia 49: 2039–2048 [DOI] [PubMed] [Google Scholar]
- Stout R. (1990) Insulin and atheroma. 20-yr perspective. Diabetes Care 13: 631–654 [DOI] [PubMed] [Google Scholar]
- Stout R., Bierma E., Ross R. (1975) Effect of insulin on the proliferation of cultured primate arterial smooth muscle cells. Circ Res 36: 319–327 [DOI] [PubMed] [Google Scholar]
- Stout R., Vallance-Owen J. (1969) Insulin and atheroma. Lancet 1: 1078–1080 [DOI] [PubMed] [Google Scholar]
- Takasawa W., Ohnuma K., Hatano R., Endo Y., Dang N., Morimoto C. (2010) Inhibition of dipeptidyl peptidase 4 regulates microvascular endothelial growth induced by inflammatory cytokines. Biochem Biophys Res Commun 401: 7–12 [DOI] [PubMed] [Google Scholar]
- The University Group Diabetes Program (1970) A study of the effects of hypoglycaemic agents on vascular complications in patients with adult-onset diabetes. Diabetes 19(Suppl. 2): 747–830 [PubMed] [Google Scholar]
- Tousoulis D., Kampoli A., Stefanadis C. (2012) Diabetes mellitus and vascular endothelial dysfunction: current perspectives. Curr Vasc Pharmacol 10: 19–32 [DOI] [PubMed] [Google Scholar]
- Triggle C., Ding H. (2010) A review of endothelial dysfunction in diabetes: a focus on the contribution of a dysfunctional eNOS. J Am Soc Hypertens 4: 102–115 [DOI] [PubMed] [Google Scholar]
- Triggle C., Ding H. (2011) The endothelium in compliance and resistance vessels. Front Biosci (Schol Ed) 3: 730–744 [DOI] [PubMed] [Google Scholar]
- Triggle C., Samuel S., Ravishankar S., Marei I., Arunachalam G., Ding H. (2012) The endothelium: influencing vascular smooth muscle in many ways. Can J Physiol Pharmacol 90: 713–738 [DOI] [PubMed] [Google Scholar]
- Tucker G., Casey C., Phillips P., Connor H., Ward J., Woods H. (1981) Metformin kinetics in healthy subjects and in patients with diabetes mellitus. Br J Clin Pharmacol 12: 235–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- UKPDS (1991) VIII. Study design, progress and performance. Diabetologia 34: 877–890 [PubMed] [Google Scholar]
- UKPDS (1998) Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group. Lancet 352: 854–865 [PubMed] [Google Scholar]
- Van Poppel P., Netea M., Smits P., Tack C. (2011) Vildagliptin improves endothelium-dependent vasodilatation in type 2 diabetes. Diabetes Care 34: 2072–2077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent M., Barrett E., Lindner J., Clark M., Rattigan S. (2003) Inhibiting NOS blocks microvascular recruitment and blunts muscle glucose uptake in response to insulin. Am J Physiol Endocrinol Metab 285: E123–E129 [DOI] [PubMed] [Google Scholar]
- Viollet B., Guigas B., Sanz Garcia N., Leclerc J., Foretz M., Andreelli F. (2012) Cellular and molecular mechanisms of metformin: an overview. Clin Sci (Lond) 122: 253–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitale C., Mercuro G., Cornoldi A., Fini M., Volterrani M., Rosano G. (2005) Metformin improves endothelial function in patients with metabolic syndrome. J Intern Med 258: 250–256 [DOI] [PubMed] [Google Scholar]
- Wang D., Jonker J., Kato Y., Kusuhara H., Schinkel A., Sugiyama Y. (2002) Involvement of organic cation transporter 1 in hepatic and intestinal distribution of metformin. J Pharmacol Exp Ther 302: 510–515 [DOI] [PubMed] [Google Scholar]
- Wang H., Wang A., Barrett E. (2011) Caveolin-1 is required for vascular endothelial insulin uptake. Am J Physiol Endocrinol Metab 300: E134–E144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Alexanian A., Ying R., Kizhakekuttu T. J., Dharmashankar K., Vasquez-Vivar J., et al. (2012) Acute exposure to low glucose rapidly induces endothelial dysfunction and mitochondrial oxidative stress: role for AMP kinase. Arterioscler Thromb Vasc Biol 32: 712–720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO (2013) Model List of Essential Medicines (18th List, updated April 2013). Available at: http://www.who.int/medicines/publications/essentialmedicines/18th_EML_Final_web_8Jul13.pdf (accessed March 2014).
- Wilcock C., Bailey C. (1994) Accumulation of metformin by tissues of the normal and diabetic mouse. Xenobiotica 24: 49–57 [DOI] [PubMed] [Google Scholar]
- Wilcock C., Wyre N., Bailey C. (1991) Subcellular distribution of metformin in rat liver. J Pharm Pharmacol 43: 442–444 [DOI] [PubMed] [Google Scholar]
- Wiryana M. (2009) The role of intensive insulin therapy in increasing superoxide dismutase (SOD) and normalizing hyperglycemia in critically ill patients. Acta Med Indones 41: 59–65 [PubMed] [Google Scholar]
- Witters L. (2001) The blooming of the French lilac. J Clin Invest 108: 1105–1107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollen N., Bailey C. (1988) Inhibition of hepatic gluconeogenesis by metformin. Synergism with insulin. Biochem Pharmacol 37: 4353–4358 [DOI] [PubMed] [Google Scholar]
- Wulffelé M., Kooy A., Lehert P., Bets D., Ogterop J., Borger van der Burg B., et al. (2003) Effects of short-term treatment with metformin on serum concentrations of homocysteine, folate and vitamin B12 in type 2 diabetes mellitus: a randomized, placebo-controlled trial. J Intern Med 254: 455–463 [DOI] [PubMed] [Google Scholar]
- Yang J., Holman G. (2006) Long-term metformin treatment stimulates cardiomyocyte glucose transport through an AMP-activated protein kinase-dependent reduction in GLUT4 endocytosis. Endocrinology 147: 2728–2736 [DOI] [PubMed] [Google Scholar]
- Yuan L., Ziegler R., Hamann A. (2002) Inhibition of phosphoenolpyruvate carboxykinase gene expression by metformin in cultured hepatocytes. Chin Med J (Engl) 115: 1843–1848 [PubMed] [Google Scholar]
- Yue L., Burchard E., Brett C., Giacomini K. (2008) Effect of genetic variation in the organic cation transporter 1, OCT1, on metformin pharmacokinetics. Clin Pharmacol Ther 83: 273–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong J., Rao X., Rajagopalan S. (2013) An emerging role of dipeptidyl peptidase 4 (DPP4) beyond glucose control: potential implications in cardiovascular disease. Atherosclerosis 226: 305–314 [DOI] [PubMed] [Google Scholar]
- Zhou G., Myers R., Li Y., Chen Y., Shen X., Fenyk-Melody J., et al. (2001) Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest 108: 1167–1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou R., Yazdi A., Menu P., Tschopp J. (2011) A role for mitochondria in NLRP3 inflammasome activation. Nature 469: 221–225 [DOI] [PubMed] [Google Scholar]
- Zimmet P. (2011) The growing pandemic of type 2 diabetes: a crucial need for prevention and improved detection. Medicographia 33: 15–21 [Google Scholar]
- Zou C., Hu H. (2013) Use of pioglitazone in the treatment of diabetes: effect on cardiovascular risk. Vasc Health Risk Manag 9: 429–433 [DOI] [PMC free article] [PubMed] [Google Scholar]