Abstract
Drug abuse is a risk factor for neurological complications in HIV infection. Cocaine has been shown to exacerbate HIV-associated brain pathology and enhance neurotoxicity of HIV-1 Tat and gp120 proteins. In this study, we found that the selective inhibitor of dopamine transporter (DAT) function, 1-[2-[bis(4- fluorophenyl) methoxy]ethyl]-4-(3-phenylpropyl) piperazine (GBR 12909, vanoxerine), but not the selective inhibitors of serotonin and norepinephrine (SERT and NET) transporters, sertraline and nizoxetine, emulated cocaine-mediated enhancement of Tat neurotoxicity in rat fetal midbrain primary cell cultures. Similar to cocaine, the significant increase of Tat toxicity in midbrain cell cultures was observed at micromolar dose (5 μM) of GBR 12909. However, different doses of another selective dopamine uptake inhibitor, WIN 35428 did not affect Tat neurotoxicity. The study supports the hypothesis that changes in control of dopamine (DA) homeostasis are important for the cocaine-mediated enhancement of HIV-1 Tat neurotoxicity. Our results also demonstrate that the inhibitors of DA uptake, which can bind to different domains of DAT, differ in their ability to mimic synergistic toxicity of cocaine and HIV-1 Tat in the midbrain cell culture.
INTRODUCTION
The nervous system is widely involved in the pathogenesis of AIDS/HIV. HIV is neuro-invasive and neuro-virulent (Manji and Miller, 2004). Invasion of the CNS by HIV-1 occurs early in the course of infection. Post-mortem examination of AIDS brain tissue reveals neuropathological changes in approximately 75-90% of the cases (Koutsiliery et al., 2002; Navia et al., 1986). Neurotoxic properties of several structural (gp120, gp41) and regulatory (Tat, Rev, Vpr) viral proteins are well documented, although the detailed mechanisms of neurotoxicity of different HIV-1 proteins are not known (Ozdener, 2005). HIV-1 transactivating protein Tat and the viral envelope protein gp120 are believed to play a significant role in the pathogenesis of HIV-associated brain pathology (Nath et al., 2002). As the advent of highly active antiretroviral therapy (HAART) has made AIDS/HIV a “manageable” disease in terms of life expectancy, the significance of NeuroAIDS as a major cause of morbidity is increasing. Growing evidence demonstrates that symptoms of HIV-related neuropathology develop faster and are more severe in drug abusing HIV patients (Nath et al., 2002; Chander et al., 2006). Cocaine is a risk factor in NeuroAIDS (Nath et al., 2002; Fiala et al., 2005). Cocaine has been shown to increase HIV-1 invasion through brain blood barrier (Fiala et al., 2005) and to enhance neurotoxicity of HIV-1 proteins Tat and gp120 (Turchan et al., 2001; Kendall et al., 2005; Aksenov et al., 2006).
Understanding of the molecular basis of cocaine participation in mechanisms of neurotoxicity of HIV-1 proteins is only beginning to emerge. In the brain, protein complexes that control levels of monoamine neurotransmitters are primary targets of cocaine. Cocaine interaction with neuronal membrane proteins affects recognition, uptake, and release of monoamine transmitters. Dysfunction of monoamine, particularly dopamine (DA), transmission is known to occur in HIV-infected brain (Nath et al, 2000; Wang et al, 2004; Silvers et al, 2006). Cocaine can bind with high affinity and inhibit dopamine, serotonin, and norepinephrine transporters (DAT, SERT, and NET). Recently published studies demonstrated that HIV-1 Tat and gp120 can disrupt DAT function in vitro (Wallace et al, 2005; Aksenova et al, 2006). Therefore, cocaine-mediated inhibition of monoamine transporter activities may overlap with molecular pathways of HIV-1 viral protein neurotoxicity. The activity cycle of a transporter involves separate steps of binding of a biogenic amine and its translocation through the cell membrane (Rudnick, 2002), both of which can be modulated by cocaine binding. The aim of the current study was to test the ability of selective inhibitors of different monoamine transporters to mimic cocaine-mediated enhancement of Tat neurotoxicity in rat midbrain cell cultures.
MATERIALS AND METHODS
Neuronal Cell Culture Preparation
The method for culturing of embryonic neurons was derived from that described by Goslin and Banker (Goslin et al., 1998). Neuronal cultures were prepared from 18-day-old Sprague-Dawley rat fetuses. Rat midbrains were dissected and incubated for 15 min in a solution of 2 mg/mL trypsin in Ca2+- and Mg2+ - free Hanks’ balanced salt solution (HBSS) buffered with 10 mM HEPES (Invitrogen, Carlsbad, CA). The tissue was then exposed for 2 min to soybean trypsin inhibitor (1 mg/mL in HBSS) and rinsed three times in HBSS. Cells were dissociated by trituration and distributed to poly-L-lysine-coated plastic culture plates (Costar, Cambridge, MA) or 35 mm glass bottom culture dishes (MatTek Corp., Ashland, MA). Initial plating densities were approximately 160-180 cells/mm2. At the time of plating, each well contained DMEM/F12 medium (Invitrogen) supplemented with 100 mL/L fetal bovine serum (Sigma Chemicals, St. Louis, MO). After a 24-hr period, the DMEM/F12 medium was replaced with 2% v/v B-27 Neurobasal medium supplemented with 2 mM GlutaMAX and 0.5% w/v D-(+) glucose (Invitrogen). Two-thirds of the Neurobasal medium was replaced with freshly prepared medium of the same composition twice a week. Cultures were used for neurotoxicity experiments after 12 days in culture and were >95% neuronal as observed by anti-MAP-2 immunostaining. The reminder (approximately 5%) of the cells was astrocytes as determined by anti-GFAP/Hoechst staining.
Treatments
In all our experiments we used recombinant Tat 1-86 produced in E-coli (Diatheva, Italy). This full length HIV-1 protein is >90% pure as determined by SDS PAGE (14 kDa protein band). All monoamine transporter inhibitor compounds were from Sigma Chemicals, St. Louis, MO.
To study the combined neurotoxicity of Tat and cocaine, cell cultures were treated with either vehicle (control), 50 nM Tat 1-86, 1.5 μM cocaine, or 50 nM Tat 1-86 + 1.5 μM cocaine. The 50 nM dose of Tat is moderately toxic and 1.5 μM cocaine alone is not toxic to cultured neurons (Aksenov et al., 2006). Appropriate vehicle controls were included for each group. The number of sister cultures (wells) per each treatment/control group was between 7 and 17. Following the addition of treatment components to the growth medium, cell cultures were incubated for 48 hours prior to analyses.
To determine whether inhibition of DAT, SERT, or NET functioning by cocaine contributed to the enhancement of neurotoxic effects of Tat, cells were exposed either to Tat or to Tat+selective monoamine transporter inhibitor compound. In these experiments we used DAT selective inhibitors GBR 12909 (vanoxerine, 1-[2-[bis(4- fluorophenyl) methoxy]ethyl]-4-(3-phenylpropyl) piperazine, and WIN 35428 (β-CFT, (−)-2-β-carbomethoxy-3-β-(4-fluorophenyl)tropane), the SERT selective inhibitor sertraline (Zoloft,(1S)-cis-4-(3,4-dichlorophenyl)-1,2,3,4-tetrahydro-N-methyl-1-naphthalenamine), and the NET selective inhibitor nisoxetine (3-(2-methoxyphenoxy)-N-methyl-3-phenylpropan-1-amine) . In a set of preliminary experiments we determined the range of nontoxic concentrations for each of the selective monoamine transporter inhibitors. Doses of GBR 12909, WIN 35428, sertraline, and nisoxetine within the range of 1-10 μM did not affect cell viability (data not shown). Non-toxic doses of each monoamine transporter inhibitor compound were selected to treat cell cultures in combination with 50 nM Tat.
Cell cultures were treated either with Tat, the selective monoamine transporter inhibitor, Tat+cocaine, or Tat+ selective monoamine transporter inhibitor. All control and treatment groups were incubated for 48 hours prior to the neurotoxicity determination.
Cell viability test
Neuronal survival was determined using a Live/Dead viability/cytotoxicity kit. (Molecular Probes, Eugene, OR). Neurons were exposed to cell-permeant calcein AM (2 μM), which is hydrolyzed by intracellular esterases, and to ethidium homodimer-1 (4 μM), which binds to nucleic acids. The cleavage product of calcein AM produces a green fluorescence (F530nm) when exposed to 494-nm light and is used to identify live cells. Bound ethidium homodimer-1 produces a red fluorescence (F645nm) when exposed to 528-nm light, allowing the identification of dead cells.). Fluorescence was measured using a Bio-Tek Synergy HT microplate reader (Bio-Tek Instruments, Inc., Winooski, VT). Each individual F530nm and F645nm value on a plate were corrected for background fluorescence (readings obtained from cell cultures (wells) that were not exposed to calcein AM and ethidium bromide) by the microplate reader KC4 software package (Bio-Tek Instruments, Inc., Winooski, VT). For each individual cell culture (well) on a plate ratios between corrected green and red fluorescence (F530nm/ F645nm, Live/Dead ratios) were calculated. All individual ratios of live and dead cells were expressed in terms of percentages of average maximum Live/Dead ratio determined for the set of non-treated control cell cultures (8-16 wells) from the same plate: [F530nm/ F645nm]well n/[ F530nm/ F645nm]average max × 100%.
Immunocytochemistry
Cell cultures were fixed in acetic alcohol (95% ethanol, 5% acetic acid) for 10 min and washed three times (5 min per wash) with Dulbecco phosphate-buffered saline (D-PBS): Na2HPO4 (1150 mg/L), KH2PO4 (200 mg/L), NaCl (8000 mg/L), KCl (200 mg/L) at pH 7.4. Fixed cultures were blocked with 5% normal horse serum (NHS) in DPBS for at least 30 min and incubated overnight with primary antibodies.
DAT immunoreactivity
Rabbit polyclonal anti-DAT antibody raised against an 18 amino acid peptide sequence near the NH2-terminus of rat brain DAT (Chemicon, Temecula, CA, catalog# AB1591P) diluted 1:200 in 1% NHS/PBS were used to immunostain cell cultures for DAT. Secondary antibodies were AlexaRed goat anti-rabbit IgG (Molecular Probes), 1:500.
SERT immunoreactivity
Rabbit polyclonal anti-SERT antibody (Chemicon, Temecula, CA, catalog# AB9322) raised against a peptide from human SERT (recognizes both rat and human SERT) was used to detect SERT immunoreactivity. Working dilution of the primary antibody was 1:200 in 1% NHS/PBS. Secondary antibodies were AlexaGreen goat anti-rabbit IgG (Molecular Probes), 1:500.
NET immunoreactivity
Rabbit polyclonal anti NET antibody (Chemicon, Temecula, CA, catalog# AB5066P) raised against first extracellular domain of rat NET was used to detect NET immunoreactivity Working dilution of the primary antibody was 1:200 in 1% NHS/PBS. Secondary antibodies were AlexaGreen goat anti-rabbit IgG (Molecular Probes), 1:500.
Primary antibodies were preliminary tested using Western blots of adult rat brain tissue extracts, crude synaptosomal fraction, and cell extracts prepared from primary midbrain cultures to ensure the detection of protein bands of appropriate molecular weight. Cells were counterstained with Hoechst (0.4 μg/ml) for 10 min. Control experiments for immunofluorescence were carried out by omitting the primary antibodies. Cell cultures treated with only secondary antibodies (“no primary antibody” controls) were exposed to Hoechst and observed in the fluorescence microscope.
Images were captured using computer-controlled inverted fluorescent microscope (Nikon Eclipse TE2000-E) with 20X magnification. “No primary antibody” controls were used to set up capturing conditions (gain factor and exposure time) for acquisition of specific immunofluorescent signals. Images of green or red immunofluorescence (specific primary/seconady AlexaGreen 488 or AlexaRed 594 antibody complex) were merged with blue (Hoechst) staining of intact cell nuclei. Cells specifically labeled with anti-monoamine transporter (anti-DAT or anti-SERT) antibodies and total numbers of Hoescht-stained nuclei in were counted using object counting option of NIS- Elements BR 2.30 imaging software package (Nikon). Estimates of relative numbers of neurons expressing certain types of transporter proteins (specific immunoreactivity vs Hoescht staining) were based on observations from at least 3 different fiends of vision.
Ligand binding
To determine the binding of selective monoamine transporter inhibitors, fetal midbrain cell cultures were prepared in 24-well plates. Cultures were rinsed and preincubated for 5 min in D-PBS. Cultures were then incubated with D-PBS containing the selective monoamine transporter ligand. Groups of 4 sister cultures were used to determine total and non-specific binding.
DAT ligand binding
Cultures were incubated with 3 nM concentration of [3H] GBR 12935 (GBR 12909 analog, 1-[2-(diphenylmethoxy) ethyl]-4-(3-phenylpropyl) piperazine, specific activity, 87 Ci/mmol; PerkinElmer, Boston, MA) 30 min at room temperature. Binding of [3H]WIN 35428 was determined under the conditions previously reported in (Xu et al., 1995; Aksenova et al., 2006). Cultures were incubated with 4 nM concentration of [3H]WIN 35428 (specific activity, 80 Ci/mmol; PerkinElmer, Boston, MA) 30 min at room temperature. Nonspecific binding of both radiolabeled DAT-selective ligands was determined in presence of 10 μM mazindol.
SERT ligand binding
Cultures were incubated with D-PBS containing 1.0 nM of [3H] Citalopram (1-[3-(dimethylamino)propyl]-1-(4-fluorophenyl)-1,3-dihydro[2]benzo furan -5-carbonitrile, specific activity, 79 Ci/mmol; PerkinElmer, Boston, MA) for 1 h at room temperature (25 C°). Non-specific binding was determined in the presence of 10 μM fluoxetine.
NET ligand binding
To determine the binding of [3H] Nisoxetine to functional norepinephrine transporters cultures were incubated with D-PBS containing 1.7 nM of [3H] Nisoxetine (specific activity, 84 Ci/mmol; PerkinElmer, Boston, MA) for 2 h at room temperature (25 C°). Non-specific binding was determined in the presence of 10 μM mazindol.
Binding of all selective monoamine transporter ligands was performed with intact cells, and was terminated by the removal of incubation buffer and 3x5 min wash with gentle agitation. Cultures were then lyzed with 0.2 ml of 50 mM Tris-HCl, 150 mM NaCl, 2 mM EDTA, 1% Triton X-100, pH 7.4. Twenty μl from each well was taken for total protein measurements. Protein concentrations were determined by BCA method (Pierce). The rest of the well content was added to scintillation fluid and used for determination of radioactive content.
Data Analysis
The significance of cocaine-mediated enhancement of Tat toxicity in the cell culture model was tested by the comparison of cell viability results between two control groups (non-treated controls and controls + cocaine) and two experimental groups (Tat-treated and Tat+cocaine). To test for the similar effects of selective inhibitors of a particular type of monoamine transporter (DAT, SERT, and NET) on Tat toxicity comparisons were made between two control groups (non-treated controls and controls+ a selective transporter inhibitor) and two experimental groups (Tat-treated and Tat+ a selective transporter inhibitor). All statistical comparisons were made using ANOVA and planned comparisons were used to determine specific treatment effects. Significant differences were set at P< 0.05.
RESULTS
The effect of cocaine on Tat 1-86 toxicity in midbrain cell cultures
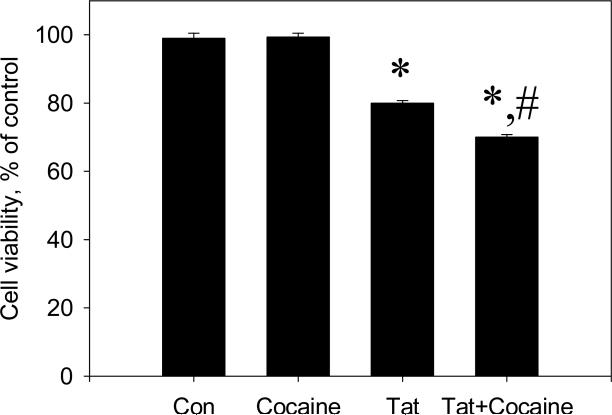
Cocaine enhanced Tat-induced neurodegeneration in rat fetal midbrain cell cultures. Following 48 hours of treatment, Live/Dead ratios in cultures treated with Tat were at 80 ± 0.7% of control. Neuronal cell viability in cultures that were treated with Tat+cocaine was at 70 ± 0.8% of control. At this time point Live/Dead ratios in Tat+cocaine –treated cultures were significantly different from those in Tat –treated cultures (P<0.05). Cocaine alone (1.5 μM dose) did not produce any changes in cell viability (Figure 1).
Figure 1.
The effect of cocaine on the toxicity of Tat 1-86 in primary rat fetal midbrain cell culture. Graph represents relative (compared to non-treated controls) changes in Live/Dead ratios 48 hours following the addition of 50 nM Tat 1-86 or 50 nM Tat 1-86 + 1.5 μM cocaine to the cell cultures. Data presented as mean values ± SEM, n of sister cultures analyzed = 7-14 per each variant of treatment. *- marks significant (P<0.05) difference between Tat-treated (50 nM Tat) group and non-treated control group; *,#-marks that this experimental group (50 nM Tat+ 1.5 μM cocaine) was significantly (P<0.05) different from non-treated control (*) and from Tat-treated group (#).
The specific binding of DAT-, SERT-, and NET –selective ligands in midbrain cell cultures
Monoamine transporters are known to be primary targets for cocaine in neurons. In the following set of experiments we determined the specific binding of ligands selective to different types of monoamine transporters in midbrain cell cultures (Figure 2).
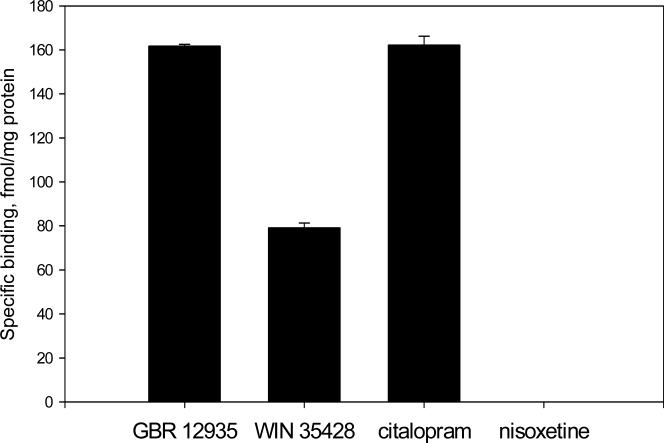
Figure 2.
The specific binding of DAT, SERT, and NET-selective ligands in rat fetal midbrain cell cultures. Data presented as mean values ± SEM, n of sister cultures analyzed = 4 per each ligand.
The specific binding of [3H]GBR 12935 (the close analog of GBR 12909) to cultured rat fetal midbrain neurons was 161.8 ± 0.8 fmol/mg. The specific binding of another DAT-selective ligand, [3H]WIN 35428, was 79.3 ± 2.0 fmol/mg protein.
The level of [3H] citalopram specific binding in midbrain cell cultures was 162.1±3.9 fmol/mg total protein.
Total binding of [3H] nisoxetine was equivalent to non-specific binding determined in presence of 10 μM mazindol. Therefore, no specific binding of nisoxetine was detected in midbrain cell cultures.
Immunostaining of rat fetal midbrain cell cultures with anti-DAT, anti-SERT and anti-NET antibodies
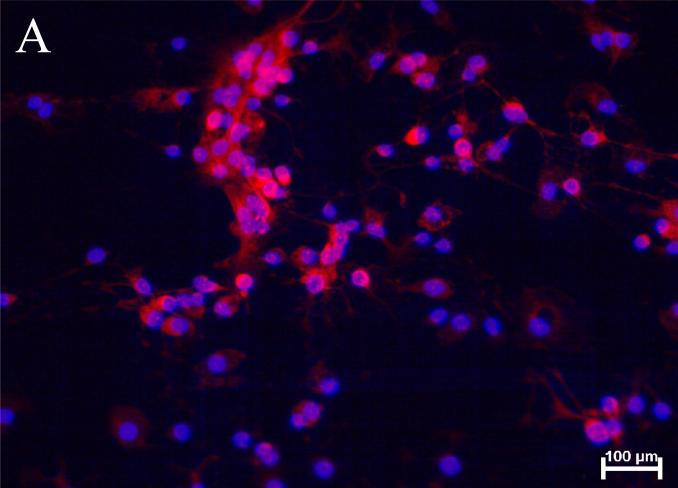
Following the determination of the specific binding of DAT, SERT, and NET – selective ligands, we performed the analysis of monoamine transporter protein expression in cultured rat fetal midbrain neurons using specific antibodies. Figure 3A shows anti-DAT immunofluorescence in cultured rat fetal midbrain neurons. Soma and processes of some neurons in midbrain cell cultures exhibited positive staining with the antibody against NH2 end of DAT. Under the experimental conditions used in this study 22-25% of the cells were DAT-positive. Similar results were obtained with anti-DAT antibody specific to external loop epitope (images not shown).
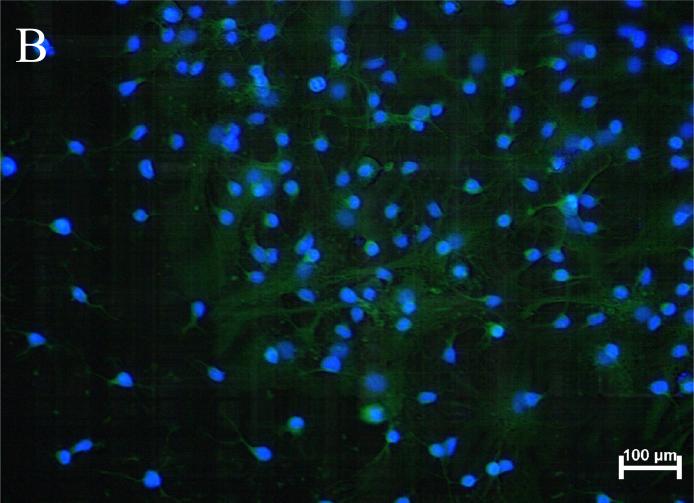
Figure 3.
Representative images of anti-DAT and anti-SERT immunoreactivity in rat fetal midbrain cell cultures.
(A) The image of anti-DAT/Hoechst staining in rat fetal midbrain cell cultures shows positive red immunofluorescence in neuronal somata and processes in 12th DIV midbrain neurons. Hoechst blue fluorescence counter stains cell nuclei. Red background fluorescence was insignificant in “no-primary antibody” controls (image not shown). (B) The image of anti-SERT/Hoechst staining in rat midbrain cell cultures shows positive anti-SERT (green) immunofluorescence in 12th DIV midbrain neurons. Hoechst staining is used to counter stain cell nuclei. Green background fluorescence was insignificant in “no-primary antibody” controls (image not shown).
Figure 3B shows SERT-immunopositive neurons in rat fetal midbrain cell cultures. Anti-SERT immunoreactivity was observed in cell bodies and processes of 20-29% of cultured rat fetal midbrain neurons.
Anti-NET antibodies failed to detect protein bands of an appropriate molecular weight in cell culture extracts and immunocytochemical analysis did not show specific anti-NET staining in midbrain cell cultures (data not shown).
Effects of monoamine transporter inhibitors on Tat 1-86 neurotoxicity in midbrain cell cultures
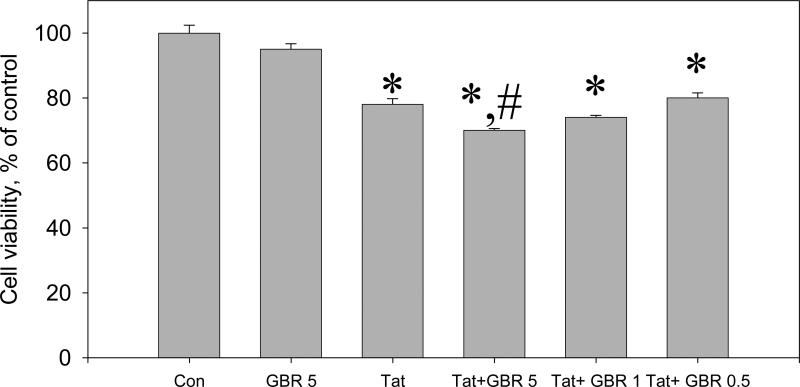
The ability of two different DAT-selective inhibitors, GBR 12909 and WIN 35428, to enhance the toxicity of Tat in rat fetal midbrain cell cultures were determined in this study. It was found that the 5 μM dose of GBR 12909 increased the toxicity of 50 nM Tat 1-86 in midbrain cell cultures. The decrease of Live/Dead ratios after 48 hr exposure of cell cultures to a combination of 50 nM Tat and 5 μM GBR 12909 (70 ± 0.6% vs control) was more pronounced (P< 0.05) than in cell cultures exposed to Tat 1-86 alone (78 ± 1.8% vs control). The increase of Tat toxicity in cultures treated with 50 nM Tat 1+86 and 1 μM GBR 12909 (74 ± 0.6% vs control) was not statistically significant (P>0.05). The cell viability decrease in cell cultures exposed to 50 nM Tat and 0.5 μM GBR 12909 (80 ± 1.6% vs control) was the same as in cell cultures treated with Tat alone (Figure 4).
Figure 4.
The effect of the selective dopamine transporter inhibitor, GBR 12909, on the toxicity of Tat 1-86 in primary rat fetal midbrain cell culture.
Results presented as mean % of Calcein/Ethidium bromide fluorescence (Live/Dead ratio)vs non-treated control ± SEM, n of sister cultures analyzed = 7-13 per each variant of treatment. *- marks significant (P<0.05) difference between the treated group (50 nM Tat or 50 nM Tat + non-toxic dose of GBR 12909) and non-treated control group; *,#-marks that this experimental group (50 nM Tat+ non-toxic dose of GBR 12909) was significantly (P<0.05) different from non-treated control (*) and from Tat-treated group (#). Similar to cocaine, 5 μM GBR 12909 significantly enhances neurotoxicity of 50 nM Tat following 48 hour-exposure. All doses of GBR 12909 used in these experiments (0.5, 1, or 5 μM) did not cause changes of Live/Dead ratios in rat fetal midbrain cell cultures. Cell viability of a group of cultures exposed to 5 μM GBR 12909 is included in the graph.
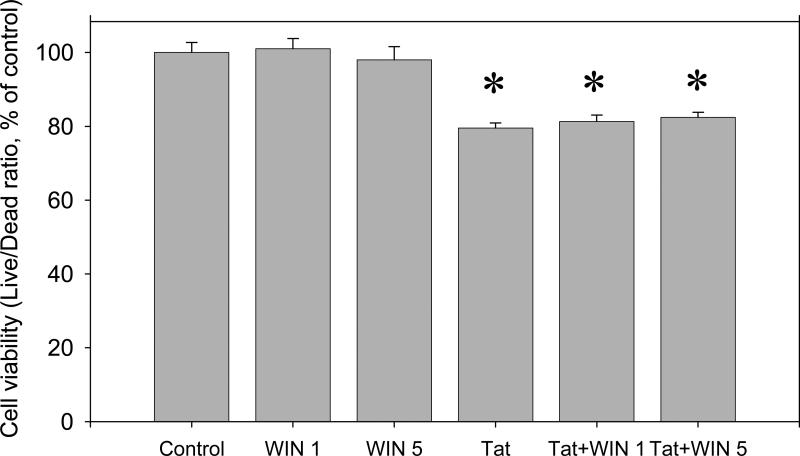
The results of 48 hour co-exposure of midbrain cell cultures to 50 nM Tat 1-86 with two different non-toxic doses (1 and 5 μM) of WIN 35428 are shown in (Figure 5). There was no statistically significant difference in Live/Dead ratios after 48 hr exposure of cell cultures to Tat 1-86 or to Tat 1-86 + WIN 35428.
Figure 5.
The effect of the selective DAT inhibitor, WIN 35428, on the toxicity of Tat 1-86 in primary rat fetal midbrain cell cultures. Results presented as mean % of Calcein/Ethidium bromide fluorescence (Live/Dead ratio)vs non-treated control ± SEM, n of sister cultures analyzed = 7-17 per each variant of treatment. Two different doses of WIN 35428 (1 and 5 μM) used in these experiments were not toxic to cultured rat fetal midbrain neurons and did not enhance the toxicity of Tat 1-86 determined after 48 hour-exposure. *- marks the significant (P<0.05) difference between Tat-treated group or Tat+WIN 35428 –treated group and non-treated control group;
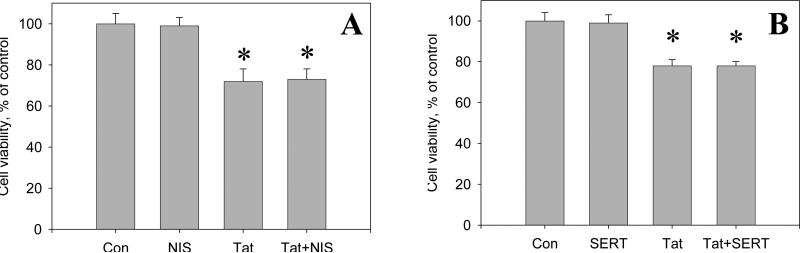
There was no statistically significant differences in the decrease of cell viability in cell cultures exposed to either Tat or Tat + any non-toxic dose of the SERT- or the NET-selective inhibitor. Figure 6 A,B illustrates the results of cell viability measurements in control (non-treated) cell cultures, cell cultures treated for 48 hours with either 5 μM nisoxetine or sertraline alone, 50 nM Tat alone, and cell cultures exposed to 50 nM Tat +5 μM sertraline or nisoxetine.
Figure 6.
The effect of the selective norepinephrine transporter inhibitor, nisoxetine (A), and the selective serotonine transporter inhibitor, sertraline (B) on the toxicity of Tat 1-86 in primary rat fetal midbrain cell culture. Results presented as mean % of Calcein/Ethidium bromide fluorescence (Live/Dead ratio)vs non-treated control ± SEM, n of sister cultures analyzed = 6-12 per each variant of treatment. *- marks significant (P<0.05) difference between the treated group (50 nM Tat or 50 nM Tat + non-toxic dose of sertraline / nisoxetine) and non-treated control group.
DISCUSSION
HIV-1 Tat is toxic to neurons in vivo and in vitro. Biologically inactive analogs of Tat consistently have been shown to be non-toxic (Aksenov et al., 2003; Self et al., 2004; Aksenov et al., 2006). Cocaine increases the neurotoxic effects of Tat in rodent and human primary cell cultures (Turchan et al., 2001; Kendall et al., 2005; Aksenov et al., 2006). However, when added to cell culture medium, cocaine does not induce neurodegeneration, even at high doses (Bennett et al., 1993). Accordingly, physiologically relevant doses of cocaine, such as the dose used in this study (1.5 μM), are non-toxic to neuronal cell cultures (Turchan et al., 2001; Aksenov et al., 2006). Combined with cocaine, even subtoxic concentrations of recombinant Tat 1-72 induce degeneration of rat fetal hippocampal neurons (Aksenov et al., 2006) and human fetal neurons in primary cell cultures (Turchan et al., 2001). In the current study we report that, consistent with the other cell culture models, cocaine enhanced Tat 1-86 toxicity in primary cultures of rat fetal midbrain neurons.
Theoretically, interactions of cocaine with any of the three types (DAT, SERT, NET) of monoamine transporter protein complexes could contribute to the cocaine-mediated increase in Tat neurotoxicity. However, the expression of different types of monoamine transporters in cultured neurons from different regions of rat fetal brain is not very well characterized. Therefore, we first determined the specific binding of selective ligands and analyzed immunoreactivities of all three different types of plasma membrane-bound monoamine transporters in our cell culture model.
The expression of functional DAT was previously demonstrated in midbrain cell cultures grown for 12-14 days in vitro (DIV) (Valchar and Hanbauer, 1995, Prasad and Amara, 2001; Aksenova et al., 2006). In this study we used tritiated DAT-selective ligands, which belong to two different groups of dopamine uptake inhibitors: substrate - like and cocaine-like compounds. GBR 12935 and its close analog GBR 12909 belong to a group of selective DAT ligands structurally related to dopamine (Van der Zee and Hespe, 1985). The binding properties of GBR 12935 and GBR 12909 are almost identical (Van der Zee and Hespe, 1985; Soucy et al., 1997). We used [3H] GBR 12935 in ligand binding experiments because this member of GBR family is readily available in tritiated form. [3H]WIN 35428, belongs to a group of DAT-selective ligands, in which the chemical structure is derived from cocaine. The detection of specific binding of GBR 12935 and WIN 35428 ligands was supported by observations of DAT immunoreactivity in cultured rat fetal midbrain neurons. Thus, in agreement with the literature, we confirmed the expression of DAT in the midbrain cell culture model.
The information about the expression of other types of monoamine transporter proteins in cultured rodent fetal midbrain cells is lacking. Autoradiographic analysis of adult rat brain sections showed SERT-specific binding in many brain regions, including the midbrain (Chen et al., 2003). Anti-SERT antibodies developed against C-end terminus of the protein label neurons in coronal sections of rat midbrain at the level of the dorsal raphe nucleus (Blakely et al., 1994). We determined that rat midbrain cell cultures contain SERT-immunopositive neurons and exhibit significant levels of specific binding of SERT-selective ligand [3H] citalopram.
In the adult rat brain, NET labeling is confined to noradrenergic neuronal somata, axons, and dendrites, including extensive arborizations within the hippocampus and cortex, but is absent from dopamine-containing neurons (Schroeter et al., 2000). In our study we did not observe specific anti-NET imunoreactivity in rat fetal midbrain cell cultures. The absence of detectable specific binding of [3H] nisoxetine also suggested that rat fetal midbrain cell cultures do not contain significant numbers of noradrenergic neurons.
Following the analysis of the pattern of monoamine transporter expression, we studied the combined toxicity of Tat and selective monoamine transporter inhibitors in our cell culture model. Since predominantly DAT- and SERT-containing neurons were present in rat fetal midbrain cell cultures, we focused on the investigation of how the selective inhibition of functioning of these two transporter proteins may contribute to the cocaine-induced enhancement of Tat neurotoxicity in our cell culture model.
We report that, much like cocaine, the DAT-selective ligand, GBR 12909, enhanced Tat 1-86 toxicity in midbrain cell cultures. The ability of GBR 12909 to increase Tat 1-86 toxicity was dose-dependent and was in agreement with the specific binding of GBR-like compounds in the rat fetal midbrain cell culture model. According to our data, cultured rat midbrain neurons exhibit significant specific binding of both dyphenylmethoxy moiety-based (GBR-like) and tropane ring-containing (WIN-like) ligands. Both GBR- and WIN-like compounds selectively bind to DAT and block DA uptake (Singh, 2000; Boos et al., 2006). However, these two groups of DA uptake inhibitors have different binding sites on DAT (Chen et al., 2004). GBR analogs bind close to NH2 terminal of DAT which is predicted to contain transmembrane domains 1 and 2. WIN-like ligands bind closer to the C terminal in a domain containing transmembrane (TM) helices 4-7 (Vaughan et al., 1999, 2007). Cocaine can bind to the same site as WIN and displaces both GBR and WIN compounds in DAT binding competition experiments (Richifield, 1991; Wilson et al., 1994). Thus, it was interesting to investigate if GBR 12909 and WIN 35428 would have the same ability to enhance Tat toxicity.
We determined that, in contrast to GBR 12909, different non-toxic concentrations of WIN 35428, the cocaine congener, did not affect Tat-induced cell death. We suggest that binding of GBR and WIN to aminoacid residues located in different functional domains of DAT may be a key to their different abilities to mimic the effect of cocaine on Tat neurotoxicity. GBR, as an analog of DA, affects DAT function through binding to the substrate recognition domain of DAT. WIN locks membrane-bound DAT in inactive state and, therefore, prevents its interaction with DA and selectively blocks DA translocation across the membrane (Ravna et al, 2003). The specific binding of GBR-like and WIN-like ligands can be differentially affected by the state of DAT protein (Chen et al., 2004). In fact, GBR and WIN bind to distinct functional form/state of DAT (Pristupa et al., 1993). WIN 35428 is known to be a very useful tool for modeling of the inhibition of DA transport across the membrane by cocaine, but it may not act exactly as cocaine in mechanisms of control of DAT function, which are not directly linked to the blockade of DA reuptake. Certain aspects of complex cocaine-mediated adaptive changes in the DAT function could be better emulated by GBR 12909 than by WIN 35428.
Interactions of hydrophobic GBR compounds with DAT in the membrane of dopaminergic neurons affect DA binding and alter protein kinase C- mediated control of DAT trafficking and surface exposure (Gorentla and Vaughan, 2005). Cocaine binding to DAT affects DAT phosphorylation, trafficking to the cell surface, and interaction with transporter-interacting proteins (Jayanthi and Ramamoorthy, 2005; Foster et al, 2006). WIN-like compounds are not known to affect DAT phosphorylation state.
In addition to uptake, the cloned transporter proteins also elicit ion channel-like currents (Ingram et al, 2002; Sulzer and Galli, 2003). Binding of substrates and substrate analogs to DAT can modulate excitability and may regulate release of neurotransmitter from midbrain DA neurons (Ingram et al, 2002). Micromolar concentrations of cocaine and GBR 12909 can induce DA release (Rouge-Pont et al., 1999; Venton et al., 2006; Thumen et al., 2002). In rat fetal mesencephalic cell cultures dose-dependent (EC50 of 0.3 μM) stimulation of DA release by cocaine was reported by (Rouge-Pont et al., 1999). Doses of GBR 12909 from 1 μM to 1 mM were shown to induce an immediate increase in extracellular DA levels in rat caudate nucleus slices (Thumen et al., 2002). There is no published evidence of DA releasing actions of WIN analogs. In this study we observed the enhancement of Tat toxicity in midbrain cell cultures in the presence of GBR 12909 dose higher than 1 μM. We cannot rule out the possibility that in our experiments GBR-induced release of DA could contribute to the ability of this compound to enhance neurotoxicity in Tat-treated midbrain cultures.
It is conceivable that GBR and WIN compounds differentially affect certain aspects of the control of DAT function. Thus, it is possible that GBR 12909 and WIN 35428 may produce different changes in extracellular DA levels following the addition to cultured midbrain neurons and, therefore, differentially affect neurotoxic properties of Tat in our cell culture model. Additional studies are necessary to compare external DA concentrations in rat fetal midbrain cell cultures exposed to GBR 12909, WIN 35428, and cocaine.
Selective blockers of SERT (citalopram, paroxetine, sertraline) bind to the domain of the serotonin transporter, which overlaps with the substrate binding site (Koe et al., 1990; Chen et al., 2005). Therefore, we used sertraline as a representative SERT-selective drug to study possible effects of SERT function inhibition on Tat neurotoxicity. Despite the presence of a significant SERT-specific ligand binding (determined using [3H] citalopram) and immunoreactivity in midbrain cell cultures, the non-toxic dose of sertraline did not change the toxicity of Tat in this cell culture model. The concentration of sertraline employed was sufficient to block SERT-mediated serotonin uptake (Koe et al, 1990). These results demonstrate that inhibition of SERT function probably does not contribute for the ability of cocaine to enhance HIV-1 Tat neurotoxicity.
The absence of any effect of NET-selective inhibitor, nisoxetine, on Tat neurotoxicity was consistent with the lack of NET-specific ligand binding and NET immunoreactivity in rat fetal midbrain cell cultures. The possible role of NET in cocaine-mediated enhancement of Tat neurotoxicity has to be tested in a different type of neuronal cell culture, and remains an open question for further investigation.
Inhibition of DA uptake and DAT-specific ligand binding was documented in cell cultures exposed to recombinant HIV-1 Tat (Wallace et al., 2006; Aksenova et al., 2006). Recently, Tat was shown to increase the potassium-evoked outflow of DA early (2 hours) after the injection into rat nucleus accumbens (Ferris et al., 2007). The protective action of the D1 receptor selective antagonist, SCH 23390, against Tat and Tat+cocaine in vitro (Aksenov et al., 2006, Silvers et al., 2007) suggested that concurrent changes in DA homeostasis induced by Tat and cocaine may contribute to their combined toxicity.
Our current study indicates that mechanisms of regulation of DA homeostasis different from direct inhibition of DA re-uptake may be important for the ability of cocaine to enhance Tat-induced neurodegenetation. Collectively, results of our experiments support the hypothesis that the disregulation of the DA transmission system plays a key role in the mechanism of synergistic toxicity of HIV-1 Tat and cocaine.
Based on the results reported in this study, we conclude that:
Cocaine enhances HIV-1 Tat 1-86 toxicity in the rat fetal midbrain cell culture, which contains neurons expressing DAT and SERT.
In Tat-exposed rat fetal midbrain cell cultures only the selective inhibitor of DAT, GBR 12909, emulates cocaine-mediated enhancement of Tat neurotoxicity. Selective inhibitors of SERT or NET do not affect the toxicity of Tat in this cell culture model.
GBR 12909 and WIN 35428, the selective DA uptake inhibitors, which bind to different domains of DAT, exhibit different effects on Tat toxicity in rat fetal midbrain cell cultures.
Acknowledgments
Support provided by: NIH grants DA 11337, DA 09160, DA 13137, HD 43680.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCE
- Aksenov MY, Hasselrot U, Wu G, Nath A, Anderson C, Mactutus CF, Booze RM. Temporal relationships between HIV-1 Tat-induced neuronal degeneration, OX-42 immunoreactivity, reactive astrocytosis, and protein oxidation in the rat striatum. Brain Res. 2003;987:1–9. doi: 10.1016/s0006-8993(03)03194-9. [DOI] [PubMed] [Google Scholar]
- Aksenov MY, Aksenova MV, Nath A, Ray PD, Mactutus CF, Booze RM. Cocaine-mediated enhancement of Tat toxicity in rat hippocampal cell cultures: the role of oxidative stress and D1 dopamine receptor. Neurotox. 2006;27:217–228. doi: 10.1016/j.neuro.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Aksenova MV, Silvers JM, Aksenov MY, Nath A, Ray PD, Mactutus CF, Booze RM. HIV-1 Tat neurotoxicity in primary cultures of rat midbrain fetal neurons: changes in dopamine transporter binding and immunoreactivity. Neurosci Lett. 2006;395:235–239. doi: 10.1016/j.neulet.2005.10.095. [DOI] [PubMed] [Google Scholar]
- Blakely RD, De Felice LJ, Hartzell HC. Molecular physiology of norepinephrine and serotonin transporters. J. Exp. Biol. 1994;196:263–281. doi: 10.1242/jeb.196.1.263. [DOI] [PubMed] [Google Scholar]
- Boos TL, Greiner E, Calhoun WJ, Prisinzano TE, Nightingale B, Dersch CM, Rothman RB, Jacobson AE, Rice KC. Structure–activity relationships of substituted N-benzyl piperidines in the GBR series: Synthesis of 4-(2-(bis(4-fluorophenyl) methoxy)ethyl)-1-(2-trifluoromethylbenzyl) piperidine, an allosteric modulator of the serotonin transporter. Bioorganic & Medicinal Chemistry. 2006;14:3967–3973. doi: 10.1016/j.bmc.2006.01.065. [DOI] [PubMed] [Google Scholar]
- Bennett BA, Hyde CE, Pecora JR, Clodfelter JE. Long-term cocaine administration is not neurotoxic to cultured fetal mesencephalic dopamine neurons. Neurosci Lett. 1993;153:210–214. doi: 10.1016/0304-3940(93)90324-e. [DOI] [PubMed] [Google Scholar]
- Chander G, Himelhoch S, Moore RD. Substance abuse and psychiatric disorders in HIV-positive patients: epidemiology and impact on antiretroviral therapy. Drugs. 2006;66:769–789. doi: 10.2165/00003495-200666060-00004. [DOI] [PubMed] [Google Scholar]
- Chen F, Rezvani AH, Lawrence AJ. Autoradiographic quantification of neurochemical markers of serotonin, dopamine and opioid systems in rat brain mesolimbic regions following chronic St.John's wort treatment. Naunyn-Schmiedeberg's Arch. Pharmacol. 2003;367:126–133. doi: 10.1007/s00210-002-0666-3. [DOI] [PubMed] [Google Scholar]
- Chen N, Zhen J, Reith ME. Mutation of Trp84 and Asp313 of the dopamine transporter reveals similar mode of binding for GBR 12909 and benztropine as opposed to cocaine. J Neurochem. 2004;86:853–864. doi: 10.1111/j.1471-4159.2004.02386.x. [DOI] [PubMed] [Google Scholar]
- Chen F, Larsen MB, Neubauer HA, Sanchez C, Plenge P, Wiborg O. Characterization of an allosteric citalopram-binding at the serotonin transporter. J Neurochem. 2005;92:21–28. doi: 10.1111/j.1471-4159.2004.02835.x. [DOI] [PubMed] [Google Scholar]
- Ferris M, Frederick-Duus D, Fadel J, Mactutus CF, Booze RM. Altered striatal dopamine transmission in rats exposed to the HIV-1 Tat protein and cocaine; Poster presented at the Society of Neuroimmune Pharmacology Meeting; Salt Lake City, UT. Apr, 2007. [Google Scholar]
- Fiala M, Eshleman AJ, Cashman J, Lin J, Lossinsky AS, Suarez V, Yang W, Zhang J, Popik W, Singer E, Chiappelli F, Carro E, Weinand M, Witte M, Arthos J. Cocaine increases human immunodeficiency virus type 1 neuroinvasion through remodeling brain microvascular endothelial cells. J Neurovirol. 2005;11:281–291. doi: 10.1080/13550280590952835. [DOI] [PubMed] [Google Scholar]
- Goslin K, Asmussen H, Banker G, Goslin K, Banker G, editors. 2nd ed. MIT Press; Cambridge, MA: 1998. Culturing nerve cells. pp. 339–370. [Google Scholar]
- Gorentla BK, Vaughan RA. Differential effects of dopamine and psychoactive drugs on dopamine transporter phosphorylation and regulation. Neuroparmacol. 2005;49:759–768. doi: 10.1016/j.neuropharm.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Foster JD, Cervinski MA, Gorentla BK, Vaughan RA. Regulation of the dopamine transporter by phosphorylation. Handb Exp Pharmacol. 2006;175:197–214. doi: 10.1007/3-540-29784-7_10. [DOI] [PubMed] [Google Scholar]
- Jayanthi LD, Ramamoorthy S. Regulation of monoamine transporters: influence of psychostimulants and therapeutic antidepressants. The AAPS Journal. 2005;7:E728–E738. doi: 10.1208/aapsj070373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram SL, Prasad BM, Amara SG. Dopamine transporter-mediated conductances increase excitability of midbrain dopamine neurons. Nature Neurosci. 2002;5:971–978. doi: 10.1038/nn920. [DOI] [PubMed] [Google Scholar]
- Kendall SL, Anderson CF, Nath A, Turchan-Cholewo J, Land CL, Mactutus CF, Booze RM. Gonadal steroids differentially modulate neurotoxicity of HIV and cocaine: testosterone and ICI 182,780 sensitive mechanism. BMC Neurosci. 2005;6:40. doi: 10.1186/1471-2202-6-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koe BK, Lebel LA, Welch WM. [3H] sertraline binding to rat brain membranes. Psychopharmacol. 1990;100:470–476. doi: 10.1007/BF02243998. [DOI] [PubMed] [Google Scholar]
- Koutsiliery E, Sopper S, Scheller C, ter Meulen V, Riederer P. Involvement of dopamine in the progression of AIDS dementia complex. J. of Neural Transm. 2002;109:399–410. doi: 10.1007/s007020200032. [DOI] [PubMed] [Google Scholar]
- Manji H, Miller R. Neurology of HIV infection. J. Neurol. Neurosurg. Psychiatry. 2004;75:29–35. doi: 10.1136/jnnp.2003.034348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nath A, Anderson C, Jones M, Maragos W, Booze R, Mactutus C. Neurotoxicity and dysfunction of dopaminergic systems associated with AIDS dementia. Journal of Psychopharmacology. 2000;14:222–227. doi: 10.1177/026988110001400305. [DOI] [PubMed] [Google Scholar]
- Nath A, Hauser KF, Wojna V, Booze RM, Maragos W, Prendergast M, Cass W, Turchan JT. Molecular basis for interactions of HIV and drugs of abuse. J Acquir. Immune Defic. Syndr. 2002;31(Suppl 2):S62–S69. doi: 10.1097/00126334-200210012-00006. [DOI] [PubMed] [Google Scholar]
- Navia BA, Cho ES, Petito CK, Price RW. The AIDS dementia complex. II. Neuropathology. Ann. Neurol. 1986;19:525–535. doi: 10.1002/ana.410190603. [DOI] [PubMed] [Google Scholar]
- Ozdener H. Molecular mechanisms of HIV-1 associated neurodegeneration. J. Biosci. 2005;30:391–405. doi: 10.1007/BF02703676. [DOI] [PubMed] [Google Scholar]
- Prasad BM, Amara SG. The dopamine transporter in mesencephalic cultures is refractory to physiological changes in membrane voltage. J Neurosci. 2001;21:7561–7567. doi: 10.1523/JNEUROSCI.21-19-07561.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pristupa ZB, Wilson JM, Hoffman BJ, Kish SJ, Niznik HB. Pharmacological heterogeneity of the cloned and native human dopamine transporter: disassociation of [3H] WIN 35428 and [3H]GBR 12935 binding. Mol Pharmacol. 1993;46:125–135. [PubMed] [Google Scholar]
- Ravna AW, Sylte I, Dahl SG. Molecular model of the neural dopamine transporter. J. of Computer-Aided Molecular Design. 2003;17:367–382. doi: 10.1023/a:1026116017725. [DOI] [PubMed] [Google Scholar]
- Richfield EK. Quantitative autoradiography of the dopamine uptake complex in rat brain using [3H]GBR 12935: binding characteristics. Brain Res. 1991;540:1–13. doi: 10.1016/0006-8993(91)90486-f. [DOI] [PubMed] [Google Scholar]
- Rouge-Pont F, Abrous DN, Le Moal M, Piazza PV. Release of endogenous dopamine in cultured mesencephalic neurons: influence of dopaminergic agonists and glucocorticoid antagonists. Euro. J. of Neuroscience. 1999;11:2343–2350. doi: 10.1046/j.1460-9568.1999.00650.x. [DOI] [PubMed] [Google Scholar]
- Rudnick G. Mechanisms of biogenic amine neurotransmitter transporters. In: Reith MEA, editor. Neurotransmitter transporters: structure, function, and regulation. Humana Press; Totowa, NJ: 2002. pp. 25–52. [Google Scholar]
- Schroeter S, Apparsundaram S, Wiley RG, Miner LH, Sesack SR, Blakely RD. Immunolocalization of the cocaine- and antidepressant-sensitive I-norepinephrine transporter. J.Comp.Neurol. 2000;420:211–232. [PubMed] [Google Scholar]
- Self RL, Mulholland PJ, Nath A, Harris BR, Prendergast MA. The human immunodeficiency virus type-1 transcription factor Tat produces elevations in intracellular Ca2+ that require function of an N-methyl-D-aspartate receptor polyamine-sensitive site. Brain Res. 2004;995:39–45. doi: 10.1016/j.brainres.2003.09.052. [DOI] [PubMed] [Google Scholar]
- Singh S. Chemistry, design, and structure-activity relationship of cocaine antagonists. Chem Rev. 2000;100:925–1024. doi: 10.1021/cr9700538. [DOI] [PubMed] [Google Scholar]
- Silvers JM, Aksenova MV, Aksenov MY, Mactutus CF, Booze RM. Neurotoxicity of HIV-1 Tat protein: Involvement of D1 dopamine receptor. Neurotox. 2007 Jul 22; doi: 10.1016/j.neuro.2007.07.005. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvers JM, Aksenov MY, Aksenova MV, Beckley J, Olton P, Mactutus CF, Booze RM. Dopaminergic marker proteins in the substantia nigra of human immunodeficiency virus type 1-infected brains. J. Neurovirol. 2006;12:140–145. doi: 10.1080/13550280600724319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soucy J-P, Mrini A, Lafaille F, Doucet G, Descarries L. Comparative evaluation of [3H]WIN 35428 and [3H]GBR 12935 as markers of dopamine innervation density in brain. Synapse. 1997;25:163–175. doi: 10.1002/(SICI)1098-2396(199702)25:2<163::AID-SYN7>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Sulzer D, Galli A. Dopamine transport currents are promoted from curiosity to physiology. Trends Neurosci. 2003;26:173–176. doi: 10.1016/S0166-2236(03)00063-8. [DOI] [PubMed] [Google Scholar]
- Thumen A, Qadri F, Sarkar R, Moser A. GBR-12909 effect on dopamine outflow depends on phosphorylation in the caudate nucleus of the rat. Synapse. 2002;46:72–78. doi: 10.1002/syn.10124. [DOI] [PubMed] [Google Scholar]
- Turchan J, Anderson C, Hauser KF, Sun Q, Zhang J, Liu Y, Wise PM, Kruman II, Maragos W, Mattson MP, Booze R, Nath A. Estrogen protects against the synergistic toxicity by HIV proteins, methamphetamine and cocaine. BMC Neurosci. 2001;2:3. doi: 10.1186/1471-2202-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Zee P, Hespe W. Interaction between substituted 1-[2-(diphenylmethoxy)ethyl] piperazines and dopamine receptors. Neuropharm. 1985;24:1171–1174. doi: 10.1016/0028-3908(85)90150-9. [DOI] [PubMed] [Google Scholar]
- Valchar M, Hanbauer I. Rat mesencephalic neuronal cells cultured for different periods as a model of dopamine transporter ontogenesis. Mol Neurobiol. 1995;11:111–119. doi: 10.1007/BF02740689. [DOI] [PubMed] [Google Scholar]
- Vaughan RA, Agoston GE, Lever JR, Newman AH. Differential binding of tropane-based photoaffinity ligands on the dopamine transporter. J Neurosci. 1999;19:630–636. doi: 10.1523/JNEUROSCI.19-02-00630.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan RA, Sakrikar DS, Parnas ML, Adkins S, Foster JD, Duval RA, Lever JR, Kulkarni SS, Hauck-Newman A. Localization of cocaine analog [125I]RTI 82 irreversible binding to transmembrane domain 6 of the dopamine transporter. J Biol Chem. 2007;282:8915–8925. doi: 10.1074/jbc.M610633200. [DOI] [PubMed] [Google Scholar]
- Wallace DR, Dodson S, Nath A, Booze RM. Estrogen attenuates gp120- and tat (1-72)-induced oxidative stress and prevents loss of dopamine transporter function. Synapse. 2005;59:51–60. doi: 10.1002/syn.20214. [DOI] [PubMed] [Google Scholar]
- Wang J-G, Chang L, Volkow N, Telang F, Logan J, Ernst T, et al. Decreased brain dopaminergic transporters in HIV-associated dementia patients. Brain. 2004;127:2452–2458. doi: 10.1093/brain/awh269. [DOI] [PubMed] [Google Scholar]
- Wilson JM, Nobrega JN, Carroll ME, Niznik HB, Shannak K, Lac ST, Pristupa Z, Dixon LM, Kish SJ. Heterogeneous subregional binding patterns of 3H-WIN 35428 and 3H-GBR 12935 are differentially regulated by chronic cocaine self-administration. J Neurosci. 1994;14:2966–2979. doi: 10.1523/JNEUROSCI.14-05-02966.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C, Coffey LL, Reith ME. Translocation of dopamine and binding of 2 beta-carbomethoxy-3 beta-(4-fluorophenyl) tropane (WIN 35,428) measured under identical conditions in rat striatal synaptosomal preparations. Inhibition by various blockers. Biomed. Pharmacol. 1995;49:339–50. doi: 10.1016/0006-2952(94)00485-5. [DOI] [PubMed] [Google Scholar]